A global perspective on the status of clinical metabolomics in laboratory medicine – a survey by the IFCC metabolomics working group
-
Elie Fux
, Marie Lenski
, James D. Otvos
and David Friedecký
Abstract
Objectives
Metabolomics aims for comprehensive characterization and measurement of small molecule metabolites (<1700 Da) in complex biological matrices. This study sought to assess the current understanding and usage of metabolomics in laboratory medicine globally and evaluate the perception of its promise and future implementation.
Methods
A survey was conducted by the IFCC metabolomics working group that queried 400 professionals from 79 countries. Participants provided insights into their experience levels, knowledge, and usage of metabolomics approaches, along with detailing the applications and methodologies employed.
Results
Findings revealed a varying level of experience among respondents, with varying degrees of familiarity and utilization of metabolomics techniques. Targeted approaches dominated the field, particularly liquid chromatography coupled to a triple quadrupole mass spectrometer, with untargeted methods also receiving significant usage. Applications spanned clinical research, epidemiological studies, clinical diagnostics, patient monitoring, and prognostics across various medical domains, including metabolic diseases, endocrinology, oncology, cardiometabolic risk, neurodegeneration and clinical toxicology.
Conclusions
Despite optimism for the future of clinical metabolomics, challenges such as technical complexity, standardization issues, and financial constraints remain significant hurdles. The study underscores the promising yet intricate landscape of metabolomics in clinical practice, emphasizing the need for continued efforts to overcome barriers and realize its full potential in patient care and precision medicine.
Introduction
Metabolomics is a discipline in which one or several analytical techniques based on mass spectrometry (MS) or nuclear magnetic resonance (NMR) spectroscopy are used to measure a large set of metabolites present in a biological sample. Highly conserved primary metabolites involved in energy metabolism (as fuels), maintenance of cell structure (as building blocks) and metabolic signaling are of particular interest in metabolomics due to the crucial roles they play in cellular function, maintenance, differentiation, growth and death. The high chemical diversity (volatility, polarity, etc.) of these metabolites as well as the wide concentration range spanning at least 11 orders of magnitude in biological samples [1] has led to the division of the discipline into several subcategories which include, for example, lipidomics, volatolomics, steroidomics and microbiome metabolomics. In addition to endogenous compounds, metabolomics is also a well-suited approach to measure xenobiotic compounds, which makes it an ideal technique for clinical toxicology. One of the greatest expected benefits of metabolomics is that it allows a holistic approach to patient health monitoring and thereby, a shift from the current “one size fits all” to patient-centric or individualized model of care [2, 3].
Two main methodological approaches can be distinguished:
Targeted metabolomics, involving analysis of a select set of chemically characterized and biochemically annotated metabolites implicated in a single or multiple pathways that in aggregate are associated with a particular pathology or clinical condition of interest.
Untargeted metabolomics, a data-driven and hypothesis-generating approach involving comprehensive profiling of as many metabolites as possible, primarily to identify novel biomarkers for subsequent targeted clinical use or disease-associated patterns that, after thorough replication and validation, might have (1) predictive value – for risk prediction, (2) diagnostic value – to identify the disease onset or development in its early stage and (3) prognostic value – to evaluate the rate of disease progression.
Clinical applications of metabolomics have been reported for disease biomarker discovery and monitoring [4], precision medicine [5] and the monitoring of treatment responses [6].
In 2021, the emerging technology division of the IFCC created a metabolomics working group with the following objectives:
Monitoring emerging trends in the field of metabolomics
Providing regular updates on the applications of metabolomics in clinical diagnosis and prognosis
Assessing the accessibility of, and barriers to, routine implementation of metabolomics approaches and methodologies in clinical laboratories
The IFCC working group on metabolomics designed a questionnaire to gain a comprehensive overview of the current use of metabolomics in clinical laboratories globally.
In this report, we summarize the findings of the survey (comprising 30 questions) that was conducted in October, 2023. Additionally, we provide some perspectives on the current state of metabolomics and issues impacting its broader clinical translation.
Methods
The questionnaire was conducted using the online survey tool SurveyMonkey (surveymonkey.com) from the 20th of October 2023 to the 5th of November 2023 and disseminated through the IFCC mailing list. In addition, participation in the survey was encouraged through social media.
Results
General description of participants (Q1–Q4)
A total of 400 professionals participated in the survey, representing 79 countries. By region, respondents were from Europe (182), Asia (110), Africa (47), South America (41), North America (15) and Oceania (5) (Figure 1).
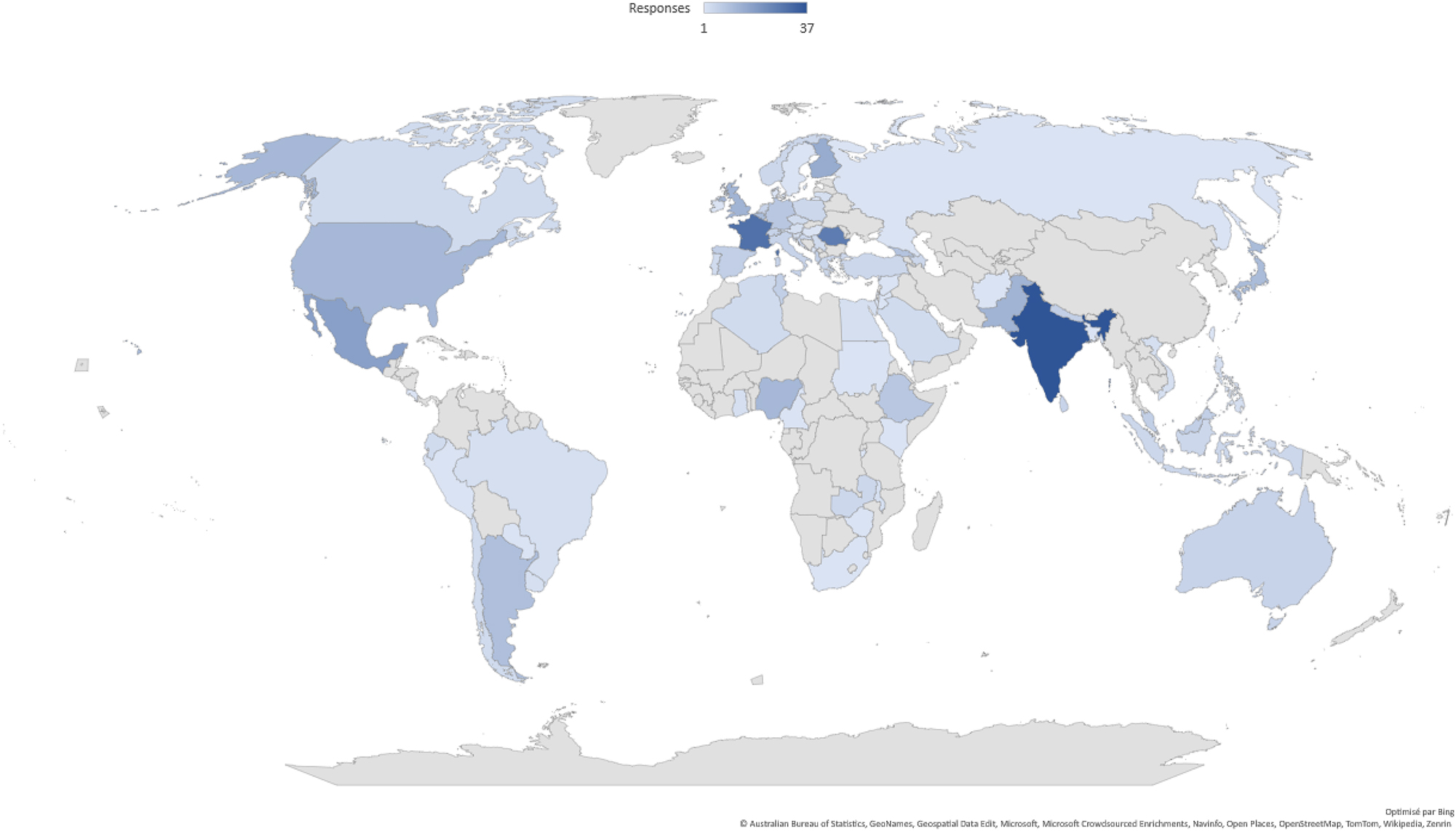
Participant demographics.
Thirty 5 % of respondents indicated they work at university hospitals, 15 % were employed by university or research institutes, and 14 % by privately owned laboratories (others: government (12 %), non-university hospital (10 %), private health care center (7 %), industry (4 %), other (4 %)).
Thirty percent were specialists in laboratory medicine, clinical biology or pathology, 29 % were lab manager/director/supervisor/coordinator, 9 % laboratory technician/medical technologist (others: 7.5 % scientist, 7 % student (graduate, PhD, resident), 6 % researcher, 2 % retired/emeritus, 1.5 % engineer, 1 % In Vitro Diagnostics industry, 8 % other).
Regarding the types of analysis performed by the respondents, 69 % indicated more than one field of activity. The breakdown was as follows: 76 % clinical biochemistry, 46 % immunology, 46 % cytology, 42 % coagulation, 34 % microbiology, 33 % molecular biology, 28 % point of care testing, 24 % inborn errors of metabolism, 21 % toxicology, 21 % therapeutic drug monitoring, 17 % transfusion medicine, 19 % other. Four percent indicated their institution does not perform any clinical analysis (Figure 2, Q2–Q4).
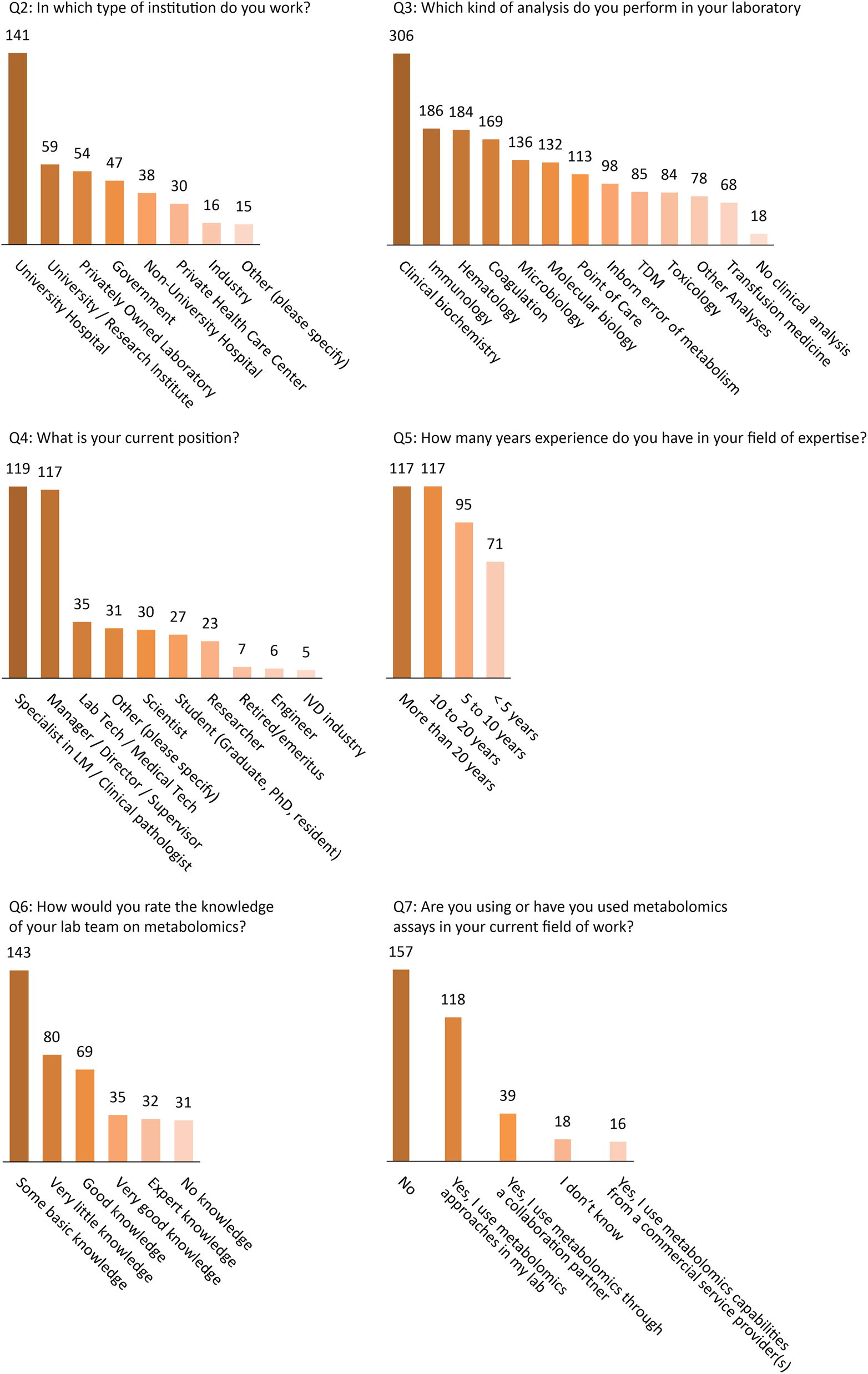
Basic characteristics of participants (Q2–Q4) and experience level of participants and their knowledge and use of metabolomics (Q5–Q7).
Participants’ experience level, knowledge and use of metabolomics (Q5–Q7)
Overall, 59 % of respondents reported having at least 10 years of experience in their fields of expertise, 24 % had 5–10 years of experience, while 18 % had less than 5 years of experience. The majority (57 %) considered themselves to have very little or only a basic knowledge of metabolomics. Twenty-seven percent reported having good or very good knowledge, while only 8 % considered themselves expert in the field. One hundred and seventy three participants (50 %) declared that they had used or were currently using metabolomics assays, either in their own labs, with collaboration partners, or from a commercial service provider (Figure 2, Q5–Q7). Of the commercial solutions, Biocrates and Chromsystems kits were the most used (11 and 9 participants, respectively).
Applications (Q8–Q17)
Among the participants who said they are or were using metabolomics (n=173), 80 % reported using it for multiple applications. Among these were research (69 %), clinical diagnostics (61 %), patient or health monitoring (42 %) and clinical prognostics (36 %). More details about subcategories of metabolomics usage within each of these four major categories is provided in the Q13–Q16 bar graphs in Figure 3.
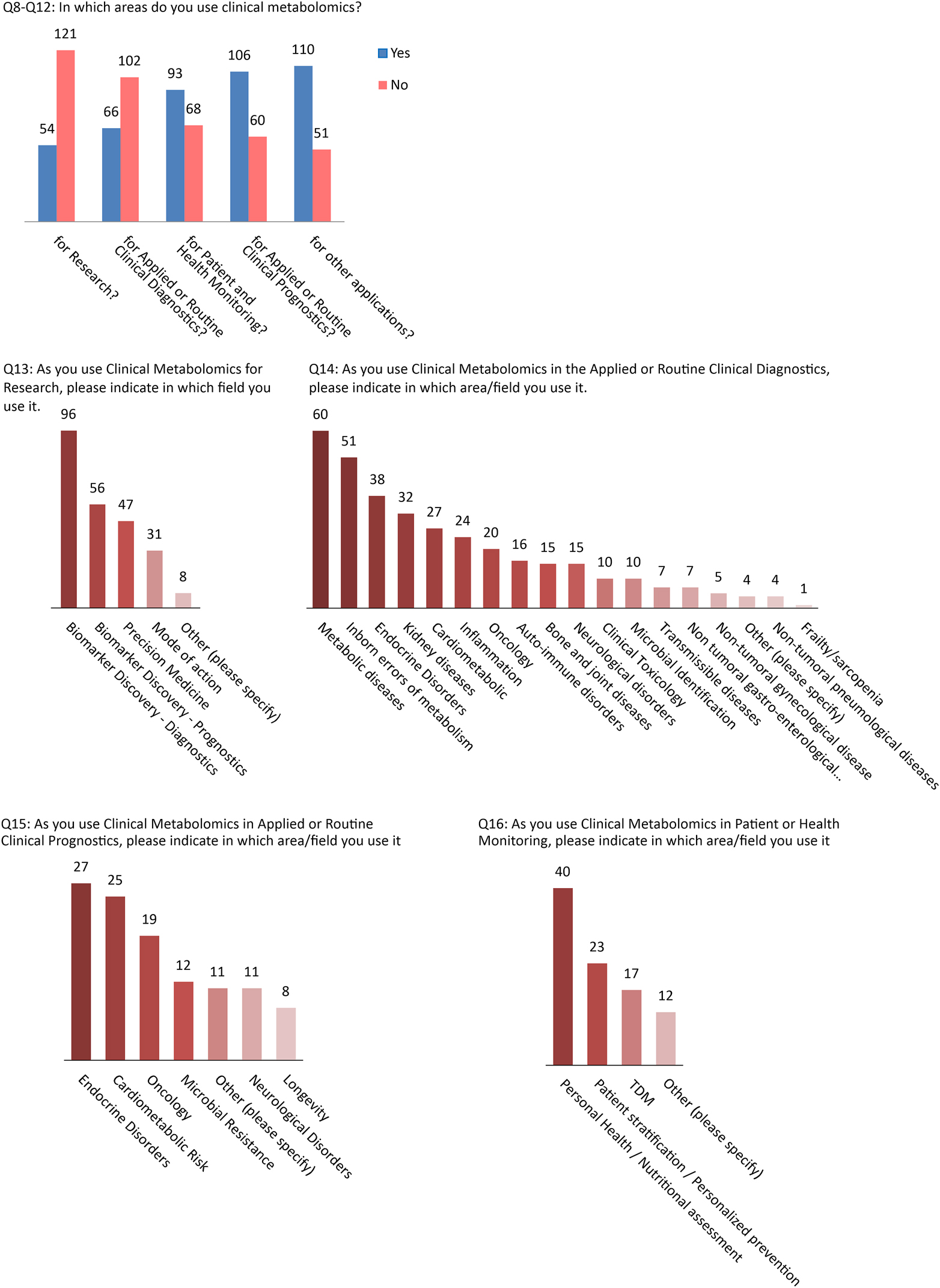
Applications of clinical metabolomics.
Targeted/untargeted approaches and other aspects of metabolomic applications (Q18–Q25)
Among respondents who said they used metabolomics (n=173), targeted and untargeted metabolomics approaches were used by 67 and 48 %, respectively (42 % used both). For those using targeted approaches, LC-MS/MS was the most frequently reported technique (83 %), whereas NMR was used much less (9 %). For untargeted applications, NMR usage was relatively greater (24 %), though still significantly lower than LC-MS/MS (64 %).
Among the 152 respondents to the question (Q23) about which types of metabolomics are employed, 51 % performed metabolite profiling using a combination of methods (for example, combination of LC selectivities or combination of techniques e.g. LC-MS + NMR), 39 % lipidomics, 29 % metabolite profiling using one generic method, 24 % steroidomics, and only 4 % volatolomics (Figure 4).
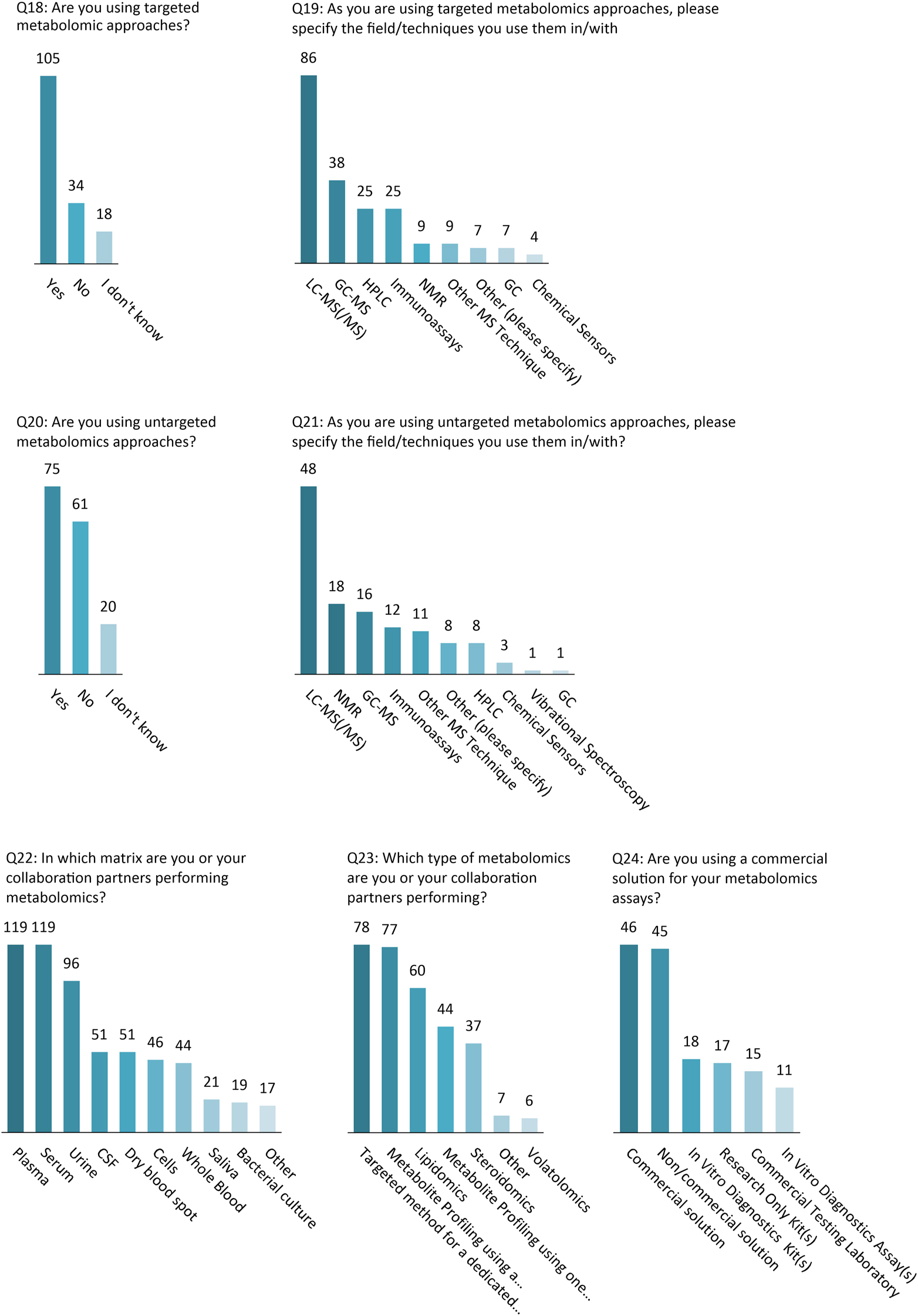
Technological approaches and methodologies used in clinical metabolomics.
Evolutions/future of metabolomics in laboratory medicine (Q26–Q30)
Almost half (49 %) of the survey participants anticipate that applications of clinical metabolomics will strongly increase for patient care, while only 2 % are expecting a decline.
The majority of the participants (60–80 %) believe that technical complexity, availability of commercial tests, the lack of assay standardization, the need for well-trained staff, the cost of the analysis as well as reimbursement should be considered as main hurdles in the implementation of metabolomics assays.
The opinions about the medical value of metabolomics-based assays were less varied, with 45 % of participants considering that metabolomics assays hold clinical utility vs. 35 % who believe there is a lack of medical value, while 20 % did not disclose their opinion (Figure 5).
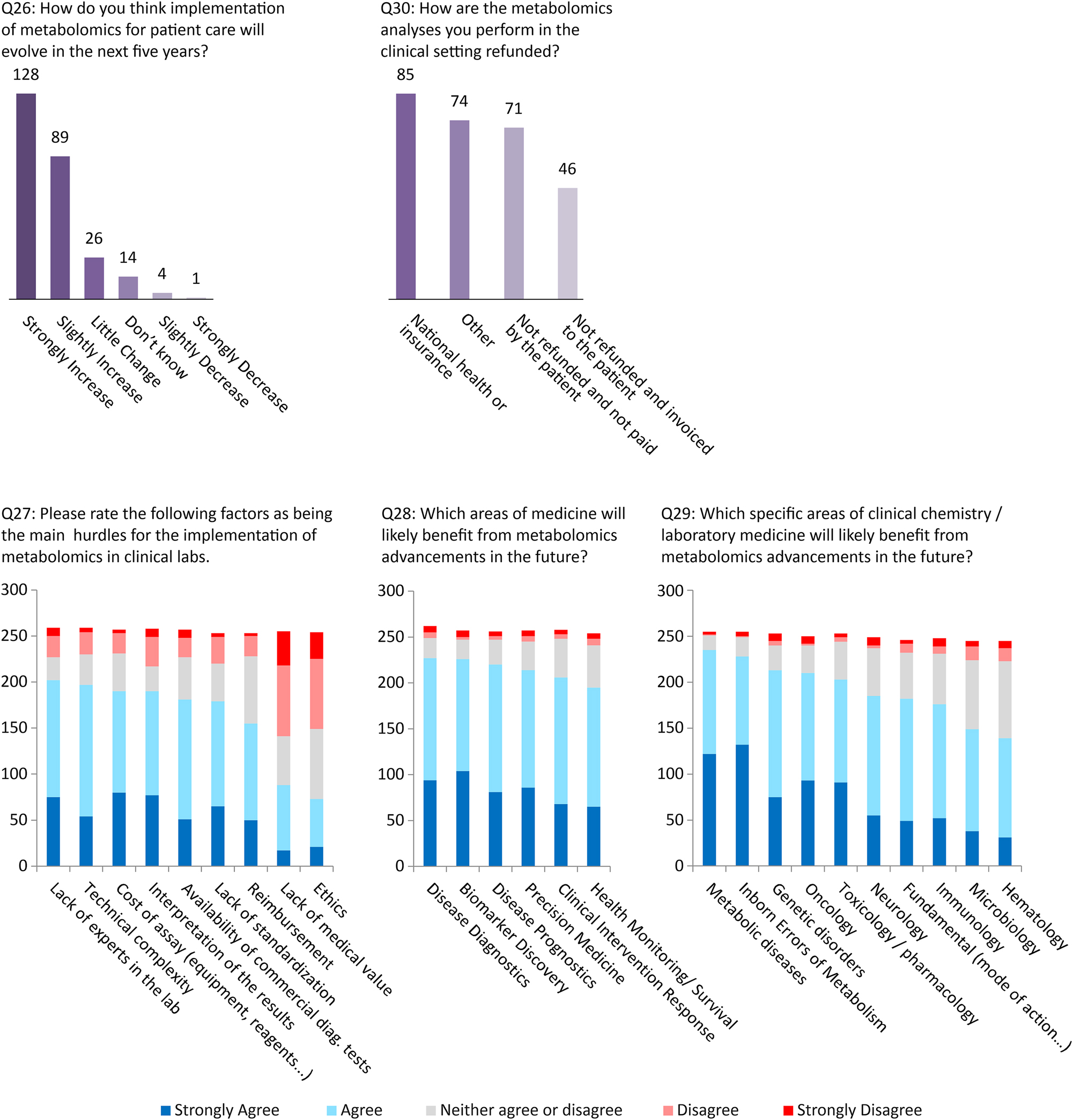
Future of clinical metabolomics (numbers next to the bars represent the number of responses).
Discussion
This survey was conducted by the IFCC metabolomics working group to evaluate the current understanding and usage of metabolomics in laboratory medicine, and the perception of the community about the promise and future implementation of this technology.
The relatively high participation rate (400 respondents) reflects the interest of the clinical community in metabolomics. The following definition of metabolomics was provided at the beginning of the survey: “Metabolomics is defined as the comprehensive characterization and simultaneous measurement of a large number of metabolites and low-molecular-weight molecules in a biological specimen. Lipidomics, volatolomics and steroidomics belong to the field of metabolomics. Users of lipidomics or other -omics of small molecules can therefore answer the questions as metabolomics users.” A fairly large percentage of our respondents (72 %) reported having at least basic knowledge of metabolomics and half of them have already used metabolomics techniques. Somewhat misleading answers were received when the participants were questioned about the extent of their experience with metabolomics. More than 25 % (n=117) reported they had more than 20 years of experience, which is an unrealistic number likely reflecting the community’s misunderstanding of the term metabolomics in general.
Approaches and methodology
There are two approaches to conduct metabolomics studies broadly defined as targeted and untargeted methods. Our survey indicates that in clinical metabolomics, targeted approaches partially dominate (n=105) vs. non-targeted approaches (n=75), while a significant number of participants are using both methods (n=59).
Targeted methods are used when specific pathways, biomarkers or classes of metabolites are of interest. The most prevalent technique used for targeted metabolomics was LC-MS (86 %), which offers significantly better sensitivity and selectivity compared to other techniques, especially when triple quadrupole MS are used in multiple reaction monitoring mode [7]. The use of GC-MS, immunoassays and HPLC techniques were also reported to a lesser extent for targeted methods.
Untargeted methods can be used for exploratory purposes to identify new biomarkers, changes in metabolic pathways or for screening the presence of xenobiotic compounds in clinical toxicology. LC-MS is also the technique that was the most commonly used for non-targeted approaches (64 %) followed by NMR (24 %) and GC-MS (21 %). Although LC-MS has the advantage of being more sensitive and of offering a larger metabolite coverage than the other techniques, it must be noted that NMR analysis has the unique benefits of being non-destructive, providing quantifiable results (also in untargeted analysis), requiring little or no separation [8]. GC-MS also has some distinct advantages over the two other techniques such as high sensitivity for volatile and semi-volatile low molecular weight compounds while providing a high chromatographic resolution, making it a powerful technique for volatolomics applications. Several participants reported the use of a combination of techniques LC-MS/GC-MS/NMR (n=7), LC-MS/NMR (n=6) and LC-MS/GC-MS (n=6) allowing them to substantially increase their metabolite coverage. The answers to the question “Which type of metabolomics are you or your collaboration partners performing?” also indicated that more than half of the respondents rely on more than one method, which includes the use of several column selectivities.
As in routine laboratory medicine, the most common matricess for metabolomics experiments reported were serum and plasma, followed by urine and whole blood, together with dry blood spots.
Applications of clinical metabolomics
The objective of the second part of the survey was to gain a more detailed understanding of the applications of metabolomics by those who reported using it in their field of work. It was not surprising given the lack of commercially offered metabolomic solutions/platforms that research was the predominant activity reported, primarily for the purpose of discovering novel diagnostic or prognostic biomarkers that could ultimately be clinically translated. However, a great number of respondents reported the use of metabolomics for applied or routine clinical testing for diagnosis or prognosis. The most prevalent areas of applications were metabolic diseases (primarily non-communicable diseases), inborn errors of metabolism, and a wide range of endocrine diseases. Current applications of metabolomics for patient monitoring were primarily for personal health/nutritional assessment and risk stratification for personalized prevention.
Applications to clinical research: from biomarker discovery to improved diagnostics, prognostics and personalized health monitoring
In our survey, 69 % of respondents apply metabolomics in the context of their research projects, consistent with their employment primarily by academic institutions, mainly university hospitals and research institutes and laboratories. The scope of the research projects in which metabolomics approaches are used ranges from fundamental biomedical research for obtaining mechanistic insights (i.e. mode of action) to applied clinical research for biomarker discovery, for improved diagnostics and prognostics of a wide panel of metabolic diseases. This is in line with the recent recognition of altered metabolism as a hallmark of multiple metabolic diseases, ranging from inborn errors of metabolism to acquired non-communicable cardiometabolic diseases (e.g. obesity, diabetes, cardiovascular diseases), to different cancer types, and neurodegenerative disorders (e.g. Alzheimer’s or Parkinson’s disease and other related cognitive impairments) [3]. Indeed, a wide range of clinical research studies, including prospective human population or epidemiological studies, and clinical intervention studies, have reported significant metabolic alterations associated with cardiometabolic risk factors such as age, sex, changes in anthropometric parameters, adiposity, diabetes or dyslipidemia markers [9, 10]. However, it remains unclear from a mechanistic standpoint whether the perturbed metabolism, particularly energy metabolism including energy utilization and storage, as well as metabolic signaling, should be seen as a cause or a consequence of disease onset, development, and progression [11], [12], [13]. Despite the lack of true mechanistic insights, there is substantial evidence that identified sets of metabolite (including lipid) markers can significantly improve the sensitivity and accuracy of disease diagnosis and prognosis (compared to traditionally applied prediction models essentially based on cholesterol and blood pressure) [14], [15], [16]. Improved diagnostics and prognostics in routine clinical practice are prerequisites for the more precise approach to patient and health monitoring that is the promise of precision medicine [2, 17], [18], [19].
Current applications in clinical research 1: metabolic diseases and endocrinology
The respondents of the survey have overwhelmingly highlighted metabolic diseases and endocrine disorders as fields where metabolomics contributes significantly to both clinical diagnostics and prognostics. Given that these conditions bear substantial consequences for public health and exert a considerable influence on overall healthcare expenditure, it logically follows that metabolomics is anticipated to play a pivotal role in their understanding and management. For instance, once the diagnosis of type 2 diabetes is established, clinicians rely on various biomarkers to predict and avoid future complications, like cardiovascular diseases and microvascular disorders including retinopathy, neuropathy, and nephropathy. Metabolomics approaches are thus expected to be major contributors to a more personalized approach to patient care [20], [21], [22], [23], [24]. In clinical practice, the simultaneous measurement of multiple steroid hormones, mainly by LC-MS/MS approaches, has already helped to provide a more holistic understanding of very complex adrenal disorders, especially in children, where symptoms might be less suggestive and sample volumes are reduced [25]. Another compelling illustration of the pivotal role metabolomics plays in endocrinology is exemplified by its thorough exploration of vitamin D metabolites. This in-depth analysis seeks to enhance our comprehension of the intricate interactions among these metabolites, elucidating the dynamic roles each one plays. This nuanced understanding not only contributes to unraveling the complexity of vitamin D metabolism but also facilitates the diagnosis of certain diseases, such as those associated with CYP24A1 mutations [26, 27].
Current applications in clinical research 2: inborn errors of metabolism
In the field of inborn errors of metabolism, targeted metabolomic approaches began to be used more than a decade ago [28]. Due to the nature of significant changes (up to 3 orders of magnitude) in biofluids, metabolomics offers an effective tool for the diagnosis of both known diseases and for the discovery of previously unrecognized ones. As one of the few areas of laboratory diagnostics in which metabolomics has already proven value, this tool has now become part of the routine diagnostic process, both at the level of targeted [29] and non-targeted [30] analysis, where it plays a growing role. A particularly important application is neonatal screening based on mass spectrometry. Because of the way these data have classically been evaluated (concentrations of individual metabolites), newborn screening can be considered as related to metabolomics, although it relies on a classical quantification of a limited number of metabolites (usually from a few to higher tens of amino acids, acylcarnitines and others; depending on national programmes).
Current applications in clinical research 3: oncology
By providing valuable new insights into the metabolic features associated with cancer risk and progression, metabolomics has emerged as a promising tool in determining the prognosis of oncological diseases. Compared to other analytical techniques such as routine molecular diagnostics, metabolomics has the advantage of providing a more holistic view of the complex and heterogeneous nature of cancer. While previous research has already correlated metabolic profiles with cancer prognosis, treatment efficacy, and early diagnosis of various cancers (e.g. gynecological and lung cancers) [31, 32], it is expected that technological advancements (e.g. LC-MS and single-cell analysis) will further facilitate the identification of specific biomarkers and provide unprecedented opportunities to investigate cancer metabolism at a cellular resolution [33]. Notwithstanding its clinical promise, the practical utility of metabolomics in cancer prognosis is still an area of active research. The next necessary step will be to thoroughly validate and translate these promising results of hundreds of research studies into routine laboratory practice.
Current applications in clinical research 4: cardiometabolic risk
Cardiometabolic disease (considered here as combined atherosclerotic cardiovascular disease [ASCVD] and diabetes mellitus [DM] linked metabolically by insulin resistance and dyslipidemia) is the leading cause of death and disability worldwide. Its prevention via personalized risk identification and tailored treatment is therefore a very high clinical priority. Given the complexity of the molecular mechanisms underlying the pathophysiology of cardiometabolic disease, metabolomic profiling is considered capable of greatly improving upon the prognostic performance of standard cholesterol and glycemia screening [34], [35], [36], [37]. Thousands of papers have been published in the last five years describing research addressing this clinical promise, but specific examples of successful clinical translation of metabolomic assays that demonstrably improve the standard of care are rare. One reason is that, so far, biomarkers of postulated novel mechanistic pathways independent of those addressed by conventional risk factors have not been found. In one example, a comprehensive study using untargeted NMR metabolic profiling identified multiple NMR features associated with subclinical atherosclerosis and future ASCVD events, but these largely overlapped with known risk factors [38]. There is, however, good reason to be optimistic that metabolomics will ultimately contribute meaningfully to clinical cardiometabolic risk assessment and management. Notably, many respondents in our survey reported they are already using metabolomics for either cardiometabolic diagnostic (n=27) or prognostic (n=25) purposes.
To illustrate a possible approach for translating omics-based cohort studies into assays for CVD risk stratification, one could look to the success story of ceramides. Ceramides are circulating bioactive lipid metabolites that were shown to be associated with increased CVD risk. Over the span of about a decade, initial discovery studies deploying a range of lipidomics approaches were translated into an assay comprising four [39] to six [14] ceramide molecular species whose concentrations in human blood correlate strongly with risk of death from CVD [27]. This panel of ceramide molecular species has been licensed by leading diagnostic providers [40]. However, the additional benefit of using a ceramide-based risk management over traditional ones remains to be demonstrated [41].
Current applications in clinical research 5: clinical toxicology
Clinical and forensic toxicology usually relies on the direct detection and/or quantification of xenobiotic substances (including medications, drugs, drug of abuse, metals, environmental toxins, and other chemical agents). However, as for the endogenous metabolites, this approach can be an analytical challenge, due to their various physicochemical properties, their low circulating concentrations, or their short detection window in usual matrices through metabolism and/or rapid elimination. The metabolome changes in response to external stimuli, making metabolomics a powerful complementary tool to those currently used in toxicology to confirm an exposure and/or an effect and/or a toxicity due to xenobiotics [42]. In the case of toxicological analysis of live patients, this is clinically relevant to improve the diagnostic sensitivity and detection window indicating drug consumption or intoxication due to acute drug intake (e.g. new psychoactive substances [43]) or drug-facilitated crime context (e.g. GHB [44]). In addictology, metabolomics is a potential alternative approach for (1) diagnosing recent consumption (2) monitoring consumption, withdrawal or relapse, and thus predicting the effectiveness of treatment [45]. In addition to the identification of biomarkers, other metabolomics studies were designed to evaluate acute and chronic toxicity mechanisms. In the post-mortem period, the metabolome undergoes time- and location-dependent modifications through degradation, accumulation or redistribution of metabolites, depending on their physico-chemical characteristics. Post-mortem metabolomics have been suggested as a potential tool for discovering new biomarkers that may assist in death investigations, and notably to improve interpretation of the causes of death or for estimating the post-mortem interval (time elapsed since death) [46, 47]. Large-scale post-mortem metabolomics has proven its ability to discriminate between different groups based on their metabolic fingerprints. In preliminary research, metabolomics was successfully applied to post-mortem samples of selected patients for screening purposes. However, this is still awaiting application in routine diagnostic practice [48].
Future of clinical metabolomics – the view of the community
The final part of the survey was devoted to the anticipated future prospects for clinical metabolomics and lipidomics by the laboratory medicine community. Besides the expected increase of the number of clinical metabolomics assays within the next five years, benefits are expected in all areas of laboratory medicine: diagnostics, prognostics, biomarker discovery, precision medicine, monitoring and clinical intervention. In particular, major benefits from metabolomics are foreseen in metabolic diseases, inborn errors of metabolism, oncology toxicology/pharmacology and neurodegenerative diseases.
However, despite such optimism, it is necessary to acknowledge and address a number of difficulties that lie ahead. Technical complexity, lack of standardization of methods and evaluation, lack of expert availability and the limited commercial solutions were reported as the main limitations for the future development of clinical metabolomics. In particular, laboratories face the major challenge of financing the purchase of devices and securing reimbursement of the assays by insurance companies or healthcare payers.
Limitation of the survey
This survey-based review aimed at the global evaluation of the metabolomics applications in the clinical environment and their potential for translation to clinical routine, with the emphasis on main hurdles and expectations (i.e. clinical utility). Thereby, the assessment of best practices, including the quality assurance and quality control strategies was out of the scope of this report. This important aspect of metabolomics workflow has been previously addressed in separate reviews and the authors encourage readers that are new in the field to familiarize themselves with these concepts (e.g. [49], [50], [51]). In the same line, the lipidomics applications could be evaluated separately, due to obvious emerging potential.
Conclusions
Overall, the authors were positively surprised with the general interest this survey generated as well as with the high numbers of participants who are already involved in metabolomics activities. Although a definition for metabolomics was provided at the beginning of the survey (see above), the frontier at which metabolomics begins and classical clinical chemistry panels ends is not defined. This can explain the high number of participants reporting the use of metabolomics as routine assays. Nevertheless, we are witnessing a number of advanced technologies (mostly based on mass spectrometry) capable of acquiring metabolomics data being established in clinical laboratories. So far, only a limited number of groups have managed to successfully transition from research use to in vitro diagnostics (IVD) assays. A decisive aspect for the successful implementation of these types of assays, based on the determination of multiple parameters, will most certainly lie in the ease of interpretation of the results for clinical decision making.
Acknowledgments
We would like to thank Mrs Silvia Colli Lanzi (IFCC Office) for her support in conducting the survey.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Elie Fux is an employee of Roche Diagnostics GmbH. All other authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Wishart, DS. Advances in metabolite identification. Bioanalysis 2011;3:1769–82. https://doi.org/10.4155/bio.11.155.Search in Google Scholar PubMed
2. Wishart, DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016;15:473–84. https://doi.org/10.1038/nrd.2016.32.Search in Google Scholar PubMed
3. Wishart, DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev 2019;99:1819–75. https://doi.org/10.1152/physrev.00035.2018.Search in Google Scholar PubMed
4. Qiu, S, Cai, Y, Yao, H, Lin, C, Xie, Y, Tang, S, et al.. Small molecule metabolites: discovery of biomarkers and therapeutic targets. Signal Transduct Targeted Ther 2023;8:132. https://doi.org/10.1038/s41392-023-01399-3.Search in Google Scholar PubMed PubMed Central
5. Everett, JR. Pharmacometabonomics in humans: a new tool for personalized medicine. Pharmacogenomics 2015;16:737–54. https://doi.org/10.2217/pgs.15.20.Search in Google Scholar PubMed
6. Dastmalchi, F, Deleyrolle, LP, Karachi, A, Mitchell, DA, Rahman, M. Metabolomics monitoring of treatment response to brain tumor immunotherapy. Front Oncol 2021;11:691246. https://doi.org/10.3389/fonc.2021.691246.Search in Google Scholar PubMed PubMed Central
7. Li, C, Chu, S, Tan, S, Yin, X, Jiang, Y, Dai, X, et al.. Towards higher sensitivity of mass spectrometry: a perspective from the mass analyzers. Front Chem 2021;9:813359. https://doi.org/10.3389/fchem.2021.813359.Search in Google Scholar PubMed PubMed Central
8. Wishart, DS. Quantitative metabolomics using NMR. Trends Anal Chem 2008;27:228–37. https://doi.org/10.1016/j.trac.2007.12.001.Search in Google Scholar
9. Huynh, K, Barlow, CK, Jayawardana, KS, Weir, JM, Mellett, NA, Cinel, M, et al.. High-throughput plasma lipidomics: detailed mapping of the associations with cardiometabolic risk factors. Cell Chem Biol 2019;26:71–84.e4. https://doi.org/10.1016/j.chembiol.2018.10.008.Search in Google Scholar PubMed
10. Mundra, PA, Barlow, CK, Nestel, PJ, Barnes, EH, Kirby, A, Thompson, P, et al.. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018;3:1–15. https://doi.org/10.1172/jci.insight.121326.Search in Google Scholar PubMed PubMed Central
11. Rinschen, MM, Ivanisevic, J, Giera, M, Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol 2019;20:353–67. https://doi.org/10.1038/s41580-019-0108-4.Search in Google Scholar PubMed PubMed Central
12. Baker, SA, Rutter, J. Metabolites as signalling molecules. Nat Rev Mol Cell Biol 2023;24:355–74. https://doi.org/10.1038/s41580-022-00572-w.Search in Google Scholar PubMed
13. Tippetts, TS, Holland, WL, Summers, SA. Cholesterol – the devil you know; ceramide – the devil you don’t. Trends Pharmacol Sci 2021;42:1082–95. https://doi.org/10.1016/j.tips.2021.10.001.Search in Google Scholar PubMed PubMed Central
14. Hilvo, M, Meikle, PJ, Pedersen, ER, Tell, GS, Dhar, I, Brenner, H, et al.. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020;41:371–80. https://doi.org/10.1093/eurheartj/ehz387.Search in Google Scholar PubMed
15. Leiherer, A, Mündlein, A, Laaksonen, R, Lääperi, M, Jylhä, A, Fraunberger, P, et al.. Comparison of recent ceramide-based coronary risk prediction scores in cardiovascular disease patients. Eur J Prev Cardiol 2022;29:947–56. https://doi.org/10.1093/eurjpc/zwab112.Search in Google Scholar PubMed
16. Summers, SA. Could ceramides become the new cholesterol? Cell Metabol 2018;27:276–80. https://doi.org/10.1016/j.cmet.2017.12.003.Search in Google Scholar PubMed
17. Després, JP. Predicting longevity using metabolomics: a novel tool for precision lifestyle medicine? Nat Rev Cardiol 2020;11:67–8. https://doi.org/10.1038/s41569-019-0310-2.Search in Google Scholar PubMed
18. Clish, CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud 2015;1:a000588. https://doi.org/10.1101/mcs.a000588.Search in Google Scholar PubMed PubMed Central
19. Beger, RD, Dunn, W, Schmidt, MA, Gross, SS, Kirwan, JA, Cascante, M, et al.. Metabolomics enables precision medicine: “a white paper, community perspective”. Metabolomics 2016;12:149. https://doi.org/10.1007/s11306-016-1094-6.Search in Google Scholar PubMed PubMed Central
20. Du, X, Yang, L, Kong, L, Sun, Y, Shen, K, Cai, Y, et al.. Metabolomics of various samples advancing biomarker discovery and pathogenesis elucidation for diabetic retinopathy. Front Endocrinol 2022;13:1037164. https://doi.org/10.3389/fendo.2022.1037164.Search in Google Scholar PubMed PubMed Central
21. Karagiannidis, E, Moysidis, DV, Papazoglou, AS, Panteris, E, Deda, O, Stalikas, N, et al.. Prognostic significance of metabolomic biomarkers in patients with diabetes mellitus and coronary artery disease. Cardiovasc Diabetol 2022;21:70. https://doi.org/10.1186/s12933-022-01494-9.Search in Google Scholar PubMed PubMed Central
22. Filla, LA, Edwards, JL. Metabolomics in diabetic complications. Mol Biosyst 2016;12:1090–105. https://doi.org/10.1039/c6mb00014b.Search in Google Scholar PubMed PubMed Central
23. Chowdhury, S, Faheem, SM, Nawaz, SS, Siddiqui, K. The role of metabolomics in personalized medicine for diabetes. Per Med 2021;18:501–8. https://doi.org/10.2217/pme-2021-0083.Search in Google Scholar PubMed
24. Zhang, J, Fuhrer, T, Ye, H, Kwan, B, Montemayor, D, Tumova, J, et al.. High-throughput metabolomics and diabetic kidney disease progression: evidence from the chronic renal insufficiency (CRIC) study. Am J Nephrol 2022;53:215–25. https://doi.org/10.1159/000521940.Search in Google Scholar PubMed PubMed Central
25. Schiffer, L, Barnard, L, Baranowski, ES, Gilligan, LC, Taylor, AE, Arlt, W, et al.. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol 2019;194:105439. https://doi.org/10.1016/j.jsbmb.2019.105439.Search in Google Scholar PubMed PubMed Central
26. Burt, LA, Kaufmann, M, Rose, MS, Jones, G, Billington, EO, Boyd, SK, et al.. Measurements of the vitamin D metabolome in the calgary vitamin D study: relationship of vitamin D metabolites to bone loss. J Bone Miner Res 2023;38:1312–21. https://doi.org/10.1002/jbmr.4876.Search in Google Scholar PubMed
27. Jones, G, Kaufmann, M. Diagnostic aspects of vitamin D: clinical utility of vitamin D metabolite profiling. JBMR Plus 2021;5:e10581. https://doi.org/10.1002/jbm4.10581.Search in Google Scholar PubMed PubMed Central
28. Janečková, H, Hron, K, Wojtowicz, P, Hlídková, E, Barešová, A, Friedecký, D, et al.. Targeted metabolomic analysis of plasma samples for the diagnosis of inherited metabolic disorders. J Chromatogr A 2012;1226:11–7. https://doi.org/10.1016/j.chroma.2011.09.074.Search in Google Scholar PubMed
29. Piskláková, B, Friedecká, J, Ivanovová, E, Hlídková, E, Bekárek, V, Prídavok, M, et al.. Rapid and efficient LC-MS/MS diagnosis of inherited metabolic disorders: a semi-automated workflow for analysis of organic acids, acylglycines, and acylcarnitines in urine. Clin Chem Lab Med 2023;61:2017–27. https://doi.org/10.1515/cclm-2023-0084.Search in Google Scholar PubMed
30. Ismail, IT, Showalter, MR, Fiehn, O. Inborn errors of metabolism in the era of untargeted metabolomics and lipidomics. Metabolites 2019;9:1–26. https://doi.org/10.3390/metabo9100242.Search in Google Scholar PubMed PubMed Central
31. Armitage, EG, Southam, AD. Monitoring cancer prognosis, diagnosis and treatment efficacy using metabolomics and lipidomics. Metabolomics 2016;12:146. https://doi.org/10.1007/s11306-016-1093-7.Search in Google Scholar PubMed PubMed Central
32. Qi, SA, Wu, Q, Chen, Z, Zhang, W, Zhou, Y, Mao, K, et al.. High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci Rep 2021;11:11805. https://doi.org/10.1038/s41598-021-91276-2.Search in Google Scholar PubMed PubMed Central
33. Danzi, F, Pacchiana, R, Mafficini, A, Scupoli, MT, Scarpa, A, Donadelli, M, et al.. To metabolomics and beyond: a technological portfolio to investigate cancer metabolism. Signal Transduct Targeted Ther 2023;8:137. https://doi.org/10.1038/s41392-023-01380-0.Search in Google Scholar PubMed PubMed Central
34. Newgard, CB. Metabolomics and metabolic diseases: where do we stand? Cell Metabol 2017;25:43–56. https://doi.org/10.1016/j.cmet.2016.09.018.Search in Google Scholar PubMed PubMed Central
35. Cheng, S, Shah, SH, Corwin, EJ, Fiehn, O, Fitzgerald, RL, Gerszten, RE, et al.. Potential impact and study considerations of metabolomics in cardiovascular health and disease: a scientific statement from the American heart association. Circ Cardiovasc Genet 2017;10:e1-13. https://doi.org/10.1161/HCG.0000000000000032.Search in Google Scholar PubMed PubMed Central
36. Iida, M, Harada, S, Takebayashi, T. Application of metabolomics to epidemiological studies of atherosclerosis and cardiovascular disease. J Atherosclerosis Thromb 2019;26:747–57. https://doi.org/10.5551/jat.rv17036.Search in Google Scholar PubMed PubMed Central
37. Nayor, M, Brown, KJ, Vasan, RS. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ Res 2021;128:287–303. https://doi.org/10.1161/circresaha.120.315890.Search in Google Scholar PubMed PubMed Central
38. Tzoulaki, I, Castagné, R, Boulangé, CL, Karaman, I, Chekmeneva, E, Evangelou, E, et al.. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J 2019;40:2883–96. https://doi.org/10.1093/eurheartj/ehz235.Search in Google Scholar PubMed PubMed Central
39. Laaksonen, R, Ekroos, K, Sysi-Aho, M, Hilvo, M, Vihervaara, T, Kauhanen, D, et al.. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–76. https://doi.org/10.1093/eurheartj/ehw148.Search in Google Scholar PubMed PubMed Central
40. Ramos, P, Meeusen, JW. Ceramide risk scores can bring lipidomics to clinical medicine. Clin Chem 2022;68:1479–80. https://doi.org/10.1093/clinchem/hvac169.Search in Google Scholar PubMed
41. Vogeser, M, Bendt, AK. From research cohorts to the patient – a role for “omics” in diagnostics and laboratory medicine? Clin Chem Lab Med 2023;61:974–80. https://doi.org/10.1515/cclm-2022-1147.Search in Google Scholar PubMed
42. Szeremeta, M, Pietrowska, K, Niemcunowicz-Janica, A, Kretowski, A, Ciborowski, M. Applications of metabolomics in forensic toxicology and forensic medicine. Int J Mol Sci 2021;22:1–16. https://doi.org/10.3390/ijms22063010.Search in Google Scholar PubMed PubMed Central
43. Olesti, E, De Toma, I, Ramaekers, JG, Brunt, TM, Carbó, ML, Fernández-Avilés, C, et al.. Metabolomics predicts the pharmacological profile of new psychoactive substances. J Psychopharmacol 2019;33:347–54. https://doi.org/10.1177/0269881118812103.Search in Google Scholar PubMed
44. Steuer, AE, Raeber, J, Simbuerger, F, Dornbierer, DA, Bosch, OG, Quednow, BB, et al.. Towards extending the detection window of gamma-hydroxybutyric acid-an untargeted metabolomics study in serum and urine following controlled administration in healthy men. Metabolites 2021;11:1–16. https://doi.org/10.3390/metabo11030166.Search in Google Scholar PubMed PubMed Central
45. Steuer, AE, Brockbals, L, Kraemer, T. Metabolomic strategies in biomarker research-new approach for indirect identification of drug consumption and sample manipulation in clinical and forensic toxicology? Front Chem 2019;7:319. https://doi.org/10.3389/fchem.2019.00319.Search in Google Scholar PubMed PubMed Central
46. Castillo-Peinado, LS, Luque de Castro, MD. Present and foreseeable future of metabolomics in forensic analysis. Anal Chim Acta 2016;925:1–15. https://doi.org/10.1016/j.aca.2016.04.040.Search in Google Scholar PubMed
47. Locci, E, Bazzano, G, Chighine, A, Locco, F, Ferraro, E, Demontis, R, et al.. Forensic NMR metabolomics: one more arrow in the quiver. Metabolomics 2020;16:118. https://doi.org/10.1007/s11306-020-01743-6.Search in Google Scholar PubMed PubMed Central
48. Chighine, A, Porcu, M, Ferino, G, Lenigno, N, Trignano, C, d’Aloja, E, et al.. Infant urinary metabolomic profile in a fatal acute methadone intoxication. Int J Leg Med 2022;136:569–75. https://doi.org/10.1007/s00414-021-02772-z.Search in Google Scholar PubMed PubMed Central
49. Mosley, JD, Schock, TB, Beecher, CW, Dunn, WB, Kuligowski, J, Lewis, MR, et al.. Establishing a framework for best practices for quality assurance and quality control in untargeted metabolomics. Metabolomics 2024;20:1–13. https://doi.org/10.1007/s11306-023-02080-0.Search in Google Scholar PubMed PubMed Central
50. Broeckling, CD, Beger, RD, Cheng, LL, Cumeras, R, Cuthbertson, DJ, Dasari, S, et al.. Current practices in LC-MS untargeted metabolomics: a scoping review on the use of pooled quality control samples. Anal Chem 2023;95:18645–54. https://doi.org/10.1021/acs.analchem.3c02924.Search in Google Scholar PubMed PubMed Central
51. Lippa, KA, Aristizabal-Henao, JJ, Beger, RD, Bowden, JA, Broeckling, C, Beecher, C, et al.. Reference materials for MS-based untargeted metabolomics and lipidomics: a review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 2022;18:1–29. https://doi.org/10.1007/s11306-021-01848-6.Search in Google Scholar PubMed PubMed Central
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Six years of progress – highlights from the IFCC Emerging Technologies Division
- IFCC Papers
- Skin in the game: a review of single-cell and spatial transcriptomics in dermatological research
- Bilirubin measurements in neonates: uniform neonatal treatment can only be achieved by improved standardization
- Validation and verification framework and data integration of biosensors and in vitro diagnostic devices: a position statement of the IFCC Committee on Mobile Health and Bioengineering in Laboratory Medicine (C-MBHLM) and the IFCC Scientific Division
- Linearity assessment: deviation from linearity and residual of linear regression approaches
- HTA model for laboratory medicine technologies: overview of approaches adopted in some international agencies
- Considerations for applying emerging technologies in paediatric laboratory medicine
- A global perspective on the status of clinical metabolomics in laboratory medicine – a survey by the IFCC metabolomics working group
- The LEAP checklist for laboratory evaluation and analytical performance characteristics reporting of clinical measurement procedures
- General Clinical Chemistry and Laboratory Medicine
- Assessing post-analytical phase harmonization in European laboratories: a survey promoted by the EFLM Working Group on Harmonization
- Potential medical impact of unrecognized in vitro hypokalemia due to hemolysis: a case series
- Quantification of circulating alpha-1-antitrypsin polymers associated with different SERPINA1 genotypes
- Targeted ultra performance liquid chromatography tandem mass spectrometry procedures for the diagnosis of inborn errors of metabolism: validation through ERNDIM external quality assessment schemes
- Improving protocols for α-synuclein seed amplification assays: analysis of preanalytical and analytical variables and identification of candidate parameters for seed quantification
- Evaluation of analytical performance of AQUIOS CL flow cytometer and method comparison with bead-based flow cytometry methods
- IgG and kappa free light chain CSF/serum indices: evaluating intrathecal immunoglobulin production in HIV infection in comparison with multiple sclerosis
- Reference Values and Biological Variations
- Reference intervals of circulating secretoneurin concentrations determined in a large cohort of community dwellers: the HUNT study
- Sharing reference intervals and monitoring patients across laboratories – findings from a likely commutable external quality assurance program
- Verification of bile acid determination method and establishing reference intervals for biochemical and haematological parameters in third-trimester pregnant women
- Confounding factors of the expression of mTBI biomarkers, S100B, GFAP and UCH-L1 in an aging population
- Cancer Diagnostics
- Exploring evolutionary trajectories in ovarian cancer patients by longitudinal analysis of ctDNA
- Diabetes
- Evaluation of effects from hemoglobin variants on HbA1c measurements by different methods
- Letters to the Editor
- Are there any reasons to use three levels of quality control materials instead of two and if so, what are the arguments?
- Issues for standardization of neonatal bilirubinemia: a case of delayed phototherapy initiation
- The routine coagulation assays plasma stability – in the wake of the new European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) biological variability database
- Improving HCV diagnosis following a false-negative anti-HCV result
Articles in the same Issue
- Frontmatter
- Editorial
- Six years of progress – highlights from the IFCC Emerging Technologies Division
- IFCC Papers
- Skin in the game: a review of single-cell and spatial transcriptomics in dermatological research
- Bilirubin measurements in neonates: uniform neonatal treatment can only be achieved by improved standardization
- Validation and verification framework and data integration of biosensors and in vitro diagnostic devices: a position statement of the IFCC Committee on Mobile Health and Bioengineering in Laboratory Medicine (C-MBHLM) and the IFCC Scientific Division
- Linearity assessment: deviation from linearity and residual of linear regression approaches
- HTA model for laboratory medicine technologies: overview of approaches adopted in some international agencies
- Considerations for applying emerging technologies in paediatric laboratory medicine
- A global perspective on the status of clinical metabolomics in laboratory medicine – a survey by the IFCC metabolomics working group
- The LEAP checklist for laboratory evaluation and analytical performance characteristics reporting of clinical measurement procedures
- General Clinical Chemistry and Laboratory Medicine
- Assessing post-analytical phase harmonization in European laboratories: a survey promoted by the EFLM Working Group on Harmonization
- Potential medical impact of unrecognized in vitro hypokalemia due to hemolysis: a case series
- Quantification of circulating alpha-1-antitrypsin polymers associated with different SERPINA1 genotypes
- Targeted ultra performance liquid chromatography tandem mass spectrometry procedures for the diagnosis of inborn errors of metabolism: validation through ERNDIM external quality assessment schemes
- Improving protocols for α-synuclein seed amplification assays: analysis of preanalytical and analytical variables and identification of candidate parameters for seed quantification
- Evaluation of analytical performance of AQUIOS CL flow cytometer and method comparison with bead-based flow cytometry methods
- IgG and kappa free light chain CSF/serum indices: evaluating intrathecal immunoglobulin production in HIV infection in comparison with multiple sclerosis
- Reference Values and Biological Variations
- Reference intervals of circulating secretoneurin concentrations determined in a large cohort of community dwellers: the HUNT study
- Sharing reference intervals and monitoring patients across laboratories – findings from a likely commutable external quality assurance program
- Verification of bile acid determination method and establishing reference intervals for biochemical and haematological parameters in third-trimester pregnant women
- Confounding factors of the expression of mTBI biomarkers, S100B, GFAP and UCH-L1 in an aging population
- Cancer Diagnostics
- Exploring evolutionary trajectories in ovarian cancer patients by longitudinal analysis of ctDNA
- Diabetes
- Evaluation of effects from hemoglobin variants on HbA1c measurements by different methods
- Letters to the Editor
- Are there any reasons to use three levels of quality control materials instead of two and if so, what are the arguments?
- Issues for standardization of neonatal bilirubinemia: a case of delayed phototherapy initiation
- The routine coagulation assays plasma stability – in the wake of the new European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) biological variability database
- Improving HCV diagnosis following a false-negative anti-HCV result

