Abstract
Objectives
Dental amalgam (50 % mercury (Hg) by weight) is a commonly used material to restore a tooth damaged by decay. In recent years, amalgam safety has become a matter of ongoing controversy. This hypothesis-testing epidemiological study evaluated the relationship between blood Hg concentrations and amalgams in American adults.
Methods
Examination of the 2015–2016 National Health and Nutrition Examination Survey (NHANES) was undertaken using SAS, version 9.4 (Cary, NC, USA), survey regression statistical modeling (with adjustments for covariates). A total of 180,811,187 weighted-Americans (n=1,377) between the ages of 18–70 years-old, with known: dental filing surface status; urinary Hg concentrations; total and blood Hg species (inorganic and methyl-Hg) concentrations; bodyweight; and urine flow rates were examined.
Results
Significant increases were found in the blood concentrations of total and inorganic Hg, when comparing adults exposed to amalgams as compared to adults not exposed to amalgams. Amalgam surfaces significantly correlated with blood inorganic Hg concentrations, and estimated daily Hg vapor doses from amalgams significantly correlated with blood total and inorganic Hg concentrations. This study supports the importance of blood as an important transport avenue for Hg, which is dose-dependently released by amalgams, to accumulate in tissues and cells throughout the body.
Conclusions
Persons with amalgams, desiring to lower their blood Hg concentrations, should consult with a dentist trained and certified in safe amalgam removal. Also, pharmaceutical treatments to reduce/render non-toxic the blood Hg concentrations from amalgams should be considered. Efforts should be made to reduce/eliminate the continued use of amalgams.
Introduction
Teeth damaged by decay or other issues are restored to appropriate structure and function by dental fillings. Dental fillings are placed on up to five different tooth surfaces [1]. Dental amalgam is a commonly used material to restore a tooth damaged by decay. Amalgams are composed of about 50 % mercury (Hg) by weight and contain several other metals, including silver, tin, copper, and zinc. Amalgams have been used in American dentistry for more than 150 years, but their use has been associated with ongoing controversy [2]. The United States (US) Food and Drug Administration (FDA) released a statement in 2020, reporting that there is a dose-dependent relationship between the number of amalgam fillings and Hg vapor exposure and that certain patients may experience adverse effects from Hg vapor exposure [3].
In this context, previous studies were undertaken to assess Hg vapor exposure from amalgams in the US population, based upon examination of National Health and Nutrition Examination Survey (NHANES) data [3], 4]. NHANES data revealed amalgams were detected in ∼58 % of adults and ∼36 % of pregnant women. Significant correlations were detected between the number of amalgam surfaces and urinary Hg concentrations. Based upon these correlations, toxicokinetic modeling was employed to estimate daily Hg vapor doses from amalgams. Overall, it was estimated 10.4 % (∼16 million) of adults and 28 % (∼600,000) of pregnant women received daily Hg vapor doses from amalgams in excess of the US Environmental Protection Agency (EPA) Hg vapor safety limit (0.048 micrograms (μg) Hg/kilogram (kg)/day). The US EPA Hg vapor safety limit is the least restrictive as compared to other Hg vapor safety limits. For example, the Hg vapor safety limit established by the state of California EPA (0.005 μg Hg/kg/day) is ∼10-fold lower than the US EPA Hg vapor safety limit [3], 4].
In light of these previous NHANES studies revealing a significant correlation between the number of amalgams and urinary Hg concentrations, an important new question to examine is the mechanism by which Hg vapor from amalgams transverses the human body. It was hypothesized that blood provides a significant avenue for Hg vapor released from amalgams to transverse the human body.
The purpose of the present hypothesis-testing epidemiological study of NHANES data was to examine the relationship between amalgams and blood speciated Hg (including: total Hg, inorganic Hg, and methyl-Hg) concentrations, as shown in Figure 1. The aims of this study were to examine the relationship between: (1) blood Hg concentrations among those with/without amalgams; (2) the number of amalgam surfaces and blood Hg concentrations; and (3) estimated daily Hg vapor doses from amalgams per kg bodyweight and blood Hg concentrations among American adults.
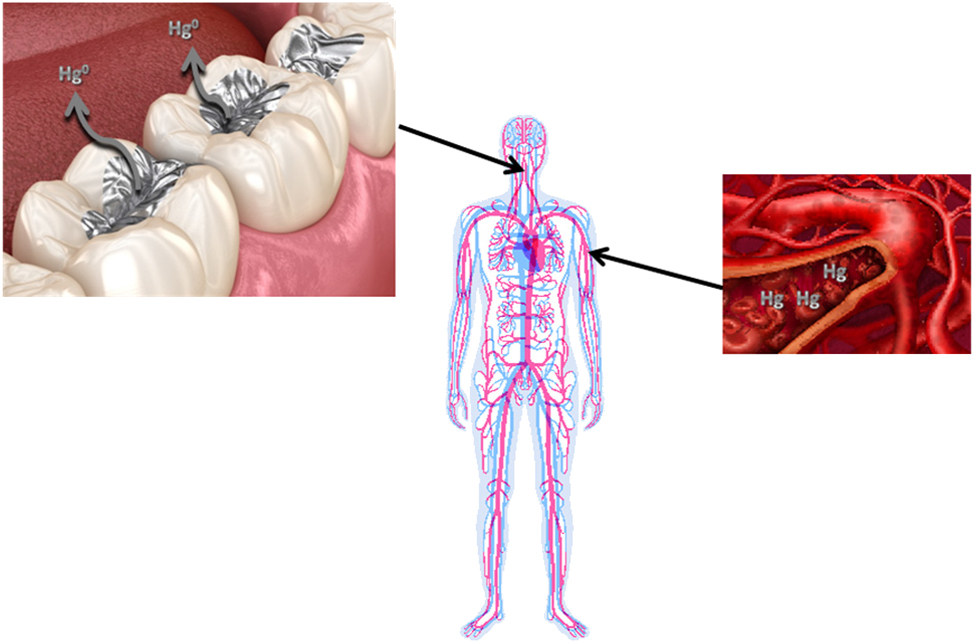
A summary of the relationship between Hg vapor released from amalgams and blood Hg concentrations investigated in this study.
Materials and methods
National health and nutrition examination survey (NHANES)
NHANES data was examined using the Statistical Analysis System (SAS) for Windows, Version 9.4 (Cary, NC, USA). NHANES data collected from 2015 to 2016 were integrated to examine demographic survey questions, oral health examinations, clinical measurements, and lab test results. NHANES data can be accessed at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
NHANES data collection methods were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB) (Protocol#2011-17). Each study subject provided informed consent to participate in the NHANES program. The health information collected in the NHANES program is kept in strictest confidence, and is only used for stated purposes.
Study participants
Figure 2 shows a schematic flowchart documenting the selection criteria utilized to assemble the group of adults examined in the NHANES data. An overall population of 316, 481, 044 weighted-persons with known gender (male or female), age in years at examination, race (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, or other – including multi-racial), and country of birth (born in the US or born outside of the US) was examined. The overall population was reduced by including only those from the age of 18–70 years-old with known dental filling surface status. Still further reductions in the number of persons examined occurred by only including those with known urinary Hg concentrations, total and blood Hg species (inorganic and methyl-Hg) concentrations, bodyweight, and urine flow rates (weighted n=180,811,187, n=1,377).
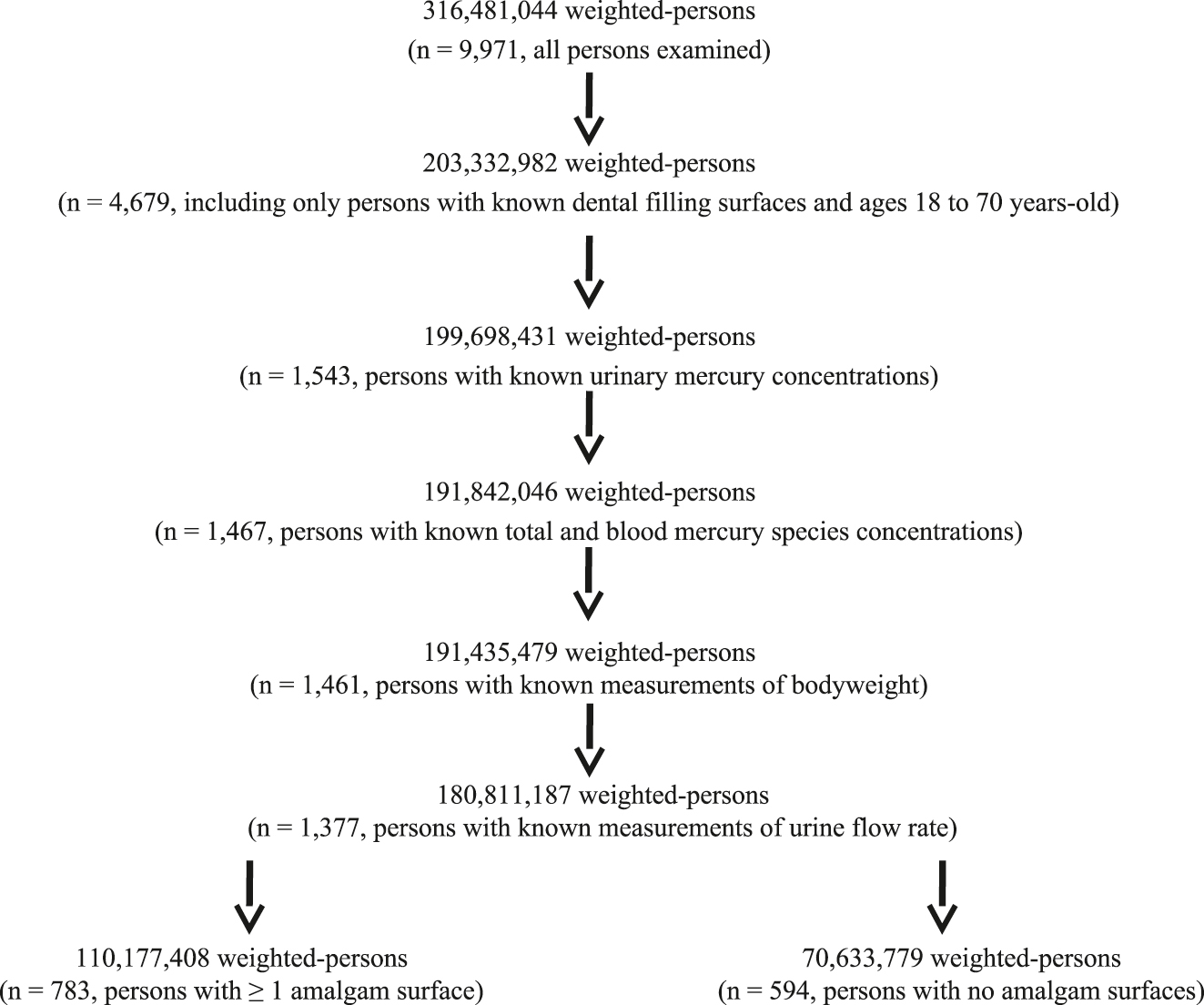
A flowchart of the data examined in this study.
The weighted number of persons was derived by applying the subsample A weight to each person examined in this study. The subsample A weight was created by the NHANES program and assigned because urinary Hg levels were only measured from a one-third subsample of NHANES participants 6 years-old or older. The subsample A weight is a measure of the number of persons in the general population that a sampled individual represents and is needed to obtain unbiased estimates of population parameters when sample participants are chosen with unequal probabilities.
Dental filling surfaces
The coronal carries assessment was conducted on each person by dental examiners, who were dentists licensed in at least one US state, and were examined in the NHANES oral health – dentition dataset. For those persons with filled surfaces, the restoration type of the filled surfaced (amalgam or other) was specified. The total number of dental amalgam filling surfaces or other dental filling surfaces was computed for each person examined. The persons examined were divided into two groups based upon amalgam status: amalgam exposed (≥1 amalgam surface) and amalgam unexposed (0 amalgam surfaces).
Daily Hg vapor exposure from amalgams
Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure urinary Hg excretion in random spot urine samples. Urinary Hg excretion was determined as µg Hg/liter (L) of urine. The integration of urinary Hg concentrations, urine flow rates, number of amalgams, bodyweight measurements (using previously described toxicokinetic modeling procedures) were utilized to estimate the daily Hg vapor exposure from amalgams per kg bodyweight for each person examined [3], [4], [5].
Blood heavy metal concentrations
ICP-MS, after a simple dilution sample preparation step, was used to measure blood total Hg concentrations (µg Hg/L blood) and blood total lead (Pb) concentrations (µg Pb/deciliter (dL) blood). Blood total Pb concentrations were examined as a control measurement – selected a priori to have no correlation with amalgam exposure status.
Quantification of blood inorganic Hg (µg inorganic Hg/L blood) and methyl-Hg (µg methyl-Hg/L blood) concentrations were determined by using a triple spike isotope dilution (TSID) method employing gas chromatography (GC) to separate the species followed by introduction into an inductively coupled plasma-dynamic reaction cell-mass spectrometry (ICP-DRC-MS) for detection. TSID is a specialized extension of the Isotope Dilution (ID) technique and provides measures of blood inorganic Hg and methyl-Hg concentrations in samples using ID principles. The lab methods utilized to measure all blood metal concentrations examined in this study remained consistent for the 2015–2016 NHANES.
Statistical analyses
The statistical package in SAS was used in all statistical analyses. A two-sided p-value <0.05 was considered statistically significant. Statistical power calculations revealed that the number of persons examined was sufficient provide adequate statistical power for the analyses undertaken in this study.
The collection of NHANES survey data involves a complex, stratified, multistage probability cluster sampling design. SAS contains specific statistical modeling procedures designed to appropriately analyze NHANES survey data, and they were employed in this study. This study employed the survey regression modeling procedure in SAS. This procedure can handle complex survey sample designs, including designs with stratification, clustering, and unequal weighting. The procedure fits linear models for survey data and computes regression coefficients and their variance-covariance matrix. It also provides significance tests for the model effects and for specified estimable linear functions of the model parameters.
The survey regression modeling procedure in SAS uses elementwise regression to compute the regression coefficient estimators by generalized least squares estimation. The procedure assumes that the regression coefficients are the same across strata and primary sampling units. To estimate the variance-covariance matrix for regression coefficients, this procedure uses either the Taylor series (linearization) method or replication (resampling) methods to estimate sampling errors of estimators, based on complex sample designs.
The null hypothesis for the statistical tests employed was that there would be: (1) no differences in the parameters examined in the amalgam exposed and amalgam unexposed groups; (2) no correlations between the number of amalgam surfaces and blood Hg (or Pb) concentrations; (3) no correlations between estimate daily Hg vapor exposure from amalgams per kg bodyweight and blood Hg (or Pb) concentrations.
Survey regression models utilized in this study were constructed without (Model I) and with adjustment for covariates (Model II). For the adjusted survey regression models (regression models require continuous variables), the categorical variables of race and country of birth were converted to continuous variables as follows: race (Hispanic=1, non-Hispanic white=2, non-Hispanic black=3, non-Hispanic Asian=4, and other – including multi-racial=5), and country of birth (born in the US=1 and born outside of the US=2). The variables of total amalgam surfaces, body mass index, and age in years were examined as continuous variables. In addition, for all the constructed survey regression models, the variables for stratum (NHANES variable: sdmvstra), cluster (NHANES variable: sdmvpsu), and weight (NHANES variable: subsample A weight) were employed.
Results
Table 1 summarizes the demographic characteristics of the 180, 811, 187 adults examined. Overall, a majority of the adults examined were members of the amalgam exposed group (60.94 %) as compared to the amalgam unexposed group (39.06 %). The gender and racial distributions, as well as the mean daily urine amounts, were similar in the amalgam exposed and unexposed groups. Adults in the amalgam exposed group were significantly older, more likely to have been born in the US, and heavier than those adults in the unexposed group.
Demographic characteristics of the adults examined.
| Parameter examined | Amalgam exposed group (weighted n=110,177,408) (unweighted n=783) |
Amalgam unexposed group (weighted n=70,633,779) (unweighted n=594) |
t-Value | p-Value |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± std | 46.8 ± 13.1 | 39.0 ± 15.8 | 7.70 | <0.0001 |
| (range) | (18–70) | (18–70) | ||
| Gender, n (%) | ||||
| Males | 52,997,321 (48.10 %) | 34,759,668 (49.21 %) | 0.25 | 0.8046 |
| Females | 57,180,088 (51.90 %) | 35,874,111 (50.79 %) | ||
| Race, n (%) | ||||
| Non-Hispanic white | 74,514,875 (67.63 %) | 38,251,110 (54.15 %) | −0.82 | 0.4228 |
| Non-Hispanic black | 11,553,087 (10.49 %) | 9,806,187 (13.88 %) | ||
| Hispanic | 15,059,501 (15.79 %) | 14,704,867 (20.82 %) | ||
| Non-Hispanic Asian | 4,249,573 (3.86 %) | 5,048,167 (7.15 %) | ||
| Other – including multi-racial | 3,746,881 (3.40 %) | 2,823,447 (4.00 %) | ||
| Country of birth, n (%) | ||||
| Born in the US | 94,284,028 (85.58 %) | 52,314,906 (74.07 %) | −4.92 | 0.0002 |
| Born outside US | 15,893,380 (14.42 %) | 18,318,873 (25.93 %) | ||
| Bodyweight, kg | ||||
| Mean ± std | 86.2 ± 21.9 | 82.9 ± 20.3 | 2.21 | 0.0434 |
| (range) | (39.8–161.9) | (39.2–178.4) | ||
| Daily urine amount, L | ||||
| Mean ± std | 1.85 ± 1.53 | 2.08 ± 3.83 | −1.67 | 0.1151 |
| (range) | (0.16–12.0) | (0.04–105) |
-
Survey regression modeling was used to compare the differences between the amalgams exposed and amalgam unexposed groups. kg, kilogram; L, liter; US, United States; std, standard deviation.
Table 2 examines blood Hg and Pb concentrations in the amalgam exposed group as compared to the amalgam unexposed group. Significant increases in the means for the number of amalgam surfaces, other surfaces, and daily amalgam Hg vapor dose were detected between the exposed and unexposed groups. In addition, the mean blood concentrations of total Hg (1.34-fold), inorganic Hg (1.33-fold), and methyl-Hg (1.26-fold) were significantly increased when comparing the amalgam exposed to the unexposed group. By contrast, no difference in the mean blood concentration of Pb was observed between the groups.
Amalgam exposure and blood concentrations of Hg and Pb among the persons examined.
| Parameter examined | Amalgam exposed group | Amalgam unexposed group | t-Value | p-Value |
|---|---|---|---|---|
| Amalgam status | ||||
| ≥1 amalgam surface | 110,177,408 (100 %) | 0 (0 %) | – | – |
| 0 amalgam surfaces | 0 (0 %) | 70,633,779 (100 %) | ||
| Number of amalgam surfaces | ||||
| Mean ± std | 7.03 ± 6.46 | 0 | 18.23 | <0.0001 |
| (range) | (1–40) | |||
| Number of other surfaces | ||||
| Mean ± std | 4.21 ± 5.45 | 2.74 ± 4.87 | 3.44 | 0.0036 |
| (range) | (0–41) | (0–33) | ||
| Daily amalgam Hg vapor dose (µg hg/kg bodyweight) | ||||
| Mean ± std | 0.030 ± 0.023 | 0 | 24.11 | <0.0001 |
| (range) | (0.003–0.16) | |||
| Blood total µg Hg/L | ||||
| Mean ± std | 1.57 ± 2.42 | 1.17 ± 2.03 | 5.27 | <0.0001 |
| (range) | (0.20–34.9) | (0.20–36.3) | ||
| Blood inorganic Hg µg/L | ||||
| Mean ± std | 0.28 ± 0.29 | 0.21 ± 0.09 | 5.46 | <0.0001 |
| (range) | (0.19–5.90) | (0.19–1.76) | ||
| Blood µg methyl-Hg/L | ||||
| Mean ± std | 1.29 ± 2.27 | 1.02 ± 2.04 | 3.65 | 0.0024 |
| (range) | (0.08–32.6) | (0.08–39.5) | ||
| Blood μg Pb/dL | ||||
| Mean ± std | 1.14 ± 1.20 | 1.05 ± 0.92 | −0.29 | 0.7736 |
| (range) | (0.05–23.5) | (0.10–19.6) |
-
Survey regression modeling with adjustments for the covariates of age, bodyweight, urine volume, ethnicity, gender, and country of birth was used to compare the differences between the amalgams exposed and amalgam unexposed groups. dL, deciliter; kg, kilogram; Pb, lead; L, liter; Hg, mercury; microgram (µg); std, standard deviation.
Correlations between the number of amalgam surfaces and blood Hg and Pb concentrations are presented in Table 3. In models I and II, a highly significant correlation between the number of amalgam surfaces and blood inorganic Hg concentrations was detected (standardized estimates=0.28–0.29), whereas a trend toward a significant correlation between the number of amalgam surfaces and blood total Hg concentrations was identified. No significant correlations were observed between the number of amalgam surfaces and blood methyl-Hg or Pb concentrations.
A summary of the correlation between amalgam surfaces and each parameter examined.
| Model | Parameter examined | β-coefficient (95 % CI) | Standardized estimate | t-Value | p-Value |
|---|---|---|---|---|---|
| Ia | |||||
| Blood total µg Hg/L | 0.051 (−0.012–0.12) | 0.14 | 1.73 | 0.104 | |
| Blood inorganic Hg µg/L | 0.011 (0.008–0.013) | 0.28 | 9.36 | <0.0001 | |
| Blood µg methyl-Hg/L | 0.033 (−0.025–0.092) | 0.093 | 1.22 | 0.2425 | |
| Blood μg Pb/dL | 0.008 (−0.018–0.034) | 0.044 | 0.64 | 0.5289 | |
| IIb | |||||
| Blood total µg Hg/L | 0.058 (−0.006–0.12) | 0.16 | 1.92 | 0.074 | |
| Blood inorganic Hg µg/L | 0.011 (0.009–0.013) | 0.29 | 10.74 | <0.0001 | |
| Blood µg methyl-Hg/L | 0.040 (−0.020–0.10) | 0.11 | 1.42 | 0.1773 | |
| Blood μg Pb/dL | 0.0054 (−0.018–0.029) | 0.030 | 0.49 | 0.6283 |
-
Survey regression modeling was utilized. Pb, lead; L, liter; Hg, mercury; microgram (µg). aNo adjustment was made for covariates. bAdjustments were made for the covariates of age, bodyweight, urine volume, ethnicity, gender, and country of birth.
Table 4 displays the correlation between daily Hg vapor dose from amalgams per kg bodyweight and blood Hg and Pb concentrations. Similar to observations in Table 3, Models I and II revealed a highly significant correlation between daily Hg vapor dose from amalgams per kg bodyweight and blood inorganic Hg concentrations (standardized estimates=0.30), but unlike Table 3, a significant correlation between daily Hg vapor dose from amalgams per kg bodyweight and blood total Hg concentrations (standardized estimates=0.17) was observed. Model II also revealed a trend toward a significant correlation between daily Hg vapor dose from amalgams per kg bodyweight and blood total methyl-Hg concentrations. Consistent with Table 3, no correlations were observed between daily Hg vapor dose from amalgams per kg bodyweight and blood total Pb concentrations.
A summary of the correlation between the estimated daily amalgam µg Hg vapor per kg bodyweight and each parameter examined.
| Model | Parameter examined | β-coefficient (95 % CI) | Standardized estimate | t-Value | p-Value |
|---|---|---|---|---|---|
| Ia | |||||
| Blood total µg Hg/L | 16.2 (0.84–31.5) | 0.17 | 2.25 | 0.0401 | |
| Blood inorganic Hg µg/L | 2.97 (2.03–3.92) | 0.30 | 6.71 | <0.0001 | |
| Blood µg methyl-Hg/L | 11.11 (to 14.5) | 0.11 | 1.61 | 0.1272 | |
| Blood μg Pb/dL | 2.78 (−3.38–8.94) | 0.059 | 0.96 | 0.3512 | |
| IIb | |||||
| Blood total µg Hg/L | 16.5 (1.46–31.6) | 0.17 | 2.34 | 0.0337 | |
| Blood inorganic Hg µg/L | 3.01 (2.21–3.82) | 0.30 | 8.01 | <0.0001 | |
| Blood µg methyl-Hg/L | 11.5 (−2.92–30.0) | 0.12 | 1.70 | 0.1096 | |
| Blood μg Pb/dL | 1.02 (−4.69–6.72) | 0.022 | 0.38 | 0.7098 |
-
Survey regression modeling was utilized. Pb, lead; L, liter; Hg, mercury; microgram (µg). aNo adjustment was made for covariates. bAdjustments were made for the covariates of age, bodyweight, urine volume, ethnicity, gender, and country of birth.
Discussion
The results of the present study provide important new data supporting the hypothesis that blood is an important avenue for the distribution of Hg vapor from amalgams across the human body. This study revealed that there were significant increases in the blood concentrations of total and inorganic Hg when comparing adults exposed to amalgams as compared to adults not exposed to amalgams. A significant correlation was observed between the number of amalgam surfaces and blood inorganic Hg concentrations. Also, a significant correlation was found between estimated daily Hg vapor doses from amalgams per kg bodyweight with blood total Hg and blood inorganic Hg concentrations. The aforementioned associations remained significant in unadjusted and adjusted (for the covariates of age, bodyweight, urine volume, ethnicity, gender, and country of birth) statistical models.
The results observed in this study are consistent with several previous studies examining the relationship between blood Hg concentrations and amalgams in adults [6], [7], [8], [9], [10]. For example, investigators observed significant associations between the placement of amalgams and acute changes in biological samples, including blood Hg concentrations [6]. Other investigators examined long-term changes in blood speciated Hg concentrations following amalgam removal [7]. These investigators observed a significant correlation between the number of amalgam filling surfaces and blood inorganic Hg concentrations, and a significant long-term decrease in blood inorganic Hg concentrations following removal of amalgams. Another study revealed blood Hg concentrations significantly correlated with the number and surface area of amalgams, and that blood Hg concentrations were significantly lower among those without amalgams as compared to those with amalgams [8]. As another example, a study was undertaken among US military personnel to determine the relationship between amalgams and blood Hg concentrations [9]. These investigators observed a significant association between the number of amalgam surfaces and blood Hg concentrations. Finally, a study of trace elements in the blood, plasma and erythrocytes of adult Slovenians revealed significant correlations between the number of amalgams and the concentrations of Hg in the blood and plasma [10].
The present study is differentiated from all these previous studies in several important aspects. First, small samples and/or non-diverse study subjects were examined. As a result, the applicability of their findings to larger and more diverse populations remains unknown. Second, no measurements of daily Hg vapor doses from amalgams per kg bodyweight were studied in any previous study. As a consequence, there is an unknown degree of imprecision regarding the dose of Hg vapor each study subject received. Third, unlike all previous studies, statistical methods were used to evaluate the data examined without consideration of the potential important impact of covariates on the results observed.
Another important consideration regarding the present study was the relationship observed between estimated daily doses of Hg vapor from amalgams and blood Hg concentrations. The present study utilized previously well described toxicokinetic modeling procedures to estimate daily Hg vapor dose from amalgams [3], [4], [5]. An important support of the validity of the toxicokinetic modeling procedures used is that significant correlations were observed between estimated daily Hg vapor doses from amalgams and blood Hg concentrations (i.e., if these relationships were not observed, it would suggest that there might be a problem with the toxicokientic modeling procedures). The results of this study also revealed blood Hg concentrations were even more sensitively correlated with estimated Hg vapor doses from amalgams than with the number of amalgam filling surfaces. All told, the observations from this study suggest that utilization of toxicokinetic modeling procedures to estimate Hg vapor from amalgams is an important additional means to assess ongoing Hg exposures from amalgams.
The results observed in this study are biologically plausible as to the importance of blood as an important transport avenue for Hg dose-dependently released by amalgams. It is known that Hg vapor is readily absorbed in the lungs (80 % absorption percentage) and quickly diffused into the blood and distributed into all body organs [11]. Since Hg vapor is in an uncharged monoatomic form of Hg (although it is rapidly oxidized), it is highly diffusible and lipid soluble, with the ability to cross the blood-brain barrier and blood-placenta barrier as well as the lipid bilayers of cellular and intracellular organellar membranes. Once inside tissues and cells, it can be oxidized and restricts the body’s ability to excrete it and potentially result in tissue-specific toxicity [12].
Unfortunately, given the aforementioned considerations, it means that blood Hg concentrations do not provide a means to determine the actual Hg concentrations present in tissues. This was demonstrated in a previous autopsy study where blood Hg concentrations did not significantly correlate with Hg concentrations in various tissues, but the number of amalgams did [13]. Thus, while the blood Hg concentrations associated with amalgams are relatively low as compared to those observed in acute Hg vapor poisoning [14], long-term exposure to Hg vapor from amalgams is of considerable concern [15], 16].
The importance of blood in the distribution of Hg vapor from amalgams may offer a means to target therapies that will reduce the accumulation/toxicity of Hg in tissues by binding and/or rendering it non-toxic in the blood. Traditionally, these have involved the use of sulfur-based chelating agents such as Dimercaptosuccinic acid (DMSA) or dimercapto-propanesulfonic acid (DMPS), but there may drawbacks associated with their clinical use [17]. A different option to explore may be new compounds such as, N,N’bis-(2-mercaptoethyl) isophthalamide (NBMI). NBMI is a lipophilic chelating agent that tightly/irreversibly binds Hg. A recent clinical trial demonstrated that NBMI dose-dependently significantly reduced plasma and urinary Hg concentration among gold miners with elevated urinary Hg concentrations [18].
Strength/limitations
In reviewing the results of the present study, it is important to consider its potential strengths and limitations.
An important potential strength of the present study is the NHANES data examined. NHANES is a unique source of data [19]. It is the only national health survey that includes health exams, laboratory tests, and dietary interviews for participants of all ages. The US Centers for Disease Control and Prevention (CDC) describe that NHANES data can help improve the health of Americans and its use has driven changes in how physicians treat patients and how public policy supports good health. Each year, the NHANES program collects data on about 5,000 adults and children in communities across the US. The NHANES sample population is selected by a random, scientific process to ensure the group of people examined can accurately represent the health and nutritional status of the US general population.
A further potential strength of this study was the study design employed. First, the data were collected independently of the study design in the present study. As such, factors such as recall bias or study participation selection were minimized. Second, NHANES employed reliable and well-established techniques to collect the data examined on the study subjects. Dental status of study subjects was determined by trained dental professionals and lab testing utilized standardized methods. Third, multiple methods of assessing exposure to Hg from amalgams were utilized. Among these were: the comparison of blood Hg concentrations between the amalgam exposed and unexposed groups, the correlation between the number of amalgam surfaces and blood Hg concentrations, and the estimation of daily Hg vapor doses from amalgams and blood Hg concentrations. All revealed increasing blood Hg concentrations were consistently associated with amalgams in unadjusted and adjusted statistical models. Fourth, the biological plausibility, consistency, and magnitude of the results observed argue strongly against the suggestion that observations made in this study are a consequence of statistical chance or unknown confounding. Fifth, blood total Pb concentrations were examined as a control measurement, and as a priori hypothesized, no correlations were observed between blood Pb concentrations and amalgams. This observation is significant because it helps to establish that there was specificity to the correlations observed from the constructed statistical models.
A potential limitation of this study was that a cross-sectional study design was employed, and there was no longitudinal follow-up of study subjects over time. It is possible by following study subjects over time that additional insights could be gleaned regarding other factors that may influence blood Hg concentrations. In the case of Hg vapor release from amalgams, it is known that daily behaviors such as eating or drinking of various foods, tooth brushing, bruxism, etc. may influence the release of Hg vapor from amalgams [20]. Environmental sources of Hg exposure overtime such as fish consumption, occupational exposure, or medicinal exposure may also impact blood Hg concentrations [3].
Another potential limitation of this study was that no information was available regarding the age or the chemical makeup of the amalgams examined. It was previously revealed that release of Hg vapor from newer as compared to older amalgams may be greater [21]. As to chemical makeup of amalgams, increasing Hg release is associated with higher copper-containing amalgams [22].
A still further limitation of this study is that only blood Hg concentrations were measured. It is possible that measurement of the concentration of Hg in other blood components such as the plasma or erythrocytes, may yield different results.
Despite all of the aforementioned potential limitations, this study was able to successfully test the hypothesis put forward. The results of this study revealed repeated, clear, and significant correlations between amalgams and blood Hg concentrations. It is recommended that future studies further evaluate the aforementioned potential limitations of this study.
Conclusions
This study revealed that blood Hg concentrations are significantly associated with amalgams. Amalgams were observed to significantly contribute to dose-dependent increases in blood total and inorganic Hg concentrations in multiple statistical models with and without adjustment for covariates. Despite the fact that elevations in blood Hg concentrations due to amalgams are not generally associated with acute Hg toxicity, this does not provide reassurance as to the safety of Hg vapor exposure from amalgams. Instead, the elevated blood Hg concentrations associated with amalgams are a cause of great concern because they reflect that Hg vapor from amalgams is being continuously transported systemically throughout the human body and is delivering Hg into many tissue and cell types. This is especially true in light of recent studies linking amalgam exposures with increased risks for outcomes such as asthma [23], arthritis [24], hearing loss [25], neurological disorders [26], [27], [28], [29], [30], [31], [32], and perinatal death [33]. Future studies should further examine the long-term consequences of amalgam exposure and explore therapeutic means to reduce blood Hg concentrations among those with amalgams. Persons with amalgams desiring to lower their blood Hg concentrations should consider consulting with a healthcare practitioner, knowledge about this topic, regarding the possibility of amalgam replacement by a dentist trained and certified in a safe amalgam protocol. Finally, every effort should be made to reduce/eliminate wherever practical, the continued use of amalgams.
-
Research ethics: NHANES data collection methods were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB) (Protocol#2011-17). The health information collected in the NHANES program is kept in strictest confidence, and is only used for stated purposes.
-
Informed consent: Each study subject provided informed consent to participate in the NHANES program.
-
Author contributions: Mr. David Geier conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Mr. Geier is director of the nonprofit Institute of Chronic Illnesses, Inc. He is a small shareholder in EmeraMed, Ltd (Dublin, Ireland), a company developing a compound to treat mercury toxicity.
-
Research funding: This work was supported by the non-profit, Institute of Chronic Illnesses, Inc. Mr. David Geier is director of the Institute of Chronic Illnesses, Inc.
-
Data availability: NHANES data can be accessed at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
1. Homme, KG, Kern, JK, Haley, BE, Geier, DA, King, PG, Sykes, LK, et al.. New science challenges old notion that mercury dental amalgam is safe. Biometals 2014;27:19–24. https://doi.org/10.1007/s10534-013-9700-9.Suche in Google Scholar PubMed PubMed Central
2. Kern, JK, Geier, DA, Bjorklund, G, King, PG, Homme, KG, Haley, BE, et al.. Evidence supporting a link between dental amalgams and chronic illness, fatigue, depression, anxiety, and suicide. Neuroendocrinol Lett 2014;35:537–52.Suche in Google Scholar
3. Geier, DA, Geier, MR. Estimated Mercury vapor exposure from amalgams among American pregnant women. Hum Exp Toxicol 2024;43:9603271241231945. https://doi.org/10.1177/09603271241231945.Suche in Google Scholar PubMed
4. Geier, DA, Geier, MR. Dental amalgam fillings and mercury vapor safety limits in American adults. Hum Exp Toxicol 2022;41:9603271221106341. https://doi.org/10.1177/09603271221106341.Suche in Google Scholar PubMed
5. Richardson, GM. Mercury exposure and risks from dental amalgam in Canada: the Canadian health measures survey 2007–2009. Hum Ecol Risk Assess 2014;20:433–47. https://doi.org/10.1080/10807039.2012.743433.Suche in Google Scholar
6. Gul, N, Khan, S, Kan, A, Nawab, J, Shamshad, I, Yu, X. Quantification of Hg excretion and distribution in biological samples of mercury-dental-amalgam users and its correlation with biological variables. Environ Sci Pollut Res Int 2016;23:20580–90. https://doi.org/10.1007/s11356-016-7266-0.Suche in Google Scholar PubMed
7. Bjorkman, L, Musial, F, Alraek, T, Werner, EL, Hamre, HJ. Mercury, silver and selenium in serious before and after removal of amalgam restorations: results from a prospective cohort study in Norway. Acta Odontol Scand 2023;81:298–310.10.1080/00016357.2022.2143422Suche in Google Scholar PubMed
8. Abraham, JE, Svare, CW, Frank, CW. The effect of dental amalgam restorations on blood mercury levels. J Dent Res 1984;63:71–3. https://doi.org/10.1177/00220345840630011801.Suche in Google Scholar PubMed
9. Kingman, A, Albertini, T, Brown, LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res 1998;77:461–71. https://doi.org/10.1177/00220345980770030501.Suche in Google Scholar PubMed
10. Stiglic, AF, Falnoga, I, Briski, AS, Zavbi, M, Osredkar, J, Skitek, M, et al.. Reference intervals of 24 trace elements in blood, plasma, and erythrocytes for the Slovenia adult population. Clin Chem Lab Med 2023;62:946–57. https://doi.org/10.1515/cclm-2023-0731.Suche in Google Scholar PubMed
11. Park, JD, Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med public Health 2012;45:344–52. https://doi.org/10.3961/jpmph.2012.45.6.344.Suche in Google Scholar PubMed PubMed Central
12. Friberg, L, Mottet, NK. Accumulation of methylmercury and inorganic mercury in the brain. Biol Trace Elem Res 1989;21:201–6. https://doi.org/10.1007/bf02917253.Suche in Google Scholar PubMed
13. Bjorkman, L, Lundekvam, BF, Laegreid, T, Bertelsen, BI, Morild, I, Lilleng, P, et al.. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health 2007;6:30. https://doi.org/10.1186/1476-069x-6-30.Suche in Google Scholar
14. Gungor, O, Ozkaya, AK, Kirik, S, Dalkiran, T, Gungor, G, Isikay, S, et al.. Acute mercury poisoning in a group of school children. Pediatr Emerg Care 2019;35:696–9. https://doi.org/10.1097/pec.0000000000001011.Suche in Google Scholar PubMed
15. Escalante, E, Semenova, Y, Peana, M, Bjorklund, G. The impact of mercury from dental amalgams on pregnancy and childhood: a health and risk assessment evaluation. Curr Med Chem in press;32:7193–212. https://doi.org/10.2174/0109298673334663250101101006.Suche in Google Scholar PubMed
16. Berlin, M. Mercury in dental amalgam: a risk analysis. Neurotoxicol 2020;81:382–6. https://doi.org/10.1016/j.neuro.2020.09.034.Suche in Google Scholar PubMed
17. Balali-Mood, M, Eizadi-Mood, N, Hassanian-Moghaddam, H, Eternad, L, Moshiri, M, Vahabzadeh, M, et al.. Recent advances in the clinical management of intoxication by five heavy metals: mercury, lead, chromium, cadmium and arsenic. Heliyon 2025;11:e42696. https://doi.org/10.1016/j.heliyon.2025.e42696.Suche in Google Scholar PubMed PubMed Central
18. Geier, DA, Geier, MR. Reductions in plasma and urine mercury concentrations following N,N’bis-(2-mercaptoethyl) isophthalamide (NBMI) therapy: a post hoc analysis of data from a randomized human clinical trial. Biometals 2024;37:433–45. https://doi.org/10.1007/s10534-023-00560-3.Suche in Google Scholar PubMed PubMed Central
19. https://www.cdc.gov/nchs/nhanes/about (Accessed 5 July 2025).Suche in Google Scholar
20. Berglund, A. Estimation by a 24-hour study of the daily dose of intra-oral Hg vapor inhaled after release from dental amalgam. J Dent Res 1990;69:1646–51. https://doi.org/10.1177/00220345900690100401.Suche in Google Scholar PubMed
21. Woods, JS, Martin, MD, Leroux, BG, DeRouen, TA, Leitao, JG, Bernardo, MF, et al.. The contribution of dental amalgam to urinary mercury excretion in children. Environ Health Perspect 2007;115:1527–31. https://doi.org/10.1289/ehp.10249.Suche in Google Scholar PubMed PubMed Central
22. Okabe, T, Elvebak, B, Carrasco, L, Ferracane, JL, Keanini, RG, Nakajima, H. Mercury release from dental amalgams into continuously replenished liquids. Dent Mater 2003;19:38–45. https://doi.org/10.1016/s0109-5641-02-00010-6.Suche in Google Scholar
23. Geier, DA, Geier, MR. Reported asthma and dental amalgam exposure among adults in the United States: an assessment of the national health and nutrition examination survey. SAGE Open Med 2021;9:20503121211048677. https://doi.org/10.1177/20503121211048677.Suche in Google Scholar PubMed PubMed Central
24. Geier, DA, Geier, MR. Dental amalgams and incidence rate of arthritis among American adults. Clin Med Insights Arthritis Musculoskelet Disord 2021;14:11795441211016261. https://doi.org/10.1177/11795441211016261.Suche in Google Scholar PubMed PubMed Central
25. Rothwell, JA, Boyd, PJ. Amalgam dental fillings and hearing loss. Int J Audiol 2008;47:770–6. https://doi.org/10.1080/14992020802311224.Suche in Google Scholar PubMed
26. Geier, DA, Kern, JK, Geier, MR. A prospective study of prenatal mercury exposure from maternal dental amalgams and autism severity. Acta Neurobiol Exp 2009;69:189–97. https://doi.org/10.55782/ane-2009-1744.Suche in Google Scholar PubMed
27. Holmes, AS, Blaxill, MF, Haley, BE. Reduced levels of mercury infirst baby haircuts of autistic children. Int J Toxicol 2003;22:277–85. https://doi.org/10.1080/10915810305120.Suche in Google Scholar PubMed
28. Khaled, EM, Meguid, NA, Bjorklund, G, Gouda, A, Bahary, MH, Hashish, A, et al.. Altered urinary porphyrins and mercury exposure as biomarkers for autism severity in Egyptian children with autism spectrum disorder. Metab Brain Dis 2016;31:1419–26. https://doi.org/10.1007/s11011-016-9870-6.Suche in Google Scholar PubMed
29. Zonouz, AT, Azar, FP, Pourzare, S, Hosinifard, H, Molaei, Z. Association between dental amalgam fillings and multiple sclerosis: a systematic review and meta-analysis. J Res Clin Med 2023;11:18. https://doi.org/10.34172/jrcm.2023.31924.Suche in Google Scholar
30. Hsu, YC, Chang, CW, Lee, HL, Chuang, CC, Chiu, HS, Li, WY, et al.. Association between history of dental amalgam fillings and risk of Parkinson’s disease: a population-based retrospective cohort study in Taiwan. PLoS One 2016;11:e0166552. https://doi.org/10.1371/journal.pone.0166552.Suche in Google Scholar PubMed PubMed Central
31. Mikhailichenko, N, Yagami, K, Chiou, JY, Huang, JY, Wang, YH, Wei, JCC, et al.. Exposure to dental filling materials and the risk of dementia: a population-based nested case control study in Taiwan. Int J Environ Res Publ Health 2019;16:3283. https://doi.org/10.3390/ijerph16183283.Suche in Google Scholar PubMed PubMed Central
32. Sun, YH, Nfor, ON, Huang, JY, Liaw, YP. Association between dental amalgam fillings and Alzheimer’s disease: a population-based cross-sectional study in Taiwan. Alzheimers Res Ther 2015;7:65. https://doi.org/10.1186/s13195-015-0150-1.Suche in Google Scholar PubMed PubMed Central
33. Bjorkman, L, Lygre, GB, Haug, K, Skaerven, R. Perinatal death and exposure to dental amalgam fillings during pregnancy in the population-based MoBa cohort. PLoS One 2018;13:e02088036. https://doi.org/10.1371/journal.pone.0208803.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

