Anti-arthritic potential of crude sulfated polysaccharide from marine macroalgae Sargassum ilicifolium (Turner) C. Agardh: Regulation of cytokine cascade
-
Lavanya Ramamoorthi
, Seethalakshmi Sankar
Abstract
Seaweeds have been utilized as food, fodder, fertilizer, and medicine since ancient times; nevertheless, they have received only a little attention. In the current work, we extracted the sulfated polysaccharide from a marine source and investigated its anti-arthritic potential in vivo. The isolated and freeze-dried polysaccharide was tested for acute oral toxicity based on OECD 423. This step was followed by investigations on clinical signs and gross pathological alterations seen. A complete Freund’s adjuvant-induced arthritis was used to test the in vivo activity in female Sprague–Dawley rats, which were divided into five groups: (1) normal control, (2) arthritic control, (3) methotrexate treatment (0.1 mg/kg), (4) crude sulfated polysaccharide (CSP) (5 mg/kg), and (5) CSP (10 mg/kg). CSP was from the marine brown algae Sargassum ilicifolium from the Gulf of Mannar. The body weight, paw volume, and biochemical markers (alanine aminotransferase, aspartate aminotransferase, creatinine, urea, and C-reactive protein levels) were also measured for each group coupled with histopathological and immunohistochemistry studies. The acute toxicity investigation indicated that the lethal dose of 50% (LD50) of the polysaccharide was more than 2,000 mg/kg. In addition, animals from the methotrexate and CSP (5 mg/kg, p.o.) groups had a substantial reduction in paw volume compared to other treatment groups. Methotrexate and CSP treatment dramatically decreased the levels of the investigated marker enzymes. Histopathology revealed that low-dose CSP (5 mg/kg, p.o.) significantly reduced the severity of synovitis, panniculitis, liver necrosis, inflammatory cell infiltration, and cortical and paracortical necrotic foci in node, compared to the high dose (10 mg/kg, p.o.). Immunohistochemical studies revealed that CSP (5 mg/kg) significantly inhibited pro-inflammatory cytokines such as tumor necrosis factor-alpha, interleukin-2, and CD4 cells. Overall, it can be concluded that a low-dose CSP (5 mg/kg) is an efficient anti-arthritic agent that confers its effects via the cytokine pathway.
Introduction
Chronic inflammatory synovitis, also known as arthritis, typically affects symmetrically distributed peripheral joints. Numerous causes, including chemical, biological, and immunological factors, contribute to its occurrence. Infiltration of CD4+ T cells and monocytes, increase in synovial lining cells and fibroblasts, neovascularization, and bone, as well as cartilage injuries are some of its distinguishing features [1]. The disease is mediated by pro-inflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) [2]. As the disease progresses, massive hypertrophy of synovium occurs with the invasion of fibrovascular tissue, known as pannus, which invades and destroys both the bone and cartilage further, leading to inflammation [3].
The acute phase is characterized by the presence of vascular permeability, increased blood flow, and recruitment of leukocytes with accumulation of inflammatory mediators such as cytokines. The chronic phase develops specific humoral and cellular immune responses to the pathogen at the tissue injury site. Of the cells involved in inflammation, some are present in the tissue itself, while others are recruited to the area of inflammation from the blood [4]. In both phases, the mediators upregulate cellular adhesion molecule expression, attracting leukocytes and controlling the activation of resident cells like fibroblasts, endothelial cells, tissue macrophages, and mast cells, as well as newly recruited inflammatory cells, including monocytes, lymphocytes, neutrophils, and eosinophils. These mediators, which are generated by macrophages, cause systemic reactions to the inflammatory process [5].
Inflammatory arthritis results from the influx of activated leukocytes into the joints to mediate the destruction seen. Typically, the pro-inflammatory cytokines and anti-inflammatory cytokines are effector phase cytokines. Native CD4 helper T-cells differentiate into T-helper 1 (Th1), the pro-inflammatory cytokines that participate in both acute and chronic inflammatory reactions as well as repair processes, and T-helper 2 (Th2) cells, the anti-inflammatory or regulatory cytokines that inhibit the inflammatory reactions [6]. In recent years, novel therapies designed against chronic inflammatory diseases like rheumatoid arthritis are targeted, decreasing the generation of auto-reactive Th1 helper cells or immune deviation in favor of Th2 cells. Therapeutic management of arthritis includes the use of non-steroidal anti-inflammatory agents like diclofenac, naproxen, ibuprofen, and disease-modifying anti-rheumatic drugs, such as methotrexate, leflunomide, sulfasalazine, and TNF-α inhibitor like etanercept. Although these drugs are effective anti-rheumatic agents, they produce severe side effects such as gastrointestinal bleeding, leucopenia, and hepatotoxicity [7,8], thus seeking new armamentariums.
Sulfated polysaccharides, particularly fructose-rich polysaccharides from brown algae, interact with P-selectin, possessing potent anti-inflammatory effects in a dose-dependent manner [9]. Similarly, it was reported in 2003 that sulfated polysaccharides from porphyridium can inhibit the movement and adhesion of polymorphonuclear leukocytes in vitro [10]. Additionally, the polysaccharide also inhibited the development of erythema in vivo and had excellent biolubricant properties that potentially can provide joint-lubricating solutions or as a substitute for hyaluronic acid to mitigate degenerative joint disorders imposed by arthritis [11,12]. Similarly, a sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura was reported to confer some anti-inflammatory activity, as seen in lipopolysaccharide (LPS)-stimulated human monocytic (THP-1) cells. Additionally, the polysaccharide exhibited significant anti-inflammatory effects acting via the toll-like receptor 4 signaling pathway [13]. Likewise, sulfated polysaccharides isolated from the edible brown seaweed, Sargassum fulvellum, were reported to have a good anti-inflammatory effect in the LPS-stimulated RAW 264.7 macrophages and zebrafish [14].
Crude sulfated polysaccharides (CSPs) are more cost-effective to extract and purify compared to highly refined sulfated polysaccharides. This is particularly important in large-scale applications and for maintaining economic viability in therapeutic settings [15]. CSP retains a complex mixture of polysaccharides, which can contribute to a broader spectrum of biological activities. This complexity can enhance the overall therapeutic efficacy, as different components of CSP may act synergistically to produce anti-inflammatory, immunomodulatory, and antioxidant effects [16]. Studies suggest that CSPs may have improved bioavailability compared to more refined polysaccharides. Due to its crude form, CSP tends to have a lower risk of side effects associated with overpurification and the presence of contaminants introduced during extensive refinement processes. This makes CSP a safer option for long-term therapeutic use [17].
Sulfated polysaccharides from Sargassum hemiphyllum have been reported to decrease ear swelling and erythema, decrease the production of myeloperoxidase, nitric oxide, IL-1β, IL-6, and TNF-α in a dose-dependent manner in arachidonic acid-induced ear inflammation [18]. However, no previous reports indicate the anti-inflammatory and anti-arthritic activities of fucose-containing sulfated polysaccharide from the brown seaweed Sargassum ilicifolium. Based on in vitro anti-inflammatory studies carried out by tissue plasminogen activator-induced inflammation on polymorphonuclear leucocytes, a sulfated polysaccharide from Sargassum ilicifolium was confirmed to possess significant anti-inflammatory [19] and free radical scavenging activities [20]. Additionally, it can inhibit the inflammatory cytokine TNF-α. Hence, an attempt was made to confirm the anti-arthritic activity of a CSP from Sargassum ilicifolium by complete Freund’s adjuvant (CFA)-induced arthritis.
Preclinical testing of anti-arthritic drugs frequently uses the rat adjuvant arthritis model, which is a trustworthy approach. The characteristics of this model include easy measurements, consistent commencement, and progression, as well as noticeable bone proliferation and resorption. It enables analysis of both the immediate inflammatory and immune responses that follow approximately 9 days later.
Methods and materials
Extraction of CSP from Sargassum ilicifolium
The extraction of polysaccharides from marine brown algae was based on the slightly modified method reported by Tako et al. [21]. Briefly, hydrochloric acid (0.05 M) was added to 10 g of dried algae, which was then agitated at room temperature for 2 h. The supernatant was filtered after centrifugation for 20 min at 3,575 rpm. The crude polysaccharide was then precipitated in two volumes of ethanol from the filtered fraction, followed by neutralization with sodium hydroxide (0.5 M). Finally, the CSP was freeze-dried after concentration in a rotary evaporator.
Acute oral toxicity study (OECD 423)
On day 0 (baseline), healthy female Sprague–Dawley rats (170 and 205 g) that were nulliparous and not pregnant were used. A step-by-step housing of the animals was conducted in a polypropylene cage with good ventilation and an artificial 12 h of light and dark photoperiod. The room was kept at a constant temperature of 22°C (±3°C) with a relative humidity of 53–60%. The animals received reverse osmosis-purified water (from Rios) and pelleted feed (from M/s. Provimi Animal Nutrition Pvt. Ltd., India) ad libitum.
Five days before dosing, the animals were housed in their cages to acclimatize to the laboratory environment. The animals underwent overnight fasting before receiving CSP and 3–4 h after. The experiment was conducted in two steps (Steps I and II). A stomach intubation needle was used in each phase to administer a dose of CSP (2,000 mg/kg) to three animals each. Observation of lethality and unusual clinical symptoms was done. The clinical symptoms were observed on the dosing day, at 30 min, 1, 2, and 4 h, as well as in the next 14 days. The body weights were measured right before dosage and weekly until the trial was over. After the observation period, significant pathological alterations were also observed [22].
Experimental animal and chemicals used for arthritic study
Three months old female Sprague–Dawley rats (150–200 g) were used in this study. CFA – 10 mg/mL (Chondrex, Inc.USA), CD4 (#SC7219, Santa Cruz, USA), interleukin-2 (IL-2) (#SC7896 Santa Cruz, USA), TGF-β (#SC-146, Santa Cruz), TNF-α (#SC52746 Santa Cruz), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, urea, and hs-CRP-kit (Bio-Systems Diagnostics Pvt. Ltd) were used.
The experimental design and animal grouping are shown in Table 1. CFA (0.1 mL) was injected in the sub-plantar region of the right hind paw on day 1. The dosing of the standard drug (methotrexate 0.1 mg/kg, i.p.) and CSP (5 and 10 mg/kg, p.o.) was started on day 9 and was continued up to day 28. The body weight and paw volume were measured at 7-day intervals for 28 days. At the end of the experimental period, blood samples (1 mL) were collected to investigate the biochemical parameters. The animals were euthanized using diethylene ether at the end of the experiment. Subsequently, the ankle joint with paw tissue of the hind limbs, liver, spleen, and popliteal lymph node (PLN) were used for histopathological studies. The ipsilateral PLN was used for the immunohistochemical study [23].
Experimental design
| Groups | Treatment |
|---|---|
| Group I | Normal control |
| Group II | Arthritic control |
| Group III | Methotrexate (0.1 mg/kg, i.p., alternate days) |
| Group IV | CSP (5 mg/kg, p.o.) |
| Group V | CSP (10 mg/kg, p.o.) |
CSP – crude sulfated polysaccharide.
Estimation of body weight
The rat’s body weight was recorded before and at 7-day intervals up to 28 days after inoculation of CFA. The difference in body weight on each day and at baseline was determined. Finally, the percentage change in body weight was calculated.
Estimation of paw volume
The volumes of injected hind paws of rats were measured before and at 7-day intervals after inoculation of CFA. Measurements were done by using a water displacement plethysmograph. Then, the percentage change in the paw volume was calculated.
Estimation of biochemical parameters
ALT, AST, creatinine, urea, and C-reactive protein (CRP) levels were estimated based on the recommended procedure provided in the biochemical kit.
Statistical analysis
Results are presented as mean ± SEM (standard error mean) from six animals per group. Statistical differences in the mean were analyzed by using a one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test. A p-value of <0.05 was deemed statistically significant. All statistical analyses were performed using GraphPad Prism Software 5 (Boston, MA, USA).
Histopathological analysis
A 10% neutral buffered formalin solution was used to fix the liver, spleen, ankle joints, paw, and PLN. The ankle joints with the paw were fixed in neutral buffered formalin for 24 h, followed by decalcification in 10% formic acid for 4 days. Once the decalcification process was completed, the digits were removed, and the ankle joints and paw were transected along the mid-sagittal plane. Representative samples of the spleen and liver tissues were removed and decalcified after 48 h. Subsequently, trimmed ankle joints and paw tissues were processed for paraffin embedment as well as sectioning and staining with hematoxylin and eosin before the microscopic analysis [24].
Immunohistochemical analysis
Tissue samples embedded in paraffin were deparaffinized in xylene and hydrated using alcohol. By using citrate buffer (pH 6.0) for TNF-α or Tris-EDTA buffer (pH 8.0) for CD4, IL-2, and TGF-β, heat-induced antigen retrieval was conducted in a microwave for 20 min at HI-90. Three washes in phosphate-buffered saline containing 0.05% tween 20 (PBST) were performed before each step as below. The first step involved quenching of endogenous peroxidase following incubation with 3% hydrogen peroxide for 30 min. Blocking was done subsequently for 30 min using 5% goat serum in 1% bovine serum albumin. The step was followed by overnight incubation of the primary antibody at 4°C. Then, the biotinylated secondary antibody was incubated for 30 min at room temperature and another 30 min in the presence of the enzyme avidin-biotin-peroxidase. The final step involved a counterstain with Harris hematoxylin for 30 s, where the 3,3′-diaminobenzidine (DAB) chromogen staining should take 10–15 min.
-
Ethical approval: The research related to animal use has complied with all the relevant national regulations and institutional policies for the care and use of animals. The Institutional Animal Ethical Committee (IAEC) of Sri Ramachandra University approved the acute oral toxicity study with the approval number IAEC/XXXVIII/SRU/351/14.
Results
Acute oral toxicity study
In the acute toxicity study of CSP on Sprague–Dawley rats, the animals were observed for lethality and abnormal signs (changes in the central and autonomic nervous systems as well as behavioral responses). The study confirmed the absence of treatment-related mortality, abnormal clinical signs, or remarkable body weight changes in both Steps I and II. No gross pathological observation was recorded in all experimental animals, suggesting that CSP was non-toxic. The 50% lethal dose (LD50) of CSP was greater than 2,000 mg/kg. Therefore, it was concluded that CSP should be classified under Globally Harmonized System hazard category 5 (Tables 2 and 3).
Body weight of experimental animals in the acute oral toxicity study
| Step/dose | No of animals | Body weight (g) | ||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | ||
| I (2,000 mg/kg) | 3 | 174.13 ± 2.49 | 183.33 ± 2.19 | 192.4 ± 4.31 |
| II (2,000 mg/kg) | 3 | 198.13 ± 5.54 | 207.73 ± 4.9 | 215.07 ± 2.3 |
“Step” refers to sequential groups of animals (each consisting of three animals) tested with the same dose of 2,000 mg/kg. The differences in body weight between Step I and Step II reflect natural biological variability among the animals in the study.
Gross pathological observation of individual animals
| Step/dose | Animal number | Organs | Observations |
|---|---|---|---|
| I (2,000 mg/kg) | H | Skin, eyes, brain, lungs, heart, liver, kidney, adrenals, spleen, and sex glands | No abnormality detected |
| B | |||
| T | |||
| II (2,000 mg/kg) | H | ||
| B | |||
| T |
Estimation of body weight
The body weight of all experimental groups was monitored throughout the study period. As depicted in Figure 1, no significant variations in body weight were observed among the groups, indicating that the treatments administered, including the standard (methotrexate) and CSP treatments, did not adversely affect the overall health and growth of the animals.

Effect of CSP on the rat’s body weight. Values are expressed as mean ± SEM; n = 6 animals. Significance was analyzed using two-way ANOVA, followed by Dunnett’s multiple comparisons test. Comparisons were made with the arthritic control group. *p < 0.05.
Estimation of paw volume
Paw swelling is an index for measuring anti-arthritic activity. In CFA-induced arthritis, the rats developed chronic swelling in multiple joints due to the presence of inflammatory cells. The inflammatory changes can ultimately result in the destruction of joint integrity and functions in the affected animal. In our study, CFA-administered rats showed only some soft tissue swelling around the ankle joints during the disease progression (Figure 2).

Paw edema in arthritic rats: (a) normal control, (b) arthritic control, (c) methotrexate treated, (d) CSP (5 mg/kg), and (e) CSP (10 mg/kg).
There was a significant increase in the paw volume of the arthritic control group that was induced with CFA on days 7, 14, 21, and 28 compared to that of the normal control group (Figure 3). Administration of CSP (5 and 10 mg/kg) both conferred a reduction in the paw volume on day 28 compared to the standard methotrexate, which showed maximum inhibition of paw volume on day 28 (Figure 4).
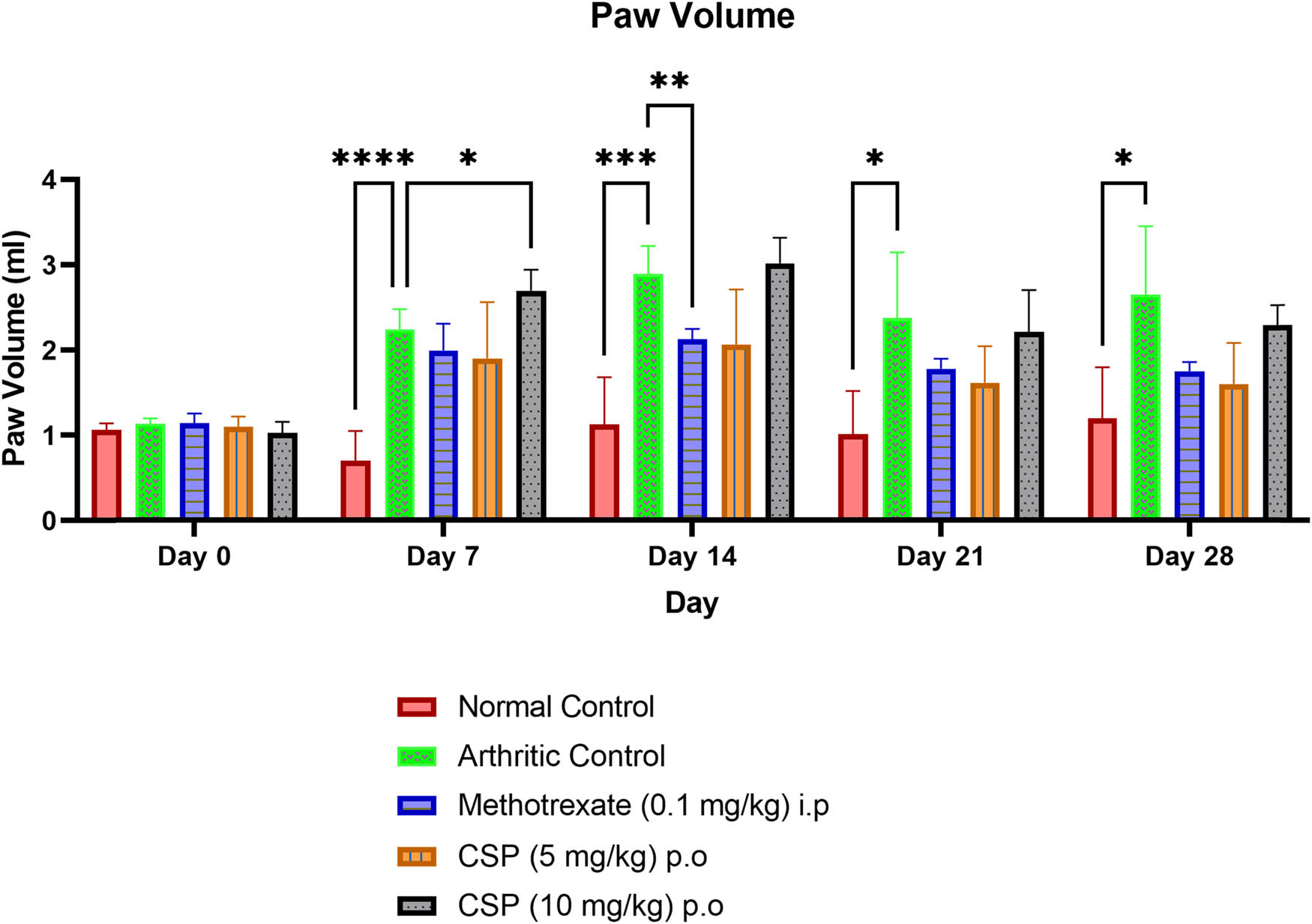
Effect of CSP on paw edema in arthritic rats. Values were expressed as mean ± SEM; n = 6 animals. Significance was analyzed using two-way ANOVA, followed by Dunnett’s multiple comparisons test. Comparisons were made with the arthritic control group. * p < 0.05, **p < 0.01, and ***p < 0.001.

Percentage inhibition of CSP on paw edema conferred by CSP (5 mg/kg) was the highest and the maximum on day 28. Values are expressed as mean ± SEM; n = 6 animals. Significance was analyzed using two-way ANOVA followed by Tukey’s multiple comparisons test. Comparisons were made with the arthritic control group.
Overall, the maximum % inhibition of paw volume was achieved in the CSP (5 mg/kg)-treated group followed by methotrexate (0.1 mg/kg) and CSP (10 mg/kg)-treated groups. When the paws were examined physically, again, the percentage inhibition of the paw was highest (39.55%) in the CSP (5 mg/kg)-treated group (Figure 4), indicating that CSP has the potential to act as an anti-inflammatory/anti-arthritic agent.
Estimation of biochemical parameters
In the present study, there was a significant (p < 0.05) increase in the ALT levels of the arthritic control group (61.67 U/L) compared to the normal control (Table 4). The changes seen were, however, significantly reversed by methotrexate (50.67 U/L) compared to the arthritic control. Similarly, a significant reduction in ALT levels of animals in the CSP (5 and 10 mg/kg)-treated groups (48.83 and 54.17 U/L, respectively) was also observed compared to the arthritic control. As expected, a significant (p < 0.05) elevation in the AST level was observed in the arthritic rats compared to the normal rats. Nevertheless, there was a significant reduction in the AST levels in animals from the CSP 5 mg/kg (p < 0.01), 10 mg/kg (p < 0.05), and methotrexate (p < 0.05)-treated groups compared to the arthritic control.
Effect of CSP on various biochemical parameters
| Group | Treatment | ALT (UL) | AST (UL) | Creatinine (mg/dL) | Urea (mg/dL) | CRP-hs (mg/dL) |
|---|---|---|---|---|---|---|
| I | Normal control | 45 ± 13.44 | 102.5 ± 29.44 | 0.58 ± 0.04 | 35.67 ± 7.4 | 2.33 ± 0.52 |
| II | Arthritic control | 61.67 ± 5.18a* | 156.00 ± 5.66a* | 0.85 ± 0.13a* | 51.33 ± 3.31a* | 3.49 ± 0.3a* |
| III | Methotrexate (0.1 mg/kg, i.p.) | 50.67 ± 7.06b* | 147.5 ± 11.96b* | 0.64 ± 0.02b* | 42.33 ± 2.9b* | 2.6 ± 0.25b** |
| IV | CSP (5 mg/kg, p.o.) | 48.83 ± 1.80b* | 113.50 ± 6.26b** | 0.62 ± 0.01b* | 44.0 ± 2.19b* | 3.1 ± 0.13b* |
| V | CSP (10 mg/kg, p.o.) | 54.17 ± 3.50b* | 135.17 ± 6.32b* | 0.60 ± 0.03b* | 48.0 ± 4.9 | 3.5 ± 0.23 |
Values are expressed in mean ± SEM; n = 6 animals. Significance was analyzed using a one-way ANOVA followed by Dunnett’s post hoc test. a) Comparisons were made between the normal control vs arthritic control groups. b) Comparisons were made between methotrexate (0.1 mg/kg) i.p., CSP (5 mg/kg) p.o., and CSP (10 mg/kg) p.o. with the arthritic control group. *p < 0.05, **p < 0.01, ns: not significant. CSP: crude sulfated polysaccharide, ALT: alanine aminotransferase, AST: aspartate aminotransferase, CRP hs: C-reactive protein (high sensitivity).
As expected, there was a significant increase in creatinine and urea levels in the arthritic control group compared to the normal control. However, there was a significant reduction in both creatinine and urea levels in the methotrexate and CSP (5 mg/kg)-treated group compared to the arthritic control. As for the CRP level, a significant (p < 0.05) elevation was observed in the arthritic rats compared to the normal rats. However, the CRP level was significantly decreased by administration of CSP at 5 mg/kg (p < 0.05) and 0.1 mg/kg methotrexate (p < 0.001) compared to the arthritic rats.
Histopathological analysis
The hematoxylin and eosin staining results of the (1) liver and ankle joints sections, (2) paw tissue, (3) spleen, and (4) popliteal node are shown in Figures 5–8, respectively. The rat’s liver from the normal control group revealed no changes, with normal histology of hepatocytes, central veins, and portal triads seen. Additionally, the ankle joint and the paw tissues from this group revealed normal histology with no signs of arthritis and associated lesions. The PLN from normal control rats revealed normal architecture, cellularity, and cortex-to-medulla ratio. The findings from the arthritic control rats, however, showed a moderate degree of hepatic necrosis with a mixed population of inflammatory cells (neutrophils and mononuclear cells) infiltration along with a moderate degree of perivascular and periportal mononuclear cell infiltration observed in the liver. The ankle joint and paw tissues of rats from the CFA-induced group revealed a severe degree of panniculitis and muscular necrosis, as characterized by infiltration of mononuclear and polymorph cells. A severe degree of synovitis characterized by the loss of trabeculae, hyperplasia, and proliferation of synovial cells with infiltration by inflammatory cells (polymorphonuclear cells, multinucleated giant cells, and mononuclear cells) was also observed. On the other hand, a moderate degree of articular cartilage erosion and synovial invasion with mononuclear cell infiltration in the eroded lesions, along with osteoclast cell infiltration in the bone components, were seen in the arthritic control group. Paw tissues revealed edema and severe infiltration by a mixed population of inflammatory cells (neutrophils and mononuclear cells) in the subcutaneous tissues. The PLN from the arthritic control rats revealed moderate to marked enlargement and distorted nodal architecture. Frequent cortical necrotic foci, marked hyperplasia of lymphocytes in B- and T-cell areas, as well as marked plasmacytosis in the paracortical and medullary regions, were observed in the CFA-induced arthritic control group. Occasionally, apoptotic bodies were noticed in germinal centers.

Histopathology findings of the liver by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control; (b) arthritic control showing hepatic necrosis with infiltration of periportal mononuclear cells; (c) methotrexate control showing mild hepatic necrosis and periportal infiltration; (d) CSP (5 mg/kg) treated showing minimal periportal infiltration; and (e) CSP (10 mg/kg) treated showing only mild necrosis.

Histopathology findings of the ankle joints by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control showing joints with synovium, (b) arthritic control showing bone erosion and synovitis, (c) methotrexate treated showing joint and synovium, (d) methotrexate treated showing panniculitis, (e) CSP (5 mg/kg) treated showing a moderate synovial proliferation to phalanges, and (f) CSP (10 mg/kg) treated showing some synovitis.

Histopathology findings of the spleen by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. The spleen from all groups did not reveal any treatment-related lesions. (a) Normal control, (b) arthritic control, (c) methotrexate control, (d) CSP (5 mg/kg), and (e) CSP (10 mg/kg).

Histopathology findings of PLN by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control showing normal nodal architecture; (b) arthritic control showing a frequent cortical necrotic foci; (c) methotrexate control showing frequent, severe coalescing necrotic foci, vacuolations in cortical and paracortical regions; (d) CSP (5 mg/kg) showing hyperplastic B-cell areas and several vacuolations in the cortical and paracortical regions; and (e) CSP (10 mg/kg) showing frequent and severe cortical and paracortical necrotic foci.
Methotrexate-treated group rats showed only mild hepatic necrosis and perivascular and periportal infiltration compared to the CFA-induced group. The ankle joint and paw tissues of rats from the methotrexate group revealed only mild to moderate degrees of synovitis and inflammatory cell infiltration, panniculitis, as well as articular cartilage erosions. A moderate degree of paw tissue inflammatory cells (mononuclear and polymorphs) infiltration was also seen. The PLN of rats from the methotrexate-treated group revealed (1) moderate to marked enlargement and distorted nodal architecture, (2) an increased number of apoptotic bodies in germinal centers, (3) moderate lymphoid hyperplasia and plasmacytosis, (4) frequent severe coalescing necrotic foci and vacuolations in the cortical and paracortical regions, (5) marked decrease in cellularity in the cortical and paracortical regions, and (6) lymphoid atrophy.
The liver of rats treated with CSP (5 mg/kg) revealed a minimal degree of necrotic foci with inflammatory cells (mononuclear cells), the absence of fatty vacuolations, a minimal degree of perivascular and periportal inflammatory infiltration, whereas the rats treated with CSP (10 mg/kg) revealed a mild degree of fatty vacuolations, a moderate degree of necrotic foci with inflammatory cell infiltration and periportal infiltration. In comparison, the ankle joint and the paw tissues of rats treated with CSP (5 mg/kg) showed a reduced severity of synovitis, panniculitis, subcutaneous inflammation, inflammatory cell infiltration in the skeletal muscles and paw tissue, and bony erosions and infiltrations compared to animals that received the higher dose CSP (10 mg/kg), indicating the low dose to be the optimal dose.
PLN, the draining node of the CFA-inoculated rat paw, treated with 5 and 10 mg/kg CSP revealed moderate enlargement and a distorted nodal architecture, with some occasional apoptotic bodies in the germinal centers, a moderate plasmacytosis and marked B-cell hyperplasia. On the other hand, the CSP (5 mg/kg)--treated group showed low numbers of cortical/paracortical necrotic foci. Severe cortical/paracortical necrotic foci were observed in nodes of rats treated with CSP (10 mg/kg). The rats’ spleen for all groups did not reveal any treatment-related lesions and remained similar to the normal control group.
Immunohistochemical analysis
Immunohistochemistry is based on the principle of antibodies binding to specific antigens in biological tissues, thereby making it possible to detect antigens in tissues. It is widely used in studies to understand the distribution of biomarkers and expressed proteins in biological tissues. Briefly, an antibody is conjugated with an enzyme that catalyzes the color-producing reaction and the antibody can be tagged to a fluorophore. The immunohistochemistry analysis for this study was carried out on the ipsilateral PLN of CFA-induced arthritis, drug-treated, and non-treated groups.
The number of CD4 cells was higher in the CFA-induced arthritic control group compared to methotrexate- and CSP-treated groups (Figure 9), indicating the effectiveness of CSP in both doses (5 and 10 mg/kg) in inhibiting CD4 cells to prevent the release of pro-inflammatory cytokines. Additionally, there was a higher expression of IL-2 in the CFA-induced arthritic control group (Figure 10) compared to methotrexate- and CSP-treated groups, which showed only a moderate expression. IL-2 is a pro-inflammatory cytokine released during chronic inflammatory conditions like rheumatoid arthritis. The immunohistochemical analysis of IL-2 reveals that both CSP doses can inhibit the release of IL-2, thereby preventing IL-2-mediated inflammatory reactions.

Immunohistochemical analysis of CD4 by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control; (b) arthritic control showing a higher number of cells; (c) methotrexate control showing fewer number of cells; (d) CSP (5 mg/kg) showing a lower number of cells; and (e) CSP (10 mg/kg) also showing a lower number of cells.

Immunohistochemical analysis of IL2 by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control; (b) arthritic control showing a marked expression; (c) methotrexate control showing a moderate expression; (d) CSP (5 mg/kg) showing a mild expression; and (e) CSP (10 mg/kg) showing a mild expression.
There was a marked expression of TNF-α, a pro-inflammatory cytokine, in the CFA-induced arthritic control group compared to methotrexate- and CSP-treated groups (Figure 11). The inhibition of TNF-α expression by both doses of CSP indicates the role of CSP in preventing the degradation of bone and cartilage. Immunohistochemical analysis of TGF-β indicates that the anti-inflammatory cytokine (Figure 12) was expressed higher in groups treated with CSP (5 mg/kg). In comparison, a mild expression was seen in the 10 mg/kg CSP and methotrexate-treated groups, although there was no significant expression of TGF-β in arthritic control rats, indicating that CSP (5 mg/kg) has a good potential as an anti-arthritic agent that should be investigated further in clinical studies.
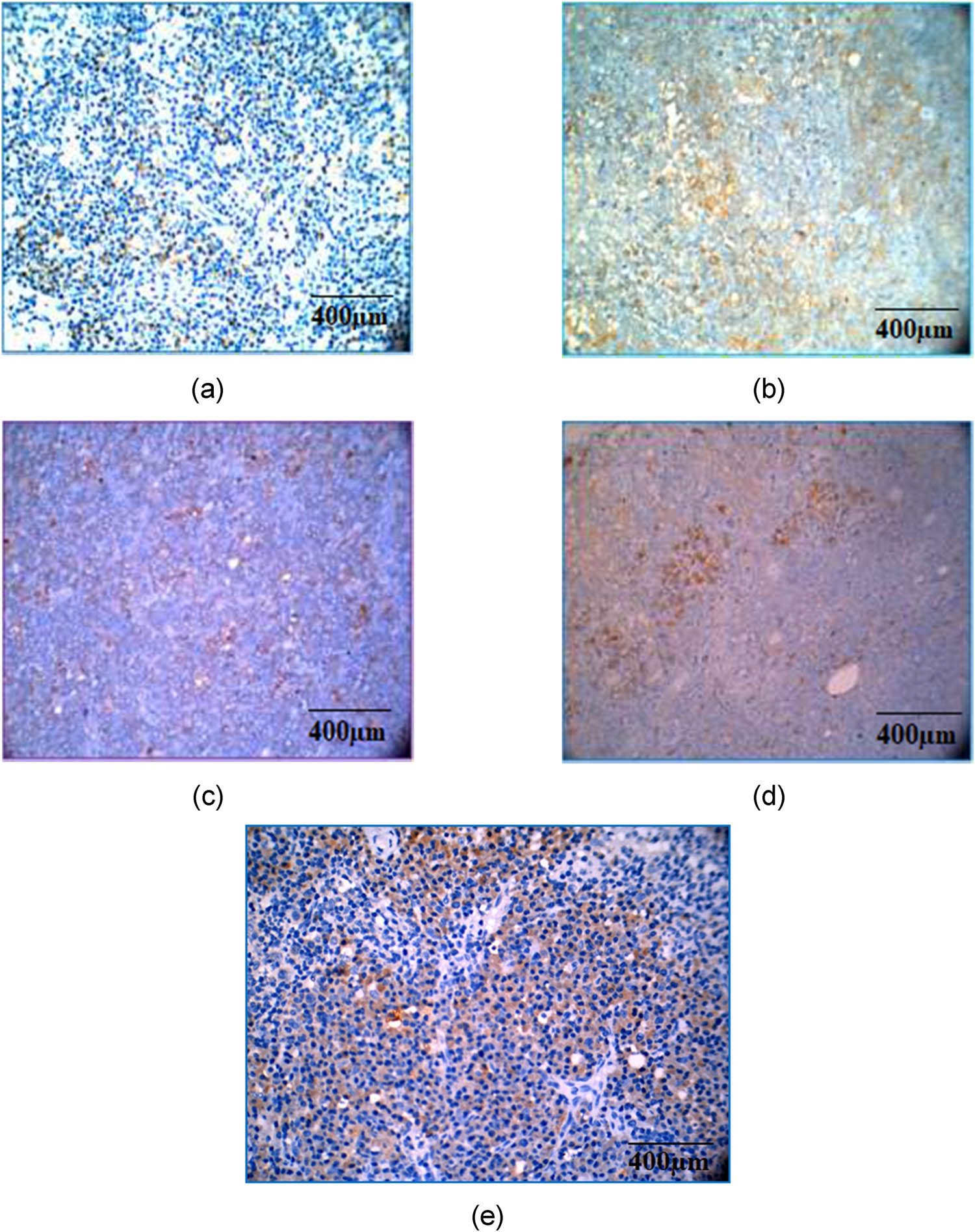
Immunohistochemical analysis of TNF-α by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control; (b) arthritic control showing a marked expression; (c) methotrexate control showing a moderate expression; (d) CSP (5 mg/kg) showing a mild expression; and (e) CSP (10 mg/kg) showing a mild expression.
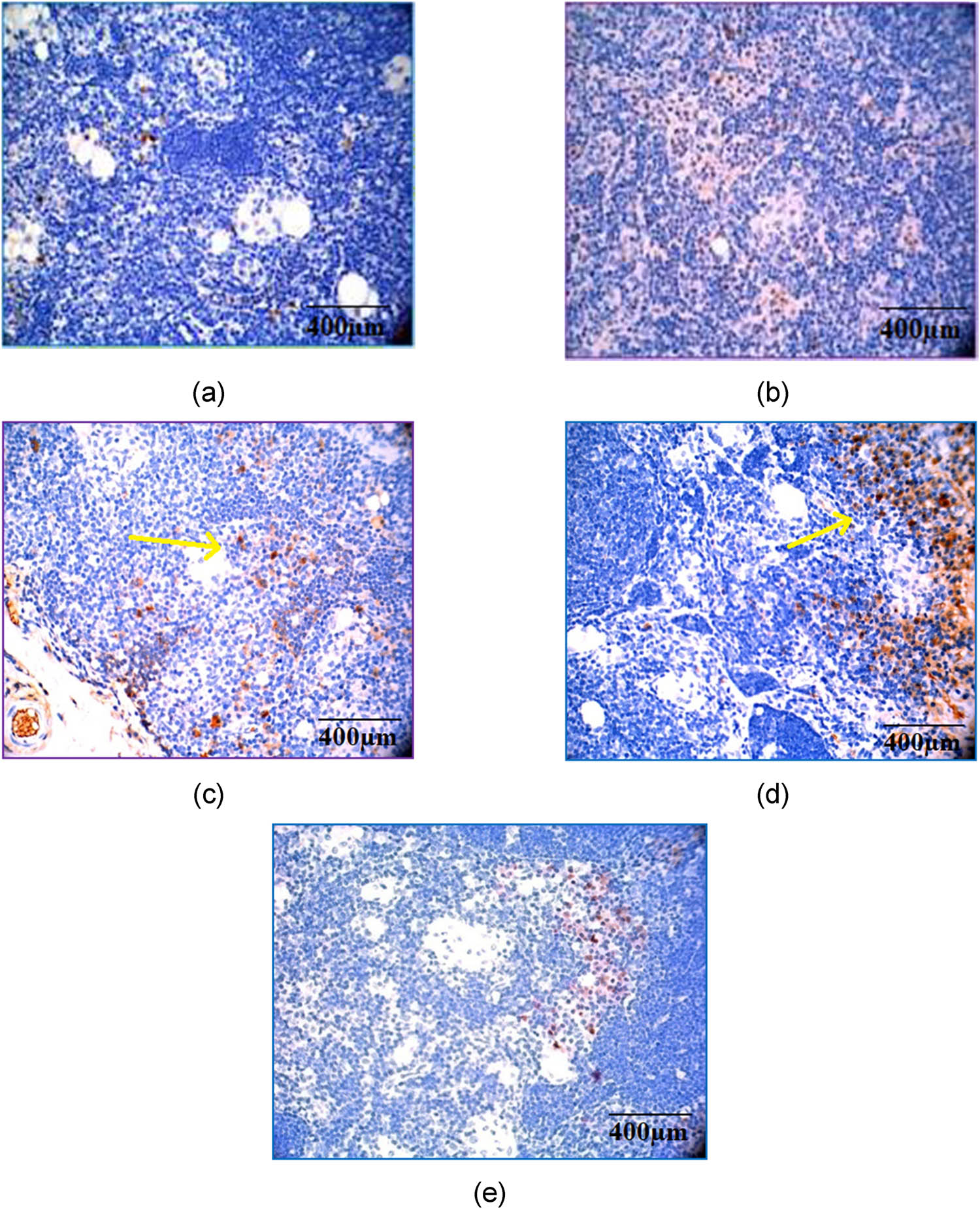
Immunohistochemical analysis of TGF-β by hematoxylin and eosin staining. Magnification and scale bar: 40× corresponds to 400 µm of different experimental groups. (a) Normal control; (b) arthritic control; (c) methotrexate control showing a moderate expression; (d) CSP (5 mg/kg) showing a marked expression; and (e) CSP (10 mg/kg) showing a mild expression.
Discussion
To our knowledge, this is the first study to confirm the anti-inflammatory effects of CSP from Sargassum ilicifolium in an in vivo model. Adjuvant-induced arthritis in rats is a chronic inflammatory model that resembles human rheumatoid arthritis and is useful in the development of potential anti-inflammatory drugs for the treatment of arthritis. At the beginning of the study, the safety profile of CSP was confirmed by an acute oral toxicity model as per OECD-423 guidelines. The isolated CSP was found to be safe (up to 2,000 mg/kg). Subsequently, the anti-arthritic activity of CSP evaluated by the CFA-induced arthritic model confirmed that a low-dose CSP showed a significant reduction in paw volume comparable with the standard drug methotrexate.
In our study, there was a significant increase in the marker enzymes in the animals from the arthritis-induced non-treated groups that were significantly ameliorated by methotrexate and CSP. ALT and AST are enzymes present in the liver and the heart cells that are released into the blood upon damage to the liver or the heart. Urea and creatinine are products of protein metabolism excreted by the kidney, with increased levels indicating kidney damage. In this study, elevated levels of blood urea and creatinine in the arthritic control group indicate some kidney dysfunction, while animals in both the methotrexate and CSP-treated groups showed a reduction in urea and creatinine levels, indicating that CSP is renoprotective.
CRP is a non-specific sensitive marker of inflammation that responds rapidly to the underlying inflammatory disease and is a general measure of disease activity in rheumatoid arthritis. Inflammation of the synovium causes the release of pro-inflammatory cytokines like TNF-α and IL-1β, which in turn leads to the release of IL-6 that stimulates the liver to secrete CRP [2,25], the level of which can be correlated with paw edema in arthritic rats. Nevertheless, the marked reduction of CRP by CSP (5 mg/kg) in arthritic rats strongly indicated its anti-inflammatory role.
Based on the histopathological studies of the tibiotarsal joints of CFA-injected rats, there were severe proliferative synovitis and infiltration of multifocal mononuclear cells with a hyperplastic synovial membrane in the arthritic control animals seen. The disease severity is further indicated by pannus invasion with severe cartilage degradation, bone erosion, severe necrosis of the liver, and coalescing necrotic foci, as well as vacuolations in the popliteal node seen. These changes were significantly less severe in animals treated with CSP and methotrexate than those observed in non-treated adjuvant-injected rats. Interestingly, rats treated with a low-dose CSP (5 mg/kg) showed a significant reduction in the severity of peri-articular inflammation with less infiltration of leukocytes and mild synovitis similar to those seen in a methotrexate-treated group, thus suggesting that CSP (5 mg/kg) can ameliorate the pathological progression of arthritis. Similarly, the liver of arthritic rats treated with 5 mg/kg CSP showed minimal necrotic foci compared to groups treated with 10 mg/kg CSP and methotrexate, which had a marked liver necrosis, indicating its hepatoprotective effect. Our findings suggest that CSP (5 mg/kg) may be an effective alternative to the existing anti-arthritic drugs, especially for individuals with pre-existing liver problems.
A network of interdependent cytokines is involved in the pathogenesis of inflammatory synovitis and rheumatoid joint destruction. TNF-α, a pro-inflammatory cytokine occupying the primary position in cytokine cascade upregulating the production of other cytokines, plays an important role in cartilage and bone degradation [26,27]. Its expression by monocyte/macrophages has been demonstrated to occur in both synovial tissue and at the cartilage-pannus junction [28]. The immune system attempts to limit inflammation and joint destruction by the homeostatic mechanism that exists in the rheumatoid joints. An initial burst of pro-inflammatory cytokines like TNF-α, IL-1, IL-6, and GM-CSF is normally followed by an increase in IL-10 synthesis [18,29]. Based on our anti-arthritic experiment on CSP, it was confirmed that CSP, especially in low doses (5 mg/kg), was more effective and may ameliorate the immune system by inhibiting the pro-inflammatory cytokines (like TNF-α and IL-2) as well as the expression of the anti-inflammatory or regulatory cytokine TGF-β. It is interesting to note from previously reported studies that the bioactivity of sulfated polysaccharides is due to their unique ability to influence the body’s regulatory systems in restoring homeostasis (balance). For this, it not only acts as an antioxidant and anticoagulant but also acts as a pro-oxidant and procoagulant [7,8,30].
In the present study, CSP behaves slightly like a hormetic agent, as it shows protective action (anti-inflammatory) at low doses and mild opposite action at high doses. This type of biphasic dose response has been demonstrated for many naturally derived phytochemicals [31], like quercetin, curcumin, and resveratrol [32,33,34]. A similar type of optimal concentration for immunostimulatory crude polysaccharide from a mollusk Chlamys farreri on phagocytic activity of RAW264.7 cells was reported by Shi et al., in which there was a slight decline in activity at high doses [35]. Further studies are required to elucidate the mechanism of this biphasic dose response.
In all of the in vivo studies conducted for CSP, both doses (5 and 10 mg/kg) were effective in controlling the inflammatory process compared to arthritic control. A slight decrease in the activity was observed with the high dose (10 mg/kg) compared to the low dose, suggesting the low dose (5 mg/kg) of CSP to be the optimal or effective concentration, as confirmed by all investigated parameters (the changes in the biochemical parameters and histopathological and immunohistochemistry analysis).
It is plausible that CSP (5 mg/kg) effectively reduces the inflammatory process by downregulating the cytokine cascade, subsequently reducing the TNF-α expression, as well as cell infiltration in the synovial membrane and confirming its protective effects by preventing cartilage and bone degradation.
The limitation of this study is the absence of purification and structural elucidation of the bioactive isolated sulfated polysaccharide. Furthermore, additional investigations are warranted to assess the pharmacokinetic properties and safety profile of the isolated sulfated polysaccharide. Finally, the development of a suitable formulation with the isolated sulfated polysaccharide is also crucial for its potential clinical application.
Conclusion
The efficacy and optimum dose for the anti-arthritic potential of the lead molecule sulfated polysaccharide from the selected algae Sargassum ilicifolium were established and reported for the first time following identification through inflammatory pathways. The isolated CSP was found to be safe (up to 2,000 mg/kg). Based on our findings, it can be concluded that the low-dose CSP has a good anti-arthritic potential and mediates its effect through the cytokine pathway.
Acknowledgments
All the authors of this manuscript are thankful to their respective Departments/Universities for successful completion of this study.
-
Funding information: The Article Processing Charge (APC) was kindly funded by INTI International University, Malaysia.
-
Author contributions: Conceptualization: L.R. and S.S.; methodology, investigation, and resources: L.R., S.S., C.D., S.J., and L.S.W.; data curation: L.R., S.S., C.D., S.J., and L.S.W.; writing – original draft preparation: L.R., S.S., C.D., S.J., and L.S.W.; writing – review and editing: L.R., S.S., M.Y.B., C.D., S.J., M.S, and L.S.W.; supervision: L.R., S.S., C.D., and S.J.; and project administration: L.R., S.S., C.D., and S.J. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Zhou J, Wang F, Qu Y, Sun H, Liu Z. Lesions of bones and joints associated with rheumatism. In: Li H, Pan S, Zhou J, editors. Radiology of infectious and inflammatory diseases – Volume 5. Singapore: Springer; 2022. 10.1007/978-981-16-5003-1_9.Search in Google Scholar
[2] Shehu S, Kurya AU, Sharma DC. Role of inflammatory cytokines in the pathogenesis of rheumatoid arthritis and novel therapeutic targets. Asian J Immunol. 2020;3:37–46.Search in Google Scholar
[3] Schuster R, Rockel JS, Kapoor M, Hinz B. The inflammatory speech of fibroblasts. Immunol Rev. 2021;302:126–46. 10.1111/imr.12971.Search in Google Scholar PubMed
[4] Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22:10–8. 10.1038/s41590-020-00816-x.Search in Google Scholar PubMed PubMed Central
[5] Colunga-Pedraza IJ, Galarza-Delgado DA, Guajardo-Jauregui N, Cardenas-de la Garza JA, Garcia-Arellano G, Arvizu-Rivera RI, et al. Association of soluble cell adhesion molecules and lipid levels in rheumatoid arthritis patients. Clin Rheumatol. 2023;42:731–9.10.1007/s10067-022-06395-6Search in Google Scholar
[6] Yap HY, Tee SZ, Wong MM, Chow SK, Peh SC, Teow SY. Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells. 2018;7:161. 10.3390/cells7100161.Search in Google Scholar PubMed PubMed Central
[7] Benjamin O, Goyal A, Lappin SL. Disease modifying anti-rheumatic drugs (DMARD). In StatPearls. Treasure Island (FL): StatPearls Publishing; 2018. PMID: 29939640.Search in Google Scholar
[8] Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, Colls J, et al. Adverse effects of low-dose methotrexate: a randomized trial. Ann Intern Med. 2020;172:369–80. 10.7326/M19-3369.Search in Google Scholar PubMed PubMed Central
[9] Wu S, Zhang X, Liu J, Song J, Yu P, Chen P, et al. Physicochemical characterization of Sargassum fusiforme fucoidan fractions and their antagonistic effect against P-selectin-mediated cell adhesion. Int J Biol Macromol. 2019;133:656–62. 10.1016/j.ijbiomac.2019.03.218.Search in Google Scholar PubMed
[10] Li S, Guo W, Zhang M, Zeng M, Wu H. Microalgae polysaccharides exert antioxidant and anti-inflammatory protective effects on human intestinal epithelial cells in vitro and dextran sodium sulfate-induced mouse colitis in vivo. Int J Biol Macromolecules. 2024 Jan;254:127811. 10.1016/j.ijbiomac.2023.127811.Search in Google Scholar PubMed
[11] De Jesus Raposo MF, De Morais RM, de Morais AM. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs. 2013 Jan;11(1):233–52. 10.3390/md11010233.Search in Google Scholar PubMed PubMed Central
[12] Arad S, Rapoport L, Moshkovich A, Van-Moppes D, Karpasas M, Golan R, et al. Superior Biolubricant from a Species of Red Microalga. Langmuir. 2006;22:7313–7. 10.1021/la060600x.Search in Google Scholar PubMed
[13] Cui M, Wu J, Wang S, Shu H, Zhang M, Liu K, et al. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int J Biol Macromol. 2019;129:377–85.10.1016/j.ijbiomac.2019.02.043Search in Google Scholar
[14] Wang L, Yang HW, Ahn G, Fu X, Xu J, Gao X, et al. In vitro and in vivo anti-inflammatory effects of sulfated polysaccharides isolated from the edible brown seaweed, Sargassum fulvellum. Mar Drugs. 2021;19:277. 10.3390/md19050277.Search in Google Scholar PubMed PubMed Central
[15] Li H-B, Cheng K-W, Wong C-C, Fan K-W, Chen F, Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007;102:771–6. 10.1016/j.foodchem.2006.06.022.Search in Google Scholar
[16] Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006;44:1144–50. 10.1016/j.fct.2006.02.002.Search in Google Scholar PubMed
[17] Rioux L-E, Turgeon SL, Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym. 2007;69:530–7. 10.1016/j.carbpol.2007.01.009.Search in Google Scholar
[18] Hwang PA, Hung YL, Chien SY. Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J Food Drug Anal. 2015;23:49–56. 10.1016/j.jfda.2014.05.004.Search in Google Scholar PubMed PubMed Central
[19] Lavanya R, Lakshmi SS, Chamundeeswari D, Gopal V. Invitro anti-inflammatory activity of Sargassum ilicifolium by HRBC membrane stabilization method. Indian Hydrobiol. 2012;14:99–101.Search in Google Scholar
[20] Lavanya R, Seethalakshmi S, Gopal V, Chamundeeswari D. Effect of crude sulfated polysaccharide from marine brown algae in TPA induced inflammation on polymorphonuclear leukocytes. Int J Pharm Pharm Sci. 2015;7:100–2.Search in Google Scholar
[21] Tako M, Uehara M, Kawashima Y, Chinen I, Hongo F. Isolation and identification of Fucoidan from Okinawamozuku (Cladosiphon okamuranus Tokida). J Appl Glycosci. 1996;43(2):143–8.Search in Google Scholar
[22] Murwanti R, Nurrochmad A, Gani AP, Sasmito E, Edwina AE, Chandra MK, et al. Acute and subchronic oral toxicity evaluation of herbal formulation: Piper crocatum Ruiz and Pav., Typhonium flagelliforme (Lodd.) Blume, and Phyllanthus niruri L. in Sprague–Dawley Rats. J Toxicol. 2023;2023(1):7511397. 10.1155/2023/7511397.Search in Google Scholar PubMed PubMed Central
[23] Tuncel J, Haag S, Hoffmann MH, Yau AC, Hultqvist M, Olofsson P, et al. Animal models of rheumatoid arthritis (I): pristane-induced arthritis in the rat. PLoS One. 2016 May;11(5):e0155936. 10.1371/journal.pone.0155936.Search in Google Scholar PubMed PubMed Central
[24] Li Y, Li N, Yu X, Huang K, Zheng T, Cheng X, et al. Hematoxylin and eosin staining of intact tissues via delipidation and ultrasound. Sci Rep. 2018 Aug;8(1):12259. 10.1038/s41598-018-30755-5.Search in Google Scholar PubMed PubMed Central
[25] Dessie G, Tadesse Y, Demelash B, Genet S, Malik T, Asmamaw Dejenie T. Evaluation of C-reactive protein and associated factors among patients suffering from rheumatoid arthritis at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Open Access Rheumatol. 2021;13:247–55. 10.2147/OARRR.S325308.Search in Google Scholar PubMed PubMed Central
[26] Kondo N, Kuroda T, Kobayashi D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2021 Oct;22(20):10922. 10.3390/ijms222010922.Search in Google Scholar PubMed PubMed Central
[27] Bedeković D, Bošnjak I, Šarić S, Kirner D, Novak S. Role of Inflammatory cytokines in rheumatoid arthritis and development of atherosclerosis: A review. Medicina. 2023 Aug;59(9):1550. 10.3390/medicina59091550.Search in Google Scholar PubMed PubMed Central
[28] Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021 Mar;22(5):2719. 10.3390/ijms22052719.Search in Google Scholar PubMed PubMed Central
[29] Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023 Jun;14:1161067. 10.3389/fimmu.2023.1161067.Search in Google Scholar PubMed PubMed Central
[30] Mukhamejanov E, Kurilenko V. Fucoidan: A nutraceutical for metabolic and regulatory systems homeostasis maintenance. Na J Adv Res Rev. 2020;6:255–64. 10.30574/wjarr.2020.6.1.0106.Search in Google Scholar
[31] Jodynis-Liebert J, Kujawska M. Biphasic dose-response induced by phytochemicals: experimental evidence. J Clin Med. 2020;9:718. 10.3390/jcm9030718.Search in Google Scholar PubMed PubMed Central
[32] Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–505. 10.1074/jbc.M708373200.Search in Google Scholar PubMed PubMed Central
[33] Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A. Hormetic effects of curcumin: What is the evidence? J Cell Physiol. 2019;234:10060–71. 10.1002/jcp.27880.Search in Google Scholar PubMed
[34] Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol. 2010;15:e134–8.Search in Google Scholar
[35] Shi F, Liu Z, Liu Y, Cheong K-L, Teng B, Khan BM, et al. Comparison of physicochemical characteristics and macrophage immunostimulatory activities of polysaccharides from Chlamys farreri. Mar Drugs. 2020;18:429. 10.3390/md18080429.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Anti-arthritic potential of crude sulfated polysaccharide from marine macroalgae Sargassum ilicifolium (Turner) C. Agardh: Regulation of cytokine cascade
- Bio-based hydrogel patches made of κ-carrageenan enriched with degalactosylated xyloglucan for wound dressing applications
- Clinical spectrum of COVID-19 patients and decreased serum level of miR-146a as a sign of inflammation
- Review Articles
- Quest for space: Tenacity of DNA, Protein, and Lipid macromolecules in intracellular crowded environment
- The impact of exercise on mitochondrial biogenesis in skeletal muscle: A systematic review and meta-analysis of randomized trials
- Communication
- Entropy and mechanistic concepts after intraovarian platelet-rich plasma: Experimental considerations for local tissue responses mediated by NF-κB and TNF-α
Articles in the same Issue
- Research Articles
- Anti-arthritic potential of crude sulfated polysaccharide from marine macroalgae Sargassum ilicifolium (Turner) C. Agardh: Regulation of cytokine cascade
- Bio-based hydrogel patches made of κ-carrageenan enriched with degalactosylated xyloglucan for wound dressing applications
- Clinical spectrum of COVID-19 patients and decreased serum level of miR-146a as a sign of inflammation
- Review Articles
- Quest for space: Tenacity of DNA, Protein, and Lipid macromolecules in intracellular crowded environment
- The impact of exercise on mitochondrial biogenesis in skeletal muscle: A systematic review and meta-analysis of randomized trials
- Communication
- Entropy and mechanistic concepts after intraovarian platelet-rich plasma: Experimental considerations for local tissue responses mediated by NF-κB and TNF-α

