External Quality Assessment (EQA) scheme for serological diagnostic test for SARS-CoV-2 detection in Sicily Region (Italy), in the period 2020–2022
-
Francesca Di Gaudio
, Giuseppina Brunacci
, Vita Giaccone
, Sonya Vasto
Abstract
Objectives
Since December 2019, worldwide public health has been exposed to a severe acute respiratory syndrome caused by Coronavirus-2. Serological testing is necessary for retrospective assessment of seroprevalence rates, and the determination of vaccine response and duration of immunity. For this reason, it was necessary to introduce a panel of tests able to identify and quantify Covid-19 antibodies.
Methods
As a Regional Reference Centre, the CRQ Laboratory (Regional Laboratory for the Quality Control) developed and conducted an External Quality Assessment (EQA) panel of assays, to evaluate the quality of various methods, that were used by 288 Sicilian laboratories, previously authorized on behalf of the Public Health Service.
Results
The performance test was based on pooled samples with different levels of concentration of antibodies. 97 , 98, and 95 % of the participating laboratories tested all samples correctly in 2020, 2021, and 2022 respectively. The best performance was observed in the test of total Ig. The general performance of laboratories improved over the years.
Conclusions
The incorrect diagnosis had and could still have important implications on vaccination cycles. Only through the effort of laboratory professionals, and the extension of the EQA scheme, a better harmonization of methods, protocols, and thus results, to guarantee a better healthcare system, will be possible.
Introduction
The COVID-19 pandemic situation of the last three years had a strong impact on laboratories’ management. The high demand for diagnostic tests determined a growing and initially uncontrolled employment of novel diagnostic assays. Different tests, with different targets, are available for diverse diagnostic purposes. In particular, among the applied methods, reverse transcription-PCR (RT-PCR), which amplifies and detects viral genome, is the primary technique for the diagnosis of acute infection [1, 2]. Serological testing, which identifies specific human antibodies, is instead recommended for retrospective assessment of seroprevalence rates for the determination of vaccine or infection response and duration of immunity and gives complementary information to the RT-PCR assay. In fact, serological assays are able to detect various types of antibodies, including IgM, IgG, IgA, total antibodies, and antibodies targeting specific components of the SARS-CoV-2 virus, such as the nucleocapsid protein, the spike protein, and the receptor binding domain (RBD) [3]. Common COVID-19 antibodies identification and quantification methods in human serum or plasma are enzyme-linked immunosorbent assays (ELISAs), chemiluminescent immunoassays (CLIAs), or chemiluminescent microparticle assays (CMIAs). The assay design variables show that large differences in test results and interpretations are likely between clinical laboratories [4, 5].
For this reason, laboratory professionals, in compliance with the requirement of ISO 15189:2012, have implemented quality assurance procedures regarding validation and verification of the performance, the definition of the criteria for the results’ interpretation, and monitoring of the test performance [6]. In this context, considering the extent of the diagnostic assays, the External Quality Assessment (EQA) became crucial in ensuring the accuracy and high quality of the diagnostic procedures to identify SARS-CoV-2 antibodies [7], [8], [9], [10], [11]. All participants in an EQA program blindly analyze the furnished samples and report their test results to an approved and accredited provider that, in case of acceptable results, certifies the laboratories’ competence. The aim of the EQA is to acknowledge the existing serological diagnostic tests and performance and to assess standard criteria and recommendations in order to improve the laboratories’ executions.
In the context of anti-SARS CoV-2 serological proficiency testing, the CRQ (Centro Regionale Qualità dei laboratori) was an Institutional Public Provider for EQA, accredited and authorized by the Regional Health Service [12]. In this report, the outcome of the three rounds per year EQA conducted in Sicily and the small surrounding islands during 2020, 2021, and 2022 is presented.
Materials and methods
EQA design
The Sicilian EQA system provides mandatory participation in the SARS-CoV-2 EQA for every public and private laboratory that intends to perform SARS-CoV-2 serological tests on patients. The EQA scheme COVS432 has been realized in the period 2020–2022, with three EQA rounds per year. The laboratories that participated in the EQA scheme COVS432 (Serologic SARS-CoV-2) in 2020–2022 were 288. 212, 194, and 209 laboratories participated in 2020, 2021, and 2022 respectively. 115 were the laboratories that took part in the EQA in all three years. The laboratories obtaining the acceptability were then authorized to perform the analysis for the Regional Health System and for COVID-19 antibodies’ profiling. The authorization was released from the Regional Government Office after a specific and public selection based on particular, structural, technological, and professional features and confirmed by the EQA results. Thus, the laboratories must have been registered at CRQ and be conformed to the quality control standards. Our research contributes to the ongoing monitoring of laboratories performance and did not involve any form of human experimentation. No human subjects were involved in our study, and thus the Ethics Committee approval was not required.
Participants
Overall, the laboratories that participated in the EQA scheme COVS432 in 2020–2022 were 288 (31 public and 257 private). 213 (24 public and 189 private), 194 (20 public and 174 private), and 209 (17 public and 192 private) laboratories participated in 2020, 2021, and 2022 respectively. Among the laboratories, there were clinical analysis centers, nursing homes, hospital units, and pharmacies. The entire process from registration and anagraphic data collection, to the evaluation of the results, was handled with an in-house platform.
Preparation of simulated samples
The SARS-CoV-2 EQA scheme COVS432 is intended for serological tests on IgG, IgM, and IgA SARS-CoV-2 antibodies. Within the period, new antibodies targeting specific components of the SARS-CoV-2 virus, such as the nucleocapsid protein (IgG nucleocapsid and Ig tot nucleocapsid), the spike protein (IgG S1), and the receptor binding domain (IgG S-RBD and IG tot S-RBD), were added to the panel of the searched antibodies. Every sample to analyze was constituted of two aliquots of 0.3 mL human serum samples (A and B). The stock blood samples of SARS-CoV-2 positive and negative patients were obtained thanks to a vaccination campaign during the COVID-19 pandemic. Three different pools of serum at three levels of concentration (high, low, and negative) of RBD were prepared from the stocked material (stored at −20 °C). Aliquots 1(A) and 1(B) were realized by mixing different ratios of the three pools in order to obtain different concentrations of each antibody. The aliquots were analyzed in triplicate to determine the type and concentration of Ig, with Shenzhen New Industries Maglumi 2000 and Bio-Rad BioPlex 2200 System. The stability of the samples was checked by measuring the antibodies concentration over three months, at the moment of the pool preparation, immediately before the beginning of the VEQ scheme, and after the VEQ scheme. The homogeneity of the samples, and commutability aspects were assessed according to the ISO 17043 guidelines. Blood collection, processing, pooling, aliquoting, capping, freezing, and storage of the serum pools were completed as quickly as possible to ensure the quality of the final product. Pools were prepared from many single donations to reduce the eventual influence of individual samples specific effects.
Data collection and statistical analysis
The CRQ’s EQA Platform engine performs statistical analysis, evaluation of the participants, and reporting. The evaluation takes into consideration the overall entered results of each sample and each antibody against target results, and it considers potential sample warnings. The acceptability of the results is based on the peer group consensus value (“trimmed mean value”) derived from all results submitted by participants in the scheme for that analyte. This approach was performed only for groups of at least 10 results. The results for groups of less than 10, were not evaluated. The mean and the standard deviation (SD) for each group were calculated, the results outside the range mean±3SD were excluded and the values recalculated. Accepted results were in the range of mean±2SD, being classified as “optimum” values in the range±0.5SD, values presenting a bias between 0.5 and 1 SD from the mean were classified as “good”, and presenting a bias between 1 and 2 SD from the mean were classified as “sufficient”. Uploaded instrument reports are also evaluated. The produced reports contain information about participants, quality tests, results, targets, and performance evaluation. In the present work, all the results ranging from sufficient to optimum are indicated as acceptable.
Results
Participants
In total, 80 laboratories participated in only one year, 93 participated in the program in two years, and 115 laboratories took part in the EQA in all three years. Participants indicated the use of a variety of methods, including chromatography, colorimetric, and immune-radiometric methods. However, chemiluminescent and immuno-enzymatic, followed by fluorimetric assays were predominant in the three years.
Overall SARS-CoV-2 serological test performance
The blind work setting was necessary to avoid false laboratory results, and thus false reports by the participating authorized laboratories. In 2020, 205 laboratories out of 212 (97 %) received a Positive Performance evaluation (Figure 2A) for the CRQ’s EQA scheme COVS432 (252 samples out of 287 processed (88 %) received an acceptable evaluation). In 2021, 191 laboratories out of 194 (98 %) received a Positive Performance evaluation for the CRQ’s EQA scheme COVS432 (1,329 samples out of 1,536 processed (87 %) received an acceptable evaluation). In 2022, 198 laboratories out of 209 (95 %) received a Positive Performance evaluation for the CRQ’s EQA scheme COVS432 (2107 samples out of 2367 processed (89 %) received an acceptable evaluation). Details about performance per year and analyte are in Figure 1. Overall, 40 % of the laboratories that were evaluated every year improved their performance over time or maintained the 100 % of acceptance (the mean of improvement was around 27 % in 2021 and around 6 % of 9 in 2022), 34 % of laboratories obtained lower acceptability in 2021 compared to 2020, but then obtained a better result or maintained the 100 % of acceptance in 2022 (the mean of worsening was around −30 % in 2021 and an improvement of around 29 % with a in 2022), in 24 % of the cases the opposite was observed (the mean of improvement was around 28 % in 2021 and a worsening of around 28 % with a in 2022), and 11 % of the laboratories worsened over time (the mean of worsening was around −13 % with a in 2021 and of around 25 % with a in 2022, Figure 2). A total rate of 0.96 for reported results/expected results was achieved, and 0.56 for evaluated results/expected results. The rate of evaluation was mainly linked to the impossibility of reaching the consensus level for certain groups. In the present study, only evaluated results are reported and discussed.
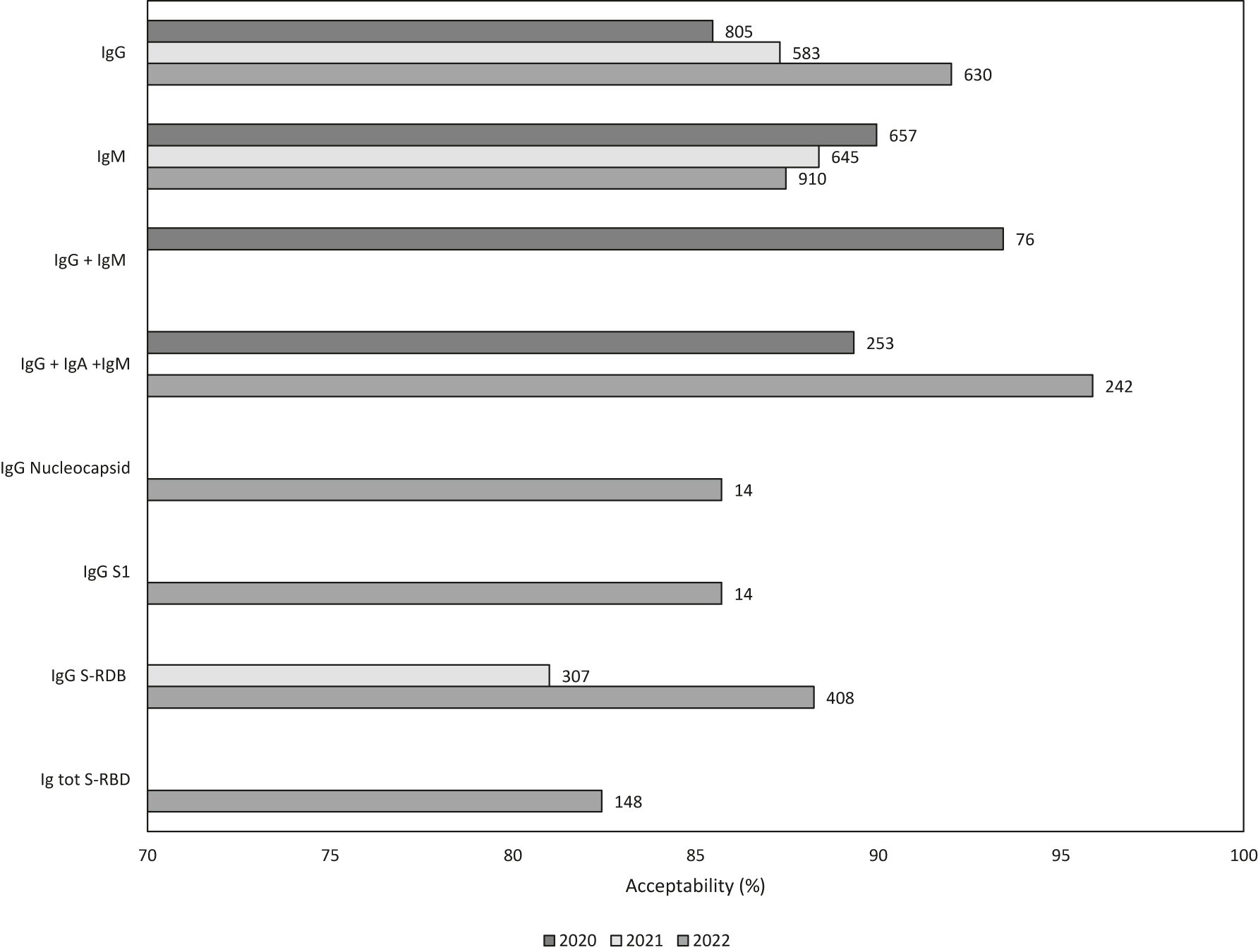
Histogram of the acceptability (%) per analyte and per year. At the top of the bars, the total number of samples is indicated.

Pie chart of the laboratories’ acceptability trend in the period 2020–2022. +/+ indicates that the acceptability was higher in 2021 compared to 2020 and in 2022 compared to 2021 or that the laboratory maintained 100 % acceptance; +/− indicates that the acceptability was higher or that the laboratory maintained 100 % acceptance in 2021 compared to 2020 and lower in 2022 compared to 2021; −/+ indicates that the acceptability was lower in 2021 compared to 2020 and higher in 2022 or that the laboratory maintained 100 % acceptance compared to 2021; −/− indicates that the acceptability was lower in 2021 compared to 2020 and in 2022 compared to 2021. The number in the chart indicates the number of laboratories for each group.
Reagents’ kits test performance
A total of 28 different reagent kits, grouped for manufacturers, were used; of them, 20 were used in 2020, 7 in 2021, and 17 in 2022 (Figure 3). The six kits used in all three years were from Abbott Diagnostics, BioMerieux Inc., DiaSorin S.p.A., Diesse Diagnostica Senese Spa, Roche Diagnostics, Shenzhen New Industries Biomedical Engineering Co, while 10, 1, and 4 kits were exclusively used, respectively, in 2020, 2021 and 2022. Four kits were used in 2020 and 2022, but not in 2021 (DEMEDITEC Diagnostics GmbH, Pantec Srl, Siemens (Siemens Healthcare), Zhejiang Orient Gene Biotech Co., Ltd.).

Venn diagram of the reactive kits used in 2020, 2021, and 2022.
The identification of the total Ig (IgG+IgA+IgM) was mainly performed with Elecsys Anti-SARS-CoV-2 kits, Roche Diagnostics. The identification of IgG was mainly performed with Abbott Diagnostics (SARS-CoV-2 IgG and SARS-CoV-2 IgG II Quant kit), BioMerieux Inc. (VIDAS SARS-CoV-2 IgG kits), Diesse Diagnostica Senese Spa (Chorus SARS-CoV-2 IgG and Enzy-well SARS-CoV-2 IgG kits), and Shenzhen New Industries Biomedical Engineering Co. (Maglumi 2019-nCoV IgG), even if the laboratories considerably reduced the use of this last kit in 2022.
Anti-SARS-COV-2 IgG+IgM analysis was mainly performed with Elecsys Anti-SARS-CoV-2 kit, Roche Diagnostics. Abbott Diagnostic (SARS-CoV-2 IgM kit), BioMerieux Inc. (VIDAS Sars-Cov-2 IgM (9COM)), Diesse Diagnostica (CHORUS SARS-CoV-2 IgM Diesse ENZYWELL SARS-CoV-2 IgM ENZY-WELL SARS-CoV-2 IgM), Shenzhen New Industries Biomedical Engineering Co. (MAGLUMI 2019-nCoV IgM) were the main kits for IgM identification and quantification. Shenzhen New Industries Biomedical Engineering Co. was also the main producer of anti-SARS-COV-2 neutralizing IgG S-RBD extraction kits MAGLUMI SARS-CoV-2 S-RBD IgG (CLIA) and MAGLUMI SARS-CoV-2 S-RBD IgG II (CLIA). Anti-SARS-COV-2 neutralizing S-RBD total Ig tests were performed only in 2022 by Diesse Diagnostica Senese Spa (CHORUS SARS-CoV-2 ‚NEUTRALIZING Ab) and Roche Diagnostics (Elecsys Anti-SARS-CoV-2 S). Further details about acceptability % are reported in Table 1.
List of the most utilized reagents and kits for each assay during the period 2020–2022.
| Assay | 2020 | 2021 | 2022 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Samples, no | Acceptability, % | Samples, no | Acceptability, % | Samples, no | Acceptability, % | Samples, no | Acceptability, % | |
| IgG | 805 | 0 | 583 | 0 | 630 | 91 | 2,018 | 88 |
|
|
||||||||
| BioMerieux Inc. | 188 | 87 | 260 | 88 | 386 | 92 | 834 | 89 |
| Abbott Diagnostics | 239 | 84 | 114 | 85 | 134 | 99 | 487 | 88 |
| Shenzhen New Industries Biomedical Engineering Co. | 243 | 93 | 109 | 82 | 12 | 100 | 364 | 90 |
| Diesse Diagnostica Senese Spa | 82 | 93 | 82 | 93 | 78 | 82 | 242 | 89 |
| DiaSorin S.p.A. | 18 | 6 | 12 | 100 | 30 | 43 | ||
| Immunostics Inc. | 12 | 83 | 12 | 83 | ||||
| NovaTec Immundiagnostica GmbH | 10 | 40 | 10 | 40 | ||||
| Beckman Coulter (Immunotech Products) | 6 | 100 | 6 | 100 | ||||
| DEMEDITEC Diagnostics GmbH | 2 | 50 | 4 | 50 | 6 | 50 | ||
| Zhejiang Orient Gene Biotech Co., Ltd. | 6 | 33 | 6 | 33 | ||||
| Guangzhou Wondfo Biotech Co., Ltd | 4 | 100 | 4 | 100 | ||||
| Siemens (Siemens Healthcare) | 4 | 50 | 4 | 50 | ||||
| Beckman Coulter (Beckman Products) | 2 | 0 | 2 | 0 | ||||
| Immunospark | 2 | 50 | 2 | 50 | ||||
| Ortho Clinical Diagnostics | 2 | 100 | 2 | 100 | ||||
| Pantec Srl | 2 | 100 | 2 | 100 | ||||
| Sentinel Diagnostics | 2 | 0 | 2 | 0 | ||||
| Shanghai Kehua Bio-Engineering Co. Ltd | 2 | 100 | 2 | 100 | ||||
| Beijing Lepu Medical Technology Co., Ltd | 1 | 0 | 1 | 0 | ||||
| Zhejiang Orient Gene Biotech Co., Ltd. | 6 | 83 | 6 | 83 | ||||
|
|
||||||||
| IgM | 657 | 90 | 645 | 88 | 910 | 89 | 2,212 | 89 |
|
|
||||||||
| Shenzhen New Industries Biomedical Engineering Co. | 254 | 91 | 221 | 87 | 296 | 86 | 771 | 88 |
| BioMerieux Inc. | 182 | 90 | 209 | 86 | 216 | 82 | 607 | 86 |
| Abbott Diagnostics | 108 | 90 | 138 | 92 | 242 | 92 | 488 | 91 |
| Diesse Diagnostica Senese Spa | 73 | 97 | 77 | 91 | 128 | 91 | 278 | 92 |
| Immunostics Inc. | 16 | 88 | 16 | 88 | ||||
| NovaTec Immundiagnostica GmbH | 10 | 70 | 10 | 70 | ||||
| AESKU.DIAGNOSTICS | 8 | 100 | 8 | 100 | ||||
| DEMEDITEC Diagnostics GmbH | 2 | 0 | 4 | 100 | 6 | 67 | ||
| Pantec Srl | 2 | 0 | 4 | 0 | 6 | 0 | ||
| Citest Diagnostics Inc. | 4 | 100 | 4 | 100 | ||||
| DiaSorin S.p.A. | 4 | 100 | 4 | 100 | ||||
| Pishtaz Teb Zaman Diagnostics | 4 | 50 | 4 | 50 | ||||
| Zhejiang Orient Gene Biotech Co., Ltd. | 4 | 100 | 4 | 100 | ||||
| Immunospark | 2 | 0 | 2 | 0 | ||||
| Sentinel Diagnostics | 2 | 100 | 2 | 100 | ||||
| Shanghai Kehua Bio-Engineering Co. Ltd | 2 | 2 | 100 | |||||
|
|
||||||||
| IgG+IgM | 76 | 93 | 76 | 93 | ||||
|
|
||||||||
| DASIT | 6 | 83 | 6 | 83 | ||||
| Roche Diagnostics | 57 | 95 | 57 | 95 | ||||
| Screen Italia | 4 | 100 | 4 | 100 | ||||
| Siemens (Siemens Healthcare) | 3 | 100 | 3 | 100 | ||||
|
|
||||||||
| IgG+IgA+IgM | 253 | 89 | 242 | 96 | 495 | 93 | ||
|
|
||||||||
| Ortho Clinical Diagnostics | 6 | 0 | 6 | 0 | ||||
| Roche Diagnostics | 247 | 91 | 242 | 96 | 489 | 94 | ||
|
|
||||||||
| IgG nucleocapsid | 14 | 86 | 14 | 86 | ||||
|
|
||||||||
| Bio-Rad Laboratories | 14 | 86 | 14 | 86 | ||||
|
|
||||||||
| IgG S1 | 14 | 86 | 14 | 86 | ||||
|
|
||||||||
| Bio-Rad Laboratories | 14 | 86 | 14 | 86 | ||||
|
|
||||||||
| IgG S-RBD | 307 | 82 | 408 | 88 | 715 | 85 | ||
|
|
||||||||
| Shenzhen New Industries Biomedical Engineering Co. | 156 | 79 | 226 | 93 | 382 | 87 | ||
| Roche Diagnostics | 90 | 80 | 66 | 88 | 156 | 83 | ||
| Abbott Diagnostics | 46 | 89 | 96 | 77 | 142 | 81 | ||
| Diesse Diagnostica Senese Spa | 13 | 92 | 13 | 92 | ||||
| Bio-Rad Laboratories | 8 | 100 | 8 | 100 | ||||
| DiaSorin S.p.A. | 6 | 100 | 6 | 100 | ||||
| DRG International, Inc. | 4 | 50 | 4 | 50 | ||||
| Beckman Coulter (Immunotech Products) | 2 | 100 | 2 | 100 | ||||
| Fujirebio Inc. | 5 | 50 | 2 | 50 | ||||
|
|
||||||||
| Ig tot S-RBD | 148 | 82 | 148 | 82 | ||||
|
|
||||||||
| Roche Diagnostics | 100 | 82 | 100 | 82 | ||||
| Diesse Diagnostica Senese Spa | 48 | 83 | 48 | 83 | ||||
|
|
||||||||
| Total | 1,791 | 1,535 | 2,366 | 5,692 | ||||
Discussion
The level of SARS-CoV-2 antibodies was and is still largely employed for the detection of late infection, monitoring of the immune response, and vaccine clinical trials. Considering the variety of available kits and instrumentation on the market, the existence of a quality assurance tool, such as the EQA scheme, is crucial. Hereby, the EQA scheme for the SARS-CoV-2 serological test assigned to Sicilian clinical laboratories in 2020, 2021, and 2022 is reported. 288 laboratories (31 public and 257 private), previously authorized by the Regional Government to perform COVID-19 serological tests, were involved. During the considered period, the SARS-CoV-2 serological test maintained its importance for COVID-19 diagnosis and vaccine response, and thus the laboratories’ participation in the EQA scheme continued to be constant over time. More, new analytes were introduced in 2021 (IgG S-RBD) and 2022 (IgG nucleocapsid, IgG S1, and Ig tot S-RBD) to better characterize individual immune responses. Overall, 97 %, 98 %, and 95 % of the participant laboratories received a positive performance evaluation in 2020, 2021, and 2022 respectively. The most used method was chemiluminescent assay (65 % of the total in 2020, 55 % in 2021, and 54 % in 2022). The performance on the analysis of total Ig and IgG improved over time. In particular, the total Ig analysis in 2022 obtained the absolute highest score of acceptability (95.87 %). On the contrary, the performance on the analysis of IgM decreased over time (89.95 %, 88.37 %, and 87.47 % in 2020, 2021, and 2022 respectively). Generally, the results on new targeted analytes (antibodies S-RBD, S1, and nucleocapsid) presented a lower score of acceptability.
The most used kits for the analysis of IgG were: Abbot, BioMerieux, Shenzhen, and Diesse. The first two kits improved their performance over time, but while the use of the first one was reduced, the use of the second one increased. The other two kits worsened their performance, with the Shenzhen kit significantly reducing their diffusion among laboratories in 2022. Shenzhen and Biomerieux were among the most used kits also for the analysis of IgM, with a reduction in performance over time. Abbot confirmed the high diffusion and performance, both improved over time, with the IgM kit. Diesse IgM kit presented a high level of acceptable results and improved its distribution among laboratories. Kits identifying both IgG and IgM were evaluated only in 2020, with Roche being the most diffused and with good performance. The most common kits for IgG S-RBD were Shenzhen, Roche, and Abbot, with the first two improving their performance and the last one significantly reducing its performance, while contemporarily doubling the number of laboratories using it. The evaluation of the analysis of Ig tot S-RBD was performed only during 2022, and it involved only Roche and Diesse kits, with modest results.
Considering the global evaluation of the laboratories, 70 % of the participant laboratories improved their performance in the last year (40 % performed better in 2021 compared to 2020, while 30 % reduced their performance in 2021 and then improved in 2022), while 30 % of the laboratories reduced their performance in 2022 or both in 2021 and 2022. This result could be linked to the lowest acceptability % on the analysis of the new analytes. The monitoring of performances, especially when dealing with new analytes and methods, is crucial in the healthcare system, highlighting once more the importance of the EQA schemes. The outcomes of the EQA, in fact, are fundamental for the improvement process of the laboratories’ performance. Furthermore, the results determine the confirmation of the laboratories’ authorization to continue their activities for the diagnosis and certification, incentivizing the laboratories in their amelioration process. The incorrect diagnosis had and could still have important implications on vaccination cycles and the now-expired green pass. Through our EQA, we were able to detect instances of non-compliance that were previously overlooked by standard health governance procedures. The participation and the positive performance of EQA schemes are required for the accreditation of laboratories, together with various guidelines and international technical standards of the sector (ISO/IEC 17025, ISO 15189, Joint Commission, etc.) [13, 14].
Even if it has to be ascribed to the amelioration process, this study presents some limitations that need to be considered. The comparison of the results over the years is affected by the variability of parameters evaluated during time (f.e. IgG+IgM and IgG+IgM+IgA were not evaluated in 2021, and nucleocapsid and RBD component quantification was evaluated only in 2022). In addition, the methods used by the laboratories were not standardized, thus a consensus level was not always reached and an evaluation of the results was not always possible. However, one of the aims of an EQA scheme is to identify the best protocols and standardize the processes. Thus, these limitations can be overcome by continuing to perform this kind of scheme.
The results of this study showed that EQA schemes should be adopted as a recognized standard for authorizing and accrediting healthcare services, as well as a means of continuously verifying compliance with necessary requirements. Only through the effort of laboratory professionals, and the extension of the EQA scheme, a better harmonization of methods, protocols, and thus results, to guarantee a better healthcare system, will be possible [15]. This approach would ensure the provision of patient-centered care and support the objectives of healthcare institutions.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Younes, N, Al-Sadeq, DW, Al-Jighefee, H, Younes, S, Al-Jamal, O, Daas, HI, et al.. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Clin Chem Lab Med 2020;59:582. https://doi.org/10.3390/v12060582.Search in Google Scholar PubMed PubMed Central
2. Mancini, F, Barbanti, F, Scaturro, M, Errico, G, Iacobino, A, Bella, A, et al.. Laboratory management for SARS-CoV-2 detection: a user-friendly combination of the heat treatment approach and rt-real-time PCR testing. Emerg Microbes Infect 2020;9:1393–6. https://doi.org/10.1080%2F22221751.2020.1775500.10.1080/22221751.2020.1775500Search in Google Scholar PubMed PubMed Central
3. Theel, ES, Slev, P, Wheeler, S, Couturier, MR, Wong, SJ, Kadkhoda, K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol 2020;58:e00797-20. https://doi.org/10.1128/JCM.00797-20.Search in Google Scholar
4. Yang, Z, Wu, J, Ye, F, Zhu, B, Guan, W, Huang, J, et al.. Expert consensus-based laboratory testing of SARS-CoV-2. J Thorac Dis 2020;12:4378. https://doi.org/10.1128/jcm.00797-20.Search in Google Scholar
5. Plebani, M, Padoan, A, Negrini, D, Carpinteri, B, Sciacovelli, L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin Chim Acta 2020;509:1–7. https://doi.org/10.1016/j.cca.2020.05.050.Search in Google Scholar PubMed PubMed Central
6. Yanikkaya-Demirel, G. ISO 15189 accreditation: requirements for quality and competence of medical laboratories, experience of a laboratory II. Clin Biochem 2009;42:279–83. https://doi.org/10.1016/j.clinbiochem.2008.09.099.Search in Google Scholar PubMed
7. Sciacovelli, L, Padoan, A, Secchiero, S, Plebani, M. Serological diagnostic for SARS-CoV-2: an experimental external quality assessment scheme. Clin Chem Lab Med 2021;59:1878–84. https://doi.org/10.1515/cclm-2021-0662.Search in Google Scholar PubMed
8. Haselmann, V, Özçürümez, MK, Klawonn, F, Ast, V, Gerhards, C, Eichner, R, et al.. Results of the first pilot external quality assessment (EQA) scheme for anti-SARS-CoV2-antibody testing. Clin Chem Lab Med 2020;58:2121–30. https://doi.org/10.1515/cclm-2020-1183.Search in Google Scholar PubMed
9. Moerman, A, Vernelen, K, China, B, Capron, A, Van Den Bossche, D, Mariën, J, et al.. Importance of anti-SARS-CoV-2 assay antigenic composition as revealed by the results of the Belgian external quality assessment (EQA) scheme. Diagn Microbiol Infect Dis 2022;102:115561. https://doi.org/10.1016/j.diagmicrobio.2021.115561.Search in Google Scholar PubMed PubMed Central
10. Pasotti, F, Pellegrinelli, L, Liga, G, Rizzetto, M, Azzarà, G, Da Molin, S, et al.. First results of an external quality assessment (EQA) scheme for molecular, serological and antigenic diagnostic test for SARS-CoV-2 detection in Lombardy region (northern Italy), 2020–2022. Diagnostics 2022;12:1483. https://doi.org/10.3390/diagnostics12061483.Search in Google Scholar PubMed PubMed Central
11. Kittel, M, Eichner, R, Aida, S, Bode, A, Ast, V, Kessler, A, et al.. Results of a European-wide External Quality Assessment (EQA) scheme for serological detection of Anti-SARS-CoV-2 (CoVimm) – pitfalls of routine application. Viruses 2022;14:1662. https://doi.org/10.3390/v14081662.Search in Google Scholar PubMed PubMed Central
12. Di Gaudio, F, Brunacci, G, Contino, F, Gallo, A, Centineo, F. Technical and health governance aspects of the External Quality Assessment Scheme for the SARS-CoV-2 molecular tests: Institutional experience performed in all clinical laboratories of a Regional Health Service. Clin Chem Lab Med 2023;61:173–9. https://doi.org/10.1515/cclm-2022-0780.Search in Google Scholar PubMed
13. ISO/IEC 17025. General requirements for the competence of testing and calibration laboratories. Geneva: ISO; 2017.Search in Google Scholar
14. ISO 15189. Medical laboratories – requirements for quality and competence. Geneva: ISO; 2012.Search in Google Scholar
15. Plebani, M. Harmonization in laboratory medicine: more than clinical chemistry? Clin Chem Lab Med 2018;56:1579–86. https://doi.org/10.1515/cclm-2017-0865.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Should APTT become part of thrombophilia screening?
- Review
- n-3 fatty acids and the risk of atrial fibrillation, review
- Guidelines and Recommendations
- Root cause analysis of cases involving diagnosis
- Opinion Papers
- What is diagnostic safety? A review of safety science paradigms and rethinking paths to improving diagnosis
- Interprofessional clinical reasoning education
- Original Articles
- Quality of heart failure registration in primary care: observations from 1 million electronic health records in the Amsterdam Metropolitan Area
- Typology of solutions addressing diagnostic disparities: gaps and opportunities
- Diagnostic errors and characteristics of patients seen at a general internal medicine outpatient clinic with a referral for diagnosis
- Cost-benefit considerations of the biased diagnostician
- Delayed diagnosis of new onset pediatric diabetes leading to diabetic ketoacidosis: a retrospective cohort study
- Monocyte distribution width (MDW) kinetic for monitoring sepsis in intensive care unit
- Are shortened aPTT values always to be attributed only to preanalytical problems?
- External Quality Assessment (EQA) scheme for serological diagnostic test for SARS-CoV-2 detection in Sicily Region (Italy), in the period 2020–2022
- Recent mortality rates due to complications of medical and surgical care in the US
- Short Communication
- The potential, limitations, and future of diagnostics enhanced by generative artificial intelligence
- Case Report – Lessons in Clinical Reasoning
- Lessons in clinical reasoning – pitfalls, myths, and pearls: a case of persistent dysphagia and patient partnership
- Letters to the Editor
- The ‘curse of knowledge’: when medical expertise can sometimes be a liability
- A new approach for identifying innate immune defects
Articles in the same Issue
- Frontmatter
- Editorial
- Should APTT become part of thrombophilia screening?
- Review
- n-3 fatty acids and the risk of atrial fibrillation, review
- Guidelines and Recommendations
- Root cause analysis of cases involving diagnosis
- Opinion Papers
- What is diagnostic safety? A review of safety science paradigms and rethinking paths to improving diagnosis
- Interprofessional clinical reasoning education
- Original Articles
- Quality of heart failure registration in primary care: observations from 1 million electronic health records in the Amsterdam Metropolitan Area
- Typology of solutions addressing diagnostic disparities: gaps and opportunities
- Diagnostic errors and characteristics of patients seen at a general internal medicine outpatient clinic with a referral for diagnosis
- Cost-benefit considerations of the biased diagnostician
- Delayed diagnosis of new onset pediatric diabetes leading to diabetic ketoacidosis: a retrospective cohort study
- Monocyte distribution width (MDW) kinetic for monitoring sepsis in intensive care unit
- Are shortened aPTT values always to be attributed only to preanalytical problems?
- External Quality Assessment (EQA) scheme for serological diagnostic test for SARS-CoV-2 detection in Sicily Region (Italy), in the period 2020–2022
- Recent mortality rates due to complications of medical and surgical care in the US
- Short Communication
- The potential, limitations, and future of diagnostics enhanced by generative artificial intelligence
- Case Report – Lessons in Clinical Reasoning
- Lessons in clinical reasoning – pitfalls, myths, and pearls: a case of persistent dysphagia and patient partnership
- Letters to the Editor
- The ‘curse of knowledge’: when medical expertise can sometimes be a liability
- A new approach for identifying innate immune defects

