Abstract
Matrix metallo-proteinases (MMPs) are a family of zinc-dependent endopeptidases, capable of degrading all the molecular components of extracellular matrix. A class of MMPs is gelatinases which includes gelatinase A or MMP-2 (72 kDa) and gelatinase B or MMP-9 (92 kDa), which have been shown to play critical roles in pathophysiology of many human disease and, in particular, cancer progression. For these reasons they obtained a great interest as potential non-invasive biomarker in providing useful clinical information in cancer diagnosis and therapy. A sensitive and unexpensive method for analysis of gelatinases is the gelatine zymography, which allows to measure the relative amounts of active and inactive enzymes in body fluids and tissue extracts. The procedure involves the electrophoretic separation of proteins under denaturing but non reducing conditions through a polyacrylamide gel containing a synthetic substrate (gelatin). The aim of this mini-review has been to describe the general principles of gelatine zymography technique, underling the main advantages and disadvantages. Even though an improvement of this method is necessary for a better applicability in laboratory medicine, gelatine zymography represents the most convenient method to detect the activity of the different gelatinases from a wide range of biological samples.
Introduction
The term “metalloprotease” encompasses esopeptidases and endopeptidases involved in many biological processes, and are grouped in 14 different clans. Some clans require only one catalytic metal ion and others require two metal ions acting co-catalytically. The divalent metal ion contained in the active site is, in the vast majority of cases, a zinc ion, but cobalt, manganese or nickel are also represented. In human, the majority of metallo-proteases are zinc metallo-endopeptidases distributed in five clans (i.e. MA, ME, MJ, MK, MM) [1]. Among these, the clan MA is the main clan of metallo-proteinases and it is characterized by the “HEXXH” zinc-binding motif with two histidines acting as a ligands of the catalytic Zn++ and the glutamate as the general basis. This clan include the following families: M3, M10, M12, M13, M41, M43, and M48. In particular, M10 family includes human zinc-endopeptidases known as “matrix metalloproteinases” (MMPs). It is becoming increasingly recognized that MMPs are a multifunctional group of biologically important molecules with diverse roles in normal cell growth and differentiation. This family consists in 25 members and numerous homologues from other species which are able to degrade basement membranes and extracellular matrix (ECM) components. Although MMPs have overlapping substrate specificities, they are divided into five groups with respect to their preferential degradation of different matrix substrates [matrilysin, collagenases, gelatinases, stromelysin and membrane MMPs (MT-MMPs)] and in eight structural classes (Table 1), three of which are membrane bound [2, 3].
Members of the MMP family.
| Structure | Name | Pseudonym | Human chromosome | Molecular weight, Da (latent/active) | Matrix substrates | Bioactive substrates |
|---|---|---|---|---|---|---|
| Minimal-domain | MMP-7 MMP-26 | Matrilysin-1 Matrilysin-2 | 11q21-q22 11p15 | 28.000/19.000 | Collagen IV, X IV, aggrecan, elastin, fibronectin, gelatin, laminin, fibrinogen, fibronectin, gelatin | TNF-α, α-PI, IGFBP-3, TNF-α, RANK ligand, IGFBP-3, fas ligand, plasminogen, E-caderin, proα-defensin, HB-EGF, MMP-9 |
| Simple hemopexin-domain | MMP-1 | Collagenase-1 | 11q22-q23 | 55.000/45.000 | Collagen I, II, III,VII,VIII, X, aggrecan, gelatin | IGFBP-3, IGFBP-2, IL-1β, MCP-3, CTGF, α2-M, α-PI, MMP-2, -9 |
| (Archetypal) | MMP-8 | Collagenase-2 | 11q21-q22 | 75.000/58.000 | Collagen I, II, III,V, VII,VIII, X, aggrecan, elastin, fibronectin, gelatin, laminin | α-PI |
| MMP-13 | Collagenase-3 | 11q22.3 | 60.000/48.000 | Collagen I, II, III, IV, IX, X, XIV, aggregan, gelatin | MCP-3, α1-antichymotrypsin, plasminogen, TGFβ | |
| MMP-18 | Collagenase-4 | – | 70.000/53.000 | Unknown | Unknown | |
| (Xenopus)a | ||||||
| MMP-3 | Stromelysin-1 | 11q23 | 57.000/45.000 | Collagen II, III, IV, IX, X, XI, aggrecan, elastin, fibronectin, gelatin, laminin | IGFBP-3, IL-1β, MCP-3, HB-EGF, E-caderin, plasminogen, uPA, α1-antichymotrypsin, α2-M, α-PI, MMP-7, -8, -13 | |
| MMP-10 | Stromelysin-2 | 11q22.3-q23 | 57.000/44.000 | Collagen III, IV, V, aggrecan, elastin, fibronectin, gelatin, laminin | MMP-1, -8 | |
| MMP-12 MMP-19 | Metallo-elastase | 11q22.2-q22.3 12q14 | 54.000/45.000 | Collagen IV, elastin, fibronectin, gelatin, laminin, aggregan, fibronectin, amelogenin | IGFBP-3, plasminogen, apolipoprotein (a), uPA, α1-antichymotrypsin, α2-M, α-PI, E-caderin, MMP-1, -7, 8, -9, -13 | |
| MMP-20 MMP-27 | Enamelysin | 11q22.3 11q24 | 54.000/22.000 | Aggregan, amelogenin, gelatin | Unknown | |
| Gelatin-binding | MMP-2 MMP-9 | Gelatinase-A Gelatinase-B | 16q13 20q11.2-q13.1 | 72.000/66.000 92.000/86.000 | Collagen I, II, III, IV, V, VII, X, XI, aggrecan, elastin, fibronectin, gelatin, laminin | IGFBP-3, -1, -5, IL-1β, ICAM-1, MCP-3, IL-2Rα, TGFβ, SDF-1, IL-8, IFNγ, FGFR-1, Kit-L, plasminogen, galectin-3 |
| Furin-activatedsecreted | MMP-11 MMP-28 | Stromelysin-3 Epilysin | 22q11.2 17q21.1 | 51.000/44.000 | Collagen IV, aggrecan, fibronectin, laminin | α2-M, α-PI, IGFBP-1, casein |
| Vitronectin-likeinsert | MMP-21 | – | 10q26.13 | 65.000/62.000 | Unknown | Unknown |
| Trans-membrane Type I (MT-MMPs Type I) | MMP-14 MMP-15 MMP-16 MMP-24 | MT1-MMP MT2-MMP MT3-MMP MT5-MMP | 14q11-q12 15q13-q21 8q21 20q11.2 | 66.000/56.000 72.000/60.000 64.000/52.000 –/62.000 | Collagen I, II, III, aggrecan, elastin, fibronectin, gelatin, laminin, vitronectin, fibrin | MCP-3, MUC-1, CD-44, transglutamatase, MMP-2, -13 |
| Glycosyl-phosphatidyl-inositol MT-MMPs (GPI-anchored MT-MMPs) | MMP-17 MMP-25 | MT4-MMP MT6-MMP | 12q24.3 16p13.3 | 57.000/53.000 | Collagen IV, fibronectin, fibrin, gelatin, laminin | Unknown |
| Trans-membrane type II (MT-MMPsType II) | MMP-23A MMP23B | CA-MMP/Femalysin | 1p36.3 | 44.000/36.000 | Gelatin | Unknown |
aNo human homologue known.
The basic structure of these enzymes is characterized by (i) a pre-peptide domain involved in the pro-enzyme secretion process, (ii) the auto-inhibitory pro-domain, (iii) the catalytic domain and (iv) the C-terminal hemopexin-like domain often involved in the recognition/positioning of substrates (Figure 1). The pro-domain, composed of about 80 residues, extends from the N-terminus to the catalytic domain and it is responsible for the enzyme latency. A cysteine sulphydryl group present in the N-terminal pro-domain (“Cys-switch”) interacts with the Zn2+ ion and blocks the active site. The pro-domain of some MMPs shows a recognition sequence for furin-like serine proteinases, which is needed for the pro-domain cleavage and, consequently, for the MMPs activation [4].

Structure of secreted MMPs and trans-membrane-type MMPs (TM-MMPs).
(A) Minimal domain MMPs: MMP-7, MMP-26; (B) archetypal MMPs (simple hemopexin-domain): MMP-1, MMP-8, MMP-13, MMP-18, MMP-3, MMP-10, MMP-12, MMP-19, MMP-20, MMP-27; (C) gelatin binding MMPs: MMP-2, MMP-9; (D) furin activated MMPs: MMP-11, MMP-28; (E) vitronectin insert MMPs: MMP-21; (F) trans-membrane-type I MMPs: MMP-14, MMP-15, MMP-16, MMP-24; (G) glycosyl-phospatidyl-Inosital (GPI-anchored) MMPs: MMP-17, MMP-25; (H) trans-membrane-type II MMPs: CA-MMP (MMP-23A,MMP-23B). Key: Pre, amino-terminal-signal sequence; Pro, pro-peptide; SH, thiol group; catalytic, catalytic domain; Zn, zinc-binding site; Fi, fibronectin; Fu, furin; Vn, vitronectin; TM, trans-menbrane domain; CY, cytoplasmic domain; GPI, glycosyl-phospatidyl-inosital; SA, N-terminal signal anchor; CA, cysteine array; Ig-like, immunoglobulin like domain.
Concerning biological function, MMPs have been shown to be critically involved in many normal biological processes, including organ development, wound healing, tissue remodeling, morphogenetic changes, etc., as well as in several pathological conditions, such as cancer, arthritis, cardiovascular diseases, nephritis, neurological diseases, breakdown of blood-brain barrier, skin ulceration, inflammation, diabetes, etc. [5]. They are important in creating an environment that support the initiation and maintenance of growth of primary and metastatic tumors, and have an important regulatory role, as they can modulate cytokines and chemokines activity by proteolytic processing [6]. Primary function of MMPs is tissue growth and remodeling by selective proteolytic degradation. Therefore, in order to avoid uncontrolled ECM turnover, inflammation, cell growth and migration, under physiological conditions, the activity of matrix metallo-proteinases is accurately regulated at the levels of transcription, zymogen activation and inhibition by endogenous inhibitors. Normally, MMPs are specifically inhibited by tissue inhibitors of metallo-proteinases (TIMPs), and the MMP/TIMP balance is considered to be a major factor in the regulation of the net proteolytic activity of the individual MMPs. In humans, four individual species of TIMPs are known: TIMP-1, -2, -3, and -4. TIMP expression is regulated during development and tissue remodeling and under pathological conditions associated with unbalanced MMPs activity [7]. All MMPs are secreted in a latent form as inactive pro-enzyme owing to the presence of a pro-domain, which binds the zinc atom in the catalytic site and inhibits enzymatic activity (Figure 1). MMPs become active after the disruption of this bond by chemical modification or by enzymatic removal of the pro-domain. This activation step may occur either intracellular (catalyzed by furin) or extracellular (by other MMPs, TIMPs or serine proteases) [3–7]. The basic action of MMPs (e.g. cleavage of proteins) has proven sufficiently sophisticated to orchestrate various functions, specific modulators of angiogenesis as well as fine-tuners of cell signaling pathways and the inflammatory response. Moreover, recent evidences indicate that MMPs may even work in a non-proteolytic manner [6].
Gelatinases
One of the most studied classes of matrix metallo-proteinases are the gelatinases or collagenases type IV, historically defined as their affinity for denatured collagen (e.g. gelatin), which are widely recognized to participate to the etiology of a plethora of neoplastic and non-neoplastic pathologies.
This class includes two members, namely (i) gelatinase A or MMP-2 (72 kDa and 62 kDa for the pro-enzyme and the active enzyme, respectively); (ii) gelatinase B or MMP-9 (92-85-82 kDa for the pro-enzyme, the intermediate form and active enzyme, respectively). Gelatinase A (MMP-2) is a no-glycosylated protein, which is strictly present as a monomer in plasma. MMP-2 is expressed in normal fibroblasts, endothelial and epithelial cells as well as in many transformed cells and in tumor-associated fibroblasts. MMP-2 digests native collagen types I, II and III in a similar manner to the collagenases. However, the collagenolytic activity of MMP-2 is much weaker than MMP-1 or other collagenases. Nevertheless, because pro-MMP-2 is recruited to the cell surface and activated by the membrane-bound MT-MMPs, it may accumulate pericellularly and express reasonable local collagenolytic activity. It may also act as a ‘collaborator’ activity, digesting collagenase-clipped collagen to smaller fragments because those fragments denature at body temperature (37 °C). Moreover, MMP-2 cleaves several different substrates, including cytokines, growth factor, receptors or binding factors. The MMP-2 activity is tightly regulated by TIMPs. In particular, TIMP-2 display relevant affinity for MMP-2 as well as TIMP-3 and -4. Therefore, their adequate secretion is required for a balanced MMP-2/TIMP ratio. The regulation of MMP-2 activity occurs at many levels, among which regulation through TIMP-2 and its cell surface receptor, MT1-MMP (MMP-14) are critically decisive. In fact, TIMP-2 forms a binary complex with MT1-MMP by binding its catalytic site, leaving no free MT1-MMP receptors. Therefore, the complex is able to recruit proMMP-2, by linking the hemopexin domain, and to inhibit its activation [8]. Moreover, the expression of TIMP-2, MMP-2 and MT1-MMP is co-regulated transcriptionally, indicating an intricate network of regulation. Pro-MMP-2 activation is also seen by complex signaling induced by ECM proteins like osteopontin or IL-8 produced by endothelial cells [9–11]. Gelatinase B (MMP-9) contains two N-glycosylated sites in the pro-domain and the catalytic domain, and a number of O-linked glycans. In plasma, MMP-9 exists as a monomer or a dimer, complexed with neutrophil gelatin-associated lipocalin (NGAL). It is expressed in normal leukocytes, macrophages, pericytes, endothelial cells as well as in transformed cells. MMP-9, as well as MMP-2, is involved in the mechanical removal of structural proteins in the extracellular matrix, but also in regulation of multiple cellular functions including cell growth, apoptosis, angiogenesis, invasion, metastasis and immune response by cleaving growth factor-precursors, cell adhesion molecules, cell surface receptors and other bioactive proteins. The structure is almost similar to MMP-2. The nascent form of MMP-9 shows an N-terminal signal sequence (pre-domain) which directs the protein to the endoplasmic reticulum. The pre-domain is followed by a pro-peptide (pre-pro-domain) that maintains enzyme-latency until it is cleaved or disrupted, and a catalytic domain that contains the conserved zinc-binding region. A hemopexin domain is connected to the catalytic domain by a hinge or linker region. The hemopexin domain is involved in NGAL, extracellular matrix components, β-hematin and TIMPs (TIMP-1 and TIMP-3) binding. Moreover, the structure also shows a series of three head-to-tail cysteine-rich repeats within its catalytic domain. These inserts resemble the collagen-binding type II repeats of fibronectin and are required to bind and cleave collagen and elastin. Like other proteolytic enzymes, MMP-9 is first synthesized as inactive proenzyme or zymogens. Activation of proMMP-9 is mediated by plasminogen activator/plasmin (PA/plasmin) system [12]. MMP-9 expression is regulated by several cytokines and growth factors, including interleukins, interferon, epidermal growth factor (EGF), nerve growth factor (NGF), basic-fibroblast growth factor (b-FGF), vascular endothelial growth factor (VEGF), platelet derived growth (PDGF), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), the extracellular matrix metalloproteinase inducer EMMPRIN and also osteopontin. Many of these stimuli induce the expression and/or activation of c-fos and c-jun proto-oncogene products, which promote the binding of activator protein-1 complex (AP-1) sites within of MMP-9 gene promoters [13]. Primary function of MMP-9 is degradation of proteins in the extracellular matrix. It proteolytically digests decorin, elastin, fibrillin, laminin, gelatin, and types IV, V, XI and XVI collagen and also activates growth factors like pro-TGF-β and pro-TNF-α. Physiologically, MMP-9 in coordination with other MMPs, play a role in normal tissue remodeling events such as neuronal growth, embryonic development, angiogenesis, ovulation, mammary gland involution and wound healing, osteoblastic bone formation and/or inhibition of osteoclastic bone reabsorption [10, 14, 15]. In chronic lymphocytic leukemia, MMP-9 promotes B cell survival in a non-proteolytic fashion via its hemopexin domain by docking to the surface receptors α4β1 and CD44, which induce intracellular signaling, involving Lyn activation and STAT3 phosphorylation, which prevents B cell apoptosis [16].
The role of MMP-2 and MMP-9 in human diseases is supported by several in vivo and in vitro evidences, even though they follow distinct and even opposite patterns, consistently with the notion that MMP-9 has a pro-inflammatory property, whereas MMP-2 has a pro-homeostatic one [17].
Nowadays, it is widely recognized that gelatinases participate to the etiology of a plethora of pathologies such as cardiovascular and auto-immune diseases which, taken altogether, represent a leading cause of mortality and morbidity in Western countries. In particular, in the case of cardiovascular diseases, gelatinases participate both to the genesis of the atherosclerotic lesions and to the acute event (i.e. stroke or myocardial infarction) [18]. In fact, high levels of MMPs have been detected in the atherosclerotic plaque, and activation of MMPs appears to facilitate atherogenesis, platelet aggregation and plaque destabilization which follow the fibrous cup rupture [19, 20].
In the auto-immune diseases, gelatinases are involved both in the generation of remnant epitopes and in the modulation of cross-talking between immune system compartments [21]. Moreover, gelatinases have been found to have an important role also in metabolic disorders, such as type 2 diabetes and obesity [22], and in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Alzheimer’s dementia (AD) [23].
Among the wide variety of physiological and pathological conditions in which gelatinases are involved, cancer progression is the most extensively studied. Initially gelatinases were thought to have a role exclusively in invasion and metastasis processes, but recent studies demonstrated that they are also critically involved in several steps in cancer formation and during the growth of tumor cells by releasing of cell membrane bound precursors of some growth factors, or the inhibition of apoptosis pathways, for example, by cleaving the Fas ligand [24]. Several studies, in fact, identified that the expression of gelatinases is enhanced in many primary tumors, such as colon-rectal cancer, breast cancer or gastric cancer [25–27]. The overexpression of gelatinases, both in neoplastic tissue and in body fluids, has been shown to correlate with grade or stage and they may also be predictor of disease-free survival after treatment, or may correlate to overall cancer-specific survival [28]. Our previous studies, in fact, identified a higher MMP-9 gelatinolytic activity in urine from bladder cancer patients [29] compared to healthy controls, as well as in sera from clear cell renal cell carcinoma (ccRCC) subjects compared to oncocytoma patients [30].
Therefore, thanks to their value as potential prognostic indicators, MMP-9 and MMP-2 represent the most promising cancer biomarkers which might offer useful clinicians information for improving cancer patients managements.
Detection of gelatinases
Several methods have been developed for analysis of active and latent forms of gelatinases A and B in biological samples. Here, we briefly describe the main techniques used for detection of MMP-2 and MMP-9, focusing on substrate-zymography and discussing the major advantages and disadvantages.
Enzyme-linked immunosorbent assay (ELISA)
In vitro ELISA kits for human, as well as other species, MMP-2 and MMP-9 are commercially available (e.g. Biotrack®, Quantikine HS® R&D Systems, etc.). However, these assays are not able to distinguish between the active and latent forms of the enzymes or between specific MMPs and their TIMP complexes. The major disadvantage of this technique is that, even if the high sensitivity, it requires two different antibodies for each individual MMP, and a separate assay plate must be used for the measurement of each MMP, making for a very time consuming assay. Also multiplexed ELISAs (e.g. Biorad Bioplex®), which allow the simultaneous determination of each of gelatinases, have been reported to exhibit high cross-reactivity among various MMPs because these proteins share common domains [31, 32].
Western blotting
For Western blot analysis, samples electrophoresed by 10% SDS–PAGE are transferred onto PVDF or nitrocellulose membranes. After incubation with a blocking buffer, saturated membrane is blotted overnight with a primary antibody anti-MMP-2 or anti-MMP-9, respectively. The following incubation of membrane with a secondary antibody and a luminescent substrate allows signal revelation which is directly related to protein amount. Compared to ELISA, Western blotting allows separation of the protein mix by size, charge, and/or conformation and allows the detection of several targets, contrary to ELISA where only one protein can be detected. However, the technique is time-consuming, requires optimizing the experimental conditions (i.e. protein isolation, buffers, type of separation, gel concentration, etc.), and most of all the detection limits are much lower than those of other methods heals, potentially leading to false negative results. Moreover immunoblotting analysis requires the availability of antibodies and the inhibition of proteolytic activity of enzymes during protein blocking and immunochemical reactions [31, 32].
High performance liquid chromatography-mass spectrometry (HPLC/MS)
Mass spectrometry-based proteomics has become a very powerful tool in MMP research. Identification of gelatinases by mass spectrometry involves enzymatic digestion after capture of the MMP using biotin-azide tags and avidin chromatography followed by analysis of the MMP-digests by HPLC/MS. This technique is able to reveals all the proteins present in a sample, and unlike classical Western blotting it is quantitative. However, despite the high sensitivity, it requires high costs and also more technically challenging to use compared to Western blotting. In addition, the method is very lengthy and still not fully reliable when it comes to identifying proteins in the digested form, because the identification often relies on the presence of only a few peptides from the digested protein [31, 32].
In situ zymography (ISZ)
Zymography in situ (ISZ) allows the localization of MMPs in tissue sections. The process provides the incubation of frozen sections of unfixed tissue sample with a specific substrate (e.g. gelatin, in case of MMP-2 and MMP-9) which will be digested by the activated MMPs in time- and dose-dependent manner. The degradation of substrate, detected by light or fluorescent microscopy as white spots on black background, directly correlates with the enzymatic activity. A big limitation of this technique is that it is not able to discriminate between different classes of MMPs and digestion process may be influenced by other proteases (e.g. serine or cysteine proteases). This limitation may be reduced by combining ISZ with immunohistochemistry (IHC). The immunolocalization of the specific MMPs, in fact, can be compared with the localization pattern of the ISZ. Moreover, the combination ISZ-HIC may be useful for co-location of proteins such as cell type markers or other proteins of interest, and can be also adapted for time-lapse analysis of MMPs activity and analysis of MMPs activity in migrating cells. However, while IHC is not able to discriminate between active and inactive forms of the enzymes, ISZ can evaluate net functional activity. A sensitive advance in this technique is the use of quenched fluorogenic substrates which allow evaluation of enzymatic activity by using confocal microscopy [33, 34].
Substrate-zymography
Substrate-zymography still represents the most simple, sensitive and quantifiable, unexpensive and functional assay for MMPs analysis, which is able to identify, simultaneously in the same sample, the entire panel of enzymes that are capable of degrading a specific substrate. In particular, the identification of gelatinases A and B activities is possible by using of gelatin as substrate (gelatin zymography). Gelatin-zymography allows determining simultaneously both active and latent forms of gelatinases A and B, in biological fluids (e.g. serum, urine, pleural effusion, cell culture medium, etc.) as well as in tissues. The proteins are separated by electrophoresis under denaturing [sodium dodecyl sulfate (SDS)] but non reducing conditions, without prior boiling. In fact, boiling cause protein precipitation of the enzymes, and reducing agents (i.e. mercaptoethanol, 1,4-dithiothreitol, urea) break the disulphide bond and inhibit the MMP refolding after electrophoresis. The separation occurs in a polyacrylamide gel containing the gelatin substrate which is co-polymerized with the acrylamide. During electrophoresis, the SDS causes the gelatinases denaturation, so they become inactive. The inactivation of gelatinases during zymography is believed to involve the dissociation of Cys73 from the zinc molecule, caused by SDS. After the gel has been run, it is incubated overnight at 37 °C in a nonionic detergent (such as Triton® X-100) which causes the exchange of the SDS, and then in an appropriate “refolding” buffer which contains Ca2+, because gelatinases are Ca-dependent enzymes. During this incubation, the enzymes partially recover their structure and activity and the latent gelatinases are auto-activated without cleavage. If the proteinases are active, the gelatin is digested and converted to low-molecular weight peptides that are washed out of the gel. Subsequently staining with Coomassie® Blue indicates the zone of lysis as a clear region on a uniform blue background of undegraded substrate (e.g. Figure 2, panels A, B and C). The clear bands in the gel can be quantified by densitometry. By using an image analysis software, it is possible to determine the molecular weight, volume and background of each band. The pixel density may be determined after background subtraction and used to calculate the integrated density of a selected band. Values of integrated density are reported in volume units of pixel intensity per mm2. The relative amounts of the different forms of both fluid and tissue gelatinases are expressed as the integrated density ×10–3 (volume) of all the pixels above the background of each band. The molecular size of bands displaying enzymatic activity is identified by comparison with pre-stained standard protein, as well as with purified gelatinase A or gelatinase B. The nature of lytic bands observed in zymograms is further confirmed by an inhibition assay, which is performed by using control gels containing either of the MMP selective inhibitors, EDTA or phenanthroline, in the sample loading buffer. Furthermore, the character of proteolytic bands is analysed by incubating the identical zymograms in PMSF, a serine protease inhibitor; or Pefabloc, an irreversible serine protease inhibitor. The immunological detection of lytic bands can be also performed by Western blotting with antibodies against MMP-2, MMP-9 which recognize the pro-forms of both enzymes (Figure 2, panel D) [33–36].
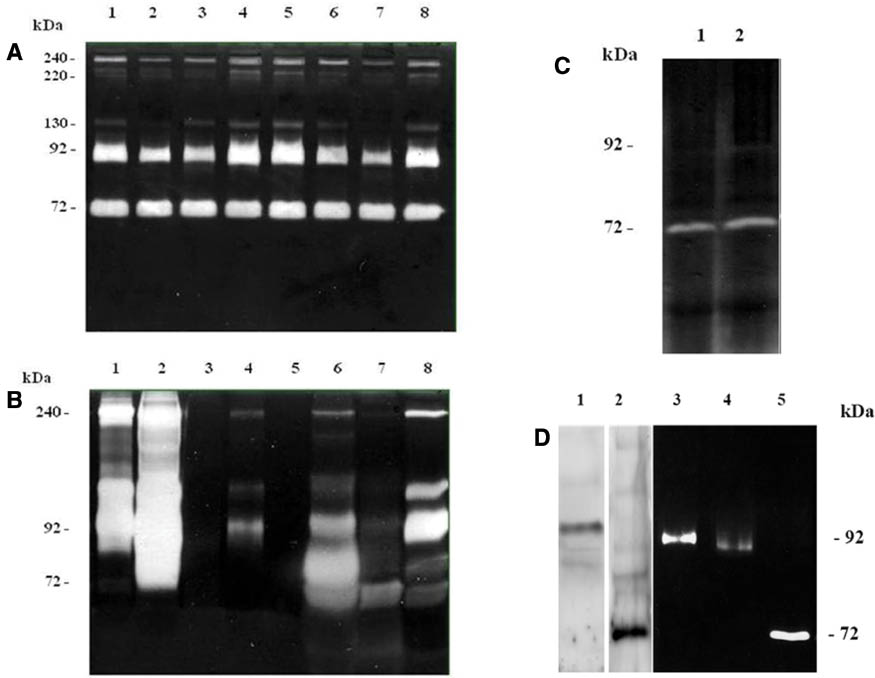
Panels of representative gelatin zymograms.
(A) Zymogram (0.6% gelatin) of sera from kidney cancer patients. In all samples 25 μg of protein was loaded onto the gel. (B) Zymogram (0.1% gelatin) of urine from kidney cancer patients. In all samples 12 μL of concentrated urine was loaded onto the gel. (C) Zymogram (0.1% gelatin) of brain tissue samples. In all samples 50 μg of protein was loaded onto the gel. (D) 1: Western blotting of pro-MMP-9 (92 kDa); 2: Western blotting of pro-MMP-2 (72 kDa); 3: Zymograms (0.6% gelatin) of purified gelatinase B (20 μU); 4: Zymograms (0.6% gelatin) of purified gelatinase B (10 μU); 5: Zymograms (0.6% gelatin) of purified gelatinase A (120 mU).
Critical step in performing substrate zymography is sample preparation. Body fluids samples, such as peripheral venous blood or first morning urine, should be used fresh and only once in order to prevent enzyme activation due to freeze-thawing processes. The discrepancy in MMP zymographic profiles between serum and plasma has been widely reported. In particular, referring to gelatinases, MMP-2 activity and concentration show no significant difference between plasma and serum, while the levels and zymographic separation of the MMP-9 is strongly affected by anticoagulants, which suggests a releasing mechanism during coagulation and fibrinolysis [37].
Regarding urine samples, hematuric samples or urine positive for leukocytes should be eliminated in order not to confound leukocytic gelatinases. Moreover, prior to analysis, urine should be concentrate by using membrane with a molecular weight cut-off of about 30,000 Da [29, 30].
Also tissue extracts should be used fresh or after rapid freezing using liquid nitrogen and stored at –80 °C. The same tissue protein extraction procedure represents a critical step in substrate-zymography, because it may cause interactions of enzymes with their respective inhibitors or may inactive them. For this reason, the extraction buffer does not have to contain protease inhibitors, EDTA or any other Zn-chelator. For many tissue types it would be preferable to purify homogenate by using gelatin-sepharose. Zang et al., in fact, demonstrated that use of gelatin-Sepharose 4B allows both the effectively separation of gelatinases from the dominance of other tissue proteins and the concentration of extracted activity from the entire tissue [38].
Substrate-zymography presents several advantages compared to other main analytical technique for studying of proteases (Table 2). The main advantage of using gelatin-zymography is that this technique does not require expensive materials (e.g. antibodies) and it is extremely sensitive because levels of 10 pg of MMP-2 and 32 pg of MMP-9 can be already detected. For this reason, zymography has been proven to be much more sensitive than Western blot analysis, because it is very difficult to identify antibodies sensitive enough to detect small amounts of MMPs.
Advantages and disadvantages of substrate zymography.
| Advantages | Disadvantages |
|---|---|
| Low cost | No discrimination between free MMPs and MMPs complexed with TIMPs and/or NGAL |
| High sensitivity, pg | No information about localization of proteolitical activity |
| Distinguish different species of enzymes due to mobility difference in same gel | Interference by other MMPs |
| Detection of active and latent form of the same protease | Long protocol (2 days) |
| No interference by gelatinases inhibitors (TIMPs) |
Quantification of proteolytic activity of samples subjected to zymography may be difficult at times because (i) the limited number of wells per gel does not allow a full standard curve and several samples to be run on the same gel and (ii) the two-step staining/destaining method is not reliable and is difficult to reproduce. Leber et al., developed an enhanced method which use a single-step staining-destaining procedure which leads to faster and more reproducible results during quantification [39].
A particular advantage of this system is also the opportunity to detect and to quantify on a single gel proteases with different molecular weights, which show activity towards the same substrate. Indeed, the pro-forms of MMPs become activated during the process of denaturation and renaturation; after gel electrophoresis, the active forms and the originally inactive forms degrade gelatin, and both forms can be detected on zymogram. Another positive aspect which needs to be considered is that during electrophoresis, SDS promotes the dissociation between MMPs and their endogenous inhibitors (TIMPs) so they do not interfere with detection of the enzymatic activity.
On the other hand, substrate zymography is not able to discriminate between free MMPs and complexed to TIMPs or other protein. In fact, MMP-9 can be associated with lipocalin-2 (NGAL) giving a band at ~130 kDa, and can also form dimer and multimer giving bands at ~220 kDa and ~240 kDa, respectively. These complexes are not dissociated in zymogram and might represent a complication during the analysis of data. Moreover, substrate zymography cannot give information about localization, in cells or tissues, of the proteolytic activity. In addition, it should be considered that other MMPs, such as MMP-1, MMP-8, and MMP-13 can also lyse the gelatin substrate.
The new advances of this method are basically focused towards two dimensional zymography which combines isoelectric focusing (IEF) with zymographic electrophoresis to achieve significant improvement in separation of the enzymatic isoforms and, most of all, to identify possible post-translational modification which can modulate enzymatic activity. For example, Chen et al., by using 2D gelatin zymography were able to detect organomercuric chemical 4-aminophenylmercuricacetate-induced activation of MMP-2 isoforms with variant pI values in the conditioned medium of human fibrosarcoma cells and also several MMP-9 pro-forms, with different pI values, in murine LPS-stimulated BV-2 cells [40].
Finally, as reported by Vandooren et al., zymography technique has been proposed as the most suitable and convenient methods in “reverse degradomics” studies for measurement of the total impact of a complex biological sample on a particular substrate, which, for example, may be linked to a particular human disease. To reach this aim, zymography has been demonstrated to be worthwhile technique in providing information on the spatial distribution of activity, an important parameter which cannot be obtained with the currently available high-throughput (reverse) degradomics platforms [36].
Concluding remarks
Matrix-metalloproteinase 2 (gelatinase A) and matrix-metalloproteinase 9 (gelatinase B) are known to have clear roles in physiological tissue turnover and remodeling, but contribute also to a number of pathological events. Therefore, they obtained a deep interest as potential non-invasive biomarkers. The study of MMP-2 and MMP-9 in physio-pathological processes requires a reliable method for the assessment of the enzymatic activity, and substrate zymography still represents a fascinating and advantageous technique in molecular biology for the study of MMPs proteolytic activity. Further improvements of zymography-based techniques will expedite the development of new diagnostic and/or prognostic tools for a wide variety of human disease.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Barret AJ, Rawlings ND, Woessner JF. The handbook of proteolytic enzymes, 2nd ed. San Diego: Academic Press, 2004.Suche in Google Scholar
2. Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 2008;40:1362–78.10.1016/j.biocel.2007.12.006Suche in Google Scholar PubMed
3. Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med 2008;29:290–308.10.1016/j.mam.2008.05.002Suche in Google Scholar PubMed PubMed Central
4. Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, et al. Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med 2012;33:119–208.10.1016/j.mam.2011.10.015Suche in Google Scholar PubMed
5. Galliera E, Tacchini L, Corsi Romanelli MM. Matrix metalloproteinases as biomarkers of diseases: updates and new insight. Clin Chem Lab Med 2015;53:349–55.10.1515/cclm-2014-0520Suche in Google Scholar PubMed
6. Kessenbrock K, PlaksV, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67.10.1016/j.cell.2010.03.015Suche in Google Scholar PubMed PubMed Central
7. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteiases: structure, function and biochemistry. Circ Res 2003;92:827–39.10.1161/01.RES.0000070112.80711.3DSuche in Google Scholar PubMed
8. Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor invasion. EMBO J 2001;20:4782–93.10.1093/emboj/20.17.4782Suche in Google Scholar PubMed PubMed Central
9. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516.10.1146/annurev.cellbio.17.1.463Suche in Google Scholar PubMed PubMed Central
10. Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvsit Y, Schneider G, et al. Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science 1999;284:1667–70.10.1126/science.284.5420.1667Suche in Google Scholar PubMed
11. Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991;5:2145–54.10.1096/fasebj.5.8.1850705Suche in Google Scholar
12. Swarnakar S, Mishra A, Chaudhuri SR. The gelatinases and their inhibitors: the structure-activity relationships. EXP 2012;103:57–82.10.1007/978-3-0348-0364-9_3Suche in Google Scholar
13. Rangaswami H, Bulbule A, Kundu GC. JNK1 differentially regulates osteopontin-induced nuclear factor-inducing kinase/MEKK1-dependent activating protein-1-mediated promatrix metalloproteinase-9 activation. J Biol Chem 2005;280:19381–92.10.1074/jbc.M414204200Suche in Google Scholar PubMed
14. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotestumor invasion and angiogenesis. Genes Dev 2000;15:163–76.10.1101/gad.14.2.163Suche in Google Scholar
15. Rangaswami H, Bulbule A, Kundu GC. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IKappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem 2004;279:38921–35.10.1074/jbc.M404674200Suche in Google Scholar PubMed
16. Redondo-Munoz J, Ugarte-Berzal E, Terol MJ, Van denSteen PE, Hernandez del Cerro M, Roderfeld M, et al. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia B cell survival through its hemopexin domain. Cancer Cell2010;17:160–72.10.1016/j.ccr.2009.12.044Suche in Google Scholar PubMed
17. Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41:271–90.10.1007/s00726-010-0689-xSuche in Google Scholar PubMed PubMed Central
18. Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS 2012;103:209–79.10.1007/978-3-0348-0364-9_7Suche in Google Scholar PubMed PubMed Central
19. Beaudeux JL, Giral P, Bruckert E, Foglietti MJ, Chapman MJ. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med 2004;42:121–31.10.1515/CCLM.2004.024Suche in Google Scholar PubMed
20. Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology 2005;56:173–80.10.1177/000331970505600208Suche in Google Scholar PubMed
21. Cauwe B, Martens E, Sagaert X, Dillen C, Geurts N, Li S, et al. Deficiency of gelatinase B/MMP-9 aggravates lpr-induced lymphoproliferation and lupus-like systemic autoimmune disease. J Autoimmun 2011;36:239–52.10.1016/j.jaut.2011.02.002Suche in Google Scholar PubMed
22. Gonçalves FM, Jacob-Ferreira AL, Gomes VA, Casella-Filho A, Chagas AC, Marcaccini, AM, et al. Increased circulating levels of matrix metalloproteinase (MMP)-8, MMP-9, and pro-inflammatory markers in patients with metabolic syndrome. Clin Chim Acta 2009;403:173–7.10.1016/j.cca.2009.02.013Suche in Google Scholar PubMed
23. Romi F, Helgeland G, Gilhus NE. Serum levels of matrix metalloproteinases: implications in clinical neurology. EurNeurol 2012;67:121–8.10.1159/000334862Suche in Google Scholar PubMed
24. Egeblad M, Werb Z. New function for the matrix metalloproteinases in cancer progression. Nature Rev 2002;2:161–74.10.1038/nrc745Suche in Google Scholar PubMed
25. Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci 2012;13:13240–63.10.3390/ijms131013240Suche in Google Scholar PubMed PubMed Central
26. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 2009;15:RA32–40.Suche in Google Scholar
27. Shen W, Xi H, Wei B, Chen L. The prognostic role of matrix metalloproteinase 2 in gastric cancer: a systematic review with meta-analysis. J Cancer Res Clin Oncol 2014;140:1003–9.10.1007/s00432-014-1630-6Suche in Google Scholar PubMed
28. Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005;87:287–97.10.1016/j.biochi.2005.01.014Suche in Google Scholar PubMed
29. Di Carlo A, Terracciano D, Mariano A, Macchia V. Urinary gelatinase activities (matrix metalloproteinases 2 and 9) in human bladder tumors. Oncol Rep 2006;15:1321–6.10.3892/or.15.5.1321Suche in Google Scholar
30. Di Carlo A. Matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in sera and urine of patients with renal carcinoma. Oncol Lett 2014;7:621–6.10.3892/ol.2013.1755Suche in Google Scholar PubMed PubMed Central
31. Lombard C, Saulnier J, Wallach J. Assay of matrix metalloproteinases (MMPs) activities: a review. Biochim 2005;87:265–72.10.1016/j.biochi.2005.01.007Suche in Google Scholar PubMed
32. Krizkowa S, Zitka O, Masarik M, Adam V, Stiborova M, Eckschlager T, et al. Assay for determination of matrix metalloproteinases and their activity. Trend Anal Chem 2011;30:1819–32.10.1016/j.trac.2011.06.016Suche in Google Scholar
33. Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005;38:73–83.10.2144/05381RV01Suche in Google Scholar
34. Kupai K, Szucs G, Cseh S, Hajdu I, Csnoka C, Csont T, et al. Matrix metalloproteinase activity assay: importance of zymography. J Pharmacol Toxicol Methods 2010;61:205–9.10.1016/j.vascn.2010.02.011Suche in Google Scholar
35. Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G. Zymography methods for visualizing hydrolytic enzymes. Nat Methods 2013;10:211–20.10.1038/nmeth.2371Suche in Google Scholar
36. Hu X, Beeton C. Detection of functional matrix metalloproteinases by zymography. J Vis Exp 2010;8:pii 2445.10.3791/2445Suche in Google Scholar
37. Mannello F, Tanus-Santos JE, Meschiari CA, Tonti GA. Differences in both matrix metalloproteinase 9 concentration and zymographic profile between plasma and serum with clot activators are due to the presence of amorphous silica or silicate salts in blood collection devices. Anal Biochem 2008;374:56–63.10.1016/j.ab.2007.11.020Suche in Google Scholar
38. Zhang JW, Gottschall PE. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. J Neurosci Methods 1997;76:15–20.10.1016/S0165-0270(97)00065-4Suche in Google Scholar
39. Leber TM, Balkwill FR. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem 1997; 249:24–8.10.1006/abio.1997.2170Suche in Google Scholar PubMed
40. Chen S, Meng F, Chen Z, Tomlinson BN, Wesley JM, Sun GY, et al. Two-dimensional zymography differentiates gelatinase isoforms in stimulated microglial cells and in brain tissues of acute brain injuries. PLOS One 2015;10:e0123852.10.1371/journal.pone.0123852Suche in Google Scholar PubMed PubMed Central
©2016 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Editorials
- Laboratory analytical quality – the process continues
- Measurement uncertainty – a revised understanding of its calculation and use
- Review
- Substrate-zymography: a still worthwhile method for gelatinases analysis in biological samples
- Opinion Papers
- Is the combination of trueness and precision in one expression meaningful? On the use of total error and uncertainty in clinical chemistry
- The problem with total error models in establishing performance specifications and a simple remedy
- Measurement uncertainty for clinical laboratories – a revision of the concept
- Uncertainty in measurement and total error – are they so incompatible?
- General Clinical Chemistry and Laboratory Medicine
- Using the hazard ratio to evaluate allowable total error in predictive measurands
- Performance of electrolyte measurements assessed by a trueness verification program
- Analytical interference by monoclonal immunoglobulins on the direct bilirubin AU Beckman Coulter assay: the benefit of unsuspected diagnosis from spurious results
- Preliminary probe of quality indicators and quality specification in total testing process in 5753 laboratories in China
- Analytical and clinical evaluation of the new Fujirebio Lumipulse®G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients
- Patient-performed extraction of faecal calprotectin
- Comparison of the clinical utility of the Elia CTD Screen to indirect immunofluorescence on Hep-2 cells
- Reference Values and Biological Variations
- Sex-related differences in the association of ghrelin levels with obesity in adolescents
- Gestation specific reference intervals for thyroid function tests in pregnancy
- Cancer Diagnostics
- SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer
- Dopamine concentration in blood platelets is elevated in patients with head and neck paragangliomas
- Letters to the Editor
- Cancer dynamics and the success of cancer screening programs
- Mother’s instinct – a rare case of multiple test interferences due to heterophile antibodies
- Sigma metric or defects per million opportunities (DPMO): the performance of clinical laboratories should be evaluated by the Sigma metrics at decimal level with DPMOs
- A national survey of preanalytical handling of oral glucose tolerance tests in pregnancy
- Updating pregnancy diabetes guidelines: is (y)our laboratory ready?
- Low serum bilirubin values are associated with pulmonary embolism in a case-control study
- Effect of Hb H on HbA1c measurements as measured by IFCC reference method and affinity HPLC
- Adipocytes in venipunctures cause falsely elevated S-100B serum values
- Earlier detection of sepsis by Candida parapsilosis using three-dimensional cytographic anomalies on the Mindray BC-6800 hematological analyzer
- Theranos phenomenon – part 4: Theranos at an International Conference
Artikel in diesem Heft
- Frontmatter
- Editorials
- Laboratory analytical quality – the process continues
- Measurement uncertainty – a revised understanding of its calculation and use
- Review
- Substrate-zymography: a still worthwhile method for gelatinases analysis in biological samples
- Opinion Papers
- Is the combination of trueness and precision in one expression meaningful? On the use of total error and uncertainty in clinical chemistry
- The problem with total error models in establishing performance specifications and a simple remedy
- Measurement uncertainty for clinical laboratories – a revision of the concept
- Uncertainty in measurement and total error – are they so incompatible?
- General Clinical Chemistry and Laboratory Medicine
- Using the hazard ratio to evaluate allowable total error in predictive measurands
- Performance of electrolyte measurements assessed by a trueness verification program
- Analytical interference by monoclonal immunoglobulins on the direct bilirubin AU Beckman Coulter assay: the benefit of unsuspected diagnosis from spurious results
- Preliminary probe of quality indicators and quality specification in total testing process in 5753 laboratories in China
- Analytical and clinical evaluation of the new Fujirebio Lumipulse®G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients
- Patient-performed extraction of faecal calprotectin
- Comparison of the clinical utility of the Elia CTD Screen to indirect immunofluorescence on Hep-2 cells
- Reference Values and Biological Variations
- Sex-related differences in the association of ghrelin levels with obesity in adolescents
- Gestation specific reference intervals for thyroid function tests in pregnancy
- Cancer Diagnostics
- SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer
- Dopamine concentration in blood platelets is elevated in patients with head and neck paragangliomas
- Letters to the Editor
- Cancer dynamics and the success of cancer screening programs
- Mother’s instinct – a rare case of multiple test interferences due to heterophile antibodies
- Sigma metric or defects per million opportunities (DPMO): the performance of clinical laboratories should be evaluated by the Sigma metrics at decimal level with DPMOs
- A national survey of preanalytical handling of oral glucose tolerance tests in pregnancy
- Updating pregnancy diabetes guidelines: is (y)our laboratory ready?
- Low serum bilirubin values are associated with pulmonary embolism in a case-control study
- Effect of Hb H on HbA1c measurements as measured by IFCC reference method and affinity HPLC
- Adipocytes in venipunctures cause falsely elevated S-100B serum values
- Earlier detection of sepsis by Candida parapsilosis using three-dimensional cytographic anomalies on the Mindray BC-6800 hematological analyzer
- Theranos phenomenon – part 4: Theranos at an International Conference

