Abstract
Laboratory medicine lies at the core of modern healthcare, enabling timely diagnosis, effective patient monitoring, and increasingly personalized therapeutic strategies. Over the past decades, automation has profoundly reshaped the role of clinical laboratories, substantially enhancing their contribution to clinical outcomes, operational efficiency, and the overall sustainability of healthcare systems. More recently, laboratory automation has emerged as a cornerstone of value-based laboratory medicine, representing not merely a technological upgrade but a strategic transformation of laboratory practice aimed at delivering measurable value to patients and healthcare stakeholders. Although automation has long been established in clinical chemistry and immunoassays, its scope is now expanding to molecular diagnostics and mass spectrometry – two disciplines that are central to precision medicine. Looking ahead, the convergence of automation, digitalization, and artificial intelligence is driving the emergence of hyperautomation in laboratory medicine. Within this paradigm, laboratories evolve from isolated testing units into integrated diagnostic hubs, in which results from multiple laboratory disciplines are harmonized and contextualized to effectively support clinical decision-making.
Introduction
Laboratory medicine is at the core of modern healthcare, enabling timely diagnosis, patient monitoring, and increasingly personalized treatment [1], 2]. Yet, laboratories are experiencing mounting pressures, including rising testing volumes, shortages of skilled personnel, the demand for 24/7 service delivery, and the imperative to deliver measurable value to patients and healthcare systems. In this evolving landscape, automation has emerged as a cornerstone of value-based laboratory medicine, not merely as a technological upgrade but as a strategic transformation of laboratory practice [1]. Beyond a purely technical upgrade, automation reshapes the role of laboratories, amplifying their contribution to clinical outcomes, efficiency, and system-wide sustainability [3]. Value-based laboratory medicine shifts the focus from the volume of tests performed to the clinical relevance and impact of laboratory information on diagnostic and therapeutic decision-making [3], 4]. By reducing manual steps, standardizing processes, and strengthening data integrity, automation ensures that laboratory information is faster, more reproducible, and more actionable, creating new opportunities for laboratories to act as true partners in patient care [3], 4]. This evolution positions laboratories as proactive partners in patient-centered care rather than passive service providers.
Impact of automation across the total testing process
The benefits of automation are well established across the total testing process (TTP) [3], 4]. Automated systems minimize human error, improving precision and safety across pre-analytical, analytical, and post-analytical phases. Continuous, end-to-end automated workflows enable laboratories to operate around the clock, ensuring consistent and predictable turnaround times that are particularly critical in emergency and acute care settings [3].
Automation also allows laboratories to manage growing workloads without proportional increases in staffing, offering a sustainable response to workforce shortages. Rather than replacing laboratory professionals, automation redefines their role, enabling highly trained staff to focus on data interpretation, clinical consultation, quality management, and innovation. This redistribution of tasks contributes to improved working conditions, reduced exposure to hazardous materials, and enhanced professional satisfaction [3], 5]. An important and often underrecognized benefit of automation is the reduction in required sample volumes. By minimizing repeat testing, optimizing analytical efficiency, and improving first-pass success rates, automated workflows support a more ethical approach to laboratory testing. This aspect is particularly relevant for vulnerable populations, such as critically ill patients, neonates, and individuals requiring frequent monitoring, where blood conservation is a clinical priority [3], 5].
Expanding automation to high-value diagnostic disciplines
While automation has long been established in clinical chemistry and immunoassays, its scope is now expanding to molecular diagnostics and mass spectrometry, two disciplines that are central to precision medicine [3], 6]. Molecular testing plays a pivotal role in infectious disease management, oncology, and genetic diagnostics but has traditionally relied on labor-intensive and complex workflows. Automation streamlines sample-to-result processes, enhances biosafety, improves reproducibility, and enables laboratories to scale testing capacity while maintaining quality and reliability.
Mass spectrometry, widely regarded as a reference method for the measurement of small molecules such as steroid hormones, vitamins, and therapeutic drugs, has historically been confined to specialized laboratories due to its operational complexity [6]. Recent advances in automated sample preparation, standardized pre-analytical workflows, and integrated analytical processes are progressively lowering these barriers. As a result, mass spectrometry is becoming more accessible within routine laboratory environments, providing highly specific and standardized measurements anchored to reference methods. This evolution supports inter-laboratory comparability and enables reliable longitudinal monitoring in clinical areas such as endocrinology, oncology, and transplantation.
By integrating molecular diagnostics and mass spectrometry into automated core laboratory workflows, laboratories significantly expand their clinical value and reinforce their role within value-based healthcare models.
Toward hyperautomation and integrated diagnostics
Looking ahead, the convergence of automation, digitalization, and artificial intelligence is driving the emergence of “hyperautomation” in laboratory medicine [5]. In this paradigm, laboratories evolve from isolated testing units into integrated diagnostic hubs, where results from multiple laboratory disciplines are combined and contextualized to support clinical decision-making. Increasingly, laboratory data are expected to be interpreted alongside clinical information and, in the future, imaging data to guide diagnostic and therapeutic pathways. This transition requires open and interoperable systems that move beyond rigid, vendor-dependent architectures [3], 4], 7]. Modular automation, end-to-end workflow optimization, and seamless data integration are essential to preserve laboratory autonomy while enabling flexibility and scalability [8]. Automation also facilitates the systematic collection of high-quality operational and clinical data, enabling continuous performance monitoring through key performance indicators such as turnaround time, error rates, throughput, specimen rejection rates, and equipment utilization (Figure 1).
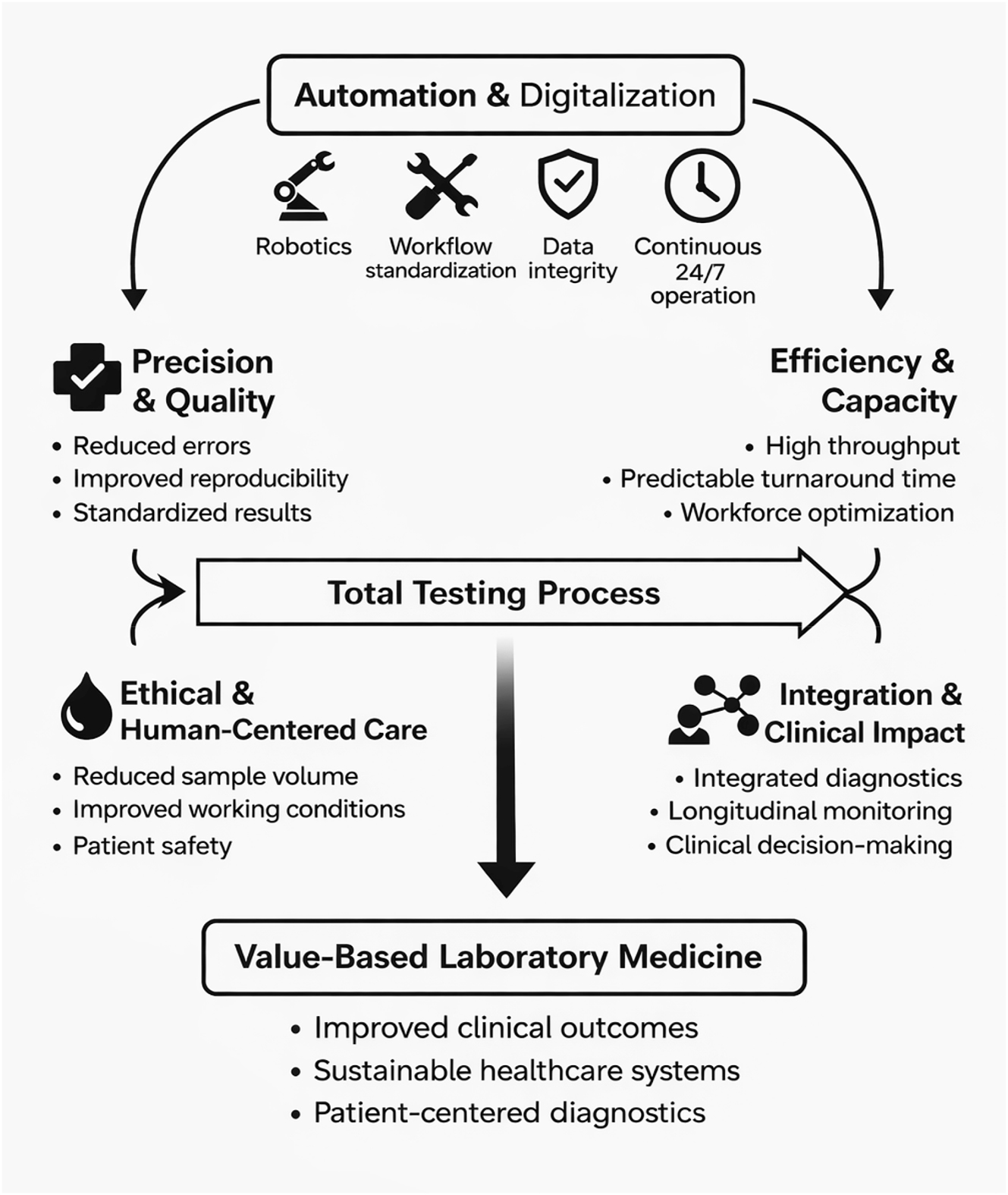
Automation as a driver of value-based laboratory medicine.
Sustainability and value-based outcomes
Sustainability must be embedded in future automation strategies. Automated laboratories have the potential to reduce waste, optimize resource utilization, and enhance resilience during periods of crisis. However, the success of automation should not be assessed solely through operational or economic metrics. In the context of value-based laboratory medicine, the true measure of success lies in demonstrable improvements in clinical outcomes, including fewer diagnostic errors, optimized therapeutic decisions, reduced hospital stays, and improved equity of access to advanced diagnostics (Tables 1–3).
General advantages of total laboratory automation.
| Domain | Advantages |
|---|---|
| Operational performance | High throughput and continuous 24/7 operation |
| Quality & safety | Reduction of pre-analytical and analytical errors |
| Efficiency | Improved workflow efficiency and predictable turnaround time |
| Flexibility | Modular and scalable design |
| Sample management | Improved and consistent sample quality |
| Clinical impact | Faster and more reliable results supporting decision-making |
| Workforce | Improved working conditions and task redistribution |
| Ethics | Reduced sample volume requirements |
| Connectivity | Integration across laboratory disciplines |
| Technology | Advanced robotics and intelligent systems |
Main challenges and limitations of laboratory automation.
| Category | Challenges |
|---|---|
| Financial | High initial investment costs |
| Technical | Maintenance requirements and potential downtime |
| Human resources | Need for training and emergence of new professional roles |
| Infrastructure | Space and layout constraints |
| IT integration | Compatibility with LIS, EMR and legacy systems |
| Change management | Adaptation of workflows and organizational culture |
-
EMR, Electronic Medical Records.
Key performance indicators (KPIs) in laboratory automation.
| KPI | Description |
|---|---|
| Automation efficiency | Proportion of tests processed through automated workflows |
| Error rate | Reduction in pre-analytical and analytical errors |
| Sample throughput | Number of samples processed per unit time |
| Turnaround time (TAT) | Time from sample receipt to result reporting |
| Equipment utilization | Effective use of automated systems |
| Specimen rejection rate | Frequency of rejected or recollected samples |
Conclusions
Automation in laboratory medicine is not an end but a strategic enabler of value-based care. By combining technological innovation with human expertise, automation enhances accuracy, capacity, and clinical relevance across the total testing process. As laboratories continue to evolve toward hyperautomation and integrated diagnostics, they will play an increasingly central role in delivering timely, meaningful, and patient-centered information for healthcare systems and society.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Plebani, M, Cadamuro, J, Vermeersch, P, Jovičić, S, Ozben, T, Trenti, T, et al.. A vision to the future: value-based laboratory medicine. Clin Chem Lab Med 2024;62:2373–87. https://doi.org/10.1515/cclm-2024-1022.Suche in Google Scholar PubMed
2. Gruson, D, Öz, TK. Trends in healthcare and laboratory medicine: a forward look into 2025. Balkan Med J 2025;42:487. https://doi.org/10.4274/balkanmedj.galenos.2025.2024-12-133.Suche in Google Scholar PubMed PubMed Central
3. Nam, Y, Park, HD. Revolutionizing laboratory practices: pioneering trends in total laboratory automation. Ann Lab Med 2025;45. https://doi.org/10.3343/alm.2024.0581.Suche in Google Scholar PubMed PubMed Central
4. Plebani, M. Total laboratory automation: fit for its intended purposes? Clin Chem Lab Med 2025. Available from: https://www.degruyterbrill.com/document/doi/10.1515/cclm-2025-0855/html?srsltid=AfmBOopX5a9Xt6Mv0x2x5kJl7zgOV--i8NRUqF1VCXFTWNbkhuAfnIbq.Suche in Google Scholar
5. Gruson, D, Magalhaes, T, Ruszanov, A, Granaldi, C, Bernardini, S, Buttigieg, SC. Hyperautomation in healthcare: perspectives from a joint IFCC – EHMA session. EJIFCC 2023;34:284.Suche in Google Scholar
6. Automation to revolutionize the use of mass spectrometry, improving lab operations and patient care, Med Lab Obs. Available from: https://www.mlo-online.com/diagnostics/article/55304261/automation-to-revolutionize-the-use-of-mass-spectrometry-improving-lab-operations-and-patient-care.Suche in Google Scholar
7. Pennestrì, F, Banfi, G. Value-based healthcare: the role of laboratory medicine. Clin Chem Lab Med 2019;57:798–801. https://doi.org/10.1515/cclm-2018-1245.Suche in Google Scholar PubMed
8. Lippi, G, Plebani, M. Reshaping laboratory medicine through technological advances. Clin Chem Lab Med 2026. https://doi.org/10.1515/cclm-2025-1733. Epub ahead of print.Suche in Google Scholar PubMed
© 2026 Walter de Gruyter GmbH, Berlin/Boston

