Abstract
Presence of moisture is very important for vegetable oils and for corresponding biodiesel because it may cause some problems or accelerate some issues that cannot be ignored. One of the main hindrances of biodiesel is its hygroscopic nature, which accelerates the corrosion of the fuel system of the engines. Thus, this study aims to investigate the effects of moisture on corn biodiesel and its susceptibility to corrosion on different automotive materials such as copper and mild carbon steel. Static immersion tests in corn biodiesel (B100) with different water concentrations (100 ppm, 500 ppm, and 700 ppm) were carried out at 90°C for 1200 h, and the results were compared to that of commercial diesel fuel (B0). After immersion tests, the surface morphology was studied by scanning electron microscopy (SEM), and corrosion products were detected by X-ray diffraction (XRD). The total acid number (TAN) was used to evaluate the changes in acidity of fuel, before and after immersion tests. It was found that under experimental conditions, corn biodiesel is more corrosive than diesel fuel, and the moisture from corn biodiesel has a strong influence on corrosion rate on metals. Copper is more susceptible to corrosion in corn biodiesel than mild carbon steel.
1 Introduction
Stringent environmental regulations, scarcity of non-renewable energy sources, and high price of petroleum and other fuels are some of the factors that make biodiesel a feasible fuel to supplement or replace petro-diesel. Although there are many advantages derived from biodiesel utilization, which include biodegradability, lower exhaust emissions, the absence of sulfur, aromatic hydrocarbons, metals, or crude oil residues, and higher flash point when compared to petro-diesel, biodiesel cannot completely replace hydrocarbon fuels because of issues concerning the availability of feed stocks, food vs. fuel debate, the storage stability strongly influenced by oxidation and auto-oxidation, high sensitivity to light, temperature, contaminants, and moisture (Haseeb et al., 2011; Atadashi et al., 2012). Several researchers (Kaul et al., 2007; Haseeb et al., 2010; Fazal et al., 2011a,b, 2012, 2013; Cursaru et al., 2014) already proved that its vulnerability to oxidation and its strong hygroscopic nature are issues, which cause the corrosive nature of biodiesel.
The largest amount of commercial biodiesel is produced from edible or non-edible vegetable oils and animal fats, in particular, through transesterification process (Antolin et al., 2002; Bari et al., 2002; Saeid et al., 2008; Demirbas, 2009; Kapilan et al., 2009).
By transesterification, the biodiesel produced is from the “first generation”. Transesterification is the reaction of triglycerides existing in vegetable oil in the presence of low-molecular weight alcohols such as methanol or ethanol, using either homogeneous catalysts (sodium hydroxide, potassium hydroxide, sulfuric acid, etc.) or heterogeneous catalysts (enzymes, resins, ion exchange resins, etc.) (Refaat, 2010; Yang et al., 2012).
The overall transesterification reaction is:

After transesterification, biodiesel is separated from glycerol by decantation. The unreacted alcohol is removed from biodiesel by vacuum distillation or flash evaporation, and contaminants such as soap, catalysts, or traces of glycerol are eliminated by water wash followed by centrifugation. The residual water in biodiesel is removed by means of an evaporator or vacuum drier (Ferella et al., 2010).
Biodiesel consists of alkyl mono-esters of fatty acids derived from parent oils or animal fats with high levels of unsaturated and polyunsaturated fatty acids, which make it susceptible to oxidation and, as a result, prone to corrosion. In fact, corrosion and oxidation symbiotically coexist and should be approached simultaneously (Ramos et al., 2009; Cursaru et al., 2013, 2014). Moisture and the hygroscopic nature of biodiesel are some of the factors that influence corrosion and, consequently, its quality. The water content accepted by the standard for biodiesel EN14214 is up to 500 ppm, but there are many situations, starting with the biodiesel synthesis and purification steps that require the presence of water. Besides, during storage and utilization in compression ignition engine, it is impossible to avoid the presence of water that remains in the final biodiesel, in dissolved or in a finely dispersed form. According to Maru et al. (2009), Demirbas (2009), and Atadashi et al. (2012), biodiesel has about 30 times higher tendency for water absorption than petro-diesel, which could be a reasonable cause for higher corrosivity of biodiesel than conventional diesel fuel. The presence of water creates difficulties in the transesterification process by reducing the conversion of triglycerides to biodiesel. In addition, it deteriorates the biodiesel quality, decreases the heat of combustion, accelerates the corrosion of fuel system, speeds up the hydrolysis reaction, favors growth of microbe colonies on tanks or injection system of diesel engines, increases the fuel consumption, decreases the engine performances, and reduces the engine life (Zhang et al., 2003; Gomes et al., 2010; Saleh et al., 2010; Atadashi et al., 2012).
For this reason, storage and utilization of biodiesel as fuel for diesel engine are strongly dependent on water content and on the possibility of decreasing the water content from all preliminary synthesis steps, essential for production and refining of biodiesel.
Thus, the main objective of this study is to evaluate the effects of water/moisture in biodiesel, synthesized from corn oil in particular, on the corrosion behavior of typical materials used for fabrication of automotive parts.
Typical feedstocks for biodiesel production are edible vegetable oils (rapeseed, palm, soy, sunflower, corn, etc.), non-edible vegetable oils (pongamia, jatropha, etc.), animal fat, waste vegetable oils and other feedstocks (algae, biomass, fungi, and bacteria) (Scott et al., 2010; Dantas et al., 2011; Siddharth et al., 2011).
Generally, corn was used as ethanol feedstock and was not considered a viable source of lipids for biodiesel production because of its low lipid content. In the last years; however, new varieties of corn that contain 4–21 wt.% oil were developed and can compete with soybean (15–20 wt.% oil) as feedstock for biodiesel production (Lu et al., 2005; Baker & Zahniser, 2006; Alptekin & Canakci, 2008; Serqueira et al., 2014; Gülüm et al., 2015).
Unfortunately, there is limited literature on the corrosion of automotive materials in corn biodiesel. Most of the researchers focus on determining the different characteristics or oxidation stability of corn biodiesel (Noureddini et al., 2004; Shi et al., 2013).
2 Raw materials for biodiesel production
2.1 Edible plant oils
The biodiesel from the first generation is predominantly produced worldwide by transesterification of edible vegetable oils (rapeseed in Canada and Europe, palm in Southeast Asia, soybean in US, sunflower in Europe, etc.) (Schlagermann et al., 2012).
Currently, these edible oils are the main resources for world biodiesel production. By analyzing the physical properties of biodiesel synthesized from these edible vegetable oils, there were no observed significant differences in density, viscosity, acid value, iodine value, flash point, copper corrosion, or pour point (Table 1). The only exceptions are for biodiesel synthesized from palm oil, which has a pour point higher than 0°C and low iodine number, which is obvious because a small value is correlated with a lower percentage of unsaturated compounds, specific to the palm methyl ester.
Main physical-chemical properties of biodiesel synthesized from different edible oils.
| Tests | Results |
Test method | |||
|---|---|---|---|---|---|
| RSME | PME | SME | SFME | ||
| Density (25°C, kg/m3) | 874 | 880 | 871 | 857 | EN ISO 3675 |
| Viscosity (40°C, mm2/s) | 4.9 | 4.6 | 3.7 | 4.2 | EN ISO 3104 |
| Acid value (mg KOH/g) | 0.22 | 0.13 | 0.11 | 0.15 | EN 14104 |
| Iodine value (g I2/100g) | 110 | 5.2 | 112 | 117 | EN 14111 |
| Pour point (°C) | −6 | +11 | −6 | −4 | ASTM D-2500 |
| Water content (mg/kg) | 121 | 143 | 163 | 144 | EN ISO-12937 |
| Methanol content (wt.%) | 0.1 | <0.1 | 0.1 | <0.1 | EN ISO-14110 |
| Cetane number | 56 | 71 | 53 | 52 | ASTM D-976 |
| Copper corrosion (3 h at 50°C) | 1 | 1 | 1 | 1 | ASTM D-130 |
| Oxidation stability (at 110°C, h) | 4.5 | 6.7 | 4.3 | 4.8 | EN ISO-14112 |
| Flash point (°C) | 132 | 169 | 184 | 176 | EN ISO-2719 |
-
RSME, Rapeseed methyl ester; PME, palm methyl ester; SME, soybean methyl ester; SFME, sunflower methyl ester.
Important differences occur when the chemical composition of biodiesel is analyzed, more precisely the fatty acid profile of the methyl esters (Table 2). The fatty acids from the composition of the biodiesel can be grouped into two categories: monounsaturated (Cn:1) and polyunsaturated with two or three double bonds (Cn:2,3). Based on these compositions Ramos et al. (2009) defined a parameter, entitled degree of unsaturation (DU), which was calculated based on equation (1).
Fatty acid composition (wt.%) of biodiesel synthesized from different edible oils.
| Fatty acids | Chemical structure | RSME | PME | SME | SFME |
|---|---|---|---|---|---|
| Lauric | CH3(CH2)10COOH | 0.0 | 0.0 | 0.0 | 0.1 |
| Myristic | CH3(CH2)12COOH | 0.0 | 0.1 | 0.4 | 0.1 |
| Palmitic | CH3(CH2)14COOH | 5.6 | 7.6 | 11.8 | 6.3 |
| Stearic | CH3(CH2)16COOH | 2.8 | 2.6 | 4.3 | 2.4 |
| Oleic | CH3(CH2)7-CH=CH-(CH2)7COOH | 64.3 | 58.9 | 24.2 | 30.4 |
| Linoleic | CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH | 17.5 | 30.5 | 54.1 | 60.1 |
| Linolenic | CH3(CH2)4-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)4COOH | 9.8 | 0.3 | 5.2 | 0.6 |
| DU [equation (1)] | 118.9 | 120.5 | 142.8 | 152.4 |
Knothe (2007), Cursaru et al. (2013), Fazal et al. (2012), and Mc Cormick et al. (2007) reported that oxidation stability of biodiesel is strongly dependent on the content of polyunsaturated methyl esters and decreases with the increase in the degree of unsaturation. A comparison of the fatty acid profile of the biodiesel synthesized from most common edible oils (Table 2) shows that sunflower biodiesel has the highest unsaturation degree, while the palm biodiesel has the lowest. In addition, Haseeb et al. (2010, 2011) and Dantas et al. (2011) stated that biodiesel with a high degree of unsaturation is more prone to corrosion, and the oxidation products are corrosive to engine chambers and may lead to blockage of the injection pumps and filters.
The continuous increase in biodiesel production from edible oils has recently been of great concern because of the competition with the food industry and the conversion of edible sources into fuels, which will compromise the supply balance in the food market, knowing that 60% of the people suffer from starvation (Balusamy & Marappan, 2007; Banapurmath et al., 2009). In this context, the researchers scrutinize alternative raw materials, even those that are genetically modified.
2.2 Non-edible plant oils
The current biodiesel synthesis from edible oils remains a controversial problem; therefore, the recent studies are focused on investigating the potential of different non-edible oils for biodiesel production. The most common sources that has extensively been reported by the literature are jatropha, karanja, tobacco, mahua, neem, rubber, sea mango, castorseed, cottonseed, and camelina (Scott et al., 2010; Dantas et al., 2011; Siddharth et al., 2011; Fazal et al., 2012; Cursaru & Neagu, 2013, 2014). The non-edible resources could be low-cost feedstock for biodiesel production. According to Mardhiah et al. (2017), non-edible-derived biodiesel possesses good prospects for its EN 14214 fuel quality with good engine performances and favorable emission characteristics. The biodiesel synthesized from the non-edible oils owns both favorable and unfavorable characteristics. Its advantages include lower sulfur content, lower aromatic content, high biodegradability, renders lower particulate matter, carbon monoxide, nitrogen oxides, and smoke emissions. Furthermore, its higher compression ratio leads to higher injection pressure and higher diffusion combustion compared to petro-diesel (Mardhiah et al., 2017). The most important disadvantages of non-edible oil-derived biodiesel include higher viscosity, lower volatility, higher percentage of carbon residue, and higher reactivity of unsaturated fatty acid chains. The feasibility of edible as well as non-edible oil-derived biodiesel depends on the fatty acid composition and on their susceptibility to corrosion. In Table 3 is presented the fatty acid composition of various non-edible oil-derived biodiesel.
Fatty acid composition (wt.%) of biodiesel synthesized from different non-edible oils.
| Fatty acids | Chemical structure | JME | KME | RsME | CsME |
|---|---|---|---|---|---|
| Lauric | CH3(CH2)10COOH | 0.0 | 0.0 | 0.0 | 0.1 |
| Myristic | CH3(CH2)12COOH | 0.0 | 0.0 | 0.0 | 0.1 |
| Palmitic | CH3(CH2)14COOH | 14.2 | 5.9 | 10.8 | 23.9 |
| Stearic | CH3(CH2)16COOH | 6.9 | 2.6 | 8.7 | 2.4 |
| Oleic | CH3(CH2)7-CH=CH-(CH2)7COOH | 43.1 | 71.3 | 24.6 | 15.8 |
| Linoleic | CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH | 34.3 | 18.3 | 39.6 | 55 |
| Linolenic | CH3(CH2)4-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)4COOH | 1.5 | 1.9 | 16.3 | 2.9 |
| DU [equation (1)] | 114.7 | 111.7 | 136.4 | 131.6 |
-
JME, Jatropha methyl ester; KME, karanja methyl ester; RsME, rubber seed methyl ester; CsME, cotton seed methyl ester.
The biodiesel derived from non-edible oils contains diverse fatty acids at different amounts. Among all these biodiesels, it was found that biodiesel from rubber seed oil has the highest unsaturation degree, comparable to that of soybean biodiesel.
2.3 Waste cooking oils, greases, and animal fat
Studies have revealed that the cost of biodiesel production is largely (about 70–80%) determined by the cost of the feedstock; therefore, less expensive feedstocks have been of interest for the last two decades (Haas et al., 2006; Singh et al., 2014; Zhang et al., 2014; Chen et al., 2018). These low-cost feedstocks include used cooking oils, greases, and animal fats. The waste feedstocks, almost always exhibiting high acid values and/or water content and presence of superfluous materials, require quality improvement prior to utilization for biodiesel synthesis (Knothe and Razon, 2017).
Animal fats have been studied in a similar context as waste cooking oils (Sims, 1985; Zheng and Hanna, 1996; Foglia et al., 1997; Bankovic et al., 2014). By comparison to vegetable oils, animal fats have more saturated fatty acids, leading to some additional handling and distribution problems because the feedstock is often solid and the resulting product tends to have poor cold flow properties, while the waste butter had approximately 20% C6–C12 methyl esters, which led to low values for flash point and poor oxidation stability (Foglia et al., 1997, Haas et al., 2010; Chakraborty et al., 2014). Waste animal fats offer twin advantages of mitigating a waste stream and generating adjacent income. As the feedstock cost is very low, the product is much less costly in spite of additional purifying steps required to remove the impurities and water from oil (Adewale et al., 2015).
2.4 Microalgae
Recently, microalgae became an interesting feedstock candidate of biodiesel production as they have a rapid growth rate, vigorous vitality, no arable land requirement, and high oil content compared to crops (Knothe, 2011). Chlorella zofingiensis, Chlorella protothecoids, Schizochytrium limacinum, Nannochloropsis salina, Phaeodactylum tricornutum, and Botryococcus braunii were well-known microalgae capable of accumulating high oil content as they could accumulate more than 50% oil of the dry body weight (Johnson & Wen, 2009; Gao et al., 2010; Mata et al., 2010; Girard et al., 2014; Tan et al., 2016; Sharma & Singh, 2017). The suitability of microalgae oil as biofuel feedstock is closely related to the length and degree of saturation of its fatty acids. A comparison of fatty acid profiles (Jiang & Gao, 2004; Huerlimann et al., 2010; Zhila et al., 2011) of some selected microalgae biodiesel, depicted in Table 4, shows that biodiesel synthesized from algae oils can contain considerably a high amount of polyunsaturated fatty acids, which favors the oxidation reactions and further promotes corrosion (Schlagermann et al., 2012).
Fatty acid composition (wt.%) of biodiesel synthesized from different types of algae.
| Fatty acids | Chemical structure | NSME | PTME | BBME |
|---|---|---|---|---|
| Lauric | CH3(CH2)10COOH | 5.0 | 0.0 | 0.7 |
| Myristic | CH3(CH2)12COOH | 0.0 | 4.5 | 0.8 |
| Palmitic | CH3(CH2)14COOH | 60.8 | 63.3 | 23.0 |
| Stearic | CH3(CH2)16COOH | 0.9 | 1.3 | 2.9 |
| Oleic | CH3(CH2)7-CH=CH-(CH2)7COOH | 11.9 | 0.0 | 3.2 |
| Linoleic | CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH | 21.4 | 5.1 | 36.4 |
| Linolenic | CH3(CH2)4-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)4COOH | 0.0 | 25.8 | 33 |
| DU [equation (1)] | 54.7 | 61.8 | 142 |
-
NSME, Nannochloropsis salina methyl ester; PTM, Phaeodactylum tricornutum methyl ester; BBME, Botryococcus braunii methyl ester.
The economic analysis studies revealed that, currently, microalgae oil cannot be considered as an alternative feedstock for biodiesel production as the cost and energy consumption of biodiesel production exceed the costs involved in biodiesel synthesis from traditional feedstock (Pimentel et al., 2009; Chen et al., 2018).
3 Automotive materials
During utilization in automobile engines, biodiesel or biodiesel blends come into contact with a wide variety of engine parts including pump, gaskets, fuel injector, filters, fuel liners, bearing, piston, piston rings, etc. All these elements of the engine are made of different materials, which behave differently in contact with the fuel. Haseeb et al. (2010, 2011) presented a classification of the typical materials used in the construction of diesel engines, which can be grouped in three categories: (i) ferrous alloys (e.g. steel and cast irons), (ii) non-ferrous alloys (e.g. aluminum alloys and copper alloys), and (iii) elastomers. Several studies are related to the compatibility of different automotive materials such as copper, leaded bronze, aluminum, zinc, brass, and cast iron with biodiesel, and some of them suggest that copper, aluminum, zinc, brass, and bronze are not compatible with biodiesel (Kaul et al., 2007; Haseeb et al., 2010, 2011). Among them, the engine parts like fuel pump, bearings, bushing, etc., manufactured by copper alloys, are mostly affected by the characteristics of the fuel.
Geller et al. (2008) observed that copper alloys are more prone to corrosion by biodiesel compared to ferrous alloys.
4 Effects of water on the quality of biodiesel fuel
Demirbas (2006), Ma et al. (1998), and Atadashi et al. (2012) noted that during the conventional catalytic transesterification of vegetable oils, the presence of water in the feedstocks reduces the conversion of triglycerides to biodiesel fuel because it favors the production of soap, consumes the catalyst and reduces its effectiveness, and results in low conversion and low yield.
One of the major hindrances of biodiesel is its degradability, which is affected by internal and external conditions. The oxidation susceptibility is linked to the presence of the unsaturated fatty acids, which is the main internal condition that affects the degradability of biodiesel. External conditions such as moisture, light, and high temperature can act as a catalyst and accelerate the oxidation of biodiesel and, consequently, its degradation.
Atadashi et al. (2012) revealed that water or moisture is the root of most of the problems encountered during production, refining, or storage of biodiesel. Therefore, the sustainability of biodiesel strongly depends on its purity and quality, and the water content should be minimized, as much as possible, in all the intermediate stages prior to commercialization.
5 Materials and methods
This study aims to investigate the corrosion behavior of copper and mild carbon steel immersed in corn biodiesel. The effects of water content, ranging from 100 ppm up to 700 ppm, from corn biodiesel on corrosion behavior of pure copper and mild carbon steel were investigated at 90°C for 1200 h, by comparing the corrosive products formed on automotive materials after immersion in biodiesel (B100) and B0 (petroleum diesel), respectively. Usually, the engine temperature is higher than 100°C, but the main purpose of the present study is to obtain useful information that may help us understand the corrosion behavior of a fuel system, which usually has an operating temperature range between 40°C and 90°C. Furthermore, the results are expected to help us determine the optimum storage conditions and to select the most compatible materials for diesel fuel systems.
5.1 Fuels
Corn biodiesel was synthesized in our labs by catalytic transesterification of corn oil (food grade) with methanol anhydrous 99.8% (provided by Sigma-Aldrich) at 60°C, using potassium hydroxide as a catalyst, via a method detailed in our previous paper (Cursaru & Neagu, 2013). After the separation of glycerol by gravity, biodiesel was purified by multiple washing with hot water in order to remove the residual catalyst and soap. The main properties of corn oil and corn methyl ester (CME) were determined according to ASTM and EN standards and are shown in Table 5.
Main physical-chemical properties of corn oil and corn methyl ester (CME).
| Tests | Results | Test method | |
|---|---|---|---|
| Corn oil | CME | ||
| Density (25°C, kg/m3) | 913 | 902 | EN ISO 3675 |
| Viscosity (40°C, mm2/s) | 31.98 | 4.5 | EN ISO 3104 |
| Lubricity WS1.4 (μm) | 220 | 232 | ASTM D-6079 |
| Acid value (mg KOH/g) | 0.13 | 0.22 | EN 14104 |
| Iodine value (g I2/100 g) | 101 | 126 | EN 14111 |
| Pour point (°C) | −8 | −11 | ASTM D-2500 |
| Water content (mg/kg) | 58 | 100 | EN ISO-12937 |
| Methanol content (wt.%) | nd | 0.1 | EN ISO-14110 |
| Cetane number | nd | 51 | ASTM D-976 |
| Copper corrosion (3 h at 50°C) | 1 | 1 | ASTM D-130 |
| Oxidation stability (at 110°C, h) | 0.9 | 1.95 | EN ISO-14112 |
| Flash point (°C) | nd | 167 | EN ISO-2719 |
| Carbon residue (wt.%) | 0.3 | 0.05 | ASTM-4530 |
-
nd, Not determined.
Petroleum diesel (B0) is an ultra-low sulfur diesel purchased from Petrotel-Lukoil Refinery, and its main physical-chemical properties are given in Table 6.
Main quality properties of diesel fuel.
| Tests | Diesel fuel | Methods |
|---|---|---|
| Density (25°C, kg/m3) | 837 | ASTM D-1298 |
| Sulfur (wt.%) | 0.0012 | ASTM D-2622 |
| Viscosity (40°C, mm2/s) | 2.7 | ASTM D-455 |
| Cetane number | 53 | ASTM D-613 |
| Cloud point (°C) | −16 | ASTM D-2500 |
| Copper corrosion (3 h at 50°C) | 1 | ASTM D-130 |
| Distillation | °C | ASTM D-86 |
| T 90% | 333.5 | |
| T 95% | 354.6 | |
| Hydrocarbon type | % vol | ASTM D-1319 |
| Aromatics | 22.3 | |
| Polyaromatics | 6.2 | |
| Lubricity WS1.4 (μm) | 528 | ASTM-D6079 |
| Acid value (mg KOH/g) | 0.15 | ASTM D-664 |
| Water content (mg/kg) | 94 | EN ISO-12937 |
As-received diesel is free of fatty acid methyl esters (FAME) and all physical-chemical properties fulfill the standard requirements.
5.2 Materials
One ferrous and one non-ferrous alloys, were chosen as test materials, respectively, mild carbon steel (C: 0.32%, Mn: 0.5%, P: 0.01, S: 0.01, Fe: balance) and copper (99.9% commercially pure). Before immersion investigations, the coupons were cut into copper strips (14 mm length, 13 mm width, and 1.9 mm thick) and mild carbon steel strips (14 mm length, 14 mm width, and 1 mm thick). A hole of 2-mm diameter was drilled near the edge of each strip to be able to hang it into the fuels. Prior to immersion investigations, the test strips were polished with silicon carbide abrasive papers (from grade 200 to 1200). The strips were cleaned with detergent and deionized water, degreased with acetone, and dried. Just after drying, the strips were immersed into fuel.
5.3 Methods
The surface morphologies and chemical composition of the metal strips after the corrosion tests were analyzed by scanning electron microscopy (SEM; Hitachi S-3400N scanning electron microscope with SE detector working at 30 kV and maximum resolution of 5 nm), while the chemical distribution of the corrosion products on metal surface exposed to fuel was investigated by X-ray diffraction (XRD). The XRD patterns of the metal specimens were explored using a Bruker D8 diffractometer with a CuKα X-ray in 2-θ range of 10–90° (λ=0.154 nm, 40 kV, and 40 mA). The specimens were scanned in the 2-θ range with a step size of 0.1° and a time step of 5 s.
Prior to SEM and XRD investigations, metal specimens were washed with acetone to remove impurities on the surface of the metals.
In order to evaluate the acidity of the fuels, previous to and after immersion tests, fuels were characterized by measuring the total acid number (TAN) according to EN 14104.
Fatty acid composition of corn biodiesel was analyzed with a Varian 450 gas chromatograph with FID detector, according to EN 14103 standard. The capillary column was an Agilent column with a length of 30 m, a film thickness of 0.25 μm and an internal diameter of 0.32 mm. One microliter of biodiesel sample was injected using a split ratio (100:1) at 24.65 psi at an inlet temperature of 250°C. Table 7 depicts the fatty acid composition (wt.%) of corn biodiesel.
Fatty acid composition (wt.%) of corn biodiesel.
| Fatty acids | Molecular formula | Chemical structure | CME |
|---|---|---|---|
| Lauric | C12H24O2 | CH3(CH2)10COOH | 0.0 |
| Myristic | C14H28O2 | CH3(CH2)12COOH | 0.0 |
| Palmitic | C16H32O2 | CH3(CH2)14COOH | 12.3 |
| Stearic | C18H38O2 | CH3(CH2)16COOH | 2.6 |
| Oleic | C18H34O2 | CH3(CH2)7-CH=CH-(CH2)7COOH | 41.3 |
| Linoleic | C18H32O2 | CH3(CH2)4-CH=CH-CH2-CH=CH-(CH2)7COOH | 43.0 |
| Linolenic | C18H30O2 | CH3(CH2)4-CH=CH-CH2-CH=CH-CH2-CH=CH-(CH2)4COOH | 0.8 |
| Saturates | 14.9 | ||
| Unsaturates | 85.1 | ||
| DUa | 128.9 |
-
aDU is degree of unsaturation (calculated with eq. bib1).
5.4 Corrosion experiment
Corrosion of copper and mild carbon steel in corn biodiesel with different water concentrations (100 ppm, 500 ppm, and 700 ppm) was investigated by static immersion test at 90°C for 1200 h.
Three duplicated strips were suspended into the glass beakers filled with 100 ml of fuel and covered by a watch glass during the evaluation tests. Before and after the immersion tests, the metal strips were weighted, and the mass loss was converted into the corrosion rate using equation (2) (Kaul et al., 2007; Haseeb et al., 2010; Cursaru et al., 2014).
whereby corrosion rate (mpy) is mils of metal (0.001 inch) per year, w is the weight loss (mg), D is the density (g/cm3), t is the exposure time (h), and A is the exposed surface area (in2). The density of the copper strip is 8.92 g/cm3, and the density of mild carbon steel is 7.87 g/cm3.
6 Results and discussion
The corrosion behavior of metals and susceptibility to oxidation or autoxidation are the most important conditions imposed to commercial biodiesel. Prior to its utilization in diesel engines, biodiesel is very sensitive to oxidative and thermal degradation, mainly due to the presence of the unsaturated fatty acids. During the oxidation of biodiesel, there appears a variety of secondary oxidation products, such as lower molecular organic acids, aldehydes, and ketones that increase the total acidity and the risk of corrosion in vehicle and fuel-handling system. Therefore, it is essential to distinguish between the cause of poor oxidation stability and corrosivity of biodiesel, especially because there are different mechanisms of action.
The degree of unsaturation (DU) was calculated based on the fatty acid composition of corn biodiesel presented in Table 7 and in equation (1).
Low oxidation stability is determined mainly by the unsaturated fatty acid methyl esters (85.1 wt.%), which include oleic (C18:1), linoleic (C18:2), and linolenic (C18:3).
The oxidation stability decreases with the increase in the content of polyunsaturated methyl esters; thus, high values of the DU indicate poor oxidation stability for biodiesel. The result calculated for corn biodiesel (128.9) indicates poor oxidation stability, which does not correlate with the total number of double bonds, but with the total number of bis-allylic sites, those that react with oxygen to form peroxide or hydroperoxide (Mc Cormick et al., 2007).
Table 5 shows the physico-chemical properties of biodiesel. The quality properties evaluated for corn biodiesel met the minimum or maximum limits on the EN 14214, except for oxidation stability. The oxidation stability was determined by the Rancimat method according to the EN 14214, showing 1.95 h, smaller than the lower limits of 6 h.
On the other hand, moisture and the hygroscopic nature of biodiesel may promote microbial growth and corrode the fuel system. Tables 5 and 6 show that the water content from corn biodiesel, which is 100 ppm and, for as-received diesel fuel, 94 ppm. These values are below the minimum limit imposed by the standards.
In order to evaluate the effect of water on the corrosion behavior of copper and mild carbon steel, 100, 500, and 700 ppm of water were added in corn biodiesel. The results were compared to those recorded after static immersion in commercial diesel fuel (B0).
Figure 1 shows the corrosion rates of copper and mild carbon steel exposed to biodiesel with 100, 500, and 700 ppm of water concentration at 90°C and 1200 h. The specimens were named B100_1 (corn biodiesel with 100 ppm of water), B100_2 (corn biodiesel with 500 ppm of water), and B100_3 (corn biodiesel with 700 ppm of water).

Corrosion rate for copper (Cu) and mild carbon steel (MCS) after immersion in B0 (diesel fuel), B100_1 (corn biodiesel with 100 ppm of water), B100_2 (corn biodiesel with 500 ppm of water), and B100_3 (corn biodiesel with 700 ppm of water) at 90°C for 1200 h.
The results indicate that corrosion rates are lower in diesel fuel than that in corn biodiesel, which also demonstrate that corn biodiesel is more corrosive than diesel fuel. These observations are not surprising because previous studies had ascribed higher stability of diesel fuel to the saturated hydrocarbons from its composition (Knothe, 2007; Hu et al., 2012; Cursaru et al., 2014). For both metals, it was found that corrosion rates increase with the increase in water concentrations in biodiesel, which indicate an acceleration of corrosion for higher water concentrations. In the same experimental conditions, corrosion of copper is more severe than that of mild carbon steel.
In order to investigate the degradation of fuels, prior to and after immersion investigations, fuels were characterized by measuring the total acid number (TAN) according to EN 14104. The degrees of unsaturation along with the acid value give important information about the oxidation stability of biodiesel and its susceptibility to corrosion. The acid number was used to determine the level of the free fatty acids and other acid types, responsible for biodiesel degradation. The oxidation products formed during biodiesel degradation, such as aldehyde, ketones, alcohols, and volatile low-molecular organic acids (i.e. acetic acid and formic acid), are highly corrosive to the metal part of the engines.
The acid number measured for corn biodiesel was 0.22 mg KOH/g and for diesel fuel, 0.15 mg KOH/g, values lower than that specified by EN 14214 standards (max: 0.5 mg KOH/g). Figures 2 and 3 show the TAN values of different fuels before and after the immersion tests.

TAN values of different fuels before immersion tests at 90°C for 1200 h. B0 (diesel fuel), B100_1 (corn biodiesel with 100 ppm of water), B100_2 (corn biodiesel with 500 ppm of water), and B100_3 (corn biodiesel with 700 ppm of water) at 90°C for 1200 h.

TAN values of different fuels after immersion tests: (A) copper, (B) MCS.
These results show that the TAN values of diesel fuel exposed to copper and mild carbon steel are similar to diesel fuel prior to immersion tests, which indicates higher stability for diesel fuel in experimental conditions. It was also found that the TAN values of biodiesel increase with water content upon exposure to both metals, but the values recorded after exposure to copper are higher (with about 12%) than those observed for biodiesel exposed to mild carbon steel. Even if the water concentration in B100_2 sample did not exceed the upper limit for water imposed by EN ISO-12937 standard, from Figure 3A and B, it is seen that after exposure to both metals, the TAN value exceeded the upper limit for acid value imposed by the standard for biodiesel.
It was found that due to exposure of copper, TAN values rise, indicating that copper is weaker in biodiesel compared to mild carbon steel. These results are in accordance with those of Fazal et al. (2012) and Kaul et al. (2007).
Figure 4 shows the XRD patterns of corrosion products on the copper and mild carbon steel exposed to diesel fuel and corn biodiesel with 100, 500, and 700 ppm water concentration at 90°C for 1200 h.

XRD pattern of copper (Cu) after immersion in B100_1 (A), B100_2 (B), B100_3 (C), B0 (D), and of MCS after immersion in B100_1 (E), B100_2 (F), B100_3 (G), B0 (H).
The XRD patterns (Figure 4A–D) mainly indicate the presence of the base metal copper (Cu). CuO and Cu(OH)2 were detected on the surface of the copper strips after immersion in diesel fuel, while CuO, Cu(OH)2, Cu(HCOO)2, Cu2O, CuCO3·Cu(OH)2, and Cu(OH)2H2O were found on the surface of copper immersed in corn biodiesel.
After the immersion of mild carbon steel in diesel fuel (Figure 4H), the concentration of iron compounds (e.g. FeO, Fe2O3, FeCO3, and FeO(OH)) are comparatively less than those in biodiesel (e.g. FeO, Fe2O3, FeO(OH), FeCO3, and C2H2FeO4·2H2O) (Figure 4E–G). These results are in agreement with those reported by Fazal et al. (2011a,b, 2013) and Karavalakis et al. (2011). In the presence of water and oxygen, copper- and iron-exposed surfaces indicate the formation of the compounds emphasized by XRD, which are precursors for corrosion. In our previous study, a reaction mechanism that might occur on copper and mild carbon steel surfaces upon exposure to sunflower biodiesel was proposed (Cursaru et al., 2014). According to this mechanism, the metal reacts with dissolved oxygen and forms different metallic oxides. In the presence of dissolved oxygen and water from biodiesel, copper leads to the formation of Cu(OH)2, while iron leads to FeO(OH). Formation of copper carbonate or iron carbonate may be initiated by the formation of the RCOO˙ radical generated from the decomposition of the esters.
However, compared to diesel fuel, biodiesel-exposed mild carbon steel surface shows a comparatively higher concentration of iron oxides and hydroxides such as FeO, Fe2O3, and FeO(OH) and the appearance of iron formate hydrate C2H2FeO4·2H2O (Figure 4A–C). The concentration of iron compounds formed on mild carbon steel exposed to corn biodiesel is higher than that in diesel fuel and increases with water concentration in biodiesel. Similar findings were observed for copper. Copper formate Cu(HCOO)2 was detected on the copper strip surface after exposure to corn biodiesel. The reason why the chemical species C2H2FeO4·2H2O and Cu(HCOO)2 were detected in corn biodiesel-exposed metal is more likely due to the high level of water from biodiesel, O2, and CO2 absorbed from air and high immersion temperature. These results are interesting because there are only a few research studies that mention the presence of formic acid as a corrosion product, although they suggested that a variation of biodiesel color, during immersion tests, from reddish yellow to pale green, is an indicator of the transformation of copper into copper carbonate (Tsuchiya et al., 2006; Haseeb et al., 2010). According to Scott (2002), the copper formate is a patina component on important statues (i.e. Statue of Liberty), and the neutral formate is freely soluble in water and is the usual product formed from the direct reaction between copper and formic acid. It is more likely to assume that formic acid and copper formate are responsible for increasing the TAN number and corrosion rate rather than copper carbonate. In order to assess the concentration of formate from all corrosion compounds, XRD measurements were coupled with Rietveld quantitative analysis. These investigations reveal that the concentration of copper formate increases from 1.45 wt.% (for copper strip immersed in B100_1) to 1.58 wt.% (for copper strip immersed in B100_3), while the iron formate concentration increases from 0.41 (for mild carbon steel strip immersed in B100_1) to 1.15 wt.% (for mild carbon steel strip immersed in B100_3). TAN and XRD coupled with Rietveld quantitative analysis proved that the presence of water in biodiesel has a strong influence on the corrosion rate.
Increasing the water content in biodiesel caused an intensification of the oxidation products such as oxides, peroxides, and acids as a result of decomposition of peroxides and salts, contributing to increase in the corrosion rate.
Further investigations of the metal surface morphologies were observed by scanning electron micrographs. Representative SEM images of each test coupon for both metals are shown in Figures 5 and 6. Figure 5 presents the SEM micrographs of copper (Cu) after immersion in diesel fuel (B0) and biodiesel (B100) with 100, 500, and 700 ppm water concentration at 90°C for 1200 h.

SEM micrographs of copper (Cu) after immersion in diesel fuel (B0) and corn biodiesel (B100) with 100, 500, and 700 ppm water concentration at 90°C for 1200 h.
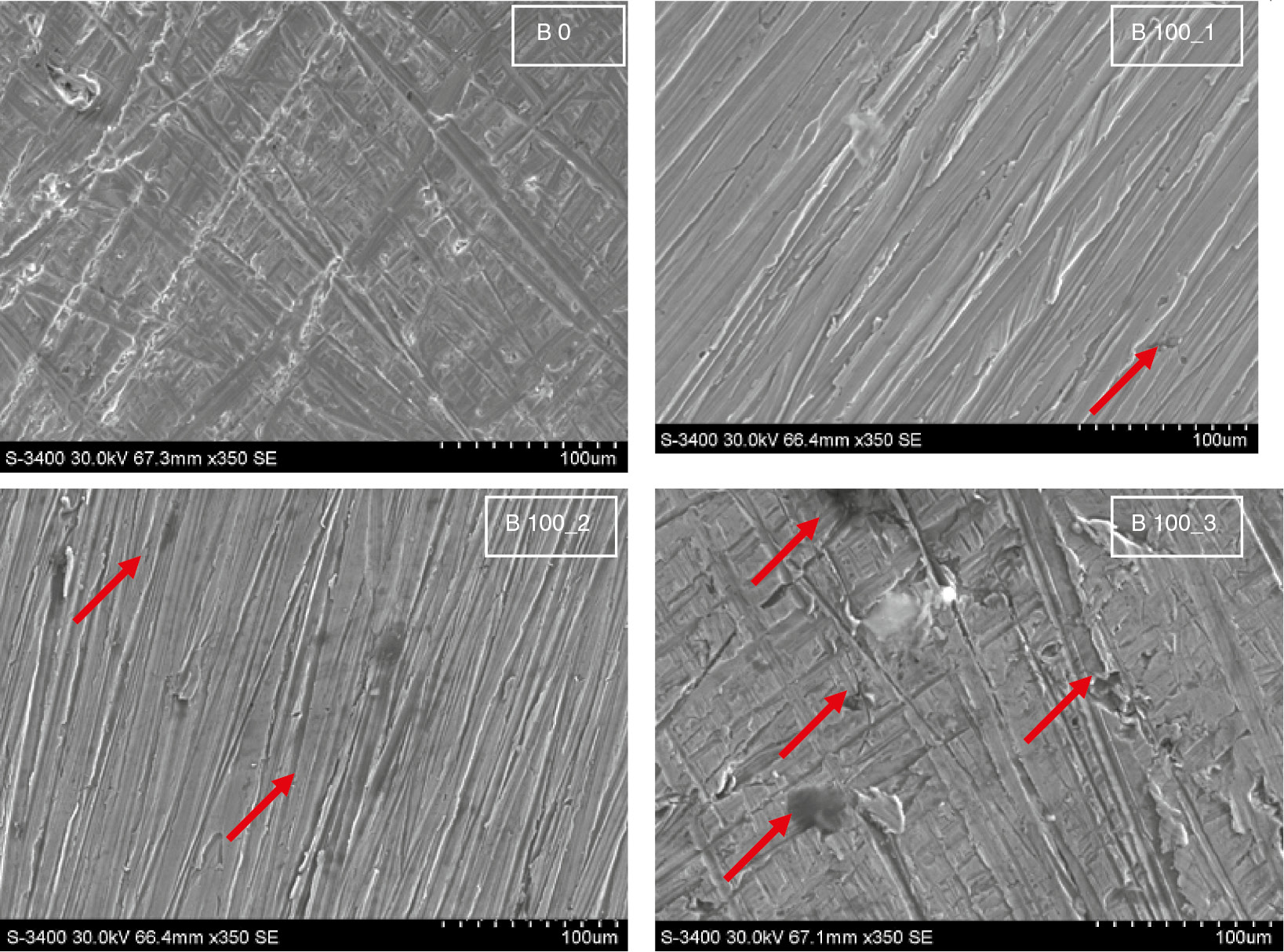
SEM micrographs of MCS after immersion in diesel fuel (B0) and biodiesel (B100) with 100, 500, and 700 ppm water concentration at 90°C for 1200 h.
After 1200-h exposure, metal strips exhibit obvious corrosion attack. It is observed that corrosion attack on diesel fuel-exposed metal surface is minor than that on biodiesel-exposed surface. Copper strips in biodiesel are subjected to higher corrosion attack.
By increasing the water content in biodiesel, it is seen as an intensification of corrosion attack on metallic surfaces. Copper strips immersed in biodiesel with the lowest water concentration show a few and small pits that seems to represent the beginning of pit formation; by increasing the moisture, an amplification of the pit formation was observed, the copper strip immersed in the biodiesel sample with 700 ppm of water being entirely corroded (the corrosion pits are highlighted by red arrows in Figure 5).
Figure 6 presents the SEM micrographs of mild carbon steel (MCS) after immersion in diesel fuel (B0) and biodiesel (B100) with 100, 500, and 700 ppm water concentration at 90°C for 1200 h.
The corrosion effects of diesel fuel on mild carbon steel were minor. After exposure to biodiesel with 100, 500, and 700 ppm of water concentration, the SEM images indicate that mild carbon steel is prone to pitting corrosion, and higher water concentrations accelerate the formation of pits on the metal surface (the corrosion pits are highlighted by red arrows in Figure 6). However, for both metals, the degradation of the surfaces exposed to corn biodiesel is comparatively higher than that in diesel fuel, but copper is less resistant in biodiesel and more prone to corrosion.
These observations are in agreement with XRD and corrosion rate results, which indicate that copper is more susceptible to corrosion than ferrous alloys.
In order to understand the appearance of the corrosion products on the metal surfaces and changes in the corrosion products due to the increase in the water content in corn biodiesel, a possible reaction mechanism to form the different compounds of copper (reactions 3–16) and iron is proposed (reactions 17–21).
6.1 Copper corrosion mechanism
The metal reacts with dissolved oxygen and absorbed moisture and form different metallic oxides and hydroxides [equations (3–6)].
According to Hernandez et al. (2010) and Fazal et al. (2013), Cu2O is unstable and very fast that it turns to more stable CuO [equation (7)] or can react with dissolved oxygen and carbon dioxide from the atmosphere and form CuCO3 [equation (8)]. Formation of copper carbonate may be also initiated by the formation of the RCOO˙ radical generated from decomposition of the esters [equations (9 and 10)]. CuO, CuCO3, and Cu(OH)2 are the main species detected by XRD investigations on copper strips exposed to corn biodiesel.
In the presence of dissolved oxygen and water present in corn biodiesel, copper leads to the formation of Cu(OH)2 [equations (11 and 12)].
Copper oxides react with organic acids and can form corresponding salts [equations (13 and 14)]
The presence of copper formate on the metallic surfaces, detected by XRD, is not surprising and can be explained based on the reactions between copper or copper oxide and formic acid [equations (15 and 16)]. Formic acid is an organic compound, easily formed during the degradation process of the biodiesel in the presence of oxygen and water.
In literature, the possible reaction mechanisms that occur during the corrosion in the presence of biodiesel did not report the presence of copper formate or iron formate on the metal surfaces. Although the XRD results did not show a clear specific peak corresponding to the copper formate, the Rietveld quantitative analyses indicate that copper formate increases from 1.45 wt.% (for copper strip immersed in B100_1) to 1.58 wt.% (for copper strip immersed in B100_3), which cannot be neglected, mainly because formic acid and its compounds are responsible with corrosion of the metal surfaces.
6.2 Iron corrosion mechanism
Because of the exposure of biodiesel to water and oxygen, the iron from mild carbon steel forms Fe2O3 and Fe(OH)2 [equations (17 and 18)]:
The presence of esters from biodiesel can generate the following reactions [equations (19 and 20)]
Equations (17–20) are in agreement with the Fazal et al. (2013) findings. XRD and Rietveld quantitative analysis reveal the presence of iron formate on the metal surfaces; its concentration increases from 0.41 (for mild carbon steel strip immersed in B100_1) to 1.15 wt.% (for mild carbon steel strip immersed in B100_3).
The presence of iron formate is believed to be due to the direct reaction between iron and formic acid [equation (21)].
Successful replacement of diesel fuel with biodiesel depends on its quality and purity. The most restrictive characteristics of biodiesel are the oxidation stability and the water content. If oxidation stability mainly depends on chemical composition of biodiesel and could be easily controlled by utilization of antioxidants, the presence of water from biodiesel is due to the production and purification processes, storage, or because of its hygroscopic nature. This study showed the evidence of the effect of water on the corrosiveness of biodiesel; therefore, it is essential to diminish the water from all steps prior to biodiesel synthesis and to avoid the exposure of biodiesel to the atmosphere because the presence of water accelerates the corrosion process. Utilization of materials with superior resistance can be also a method to reduce corrosion attacks.
7 Conclusions
Nowadays, there is a strong tendency to replace the conventional diesel fuel with more environmentally friendly fuels, for example, biodiesel. Unfortunately, corrosion and a high susceptibility to oxidation or autoxidation are the main concerns associated to biodiesel compatibility issues.
The standard for biodiesel EN 14214 has been developed based on the standard for petro- diesel; therefore, the specific method to investigate the corrosive proclivity is the same for the diesel, biodiesel, and biodiesel blends. The standard for biodiesel imposes as a method to measure the corrosion of biodiesel by strip tarnish test according to ASTM D130. This test method covers the detection of corrosiveness of copper to biodiesel, biodiesel blends, or diesel fuel. Unfortunately, the results of this test indicate only a slight tarnish for diesel fuel and biodiesel, which usually associate these fuels to the first corrosion class. This method indicates only marginal corrosion and cannot make a measurable difference between diesel fuel and different concentrations of biodiesel in blends.
It was already proved that biodiesel (because of its hygroscopic nature and the content of polyunsaturated methyl esters) is more prone to corrosion; therefore, in order to have a complete overview on the corrosiveness of biodiesel, it is not enough to examine the copper strip corrosion results, but also the water content, the acid value, the oxidation stability, and polyunsaturated methyl ester content.
The experimental aspects of this paper were to investigate the corrosion behavior of copper and mild carbon steel used as automotive materials, after exposure to corn biodiesel. The effect of water from corn biodiesel on the corrosion behavior of metal materials was studied by comparing corrosive products formed on the copper and mild carbon steel after static immersion in corn biodiesel and commercial diesel fuel.
The corrosion attacks on diesel-exposed metal surfaces are comparatively less than that on corn biodiesel-exposed metal surfaces.
Upon exposure to corn biodiesel, copper is subjected to higher corrosion attack followed by mild carbon steel.
The corrosion attacks on the surface of metal strips become more serious with increasing water content in corn biodiesel. An increase in the TAN value after immersion test indicates intensification of the oxidation and further corrosion.
The corrosion mechanism should be associated to the chemical corrosion. The corrosion products were mainly oxidation products such as CuO, Cu(OH)2, CuCO3, and also, a small amount of copper formate was detected on the metal surface.
On mild carbon steel surface, Fe2O3, Fe(OH)2 and FeCO3 are the main oxidation products detected, but a small amount of iron formate was found to be formed on the metal surface exposed to corn biodiesel.
The concentration of the copper or iron compounds formed on the metal surfaces exposed to diesel fuel are comparatively less than that to biodiesel, and the degradation of the metal surfaces exposed to corn biodiesel is higher than that in diesel. The presence of water in biodiesel seems to accelerate the corrosion of the metal surfaces, as well as the degradation of fuel characteristics.
Further study should be done to understand the appearance of the cooper and iron formate and their role in the corrosion attack on the metal surfaces.
Biodiesel is not a “young” product; it celebrates over 100 years of existence. It came back into light in the 1980s, after the world oil crisis of the 1970s, as a result of the struggle to discover renewable energy sources, alternative fuels to fossil fuel. Despite the many benefits related to biodiesel utilization, there are still key issues that need to be overcome so that biodiesel gets mature and can be, in the near future, a viable substitute for petro diesel. In order to make biodiesel a sustainable biofuel, it is imperative to find alternative raw materials, with production that does not use arable land and does not compete with the food industry. It is also mandatory to increase the economic perspectives, by reducing the production costs, especially the costs for raw materials, to decrease the amount of the byproduct – glycerol, or to find alternative methods for diversification of the products derived from glycerol. Issues as low oxidation stability or high susceptibility to oxidation or autoxidation should be overcome using antioxidant additives, by selecting raw materials with a low content of polyunsaturated fatty acids and by avoiding the contact between biodiesel and moisture or other impurities, which can promote oxidation and further corrosion.
References
Adewale P, Dumont MJ, Ngadi M. Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew Sustain Energy Rev 2015; 45: 574–588.10.1016/j.rser.2015.02.039Search in Google Scholar
Alptekin E, Canakci M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew Energy 2008; 33: 2623–2530.10.1016/j.renene.2008.02.020Search in Google Scholar
Atadashi IM, Aroua MK, Abdul Aziz AR, Sulaiman NMN. The effect of water on biodiesel production and refining technologies: a review. Renew Sustain Energy Rev 2012; 16: 3456–3470.10.1016/j.rser.2012.03.004Search in Google Scholar
Antolin G, Tinaut FV, Briceno Y, Castano V, Perez C, Ramirez Al. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour Technol 2002; 83: 111–114.10.1016/S0960-8524(01)00200-0Search in Google Scholar
Baker A, Zahniser S. Ethanol reshapes the corn market. Amber Waves 2006; 4: 30–35.Search in Google Scholar
Balusamy T, Marappan R. Perfromance evaluation on direct injection diesel engines with blend of Thevetia peruviana seed oil and diesel. J Sci Ind Res 2007; 66: 1035–1040.Search in Google Scholar
Banapurmath NR, Tewari PG, Yaliwal VS, Kambalimath S, Basavarajappa YH. Combustion characteristics of a 4-stroke CI engine operated on Honge oil, neem and rice bran oil when directed injected and dual fuelled with producer gas injector. Renew Energy 2009; 34: 1877–1884.10.1016/j.renene.2008.12.031Search in Google Scholar
Bankovic IB, Stojkovic IJ, Stamenkovic OS, Veljkovic VB, Hung YT. Waste animal fats as feedstocks for biodiesel production. Renew Sustain Energy Rev 2014; 32: 238–254.10.1016/j.rser.2014.01.038Search in Google Scholar
Bari S, Lim TH, Yu CW. Effects of preheating of crude palm oil on injection system, performance and emission of a diesel engine. Renew Energy 2002; 27: 339–351.10.1016/S0960-1481(02)00010-1Search in Google Scholar
Chakraborty R, Gupta AK, Chowdhury R. Conversion of slaughterhouse and poultry farm animal fats and wastes to biodiesel: parametric sensitivity and fuel quality assessment. Renew Sustain Energy Rev 2014; 29: 120–134.10.1016/j.rser.2013.08.082Search in Google Scholar
Chen J, Li J, Dong W, Zhang X, Tyagi RD, Drogui P, Surampalli RY. The potential of microalgae in biodiesel production. Renew Sustain Energy Rev 2018; 90: 336–346.10.1016/j.rser.2018.03.073Search in Google Scholar
Cursaru D, Neagu M. Effect of biodiesel from sunflower oil on diesel engine performances and exhaust emissions. Rev Chim 2013; 64: 317–320.Search in Google Scholar
Cursaru D, Neagu M, Bogatu L. Investigations on the oxidation stability of biodiesel synthesized from different vegetable oils. Rev Chim 2013; 64: 438–441.Search in Google Scholar
Cursaru D, Brănoiu Gh, Ramadan I, Miculescu F. Degradation of automotive materials upon exposure to sunflower biodiesel. Ind. Crops Prod 2014; 54: 149–158.10.1016/j.indcrop.2014.01.032Search in Google Scholar
Dantas MB, Albuquerque AR, Barros AK, Rodrigues Filho MG. Evaluation of the oxidative stability of corn biodiesel. Fuel 2011; 90: 773–778.10.1016/j.fuel.2010.09.014Search in Google Scholar
Demirbas A. Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers Manage 2006; 47: 2271–2282.10.1016/j.enconman.2005.11.019Search in Google Scholar
Demirbas A. Progress and recent trends in biodiesel fuels. Energy Convers Manage 2009; 50: 14–34.10.1016/j.enconman.2008.09.001Search in Google Scholar
Fazal MA, Haseeb ASMA, Masjuki HH. Effect of temperature on the corrosion behavior of mild steel upon exposure to palm diesel. Energy 2011a; 36: 3328–3334.10.1016/j.energy.2011.03.028Search in Google Scholar
Fazal MA, Haseeb ASMA, Masjuki HH. Effect of different corrosion inhibitors on the corrosion of cast iron in palm biodiesel. Fuel Process Technol 2011b; 92: 2154–2159.10.1016/j.fuproc.2011.06.012Search in Google Scholar
Fazal MA, Haseeb ASMA, Masjuki HH. Degradation of automotive materials in palm biodiesel. Energy 2012; 40: 76–83.10.1016/j.energy.2012.02.026Search in Google Scholar
Fazal MA, Haseeb ASMA, Masjuki HH. Corrosion mechanism of copper in palm biodiesel Corros Sci 2013; 67: 50–59.10.1016/j.corsci.2012.10.006Search in Google Scholar
Ferella F, Mazziotti Di Celso G, De Michelis I, Stanisci V, Veglio F. Optimization of the transesterification reaction in biodiesel production. Fuel 2010; 89: 36–42.10.1016/j.fuel.2009.01.025Search in Google Scholar
Foglia TA, Nelson LA, Dunn RO, Marmer W. Low-temperature properties of alkyl esters of tallow and grease. J Am Oil Chem Soc 1997; 74: 951–955.10.1007/s11746-997-0010-7Search in Google Scholar
Gao C, Zhai Y, Ding Y, Wu Q. Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy 2010; 87: 756–761.10.1016/j.apenergy.2009.09.006Search in Google Scholar
Geller DP, Adams TT, Goodrum J, Pendergrass J. Storage stability of poultry fat and diesel fuel mixtures: specific gravity and viscosity. Fuel 2008; 87: 92–102.10.1016/j.fuel.2007.03.043Search in Google Scholar
Girard JM, Roy ML, Hafsa MB, Gagnon J, Faucheux N, Heitz M. Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res 2014; 5: 241–248.10.1016/j.algal.2014.03.002Search in Google Scholar
Gomes MCS, Pereira NC, Davantel de Barros ST. Separation of biodiesel and glycerol using ceramic membranes. J Membr Sci 2010; 352: 271–276.10.1016/j.memsci.2010.02.030Search in Google Scholar
Gülüm M, Bilgin A. Density, flash point and heating value variations of corn oil biodiesel–diesel fuel blends. Fuel Process Technol 2015; 134: 456–464.10.1016/j.fuproc.2015.02.026Search in Google Scholar
Haas MJ, McAloon AJ, Yee WC, Foglia TA. A process model to estimate biodiesel production costs. Bioresour Technol 2006; 97: 671–678.10.1016/j.biortech.2005.03.039Search in Google Scholar PubMed
Haas MJ, Adawi N, Berry WW, Feldman E, Kasprzyk S, Ratigan B. Butter as a feedstock for biodiesel production. J Agric Food Chem 2010; 58: 7680–7684.10.1021/jf1003754Search in Google Scholar PubMed
Haseeb ASMA, Masjuki HH, Ann LJ, Fazal MA. Corrosion characteristics of copper and leaded bronze in palm biodiesel. Fuel Process Technol 2010; 91: 329–334.10.1016/j.fuproc.2009.11.004Search in Google Scholar
Haseeb ASMA, Fazal MA, Jahirul MI, Masjuki HH. Compatibility of automotive materials in biodiesel: a review. Fuel 2011; 90: 922–931.10.1016/j.fuel.2010.10.042Search in Google Scholar
Hernandez RPB, Paszti Z, de Melo HG, Aoki IV. Chemical characterization and anticorrosion properties of corrosion products formed on pure copper in synthetic rainwater of Rio de Janeiro and Sao Paolo. Corros Sci 2010; 52: 826–837.10.1016/j.corsci.2009.11.003Search in Google Scholar
Hu E, Xu Y, Hu X, Pan L, Jiang S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew Energy 2012; 37: 371–378.10.1016/j.renene.2011.07.010Search in Google Scholar
Huerlimann R, de Nys R, Heimann K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 2010; 107: 245–257.10.1002/bit.22809Search in Google Scholar PubMed
Jiang H, Gao K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 2004; 40: 651–654.10.1111/j.1529-8817.2004.03112.xSearch in Google Scholar
Johnson MB, Wen Z. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 2009; 23: 5179–5183.10.1021/ef900704hSearch in Google Scholar
Kapilan N, Ashok Babu TP, Reddy RP. Technical aspects of biodiesel and its oxidation stability. Int J Chemtech Res 2009; 1: 278–282.Search in Google Scholar
Karavalakis G, Hilari D, Givalou L, Karonis D, Stournas S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 2011; 36: 369–374.10.1016/j.energy.2010.10.029Search in Google Scholar
Kaul S, Saxena RC, Kumar A, Negi MS, Bhatnagar AK, Goyal HB, Gupta AK. Corrosion behavior of biodiesel from seed oils of Indian origin on diesel engine parts. Fuel Process Technol 2007; 88: 303–307.10.1016/j.fuproc.2006.10.011Search in Google Scholar
Knothe G. Some aspects of biodiesel oxidative stability. Fuel Process Technol 2007; 88: 669–677.10.1016/j.fuproc.2007.01.005Search in Google Scholar
Knothe G. A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae-derived biodiesel perform? Green Chem 2011; 13: 3048–3065.10.1039/c0gc00946fSearch in Google Scholar
Knothe G, Razon LF. Biodiesel fuels. Prog Energy Combust Sci 2017; 58: 36–59.10.1016/j.pecs.2016.08.001Search in Google Scholar
Lu J, Khot S, Wool RP. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005; 46: 71–80.10.1016/j.polymer.2004.10.060Search in Google Scholar
Ma F, Clements LD, Hanna MA. The effects of catalysts, free fatty acids and water on transesterification of beef tallow. Trans ASAE 1998; 41: 1261–1264.10.13031/2013.17292Search in Google Scholar
Mardhiah HH, Ong HC, Masjuki HH, Lim S, Lee HV. A review on latest developments and future prospects on heterogeneous catalyst in biodiesel production from non-edible oils. Renew Sustain Energy Rev 2017; 67: 1225–1236.10.1016/j.rser.2016.09.036Search in Google Scholar
Maru M, Lucchse M, Legnani C, Quirino W, Balbo A, Aranha I, Costa L, Vilani C, Sena L, Damasceno J, Cruz T, Lidizio L, Silva F, Jorio A, Achete C. Biodiesel compatibility with carbon steel and HDPE parts. Fuel Process Technol 2009; 90: 1175–1182.10.1016/j.fuproc.2009.05.014Search in Google Scholar
Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 2010; 14: 217–232.10.1016/j.rser.2009.07.020Search in Google Scholar
Mc Cormick RL, Ratcliff M, Moens L, Lawrence R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process Technol 2007; 88: 651–657.10.1016/j.fuproc.2007.01.006Search in Google Scholar
Noureddini H, Gao X, Philkana RS. Immobilized Pseudornonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour Technol 2004; 96: 769–777.10.1016/j.biortech.2004.05.029Search in Google Scholar PubMed
Pimentel D, Marklein A, Toth MA, Karpoff MN, Paul GS, McCormack R, Kyriazis J, Krueger T. Food versus biofuels: environmental and economic costs. Hum Ecol 2009; 37: 1–12.10.1007/s10745-009-9215-8Search in Google Scholar
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 2009; 100: 261–268.10.1016/j.biortech.2008.06.039Search in Google Scholar PubMed
Refaat AA. Different techniques for the production of biodiesel from waste vegetable oil. Int J Environ Sci Technol 2010; 7: 183–213.10.1007/BF03326130Search in Google Scholar
Saeid B, Aroua MK, Abdul Raman A, Sulaiman NMN. Density of palm oil-based methy ester. J Chem Eng Data 2008; 53: 877–880.10.1021/je700682dSearch in Google Scholar
Saleh J, Tremblay AY, Dube M. Glycerol removal from biodiesel using membrane separation technology. Fuel 2010; 89: 2260–2266.10.1016/j.fuel.2010.04.025Search in Google Scholar
Schlagermann P, Göttlicher G, Dillschneider R, Rosello-Sastre R, Posten C. Composition of algal oil and its potential as biofuel. J Combust 2012; 2012: Article ID 285185, 14 pages. https://doi.org/10.1155/2012/285185.10.1155/2012/285185Search in Google Scholar
Scott DA. Copper and bronze in art. Corrosion, colorants, conservation, Los Angeles: Getty Publications, 2002.Search in Google Scholar
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG. Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 2010; 21: 277–286.10.1016/j.copbio.2010.03.005Search in Google Scholar PubMed
Serqueira DS, Fernandes DM, Cunha RR, Squissato AL, Santos DQ, Richter EM, Munoz RAA. Influence of blending soybean, sunflower, colza, corn, cottonseed, and residual cooking oil methyl biodiesels on the oxidation stability. Fuel 2014; 118: 16–20.10.1016/j.fuel.2013.10.028Search in Google Scholar
Sharma YC, Singh V. Microalgal biodiesel: a possible solution for India’s energy security. Renew Sustain Energ Rev 2017; 67: 72–88.10.1016/j.rser.2016.08.031Search in Google Scholar
Shi A, Du Z, Ma X, Cheng Y, Min M, Deng S, Chen P, Li D, Ruan R. Production and evaluation of biodiesel and bioethanol from high oil corn using three processing routes. Bioresour Technol 2013; 128: 100–106.10.1016/j.biortech.2012.10.007Search in Google Scholar PubMed
Siddharth J, Sharma MP. Long term storage stability of Jatropha curcas biodiesel. Energy 2011; 36: 5409–5415.10.1016/j.energy.2011.06.055Search in Google Scholar
Sims REH. Tallow esters as an alternative diesel fuel. Trans ASAE 1985; 28: 716–721.10.13031/2013.32326Search in Google Scholar
Singh B, Guldhe A, Rawat I, Bux F. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew Sustain Energy Rev 2014; 29: 216–245.10.1016/j.rser.2013.08.067Search in Google Scholar
Tan CH, Chen CY, Show PL, Ling TC, Lam HL, Lee DJ. Strategies for enhancing lipid production from indigenous microalgae isolates. J Taiwan Inst Chem Eng 2016; 63: 189–194.10.1016/j.jtice.2016.02.034Search in Google Scholar
Tsuchiya T, Shiotani H, Goto S, Sugiyama G, Maeda A. Japanese standards for diesel fuel containing 5% FAME: investigation of acid generation in FAME blended diesel fuels and its impact on corrosion, SAE Technical Paper 2006-01-3303, 2006, https://doi.org/10.4271/2006-01-3303.10.4271/2006-01-3303Search in Google Scholar
Yang CY, Li ZFB, Long YF. Review and prospects of Jatropha biodiesel industry in China. Renew Sustain Energy Rev 2012; 16: 2178–2190.10.1016/j.rser.2012.01.043Search in Google Scholar
Zhang Y, Dubei MA, McLean DD, Kates M. Biodiesel production from waste cooking oil. 1: Process design and technological assessment. Bioresour Technol 2003; 89: 1–16.10.1016/S0960-8524(03)00040-3Search in Google Scholar PubMed
Zhang Y, Wong WT, Yung KF. Biodiesel production via esterification of oleic acid catalyzed by chlorosulfonic acid modified zirconia. Appl Energy 2014; 116: 191–198.10.1016/j.apenergy.2013.11.044Search in Google Scholar
Zheng D, Hanna MA. Preparation and properties of methyl esters of beef tallow. Bioresour Technol 1996; 57: 137–142.10.1016/0960-8524(96)00062-4Search in Google Scholar
Zhila NO, Kalacheva GS, Volova TG. Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J Appl Phycol 2011; 23: 47–52.10.1007/s10811-010-9532-8Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Cathodic modification of stainless steels with ruthenium: a review of recent advances in making the cheaper option cheaper
- General properties and comparison of the corrosion inhibition efficiencies of the triazole derivatives for mild steel
- Original articles
- Fabrication of Zn-Ni-Mn alloy by electrodeposition and its characterization
- Impact of moisture on the corrosion behavior of copper and mild carbon steel in corn biodiesel
- Oil-in-water emulsion of a heterocyclic adduct as a novel inhibitor of API X52 steel corrosion in acidic solution
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Cathodic modification of stainless steels with ruthenium: a review of recent advances in making the cheaper option cheaper
- General properties and comparison of the corrosion inhibition efficiencies of the triazole derivatives for mild steel
- Original articles
- Fabrication of Zn-Ni-Mn alloy by electrodeposition and its characterization
- Impact of moisture on the corrosion behavior of copper and mild carbon steel in corn biodiesel
- Oil-in-water emulsion of a heterocyclic adduct as a novel inhibitor of API X52 steel corrosion in acidic solution

