Congress of Laboratory Medicine and Clinical Chemistry 7th Annual Meeting of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC)
Under the auspices of
European Federation of Clinical Chemistry and Laboratory Medicine (EFLM)
International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
World Association of Societies of Pathology and Laboratory Medicine (WASPaLM)
Co-operating Societies
Österreichische Gesellschaft für Gute Analysen- und Laborpraxis (GALP)
Österreichische Gesellschaft für Qualitätssicherung und Standardisierung
medizinisch-diagnostischer Untersuchungen (ÖQUASTA)
European Autoimmunity Standardisation Initiative (EASI)
Congress President
Alexander Haushofer (Wels-Grieskirchen, Austria)
Congress Secretary
Gregor Hörmann (Wien, Austria)
Scientific Committee
Baumann Gabriele, Steyr; Einwallner Elisa Franziska, Wien; Endler Georg, Wien; Griesmacher Andrea, Innsbruck; Haushofer Alexander, Wels-Grieskirchen;
Herold Manfred, Innsbruck; Hörmann Gregor, Wien; Kessler Harald H., Graz;
Leixner Georg, St. Pölten; Mustafa Hans Georg, Salzburg;
Orth Matthias, Stuttgart; Perné Andrea, Klagenfurt; Renner Wilfried, Graz;
Schweiger Christian, Wien; Seger Christoph, Schaan; Stimpfl Thomas, Wien;
Sunder-Plassmann Raute, Wien; Wagner Oswald, Wien;
CLINICAL CHEMISTRY
A13
Pseudo-High-T3-syndrome
W Prokop, A Griesmacher, M Anliker, V Fux
Landeskrankenhaus Innsbruck, 6020 Innsbruck, Austria
Background: High-T3-syndrome is characterized by isolatedly elevated fT3 while fT4 and TSH are in the norm and is a rare course of non-thyroidal-illness-syndrome. It appears with an increased activity of the peripheral deiodinase, which can be observed in patients with post-traumatic stress disorder (1). High-T3-syndrome is described as a rare condition in the literature. The true prevalence of the disease could not be found and is strongly dependent on the study group. Aim: Verification of isolated elevated fT3-results whether they are attributable to high-T3-syndrome or can be related to the methodological issues of fT3 testing. Methods: TSH, fT4 and fT3 were routinely measured on Elecsys (Roche Diagnostics). Architect (Abbot) and Access II (Beckman Coulter) were applied as alternative methods for fT3 testing. In detail, all samples with isolatedly elevated fT3 on Elecsys were subsequently remeasured on Architect. In case of a significantly different result between these methods we would repeat the measurement of fT3 with a third method (Access II). We defined a significant discrepancy when fT3-results on Architect were below 67% of the results measured on Elecsys. In each case with fT3-discrepancy the result was validated by a laboratory doctor. Results: In the years 2015, 2016 and 2017 we analyzed nearly 100,000 samples for fT3. 780 samples (0.78%) showed an elevated fT3 on the Elecsys platform while fT4 and TSH were within the reference range.
Of these, 500 samples (66.7%) showed a concordant fT3 result on the Architect platform.
However, 250 samples (33.3%) showed discrepant results and were additionally tested on the Access II platform. Of these 250 strongly discrepant samples, results from Access II were issued in 170 cases. In the end only 750 initially isolatedly elevated fT3-constellations were issued, whereas isolated fT3 elevation could not be confirmed in 30 cases. Conclusions: Isolated elevation of fT3 is not always related to a High-T3-syndrome, but can presumably be a frequent methodological query of the fT3 analytics. Diverse methodologies with insufficient standardization and missing international reference material currently do not lead to better results (2, 3, 5, 6, 7, 8, 9 ,10, 11). According to the company Roche Diagnostics the cause for falsely elevated fT3-results are disturbing antibodies of the IgG-type of the sample against monoclonal anti-T3-antibody (of sheep) of the reagent (4). Manufacturers are endeavoured to reduce the attack surface of the monoclonal reagent-antibody by using truncated antibody-fragments. Other laboratories that perform thyroid hormone analysis on the Elecsys platform should be aware of this condition.
1. Chatzitomaris et al., 2017. Thyroid Allostasis–Adaptive Responses of Thyrotropic Feedback Control to Conditions of Strain, Stress, and Developmental Programming. Front. Endocrinol. 8:163. doi: 10.3389/fendo.2017.00163. PMID 28775711
2. TSH-Packungsbeilage der Fa. Roche Diagnostics
3. FT4-leaflet from Roche Diagnostics
4. FT3-leaflet from Roche Diagnostics
5. FT3-leaflet from Abbott
6. FT3-leaflet from Beckman Coulter
7. Product Information on fT3 III from Roche Diagnostics
8. Veitl et al. Comparison of immunoassays for reproduction- and thyroid-hormones performed on five automated analysers: ARCHITECT, Axsym, Centaur, Elecsys 2010 and IMMULITE 2000. J Lab Med. 2002;26(3/4):191-202.
9. Lewandowski et al. Case report: When measured free T4 and free T3 may be misleading. Interference with freee thyroid hormones measurements on Roche® and Siemens® platforms. Thyroid Research 2012,5:11
10. Fillée et al. Comparison of three free T4 (FT4) and free T3 (FT3) immunoassays in healthy subjects and patients with thyroid diseases and severe non-thyroidal illnesses. Clin Lab. 2012;58(7-8):725-36.
11. Korte et al. Performance evaluation of the Access FT3 and FT4 assays, comparison with Immulite and Axsym, and the relationship to TSH values. Clin Lab. 2012;58(7-8):645-57.
A14
HBV Pre-S deletion – a case report and literature review
V Fux, A Griesmacher, M Anliker, H Zoller
Central institute of medical and chemical laboratory diagnostics; hepatological laboratory – department for internal medicine I; Innsbruck university clinic, Innsbruck, Austria
Background: Hepatitis B virus (HBV) has a double-stranded relaxed circular DNA genome (1). HBV infection is still a health issue that affects close to 240 million people in the world (2). Patients can be in an asymptomatic carrier state (3) or manifest with acute and chronic hepatitis, liver failure, liver cirrhosis and hepatocellular carcinoma (HCC) (4). The genetic variability of the hepatitis B virus has an effect on many aspects of HBV infections including the severity of the disease, the response of the host‘s immune system, the response to therapy and the requirement for more intensive follow-up strategies (10). Design: Case presentation. Patient: During pregnancy screening a 42-year-old female patient showed highly positive for HBsAg and anti-HBs, while also positive for anti-HBc (IgG), negative for anti-HBc (IgM), negative for HBe-Ag and positive for HBe-AK. A co-infection with HDV, HCV and HEV could be ruled out. DNA-sequencing revealed HBV pre-S deletion, explaining the positivity for both HBsAg and anti-HBs. Conclusion: The importance of pre-S/S variants has gained increased awareness among clinicians. Different variants favour the progression to liver disease through distinct means (6). Commonly occurring hepatitis B virus pre-S/S variants have been described and show an association with progressive liver diseases, especially with the development of HCC (5). It comes into sight that patients with chronic hepatits B infection and unclear hepatitis B serology should undergo screening for pre-S/S mutations to identify those patients who require an appropriate treatment and more in-depth follow-up scheme as these variants may be undetectable by commercially available HBsAg assays (6).
1. Weifan G, Jianming H. J Virol 2007; 81:6164-6174
2. Noele N, Philippa E, Brian M. Clin Liver Dis 2016; 20: 607-628
3. Maria M, Isabel P. GE Port J Gastroenterol 2015; 22: 47-51
4. Jake L. Hepatology 2009; 49:13-21
5. Zheng Z. World J Gastroenterol. 2014; 20:7996-7706
6. Pollicino T, Irene C, Francesca S, Giovanni R. Journal of Hepatology 2014:61: 408-417
CLINICAL CHEMISTRY & IMMUNOLOGY
A02
Association between increased plasma homocysteine levels and depression observed in individuals with primary lactose malabsorption
D Enko1,2, A Meinitzer2, W Brandmayr3, G Halwachs-Baumann1, Gernot Kriegshäuser1,2
1Institute of Clinical Chemistry and Laboratory Medicine, General Hospital Steyr, Steyr, Austria;
2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria;
3Department of Psychiatry and Psychotherapeutic Medicine, General Hospital Steyr, Steyr, Austria
Introduction: Homocysteine (HCY) is a non-proteogenic thiol amino acid, which is generated from the methionine metabolism through demethylation. Current literature reports associations between HCY, folic acid (FA), vitamin B12 metabolism and depression. However, the exact underlying biological mechanisms remain unclear. This study aimed at evaluating a possible link between primary adult-type lactose malabsorption (PALM), HCY, FA, vitamin B12 metabolism and depressive disorder.
Methods: Plasma levels of HCY, FA and vitamin B12 were determined in 78 patients with PALM and 160 individuals with lactase persistence sub-grouped by the presence or absence of major depression.
Results: In 78 patients with PALM, the subgroup of 22 individuals with major depression showed significantly higher median (interquartile range) HCY (10.10 [8.46 – 12.03] vs. 8.9 [7.54 – 9.86] μmol/L, p=0.029) and lower plasma FA levels (5.7 [4.68 – 9.14] vs. 6.95 [5.24 – 10.56] μmol/L, p=0.272) compared to the subgroup of 56 individuals without depression, respectively. No such associations could be observed for those 160 individuals without PALM (i.e., lactase persistence).
Plasma HCY levels were positively correlated with depressive symptoms (p=0.052), and showed negative correlations with FA (p=< 0.001) and vitamin B12 (p=0.029), respectively.
Conclusions: Depressed individuals with PALM showed significantly higher HCY and lower FA levels compared to non-depressed individuals with PALM. This association was absent in the subgroup of lactase persistent individuals. Present data suggest an association between increased HCY levels, lactose malabsorption and depression.
A03
Performance evaluation of a liquid chromatography-mass spectrometry method for quantitative determination of serum hepcidin-25 levels
D Enko1,2, S Zelzer2, M Herrmann2, Günter Fauler2
1Institute of Clinical Chemistry and Laboratory Medicine, General Hospital Steyr, Steyr, Austria;
2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria
Introduction: In the last decade the knowledge of hepcidin-25 as one of the key regulators of the human iron homeostasis has increased. The quantitative analysis of this acute-phase protein is of great interest. Nevertheless, the implementation of liquid chromatography-tandem mass-spectrometry LC-MS/MS assays for protein analysis in a clinical routine laboratory setting is still uncommon. The present study aimed at evaluating the analytical and pre-analytical performance of the Hepcidin-25 LC-MS/MS Kit (Immundiagnostic AG, Bensheim, Germany) for quantification of this protein.
Methods: The LC-MS/MS method was evaluated according to international guidelines. The two-point calibration proposed by the manufacturer was replaced by an in-house six-point calibration curve. We investigated sample stability at room temperature (RT) and after repeated freeze-/thaw-cycles. Additionally, we assessed serum hepcidin-25 levels of 165 healthy adult individuals referred for a medical check-up.
Results: The hepcidin-25 LC-MS/MS assay was linear over the concentration range of 3 – 200 ng/mL. The limit of detection (LOD) and the limit of quantification (LOQ) were 1 and 3 ng/mL. Within- and between-run precision ranged between 1.9 – 8.6% and 5.1 – 12.4%. The observed average recovery was 96.7%, respectively. At RT samples were stable for 3 h (mean increase: + 0.29%). After two and three freeze-/thaw-cycles hepcidin-25 concentrations showed an increase of + 8 and + 20%. Out of 165 healthy adults, 109 females had a significantly lower median of 8.42 (range: 1 – 60.1) ng/mL compared to 56 males with 15.76 (range: 1.5 – 60.5) ng/mL (p=0.002), respectively.
Conclusions: The hepcidin-25 LC-MS/MS assay evaluated here has a broad analytical range with reliable analytical performance. Serum samples can be stored at RT for at least 3 h and resist up to 2 freeze-/thaw-cycles. However, a multi-point calibration is recommended in order to provide optimized precision.
A04
Associations between Helicobacter pylori status, eradication therapy and different biomarkers of iron metabolism
D Enko1,2, H Wagner3, G Kriegshäuser1,2, G Halwachs-Baumann1, WJ Schnedl4, S Zelzer2, G Fauler2, M Herrmann2, Andreas Meinitzer2
1Institute of Clinical Chemistry and Laboratory Medicine, General Hospital Steyr, Steyr, Austria
2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University Graz, Graz, Austria
3Department of Applied Statistics, Johannes Kepler University Linz, Linz Austria
4Practice for General Internal Medicine, Bruck an der Mur, Austria
Introduction: A number of previously published studies evaluated a possible link between H. pylori infection and low plasma ferritin levels. However, study designs, which investigate possible associations between H. pylori status and alternative laboratory biomarkers of iron metabolism, are still lacking. Therefore, this study aimed at investigating iron deficiency (ID) with conventional (ferritin, transferrin saturation [TSAT]) and less commonly used iron biomarkers (soluble transferrin receptor [sTfR], sTfR/log ferritin, reticulocyte haemoglobin content [CHr], hepcidin-25) in patients sub-grouped by the presence or absence of H. pylori infection.
Methods: In total, 200 outpatients, who were referred for the H. pylori 13C-urea breath test (13C-UBT), underwent blood testing for ID. Anamnesis was carried out about completed eradication therapy. Thomas-Plot (TP)-analyses (sTfR/log ferritin, CHr) were calculated. Additionally, logistic regression models including the covariates age, female sex, positive H. pylori 13C-UBT and completed eradication therapy were fitted for different definitions of ID.
Results: Fifty-three patients with H. pylori infection showed significantly higher median plasma levels (ranges) of sTfR (1.22 [0.78 – 3.6] vs. 1.11 [0.62 – 4.57] mg/L, P=0.02) and sTfR/log ferritin ratios (0.78 [0.34 – 7.55] vs. 0.64 [0.32 – 9.58], P=0.02), and in tendency lower ferritin (39 [3 – 362] vs. 54 [3 – 317] μg/L, P=0.09) and Hb-values (13.6 [8.3 – 16.4] vs. 14 [7.8 – 16.9] g/dL, P=0.05) compared to 147 H. pylori-uninfected individuals. Based on a ferritin<30 μg/L and/or a TSAT<20%, 88/200 patients (25 with positive H. pylori 13C-UBT) (44.0%) were identified with ID, based on TP-analyses, 27/200 individuals (9 with positive H. pylori 13C-UBT) showed ID, respectively. Overall, 88/200 (43.5%) individuals had completed a triple therapy (proton pump inhibitor + 2 antibiotics). Logistic regression analysis for the probability of ID according to TP showed significant effects of eradication therapy (P=0.019). Interestingly, 8/9 (88.9%) individuals with functional ID (CHr<28 pg) had completed such a kind of therapy, respectively.
Conclusions: H. pylori infection was associated with significantly higher plasma sTfR and sTfR/log ferritin levels and tendentiously lower ferritin and Hb-values. Patients with H. pylori eradication therapy were more often detected with functional ID compared to patients without therapy. To get better insight in the relationship between H. pylori therapy and functional ID, we suggest conducting further prospective studies including longitudinal TP-analyses before and after eradication procedures.
A10
Comparison of ferritin values obtained from capillary self-sampling versus conventional venipuncture
C Conrad-Billroth1, L Diem1, Ch Bisanz1, M Exner2,3, G Endler G2,3
1Nevos, Vienna, Austria
2Labors.at, Vienna, Austria
3Sigmund Freud University, Vienna, Austria
Background: Iron deficiency is the major cause of chronic anemia, affects up to 30% of the Austrian population and is highly underdiagnosed. Especially younger women are at higher risk of developing iron deficiency. Self-sampling at home of capillary blood for ferritin determination could lead to a faster and more accurate diagnosis of iron deficiency. In this study, we evaluated the suitability of self-sampled capillary blood versus conventional venipuncture for ferritin determination.
Patients and methods: 61 apparently healthy individuals (mean age 33 years, 46 females) sampled capillary blood at home and subsequently had a venipuncture within 6 hours for simultaneous ferritin determination. Ferritin in both samples was determined simultaneously turbidimetricaly on an automated clinical chemistry analyzer (Roche Cobas 8000, Roche Inc., Switzerland).
The study was approved by the local ethic committee and all volunteers gave their written informed consent.
Results and discussion: 10 capillary samples had to be discarded due to severe hemolysis and 4 due to insufficient sample volume. However, our data indicates the high rate of inadequate samples can be reduced by improving instructions to the probands. Both samples showed an excellent correlation (R=0,98, p<0,001), however overall ferritin levels were slightly higher in capillary samples (mean 58 μg/l ±6 μg/l) than in venous samples (mean 51 μg/l ±6 μg/l), which seems acceptable due to the intraindividual day by day variability of Ferritin which is ~15%. According to the WHO definition for iron deficiency, which is defined as <30 μg/l, 18 of 47 individuals (38%) were to be considered iron deficient which is comparable to the reported prevalence. 72% of these individuals had concordant results in both samples whereas the others had values slightly above the cutoff levels in the capillary sample.
Conclusion: If performed correctly, capillary self-sampling is an excellent, low-threshold alternative for broad screening for iron deficiency. Ferritin values showed a very good correlation compared to venous sampling. However, due to a tendency towards higher ferritin levels in capillary samples a grey value (30-45 μg/l) should be defined to identify all individuals with latent iron deficiency.
A11
Retrospective Evaluation of the QuantiFERON®-TB Gold-ELISA Assay as a Part of the In-vitro Diagnostic Process for Mycobacterium Tuberculosis
M Kompiller1, G Weigl2, E Mara1, W Hübl2
1Fachhochschule Wiener Neustadt, Wiener Neustadt, Austria
2Inst. f. Labormedizin, Otto-Wagner-Spital, Vienna, Austria
This investigation comprises a retrospective evaluation of the QuantiFERON®-TB Gold-ELISA Interferon-Gamma-Release-Assay (IGRA) in the in-vitro diagnostic process of Mycobacterium tuberculosis (MTB) as performed in a routine clinical laboratory. Overall, 10,226 feasible IGRA results (period 1.1.2011 to 31.10.2016) from the LIS database of the Institute of Laboratory Medicine, Otto-Wagner-Hospital, Vienna, Austria, were used and statistically evaluated together with respective Auramin/Ziehl-Neelsen (A/ZN) sputum staining and MTB-PCR results. On the other hand, a logistic regression model was established comprising age and sex of patients together with the laboratory tests mentioned and a positive MTB diagnosis as coded in the overall patient report as the dependent variable for this model. With regard to A/ZN screening the sensitivity of the IGRA was 76% and the specificity was 65%. The diagnostic performance of the IGRA as compared to MTB-PCR showed a sensitivity of 92% and a specificity of 64%. Logistic regression analysis showed a statistically significant relation between a positive clinical MTB outcome and the following independent variables: patient’s age, qualitative A/ZN screening results, qualitative MTB-PCR results, and both qualitative as well as quantitative IGRA test results (AUC 0.851; p<0.0001). Furthermore, the logistic regression model demonstrated that positive A/ZN screening results showed a 3.3 fold increased risk for a positive MTB outcome (CI-95 % 2.0173 – 5.2382), whereas positive MTB-PCR results increased this risk with a factor of 3.7 (CI-95 % 2.1829 – 6.1717) and positive IGRA results with a factor of 4.8 (CI-95 % 3.0507 – 7.5153). In conclusion, the data presented show that false negative IGRA results as compared to available results of in-vitro MTB diagnostic tests were approximately within the scope as respective data from scientific literature studies (false negative IGRA rates vary from 8 to 19%). On the other hand, the specificity of the IGRA analytics was poor and the method seems not to be feasible to demonstrate a relationship with a positive bacteriological outcome (A/ZN screening, MTB-PCR) as presented in this context.
A12
Method Comparison of two Automatized In-vitro Test Systems for Serologic Hepatitis-B Diagnostics
S Dieterich1, G Weigl2, M Krutz2, S Pauschenwein-Frantsich1, W Hübl2
1Fachhochschule Wiener Neustadt, Wiener Neustadt, Austria
2Inst. f. Labormedizin, Otto-Wagner-Spital, Vienna, Austria
The aim of this investigation was the evaluation of the diagnostic performance of two chemiluminescence immunoassay systems, the Siemens ADVIA Centaur XPT, and the DiaSorin LIAISON, regarding assays for the detection of Hepatitis B (HB) virus (HBV) surface antigen (HBs-AG), and antibodies against HBs (anti-HBs) and core antigen (anti-HBc). Eligible routine samples of the Institute of Laboratory Medicine, Otto-Wagner-Hospital, Vienna, Austria, from 99 patients were collected, frozen, and stored until analysis in batch (84 samples were tested for HBs-AG and anti-HBc, 97 samples were analyzed for anti-HBs on both systems). To evaluate potential effects of the freezing process, 97 of the original tests results reported to clinicians were available for comparison with the results measured for this investigation. Furthermore, HBV-PCR results of 27 patients and the medical history (serological HBV-parameters) of 27 patients were collected from the laboratory information system. For the comparison of the diagnostic performance, the CENTAUR system used in daily routine processing was defined as the “reference system”. The diagnostic efficiency regarding the HBs-AG assays was 100%. The comparison of the anti-HBs assays, also including the available medical history of the patients, resulted in a specificity of 98%, a sensitivity of 100%, and a diagnostic efficiency of 99%. Quantitative anti-HBs analytics showed an overall poor correlation of measurements (r=0.54; n=97) and CENTAUR measurements were approximately 25% lower than respective LIAISON measurements (Passing-Bablok regression; Slope=0.7568) with a statistically significant difference of sample medians of 4.25 mIE/mL (IQR 3.1000 – 222.8950) and 6.57 mIE/mL (IQR 3.0000 – 301.2500) for the CENTAUR and the LIAISON system, respectively (Wilcoxon test; p=0.0023). Regarding the anti-HBc assay, the diagnostic performance resulted in a specificity of 96%, a sensitivity of 90% and a diagnostic efficiency of 93%. There was no significant difference between the assays regarding the number of test results within the quantitative gray zones as defined by the respective reagent manufacturer (anti-HBs, p=0.68; anti-HBc, p=0.46; Fisher-Exact-Test). This concludes that neither system has a notable advantage with regard to this aspect. Comparing the original test results of the CENTAUR with the tests performed for this investigation, particular cases showed a decrease of antibody concentration what might be associated with the freezing process. Direct comparison of the assays showed that especially qualitative results of the anti-HBc assay showed differences between the test systems evaluated in a handful of cases, leading to the necessity of re-evaluating distinct cases with dubious or implausible results (eg. gray zone results) with an alternative test system.
A16
Heavy / light chain assay versus other quantitative methods for the diagnosis of IgM multiple myeloma Patients
J Radek1, P Birkner-Krackowizer1, I Gruber1, E Ambrus1, D Schuhmeister1, G Leixner1, P Hopmeier1, AC Haushofer2, D Trubert-Exinger1
1University Hospital St. Pölten, Institute of Laboratory Medicine, St. Pölten, Austria
2Institute of Laboratory Medicine & Blood Bank Hospital Wels-Grieskirchen, Austria
Background: The heavy / light chain (HLC) immunoassays (Hevylite®, The Binding Site, UK) separately identify and quantify the immunoglobulin isotypes like IgM-Kappa and IgM-Lambda. This enables the calculation of the heavy / light chain Kappa/Lambda ratio (HLCR) which provides additional information about the underlying disease. The HLC assays are used for diagnosis, monitoring, response assessment and prognosis of monoclonal gammopathies.
Objectives: The aim of the study was to assess the correlation between HLCR and other standard laboratory methods. Results were compared retrospectively with serum immunofixation electrophoresis (sIFE), total IgM, total light chains and serum free light chains (sFLC).
Patients and Methods: Sera from 42 IgM myeloma patients, selected on the basis of positive sIFE results, were studied. sFLC (Freelite®, The Binding Site, UK) and HLC were quantified using polyclonal antisera assays on the BNTMII nephelometer (Siemens Healthcare Diagnostics Inc). Total IgM and total light chains were measured on the BNTMII. sIFE was performed using the Sebia Hydrasys® 2 system.
Results: An abnormal IgM-HLCR was present in 85,7 % of the IgM samples with a positive sIFE result. Total IgM was abnormal elevated in 76,2 % and total light chain ratio was abnormal in 47,6 % of the cases.
An abnormal sFLC ratio was found in 100 % of the sIFE positive samples.
Two patients with a biclone (IgM-Lambda, IgG-Kappa) which presented with normal values of total IgM and normal total light chain ratio but abnormal HLCR had been identified.
Conclusion: A 85,7% and a 100 % agreement with positive sIFE was found with an abnormal IgM-HLCR as well with an abnormal FLC ratio. HLCR was more sensitive than total IgM or total light chain ratio. The separate analysis of involved and uninvolved HLC could give additional information in case of biclonality.
Hevylite and Freelite are registered trademarks of The Binding Site Group Ltd (Birmingham, UK) in certain countries; BNII is a trademark of Siemens Healthcare Diagnostics Inc.; Sebia Electrophoresis is a registered trademark of Sebia, Inc.
A18
Tryptophan, kynurenine and neopterin serum levels during dengue virus infection
S Geisler1, S Lytton2, NL Toan3, TH Nghia4, NM Nam4, HV Hung4, DT Anh4, HT Tuyen4, TV Tien4, NT Thai Son4, HV Tong3, TP Velavan3, JM Gostner5, D Fuchs1
1,5Medical University of Innsbruck, 2SeraDiaLogistics, 3,4Vietnam Military Medical University
1Division of Biological Chemistry, Biocentre, 3Department of Pathophysiology, 4103 Military Hospital, 5Division of Medical Biochemistry
1,5Innsbruck, Austria, Munich, Germany, 3,4Ha Dong, Hanoi, Vietnam
Dengue virus (DENV) disease is one of the most important arboviral diseases around the world. DENV infection induces immune system activation including the production of the immune system biomarker neopterin [1] and the activation of the tryptophan (Trp) degrading enzyme indoleamine 2,3 dioxygenase-1 (IDO-1) [2,3] as is indicated by an increased kynurenine to tryptophan ratio (Kyn/Trp) in the blood of infected patients. In this study, serum samples from seventy-four patients with indication of DENV infection were screened for serum neopterin, tryptophan and kynurenine levels. Compared to healthy blood donors, the mean neopterin concentration (33.8 ± 13.3 nmol/L) was significantly elevated. Likewise, the mean Kyn/Trp (93.4 ± 57.4 μmol/mmol) was significantly increased. Fifty-six patients were confirmed positive for DENV by RT-PCR. This group presented with significantly higher neopterin levels (36.5 ± 11.7 nmol/L) and Kyn/Trp (102.0 ± 61.3 μmol/mmol) compared to individuals with DENV negative testing results. The association between neopterin and Kyn/Trp concentrations was strongly positive but interestingly was even stronger in the DENV- (rl=0.886, p <0.001) than in the DENV+ patients (rl=0.366, p <0.01). Moreover, in a subgroup of 13 patients the course of neopterin levels and Kyn/Trp ratios were monitored during follow-up and a significant decrease of neopterin levels and Kyn/Trp ratios was observed within 10 days after hospital entry. Our study confirms and extends earlier findings about an important role of neopterin and tryptophan metabolism in the course of DENV infection, both the biochemical pathways are strongly inducible by the Th1-type cytokine interferon-γ. The strong correlation between neopterin and Kyn/Trp concentrations observed in the DENV- group of patients may indicate that they suffered from infections other than DENV, probably viral, which have not been screened for but evoked the immune response which underlies the parallel increase of neopterin and Kyn/Trp concentrations. The abnormal tryptophan metabolism in DENV patients could also influence serotonin availability in platelets and play a role in the pathogenesis of hemorrhagic fever. Future follow up studies will show whether the monitoring of neopterin and Kyn/Trp concentrations is able to reflect the course of infections in the patients and to predict outcome. Moreover, especially the changes of tryptophan metabolism are likely to influence serotonin production and may relate to the neuropsychiatric symptomatology in the patients with DENV infection.
[1] Chan CP, Choi JW, Cao KY, Wang M, Gao Y, Zhou DH, Di B, Xu HF, Leung MF, Bergmann A, Lehmann M, Nie YM, Cautherley GW, Fuchs D, Renneberg R, Zheng BJ. J Infect 2006;53:152-8
[2] Becerra A, Warke RV, Xhaja K, Evans B, Evans J, Martin K, de Bosch N, Rothman AL, Bosch I. J Gen Virol 2009;90:810-7
[3] Villar LÁ, Gélvez RM, Rodríguez JA, Salgado D, Parra B, Osorio L, Bosch I .Biomedica 2013;33 Suppl 1:108-16
A24
Incidence and outcome of secondary hemophagocytic lymphohistiocytosis among SIRS patients on standard care wards
F Ratzinger1*, G Gualdoni2*, G A Hofmann2, P Wohlfarth3, H Haslacher1, H Agis4, H-M Winkler2, S Winkler2, H Burgmann2; *contributed equally
1Department of Laboratory Medicine, Division of Medical and Chemical Laboratory Diagnostics, Medical University Vienna
2Department of Medicine I, Division of Infectious Diseases and Tropical Medicine, Medical University Vienna
3Department of Medicine I, Division of Blood and Marrow Transplantation, Medical University Vienna
4Department of Medicine I, Division of Oncology, Medical University Vienna
Background: Hemophagocytic lymphohistiocytosis (HLH) is rare but potentially fatal, characterized by erratic immune-activation. Although, reactive HLH is often suspected in SIRS patients, particularly with underlying malignant or autoimmune diseases, to date, there is no precise data concerning the incidence and disease course in this patient group. For diagnostic purposes, the HLH-2004 score criteria are mostly used, which were primarily designed for detecting hereditary HLH (1). Further, the H-score for assessing the probability of reactive HLH were established (2).
| HLH-2004 score (cut-off: 5 points) | H-score (cut-off: 168 points) |
| Fever Splenomegaly Cytopenias (affecting ≥2 of 3 lineages): hemoglobin <100 g/L) Platelets <100 x 109/L Neutrophils <1.0 x 109/L Hypertriglyceridemia and/or hypofibrinogenemia: Fasting triglycerides ≥3.0 mmol/L (i.e., ≥265 mg/dl) Fibrinogen ≤1.5 g/L Hemophagocytosis in bone marrow or spleen or lymph nodes Low or absent NK-cell activity Ferritin ≥500 mg/L Soluble CD25 ≥2,400 U/ml | Known underlying immune suppression 0 (no) or 18 (yes) Temperature 0 (<38.4), 33 (38.4–39.4), or 49 (>39.4) Organomegaly 0 (no), 23 (hepatomegaly or splenomegaly), or 38 (hepato- and splenomegaly) Nr. of cytopenias 0 (1 lineage), 24 (2 lineages), or 34 (3 lineages) Hypertriglyceridemia (mmol/L) 0 (<1.5), 44 (1.5–4), or 64 (>4) Hypofibrinogenemia (g/L) 0 (>2.5) or 30 (≤2.5) Hemophagocytosis in bone marrow aspirate 0 (no) or 35 (yes) Ferritin 0 (<2,000), 35 (2,000–6,000), or 50 (>6,000) Serum glutamic oxaloacetic transaminase (IU/L) 0 (<30) or 19 (>30) |
Methods: We conducted a prospective cohort study on standard care wards, including 468 patients fulfilling two or more SIRS criteria. On the first day after onset of SIRS symptoms, the H-score and the HLH-2004 criteria was assessed.
Results: In 468 standard care SIRS patients (27.6% bacteraemia rate), two patients had high H-scores (223 and 204) and fulfilled 4 HLH-2004 criteria, suggesting incipient or HLH-like disease. In this cohort, the median HLH-2004 score was 1 (IQR: 1) and the median H-score was 63.5 (IQR: 62.25). Interestingly, both scores correlated only in a moderate range (rs= 0.556). Patients with a H-score less than 92 presented with a significantly better 3 year survival probability (p= 0.001) when adjusted for patients´ age and sex.
Conclusion: We show that reactive HLH is a rare but relevant condition among SIRS patients. Further studies are needed to better establish diagnostic criteria and screening biomarkers in this setting.
References:
(1) Henter J-I, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2006, 48(2):124–131.
(2) Fardet L, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014, 66(9):2613–2620.
A26
Reference intervals for the Roche ECLIA serum ferritin assay in seniors: results from the prospective SENIORLAB study
1M Risch, 2,3W Hermann, 3AJ Campos, 3UE Nydegger, 3B Sakem, 3F Flahaut, 2-4L Risch
1Central Laboratory, Kantonsspital Graubünden, Chur, Switzerland
2Private University in the Principality of Liechtenstein, Triesen, Liechtenstein
3Labormedizinisches zentrum Dr. Risch, Buchs & Liebefeld Switzerland
4Center of Laboratory Medicine, University Institute of Clinical Chemistry, University Hospital, Inselspital, and University of Bern, Bern, Switzerland
Background and aim: Ferritin represents a frequently measured parameter in seniors. There is considerable heterogeneity of ferritin reference intervals (RI) among different laboratories, even when using the same method (e.g. RI in Switzerland for ECLIA vary from 10-120 mg/mL to 30-400 mg/mL, with or without gender and age specific stratification) and there is not much known on accurate ferritin RI in seniors. The aim of the present analysis was to determine RI for ferritin in Caucasian individuals aged 60 years and more.
Methods: From a total of 1467 study participants reporting subjective wellbeing at baseline examination in the SENIORLAB study, serum samples were drawn in fasting state and processed under optimal and standardized preanalytical circumstances. The following individuals were excluded from analysis: death at first follow-up for participants <80 years of age (mean follow-up time 3.7 +/- 0.7 years), survival of less than 3 years between age 80-84, survival of less than 2 years between age 85-89, and survival of less than 1 year for age 90 and older. Further exclusion criteria known to affect ferritin levels were: systemic inflammation (CRP>10 mg/L), hepatic cell damage (ALAT>35 U/L), diabetes mellitus (fasting glucose>7.0 mmol/L or known diabetes), iron overload (transferrin saturation >50%), and antiplatelet or anticoagulation therapy. Double sided 95% RI together with the 90% confidence intervals (CI) were evaluated according to CLSI guideline EP28-3c. Transference of the results originally obtained from another analytic platform (i.e. Abbott Architect) to the Roche Cobas 6000 ECLIA platform was done. Results: A total of 854 study participants (318 males, 536 females, age range 60-99, median age 70 years) had measurements available and were included into the present analysis. Median ferritin concentrations were significantly lower in female than in male study participants (103 vs. 186 mg/mL; p<0.0001). In contrast to male study participants (p=0.13), there was a significant correlation of ferritin concentrations with age in female study participants (p=0.02). For males (n=311), an age independent robust RI was 25 90% CI [20,33] to 502 90% CI [470,534] mg/mL. For females, age stratified RI were: 19 90% CI [16,24] to 283 90% CI [263,302] mg/mL for women aged 60-72 years (n=310), and 33 90% CI [30,37] to 313 90% CI [283,345] mg/mL for women aged 73 years and older (n=215). Conclusion: In this carefully selected reference population of seniors, ferritin RI exhibit a gender-specific behavior. Whereas age independent RI can be employed for male seniors, age stratification of RI is suggested for females. This contrasts to the current practice among medical laboratories.
A33
Interference of fulvestrant in in-vitro diagnostics for determination of estrogen – Analysis of field safety notices published by the BfArM in Germany
R Siekmeier1, J Hannig1, T Grammer2, W März2,3,4
1Drug Regulatory Affairs, Pharmaceutical Institute, University Bonn, Germany
2Medical Clinic V, Mannheim Medical Faculty, University of Heidelberg, Germany
3Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University Graz, Graz, Austria
4Synlab Academy, Synlab Holding Deutschland GmbH, Mannheim, Germany
Introduction: Since 1998 the European Directive 98/79/EC on In-vitro Diagnostics (IVD) regulates marketing and post market surveillance of IVD in the European Economic Area. In cases of incidents and field safety corrective actions (FSCA) manufacturers have to inform responsible Competent Authority (D: BfArM, AU: BASG) and public by field safety notices (FSN). Type and content of FSN are regulated by MEDDEV criteria. In Germany FSCA/FSN are published by the BfArM (http://www.bfarm.de/DE/Medizinprodukte/riskinfo/kundeninfo/functions/kundeninfo-node.html). Here we evaluated FSCA/FSN for IVD regarding drug interference caused by the estrogen receptor antagonist fulvestrant serving for treatment of women with breast cancer.
Materials and Methods: Analysis was made for FSCA/FSN for IVD published between begin 2015 and end of July 2018 by the BfArM. Interferences by other molecules than fulvestrant, e. g. pharmaceuticals, nutrition supplements, biological molecules and sample additives were excluded.
Results: 817 FSCA were published 2015-2018 for IVD (since late 2004 totally 2538 FSCA). Of these 43 FSCA addressed interferences by drugs/nutrition supplements. Of these 8 FSCA adressed fulvestrant interference (all within few months in 2016). However, the number of affected tests/methods is higher because some FSCA include several tests/platforms. Fulvestrant interference in all FSN is followed by increased test results for estrogen and in consequence, a potentially incorrect classification of menopausal status (pre-/postmenopausal) relevant for therapy. 6 FSN described a cross-reactivity to be the underlying cause. 7 FSN recommended to stop the use of the tests in patients treated with fulvestrant and 4 recommended the use of alternate methods (e. g. LC-MS). 7 FSN advised customers to discuss prior obtained results, however retesting was explicitly recommended in 3 FSN only. FSN differ strongly regarding type and quality of information describing the intensity of interference (e. g. measurement of spiked samples) and description of risks for patients (incorrect treatment). Only few FSN announced a future modification of the IFU (1) or provided information that there is no interference by a number of other tested pharmaceuticals (1).
Conclusions: The number of FSN reporting fulvestrant interference in IVD for estrogen determination is small. Erroneous results bear a risk for misinterpretation of the menopausal status of breast cancer patients followed by incorrect treatment. Therefore, this interference was subject of intense discussion and several publications from major medical laboratories and safety communications from health agencies (e. g. MHRA; https://mhra.filecamp.com/public/file/2rr9-sfhg05r4; no more available) since 2016. However, our analysis of FSN/FSCA investigates another aspect, namely the quality of FSN of manufacturers to inform users/physicians about product related risks and fulfilment of MEDDEV criteria. Although most FSN comply with formal MEDDEV criteria (e. g. name of manufacturer, type of action, specific details for product identification, contact data), major differences in respect to their type and quality were found. As FSN play a central role for risk reduction in FSCA their quality should be further improved.
A34
Interference of biotin in in-vitro diagnostics – Analysis of field safety notices published by the BfArM in Germany
R Siekmeier1, J Hannig1, T Grammer2, W März2,3,4
1Drug Regulatory Affairs, Pharmaceutical Institute, University Bonn, Germany
2Medical Clinic V, Mannheim Medical Faculty, University of Heidelberg, Germany
3Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University Graz, Graz, Austria
4Synlab Academy, Synlab Holding Deutschland GmbH, Mannheim, Germany
Introduction: Since 20 years the European Directive 98/79/EC on In-vitro Diagnostics (IVD) regulates marketing and post market surveillance of IVD in the European Economic Area. In cases of incidents and field safety corrective actions (FSCA) manufacturers have to inform responsible Competent Authority (D: BfArM, AU: BASG) and public by field safety notices (FSN). Type and content of these are regulated by MEDDEV criteria. In Germany FSCA/FSN are published on the BfArM homepage (http://www.bfarm.de/DE/Medizinprodukte/riskinfo/kundeninfo/functions/kundeninfo-node.html). Here we evaluated FSCA/FSN for IVD regarding drug interference caused by biotin (vitamin B7) frequently administered in high doses by pharmaceuticals (e. g. up to 600 mg/day in multiple sclerosis studies) and nutrition supplements (the latter without knowledge of health care providers).
Materials and Methods: Analysis was made for FSCA/FSN published between begin 2015 and end of July 2018 on the BfArM homepage. Interferences by other molecules than biotin, e. g. pharmaceuticals, nutrition supplements, biological molecules and sample additives were excluded.
Results: 817 FSCA were published 2015-2018 for IVD (since late 2004 totally 2538 FSCA). Of these 43 FSCA addressed interferences by drugs/nutrition supplements. Of these 7 FSCA described biotin interference (first in May 2016). However, the number of affected tests/methods is higher because most manufacturers include several tests/platforms in one FSCA. Affected were analytes with low serum concentrations, e. g. hormones (TSH, T3, T4, fT4), proteins/proteohormones (prolactin, FSH, chromogranin, thyreoglobulin, CK-MB mass, free PSA, TnI, TnI-ultra, HBc IgM, allergen specific IgG4) and others (e. g. DHEA-SO4, folate, sirolimus, cyclosporine). In their FSN manufacturers provided a description of the interference (increase/decrease, dependent on the test design competitive/sandwich assay). Only a subset of manufacturers provided borderline concentrations of biotin without relevant effects and/or data regarding the size of the bias dependent from the biotin concentration. Most manufacturers announced a modification of the instructions for use of their tests.
Conclusions: The number of FSN reporting biotin interference in IVD is small. Affected are tests based on the streptavidin-biotin system in the detection reaction. Erroneous results of some analytes (e. g. TnI, cyclosporine) bear a risk for direct medical consequences in patients. Therefore, this interference was subject of intense discussion and several publications (e. g. JAMA 2017;318(12):1150-1160) and safety communications from health agencies (e. g. FDA; https://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm586505.htm) since 2017. However, our analysis of FSN/FSCA investigates another aspect, namely the quality of FSN of manufacturers to inform users/physicians about product related risks and fulfilment of MEDDEV criteria. Although most FSN comply with MEDDEV criteria there were major differences in respect to their type and quality. As FSN play a central role for risk reduction in FSCA their quality should be further improved.
IMMUNOLOGY
A22
Experience using AC-XX for unclassified autoantibody patterns
W Klotz1, S Blunder2, H Naschberger2, M Herold1,2
1Medical University of Innsbruck, Department of Internal Medicine II, Innsbruck, Austria
2Tirol-Kliniken, Landeskrankenhaus Innsbruck, Innsbruck, Austria
Based on the international consensus ICAP started in 2014 (1), autoantibody patterns on HEp2-cells are classified from AC-0 to AC-29 (www.anapatterns.org). Patterns which cannot assigned to one of these defined 30 patterns may be reported as AC-XX (2).
The study was aimed to evaluate the frequency of AC-XX reports and to check which are the most common accompanying patterns in our database.
929 (8.2 %) out of 11301 (Jan 2017 – June 2018) consecutive serum samples sent to our laboratory for antinuclear antibody (ANA) screening were classified as AC-XX either alone or in combination with other AC-patterns. 599 (64.5 %) AC-XX samples were ANA negative (titer<1:100 or cytoplasmic only), 330 (35.5 %) samples were ANA positive. 33 samples were described as a cell cycle dependent pleomorph pattern different from AC-13 and AC-14. Most AC-XX samples (896; 96.4 %) were based on a cytoplasmatic pattern verbally described as fine reticular. 626 (67.4 %) of all AC-XX samples were categorized without any other pattern. Alone or in combination AC-1 (55.4 %) and AC-4 (50.1 %) were far the most common patterns together with cytoplasmic fine reticular AC-XX.
AC-XX was most often used to code the cytoplasmic fine reticular pattern and in few cases of true ANAs for pleomorphic patterns. Recurrent incidence of same descriptions of certain AC-XX patterns like “cell cycle dependent pleomorph” or “fine reticular cytoplasmatic” might result in new ICAP classifications.
AC-XX in general may be an indication of an autoimmune disease though the target antibody is unknown. In computer based systems coded entries like AC-XX can help to find similar reports and possible disease relations.
Literature: 1. Chan EKL et al. Front Immunol. 2015;6:412. doi: 10.3389/fimmu.2015
2. Herold M et al. Clin Chem Lab Med. 2018. doi: 10.1515/cclm-2018-0052
A27
Overexpression of PDE4A acts as central checkpoint inhibitor against cAMP-mediated immunosuppression in human T-cells
KG Schmetterer, K Goldhahn, L Ziegler, MC Gerner, RLJ Schmidt, M Themanns, E Zebedin-Brandl, D Trapin, J Leitner, WF Pickl, P Steinberger, I Schwarzinger and R Marculescu
Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
Malignant cells acquire physiological mechanisms of immunosuppression to escape immune surveillance. Strategies to counteract this suppression could help to improve adoptive immunotherapy regimen. The intracellular second messenger cyclic AMP (cAMP) acts as a potent immunosuppressive signaling molecule in T-cells and is up-regulated by multiple tumor-relevant suppressive entities including prostaglandin E2 (PGE2), adenosine and the functions of regulatory T-cells. Consequently, we aimed to abrogate cAMP signaling in human T-cells by ectopic overexpression of phosphodiesterase 4A (PDE4A). We could show that retroviral transduction of PDE4A into T-cells led to efficient degradation of cAMP in response to induction of adenylate cyclase. Retroviral transduction of PDE4A into CD4+ and CD8+ T-cells restored proliferation, cytokine secretion as well as cytotoxicity under immunosuppression by PGE2 and A2A-R agonists. PDE4A-transgenic T-cells were also partially protected from suppression by regulatory T-cells. Furthermore, PGE2-mediated upregulation of the inhibitory surface markers CD73 and CD94 on CD8+ T-cells was efficiently counteracted by PDE4A. Importantly, no differences in the functionality under non-suppressive conditions between PDE4A- and control-vector transduced T-cells were observed, indicating that PDE4A does not interfere with T-cell activation per se. Similarly, expression of surface markers associated with T-cell exhaustion were not influenced by PDE4A overexpression in long term cultures. Thus, we provide first evidence that PDE4A can be exploited as central immune checkpoint inhibitor against multiple suppressive entities.
ENDOCRINOLOGY
A19
Evaluation of intact and biointact PTH assays in chronic hemodialysis patients
C Berner1, R Marculescu2, H Sliwka3, C Bieglmayer2, M Hecking1
1Nephrology and Dialysis, 2Laboratory Medicine, 3Coordination Centre for Clinical Studies;
Medical University of Vienna, Austria
Background: Clinical management of chronic kidney disease - mineral and bone disorder is largely based on parathyroid hormone (PTH) concentrations, measured at regular intervals. The existence of PTH fragments, detected by first and second generation (“intact”) PTH (i-PTH) assays, led to the development of third generation (“biointact”) whole PTH (w-PTH) assays detecting only full length PTH. Despite efforts to use only w-PTH assays, i-PTH assays, which are usually less costly, are still being used and marketed, such that it may be indispensable to convert PTH values from one assay to another. We aimed at assessing the technical performance of a novel i-PTH assay, in comparison to 2 widely used w-PTH assays and to another i-PTH assay.
Methods: PTH levels of 134 patients on chronic hemodialysis were measured at 2 timepoints within 3 months, using the novel i-PTH assay by Siemens Healthcare (SH) and Roche Diagnostic (RD), respectively and the w-PTH assay by RD and DiaSorin (DS), respectively. All concentrations were measured in pg/ml by automated analyzers: Advia Centaur CP (SH), Cobas e 602 (RD) and Liaison XL (DS). Statistical methods included Passing-Bablok regression analyses and Bland Altmann plots.
Results: The i-PTH SH was slightly higher calibrated than i-PTH RD. Compared with w-PTH assays, the i-PTH SH assay yielded 2.5-fold higher results, while i-PTH RD was about 2-fold higher. Among the w-PTH(1-84) assays a rather good concordance was observed, with a mean bias of 12 ± 45.6 pg/mL (±2 SD) by Bland Altmann plots. In contrast, there was a substantial bias of -88.7 ± 183.4 pg/mL between the two iPTH assays (RD minus SH).
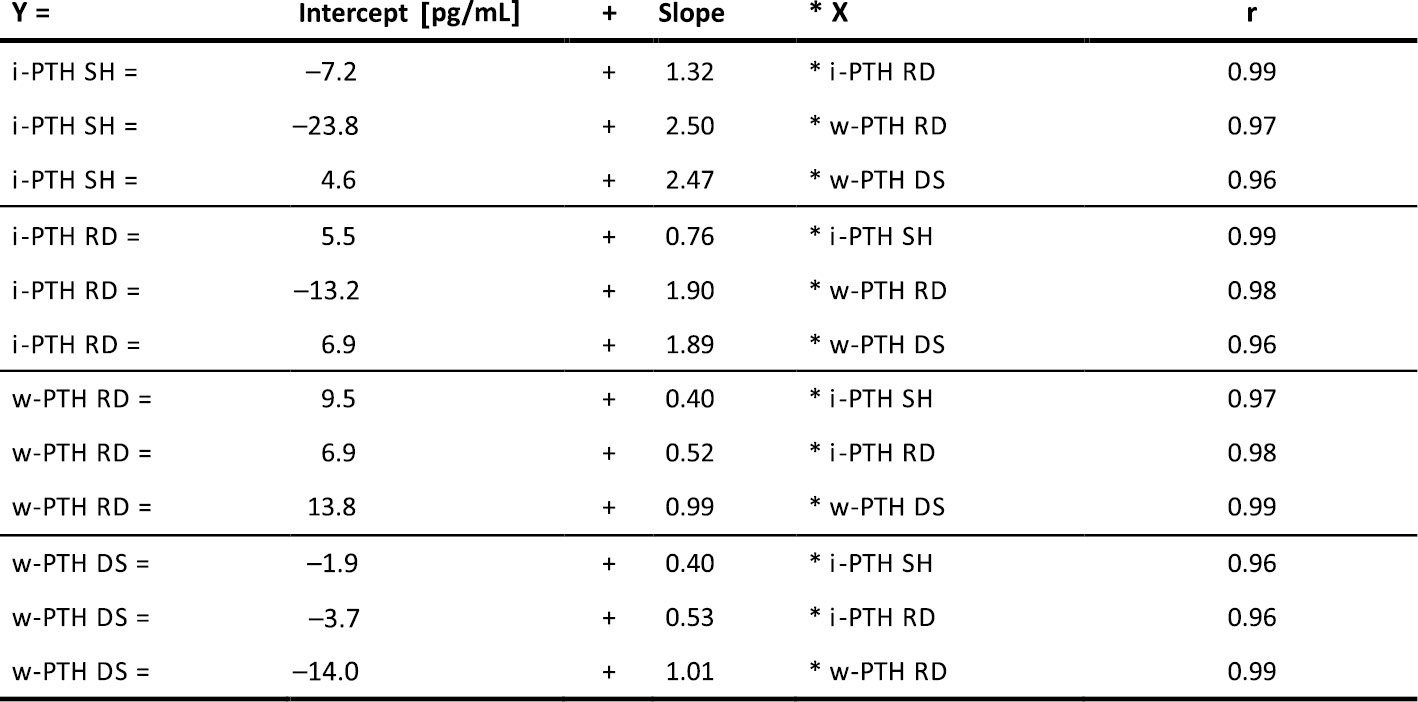
Conclusion: The table provided above enables method conversions, which are of interest, if a laboratory switches the assay or if a patient changes the treatment center.
A20
Iron rather than erythropoietin explains the role of fibroblast growth factor 23 in renal anemia in humans
B Bielesz1, T Reiter1, FP Hammerle1, W Winnicki1, M Bojic1, A Gleiss2, H Kieweg3, F Ratzinger3, G Sunder-Plassmann1 and R Marculescu3
1Division of Nephrology and Dialysis, Department of Medicine III, 2Centre for Medical Statistics, Informatics, and Intelligent Systems, 3Department of Laboratory Medicine
Medical University of Vienna, Austria
Background: Renal anemia is an almost universal complication of chronic kidney disease (CKD). In animal experiments, fibroblast growth factor 23 (FGF23), a well-known key-player in mineral metabolism, has been causally implicated in the development of anemia by suppressing erythropoietin. We assessed whether FGF23 might contribute to anemia by inducing relative erythropoietin deficiency.
Methods: Hemoglobin, erythropoietin, iron, and mineral metabolism parameters, including both intact and c-terminal-FGF23 (cFGF23) were measured in 225 non-dialysis CKD patients (stage 1-5, median eGFR: 30 ml/min/1.73m2) not on erythropoiesis stimulating agent or intravenous iron therapy. To approximate relative erythropoietin deficiency, gender-specific and hemoglobin dependent erythropoietin-standard deviation scores (EPO-SDS) were calculated. Analysis was performed by multiple linear regression.
Results: Both intact and c-terminal FGF23 were associated with hemoglobin in univariate analysis but only cFGF23 remained significant after adjustment for eGFR and other important confounders. Inclusion of EPO-SDS did not alter the association of cFGF23 with hemoglobin, which is rather displaced by iron indices. Inflammatory markers did not play a role in this context.
Conclusions: In patients with predominantly moderate to severe renal function impairment relative erythropoietin deficiency fails to explain the association of cFGF23 with anemia, which seems to be mediated by iron metabolism.
A23
Stress hormones for survival prediction in standard care SIRS patients
F Ratzinger1, H Haslacher1, T Perkmann1, A Makristathis2, H Burgmann3, R Marculescu1
1Department of Laboratory Medicine, Division of Medical and Chemical Laboratory Diagnostics, Medical University Vienna
2Department of Laboratory Medicine, Division of Clinical Microbiology, Medical University Vienna
3Department of Medicine I, Division of Infectious Diseases and Tropical Medicine, Medical University Vienna
Background: Severe bacterial infections causing a systemic inflammatory response (SIRS) also induce a neuro-endocrine stress response. Thus, reliable stress markers might be used for prognostic purposes.
Methods: In a prospective observational study, SIRS patients with suspected bacteraemia treated on standard care wards were included. A panel of stress markers and related peptides was assessed on the first and on the third day after onset of SIRS symptoms for short term (<28 days) and long term survival (>28 days – 1850 days/5 years). The cumulative/dynamic area under the curve (AUC)-estimator according to Uno was used to assess the prognostic capacity of age and sex adjusted stress markers (1). Moreover, stepwise Cox regression models were applied. Optimal cut-off values were identified by using the maximum selected rank statistics.
Results: All assessed stress markers presented with a significant prognostic capacity. In short term survival, the highest capacity was found for free cortisol on the first day (AUC: 0.792) and cortisol binding protein on the third day (AUC: 0.795). For the long term survival rate, copeptin presented as the best marker with an AUC of 0.624 on the first day and an AUC of 0.630 on the third day. Elevated free cortisol and higher copeptin levels presented as independent risk factors for an impaired short term and long term survival rate. The optimal cut-off points of free cortisol to identify high risk group patients were 12.50 ng/mL / 12.30 ng/mL (day 1 / day 3) for short term survival and the optimal cut-off points of copeptin were 7.90 pmol/L / 8.10 pmol/L (day 1 / day 3) for the long term survival rate.
Conclusion: Markers of the neuro-endocrine stress response, including free cortisol or copeptin might be valuable options to predict the short term and long term survival rates of SIRS patients.
References
(1) Uno H, Cai T, Tian L, and Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Association 2007; 102:527-537.
ENDOCRINOLOGY, MASS SPECTROMETRY & THERAPEUTIC DRUG MONITORING
A06
Berry leaves attenuate postprandial hyperglycemia
I Takács1, Á Takács2, M Mézes3, KJ Kovács4, S Ferenczi4, L Lakatos5
1Department of Microbiology, University of Szeged, Hungary
2Department of Food Engineering, University of Szeged, Hungary
3Department of Nutrition, Szent István University Gödöllő, Hungary
4Laboratory of Molecular Neuroendocrinology, Institute of Experimental Medicine, Budapest, Hungary
5Institute of Pharmacognosy, Faculty of Pharmacy, University of Szeged, Hungary
The treatment of hyperglycemia is crucial in the management of metabolic syndromes such as type II diabetes. The α-amylase and α-glucosidase as digestive enzymes have an essential role in glucose release as they hydrolyse starch. Consequently having a vital role in diabetes research as these enzymes are potential targets for antidiabetic drug research. The inhibition of these enzymes notably postpone the adsorption of glucose along with the postprandial hyperglycemia.
Natural extracts have long been used as natural source of antidiabetic agents. In our research wild strawberry (Fragaria vesca L.), blackberry (Rubus fruticosus L. ex Dierb.) and European blueberry (Vaccinium myrtillus L.) leaf extracts was used.
The aim of this study was to test the herb extracts in vivo in STZ induced and HFHS diet induced diabetic rats and mapping the possible molecules with mass spectrometric method.
For the in vivo model STZ induced CD1 mice were used. Five days after STZ treatment administration of starch and plant extracts or Acarbose® followed by glucose level evaluation. Glucose levels of mice treated with Acarbose® remained between normal values. Glucose levels of mice treated with plant extracts also remained between normal values. The blood glucose level was measured five times consequently and data showed no significant difference between plant extract and Acarbose®. For further diabetic mice model C57B16 mice were used. This strain of mice susceptible to diet induced obesity. The control group for type II diabetes model had the same age and fed with normal diet. This experimental group showed no difference after administration of starch and plant extracts or Acarbose®. The starting blood glucose level of this group were notably lower compared to the diabetic mice. The blood glucose level of HFHS diet induced mice had a significant increase in 30 min and 60 min after the administration of starch. After dispensation of starch and Acarbose® or starch and plant extract in both designs the blood glucose level remained stabile throughout the experiment. Results showed that the inhibition of Acarbose® and plant extracts were not differed significantly.
The high inhibition capacity is not associated with one molecule rather with certain groups. The MALDI-TOF MS reassured this assumption of which several active compounds were present. The compounds we summarised in our work are different flavonoids with numerous known biological activity, although these compounds as glycosidase inhibitors have not been studied profoundly. Results showed that high amount of polyphenolic substances were found especially tannins, ellagic tannins, flavonoids and its derivatives. The compounds are mainly present in glycosylated form consequently the presence of these glycosides is strongly hypothesised. These are the followings kaempferol, luteolin, apigenin, hyperoside, myricetin etc. These compounds alone have great effect on the development of diabetes but the synergist effect of several compounds pose higher effectiveness. With this inhibitory tendency of this mixture we assume the effectiveness of these extract.
A07
Comparison of thyroid-stimulating hormone concentrations among fingerstick capillary and venous blood samples
J Asenbaum1, K Grohs1, B Stöckelmeier1, D Brunner2, M Födinger1,3
1Kaiser Franz Josef Hospital, Institute of Laboratory Diagnostics, Vienna, Austria
2Kaiser Franz Josef Hospital, Department of Pediatrics, Vienna, Austria
3Sigmund Freud University, Medical Faculty, Vienna, Austria
PURPOSE: The purpose of this study was to evaluate the suitability of fingerstick capillary blood samples as compared to venous blood sampling for determination of thyroid-stimulating hormone (TSH) concentrations.
METHODS: Venous and capillary blood samples were obtained from 25 adults (mean age: 43 year (range: 27- 63); 12% male). Following centrifugation TSH serum concentrations were simultaneously analyzed on a Roche Cobas 8000 e602 module. Test results were subjected to statistical analysis using Passing-Bablok-Regression and Bland-Altman plot analysis.
RESULTS: Five capillary blood specimens showed insufficient sample volumes after centrifugation and thus had to be excluded from further analyses. In venous blood samples the mean TSH concentration was 1,8318 μU/mL (range: 0,018 - 4,64 μU/mL). In capillary blood mean TSH concentration was 1,76305 μU/mL (range: 0,015 – 4,3 μU/mL). Passing Bablok-Regression analysis revealed a correlation coefficient R2 of 1. The Bland-Altman bias plot between venous and capillary blood samples showed a mean difference of 0,07 with a tendency for slightly higher TSH concentrations among venous blood samples.
CONCLUSION: Our study indicates a good concordance of TSH concentrations between venous and capillary blood in a small pool of samples.
A25
Shrunken pore syndrome (SPS), preeclampsia (PE), and markers of NO-metabolism in pregnant women at the end of the first trimester
1AJ Campos, 1L Risch, 2M Baumann, 1MT Purde, 3D Sonntag, 4H Renz, 2B Mosimann, 2L Raio, 5M Mohaupt, 2D Surbek, 6M Risch
1Labormedizinisches zentrum Dr. Risch, Vaduz, Liechtenstein
2Department of Obstetrics and Gynecology, Inselspital, University of Bern, Bern, Switzerland
3BIOCRATES Life Sciences AG, Innsbruck, Austria
4Institute of Laboratory Medicine, Philipps University Marburg, Marburg, Germany
5Sonnenhofspital, Bern, Switzerland
6Zentrallabor, Kantonsspital Graubünden, Chur, Switzerland
Background: We recently identified a subgroup of pregnant women with shrunken pore syndrome (SPS), a condition with decreased renal clearance of low molecular weight proteins and normal clearance of creatinine, a circumstance presumably resulting from shrinking of glomerular pores. Women with SPS already during the first trimester have been demonstrated to be at considerable risk to develop subsequent preeclampsia (PE) later in pregnancy. The NO-metabolism markers arginine and ADMA and especially their ratio (Arg/ADMA) are recognized as markers of endothelial dysfunction. Aim: To investigate markers of NO-metabolism in women already showing evidence of SPS during the first trimester. Methods: We conducted a nested case-control study within the PRADO cohort. Women with PE having evidence of SPS at baseline during the first trimester (n=5) were compared to PE-cases without evidence of SPS (n=33), women with evidence of SPS at baseline without subsequent PE (n=3), and women without evidence of either PE or SPS (n=33). Double-sided 95% reference intervals according to CLSI guideline EP28-A3c were determined within the group of women with neither PE nor SPS. Arginine and ADMA were measured from serum obtained during gestational age 11+0 to 13+6 weeks using mass spectrometry. Results: Reference intervals for arginine (Arg), ADMA and the Arg/ADMA ratio were 95.2, 95% confidence interval, [81.3-111.2], to 228.8 [210.5-245.3] μmol/L, 0.13 [0.11-0.16] to 0.56 [0.47-0.65] μmol/L, and 214 [166-284] to 1269 [1073-1467], respectively. There were no significant differences in Arg and ADMA concentrations between women with neither PE nor SPS, women with PE and without SPS, women with SPS and no PE, and women with SPS and PE. However, women with PE and SPS had a significantly lower Arg/ADMA ratio than the other 3 groups (p=0.02). The other 3 groups did not have significant differences of Arg/ADMA ratios. Conclusions: Our findings show that SPS in the first trimester with subsequent PE is associated with another pathophysiological condition, i.e. lower NO-production leading to a subsequent increase in vessel tone. The observed association offers an explanation how SPS could lead to PE. Further, it raises the question, whether women with SPS during the first trimester would benefit from supplementation with arginine and/or an NO-donor drug (e.g. sildenafil) in order to mitigate the risk for developing PE.
General Topics in Clinical Chemistry and Laboratory Medicine including Quality Management
A21
Application of the reference limit estimator on different populations
S Michaelis, J Stroppa, C Grabher, P Fraunberger and M Hubmann
Medizinisches Zentrallaboratorium, Feldkirch, Austria
Reference values given by manufacturer’s instructions are not always suitable for application on individual populations and their own determination is a great challenge to most medical laboratories. The selection of an adequate quantity of appropriate individuals (e.g. CLSI guidelines require 120 apparently healthy individuals), the application of testing procedures and of statistical methods are not part of routine diagnostic procedures and are therefore time and cost consuming.
In order to solve this problem, the working group “Richtwerte” of the “Deutsche Gesellschaft für Klinische Chemie2 (DGKL) is providing a statistics tool for the calculation of reference values - the “Reference Limit Estimator” (RLE) - which reduces the efforts tremendously by using routinely generated patient data.
The aim of this study was to determine reference values for the local population in Vorarlberg (Austria), to compare these results to the manufacturer´s publications and to evaluate the effect of two different collectives - hospitalized patients vs patients from extramural practitioners - on the values generated by the RLE.
We investigated 25 common analytes without changes in the analytical procedures between 2012 and 2016. Data from 18 to 65 years old male and female patients were included, except from clinical divisions with high percentage of pathologic values, e.g. ICU, oncology or dialysis. The number of measurements included varied for each parameter, but for correct calculation at least 4000 per sex were necessary. Patients were first classified by the RLE in pathologic or non-pathologic and only the non-pathologic group was used to calculate reference values based on 2,5 to 97,5 percentiles. Analytes showing a significant age- and sex-dependence were selected and evaluated separately.
Electrolytes like sodium, potassium and calcium showed a very good accordance to the published data. In contrast, strong discrepancies occured for haemoglobin, ALAT and GGT.The RLE was not able to calculate values for ferritin, supposedly due to an inapplicable distribution of data. In general, results of extramural patients corresponded better to the manufacturer´s data.
The RLE is a useful tool, its installation and handling is easy and intuitive. The deviation of hospitalized and extramural patients indicate a considerable influence of selection and general health status of patients. Collectives of extramural practitioners appear to be more homogenous in terms of their state of health and thus more appropriate for determining reference values using the RLE. However, the preanalytical stability has to be considered and provided in special cases, e.g. coagulation or hormons.
Genetics including Pharmacogenetics and Liquid Biopsy
A08
SFRP1 promotor methylation analysis in colorectal adenoma using genomic DNA isolated from FTA cards
G Kriegshäuser1,2, A Reiner3, C Sebesta4, K Müllner-Ammer4, S Kriwanek5, C Ausch6, V Buxhofer-Ausch7
1Institute of Clinical Chemistry and Laboratory Medicine, General Hospital, Steyr, Austria
2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University, Graz, Austria
3Department of Pathology, Donauspital – SMZO, Vienna, Austria
4Department of Internal Medicine 2, Donauspital – SMZO, Vienna, Austria
5Department of Surgery, Donauspital – SMZO, Vienna, Austria
6Department of Surgery, General Hospital, Steyr, Austria
7Department of Internal Medicine I, Hospital Elisabethinen, Linz, Austria
Introduction: Formalin-fixed paraffin-embedded (FFPE) tissue is widely used for sample preparation and archiving. However, DNA extraction from FFPE samples has proven challenging in terms of formaldehyde cross-linking, degradation and the presence of PCR inhibitors. For this reason genomic DNA isolated from Whatman FTA cards has been used for a multitude of applications such as mutation detection by real-time PCR or sequencing, identity testing and DNA banking. So far, the literature lacks reports on the epigenetic analysis of FTA card-derived DNA from colonic tissue. Therefore, this study aimed at demonstrating that punches from colorectal adenoma preserved on FTA filter cards are suitable for methylation analysis by real-time methylation-specific PCR (MSP).
Materials and methods: A total of 40 sporadic colorectal adenoma samples stored on Whatman FTA cards were available. Genomic DNA was isolated from a single punch and bisulfite-treated using a commercial kit. Deaminated DNA was then analyzed by SYBR Green real-time MSP using primers specific for methylated or unmethylated promotor sequences of the secreted frizzled-related protein 1 (SFRP1) gene.
Results: Amplifiable DNA could be isolated from all FTA punches (n=40) while SFRP1 promotor methylation was present in 34/40 (85.0%) DNA samples as judged by real-time MSP using primers specific for unmethylated and methylated SFRP1, respectively.
Conclusion: Genomic DNA isolated from colorectal tissue samples preserved on FTA cards is suitable for downstream methylation detection methodologies such as MSP.
A09
Reverse-hybridization resolves an unclear HFE genotype obtained by real-time PCR and melting curve analysis in a patient with hyperferritinemia and alcoholic liver disease
D Enko1,2, M Novy3, C Oberkanins3, G Halwachs-Baumann1, G Kriegshäuser1,2
1Institute of Clinical Chemistry and Laboratory Medicine, General Hospital Steyr, Steyr, Austria
2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University Graz, Graz, Austria
3ViennaLab Diagnostics GmbH, 1120 Vienna, Austria
Introduction: Diagnostic approaches to the genetic heterogeneity of hereditary hemochromatosis (HH) may be a challenge, especially in patients with other liver diseases and comorbidities.
Materials and methods: We report the case of a 47-year old Caucasian male admitted to our hospital with chronic fatigue, jaundice, lack of appetite and abdominal fullness during the last half year. Medical history included chronic alcoholic liver disease (ALD). Iron parameters were highly elevated with a serum ferritin of 3070 (normal 30 – 300) μg/L and a transferrin saturation of (normal 20-45) 87.6%, respectively.
Results: Real-time PCR followed by melting curve analysis identified the patient to be wild-type homozygous and heterozygous for homo sapiens homeostatic iron regulator gene (HFE) mutations C282Y and H63D, respectively. However, an atypical melting curve with a shifted Tm peak was obtained for HFE mutation S65C therefore rendering the sample untypable. A second-tier assay based on multiplex PCR and reverse-hybridization identified the patient to be compound heterozygous for HFE mutations V59M/H63D.
Conclusion: Here we describe a rare HFE genotype identified in a patient with hyperferritinemia and ALD. This case not only illustrates the pitfalls associated with HH mutation analysis but also highlights the complexity of differentially diagnosing iron status alterations in humans.
GENETICS; HEMATOLOGY
A17
Molecular Quantification of Tissue Mast Cell Burden in Systemic Mastocytosis: a New Approach for Diagnostics
G Greiner1, M Gurbisz1, F Ratzinger1, N Witzeneder1,2, SV Class3, I Simonitsch-Klupp3, H Esterbauer1, M Mayerhofer4, L Müllauer3, WR Sperr2, P Valent2, and G Hoermann1,5
1Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
2Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Vienna, Austria
3Department of Pathology, Medical University of Vienna, Vienna, Austria
4Ludwig Boltzmann Institute of Osteology, Hanusch Hospital, Vienna, Austria
5MLL Munich Leukemia Laboratory, Munich, Germany
Background: The somatic KIT D816V mutation is a diagnostic criterion for systemic mastocytosis (SM). Molecular techniques with a high analytical sensitivity detect the KIT mutation in peripheral blood (PB) and bone marrow (BM) aspirates.1 In most of the patients, only a few KIT D816V+ mast cells (MC) or MC precursors, are found in liquid specimen (PB and BM aspirates). In contrast, the MC count in BM biopsies is much higher. We have previously proposed digital PCR (dPCR) as a new method of KIT D816V testing that can also reliably quantify the variant allele fraction (VAF) in formalin-fixed paraffin-embedded (FFPE) material.3 While multilineage involvement of KIT D816V indicated by a VAF in PB was associated with an aggressive clinical course,4-7 the mutant allele burden in the tissue has not been studied in a large patient cohort.
Methods: The VAF of KIT D816V in the tissue was assessed by dPCR (PrimePCR ddPCR, Biorad) on DNA isolated from 211 FFPE BM sections from 116 patients with SM (91 indolent SM, 25 advanced SM).
Results: The KIT D816V VAF in the tissue showed marked differences between patients ranging from 0.027 to 60% (median: 1.9%) and was largely independent from that in liquid specimen. Especially indolent SM patients showed higher VAF levels in tissue than in liquid specimen. In line with this, a higher correlation of KIT D816V mutation burden to BM MC infiltration and serum tryptase levels was observed for FFPE tissue (r=0.68 and r=0.68 respectively) than for liquid specimen (r=0.48 and r=0.58 respectively). When analyzing subgroups of SM, patients with advanced SM had a significantly higher KIT D816V VAF (median 23.40%) compared to indolent SM (median 1.65%; p<0.0001).
Conclusion: dPCR is a sensitive method to detect KIT D816V and reliably quantify the VAF in FFPE BM sections of SM patients. The mutational burden in the tissue represents a new molecular marker of the disease burden in SM since it reflects both the multilineage involvement of KIT D816V and the MC burden while the mutation burden in PB primarily reflects the KIT D816V multilineage involvement. Although the tissue mutational burden in patients with advanced SM is higher compared to ISM, the prognostic value of KIT D816V VAF measurement in SM needs to be redefined for BM sections. Potential future applications of KIT D816V quantification in FFPE BM sections include treatment response monitoring and definition of high disease burden in smoldering SM (SSM). We propose to include dPCR-based KIT D816V VAF measurement in BM tissue as a new biomarker for disease burden in SM in future clinical trials.
HEMATOLOGY
A15
The different faces of celiac disease a many-headed hydra – a case report and a review on its hematological manifestations
V Fux, A Griesmacher, M Anliker
Central institute of medical and chemical laboratory diagnostics – Innsbruck university clinic, Austria
Background: In a lecture with the title „On the coeliac affection“ hold by Dr. Samuel Gee in London a disease was described we now relate to celiac disease (CD) (1). CD is an immune-based reaction to dietary gluten and related prolamins that primarily affect the small intestine (2). It has a world-wide prevalence of about 1% (2) turning out to be more common than previously thought and is one of the most common causes of malabsorption syndromes (3). A gluten-free diet is still considered as the cornerstone of treatment relieving symptoms and reducing complications (2). Gluten can be found in wheat, barley and rye. CD has genetic, immunogenic and environmental components with systemic impacts and is not only limited to the small intestine (4). CD can present with a variety of symptoms: diarrhea, irritable bowel syndrome, osteoporosis, neurological and psychiatric diseases, thyroid disease, dermatitis herpetiformis, liver abnormalities, growth delay, splenic dysfunction, oral ulcerations or malignancy (2,4). Patients with CD may also be directed to hematologists for evaluation prior to showing gastrointestinal symptoms or being diagnosed with CD (5). Common hematological manifestations of CD may be anemia related to iron, folic acid and vitamin B12 deficiency, bleeding tendency, thrombocytosis, thrombocytopenia, leukopenia, hyposplenism, venous thromboembolism and IgA deficiency. However, anemia remains the most common symptom and can be the only one presenting manifestation (6). It is well known that patients diagnosed with CD have a significant increased risk for T-cell type lymphoma (2). Design: Case presentation and review of the literature on the hematological manifestations of CD Patient: Here we present a 54 year old patient manifesting with severe diarrhea, recurrent gastrointestinal pain, weight loss, anemia, thrombocytopenia, moderately elevated IgA- and IgG antibodies against deamidated gliadin petides and tissue transglutaminase (IgA). Pathological findings from duodenal biopsies revelead Marsh score at stage 4 compatible with celiac disease. Differential blood test showed partly atypical lymphocytes, which were further clarified by flow cytometry, which revelead pathological T-cells with abnormal markers (CD2+, CD3-, CD4-, CD5-, CD8-, CD7+, CD52+, CD103+, cytCD3+). Molecular T-cell-receptor rearrangement showed T-cell clonality. Immunhistological findings from the duodenal mucous membrane showed low count of CD8+ lymphocytes comparable with marker loss seen in refractory celiac disease patients. In summary of all findings the diagnosis of enteropathy-associated t-cell lymphoma with leukemic washout as a rare complication of celiac disease was made. Conclusion: CD can appear with a variety of symptoms, atypical in some cases and at any age (2). Atypical symptoms may delay diagnosis (2). CD remains vastly underdiagnosed and is commonly more found in adults than in children (2). Patients with ferropenic anemia, increased transaminases, thyroid issues and unexplained and isolated hematological findings should be screened for CD (2). T-cell lymphoma is a rare complication of CD and shows a very aggressive progression (2). Most cases are verily not limited to the enteropathy-type T cell lymphoma and include other types of lymphoma (2). Early detection and adherence to a gluten-free diet may improve the patients’ outcome (2). Clinicians and laboratory doctors should rule out CD in patients presenting with hematological abnormalities of unknown causes.
1. Dowd B, Walker-Smith J. Br Med J 1974; 2:45-47
2. Rubio-Tapia A, Hill ID, Kelly CP, Calderwood Ah, Murraj J. Am J Gastroenterol 2013; 108: 656-76
3. Di Sabatino A, Corazza G. Coeliac disease. Lancet 2009; 373:1480-93
4. Armin A, Peter H. Ann Intern Med 2005; 142:289-298
5. Thorvardur R, Mark L, Joseph M. Blood 2007; 109:412-421
6. Baydoun A, Maakaron JE, Halawi H, Abou Rahal J, Taher AT. Scand J Gastroenterol 2012; 47:1401-11
HEMATOLOGY, HEMOSTASEOLOGY & TRANSFUSION MEDICINE
A05
Effect of wheatgrass on clotting time
I Takács1, M Urkon2, A Szabó3,4, G Oszlánczi3,4, A Lukács3,4, ZP Zomborszki2,4, D Csupor2,4, T Kiss2,4
1Department of Microbiology, University of Szeged, Hungary
2Department of Pharmacognosy, University of Szeged, Hungary
3Department of Public Health, University of Szeged, Hungary
4Interdisciplinary Centre for Natural Products / Eötvös, University of Szeged, Hungary
Wheatgrass (young shoots of Triticum aestivum L.) has been used from the early 20th century as a feed additive for animals. It was supposed that young grass contains a water-soluble growth-promoting substance, which is distinct from all other known nutritional essentials. However, the chemical identity of the “grass juice factor” remained unknown. Nevertheless, a special wheatgrass product, Cerophyl became very popular also for human use before the era of synthetic multivitamins. Later, the use of Cerophyl was broadened: according to case studies, this product was used with success in the therapy of haemorrhages of different origin. Nowadays, wheatgrass is gaining popularity primarily due to its supposed antioxidant effect.
The aim of our work was to study the antioxidant capacity of wheatgrass and to analyse the effect of wheatgrass juice on blood clotting, a potential source of adverse events in case of prolonged use.
Wheatgrass powder was subjected to column chromatography (polyamide, gradient elution using 20%, 40%, 60%, 80%, and 100% aqueous methanol) and the fractions’ antioxidant capacity was determined by DPPH and ORAC assays. The effect on blood clotting was investigated by in vivo experiment. Male Whistar rats were treated with wheatgrass (137.5 mg/kg b.w.), warfarin (0.5 mg/kg b.w.) and with the combination of warfarin and wheatgrass (137.5 mg + 0.5 mg/kg. b.w.) per os for five days. The prothrombin time was determined by parallel measurements using Techrom IV plus (Teco GmBH, Germany) and Merlin MC1 (Merlin Medical, Germany) coagulometers. The statistical analysis was carried out on R 3.5.1 and GraphPad Prism 6.0.
The crude methanolic extract and fractions obtained by column chromatography exerted very low antioxidant activity (DPPH: EC50=3.21-46.29 μg/ml, ORAC: 0.46-9.43 μmolTE/g). The lyophilised powder of wheatgrass juice significantly altered (p<0.001) the effect of warfarin (e.g. increased the prothrombin time) in rats compared to those fed with combination of warfarin and wheatgrass.
Our experiments do not support the high antioxidant activity of wheatgrass. Moreover, our results suggest that wheatgrass is not only ineffective in the treatment of haemorrghages, but it may also increase the efficacy of warfarin by elevating prothrombin time.
A28
Quick and easy in-house ADAMTS13-assay – field experience in two hospital centers
J Rabensteiner1, P Knoebl2, J Cadamuro J3, F Prüller1
1Clinical Institute of Medical and Chemical Laboratory Diagnostics, University Hospital Graz, Medical University of Graz, Graz, Austria
2Division of Hematology and Hemostasis, Department of Medicine 1, Medical University of Vienna, Vienna, Austria
3Department of Laboratory Medicine, Paracelsus Medical University, Salzburg, Austria
Rapid quantification of ADAMTS13 activity in life threatening diseases such as thrombotic thrombocytopenic purpura (TTP) or atypical haemolytic uremic syndrome (aHUS) is favourable. However, currently only non-automated time consuming and laborious ELISA based assays are commercially available. Based on Furlan’s concept of degrading ultralarge von Willebrand factor (VWF) multimers, acquired and inborn ADAMTS13 deficiencies can easily be detected with modern automated VWF activity assays based on recombinant GP-Ib fragments.
In two Austrian hospital laboratories this in-house method, previously presented (1), is applied, based on the measurement of residual VWF activity.
Adding VWF from a plasmatic source in excess and degrading VWF with barium chloride activated ADAMTS13 from the patient’s sample allows the quantification of ADAMTS13activity by measuring the amount of remaining vWF activity.
In both institutions the in-house ADAMTS13 activity measurements correlate excellent with commercially available assays (r2>0,9) and even allows to detect the total absence of ADAMTS13 activity (measuring range 0-150%).
The hospital centres participate in external quality control assessment programmes with outstanding accuracy rate.The test can be requested 24/7 and time to result is 90 minutes, fulfilling the necessary requirements for emergency testing.
The results of this in-house VWF-activity based ADAMTS13 method provides accurate and fast ADAMTS13 activity measurements in everyday usage. This allows for faster
diagnosing and treating life-threatening diseases, as TTP and aHUS disease.
(1) Prüller F, Koder S, Knoebl P. Hämostaseologie 2018, 38: A3
A29
Simplifying Coagulation measurements in turbulent times of NOAC usage
J Rabensteiner, F Prüller
Clinical Institute of Medical and Chemical Laboratory Diagnostics University Hospital Graz, Medical University of Graz, Graz, Austria
Introduction
Non-Coumarin Oral Anti-Coagulants (NOACs) influence standard coagulation assays and thrombophilia testing [1]. Commercially available drug specific tests are on the market to quantify the concentration of all new NOACS and pricey charcoal tablets are available to remove the interfering anticoagulation (i.e. Dabigatran, Rivaroxaban, Apixaban, Edoxaban and others in the pipeline) [2]. At least, in emergency cases the NOAC concentration is requested and providing these tests for all new drugs is a burden on coagulation laboratories.
Method
Widely available anti-Xa-assays calibrated only for low molecular weight heparin in combination with purified medical charcoal will give the expected [prolonged due to anticoagulation] and the corrected [normalized due to absorption] coagulation results.
Simple loose medicinal charcoal is put into no additive tubes [using a spatula] and anti- coagulated patient plasma is added. After 5 minutes incubation at room temperature the tube is centrifugated and the requested coagulation assays performed with routine coagulation analysers.
Results
Anti-X-activities based on low molecular weight heparin calibration (AXaA-LMWH) allow for easy calculation of NOAC concentrations using predefined conversion factors (r2 >0,95):
Rivaroxaban (ng/ml)=AXaA-LMWH * 105 (Siemens BCS XP, Hyphen BIOPHEN Heparin 6 and Rivaroxaban Calibrator, in-house)
Apixaban (ng/ml)=AXaA-LMWH * 55 (Siemens BCS XP, Hyphen BIOPHEN Heparin 6 and Apixaban Calibrator, in-house)
Edoxaban (ng/ml)=AXaA-LMWH * 50 [3]
Medicinal charcoal was found to remove all present types of NOACS from citrate anti- coagulated plasmas and allows to discriminate between heparin and NOAC impact, and to test for thrombophilia while on NOACs, respectively.
Conclusion
Heparin based anti-Xa-activities will allow for easy monitoring and for rapid results in emergency cases (e.g. stroke and lysis) and loose medicinal charcoal is as effective as commercially available disposable compressed charcoal providing correct coagulation results.
[1] Halbmayer WM, Weigel G, Quehenberger P, et al. Clin Chem Lab Med 2012, 50: 1601-1605
[2] Exner T, Michalopoulos N, Pearce J, et al. Thromb. Res. 2018, 163: 117-122.
[3] Ruff CT, Giugliano RP, Braunwald E, et al. Lancet 2015, 385: 2288-2295
A30
A case of pregnancy associated acquired haemophilia A
J Rabensteiner1, P Neumeister2, A Mulabecirovic2, EC Weiss3, IC Lakovschek3, F Prüller1
1Clinical Institute of Medical and Chemical Laboratory Diagnostics; 2Department of Internal Medicine, Division of Haematology; 3Department of Obstetrics and Gynaecology;
University Hospital Graz, Medical University Graz, Graz, Austria
A 32-year-old woman presented in labour after 39 weeks gestation. So far her medical history was without pathological findings: no diagnosis of autoimmune diseases or other chronic disease, only mild nicotine consumption of 5 cigarettes per day was reported. The pregnancy was uncomplicated, as was the vacuum- assisted vaginal delivery. On postpartum day 10, profuse vaginal bleeding occurred, which could be stopped by curettage of the uterus. Due to the massive blood loss, postoperative haemoglobin dropped from 9,3 g/dL to 5,8g/dL, the transfusion of two packed red blood cell concentrates was necessary. After stabilizing haemoglobin levels and the woman could be discharged on the following day.
Three days later, she presented again with profuse vaginal bleeding in the obstetric emergency department. This time, a cavum balloon was inserted and laboratory results revealed a prolonged activated partial thromboplastin time of 58 seconds. Urgent factor VIII level was determined and showed activity of only 5,8%. The simultaneously performed mixing assay showed only a weak correction to 30% activity of factor VIII levels corresponding to the presence of low factor VIII inhibitor – a subsequent performed Bethesda assay showed 1,15 Bethesda Units/mL. Lupus anticoagulant was detectable.
Immediately, the patient was transferred to ICU for substitution of factor VIII concentrates; due to the low inhibitor concentration no bypassing agent was primarily used. Furthermore tranexamic acid as well as prednisone 125 mg p.o. was administered. In the following 4 days at the ICU, factor VIII was administered every 8 hours in combination with prednisone and a onetime substitution of factor XIII, leading to a reduction of bleeding complications and stabilized the patient, allowing a transfer to the haematology ward for further care. Factor VIII levels increased from about 30% at the ICU to stable concentrations of about 70% without the need for further factor VIII substitution.
Prednisone was slowly tapered due to a relapse three months after delivery and a stable factor VIII activity of>70% was reached four months after delivery showing no signs and symptoms of bleeding.
In the literature low inhibitor titres (<5 Bethesda units) tend to disappear spontaneously after a median of 30 months and usually do not recur in the following pregnancies.
An interdisciplinary care (gynaecology, anaesthesiology, haematology and laboratory medicine) is crucial for a rapid diagnostic and therapeutic successful management, as the reported mortality rate is still above 20% for acquired haemophilia A (1).
1. Scully MF, Shublaq W, Oliver GD. J Obstet Gynaecol Can 2002, 24: 430-2.
A35
A case of an acquired F VIII inhibitor
J Radek1, D Trubert-Exinger1, P Quehenberger2, P Hopmeier1
1Universitätsklinikum St. Pölten, Austria
2Klin. Institut für Medizinische und Chemische Labordiagnostik, MUW, Wien, Austria
A 78year old female patient underwent laprascopic cholecystectomy due to gallstones and recurrent pancreatitis. There was no history of post-operative bleeding, with a hysterectomy in 2005 and two cataract operations in 2014 performed without complications. She had never received factor concentrates nor any other type of procoagulant or anticoagulant therapy. However, according to her physician she had recently developed a tendency to mild bruising. Laprascopic cholecystectomy was performed successfully with no factor concentrates or plasma substitution required. By the 3rd post-operative day a haematoma had formed at the operation site which was removed by surgical intervention. Three weeks later the patient was again hospitalized with several haematomas on the neck and chest as well as a large one of the rectus sheath which all required surgical treatment. Coagulation screening tests showed a normal PT and a clearly prolonged aPTT. F VIII clotting activity was<1 %, while VWF-antigen, fibrinogen and F IX clotting activity were in normal range. Since the presence of an acquired F VIII inhibitor was suspected, treatment with FEIBA (5.000 IU twice a day) and methylprednisolone was initiated. Subsequent inhibitor testing revealed a F VIII inhibitor concentration of 68.1 BU/ml. Inhibition kinetics showed a type 2 pattern consistent with an acquired F VIII inhibitor. Treatment was switched to NovoSeven® (8 mg every 3 then every 4 hours) and subsequently 180 mg Emicizumab (Hemlibra®) per week, with Rituximab started 4 weeks later. As expected, during Emicizumab off-label use F VIII clotting activity and aPTT returned to normal whereas F VIII chromogenic activity (Biophen FVIII:C assay, Hyphen BioMed) remained low (approx. 5 %). No further bleeding episodes occurred during treatment. In up to 50 % of patients with acquired F VIII inhibitors an association to certain diseases (particularly lupus erythematosus, rheumatoid arthritis and various malignancies) has been described. However, in the present case none of these triggers for antibody formation was identified. It is also noteworthy that the bleeding pattern differed significantly to bleeding in haemophilia A, and rather resembled the haemorrhage pattern seen in compromised wound healing.
A36
PNH hemolysis assay: a sensitive tool for in vitro investigation of the hemolytic potential of therapeutic antibodies
L Loacker1, CQ Schmidt3, B Höchsmann2, I von Zabern, C Weinstock2, H Schrezenmeier2, A Griesmacher1, M Anliker1
1Central Institute for Med. Chem. Laboratory Diagnostics, University Hospital Innsbruck, Innsbruck, Austria
2Institute of Clinical Transfusion Medicine and Immunogenetics Ulm, German Red Cross Blood Transfusion Service Baden-Württemberg-Hessen, and Institute of Transfusion Medicine, University of Ulm, Ulm, Germany
3Institute of Pharmacology of Natural Products and Clinical Pharmacology, University of Ulm, Ulm, Germany
Background Complement activation by human alloantibodies (e.g. transfusion related) and cold agglutinins have been studied successfully with a particularly sensitive assay (PNH hemolysis test) using RBCs of patients with paroxysmal nocturnal hemoglobinuria (PNH).1 PNH RBCs are particularly susceptible for hemolysis by complement due to their deficiency of GPI-linked proteins including the two crucial complement regulators CD55 and CD59. Thus PNH RBCs are a useful and sensitive tool for the investigation of anti-RBC-antibodies. The therapeutic IgG1 anti-CD38 antibody Daratumumab binds to RBCs and is known to interfere with blood compatibility testing by causing panagglutination.2 The aim of the present study was to investigate whether hemolytic capacity of Daratumumab can be demonstrated in vitro. Methods Blood samples of several PNH patients with large (20-50%) PNH III clones were obtained from the IKT Ulm. Daratumumab dilutions were prepared from a 20mg/ml solution in PBS. Dilutions of Daratumumab in PBS were incubated with PNH RBCs at 37 °C for 20 min (controls received PBS instead); after washing of the RBCs, pooled normal human AB serum (NHS) was added as source of complement and incubated 1.5 hours at 37 °C; after washing RBCs were analysed by flow cytometry using a monoclonal anti-CD59. Results PNH RBCs preincubated with Daratumumab showed moderate dose dependant lysis of PNH III RBCs. This effect was more pronounced when increasing concentrations of a specific complement factor H inhibitor (FactorH-18-20) or anti-P alloantibody containing serum were added, causing an augmentation of complement activation. Conclusions Daratumumab is known to cause complement dependent cytotoxicity towards myeloma cells. At present, neither transfusion related adverse events nor hemolysis have been reported.3 The binding of Daratumumab to RBCs which becomes obvious in blood compatibility testing led us to investigate the complement dependent lytic capacity in a sensitive test system. The overall only moderate lysis of PNH III RBCs is in agreement with the observation that no severe hemolytic events have been reported. However, we found an enhancement of hemolysis when alloantibodies or other complement activating agents were applied together with Daratumumab. Therefore, it may not be excluded that these in vitro observed effects might become relevant as breakthrough hemolysis in vivo, when patients suffering from ongoing pathological complement activation are treated with Daratumumab.
1 Anliker M et al. Transfusion 2018 in press.
2 Chapuy CI et al. Transfusion 2015; 55: 545-54.
3 Plesner T et al. Front Immunol 2018;9: 1228.
HEMOSTASEOLOGY
A01
Von Willebrand disease: diagnoses in the central laboratory of Innsbruck from 2012 to 2018.
C Irsara, L Loacker, A Griesmacher
Univ. clinic of Innsbruck, central laboratory, Innsbruck, Austria
Introduction. Von Willebrand Disease (vWD) is the most common inherited bleeding disorder. It affects up to 1 percent of the population, although only a minority of these individuals are symptomatic (1). Von Willebrand factor (vWF) circulates at different multimer sizes, the large vWF multimers being the most active. vWF mediates the binding of platelets to injured [sub]endothelial structures and adjacent platelets, particularly at areas of high shear stress. It also serves as carrier protein for factor VIII, prolonging its half-life time substantially. As vWF has important implications in both primary and secondary haemostasis, vWD can lead to different bleeding phenotypes, depending on the vWD subtype (2). Type 1 vWD is due to a partial quantitative loss of vWF. Type 2 vWD summarizes different qualitative defects of vWF (2A, 2B, 2M, 2N). In the most severe form, type 3 vWD, vWF is completely absent. Furthermore, different diseases (e.g. aortic valve stenosis) and conditions (e.g. patients on extracorporeal membrane oxygenation) can cause acquired qualitative vWD. Methods. From 2012 (Jan) to 2018 (July) 256 patients were analysed. VWD was diagnosed based on the results of platelet function analyzer-100 EPI/ADP, factor VIII activity, vWF antigen, vWF glycoprotein I b binding activity, vWF ristocetin cofactor activity, vWF collagen binding activity, vWF multimer analysis and in several cases Born aggregometry. Results. Out of the 256 patients, 89 were diagnosed with hereditary vWD and 18 with acquired vWD. The distribution of hereditary vWD diagnoses was: 60 (67,4%) type 1, 27 (30,3%) type 2, 2 (2,2 %) type 3. The 27 patients diagnosed with type 2 vWD were sub-classified into 15 type 2A, 2 type 2B, 3 type 2M, 1 type 2N and 6 not otherwise classified. The gender distribution women: men was 164:92 (64:36%) for all analyses and 65:42 (61:39%) for vWD diagnosed patients. Conclusion. vWD is the most frequent clinically relevant bleeding disorder. The distribution of vWD subtypes in our examined population is mostly in line with published data (3).
1. Leebeek FW, Eikenboom JC. Von Willebrand’s Disease. N Engl J Med. 2016; 375:2067-2080.
2. Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14:171-232.
3. Budde U, Drewke E, Mainusch K, Schneppenheim R. Laboratory diagnosis of congenital von Willebrand disease. Semin Thromb Hemost. 2002;28:173-90.
MASS SPECTROMETRY
A32
A new HPLC-MS approach for sensitive analyses of new oral anticoagulants
T Panic-Jankovic1, F Ratzinger1, P Quehenberger1 G Mitulovic1, 2
1Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna, Austria
2Proteomics Core Facility, Medical University of Vienna, Austria
Rivaroxaban, together with other oral direct factor Xa inhibitors, like edoxaban and apixaban has been successfully used in the treatment and prevention of thromboembolic disorder for almost twenty years. Thromboembolic disorders are the most common causes of comorbidity and mortality worldwide. Due to their predictable pharmacokinetics and pharmacodynamics, in comparison to vitamin K antagonists, there is no necessity for routine monitoring of new oral anticoagulants’ plasma concentrations. However, an increased need for monitoring of rivaroxaban levels in plasma has been reported for certain situations. Monitoring of novel oral anticoagulants is desired especially in clinical circumstances such as acute renal failure, bleeding complications or thrombosis during treatment, prior to urgent surgery, when life-threatening bleeding occurs, stroke, overdose and suspected accumulation of drug, and during pregnancy and lactation.
In our present work, we developed and validated a rapid, sensitive high-performance liquid chromatography method coupled with tandem mass spectrometry for precise plasma determination of new oral anticoagulants. The developed method is characterized through easy sample preparation including only protein precipitations from a small amount of plasma samples by using acetonitrile, and fast HPLC separation performed on a nano HPLC and separeted by micro-Pillar-Arrayed Column (μPAC). Detection was performed using both UV and positive nanoelectrospray within a range from 10 to 1000 ng/mL.
MASS SPECTROMETRY / PROTEOMICS
A31
Detection of Coxiella burnetii’s Proteins in Human Cells
G Mitulović1, M Dantchenko2, G Flores-Ramirez2
1Clinical Institute of Laboratory Medicine, Medical University of Vienna, Austria
2Slovak Academy of Sciences Bratislava, Slovakia
Introduction
Coxiella burnetii, a small coccobacillus is an intracellular pathogen that causes Q fever, a zoonotic disease found worldwide. It is transmitted to humans by the inhalation of aerosols or contaminated dust containing bacteria from infected livestock. C. burnetii has a potential to be a bioterrorism agent, because only a small dose is necessary for infection.
The micro Pillar Array Column (PAC) show an almost perfect order of the pillars as opposed to packed columns where the column packing never can be perfect. The column shows high permeability as there are no pores that need to be flushed.
Methods
The C. burnetii strain Herzenling 331 (phase II) was grown in ACCM-2 host-free axenic medium. For 7 days, every 24 h, fresh and sterile antibiotic was added to DOX (doxycycline) treatments. After that, the cells were pelleted and washed with phosphate-buffered saline (PBS), centrifuged at 17 000 g for 20 min at 4 °C and stored at -80 °C. The cell lysis was performed using the AllPrep RNA/prot/DNA kit (QIAGEN). The proteins were precipitated overnight with cold acetone and digestion was performed with trypsin by filter-aided sample preparation protocol.
NanoRSLC was used for peptide separation on a μPAC column. MS detection was performed using Orbitrap Q-Exactive plus. Data analysis was performed using thegpm.org and the
Results
The use of μPAC columns resulted in identification of more than 900 proteins within the single run. An excellent reproducibility for both treated and untreated samples was achieved was achieved on the μPAC column combined with the trap column. C. burnetii proteins were identified in samples not treated with Doxocycline suggesting efficacy of thi s agent against the C. burnetii infection.
MICROBIOLOGY& VIROLOGY
A37
A workflow implementation for further investigation of HPV-positive women in routine diagnostics
R Reitinger, A Trauner, M Weik, C Schausberger, L Mustafa
Labor Dr. Mustafa Dr. Richter, Salzburg, Austria
Background: Primary screening based on high risk HPV (hrHPV) testing for cervical cancer prevention has provided a significant increase in sensitivity and reproducibility compared to the Pap test. In Austria a new guideline converted recently the recommendation of the currently performed PAP-screening to a hrHPV-screening for women aged>30 years. To determine the risk for cervical cancer of hrHPV positive women with equivocal cytology, immunocytochemical staining of the tumor suppression marker p16 and the proliferation marker Ki67 (CINtec® Plus) could be performed to improve disease management of HPV related cervical lesions. Material and Methods: Data were collected from 80 women aged 17 to 77 (Ø=41.4), who were tested for hrHPV followed by liquid based cytology (LBC) screening. Immunocytochemical staining was performed of cervical cell samples which were tested positive for hrHPV genotyping and/or had abnormal results according to the Bethesda system. All samples were preserved in BD SurePath™ collection vials. The hrHPV was detected with BD Viper ™ LT real-time PCR. LBC was prepared using the BD PrepStain® System. For the immunocytochemical staining was used the CINtec® Plus test (Roche). Results: The results of hrHPV typing and LBC were consistent in 82.5% of the cases, 51 cases (51/80; 63.7%) were hrHPV and LBC negative. 15 cases (15/80; 18.8%) were hrHPV positive and were scored between low- grade (LSIL) and high-grade lesions (HSIL). 7 cases with LBC high- grade lesions were tested CINtec® Plus positive. The LBC of one case was scored as atypical squamous cells of undetermined significance (ASC-US) and was tested CINtec® Plus negative. Seven cases scored as low grad lesions were CINtec® Plus test negative. The remaining 14 cases (14/80; 17.5%) had discrepant results: seven cases were hrHPV negative and were scored between LSIL (6/7) and one atypical squamous cell cannot exclude high grade lesion (ASC-H), which was also tested CINtec® Plus negative. Seven cases were hrHPV positive and scored as normal for the LBC and were confirmed CINtec® Plus negative. Conclusion: Our findings indicate that the approach to triage hr HPV genotyping with liquid based cytology and subsequent p16/k67 immunochemical staining in hrHPV positive women is an efficient option to determinate the severity of low grade lesion and a useful tool to identify women at high risk for serious cervical disease.
*)These abstracts have been reproduced directly from the material supplied by the authors, without editorial alteration by the staff of this Journal. Insufficiencies of preparation, grammar, spelling, style, syntax and usage are the authors’ responsibility.
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Observing an analyzer’s operational life cycle: a useful management tool for clinical laboratories
- Reviews
- Personalized laboratory medicine: a patient-centered future approach
- Circular RNAs: a new class of biomarkers as a rising interest in laboratory medicine
- Mini Review
- Impact of interactions between drugs and laboratory test results on diagnostic test interpretation – a systematic review
- Opinion Paper
- Uncertainty in measurement and total error: different roads to the same quality destination?
- Guidelines and Recommendations
- Joint EFLM-COLABIOCLI Recommendation for venous blood sampling
- General Clinical Chemistry and Laboratory Medicine
- Evidence for the positive impact of ISO 9001 and ISO 15189 quality systems on laboratory performance – evaluation of immunohaematology external quality assessment results during 19 years in Austria
- Effects of high-dose, intravenous lipid emulsion on laboratory tests in humans: a randomized, placebo-controlled, double-blind, clinical crossover trial
- Commutability of the certified reference materials for the standardization of β-amyloid 1-42 assay in human cerebrospinal fluid: lessons for tau and β-amyloid 1-40 measurements
- Failure rate prediction of equipment: can Weibull distribution be applied to automated hematology analyzers?
- Evaluation of serum alkaline phosphatase measurement through the 4-year trueness verification program in China
- Increased serum concentrations of soluble ST2 predict mortality after burn injury
- The clinical significance of borderline results of the Elia CTD Screen assay
- Reference Values and Biological Variations
- Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative
- Cancer Diagnostics
- BCL2L12 improves risk stratification and prediction of BFM-chemotherapy response in childhood acute lymphoblastic leukemia
- Cardiovascular Diseases
- The correlation between glucose fluctuation from self-monitored blood glucose and the major adverse cardiac events in diabetic patients with acute coronary syndrome during a 6-month follow-up by WeChat application
- Diabetes
- Impact of blood cell counts and volumes on glucose concentration in uncentrifuged serum and lithium-heparin blood tubes
- Letters to the Editor
- Standard process-oriented workflow introduces pre-analytical error when used in large study sample batches
- Comparison of three staining methods in the automated digital cell imaging analyzer Sysmex DI-60
- Detection of Plasmodium falciparum using automated digital cell morphology analyzer Sysmex DI-60
- Serum ischemia-modified albumin concentration may reflect long-term hypoxia in chronic respiratory disease: a pilot study
- Wet absorptive microsampling at home for HbA1c monitoring in diabetic children
- Serum endocan levels in patients with chronic obstructive pulmonary disease: a potential role in the evaluation of susceptibility to exacerbation
- Analytical and clinical validation of the new Roche Elecsys Vitamin D Total II assay
- Analytical validation of two second generation thyroglobulin immunoassays (Roche and Thermo Fisher)
- Omission of preservatives during 24-h of urine collection for the analysis of fractionated metanephrines enhance patient convenience
- Transient monoclonal gammopathy in a 2-year-old child with combined viral and bacterial infection
- Nephelometric assay of urine free light chains: an alternative and early clinical test for Bence-Jones protein quantification
- Congress Abstracts
- Congress of Laboratory Medicine and Clinical Chemistry 7th Annual Meeting of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC)
- 50th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC – Laboratory Medicine)
- Revolution drives Evolution – from measuring to understanding: Annual meeting of Swiss Society of Clinical Chemistry (SSCC) in Bern, November 15-16 2018
Articles in the same Issue
- Frontmatter
- Editorial
- Observing an analyzer’s operational life cycle: a useful management tool for clinical laboratories
- Reviews
- Personalized laboratory medicine: a patient-centered future approach
- Circular RNAs: a new class of biomarkers as a rising interest in laboratory medicine
- Mini Review
- Impact of interactions between drugs and laboratory test results on diagnostic test interpretation – a systematic review
- Opinion Paper
- Uncertainty in measurement and total error: different roads to the same quality destination?
- Guidelines and Recommendations
- Joint EFLM-COLABIOCLI Recommendation for venous blood sampling
- General Clinical Chemistry and Laboratory Medicine
- Evidence for the positive impact of ISO 9001 and ISO 15189 quality systems on laboratory performance – evaluation of immunohaematology external quality assessment results during 19 years in Austria
- Effects of high-dose, intravenous lipid emulsion on laboratory tests in humans: a randomized, placebo-controlled, double-blind, clinical crossover trial
- Commutability of the certified reference materials for the standardization of β-amyloid 1-42 assay in human cerebrospinal fluid: lessons for tau and β-amyloid 1-40 measurements
- Failure rate prediction of equipment: can Weibull distribution be applied to automated hematology analyzers?
- Evaluation of serum alkaline phosphatase measurement through the 4-year trueness verification program in China
- Increased serum concentrations of soluble ST2 predict mortality after burn injury
- The clinical significance of borderline results of the Elia CTD Screen assay
- Reference Values and Biological Variations
- Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative
- Cancer Diagnostics
- BCL2L12 improves risk stratification and prediction of BFM-chemotherapy response in childhood acute lymphoblastic leukemia
- Cardiovascular Diseases
- The correlation between glucose fluctuation from self-monitored blood glucose and the major adverse cardiac events in diabetic patients with acute coronary syndrome during a 6-month follow-up by WeChat application
- Diabetes
- Impact of blood cell counts and volumes on glucose concentration in uncentrifuged serum and lithium-heparin blood tubes
- Letters to the Editor
- Standard process-oriented workflow introduces pre-analytical error when used in large study sample batches
- Comparison of three staining methods in the automated digital cell imaging analyzer Sysmex DI-60
- Detection of Plasmodium falciparum using automated digital cell morphology analyzer Sysmex DI-60
- Serum ischemia-modified albumin concentration may reflect long-term hypoxia in chronic respiratory disease: a pilot study
- Wet absorptive microsampling at home for HbA1c monitoring in diabetic children
- Serum endocan levels in patients with chronic obstructive pulmonary disease: a potential role in the evaluation of susceptibility to exacerbation
- Analytical and clinical validation of the new Roche Elecsys Vitamin D Total II assay
- Analytical validation of two second generation thyroglobulin immunoassays (Roche and Thermo Fisher)
- Omission of preservatives during 24-h of urine collection for the analysis of fractionated metanephrines enhance patient convenience
- Transient monoclonal gammopathy in a 2-year-old child with combined viral and bacterial infection
- Nephelometric assay of urine free light chains: an alternative and early clinical test for Bence-Jones protein quantification
- Congress Abstracts
- Congress of Laboratory Medicine and Clinical Chemistry 7th Annual Meeting of the Austrian Society for Laboratory Medicine and Clinical Chemistry (ÖGLMKC)
- 50th National Congress of the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC – Laboratory Medicine)
- Revolution drives Evolution – from measuring to understanding: Annual meeting of Swiss Society of Clinical Chemistry (SSCC) in Bern, November 15-16 2018

