Abstract
Acute intestinal ischemic injury (i3) is a life-threatening condition with disastrous prognosis, which is currently difficult to diagnose at the early stages of the disease; a rapid diagnosis is mandatory to avoid irreversible ischemia, extensive bowel resection, sepsis and death. The overlapping protein expression of liver and gut related to the complex physiopathology of the disease, the heterogeneity of the disease and its relative rarity could explain the lack of a useful early biochemical marker of i3. Apart from non-specific biological markers of thrombosis, hypoxia inflammation, and infection, several more specific biomarkers in relation with the gut barrier dysfunction, the villi injury and the enterocyte mass have been used in the diagnosis of acute i3. It includes particularly D-lactate, intestinal fatty acid-binding protein (FABP) and citrulline. Herein, we will discuss leading publications concerning these historical markers that point out the main limitations reagrding their use in routine clinical practice. We will also introduce the first and limited results arising from omic studies, underlying the remaining effort that needs to be done in the field of acute i3 biological diagnosis, which remains a challenge.
Introduction
Acute intestinal ischemic injury (i3) is a life-threatening condition associated with a high short-term mortality rate ranging from 32% to 86%. Such a prognosis remains unchanged through decades despite significant improvements in vascular surgery, interventional radiology and resuscitation. In the emergency setting, these catastrophic outcomes are closely linked to delays in diagnosis [1] and treatment, which is of major concern since the early presentation of acute i3 is potentially fully reversible when using a specific multimodal management that includes revascularization [1], [2]. However, at this stage, the clinical presentation is dominated by acute non-specific abdominal pain without any other discriminating clinical or biological characteristics [3]. As a result, early diagnosis may only be achieved by a high degree of clinical suspicion and a prompt confirmation by an abdominal computed tomography (CT) scan angiography identifying features of both splanchnic vascular insufficiency and intestinal injury [4]. However, selection of patients requiring CT evaluation remains a challenge due to the lack of an available diagnostic sign or biomarker. As a consequence, physicians have long sought to identify a biomarker or a combination of markers to ensure a sensitive, specific and early diagnosis of acute i3. In this study, we will review the basis of the pathophysiology of i3, the conventional past and present strategies for biomarker discovery and their remaining gaps and introduce the new perspectives opened by the “-omics” technologies. We focused in the first part of this review on human clinical studies, then we presented the pre-clinical research in the second part since we did not find enough published materials to limit our presentation to humans.
Pathophysiology of i3
Definition of i3
i3 is an digestive injury related to intestinal vascular insufficiency, occlusion or low splanchnic-mesenteric flow. The pathophysiology of i3 responds to a multi-step process that begins with an intermittent or continuous, complete or incomplete decrease in digestive blood flow. Subsequent mucosal/submucosal ischemia evolves into transmural ischemia, often acute, followed by intestinal necrosis and death without treatment [5].
Several theories have been proposed to explain how this non-infectious vascular disease could lead to systemic inflammatory response syndrome (SIRS) followed by sepsis and multi-visceral failure [6].
Multistep pathophysiology
Acute mesenteric ischemia (AMI) should be considered as one of the stages of the i3 process (Figure 1), which starts from digestive vascular insufficiency to intestinal necrosis. Ischemia begins early and superficially and then spreads deep and in the surface of the intestinal wall. Vascular insufficiency is initially responsible for an inadequacy between inputs and requirements for energy substrates by overcoming the adaptive processes of a digestive territory. This loss of homeostasis results from a sudden decrease or interruption of the splanchnic-mesenteric blood flow. The decrease in splanchnic blood flow in the proximal circulation induces a deep extension of the ischemia which then becomes transmural and gangrenous. Conversely, when perfusion abnormalities relate to intra-parietal arterioles, lesions of ischemia remain superficial.
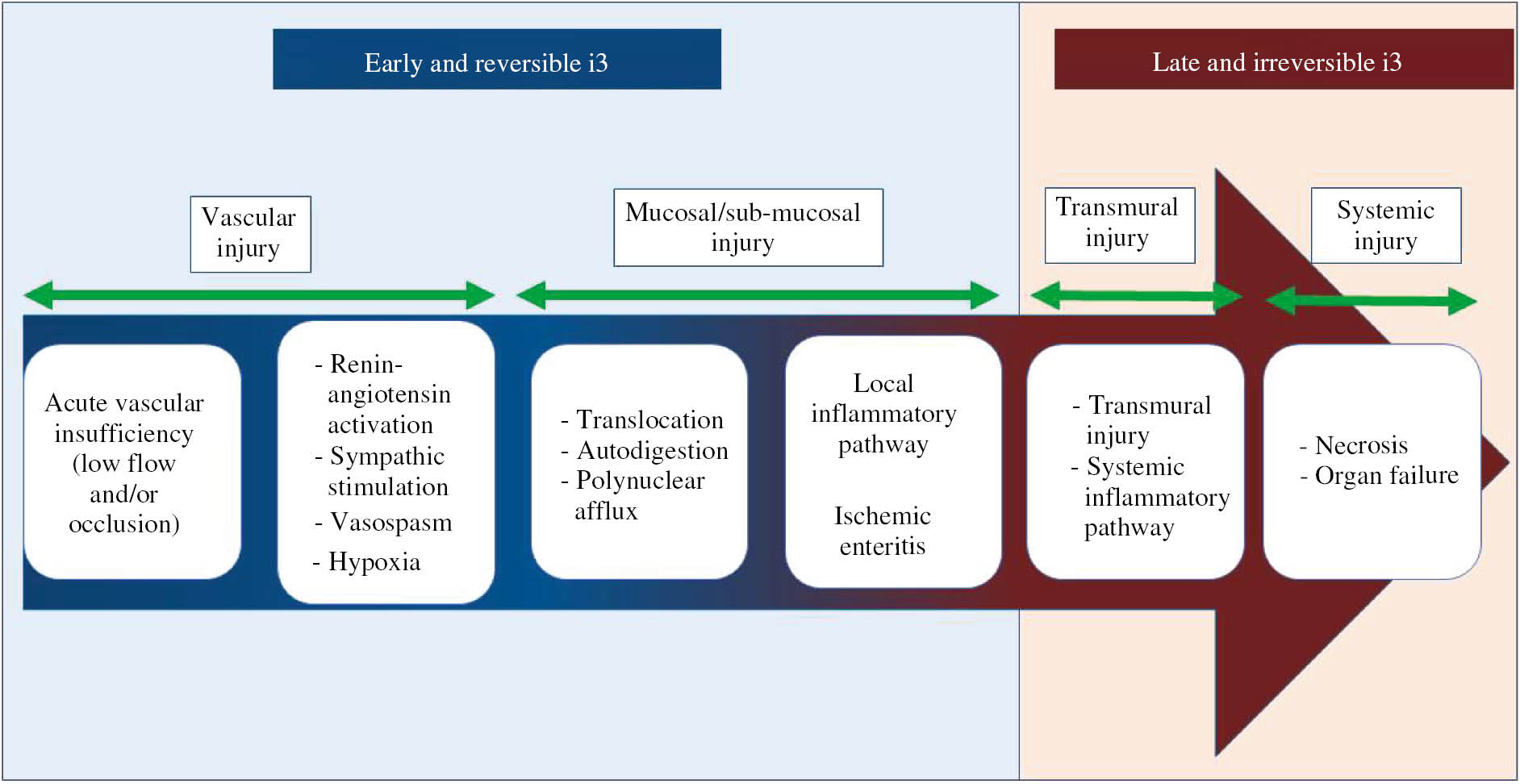
Schematic representation of the time course and physiopathology of i3.
i3 is the result of a multistep process that is initially limited, reversible and then became systemic and irreversible and led to death. The pathophysiological process can be divided into two main stages related to the prognosis, the early and reversible phase and the late and irreversible phase.
Intestinal vascular insufficiency leads to hypoxia, first with mucosal and submucosal consequences. The hypoperfusion of the intestinal mucosa is responsible for an early hypoxic cellular desquamation of the intestinal villi. Polymorphonuclear neutrophils are early major lesional actors that adhere and migrate to the ischemic site to ensure the removal of tissue debris during necrosis. Mucosal and submucosal cells switch to anaerobic glycolysis with local production of lactate initially fully metabolized by the liver. The increase in intracellular acidosis blocks anaerobic metabolism and the membrane pumps of ionic and acid-base regulation. This leads to a profound alteration of cellular homeostasis and, ultimately, to cell death by apoptosis [7], [8], [9]. Initially, there is a dissociation between high porto-mesenteric blood lactate levels and normal peripheral blood lactate levels due to the active liver metabolism [1]. Systemic lactic acidosis is, therefore, a late phenomenon, which often indicates intestinal necrosis and the onset of a multi-visceral failure [10]. Associated endothelial lesions can lead to platelet, pro- and anti-thrombotic agent (protein C, S, and antithrombin) consumption, which causes the hemorrhagic syndrome.
Furthermore, the intestinal neuro-hormonal regulation of vasomotricity is associated with the activation of the renin-angiotensin-aldosterone system, which maintains the mucosal oxygen extraction rate. This induces a reflex splanchnic arterial vasospasm, irrespective of the initial vascular mechanism, that may prolong and worsen ischemia despite therapeutic revascularization. This vasoconstriction accompanies, for example, situations of hypovolemia, during which digestive ischemia develops before clinical hemodynamic instability [11], [12].
The disruption of the epithelial barrier resulting from mucosal alterations leads to interactions among microorganisms, bacterial antigens, endotoxins of the intestinal lumen with the mucosal and submucosal immune system. The stimulation of innate immunity will result in local then systemic inflammatory pathways activation such as TLR, NF-κB or TNF [13], [14]. Through the bloodstream, bacteria, endotoxins, cells degradation products and activated immune cells translocate and promote SIRS. Cytokines, chemokines, cellular and bacterial debris can also reach the pulmonary circulation from the lymphatic circulation and thus cause acute respiratory distress syndromes [15], [16]. The absence of a rapid recovery of a sufficient digestive perfusion leads to irreversible transmural necrosis and then to peritonitis. Without intestinal resection, the SIRS evolves to multiple organ syndrome and death [16].
In the model of the “gut origin of sepsis”, the gut was considered to be the “motor” of multi-organ failure [11], [15], [16], [17]. Aside from its barrier function, the gut contains growth factors, adenosine and hormones, which are potential mediators of the modulation of intestinal inflammation and repair, due to their roles in cellular proliferation, differentiation, migration, apoptosis and autophagy [18], [19], [20], [21], [22]. Physiologically, the gut could initiate and propagate sepsis due to the ability of bacteria, endotoxins, and other antigens to translocate, along with the production of pro-inflammatory cytokines and toxins [11]. In the “three hits model”, Deitch [23] added the phenomenon of reperfusion injury. In the “gut-lymph” theory, bacteria, cellular components, immune cells, cytokines and chemokines generated by the injured gut travel via the lymphatics to reach the pulmonary circulation, activating alveolar macrophages and contributing to acute lung injury, acute respiratory distress syndrome and multi-organ failure related to AMI [15], [16], [24].
Intestinal autodigestion
This quite recent concept describes the effect of pancreatic enzymes on the intestinal barrier altered by ischemia. Self-digestion contributes to the worsening of i3 lesions and the development of the related systemic inflammatory response. Degradation products of pancreatic enzymes, residues of bacterial products pass through the lymphatic, hematogenous or peritoneal barrier and are likely to induce not only a loco-regional but also a systemic reaction [19], [20]. In animal models, inhibition of these enzymes results in a decrease in intra-parietal micro-bleeding, systemic inflammatory response, and even in mortality in some studies [21]. The action of these enzymes would involve degradation of inter-enterocytic tight junction’s proteins such as E-cadherin. Moreover, these enzymes would also induce a cleavage of the pro-metalloproteinases into active metalloproteinases [22].
The systemic consequences of bowel ischemia and necrosis are lethal in most patients in the absence of curative treatment including revascularization [25], [26]. However, reoxygenation of the digestive mucosa can also paradoxically worsen epithelial and vascular lesions, due to an oxidative burst mechanism causing the influx and death of neutrophils with the formation of neutrophil extracellular traps and the secretion of their granular content [27].
Clinical unmet needs in the diagnosis workup of i3: urgent need for a biomarker
Early diagnosis of i3 requires a high degree of suspicion faced with any abdominal pain, especially when the pain is sudden or rapidly growing (“vascular-like”), unusual, intense and requiring opioids. Other clinical and biological associated signs (vomiting, diarrhea, gastrointestinal hemorrhage, hyperleukocytosis, lactic acidosis) are not constant or appear too late in the course of the disease and have no diagnostic value [1]. In our retrospective experience of a cohort of 221 patients, peritoneal signs, organ failure and serum lactate elevation were initially lacking in 85%, 77% and 57% of the cases, respectively [28]. When unrecognized at this stage, the diagnosis was carried out later at the stage of necrosis and complications, explaining why 184/221 (83%) of the patients required intestinal resection, resulting in short bowel syndrome in 148/184 (80%).
Improving the prognosis of i3 requires the discovery of early, sensitive and specific diagnostic biomarkers. In the last decade, some potential biomarkers have emerged from the literature. Some of these markers have been studied with particular interest, because of their higher presumed enterocyte specificity.
Materials and methods
Searching strategy
In an attempt to identify all studies that evaluate markers for human intestinal ischemia, two methods were used to retrieve information for this review. First, we conducted search onto the on-line databases PubMed and Google. The search was conducted using the following terms: (((“mesenteric ischemia” [MeSH terms] OR (“mesenteric” [all fields] AND “ischemia” [all fields]) OR “mesenteric ischemia” [all fields]) OR AMI [all fields] OR ((“intestines” [MeSH terms] OR “intestines” [all fields] OR “intestinal” [all fields]) AND (“ischaemia” [all fields] OR “ischemia” [MeSH terms] OR “ischemia” [all fields]))) AND (“diagnosis” [subheading] OR “diagnosis” [all fields] OR “diagnosis” [MeSH terms]) AND (“biomarkers” [MeSH terms] OR “biomarkers” [all fields] OR “marker” [all fields]) OR (“mesenteric ischemia” [MeSH terms] AND proteomic [all fields]) OR (“mesenteric ischemia” [MeSH terms] AND “metabolomic” [all fields]) OR (“mesenteric ischemia” [MeSH terms] AND “genetics” [all fields] OR (“mesenteric ischemia” [MeSH terms] AND “genetic variant” [all fields])).
The second method was to examine the references of the articles found by the electronic searches methods for additional citations.
With the use of retrieval mentioned above method, all English-language studies describing human subjects were considered for this review.
Selection
Two authors performed the searches. Any duplicates were removed. Two authors selected resulting articles independently. A first selection was made by screening the titles and abstracts of all articles. Next, full articles were read to make a final selection. Where there was no consensus, the manuscript was discussed with all authors to make a final decision about inclusion.
Non-specific biomarkers
Common laboratory findings
Variations in common biological blood parameters (base deficit, lactate dehydrogenase, aspartate aminotransferase, creatinine phosphokinase, alkaline phosphatase, phosphate, amylase) have frequently been observed [29], [30]. Metabolic acidosis is commonly observed as presented earlier.
Regarding hematological parameters, attention has been given to platelet indices, particularly platelets volume, but also to the various combination of neutrophils, lymphocytes and platelets ratios [31]. Budak et al. [32] proposed in a systematic review that low platelets volume could be used in the diagnosis, but considered that high platelet volume would be a poor prognosis indicator. As a whole, the use of such indices appears to be difficult to translate into clinical practice.
Biological markers of thrombosis
D-dimer, an enzymatic degradation product of fibrin, has been found to be the most consistent highly sensitive early marker, but has low specificity. Moreover, D-dimers are usually increased either in arterial or venous occlusive forms (A. Nuzzo and O. Corcos, personal communication), although they remain in the normal range in non-occlusive acute i3 [33]. In a meta-analysis, Cudnik et al. [34] evidenced that L-lactate and D-dimers exhibited a good pooled sensitivity (96%), although both were not specific enough (40%) to be used as diagnostic markers. The inclusion of both occlusive and non-occlusive forms of i3, as discussed in the larger paper of Matsumoto et al. [33], could lead, however, to high heterogeneity in patients. However, a recent meta-analysis estimated the area under the curve (AUC) of the receiver operating curve (ROC) of D-dimer to 0.81, underlying its potential utility in clinical practice [35].
Biological markers of hypoxia and oxidative stress
L-lactate is a ubiquitous product of glycolysis in the context of anaerobia. In 1994, Lange and Jackel [36] qualified L-lactate as the best marker of intestinal ischemia, regarding its negative predictive value. However, as expected, L-lactate elevation in plasma could not differentiate intestinal ischemia from the other etiologies of abdominal emergencies or intensive care diseases [37], [38], [39]. Its elevation better reflects the late stage of the disease, with extensive transmural necrosis, anaerobic metabolism due to systemic hypoperfusion [10]. Hence, it should not be used anymore as an early diagnostic marker of AMI [40].
Glutathione S-transferases (GST) are enzymes involved in the detoxification of a wide variety of endo- and xeno-biotics, conjugating them to glutathione. These enzymes are sensitive biomarkers of cytolysis, with a very short half-life, and are currently used in the diagnosis of hepatic cytolysis [41]. A-GST showed a significant increase in 50% of acute i3 patients, as compared with 12 other types of unclear acute abdominal pain suspected as being ischemic, with a negative predictive value of 100% [42]. In the analysis published by Evennett et al. [30], the pooled estimate of sensitivity was 68% and the pooled estimate of specificity was 85%. However, A-GST also increases in non-specific hypotensive patients with multiple organ failures [43].
During acute ischemic conditions, albumin’s metal-binding capacity is reduced, leading to the appearance of a metabolic variant known as ischemia-modified albumin (IMA). It is a sensitive but non-specific marker of myocardial and muscle ischemia, pulmonary embolism and stroke [44]. IMA is usually measured in the plasma or serum by enzyme-linked immunosorbent assay (ELISA) or using an assay based on a spectrophotometric method that measures altered cobalt-human serum albumin binding. Significantly increased plasmatic concentrations were found at admission time of seven patients with acute i3, as compared to healthy controls [45]. Another small study reported 100% sensitivity and 86% specificity in the detection of 12 intestinal ischemia in preoperative plasmas of 26 patients scheduled for exploratory laparotomy of mesenteric ischemia suspicion [46].
Biomarkers of inflammation
The C-reactive protein is commonly increased in acute i3. Acute inflammatory mediators such as interleukin-2 and 6 and tumor necrosis factor are non-specific of intestinal injury, although interleukin-6 (IL-6) has been proposed to be both sensitive and specific in a small cohort of 10 AMI [47].
Biomarker of infection
Acute i3 is usually associated with an increase in leukocytosis that can exceed 20 G/L [48]. Procalcitonin (PCT) has been proposed for the diagnosis of AMI. This precursor of the calcitonin is currently used in clinical practice for the differential diagnosis of infection of bacterial origin. According to a systematic review by Cosse et al. [49], PCT’s positive and negative predictive values for the diagnosis of acute i3 ranged between 27% and 90% and between 81% and 100%, respectively. As underlined by the authors, PCT is usually elevated during sepsis, in specific bacterial infections, and in various types of ischemias. As a whole, the use of PCT for the diagnosis of acute i3 lacks specificity.
Finally, none of the parameters cited above show enough clinical accuracy as a diagnostic marker of early, limited and reversible i3, which is mandatory to prevent the occurrence of intestinal necrosis and reduce the mortality rate. Therefore, the markers cited above, which can be assessed through current laboratory assays, show acceptable sensitivities, but none of them are specific enough to be used as a diagnostic marker.
Promising candidate biomarkers
In a candidate approach, some markers related to the pathophysiology of ischemia, intestinal insufficiency and gut barrier failure would be of interest in the biological diagnosis. Selected clinical studies concerning these biomarkers are presented in Table 1.
Selected clinical studies about citrulline, I-FABP and D-lactate as biomarkers of acute i3.
| First author, year | Sample size | Clinical condition | Biomarker | Tissue | Mean value | Range | Cut-off value | Sensitivity, % | Specificity, % | LR + | LR − | PPV | NPV | Experimental procedure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murray et al. 1994 [50] | 41 | Thirty-one patients undergoing laparotomy for an acute abdominal emergency, including patients with acute mesenteric ischemia (n=9), with small bowel obstruction (SBO) (n=5) or patient with only acute abdomen condition (n=17). Ten healthy controls were also included | D-lactate | Plasma | 32.37 μg/L | 11.71–48.66 μg/L | – | 90 | 87 | – | – | 70 | 96 | Spectrophotometric method on protein free plasma |
| Poeze et al. 1998 [51] | 45 | Ruptured abdominal aortic aneurysm (AAA) patients with (n=11) or without (n=13) ischemic complication were compared with controls (n=21) | D-lactate | Serum | – | – | 0.20 mmol/L | 82 | 77 | – | – | 83 | 75 | Spectrophotometric method on protein free plasma |
| Thuijls et al. 2011 [37] | 46 | Forty-six patients suspected for intestinal ischemia, including 22 that were diagnosed with intestinal ischemia and 24 patients that were diagnosed with other diseases | I-FABP | Plasma | 0.653 ng/mL | 0.04–74.711 ng/mL | 0.268 ng/mL | 68 | 71 | 23.4 | 45 | – | – | ELISA (Hycult Biotech, Uden, The Nederlands) |
| I-FABP | Urine | 3.377 ng/mL | 0.142–442.795 ng/mL | 0.551 ng/mL | 90 | 89 | 81.8 | 11 | – | – | ||||
| Güzel et al. 2014 [52] | 77 | Thirty patients diagnosed for intestinal ischemia were compared with 27 patients with other types of acute abdomen and with 20 healthy controls | I-FABP | Serum | 0.421 ng/mL | 0.04–5 ng/mL | 0.09 ng/mL | 90 | 100 | – | – | 100 | 87 | ELISA (Hycult Biotech, Uden, The Nederlands) |
| Kittaka et al. 2014 [53] | 37 | Thirty-seven patients diagnosed with SBO including 21 that were diagnosed with strangulated SBO and 16 patients that were diagnosed with simple SBO | I-FABP | Serum | 18.5 ng/mL | – | 6.5 ng/mL | 71.4 | 93.8 | – | – | 93.8 | 71.4 | ELISA |
| Matsumoto et al. 2014 [33] | 208 | Two hundred and eight patients including 24 patients with vascular, intestinal ischemia. Sixty-two patients with non-vascular ischemia and 122 with the non-ischemic disease | I-FABP | Serum | 31 ng/mL | 1.1–498.4 ng/mL | 9.1 ng/mL | 83.3 | 89.1 | – | – | 97.6 | 50 | ELISA |
| Schellekens et al. 2014 [54] | 32 | Human ischemia-reperfusion model | I-FABP | Arterioveinous difference | 463 ng/mL | – | 1.3 ng/mL | 90 | 100 | – | – | – | – | ELISA |
| Shi et al. 2015 [55] | 309 | Three hundred and nine patients including 39 patients with ischemic acute abdomen. Two hundred and thirty-three patients with non-ischemic acute abdomen and 37 healthy controls | I-FABP | Serum | 149.74 ng/mL | – | 93.07 ng/mL | 76.2 | 74.8 | 3.25 | 0.24 | 32.1 | 96.3 | ELISA |
| D-lactate | Serum | 52.73 μg/mL | – | 34.28 μg/mL | 66.7 | 85.9 | 2.82 | 0.31 | 86.3 | 72.6 | ||||
| Kulu et al. 2016 [56] | 48 | Forty-eight patients suspected for intestinal ischemia including 23 that were diagnosed with intestinal ischemia and 25 patients that were diagnosed with other diseases | Citrulline | Plasma | 21.7 nmol/L | – | 15.82 mmol/L | 39.13 | 100 | – | 0.61 | 100 | 64.1 | Ion-exchange chromatography method coupled with LC-MS/MS (amino acids LC-MS/MS analysis kit in biological, Zivak Technologies, Istambul, Turkey) |
| Salim et al. 2017 [57] | 18 | Eighteen patients suspected AMI among whom 13 were diagnosed with AMI. The five left were used as controls | I-FABP | Serum | 9 ng/mL | – | 0.69 ng/mL | 92.3 | 40 | 1.54 | 0.19 | – | – | ELISA (R&D Systems DuoSet., MN, USA) |
| I-FABP | Urine | 7 ng/mL | – | 2.52 ng/mL | 91.7 | 80 | 4.58 | 0.1 | – | – |
For simplification, the units were harmonized. LR+, likelihood ratio +; LR−, likelihood ratio −; PPV, positive predictive value; NPV, negative predictive value; n, number of patients.
D-lactate, a biomarker of gut barrier dysfunction
D-lactate, the second stereoisomer of lactate, is a byproduct of bacterial fermentation, with only a small amount being produced by human cells [58]. It can be found in the circulation after ischemic injury, increased intestinal permeability, or bacterial overgrowth. i3 is associated with growth of the resident bacterial microbiota that releases D-lactate into portal and systemic circulations. The analysis of D-lactate concentration requires strict preanalytical conditions, which are comparable to the one needed for the assessment of L-lactate concentration. D-lactate is usually assayed using an enzymatic UV spectrophotometric method on deproteinized plasma [59]. This latter method could be automated [60]. In the last decades, few studies were focused on the use of seric D-lactate in the biological diagnosis of i3. In 2006, in a prospective study, Collange et al. [61] compared D-lactate concentrations in 29 surgical abdominal aortic aneurysms patients (AAA), which may be associated with i3. D-lactate levels were increased in AAA patients for whom the inferior mesenteric artery was hypoperfused during the surgery (n=6, 0.13 mmol/L), as compared with AAA patients without hypoperfusion (n=23, 0.03 mmol/L, p=0.007). In 2015, Shi et al. [55] showed that serum D-lactate levels were increased in i3 patients (52.73±26.46 μg/mL in i3 vs. 15.58±5.17 μg/mL in non-intestinal ischemia) and could participate in the diagnosis. As a whole, according to the chosen threshold, the sensitivity of this marker in either plasma or serum ranged between 67% and 90%, whereas the specificity reached 87%. The design of the studies and their definition of intestinal ischemia were, however, heterogeneous. Therefore, there is a need for larger studies with well-characterized populations to confirm the potential use of D-lactate. This could be a promising track since the assay could be easily automated.
Fatty acid-binding proteins (FABP), biomarkers of villi injury
FABP are cytosolic proteins involved in the uptake and intracellular transport of fatty acids. The mature enterocyte expresses three isoforms: intestinal FABP (I-FABP), ileal bile acid-binding protein (I-BABP) and liver FABP (L-FABP).
The intestine, liver and kidneys express L-FABP, whereas I-BABP is specific of the ileum. I-FABP is a 15-kDa soluble protein expressed by enterocytes located at the tips of the intestinal mucosal villi, the anatomical region that is first affected by ischemic injuries. In physiological conditions, I-FABP is low in peripheral circulation and is cleared via the urine [62], [63]. After mucosal tissue injury, and especially enterocyte necrosis, the protein is quickly released into the bloodstream [64]. The liberation of the biomarker has been shown in rat to be concomitant with the ischemia [62] underlying its potential interest as a very early diagnosis marker [64].
Many studies have reported a relationship between blood I-FABP concentration and small intestinal diseases, in acute i3, critically ill patients, or post cardiac surgery, in which i3 represents roughly 1% of common complications [65]. Most of them are presented in Table 1.
In clinical settings, I-FABP concentrations measured in peritoneal fluid, plasma and urine are significantly higher in patients with i3 than in healthy controls and patients with other causes of the acute abdomen [66], [67], [68]. In peritoneal fluid, whose presence reveals late and severe disease, high levels of I-FABP were detected in patients with intestinal diseases [68].
In the study by Kanda et al. [67], elevated levels of serum I-FABP upon admission at the hospital were associated with 2/8 patients presenting with a strangulated bowel obstruction and 5/5 patients suffering from acute i3, whereas ranges were normal for healthy subjects (n=35) and patients with abdominal pain of other etiologies (n=48).
In a human experimental model of ischemia-reperfusion, Schellekens et al. [54] evidenced a correlation between the duration of ischemia and the increase of serum I-FABP. Kittaka et al. [53] found that serum I-FABP concentrations were significantly increased in patients with strangulated bowel obstruction (n=21; 18.5 ng/mL), as compared with patients with simple obstruction (n=16; 1.6 ng/mL).
Urine I-FABP could be a biomarker with high specificity and sensitivity (area under the receiver operating curve=0.88) for the diagnosis of acute i3 in 18 patients with suspected i3 [57].
Large variations are observed among studies regarding mean values and ranges. The comparison between seric and plasmatic values has not yet been published. Moreover, different ELISA kits are used participating to the variability of the results. For example, Shi et al. [55] described higher mean values and a higher cut-off value than other studies. As they do not mention the use of Hycult ELISA, which is used in all other studies, we can reasonably speculate that this difference could be related to the assay that was used.
A recent meta-analysis evidenced a pooled sensitivity of 80% for serum I-FABP, a pooled specificity of 85%, and an area under the ROC curve of 0.86 in the diagnosis of acute i3 [69].
Citrulline, a biomarker of enterocyte mass and intestinal failure
Citrulline is a non-proteinogenic amino acid synthesized from glutamine by small bowel enterocytes. This amino acid is a precursor of nitrogen oxide and participates in the transformation of ammonia into urea, and in the synthesis of arginine. Its plasmatic concentration depends on gut synthesis and renal elimination, decreasing in short bowel conditions and thus known as a functional marker of enterocyte mass, correlated with remnant small bowel length and home parenteral nutrition dependence [70]. Citrulline is usually measured in plasma or serum, using ELISA methods, high-performance liquide chromatography or mass spectrometry. Critically ill patients with shock may have an acute non-occlusive i3 resulting in a reduction of enterocyte mass and related citrulline synthesis, leading to low plasma citrulline concentrations [71]. In a study by Piton et al. [71], plasmatic citrulline concentration was shown to decrease in the first hours of shock in 24/55 critically ill patients and was correlated with mortality within 28 days.
In 2016, Kulu et al. [56] found that the concentration of plasmatic citrulline was significantly decreased in 23 patients with acute abdominal findings preoperatively attributed to AMI (mean: 0.72 mmol/L; range 0.57–0.84), as compared to those in patients with other acute abdominal conditions. Acute renal failure induces high plasmatic citrulline concentrations by decreasing renal clearance and citrulline transformation into arginine [72], which may complicate the interpretation of the results in severe patients with multiorgan failures. Moreover, post prandial samples are associated with a 10%–20% decrease in blood concentration [72]. Moreover, some inter-ethnic variations have been described in reference ranges.
These results suggest that citrulline is probably more promising as a prognostic than a diagnostic marker of AMI. Moreover, the interpretation of a ratio between plasmatic citrulline and creatinine could help minimize the effect of acute renal failure leading to possible false-negative results.
Overview on current biomarkers
Two recent systematic reviews have presented similar data as ours. Derikx et al. [73] presented D-dimer, I-FABP and α-GST as the most promising biomarkers. Treskes et al. [74] calculated combined AUC for IFABP, D-lactates and α-GST and α-GST appeared to be the more accurate, before IFABP and D-lactates, with only discrete differences between AUC (0.876, 0.84 and 0.814), respectively. Overall, on the basis of available studies, IFABP, α-GST, D-dimers and D-lactates appear to be useful in the diagnosis of acute i3, none of this marker being performant enough to be use solely. Moreover, a recent study by Schellekens et al. [75] proposed the smooth muscle of 22 kDa (SM22) as a potential marker of transmural intestinal ischemia in patients, adding to the spectra of potential biomarkers in i3.
Extensive efforts are still needed to design studies with increased population size, well-characterized in terms of phenotypic groups, and with standardized preanalytical and analytical conditions. On the other hand, more performant biomarkers could perhaps emerge from omics studies.
Towards promising novel approaches for finding biomarkers
The lack of suitably validated markers for the diagnosis of i3 pushes the scientific community to continue their investigations on the search for the molecule that will help for the early diagnostic of i3 and that will prevent the complications and the need for surgery. The emerging use of omics in the discovery of biomarkers opens new ways.
Regarding genomic data, no clue arises from genetic studies in acute i3. However, no large genetic study has been published in this disease. The genetic variants predisposing to venous thromboembolism (i.e. factors V and II Leiden, MTHFR variants) could participate in the physiopathological process of the disease, although descriptions are rare and related to private patients [76].
Transcriptomic studies in models of acute i3 are scarce. In a pig model with induced proximal arterial occlusion of the superior mesenteric artery, Block et al. identified a panel of up- and down-regulated mRNA (157 and 57 transcripts, respectively). Up-regulated mRNA included monocyte chemoattractant protein 1 (MCP1) and acyl CoA synthetase long-chain family member 4 [77]. In a preclinical study in swine, a microRNA signature was identified in an i3 model related to hypothermic circulatory arrest associated with the gut barrier dysfunction. This signature included a noticeable decrease in mRNA-31, which interacts with the hypoxia-inducible factor HIF function [78].
Proteomic studies investigate the pool of full-length, truncated and post-traductionally modified proteins and peptides, whereas metabolomics explores the whole metabolic process of peptides, saccharides and lipids. Both approaches have been increasingly used for biomarker discovery in the last decade, thanks to the development of mass spectrometry and bioinformatics.
Experimental studies for acute i3 biomarkers search using both proteomic and metabolomics approaches are summarized in Table 2. We found only five studies using heterogeneous techniques and models, and no common marker was evidenced.
Experimental animal studies using either metabolomics or proteomic approaches.
| First author, year | Omic | Tissue | Molecule | Results | Model | Specie | Procedure |
|---|---|---|---|---|---|---|---|
| Solligard et al. 2005 [79] | Metabolomic | Gut lumen microdialysate | Glycerol | Increase | Mesenteric ischemia-reperfusion model (ligature of the superior mesenteric artery for 60 or 120 min followed by 3 h of reperfusion | Pig | Microdialysis |
| Li et al. 2010 [80] | Proteomic/metabolomic | Intestinal mucosa | Phosphoglycerate mutase 1 Cytochrome b-c1 complex | Decrease Decrease | Ischemia/reperfusion model | Rat | 2D Gel electrophoresis coupled with MALDI-TOF |
| Pyruvate kinase | Decrease | ||||||
| Cytoplasmic aconitate hydratase | Decrease | ||||||
| Glutamate dehydrogenase | Decrease | ||||||
| Enoyl coenzyme A hydratase 1 | Decrease | ||||||
| Isocitrate dehydrogenase | Decrease | ||||||
| Glyceraldehyde-3-phosphate dehydrogenase | Decrease | ||||||
| Aldose reductase | Decrease | ||||||
| Aldehyde dehydrogenase | Decrease | ||||||
| Protein disulfide isomerase A3 | Decrease | ||||||
| Tubulin a-1B chain | Increase | ||||||
| Intelectin 1 | Decrease | ||||||
| Retinol binding protein 2 | Decrease | ||||||
| Albumin precursor | Increase | ||||||
| Annexin A2 | Increase | ||||||
| Birke-Sorensen and Andersen 2010 [81] | Metabolomic | Intestinal segments | Glucose Lactate | Decrease Increase | Mesenteric ischemia-reperfusion model (ligature of the superior mesenteric artery for 60 min followed by 2 h of reperfusion) | Pig | Microdialysis |
| Fahrner et al. 2012 [82] | Metabolomic | Serum | Arabinose Xylose | Decrease Decrease | Mesenteric ischemia model (ligature of the superior mesenteric artery for 2–4 h) | Mouse | GC/MS |
| Glucose | Decrease | ||||||
| Ribose | Decrease | ||||||
| Stearic acid | Decrease | ||||||
| Urea | Increase | ||||||
| Threonic acid | Increase | ||||||
| Inorganic phosphate | Increase | ||||||
| Steelman et al. 2014 [83] | Metabolomic | Serum | Citrulline | Decrease | Horse laminitis model | Horse | GC-LC/MS |
GC/MS, Gaz chromatography/mass spectrometry.
Steelman et al. [83] confirmed in the horse the interest of citrulline as a biomarker. None of the proteomic studies identified in either pig or rodent FABP proteins. However, this could be due to imperfect animal models and interspecies variability. Regarding metabolomic studies, the only common observation was a decrease in glucose observed in two rodent models.
Conclusions
Acute i3 is a highly severe condition, with a high mortality rate and major anatomical and functional consequences in case of survival. Acute i3 represents a gut and life-threatening emergency for which the main identified prognosis factor is the precocity of the diagnosis and treatment.
A large number of molecules have been proposed as potential biomarkers of i3. However, conflicting findings from different studies had very unsatisfactory results. We mainly chose to focus our review on the three promising biomarkers: I-FABP, D-lactate and citrulline. Even though these molecules are interesting candidates, the reported findings highlighted many limitations. In the clinical setting, most studies were performed in small populations, with high pre-test probability of high suspicion of intestinal ischemia, and at a late stage. Studies in early and less severe disease are still lacking. As observed in numerous diseases, the combination of several biomarkers rather than the use of a single marker is probably a better paradigm to explore. Moreover, the use of omics studies on well-phenotyped patients could be another promising track. Indeed, several works that used high-scale proteomics and metabolomics studies have emerged in the last decade. The advantage of such a technique is to allow the identification of completely novel candidates that have not been hypothesized yet on the basis of physiopathology.
Nevertheless, in 2017, the need for early and robust biomarkers for i3 diagnosis remains.
Acknowledgments
The authors would like to acknowledge MSD-France (Merck Sharp & Dohme) for funding the SURVI project.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: MS-France: SURVI.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Corcos O, Nuzzo A. Gastro-intestinal vascular emergencies. Best Pract Res Clin Gastroenterol 2013;27:709–25.10.1016/j.bpg.2013.08.006Search in Google Scholar PubMed
2. Nuzzo A, Corcos O. Reversible acute mesenteric ischemia. N Engl J Med 2016;375:e31.10.1056/NEJMicm1509318Search in Google Scholar PubMed
3. Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med 2016;374:959–68.10.1056/NEJMra1503884Search in Google Scholar PubMed
4. Menke J. Diagnostic accuracy of multidetector ct in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010;256:93–101.10.1148/radiol.10091938Search in Google Scholar PubMed
5. Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol 1986;250:G749–53.10.1152/ajpgi.1986.250.6.G749Search in Google Scholar PubMed
6. Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 2007;28:384–93.10.1097/shk.0b013e31805569dfSearch in Google Scholar PubMed PubMed Central
7. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970;101:478–83.10.1001/archsurg.1970.01340280030009Search in Google Scholar PubMed
8. Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 1992;216:117–34.10.1097/00000658-199208000-00002Search in Google Scholar PubMed PubMed Central
9. Zimmerman BJ, Granger DN. Mechanisms of reperfusion injury. Am J Med Sci 1994;307:284–92.10.1097/00000441-199404000-00009Search in Google Scholar PubMed
10. Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol 2017;112:597–605.10.1038/ajg.2017.38Search in Google Scholar PubMed
11. Boley SJ, Brandt LJ, Sammartano RJ. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin North Am 1997;77:275–88.10.1016/S0039-6109(05)70548-XSearch in Google Scholar
12. Boley SJ, Sprayregan S, Siegelman SS, Veith FJ. Initial results from an agressive roentgenological and surgical approach to acute mesenteric ischemia. Surgery 1977;82:848–55.Search in Google Scholar
13. Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med 1997;25:1813–9.10.1097/00003246-199711000-00018Search in Google Scholar PubMed
14. Hietbrink F, Besselink MG, Renooij W, de Smet MB, Draisma A, van der Hoeven H, et al. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock 2009;32:374–8.10.1097/SHK.0b013e3181a2bcd6Search in Google Scholar PubMed
15. Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced sirs and mods: the gut-lymph hypothesis, a review. Front Biosci 2006;11:520–8.10.2741/1816Search in Google Scholar PubMed
16. Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma 2006;60:958–65; discussion 65–7.10.1097/01.ta.0000215500.00018.47Search in Google Scholar PubMed
17. Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001;345:1683–8.10.1056/NEJMra010076Search in Google Scholar PubMed
18. Elkrief L, Corcos O, Bruno O, Larroque B, Rautou PE, Zekrini K, et al. Type 2 diabetes mellitus as a risk factor for intestinal resection in patients with superior mesenteric vein thrombosis. Liver Int 2014;34:1314–21.10.1111/liv.12386Search in Google Scholar PubMed
19. Fishman JE, Sheth SU, Levy G, Alli V, Lu Q, Xu D, et al. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg 2014;260:1112–20.10.1097/SLA.0000000000000631Search in Google Scholar PubMed PubMed Central
20. Pontell L, Sharma P, Rivera LR, Thacker M, Tan YH, Brock JA, et al. Damaging effects of ischemia/reperfusion on intestinal muscle. Cell Tissue Res 2011;343:411–9.10.1007/s00441-010-1096-zSearch in Google Scholar PubMed
21. Chang M, Kistler EB, Schmid-Schonbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock 2012;37:297–305.10.1097/SHK.0b013e318240b59bSearch in Google Scholar PubMed PubMed Central
22. Altshuler AE, Richter MD, Modestino AE, Penn AH, Heller MJ, Schmid-Schonbein GW. Removal of luminal content protects the small intestine during hemorrhagic shock but is not sufficient to prevent lung injury. Physiol Rep 2013;1:e00109.10.1002/phy2.109Search in Google Scholar
23. Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon 2012;10:350–6.10.1016/j.surge.2012.03.003Search in Google Scholar
24. Badami CD, Senthil M, Caputo FJ, Rupani BJ, Doucet D, Pisarenko V, et al. Mesenteric lymph duct ligation improves survival in a lethal shock model. Shock 2008;30:680–5.10.1097/SHK.0b013e318173edd1Search in Google Scholar
25. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease etiology. Br J Surg 2004;91:17–27.10.1002/bjs.4459Search in Google Scholar
26. Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg 2008;393:163–71.10.1007/s00423-007-0263-5Search in Google Scholar
27. Parks DA, Jacobson ED. Physiology of the splanchnic circulation. Arch Intern Med 1985;145:1278–81.10.1001/archinte.1985.00360070158027Search in Google Scholar
28. Nuzzo A, Maggiori L, Ronot M, Becq A, Cazals-Hatem D, Plessier A, et al. Intestinal resection in acute mesenteric ischemia: predictive factors in 221 consecutive patients followed in an intestinal stroke center. Gastroenterology 2016;150:S692.10.1016/S0016-5085(16)32358-7Search in Google Scholar
29. Kurland B, Brandt LJ, Delany HM. Diagnostic tests for intestinal ischemia. Surg Clin North Am 1992;72:85–105.10.1016/S0039-6109(16)45629-XSearch in Google Scholar
30. Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg 2009;33:1374–83.10.1007/s00268-009-0074-7Search in Google Scholar PubMed
31. Degerli V, Ergin I, Duran FY, Ustuner MA, Duran O. Could mean platelet volume be a reliable indicator for acute mesenteric ischemia diagnosis? A case-control study. Biomed Res Int 2016;2016:9810280.10.1155/2016/9810280Search in Google Scholar PubMed PubMed Central
32. Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb) 2016;26:178–93.10.11613/BM.2016.020Search in Google Scholar PubMed PubMed Central
33. Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, et al. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg 2014;101:232–8.10.1002/bjs.9331Search in Google Scholar PubMed
34. Cudnik MT, Darbha S, Jones J, Macedo J, Stockton SW, Hiestand BC. The diagnosis of acute mesenteric ischemia: a systematic review and meta-analysis. Acad Emerg Med 2013;20:1087–100.10.1111/acem.12254Search in Google Scholar PubMed
35. Sun DL, Li SM, Cen YY, Xu QW, Li YJ, Sun YB, et al. Accuracy of using serum d-dimer for diagnosis of acute intestinal ischemia: a meta-analysis. Medicine (Baltimore) 2017;96:e6380.10.1097/MD.0000000000006380Search in Google Scholar PubMed PubMed Central
36. Lange H, Jackel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994;160:381–4.Search in Google Scholar
37. Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, et al. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg 2011;253:303–8.10.1097/SLA.0b013e318207a767Search in Google Scholar PubMed
38. Acosta S, Nilsson TK, Malina J, Malina M. L-lactate after embolization of the superior mesenteric artery. J Surg Res 2007;143:320–8.10.1016/j.jss.2007.02.003Search in Google Scholar PubMed
39. Chiu YH, Huang MK, How CK, Hsu TF, Chen JD, Chern CH, et al. D-dimer in patients with suspected acute mesenteric ischemia. Am J Emerg Med 2009;27:975–9.10.1016/j.ajem.2009.06.006Search in Google Scholar PubMed
40. Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis 2012;33:355–61.10.1007/s11239-011-0660-zSearch in Google Scholar PubMed
41. Vaubourdolle M, Chazouilleres O, Briaud I, Legendre C, Serfaty L, Poupon R, et al. Plasma alpha-glutathione s-transferase assessed as a marker of liver damage in patients with chronic hepatitis c. Clin Chem 1995;41:1716–9.10.1093/clinchem/41.12.1716Search in Google Scholar
42. Delaney CP, O’Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione s-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg 1999;86:1349–53.10.1046/j.1365-2168.1999.01245.xSearch in Google Scholar PubMed
43. van den Heijkant TC, Aerts BA, Teijink JA, Buurman WA, Luyer MD. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol 2013;19:1338–41.10.3748/wjg.v19.i9.1338Search in Google Scholar
44. Keating L, Benger JR, Beetham R, Bateman S, Veysey S, Kendall J, et al. The prima study: presentation ischaemia-modified albumin in the emergency department. Emerg Med J 2006;23:764–8.10.1136/emj.2006.036269Search in Google Scholar
45. Gunduz A, Turedi S, Mentese A, Karahan SC, Hos G, Tatli O, et al. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med 2008;26:202–5.10.1016/j.ajem.2007.04.030Search in Google Scholar
46. Polk JD, Rael LT, Craun ML, Mains CW, Davis-Merritt D, Bar-Or D. Clinical utility of the cobalt-albumin binding assay in the diagnosis of intestinal ischemia. J Trauma 2008;64:42–5.10.1097/TA.0b013e31815b846aSearch in Google Scholar
47. Sgourakis G, Papapanagiotou A, Kontovounisios C, Karamouzis MV, Lanitis S, Konstantinou C, et al. The value of plasma neurotensin and cytokine measurement for the detection of bowel ischaemia in clinically doubtful cases: a prospective study. Exp Biol Med (Maywood) 2013;238:874–80.10.1177/1535370213494663Search in Google Scholar
48. Block T, Nilsson TK, Bjorck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest 2008;68:242–8.10.1080/00365510701646264Search in Google Scholar
49. Cosse C, Sabbagh C, Kamel S, Galmiche A, Regimbeau JM. Procalcitonin and intestinal ischemia: a review of the literature. World J Gastroenterol 2014;20:17773–8.10.3748/wjg.v20.i47.17773Search in Google Scholar
50. Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum d(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg 1994;167:575–8.10.1016/0002-9610(94)90101-5Search in Google Scholar
51. Poeze M, Froon AH, Greve JW, Ramsay G. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg 1998;85:1221–4.10.1046/j.1365-2168.1998.00837.xSearch in Google Scholar PubMed
52. Guzel M, Sozuer EM, Salt O, Ikizceli I, Akdur O, Yazici C. Value of the serum i-fabp level for diagnosing acute mesenteric ischemia. Surg Today 2014;44:2072–6.10.1007/s00595-013-0810-3Search in Google Scholar PubMed
53. Kittaka H, Akimoto H, Takeshita H, Funaoka H, Hazui H, Okamoto M, et al. Usefulness of intestinal fatty acid-binding protein in predicting strangulated small bowel obstruction. PLoS One 2014;9:e99915.10.1371/journal.pone.0099915Search in Google Scholar PubMed PubMed Central
54. Schellekens DH, Grootjans J, Dello SA, van Bijnen AA, van Dam RM, Dejong CH, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol 2014;48:253–60.10.1097/MCG.0b013e3182a87e3eSearch in Google Scholar PubMed
55. Shi H, Wu B, Wan J, Liu W, Su B. The role of serum intestinal fatty acid binding protein levels and d-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol 2015;39:373–8.10.1016/j.clinre.2014.12.005Search in Google Scholar PubMed
56. Kulu R, Akyildiz H, Akcan A, Ozturk A, Sozuer E. Plasma citrulline measurement in the diagnosis of acute mesenteric ischaemia. ANZ J Surg 2016.10.1111/ans.13524Search in Google Scholar PubMed
57. Salim SY, Young PY, Churchill TA, Khadaroo RG. Urine intestinal fatty acid-binding protein predicts acute mesenteric ischemia in patients. J Surg Res 2017;209:258–65.10.1016/j.jss.2016.07.017Search in Google Scholar PubMed
58. Ewaschuk JB, Naylor JM, Zello GA. D-lactate in human and ruminant metabolism. J Nutr 2005;135:1619–25.10.1093/jn/135.7.1619Search in Google Scholar PubMed
59. Marti R, Varela E, Segura RM, Alegre J, Surinach JM, Pascual C. Determination of d-lactate by enzymatic methods in biological fluids: study of interferences. Clin Chem 1997;43:1010–5.10.1093/clinchem/43.6.1010Search in Google Scholar
60. Sapin V, Nicolet L, Aublet-Cuvelier B, Sangline F, Roszyk L, Dastugue B, et al. Rapid decrease in plasma d-lactate as an early potential predictor of diminished 28-day mortality in critically ill septic shock patients. Clin Chem Lab Med 2006;44:492–6.10.1515/CCLM.2006.086Search in Google Scholar PubMed
61. Collange O, Tamion F, Meyer N, Quillard M, Kindo M, Hue G, et al. Early detection of gut ischemia-reperfusion injury during aortic abdominal aneurysmectomy: a pilot, observational study. J Cardiothorac Vasc Anesth 2013;27:690–5.10.1053/j.jvca.2013.01.018Search in Google Scholar PubMed
62. Gollin G, Marks C, Marks WH. Intestinal fatty acid binding protein in serum and urine reflects early ischemic injury to the small bowel. Surgery 1993;113:545–51.Search in Google Scholar
63. Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest 1974;54:326–38.10.1172/JCI107768Search in Google Scholar PubMed PubMed Central
64. Piton G, Capellier G. Biomarkers of gut barrier failure in the icu. Curr Opin Crit Care 2016;22:152–60.10.1097/MCC.0000000000000283Search in Google Scholar
65. Guillaume A, Pili-Floury S, Chocron S, Delabrousse E, De Parseval B, Koch S, et al. Acute mesenteric ischemia among postcardiac surgery patients presenting with multiple organ failure. Shock 2017;47:296–302.10.1097/SHK.0000000000000720Search in Google Scholar
66. Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery 1997;121:335–42.10.1016/S0039-6060(97)90363-9Search in Google Scholar
67. Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996;110:339–43.10.1053/gast.1996.v110.pm8566578Search in Google Scholar
68. Sonnino R, Ereso G, Arcuni J, Franson R. Human intestinal fatty acid binding protein in peritoneal fluid is a marker of intestinal ischemia. Transplant Proc 2000;32:1280.10.1016/S0041-1345(00)01225-2Search in Google Scholar
69. Sun DL, Cen YY, Li SM, Li WM, Lu QP, Xu PY. Accuracy of the serum intestinal fatty-acid-binding protein for diagnosis of acute intestinal ischemia: a meta-analysis. Sci Rep 2016;6:34371.10.1038/srep34371Search in Google Scholar PubMed PubMed Central
70. Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000;119:1496–505.10.1053/gast.2000.20227Search in Google Scholar PubMed
71. Piton G, Manzon C, Monnet E, Cypriani B, Barbot O, Navellou JC, et al. Plasma citrulline kinetics and prognostic value in critically ill patients. Intens Care Med 2010;36:702–6.10.1007/s00134-010-1751-6Search in Google Scholar PubMed
72. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008;27:328–39.10.1016/j.clnu.2008.02.005Search in Google Scholar PubMed
73. Derikx JP, Schellekens DH, Acosta S. Serological markers for human intestinal ischemia: a systematic review. Best Pract Res Clin Gastroenterol 2017;31:69–74.10.1016/j.bpg.2017.01.004Search in Google Scholar PubMed
74. Treskes N, Persoon AM, van Zanten AR. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med 2017.10.1007/s11739-017-1668-ySearch in Google Scholar PubMed PubMed Central
75. Schellekens D, Reisinger KW, Lenaerts K, Hadfoune M, Olde Damink SW, Buurman WA, et al. Sm22 a plasma biomarker for human transmural intestinal ischemia. Ann Surg 2017.10.1097/SLA.0000000000002278Search in Google Scholar PubMed
76. Karmacharya P, Aryal MR, Donato A. Mesenteric vein thrombosis in a patient heterozygous for factor v leiden and g20210a prothrombin genotypes. World J Gastroenterol 2013;19:7813–5.10.3748/wjg.v19.i43.7813Search in Google Scholar PubMed PubMed Central
77. Block T, Isaksson HS, Acosta S, Bjorck M, Brodin D, Nilsson TK. Altered mrna expression due to acute mesenteric ischaemia in a porcine model. Eur J Vasc Endovasc Surg 2011;41:281–7.10.1016/j.ejvs.2010.09.012Search in Google Scholar PubMed
78. Lin WB, Liang MY, Chen GX, Yang X, Qin H, Yao JP, et al. Microrna profiling of the intestine during hypothermic circulatory arrest in swine. World J Gastroenterol 2015;21:2183–90.10.3748/wjg.v21.i7.2183Search in Google Scholar PubMed PubMed Central
79. Solligard E, Juel IS, Bakkelund K, Jynge P, Tvedt KE, Johnsen H, et al. Gut luminal microdialysis of glycerol as a marker of intestinal ischemic injury and recovery. Crit Care Med 2005;33:2278–85.10.1097/01.CCM.0000178187.84732.6CSearch in Google Scholar
80. Li YS, Wang ZX, Li C, Xu M, Li Y, Huang WQ, et al. Proteomics of ischemia/reperfusion injury in rat intestine with and without ischemic postconditioning. J Surg Res 2010;164: e173–80.10.1016/j.jss.2009.10.003Search in Google Scholar PubMed
81. Birke-Sorensen H, Andersen NT. Metabolic markers obtained by microdialysis can detect secondary intestinal ischemia: an experimental study of ischemia in porcine intestinal segments. World J Surg 2010;34:923–32.10.1007/s00268-010-0502-8Search in Google Scholar PubMed
82. Fahrner R, Beyoglu D, Beldi G, Idle JR. Metabolomic markers for intestinal ischemia in a mouse model. J Surg Res 2012;178:879–87.10.1016/j.jss.2012.08.011Search in Google Scholar PubMed PubMed Central
83. Steelman SM, Johnson P, Jackson A, Schulze J, Chowdhary BP. Serum metabolomics identifies citrulline as a predictor of adverse outcomes in an equine model of gut-derived sepsis. Physiol Genomics 2014;46:339–47.10.1152/physiolgenomics.00180.2013Search in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Analytical quality: an unfinished journey
- Reviews
- Update in diagnosis and management of primary aldosteronism
- Diagnosis biomarkers in acute intestinal ischemic injury: so close, yet so far
- Opinion Papers
- Irregular analytical errors in diagnostic testing – a novel concept
- A Black Swan in clinical laboratory practice: the analytical error due to interferences in immunoassay methods
- General Clinical Chemistry and Laboratory Medicine
- Reaching consensus on communication of critical laboratory results using a collective intelligence method
- Stability of routine biochemical analytes in whole blood and plasma/serum: focus on potassium stability from lithium heparin
- GFR estimation based on standardized creatinine and cystatin C: a European multicenter analysis in older adults
- Binding of bromocresol green and bromocresol purple to albumin in hemodialysis patients
- Interlaboratory variability of urinary iodine measurements
- The venous thromboembolic risk and the clot wave analysis: a useful relationship?
- Hematology and Coagulation
- Autovalidation and automation of the postanalytical phase of routine hematology and coagulation analyses in a university hospital laboratory
- Reference Values and Biological Variations
- Indirect method for validating transference of reference intervals
- Differences in levels of albumin, ALT, AST, γ-GT and creatinine in frail, moderately healthy and healthy elderly individuals
- Cancer Diagnostics
- Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma
- Intragenic hypomethylation of DNMT3A in patients with myelodysplastic syndrome
- Cardiovascular Diseases
- Evaluation of analytical performance of a new high-sensitivity immunoassay for cardiac troponin I
- MEF2C loss-of-function mutation associated with familial dilated cardiomyopathy
- Letter to the Editor
- Hyperuricemia does not seem to be an independent risk factor for coronary heart disease
- Reply to: Hyperuricemia does not seem to be an independent risk factor for coronary heart disease
- Preanalytics of ammonia: stability, transport and temperature of centrifugation
- Influence of delayed separation of plasma from whole blood on Cu, I, Mn, Se, and Zn plasma concentrations
- Copeptin as a diagnostic and prognostic biomarker in patients admitted to Emergency Department with syncope, presyncope and vertiginous syndrome
- Development of an internally controlled quantitative PCR to measure total cell-associated HIV-1 DNA in blood
- Selective changes in cholesterol metabolite levels in plasma of breast cancer patients after tumor removal
- Athletes beware before throwing towels to audiences
Articles in the same Issue
- Frontmatter
- Editorial
- Analytical quality: an unfinished journey
- Reviews
- Update in diagnosis and management of primary aldosteronism
- Diagnosis biomarkers in acute intestinal ischemic injury: so close, yet so far
- Opinion Papers
- Irregular analytical errors in diagnostic testing – a novel concept
- A Black Swan in clinical laboratory practice: the analytical error due to interferences in immunoassay methods
- General Clinical Chemistry and Laboratory Medicine
- Reaching consensus on communication of critical laboratory results using a collective intelligence method
- Stability of routine biochemical analytes in whole blood and plasma/serum: focus on potassium stability from lithium heparin
- GFR estimation based on standardized creatinine and cystatin C: a European multicenter analysis in older adults
- Binding of bromocresol green and bromocresol purple to albumin in hemodialysis patients
- Interlaboratory variability of urinary iodine measurements
- The venous thromboembolic risk and the clot wave analysis: a useful relationship?
- Hematology and Coagulation
- Autovalidation and automation of the postanalytical phase of routine hematology and coagulation analyses in a university hospital laboratory
- Reference Values and Biological Variations
- Indirect method for validating transference of reference intervals
- Differences in levels of albumin, ALT, AST, γ-GT and creatinine in frail, moderately healthy and healthy elderly individuals
- Cancer Diagnostics
- Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma
- Intragenic hypomethylation of DNMT3A in patients with myelodysplastic syndrome
- Cardiovascular Diseases
- Evaluation of analytical performance of a new high-sensitivity immunoassay for cardiac troponin I
- MEF2C loss-of-function mutation associated with familial dilated cardiomyopathy
- Letter to the Editor
- Hyperuricemia does not seem to be an independent risk factor for coronary heart disease
- Reply to: Hyperuricemia does not seem to be an independent risk factor for coronary heart disease
- Preanalytics of ammonia: stability, transport and temperature of centrifugation
- Influence of delayed separation of plasma from whole blood on Cu, I, Mn, Se, and Zn plasma concentrations
- Copeptin as a diagnostic and prognostic biomarker in patients admitted to Emergency Department with syncope, presyncope and vertiginous syndrome
- Development of an internally controlled quantitative PCR to measure total cell-associated HIV-1 DNA in blood
- Selective changes in cholesterol metabolite levels in plasma of breast cancer patients after tumor removal
- Athletes beware before throwing towels to audiences

