Status of the implementation of pharmacogenetics in clinical practice in Spain: from regional to national initiatives
-
Maria Apellaniz-Ruiz
, Jordi Barrachina
, Rocio Nunez-Torres
and On behalf of the Junior Group of the Spanish Society of Pharmacogenetics and Pharmacogenomics
Abstract
Introduction
Pharmacogenetics (PGx) has the potential to improve patient care, allowing to transform medical interventions by providing personalized therapeutic strategies. Scientific evidence supports the use of PGx in clinical practice and international organizations are developing clinical guidelines to facilitate the utilization of PGx testing. However, clinical implementation of PGx is limited and unequal worldwide.
Content
This review summarizes regional and national Spanish initiatives to implement PGx in the clinical practice.
Summary and Outlook
Diverse strategies to implement PGx in healthcare are applied across countries or even in the different regions of a specific country. Such was the case of Spain, a European country with 17 Autonomous Regions and two Autonomous Cities, each one with capacity to manage their own healthcare systems. Nevertheless, during the past years, many initiatives and strategies have been launched in Spain to develop different aspects of PGx. Importantly, the National Healthcare System has approved a PGx testing catalogue. This review highlights the crucial work and efforts of scientific societies (like the Spanish Society of Pharmacogenetics and Pharmacogenomics), of experts in PGx, of healthcare providers and of governmental parties in the implementation of PGx to personalize patient therapy, focused in Spain.
Introduction
Pharmacogenetics (PGx) has the potential to immediately impact the care of patients in a clinically meaningful manner. The scientific evidence supporting the use of PGx in clinical practice is high and there is an important demand for its clinical implementation [1]. Personalised Medicine (PM), based on comprehensive genomic and diagnostic characterization, has the potential to transform medical interventions by providing effective and tailored therapeutic strategies, and it has become a real need [2]. Unfortunately, during the past decades, the implementation of PGx in clinical practice has been limited due to the lack of consistency in clinical recommendations. To bridge the knowledge gap in PGx, several organizations have developed valuable information under the form of clinical guidelines, including the Clinical Pharmacogenetics Implementation Consortium (CPIC) [3], the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) [4], the Dutch Pharmacogenetics Working Group (DPWG) [5] and the French National Network of Pharmacogenetics (RNPGx) [6]. Nevertheless, implementation of PGx is unequal worldwide, with different strategies applied across the countries or even in the different regions of a specific country. Spain, with 17 Autonomous Regions and two Autonomous Cities, each one with capacity to manage their own healthcare systems, was an example of the latter. Although some regions started PGx initiatives years ago, it has been recently when many strategies have been launched to develop different aspects regarding PGx implementation in the clinical practice across the country. Importantly, a national strategy for PM, serving as a general framework for its implementation in the Spanish National Healthcare System (NHS) is crucial to guarantee technical quality, equal access of all citizens to the best practices and the sustainability of the healthcare system. Therefore, in 2018 the Spanish Senate delivered a proposal asking for a National Strategy on Genomics and Personalised Genomic Medicine [7]. As a consequence, in 2020 the national government planned to invest €25 million into Predictive Medicine, Data Science and Genomic Medicine over the following three years under a project called Infrastructure of Precision Medicine associated with Science and Technology (IMPaCT) [8]. The effort is resulting in the creation of a national cohort for research, as well as an integrated clinical and genomic data infrastructure at the national level.
Moreover, the primary professional scientific non-profit society for pharmacogenetics and pharmacogenomics in Spain, the Spanish Society of Pharmacogenetics and Pharmacogenomics (Sociedad Española de Farmacogenética y Farmacogenómica–SEFF, https://seff.es/), founded in 2005, is actively working on the translation of PGx information into today’s healthcare, as well as supporting clinical implementation both on a national and clinical laboratory level. In the last few years, the SEFF has been working on the elaboration of clinical guidelines in order to offer PGx counselling for Spanish speaking healthcare professionals in Spain and also in Latin America. In this sense, the key role of clinical pharmacology, hospital pharmacy, biochemistry and genetics societies in leading PGx initiatives has been previously described [9]. Thus, SEFF is bringing together the efforts of experts from a variety of fields–clinical pharmacologists, geneticists, clinicians, and pharmacists–to provide the necessary guidance to bridge the gap between PGx research and the clinic.
It’s also worth mentioning that early this year thanks to the efforts of several experts, representatives from the Autonomous Communities and Spanish scientific societies, a national common portfolio of services in the area of genetics has been presented by the NHS in order to guarantee a more homogeneous and equitable access. Indeed, this portfolio contains the first PGx catalogue approved at a national healthcare level in Europe.
This review sets the focus on regional and national Spanish initiatives to implement PGx in the clinical practice.
Methods
The information included in this review has been collected from different sources [1]: PubMed database [2]; Institutional resources and web pages [3]; Spanish hospitals’ service portfolio – when available in hospital’s website, PGx information has been obtained and included in the revision. Please note that this information is subjected to be the latest update of the Hospital’s webpage [3]; Direct contact with healthcare professionals at Spanish hospitals – when possible, direct contact with the Pharmacy or Clinical Pharmacology or Clinical Analysis units from hospitals has been established in order to collect first-hand updated information. All the information provided by the Hospitals’ units have been included. Available information regarding PGx tests is indicated in Table 1 and represent information as of 2023, before the presentation of the national genetic catalogue in January 2024.
Spanish centers of the public National Healthcare System with pharmacogenetic implementation prior to the NHS PGx approval.
| Spanish region | City | Hospital/Centre | Department | Laboratory | <5 genes | 5–10 genes | >10 genes | PGx report | External service | Source of data |
|---|---|---|---|---|---|---|---|---|---|---|
| Andalusia | Sevilla | Hospital Universitario Virgen del Rocío | Pharmacy/Pharmacology | Clinical Analysis | ● | Provided by Hospital | ||||
| Andalusia | Sevilla | Hospital Universitario Virgen Macarena | Pharmacy | Biochemistry | ● | Provided by Hospital | ||||
| Andalusia | Cádiz | Hospital Puerta del Mar | Pharmacy | Clinical Analysis | ● | Hospital’s service portfolio | ||||
| Andalusia | Granada | Hospital Universitario Virgen de las Nieves | Pharmacy | Pharmacogenetic | ● | ● | Provided by Hospital | |||
| Andalusia | Granada | Hospital Universitario Clínico San Cecilio | Pharmacy | Genyo | ● | ● | Provided by Hospital | |||
| Andalusia | Malaga | Hospital Universitario Carlos Haya | Pharmacy | ● | ● | Provided by Hospital | ||||
| Aragon | Zaragoza | Hospital Miguel Servet | Pharmacy | Genetics | ● | Provided by Hospital | ||||
| Asturias | Oviedo | Hospital Universitario Central de Asturias | Genetics and Molecular Oncology | ● | Hospital’s service portfolio | |||||
| Basque country | Bilbao | Hospital Universitario Cruces | Clinical Analysis | ● | Provided by Hospital | |||||
| Basque country | Donostia-San Sebastián | Hospital Universitario Donostia | Clinical Analysis | ● | Provided by Hospital | |||||
| Castile-La Mancha | Toledo | Complejo Hospitalario Universitario de Toledo | Clinical Analysis | ● | Provided by Hospital | |||||
| Castile-La Mancha | Talavera de la Reina | Hospital Nuestra Señora del Prado | Clinical Analysis | ● | Provided by Hospital | |||||
| Castile-La Mancha | Ciudad Real | HU de Ciudad Real | Clinical Analysis | ● | Provided by Hospital | |||||
| Castile-La Mancha | Cuenca | Hospital Virgen de la Luz | Clinical Analysis | ● | Provided by Hospital | |||||
| Castile-La Mancha | Albacete | Complejo Hospitario Universitario de Albacete | Clinical Analysis | ● | Provided by Hospital | |||||
| Catalonia | Barcelona | Hospital de la Santa Creu i Sant Pau | Pharmacy/Genetics | Genetics | ● | ● | ● | Provided by Hospital | ||
| Catalonia | Barcelona | Hospital de la Vall d’Hebron | Pharmacokinetics and Pharmacogenetics Unit (Pharmacy) | Clinical Analysis | ● | ● | ● | Provided by Hospital | ||
| Catalonia | Barcelona | Hospital Parc de Salut Mar | Pharmacy/Oncology | Molecular Biology Laboratory | ● | ● | ● | Provided by Hospital | ||
| Catalonia | Barcelona | Hospital Clínic de Barcelona | Toxicology & Pharmacogenetics lab | Toxicology and Pharmacogenetics lab | ● | ● | ● | Provided by Hospital | ||
| Catalonia | Badalona | Hospital Germans Trias i Pujol | Pharmacogenetics Unit | Clinical Analysis | ● | ● | Provided by Hospital | |||
| Catalonia | L’Hospitalet de Llobregat | Hospital Universitari de Bellvitge | Laboratory/Pharmacy | Clinical Analysis | ● | ● | Provided by Hospital | |||
| Catalonia | L’Hospitalet de Llobregat | Institut Català d’Oncologia - Hospitalet | Laboratory/Pharmacy | Molecular Biology Laboratory | ● | ● | Provided by Hospital | |||
| Catalonia | Terrassa | Hospital Universitari Mútua de Terrassa | Pharmacy/Genetics | Genetics | ● | ● | ● | Provided by Hospital | ||
| Cantabria | Santander | Hospital Universitario Marqués de Valdecilla | Pharmacology | ● | Provided by Hospital | |||||
| Extremadura | Several cities | Hospitals, Mental Health and Primary Care Centres | Various services /Units | CICAB | ● | ● | For the full list of centres involved in MedeA Project consult: https://www.proyectomedea.es/equipo/ | |||
| Extremadura | Badajoz | University Hospital of Badajoz | Pharmacogenetic | CICAB | ● | ● | MedeA Project | |||
| Extremadura | Coria | Hospital of Coria | Internal Medicine | CICAB | ● | ● | MedeA Project | |||
| Extremadura | Don Benito | Hospital Don Benito-Villanueva | Oncology | CICAB | ● | ● | MedeA Project | |||
| Extremadura | Llerena | General Hospital of Llerena | Psychiatry | CICAB | ● | ● | MedeA Project | |||
| Extremadura | Mérida | Hospital of Mérida | Rheumatology | CICAB | ● | ● | MedeA Project | |||
| Extremadura | Plasencia | Hospital Virgen del Puerto de Plasencia | Nephrology | CICAB | ● | ● | MedeA Project | |||
| Galicia | Santiago de Compostela | Fundación Pública Galega de Medicina Xenómica | Pharmacogenetics | Pharmacogenetics | ● | ● | ● | Provided by FPGMX | ||
| La Rioja | Logroño | Hospital Universitario San Pedro | Clinical Analysis | ● | ● | Provided by Hospital | ||||
| Madrid | Madrid | Hospital Universitario La Princesa | Pharmacology | Pharmacogenetic | ● | ● | ● | Provided by Hospital | ||
| Madrid | Madrid | Hospital Universitario Gregorio Marañon | Pharmacy | Pharmacogenetic | ● | ● | ● | Provided by Hospital and Portfolio | ||
| Madrid | Madrid | Hospital Universitario La Paz | Pharmacogenetic | ● | ● | ● | Provided by Hospital | |||
| Madrid | Madrid | Hospital Universitario Ramón y Cajal | Genetics | ● | Provided by Hospital | |||||
| Madrid | Madrid | Hospital Clinico San Carlos | Laboratory/Pharmacology | ● | Provided by Hospital | |||||
| Madrid | Madrid | Hospital Severo Ochoa | Biochemistry | ● | Provided by Hospital | |||||
| Madrid | Madrid | Spanish National Cancer Research Centre (CNIO) | Human Genotyping Unit | Human Genotyping Unit | ● | ● | ● | Provided by CNIO | ||
| Murcia | Cartagena | Hospitalario Universitario de Cartagena | Molecular Diagnostic laboratory | ● | ● | Provided by Hospital | ||||
| Murcia | Murcia | Hospital Morales Meseguer | Haematology | ● | ● | Provided by Hospital and Portfolio | ||||
| Murcia | Murcia | Hospital Virgen Arrixaca | Clinical Analysis | ● | Provided by Hospital | |||||
| Navarra | Pamplona | Hospital Universitario de Navarra | Genetics | ● | ● | Provided by Hospital | ||||
| Valencian region | Valencia | Consorcio Hospital General Universitario de Valencia | Clinical Analysis | Genetics and molecular biology | ● | ● | ● | Provided by Hospital | ||
| Valencian region | Valencia | Hospital de Sagunto | Clinical Analysis | Clinical Analysis | ● | ● | Provided by Hospital | |||
| Valencian region | Valencia | Hospital la Fe | Clinical Analysis | Clinical Analysis | ● | ● | Hospital’s service portfolio | |||
| Valencian region | Valencia | Hospital Arnau de Vilanova | Clinical Analysis | Clinical Analysis | ● | ● | Provided by Hospital |
Spanish regional initiatives
In this section, an overview of the main Spanish regional PGx initiatives is given.
Andalusia
Andalusia had an unequal implementation of PGx across the region without any regional initiative in the Andalusian Health System. In western Andalusia, Hospital Universitario Virgen Macarena (HUVM) [10] and Hospital Universitario Virgen del Rocío (HUVR) in Seville had implemented PGx of some genes in the clinical practice (Table 1). Additionally, HUVM was involved in a multi-centric PGx pilot project, in collaboration with the Human Genotyping Unit at the Spanish National Cancer Research Centre (CEGEN-CNIO, Madrid), to evaluate the barriers in the implementation of PGx through the complete characterization of a subset of the Andalusian population [11].
On the contrary, in eastern Andalusia, there were two specific PGx units in public hospitals, one in San Cecilio Clinical University Hospital (HUCS) and another in Virgen de las Nieves University Hospital (HUVN) with different levels of implementation (Table 1). Of note, the Pharmacy Service of HUCS had been part of the H2020 project entitled “Ubiquitous Pharmacogenomics (U-PGx): Making actionable pharmacogenomic data and effective treatment optimization accessible to every patient” in which around 8,100 patients from 7 European countries were recruited and treatments adjusted based on PGx information [12].
Additionally, the Andalusian community developed an ambitious Program of Personalized Medicine [13]. This program started in 2010 with the Medical Genome Project whose aim was to address the sequencing of human genomes of phenotyped patients and control individuals [14]. This program allowed the creation of the Collaborative Spanish Variant Server (CSVS), a reference database of the genomic variation in the Spanish population [15]. Recently, PGx information have been included in CSVS to use as reference of the PGx alleles in this population. The Program of Personalized Medicine aimed at using patients’ genomic data in the clinical practice thorough the development of a bioinformatics structure to manage genomic data in a scalable, equitable and affordable manner, compatible with the General Data Protection Regulation (GDPR). Lastly, Andalusia has developed an ambitious training program in Precision Medicine (PanMEP) [16].
Basque Country
At the administrative level, there is great interest in the implementation of personalized medicine (PM). This is reflected in the creation of the Planning and Management Committee for Personalized Medicine in the Basque Country in 2021 [17], the Basque Health Research and Innovation Strategy 2022–2025 [18], the Science, Technology and Innovation Plan 2030 (PCTI 2030) of the Basque Government [19], and the Basque Oncology Plan 2018–2023 [20].
PGx testing for DPYD (5-fluorouracil, in cancer), CYP2C9 (siponimod, in multiple sclerosis), UGTA1 (irinothecan, in cancer), and CYP2D6 (eliglustat, Gaucher disease) were performed at the University Hospital Cruces and/or the University Hospital Donostia (Table 1). These tests were included in the service portfolio of Osakidetza, the Basque Health Service, and all the hospitals in the network had access to them. All petitioner physicians in the network also had access to the information about the test and the meaning of the variants. In some cancer types, tumour molecular testing was also available for treatment personalization. These techniques were implemented at the proposal of the interested services if there was a level of evidence and cost-effectiveness.
Canary Islands
The Canary medical council created in 2022 a specific commission for the development of PM. The PGx testing was included in the service portfolio of the Hospital Universitario de Canarias and the Hospital Universitario Doctor Negrín. The Institute of Technology and Renewable Energies (ITER) manages the supercomputer Teide–High Performance Computing and has a Genomics division specialized in next generation sequencing (panels, exomes and genomes) [21], 22]. ITER collaborates with the Hospital Universitario Nuestra Señora de La Candelaria (HUNSC) in research projects related to the development of PM in the Canary Islands. The project PHARMA-CAN aimed at improving drug prescription by taking into account the PGx allelic variation found in the Canarian population, using whole exome sequencing [23]. In addition, other projects focused on evaluating PGx using NGS were in progress. Besides, the Canary health service also collaborates with the IMPaCT project [8].
Castile and Leon
Castile and Leon was one of the most advanced regions in the implementation of PM in Spain at a regional level. In the clinical practice, two different protocols had been implemented: one protocol applied to diagnostics (particularly for rare diseases) with a network operation and a Regional Reference Unit on the Advanced Diagnostic of Rare Diseases (DiERCyL) [24]. And the second protocol, the 5SPM model (5 steps in PM) for the application of PGx in polymedication, carried out at the Complejo Hospitalario de Salamanca, where the specific Reference Unit for Pharmacogenetics and Precision Medicine in Castile and Leon is located [24].
In this Unit, more than 2000 cases of patients with adverse effects or therapeutic failure had been already analyzed, not only regarding their genetic factors, but also other characteristics that allow the personalisation of the therapy. This Unit has published in international journals good practice recommendations for PM and collected economic data indicate the benefit of using PM in therapy [25], 26].
In addition, this region set up the Network of Precision Oncopathology and was preparing the Strategic Plan for Personalised Precision Medicine, with the aim of implementing PGx. Castile and Leon is also involved in the IMPaCT Project, coordinating the Pharmacogenetics work package of the Genomic Medicine axis [8]. Also, the University Hospital of Burgos and the Foundation for Health Research (FBIS) participate in IPHARMGx project, a collaborative study on the implementation of PGx (see further details in the Madrid section).
Regarding research, the Instituto de Investigación Biomédica de Salamanca (IBSAL) has recently started a multicentric project on PGx in mental and cardiovascular health, funded by the call “Medicina Personalizada de Precisión” from the Health Strategic Action (AES) by the Spanish public health research institute Carlos III.
Catalonia
Catalonia had an unequal implementation of PGx across the region. Most hospitals that perform PGx studies were located in Barcelona. Some of them offered their services to external users, both for clinical and investigational purposes. The inequality was probably due to the lack of governmental initiatives for the implementation of germline PGx in Catalonia. In this sense, each hospital performed its own analyses. Whereas DPYD was genotyped in most Catalan hospitals, other interesting genes such as the ones encoding cytochromes, were determined only in a small number of hospitals. Conversely, the situation for the determination of somatic biomarkers is better established. In 2021, the ‘precision oncology program’ was implemented in Catalonia (Servei Català de la Salut) [27]. This program is an approach to the diagnosis and treatment of cancer focused on the identification of subgroups of patients with certain cancers carrying specific molecular characteristics that can be eligible to receive targeted treatments with greater effectiveness. Its main objective is to guarantee equitable access to molecular determinations and targeted treatments for all patients in the region. This program has a field of action that includes all molecular or genetic alterations that are prognostic markers or predictors of therapeutic response. The identification of these markers determines the targets that should be considered in the development of more precise drugs, and in the indication of selected treatments for certain patients. The program is divided into four different areas: haematological tumours, solid tumours, paediatric tumours, and hereditary cancer.
More recently, on the 22nd February 2023, the document entitled ‘Proposals to advance in the precision personalised medicine model in Catalonia’ was presented (Consorsi de Salut I Social de Catalunya) [28]. More than 30 healthcare professionals from institutions and centers belonging to the Catalan public health system participated in it. The aim of this work was to identify the challenges to implement a PM model in this region, and to describe the measures needed to succeed.
Extremadura
In Extremadura, the implementation of PGx was being accomplished with a centralized initiative called “MedeA–Clinical implementation of pharmacogenetics and personalized drug prescription based on e-health” [29]. MedeA is a PM strategy which integrates PGx and relevant clinical data, to develop a decision-supporting tool to be used for individualized drug prescription during regular clinical practice within the context of e-health [30].
This initiative also sought the involvement of private companies through the use of innovative public procurement as an instrument to promote research, development and innovation in companies and within the framework of the Open Innovation Model promoted by the Extremadura Health Care Service and the Spanish Ministry of Science and Innovation. MedeA was designed and developed at the Clinical Research Center of the Extremadura University Institute for Biomedical Research (INUBE), where the PGx and Personalized Medicine Unit was created and is coordinating the project. MedeA involves many services/units from all hospitals in the regional Health Care Service, as well as numerous mental health and primary care centres across Extremadura (Table 1; and for the full list of hospitals and centers participating, consult the following link: https://www.proyectomedea.es/equipo/).
MedeA program main aims are to develop: 1) a personalized prescription program incorporated into the electronic medical records (EMR), 2) an artificial intelligence algorithm for the translation of individual’s genetic information into phenotypes to facilitate drug prescription, 3) the necessary analytical and laboratory methods, and 4) methodologies to evaluate the pharmacological effects during patients’ daily lives.
At the end, the project will allow clinicians to consult automatically information regarding the PGx background of the patients within the EMR of the regional Healthcare System, allowing them to select the most appropriate drug treatment options. In addition, as the first project in this sense, it would also allow validating this approach under real clinical conditions.
Galicia
The Galician Public Foundation of Genomic Medicine (FPGMX) centralizes the genetic testing from all hospitals within the Galician Health Service (SERGAS), thus covering 3.5 million inhabitants. FPGMX is specialized in genetics of hereditary diseases, oncohematology, prenatal diagnosis, genetic counseling, PGx and research activities. PGx testing was incorporated into the Galician health care portfolio in 2004 [31], since then clinicians are allowed to request this service. A number of PGx biomarker analyses to predict drug response had been implemented (Table 1). Once a specific analysis was performed a detailed PGx report was uploaded into the EMR of the patient. Additionally, since 2021, the FPGMX was entrusted with the management of an Implementation Plan of Pharmacogenetics in Psychiatry by SERGAS, as part of the Galician Mental Health Plan post-COVID-19 [32]. This plan aimed to implement the use of PGx in psychiatry, in order to reduce hospital admissions and adverse effects of treatment, as well as increase the efficacy of the treatment [33], [34], [35]. The FPGMX not only conducts PGx analyses in relation to long-acting antipsychotics, but also extends the scope to include other drugs [34], [35], [36].
From a research point of view, the FPGMX is actively working on establishing long-lasting multidisciplinary collaborations with medical and pharmaceutical teams to promote and coordinate translational projects in PGx. These activities are developed in collaboration with different entities, such as the Santiago de Compostela Health Research Institute (IDIS), the Genomic Medicine Group, the Universidad de Santiago de Compostela (USC) and the Biomedical Research Network on Rare Diseases (CIBER).
At present, the FPGMX leads projects in different areas being some of the most recent: PM for the administration of methadone based on PGx profiles, optimization of schizophrenia treatment using OMIC data and systems biology (SchizOMICS), and omnigenic view of genetic susceptibility to severe COVID19 [31].
FPGMX is a member center of the Genomic Analysis Network within the Genomic Medicine Axis of the IMPaCT Platform [8]. Also, it is important to highlight that the FPGMX obtained funding and takes part in the Precision Medicine Research Projects (ISCIII) such as “Evaluation of a Proposal for the Implementation of Pharmacogenetics in the National Health System for Mental and Cardiovascular Health (BioFRAM)” and participates at “Clinical and PGx interventions in childhood and juvenile population (INGENIA)”.
La Rioja
In order to advance in the implementation of PM, in 2016, La Rioja healthcare service created a Committee on Genetic Counselling to unify the petition and approval of genetic tests requested by the clinicians. The Clinical Analysis Laboratory at Hospital Universitario San Pedro performed PGx testing for DPYD (for fluoropyrimidine drugs), UGT1A1 (irinotecan), CYP3A5 (tacrolimus), CYP2C9 (siponimod) and SLCO1B1 (statins) [37]. Physicians in the public network were able to request these tests and also tumor molecular testing for treatment tailoring in certain tumor types. Moreover, the Hospital announced the creation of a Precision and Personalized Medicine Unit [38]. This Autonomous Community uses the EMR for each patient which also includes the genetic information that can be securely accessed by the specialist.
Regarding Research and Innovation Projects, the region launched the “Smart Specialization Strategy (RIS3, 2014–2020), the “V Plan Riojano de I+D+I” (2017–2020) and the “I Strategic Plan of Innovation in Health” (2017–2022) [39], 40]. Within these plans, PM is recognized as one of the future areas and is established as a funding priority. However, the specific objectives and measures to implement it need to be further developed. La Rioja also has a Biomedical Research Center (CIBIR) with research lines focusing on rare diseases, oncology, infectious diseases and neurodegeneration using next generation technologies and Big Data [41]. The CIBIR also counts with several Genomics & Bioinformatics and Biomedical Research platforms.
Madrid
In the region of Madrid, there is a regional initiative for the implementation of PGx under development. The main PGx units in public hospitals in this region are located in University Hospital (U.H.) La Princesa, U.H. Gregorio Marañón and U.H. La Paz (Table 1) [42], [43], [44]. These units also offer their services to external users, both for clinical and investigational purposes. In addition, some of these PGx units have demonstrated the utility of the implementation of pre-emptive PGx through research studies. Noteworthy, U.H. La Paz presented their experience after 3 years of pre-emptive PGx testing [45] and U.H. La Princesa is developing a multidisciplinary initiative named “PriME-PGX” to implement PGx in their hospital [46], and also contributing to the expansion of PGx knowledge among professionals, the general population and throughout the field of clinical trials. Other hospitals in the region that offered PGx tests in their service portfolios include U.H. Ramón y Cajal, U.H. Clínico San Carlos, U.H. Severo Ochoa and U.H. Fuenlabrada. The detailed level of PGx implementation of each hospital is showed in Table 1. In all cases, PGx reports include both genetic determination result and treatment recommendations (indication and or dose adjustments) for relevant gene-drug pairs. Moreover, research projects on PGx are developed at different hospitals and research centers such as IPHARMGx [47]. This is a National collaborative study, coordinated by the Clinical Pharmacology Department at U.H. La Paz, to evaluate the efficacy and cost-effectiveness of the implementation of PGx biomarkers through an anticipated genotyping strategy in the NHS. It involves more than 60 researchers from 11 hospitals (also in Madrid: U.H. Gregorio Marañón, Health Research Institute Gregorio Marañón and the Nephrology department at U.H. La Paz). Some of the axes of this study include PGx in kidney transplant patients and in high cardiovascular risk patient, among others. Additionally, Human Genotyping-CEGEN Unit at the Spanish National Cancer Research Centre (CNIO) offers a comprehensive PGx analysis, which includes 17 genes and 59 drugs to public hospitals of this region or other Spanish regions, facilitating their access to PGx analysis [48]. In this sense, a multicentric PGx pilot project in collaboration with different hospitals from Madrid, Balearic Islands and Andalusia is ongoing to evaluate the barriers of PGx implementation across the country in more than 300 Spanish patients. In addition, a recent study accomplished by CEGEN-CNIO in collaboration with the Clinical Bioinformatics Unit from HUVR has performed a comprehensive analysis of the genetic variability of 21 pharmacogenes in more than 3,000 Spanish individuals, evaluated the clinical impact on 64 drugs as well as determined PGx CNVs [49], 50]. Genetic data is available through the CSVS portal (http://csvs.babelomics.org/) [15].
Navarra
Until recently, the use and implementation of PGx in Navarre was mainly focused on the determination of tumor alterations (e.g. ALK translocations, BRAF and EGFR mutations, BCR-ABL fusion) and HLA-B typing. These tests were performed by the Anatomical Pathology, the Medical Genetics and the Immunology Departments of the University Hospital of Navarra (HUN) from the public healthcare system–Osasunbidea. Testing of CYP2C9 and DPYD have also been incorporated into the clinical practice (Table 1).
The Navarre regional government has promoted strategic projects on Personalized Medicine (RETO GEMA) [51] and since 2016, the HUN and Navarrabiomed have pursued the implementation of genome sequencing as a clinical tool to develop personalized medicine in the public healthcare system (Nagen program [52]). Specifically to this review, the Pharmacy Service of the HUN and the Genomics Medicine Unit of Navarrabiomed developed PharmaNAGEN project [53]. This study evaluated the use of NGS as a clinical tool to obtain PGx data to improve treatment decisions in more than 250 individuals. The initiative aimed to develop a pharmacogenetic alert system implemented into the electronic clinical decision support system of the public healthcare. The prescription assistance tool, based on the individual PGx profile, will offer recommendations to clinicians to individualize the therapies. This will include recommendations for 52 drugs affected by the genotype of 13 genes. PharmaNAGEN paved the way for the implementation of PGx data in the healthcare system, acquiring the knowledge, developing the tools and training of clinical experts so that patients can benefit from personalized drug prescription [54], 55]. In addition, clinicians and researchers from the Biomedical Center of Navarre (Navarrabiomed), Navarra Services and Technologies (NASERTIC) and HUN are also participating in IMPaCT projects [8].
Valencian Community
In the province of Valencia, the University General Hospital of Valencia, Sagunto Hospital, La Fe Hospital and Arnau de Vilanova Hospital performed PGx testing. Each hospital performed its own analyses, ranging from a few genes to more than 10 genes, and provide with PGx reports. Moreover, various projects are currently being carried out for the implementation of PGx in clinical practice. IMPaCT project includes PGx within the IMPaCT-GENÓMICA program and its objective is to lay the foundations for the implementation of PGx tests in the NHS [8]. La Fe Hospital/Health Research Institute (IIS) in Valencia participate in this project in collaboration with other national centers [8]. Aligned with IMPaCT, the BioFRAM project aims at individualizing the prescription of drugs used in mental and cardiovascular diseases based on PGx markers for the prevention of adverse reactions. In this region, Hospital La Fe, Hospital Clínico, Hospital Doctor Peset and primary care centers are involved in this collaborative study.
In addition, the Pediatric Oncology Unit of Hospital la Fe together with the PGx Unit of IIS La Fe is carrying out, as part of the neuroblastoma European project ‘HR-NBL2/SIOPEN’, an independent work on the PGx of high-risk neuroblastoma patients to establish associations with drug side effects [56]. The abovementioned PGx Platform at IIS La Fe also investigates the association of PGx with immunosuppressants in organ transplantation, as well as it offers services to design PGx panels and validate PGx markers [57].
The PGx Platform of the Alicante Health and Biomedical Institute (ISABIAL) is currently participating in the project called IPHARMGx [47]. ISABIAL is also conducting a clinical trial (EudraCT Number: 2021-001238-21) entitled “Gender biases in pain medicine: from omics to healthcare” that integrates PGx, epigenetic, and metabolomic markers to understand the gender differences observed in the analgesic response to opioids.
Also, at Miguel Hernández University, a research project is being developed focused on the genetic variation and functional analysis of cytidine deaminase enzyme (CDA) activity to categorize it as a biomarker of oncological treatment toxicity [58].
Other Spanish regions
Some Spanish regions did not present a PGx clinical implementation structure although the main hospitals of these regions performed PGx testing for some genes (e.g. DPYD, TPMT or UGT1A1).
This was the case of Aragon, although they showed interest in PGx and PM since 2010 [59], the implementation of PGx began at the end of 2020 with the DPYD alert from the Spanish Agency of Medicines and Medical Devices (AEMPS). Since then, genotyping of the population of the entire region was centralized at the Miguel Servet Hospital, located in Zaragoza, specifically at the Genetics Unit. The Pharmacy Department prepares the reports for each genotyped patient (Table 1).
In Asturias, the Genetics and the Molecular Oncology Units at the Central University Hospital of Asturias (HUCA) screen for CYP2C9, CYP3A4 and UGT1A as per the hospital portfolio [60]. Also, the University of Oviedo and the Healthcare Research Institute of Asturias are participating in the IMPaCT project [8].
In Balearic Islands, the Son Espases Hospital set up the Genetic and Genomic Unit, which aims at acting as the diagnostic reference for genomic analysis in the regional healthcare service. Furthermore, the Clinical Analysis Unit of the Hospital includeed PGx testing in the portfolio, although it was not yet included in other hospitals. The region also collaborates with the IMPaCT project through the Health Research Institute of the Balearic Islands (IdISBa) [8].
In Castile-La Mancha, the main hospitals of Toledo, Ciudad Real, Cuenca and Albacete had implemented the PGx testing through their Clinical Analysis Units. They mainly performed the analysis of DPYD but also CYP2C9 and HLA-B in some cases (Table 1). Also, researchers and clinicians from the Tomelloso Hospital and the Health Research Institute of Castile-La Mancha participate in the iPHARMGx project (already mentioned in the Alicante region) [47].
In Cantabria region, at the Marqués de Valdecilla University Hospital, TPMT polymorphism determinations were carried out in patients treated with azathioprine and MTHFR polymorphism determinations for hematological diseases. Negotiations were underway between the Molecular Genetics Service and Clinical Pharmacology of the same hospital to carry out laboratory tests by the Molecular Genetics Service, while the Clinical Pharmacology Service would prepare clinical reports for patients. Also, researchers and clinicians from the Marqués de Valdecilla University Hospital and Valdecilla Research Institute (IDIVAL) participate in the iPHARMGx project [47].
In Murcia, the Molecular Diagnostic Laboratory of the University Hospital Complex of Cartagena analyses DPYD, UGT1A1, IL28, CYP2C9, CYP2C19 genes and the Genomics Laboratory of the Hematology Service of the University General Hospital Morales Meseguer studies UGT1A1 and DPYD (Table 1) [61]. Both hospitals screen for somatic alterations with predictive value in neoplasias (e.g., BCR-ABL, BRAF, EGFR, TP53). Hospitals from this region also collaborate with the IMPaCT project [8].
Spanish national initiatives
In this section, we provide information on the most important national PGx initiatives.
IMPaCT project
IMPaCT is the Precision Medicine Infrastructure associated with Science and Technology [8]. This infrastructure is designed to provide service to the research, development and innovation system oriented to PM, to promote the generation and transfer of high-quality knowledge in the NHS, ensuring scientific-technical excellence, equity and efficiency in the use of the available resources. The IMPaCT Strategic Plan is configured around three strategic axes (Predictive Medicine, Data science and Genomic Medicine) and two transversal strategic lines. The strategic axes are arranged in specific actions and work packages, with compliance indicators that should allow verifying the effectiveness of infrastructure deployment (Figure 1).
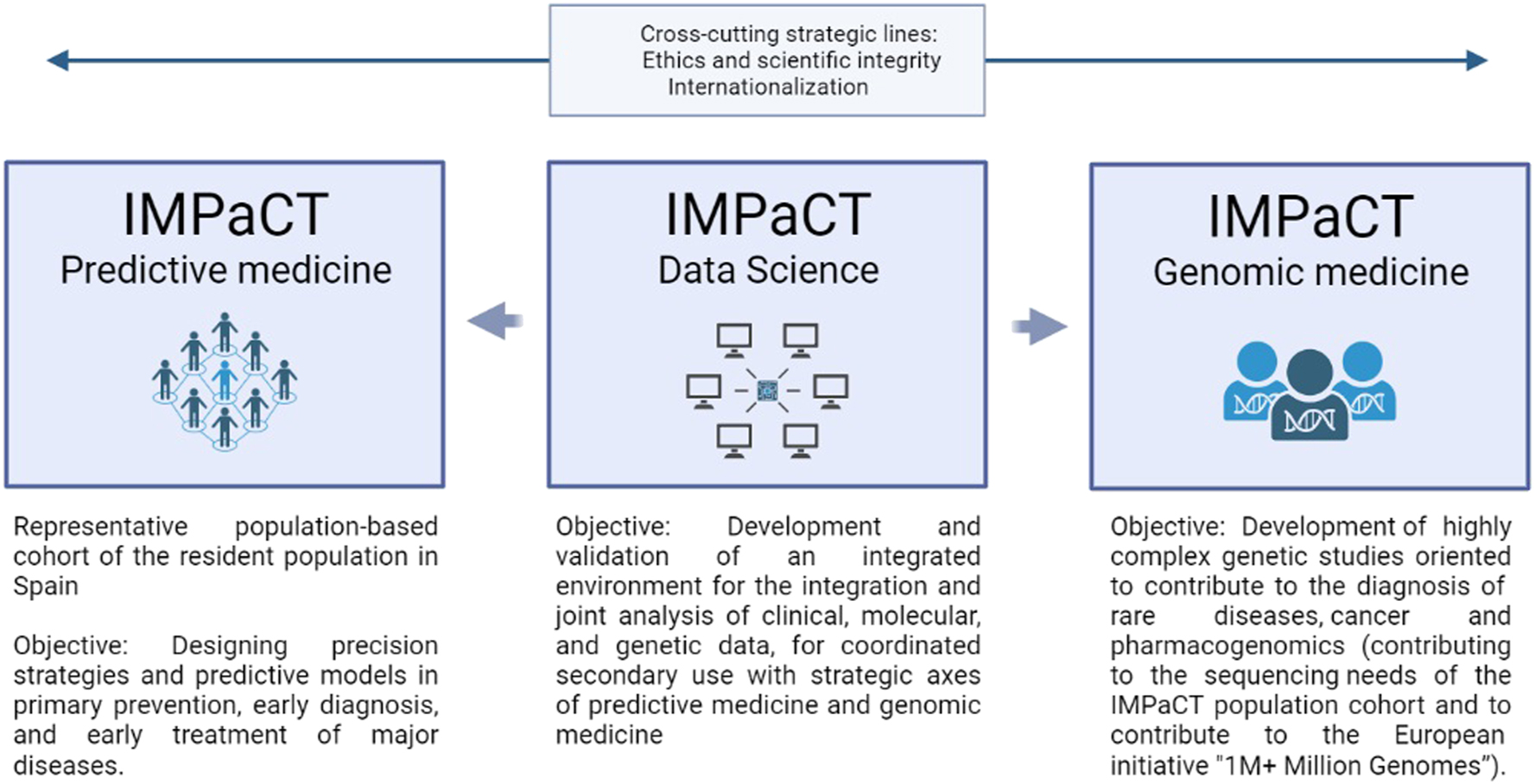
Strategic axis of IMPACT project.
In a complementary way, the two transversal strategic lines are common to the above mentioned three axes and they provide internal coherence. The first transversal line refers to Ethics and scientific integrity, and the second one to Internationalization. This later aiming to position Spain as an international benchmark in the field of PM.
Specifically to the topic of this review, in the Genomic Medicine axis, one of the areas of application was PGx [62]. The main objectives of this part were to lay the foundation to allow PGx testing implementation in the NHS, and also to provide with genomic diagnostic services equitably across the country to individuals with serious drug or vaccine adverse events. This working group put all their efforts to: 1) connect with the Pharmacovigilance network and establish the samples workflow, 2) create Expert and Result Interpretation Committees, 3) establish and renew standardized laboratory protocols for drug adverse events, 4) elaborate Clinical Guidelines and a clinical report template, 5) provide diagnostic genomic services in a fair manner across Spain, 6) federated data sharing and data discovery, and 7) interrelate with the Predictive Medicine axis.
SEFF implementation strategy
There are many successful cases in which PGx was implemented in the clinical practice, where, based on the clinical guidelines established by the Clinical Pharmacogenetics Implementation Consortium (CPIC), genotyping began to be carried out prior to the prescription of certain drugs such as warfarin, clopidogrel, codeine, antidepressants and antiepileptics [3], [63], [64], [65], [66], [67].
Similarly to other national/international PGx groups, the Spanish Society of Pharmacogenetics and Pharmacogenomics (SEFF) is a national multidisciplinary scientific society actively working on the development of PGx guidelines in Spanish. The SEFF developed the General Strategy for the Clinical Implementation of Pharmacogenetics with the overall objective of generating recommendations for the implementation of the PGx to optimise the safety and efficacy of the pharmacological treatments taken by patients [68]. More than 50 experts in the field are working together organized in working groups (WG1. PGx regulation–List of recommended drugs; WG2. Methodology and analytical interpretation; WG3. Clinical recommendations; WG4. Solid tumors) and programmes (1. Proficiency testing, 2. Teaching-training, and 3. Map of the labs and PGx services in Spain).
The advancement in PGx must be in line with therapeutic guidelines and regulatory agencies; however, progress in this area is often slow. As a consequence of this situation, the SEFF is working on the development of clinical recommendations based on the existing evidence, so that these can serve as a basis on which to start implementing PGx in the clinic [69]. So far, it has generated clinical recommendation documents for 12 gene – drug pairs that are available at the SEFF website (https://seff.es/), as well as published a consensus paper in collaboration with the Spanish Society of Medical Oncology for the genotyping of DPYD in cancer patients who are candidates for treatment with fluoropyrimidines [70]. More documents are in progress. With this strategy, SEFF wants to become a fundamental element in the Spanish Strategy for Personalised Medicine promoted by the National Government and to facilitate the implementation of the current NHS PGx portfolio.
National PGx implementation strategy
At the national level, a Working Group of experts reporting to the Healthcare Ministry has been working on updating and specifying a common portfolio of services in the area of genetics for the NHS in order to guarantee a more homogeneous and equitable access to all individuals who require these tests within the NHS.
The first version of the catalogue, that will need continuous updating, was approved in the plenary session of the Interterritorial Council of the NHS on June 23rd 2023, and presented it on January 23rd 2024 by the Healthcare Ministry [71]. The proposed catalogue was developed by eight subgroups, with the participation of representatives from the Autonomous Communities, Spanish scientific societies, the health research institute Carlos III (ISCIII) and the Network of Agencies for Assessing NHS Technologies and Performance, for these prioritized areas: adult onco-hematology, pediatric onco-hematology, pharmacogenomics, heart disease and disorders of the circulatory system, ophthalmological diseases, hereditary metabolic and mitochondrial diseases, neurological diseases and neuromuscular/neurodevelopmental disorders including cognitive deficits.
It is worth mentioning that this is the first PGx catalogue approved at a national healthcare level in Europe. It includes 12 genes, for 22 main drugs and the clinical indication criteria for each test (Table 2 and in the link: https://cgen.sanidad.gob.es/#/consulta-general). Information on the type of technique and the indispensable variants is also included, and it is warned that the obtained genetic information can be used for the adjustment of other treatments that also have specific recommendations. In addition, the Spanish Agency for Medicines and Medical Devices (AEMPS, abbreviation in Spanish) has recently published a database with PGx biomarkers to facilitate the implementation of PGx in the clinics (https://www.aemps.gob.es/medicamentos-de-uso-humano/base-de-datos-de-biomarcadores-farmacogenomicos/). This resource provides with the regulatory information included in drug data sheets, and in the near future, it will also contain the established recommendations for each active compound.
Recommended pharmacogenetic tests in the Spanish Public National Healthcare System.
| Drug | Gene | Essential alleles |
|---|---|---|
| Abacavir | HLA-B | HLA-B*57:01 |
| Allopurinol | HLA-B | HLA-B*58:01 |
| Atazanavir | CYP2C19 | *2, *3, *4, *17 |
| Azathioprine | NUDT15 | *2, *3 |
| Azathioprine | TPMT | *2, *3A, *3B, *3C, *4 |
| Capecitabine | DPYD | NM_000110.3(DPYD): c.1905+1G>A (*2A), c.1679T>G (*13), c.2846A>T, [c.1129–5923C>G/c.1236G>A](HapB3) |
| Carbamazepine | HLA-A | HLA-A*31.01 |
| Carbamazepine | HLA-B | HLA-B*15:02 |
| Clopidogrel | CYP2C19 | *2, *3, *4, *17 |
| Eliglustat | CYP2D6 | *3, *4, *5, *6, *9, *10, *17, *29, *36, *41, copy number variants |
| Fluorouracil | DPYD | NM_000110.3(DPYD): c.1905+1G>A (*2A), c.1679T>G (*13), c.2846A>T, [c.1129–5923C>G/c.1236G>A](HapB3) |
| Irinotecan | UGT1A1 | *28 |
| Ivacaftor | CFTR | NP_000483.3(CFTR): p.F508del (c.1521_1523delCTT), p.R117H (c.350G>A), p.G178R (c.532G>A), p.S549R (c.1645A>C), p.S549 N (c.1646G>A), p.G551S (c.1651G>A), p.G551D (c.1652G>A), p.G1244E (c.3731G>A), p.G1349D (c.4046G>A), p.S1251 N (c.3752G>A), p.S1255P (c.3763T>C) |
| Mercaptopurine | NUDT15 | *2, *3 |
| Mercaptopurine | TPMT | *2, *3A, *3B, *3C, *4 |
| Omeprazole | CYP2C19 | *2, *3, *4, *17 |
| Oxcarbazepine | HLA-B | HLA-B*15:02 |
| Phenytoin | HLA-B | HLA-B*15:02 |
| Pimozide | CYP2D6 | *3, *4, *5, *6, *9, *10, *17, *29, *36, *41, copy number variants |
| Rasburicase | G6PD a | NM_001360016.2(G6PD): c.563C>T, c.844G>C, c.376A>G/c.680G>T, c.376A>G/c.202G>A, c.376A>G/c.968T>C, c.376A>G/c.95A>G, c.1360C>T, c.1376G>T |
| Simvastatin | SLCO1B1 | NM_006446.5(SLCO1B1): c.521T>C (*5 or *15) |
| Siponimod | CYP2C9 | *2, *3 |
| Tegafur | DPYD | NM_000110.3(DPYD): c.1905+1G>A (*2A), c.1679T>G (*13), c.2846A>T, [c.1129–5923C>G/c.1236G>A](HapB3) |
| Tetrabenazine | CYP2D6 | *3, *4, *5, *6, *9, *10, *17, *29, *36, *41, copy number variants |
| Voriconazole | CYP2C19 | *2, *3, *4, *17 |
-
aThe frequencies of reduced function alleles in G6PD vary depending on the country and ethnic group. Therefore, G6PD genotyping must be studied individually. Some of G6PD alleles are included in this Table. Further information on the pharmacogenetic tests included in the catalogue of the Spanish National Healthcare System can be consulted in the link: https://cgen.sanidad.gob.es/#/consulta-general.
Once the appropriate regulatory development is in place, each Autonomous Community will need to establish the necessary circuits to ensure adequate patient access. To this end, some regional groups have already started working, and important developments will come throughout this year.
Overview of PGx practice in other countries
Although this review focuses in Spain, PGx implementation approaches have been launched and developed worldwide since the early 2000s. Therefore, we deemed necessary to give a broader overview of these initiatives (summary tables with main worldwide PGx initiatives are available in [72], 73]).
In the United States, several institutions are involved in projects such as the Electronic Medical Records and Genomics Network–Pharmacogenomics Study (eMERGE-PGx) [74], Implementing GeNomics In practice (IGNITE) [75], the St. Jude Children’s Research Hospital institution-wide preemptive pharmacogenomics program (PG4KDS) [76], the Pharmacogenomics Research Network (PGRN) [77] or the Pharmacogenomics Resource for Enhanced Decisions in Care and Treatment (PREDICT) [78], among others. In Europe and Canada pre-emptive single gene testing have been implemented as examples of clinically actionable PGx testing with sufficient clinical evidence for patient benefit. One example being DPYD genotyping in patients prior to fluoropyrimidine chemotherapy [79], [80], [81]. Moreover, in the European Union, the Ubiquitous Pharmacogenomics Consortium has recently published the results of the PREPARE study [73], 82]. An open-label, multicentre, controlled, cluster-randomised, crossover implementation study of a 12-gene pharmacogenetic panel in seven European countries (Austria, Greece, Italy, the Netherlands, Slovenia, Spain, and the UK) demonstrating that genotype-guided treatment significantly reduced the incidence of clinically relevant adverse drug reactions and was feasible across diverse European health-care system organisations and settings [82].
Moving to Asia, the South East Asian Pharmacogenomics Research Network (SEAPharm) program was established by Korea, Indonesia, Malaysia, Taiwan, and Thailand, to conduct trial studies of adverse drug effects and develop guidelines adapted to Asian populations, which could guide drug use and prove useful in disease prediction/diagnosis [83], 84]. Other countries include Qatar, with the Qatar Pharmacogenetics Clinical Applications and Research Enhancement Strategies aiming to set a roadmap for optimizing PGx research and clinical implementation on a national scale [85]; Lebanon, with a handful of places performing PGx testing and/or research (e.g. the American University of Beirut Medical Center Molecular Diagnostics Laboratory) and participating in online Pharmacogenomics and Personalized Medicine (OPPM) courses in collaboration European partners and Egyptian institutions [86]; Australia, that recently conducted a national consultation commissioned by the Australian Genomics Health Alliance to identify and develop recommendations for research priorities for implementation of PGx testing in in the country [87], 88] or United Kingdom, where the UK Pharmacogenetics and Stratified Medicine Network, NHS England and Genomics England invited experts from academia, the healthcare sector, industry and patient representatives to come together to discuss the opportunities and challenges on the potential for implementing PGx into the NHS [89].
The abovementioned initiatives present a snapshot of the different stages of PGx implementation worldwide.
Challenges of PGx implementation
Although the clinical implementation of PGx has made significant progress and most agree on the relevance of PGx, a number of hurdles still need to be overcome. It is therefore crucial to outline the current problems to be solved and the challenges ahead. Some of these issues are related to the lack of pharmacogenetic knowledge and experience to interpret PGx results among the healthcare professionals [90], 91]. An extensive survey performed by Dutch pharmacists showed that, despite the general approval of the concept of PGx (99.7 %), less than 20 % of them had used PGx testing or felt adequately informed about PGx testing. Nevertheless, most presented a global interest (88.8 %) in receiving additional training on PGx [92]. Therefore, this aspect is considered a key barrier to translate PGx from research to clinical setting [93]. This could be circumvented with specific PGx education for clinicians, as shown in the U-PGx project where a variety of communication channels were needed throughout the PGx implementation [94]. Another example, in Spain, the SEFF teaching-training programme has developed a series of webinars in Spanish focused on PGx training for clinicians including information for the main genes and drugs used in the clinical setting [68].
Other important barrier to overcome is the lack of clear consensus guidelines in product labels and recommendations from different pharmacogenomic expert groups. Consensus of actionable pharmacogenomic labeling between the FDA and the EMA/FM has been identified only in 54 % out of 180 drugs with PGx information in both entities [95]. Therefore, this issue affects how PGx results are reported, as different guidelines may be applied by different hospitals in the same country. In addition, there is the absence of a standardized reporting format for PGx testing results [77], which seems to be mandatory to facilitate clinicians the understanding of PGx reports. In addition, other problem reported in the clinical setting is an unclear allocation of responsibilities among healthcare practitioners about who should discuss PGx with the patients and apply PGx results in their healthcare [96]. Therefore, a well-defined workflow is needed in any healthcare system to clearly stablish the way in which the PGx testing is ordered, interpreted, incorporated into the patient’s clinical records, explained to them and applied in the routine practice.
On the other hand, on May 26, 2022, after a transitional period of 5 years, the new Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) will fully replace Directive 98/79/EC on in vitro diagnostic medical devices (IVDD), aiming to enhance the safety, performance, and market regulation of IVDs, including genetic tests [97]. Therefore, PGx testing laboratories must prepare for significant changes, including stricter requirements for in-house devices (IH-IVDs), presenting challenges for innovation and the use of lab-developed tests. This new regulation could delay the implementation of the PGx setting in those laboratories not adapted enough.
Lastly, regarding the economic side, a complete review about the cost-effectiveness of PGx testing showed that 57 % drew conclusions in favour of PGx testing, of which 30 % were cost-effective (more effective at an acceptable additional cost) and 27 % were cost-saving (more effective at a lower cost). In addition, if genetic information was freely available, 75 % of economic evaluations would support PGx-guided treatment, of which 25 % would be cost-effective and 50 % would be cost-saving [98]. In this line, pre-emptive genotyping strategy using a pharmacogenetic panel has shown effectiveness and feasibility across diverse European health-care system organisations and settings [82].
These are some of the main hurdles obstructing the implementation of PGx, some of which are currently being addressed by the ongoing initiatives. Moreover, legal and regulatory frameworks as well as ethical considerations (e.g., data protection, confidentiality, health inequalities and genetic diversity) need to be taken into account and further discussed among all parties involved (i.e., healthcare managers, providers, patient representative bodies, the general public, regulatory agencies and governments).
Conclusions
PGx is one of the core elements of PM, which is transforming clinical and biomedical research. Implementation of PGx is likely to be a major driver to mainstream genomics into clinical practice. Nevertheless, the use of PGx testing in routine healthcare is extremely slow and unequal worldwide due to several barriers such as the knowledge gap in PGx in healthcare providers, the modification of the current clinical workflows and infrastructure, as well as the cost effectiveness. In Spain, although the first autonomic communities developing PGx implementation date since 2004, it was not until recently that we experienced an increase in the development of PGx and PM implementation programs and studies. The application of PGx in clinical routine had remained limited due to the fact that Spain has 17 different Autonomous Communities, each one with capacity to manage their own healthcare system. However, thanks to the work and efforts of scientific societies such as the SEFF, of experts in PGx, of healthcare providers and of regional and national governmental parties, the adoption and implementation of PGx in Spain to personalize patient therapy is now a reality with the approval of a national PGx catalogue by the NHS.
Acknowledgments
We are grateful to all the healthcare providers and researchers that have helped us providing information, and to SEFF senior members and SEFF board of directors for their support.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Conceptualization, methodology, data collection and writing original draft: all authors. Review and editing: MA-R, AER-V and RN-T. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interests: The authors state no conflict of interest.
-
Research funding: MA-R received a Postdoctoral Junior Leader–INCOMING Fellowship from “la Caixa” Foundation (ID: 100010434) and from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska Curie grant agreement No. 847648 (fellowship code: LCF/BQ/PI21/11830009). PR has a Juan Rodés fellowship funded by the Instituto de Salud Carlos III (code: JR20/00008). AV-R received a Doctoral Grant FPU by the Spanish Ministry of Science and Innovation (FPU17-04877). The rest of the authors declare no funding.
-
Data availability: Not applicable.
References
1. Roden, DM, McLeod, HL, Relling, MV, Williams, MS, Mensah, GA, Peterson, JF, et al.. Pharmacogenomics. Lancet 2019;394:521–32. https://doi.org/10.1016/s0140-6736(19)31276-0.Search in Google Scholar
2. Doble, B, Schofield, DJ, Roscioli, T, Mattick, JS. Prioritising the application of genomic medicine. NPJ Genom Med 2017;2:35. https://doi.org/10.1038/s41525-017-0037-0.Search in Google Scholar PubMed PubMed Central
3. Relling, MV, Klein, TE. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther 2011;89:464–7. https://doi.org/10.1038/clpt.2010.279.Search in Google Scholar PubMed PubMed Central
4. Ross, CJD, Visscher, H, Sistonen, J, Brunham, LR, Pussegoda, K, Loo, TT, et al.. The Canadian pharmacogenomics network for drug safety: a model for safety pharmacology. Thyroid 2010;20:681–7. https://doi.org/10.1089/thy.2010.1642.Search in Google Scholar PubMed
5. Swen, JJ, Nijenhuis, M, de Boer, A, Grandia, L, Maitland-van der Zee, AH, Mulder, H, et al.. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther 2011;89:662–73. https://doi.org/10.1038/clpt.2011.34.Search in Google Scholar PubMed
6. Picard, N, Boyer, JC, Etienne-Grimaldi, MC, Barin-Le Guellec, C, Thomas, F, Loriot, MA, et al.. Pharmacogenetics-based personalized therapy: levels of evidence and recommendations from the French Network of Pharmacogenetics (RNPGx). Therapie 2017;72:185–92. https://doi.org/10.1016/j.therap.2016.09.014.Search in Google Scholar PubMed
7. Boletin Oficial de las Cortes Generales Senado. Informe de la ponencia de estudio sobre genómica, constituida en el seno de la Comisión de Sanidad. Consumo y Bienestar Social; 2019. [Internet] [cited 2024 May 3]. Available from: https://www.senado.es/legis12/publicaciones/pdf/senado/bocg/BOCG_D_12_341_2574.PDF.Search in Google Scholar
8. IMPACT strategic plan [Internet]. [cited 2024 Sep 22]. Available from: https://impact.isciii.es/.Search in Google Scholar
9. Amstutz, U, Carleton, BC. Pharmacogenetic testing: time for clinical practice guidelines. Clin Pharmacol Ther 2011;89:924–7. https://doi.org/10.1038/clpt.2011.18.Search in Google Scholar PubMed
10. Hospital pharmacy service at virgen Macarena university hospital [internet]. [cited 2023 Jun 3]. Available from: https://www.hospitalmacarena.es/entrada-blog/cartera-de-servicios-farmacia/.Search in Google Scholar
11. Amaro Álvarez, L, Cordero Ramos, J, González Díaz, A, Martínez Escudero, A, Núñez Torres, R. Cribado farmacogenético preventivo, desarrollo y registro en la historia electrónica. In: 18o congreso de la sociedad andaluza de farmacéuticos de hospitales y centros sanitarios. Malaga: Sociedad Andaluza de Farmacéuticos de hospitales y centros Sociosanitarios; 2023. Available from: https://18congreso.safh.org/wp-content/uploads/2023/04/libro-comunicaciones-SAFH18.pdf.Search in Google Scholar
12. Manson, LE, van der Wouden, CH, Swen, JJ, Guchelaar, HJ. The Ubiquitous Pharmacogenomics consortium: making effective treatment optimization accessible to every European citizen. Pharmacogenomics 2017;18:1041–5. https://doi.org/10.2217/pgs-2017-0093.Search in Google Scholar PubMed
13. Andalusian plan for precision and personalized medicine 2023-2027 [Internet]. [cited 2024 Jan 30]. Available from: https://www.juntadeandalucia.es/organismos/saludyconsumo/consejeria/transparencia/planificacion-evaluacion-estadistica/planes/detalle/447883.html.Search in Google Scholar
14. The medical genome project [Internet]. [cited 2024 Jan 2]. Available from: https://www.clinbioinfosspa.es/content/medical-genome-project.Search in Google Scholar
15. Peña-Chilet, M, Roldán, G, Perez-Florido, J, Ortuño, FM, Carmona, R, Aquino, V, et al.. CSVS, a crowdsourcing database of the Spanish population genetic variability. Nucleic Acids Res 2021;49:D1130–7. https://doi.org/10.1093/nar/gkaa794.Search in Google Scholar PubMed PubMed Central
16. Training on precision and personalized medicine (PANMEP) [internet]. [cited 2023 Jul 1]. Available from: https://medicinaprecisionandalucia.iavante.es/.Search in Google Scholar
17. Basque country planning and management committee for personalized medicine [Internet]. [cited 2023 Sep 1]. Available from: https://www.legegunea.euskadi.eus/eli/es-pv/o/2021/03/01/(2)/dof/spa/html/webleg00-contfich/es/.Search in Google Scholar
18. Basque country health research and innovation strategy 2022-2025 [Internet]. [cited 2023 Sep 3]. Available from: https://www.euskadi.eus/estrategia-de-investigacion-e-innovacion-en-salud-2022-2025/web01-a2ikerpr/es/.Search in Google Scholar
19. Basque Country Science. Technology and innovation plan 2030. [Internet]. [cited 2023 Sep 3]. Available from: https://www.euskadi.eus/pcti-2030/web01-a2pcti30/es/.Search in Google Scholar
20. Basque country oncology plan 2018-2023 [Internet]. [cited 2023 Sep 1]. Available from: https://www.euskadi.eus/plan-oncologico-de-euskadi-2018-2023/web01-a2gaixo/es/.Search in Google Scholar
21. Institute of technology and renewable energies – high performance computing [Internet]. [cited 2024 Jan 10]. Available from: https://www.iter.es/portfolio-items/teide-hpc/.Search in Google Scholar
22. Genomics area at institute of Technology and renewable Energies [Internet]. [cited 2024 Jan 10]. Available from: https://www.iter.es/areas/area-genomica/.Search in Google Scholar
23. PHARMA-can project [Internet]. [cited 2024 Jan 10]. Available from: https://www.iter.es/portfolio-items/analisis-de-variantes-farmacogenomicas-utilizando-datos-de-secuenciacion-de-nueva-generacion/.Search in Google Scholar
24. Reference units – healthcare complex of salamanca [Internet]. [cited 2024 Jan 10]. Available from: https://www.saludcastillayleon.es/CASalamanca/es/hospital-universitario-salamanca-nuevo-edificio/acceso-servicios/analisis-clinicos#marco3.Search in Google Scholar
25. Peña-Martín, MC, García-Berrocal, B, Sánchez-Martín, A, Marcos-Vadillo, E, García-Salgado, MJ, Sánchez, S, et al.. Ten years of experience support pharmacogenetic testing to guide individualized drug therapy. Pharmaceutics 2022;14:160. https://doi.org/10.3390/pharmaceutics14010160.Search in Google Scholar PubMed PubMed Central
26. Carrascal-Laso, L, Franco-Martín, MÁ, García-Berrocal, MB, Marcos-Vadillo, E, Sánchez-Iglesias, S, Lorenzo, C, et al.. Application of a pharmacogenetics-based precision medicine model (5SPM) to psychotic patients that presented poor response to neuroleptic therapy. J Pers Med 2020;10:289. https://doi.org/10.3390/jpm10040289.Search in Google Scholar PubMed PubMed Central
27. Catalonia precision oncology program [Internet]. [cited 2023 Sep 1]. Available from: https://scientiasalut.gencat.cat/handle/11351/5692.Search in Google Scholar
28. Catalonia plan to advance in precision and personalized medicine [Internet]. [cited 2023 Aug 1]. Available from: http://www.consorci.org/media/upload/arxius/publicacions/WEB_informe_MPP_v01_2022_02_17.pdf.Search in Google Scholar
29. Llerena, A, Peñas-Lledó, E, de Andrés, F, Mata-Martín, C, Sánchez, CL, Pijierro, A, et al.. Clinical implementation of pharmacogenetics and personalized drug prescription based on e-health: the MedeA initiative. Drug Metab Pers Ther 2020;0. https://doi.org/10.1515/dmpt-2020-0143.Search in Google Scholar PubMed
30. MEDEA project [Internet]. [cited 2024 Jan 10]. Available from: https://www.proyectomedea.es/.Search in Google Scholar
31. Galician genomics medicine group [Internet]. [cited 2023 Oct 10]. Available from: https://www.xenomica.eu/.Search in Google Scholar
32. Galician plan to implement pharmacogenomics in psychiatry [Internet]. [cited 2023 Oct 1]. Available from: https://www.xunta.gal/dog/Publicados/2021/20210621/AnuncioC3K1-110621-0002_es.html.Search in Google Scholar
33. Facal, F, Portela, B, Gil-Rodríguez, A, Barros, F, Maroñas, O, Carracedo, A. Deletion of the CYP2D6 gene as a likely explanation for the serious side effects of the antipsychotic drug pimozide: a case report. Front Pharmacol 2023;14. https://doi.org/10.3389/fphar.2023.1237446.Search in Google Scholar PubMed PubMed Central
34. Ruan, CJ, Olmos, I, Ricciardi, C, Schoretsanitis, G, Vincent, PD, Anıl Yağcıoğlu, AE, et al.. Exploring low clozapine C/D ratios, inverted clozapine-norclozapine ratios and undetectable concentrations as measures of non-adherence in clozapine patients: a literature review and a case series of 17 patients from 3 studies. Schizophr Res 2023;268:293–301. https://doi.org/10.1016/j.schres.2023.07.002.Search in Google Scholar PubMed
35. Schoretsanitis, G, Anıl Yağcıoğlu, AE, Ruan, CJ, Eap, CB, Molden, E, Baptista, T, et al.. Clozapine ultrarapid metabolism during weak induction probably exists but requires careful diagnosis. A literature review, five new cases and a proposed definition. Schizophr Res 2024;268:302–7. https://doi.org/10.1016/10.1016/j.schres.2023.05.010.Search in Google Scholar
36. Abad-Santos, F, Aliño, SF, Borobia, AM, García-Martín, E, Gassó, P, Maroñas, O, et al.. Developments in pharmacogenetics, pharmacogenomics, and personalized medicine. Pharmacol Res 2024;200. https://doi.org/10.1016/j.phrs.2024.107061.Search in Google Scholar PubMed
37. Clinical analysis department at La Rioja healthcare system [Internet]. [cited 2023 Jun 1]. Available from: https://www.riojasalud.es/servicios/analisis-clinicos.Search in Google Scholar
38. Precision and personalized medicine unit at San Pedro Hospital La Rioja [Internet]. [cited 2023 May 1]. Available from: https://www.riojasalud.es/institucion/actualidad/2022/11/el-hospital-universitario-san-pedro-contara-proximamente-con-una-unidad-funcional-de-medicina-personalizada-y-de-precision.Search in Google Scholar
39. La Rioja health strategic plans [Internet]. [cited 2023 Jul 1]. Available from: https://www.riojasalud.es/institucion/planes-estrategicos.Search in Google Scholar
40. Roche Institute. Precision personalized medicine in Spain: map of autonomous communities [internet]; 2019. [cited 2023 Jun 1]. Available from: https://www.institutoroche.es/recursos/publicaciones/181/medicina_personalizada_de_precision_en_espana_mapa_de_comunidades.Search in Google Scholar
41. La Rioja biomedical research center (CIBIR) [Internet]. [cited 2023 May 1]. Available from: https://www.cibir.es/es/grupos-de-investigacion.Search in Google Scholar
42. Clinical pharmacology and pharmacogenetics department at la princesa university hospital [internet]. [cited 2023 May 1]. Available from: https://www.iis-princesa.org/investigacion/area-2/linea-3-grupo-32/.Search in Google Scholar
43. Pharmacogenetics unit at the Gregorio Marañón general university hospital [Internet]. [cited 2023 May 3]. Available from: https://www.comunidad.madrid/hospital/gregoriomaranon/profesionales/monitorizacion-farmacogenetica.Search in Google Scholar
44. Clinical pharmacology service at La Paz university hospital [Internet]. [cited 2023 Jun 3]. Available from: https://www.comunidad.madrid/hospital/lapaz/profesionales/servicios-centrales/farmacologia-clinica.Search in Google Scholar
45. Borobia, AM, Dapia, I, Tong, HY, Arias, P, Muñoz, M, Tenorio, J, et al.. Clinical implementation of pharmacogenetic testing in a hospital of the Spanish national health system: strategy and experience over 3 years. Clin Transl Sci 2018;11:189–99. https://doi.org/10.1111/cts.12526.Search in Google Scholar PubMed PubMed Central
46. Zubiaur, P, Mejía-Abril, G, Navares-Gómez, M, Villapalos-García, G, Soria-Chacartegui, P, Saiz-Rodríguez, M, et al.. PriME-PGx: La Princesa university hospital multidisciplinary initiative for the implementation of pharmacogenetics. J Clin Med 2021;10:3772. https://doi.org/10.3390/jcm10173772.Search in Google Scholar PubMed PubMed Central
47. iPHARMGx project. [cited 2023 Jan 15]; Available from: https://isabial.portalinvestigacion.com/proyectos/22498.Search in Google Scholar
48. Human genotyping-CEGEN unit at CNIO [Internet]. [cited 2023 Oct 1]. Available from: https://www.cnio.es/en/research-innovation/scientific-programmes/human-cancer-genetics-programme/human-genotyping-cegen-unit/.Search in Google Scholar
49. Nunez-Torres, R, Pita, G, Peña-Chilet, M, López-López, D, Zamora, J, Roldán, G, et al.. A comprehensive analysis of 21 actionable pharmacogenes in the Spanish population: from genetic characterisation to clinical impact. Pharmaceutics 2023;15:1286. https://doi.org/10.3390/pharmaceutics15041286.Search in Google Scholar PubMed PubMed Central
50. López-López, D, Roldán, G, Fernández-Rueda, JL, Bostelmann, G, Carmona, R, Aquino, V, et al.. A crowdsourcing database for the copy-number variation of the Spanish population. Hum Genom 2023;17:20. https://doi.org/10.1186/s40246-023-00466-8.Search in Google Scholar PubMed PubMed Central
51. Navarre personalized medicine strategy [Internet]. [cited 2023 Dec 1]. Available from: https://medicinapersonalizada.navarra.es/es/proyectos-estrat%C3%A9gicos-gema.Search in Google Scholar
52. NAGEN program [Internet]. [cited 2023 Jul 1]. Available from: https://www.navarrabiomed.es/en/nagen.Search in Google Scholar
53. Pharmanagen project [Internet]. [cited 2023 Sep 3]. Available from: https://www.navarrabiomed.es/es/investigacion/proyectos/pharmanagen.Search in Google Scholar
54. ICPerMed “best practice in personalised medicine” recognition 2021 [Internet]. [cited 2023 May 15]. Available from: https://www.icpermed.eu/en/icpermed-best-practice-in-personalised-medicine-recognition-2021-winners-941.php.Search in Google Scholar
55. Beloqui-Lizaso, JJ, Teijido, O, Delgado de Mora, L, Maillo, A, Vicuña Arregui, M, Gomez-Cabrero, D, et al.. “PharmaNAGEN”: implementation of pharmacogenomics in the clinical routines of the public health system based on next generation sequencing. In: Personalized and Precision Medicine International Conference. PEMED; 2020. Available from: https://premc.org/doc/PEMED2020/PEMED2020_Book_Of_Abstracts.pdf.Search in Google Scholar
56. Clinical investigation projects AES 2021 call [Internet]. [cited 2024 Feb 3]. Available from: https://firmadoc.isciii.es/firmadoccontroller?action=download&id=16/12/2021-d3aa84e35b.Search in Google Scholar
57. Pharmacogenetics and gene therapy unit at IIS La Fe [Internet]. [cited 2024 Jan 15]. Available from: https://www.iislafe.es/es/apoyo-a-la-investigacion/plataformas-cientifico-tecnologicas/unidad-farmacogenetica/.Search in Google Scholar
58. Castro-Sánchez, P, Talens-Bolós, MA, Prieto-Castelló, MJ, Pitaluga-Poveda, L, Barrera-Ramírez, JA, Corno-Caparrós, A. Genetic variants and enzyme activity in citidin deaminase: relationship with capecitabine toxicity and recommendation for dose adjustment. Farm Hosp 2023;47:127–32. https://doi.org/10.1016/j.farma.2023.03.004.Search in Google Scholar PubMed
59. Pharmacy Department of Miguel Servet University Hospital, Roche Institute. Seminar on clinical implementation and legal, ethical and economic aspects of Pharmacogenetics and Genomics Medicine. [Internet]. Zaragoza; [cited 2010 Jun 18]. Available from: https://s3-eu-west-1.amazonaws.com/contenidos.institutoroche.es/doc/NP_Farmacogenetica_Zaragoza.doc.Search in Google Scholar
60. Central university hospital of Asturias hospital portfolio [Internet]. [cited 2024 Sep 3]. Available from: https://huca.sespa.es/huca/web/contenidos/websdepartam/Cartera%20Laboratorios/CS_Oncologia_Molecular_201706.pdf.Search in Google Scholar
61. University general hospital Morales meseguer portfolio [Internet]. [cited 2024 Sep 3]. Available from: https://www.hematoncologia.com/cartera-de-servicios/laboratorio-de-genomica.Search in Google Scholar
62. Genomic-IMPACT pharmacogenomics [Internet]. [cited 2024 Sep 22]. Available from: https://genomica-impact.es/farmacogenomica/introduccion/.Search in Google Scholar
63. Phillips, EJ, Sukasem, C, Whirl-Carrillo, M, Müller, DJ, Dunnenberger, HM, Chantratita, W, et al.. Clinical pharmacogenetics implementation consortium guideline for HLA genotype and use of carbamazepine and oxcarbazepine: 2017 update. Clin Pharmacol Ther 2018;103:574–81. https://doi.org/10.1002/cpt.1004.Search in Google Scholar PubMed PubMed Central
64. Hicks, JK, Sangkuhl, K, Swen, JJ, Ellingrod, VL, Müller, DJ, Shimoda, K, et al.. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther 2017;102:37–44. https://doi.org/10.1002/cpt.597.Search in Google Scholar PubMed PubMed Central
65. Crews, KR, Monte, AA, Huddart, R, Caudle, KE, Kharasch, ED, Gaedigk, A, et al.. Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin Pharmacol Ther 2021;110:888–96. https://doi.org/10.1002/cpt.2149.Search in Google Scholar PubMed PubMed Central
66. Lee, CR, Luzum, JA, Sangkuhl, K, Gammal, RS, Sabatine, MS, Stein, CM, et al.. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther 2022;112:959–67. https://doi.org/10.1002/cpt.2526.Search in Google Scholar PubMed PubMed Central
67. Johnson, JA, Caudle, KE, Gong, L, Whirl-Carrillo, M, Stein, CM, Scott, SA, et al.. Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther 2017;102:397–404. https://doi.org/10.1002/cpt.668.Search in Google Scholar PubMed PubMed Central
68. SEFF general strategy for the clinical implementation of pharmacogenetics [Internet]. [cited 2024 Jan 4]. Available from: https://seff.es/wp-content/uploads/2023/01/ESTRATEGIA-DE-IMPLAMENTACION-DE-LA-SEFF.pdf.Search in Google Scholar
69. PGx clinical recommendations from the Working Groups of SEFF [Internet]. [cited 2024 Jan 4]. Available from: https://seff.es/recomendaciones-grupos-de-trabajo-de-la-seff/.Search in Google Scholar
70. García-Alfonso, P, Saiz-Rodríguez, M, Mondéjar, R, Salazar, J, Páez, D, Borobia, AM, et al.. Consensus of experts from the Spanish Pharmacogenetics and Pharmacogenomics Society and the Spanish Society of Medical Oncology for the genotyping of DPYD in cancer patients who are candidates for treatment with fluoropyrimidines. Clin Transl Oncol 2022;24:483–94. https://doi.org/10.1007/s12094-021-02708-4.Search in Google Scholar PubMed PubMed Central
71. Catalogue of genetic tests of the portfolio of common services of the NHS. 2023 [Internet] [cited 2024 Jan 15]. Available from: https://www.sanidad.gob.es/organizacion/consejoInterterri/docs/1553.pdf.Search in Google Scholar
72. Krebs, K, Milani, L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Hum Genom 2019;13:39. https://doi.org/10.1186/s40246-019-0229-z.Search in Google Scholar PubMed PubMed Central
73. van der Wouden, C, Cambon‐Thomsen, A, Cecchin, E, Cheung, K, Dávila‐Fajardo, C, Deneer, V, et al.. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther 2017;101:341–58. https://doi.org/10.1002/cpt.602.Search in Google Scholar PubMed
74. Gottesman, O, Kuivaniemi, H, Tromp, G, Faucett, WA, Li, R, Manolio, TA, et al.. The electronic medical records and genomics (eMERGE) network: past, present, and future. Genet Med 2013;15:761–71. https://doi.org/10.1038/gim.2013.72.Search in Google Scholar PubMed PubMed Central
75. Weitzel, KW, Alexander, M, Bernhardt, BA, Calman, N, Carey, DJ, Cavallari, LH, et al.. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genom 2015;9:1. https://doi.org/10.1186/s12920-015-0162-5.Search in Google Scholar PubMed PubMed Central
76. Hoffman, JM, Haidar, CE, Wilkinson, MR, Crews, KR, Baker, DK, Kornegay, NM, et al.. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet 2014;166:45–55. https://doi.org/10.1002/ajmg.c.31391.Search in Google Scholar PubMed PubMed Central
77. Shuldiner, AR, Relling, MV, Peterson, JF, Hicks, K, Freimuth, RR, Sadee, W, et al.. The pharmacogenomics research network translational pharmacogenetics program: overcoming challenges of real-world implementation. Clin Pharmacol Ther 2013;94:207–10. https://doi.org/10.1038/clpt.2013.59.Search in Google Scholar PubMed PubMed Central
78. Pulley, JM, Denny, JC, Peterson, JF, Bernard, GR, Vnencak-Jones, CL, Ramirez, AH, et al.. Operational implementation of prospective genotyping for personalized medicine: the design of the vanderbilt PREDICT project. Clin Pharmacol Ther 2012;92:87–95. https://doi.org/10.1038/clpt.2011.371.Search in Google Scholar PubMed PubMed Central
79. Wu, A, Anderson, H, Hughesman, C, Young, S, Lohrisch, C, Ross, CJD, et al.. Implementation of pharmacogenetic testing in oncology: DPYD-guided dosing to prevent fluoropyrimidine toxicity in British Columbia. Front Pharmacol 2023;14. https://doi.org/10.3389/fphar.2023.1257745.Search in Google Scholar PubMed PubMed Central
80. Medwid, S, Kim, RB. Implementation of pharmacogenomics: where are we now? Br J Clin Pharmacol 2024;90:1763–81. https://doi.org/10.1111/bcp.15591.Search in Google Scholar PubMed
81. de With, M, Sadlon, A, Cecchin, E, Haufroid, V, Thomas, F, Joerger, M, et al.. Implementation of dihydropyrimidine dehydrogenase deficiency testing in Europe. ESMO Open 2023;8. https://doi.org/10.1016/j.esmoop.2023.101197.Search in Google Scholar PubMed PubMed Central
82. Swen, JJ, van der Wouden, CH, Manson, LE, Abdullah-Koolmees, H, Blagec, K, Blagus, T, et al.. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 2023;401:347–56. https://doi.org/10.1016/s0140-6736(22)01841-4.Search in Google Scholar
83. Ang, HX, Chan, SL, Sani, LL, Quah, CB, Brunham, LR, Tan, BOP, et al.. Pharmacogenomics in Asia: a systematic review on current trends and novel discoveries. Pharmacogenomics 2017;18:891–910. https://doi.org/10.2217/pgs-2017-0009.Search in Google Scholar PubMed
84. Chumnumwat, S, Lu, ZH, Sukasem, C, Winther, MD, Capule, FR, Abdul Hamid, AA, et al.. Southeast asian pharmacogenomics research network (SEAPharm): current status and perspectives. Public Health Genomics 2019;22:132–9. https://doi.org/10.1159/000502916.Search in Google Scholar PubMed
85. Abdel-latif, R, Badji, R, Mohammed, S, Al-Muftah, W, Mbarek, H, Darwish, D, et al.. QPGx-CARES: Qatar pharmacogenetics clinical applications and research enhancement strategies. Clin Transl Sci 2024;17:e13800. https://doi.org/10.1111/cts.13800.Search in Google Scholar PubMed PubMed Central
86. Chidiac, L, Yazbeck, H, Mahfouz, R, Zgheib, NK. Pharmacogenomics in Lebanon: current status, challenges and opportunities. Pharmacogenomics J 2024;24:16. https://doi.org/10.1038/s41397-024-00336-z.Search in Google Scholar PubMed
87. Royal Australian College of General Practitioners. Genomics in general practice [Internet] 2023. [cited 2024 Sep 3]. Available from https://www.racgp.org.au/getattachment/a7b97d5a-5b5f-4d4b-ab3b-efa9c08b1d6d/Genomics-in-general-practice.aspx.Search in Google Scholar
88. Australian Genomics. Research priorities for Australian pharmacogenomics. Final report from the pharmacogenomics incubator project working group; 2022. [Internet] [cited 2024 Sep 3]. Available from https://www.australiangenomics.org.au/wp-content/uploads/2022/11/Pharmacogenomics-Incubator-Project-Report_30Aug2022-.pdf.Search in Google Scholar
89. Turner, RM, Newman, WG, Bramon, E, McNamee, CJ, Wong, WL, Misbah, S, et al.. Pharmacogenomics in the UK national health service: opportunities and challenges. Pharmacogenomics 2020;21:1237–46. https://doi.org/10.2217/pgs-2020-0091.Search in Google Scholar PubMed
90. Ewasiuk, E, Emery, J, Reid, G, Saya, S. Implementing pharmacogenomic testing in Australian general practice: an exploratory qualitative study. Pharmacogenomics 2024;25:1–13. https://doi.org/10.1080/14622416.2024.2382078.Search in Google Scholar PubMed PubMed Central
91. Rafi, I, Crinson, I, Dawes, M, Rafi, D, Pirmohamed, M, Walter, FM. The implementation of pharmacogenomics into UK general practice: a qualitative study exploring barriers, challenges and opportunities. J Commun Genet 2020;11:269–77. https://doi.org/10.1007/s12687-020-00468-2.Search in Google Scholar PubMed PubMed Central
92. Bank, PC, Swen, JJ, Guchelaar, HJ. A nationwide survey of pharmacists’ perception of pharmacogenetics in the context of a clinical decision support system containing pharmacogenetics dosing recommendations. Pharmacogenomics 2017;18:215–25. https://doi.org/10.2217/pgs-2016-0138.Search in Google Scholar PubMed
93. Lauschke, VM, Ingelman-Sundberg, M. Emerging strategies to bridge the gap between pharmacogenomic research and its clinical implementation. NPJ Genom Med 2020;5:9. https://doi.org/10.1038/s41525-020-0119-2.Search in Google Scholar PubMed PubMed Central
94. Blagec, K, Swen, JJ, Koopmann, R, Cheung, KC, Crommentuijn – van Rhenen, M, Holsappel, I, et al.. Pharmacogenomics decision support in the U-PGx project: results and advice from clinical implementation across seven European countries. PLoS One 2022;17:e0268534. https://doi.org/10.1371/journal.pone.0268534.Search in Google Scholar PubMed PubMed Central
95. Shekhani, R, Steinacher, L, Swen, JJ, Ingelman-Sundberg, M. Evaluation of current regulation and guidelines of pharmacogenomic drug labels: opportunities for improvements. Clin Pharmacol Ther 2020;107:1240–55. https://doi.org/10.1002/cpt.1720.Search in Google Scholar PubMed PubMed Central
96. Lanting, P, Drenth, E, Boven, L, van Hoek, A, Hijlkema, A, Poot, E, et al.. Practical barriers and facilitators experienced by patients, pharmacists and physicians to the implementation of pharmacogenomic screening in Dutch outpatient hospital care–an explorative pilot study. J Pers Med 2020;10:293. https://doi.org/10.3390/jpm10040293.Search in Google Scholar PubMed PubMed Central
97. Lubbers, BR, Schilhabel, A, Cobbaert, CM, Gonzalez, D, Dombrink, I, Brüggemann, M, et al.. The new EU regulation on in vitro diagnostic medical devices: implications and preparatory actions for diagnostic laboratories. Hemasphere 2021;5:e568. https://doi.org/10.1097/hs9.0000000000000568.Search in Google Scholar
98. Verbelen, M, Weale, ME, Lewis, CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J 2017;17:395–402. https://doi.org/10.1038/tpj.2017.21.Search in Google Scholar PubMed PubMed Central
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- ‘Pharmacogenetics, health and ethnicity in Latin American populations’ call for the “Dr José María Cantú Award 2024”
- Reviews
- Current developments and advancements of 3-dimensional printing in personalized medication and drug screening
- Status of the implementation of pharmacogenetics in clinical practice in Spain: from regional to national initiatives
- Minireview
- CYP2C19 genotype-phenotype correlation: current insights and unanswered questions
- Original Articles
- Pediatric pharmacogenetics: profiling CYP2C8 polymorphisms at King Abdulaziz University Dental Clinic
- Optimizing tacrolimus therapeutic drug monitoring in Tunisian kidney transplant recipients: exploring the variability in bioavailability and the correlation between pharmacokinetic parameters
- Efficacy and safety of a polyherbal formulation in the management of Escherichia coli urinary tract infection
- UHPLC-MS/MS standardized extract of Vernonia amygdalina leaf inhibits CYP2C9 and CYP3A4 activities in hepatic cells of control and streptozotocin-induced diabetic rats
Articles in the same Issue
- Frontmatter
- Editorial
- ‘Pharmacogenetics, health and ethnicity in Latin American populations’ call for the “Dr José María Cantú Award 2024”
- Reviews
- Current developments and advancements of 3-dimensional printing in personalized medication and drug screening
- Status of the implementation of pharmacogenetics in clinical practice in Spain: from regional to national initiatives
- Minireview
- CYP2C19 genotype-phenotype correlation: current insights and unanswered questions
- Original Articles
- Pediatric pharmacogenetics: profiling CYP2C8 polymorphisms at King Abdulaziz University Dental Clinic
- Optimizing tacrolimus therapeutic drug monitoring in Tunisian kidney transplant recipients: exploring the variability in bioavailability and the correlation between pharmacokinetic parameters
- Efficacy and safety of a polyherbal formulation in the management of Escherichia coli urinary tract infection
- UHPLC-MS/MS standardized extract of Vernonia amygdalina leaf inhibits CYP2C9 and CYP3A4 activities in hepatic cells of control and streptozotocin-induced diabetic rats

