Abstract
A sniffer (detecting) dog is conventionally defined as an animal trained to use its olfactory perceptions for detecting a vast array of substances, mostly volatile organic compounds (VOCs), including those exceptionally or exclusively generated in humans bearing specific pathologies. Such an extraordinary sniffing performance translates into the capability of detecting compounds close to the femtomolar level, with performance comparable to that of current mass spectrometry-based laboratory applications. Not only can dogs accurately detect “abnormal volatilomes” reflecting something wrong happening to their owners, but they can also perceive visual, vocal and behavioral signals, which altogether would contribute to raise their alertness. Although it seems reasonable to conclude that sniffer dogs could never be considered absolutely “diagnostic” for a given disorder, several lines of evidence attest that they may serve as efficient screening aids for many pathological conditions affecting their human companions. Favorable results have been obtained in trials on cancers, diabetes, seizures, narcolepsy and migraine, whilst interesting evidence is also emerging on the capability of early and accurately identifying patients with infectious diseases. This would lead the way to proposing an “olfactory fingerprint” loop, where evidence that dogs can identify the presence of human pathologies provides implicit proof of the existence of disease-specific volatilomes, which can be studied for developing laboratory techniques. Contextually, the evidence that specific pathologies are associated with abnormal VOC generation may serve as reliable basis for training dogs to detect these compounds, even (or especially) in patients at an asymptomatic phase.
Introduction
The domestic dog (also known as Canis lupus familiaris) is a member of the genus Canis (canines), belonging to the family of wolf-like canids [1]. According to the most recent theory, dogs very likely descended from some extinct late Pleistocene grey wolf (Canis lupus) exemplars, which tended to live in East Asia approximately 100,000 years ago [2]. A remarkable post-divergence gene flow has since occurred between wolves and dogs, accompanied by expansion, contraction and replacement of the two populations around the world, so that modern wolves shall no longer be considered direct ancestors of domestic dogs which, in turn, have rapidly evolved taking profit from a mutually beneficial relationship with humans [3].
Although a definitive date for the first dog domestication cannot be accurately set, it is commonly thought that the human-dog relationships commenced some 35,000–37,000 years ago, much earlier than any other animal or plant domestication [4]. The most accredited scenario is that of grey wolf ancestors being attracted by human meal leftovers, which led to a gradual engagement in following human hunters for purposes of easier pickings. The increasing presence of these animals near human settlements became a deterrent for other dangerous carnivores from straying close to humans, thus generating a kind of mutually beneficial partnership for wolves and for their new (human) companions. Such an unconscious process of “proto”-domestication has then been followed by a more strict and real domestication, which probably commenced some 10,000–14,000 years ago [4]. During this second domestic process, an intense selective pressure was started, which led to the generation of smaller sized animals, with partially mutated phenotypes and expressing more friendly and docile behaviors [2]. A third domestication phase then initiated a few hundred years ago (nearly 200–300), characterized by systematic breeding practices which led to the appearance of hundreds of different breeds. Notably, genome-wide single nucleotide polymorphism (SNP) array studies now show that the modern dog breeds carry a kaleidoscope of differing genomic signatures compared to their ancestral progenitors, as well as an enhanced haplotype homozygosity and linkage disequilibrium, whose characteristics have enriched the Dog 10K Genomes Project and Dog Genome SNP Database (DoGSD). Overall, the broad degree of genetic diversity that has finally emerged across different dog populations drives the conclusion that dogs shall no longer be regarded as a single group when performing demographic inferences, whereby they can vary for sheer size, body, limb and skull proportions [5]. It is hence not surprising that dogs now display a much larger phenotypic heterogeneity than that observed throughout the whole order of carnivores, whereby their breeds span from the dolichocephalic, short-limbed dachshund to the wolf-like Alaskan malamute, from the 0.5 kg poodle to the over 90 kg mastiff, and even from the 20-cm-high Chihuahua to the over 80-cm-high Great Dane.
Regardless of their structural (and functional) heterogeneity, there is now little doubt that dogs have become “man’s best friend” and have been – and are still involved – in many different activities alongside their human companions. These basically include being “simple pets”, or else working as guard dogs, hunting dogs, herding dogs, guide dogs (e.g. for blind people), police dogs and – last but not least – sniffer (detection) dogs [6].
The mammal olfactory system
One of the most widely accepted definitions of “olfaction” is that of a sense mediating the perception of volatile chemicals, whose combination can also be referred to as “volatilome”. Irrespective of structural heterogeneities, a chemosensory system can be found in almost every animal species and has the main function of detecting changes in the biochemical structure of odorant molecules, in their concentration, as well as in their specific combination, thus enabling to garner vital information on the surrounding environment [7]. Briefly, the olfactory system in mammals is conventionally organized in two distinct organs within the nasal cavity, i.e. the main olfactory epithelium (MOE), containing olfactory receptors of class I and II (ORs class I and ORs class II), which mostly reacts to volatile odorants and is hence responsible for the conscious odor perception, along with the vomeronasal organ (VNO), containing vomeronasal receptors type 1 and 2 (V1R and V2R) and formyl peptide receptor-like proteins (FPRs), which preferentially reacts to pheromones and mediates a large number of neuroendocrine and behavioral responses [8]. Despite a basically similar structure, the olfactory system has evolved independently and rather differently in the various animal species, so that the functional odor perception now varies rather widely. A comparison of the main characteristics of olfactory system in humans and dogs is summarized in Table 1 [9], [10]. Compared to humans, dogs possess ~3-fold more genes encoding for olfactory receptors, an up to 30-fold larger olfactory epithelium extension, a nearly 50-fold higher number of olfactory receptors and a 3-fold bigger olfactory bulb size. Yet, dogs are not the best-smelling species in the animal kingdom, whereby the ratio between olfactory bulb to total encephalic volume is ~2% in mice, then followed by dogs (~0.3%) and goats (~0.2%). Notably, that same olfactory bulb to encephalic volume ratio is only ~0.1% in macaques and ~0.01% in humans, respectively [11].
Main characteristics of olfactory system in humans and dogs.
| Parameter | Humans | Dogs |
|---|---|---|
| Genes encoding for olfactory receptors | ~350 | >1100 |
| Olfactory epithelium extension | 3–4 cm2 | 18–150 cm2 |
| Number of olfactory receptors | 5–6 million | 150–300 million |
| Olfactory bulb size | ~1 cm | ~3 cm |
The potential of olfactory receptors in recognizing a kaleidoscope of different odorant molecules is mostly attributable to the presence of hyper-variable domains located within some of their transmembrane domains [12]. Another interesting aspect is that the different dog breeds share some notable difference in the structure of their olfactory system. For example, the number of olfactory receptors approximates 150 million in the Dachshund, around 225 million in Beagles and German Shepherds, but is as high as 300 million in Bloodhounds. Not only the number but also the structure of olfactory receptors varies across dog breeds. Robin et al. sequenced nearly 100 olfactory receptor genes, which were representative enough of the entire canine repertoire [13]. Overall, a consistent number of SNPs was found in over 50% olfactory receptor genes in six different dog breeds, whereas the remaining genes were free of SNPs or were only modestly polymorphic. Interestingly, up to 25% of the observed SNPs were classified as being breed-specific, and half of these encoded for receptors with a modified ligand-binding capacity. Further studies confirmed that the genetic heterogeneity is associated with different smelling capabilities not only among the different dog breeds, but also in dogs belonging to the same breed [14], [15], [16].
Comparing the analytical sensitivity and instrumental approach of “electronic” and dog noses
The electronic nose, or e-Nose, was first formally defined in 1994 by Gardner and Bartlett, as “an instrument which comprises an array of electronic chemical sensors with partial specificity and appropriate pattern recognition system, capable of recognizing simple or complex odors” [17]. In defining this term, the authors discussed assay intricacy, limits of detection (LoD), and time-constraints on using conventional measurement techniques (e.g. gas chromatography-mass spectrometry, GC-MS) for the analysis of complex odor profiles. This defined a need to mimic the mammalian olfactory system in a manner which provided a rapid and responsive answer to the defined analytical question. To date, an array of technologies has been developed for mimicking the olfactory system to detect volatile chemicals including but not limited to acoustic, electrochemical, infrared and optical sensors [18]. Many of these developments have been applied within settings such as environmental and food science (e.g. [19], [20]); however, there has been a perpetuating interest over recent years in the human volatilome, with contributions in its make-up generated from exhaled breath, sweat, saliva, urine and feces [21]. To date, the study of this human “smell print” has been widely applied in applications as diverse as clinical investigations [22], psychological stress [23], sport and exercise science [24], and even for the potential purpose of locating trapped survivors of natural disasters [25]. These interests have led to a rapid rise in the application of technical “smelling” devices (i.e. e-Noses), with a 5-fold increase in annual publications on this topic in the previous decade (source: PubMed, December 2019).
Much of the research into human volatilome has been performed using offline MS-based assays (e.g. GC-MS) or have employed online/real-time measurements using large footprint and expensive MS instruments such as proton transfer reaction (PTR)-MS and selected ion flow tube (SIFT)-MS [26]. However, a recent push toward transportable MS systems for online measurements has provided excellent specificity and sensitivity without time-consuming sample preparation, chromatographic separation and high cost instrumentation, thus providing a sample delivery more akin to that entering a biological nose and thus more translatable to that which is inhaled by the “sniffer dog”. Examples of this include compact quadrupole mass spectrometers for direct analysis of exhaled breath [27], headspace analysis (i.e. volatile ‘smells’ above a sample) [28] and a direct-off-surface sampling approach for volatile compounds present in dried blood spot samples [29]. These compact MS instruments (e.g. Advion Expression CMS) can be set up on mobile trolley systems with the necessary infrastructure (e.g. N2 generator and rotary pumps) to be transported to the clinical bedside or patient residences and are currently being assessed for clinical suitability of in-situ sampling and analysis [30]. In addition to this, developments of innovative MS ionization techniques have provided novel avenues for the direct measurement of non-volatile and semi-volatile molecules in biofluid samples, with semi-volatile molecules shown to be highly detectable by the canine nose and therefore relevant to the responses observed from bio-detection dogs [31]. A prominent example of this is paper spray ionization-MS where a biofluid can be spotted onto a filter paper “triangle”, and a high voltage is then applied which desorbs the molecules off the paper and into an adjacent MS instrument [32]. This technique is currently being applied to clinical cohorts for potential translation to everyday workflows [33], and can be combined to miniature MS instruments that can be carried around by a handle [34]. Furthermore, probe electrospray ionization-MS has shown excellent capability of instantaneous and direct measurement of biofluids for clinical purposes [35]. This technique uses an electrospray probe as a “dipstick”, causing molecules from the biosamples to adhere to the probes’ surface which are subsequently desorbed rapidly into the MS system following the application of a high voltage [36]. This approach has the potential for rapid diagnostic information on easily obtainable biofluids such as urine, saliva and sweat, thus allowing the expansion to investigate beyond the analysis of only volatile chemicals [37]. For these reasons, the application of a full spectrum analysis of chemical compounds, i.e. volatile, semi-volatile and non-volatile, using adapted e-Nose-style MS systems provides a fantastic analytical opportunity to both complement and add to the exciting prospect of resident “medical-detection” dog companions.
One of the most essential aspects that needs to be clarified before defining the analytical sensitivity of the canine olfactory apparatus is that the olfactory detection thresholds for all the different odorant molecules is strongly dependent on a genetic basis, as previously discussed [12]. The sensibility of different animal species to different chemical substances is hence extremely heterogeneous. For example, it has been now clearly established that dogs are extremely sensitive to certain amino acids (e.g. cysteine and methionine), aliphatic aldehydes (e.g. bourgeonal), acetate esters (e.g. amyl acetate) and sulfur-containing volatiles (e.g. butanethiol and phenylethyl sulfide), whilst they are virtually insensitive to other compounds that instead are strongly perceived by humans (e.g. aliphatic alcohols and benzene derivatives) [7]. Therefore, although it is common perception that dogs may be 10,000–100,000 more efficient than humans in their general sniffing capacity [38], it is not essentially true that dogs are always more sensitive than humans in smelling substances. It may also be hypothetically misleading to set a universal sensitivity threshold for the canine olfactory apparatus, whilst it seems more appropriate to use some practical examples for establishing “how low dogs could go” in detecting certain compounds. Walker et al. published the results of an interesting study, where the olfactory sensitivity of two different dogs (i.e. a Standard Schnauzer and a Rottweiler) for n-amyl acetate was comprehensively assessed [39]. After systematically lowering the n-amyl acetate concentration within Teflon boxes during several weeks, the lowest sniffing sensitivity (i.e. equaling the LoD of conventional laboratory methods) of the two dogs for the chemical compound was found to be as low as 1.14 and 1.90 parts per trillion (ppt), respectively. Considering that the molecular weight of n-amyl acetate is ~130 Da, these values will roughly correspond to a LoD comprised between 8.8 and 14.6 fmol/L. A virtually identical lower limit of sensitivity (i.e. 1.5 ppt, corresponding to ~11.5 fmol/L) has also been more recently identified by Concha et al. using 10 different dogs (3 Labrador Retrievers, 3 Working Cocker Spaniels, 2 English Springer Spaniels and 2 Border Collies) [40], though the animals displayed a high inter-individual response. Such an extraordinary analytical sensitivity almost translates into the capability of identifying one drop of a given liquid within nearly 20 Olympic-size (i.e. 760 m2) swimming pools [41]. Recent technical and analytical developments in MS-based applications have now made it possible to detect (and measure) metabolites at concentrations lower than pmol/L, approximating the femtomolar level (Figure 1) [42].
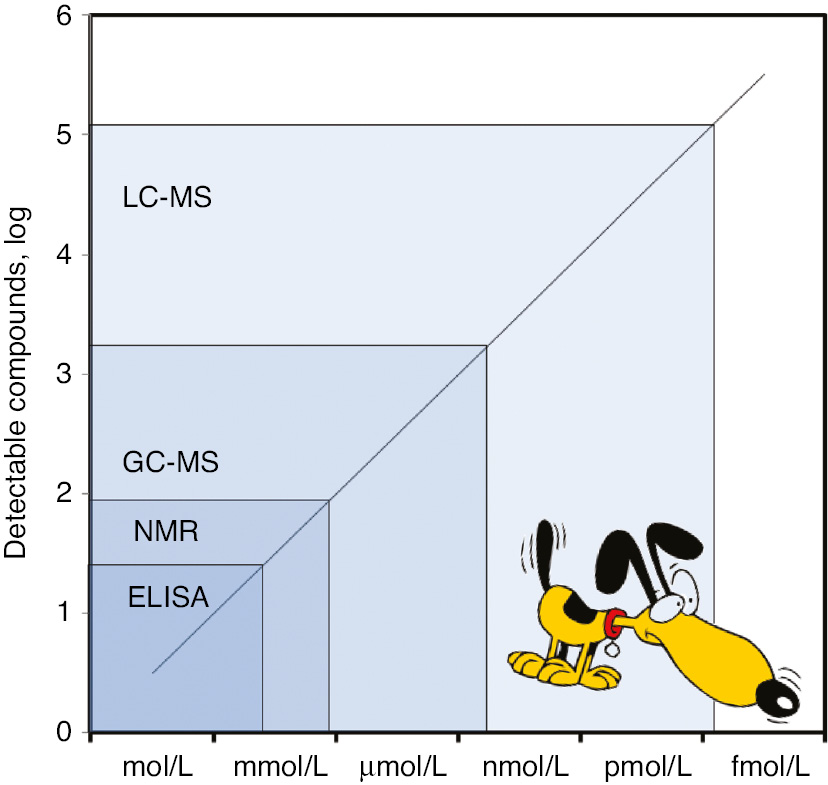
Lower limit of detection for certain compounds of some laboratory techniques compared with dogs.
ELISA, enzyme-linked immunosorbent assays; NMR, nuclear magnetic resonance; GC-MS, gas chromatography coupled with mass spectrometry; LC-MS, liquid chromatography coupled with mass spectrometry.
Taken together, this evidence would lead us to conclude that the “analytical sensitivity” of canine olfactory apparatus for certain compounds is perhaps as low as that of the most sensitive laboratory techniques, thus opening intriguing scenarios for many civilian, military and forensic applications of canine olfactory detection, whereby the so-called “sniffer” (detection) dogs can be trained for detecting substances such as foods, humans, explosives, illegal drugs, wildlife scat, currency, contraband electronics (e.g. illicit mobile phones) and – last but not the least – human diseases.
Clinical trials with sniffer dogs
A sniffer dog is an animal trained to use its olfactory sense for detecting a vast array of substances, mostly volatile organic compounds (VOCs; mostly represented by low molecular weight chemicals, which evaporate easily under normal temperature and pressure conditions), also including those exceptionally or exclusively generated in patients with specific human diseases, whose variable association contribute to generate multiple specific volatilomes [41], [43]. Although the use of the so-called “bio-detection” or “medical-detection” dogs is just in embryo, being still far from regarded as routine replacement for other and more validated diagnostic investigations, the results of some encouraging studies will be reviewed and summarized in the following part of this article. Notably, some consistent evidence has already been garnered concerning some specific human diseases such as cancer, diabetes and seizures, promising findings are emerging for others such as narcolepsy and migraine, whilst preliminary evidence has also been recently published that sniffer dogs may be used for screening infectious diseases (Table 2).
Current and future (potential) clinical applications of sniffer dogs.
| – | Cancers |
| – | Diabetes |
| – | Seizures |
| – | Narcolepsy |
| – | Migraine |
| – | Infectious diseases |
Potential advantages and drawbacks of sniffer (detection) dogs and mass-spectrometry (MS)-based laboratory methods for screening and/or diagnosing human diseases.
| Characteristics | Dogs | MS |
|---|---|---|
| Initial cost | ++ | +++ |
| Operating costs | + | ++ |
| Environment adaptation | + | +++ |
| Portability | +++ | −/+ |
| Invasiveness of testing | −/+ | −/+++ |
| Pre-analytical sample precautions | + | +++ |
| Efforts for calibration (or dog training) | +++ | + |
| Standardization | − | +/++ |
| Possibility of quality control assessment | −/+ | +++ |
| Early diagnosis | ++a | + |
| Overall diagnostic performance | +++a | +++ |
| Inter-assay (or individual) imprecision | +++ | + |
| Companionship, loyalty and devotion | +++ | − |
aVaries widely among breeds and within dogs of the same breed. MS, mass-spectrometry.
Cancer detection
The very first anecdotal evidence that dogs are somehow capable of smelling (and thus detecting) cancers was published by Williams and Pembroke, in 1989 [44]. Briefly, the authors described the case of a 44-year-old woman, who had been referred to a dermatology clinic for a lesion on the left thigh, which was finally diagnosed as being a melanoma. Notably, the patient was first alerted on the possible malignant origin of that lesion because her dog, a cross between a Doberman and a Border Collie, was spending a lot of time intensely sniffing at the lesion, while showing no interest for other parts of the woman’s body. Many other case reports and clinical trials then followed this original case report, which provided favorable outcomes occasionally mixed with controversial findings. As a rule of thumb, generalization of data may be challenging, as the several published studies are heterogeneous in terms of experimental design, odor samples (urine, cancer tissue, serum or plasma, exhaled air), presence of confounding odors, specimen collection and storage, dog breed and characteristics (i.e. age, sex), as well as for the technique and amount of dog training [45]. Irrespective of these important drawbacks, it now seems evident that dogs can effectively smell the presence of certain malignancies in a multitude of biospecimens [46].
Amongst some of the most significant studies which have been recently published, Fischer-Tenhagen et al. showed that trained dogs are capable of identifying absorbed breath samples of lung cancer patients with a 0.89–1.00 sensitivity and a 0.40–0.80 specificity [47]. Interestingly, it could also be demonstrated that the dogs especially reacted to the presence of 1-butanol, 2-butanone, 2-pentanone and hexanal, whose concentration is exceptionally increased in the exhaled air of lung cancer patients. Important evidence has also been recently provided by Junqueira et al., who showed that trained dogs may be capable of identifying blood samples from lung cancer patients with a 0.97 sensitivity and a 0.98 specificity [48]. Almost identical data have then been published by Guirao Montes et al. who specifically trained a dog to identify exhaled gas from lung cancer patients [49], and showed that the animal was effective in detecting early lung cancer samples (785 tests overall using cancerous and non-cancerous samples) with sensitivity and specificity as high as 0.95 and 0.98, respectively. A myriad of articles has then been published on dogs’ capability to identify patients with prostate cancer [9]. Among these, Pacik et al. recently published the results of a study where German Shepherd dogs were capable of detecting urines collected from prostate cancer patients with a 94% sensitivity and a 92% specificity, as well as the presence of urinary sarcosine, a well-established biomarker of prostate cancer, with a 90% sensitivity and a 95% specificity [50]. As regards the potential for dog recognition of other cancer types, interesting data have been published in patients with malignancies of the skin, and in breast, pancreas, colorectal, ovary and bladder cancers, as comprehensively reviewed elsewhere [9], [45]. Overall, the diagnostic performance of dogs in detecting different cancerous samples ranged between 0.30 and 0.99 for sensitivity and 0.08–0.99 for specificity, respectively, with the highest detection rates found with the analysis of exhaled air specimens.
Detection of hyper- and hypoglycemia
Several lines of evidence now attest that dogs may be capable of predicting and thus potentially preventing, the onset of hyper- and hypoglycemic crises in their owners, as recently reviewed elsewhere [51], [52]. Basically, cumulative evidence suggests that the so-called diabetes alerting dogs (DADs) may display a sensitivity of up to 0.80 for detecting hyperglycemia, with a specificity of around 0.50, whilst they can identify hypoglycemia with up to 0.78 sensitivity and 0.96 specificity [52]. Nevertheless, it has also been clearly highlighted that individual canine performance may be extremely heterogeneous, with some animals displaying up to 2-fold better sensitivity and specificity than others. An interesting article has been recently published by Rooney et al. [53], who asked 27 routine dog owners to record their pets’ behaviors during out-of-range episodes of blood glucose. A total number of nearly 4000 out-of-range episodes were recorded during the study period, with dogs exhibiting a median sensitivity of 0.83 (interquartile range [IQR], 66%–94%) and 0.67 (IQR, 17%–91%) for hypoglycemic and hyperglycemic episodes, respectively. Overall, the likelihood that animals were capable of alerting their owners when blood glucose values were outside the target range was 2.8-fold and 2.3-fold during hypoglycemic and hyperglycemic episodes, respectively. The best predictors of dogs’ alert reaction were new accreditation (vs. unaccredited dogs), time since accreditation, along with animals’ motivation and enjoyment of the task. The same team of authors had previously published the results of another interesting study, where the psychosocial benefits of having a DAD was assessed in 17 owners [54]. Since the time when the dog had been originally introduced, 94% declared that quality of life was enhanced and 88% that independency was increased. Even more importantly, 88% and 81% of these dog owners claimed that they had complete faith in the capability of their pet to alert hypoglycemic and hyperglycemic episodes.
Alerting seizures
The first article on the so-called seizure-alert dogs (SADs) was published by Strong et al., in 1993 [55], who provided anecdotal evidence that dogs may be capable of predicting and even responding to the onset of seizures in their owners, by becoming apprehensive, anxious or restless. Since then a myriad of other studies has been published, as recently reviewed by Catala et al. [56]. This scoping review concluded that dogs’ accuracy in anticipating owners’ seizures was comprised between 0.70 and 0.85 (irrespective of the type of seizures), the reported times of alert before the onset of seizures ranged between 10 s and 5 h, whilst alerting behaviors mostly encompassed attention-getting. Interestingly, another study has been recently published by Martos Martinez-Caja and colleagues [57], based on dissemination of an online questionnaire to as many as 227 dog owners who suffered from different types of epilepsy. Overall, nearly 70% of dogs were reported to display some types of alerting behaviors, all occurring between 10 and 60 min before the seizure episode in seizure-trained dogs, and between 0 and 10 min before the seizure episode in untrained dogs, respectively. The most frequent alerting behaviors encompassed hands or face licking, staring and sitting. Regarding the type of signals that dogs may detect before the onset of a seizure episode affecting their human companion, it has been hypothesized that the animal could probably perceive a mixture of different signals, such as heart rate variation (which often anticipate the onset of seizures), release of hormones or neurotransmitters, as well as behavioral or postural alterations [56]. Notably, a recent study published by Catala and colleagues emphasized that dogs may be capable of detecting a highly specific, but still virtually undefined, epileptic seizure odor signature [58], thus paving the way for further research aimed at developing laboratory assays for measuring or monitoring these compounds and helping to prevent the onset of seizure episodes in predisposed individuals.
Narcolepsy
A preliminary report on the potential usefulness of dogs in detecting narcolepsy has recently been published by Dominguez-Ortega [59]. Two dogs trained to identify narcoleptic sweat samples were challenged with sweat samples collected from 12 narcoleptic and 22 healthy controls. The cumulative diagnostic sensitivity and specificity of the two dogs in identifying specimens collected from narcoleptic individuals were 0.92 and 0.86, respectively, averaging an area under the curve (AUC) of 0.89.
Migraine
Interesting evidence has also emerged from the analysis of studies based on patients suffering from migraine, a very limiting disorder whose prevalence is incessantly escalating around the world [60]. The very first evidence has been published by Dawn A. Marcus [61], who described eight cases of migraineurs attesting that their dogs developed certain types of migraine-alerting behaviors finalized to attract the attention of the owner even earlier than the painful symptoms of migraine onset. The most frequent abnormal behaviors included excessive attention to the owner and denial to leave the patient’s side, beginning minutes to hours in advance of the headache episode. Marcus and Bhowmick then published the results of a study based on the dissemination of an online survey to over 1000 adult migraineurs (94.9% women) [62]. A clearly acknowledgeable abnormality of their dog’s behavior before or during the initial phase of a migraine episode could be detected by nearly 60% of all participants, generally 2 h before the headache began. The most frequent alert behaviors encompassed denial to leave the patient’s side, unusual attentiveness to the owner and staring. Notably, up to 36% of responders declared that they began migraine therapy after recognizing an abnormal dog’s behavior, thus earlier than migraine symptoms had even started.
Infectious diseases
The potential use of sniffer dogs as mobile bio-detection tools has recently been highlighted in some interesting articles.
Maurer et al. published the results of a double-blinded, case-control study, where five dogs were trained to distinguish urine samples positive for bacteriuria [63]. The dogs were then challenged with as many as 687 urine samples, 231 (33.6%) testing positive for bacteriuria (191 for Escherichia coli, 19 for Klebsiella, 11 for Staphylococcus aureus, and 10 for Enterococcus, respectively). The five dogs exhibited an exceptional diagnostic performance for identifying positive urine samples, displaying 1.00 sensitivity and 0.91–0.96 specificity, respectively. No major differences could be observed in the capability of identifying different bacterial species.
Guest et al. collected nylon socks (and relative foot odors) of children aged between 5 and 13 years, presenting with or without symptoms of falciparum malaria [64]. Two dogs trained to identify malaria were then challenged with the children’s socks, displaying sensitivities and specificities comprised between 0.70–0.73 and 0.90–0.91, respectively. These results are not really unexpected, whereby the exhaled air of patients with Plasmodium falciparum infection is characterized by a clearly specific volatilome signature (i.e. the concentration of isoprene, acetone, benzene, carbon dioxide, cyclohexanone and four other thioethers varies considerably in people with active malarial infection) [65].
An interesting study has also been published by Angle et al. [66]. Briefly, the authors trained two adult male Labrador Retrievers to identify cell cultures infected with bovine viral diarrhea virus (BVDV), and then assessed their capability in distinguishing BVDV-infected cell cultures from those infected with bovine herpes virus 1 (BHV 1), bovine parainfluenza virus 3 (BPIV 3) or from uninfected cell cultures. Overall, the sensitivity and specificity of these two dogs in accurately identifying BVDV-infected cell cultures ranged between 0.85–0.97 and 0.98–0.99, respectively. This evidence would finally persuade us to conclude that “bio-detection” dogs could be trained in identifying specific pathogen targets, thus potentially serving as a highly accurate and mobile sensory means for rapid microbiological diagnosis and even anti-bioterrorism weapons.
Conclusions
The many important findings described in the previous sections of this article would hence cumulatively contribute to depict the dog smelling capacity applied to diagnostic medicine as a kind of “olfactory fingerprint” loop, which can be initiated at virtually any stage, as shown in Figure 2. It may start with the dog, as the evidence that the animal can sniff (and thereby anticipate or detect) a certain human pathology provides implicit evidence that the disease is associated with a specific volatilome, which can in turn be identified and dissected, paving the way to develop laboratory techniques for its routine assessment. On the other hand, the evidence that specific pathologies are associated with enhanced generation of certain VOCs may serve as a reliable basis for specifically training dogs to detect these compounds, even (or especially) in patients at an asymptomatic phase of disease. Due to their extremely fine analytical sensitivity, dogs may hypothetically be able to identify human diseases even earlier than present routine diagnostic investigations could do, using a substantially non-invasive approach, overcoming the many challenges associated with pre-analytical biospecimen management, while contextually providing companionship, loyalty and full devotion to their owners [67], [68]. Dogs have also very limited needs in terms of environment adaptation and are “fully portable”, even more easily than some point-of-care instruments. Unlike current laboratory techniques, their diagnostic efficiency is perhaps magnified by the fact that they would be capable of recognizing an entire olfactory fingerprint rather than a single compound. Dogs can then use a rich variety of signals to figure out what is probably happening to their owners. Not only can they accurately detect an “abnormal volatilome” as an index of something wrong occurring to their human companions, but they can also perceive some other visual, vocal and behavioral signals, which altogether would contribute to raise their alertness. This seems especially frequent in certain pathologies such as diabetes and seizures, whereby changes of posture or expression of owners are clear signs that the dog perceives and, perhaps unconsciously, translates into the conclusion that something bad or harmful will be happening soon [52]. A deep investigation of this innate dog’s ability can hence substantially expedite the development of new laboratory technology and instrumentation (i.e. biosensors and online MS-based e-Noses) aimed at reproducing the animal detection performance (Figure 2, Table 3). Although many future studies would be needed for more accurately identifying what trained and non-trained dogs are actually smelling (or – more generally – perceiving) on their owners, some automatic devices have been recently constructed for facilitating the training of dog sniffing capacity [69], and which may be ultimately effective in accelerating and improving the accuracy of detection.
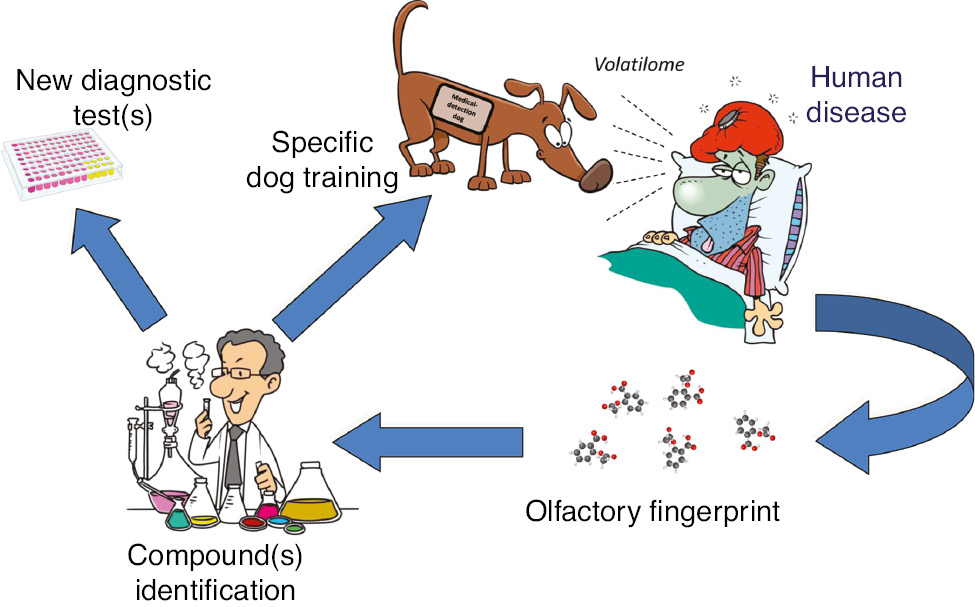
Synergy between dogs and laboratory testing for improving medical diagnostics.
That said, the most logical question here is whether we can envision a future with “bio-detection” or “medical-detection” (sniffer) dogs populating our hospitals, wearing lab equipment and performing complex diagnostics tasks (Figure 3). Neither would a crystal ball help predicting the possible scenarios, but some additional reflections can be made. First, the training of sniffer dogs, especially of “bio-detection” animals, is still a challenging and expensive activity. There are no official courses that have been established, nor have official guidelines been published on how these pets shall be educated. Then, despite some breeds being unquestionably better than others in smelling capability and the consequent disease detection (e.g. breeds selected for scent works outperform non-scent and short-nosed) [70], the inter-individual performance is also extremely variable, with some dogs capable of identifying cancerous specimens with a 1.00 sensitivity, whilst others performing only modestly better than chance.

Exemplar of “bio-detection”, “medical-detection” (sniffer) dog.
It seems hence reasonable to conclude that sniffer dogs will never be completely “diagnostic” for a given human disease, similar to the fact that when police dogs point a specific baggage this does not automatically translate into a final sentence of guilt, at least until illicit or unfair goods (drugs, tobacco, food, money, etc.) are objectively detected by policemen. Similarly, dogs could only serve for screening diseases, as additional diagnostic investigations would then be needed for confirming the final diagnosis and for staging the pathology. Nevertheless, there is now virtually incontrovertible evidence that man’s best friends can really improve the quality of life of many diseased people, whereby accurately trained dogs would be extremely efficient in alerting their owners on some imminent life-threatening conditions, such as hypo- or hyperglycemia crises, seizures, and even on migraine episodes. Although no clinical trials have been published so far, to the best of our knowledge, on dogs’ capability to anticipate an acute ischemic event, the results of a recent survey on as many as 181,696 patients with a previous episode of acute myocardial infarction (AMI) and 154,617 patients with a previous episode of ischemic stroke [71], revealed that patients owning dogs had a ~30% lower risk of death after hospitalization for AMI (hazard ratio, 0.67; 95% confidence interval, 0.61–0.75) or ischemic stroke (hazard ratio, 0.73; 95% confidence interval, 0.66–0.80). This would finally reinforce the concept that dogs’ friendship, loyalty and devotion, coupled with their extraordinary sensory capability, shall be seen as an exceptional boon for human health.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005;438:803–19.10.1038/nature04338Search in Google Scholar PubMed
2. Wayne RK, vonHoldt BM. Evolutionary genomics of dog domestication. Mamm Genome 2012;23:3–18.10.1007/s00335-011-9386-7Search in Google Scholar PubMed
3. Freedman AH, Wayne RK. Deciphering the origin of dogs: from fossils to genomes. Annu Rev Anim Biosci 2017;5:281–307.10.1146/annurev-animal-022114-110937Search in Google Scholar PubMed
4. Galibert F, Quignon P, Hitte C, André C. Toward understanding dog evolutionary and domestication history. C R Biol 2011;334:190–6.10.1016/j.crvi.2010.12.011Search in Google Scholar PubMed
5. Ostrander EA, Wayne RK, Freedman AH, Davis BW. Demographic history, selection and functional diversity of the canine genome. Nat Rev Genet 2017;18:705–20.10.1038/nrg.2017.67Search in Google Scholar PubMed
6. Yamamoto M, Hart LA. Professionally- and self-trained service dogs: benefits and challenges for partners with disabilities. Front Vet Sci 2019;6:179.10.3389/fvets.2019.00179Search in Google Scholar PubMed PubMed Central
7. Wackermannová M, Pinc L, Jebavý L. Olfactory sensitivity in mammalian species. Physiol Res 2016;65:369–90.10.33549/physiolres.932955Search in Google Scholar PubMed
8. Rouquier S, Giorgi D. Olfactory receptor gene repertoires in mammals. Mutat Res 2007;616:95–102.10.1016/j.mrfmmm.2006.11.012Search in Google Scholar PubMed
9. Palmieri B, Nardo B, Lippi G, Palmieri L, Vadalà M, Laurino C. Dogs’ olfactory diagnostics applied on human species: state of the art and clinical perspectives. Clin Ter 2016;167:e78–84.Search in Google Scholar
10. Padodara RJ, Jacob N. Olfactory sense in different animals. Indian J Vet Sci 2014;2:1–14.Search in Google Scholar
11. McGann JP. Poor human olfaction is a 19th-century myth. Science 2017;356. pii: eaam7263. doi: 10.1126/science.aam7263.Search in Google Scholar PubMed PubMed Central
12. Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci 2005;25:1806–15.10.1523/JNEUROSCI.4723-04.2005Search in Google Scholar PubMed PubMed Central
13. Robin S, Tacher S, Rimbault M, Vaysse A, Dréano S, AndréC, et al. Genetic diversity of canine olfactory receptors. BMC Genomics 2009;10:21.10.1186/1471-2164-10-21Search in Google Scholar
14. Quignon P, Rimbault M, Robin S, Galibert F. Genetics of canine olfaction and receptor diversity. Mamm Genome 2012;23:132–43.10.1007/s00335-011-9371-1Search in Google Scholar
15. Hall NJ, Glenn K, Smith DW, Wynne CD. Performance of pugs, German shepherds, and greyhounds (Canis lupus familiaris) on an odor-discrimination task. J Comp Psychol 2015;129:237–46.10.1037/a0039271Search in Google Scholar
16. Yang M, Geng GJ, Zhang W, Cui L, Zhang HX, Zheng JL. SNP genotypes of olfactory receptor genes associated with olfactory ability in German Shepherd dogs. Anim Genet 2016;47:240–4.10.1111/age.12389Search in Google Scholar
17. Gardner JW, Bartlett PN. A brief history of electronic noses. Sens Actuators B Chem 1994;18:211–20.10.1016/0925-4005(94)87085-3Search in Google Scholar
18. Wilson AD, Baietto M. Applications and advances in electronic-nose technologies. Sensors 2009;9:5099–148.10.3390/s90705099Search in Google Scholar PubMed PubMed Central
19. Herrero JL, Lozano J, Santos JP, Suárez JI. On-line classification of pollutants in water using wireless portable electronic noses. Chemosphere 2016;152:107–16.10.1016/j.chemosphere.2016.02.106Search in Google Scholar PubMed
20. Shi H, Zhang M, Adhikari B. Advances of electronic nose and its application in fresh foods: a review. Crit Rev Food Sci Nutr 2018;58:2700–10.10.1080/10408398.2017.1327419Search in Google Scholar PubMed
21. Amann A, Costello Bde L, Miekisch W, Schubert J, Buszewski B, Pleil J, et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res 2014;8:034001.10.1088/1752-7155/8/3/034001Search in Google Scholar PubMed
22. Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017;49:1600965.10.1183/13993003.00965-2016Search in Google Scholar PubMed
23. Martin HJ, Turner MA, Bandelow S, Edwards L, Riazanskaia S, Thomas CL. Volatile organic compound markers of psychological stress in skin: a pilot study. J Breath Res 2016;10:046012.10.1088/1752-7155/10/4/046012Search in Google Scholar PubMed
24. Heaney LM, Lindley MR. Translation of exhaled breath volatile analyses to sport and exercise applications. Metabolomics 2017;13:139.10.1007/s11306-017-1266-zSearch in Google Scholar
25. Huo R, Agapiou A, Bocos-Bintintan V, Brown LJ, Burns C, Creaser CS, et al. The trapped human experiment. J Breath Res 2011;5:046006.10.1088/1752-7155/5/4/046006Search in Google Scholar PubMed
26. Smith D, Španěl P, Herbig J, Beauchamp J. Mass spectrometry for real-time quantitative breath analysis. J Breath Res 2014;8:027101.10.1088/1752-7155/8/2/027101Search in Google Scholar PubMed
27. Heaney LM, Ruszkiewicz DM, Arthur KL, Hadjithekli A, AldcroftC, Lindley MR, et al. Real-time monitoring of exhaled volatiles using atmospheric pressure chemical ionization on a compact mass spectrometer. Bioanalysis 2016;8:1325–36.10.4155/bio-2016-0045Search in Google Scholar PubMed
28. Perez-Hurtado P, Palmer E, Owen T, Aldcroft C, Allen MH, Jones J, et al. Direct analysis of volatile organic compounds in foods by headspace extraction atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 2017;31:1947–56.10.1002/rcm.7975Search in Google Scholar PubMed PubMed Central
29. Rankin-Turner S, Turner MA, Kelly PF, King RS, Reynolds JC. Transforming presumptive forensic testing: in situ identification and age estimation of human bodily fluids. Chem Sci 2018;10:1064–9.10.1039/C8SC04133DSearch in Google Scholar PubMed PubMed Central
30. Heaney LM, Jones DJ, Suzuki T. Mass spectrometry in medicine: a technology for the future? Future Sci OA 2017;3:FSO213.10.4155/fsoa-2017-0053Search in Google Scholar PubMed PubMed Central
31. Pleil JD, Wallace MA, McCord J, Madden MC, Sobus J, FergusonG. How do cancer-sniffing dogs sort biological samples? Exploring case-control samples with non-targeted LC-Orbitrap, GC-MS, and immunochemistry methods. J Breath Res 2019;14:016006.10.1088/1752-7163/ab433aSearch in Google Scholar PubMed PubMed Central
32. Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, et al. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem 2011;83:1197–201.10.1021/ac103150aSearch in Google Scholar PubMed PubMed Central
33. Skaggs CL, Ren GJ, Elgierari ET, Sturmer LR, Shi RZ, ManickeNE, et al. Simultaneous quantitation of five triazole anti-fungal agents by paper spray-mass spectrometry. Clin Chem Lab Med 2020; doi: 10.1515/CCLM.2019.0895.Search in Google Scholar
34. Gao L, Song Q, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Anal Chem 2006;78:5994–6002.10.1021/ac061144kSearch in Google Scholar PubMed
35. Johno H, Yoshimura K, Mori Y, Kimura T, Niimi M, Yamada M, et al. Detection of potential new biomarkers of atherosclerosis by probe electrospray ionization mass spectrometry. Metabolomics 2018;14:38.10.1007/s11306-018-1334-zSearch in Google Scholar PubMed
36. Hiraoka K, Nishidate K, Mori K, Asakawa D, Suzuki S. Development of probe electrospray using a solid needle. Rapid Commun Mass Spectrom 2007;21:3139–44.10.1002/rcm.3201Search in Google Scholar PubMed
37. Rankin-Turner S, Ninomiya S, Reynolds JC, Hiraoka K. Sheath-flow probe electrospray ionization (sfPESI) mass spectrometry for the rapid forensic analysis of human body fluids. Anal Methods 2019;11:3633–40.10.1039/C9AY00698BSearch in Google Scholar
38. Jenkins EK, DeChant MT, Perry EB. When the nose doesn’t know: canine olfactory function associated with health, management, and potential links to microbiota. Front Vet Sci 2018;5:56.10.3389/fvets.2018.00056Search in Google Scholar PubMed PubMed Central
39. Walker DB, Walker JC, Cavnar PJ, Taylor JL, Pickel DH, Hall SB, et al. Naturalistic quantification of canine olfactory sensitivity. Appl Anim Behav Sci 2006;97:241–54.10.1016/j.applanim.2005.07.009Search in Google Scholar
40. Concha AR, Guest CM, Harris R, Pike TW, Feugier A, Zulch H, et al. Canine olfactory thresholds to amyl acetate in a biomedical detection scenario. Front Vet Sci 2019;5:345.10.3389/fvets.2018.00345Search in Google Scholar PubMed PubMed Central
41. Angle C, Waggoner LP, Ferrando A, Haney P, Passler T. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci 2016;3:47.10.3389/fvets.2016.00047Search in Google Scholar PubMed PubMed Central
42. Goldansaz SA, Guo AC, Sajed T, Steele MA, Plastow GS, Wishart DS. Livestock metabolomics and the livestock metabolome: a systematic review. PLoS One 2017;12:e0177675.10.1371/journal.pone.0177675Search in Google Scholar PubMed PubMed Central
43. Moser AY, Bizo L, Brown WY. Olfactory generalization in detector dogs. Animals (Basel) 2019;9. pii: E702. doi: 10.3390/ani9090702.Search in Google Scholar
44. Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet 1989;1:734.10.1016/S0140-6736(89)92257-5Search in Google Scholar
45. Jezierski T, Walczak M, Ligor T, Rudnicka J, Buszewski B. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. J Breath Res 2015;9:027001.10.1088/1752-7155/9/2/027001Search in Google Scholar PubMed
46. Lippi G, Cervellin G. Canine olfactory detection of cancer versus laboratory testing: myth or opportunity? Clin Chem Lab Med 2012;50:435–9.10.1515/cclm.2011.672Search in Google Scholar
47. Fischer-Tenhagen C, Johnen D, Nehls I, Becker R. A proof of concept: are detection dogs a useful tool to verify potential biomarkers for lung cancer? Front Vet Sci 2018;5:52.10.3389/fvets.2018.00052Search in Google Scholar PubMed PubMed Central
48. Junqueira H, Quinn TA, Biringer R, Hussein M, Smeriglio C, Barrueto L, et al. Accuracy of canine scent detection of non-small cell lung cancer in blood serum. J Am Osteopath Assoc 2019 Jun 17. doi: 10.7556/jaoa.2019.077. [Epub ahead of print].Search in Google Scholar PubMed
49. Guirao Montes Á, Molins López-Rodó L, Ramón Rodríguez I, Sunyer Dequigiovanni G, Viñolas Segarra N, Marrades Sicart RM, et al. Lung cancer diagnosis by trained dogs. Eur J Cardiothorac Surg 2017;52:1206–10.10.1093/ejcts/ezx152Search in Google Scholar PubMed
50. Pacik D, Plevova M, Urbanova L, Lackova Z, Strmiska V, Necas A, et al. Identification of sarcosine as a target molecule for the canine olfactory detection of prostate carcinoma. Sci Rep 2018;8:4958.10.1038/s41598-018-23072-4Search in Google Scholar PubMed PubMed Central
51. Lippi G, Cervellin G, Dondi M, Targher G. Hypoglycemia alert dogs: a novel, cost-effective approach for diabetes monitoring? Altern Ther Health Med 2016;22:14–8.Search in Google Scholar
52. Lippi G, Plebani M. Diabetes alert dogs: a narrative critical overview. Clin Chem Lab Med 2019;57:452–8.10.1515/cclm-2018-0842Search in Google Scholar PubMed
53. Rooney NJ, Guest CM, Swanson LC, Morant SV. How effective are trained dogs at alerting their owners to changes in blood glycaemic levels? Variations in performance of glycaemia alert dogs. PLoS One 2019;14:e0210092.10.1371/journal.pone.0210092Search in Google Scholar PubMed PubMed Central
54. Rooney NJ, Morant S, Guest C. Investigation into the value of trained glycaemia alert dogs to clients with type I diabetes. PLoS One 2013;8:e69921.10.1371/journal.pone.0069921Search in Google Scholar PubMed PubMed Central
55. Edney AT. Dogs and human epilepsy. Vet Rec 1993;132: 337–8.10.1136/vr.132.14.337Search in Google Scholar PubMed
56. Catala A, Cousillas H, Hausberger M, Grandgeorge M. Dog alerting and/or responding to epileptic seizures: a scoping review. PLoS One 2018;13:e0208280.10.1371/journal.pone.0208280Search in Google Scholar PubMed PubMed Central
57. Martos Martinez-Caja A, De Herdt V, Boon P, Brandl U, Cock H, Parra J, et al. Seizure-alerting behavior in dogs owned by people experiencing seizures. Epilepsy Behav 2019;94:104–11.10.1016/j.yebeh.2019.02.001Search in Google Scholar PubMed
58. Catala A, Grandgeorge M, Schaff JL, Cousillas H, Hausberger M, Cattet J. Dogs demonstrate the existence of an epileptic seizure odour in humans. Sci Rep 2019;9:4103.10.1038/s41598-019-40721-4Search in Google Scholar PubMed PubMed Central
59. Dominguez-Ortega L, Díaz-Gállego E, Pozo F, Cabrera García-Armenter S, Serrano Comino M, Dominguez-Sanchez E, et al. Narcolepsy and odor: preliminary report. Semergen 2013;39:e41–6.10.1016/j.semerg.2013.06.002Search in Google Scholar PubMed
60. Mattiuzzi C, Lippi G. Updates on migraine epidemiology. Eur J Neurol 2019 Nov 9. doi: 10.1111/ene.14120. [Epub ahead of print].Search in Google Scholar PubMed
61. Marcus DA. Canine responses to impending migraines. J Altern Complement Med 2012;18:106–8.10.1089/acm.2011.0773Search in Google Scholar PubMed
62. Marcus DA, Bhowmick A. Survey of migraine sufferers with dogs to evaluate for canine migraine-alerting behaviors. J Altern Complement Med 2013;19:501–8.10.1089/acm.2012.0234Search in Google Scholar PubMed PubMed Central
63. Maurer M, McCulloch M, Willey AM, Hirsch W, Dewey D. Detection of bacteriuria by canine olfaction. Open Forum Infect Dis 2016;3:ofw051.10.1093/ofid/ofw051Search in Google Scholar PubMed PubMed Central
64. Guest C, Pinder M, Doggett M, Squires C, Affara M, Kandeh B, et al. Trained dogs identify people with malaria parasites by their odour. Lancet Infect Dis 2019;19:578–80.10.1016/S1473-3099(19)30220-8Search in Google Scholar
65. Berna AZ, McCarthy JS, Wang RX, Saliba KJ, BravoFG, CassellsJ, et al. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J Infect Dis 2015;212:1120–8.10.1093/infdis/jiv176Search in Google Scholar PubMed PubMed Central
66. Angle TC, Passler T, Waggoner PL, Fischer TD, Rogers B, Galik PK, et al. Real-time detection of a virus using detection dogs. Front Vet Sci 2016;2:79.10.3389/fvets.2015.00079Search in Google Scholar PubMed PubMed Central
67. Lippi G, Betsou F, Cadamuro J, Cornes M, Fleischhacker M, Fruekilde P, et al. Preanalytical challenges – time for solutions. Clin Chem Lab Med 2019;57:974–81.10.1515/cclm-2018-1334Search in Google Scholar PubMed
68. Lippi G, Simundic AM. The preanalytical phase in the era of high-throughput genetic testing. What the future holds. Diagnosis (Berl) 2019;6:73–4.10.1515/dx-2018-0022Search in Google Scholar PubMed
69. Edwards TL. Automated canine scent-detection apparatus: technical description and training outcomes. Chem Senses 2019;44:449–55.10.1093/chemse/bjz039Search in Google Scholar PubMed
70. Polgár Z, Kinnunen M, Újváry D, Miklósi Á, Gácsi M. A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS One 2016;11:e0154087.10.1371/journal.pone.0154087Search in Google Scholar PubMed PubMed Central
71. Mubanga M, Byberg L, Egenvall A, Ingelsson E, Fall T. Dog ownership and survival after a major cardiovascular event: a register-based prospective study. Circ Cardiovasc Qual Outcomes 2019;12:e005342.10.1161/CIRCOUTCOMES.118.005342Search in Google Scholar PubMed
©2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Advancements in mass spectrometry as a tool for clinical analysis: part II
- Quantitative protein assessment
- Complexity, cost, and content – three important factors for translation of clinical protein mass spectrometry tests, and the case for apolipoprotein C-III proteoform testing
- Vedolizumab quantitation using high-resolution accurate mass-mass spectrometry middle-up protein subunit: method validation
- Development and evaluation of an element-tagged immunoassay coupled with inductively coupled plasma mass spectrometry detection: can we apply the new assay in the clinical laboratory?
- MALDI-MS for the clinic
- Matrix-assisted laser desorption ionisation (MALDI) mass spectrometry (MS): basics and clinical applications
- Clinical use of mass spectrometry (imaging) for hard tissue analysis in abnormal fracture healing
- Cellular resolution in clinical MALDI mass spectrometry imaging: the latest advancements and current challenges
- Bacterial identification by lipid profiling using liquid atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry
- Clinical application of ’omics technologies
- Individualized metabolomics: opportunities and challenges
- Diagnostic amyloid proteomics: experience of the UK National Amyloidosis Centre
- The “olfactory fingerprint”: can diagnostics be improved by combining canine and digital noses?
- Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis
- The influence of hypoxia on the prostate cancer proteome
- Laboratory automation and kit-based approaches
- Mass spectrometry and total laboratory automation: opportunities and drawbacks
- The pathway through LC-MS method development: in-house or ready-to-use kit-based methods?
- Evaluation of the 25-hydroxy vitamin D assay on a fully automated liquid chromatography mass spectrometry system, the Thermo Scientific Cascadion SM Clinical Analyzer with the Cascadion 25-hydroxy vitamin D assay in a routine clinical laboratory
Articles in the same Issue
- Frontmatter
- Editorial
- Advancements in mass spectrometry as a tool for clinical analysis: part II
- Quantitative protein assessment
- Complexity, cost, and content – three important factors for translation of clinical protein mass spectrometry tests, and the case for apolipoprotein C-III proteoform testing
- Vedolizumab quantitation using high-resolution accurate mass-mass spectrometry middle-up protein subunit: method validation
- Development and evaluation of an element-tagged immunoassay coupled with inductively coupled plasma mass spectrometry detection: can we apply the new assay in the clinical laboratory?
- MALDI-MS for the clinic
- Matrix-assisted laser desorption ionisation (MALDI) mass spectrometry (MS): basics and clinical applications
- Clinical use of mass spectrometry (imaging) for hard tissue analysis in abnormal fracture healing
- Cellular resolution in clinical MALDI mass spectrometry imaging: the latest advancements and current challenges
- Bacterial identification by lipid profiling using liquid atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry
- Clinical application of ’omics technologies
- Individualized metabolomics: opportunities and challenges
- Diagnostic amyloid proteomics: experience of the UK National Amyloidosis Centre
- The “olfactory fingerprint”: can diagnostics be improved by combining canine and digital noses?
- Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis
- The influence of hypoxia on the prostate cancer proteome
- Laboratory automation and kit-based approaches
- Mass spectrometry and total laboratory automation: opportunities and drawbacks
- The pathway through LC-MS method development: in-house or ready-to-use kit-based methods?
- Evaluation of the 25-hydroxy vitamin D assay on a fully automated liquid chromatography mass spectrometry system, the Thermo Scientific Cascadion SM Clinical Analyzer with the Cascadion 25-hydroxy vitamin D assay in a routine clinical laboratory


