Abstract
Large defects in bone tissue due to trauma, tumors, or developmental abnormalities usually require surgical treatment for repair. Numerous studies have shown that current bone repair and regeneration treatments have certain complications and limitations. With the in-depth understanding of bone regeneration mechanisms and biological tissue materials, a variety of materials with desirable physicochemical properties and biological functions have emerged in the field of bone regeneration in recent years. Among them, hydrogels have been widely used in bone regeneration research due to their biocompatibility, unique swelling properties, and ease of fabrication. In this paper, the development and classification of hydrogels were introduced, and the mechanism of hydrogels in promoting bone regeneration was described in detail, including the promotion of bone marrow mesenchymal stem cell differentiation, the promotion of angiogenesis, the enhancement of the activity of bone morphogenetic proteins, and the regulation of the microenvironment of bone regeneration tissues. In addition, the future research direction of hydrogel in bone tissue engineering was discussed.
Introduction
The skeleton is one of the major tissues in the human body with multiple physiological functions and plays an important role in maintaining the stability of the internal environment and releasing minerals [1]. Bone defects usually imply the destruction of bone structure and are mostly caused by infection, trauma, tumors, and surgery [2]. Despite the inherent regenerative potential of bone tissue, bone defects exceeding a critical size are difficult to heal naturally and take a long time to heal [3]. The main treatment for severe bone defects is bone grafting, including autologous bone grafting, allogeneic bone grafting, xenografting, and synthetic bone grafting [4]. Autologous bone grafting is considered the gold standard for the treatment of bone defects. However, autologous bone grafts are expensive and may also cause other symptoms such as infection and inflammation [5]. On the other hand, the use of allogeneic bone grafts is limited by factors such as tissue source, bone defects at the donor site, immune response, and infection [6].
Bone regeneration (Figure 1) is a highly complex bio regulatory process involving multiple cell types and coordination of different signaling pathways [7]. The factors affecting bone regeneration mainly include primitive cells with the ability to differentiate and proliferate, various cytokines that regulate bone formation, a microenvironment that is suitable for cell growth and conducive to the restoration of the original bone morphology and continuity, as well as an adequate blood supply and nutrients [8], [9], [10]. The mechanisms of bone regeneration mainly include three types: osteogenesis (cells carried by the graft can form new bone in the area of grafted bone), inducing osteogenesis (bioactive substances in the graft can induce host mesenchymal stem cells (MSCs) to aggregate and differentiate into osteoblasts to form new bone), and conductive osteogenesis (the graft can provide a scaffold structure to guide the growth of blood vessels and osteoblasts toward the grafted bone and form new bone) [11], [12], [13], [14]. With the development of biomaterials, stem cells, and bone tissue engineering technology, various materials with good biological functions have appeared in the field of bone regeneration in recent years [15]. Ideal bone regeneration materials should exhibit excellent cytocompatibility, biodegradability, bioactivity, and appropriate biomechanical characteristics, as well as promote cell adhesion and proliferation [16].
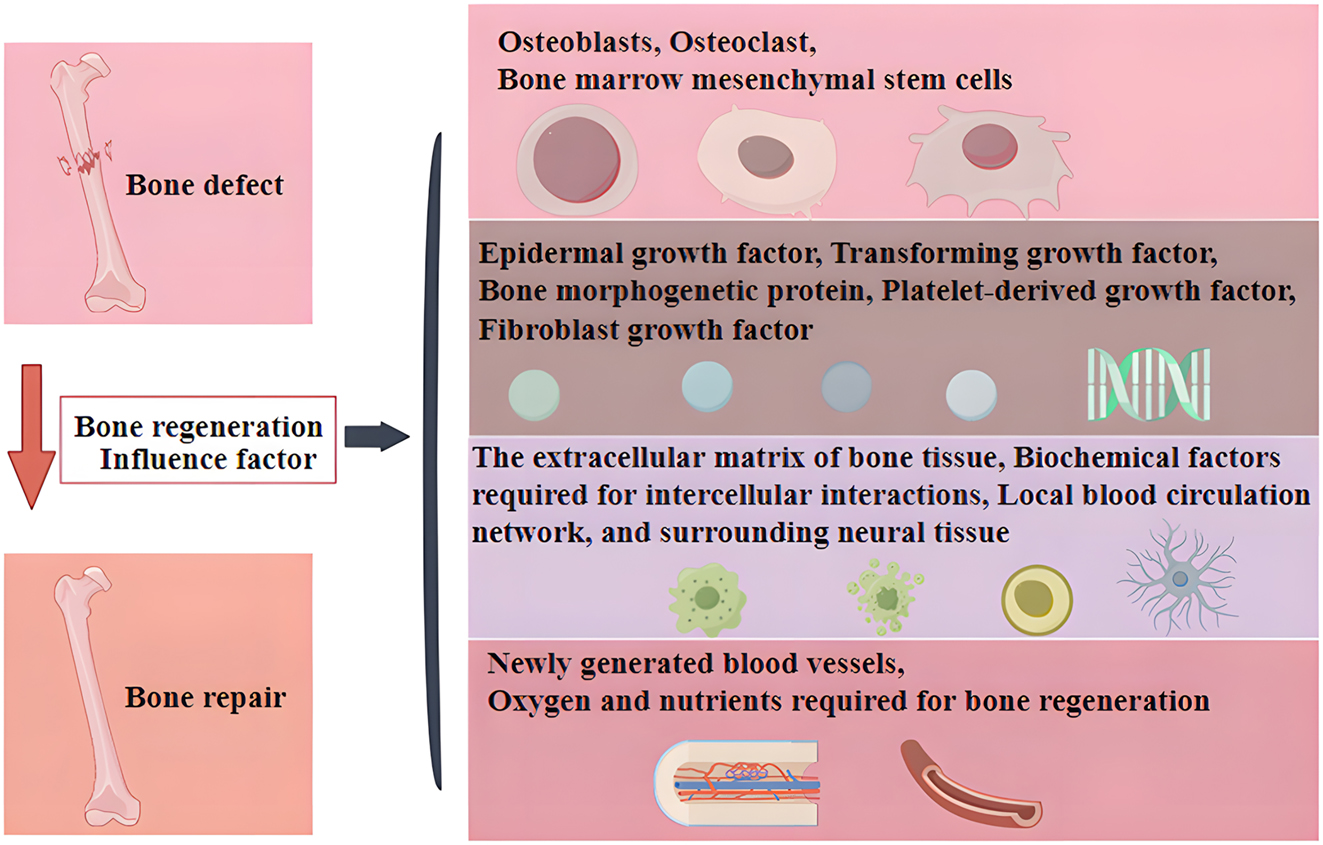
The main factors affecting bone regeneration.
Hydrogels are extremely hydrophilic three-dimensional network gel formed by physical or chemical crosslinking of hydrophilic polymers [17]. They are widely used in bone tissue engineering because of their good biocompatibility, flexibility, designability and versatility, can be formed into different shapes and sizes to meet specific requirements under different conditions [18]. The water content of hydrogel exceeds 90 % compared to other types of gels. When the polymer network is permeated by water, hydrogels become flexible and deformable like a fluid [19]. In addition, hydrogel has no side effects on biological tissues. Its softness and high water content provide a microenvironment similar to the natural extracellular matrix, which provides nutrients to cells and serves as a platform for cell proliferation and differentiation [20]. The development of hydrogels can be divided into four generations (Table 1). The first-generation hydrogels include polymers formed by free radical-induced chain reaction of vinyl monomers, covalently crosslinked hydrophilic polymers, and cellulose-based hydrogels. The second-generation hydrogels are mainly polyethylene glycol (PEG)/polyester block copolymers. The third-generation hydrogels mainly modulate the mechanical and degradation properties of hydrogels through stereo complexation, inclusion, and metal-ligand coordination. The fourth-generation hydrogels combine organic, polymer chemistry, and nanotechnology for more precise targeting properties [38], 39].
The development of hydrogels.
| Hydrogel | Classification | Representation | References |
|---|---|---|---|
| First generation hydrogel | A polymer synthesized from an olefinic monomer | Polyacrylamide (PAM), polyhydroxyethyl methacrylate (pHEMA) | [21], 22] |
| Covalently crosslinked hydrophilic polymer | Polyvinyl alcohol (PVA), PEG | [23], 24] | |
| Hydrogels based on cellulose | Methylcellulose (MC), carboxymethyl cellulose (CMC), hydroxypropyl cellulose (HPC) | [25], [26], [27] | |
| Second generation hydrogel | Temperature-responsive thermosensitive hydrogel | poly(N-isopropylacrylamide) (PNIPA), poly(n,N-diethylacrylamide) (PDEAM) | [28], 29] |
| pH sensitive hydrogel | Polyacrylic acid (PAA), polyacrylamide, chitosan | [30], [31], [32] | |
| Biomolecular sensitive hydrogel | Glucose oxidase hydrogel | [33] | |
| Third generation hydrogel | Synthesis through stereo coordination, inclusion complexes, metal ligand coordination, and peptide chain synthesis | Poly-l-Lactic acid (PLLA), poly (d-lactic acid) (PDLA) | [34], 35] |
| Fourth generation hydrogel | Combining organic and polymer chemistry with nanotechnology | Polymer nanocomposite hydrogel | [36], 37] |
Hydrogels can be categorized into natural hydrogels and synthetic hydrogels according to their origin [40]. Natural hydrogels are obtained directly from nature, while synthetic hydrogels need to be prepared by chemical reactions (Table 2). Hydrogels from natural sources include proteins (gelatin, collagen, fibrin, and fibroin) and polysaccharide (hyaluronic acid, chondroitin sulfate, alginate, and chitosan). In addition, DNA, peptides and proteins hydrogels, as one kind of natural hydrogels, are also widely used in disease treatment due to their unique advantages [56], [57], [58]. Synthetic hydrogels mainly include poly (2-hydroxyethyl methacrylate), PEG, PVA, poly (N-isopropylacrylamide), and polyacrylamide [59]. Compared with synthetic hydrogels, natural hydrogels are biocompatible, environmentally sensitive, and inexpensive, but less stable. To better realize the application of hydrogels in the fields of drug delivery and tissue regeneration, intelligent hydrogels have emerged [60]. The so-called intelligent hydrogels are hydrogels that can respond to the external environment (such as pH and temperature) to achieve hydrogel controllability. Among them, temperature-responsive hydrogels include poly (N-isopropylacrylamide) based hydrogels, poloxamer, etc., and pH-responsive hydrogels include poly (dimethylaminoethyl methacrylate) based hydrogels, poly (allyl acetate) based hydrogels, hydrazone linked hydrogels, etc. [61], 62]. Hydrogels have been widely used in the biomedical field for the study of physiopathological mechanisms and the treatment of regenerative diseases, among which more studies have been conducted on the application of hydrogel in the field of bone regeneration. This article reviewed the application mechanism of hydrogels in bone regeneration (Figure 2).
Hydrogel classification, advantages and disadvantages.
| Natural hydrogels | Advantages | Disadvantages | References |
|---|---|---|---|
| Gelatin | Biocompatibility, non-toxic, adjustable degradation, and easy modification | Risk of triggering immunogenic reactions | [41] |
| Collagen | Biocompatibility, biodegradability, and good bone conduction activity | Poor mechanical performance, unsatisfactory processing conditions, and risk of denaturation during the processing | [42] |
| Fibrin | Good biocompatibility and biodegradability, renewability, flexibility, and high mechanical strength | Poor solubility | [43] |
| Silk heart protein | Good mechanical properties, biocompatibility, and bio-absorbability | Brittleness is high | [44] |
| Hyaluronic acid | Non-toxic, non-allergenic, biocompatible, and biodegradable | Easy to induce allergic reactions | [45] |
| Chondroitin sulfate | Good biocompatibility, low toxicity, and good biodegradability | Poor mechanical performance | [46] |
| Alginate | Biocompatibility, lack of immunogenicity, mild cross-linking, adjustable mechanical properties | Lack of cell adhesion sites and slow degradation | [47] |
| Chitosan | Biocompatibility, ease of processing, antibacterial properties, adjustable degradation rate | Poor mechanical performance and stability | [48] |
| Agarose | Good biodegradability, biocompatibility, antibacterial properties, and high hydrophilicity | High gelation temperature and high rigidity | [49] |
| Proteins and peptides | Superior biocompatibility and lower immunogenicity | Poor mechanical performance | [50] |
| DNA | Good stability, biocompatibility, biodegradability, and adjustable functionality | Low sensitivity and quantitative barriers | [51] |
|
|
|||
| Synthetic hydrogel | Advantage | Disadvantage | References |
|
|
|||
| 2-Hydroxyethyl methacrylate | Hydrophilicity, biocompatibility, and non-toxicity | Has allergenicity that irritates the skin and mucous membranes | [52] |
| Polyethylene glycol(PEG) | Biocompatibility, non-toxic, easy to functionalize and modify | Slow degradation rate, able to resist protein and cell adhesion | [53] |
| Poly (N-isopropylacrylamide) | Good biocompatibility and temperature sensitivity | Low mechanical strength, limited drug loading, and low biodegradability | [54] |
| PVA | Biodegradable, biocompatible, and easy to functionalize | Lack of cell adhesion sites | [55] |
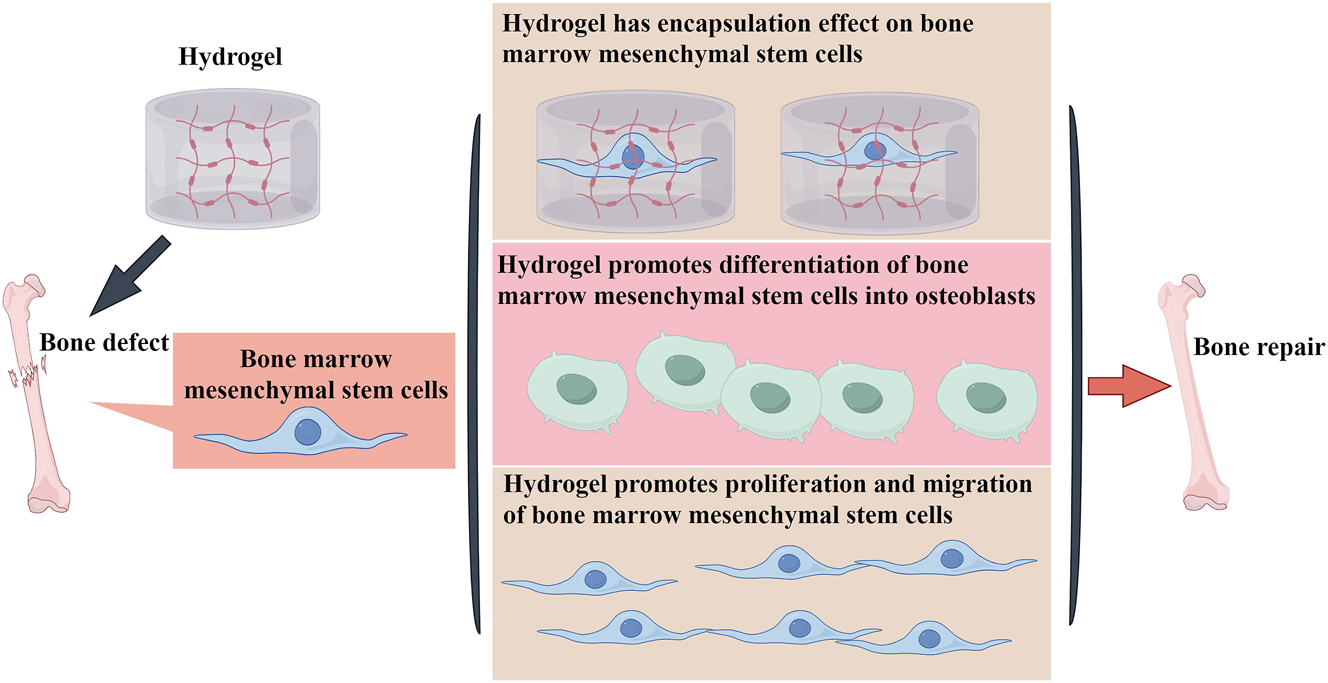
Hydrogel promotes the differentiation of bone marrow mesenchymal stem cells into osteoblasts to achieve bone regeneration.
Mechanism of hydrogel in bone regeneration
Promoting the differentiation of MSCs into osteoblasts
Although bone marrow MSCs have the potential for multi-directional differentiation, they do not differentiate into osteocytes on their own and need to be stimulated to complete the transformation into chondrocytes [63]. Hydrogel is capable of encapsulating bone marrow MSCs and has no toxic effects. It can promote the aggregation of bone marrow hematopoietic stem cells and enhance cell migration and proliferation [64]. In addition, the reticular structure inside the hydrogel facilitates the directional transport of cells and accelerates the osteogenic differentiation ability and speed of stem cells [65]. A recent study on hydrogels in small animals found that MSCs encapsulated in hydrogels had good temperature sensitivity and could promote bone regeneration and repair of cranial defects [66]. Wise et al. utilized collagen/chitosan hydrogels to encapsulate bone marrow MSCs from different sources and compared the osteogenic ability of the stem cells. The results showed that both freshly isolated rat bone marrow MSCs and purified cultured rat MSCs could be well encapsulated by the hydrogel and produced large amounts of osteocalcin within 21 days, increasing calcium deposition and phosphate mineralization with a similar degree of osteogenesis [67]. A study on the use of a novel hydrogel found that polysaccharide hybrid hydrogels can promote the proliferation and migration of rabbit bone marrow MSCs and enhance the aggregation of bone marrow MSCs in vitro [67]. Another study found that a novel temperature-sensitive hydrogel fortified with platelet-rich plasma can capture adipose-derived stem cells, induce their osteogenesis, promote calcium deposition and extracellular matrix mineralization, and upregulate the expression of osteogenic marker genes by increasing the cell proliferation rate and alkaline phosphatase activity [68]. Meanwhile, hydrogels were implanted into rabbit cranial defects, and new bone formation was found at the defects, confirming that temperature-sensitive composite hydrogels could promote osteogenesis of rabbit adipose-derived stem cells [69].
In addition, the specific properties of hydrogel can directly affect the signaling pathway of osteoblastic differentiation, thus promoting the differentiation of bone marrow MSCs into osteoblasts. A study on phenolic alginate gel revealed that it contains collagen nanoparticles and hydroxyapatite, with good mechanical characteristics. The addition of hydroxyapatite reduces collagen degradation, increases the swelling rate and stiffness of the hydrogel in tandem, and reduces elasticity and pore size, which is beneficial for the differentiation of bone marrow MSCs into osteoblasts. Various experimental methods confirmed that Wnt signal pathway related protein expression is induced during the process of phenolic alginate saline gel inducing stem cells to osteoblasts, indicating that the Wnt signal is involved in this process [70]. Ji et al. explored the osteogenic induction ability of newly synthesized temperature-sensitive hydroxypropyl chitacean hydrogel (HPCH). It was found to be non-toxic to cells and has good mechanical properties, which can stimulate stem cells to secrete angiogenic factors and osteogenic growth factors. By loading bone marrow MSCs and macrophages to the hydrogel, stem cells can promote the transformation of macrophages to anti-inflammatory M2 type, inhibit the secretion of inflammatory factors, upregulate the expression of osteogenic proteins osteocalcin, osteopontin and runt-related transcription factor 2, and promote cartilage solidification and the generation of angiogenic factors. Moreover, its unique 3D structure shows excellent compressive strength, creating a favorable microenvironment for vascularization and osteogenesis [71]. A study on multifunctional hydrogel found voltage and mechanical signaling effects. It can activate the downstream p-extracellular signal-regulated kinase (ERK) signaling pathway through multiple signaling proteins like mitogen-activated protein kinases (MAPK), c-Jun N-terminal kinase (JNK), P38, and transforming growth factor beta (TGF-β), ultimately promoting the differentiation of bone marrow MSCs into osteoblasts [72]. Yang et al. prepared collagen hydrogel with electrical properties by using methacrylic anhydride and succinic anhydride, this hydrogel has a triple helical structure and electrochemical characteristics. It can make bone marrow MSCs better adhere and spread on the hydrogel surface by affecting hydrophilicity, protein diffusion rate, and binding speed. It also promotes bone marrow MSCs to secrete sulfate mucopolysaccharide (sGAG) and further cartilage differentiation by improving integrin gene expression. Similar results were observed for in vivo subcutaneous implantation of this hydrogel, where significantly higher expression levels of sGAG, SOX9, and type II collagen were found in collagen hydrogels with higher negative charge [73]. In conclusion, hydrogels from various sources can promote the differentiation of bone marrow MSCs into osteoblasts, thus achieving bone regeneration (Figure 2).
Promote neovascularization in bone tissue
The vascular system is the body’s pipeline system in the human body and plays a crucial role in bone regeneration through its main functions of transporting oxygen and nutrients, maintaining blood flow and blood pressure, participating in immune reactions, recruiting cells in bone tissue, and regulating osteogenesis [74]. The key to successful bone regeneration is the establishment of a strong vascular network at the repair site to deliver the necessary nutrients and oxygen to the injured site. Thus, angiogenesis is a prerequisite and necessary factor for bone regeneration. since bone is a calcified tissue it has a limited blood supply and if blood supply is disturbed, new growth of blood vessels is time sensitive as it requires bone remodeling [75]. Therefore, the development of biomaterials that can promote the construction of functional vascular networks at defect sites is crucial in bone regeneration research. Another mechanism by which hydrogels promote bone regeneration is to promote angiogenesis in bone tissue [76]. Hydrogels have no toxic effect on vascular endothelial cells and can serve as vascular scaffolds and protective layers to protect neovascularization from damage and provide better protection against risk factors in the microenvironment [77]. Hydrogels can carry cells that promote angiogenesis and crosslink various cellular particles and cytokines. In addition, hydrogels can form a biphasic system with stem cells, which act directly on the bone defect site to simulate the normal biological environment around the bone and achieve bone and vascular regeneration, thus accelerating the repair of bone defects [76]. A study showed that implanting hydrogels co-cultured with human umbilical vein endothelial cells (HUVEC) into bone defect sites could enhance the formation of endothelial cell tubular structures and the number of branches, stimulate angiogenesis, and better promote bone tissue regeneration [78]. At the same time, Zhang et al. encapsulated exosomes derived from umbilical cord MSCs in hyaluronic acid hydrogel and combined them with customized nano scaffolds to repair cranial defects in rats. Imaging and histological evaluations indicated that this composite can enhance the proliferation, migration, and differentiation of vascular endothelial cells, further promoting bone regeneration [79]. Furthermore, many studies on the mechanism of bone regeneration and repair have shown that the novel hydrogel obtained after the implantation of multiple metal ions in the hydrogel has a better role in promoting angiogenesis. For example, when magnesium ions were implanted into hydrogels to repair cranial defects in rats, the novel hydrogel significantly promoted angiogenesis and bone regeneration in bone tissue, which was characterized by the upregulation of the expression of platelet endothelial cell adhesion molecule (CD31) and platelet endothelial cell adhesion molecule (VEGF), and the increase in vascular density [80], [81], [82].
Chen et al. conducted MgO nanoparticles were introduced into a newly synthesized aqueous water-soluble phosphocreatine-functionalized chitosan (CSMP) solution to form an injectable CSMP–MgO hydrogel. The combination of phosphate groups in CSMP and magnesium in MgO nanoparticles promotes angiogenesis in bone tissue and ultimately osteogenic differentiation. HUVEC cells were co-cultured with the hydrogel to evaluate its pro-angiogenic effect in vitro by observing the formation of a capillary network. It was found that HUVEC cells in the CSMP–MgO hydrogel group showed a rich vascular tubular network structure and revealed the largest capillary length, confirming the pro-angiogenic effect of the CSMP–MgO hydrogel [83]. Another study on multifunctional piezoelectric hydrogel discovered that it not only generates electricity but also promotes angiogenesis and bone regeneration by regulating the immune response. In this study, the effect of different stimulations on the angiogenic capacity of HUVEC cells was tested. It was found that the piezoelectric hydrogel promoted the chemotaxis and migration of HUVEC cells by inhibiting the immune response. Moreover, it enhanced the tube-forming ability of HUVEC cells by increasing vascular branch and tube formation and increased the expression of vascular function related protein CD31. All these results confirm the pro-angiogenic effect of the piezoelectric hydrogel [84]. Gao et al. successfully constructed a silk fibroin hydrogel compounded with nanoparticles and applied it to bone regeneration research. This hydrogel has excellent bioactivity and can promote the adhesion and spreading of HUVEC cells. At the same time, it can enhance angiogenesis and osteogenic differentiation. In the rat cranial regeneration model, transplanting the hydrogel significantly accelerated the cranial regeneration process. After 8 weeks of implantation, the healing rate can reach 86.77 ± 8.91 %, the pro-angiogenic effect shows that the number and branches of new blood vessels in HUVEC cells in the hydrogel group increase. Among them, the number of new blood vessels increases by 1.77 times, increased expression of VEGF and CD31 proteins. The results confirm that the hydrogel can accelerate bone regeneration by promoting angiogenesis [85]. Therefore, accelerating angiogenesis in bone tissue is another important mechanism by which hydrogels promote bone regeneration (Figure 3).
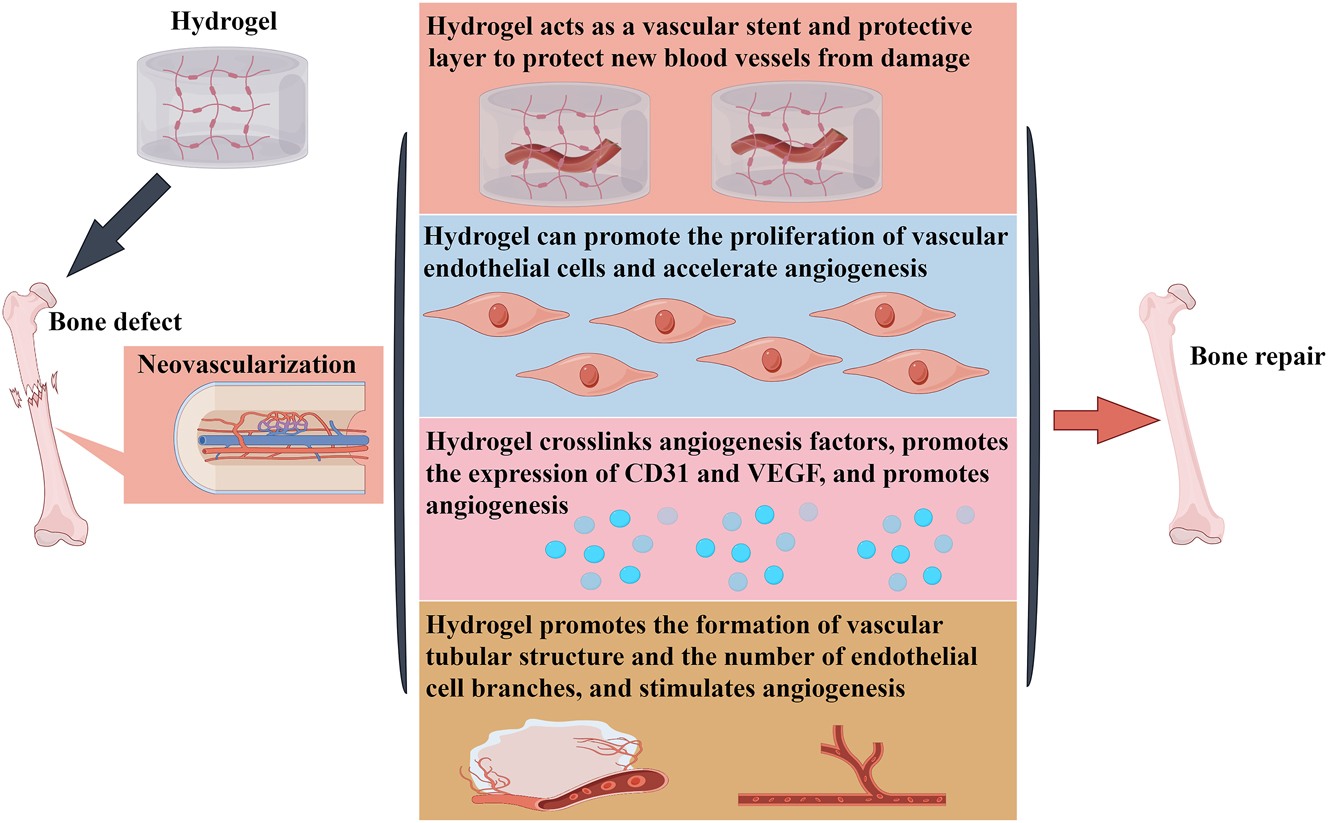
Accelerating angiogenesis in bone tissue is the main mechanism of hydrogel to promote bone regeneration.
Enhance bone morphogenetic protein (BMP) activity
BMP are a class of transforming growth factors involved in various processes of bone tissue repair and are capable of inducing the differentiation of bone marrow mesenchymal cells into osteoblasts and chondrocytes [86]. Among them, BMP2 is the most important regulator of bone formation, inducing the differentiation of bone precursor cells into mature stem cells [87], 88]. As a growth factor, BMP has a short half-life, is prone to inactivation and degradation in vivo, and requires excessive physiological doses to achieve the effect of promoting bone formation. However, excessive amounts of BMP can lead to side effects such as bone swelling, osteolysis, and bone resorption [89]. Therefore, there is a need for a biological delivery material in combination with BMP that reduces the dosage of BMP while increasing the activity and stability of BMP to better achieve the effect of promoting bone regeneration. It has been confirmed that combining BMP with hydrogel for bone defects not only maintains the stability of BMP and delays the degradation time of BMP, but also prolongs the activity time of osteoblasts and promotes bone regeneration [90]. Another study also found that by increasing or decreasing the number and type of anions in the hydrogel composition and changing the solid-liquid transition period and internal pore size, BMP2 can be released slowly and continuously, thus better promoting bone regeneration [91]. For example, when BMP2 was implanted into fibrin hydrogel containing macromolecular heparin, BMP2 could adhere well to the hydrogel to maintain its stability and activity, avoiding its sudden release and prolonging the action time of BMP2, thus enabling BMP2 to act directly on mesenchymal cells, accelerating their differentiation to osteoblasts, and promoting bone regeneration [92]. Similarly, Xu et al. first combined BMP2 with microspheres to prepare BMP2 microspheres and then implanted the microspheres into chitosan/polyethylene glycol hydrogels to prepare composite hydrogels. This composite hydrogel provides an environment and space for new bone growth, and the pore diameter of the hydrogel scaffold also provides exchange space for materials needed for bone regeneration. Microspheres can slowly release BMP2 and generate large amounts of calcium and phosphate ions to promote bone formation [93]. In addition, BMP7 and BMP9 can also be slowly released in the hydrogel, accelerating the aggregation, proliferation, and differentiation of osteoblast at the bone defects, contributing to the fusion of newly formed bone and surrounding bone tissues, and upregulating the expression of bone-related factors, and ultimately achieve the purpose of promoting bone regeneration [94], 95].
Heparin is a highly sulfated anionic polysaccharide composed of repeating glycoamine and uronic acid residues and has anticoagulant function. Due to the presence of a large number of negatively charged sulfate and carboxyl groups, it can bind to various proteins and positively charged cationic compounds. The heparin-conjugated fibrin (HCF) hydrogel is synthesized from adipose-derived pericytes (ADPs) and BMP2. The heparin sulfate groups of this hydrogel interact with the BMP2 protein to form a stable strong polyanionic complex, which not only protects heparin from hydrolysis but also delays the release of BMP2 from the hydrogel and maintains its biological activity. In vitro release experiments show that the continuous release time of BMP2 from the HCF hydrogel exceeds 4 weeks and can enhance the activity of alkaline phosphatase (ALP) protein in newly generated osteoblasts of rats and induce osteogenesis of osteoblasts in rat skull [96]. Alginate is a natural biopolymer. In the presence of divalent cations, alginate is easily ionically cross-linked to form a stable hydrogel. Although alginate biomaterials lack osteoconductivity and osteoinductivity, the addition of BMP2 can enhance its osteogenic induction ability. A new type of hydrogel synthesized from BMP2-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles and alginate shows excellent mechanical properties and can continuously release BMP2 for up to 2 weeks. In addition, this hydrogel can significantly enhance calcium deposition and alkaline phosphatase activity and upregulate the expression of osteogenic genes in MSCs [97]. In addition, a 3D-printed hydrogel scaffold is used to encapsulate BMP2-engineered human MSCs cells. MSCs cells can continuously express BMP2 and differentiate into osteoblasts. When implanted into severely immunodeficient mice, at 14 days after implantation, bone formation in mice increases, and mature trabecular bone structure and a large number of new blood vessels appear [97]. Chitosan has biocompatibility and biodegradability and can be used for bone transplantation. The presence of amino and hydroxyl groups in chitosan improves the absorption capacity of drugs, ions and growth factors. These groups are also modifiable positions for connecting additional functional groups to further enhance covalent adsorption. Methyl acrylate-modified chitosan was used to deliver BMP2 for bone regeneration research. The results showed that about 30 % of BMP2 was released on the first day, and then 70 % was released in the following week. The tibial defect in rats recovered by 3 mm within 4 weeks, indicating that chitosan-delivered BMP2 has important value in bone repair. In addition, sulfated chitosan can maintain a beneficial immune microenvironment by regulating immune function, enhance the connection between immune cells and stem cells, promote the osteogenic induction ability of stem cells and BMP2, and promote the chemotaxis of bone marrow stromal cells by upregulating bone morphogenetic protein receptor IA (BMPR-IA) expression and amplifying BMP2/Smad signal transduction [98]. Therefore, the enhancement of BMP2 activity is another mechanism by which hydrogels promote bone regeneration (Figure 4).
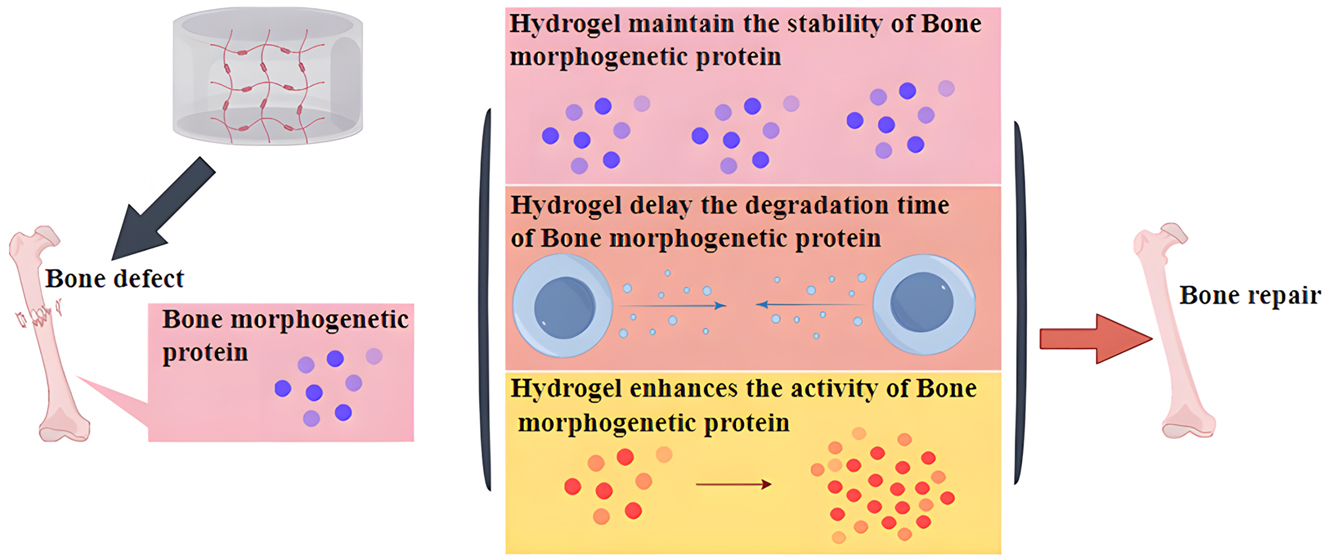
Enhancement of BMP2 activity is another mechanism by which hydrogels promote bone regeneration.
Regulating the microenvironment of bone regeneration tissue
Bone injuries from various causes can lead to changes in the microenvironment of bone defects. Among them, the hypoxic environment caused by blood interruption due to microvascular rupture at the defect site is the main reason affecting bone regeneration [99]. It is well known that hypoxia not only affects the proliferation and activity of bone marrow MSCs, but also inhibits their metabolic transformation and tube-forming ability, and further reduces the expression of osteogenic markers and mineralization [100]. In addition to directly decreasing cell activity, hypoxia increases the release of reactive oxygen species (ROS). High concentrations of ROS induce the death of osteoblast precursor cells and mature osteoblasts and inhibit the expression of ALP/BMP and tubular markers [101]. In addition, short-term over-oxygenation can lead to high oxygen tension and affect osteoblast activity [102]. Therefore, reasonable oxygenation and removal of ROS are important for promoting bone tissue regeneration. Hydrogels can improve the microenvironment of bone defects. On the one hand, specific components in the microenvironment activate the release of drugs and growth factors loaded in hydrogels. On the other hand, hydrogels can improve the stability of the microenvironment, promote osteoblast differentiation, and accelerate osteogenesis [103]. Sun et al. designed a composite hydrogel to meet the needs of bone defects, which has the potential to responsively clear ROS and prolong the duration of oxygen supply, thereby generating large amounts of oxygen to improve the hypoxic microenvironment at the site of bone defects, and also promotes the regeneration and repair of bone tissues by facilitating the formation of vascular rings and osteoblast differentiation, as well as by inhibiting osteoclast differentiation [104]. Zhou et al. also developed an antioxidant hydrogel to accelerate bone tissue regeneration through a similar mechanism of action [105]. Notably, due to the presence of hyperglycemia, the blood vessels of diabetic patients usually exhibit abnormal function and are prone to hypoxia and ROS overproduction, which induces the production of inflammatory factors and harmful substances resulting in the disturbance of the microenvironment at the bone defects. Therefore, bone regeneration in diabetic patients faces greater challenges. Li et al. designed a dual-network adaptive hydrogel composed of phenylboronic acid crosslinked polyvinyl alcohol and gelatin gel. This network hydrogel reversibly decomposed upon exposure to a high glucose environment and ROS, decreased the ROS content in the microenvironment, increased oxygen content by inhibiting inflammatory reactions, and improved the imbalanced microenvironment, which in turn promoted bone regeneration [106].
Hydrogels can bind to host cells through specific molecular pathways and regulate the bone regeneration microenvironment to promote bone hyperplasia. Sun et al. developed a smart responsive hydrogel. It can increase the expression of the brain and muscle arntlike 1(BMAL1) gene in osteoblasts by releasing oxygen, upregulate the expression levels of autophagy related proteins Beclin1 and LC3, and regulate the microenvironment in the bone regeneration process to promote osteogenesis [107]. Zhang et al. developed a new type of ultrasound-triggered biomimetic ultrashort peptide nanofiber hydrogel. This hydrogel can promote the healing of bone defects by constructing a microenvironment conducive to bone regeneration. Exploring its mechanism reveals that this hydrogel can change the polarization state of macrophages, reduce the number of M1 types and increase the number of M2 types. At the same time, interleukin-4 activates the transcription factor Signal Transducer and Activator of Transcription 6 (STAT6), which further binds to specific target genes and increases the number of peroxisome proliferator-activated receptor gamma (PPAR-γ) heterodimerization and M2 macrophages. More interestingly, the nanofibers released by this hydrogel can also inhibit the release of ROS by regulating macrophage mitochondrial energy metabolism and secrete BMP2 and insulin-like growth factor 1(IGF-1) proteins to accelerate the osteogenic differentiation ability of bone marrow MSCs [108]. A study on immunomodulatory blood-derived hybrid hydrogel found that it can regulate the bone regeneration microenvironment in multiple ways to enhance bone regeneration ability. This double-network hybrid hydrogel is composed of platelet-rich fibrin and polycaprolactone/hydroxyapatite composite nanofibers, with polydopamine (PDA) as an anchor. The polycaprolactone in hydrogel has good mechanical properties to stimulate osteoblast differentiation in an appropriate biomechanical microenvironment. PDA can not only improve the adhesion ability of growth factors but also continuously create a suitable biochemical and immune microenvironment for osteoblasts and promote bone healing by inducing M2 macrophage polarization [109]. Yang et al. developed a 3D printed composite hydrogel scaffold, this hydrogel can provide a suitable microenvironment for bone regeneration and angiogenesis. In addition, the CS (Ca and Si) molecules released from this 3D printed composite hydrogel scaffold endow the scaffold with multiple functions. Firstly, it promotes the differentiation of bone mesenchymal MSCs into osteoblasts and blood vessels. Secondly, it induces the transformation of macrophages to M2 type by inhibiting the expression of Smad six and Smad seven proteins. At the same time, it remodels the immune microenvironment conducive to bone and angiogenesis. The rabbit skull defect experiment confirmed that the scaffold can promote osteogenesis and neovascularization by regulating the bone regeneration microenvironment. In general, the strong osteoinductive ability and good bone immune regulation characteristics of the scaffold are considered a promising strategy for treating bone defects [110]. Therefore, regulating the microenvironment of bone tissue is another mechanism by which hydrogels promote bone regeneration (Figure 5).
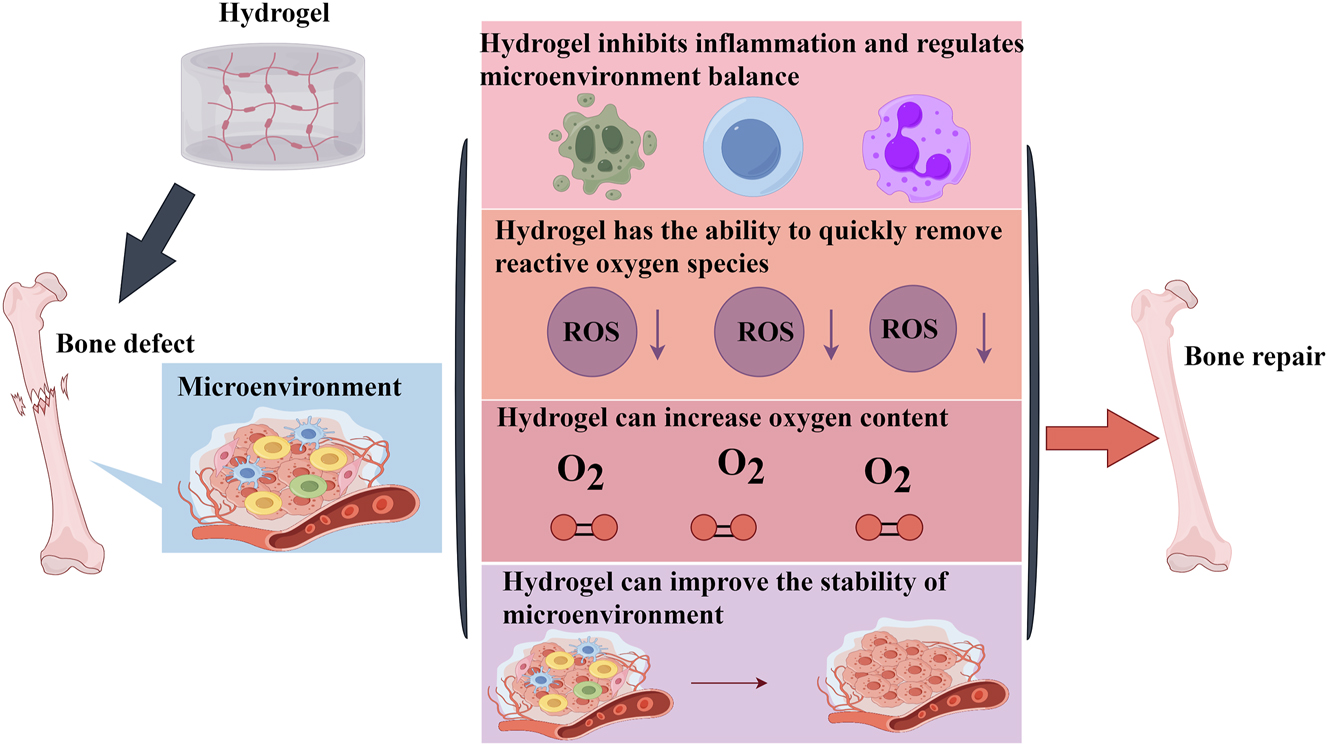
Regulating the microenvironment of bone tissue is another mechanism by which hydrogels promote bone regeneration.
Summary
Repairing bone tissue defects has long been a major challenge in the field of bone regeneration, and biomaterials for bone regeneration need high biocompatibility and low immunogenicity. With in-depth research on the mechanism of bone regeneration and the application of bio-tissue materials, a variety of bio-functional materials with excellent performance have appeared in the field of bone regeneration, which has brought new hope for the repair of large-scale bone defects. Because of their unique physical and biological properties, hydrogels play an important role in numerous diseases such as tumors, wound healing, neurological diseases, digestive diseases, and motor system diseases. Among them, hydrogels show significant advantages in the fields of bone regeneration and repair due to their good biocompatibility and modifiable properties. This article summarized the main mechanisms of hydrogel to promote bone regeneration, including inducing the differentiation of bone marrow MSCs to osteoblasts and angiogenesis, enhancing the activity of bone morphogenetic proteins, and improving the microenvironment of bone regeneration tissues. The different mechanisms interact with each other to jointly promote bone regeneration and repair. Through an in-depth understanding of the mechanism of hydrogel promoting bone regeneration, this paper provides a research direction for the development of novel hydrogels and other bio-tissue materials. Certainly, there are still more mechanisms and functions of hydrogels for bone regeneration that need to be further summarized and studied.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Conceptualization: Zengguang Ke; writing and original draft preparation: Yuanyuan Zheng; writing and editing: Guofeng Hu and Songlin Tong. All authors have read and agreed to the published version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: Not applicable.
-
Data availability: Not applicable.
References
1. Maresca, JA, DeMel, DC, Wagner, GA, Haase, C, Geibel, JP. Three-dimensional bioprinting applications for bone tissue engineering. Cells 2023;12:1230. https://doi.org/10.3390/cells12091230.Suche in Google Scholar PubMed PubMed Central
2. Han, Z, Xiong, J, Jin, X, Dai, Q, Han, M, Wu, H, et al.. Advances in reparative materials for infectious bone defects and their applications in maxillofacial regions. J Mater Chem B 2024;12:842–71. https://doi.org/10.1039/d3tb02069j.Suche in Google Scholar PubMed
3. Wang, J, Liu, M, Yang, C, Pan, Y, Ji, S, Han, N, et al.. Biomaterials for bone defect repair: types, mechanisms and effects. Int J Artif Organs 2024;47:75–84. https://doi.org/10.1177/03913988231218884.Suche in Google Scholar PubMed
4. Wang, C, Min, S, Tian, Y. Injectable and cell-laden hydrogel in the contained bone defect animal model: a systematic review. Tissue Eng Regen Med 2023;20:829–37. https://doi.org/10.1007/s13770-023-00569-2.Suche in Google Scholar PubMed PubMed Central
5. Ruiz-Muñoz, M, Martinez-Barrios, FJ, Fernandez-Torres, R, Lopezosa-Reca, E, Marchena-Rodriguez, A. Autologous platelet-rich plasma (APRP) in diabetes foot disease: a meta-analysis. J Diabet Complicat 2024;38. https://doi.org/10.1016/j.jdiacomp.2024.108690.Suche in Google Scholar PubMed
6. Asserson, DB. Allogeneic mesenchymal stem cells after in vivo transplantation: a review. Cell Reprogr 2023;25:264–76. https://doi.org/10.1089/cell.2023.0084.Suche in Google Scholar PubMed
7. Zorrón, M, Cabrera, AL, Sharma, R, Radhakrishnan, J, Abbaszadeh, S, Shahbazi, MA, et al.. Emerging 2D nanomaterials-integrated hydrogels: advancements in designing theragenerative materials for bone regeneration and disease therapy. Adv Sci 2024;11:e2403204. https://doi.org/10.1002/advs.202403204.Suche in Google Scholar PubMed PubMed Central
8. Xin, L, Wen, Y, Song, J, Chen, T, Zhai, Q. Bone regeneration strategies based on organelle homeostasis of mesenchymal stem cells. Front Endocrinol 2023;14. https://doi.org/10.3389/fendo.2023.1151691.Suche in Google Scholar PubMed PubMed Central
9. Lu, Y, Mai, Z, Cui, L, Zhao, X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res Ther 2023;14:55. https://doi.org/10.1186/s13287-023-03275-x.Suche in Google Scholar PubMed PubMed Central
10. Wang, X, Yu, F, Ye, L. Epigenetic control of mesenchymal stem cells orchestrates bone regeneration. Front Endocrinol 2023;14. https://doi.org/10.3389/fendo.2023.1126787.Suche in Google Scholar PubMed PubMed Central
11. Hirakawa, H, Gao, L, Tavakol, DN, Vunjak-Novakovic, G, Ding, L. Cellular plasticity of the bone marrow niche promotes hematopoietic stem cell regeneration. Nat Genet 2023;55:1941–52. https://doi.org/10.1038/s41588-023-01528-2.Suche in Google Scholar PubMed
12. Bai, L, Song, P, Su, J. Bioactive elements manipulate bone regeneration. Biomater Transl 2023;4:248–69. https://doi.org/10.12336/biomatertransl.2023.04.005.Suche in Google Scholar PubMed PubMed Central
13. Liu, X, Huang, H, Zhang, J, Sun, T, Zhang, W, Li, Z. Recent advance of strontium functionalized in biomaterials for bone regeneration. Bioengineering (Basal) 2023;10:414. https://doi.org/10.3390/bioengineering10040414.Suche in Google Scholar PubMed PubMed Central
14. Luo, Y, Liu, H, Zhang, Y, Liu, Y, Liu, S, Liu, X, et al.. Metal ions: the unfading stars of bone regeneration-from bone metabolism regulation to biomaterial applications. Biomater Sci 2023;11:7268–95. https://doi.org/10.1039/d3bm01146a.Suche in Google Scholar PubMed
15. Jahangirnezhad, M, Mahmoudinezhad, SS, Moradi, M, Moradi, K, Rohani, A, Tayebi, L. Bone scaffold materials in periodontal and tooth-supporting tissue regeneration: a review. Curr Stem Cell Res Ther 2024;19:449–60. https://doi.org/10.2174/1574888x18666221227142055.Suche in Google Scholar
16. Gisbert-Garzarán, M, Gómez-Cerezo, MN, Vallet-Regí, M. Targeting agents in biomaterial-mediated bone regeneration. Int J Mol Sci 2023;24:2007. https://doi.org/10.3390/ijms24032007.Suche in Google Scholar PubMed PubMed Central
17. Ho, TC, Chang, CC, Chan, HP, Chung, TW, Shu, CW, Chuang, KP, et al.. Hydrogels: properties and applications in biomedicine. Molecules 2022;27:2902. https://doi.org/10.3390/molecules27092902.Suche in Google Scholar PubMed PubMed Central
18. Sun, L, Xu, Y, Han, Y, Cui, J, Jing, Z, Li, D, et al.. Collagen-based hydrogels for cartilage regeneration. Orthop Surg 2023;15:3026–45. https://doi.org/10.1111/os.13884.Suche in Google Scholar PubMed PubMed Central
19. Xu, Y, Wang, JY, Meng, T, Ma, XW, Li, H, Li, K. Role of hydrogels in osteoarthritis: a comprehensive review. Int J Rheum Dis 2023;26:2390–401. https://doi.org/10.1111/1756-185x.14968.Suche in Google Scholar PubMed
20. Gutierrez, AM, Frazar, EM, X Klaus, MV, Paul, P, Hilt, JZ. Hydrogels and hydrogel nanocomposites: enhancing healthcare through human and environmental treatment. Adv Healthcare Mater 2022;11:e2101820. https://doi.org/10.1002/adhm.202101820.Suche in Google Scholar PubMed PubMed Central
21. Chan, D, Maikawa, CL, d’Aquino, AI, Raghavan, SS, Troxell, ML, Appel, EA. Polyacrylamide-based hydrogel coatings improve biocompatibility of implanted pump devices. J Biomed Mater Res 2023;111:910–20. https://doi.org/10.1002/jbm.a.37521.Suche in Google Scholar PubMed PubMed Central
22. Tuancharoensri, N, Sonjan, S, Promkrainit, S, Daengmankhong, J, Phimnuan, P, Mahasaranon, S, et al.. Porous poly(2-hydroxyethyl methacrylate) hydrogel scaffolds for tissue engineering: influence of crosslinking systems and silk sericin concentration on scaffold properties. Polymers 2023;15:4052. https://doi.org/10.3390/polym15204052.Suche in Google Scholar PubMed PubMed Central
23. Malka, E, Margel, S. Engineering of PVA/PVP hydrogels for agricultural applications. Gels 2023;9:895. https://doi.org/10.3390/gels9110895.Suche in Google Scholar PubMed PubMed Central
24. Eddine, MA, Carvalho, A, Schmutz, M, Salez, T, de Chateauneuf-Randon, S, Bresson, B, et al.. Sieving and clogging in PEG-PEGDA hydrogel membranes. Langmuir 2023;39:15085–94. https://doi.org/10.1021/acs.langmuir.3c02153.Suche in Google Scholar PubMed
25. Niemczyk-Soczynska, B, Gradys, A, Kolbuk, D, Krzton-Maziopa, A, Rogujski, P, Stanaszek, L, et al.. A methylcellulose/agarose hydrogel as an innovative scaffold for tissue engineering. RSC Adv 2022;12:26882–94. https://doi.org/10.1039/d2ra04841h.Suche in Google Scholar PubMed PubMed Central
26. Enoch, K, Somasundaram, AA. Rheological insights on Carboxymethyl cellulose hydrogels. Int J Biol Macromol 2023;253. https://doi.org/10.1016/j.ijbiomac.2023.127481.Suche in Google Scholar PubMed
27. Filip, D, Macocinschi, D, Zaltariov, MF, Ciubotaru, BI, Bargan, A, Varganici, CD, et al.. Hydroxypropyl cellulose/pluronic-based composite hydrogels as biodegradable mucoadhesive scaffolds for tissue engineering. Gels 2022;8:519. https://doi.org/10.3390/gels8080519.Suche in Google Scholar PubMed PubMed Central
28. Su, R, Xiao, X, Li, G. Thermosensitive poly(N-isopropylacrylamide) hydrogel/highly internal phase emulsion porous polymer tube tip solid-phase extraction for the determination of methylimidazole in beverage. J Chromatogr A 2023;1712. https://doi.org/10.1016/j.chroma.2023.464476.Suche in Google Scholar PubMed
29. Zhou, D, Fu, P, Lin, WT, Li, WL, Xu, ZK, Wan, LS. Poly(N,N-diethylacrylamide)-endowed spontaneous emulsification during the breath figure process and the formation of membranes with hierarchical pores. Soft Matter 2024;20:1905–12. https://doi.org/10.1039/d3sm01603j.Suche in Google Scholar PubMed
30. Liu, J, Hu, N, Xie, Y, Wang, P, Chen, J, Kan, Q. Polyacrylic acid hydrogel coating for underwater adhesion: preparation and characterization. Gels 2023;9:616. https://doi.org/10.3390/gels9080616.Suche in Google Scholar PubMed PubMed Central
31. Yi, FL, Guo, FL, Li, YQ, Wang, DY, Huang, P, Fu, SY. Polyacrylamide hydrogel composite E-skin fully mimicking human skin. ACS Appl Mater Interfaces 2021;13:32084–93. https://doi.org/10.1021/acsami.1c05661.Suche in Google Scholar PubMed
32. Wang, X, Song, R, Johnson, M, A, S, Shen, P, Zhang, N, et al.. Chitosan-based hydrogels for infected wound treatment. Macromol Biosci 2023;23:e2300094. https://doi.org/10.1002/mabi.202300094.Suche in Google Scholar PubMed
33. Ko, Y, Oh, Y, Park, CH, Kim, SH. Designing tough hydrogel shells for glucose sensing. Small 2024;20:e2310283. https://doi.org/10.1002/smll.202310283.Suche in Google Scholar PubMed
34. Christen, MO. Collagen stimulators in body applications: a review focused on poly-L-lactic acid (plla). Clin Cosmet Invest Dermatol 2022;15:997–1019. https://doi.org/10.2147/ccid.s359813.Suche in Google Scholar
35. Liffland, S, Kumler, M, Hillmyer, MA. High performance star block aliphatic polyester thermoplastic elastomers using PDLA-b-PLLA stereoblock hard domains. ACS Macro Lett 2023;12:1331–8. https://doi.org/10.1021/acsmacrolett.3c00437.Suche in Google Scholar PubMed
36. Trombino, S, Sole, R, Di Gioia, ML, Procopio, D, Curcio, F, Cassano, R. Green chemistry principles for nano- and micro-sized hydrogel synthesis. Molecules 2023;28:2107. https://doi.org/10.3390/molecules28052107.Suche in Google Scholar PubMed PubMed Central
37. Zhao, Q, Yue, X, Miaomiao, L, Yanming, W, Wu, G. Nano-injectable pH/NIR-responsive hydrogel for chemo-photothermal synergistic drug delivery. J Biomater Appl 2023;38:614–28. https://doi.org/10.1177/08853282231209653.Suche in Google Scholar PubMed
38. Conley, BM, Yang, L, Bhujel, B, Luo, J, Han, I, Lee, KB. Development of a nanohybrid peptide hydrogel for enhanced intervertebral disc repair and regeneration. ACS Nano 2023;17:3750–64. https://doi.org/10.1021/acsnano.2c11441.Suche in Google Scholar PubMed
39. Kuo, CY, Lin, TY, Yeh, YC. Hydrogel-based strategies for the management of osteomyelitis. ACS Biomater Sci Eng 2023;9:1843–61. https://doi.org/10.1021/acsbiomaterials.2c01057.Suche in Google Scholar PubMed
40. Jurczak, P, Lach, S. Hydrogels as scaffolds in bone-related tissue engineering and regeneration. Macromol Biosci 2023;23:e2300152. https://doi.org/10.1002/mabi.202300152.Suche in Google Scholar PubMed
41. Mohanto, S, Narayana, S, Merai, KP, Kumar, JA, Bhunia, A, Hani, U, et al.. Advancements in gelatin-based hydrogel systems for biomedical applications: a state-of-the-art review. Int J Biol Macromol 2023;253. https://doi.org/10.1016/j.ijbiomac.2023.127143.Suche in Google Scholar PubMed
42. Su, H, Karin, M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer 2023;9:764–73. https://doi.org/10.1016/j.trecan.2023.06.002.Suche in Google Scholar PubMed
43. Sanz-Horta, R, Matesanz, A, Gallardo, A, Reinecke, H, Jorcano, JL, Acedo, P, et al.. Technological advances in fibrin for tissue engineering. J Tissue Eng 2023;14. https://doi.org/10.1177/20417314231190288.Suche in Google Scholar PubMed PubMed Central
44. Sultan, MT, Hong, H, Lee, OJ, Ajiteru, O, Lee, YJ, Lee, JS, et al.. Silk fibroin-based biomaterials for hemostatic applications. Biomolecules 2022;12:660. https://doi.org/10.3390/biom12050660.Suche in Google Scholar PubMed PubMed Central
45. Xu, Z, Liu, G, Liu, P, Hu, Y, Chen, Y, Fang, Y, et al.. Hyaluronic acid-based glucose-responsive antioxidant hydrogel platform for enhanced diabetic wound repair. Acta Biomater 2022;147:147–57. https://doi.org/10.1016/j.actbio.2022.05.047.Suche in Google Scholar PubMed
46. Shen, Q, Guo, Y, Wang, K, Zhang, C, Ma, Y. A review of chondroitin sulfate’s preparation, properties, functions, and applications. Molecules 2023;28:7093. https://doi.org/10.3390/molecules28207093.Suche in Google Scholar PubMed PubMed Central
47. Tomić, SL, Babić Radić, MM, Vuković, JS, Filipović, VV, Nikodinovic-Runic, J, Vukomanović, M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar Drugs 2023;21:177. https://doi.org/10.3390/md21030177.Suche in Google Scholar PubMed PubMed Central
48. Zhao, J, Qiu, P, Wang, Y, Wang, Y, Zhou, J, Zhang, B, et al.. Chitosan-based hydrogel wound dressing: from mechanism to applications, a review. Int J Biol Macromol 2023;244. https://doi.org/10.1016/j.ijbiomac.2023.125250.Suche in Google Scholar PubMed
49. Jiang, F, Xu, XW, Chen, FQ, Weng, HF, Chen, J, Ru, Y, et al.. Extraction, modification and biomedical application of agarose hydrogels: a review. Mar Drugs 2023;21:299. https://doi.org/10.3390/md21050299.Suche in Google Scholar PubMed PubMed Central
50. Li, S, Yu, Q, Li, H, Chen, M, Jin, Y, Liu, D. Self-assembled peptide hydrogels in regenerative medicine. Gels 2023;9:653. https://doi.org/10.3390/gels9080653.Suche in Google Scholar PubMed PubMed Central
51. Khajouei, S, Ravan, H, Ebrahimi, A. DNA hydrogel-empowered biosensing. Adv Colloid Interfaces Sci 2020;275. https://doi.org/10.1016/j.cis.2019.102060.Suche in Google Scholar PubMed PubMed Central
52. Simeonov, M, Kostova, B, Vassileva, E. Interpenetrating polymer networks of poly(2-hydroxyethyl methacrylate) and poly(N, N-dimethylacrylamide) as potential systems for dermal delivery of dexamethasone phosphate. Pharmaceutics 2023;15:2328. https://doi.org/10.3390/pharmaceutics15092328.Suche in Google Scholar PubMed PubMed Central
53. Fattahi, N, Reed, J, Heronemus, E, Fernando, P, Hansen, R, Parameswaran, P. Polyethylene glycol hydrogel coatings for protection of electroactive bacteria against chemical shocks. Bioelectrochemistry 2024;156. https://doi.org/10.1016/j.bioelechem.2023.108595.Suche in Google Scholar PubMed
54. Xu, X, Liu, Y, Fu, W, Yao, M, Ding, Z, Xuan, J, et al.. Poly(N-isopropylacrylamide)-Based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020;12:580. https://doi.org/10.3390/polym12030580.Suche in Google Scholar PubMed PubMed Central
55. Vishwanath, K, McClure, SR, Bonassar, LJ. Polyacrylamide hydrogel lubricates cartilage after biochemical degradation and mechanical injury. J Orthop Res 2023;41:63–71. https://doi.org/10.1002/jor.25340.Suche in Google Scholar PubMed
56. Chen, Z, Li, J, Li, T, Fan, T, Meng, C, Li, C, et al.. A CRISPR/Cas12a-empowered surface plasmon resonance platform for rapid and specific diagnosis of the Omicron variant of SARS-CoV-2. Natl Sci Rev 2022;9. https://doi.org/10.1093/nsr/nwac104.Suche in Google Scholar PubMed PubMed Central
57. Zheng, F, Chen, Z, Li, J, Wu, R, Zhang, B, Nie, G, et al.. A highly sensitive CRISPR-empowered surface plasmon resonance sensor for diagnosis of inherited diseases with femtomolar-level real-time quantification. Adv Sci 2022;9:e2105231. https://doi.org/10.1002/advs.202105231.Suche in Google Scholar PubMed PubMed Central
58. Chen, Z, Wu, C, Yuan, Y, Xie, Z, Li, T, Huang, H, et al.. CRISPR-Cas13a-powered electrochemical biosensor for the detection of the L452R mutation in clinical samples of SARS-CoV-2 variants. J Nanobiotechnol 2023;21:141. https://doi.org/10.1186/s12951-023-01903-5.Suche in Google Scholar PubMed PubMed Central
59. Choi, C, Yun, E, Cha, C. Emerging technology of nanofiber-composite hydrogels for biomedical applications. Macromol Biosci 2023;23:e2300222. https://doi.org/10.1002/mabi.202300222.Suche in Google Scholar PubMed
60. Zhao, Z, Fan, X, Li, X, Qiu, Y, Yi, Y, Wei, Y, et al.. All-natural injectable antibacterial hydrogel enabled by chitosan and borneol. Biomacromolecules 2024;25:134–42. https://doi.org/10.1021/acs.biomac.3c00874.Suche in Google Scholar PubMed
61. Xu, K, Weng, J, Li, J, Chen, X. Advances in intelligent stimuli-responsive microneedle for biomedical applications. Macromol Biosci 2023;23:e2300014. https://doi.org/10.1002/mabi.202300014.Suche in Google Scholar PubMed
62. He, T, Lv, S, Wei, D, Feng, R, Yang, J, Yan, Y, et al.. Photothermal conversion of hydrogel-based biomaterial. Chem Rec 2023;23:e202300184. https://doi.org/10.1002/tcr.202300184.Suche in Google Scholar PubMed
63. Han, F, Wang, C, Cheng, P, Liu, T, Wang, WS. Bone marrow mesenchymal stem cells derived exosomal miRNAs can modulate diabetic bone-fat imbalance. Front Endocrinol 2023;14. https://doi.org/10.3389/fendo.2023.1149168.Suche in Google Scholar PubMed PubMed Central
64. Wang, H, Xu, Y, Wang, P, Ma, J, Wang, P, Han, X, et al.. Cell-mediated injectable blend hydrogel-BCP ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater 2021;123:364–78. https://doi.org/10.1016/j.actbio.2020.12.056.Suche in Google Scholar PubMed
65. Bai, H, Zhao, Y, Wang, C, Wang, Z, Wang, J, Liu, H, et al.. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 2020;10:4779–94. https://doi.org/10.7150/thno.43736.Suche in Google Scholar PubMed PubMed Central
66. Liu, T, Li, J, Shao, Z, Ma, K, Zhang, Z, Wang, B, et al.. Encapsulation of mesenchymal stem cells in chitosan/β-glycerophosphate hydrogel for seeding on a novel calcium phosphate cement scaffold. Med Eng Phys 2018;56:9–15. https://doi.org/10.1016/j.medengphy.2018.03.003.Suche in Google Scholar PubMed
67. Wise, JK, Alford, AI, Goldstein, SA, Stegemann, JP. Comparison of uncultured marrow mononuclear cells and culture‐expanded mesenchymal stem cells in 3D collagen‐chitosan microbeads for orthopedic tissue engineering. Tissue Eng 2014;20:210–24. https://doi.org/10.1089/ten.tea.2013.0151.Suche in Google Scholar PubMed PubMed Central
68. Pu, X, Tong, L, Wang, X, Liu, Q, Chen, M, Li, X, et al.. Bioinspired hydrogel anchoring 3DP GelMA/HAp scaffolds accelerates bone reconstruction. ACS Appl Mater Interfaces 2022;14:20591–602. https://doi.org/10.1021/acsami.1c25015.Suche in Google Scholar PubMed
69. Zhao, X, Chen, X, Deng, Y, Wu, C, Ruan, Z, Li, C, et al.. A novel adhesive dual-sensitive hydrogel for sustained release of exosomes derived from M2 macrophages promotes repair of bone defects. Mater Today Bio 2023;23. https://doi.org/10.1016/j.mtbio.2023.100840.Suche in Google Scholar PubMed PubMed Central
70. Saghati, S, Avci, ÇB, Hassani, A, Nazifkerdar, S, Amini, H, Saghebasl, S, et al.. Phenolated alginate hydrogel induced osteogenic properties of mesenchymal stem cells via Wnt signaling pathway. Int J Biol Macromol 2023;253. https://doi.org/10.1016/j.ijbiomac.2023.127209.Suche in Google Scholar PubMed
71. Ji, X, Yuan, X, Ma, L, Bi, B, Zhu, H, Lei, Z, et al.. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone)/nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics 2020;10:725–40. https://doi.org/10.7150/thno.39167.Suche in Google Scholar PubMed PubMed Central
72. He, X, Liu, Y, Dai, Z, Chen, Y, Liu, W, Dai, H, et al.. Yoda1 pretreated BMSC derived exosomes accelerate osteogenesis by activating phospho-ErK signaling via Yoda1-mediated signal transmission. J Nanobiotechnol 2024;22:407. https://doi.org/10.1186/s12951-024-02669-0.Suche in Google Scholar PubMed PubMed Central
73. Yang, J, Xiao, Y, Tang, Z, Luo, Z, Li, D, Wang, Q, et al.. The negatively charged microenvironment of collagen hydrogels regulates the chondrogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Mater Chem B 2020;8:4680–93. https://doi.org/10.1039/d0tb00172d.Suche in Google Scholar PubMed
74. Dudley, AC, Griffioen, AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 2023;26:313–47. https://doi.org/10.1007/s10456-023-09876-7.Suche in Google Scholar PubMed PubMed Central
75. Irfan, D, Ahmad, I, Patra, I, Margiana, R, Rasulova, MT, Sivaraman, R, et al.. Stem cell-derived exosomes in bone healing: focusing on their role in angiogenesis. Cytotherapy 2023;25:353–61. https://doi.org/10.1016/j.jcyt.2022.08.008.Suche in Google Scholar PubMed
76. Zhong, M, Wei, D, Yang, Y, Sun, J, Chen, X, Guo, L, et al.. Vascularization in engineered tissue construct by assembly of cellular patterned micromodules and degradable microspheres. ACS Appl Mater Interfaces 2017;9:3524–34. https://doi.org/10.1021/acsami.6b15697.Suche in Google Scholar PubMed
77. Li, J, Cui, X, Lindberg, G, Alcala-Orozco, C, Hooper, G, Lim, K, et al.. Hybrid fabrication of photo-clickable vascular hydrogels with additive manufactured titanium implants for enhanced osseointegration and vascularized bone formation. Biofabrication 2022;14:034103. https://doi.org/10.1088/1758-5090/ac6051.Suche in Google Scholar PubMed
78. Nulty, J, Freeman, F, Browe, D, Burdis, R, Ahern, D, Pitacco, P, et al.. 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater 2021;126:154–69. https://doi.org/10.1016/j.actbio.2021.03.003.Suche in Google Scholar PubMed
79. zhang, Y, Xie, Y, Hao, Z, Zhou, P, Wang, P, Fang, S, et al.. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces 2021;13:18472–87. https://doi.org/10.1021/acsami.0c22671.Suche in Google Scholar PubMed
80. Chen, R, Chen, HB, Xue, PP, Yang, WG, Luo, LZ, Tong, MQ, et al.. HA/MgO nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J Mater Chem B 2021;9:1107–22. https://doi.org/10.1039/d0tb02553d.Suche in Google Scholar PubMed
81. Xu, Y, Xu, C, He, L, Zhou, J, Chen, T, Ouyang, L, et al.. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact Mater 2022;16:271–84. https://doi.org/10.1016/j.bioactmat.2022.02.024.Suche in Google Scholar PubMed PubMed Central
82. Zhang, X, Huang, P, Jiang, G, Zhang, M, Yu, F, Dong, X, et al.. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater Sci Eng C 2021;121:111868–10.1016. https://doi.org/10.1016/j.msec.2021.111868.Suche in Google Scholar PubMed
83. Chen, Y, Sheng, W, Lin, J, Fang, C, Deng, J, Zhang, P, et al.. Magnesium oxide nanoparticle coordinated phosphate-functionalized chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. ACS Appl Mater Interfaces 2022;14:7592–608. https://doi.org/10.1021/acsami.1c21260.Suche in Google Scholar PubMed
84. Wu, P, Shen, L, Liu, HF, Zou, XH, Zhao, J, Huang, Y, et al.. The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine. Mil Med Res 2023;10:35. https://doi.org/10.1186/s40779-023-00469-5.Suche in Google Scholar PubMed PubMed Central
85. Gao, J, Ren, J, Ye, H, Chu, W, Ding, X, Ding, L, et al.. Thymosin beta 10 loaded ZIF-8/sericin hydrogel promoting angiogenesis and osteogenesis for bone regeneration. Int J Biol Macromol 2024;267. https://doi.org/10.1016/j.ijbiomac.2024.131562.Suche in Google Scholar PubMed
86. Hettiaratchi, M, Rouse, T, Chou, C, Krishnan, L, Stevens, H, Li, M, et al.. Enhanced in vivo retention of low dose BMP-2 via heparin microparticle delivery does not accelerate bone healing in a critically sized femoral defect. Acta Biomater 2017;59:21–32. https://doi.org/10.1016/j.actbio.2017.06.028.Suche in Google Scholar PubMed PubMed Central
87. Zhou, X, Chen, J, Sun, H, Wang, F, Wang, Y, Zhang, Z, et al.. Spatiotemporal regulation of angiogenesis/osteogenesis emulating natural bone healing cascade for vascularized bone formation. J Nanobiotechnol 2021;19:420. https://doi.org/10.1186/s12951-021-01173-z.Suche in Google Scholar PubMed PubMed Central
88. Seo, B, Koh, J, Song, S. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017;122:91–104. https://doi.org/10.1016/j.biomaterials.2017.01.016.Suche in Google Scholar PubMed
89. Cheng, L, Chen, Z, Cai, Z, Zhao, J, Lu, M, Liang, J, et al.. Bioinspired functional black phosphorus electrospun fibers achieving recruitment and biomineralization for staged bone regeneration. Small 2020;16:e2005433. https://doi.org/10.1002/smll.202005433.Suche in Google Scholar PubMed
90. Krishnan, L, Priddy, L, Esancy, C, Klosterhoff, B, Stevens, H, Tran, L, et al.. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater 2017;49:101–12. https://doi.org/10.1016/j.actbio.2016.12.012.Suche in Google Scholar PubMed PubMed Central
91. Chen, X, Tan, B, Bao, Z, Wang, S, Tang, R, Wang, Z, et al.. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials 2021;277. https://doi.org/10.1016/j.biomaterials.2021.121117.Suche in Google Scholar PubMed
92. Ao, Q, Wang, S, He, Q, Ten, H, Oyama, K, Ito, A, et al.. Fibrin glue/fibronectin/heparin-based delivery system of BMP2 induces osteogenesis in mc3t3-E1 cells and bone formation in rat calvarial critical-sized defects. ACS Appl Mater Interfaces 2020;12:13400–10. https://doi.org/10.1021/acsami.0c01371.Suche in Google Scholar PubMed
93. Xu, H, Luo, H, Chen, J, Chen, G, Yu, X, Ye, Z. BMP-2 releasing mineral-coated microparticle-integrated hydrogel system for enhanced bone regeneration. Front Bioeng Biotechnol 2023;11. https://doi.org/10.3389/fbioe.2023.1217335.Suche in Google Scholar PubMed PubMed Central
94. Mantripragada, VP, Jayasuriya, AC. Injectable chitosan microparticles incorporating bone morphogenetic protein-7 for bone tissue regeneration. J Biomed Mater Res 2014;102:4276–89. https://doi.org/10.1002/jbm.a.35100.Suche in Google Scholar PubMed PubMed Central
95. Song, X, Li, X, Wang, F, Wang, L, Lv, L, Xie, Q, et al.. Bioinspired protein/peptide loaded 3D printed PLGA scaffold promotes bone regeneration. Front Bioeng Biotechnol 2022;10. https://doi.org/10.3389/fbioe.2022.832727.Suche in Google Scholar PubMed PubMed Central
96. Kudaibergen, G, Mukhlis, S, Mukhambetova, A, Issabekova, A, Sekenova, A, Sarsenova, M, et al.. Repair of rat calvarial critical-sized defects using heparin-conjugated fibrin hydrogel containing BMP-2 and adipose-derived pericytes. Bioeng (Graefelf) 2024;11:437. https://doi.org/10.3390/bioengineering11050437.Suche in Google Scholar PubMed PubMed Central
97. Qi, J, Wu, H, Liu, G. Novel strategies for spatiotemporal and controlled BMP-2 delivery in bone tissue engineering. Cell Transplant 2024;33:. https://doi.org/10.1177/09636897241276733.Suche in Google Scholar PubMed PubMed Central
98. Yoon, SJ, Yoo, Y, Nam, SE, Hyun, H, Lee, DW, Um, S, et al.. The cocktail effect of BMP-2 and TGF-β1 loaded in visible light-cured glycol chitosan hydrogels for the enhancement of bone formation in a rat tibial defect model. Mar Drugs 2018;16:351. https://doi.org/10.3390/md16100351.Suche in Google Scholar PubMed PubMed Central
99. Teh, SW, Koh, AE, Tong, JB, Wu, X, Samrot, AV, Rampal, S, et al.. Hypoxia in bone and oxygen releasing biomaterials in fracture treatments using mesenchymal stem cell therapy: a review. Front Cell Dev Biol 2021;9. https://doi.org/10.3389/fcell.2021.634131.Suche in Google Scholar PubMed PubMed Central
100. Suvarnapathaki, S, Wu, X, Zhang, T, Nguyen, MA, Goulopoulos, AA, Wu, B, et al.. Oxygen generating scaffolds regenerate critical size bone defects. Bioact Mater 2022;13:64–81. https://doi.org/10.1016/j.bioactmat.2021.11.002.Suche in Google Scholar PubMed PubMed Central
101. Ejtehadifar, M, Shamsasenjan, K, Movassaghpour, A, Akbarzadehlaleh, P, Dehdilani, N, Abbasi, P, et al.. The effect of hypoxia on mesenchymal stem cell biology. Adv Pharmaceut Bull 2015;5:141–9. https://doi.org/10.15171/apb.2015.021.Suche in Google Scholar PubMed PubMed Central
102. Stegen, S, Stockmans, I, Moermans, K, Thienpont, B, Maxwell, PH, Carmeliet, P, et al.. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat Commun 2018;9:2557. https://doi.org/10.1038/s41467-018-04679-7.Suche in Google Scholar PubMed PubMed Central
103. Huang, YC, Zhu, HM, Cai, JQ, Huang, YZ, Xu, J, Zhou, Y, et al.. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J Cell Biochem 2012;113:1407–15. https://doi.org/10.1002/jcb.24014.Suche in Google Scholar PubMed
104. Sun, H, Xu, J, Wang, Y, Shen, S, Xu, X, Zhang, L, et al.. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact Mater 2023;24:477–96. Published 2023 Jan 9. https://doi.org/10.1016/j.bioactmat.2022.12.021.Suche in Google Scholar PubMed PubMed Central
105. Zhou, J, Li, Y, He, J, Liu, L, Hu, S, Guo, M, et al.. ROS scavenging graphene-based hydrogel enhances type H vessel formation and vascularized bone regeneration via ZEB1/notch1 mediation. Macromol Biosci 2023;23:e2200502. Epub 2023 Jan 22. PMID: 36637816. https://doi.org/10.1002/mabi.202200502.Suche in Google Scholar PubMed
106. Li, D, Chen, K, Tang, H, Hu, S, Xin, L, Jing, X, et al.. A logic-based diagnostic and therapeutic hydrogel with multistimuli responsiveness to orchestrate diabetic bone regeneration. Adv Mater 2022;34:e2108430. https://doi.org/10.1002/adma.202108430.Suche in Google Scholar PubMed
107. Sun, H, Xu, J, Wang, Y, Shen, S, Xu, X, Zhang, L, et al.. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact Mater 2023;24:477–96. https://doi.org/10.1016/j.bioactmat.2022.12.021.Suche in Google Scholar
108. Zhang, F, Lv, M, Wang, S, Li, M, Wang, Y, Hu, C, et al.. Ultrasound-triggered biomimetic ultrashort peptide nanofiber hydrogels promote bone regeneration by modulating macrophage and the osteogenic immune microenvironment. Bioact Mater 2024;31:231–46. https://doi.org/10.1016/j.bioactmat.2023.08.008.Suche in Google Scholar PubMed PubMed Central
109. Li, N, Liu, L, Wei, C, Ren, S, Liu, X, Wang, X, et al.. Immunomodulatory blood-derived hybrid hydrogels as multichannel microenvironment modulators for augmented bone regeneration. ACS Appl Mater Interfaces 2022;14:53523–34. https://doi.org/10.1021/acsami.2c16774.Suche in Google Scholar PubMed
110. Yang, SY, Zhou, YN, Yu, XG, Fu, ZY, Zhao, CC, Hu, Y, et al.. A xonotlite nanofiber bioactive 3D-printed hydrogel scaffold based on osteo-/angiogenesis and osteoimmune microenvironment remodeling accelerates vascularized bone regeneration. J Nanobiotechnol 2024;22:59. https://doi.org/10.1186/s12951-024-02323-9.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review
- Hydrogel promotes bone regeneration through various mechanisms: a review
- Research Articles
- Wear investigation of implant-supported upper removable prothesis with electroplated gold or PEKK secondary crowns
- Straight and helical plating with locking plates for proximal humeral shaft fractures – a biomechanical comparison under physiological load conditions
- Integration of neuromuscular control for multidirectional horizontal planar reaching movements in a portable upper limb exoskeleton for enhanced stroke rehabilitation
- Recognition analysis of spiral and straight-line drawings in tremor assessment
- Combination of edge enhancement and cold diffusion model for low dose CT image denoising
- High-performance breast cancer diagnosis method using hybrid feature selection method
- A multimodal deep learning-based algorithm for specific fetal heart rate events detection
Artikel in diesem Heft
- Frontmatter
- Review
- Hydrogel promotes bone regeneration through various mechanisms: a review
- Research Articles
- Wear investigation of implant-supported upper removable prothesis with electroplated gold or PEKK secondary crowns
- Straight and helical plating with locking plates for proximal humeral shaft fractures – a biomechanical comparison under physiological load conditions
- Integration of neuromuscular control for multidirectional horizontal planar reaching movements in a portable upper limb exoskeleton for enhanced stroke rehabilitation
- Recognition analysis of spiral and straight-line drawings in tremor assessment
- Combination of edge enhancement and cold diffusion model for low dose CT image denoising
- High-performance breast cancer diagnosis method using hybrid feature selection method
- A multimodal deep learning-based algorithm for specific fetal heart rate events detection


