Abstract
Phosphoric acid has been used in many industrial applications and can be in contact with a lot chemical and petrochemical equipment. Most of this equipment is made of steel alloys that may be damaged because of contact with acid. Corrosion inhibitors can be used for to protect steel alloys against several forms and various environments of corrosion. In this paper, the efficiency and applications of organic and inorganic inhibitors in different environments of phosphoric acid are investigated. More than 40 papers were collected and reviewed. Surveys of the stated works in phosphoric acid environments are documented. It was concluded that organic inhibitors performed as good corrosion inhibitors and are used widely. The use of inorganic inhibitors was limited. Most of studied inhibitors follow Langmuir’s adsorption isotherm, and the mechanism of the inhibition can be ascribed to the mixed effect on cathodic and anodic reactions. The synergistic effect was also addressed in the present work.
1 Introduction
In nature, most metals that originate in a chemically joint state are known as ore. Ores may be oxides, sulfides, carbonates, or other complex compounds. In order to separate a metal such as iron from one of its ores, e.g. iron oxide, a large amount of energy is essential. Therefore, metals in their uncombined condition are usually in high-energy states. Thermodynamic laws tell us that there is a high trend for high-energy states to convert into low-energy states. It is this tendency of metals to recombine with components of the environment that yields to the phenomenon known as corrosion. A good definition of corrosion is as follows: “Corrosion is the degradation of a metal by an electrochemical reaction with its environment” (Trethewey and Chamberlain, 1996). The significance of corrosion studies is threefold. The most significance is economic, including the objective of reducing material losses resulting from the corrosion of piping, tanks, metal parts of machines, ships, etc. The second is enhanced safety of operating equipment, which through corrosion may fail with terrible consequences, such as pressure vessels and boilers. The third is safeguarding, applied mainly to metal resources whose world supply is limited and their depletion includes corresponding losses of energy and water reserves related with the production and manufacture of metal structures. Losses contributed by industry, by the military and by municipalities amount to many billions of dollars annually (Uhlig, 1971). Steel and steel alloys are subjected to the action of acids in many different ways and by many different causes. The exposure can be very severe, but in many cases, the corrosion can be controlled by means of anticorrosion chemicals. Processes in which acids play a very significant role are as follows (George, 1974): Acid pickling is the removal of unwanted oxide from metals and for preparing the surface for further operations, such as enameling and painting. Industrial acid cleaning is applied essentially for the removal of scale and undesirable deposits from steam-generating equipment and from chemical and petrochemical reaction vessels. Hydrochloric acid is widely used. Sulfuric and phosphoric acids are also used for chemical cleaning. Oil well acidizing is used for simulations, wherein large quantities of acid are pumped at high rates of flow through the oil well tubing in the production process. The primary objective is to act on the formation in such a way as to simulate the oil flow. One of the used acids in these processes is the phosphoric acid. Also, phosphoric acid solutions are an efficient catalyst for organic reactions such as the alkylation of aromatic hydrocarbons with olefins, the isomerization of olefins, and the polymerization of normally gaseous olefins (Khadom, 2000). Therefore, it is very important to study the corrosion and corrosion prevention of carbon steel by phosphoric acid. The present work is a step in the direction toward understanding the corrosion of carbon steel by phosphoric acid (H3PO4) and its inhibition by means of a literature review of previous and recent works. Mass loss and potentiostatic polarization techniques were used. Corrosion rates, inhibition efficiency, and other corrosion parameters were obtained.
2 Corrosion inhibition via organic inhibitors
The use of organic materials as corrosion inhibitors is one of the most useful methods for protecting against corrosion, and it is becoming progressively more common. The existing data show that organic inhibitors act by adsorption on the metal surface and creation of a protective layer. The adsorption of organic inhibitors at the metal/solution interface takes place through the replacement of water molecules by the organic inhibitor materials according to the following equation (Ramazan et al., 2011):
where Org(sol) and Org(ads) are organic molecules in the solution and organic molecules adsorbed on the metal surface, respectively, and x is the number of water molecules replaced by the organic molecules. Generally, acid inhibitors are organic compounds that contain nitrogen, sulfur, oxygen or phosphorus with the structure of aromatic ring or triple bonds. The efficiency of inhibition of corrosion decreases according to the following order: O<N<S<P (Subramanyam et al., 1993). It has also been found that the molecules containing both nitrogen and sulfur in their molecular structure have shown better corrosion inhibition efficiency in comparison with those that only have one of these atoms (Behpour et al., 2009).
Poornima et al. (2011) studied the effect of 4-(N,N-diethylamino)benzaldehyde thiosemicarbazone (DEABT) and its corrosion inhibition on the corrosion of a steel alloy in 0.67 m H3PO4 solution at 30–50°C by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and weight loss methods. Inhibition effectiveness of DEABT was found to rise with the increase in DEABT concentration and decline with the increase in temperature. The activation energy Ea and other thermodynamic factors have been assessed and discussed. The adsorption of DEABT on the steel surface follows the Langmuir adsorption isotherm model, and the inhibitor showed mixed-type inhibition performance.
Yaro et al. (2013) investigated the corrosion inhibition of low-carbon steel in 1 m phosphoric acid using apricot juice at variable temperatures in accordance to the mass loss method. Adsorption, activation, and statistical studies were reviewed in this work. Adsorption studies showed that the inhibitor adsorbed on metal surface in relation to the Langmuir isotherm. The average value of heat of adsorption was −14.93 kJ/mol that shows a spontaneous physical adsorption on a metal surface. Activation parameters did not change by the addition of the inhibitor, indicating that there is no change in the reaction mechanism. Analysis of variance was also applied. This analysis showed that the corrosion rate is influenced by temperature, inhibitor concentration, and the combined interaction of both.
Li et al. (2011) studied the inhibition effect of benzyltrimethylammonium iodide (BTAI) on the corrosion of steel in 7.0 m H3PO4 produced by a dihydrate wet method process using weight loss, potentiodynamic polarization, EIS, and scanning electron microscopy (SEM) methods. The outcomes show that BTAI is a decent inhibitor, and the adsorption of BTAI follows the Langmuir adsorption isotherm. Polarization data show that BTAI acts as a mixed-type inhibitor. EIS spectra exhibit one capacitive loop and confirm BTAI’s inhibitive ability. The inhibition action of BTAI is also evidenced by SEM images. They attributed the action of the inhibitor to the ionization of BTAI in the acid according to the following equation:
Therefore, in aqueous acidic media, BTAI exists as the organic part (BTA+) and the anion inorganic part (I−). Furthermore, the steel surface can be charged positively in an acid solution (Li et al., 2009a,b), so it is difficult for BTA+ to adhere to the steel surface because of repulsion. However, I− can be oxidized by oxygen dissolved in acidic media and turns to the yellowish tri-iodide. The oxidization reactions of iodide ions can be described as follows (Feng et al., 1999):
The I−3 ions could accumulate slowly to the steel/solution interface and be adsorbed, and the BTA+ may be adsorbed through electrostatic interactions between the positively charged molecules and the negatively charged metal surface. In other words, there may be a synergism between BTA+ and I−3.
Jianguo et al. (1995) demonstrated that both polyvinylpyrrolidone and polyethylenimine are powerful for the inhibition of low-carbon steel over a wide concentration range of aqueous H3PO4 solutions. Both polymers retard the anodic and cathodic corrosion reactions with emphasis on the former. The outcomes for uninhibited acid confirm the kinetic expression proposed by Mathur and Vasudevan (1982).
Benabdellah et al. (2006) extracted artemisia oil (Ar) from Artemisia herba alba, and it was confirmed as a corrosion inhibitor of steel in 2 m phosphoric acid using weight loss, electrochemical polarization, and EIS procedures. The natural oil decreases the corrosion rate. The inhibition efficiency was found to increase with oil concentration to approach 79% at 6 g/l. Artemisia oil behaves as a cathodic inhibitor. The influence of temperature on the corrosion mechanism of steel indicates that the inhibition efficiency of the natural substance decreases with the rise of temperature. The adsorption isotherm of the natural product on the steel has been determined. They proposed that the adsorption of heterocyclic compounds happens when the aromatic rings are mostly perpendicular to the steel surface at a low concentration of inhibitor, but at elevated inhibitor concentration, the molecules are reoriented to the parallel mode, and this result agrees with the work of Bockris and Yang (1991).
Dadgarinezhad and Baghaei (2009) studied the effect of 2-phenyl-1-hydrazine carboxamide on the corrosion of low-carbon steel in solutions of H3PO4. The results obtained revealed that this compound is a good mixed-type inhibitor. Increasing the acid concentration increased the metal corrosion but did not affect the inhibitor efficiency. The optimum inhibitor concentration remained at 150 ppm for various concentrations of phosphoric acid. The surface morphologies of specimens after 1 h of immersion in various solutions were studied by SEM. The Langmuir adsorption isotherm fitted well with the experimental data. They also concluded that the performance of the inhibitor is attributed to the presence of electron donor groups (N, O, and the aromatic ring) in the structure of 2-phenyl-1-hydrazine carboxamide, which leads to the adsorption on the metal surface.
Ben Hmamou et al. (2013) investigated the inhibition effect of alizarin red on the corrosion of carbon steel in 2 m phosphoric acid solutions at 298 K by using EIS, potentiodynamic polarization, and weight loss techniques. A main decrease in the corrosion rate of steel was noticed in the presence of the investigated alizarin red. Potentiodynamic polarization plots exposed that this inhibitor acted as a mixed-type inhibitor, affecting both cathodic and anodic corrosion processes. The adsorption of the inhibitor on the metal surface was found to follow the Langmuir adsorption isotherm. Thermodynamic adsorption parameters of the examined inhibitor were calculated from the linear formula of the Langmuir adsorption isotherm.
Li et al. (2017) studied the inhibition effect of the cationic surfactant of tetradecylpyridinium bromide (TDPB) on the corrosion of steel in 7 m H3PO4 by weight loss and potentiodynamic polarization methods and EIS and SEM. Quantum chemical calculation was applied to clarify the adsorption type of TDPB molecules on a steel surface. The results show that TDPB acts as a good inhibitor, and its maximum inhibition efficiency is higher than 90% even at low concentrations. The adsorption of TDPB obeys the Langmuir adsorption isotherm. Polarization curves indicate that TDPB bromide acts as a mixed-type inhibitor in acid. EIS studies show one capacitive loop, which indicates that the corrosion reaction is controlled by charge transfer process. They also classified TDPB as 1:1 electrolyte, and it ionized as [TDP]+ and [Br]−, which agrees with the explanation of Li et al. (2011).
Wang et al. (2012) studied the corrosion inhibition by a triazole derivative on low-carbon steel in H3PO4 solution by weight loss and polarization methods. The experimental results state that the material has an important inhibiting effect on the corrosion of steel in phosphoric acid solution. It also shows good corrosion inhibition at the upper concentration level of phosphoric acid. Potentiodynamic polarization results have shown that the compound acts as a mixed-type inhibitor, hindering the anodic and cathodic corrosion reactions with the largest effect on the cathodic reaction. The values of inhibition efficiency attained from weight loss and polarization measurements are in good agreement. The adsorption of this material is found to follow the Langmuir adsorption isotherm. Some kinetic and thermodynamic parameters such as apparent activation energy, frequency factor, and adsorption free energy have been estimated and discussed. The inhibitor efficiency decreases slightly with increasing H3PO4 concentration; this conclusion agrees with the results of Dadgarinezhad and Baghaei (2009).
The inhibition effect of dimethylaminobenzylidene acetone on low-carbon steel corrosion in 1 m H3PO4 solution was investigated by Saratha and Meenakshi (2011). The data showed that dimethylaminobenzylidene acetone was a steel corrosion inhibitor in H3PO4 solution, and the maximum inhibition performance achieved was 94.9% at the concentration of 0.04% (w/v) for the immersion period of 12 h. Temperature studies were carried out, and thermodynamic parameters such as change in enthalpy, change in entropy, and change in free energy were evaluated. Adsorption of dimethylaminobenzylidene acetone on the steel surface in 1 m H3PO4 solution followed the Temkin adsorption isotherm. EIS measurements showed an increase in charge transfer resistance. The potentiodynamic study showed it as a mixed type of inhibitor, controlling both the anodic and cathodic reactions. Surface analysis by Fourier transform infrared spectroscopy (FTIR) and SEM confirmed the formation of protective coating on the mild steel surface.
Belghiti et al. (2016b) tested three hydrazine derivatives, namely, 1,2-bis(pyrrol-2-ylidenemethyl)hydrazine (HZ1), 1,2-bis(thiophen-2-ylidenemethyl)hydrazine (HZ2), and 1,2-bis(furyl-2-ylidenmethyl)hydrazine (HZ3) as corrosion inhibitors of mild steel in phosphoric acid using experimental and theoretical methods. In the theoretical part, quantum chemical calculations based on density functional theory (DFT) and quantitative structure-activity relationship methods were done to determine the connection between the molecular structure of hydrazines and their inhibition efficiencies. The quantum chemical parameters such as the localization of frontier molecular orbitals, EHOMO, ELUMO, energy gap (∆E), dipole moment (μ), hardness (ŋ), softness (S), the fractions of electrons transmit (∆N), electrophilicity index (χ), and total energy charge were calculated and used to clarify the electron transfer mechanism between the inhibitor molecules and the steel surface. Furthermore, statistical equations were proposed using the multiple-linear and the nonlinear regression analyses. In the experimental part (Belghiti et al., 2016a), the inhibitory effects of three hydrazine derivatives were studied using weight loss, electrochemical impedance spectroscopy, and polarization techniques. It was found that the inhibition efficiency of the compounds increases with the increasing inhibitor concentration. The adsorption of inhibitors on the steel surface obeys the Langmuir isotherm. The effect of temperature on the corrosion behavior of mild steel in phosphoric acid solution in the absence and presence of the inhibitors was also studied. The inhibitor’s efficiency decreased with the rise in temperature. Polarization curves showed that HZ1 and HZ3 behave as mixed-type corrosion inhibitors in phosphoric acid solution. The inhibition efficiencies of the inhibitors follows the order of HZ2>HZ3>HZ1. These results agree with the research that the number of N-heterocyclic compounds in the aromatic or long carbon chain system have effective inhibitor performance (Dafali et al., 2002). The notable inhibitory influence is supported by the presence of heteroatoms such as sulfur (S), oxygen (O), and nitrogen (N) in the ring that simplifies the adsorption on the metal surface following the sequence of -S->=N->-O-> (Dafali et al., 2003).
Zarrok et al. (2014) investigated the inhibition of corrosion of carbon steel in phosphoric acid solution by the use of l-cysteine methyl ester hydrochloride by weight loss and electrochemical procedures. The performance of inhibition was increased by increasing the concentration of the inhibitor and reduced by increasing the temperature. Polarization data showed that l-cysteine methyl ester hydrochloride behaves as a mixed-type inhibitor. Electrochemical impedance spectroscopy showed that dissolution of the steel occurs as a result of charge transfer. Adsorption of the inhibitor obeyed the Langmuir adsorption isotherm. Kinetic and thermodynamic data were also estimated.
Sivaraju and Kannan (2010) studied the inhibition effect of Acalypha indica L. alcoholic extract (AIAE) on mild steel corrosion in 1 N H3PO4 by weight loss and polarization methods in a temperature range of 303–333 K. The inhibition efficiency increased with increase in the concentration of AIAE. The corrosion rate increased with increase in temperature and decreased with increase in the concentration of inhibitor compared with the inhibitor-free solution. The adsorption of inhibitor on the mild steel surface has been found to obey the Temkin adsorption isotherm. Potentiostatic polarization results revealed that the extract acts as a mixed-type inhibitor. The values of the activation energy (Ea), free energy of adsorption (ΔGads), heat of adsorption (Qads), enthalpy of adsorption (ΔH), and entropy of adsorption (ΔS) were calculated. Surface analyses (FTIR and SEM) were also achieved to establish the mechanism of corrosion inhibition on the mild steel corrosion in H3PO4 solution.
Noyel et al. (2015) investigated the adsorption and corrosion inhibition property of the alcoholic Psidium guajava (guava) leaf extract on mild steel in 1 m H3PO4 solution medium by mass loss, potentiodynamic polarization, and electrochemical impedance spectroscopy techniques. The studies showed that the inhibition efficiency increases with inhibitor concentration up to 800 ppm and decreases slightly at 1200 ppm. A minor fall in the inhibitor performance at 1200 ppm concentration may be due to the desorption of the inhibitor molecules into the bulk on approaching the critical concentration. Desorption declines the metal-inhibitor interaction resulting in a drop in inhibition efficiency. The adsorption obeys both the Langmuir and Temkin adsorption isotherm formulas. The kinetic and thermodynamic parameters were estimated. The adsorption was found to follow a comprehensive-type adsorption dominated by chemisorption. Potentiodynamic polarization studies showed that the inhibitor acted as a mixed-type inhibitor.
Balanaga Karthik (2011) tested the influence of allyl methyl sulfide on the corrosion of mild steel in a H3PO4 solution by the weight loss method in relation to the concentration of the inhibitor, acid, and temperature. At room temperature, the efficiency of the inhibition increases with the increase in the concentration of allyl methyl sulfide for mild steel. The corrosion rate is at a maximum for an uninhibited solution as compared with that of the inhibited solution at all temperatures. The corrosion rate is increasing with the increase of temperature. For 1×10−2m of allyl methyl sulfide concentration, the percentage efficiency decreases with temperature increase because of decreasing adsorption with increase in temperature. Low heat of adsorption indicates the physical adsorption. By increasing the temperature, the free energy change values decrease; this may be ascribed to the increase in temperature favoring the corrosion process. As the concentration of the inhibitor decreases, the free energy change increases. This shows that with the decrease in the concentration of allyl methyl sulfide, the corrosion process becomes more and easier.
Arab and Al-Turkustani (2006) investigated the corrosion inhibition of low-carbon steel in 0.67 m phosphoric acid by phenacyl dimethylsulfonium bromide and six of its p-substituted derivatives using different chemical, electrochemical, and SEM methods. The order of increasing inhibition efficiency was correlated with its p-substitution through the Hammett relation. Potentiodynamic polarization curves indicated that the compounds acted primarily as mixed-type inhibitors. Electrochemical impedance spectroscopy showed that the steel dissolution is organized by a charge-transfer mechanism. The kinetic-thermodynamic model of adsorption isotherm described the experimental findings. The number of active sites, binding constant, and change of free energy were computed for all the studied compounds. Depending on the inhibitor, it was found that each organic molecule substituted one or two adsorbed water molecules from the steel surface. The adsorption center was suggested to be the π electrons of the phenyl ring, and a flat configuration adsorption of the molecule may occur.
Wang et al. (2000) used mass loss and polarization techniques to show that 2-mercaptobenzoxazole is effective for the inhibition of low-carbon steel over a wide concentration range of aqueous H3PO4 solutions. The inhibitor hinders the anodic and cathodic corrosion reaction with an emphasis on the former. Results for uninhibited acid solution confirm the kinetic expression proposed by Mathur.
The corrosion behavior of steel alloy in phosphoric acid was investigated by various electrochemical methods. The action of propargyl alcohol as an inhibitor was also studied over a wide range of acid concentrations and solution temperatures (Morad, 1999). The results obtained from both polarization curves and EIS proved that propargyl alcohol greatly inhibits the hydrogen evolution reaction on mild steel in phosphoric acid solutions at temperatures ≥40°C and inhibition occurs by adsorption of propargyl alcohol molecules. The most favorable adsorption style is one in which the carbon atoms forming the triple bond interact with the iron atoms. Inhibition of the cathodic reaction proposes that adsorption of the inhibitor is most favorable at a surface partially covered by hydrogen. A similar mode of inhibition by propargyl alcohol was suggested by Bartos et al. (1993), Kuteja et al. (1995), and Bockris and Yang (1991). The results of this work present no indications of the formation of a polymer film of propargyl alcohol on the metal surface in the presence of propargyl alcohol.
Noor (2005) determined the corrosion rates of steel in concentrated phosphoric acid (1.0–11.0 m) by the mass loss technique, at three temperatures 298, 308, and 323 K. The corrosion rate increases with both acid concentration and temperature. The activation energies, enthalpies, and entropies of the dissolution process were determined. The effect of some quaternary N-heterocyclic compounds as corrosion inhibitors was evaluated. Results obtained showed that these compounds are good mixed-type inhibitors without changing the mechanism of the corrosion reaction. General, at constant acid concentration, inhibitor performance increased with the concentration of the inhibitor. On the other hand, at constant inhibitor concentration, inhibitor efficiency decreased with the concentration of the acid up to a critical concentration above which it started to increase. The studied compounds seemed to function through general adsorption following the thermodynamic-kinetic adsorption isotherm.
Messali et al. (2017) studied the effect of guar gum as a water-soluble, green, biodegradable, and biocompatible inhibitor for the corrosion inhibition of carbon steel in 2 m H3PO4 solution. The characteristic effect of guar gum on the steel corrosion was studied at concentration ranges from 0.1 to 1.0 g/l at 298 to 328 K by weight loss and electrochemical methods. The results showed that the inhibition efficiency of guar gum decreased slightly when the temperature increased and increased by increasing the inhibitor concentration reaching the maximum value at 1.0 g/l. The adsorption of guar gum on the metal surface obeyed the Temkin adsorption isotherm. EIS measurements indicate that the values of the polarization resistance of carbon steel in the presence of guar gum are significantly higher than that of the untreated surface. A steel surface coated with guar gum was analyzed by SEM, FTIR, and X-ray diffraction. The quantum calculations using the DFT method and molecular dynamic simulations were achieved to define the relationship between the inhibition performance of the investigated compound and their molecular structure. The performance of guar gum is due to the adsorption on the metal surface that depends largely on the molecular structure of the tested inhibitor, which contains reactive sites such as oxygen atoms and -OH groups. Furthermore, the steel corrosion in acidic medium mainly contains two simultaneous electrochemical reactions (anodic and cathodic reactions). From the observations reported in the electrochemical, theoretical, and surface analysis results, it can be determined that the guar gum is possibly adsorbed on the anodic sites of the metal surface by chelate formation with Fe2+ present on those sites involving an endocyclic “O” atom of the mannose unit together with the oxygen atom of the same monosaccharide unit. The -OH groups are mainly responsible for adsorption of tested guar on the cathodic sites of the steel surface.
The summary of the above literature review is shown in Table 1. It is seen that the inhibition performance of sulfur-containing compounds is greater than that of nitrogen-containing compounds. This may be attributed to the presence of two electron pairs available for organization. Furthermore, iron is well known for its coordination affinity to ligands possessing a heteroatom. An increase in inhibition performance with an increase in inhibitor concentration shows that the inhibition action is due to the adsorption on the steel surface, and this agrees with the conclusion of Ebenso et al. (1999).
Summary of organic corrosion inhibitor for steel in phosphoric acid in terms of steel type, acid concentration (C), inhibitor type, temperature range (T), adsorption isotherm, and maximum efficiency (IE).
| Steel type | C | Inhibitor | T (°C) | Adsorption type/isotherm | IE (%) | References |
|---|---|---|---|---|---|---|
| Aged maraging steel (0.015 wt% C) | 0.67 m | 4-(N,N-Diethylamino)benzaldehyde thiosemicarbazone |
30–50 | Predominantly physical adsorption/Langmuir | 92.17 | Poornima et al. (2011) |
| Mild steel (0.199 wt% C) | 1 m | Plant extract/apricot juice | 30–60 | Physical adsorption/Langmuir | 75 | Yaro et al. (2013) |
| Cold rolled steel (0.07 wt% C) | 7 m | Benzyltrimethylammonium iodide |
20–50 | Comprehensive adsorption/Langmuir | 98 | Li et al. (2011) |
| 1010 grade low-carbon steel | 1–9 m | Polyvinylpyrrolidone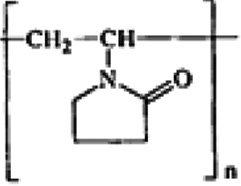  |
30–70 | Physical adsorption/Langmuir | 89 90 |
Jianguo et al. (1995) |
| Steel (0.21 wt% C) | 2 m | Plant extract/artemisia oil |
25–75 | Physical adsorption/Langmuir | 79 | Benabdellah et al. (2006) |
| Mild steel (0.05 wt% C) | 1–3 m | 2-Phenyl-1-hydrazine carboxamide | 25 | Langmuir | 98.9 | Dadgarinezhad and Baghaei (2009) |
| Carbon steel (0.37 wt% C) | 2 m | Alizarin red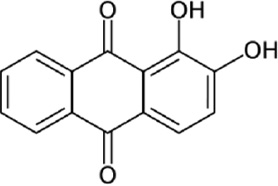 |
25 | Comprehensive adsorption/Langmuir | 93.6 | Ben Hmamou et al. (2013) |
| Cold rolled steel (0.07 wt% C) | 7 m | Tetradecylpyridinium bromide |
20–50 | Comprehensive adsorption/Langmuir | 92.2 | Li et al. (2017) |
| Mild steel (0.05 wt% C) | 2, 7, 9 mole/dm3 | 3-Phenyl-4-amino-5-mercapto-1,2,4-triazole |
30 | Predominantly chemical adsorption/Langmuir | 97 | Wang et al. (2012) |
| Mild steel (0.039 wt% C) | 1 m | Dimethylaminobenzylidene |
30–70 | Predominantly chemical adsorption/Temkin | 90 | Saratha and Meenakshi (2011) |
| Mild steel (0.21 wt% C) | 2 m | Hydrazine derivatives   |
35 | Comprehensive adsorption/Langmuir | 88.14 (HZ1) 89.42 (HZ2) 88.5 (HZ3) | Belghiti et al. (2016a, b) |
| Carbon steel (0.37 wt% C) | 2 m |
l-Cysteine methyl ester hydrochloride |
35–55 | Physical adsorption/Langmuir | 93.5 | Zarrok et al. (2014) |
| Mild steel (0.017 wt% C) | 1 N | Plant extract/Acalypha indica L. | 30–60 | Physical adsorption/Temkin | 90.38 | Sivaraju and Kannan (2010) |
| Mild steel (0.2 wt% C) | 1 m | Plant extract/Psidium guajava (guava) | 30–80 | Comprehensive adsorption/Langmuir and Temkin | 89 | Noyel et al. (2015) |
| Mild steel (0.07 wt% C) | 1 m | Allyl methyl sulfide |
30–65 | Physical adsorption/Langmuir | 96.87 | Balanaga Karthik (2011) |
| Mild steel (0.17 wt% C) | 0.67 m | Phenacyl dimethyl sulfonium bromide (A) |
30 | Physical adsorption/kinetic-thermodynamic | 66.39 (A) 84.71 (B) 69.41 (C) 74.96 (D) 79.83 (E) 67.90 (F) |
Arab and Al-Turkustani (2006) |
4-Methylphenacyl dimethyl sulfonium bromide (B) |
||||||
4-Chlorophenacyl dimethyl sulfonium bromide (C) |
||||||
4-Bromophenacyl dimethyl sulfonium bromide (D)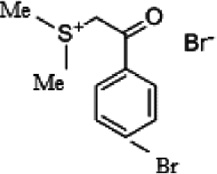 |
||||||
4-Nitrophenacyl dimethyl sulfonium bromide (E) |
||||||
4-Methoxyphenacyl dimethyl sulfonium bromide (F) |
||||||
| 1010 grade low-carbon steel | 3 m | 2-Mercaptobenzoxazole | 30 | N/A | 94 | Wang et al. (2000) |
| Mild steel | 1–9 m | Propargyl alcohol | 35–50 | N/A | 97.2 | Morad (1999) |
| Mild steel (0.21 wt% C) | 2 m | N-Heterocyclic compounds: | 30–70 | Physical adsorption/Langmuir | 96.49 (I) 95.91 (II) 99.95 (III) |
Noor (2005) |
 |
||||||
 |
||||||
 |
||||||
| Carbon steel (0.37 wt% C) | 2 m | Plant extract/guar gum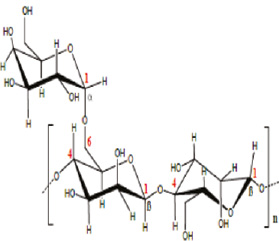 |
25–55 | Comprehensive adsorption/Temkin | 95.8 | Messali et al. (2017) |
Summary of inorganic corrosion inhibitor for steel in phosphoric acid in terms of steel type, acid concentration (C), inhibitor type, temperature range (T), adsorption isotherm, and maximum efficiency (IE).
| Steel type | C (m) | Inhibitor | T (°C) | Adsorption type/isotherm | IE (%) | References |
|---|---|---|---|---|---|---|
| Mild steel (0.05 wt% C) | 0.33 | Phosphate of aluminum, AlPO4 | 25–65 | Physical/Langmuir | 85 | Alaoui et al. (2008) |
| Carbon steel (0.041 wt% C) | 2.5 | Potassium iodide, KI | 30–60 | N/A (mathematical modeling) | 98 | Khadom (2012) |
| Carbon steel (0.041 wt% C) | 2.5 | Potassium iodide, KI | 30–60 | Chemical/Langmuir | 99.95 | Khadom and Yaro (2011) |
| Carbon steel (0.041 wt% C) | 2.5 | Potassium iodide, KI | 30–60 | N/A (electrochemical modeling) | 99 | Yaro and Khadom (2010) |
Summary of synergistic effect corrosion inhibitor for steel in phosphoric acid in terms of steel type, acid concentration (C), inhibitor type, temperature range (T), adsorption isotherm, and maximum efficiency (IE).
| Steel type | C (m) | Inhibitor | T (°C) | Adsorption type/isotherm | IE (%) | References |
|---|---|---|---|---|---|---|
| Cold rolled steel (0.1 wt% C) | 1 |
o-Phenanthroline+sodium chloride |
30–45 | Chemical/Langmuir | 97.3 | Guan et al. (2004) |
| Cold rolled steel (0.1 wt% C) | 0.1 | Dodecylamine+potassium iodide |
20 | N/A | 98.4 | Qu et al. (2008) |
| Cold rolled steel (0.1 wt% C) | 1–4 | Cetyltrimethylammonium bromide+sodium chloride |
40 | N/A | <90 | Li et al. (2008) |
| Cold rolled steel (0.07 wt% C) | 1–10 | Red tetrazolium+uracil |
20–50 | Physical/Freundlich | 98.8 | Li et al. (2009b) |
3 Corrosion inhibition via inorganic inhibitors
Inorganic inhibitors can be categorized as anodic and cathodic inhibitors. Anodic inhibitors (also called passivation inhibitors) act by reducing the anodic reaction that blocks the anode reaction and supports the natural formation of a passivation metal surface, also due to the formation of a layer adsorbed on the metal surface. In general, the inhibitors react with the corrosion product, initially formed, resulting in a solid and insoluble layer on the steel surface. Cathodic corrosion inhibitors inhibit the cathodic reaction of the metal from happening. These inhibitors have metal ions able to produce a cathodic reaction due to alkalinity, thus producing insoluble products that precipitate selectively on cathodic sites. A compact and adherent layer deposits over the metal, restricting the diffusion of reducible species at these sites and thus increasing the impedance of the surface and the diffusion restriction of the reducible species, that is, the oxygen diffusion and electrons conductive in these areas. These anticorrosion chemicals cause high cathodic inhibition (Roberge, 1999; Talbot & Talbot, 2000).
Alaoui et al. (2008) studied the influence of phosphate of aluminum on the corrosion inhibition of low-carbon steel in H3PO4 solution using the mass loss and electrochemical methods. The inhibition efficiency of inhibitor increases with concentration to attain 84% at 10−2m of phosphate of aluminum in 0.33 m H3PO4. Polarization studies proved that phosphate of aluminum is a mixed-type inhibitor and acts both on the cathodic and anodic reactions without changing the mechanism of the hydrogen evolution reaction. The inhibition efficiency of phosphate of aluminum is temperature-dependent. Phosphate of aluminum adsorbs on the steel surface according to the Langmuir adsorption isotherm via physical adsorption.
Khadom (2012) evaluated the corrosion of mild steel in H3PO4 at different temperatures in the absence and presence of potassium iodide (KI) as a corrosion inhibitor. Mass loss and polarization methods were used to estimate the corrosion rate data. Two mathematical models were suggested to represent the corrosion rate data. Polynomial and exponential models were used. A computer program was used to estimate the constants of these models. Both models successfully represented the corrosion rate data with high correlation coefficients.
Khadom and Yaro (2011) studied the corrosion of low-carbon steel in 2.5 m H3PO4 at a temperature range of 30–60°C in the presence and absence of potassium iodide as an inorganic inhibitor in the range of 0.02–0.05 m at static conditions. Mass loss and polarization techniques were employed in this investigation. The maximum value of inhibitor efficiency obtained was 99.95% at 50°C in the presence of 0.05 m inhibitor concentration. The fraction of surface covered calculated from corrosion rates obeyed the Langmuir adsorption isotherm. Polarization experiments showed that for a given temperature, the curves were shifted toward the potential axis, leading to lower corrosion rates as the inhibitor concentration increased. Potassium iodide inhibits carbon steel corrosion in phosphoric acid by altering both anodic and cathodic partial reactions with the anodic reaction being more prevailing.
Yaro and Khadom (2010) discussed the nonlinear region of the polarization curve near the corrosion potential, depending on potential data as a function of current densities. These data were analyzed by suggesting a mathematical model, taking into account the effect of mass transfer on the activation process. This model was used to evaluate the values of polarization resistance in the presence and absence of potassium iodide as a corrosion inhibitor of mild steel in H3PO4 at different temperatures. The values of polarization resistance obtained by the mathematical model were compared with those obtained by Tafel parameters. The maximum value of polarization resistance was 5049.13 Ω cm2 at 30°C in the presence of 0.05 m KI, while the minimum value was 1.885 Ω cm2 at 60°C in the absence of KI. These results indicate that the polarization resistance values increased with the increase of KI concentration and decreased with the increase of temperature. The summary of inorganic corrosion inhibitor for steel in phosphoric acid was shown in Table 2.
4 Corrosion inhibition via synergistic effect inhibitors
Promotion of the inhibition effect of organic inhibitors is another important criteria, as most of these inhibitors are very definite in their action and the corrosion process is complicated. Extensive studies have thus been undertaken to identify the synergistic effects with other additives (Onuchukwu & Lori, 1984; Zucchi et al., 1994; Aramaki, 2002; Jabeera et al., 2006). Synergism is described as a joint action of compounds with greater in total effect than the sum of the individual effects. For corrosion inhibitor systems, synergism usually arises either as a consequence of the interaction between components of the inhibitor formulation or due to the interaction between the inhibitor and one of the ions present in the aqueous media. The synergistic effect can be explained according to synergistic parameter, Sθ, obtained from the surface coverage values (θ) of the anion, cation, and both. Khadom (2015) calculated the synergism parameter, Sθ, using the following equation:
where θ1+2=(θ1+θ2)−(θ1θ2), θ1 is the surface coverage by anion, θ2 is the surface coverage by cation, and θ*1+2 is the measured surface coverage by both the anion and the cation. If Sθ approaches 1, no interaction between inhibitors exists, while S>1 refers to a synergistic effect. While if S<1, the antagonistic interaction prevails, which may be attributed to competitive adsorption (Khadom, 2015). In competitive adsorption, the anion and cation are adsorbed at different sites on the metal surface, while in cooperative adsorption; the anion is adsorbed on the metal surface and followed by the adsorption of the cation on a layer of anion.
The effects of single o-phenanthroline and the mixture of various concentrations of NaCl and 0.0002 mo-phenanthroline on the corrosion of steel in H3PO4 were investigated by Guan et al. (2004). The research showed that steel in phosphoric acid was more efficiently inhibited by o-phenanthroline in the presence of NaCl than single o-phenanthroline, and inhibition efficiency increases with increasing concentration of NaCl in the presence of 0.0002 mo-phenanthroline. At a higher concentration of NaCl, there is the synergistic influence between o-phenanthroline and NaCl. The adsorption of inhibitor follows the Langmuir adsorption isotherm. Polarization results showed that the inhibitor mainly acts as a mixed-type inhibitor for steel in 1.0 m H3PO4. The results obtained from weight loss and polarization methods are in good agreement. They attributed the synergistic effect to the smaller degree of hydration of chloride ions, and it can bring excess negative charges in the area of the interface due to the adsorption, then positively charged ions may adsorb onto the metal surface. o-Phenanthroline may be protonated in the acid solution as follows:
Subsequently, the steel surface contains positive charges in the acid solution, and the results can be described on the assumption that in the presence of Cl−, the negatively charged Cl− would align to the positively charged surface. Then, near the interface, the concentrations of Cl− and protonated o-phenanthroline are much higher than those in the bulk solution. The protonated o-phenanthroline do not attach directly to the positively charged steel surface because of the repulsive interaction between the protonated o-phenanthroline and the positively charged steel surface. The protonated o-phenanthroline, however, can attach to the steel surface by means of electrostatic interaction between Cl− and protonated o-phenanthroline.
Qu et al. (2008) investigated the corrosion inhibition of cold rolled steel in 0.1 m aerated H3PO4 in the presence of dodecylamine (DDA) and potassium iodide (KI) by the Tafel polarization curve and EIS at 20°C. The results achieved from the polarization curve show that the inhibition efficiency of DDA with and without KI increases with the increase in concentration of DDA, but the inhibition efficiency of DDA without KI is lower. A synergistic effect exists when DDA and KI are combined together to avoid steel corrosion in 0.1 m H3PO4. All impedance spectra in EIS tests exhibit one capacitive loop that indicates that the corrosion reaction is controlled by a charge-transfer process. Inhibition efficiencies obtained from Tafel polarization and transfer resistance (Rt) are reliable. Clearly, dodecylamine is a nitrogen-containing organic compound, which contains unshared electron pair and p electrons. Therefore, dodecylamine can be absorbed onto the surface of metal through the transference of lone-pair electrons of nitrogen atoms to the d orbital in the iron atom, but in acid solution, dodecylamine may be protonated, leading to positive charge in the molecule as follows:
The steel surface contains a positive charge in an acid solution; thus, it is difficult for the positively charged dodecylamine to reach the positively charged steel surface because of repulsion forces, and for this reason, the single dodecylamine cannot act as excellent inhibitor for steel corrosion in 0.1 m H3PO4 solution without KI. The added KI can facilitate the adsorption process of dodecylamine and hence improve its performance. This explanation is in agreement with the previous discussion (Guan et al., 2004).
Li et al. (2008) studied the effect of chloride ions on the inhibitive performance of cetyltrimethylammonium bromide in 1–4 m of phosphoric acid for steel using weight loss and polarization techniques. The influence of acid concentration on corrosion inhibition has been examined. The results reveal that a synergistic effect has been observed for cetyltrimethylammonium bromide with NaCl at each acid concentration. In 1 m phosphoric acid, the polarization curves show that the inhibitor-NaCl blend is a mixed-type inhibitor. The explanation of cetyltrimethylammonium bromide-NaCl action was in good agreement with the works of Guan et al. (2004) and Qu et al. (2008).
Li et al. (2009b) investigated the synergistic inhibition effect of red tetrazolium and uracil on the corrosion of cold rolled steel (CRS) in 1.0–10.0 m H3PO4 solution by weight loss and potentiodynamic polarization methods. Atomic force microscopy provided the CRS surface conditions. The results showed that red tetrazolium had a moderate inhibitive effect, and the adsorption of red tetrazolium followed the Freundlich adsorption isotherm. Polarization curves proved that red tetrazolium was a mixed-type inhibitor in phosphoric acid. For uracil, it had a weak inhibition effect and acted as a cathodic inhibitor. However, a combination of red tetrazolium with uracil significantly improved the inhibition performance. The inhibition efficiency (IE) for red tetrazolium in combination with uracil was higher than the summation of IE for a single red tetrazolium and a single uracil, which was synergistic in nature. Polarization studies revealed that the red tetrazolium/uracil mixture acted as a mixed-type inhibitor, which extremely inhibited both anodic and cathodic reactions. The synergistic inhibition effect of red tetrazolium and uracil could also be evidenced by atomic force microscopy images. Depending on the results, the synergism mechanism was discussed from the viewpoint of co-adsorption. The combination of red tetrazolium and uracil significantly improved inhibition efficiency, and all the values of synergism parameter (Sθ) are higher than unity, which specifies that there is a real synergistic effect of red tetrazolium and uracil in phosphoric acid. A positively charged steel surface in acidic media was also stated. When red tetrazolium was mixed with uracil in phosphoric acid, Cl− (the anion part of red tetrazolium) may first adsorb on the positively charged steel surface by electrostatic attraction. Then the protonated red tetrazolium and uracil could easily approach the steel surface, followed by transference of electrons from nitrogen heteroatoms and oxygen atoms to the d orbital of the iron atom (chemical adsorption) at the steel/solution interface. The summary of synergistic effect of corrosion inhibitor for steel in phosphoric acid was shown in Table 3.
5 Conclusion
Phosphoric acid is one of the most important mineral acids. Corrosion control using inhibitors is a powerful way to protect steel and its alloys in different environments of phosphoric acid. Literature on the effects of organic and inorganic inhibitors and synergistic effect of some components was reviewed, and the following conclusions were obtained:
Corrosion of steel in phosphoric acid increased with temperature and acid concentration and decreased with inhibitor concentration.
Phosphoric acid concentration has no significant effect on inhibitor performance.
Organic inhibitors are widely used in different concentrations of phosphoric acid, and these inhibitors can be adsorbed physically, chemically, or comprehensively on steel surfaces, forming a barrier to the corrosive environment. Physical adsorption was more applied.
Generally, the adsorption follows the Langmuir adsorption isotherm, suggesting a formation of a monolayer on the metal surface.
Plant extracts were also used as clean, green, cheap, and environmentally friendly corrosion inhibitors.
Inorganic inhibitors are used to a lesser extent. Potassium iodide was one of the most efficient corrosion inhibitor with maximum inhibitor efficiency approaching 99.8%.
The synergistic effect between organic corrosion inhibitors and other components improved the inhibitor performance.
In general, the efficiency of inhibitors increased with inhibitor concentration and decreased with a rise in temperature.
Acknowledgments
Special thanks go to Prof. Dr. Aprael S. Yaro for his continuous support and encouragement.
References
Alaoui LM, Kertit S, Bellaouchou A, Guenbour A, Benbachir A, Hammouti B. Phosphate of aluminum as corrosion inhibitor for steel in H3PO4. Port Electrochim Acta 2008; 26: 339–347.10.4152/pea.200804339Search in Google Scholar
Arab T, Al-Turkustani AM. Corrosion inhibition of steel in phosphoric acid by phenacyldimethyl sulfonium bromide and some of its p-substituted derivatives. Port Electrochim Acta 2006; 24: 53–69.10.4152/pea.200601053Search in Google Scholar
Aramaki K. Synergistic inhibition of zinc corrosion in 0.5 m NaCl by combination of cerium(III) chloride and sodium silicate. Corros Sci 2002; 44: 871–886.10.1016/S0010-938X(01)00087-7Search in Google Scholar
Balanaga Karthik B. Inhibition of mild steel corrosion in phosphoric acid using allyl methyl sulfide. Asian J Chem 2011; 23: 4276–4278.Search in Google Scholar
Bartos M, Kapusta SD, Hackerman N. A study of polymerization of propargyl alcohol on steel. J Electrochem Soc 1993; 140: 2604–2605.10.1149/1.2220870Search in Google Scholar
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M. The inhibitive effect of some bis-N,S-bidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution. Corros Sci 2009; 51: 1073–1082.10.1016/j.corsci.2009.02.011Search in Google Scholar
Belghiti M, Tighadouini S, Karzazi Y, Dafali A, Hammouti B, Radi S, Solmaz R. New hydrazine derivatives as corrosion inhibitors for mild steel protection in phosphoric acid medium. Part A: Experimental study. J Mater Environ Sci 2016a; 7: 337–346.Search in Google Scholar
Belghiti M, Karzazi Y, Tighadouini S, Dafali A, Jama C, Warad I, Hammouti B, Radi S. New hydrazine derivatives as corrosion for mild steel in phosphoric acid medium. Part B: Theoretical investigation. J Mater Environ Sci 2016b; 7: 956–967.Search in Google Scholar
Ben Hmamou D, Salghi R, Zarrouk A, Zarrok H, Assouag M, Hammouti B, Al-Deyab S, Hezzat M. Inhibition of carbon steel corrosion in phosphoric acid solution by Alizarin red. Pharm Lett 2013; 5: 135–142.Search in Google Scholar
Benabdellah M, Benkaddour M, Hammouti B, Bendahhou M, Aouniti A. Inhibition of steel corrosion in 2 m H3PO4 by artemisia oil. Appl Surf Sci 2006; 252: 6212–6217.10.1016/j.apsusc.2005.08.030Search in Google Scholar
Bockris JO’M, Yang B. The mechanism of corrosion inhibition of iron in acid solution by acetylenic alcohols. J Electrochem Soc 1991; 138: 2237–2252.10.1149/1.2085956Search in Google Scholar
Dadgarinezhad A, Baghaei F. The inhibition mild steel corrosion in phosphoric acid solutions by 2-phenyl-1-hydrazine carboxamide. J Chil Chem Soc 2009; 54: 208–211.10.4067/S0717-97072009000300002Search in Google Scholar
Dafali A, Hammouti B, Touzani R, Kertit S, Ramdani A, Elkacemi K. Corrosion inhibition of copper in 3 per cent NaCl solution by new bipyrazolic derivatives. Anti-Corros Methods Mater 2002; 49: 96–104.10.1108/00035590210419335Search in Google Scholar
Dafali A, Hammouti B, Mokhlisse R, Kertit S, Elkacemi K. Substituted uracils as corrosion inhibitors for copper in 3% NaCl solution. Corros Sci 2003; 45: 1619–1630.10.1016/S0010-938X(02)00255-XSearch in Google Scholar
Ebenso E, Ekpe U, Ita B, Offiong O, Ibok U. Effect of molecular structure on the efficiency of amides and thiosemicarbazones used for corrosion inhibition of mild steel in hydrochloric acid. Mater Chem Phys 1999; 60: 79–90.10.1016/S0254-0584(99)00074-7Search in Google Scholar
Feng Y, Siow KS, Teo WK, Hsieh AK. The synergistic effects of propargyl alcohol and potassium iodide on the inhibition of mild steel in 0.5 m sulfuric acid solution. Corros Sci 1999; 41: 829–852.10.1016/S0010-938X(98)00144-9Search in Google Scholar
George G. Corrosion inhibitors. Houston, TX: NACE, 1974.Search in Google Scholar
Guan N, Xueming Li, Fei Li. Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater Chem Phys 2004; 86: 59–68.Search in Google Scholar
Jabeera B, Shibli S, Anirudhan T. Synergistic inhibitive effect of tartarate and tungstate in preventing steel corrosion in aqueous media. Appl Surf Sci 2006; 252: 3520–3524.10.1016/j.apsusc.2005.05.029Search in Google Scholar
Jianguo Y, Lin W, Otieno-Alego V, Schweinsberg D. Polyvinylpyrrolidone and polyethylenimine as inhibitors for the corrosion of a low carbon steel in phosphoric acid. Corros Sci 1995; 37: 975–985.10.1016/0010-938X(95)00008-8Search in Google Scholar
Khadom A. The influence of temperature on corrosion inhibition of carbon steel in phosphoric acid. MSc thesis, University of Baghdad, 2000.Search in Google Scholar
Khadom A. Mathematical models for prediction of corrosion inhibition rates of steel in acidic media. The First National Conference for Engineering Sciences, November 7–8, Baghdad, Iraq. Baghdad, Iraq: IEEE, 2012.10.1109/NCES.2012.6740458Search in Google Scholar
Khadom A. Kinetics and synergistic effect of iodide ion and naphthylamine for the inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 2015; 115: 463–481.10.1007/s11144-015-0873-9Search in Google Scholar
Khadom A, Yaro A. Protection of low carbon steel in phosphoric acid by potassium iodide. Prot Met Phys Chem Surf 2011; 47: 662–669.10.1134/S2070205111050078Search in Google Scholar
Kuteja P, Vosta J, Pancir J, Hackerman N. Electrochemical and quantum chemical study of propargyl alcohol adsorption on iron. J Electrochem Soc 1995; 142: 1847–1850.10.1149/1.2044204Search in Google Scholar
Li XM, Tang LB, Liu HC, Mu GN, Liu GH. Influence of halide ions on inhibitive performance of cetyl trimethyl ammonium bromide in various concentrations of phosphoric acid for cold rolled steel. Mater Lett 2008; 62: 2321–2324.10.1016/j.matlet.2007.11.080Search in Google Scholar
Li XH, Deng SD, Fu H, Li T. Adsorption and inhibition of 6-benzylaminopurine on cold rolled steel in 1.0 m HCl. Electrochim Acta 2009a; 54: 4089–4098.10.1016/j.electacta.2009.02.084Search in Google Scholar
Li XH, Deng SD, Fu H. Synergistic inhibition effect of red tetrazolium and uracil on the corrosion of cold rolled steel in H3PO4 solution: weight loss, electrochemical, and AFM approaches. Mater Chem Phys 2009b; 115: 815–824.10.1016/j.matchemphys.2009.02.025Search in Google Scholar
Li XH, Deng SD, Fu H. Benzyltrimethylammonium iodide as a corrosion inhibitor for steel in phosphoric acid produced by dihydrate wet method process. Corros Sci 2011; 53: 664–670.10.1016/j.corsci.2010.10.013Search in Google Scholar
Li XH, Deng SD, Xie XG. Inhibition effect of tetradecylpyridinium bromide on the corrosion of cold rolled steel in 7.0 m H3PO4. Arabian J Chem 2017; 10: S3715–S3724.10.1016/j.arabjc.2014.05.004Search in Google Scholar
Mathur PB, Vasudevan T. Reaction rate studies for the corrosion of metals in acids. I. Iron in mineral acids. Corros 1982; 38: 171–178.10.5006/1.3579270Search in Google Scholar
Messali M, Lgaz H, Dassanayake R, Salghi R, Jodeh S, Abidi N, Hamed O. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: electrochemical, surface, DFT and MD simulations studies. J Mol Struct 2017; 1145: 43–54.10.1016/j.molstruc.2017.05.081Search in Google Scholar
Morad MS. Influence of propargyl alcohol on the corrosion behaviour of mild steel in H3PO4 solutions. Mater Chem Phys 1999; 60: 188–195.10.1016/S0254-0584(99)00090-5Search in Google Scholar
Mu GN, Li XM, Li F. Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater Chem Phys 2004; 86: 59–68.10.1016/j.matchemphys.2004.01.041Search in Google Scholar
Noor A. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros Sci 2005; 47: 33–55.10.1016/j.corsci.2004.05.026Search in Google Scholar
Noyel V, Rohith P, Manivannan R. Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int J Electrochem Sci 2015; 10: 2220–2238.10.1016/S1452-3981(23)04842-3Search in Google Scholar
Onuchukwu A, Lori J. The mechanism of the corrosion inhibition of carbon steel in neutral medium by chromate and nickel ions. Corros Sci 1984; 24: 833–841.10.1016/0010-938X(84)90122-7Search in Google Scholar
Poornima T, Jagannath N, Nityananda S. Effect of 4-(N,N-diethylamino)benzaldehyde thiosemicarbazone on the corrosion of aged 18 Ni 250 grade maraging steel in phosphoric acid solution. Corros Sci 2011; 53: 3688–3696.10.1016/j.corsci.2011.07.014Search in Google Scholar
Qu Q, Hao Z, Jiang S, Li L, Bai W. Synergistic inhibition between dodecylamine and potassium iodide on the corrosion of cold rolled steel in 0.1 m phosphoric acid. Mater Corros 2008; 59: 883–888.10.1002/maco.200804176Search in Google Scholar
Ramazan S, Ece A, Gülfeza K. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4-thiadiazol-2-ylimino)methyl)phenol Schiff base on mild steel. Mater Chem Phys 2011; 125: 796–801.10.1016/j.matchemphys.2010.09.056Search in Google Scholar
Roberge R. Handbook of corrosion engineering, 1st ed., New York, NY: McGraw Hill, 1999.Search in Google Scholar
Saratha R, Meenakshi R. Dimethylaminobenzylidene acetone as corrosion inhibitor for mild steel in acid medium. Rasayan J Chem 2011; 4: 251–263.Search in Google Scholar
Sivaraju M, Kannan K. Inhibitive properties of plant extract (Acalypha indica L.) on mild steel corrosion in 1 N phosphoric acid. Int J ChemTech Research 2010; 2: 1243–1253.Search in Google Scholar
Subramanyam NC, Sheshardi BS, Mayanna SA. Thiourea and substituted thioureas as corrosion inhibitors for aluminium in sodium nitrite solution. Corros Sci 1993; 34: 563–571.10.1016/0010-938X(93)90272-ISearch in Google Scholar
Talbot D, Talbot J. Corrosion science and technology. Boca Raton, FL: CRC Press, 2000.Search in Google Scholar
Trethewey KR, Chamberlain J. Corrosion for science and engineering, 2nd ed., Harlow, UK: Longman, 1996.Search in Google Scholar
Uhlig HH. Corrosion and corrosion control, 2nd ed., London: John Wiley & Sons, Inc., 1971.Search in Google Scholar
Wang G, Yin J, Zhang Q, Pu X. Corrosion inhibition of low-carbon steel in phosphoric acid solution by 2-mercaptobenzoxazole. Corrosion 2000; 56: 1083–1085.10.5006/1.3294392Search in Google Scholar
Wang L, Zhu MJ, Yang FC, Gao CW. Study of a triazole derivative as corrosion inhibitor for mild steel in phosphoric acid solution. Int J Corros 2012; 2012: 1–6/573964.10.1155/2012/573964Search in Google Scholar
Yaro A, Khadom A. Polarisation resistance behaviour of corrosion inhibition of low carbon steel in H3PO4 acid. Int J Surf Sci Eng 2010; 4: 429–438.10.1504/IJSURFSE.2010.035145Search in Google Scholar
Yaro A, Khadom A, Wael R. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng J 2013; 52: 129–135.10.1016/j.aej.2012.11.001Search in Google Scholar
Zarrok H, Zarrouk A, Salghi R, Hammouti B, Elbakri M, Touhami M, Bentiss F, Oudda H. Study of a cysteine derivative as a corrosion inhibitor for carbon steel in phosphoric acid solution. Res Chem Intermed 2014; 40: 801–815.10.1007/s11164-012-1004-0Search in Google Scholar
Zucchi F, Trabanelli G, Brunoro G. Iron corrosion inhibition in hot 4 m HCl solution by t-cinnamaldehyde and its structure-related compounds. Corros Sci 1994; 36: 1683–1690.10.1016/0010-938X(94)90123-6Search in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Technological applications of volatile corrosion inhibitors
- Performance of green corrosion inhibitors from biomass in acidic media
- Corrosion inhibition of steel in phosphoric acid
- Original articles
- Effect of pearlite on stress corrosion cracking of carbon steel in fuel-grade ethanol
- Gravimetric and electrochemical methods to evaluate the performance of corrosion inhibitors for galvanized steel strips
- Zeolites as reservoirs for Ce(III) as passivating ions in anticorrosion paints
Articles in the same Issue
- Frontmatter
- In this issue
- Reviews
- Technological applications of volatile corrosion inhibitors
- Performance of green corrosion inhibitors from biomass in acidic media
- Corrosion inhibition of steel in phosphoric acid
- Original articles
- Effect of pearlite on stress corrosion cracking of carbon steel in fuel-grade ethanol
- Gravimetric and electrochemical methods to evaluate the performance of corrosion inhibitors for galvanized steel strips
- Zeolites as reservoirs for Ce(III) as passivating ions in anticorrosion paints

