Increased specificity of the “GFAP/UCH-L1” mTBI rule-out test by age dependent cut-offs
-
Aurélie Ladang
, George Vavoulis
and Konstantinos Makris
Abstract
Objectives
Mild traumatic brain injury (mTBI) remains challenging to diagnose effectively in the emergency department. Abbott has developed the “GFAP/UCH-L1” mTBI test, to guide the clinical decision to perform a computed tomography (CT) head scan by ruling out the presence of mTBI. We evaluated the diagnostic accuracy of the “GFAP/UCH-L1” mTBI test in a Greek cohort and established age-dependent cut-offs.
Methods
A total of 362 subjects with suspected mTBI and admitted to the Emergency department of the KAT General Hospital of Athens, Greece were recruited for the study. All subjects underwent a CT head scan to establish the diagnosis of mTBI. GFAP and UCH-L1 were measured using Alinity I (Abbott). 163 healthy subjects served as controls.
Results
Using the manufacturer’s cut-offs (35 ng/L for GFAP and 400 ng/L for UCH-L1), the “GFAP/UCH-L1” mTBI test had a sensitivity of 99.1 % and a specificity of 40.6 %. However, the specificity dropped to 14.9 % in patients older than 65 years old. By defining a new cut-off of 115 ng/L for GFAP and 335 ng/L specifically for patients older than 65 years, specificity was increased up to 30.6 % without changing test sensitivity and the number of CT head scans avoided was doubled in this subgroup.
Conclusions
The “GFAP/UCH-L1” mTBI test is an efficient “rule-out test” to exclude patients suffering from mTBI. By adjusting the cut-offs in patients older than 65 years old, we could significantly increase the number of CT head scans avoided without affecting the sensitivity. These new cut-offs should be externally validated.
Introduction
Traumatic brain injuries (TBIs) are classified into 3 categories based on the severity of the event. Severe TBI accounts for 3 % of the TBI and is diagnosed when the patient has a Glasgow Coma Scale (GCS) score of 3–8 and severe neurological deficits following the trauma [1]. Moderate TBI accounts for 2 % of the TBI and is diagnosed when the patient has a GCS of 9–12 [1]. Finally, mild traumatic brain injury (mTBI) is the less severe category of TBI [1] and is defined by the French Society of Neurosurgery as “a brain trauma secondary to the transmission of a kinetic energy to the head responsible for a transient cerebral dysfunction” [2].
mTBI is a serious public health problem and though being the least severe of all brain injuries, identification is the most challenging as mTBI is often missed at diagnosis [3]. Worldwide, about 45 million people experience mTBI at least once each year and in Europe, mTBI affects 2.25 million patients [4], 5]. The leading cause of mTBI varies from traffic accidents in young adults to accidental falls in children and older adults [5] which explains why children, young men, and older women are the most at risk of mTBI [5], [6], [7].
Major barriers to mTBI identification are the wide variability in diagnostic criteria and the lack of sensitive standardized measures to identify mTBI manifestations, which are typically subtle and rapidly resolving. Indeed, the gold standard diagnostic tools for TBI are the clinical judgment as assessed by the 15-point GCS and the computed tomography scan (CT head scan) [4]. However, multiple CT head scans increase the risk of radiation-related disease [6], 8]. Additionally, knowing that many older adults fall regularly and that the risk of poor outcome is also age-related [6], 8], strategies have been developed to reduce the CT head scan overuse. The first strategy is to exclude suspicious cases based on clinical decision criteria, as it is the case in the Canadian CT head rule (CCTHR) guidelines [9]. However, the effectiveness of the CCTHR guidelines is limited in the older population as being older than 65 years old is an indication for a CT head scan [9].
Despite CCTHR efforts to clarify and improve mTBI algorithmic decision making, the clinical decision to perform a CT head scan, while effectively using hospital sources, reducing the patient exposure to radiation and ensuring the patient’s safe return home, remains challenging. Biomarkers could provide a rapid, definitive, noninvasive, and cost-effective diagnostic test for brain injury that would guide the implementation of appropriate triage and medical management. The use of biomarkers is particularly relevant in specific populations such as the elderly that falls regularly and radiation sensitive populations like children and pregnant women.
Two tests are commonly cited to allow the rule-out of mTBI based on biomarkers: measurement of S100B or the “GFAP/UCH-L1” mTBI test. Both tests have shown similar performance [10] but the “GFAP/UCH-L1” mTBI test has the advantage that it can be performed up to 12 h post-concussion compared to 3 h for S100B [11].
The “GFAP/UCH-L1” mTBI test for the rule-out of mTBI is based on the quantitative measurement of glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase L-1 (UCH-L1) [12]. GFAP is a monomeric intermediate filament protein that represents the major component of the astroglial cytoskeleton. It is mainly found in the central nervous system. UCH-L1 is an enzyme highly abundant in neurons, representing between 1 and 5 % of total soluble brain protein. It has been suggested that UCH-L1 plays an important role in the removal of excessive, oxidized, or misfolded proteins in both normal and neuropathological conditions including neurodegenerative disorders. This protein is released into the extracellular space as a result of cell destruction under pathological conditions [13]. These two proteins show a different temporal expression in the first hours after head trauma [14]. The “GFAP/UCH-L1” mTBI test takes advantage of these physiopathological properties to provide an assay that is selectively elevated in case of traumatic brain injuries but also in many other conditions not related to head trauma. Therefore, this assay is not designed for the diagnosis of mTBI but only to exclude it.
In this context, this study aims to evaluate the accuracy of the “GFAP/UCH-L1” mTBI test in the white European population. Additionally, this study aims to decipher impact of age and anticoagulant use on test accuracy and to provide age-dependent cut-offs.
Materials and methods
Study population
This prospective study was conducted between 2022 and 2023 at the KAT General Hospital in Kifissia, Greece. All procedures were compliant with the Hospitals standard protocol for the management of patient with TBI, and the admission and discharge of each patient was decided after clinical evaluation and analysis of CT head scan report by the attending neurosurgeon. CT head scans were performed on all subjects with suspected TBI upon admission at the Emergency Department (ED) by an experienced attending radiologist using a Philips ingenuity 5,000 CT scanner. All clinicians who evaluated the patients at the ED were blinded to mTBI test results throughout the study (Figure 1).

Diagram showing the flow and evaluation of patients in our study.
In addition, healthy control subjects matched to the patients (age and sex) were additionally recruited from a population of healthy volunteers participating in a study on reference ranges. The control subjects had the same exclusion criteria as the mTBI subjects.
For each enrolled patient, a K2-EDTA blood sample was collected and sent to Clinical Biochemistry department. Samples were centrifuged immediately and plasma aliquoted and stored at −80 °C until tested. Blood samples were collected as close as possible to the time of evaluation, but no later than 12 h after the reported time of head injury.
All participants or legal guardians were granted written informed consent to participate. The study was approved by the KAT-Hospital Scientific and Ethical Committee under the number 746/10-9-2021.
Inclusion and exclusion criteria were as follows:
Inclusion criteria
>18 years of age
GCS 13–15
Indication of brain CT head scan: neurological focal deficit; anterograde amnesia; GCS <15 after 2 h post-TBI; suspicion of vault depression fracture; fracture of the basal skull; persisting nausea, vomiting or headache; post-TBI seizures; pre-injury treatment with antithrombotic drugs; loss of consciousness or amnesia with age >65 years, fall >1 m or hit pedestrian.
Available CT head scan and CT report
Exclusion criteria
Children
GCS 3–12 on admission
Primary admission for non-traumatic neurological disorder (e.g., stroke, spontaneous intracranial hematoma) as well as subject diagnosed neurodegenerative disease or other neurological disorder including dementia, Parkinson disease, multiple sclerosis, seizure disorder, or brain tumors
Time of injury cannot be determined or >12 h
Primary diagnosis of ischemic or hemorrhagic stroke or transient ischemic attack (TIA) within the last 6 months) or history of neurosurgery procedure within the last 90 days
Blood collection not feasible (i.e., skin integrity compromised at the venipuncture sites, blood vessel calcification (i.e., IV drug users, advanced atherosclerosis) both upper limbs missing (congenital or amputee))
Participating in an interventional, or therapeutic clinical study that may affect the results of this study
Penetrating head trauma
Patient with mechanical ventilation
Administration of blood transfusion after head injury at the admission and prior to the study blood draw
The subject is a female who is pregnant or lactating
Laboratory measurements
We used the Abbott’s “GFAP/UCH-L1” mTBI test on the automated Alinity I system (Abbott Park, IL, USA) to evaluate all patients and normal controls. The “GFAP/UCH-L1” mTBI test is a panel of in vitro diagnostic chemiluminescent microparticle immunoassays (CMIA) used for the quantitative measurement of GFAPand UCH-L1, in human plasma and serum. Although each biomarker is calibrated separately and provides quantitative results in ng/L, the “GFAP/UCH-L1” mTBI test combines both results to provide a semiquantitative interpretation of the test results derived from these measurements. The measurement of both biomarkers was performed according to manufacturer’s instructions on plasma EDTA and the mTBI result was evaluated as follows and according to the manufacturer’s datasheet: The mTBI test is considered as negative if GFAP is below 35 ng/L and UCH-L1 below 400 ng/L. In case, either GFAP or UCH-L1 or both are above these cut-offs, the mTBI test is considered as positive. The analytical coefficients of variation (CVs), determined according to CLSI guidelines, were 3.9 % for UCH-L1 and 3.4 % for GFAP.
Statistical analysis
Variables were assessed for normality using the Shapiro–Wilk test and plots. Since none of the continuous variables showed a normal distribution, nonparametric tests were used. For descriptive statistics, categorical data are reported as absolute numbers, whereas continuous variables are expressed as median, interquartile range (IQR). Given the usual clinical practice, we partitioned the mTBI cohort into patient younger or older than 65 years old when subgroups were investigated. Between groups comparison were performed using the Mann-Whitney test. Fitting curve in healthy subjects was drawn with LOESS smoothing with a span of 80 %.
Receiver operating characteristics (ROC) curves were drawn to calculate the area under the curve (AUC) and to define the new cut-offs. Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were calculated using cut-offs provided by Abbott and our own cut-offs.
For all statistical tests, the level of significance was fixed at 0.05. Medcalc statistical software (Medcalc, Belgium) was used for all statistical analyses.
Results
Healthy controls
To evaluate the potential need for age-dependent cut-offs, we first evaluated the GFAP and UCH-L1 trend according to age in a healthy control population. The fitting curve in healthy controls showed an inflection point around 50 years old upon visual inspicetion (Figure 2A and B). A statistically significant increase was found between subjects <50 years old, subjects comprised between 50 and 64 years old and subjects ≥65 years old for both UCH-L1 and GFAP (p<0.0001) (Table 1).

Fitting curve of GFAP (A) and UCH-L1(B) according to age.
GFAP and UCH-L1 concentration in healthy controls.
| Healthy controls | n | Median | Percentile 5 | Percentile 95 | |
|---|---|---|---|---|---|
| GFAP | <50 years old | 80 | 16.6 | 9.8 | 30.1 |
| 50≤ age <65 years old | 65 | 21.4 | 12.5 | 52.0 | |
| ≥65 years old | 16 | 34.1 | 18.3 | 87.6 | |
| UCH-L1 | <50 years old | 80 | 73.9 | 52.6 | 127.2 |
| 50≤ age <65 years old | 65 | 89.2 | 57.5 | 175.9 | |
| ≥65 years old | 16 | 112.3 | 73.1 | 173.1 | |
mTBI cohort description
The cohort recruitment scheme is presented in Figure 1 and patients consisted of 362 individuals, 38.4 % of whom were women and 47.2 % were older than 65 years old (Table 2). A total of 37.0 % of the cohort reported to have anticoagulant treatment.
Cohort description.
| Total n=362 |
Negative CT-scan n=249 |
Positive CT-scan n=113 |
|
|---|---|---|---|
| Median age, years (IQR) |
63 (40.5) | 64 (39.5) | 55 (41) |
| Gender, n | |||
| Male | 219 | 141 | 78 |
| Female | 139 | 105 | 34 |
| Not specified | 4 | 3 | 1 |
| Mechanism of injury | |||
| Fall (stairs or same height) | 185 | 143 | 42 |
| Traffic related accident | 137 | 75 | 62 |
| Other | 40 | 31 | 9 |
| Type of lesions | |||
| SDH | 20 | 0 | 20 |
| Epidural Hematoma | 12 | 0 | 12 |
| SAH | 29 | 0 | 29 |
| Contusion | 36 | 0 | 36 |
| Fracture | 12 | 0 | 12 |
| Contusion + SDH | 2 | 0 | 2 |
| Fracture + SAH | 2 | 0 | 2 |
| Anticoagulant | |||
| Yes | 134 | 92 | 42 |
| No | 212 | 148 | 64 |
| Not specified | 16 | 9 | 7 |
| Median GFAP (IQR) | |||
| Entire population | 77.5 (246.0) | 44.0 (71.3) | 474.0 (859.5) |
| <65 years old | 42.0 (275.0) | 26.1 (28.2) | 396.0 (739.5) |
| ≥65 years old | 124.0 (207.1) | 79.3 (93.5) | 776.0 (1,580.0) |
| Median UCH-L1 (IQR) | |||
| Entire population | 340.0 (408.0) | 261.0 (245.3) | 774.6 (1,248.2) |
| <65 years old | 250.0 (418.9) | 182.8 (188.2) | 774.6 (833.5) |
| ≥65 years old | 426.0 (382.0) | 327.0 (266) | 868.0 (1,331.1) |
-
IQR, interquartile range; SDH, subdural hematoma; SAH, subarachnoïdal hematomat.
A total of 68.8 % of individuals had a negative CT head scan, whereas 31.2 % of subjects were positive on CT head scan with a GCS ≥13.
Median GFAP was significantly higher in individuals with positive CT head scan compared to those with a negative CT head scan (p<0.001). Additionally, median GFAP was significantly increased in subjects ≥65 years old, not only in the overall population (p<0.001), but also in the CT-scan negative population (p<0.001) compared to their younger counterparts (Table 2). The median GFAP in subjects ≥65 years and with negative CT head scan was almost twice the manufacturer’s cut-off for GFAP.
Similarly, to GFAP, UCH-L1 was elevated in people ≥65 years old in both the overall population and the CT-scan negative population (both p<0.001). Still, median UCH-L1 for the negative CT head scan population was below the cut-off suggested by the manufacturer (Table 2).
mTBI test performances
In our population, the sensitivity of the “GFAP/UCH-L1” mTBI test was 99.1 % and the NPV was 99 % while both GFAP and UCH-L1 sensitivity were decreased when considered separately (Table 3). Only one subject was negative on the “GFAP/UCH-L1” mTBI test while being positive on the CT head scan (Table 3). For this 27 years old patient whose CT head scan was objectivating a skull fracture, the GFAP concentration was 31 ng/L and the UCH-L1 concentration was 312 ng/L.
Accuracy of the “GFAP/UCH-L1” mTBI test according to Abbott cut-offs.
| Whole population n=362 |
<65 years old n=191 |
≥65 years old n=171 |
||
|---|---|---|---|---|
| “GFAP/UCH-L1” mTBI test | Sensibility | 99.1 | 98.4 | 100 |
| Specificity | 40.6 | 64.8 | 14.9 | |
| PPV | 43.1 | 57.9 | 32.7 | |
| NPV | 99 | 98.8 | 100 | |
| GFAP | Sensibility | 96.5 | 93.7 | 100 |
| Specificity | 42.6 | 68 | 15.7 | |
| PPV | 43.3 | 59 | 32.9 | |
| NPV | 96.4 | 95.6 | 100 | |
| UCH-L1 | Sensibility | 82.3 | 82.5 | 82 |
| Specificity | 74.3 | 88.3 | 59.5 | |
| PPV | 59.2 | 77.6 | 45.6 | |
| NPV | 90.2 | 91.1 | 88.9 | |
-
PPV, predictive positive value; NPV, predictive negative value.
On the other hand, the specificity of the “GFAP/UCH-L1” mTBI test was less than 50 % in our cohort. The test specificity was driven by age as the specificity was significantly decreased in individuals older than 65 years old (Table 3). To better understand the impact of age on the number of positive tests, we further partitioned the cohort by 10 years. Although the number of CT head scans was constant regardless of the age, the number of positive tests increased from 45.5 % in the youngest adults to reach 96.5 % after 80 years of age (Figure 3A and B). Additionally, these patients were positive for GFAP in 40.0 % of the first two age groups and 96.5 % in the last two age groups whereas for UCH-L1, it was 34.5 % for the youngest and 56.5 % for the oldest (Figure 3C and D).

Positivity rate of the “GFAP/UCH-L1” mTBI test is age-dependent. Positivity rate of CT head scan (A), “GFAP/UCH-L1” mTBI test (B), GFAP (C) and UCH-L1 (D) when the cohort is partitioned by 10 years.
To evaluate the impact of anticoagulant use, we selected the subjects older than 65 years old since the test performance is age-related and these medications are predominantly prescribed to the elderly. In this subgroup, 118 subjects were taking anticoagulant drugs while 40 did not (and 12 have unknown status). Among these 158 individuals, positive CT head scans increased, as expected, from 15 % in those not on anticoagulant medication to 31.4 % in those on anticoagulant medication (Figure 4A). However, the percentage of positive “GFAP/UCH-L1” mTBI tests was identical in both cases (90 and 89.8 %, respectively) (Figure 4B). Additionally, sensitivity and NPV were of 100 % for subjects older than 65 years old with or without anticoagulant intake while specificity was 14.8 and 16.7 % and PPV was 34.9 and 28.6 % for subjects older than 65 years old with or without anticoagulant intake, respectively. Specificity and PPV were not statistically different in both groups.
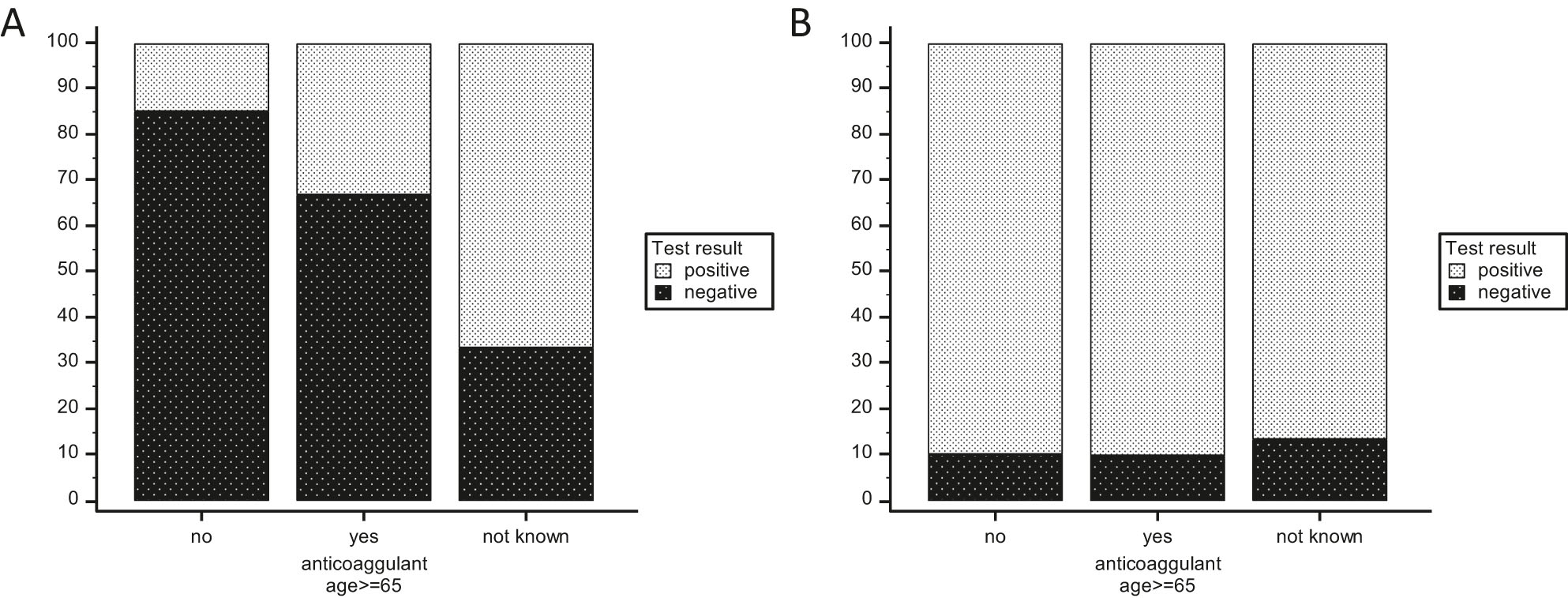
Positivity rate of the “GFAP/UCH-L1” mTBI test is not modified by anticoagulant use: Positivity rate of CT head scan (A) and “GFAP/UCH-L1” mTBI test (B) when the cohort is divided based on reported anticoagulant use.
Age-dependent cut-offs for mTBI
Given the impact of age on test accuracy, we evaluated whether age-dependent reference ranges could increase test specificity. Receiver operating characteristic (ROC) curves were generated for this purpose. GFAP had an area under the curve (AUC) of 0.904 while the AUC for UCH-L1 was 0.864 when the entire population was evaluated. When only individuals older than 65 years old were used to generate ROC curves, the AUCs were as good as for the entire population (GFAP AUC: 0.928 and UCH-L1 AUC: 0.842), indicating that the same diagnostic performance can be achieved in the oldest population. To do so, we selected a sensitivity of 96 % for GFAP and 90 % for UCH-L1 in the subset of individuals >65 years old according to the Youden index. By taking advantage of both sensitivities, we obtained a “GFAP/UCH-L1” mTBI test sensitivity of 100 % as well as a NPV of 100 % with an increased specificity and PPV in this specific group (Table 4). Overall, the test accuracy with the new age-dependent cut-offs was 99.1 % sensitivity and 48.2 % specificity (Table 4). By applying these cut-offs, an additional 19 unnecessary CT head scans were avoided, doubling the number of ruled-out patients in patients older than 65 years old.
Accuracy of the “GFAP/UCH-L1” mTBI test according to our new cut-offs.
| Whole population n=362 |
<65 years old n=191 |
≥65 years old n=171 |
||
|---|---|---|---|---|
| New cut-offs | GFAP | Age dependent | 35 | 115 |
| UCH-L1 | Age dependent | 400 | 335 | |
| “GFAP/UCH-L1” mTBI test | Sensibility | 99.1 | 98.4 | 100 |
| Specificity | 48.2 | 64.8 | 30.6 | |
| PPV | 46.5 | 57.9 | 37.3 | |
| NPV | 99.2 | 98.8 | 100 | |
-
PPV, predictive positive value; NPV, predictive negative value.
Discussion
This study confirms the good accuracy of the “GFAP/UCH-L1” mTBI test to exclude mTBI. It further highlights that the accuracy of the test is age-dependent but not modified by anticoagulant use. Moreover, we demonstrated that the specificity of the test can be increased by age-dependent cut-offs.
The “GFAP/UCH-L1” mTBI test has recently received FDA approval in the USA for the rule-out of mTBI. However, this approval was based on studies primarily conducted in the United States [12]. To further drive the adoption of the “GFAP/UCH-L1” mTBI test, European data were awaited [15]. Recent studies in the Netherlands, France and Croatia have shown comparable test accuracy with test sensitivity always above 97 % but test specificity around 30 %, suggesting that the use of the “GFAP/UCH-L1” mTBI test as a rule-out test could reduce the use of CT head scans by one third [10], 16], 17]. Our results are consistent with these studies.
Excellent sensitivity is essential for a rule-out test. However, it is the specificity of the test that drives the number of patients ruled-out, the number of unnecessary CT head scans avoided and thus, determine the cost-effectiveness of the test. In this context, it is important to understand if and how test specificity can be increased without altering test sensitivity and NPV. Two approaches have been proposed to optimize the specificity. Chayoua et al. suggested that specificity could be increased by integrating loss of consciousness and time of sampling into the algorithm [16]. Alternatively, Ward and colleagues, by showing a decreased specificity of the test in older patients, suggested the hypothesis that test performances could be improved by age-dependent cut-offs [18].
Our results in healthy controls showed that GFAP and UCH-L1 increased with age. Blood-based neurological biomarkers have been widely described as age-dependent [19] and biomarkers such as neurofilament light chain (NfL) have been recognized to require age-dependent reference ranges [20]. Additionally, by focusing on confounding factors, we have previously shown that, with the current cut-offs, more than 80 % of subjects above 80 years old were GFAP positive without any report of recent trauma [21]. This further highlights the relevance of age-dependent cut-offs. Although we are the first to provide data based on age-dependent cut-offs for the rule-out of mTBI, this strategy was already applied to optimize performances of S100B, the “historical” biomarker for the rule-out of mTBI [22].
In the near future, new studies and guidelines will need to specifically address the question of the usefulness of the “GFAP/UCH-L1” mTBI test in the elderly. Indeed, this population is the most at risk for mTBI due to a higher risk of falls and frequent comorbidities, but this is also the population that cannot be excluded by clinical examination as suggested by the Canadian guidelines [9]. However, not only is age a confounding factor in the “GFAP/UCH-L1” mTBI test but so are also all-cause dementia and neurological disorders [19]. Notably, GFAP is also known to be a good biomarker for Alzheimer’s disease [23]. Therefore, the needs in this population are different and should be addressed in specific studies.
Our study has the strength of including a large proportion of subjects older than 65 years old (47 %). Regarding limitation, the cohort is a mono-centric cohort of medium size and other confounding factors such as renal function or BMI were not assessed. Further studies should be dedicated to confirm the accuracy of these new cut-offs in other larger cohorts from other countries. Additionally, since GFAP and UCH-L1 measurements are not standardized methods, it remains to be determined whether these cut-offs are applicable in other platforms. Nevertheless, no bias was reported between measurements performed on i-STAT or on Alinity I [24], suggesting that these cut-offs may be applicable to the i-STAT platform after confirmatory studies.
In conclusion, data from our prospective cohort show good diagnostic accuracy for the “GFAP/UCH-L1” mTBI test while accuracy of the test is increased by age dependent cut-offs. Further studies should be dedicated specifically to the elderly to evaluate and optimize the accuracy of the “GFAP/UCH-L1” mTBI test in this population.
Funding source: Clinical Biochemistry Department – KAT General Hospital, Kifissia, Athens Greece
-
Research ethics: The mTBI study was approved by the KAT-Hospital Scientific and Ethical Committee. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
-
Informed consent: All participants or legal guardians were granted written informed consent to participate.
-
Author contributions: Design of the study: AL, GV, IT, KK, AM, KV, EC, KM. Experiments: IT, KM. Statistical analysis: AL, EmC, KM. Cohort design and sampling: GV, IT, KK, AM, KV, KM. Writing and reviewing: AL, GV, IT, EmC, KK, AM, KV, EC, KM. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This study was supported by (3) Clinical Biochemistry Department – KAT General Hospital, Kifissia, Athens Greece.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Korley, FK, Kelen, GD, Jones, CM, Diaz-Arrastia, R. Emergency department evaluation of traumatic brain injury in the United States, 2009–2010. J Head Trauma Rehabil 2016;31:379–87. https://doi.org/10.1097/htr.0000000000000187.Search in Google Scholar PubMed PubMed Central
2. Aghakhani, N, Decq, P. Mild traumatic brain injury: an update. Report of the French society of neurosurgery and the French-speaking neurosurgical society. Neurochirurgie 2021. https://doi.org/10.1016/j.neuchi.2021.04.002.Search in Google Scholar PubMed
3. Ruff, RM, Iverson, GL, Barth, JT, Bush, SS, Broshek, DK, the NAN Policy and Planning Committee. Recommendations for diagnosing a mild traumatic brain injury: a national academy of neuropsychology education paper. Arch Clin Neuropsychol 2009;24:3–10. https://doi.org/10.1093/arclin/acp006.Search in Google Scholar PubMed
4. Gil-Jardiné, C, Payen, J-F, Bernard, R, Bobbia, X, Bouzat, P, Catoire, P, et al.. Management of patients suffering from mild traumatic brain injury 2023. Anaesth Crit Care Pain Med 2023;42:101260. https://doi.org/10.1016/j.accpm.2023.101260.Search in Google Scholar PubMed
5. Lefevre-Dognin, C, Cogné, M, Perdrieau, V, Granger, A, Heslot, C, Azouvi, P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 2021;67:218–21. https://doi.org/10.1016/j.neuchi.2020.02.002.Search in Google Scholar PubMed
6. Laic, RAG, Vander Sloten, J, Depreitere, B. Traumatic brain injury in the elderly population: a 20-year experience in a tertiary neurosurgery center in Belgium. Acta Neurochir 2022;164:1407–19. https://doi.org/10.1007/s00701-022-05159-0.Search in Google Scholar PubMed
7. Cancelliere, C, Coronado, VG, Taylor, CA, Xu, L. Epidemiology of isolated versus nonisolated mild traumatic brain injury treated in emergency departments in the United States, 2006–2012: sociodemographic characteristics. J Head Trauma Rehabil 2017;32:E37–46. https://doi.org/10.1097/htr.0000000000000260.Search in Google Scholar PubMed PubMed Central
8. Sercy, E, Orlando, A, Carrick, M, Lieser, M, Madayag, R, Vasquez, D, et al.. Long-term mortality and causes of death among patients with mild traumatic brain injury: a 5-year multicenter study. Brain Inj 2020;34:556–66. https://doi.org/10.1080/02699052.2020.1725981.Search in Google Scholar PubMed
9. Stiell, IG, Wells, GA, Vandemheen, K, Clement, C, Lesiuk, H, Laupacis, A, et al.. The Canadian CT head rule for patients with minor head injury. Lancet 2001;357:1391–6. https://doi.org/10.1016/s0140-6736(00)04561-x.Search in Google Scholar PubMed
10. Oris, C, Bouillon-Minois, J-B, Kahouadji, S, Pereira, B, Dhaiby, G, Defrance, VB, et al.. S100B vs. “GFAP and UCH-L1” assays in the management of mTBI patients. Clin Chem Lab Med (CCLM). De Gruyter 2024;62:891–9. https://doi.org/10.1515/cclm-2023-1238.Search in Google Scholar PubMed
11. Backus, BE, Moustafa, F, Skogen, K, Sapin, V, Rane, N, Moya-Torrecilla, F, et al.. Consensus paper on the assessment of adult patients with traumatic brain injury with Glasgow Coma Scale 13–15 at the emergency department: a multidisciplinary overview. Eur J Emerg Med 2024;31:240–9. https://doi.org/10.1097/mej.0000000000001140.Search in Google Scholar PubMed
12. Bazarian, JJ, Biberthaler, P, Welch, RD, Lewis, LM, Barzo, P, Bogner-Flatz, V, et al.. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol 2018;17:782–9. https://doi.org/10.1016/s1474-4422(18)30231-x.Search in Google Scholar
13. Sapin, V, Gaulmin, R, Aubin, R, Walrand, S, Coste, A, Abbot, M. Blood biomarkers of mild traumatic brain injury: state of art. Neurochirurgie 2021;67:249–54. https://doi.org/10.1016/j.neuchi.2021.01.001.Search in Google Scholar PubMed
14. Papa, L, Lewis, LM, Falk, JL, Zhang, Z, Silvestri, S, Giordano, P, et al.. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med 2012;59. https://doi.org/10.1016/j.annemergmed.2011.08.021.Search in Google Scholar PubMed PubMed Central
15. Oris, C, Kahouadji, S, Bouvier, D, Sapin, V. Blood biomarkers for the management of mild traumatic brain injury in clinical practice. Clin Chem 2024:hvae049.10.1093/clinchem/hvae049Search in Google Scholar PubMed
16. Chayoua, W, Visser, K, De Koning, ME, Beishuizen, A, Ijmker, R, Van Der Naalt, J, et al.. Evaluation of glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 using a rapid point of care test for predicting head computed tomography lesions after mild traumatic brain injury in a Dutch multi-center cohort. J Neurotrauma 2024;41:e1630–40. https://doi.org/10.1089/neu.2023.0491.Search in Google Scholar PubMed
17. Lapić, I, Rogić, D, Lončar Vrančić, A, Gornik, I. Exploratory analysis of glial fibrillary acidic protein and ubiquitin C-terminal hydrolase L1 in management of patients with mild neurological symptoms undergoing head computed tomography scan at the emergency department: a pilot study from a Croatian tertiary hospital. Lab Med 2024;55:492–7. https://doi.org/10.1093/labmed/lmad116.Search in Google Scholar PubMed
18. Ward, MD, Weber, A, Merrill, VD, Welch, RD, Bazarian, JJ, Christenson, RH. Predictive performance of traumatic brain injury biomarkers in high-risk elderly patients. J Appl Lab Med 2020;5:91–100. https://doi.org/10.1093/jalm/jfaa039.Search in Google Scholar PubMed
19. Pichet Binette, A, Janelidze, S, Cullen, N, Dage, JL, Bateman, RJ, Zetterberg, H, et al.. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimer’s Dementia 2023;19:1403–14. https://doi.org/10.1002/alz.12787.Search in Google Scholar PubMed PubMed Central
20. Ladang, A, Kovacs, S, Lengelé, L, Locquet, M, Reginster, J-Y, Bruyère, O, et al.. Neurofilament light chain concentration in an aging population. Aging Clin Exp Res 2022;34:331–9. https://doi.org/10.1007/s40520-021-02054-z.Search in Google Scholar PubMed PubMed Central
21. Calluy, E, Beaudart, C, Alokail, MS, Al-Daghri, NM, Bruyère, O, Reginster, J-Y, et al.. Confounding factors of the expression of mTBI biomarkers, S100B, GFAP and UCH-L1 in an aging population. Clin Chem Lab Med (CCLM) [Internet] 2024. [cited 2024 Jul 25];0. Available from: https://www.degruyter.com/document/doi/10.1515/cclm-2024-0194/html.10.1515/cclm-2024-0194Search in Google Scholar PubMed
22. Oris, C, Bouillon-Minois, J-B, Pinguet, J, Kahouadji, S, Durif, J, Meslé, V, et al.. Predictive performance of blood S100B in the management of patients over 65 years old with mild traumatic brain injury. J Gerontol: Series A 2021;76:1471–9. https://doi.org/10.1093/gerona/glab055.Search in Google Scholar PubMed
23. Hansson, O, Blennow, K, Zetterberg, H, Dage, J. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat Aging 2023;3:506–19. https://doi.org/10.1038/s43587-023-00403-3.Search in Google Scholar PubMed PubMed Central
24. Oris, C, Khatib-Chahidi, C, Pereira, B, Bailly Defrance, V, Bouvier, D, Sapin, V. Comparison of GFAP and UCH-L1 measurements using two automated immunoassays (i-STAT® and Alinity®) for the management of patients with mild traumatic brain injury: preliminary results from a French single-center approach. IJMS 2024;25:4539. https://doi.org/10.3390/ijms25084539.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Are the benefits of External Quality Assessment (EQA) recognized beyond the echo chamber?
- Reviews
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part I – EQA in general and EQA programs in particular
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part II – EQA cycles
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part III – EQA samples
- Behind the scenes of EQA–characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part IV – Benefits for participant laboratories
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part V – Benefits for stakeholders other than participants
- Opinion Papers
- Not all biases are created equal: how to deal with bias on laboratory measurements
- Krebs von den Lungen-6 (KL-6) as a diagnostic and prognostic biomarker for non-neoplastic lung diseases
- General Clinical Chemistry and Laboratory Medicine
- Evaluation of performance in preanalytical phase EQA: can laboratories mitigate common pitfalls?
- Point-of-care testing improves care timeliness in the emergency department. A multicenter randomized clinical trial (study POCTUR)
- The different serum albumin assays influence calcium status in haemodialysis patients: a comparative study against free calcium as a reference method
- Measurement of 1,25-dihydroxyvitamin D in serum by LC-MS/MS compared to immunoassay reveals inconsistent agreement in paediatric samples
- Knowledge among clinical personnel on the impact of hemolysis using blood gas analyzers
- Quality indicators for urine sample contamination: can squamous epithelial cells and bacteria count be used to identify properly collected samples?
- Reference Values and Biological Variations
- Biological variation of cardiac biomarkers in athletes during an entire sport season
- Increased specificity of the “GFAP/UCH-L1” mTBI rule-out test by age dependent cut-offs
- Cancer Diagnostics
- An untargeted metabolomics approach to evaluate enzymatically deconjugated steroids and intact steroid conjugates in urine as diagnostic biomarkers for adrenal tumors
- Cardiovascular Diseases
- Comparative evaluation of peptide vs. protein-based calibration for quantification of cardiac troponin I using ID-LC-MS/MS
- Infectious Diseases
- The potential role of leukocytes cell population data (CPD) for diagnosing sepsis in adult patients admitted to the intensive care unit
- Letters to the Editor
- Concentrations and agreement over 10 years with different assay versions and analyzers for troponin T and N-terminal pro-B-type natriuretic peptide
- Does blood tube filling influence the Athlete Biological Passport variables?
- Influence of data visualisations on laboratorians’ acceptance of method comparison studies
- An appeal for biological variation estimates in deep immunophenotyping
- Serum free light chains reference intervals for the Lebanese population
- Applying the likelihood ratio concept in external quality assessment for ANCA
- A promising new direct immunoassay for urinary free cortisol determination
Articles in the same Issue
- Frontmatter
- Editorial
- Are the benefits of External Quality Assessment (EQA) recognized beyond the echo chamber?
- Reviews
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part I – EQA in general and EQA programs in particular
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part II – EQA cycles
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part III – EQA samples
- Behind the scenes of EQA–characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part IV – Benefits for participant laboratories
- Behind the scenes of EQA – characteristics, capabilities, benefits and assets of external quality assessment (EQA): Part V – Benefits for stakeholders other than participants
- Opinion Papers
- Not all biases are created equal: how to deal with bias on laboratory measurements
- Krebs von den Lungen-6 (KL-6) as a diagnostic and prognostic biomarker for non-neoplastic lung diseases
- General Clinical Chemistry and Laboratory Medicine
- Evaluation of performance in preanalytical phase EQA: can laboratories mitigate common pitfalls?
- Point-of-care testing improves care timeliness in the emergency department. A multicenter randomized clinical trial (study POCTUR)
- The different serum albumin assays influence calcium status in haemodialysis patients: a comparative study against free calcium as a reference method
- Measurement of 1,25-dihydroxyvitamin D in serum by LC-MS/MS compared to immunoassay reveals inconsistent agreement in paediatric samples
- Knowledge among clinical personnel on the impact of hemolysis using blood gas analyzers
- Quality indicators for urine sample contamination: can squamous epithelial cells and bacteria count be used to identify properly collected samples?
- Reference Values and Biological Variations
- Biological variation of cardiac biomarkers in athletes during an entire sport season
- Increased specificity of the “GFAP/UCH-L1” mTBI rule-out test by age dependent cut-offs
- Cancer Diagnostics
- An untargeted metabolomics approach to evaluate enzymatically deconjugated steroids and intact steroid conjugates in urine as diagnostic biomarkers for adrenal tumors
- Cardiovascular Diseases
- Comparative evaluation of peptide vs. protein-based calibration for quantification of cardiac troponin I using ID-LC-MS/MS
- Infectious Diseases
- The potential role of leukocytes cell population data (CPD) for diagnosing sepsis in adult patients admitted to the intensive care unit
- Letters to the Editor
- Concentrations and agreement over 10 years with different assay versions and analyzers for troponin T and N-terminal pro-B-type natriuretic peptide
- Does blood tube filling influence the Athlete Biological Passport variables?
- Influence of data visualisations on laboratorians’ acceptance of method comparison studies
- An appeal for biological variation estimates in deep immunophenotyping
- Serum free light chains reference intervals for the Lebanese population
- Applying the likelihood ratio concept in external quality assessment for ANCA
- A promising new direct immunoassay for urinary free cortisol determination

