Prospective urinary albumin/creatinine ratio for diagnosis, staging, and organ response assessment in renal AL amyloidosis: results from a large cohort of patients
-
Marco Basset
Abstract
Objectives
Quantification of 24 h-proteinuria is the gold standard for diagnosing, staging, and monitoring of patients with renal AL amyloidosis. However, 24 h-urine collection is cumbersome and may result in preanalytical error. In this prospective study, we investigated the role of urinary albumin/creatinine ratio (UACR) (cut-off: 300 mg/g) identifying renal involvement, evaluated a UACR-based staging system (UACR cut-off: 3,600 mg/g) and assessed whether UACR response (UACR decrease >30% without worsening in eGFR >25%) predicts renal outcome in 531 patients with newly-diagnosed AL amyloidosis.
Methods
From October 2013 paired 24 h-proteinuria and UACR (on first morning void) were measured in all newly-diagnosed patients with AL amyloidosis. Correlation between 24 h-proteinuria and UACR at baseline was assessed by Pearson’s r test. Impact of UACR response on renal outcome was assessed in randomly created testing (n=354) and validation (n=177) cohorts.
Results
A strong linear correlation was found between 24 h-proteinuria and UACR at baseline (r=0.90; p<0.001). After a median follow-up of 31 months, 57 (11%) patients required dialysis. A UACR-based renal staging system identified three stages with significantly higher dialysis rate at 36 months comparing stage I with stage II and stage II with stage III. Achieving a renal response, according to a UACR-based criterion, resulted in lower dialysis rate in both testing and validation cohorts.
Conclusions
UACR is a reliable marker for diagnosis, prognosis, and organ response assessment in renal AL amyloidosis and can reliably replace 24 h-proteinuria in clinical trials and individual patients’ management.
Introduction
Immunoglobulin light chain (AL) amyloidosis, is caused by a misfolded monoclonal free light chain (FLC), that deposits as amyloid fibrils causing dysfunction and damage in target organs [1]. Serum and urinary biomarkers are the cornerstone for patient management and the clinical laboratory has a key role in the diagnosis, prognostic evaluation, and response assessment in this disease [2].
Renal involvement is frequent in AL amyloidosis, being present in approximately two thirds of patients [1] and although it does not have a major impact on patients’ survival, it can result in end-stage renal failure, hampering quality of life and limiting access to treatment and interfering with the interpretation of key clinical chemistry tests [3, 4]. 24 h-proteinuria is currently required for diagnosis, staging, and response assessment in AL amyloidosis [5]. However, 24 h-urine collection is a cumbersome procedure that can cause discomfort to patients and may introduce a preanalytical error [6, 7]. Moreover, urine total protein quantification presents analytical limitations compared to albumin measurement [8]. Urinary albumin/creatinine ratio (UACR) represents a valid alternative to estimate urine protein loss in various chronic kidney diseases [9], [10], [11], [12]. In the last years, the use of this biomarker in AL amyloidosis was largely discussed and recently the Mayo Clinic group proposed UACR cut-offs for identification of renal involvement, prognostication of renal survival at diagnosis and definition of renal response [13]. However, in this large study (575 patients included) UACR and 24 h-proteinuria samples were collected on the same day only in a half of cases and paired samples at diagnosis were available only in 155 patients (of whom 109 with renal involvement). In the Mayo Clinic study, UACR sample was collected on a random spot urine. More importantly, their study did not include a validation set and UACR-based response criteria were not evaluated on renal outcome. Here we present a large prospective study on 531 patients with newly-diagnosed AL amyloidosis and paired 24 h-proteinuria and UACR (first morning void) samples at baseline and at response assessment in order to define and validate the possibility to replace the 24 h-urine collection with a simpler test in the diagnosis, staging, and response assessment of renal AL amyloidosis.
Materials and methods
From October 2013 we started evaluating systematically both 24 h-proteinuria and UACR in all patients with newly diagnosed AL amyloidosis evaluated at the Amyloidosis Research and Treatment Center of Pavia. Patients were asked to bring at first evaluation and at each follow-up visit the 24 h-urine collection as well as the first urine morning void. All subjects received oral and written instructions on appropriate 24 h collection. Data were prospectively collected in 531 patients until December 2018. All subjects gave written informed consent for their clinical data to be used in accordance with the Declaration of Helsinki.
AL amyloidosis was diagnosed after histological identification and typing of amyloidogenic light chains in tissue biopsies by immunoelectron microscopy or mass spectrometry [14], [15], [16], and organ involvement and severity of organ dysfunction were assessed per current criteria and according current validated staging systems [5, 17], [18], [19]. Renal involvement was defined by the presence of 24 h-albuminuria >0.5 g/24 h, and severity of renal involvement was assessed with the current validated staging system based on 24 h-proteinuria (cutoff: 5 g/24 h) and eGFR (cut-off: 50 mL/min) [5, 17].
We tested in the overall population the UACR cut-offs identified by the Mayo Clinic investigators for identification of renal involvement (300 mg/g, corresponding to the 24 h-proteinuria cut-off of 0.5 g/24 h), renal staging (3,600 mg/g, corresponding to the 24 h-proteinuria cut-off of 5 g/24 h) and renal response (decrease in UACR from baseline >30%, corresponding to a decrease in 24 h-proteinuria >30%) [13]. The study population was further divided in a testing (354 patients) and in an internal validation cohort (177 patients) to explore and validate the benefit of the proposed UACR-based renal response criterion – i.e. reduction in UACR from baseline >30% in absence of a reduction in eGFR >25% at 6 months – on renal survival. Patients were randomly assigned to one of these cohorts with a rate of 2:1. Only patients evaluable for UACR-based renal response – defined as a baseline UACR >300 mg/g – were included in this analysis.
Fisher’s exact test and Mann–Whitney test were used to assess differences in nominal and continuous variables between testing and validation cohort. Median and interquartile range (IQR) were reported for continuous variables. Correlation between 24 h-proteinuria and UACR at baseline was assessed by Pearson’s r test. Renal survival was defined as time from diagnosis of AL amyloidosis to dialysis or death/last contact. Renal survival curves were plotted according to Kaplan Meier, and differences in survival were tested for significance with the log-rank test. In order to make the renal survival analysis comparable with other published analysis – including the Mayo Clinic paper and the manuscript validating the currently used renal staging system and renal response criteria [5, 13] – patients who died before progression to dialysis were censored at time of death. In parallel, the cumulative incidence of dialysis was estimated with Kalbfleisch & Prentice method in a competing-risk approach. Comparison of cumulative incidence between groups of patients was tested via Pepe & Mori test. A 6 months landmark analysis was performed to evaluate the benefit of a UACR-based renal response on renal survival. MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014) and Stata 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC) were used for computation.
Results
From October 2013 to December 2018, 531 consecutive patients, newly-diagnosed at the Amyloidosis Research and Treatment Center of Pavia with AL amyloidosis were included in the study. Patients’ characteristics are reported in Table 1. Renal involvement, defined as a baseline 24 h-proteinuria >0.5 g/24 h, was present in 328 patients (62%). In patients with renal AL amyloidosis, renal stage, based on 24 h-proteinuria and eGFR, was I in 93 (28%), II in 161 (49%) and III in 74 (23%) patients, respectively. In the overall population, baseline median 24 h-proteinuria and UACR were 1.30 g/24 h (IQR: 0.24–5.68) and 1,185 mg/g (IQR: 90–5,990), respectively. No statistically significant differences were observed between the internal testing and validation cohorts (Supplemental Table 1).
Patients characteristics.
| Variables | Overall population 531 patients n (%) – median (IQR) |
|---|---|
| Age, years | 66 (57–73) |
| Sex, male | 304 (57%) |
| Organ involvement | |
| Heart | 393 (74%) |
| Kidney | 328 (62%) |
| Liver | 46 (9%) |
| ST | 92 (17%) |
| PNS | 35 (7%) |
| ANS | 26 (5%) |
| Mayo stage | n=482 |
| I | 71 (15%) |
| II | 185 (38%) |
| IIIa | 132 (27%) |
| IIIb | 94 (20%) |
| Renal staging | |
| I | 236 (44%) |
| II | 221 (42%) |
| III | 74 (14%) |
| Proteinuria, g/24 h | 1.30 (0.24–5.68) |
| eGFR, mL/min × 1.73 m2 | 60 (35–85) |
| UACR, mg/g | 1,185 (89.8–5,990) |
| Monoclonal component | |
| IgG | 184 (35%) |
| IgA | 60 (11%) |
| IgM | 28 (6%) |
| IgD | 3 (1%) |
| FLC | 251 (47%) |
| LC isotype | |
| Kappa | 112 (21%) |
| Lambda | 419 (79%) |
| dFLC, mg/L | 170 (66–438) |
| dFLC <50 mg/L | 104 (20%) |
| BMPC, % | 10 (7–15) |
| Treatment | |
| CyBorD | 274 (52%) |
| BMDex | 92 (17%) |
| BDex | 22 (4%) |
| MDex | 64 (12%) |
| IMiDs | 28 (5%) |
| Rituximab | 20 (3%) |
| Othera | 10 (2%) |
-
aHigh dose dexamethasone in 8, autologous stem cell transplant in 1 and daratumumab, bortezomib and dexamethasone in 1 case, respectively. ST, soft tissues; PNS, peripheral nervous system; ANS, autonomic nervous system; eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio; FLC, free light chain; LC, light chains; dFLC, difference between involved and uninvolved free light chains; BMPC, bone marrow plasma cells; CyBorD, cyclophosphamide, bortezomib and dexamethasone; BMDex, bortezomib, melphalan and dexamethasone; BDex, bortezomib and dexamethasone; MDex, melphalan and dexamethasone; IMiDs, immunomodulatory drugs.
In the overall population, there was a strong linear correlation between 24 h-proteinuria and UACR at baseline, with a correlation coefficient r of 0.90 (95% CI: 0.89–0.92; p<0.001; Figure 1). The Mayo Clinic UACR cut-off (300 mg/g) classified as having renal involvement 340 (64%) patients, with a 90% concordance (95% CI: 87–92%) with the 24 h-proteinuria cut-off (Figure 2).

Correlation between 24 h-proteinuria and UACR.
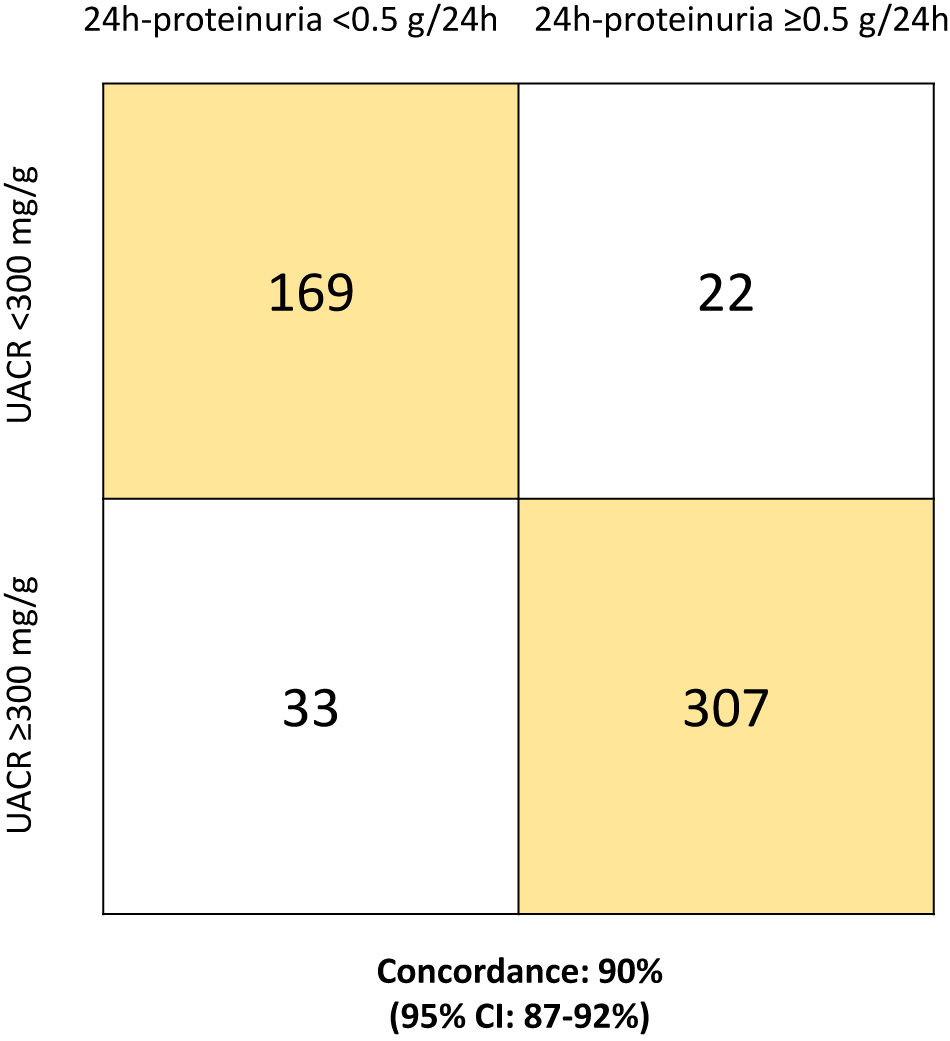
Concordance in identification of renal involvement between 24 h-proteinuria and UACR.
After a median follow-up of living patients of 31 months, 57 (11%) had a progression of renal dysfunction to end stage renal failure requiring dialysis. We explored whether a UACR-based renal staging system (replacing the 24 h-proteinuria of 5 g/24 h cut-off with the UACR cut-off of 3,600 mg/g) was able to stratify patients with renal involvement in three stages with statistically significant different risk of progression to dialysis. Of 328 patients with renal involvement, 65 (20%) were stage I, 165 (50%) stage II and 98 (30%) stage III. Rate of dialysis at 36 months was significantly lower in stage I patients compared to stage II (0 vs. 20%; p=0.002) and in stage II subjects compared to stage III (20 vs. 44%; p<0.001) (Figure 3A). Similar results were observed when analysis was performed with a competitive risk model (Figure 3B), with a significantly lower 36 months dialysis-rate in stage I compared to stage II (0 vs. 16%; p<0.001) and in stage II compared to stage III (16 vs. 36%; p<0.001). No stage I patient required dialysis during the study period. According to the validated 24 h-proteinuria-based renal staging system, of the same 328 patients with renal involvement, 93 (28%) were stage I, 161 (49%) stage II, and 74 (23%) stage III (Figure 3C, D). Three stage I patients progressed to end stage renal failure. The 24 h-proteinuria-based and UACR-based renal staging systems were concordant in 82% (95% CI 77–86%) of cases (Figure 3E). Interestingly, three of the 31 subjects who were re-classified as renal stage II from renal stage I according to the UACR-based renal stage required dialysis initiation.

UACR-based compared to 24 h-proteinuria-based for renal staging system.
Renal staging system based on UACR (cut-off 3,600 mg/g) and eGFR (cut-off 50 mL/min), censoring patients who died before progression to dialysis (A); renal staging system based on UACR and eGFR with a competitive risk model (B); renal staging system based on 24 h-proteinuria (cut-off 5 g/24 h) and eGFR (cut-off 50 mL/min), censoring patients who died before progression to dialysis: rate of dialysis at 36 months was 10% in stage I, 20% in stage II and 49% in stage III (C); renal staging system based on 24 h-proteinuria (cut-off 5 g/24 h) and eGFR (cut-off 50 mL/min) with a competitive risk model: rate of dialysis at 36 months was 5% in stage I, 16% in stage II and 41% in stage III (D); concordance between renal staging system based on 24 h-proteinuria (cut-off 5 g/24 h) and that one based on UACR (cut-off 3,600 mg/g) (E).
A UACR-based renal response defined as a reduction of UACR >30% from baseline at 6 months in the absence of a concomitant reduction of eGFR >25%, was observed in 58/220 (26%) evaluable patients. We evaluated whether this UACR renal response criterion was able to predict renal survival in the testing (n=354 patients) and in the validation cohort (n=177 patients). Details about first-line treatment are reported in Table 1. UACR-based renal response was observed in 41/153 (27%) evaluable patients in the testing cohort and in 17/67 (25%) evaluable cases in the validation cohort. The 6-months landmark analysis showed that patients who achieved a renal response according to this UACR-based criterion had a statistically significant lower dialysis-rate at 36 months both in the testing (0 vs. 17%; p=0.004; Figure 4A) and in the validation cohort (0 vs. 31%; p=0.006; Figure 4B). Also in this case, these results were confirmed when the analysis was conducted with the competitive risk model. The 36 months dialysis rate was 0% for UACR-responders and 16% for no-responders (p<0.001) in the testing cohort and 0% for UACR-responders and 36% for no-responders (p<0.001) in the validation cohort. Concordance between UACR-based renal response and 24 h-proteinuria-based renal response was 85% (95% CI 79–89%). Comparison of renal response rate according to 24 h-proteinuria and UACR-based criteria and rate of dialysis in renal responders is reported in Table 2. Four patients who resulted as renal responders according to the 24 h-proteinuria criterion progressed to end-stage renal failure. Importantly, these subjects did not achieve a UACR-based renal response. No patient who achieved a UACR-based renal response progressed to end-stage renal failure requiring dialysis, both in the testing and in the validation cohort.

Impact of UACR-based renal response at 6 months on renal survival.
UACR-based renal response was defined as reduction in UACR >30% from baseline, in absence of a concomitant decrease in eGFR >25%. UACR-based renal response in the testing cohort, censoring patients who died before progression to dialysis (A); UACR-based renal response in the validation cohort, censoring patients who died before progression to dialysis (B); UACR-based renal response in the testing cohort with a competitive risk model (C); UACR-based renal response in the validation cohort with a competitive risk model (D). Analysis was performed with a 6 months landmark.
Renal response according to 24 h-proteinuria and UACR-based criteria.
| Variables | 24 h-proteinuria-based criteria 222 evaluable patients |
UACR-based criteria 220 evaluable patients |
|---|---|---|
| Renal response rate at 6 months | 32% (95% CI 26–38%) | 26% (95% CI 21–33%) |
| Rate of dialysis at 36 months from diagnosis in renal respondersa | 7% (95% CI 2–13%) | 0% (95% CI 0–5%) |
-
aThe analysis gave the same results both with censoring and competing risk approach. UACR, urinary albumin to creatinine ratio.
Discussion
The present study explores the use of UACR instead of 24 h-proteinuria in the diagnosis, staging and monitoring of renal AL amyloidosis in a large prospective case series. In agreement with the Mayo Clinic study, we observed a remarkably good concordance between UACR and 24 h-proteinuria at baseline. We tested the UACR cut-off identified by Mayo Clinic investigators in an independent and larger population. We found that the proposed diagnostic cut-off (300 mg/g) can identify renal involvement with a 90% concordance with the 24 h-proteinuria cut-off. We observed that replacing 24 h-proteinuria with UACR (3,600 mg/g) in the renal staging systems allows discrimination of three groups with significantly different survival. Indeed, in our study, the patients who were reclassified from stage I to stage II eventually progressed to dialysis, indicating that the UACR-based staging system might even improve the discriminating ability of the standard staging system, possibly because of lower probability of preanalytical error. Importantly, in the present study UACR was measured on the first morning void, that is considered more reliable than random spot urine samples for the assessment of microalbuminuria [20]. Finally, for the first time we showed prospectively that UACR-based renal response, defined as a UACR reduction >30% at 6 months from baseline without a worsening of eGFR >25%, can predict renal outcomes. Because the Mayo Clinic investigators did not explore the impact of UACR-based renal response in their study, we provided an internal validation and UACR renal responders had a significantly longer renal survival both in the testing and in the validation cohort. Importantly, the patients who achieved a renal response assessed with 24 h-proteinuria and that eventually progressed to dialysis were reclassified as non-responders according to the UACR-based renal response criterion, indicating a better discriminating ability of UACR even in renal response assessment.
The availability of a renal marker that is independent of 24 h-urine collection is relevant in AL amyloidosis, since patients with this rare disease have often to travel to national referral centers for diagnosis and response to therapy assessment that requires frequent evaluations (every 1–3 months). In this setting, the 24 h-urine collection is uncomfortable and exposed to a potential preanalytical error, due to an incorrect collection.
In conclusion, our study prospectively validates the use of UACR as a biomarker for the identification and staging of renal involvement in AL amyloidosis and demonstrates that renal response assessment with UACR consistently predicts renal outcomes. The Mayo Clinic study and ours allow replacement of 24 h-proteinuria with UACR in individual patients’ management and in the design of clinical trials.
Funding source: CARIPLO http://dx.doi.org/10.13039/501100002803
Award Identifier / Grant number: 2018-0257
Funding source: Italian Ministry of Health http://dx.doi.org/10.13039/501100003196
Award Identifier / Grant number: GR-2018-12368387
Funding source: Cancer Research UK, FCAECC and AIRC http://dx.doi.org/10.13039/501100002704
Award Identifier / Grant number: Accelerator Award 2017
-
Research funding: This study was supported by a grant from CARIPLO “Harnessing the plasma cell secretory capacity against systemic light chain amyloidosis” (n. 2018-0257), by a grant from the Italian Ministry of Health “Towards effective, patient-tailored anti-plasma cell therapies in AL amyloidosis: Predicting drug response and overcoming drug resistance” (GR-2018-12368387), and by a grant from Cancer Research UK, FCAECC and AIRC under the Accelerator Award 2017 Program “Early detection and intervention: Understanding the mechanisms of transformation and hidden resistance of incurable haematological malignancies”.
-
Author contributions: G.P. designed the study; M.B and G.P. evaluated patients, collected data, analyzed data, wrote the manuscript and gave final approval; P.M., M.N., A.F., F.B., Ma.Na., F.R. and G.M. evaluated patients, critically reviewed the manuscript and gave final approval; Ma.Bo., J.R., M.S., T.B and R.A critically reviewed the manuscript and gave final approval. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: M.B. reports honoraria for lectures from Jannsen-Cilag; P.M. reports honoraria for lectures from Pfizer, Jannsen-Cilag, and travel support from Celgene; M.N. reports honoraria for lectures from Jannsen-Cilag; A.F. reports honoraria for lectures from Jannsen-Cilag; G.P. reports honoraria for lectures from and membership on advisory boards with Jannsen-Cilag.
-
Informed consent: All subjects gave written informed consent for their clinical data to be used in accordance with the Declaration of Helsinki.
-
Ethical approval: The study was approved by Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico S. Matteo’s Ethical Committee.
References
1. Merlini, G, Dispenzieri, A, Sanchorawala, V, Schönland, SO, Palladini, G, Hawkins, PN, et al.. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Prim 2018;4:38. https://doi.org/10.1038/s41572-018-0034-3.Search in Google Scholar PubMed
2. Palladini, G, Milani, P, Merlini, G. Management of AL amyloidosis in 2020. Blood 2020;136:2620–7. https://doi.org/10.1182/blood.2020006913.Search in Google Scholar PubMed
3. Palladini, G, Foli, A, Milani, P, Russo, P, Albertini, R, Lavatelli, F, et al.. Best use of cardiac biomarkers in patients with AL amyloidosis and renal failure. Am J Hematol 2012;87:465–71. https://doi.org/10.1002/ajh.23141.Search in Google Scholar PubMed
4. Palladini, G, Milani, P, Foli, A, Basset, M, Russo, F, Bosoni, T, et al.. The impact of renal function on the clinical performance of FLC measurement in AL amyloidosis. Clin Chem Lab Med 2016;54:939–45. https://doi.org/10.1515/cclm-2015-0985.Search in Google Scholar PubMed
5. Palladini, G, Hegenbart, U, Milani, P, Kimmich, C, Foli, A, Ho, AD, et al.. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014;124:2325–32. https://doi.org/10.1182/blood-2014-04-570010.Search in Google Scholar PubMed
6. Benz-de Bretagne, I, Perrier, F, Piéroni, L, Plouvier, E, Caussé, E, groupe de travail SFBC SFN, S. N. P. Preanalytical step of urinary protein measurement: from urine sampling to preparation for analysis of the specimen. Ann Biol Clin (Paris) 2018;76:609–16. https://doi.org/10.1684/abc.2018.1387.Search in Google Scholar PubMed
7. Bottini, PV, Garlipp, CR, Lima, PRM, Brito, IT, Carvalho, LMG. Are patients adequately informed about procedures for 24-h urine collection? Clin Chem Lab Med 2020;58:e32–5. https://doi.org/10.1515/cclm-2019-0368.Search in Google Scholar PubMed
8. Lamb, EJ, MacKenzie, F, Stevens, PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009;46:205–17. https://doi.org/10.1258/acb.2009.009007.Search in Google Scholar PubMed
9. Jensen, JS, Clausen, P, Borch-Johnsen, K, Jensen, G, Feldt-Rasmussen, B. Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant 1997;12(2 Suppl):6–9.Search in Google Scholar
10. Mykkänen, L, Haffner, SM, Kuusisto, J, Pyorälä, K, Laakso, M. Microalbuminuria precedes the development of NIDDM. Diabetes 1994;43:552–7.10.2337/diab.43.4.552Search in Google Scholar PubMed
11. Nisell, H, Trygg, M, Bäck, R. Urine albumin/creatinine ratio for the assessment of albuminuria in pregnancy hypertension. Acta Obstet Gynecol Scand 2006;85:1327–30. https://doi.org/10.1080/00016340600808747.Search in Google Scholar PubMed
12. James, MT, Grams, ME, Woodward, M, Elley, CR, Green, JA, Wheeler, DC, et al.. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015;66:602–12. https://doi.org/10.1053/j.ajkd.2015.02.338.Search in Google Scholar PubMed PubMed Central
13. Visram, A, Al Saleh, AS, Parmar, H, McDonald, JS, Lieske, JC, Vaxman, I, et al.. Correlation between urine ACR and 24-h proteinuria in a real-world cohort of systemic AL amyloidosis patients. Blood Cancer J 2020;10:124. https://doi.org/10.1038/s41408-020-00391-2.Search in Google Scholar PubMed PubMed Central
14. Fernández de Larrea, C, Verga, L, Morbini, P, Klersy, C, Lavatelli, F, Foli, A, et al.. A practical approach to the diagnosis of systemic amyloidoses. Blood 2015;125:2239–44. https://doi.org/10.1182/blood-2014-11-609883.Search in Google Scholar PubMed
15. Brambilla, F, Lavatelli, F, Di Silvestre, D, Valentini, V, Rossi, R, Palladini, G, et al.. Reliable typing of systemic amyloidoses through proteomic analysis of subcutaneous adipose tissue. Blood 2012;119:1844–7. https://doi.org/10.1182/blood-2011-07-365510.Search in Google Scholar PubMed
16. Lavatelli, F, Palladini, G, Merlini, G. Perspectives in developments of mass spectrometry for improving diagnosis and monitoring of multiple myeloma and other plasma cell disorders. Clin Chem Lab Med 2021;59:633–5. https://doi.org/10.1515/cclm-2021-0181.Search in Google Scholar PubMed
17. Gertz, MA, Comenzo, R, Falk, RH, Fermand, JP, Hazenberg, BP, Hawkins, PN, et al.. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005;79:319–28. https://doi.org/10.1002/ajh.20381.Search in Google Scholar PubMed
18. Dispenzieri, A, Gertz, MA, Kyle, RA, Lacy, MQ, Burritt, MF, Therneau, TM, et al.. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 2004;22:3751–7. https://doi.org/10.1200/jco.2004.03.029.Search in Google Scholar PubMed
19. Palladini, G, Sachchithanantham, S, Milani, P, Gillmore, J, Foli, A, Lachmann, H, et al.. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015;126:612–5. https://doi.org/10.1182/blood-2015-01-620302.Search in Google Scholar PubMed
20. Witte, EC, Lambers Heerspink, HJ, de Zeeuw, D, Bakker, SJ, de Jong, PE, Gansevoort, R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 2009;20:436–43. https://doi.org/10.1681/asn.2008030292.Search in Google Scholar PubMed PubMed Central
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cclm-2021-0912).
© 2022 Marco Basset et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Obituary
- Dr Per Hyltoft Petersen: an appreciation
- Editorials
- Extrapolated normative GFR data for living kidney donation
- Effectiveness of interventions to improve test appropriateness
- Reviews
- The pathogenesis, epidemiology and biomarkers of susceptibility of pulmonary fibrosis in COVID-19 survivors
- S100B in cardiac surgery brain monitoring: friend or foe?
- Mini Review
- Blood lactate concentration in COVID-19: a systematic literature review
- Opinion Paper
- Commercial immunoassays for detection of anti-SARS-CoV-2 spike and RBD antibodies: urgent call for validation against new and highly mutated variants
- General Clinical Chemistry and Laboratory Medicine
- A randomized controlled study of biochemical tests in primary care: interventions can reduce the number of tests but usage does not become more appropriate
- A national surveillance program for evaluating new reagent lots in medical laboratories
- External quality assessment providers’ services appear to more impact the immunohaematology performance of laboratories than national regulatory and economic conditions
- International collaborative study to evaluate and calibrate two recombinant L chain Ferritin preparations for use as a WHO International Standard
- Method comparison of three serum free light chain assays on the Roche Cobas 6000 c501 chemistry analyzer
- Prospective urinary albumin/creatinine ratio for diagnosis, staging, and organ response assessment in renal AL amyloidosis: results from a large cohort of patients
- Analytical evaluation of the Nittobo Medical tartrate resistant acid phosphatase isoform 5b (TRACP-5b) EIA and comparison with IDS iSYS in different clinically defined populations
- Reference Values and Biological Variations
- Age-adapted percentiles of measured glomerular filtration in healthy individuals: extrapolation to living kidney donors over 65 years
- Indirectly determined hematology reference intervals for pediatric patients in Berlin and Brandenburg
- Hematology and Coagulation
- Monocyte distribution width (MDW): a useful biomarker to improve sepsis management in Emergency Department
- Diabetes
- A step towards optimal efficiency of HbA1c measurement as a first-line laboratory test: the TOP-HOLE (Towards OPtimal glycoHemOgLobin tEsting) project
- Labile glycated hemoglobin: an underestimated laboratory marker of short term glycemia
- Infectious Diseases
- Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays
- Letters to the Editors
- Molecular detection of SARS-CoV-2 eta VOI in Northern Italy: a case report
- Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role?
- Measurement in different sample types may aid in detecting interferences and macrocomplexes affecting cardiac troponin measurements
- Which method to detect macrotroponin?
- Marked geographical variation in the prevalence of macroprolactinemia
- Pancreatic lipase assays: time for a change towards immunoassays?
- Analytical evaluation of the performances of a new procalcitonin immunoassay
- Necessity of harmonization of tissue transglutaminase IgA assays to align clinical decision making in coeliac disease
- A fortuitous but characteristic blood smear observation allowing a late diagnosis of MPS-VII
- Prognostic significance of blood-based multi-cancer detection in plasma cell-free DNA
Articles in the same Issue
- Frontmatter
- Obituary
- Dr Per Hyltoft Petersen: an appreciation
- Editorials
- Extrapolated normative GFR data for living kidney donation
- Effectiveness of interventions to improve test appropriateness
- Reviews
- The pathogenesis, epidemiology and biomarkers of susceptibility of pulmonary fibrosis in COVID-19 survivors
- S100B in cardiac surgery brain monitoring: friend or foe?
- Mini Review
- Blood lactate concentration in COVID-19: a systematic literature review
- Opinion Paper
- Commercial immunoassays for detection of anti-SARS-CoV-2 spike and RBD antibodies: urgent call for validation against new and highly mutated variants
- General Clinical Chemistry and Laboratory Medicine
- A randomized controlled study of biochemical tests in primary care: interventions can reduce the number of tests but usage does not become more appropriate
- A national surveillance program for evaluating new reagent lots in medical laboratories
- External quality assessment providers’ services appear to more impact the immunohaematology performance of laboratories than national regulatory and economic conditions
- International collaborative study to evaluate and calibrate two recombinant L chain Ferritin preparations for use as a WHO International Standard
- Method comparison of three serum free light chain assays on the Roche Cobas 6000 c501 chemistry analyzer
- Prospective urinary albumin/creatinine ratio for diagnosis, staging, and organ response assessment in renal AL amyloidosis: results from a large cohort of patients
- Analytical evaluation of the Nittobo Medical tartrate resistant acid phosphatase isoform 5b (TRACP-5b) EIA and comparison with IDS iSYS in different clinically defined populations
- Reference Values and Biological Variations
- Age-adapted percentiles of measured glomerular filtration in healthy individuals: extrapolation to living kidney donors over 65 years
- Indirectly determined hematology reference intervals for pediatric patients in Berlin and Brandenburg
- Hematology and Coagulation
- Monocyte distribution width (MDW): a useful biomarker to improve sepsis management in Emergency Department
- Diabetes
- A step towards optimal efficiency of HbA1c measurement as a first-line laboratory test: the TOP-HOLE (Towards OPtimal glycoHemOgLobin tEsting) project
- Labile glycated hemoglobin: an underestimated laboratory marker of short term glycemia
- Infectious Diseases
- Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays
- Letters to the Editors
- Molecular detection of SARS-CoV-2 eta VOI in Northern Italy: a case report
- Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role?
- Measurement in different sample types may aid in detecting interferences and macrocomplexes affecting cardiac troponin measurements
- Which method to detect macrotroponin?
- Marked geographical variation in the prevalence of macroprolactinemia
- Pancreatic lipase assays: time for a change towards immunoassays?
- Analytical evaluation of the performances of a new procalcitonin immunoassay
- Necessity of harmonization of tissue transglutaminase IgA assays to align clinical decision making in coeliac disease
- A fortuitous but characteristic blood smear observation allowing a late diagnosis of MPS-VII
- Prognostic significance of blood-based multi-cancer detection in plasma cell-free DNA

