Abstract
This study integrates digital technology into the teaching of redox reaction concepts based on the Technological Pedagogical Content Knowledge (TPACK) framework, aiming to addresses the cognitive difficulties in redox reactions. The teaching process involved context introduction, experimental inquiry, conceptual modeling, and concept consolidation. Specifically, fruit batteries serve as authentic problem contexts to characterize the electron transfer process of redox reaction and provide visual evidence for students. Subsequently, relying on digital tools (e.g., current/pH sensors), experiments are designed to conduct multidimensional correlation analysis of observable phenomena (macroscopic), particle behavior (microscopic), symbolic representations (reaction equations), and sensor data, enabling multi-level interpretation of redox reaction processes. This teaching model effectively enabled students to develop an essential understanding of electron transfer in redox reactions and cultivates their chemical core competencies through the integrated cognitive processing of macroscopic phenomena, microscopic mechanisms, symbolic representations, and sensor data analysis. This study provides a new paradigm empowered by technology for the teaching of core concepts in middle school chemistry, which has important theoretical and practical significance.
1 Introduction
As a core concept in secondary school chemistry curricula, oxidation-reduction (redox) reactions underpin numerous chemical and biological processes, such as photosynthesis, electrochemical cell operation, and metallic corrosion. 1 , 2 , 3 Despite this, chemistry education research indicates that teaching and learning redox concepts pose persistent difficulties in secondary chemistry. 4 The topic’s abstract theoretical nature renders it particularly challenging. 5 Consequently, instructors struggle to effectively demonstrate reaction mechanisms, while students find identifying reactions, assigning oxidation states, and balancing equations difficult. 6
Redox reactions entail complex interactions among multiple species, necessitating an integrated grasp of macroscopic and microscopic principles. Conventional teaching approaches often emphasize theoretical abstraction rather than explicit electron transfer analysis. This hinders students’ ability to visualize the fundamental mechanisms driving these processes. To improve student comprehension of redox concepts, educators have explored diverse pedagogical approaches. Research suggests that framing chemical knowledge within the three representational levels (macroscopic, submicroscopic, and symbolic) promotes deep conceptual understanding. Yet, students frequently struggle to integrate these levels seamlessly, consequently hindering their learning of chemistry, particularly redox concepts. 7 , 8 While existing pedagogy for redox reactions emphasizes symbolic representation (e.g., chemical equations), 9 , 10 instruction solely focused at this level often encourages rote manipulation of equation formalism and oxidation rules. 11 , 12 Given the essential role of experimentation in chemistry education, redox-focused learning activities should prioritize inquiry-based approaches. This enables direct observation of macroscopic manifestations, exposing fundamental reaction mechanisms. 13 Wang et al. developed a mixed-reality model bridging traditional experiments with immersive visualization technologies, enabling dynamic molecular visualization of redox processes. 4 Their research showed that integrating bonding concepts strengthens students’ macroscopic-symbolic representational connections to enhance conceptual learning, while dynamic electron transfer visualization deepens mechanistic understanding.
The Technological Pedagogical and Content Knowledge (TPACK) framework, which synthesizes technological, pedagogical, and content knowledge, has gained substantial research attention in recent years. 14 , 15 This integration empowers the application of contemporary educational technologies (e.g., virtual simulations, digital sensors) to clarify complex reaction principles. 16 Empirical evidence confirms that teacher mastery of technological-pedagogical-content knowledge synthesis enables more effective instructional design, ultimately improving learning outcomes. 17 Molecular simulations in TPACK-informed instruction enhance students’ understanding of dynamic equilibrium beyond traditional lectures. 18 Effective technology integration, however, demands synergistic synthesis of technological, pedagogical, and content knowledge rather than isolated domain expertise. As Pamuk notes, technology’s pedagogical value derives not from mere availability, but from strategic integration with disciplinary logic and instructional design. 19 Crucially, TPACK emphasizes synergistic integration rather than additive combination of knowledge domains. 20 This interdependence transcends technical proficiency, requiring educators to orchestrate technology, pedagogy, and content into unified instructional systems. 21
To address these challenges and improve the teaching and learning of redox reaction concepts, this research aims to explore the following questions:
How can digital technologies be pedagogically integrated within the TPACK framework to enhance redox reaction instruction?
What are the implementation outcomes of a TPACK-aligned digital approach for teaching redox concepts?
2 Theoretical framework
2.1 The TPACK framework
Originally conceptualized by Koehler and Mishra, 23 the TPACK framework builds on Shulman’s foundational Pedagogical Content Knowledge (PCK) model through innovative integration of educational technology. 15 By systematically combining technological dimensions with established educational theories, the TPACK framework marks a paradigm shift in technology-enhanced pedagogy. 24 The TPACK framework integrates three core domains: TK encompassing digital tools and resources, PK addressing teaching methodologies, and CK representing subject-specific expertise. Its critical innovation lies in four intersecting knowledge dimensions: Technological Content Knowledge (TCK) connecting technology with subject matter, Technological Pedagogical Knowledge (TPK) bridging technology and instructional strategies, PCK maintaining Shulman’s original concept of discipline-specific teaching approaches, and TPACK synergistically integrating the three core components. This multidimensional structure is visually represented in Figure 1 through overlapping knowledge domains. The TPACK framework emphasizes the dynamic equilibrium required between technological implementation, pedagogical effectiveness, and content mastery.
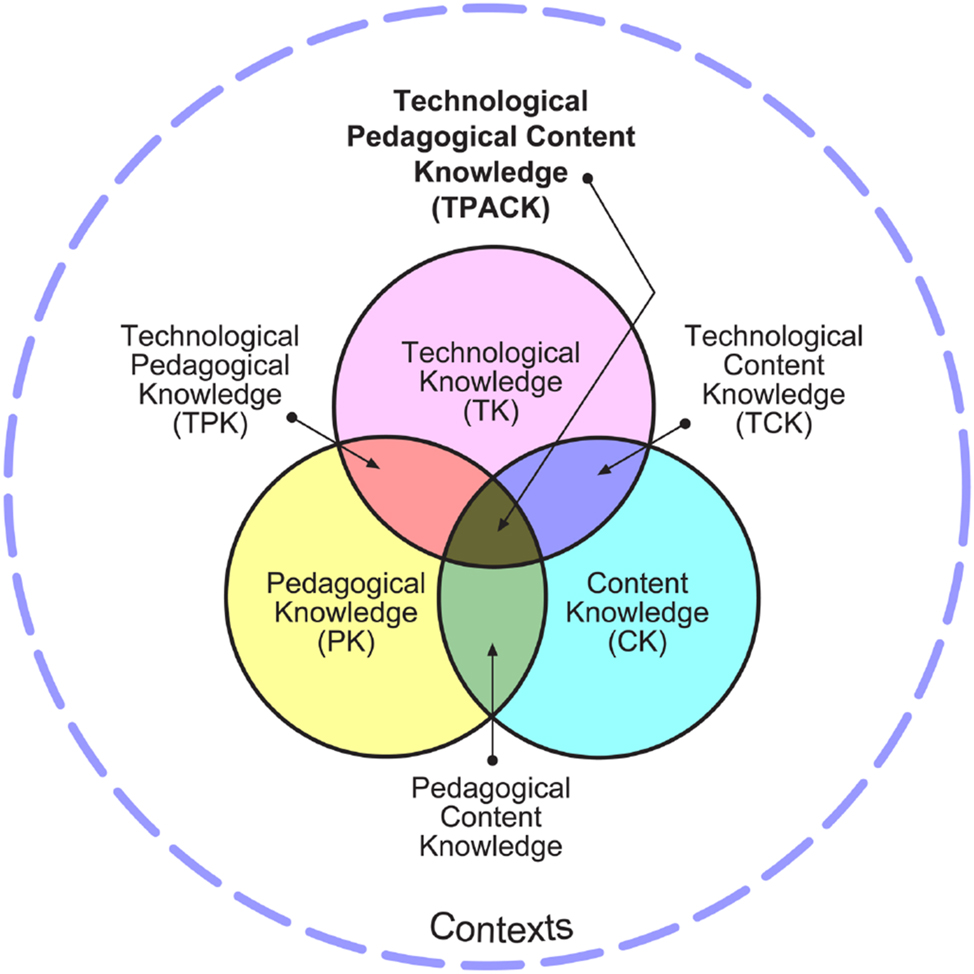
The TPACK framework. 22
2.2 Redox reaction
As a foundational conceptual principle in secondary chemistry curricula, redox reactions permeate the discipline. 25 Redox reactions inherently comprise multiple interrelated and complementary abstract concepts, such as oxidizing/reducing agents and oxidized/reduced species. 26 These concepts establish a cognitive framework for understanding particle-level changes. This framework not only underpins the systematic study of elemental and compound properties and applications, but also necessitates mastery of core competencies, including: judging redox reactions and valence states, identifying oxidizing/reducing agents, analyzing electron transfer quantities, balancing equations, and applying principles to solve practical problems. 27
Traditional teaching approaches typically introduce redox reaction analysis through the direct application of either the oxygen gain-loss model or equation-based models (Figure 2), 28 aiming to establish a unified conceptual framework for students (Figure 3). However, this pedagogical approach exhibits significant shortcomings. Firstly, it omits essential transitional steps: guiding students from macroscopic substance identification to microscopic particle transformation analysis and its correspondence with symbolic representations. More critically, it neglects the contextual nature of inter-particle electron transfer and fails to demonstrate the formation process of the redox concept’s essence. Consequently, students lack a robust mental model for visualizing electron transfer. 28 students frequently develop misconceptions about the core nature and attributes of redox reactions, resulting in merely superficial understanding. Specifically, their grasp of core concepts, including oxidation state changes, electron transfer quantities, oxidizing/reducing agents, and oxidation/reduction products, remains vague and confused. This conceptual ambiguity, compounded by an inadequate grasp of microscopic processes, inevitably erodes student interest in chemistry. Confronted with such abstraction, students primarily rely on rote memorization of mnemonics. Confronted with specific problems, students mechanically apply these mnemonics. Yet the highly abstract concepts and prevalent lack of conceptual understanding hinder the establishment of a fundamental oxidation state-redox reaction linkage, perpetuating conceptual confusion and impeding product prediction and reaction analysis. Additionally, underdeveloped abstract reasoning complicates comprehension of the microscopically grounded stoichiometric equivalence between electrons gained/lost and total transferred electrons. These cumulative cognitive barriers ultimately undermine students’ chemistry learning confidence. 29

The traditional teaching idea of “redox reaction”.
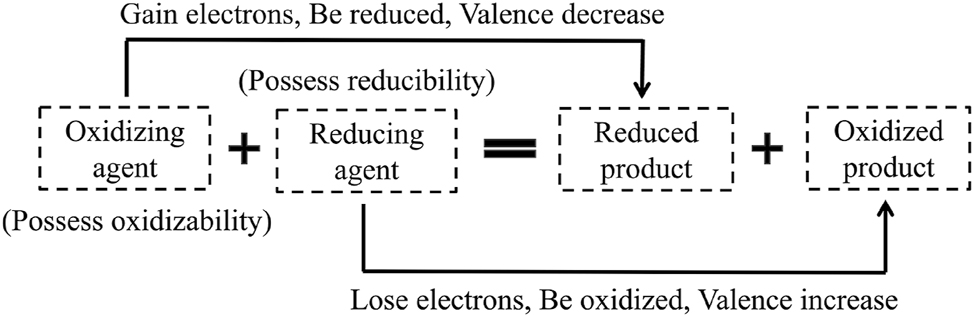
The conceptual framework of redox reaction.
2.3 TPACK-based redox reaction teaching
The application of the TPACK framework aligns with the advancement of educational informatization, thus effectively addressing contemporary learners’ needs. 30 The TPACK-based knowledge framework for redox reactions (Figure 4) necessitates that teachers systematically master subject content while proficiently utilizing technologies like current sensors, pH sensors, and multimedia. This integration transforms subject knowledge into technologized content knowledge (TCK). For instance, sensors can monitor real-time current and pH changes during reactions, while multimedia animations visually demonstrate microscopic processes, enhancing conceptual clarity. Building upon this TCK foundation, teachers must then integrate appropriate pedagogical approaches, such as experimental demonstrations and problem-driven instruction, to develop technological pedagogical knowledge (TPK). This involves, for example, employing multimedia to create problem-solving scenarios or designing sensor-based experiments structured around problem chains. Furthermore, instructional activities should be strategically designed by aligning specific teaching methods with the unique characteristics of redox reaction concepts. Typical experimental demonstrations combined with guided problem-solving can effectively scaffold student understanding and address learning difficulties. Ultimately, this approach achieves comprehensive TPACK integration, where subject knowledge, pedagogy, and technology dynamically interact and mutually reinforce each other.

The knowledge frame of TPACK-based redox reaction.
3 Instructional design and implementation
3.1 Learning goals
Integrating educational research, subject expertise, and practitioner insights, our research team has developed a learning objective system rigorously aligned with the conceptual framework of redox reactions. This framework was systematically developed through rigorous curriculum standard analysis and multidimensional learning characteristic assessment. 4 The specific objectives are as follows:
Students can identify the atomic components and determine oxidation/reduction reactions through oxidation state changes, establishing the relationship between oxidation state variation and electron transfer.
Students can apply the half-reaction method to analyze electron transfer in chemical reactions, recognize electron transfer as the essence of redox reactions, and construct conceptual redox models.
Students can simulate electron gain/loss and the shift of shared electron pairs from the atomic structure perspective, identify the characteristics of redox reactions, and establish their conceptual model.
3.2 Teaching process
This study, with the TPACK framework as its theoretical foundation, focuses on the cognitive difficulties in redox reactions and explores new pathways for teaching empowered by digital technologies. The teaching process for redox reactions based on the TPACK framework is presented in Table 1.
Teaching process of “Redox Reactions” based on the TPACK framework.
| Teaching segments | Teacher’s activities | Students’ activities | Design intent |
|---|---|---|---|
| Context Introduction– Exploration of fruit batteries | (1) Demonstration: Insert zinc and copper sheets into fruit, and connect them to a galvanometer with wires to make its pointer deflect. (2) Question: What changes occur to the metal sheets? |
(1) Observe the deflection of the galvanometer pointer (relating “energy conversion” to “chemical reactions”). (2) Based on the metal activity series, analyze the direction of electron transfer (Zn→Cu). |
Stimulate cognitive conflicts through real situations; connect with life experiences to introduce the core concept of electron transfer. |
| Experimental Inquiry– Revealing the essence of chemical reactions | (1) Demonstrate the battery reaction between zinc and dilute sulfuric acid (copper/zinc sheets inserted into dilute sulfuric acid). (2) Connect current sensors and pH sensors to display changes in current and pH data. (3) Flash demonstration of electrode reactions: Zn loses electrons → Zn2+ enters the solution; H+ gains electrons at the copper sheet → H2 bubbles are generated. |
(1) Simultaneously mark electrode phenomena (zinc sheet dissolving, bubbles of gas forming on the copper sheet). (2) Analyze the changes in current-time and pH-time curves. (3) Write the ionic equations for the electrode reactions,zinc electrode: Zn – 2e− → Zn2+; copper electrode: 2H+ + 2e− → H2; overall reaction: Zn + H+ → Zn2+ + H2. |
Utilize digital technologies to visualize microscopic electron changes and establish the cognition that redox reactions are accompanied by electron transfer. |
| Conceptual Modeling– Introducing Electron Transfer | (1) Guide students to analyze the microscopic essence of the reaction and establish concepts related to redox reactions: oxidizing agent (H+), reducing agent (Zn), oxidation reaction, reduction reaction, oxidation product, and reduction product. (2) Display diagrams of the formation of sodium chloride and hydrogen chloride, and compare the differences in electron transfer between the formation processes of NaCl (ionic) and HCl (covalent). (3) Organize discussions on the determination of oxidation numbers in ionic compounds and covalent compounds. |
(1) Listen and learn chemical terminology. (2) Discuss and analyze to establish the relationship between electron gain/loss and changes in valence: the valence of Zn increases (losing electrons → oxidation reaction, reducing agent), and Zn2+ is the oxidation product; the valence of H+ decreases (gaining electrons → reduction reaction, oxidizing agent); H2 is the reduction product. (3) Understand that electron transfer occurs in various forms such as “gain/loss” and “shift”, and apply valence to determine oxidation numbers. |
Develop students’ particle-based thinking and establish the relationship between electron gain/loss and changes in valence. |
| Concept Consolidation– Deepening the characteristics of electron transfer | (1) Digital platform demonstrates the double-line bridge notation method and displays chemical reaction equations. (2) Concept discrimination: Contrast non-redox reactions and emphasize the unchanged valence of elements. |
(1) Draw double-line bridges and mark the number of electrons gained or lost. (2) Interactive Venn diagram correlating the four basic reaction types with redox reactions. |
Analyze and summarize the relationship between valence changes and electron transfer, and establish criteria for classifying chemical reactions |
Section 1: Context Introduction– Exploration of fruit batteries
【Creating situation】The teacher demonstrates inserting zinc and copper electrodes into a fruit and connecting them to a galvanometer (Figure 5), then asks students to explain why the galvanometer needle deflects and what chemical changes occur on the metal surfaces.

Diagram of fruit batteries.
【Communication and discussion】Students observe the galvanometer deflection, correlate the energy conversion (from chemical to electrical) with redox processes, and deduce the direction of electron transfer (Zn → Cu) using the metal reactivity series.
【Explanation】The teacher explains that organic acids are present in fruits, then substitutes sulfuric acid for the fruit’s acid in the electrochemical system to investigate the microscopic reaction mechanism, thereby facilitating mechanistic analysis of electron transfer processes.
【Design intent】 Using a fruit batteries in a real-world scenario triggers cognitive conflict and stimulates inquiry. Connecting to students’ life experiences with everyday objects (e.g., metals, fruits) naturally introduces the core concept of electron transfer, establishing a conceptual bridge for knowledge integration.
Section 2: Experimental Inquiry– Revealing the essence of chemical reactions
【Experimental demonstration】The teacher demonstrates a galvanic cell (Figure 6) by immersing zinc and copper electrodes in dilute sulfuric acid while initiating real-time sensor data acquisition.
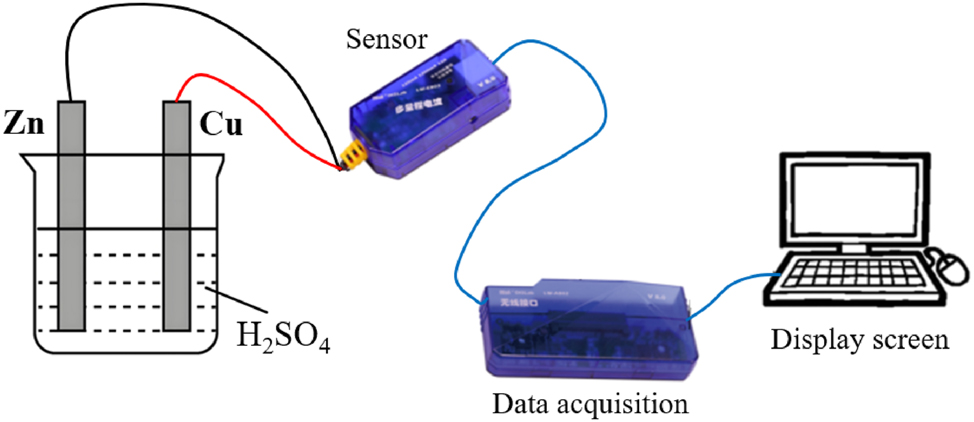
Schematic of a galvanic cell setup (Zn/Cu electrodes in H2SO4).
【Question】The instructor reminds students to carefully observe the phenomena at the zinc and copper electrodes and explain the trends in data synchronously collected by the current sensor and pH sensor.
【Response】Students recorded the following experimental observations: the zinc electrode gradually dissolved, while gas evolved at the copper electrode. Synchronous sensor data showed that the current value decreased over time (Figure 7a), indicating a reduction in the rate of electron transfer and, consequently, a decline in reaction rate. Meanwhile, the increasing pH value confirmed a decrease in H+ concentration, signifying reduced solution acidity (Figure 7b and c).

Monitored reaction data for the Zn-Cu cell. (a) Time-current curve, (b) initial pH value, (c) pH after a reaction period.
【Further question】The teacher uses a Flash animation (Figure 8) to demonstrate the electrode reaction process, clearly illustrating the microscopic changes: zinc loses electrons to form Zn2+ ions that enter the solution, while H+ ions gain electrons at the copper electrode to generate H2 gas bubbles. Key steps are replayed to facilitate student understanding. Based on this microscopic animation, students are prompted to consider how to write the ionic equations for the electrode reactions. From the perspective of microscopic particle changes (electron transfer and ion transformations), they are guided to derive the ionic equations for the zinc and copper electrodes, then the overall reaction, with instruction to label the changes in element valences before and after the reaction.

Visualization of the microscopic reaction mechanism for Zn/Cu electrodes in H2SO4.
【Communication and discussion】Guided by the instructor and Flash demonstration, students formulate ionic equations for the electrode and overall reactions (Figure 9), labeling valency changes, and establish connections between microscopic reactions and macroscopic phenomena through this exercise.

Equations of electrode reactions.
【Extended question】The teacher continues: If other metals (e.g., iron) are used in combination with dilute sulfuric acid and a copper sheet, how would the reaction and electron transfer change? This guides students to transfer and apply their knowledge, deepening their understanding that redox reactions involve the directional flow of electrons.
【Response】Iron is more reactive than copper. It reacts with dilute sulfuric acid: iron atoms lose 2 electrons to form Fe2+, with their oxidation state increasing from 0 to +2. Hydrogen ions gain 2 electrons to form H2, with their oxidation state decreasing from +1 to 0. Copper does not participate in the reaction in this system, and the direction of electron transfer is from iron to hydrogen ions.
【Design intent】The teacher guides observation and explanation to support students in effectively labeling phenomena and analyzing data curves, achieving the objective of “transforming abstract concepts (electron flow, pH) into observable data using sensors.” This exposes students to experimental data and ensures mastery of the reaction’s underlying principles. Using Flash animations and guided derivation, students translate microscopic processes into symbolic representations. Through writing, annotation, and connecting multiple representations, they achieve the “quadruple representation” (from macroscopic phenomena to data interpretation, then to submicroscopic understanding, and finally to symbolic expression). Analysis of valence changes establishes the core concept that “redox reactions involve directional electron flow.” Scaffolded questioning then deepens understanding, enabling students to not only grasp the specific reaction but also transfer core knowledge flexibly. This progressive design ensures deep comprehension and application.
Section 3: Conceptual Modeling– Introducing electron transfe
【Concept Introduction】The teacher guided students in reviewing the experimental process: from observing phenomena and collecting/analyzing data, to understanding microscopic animations and writing symbolic representations. This established connections between directional electron flow (Zn loses electrons, H+ gains electrons) and the essence of chemical reactions (electron transfer triggers changes in both matter and energy). This led to the introduction of oxidation-reduction reaction concepts. Zinc acts as the reducing agent, undergoing oxidation (loss of electrons) to form Zn2+. Conversely, H+ acts as the oxidizing agent, undergoing reduction (gain of electrons) to form H2. The teacher then clearly defined and explained each key term, including oxidizing agent, reducing agent, oxidation reaction, reduction reaction, oxidation product, and reduction product.
【Projection display】The teacher presents a diagram (Figure 10) demonstrating the formation of sodium chloride (NaCl) and hydrogen chloride (HCl), depicting electron transfer in the ionic compound and electron sharing in the covalent compound. Using the diagram and knowledge of oxidation states, the teacher guides students to analyze electron transfer. For NaCl, sodium’s oxidation state changes from 0 to +1 (losing an electron), while chlorine’s changes from 0 to −1 (gaining an electron). For HCl, the shared electron pair is polarized towards chlorine, resulting in oxidation states of −1 for Cl and +1 for H. This demonstrates that electron transfer encompasses both complete transfer (gain/loss) and polarization (sharing).

Models of atomic structure in chemical change processes.
【Observational learning】Students observe atomic structure models and formation diagrams while listening to the teacher explain key chemical terminology (e.g., electron gain/loss, electron pair polarization). They learn to describe electron transfer using precise chemical language. By correlating changes in oxidation states, they analyze electron transfer in NaCl and HCl, distinguishing electron gain/loss in ionic compounds from electron pair polarization in covalent compounds. This builds their understanding of the diverse forms of electron transfer.
【Question】The teacher raises questions: How to determine the oxidation number (valence) in covalent compounds (such as HCl, H2O, etc.)? What is the relationship between electron pair shift and changes in oxidation number?
【Communication and discussion】Working in groups, students discuss how oxidation states indicate electron transfer. They clarify the relationship between electron pair polarization, oxidation state changes, and electron transfer in covalent compounds, contrasting this with electron gain/loss in ionic compounds. This consolidates their understanding of the diverse forms of electron transfer, namely complete transfer (gain or loss) and polarization, and their connection to changes in oxidation states.
Section 4: Concept Consolidation– Deepening the characteristics of electron transfer
【Explanation】Take the reactions (3CO + Fe2O3 = 2Fe + 3CO2, KClO3 + 6HCl = KCl + 3Cl2↑ + 3H2O) as examples (Figure 11), the teacher demonstrates the double-bridge notation process for redox reactions. The teacher explains the key specifications and principles of double-bridge notation, including the non-crossing rule of oxidation states: When atoms of the same element in different oxidation states undergo a redox reaction, the higher state must decrease and the lower state must increase, forming an intermediate oxidation state between them.
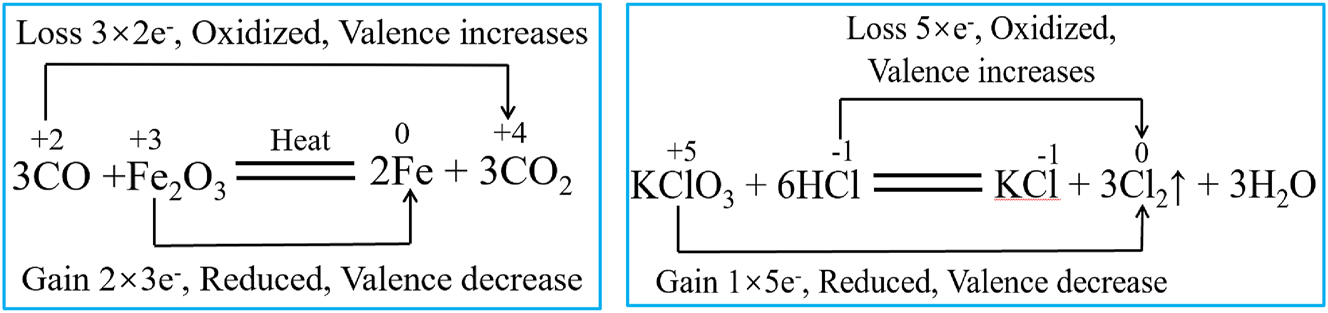
Double-line bridge method represents the reaction equation.
【Observational learning】Students review double-bridge notation examples to learn the labeling methodology and standards. Clearly identify how the notation demonstrates the correspondence between electron transfer (gain/loss or polarization) and changes in oxidation state.
【Task Arrangement】Diverse reaction types, including those with ionic compounds and covalent compounds involving electron gain/loss, or shift, are selected. Students practice using double-line bridge notation to track electron transfer counts and valence changes, reinforcing their understanding of electron transfer mechanisms. Teachers circulate to guide, correct nonstandard notations, and address questions. Through this practice, students consolidate knowledge of electron transfer forms, deepen comprehension of valence-electron relationships, refine notation skills, and build a conceptual framework for redox reactions.
【Design intent】Using atomic structure models, students deepen their understanding of diverse electron transfer forms (gain/loss or sharing) and their relationship to valence changes. Digital platforms demonstrate double-line bridge notation, analyzing electron transfer mechanisms to reinforce students’ grasp of its characteristics and valence linkages. This enhances chemical representation skills and microscopic thinking.
【Question】The teacher presents a non-redox reaction example, guiding students to analyze pre-/post-reaction valence states. This highlights unchanged element valences, contrasting redox reactions (electron transfer → valence change) with non-redox reactions (no electron transfer → stable valences). Students then recall the four basic reaction types. Using interactive Venn diagrams, they categorize these types (combination, decomposition, displacement, double displacement) based on electron transfer presence (indicated by valence changes).
【Response】Students engaged in group discussions to summarize the characteristics of redox and non-redox reactions. Guided by the teacher, they explored the connections between the four fundamental reaction types and redox reactions, creating an interactive Venn diagram to establish their relationship (Figure 12).
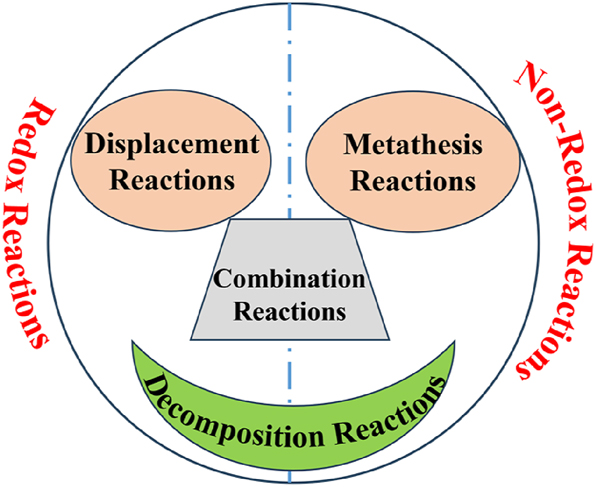
Relationship between redox reactions and basic reaction types.
【Design intent】By creating an interactive Venn diagram to connect the four fundamental reaction types with redox reactions, students consolidate the classification criteria (based on electron transfer) and gain a holistic understanding of chemical reaction categories. While analyzing electron transfer (reflected in changes in oxidation states) across reaction types, they consistently apply a particulate-level perspective. This reinforces their grasp of the relationship between electron gain/loss and changes in oxidation state, helping them build a systematic framework for classifying reactions and develop chemical thinking.
4 Assessments
4.1 Participants
To evaluate the effectiveness of the TPACK-based redox reaction teaching approach, this study conducted a controlled experiment with two parallel first-year high school classes of comparable academic standing. The experimental and control classes were comparable in size (experimental: n=59; control: n=60). The experimental class received the technology-integrated teaching method, while the control class maintained a traditional approach, with both taught by the same instructor. All participants completed identical post-intervention tests simultaneously to ensure assessment consistency.
4.2 Methods
Following the intervention, both classes completed a pen-and-paper assessment on the target redox reaction concepts. The assessment, developed by the research team and validated by two chemistry education experts, was administered under identical time conditions. After anonymous scoring by the instructor using a predefined rubric, data were analyzed with SPSS 20.0.
4.3 Results and discussion
We compared the pre-test chemistry scores (recent monthly exam results) of the experimental and control classes. As shown in Table 2, the classes had comparable sample sizes (N=60 vs. N=59) and nearly identical mean scores (62.47 vs. 62.49), indicating comparable initial performance. To statistically assess any significant difference, an independent samples t-test was conducted. Table 3 shows Levene;s test for equality of variances was not significant (p = 0.59 > 0.05). Therefore, the results assuming equal variances were interpreted, revealing a non-significant two-tailed p-value (0.99 ≥ 0.05). This confirms that the experimental and control groups had similar prior knowledge levels at baseline, with no statistically significant difference. Consequently, these classes were deemed suitable for the subsequent experiment.
Analysis of pre-test chemistry scores.
| Class | N | Mea | Std. deviation | Std. error mean |
|---|---|---|---|---|
| Control class | 60 | 62.47 | 8.18 | 1.06 |
| Experimental class | 59 | 62.49 | 8.39 | 1.09 |
Independent samples t-test of pre-test scores.
| Levene’s test | t-test for equality of means | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean difference | Std. error difference | 95 % confidence interval of the difference | ||
| Lower | Upper | ||||||||
| Equal variances assumed | 0.30 | 0.59 | −0.16 | 117 | 0.99 | −0.25 | 1.52 | −3.03 | 2.98 |
| Equal variances not assumed | −0.16 | 116.8 | 0.99 | −0.25 | 1.52 | −3.04 | 2.99 | ||
Following the teaching experiment, post-test scores of the experimental and control classes were analyzed. As presented in Table 4, the experimental class achieved a significantly higher mean score (48.83) than the control class (42.45), indicating a performance difference. To statistically verify this difference, an independent samples t-test was conducted. Table 5 shows Levene’s Test for Equality of Variances was not significant (F = 0.20, p = 0.66 > 0.05), confirming homogeneity of variance. Consequently, the t-test results assuming equal variances were interpreted, revealing a statistically significant difference (two-tailed p = 0.03 < 0.05) in post-test scores between the classes. This significant difference suggests that the TPACK-based instructional design had a positive effect on student learning outcomes.
Analysis of post-test chemistry scores.
| Class | N | Mea | Std. deviation | Std. error mean |
|---|---|---|---|---|
| Control class | 60 | 42.45 | 16.76 | 2.16 |
| Experimental class | 59 | 48.83 | 14.79 | 1.93 |
Independent samples t-test of post-test scores.
| Levene’s test | t-test for equality of means | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean difference | Std. error difference | 95 % confidence interval of the difference | ||
| Lower | Upper | ||||||||
| Equal variances assumed | 0.20 | 0.66 | −2.20 | 117 | 0.03 | −6.38 | 2.90 | −12.12 | −0.63 |
| Equal variances not assumed | −2.20 | 115.7 | 0.03 | −6.38 | 2.90 | −12.11 | −0.64 | ||
4.4 Instructor reflections
Teachers effectively restructured courses using digital technologies to prioritize individual learning experiences. By employing sensors (e.g., current, pH), abstract concepts like electron transfer and ion concentration changes were transformed into real-time visual data, addressing the teaching challenge of visualizing microscopic processes in redox reactions. Flash animations dynamically visualized electrode reactions, vividly illustrating the microscopic processes of electron transfer and ion migration. This approach overcame the static limitations of traditional chalkboard instruction, thus significantly reducing students’ learning barriers in understanding half-reaction notation and electrode reaction equations. Many students demonstrated performance exceeding course expectations and learning objectives. While collaborative learning proved beneficial for students, those with weaker foundational knowledge faced challenges in processing experimental data and comprehending microscopic concepts. Higher-order cognitive tasks, such as designing electrode configurations or explaining current variations, were predominantly led by high-achieving students, limiting others’ full engagement in the complete “variable control-evidence reasoning” process. Although real-time sensor data enhanced perceptual accessibility, an exclusive focus on graphical trends (e.g., current intensity values) among some students distracted from deeper mechanistic reasoning. While Flash animations depicted predetermined electron transfer paths, they constrained students’ understanding of electron motion randomness (e.g., omitting random ion collisions in solution), potentially fostering a mechanistic view of “directed current flow.” Visualizing electron redistribution during covalent bond cleavage/formation, essential for concepts like oxidation state determination in HCl, remained challenging, often relying on teacher explanation due to limited dynamic representation tools. Restricted sensor availability limited hands-on instrumentation experience for some students, diminishing technology’s cognitive benefits. Furthermore, compressed lesson time curtailed deep analysis of experimental data, preventing thorough exploration of underlying causes, such as polarization effects, for phenomena like the nonlinear relationship between electrode activity differences and current intensity.
5 Conclusions
This study implemented digital technology within the TPACK framework to enhance the teaching of redox reaction concepts. This approach enhanced representational competence across macroscopic, microscopic, symbolic, and data domain. By organically combining the essence of disciplinary teaching knowledge, teaching methods, and technological knowledge, we have created an innovative and effective teaching experience. Using digital sensors to design engaging experiments, it could offer a vivid and intuitive understanding of redox reactions. This not only enhances the timeliness, thoughtfulness, and cutting-edge nature of the teaching content but also transcends mere knowledge transmission. Students are encouraged to learn from multiple perspectives, including knowledge acquisition, critical thinking, and methodological skills. In the teaching practice, teachers are expected to deeply explore both disciplinary teaching knowledge and pedagogical knowledge while skillfully applying technological knowledge. This process helps to solidify and complete their TPACK framework, laying a robust foundation for future TPACK-based teaching.
Funding source: Research Project on Teaching Reform of Hubei Normal University
Award Identifier / Grant number: 2024020
Acknowledgments
The authors gratefully acknowledge the participation and valuable contributions of the students and chemistry educators involved in this pedagogical research. Special thanks are extended to Hubei Normal University for providing the experimental facilities and technical support essential for implementing digital sensor-based activities. We also recognize the pioneering researchers in TPACK and chemistry education whose foundational studies informed the theoretical framework of this investigation.
-
Research ethics: No ethical aspects are relevant in this development project.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Use of Large Language Models, AI and Machine Learning Tools: No AI or machine learning tools were used in the design, analysis, or writing of this study.
-
Conflict of interest: No potential conflict of interest was reported by the authors.
-
Research funding: This work was partially supported by the Research Project on Teaching Reform of Hubei Normal University (2024020).
-
Data availability: All data generated or analyzed during this study are included in this published article.
References
1. Goes, L. F.; Fernandez, C.; Eilks, I. The Development of Pedagogical Content Knowledge about Teaching Redox Reactions in German Chemistry Teacher Education. Educ. Sci. 2020, 10 (7), 170; https://doi.org/10.3390/educsci10070170.Suche in Google Scholar
2. Chen, D.; Huang, R.; Gong, W. Study on Concept of Chemistry Key Literacy Education Based on Subject Understanding. Chin. Educ. Chem. 2025, 2, 39–44.Suche in Google Scholar
3. Soudani, M.; Sivade, A.; Cros, D.; Mèdimagh, M. S. Transferring Knowledge from the Classroom to the Real World: Redox Concepts. Sch. Sci. Rev. 2000, 82, 65–92.Suche in Google Scholar
4. Wang, L.; Hodges, G.; Lee, J. Connecting Macroscopic, Molecular, and Symbolic Representations with Immersive Technologies in High School Chemistry: The Case of Redox Reactions. Educ. Sci. 2022, 12, 428; https://doi.org/10.3390/educsci12070428.Suche in Google Scholar
5. Österlund, L. L.; Berg, A.; Ekborg, M. Redox Models in Chemistry Textbooks for the Upper Secondary School: Friend or Foe? Chem. Educ. Res. Pract. 2010, 11, 182–192; https://doi.org/10.1039/c005467b.Suche in Google Scholar
6. Gao, J.; Wang, F. F.; Chen, P.; Du, H.; Liao, Z. G. Investigation on TSPCK Level of Chemistry Teachers in Senior High School with the Topic of Redox Reaction. Chin. J. Chem. Educ. 2024, 45 (15), 88–95.Suche in Google Scholar
7. Johnstone, A. H. The Development of Chemistry Teaching: A Changing Response to Changing Demand. J. Chem. Educ. 1993, 70, 701–705; https://doi.org/10.1021/ed070p701.Suche in Google Scholar
8. Talanquer, V. Macro, Submicro, and Symbolic: The Many Faces of the Chemistry “Triplet”. Int. J. Sci. Educ. 2011, 33, 179–195; https://doi.org/10.1080/09500690903386435.Suche in Google Scholar
9. Garnett, P. J.; Treagust, D. F. Conceptual Difficulties Experienced by Senior High School Students of Electrochemistry: Electric Circuits and Oxidation-Reduction Equations. J. Res. Sci. Teachnol. 1992, 29, 1079–1099; https://doi.org/10.1002/tea.3660290204.Suche in Google Scholar
10. De Jong, O.; Acampo, J.; Verdonk, A. Problems in Teaching the Topic of Redox Reactions: Actions and Conceptions of Chemistry Teachers. J. Res. Sci. Teachnol. 1995, 32, 1097–1110; https://doi.org/10.1002/tea.3660321008.Suche in Google Scholar
11. Brandriet, A. R.; Bretz, S. L. The Development of the Redox Concept Inventory as a Measure of students’ Symbolic and Particulate Redox Understandings and Confidence. J. Chem. Educ. 2014, 91, 1132–1144; https://doi.org/10.1021/ed500051n.Suche in Google Scholar
12. Barke, H. D.; Hazari, A.; Yitbarek, S. Misconceptions in Chemistry: Addressing Perceptions in Chemical Education; Springer: Berlin/Heidelberg, Germany, 2009; pp. 21–36.Suche in Google Scholar
13. Kennepohl, D. Laboratory Activities to Support Online Chemistry Courses: A Literature Review. Can. J. Chem. 2021, 99, 851–859; https://doi.org/10.1139/cjc-2020-0506.Suche in Google Scholar
14. Koehler, M. J.; Mishra, P.; Cain, W. What is Technological Pedagogical Content (TPACK)? J. Educ. 2013, 193 (3), 13–19; https://doi.org/10.1177/002205741319300303.Suche in Google Scholar
15. Shulman, L. S. Those who Understand: Knowledge Growth in Teaching. Educ. Res. 1986, 15 (2), 4–14; https://doi.org/10.2307/1175860.Suche in Google Scholar
16. Niess, M. L. Investigating TPACK: Knowledge Growth in Teaching with Technology. J. Educ. Comput. Res. 2011, 44 (3), 299–317; https://doi.org/10.2190/ec.44.3.c.Suche in Google Scholar
17. Harris, J.; Mishra, P.; Koehler, M. Teachers’ Technological Pedagogical Content Knowledge and Learning Activity Types. J. Res. Technol. Educ. 2009, 41 (4), 393–416; https://doi.org/10.1080/15391523.2009.10782536.Suche in Google Scholar
18. Donnelly, D. F.; Linn, M. C.; Ludvigsen, S. Impacts and Characteristics of Computer-Based Science Inquiry Learning Environments for Precollege Students. Rev. Educ. Res. 2014, 84 (4), 572–608; https://doi.org/10.3102/0034654314546954.Suche in Google Scholar
19. Pamuk, S.; Ergun, M.; Cakir, R.; Yilmaz, H. B.; Ayas, C. Exploring Relationships Among TPACK Components and Development of the TPACK Instrument. Educ. Inf. Technol. 2015, 20, 241–263; https://doi.org/10.1007/s10639-013-9278-4.Suche in Google Scholar
20. Koehler, M. J.; Mishra, P.; Kereluik, K.; Shin, T. S.; Graham, C. R. The Technological Pedagogical Content Knowledge Framework. In Handbook of Research on Educational Communications and Technology; Spector, J.; Merrill, M.; Elen, J.; Bishop, M., Eds.; Springer: New York, 2014.10.1007/978-1-4614-3185-5_9Suche in Google Scholar
21. Voogt, J.; Erstad, O.; Dede, C.; Mishra, P. Challenges to Learning and Schooling in the Digital Networked World of the 21st Century. J. Comput. Assist. Lear. 2013, 29 (5), 403–413; https://doi.org/10.1111/jcal.12029.Suche in Google Scholar
22. Ait Ali, D.; El Meniari, A.; El Filali, S.; Morabite, O.; Senhaji, F.; Khabbache, H. Empirical Research on Technological Pedagogical Content Knowledge (TPACK) Framework in Health Professions Education: A Literature Review. Med. Sci. Educ. 2023, 33 (3), 791–803; https://doi.org/10.1007/s40670-023-01786-z.Suche in Google Scholar PubMed PubMed Central
23. Mishra, P.; Koehler, M. J. Technological Pedagogical Content Knowledge: A Framework for Teacher Knowledge. Teach. Coll. Rec. 2006, 108 (6), 1017–1054; https://doi.org/10.1111/j.1467-9620.2006.00684.x.Suche in Google Scholar
24. Rosenberg, J. M.; Koehler, M. J. Context and Technological Pedagogical Content Knowledge (TPACK): A Systematic Review. J. Res. Technol. Educ. 2015, 47 (3), 186–210; https://doi.org/10.1080/15391523.2015.1052663.Suche in Google Scholar
25. Macciò, D.; Ottonelli, M.; Alloisio, M. Solving Redox Reactions: The Advantages of the Thermodynamic Method. J. Chem. Educ. 2023, 100 (6), 2215–2223; https://doi.org/10.1021/acs.jchemed.2c01151.Suche in Google Scholar
26. Mai, Y. H.; He, Q. H.; Xiao, X. Analysis of Senior High School Students’ Learning of Scientific Principles Based on the Knowledge Space Theory: A Case of Oxidation-Reduction Reaction. Chin. J. Chem. Educ. 2018, 39 (19), 34–40.Suche in Google Scholar
27. Li, X. G.; Yang, R. M. Core Literacy-Oriented Teaching of Scientific Concept: Oxidation-Reduction Reaction. Chin. J. Chem. Educ. 2022, 43 (13), 42–46.Suche in Google Scholar
28. Zhen, W. C. Diagnosis of Learning Difficulties and Teaching Suggestions on Related Concepts of Redox Reaction. Chin. J. Chem. Educ. 2016, 37 (9), 8–15.Suche in Google Scholar
29. Ru, Z. Y.; Liu, W. D.; Zhu, L. Y. Chemical Escape Room Design Based on PowerPoint: Redox Reaction. Chin. J. Chem. Educ. 2023, 44 (15), 95–102.Suche in Google Scholar
30. Valtonen, T.; Eriksson, M.; Kärkkäinen, S.; Tahvanainen, V.; Turunen, A.; Vartiainen, H.; Kukkonen, J.; Sointu, E. Emerging Imbalance in the Development of TPACK-A Challenge for Teacher Training. Educ. Inf. Technol. 2023, 28 (5), 5363–5383; https://doi.org/10.1007/s10639-022-11426-5.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

