Use of external quality assessment in extra-analytical phases in clinical laboratories in Spain: a survey by the Spanish Society of Laboratory Medicine (SEQCML)
-
Andrea Caballero Garralda
, Immaculada Comas Reixach
, Carlos García Miralles
, Rubén Gómez Rioja
, María Antonia Llopis Díaz
, Débora Martínez Espartosa
, Reyes Nicolás de Blas
, Mariona Panadès Turró
, Laura Rodelgo Jiménez
and Emma Ventura Orriols
ABSTRACT
Objectives
Quality indicators (QIs) are a valuable tool for monitoring and improving extra-analytical phase performance in the clinical laboratory (CL). For that reason, the Spanish Society of Laboratory Medicine (SEQCML) launched its Pre-analytical Phase EQA Program in 2001.
Methods
In 2023, a survey was conducted to assess the level of participation in the EQA Program, identify its potential improvements, and enhance the understanding of the collection and estimation processes of the extra-analytical QI.
Results
In total, 51 % of respondents (124 responses in total) were enrolled in the program. The primary reason for participating was the need of monitoring of the extra-analytical phases (38 %). In contrast, the main reason for not taking part in the EQA Program was the difficulty in collecting data (32 %). As to potential points for improvement, 56 % considered that QI should be analyzed disaggregated by type of extraction (ambulatory vs. urgent). As many as 86 % of respondents would be interested in participating in a post-analytical phase EQA Program. Most of the suggestions about indicators to be included were related to events regarding temperature and transport times from peripheral extraction sites.
Conclusions
Although interest in QIs for pre- and post-analytical phases is growing, the percentage of laboratories that collect data regularly is still limited. The findings of this survey have led the future directions for the EQA Pre-analytical Program, updating and adapting it to the current needs of CLs.
Introduction
Most errors in the clinical laboratory (CL) occur in the pre- and post-analytical phases of the total testing process (TTP), which jeopardizes patient safety [1]. Although the concept of brain-to-brain loop was first described over 43 years ago [2], it has not been until recently that awareness and consensus about the impact of extra-analytical phases on laboratory results became widespread [3], 4]. Continuous monitoring and assessment of the entire TTP are essential for quality laboratory results to be obtained [5].
The implementation of an internal quality monitoring system [6] and participation in external quality assurance (EQA) programs are crucial for continuous improvement [7]. The integration of quality indicators (QI) in ongoing improvement schemes has been recognized to be a useful tool for monitoring and improving extra-analytical performance [8], 9]. According to the ISO 15189:2023 standard, the identification and use of QI throughout the TTP is an essential requirement for accreditation [10], including the use of QI to assess performance in pre- and post-analytical phases [11].
For that reason, in 2001, the Spanish Society of Laboratory Medicine (SEQCML) launched the Pre-Analytical EQA Program, which initially consisted of cross-sectional studies [12], 13]. From 2014, this Program was transformed into a Pre-analytical QI Benchmarking Program. The Program explores a reduced number of QI based on the most prevalent events detected in the previous phase of the Program. Special focus is placed on the reasons for sample rejection at the laboratory. In this Program, only routine test results are considered, whereas urgent test results are excluded. The denominator used is the most common type of test used for each type of specimen (i.e. creatinine for serum; CBC for EDTA; and prothrombin time for citrate; the total number of tubes is only used for urine). These QI are specially focused on the sample extraction (venous puncture) and collection process. These processes can be improved by enhancing the training of personnel involved in the proper monitoring of processes.
The purpose of this Program is to improve pre-analytical phases by reducing errors with a potential impact on patient safety. In general terms, the same QI have been used for years, which has facilitated the development of specifications based on the state-of-the-art, as suggested in the European Federation of Laboratory Medicine (EFLM) recommendations (Task Finish Group – Performance specifications for the extra-analytical phases). According to these recommendations, percentiles p25, p50 and p75 from participant results are adopted as optimal, desirable and minimum specifications, respectively.
On another note, CLs are striving for laboratory results to have the greatest possible impact on patient outcomes. Most recently, CLs have placed the focus on the post-analytical phase and how the laboratory can add value to laboratory results (interpretative comments, critical result alerts, turnaround times, among others) [14], 15]. In this sense, the Working Group for Post-analytics of the Croatian Society of Medical Biochemistry and Laboratory Medicine conducted a survey to assess post-analytical practices and evaluate national recommendations [16].
In addition, the advent of new technologies, laboratory information systems (LIS), and increasing process automation makes it necessary to review the validity of the QI currently used for monitoring laboratory performance. The EFLM Working Group for the Pre-analytical Phase conducted a survey to assess European laboratory practices in monitoring the pre-analytical phase [17], 18]. The survey revealed that 71 % of laboratories were enrolled in an extra-analytical EQA program. However, a very low number of the laboratories engaged in a SEQCML Quality Assurance Program (PGCLC) participated in the Extra-Analytical Quality Assurance Program.
For this reason, a survey was conducted among CLs taking part in any PGCLC scheme. The aim of the Survey was to assess the level of participation in the Program and identify potential points for improvement. Other objectives included assessing the procedures used and difficulties faced by laboratories in relation to the collection and estimation of extra-analytical QI. Finally, the Survey examined whether the QI currently used meet the needs of increasingly automated and complex CL.
Materials and methods
Survey design
The Survey was designed by the members of the SEQCML Extra-analytical Quality Commission. It consisted of 48 dichotomous/multiple-choice/open questions and was developed on the Google forms platform. The Survey was distributed as a pilot to the members of the Extra-analytical Quality Commission, whose contributions were used to refine its design. All questions and answer options are shown in Table 1 of the Supplementary Material.
Summary of the main results of the survey.
| Annual requests | |
|---|---|
| Responses (n=124) | % |
| <25,000 | 17 |
| 25,000–300,000 | 41 |
| >300,000 | 42 |
|
|
|
| Types of requests | |
|
|
|
| Responses (n=124) | % |
| Routine ambulatory | 31 |
| Ambulatory ED | 17 |
| Routine hospital | 26 |
| Urgent hospital | 26 |
|
|
|
| Percentage of external samples | |
|
|
|
| Responses (n=124) | % |
| <33 % | 26 |
| 33–66 % | 43 |
| >66 % | 31 |
|
|
|
| Review of indicators disaggregated by type of extraction | |
|
|
|
| Responses (n=124) | % |
| Yes | 56 |
| No | 44 |
|
|
|
| Interest in including sigma | |
|
|
|
| Responses (n=120) a | % |
| Yes | 74 |
| No | 26 |
|
|
|
| Interest in the post-analytical phase | |
|
|
|
| Responses (n=124) | % |
| Yes | 86 |
| No | 14 |
|
|
|
| Interest in including ED laboratory samples | |
|
|
|
| Responses b | % |
| Yes, combining urgent and routine requests | 37 |
| Yes, separating urgent and routine requests | 44 |
| No | 19 |
-
aOf laboratories taking part in other programs, 1 yes has sigma and 3 no. bThe four remaining responses are from laboratories engaged in other programs. All received urgent requests.
Survey distribution
The Survey was distributed to PGCLC-participating laboratories (n=2,409) via e-mail and a post on LinkedIn, both including a link to complete the questionnaire on Google forms. The questionnaire was left open for two months and a half, from June 19, 2023 to August 31, 2023.
Survey analysis
Graphs were created to analyze the results of close-ended questions. Responses to open questions were analyzed one by one, grouped by themes.
Results
A total of 124 questionnaires were returned, accounting to a 5 % response rate, which is consistent with the response rate of previous surveys conducted by the Commission [19]. Table 1 summarizes the most relevant findings.
In relation to the characteristics of respondents, 42 % were members of large laboratories receiving over 300,000 requests on an annual basis; 41 % belonged to medium laboratories (25,000–300,000), and the remaining worked at small laboratories receiving less than 25,000 annual requests. Laboratories reported that they receive a similar number of routine and urgent outpatient and inpatient test orders. In most laboratories (43 %), 33–66 % of samples were external (samples collected at a center other than the laboratory’s).
Regarding the participation in the EQA Program, 51 % percent of respondents were engaged in the Pre-analytical EQA Program, 46 % did not participate in it, and 3 % were engaged in EQA Programs offered by other entities. The main reason to participate in the Programs was that they considered it necessary to monitor extra-analytical performance (38 %), harmonize QIs (25 %), and establish performance specifications (22 %). The least frequent response was the need to participate in the Program due to accreditation requirements. In total, 40 % of participants considered that the main advantage of participating in the program was that it provides information about the position of the laboratory in the ranking and provides an insight into the level of compliance of specifications with respect to similar laboratories (40 %), followed by patient safety (19 %) (Figure 1). In contrast, the main reason stated by non-participating laboratories (57) for not being engaged in the Program was the difficulty in collecting data (32 %), followed by unawareness of the availability of this Program (23 %) (Figure 2).
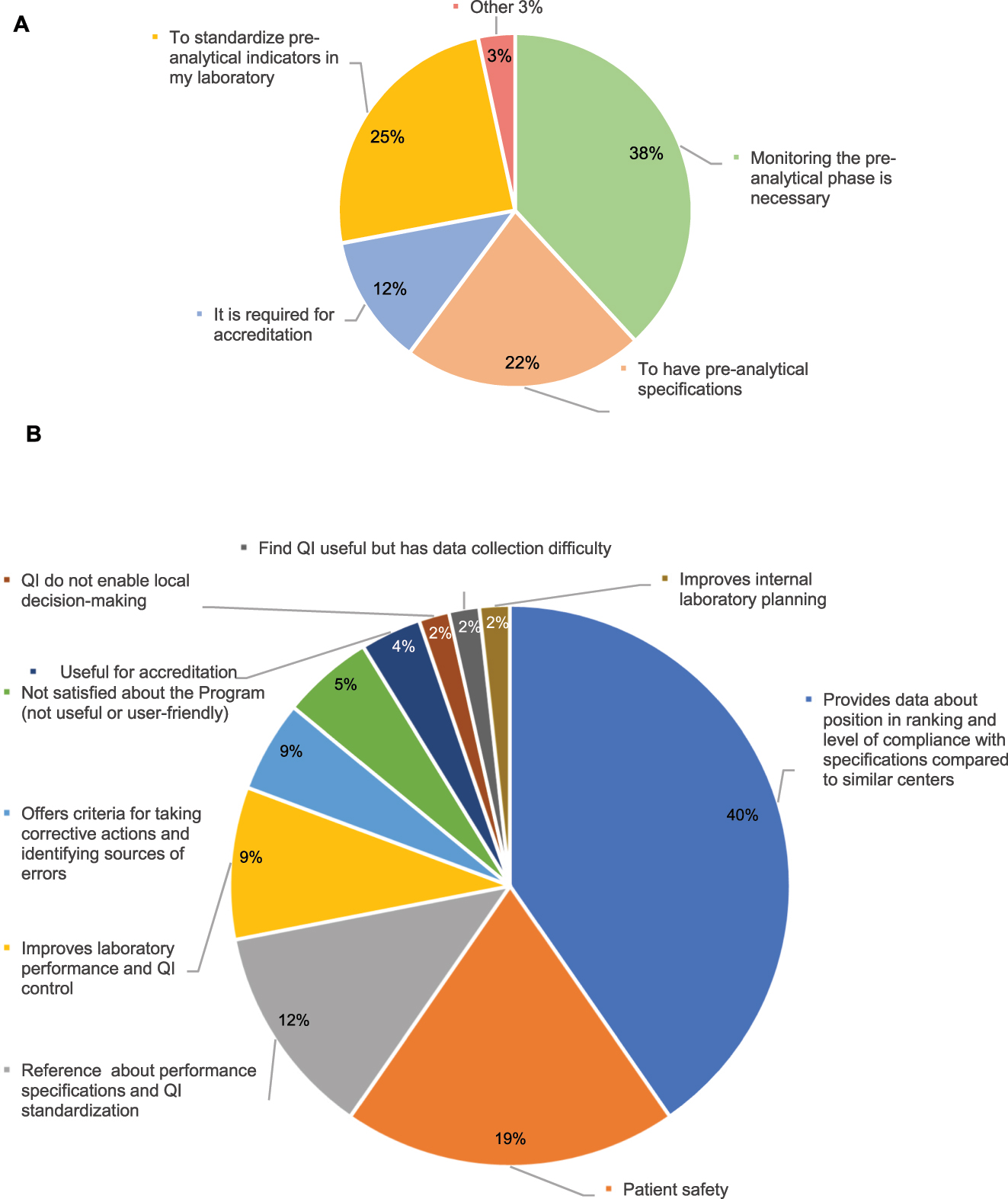
Results for the participating laboratories in the pre-analytical program (63 laboratories). (A) Reasons for taking part. (B) Main contributions of the program.

Reasons for laboratories not participating in the pre-analytical program (57 laboratories). *Other: (1) indices are not available on the LIS. (2) The laboratory company does not participate. (3) We would like our customers to participate. (4) No specific reason. (5) I took part some time ago but did not agree with the reported results. (6) We only take part in the fecal occult blood program. (7) I work at the ED laboratory. (8) Lack of time. (9) Only internal pre-analytical quality monitoring is performed. (10) We did not receive any offer from the organizing entity. (11) The performance specifications used. (12) Serum indices are not available in the laboratory and an automated request and report system is not available.
In relation to potential improvements to the Program, 56 % suggested that disaggregation of QI by type of extraction (inpatient vs. outpatient) would be useful. More specifically, most participants supported the inclusion of urgent test requests in QI assessment, separated from routine test requests. Conversely, 37 % of respondents would combine them with routine test, and 19 % would exclude them. Urgent samples are considered in all extra-analytical EQA Programs other than the SEQCML Program. In total, 74 % would include Sigma estimates. Notably, Sigma estimates are included in only one of the four non-SEQCML programs available. Interestingly, 86 % of respondents showed interest in participating in a post-analytical quality assessment program, one of the open-ended questions surveyed which post-analytical QIs (Figure 3). The most selected indicators included the percentage of critical alerts raised with respect to the total critical result alerts and the number of reports delivered out of the turnaround time. Other indicators frequently selected by respondents were related to report modifications, interpretative comments, result transcription and the delivery of reports containing errors.

Interest in post-analytical indicators. TAT: turnaround time from receipt at the laboratory to the communication of results for urgent samples. *Other: (1) indicators related to consultations from clinicians to the laboratory and with result alerts; (2) routine inpatient and outpatient samples; (3) percentage of reports modified/total of reports; (4) critical rejections timely communicated/total of critical rejections detected; (5) number of poorly sent-validated reports/total reports (6) turnaround time from receipt at the laboratory to the communication of results for venous and arterial blood gas tests; (7) indicators related to the communication of results for procalcitonin in urgent samples; (8) interpretative results/diagnostic opinion (% of lack of correlation between conclusive results and % of correlation between clinical picture and the tests performed); (9) indicators related to the transcription of results (% of errors in result transcription and % of untimely transcription of results).
The second part of the Survey was focused on QI data collection and estimation by laboratories. In 73 % of cases, this process is performed via the LIS, followed by manual counting (on an Excel spreadsheet or similar) (19 %), with the rest of laboratories using dedicated software. LIS suppliers included Werfen Modulab® (23 %), Roche Infinity® (19 %) and Siemens Healthineers Servolab® (14 %), with other suppliers accounting for less than 10 %.
Finally, interest was shown for some QI not assessed in the Program, related to events regarding temperatures and transport times from peripheral extraction centers. Also, suggestions were made about applying some QI already included in the Program to other types of samples (blood gas test syringes, urine for urine analysis, other microbiology samples, among others). Respondents showed interest in QI related to sample transport, since 33–66 % of samples received by laboratories are external. The management of such a high percentage of external samples requires close coordination with external centers, the inclusion of ED laboratory data and clearer instructions for QI collection, along with a modified denominator for calculating QI, and the inclusion of other types of samples.
Discussion
Participation in external quality assurance programs for the pre-analytical phase facilitates the identification and prioritization of areas for improvement. The participants, most being large laboratories receiving over 300,000 requests a day, indicated that the SEQCML External Quality Assurance Program for the Pre-Analytical Phase is a valuable tool.
The monitoring of pre-analytical errors through robust QI is essential for their proper management. Additionally, external benchmarking enables laboratories to assess their performance against standard specifications. As a result, the number of errors decreases, thereby improving patient safety.
The results of this Survey demonstrate that participation of clinical laboratories in Extra-Analytical Programs is still low. Only half of clinical laboratories in Spain participate in these Programs, compared to 71 % in Europe, as shown in a similar Survey (17). The main reason for not taking part in these programs is the difficulty in IQ data collection, followed by unawareness of the availability of these Programs. Another possible reason for such a low level of participation in Extra-Analytical Quality Programs is the reluctance to disclose organizational data, which may negatively affect their image or relationship with customers. Although confidentiality is ensured in this Program, a negative bias in participation may arise for this reason.
Based on the results of this Survey, a campaign was launched on the PGCLC website and SEQCML social networks. The results of the Survey highlight the need for cooperating with the leading LIS suppliers to create modules for a simpler, standardized, automated, real-time calculation of QI that can be easily shared and assessed. The Survey revealed that 19 % of laboratories still rely on manual methods for QI calculation, which poses the opportunity to implement more automated LIC-based solutions.
Based on the interest shown by participants in including urgent samples along with post-analytical and sample transport QI, the SEQCML Extra-analytical Quality Commission and the External Quality Programs Committee conducted a pilot study among its members. Following the obtained results, they both decided to further include urgent samples for all Program participants over 2025. For such purpose, the number of QI doubled. Participants will receive two reports, one for routine samples and another for urgent samples. Separated reports will allow participating laboratories to compare the two processes and establish targeted corrective measures.
In a second phase, two more QI will be integrated in the Program, in response of the answers obtained, such as the generalized interest in the post-analytical phase and the addition of more pre-analytical QI different from the ones currently used. The two new QI include the compliance with the turnaround time and critical result alerts. Furthermore, another pre-analytical QI about transport events from peripheral extraction samples will be included, following the same scheme as the pilot study with a progressive implementation over 2026.
With respect to other programs, the Working Group Laboratory Errors and Patient Safety (WG-LEPS) of the International Federation of Clinical Chemistry (IFCC) has not released any further publication since 2019 [20] nor has the Australian key incident monitoring and management system program (KIMMS) of the Royal College of Australasian Pathologist (RCPA) [21] since 2020. The results of these programs were already compared against the SEQCML Preanalytical Program in a publication of 2022 [22]. Notably, two recent experiences deserve special attention, one of the Argentine Foundation for Biochemistry [23] and another in China [24]. The first one was a quarterly survey where only outpatient samples and four QI were included. It was conducted in the framework of a larger program and indicators were entered onto an Excel spreadsheet. Participation was voluntary, resulting in a varying percentage of laboratories, with differences according to the QI. Results are provided for the 2021–2022 period. Between 100 and 200 out of a total of 400 laboratories returned the questionnaire (25–50 %), with similar results if percentiles are compared against those of the IFCC (23). In the Survey of the National Centre for Clinical Laboratories of China, two questionnaires assessing six QI were distributed annually via email over the 2017–2019 period. In total, 434 laboratories of the province of Zhejiang responded to the six runs. The rate of rejection was very low, as compared to the Q-Probes and Q-Tracks Program of the College of American Pathologist (CAP).
A significant limitation of the Survey conducted with SEQCML laboratories is the low participation. Although this is not a particularity of this Survey and the reasons cannot be clearly identified (overexposure to surveys? Limited time of laboratory staff? Low interest in this issue?), a change of format should be considered for future surveys. Other strategies to be adopted include increasing survey dissemination and improving access through different channels to enhance response rates and the power of the studies.
There is a growing interest in QIs related to the pre- and post-analytical phases. However, a reduced proportion of laboratories collect data regularly. The findings of this Survey suggest future directions for new developments and optimization of the SEQCML Pre-analytical EQA Program to better adapt to the current needs of clinical laboratories. Data collection difficulty is the main reason for such a low level of participation. Hence, further collaboration with LIS suppliers is necessary for the development of new, simpler data collection tools. Furthermore, it is the responsibility of laboratories to allocate more resources, personnel and funds to the extra-analytical phase.
In conclusion, the monitoring of pre-analytical errors through robust QI is essential for their proper management. Additionally, external benchmarking enables laboratories to assess their performance against standard specifications. As a result, the number of errors decrease and patient safety is improved.
Acknowledgments
SEQCML External Quality Control Assurance.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
-
Article Note: The original article can be found here: https://doi.org/10.1515/almed-2024-0160.
References
1. Plebani, M, Astion, ML, Barth, JH, Chen, W, De Oliveira Galoro, CA, Escuer, MI, et al.. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52:951–8. https://doi.org/10.1515/cclm-2014-0142.Search in Google Scholar PubMed
2. Lundberg, G. Managing the patient-focused laboratory. Reinhold VNost, editor. Medical Economics Co; 1975:379 p.Search in Google Scholar
3. Plebani, M, Sciacovelli, L, Aita, A, Pelloso, M, Chiozza, ML. Performance criteria and quality indicators for the pre-analytical phase. Clin Chem Lab Med 2015;53:943–8. https://doi.org/10.1515/cclm-2014-1124.Search in Google Scholar PubMed
4. Plebani, M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med 2006;44:750–9. https://doi.org/10.1515/cclm.2006.123.Search in Google Scholar PubMed
5. Plebani, M, Chiozza, ML, Sciacovelli, L. Towards harmonization of quality indicators in laboratory medicine. Clin Chem Lab Med 2013;51:187–95. https://doi.org/10.1515/cclm-2012-0582.Search in Google Scholar PubMed
6. Marzana Sanz, I, Ibarz Escuer, M, Llopis Diaz, MA, Barba Meseguer, N, Alsina Kirchner, MJ, Martínez Espartosa, D, et al.. Recomendaciones para el diseño e implementación de un programa de aseguramiento de la calidad de la fase preanalítica. Revista Del Lab Clínico 2019;12:e54–65. https://doi.org/10.1016/j.labcli.2019.01.003.Search in Google Scholar
7. Llopis, MA, Bauça, JM, Barba, N, Álvarez, V, Ventura, M, Ibarz, M, et al.. Spanish preanalytical quality monitoring program (SEQC), an overview of 12 years’ experience. Clin Chem Lab Med 2017;55:530–8. https://doi.org/10.1515/cclm-2016-0382.Search in Google Scholar PubMed
8. Sciacovelli, L, Lippi, G, Sumarac, Z, West, J, Garcia, I, Furtado Vieira, K, et al.. Quality indicators in laboratory medicine: the status of the progress of IFCC working group “laboratory errors and patient safety” project. Clin Chem Lab Med 2017;55:348–57. https://doi.org/10.1515/cclm-2016-0929.Search in Google Scholar PubMed
9. Sciacovelli, L, Aita, A, Plebani, M. Extra-analytical quality indicators and laboratory performances. Clin Biochem 2017;50:632–7. https://doi.org/10.1016/j.clinbiochem.2017.03.020.Search in Google Scholar PubMed
10. AENOR. Laboratorios clínicos. Requisitos particulares para la calidad y la competencia (ISO 15189:2023). UNE-EN ISO 15189; 2023:61 p.Search in Google Scholar
11. Lippi, G, Becan-McBride, K, Behúlová, D, Bowen, RA, Church, S, Delanghe, J, et al.. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med 2013;51:229–41. https://doi.org/10.1515/cclm-2012-0597.Search in Google Scholar PubMed
12. Alsina, MJ, Álvarez, V, Barba, N, Bullich, S, Cortés, M, Escoda, I, et al.. Revision de los resultados del Programa de Evaluacion Externa de la Calidad Preanalitica (resumen 2001-2005). Quimica Clinica 2006;26:325–31.Search in Google Scholar
13. Alsina, M, Virtudes, A, Barba, N, Bullich, S, Cortés, M, Escoda, I, et al.. Preanalytical quality control program – an overview of results (2001–2005 summary). Clin Chem Lab Med 2008;46:849–54. https://doi.org/10.1515/cclm.2008.168.Search in Google Scholar PubMed
14. Ajzner, É. Adding value in the postanalytical phase. EJIFCC 2016;27:166–73.Search in Google Scholar
15. Lundberg, GD. Adding outcome as the 10th step in the brain-to-brain laboratory test loop. Am J Clin Pathol 2014;141:767–9. https://doi.org/10.1309/ajcp5ksxwti2dmcc.Search in Google Scholar PubMed
16. Jokic, A, Rimac, V, Tanaskovic, JV, Podolar, S, Honovic, L, Krleza, JL. The concurrence of the current postanalytical phase management with the national recommendations: a survey of the working group for postanalytics of the Croatian society of medical biochemistry and laboratory medicine. Biochem Med 2021;31:1–9.10.11613/BM.2021.030704Search in Google Scholar PubMed PubMed Central
17. Cadamuro, J, Lippi, G, von Meyer, A, Ibarz, M, van Dongen-Lases, E, Cornes, M, et al.. European survey on preanalytical sample handling – Part 1: how do european laboratories monitor the preanalytical phase? on behalf of the european federation of clinical chemistry and laboratory medicine (EFLM) working group for the preanalytical pase. Biochem Med 2019;29:322–33.10.11613/BM.2019.020704Search in Google Scholar PubMed PubMed Central
18. Cornes, M, Simundic, AM, de la Salle, B, Kristensen, GBB, Guimaraes, JT, Grankvist, K, et al.. European survey on preanalytical sample handling – Part 2: practices of European laboratories on monitoring and processing haemolytic, icteric and lipemic samples. On behalf of the European Federation of Clinical Chemistry and Laboratory Medicine. Biochem Med 2019;29:334–45. https://doi.org/10.11613/bm.2019.020705.Search in Google Scholar
19. García-del-Pino, I, Bauça, JM, Gómez, C, Caballero, A, Ibarz, M, Marzana, I, et al.. Preanalytical issues related to routine and diagnostic glucose tests: results from a survey in Spain. Biochem Med 2020;30:1–8.10.11613/BM.2020.010704Search in Google Scholar PubMed PubMed Central
20. Sciacovelli, L, Lippi, G, Sumarac, Z, del Pino Castro, IG, Ivanov, A, De Guire, V, et al.. Pre-analytical quality indicators in laboratory medicine: performance of laboratories participating in the IFCC working group “laboratory errors and patient safety” project. Clin Chim Acta 2019;497:35–40. https://doi.org/10.1016/j.cca.2019.07.007.Search in Google Scholar PubMed
21. Gay, S, Badrick, T. Changes in error rates in the australian key incident monitoring and management system program. Biochem Med 2020;30:1–8. https://doi.org/10.11613/bm.2020.020704.Search in Google Scholar
22. Caballero, A, Gómez-Rioja, R, Ventura, M, Llopis, MA, Bauça, JM, Gómez-Gómez, C, et al.. Evaluation of 18 quality indicators from the external quality assurance preanalytical programme of the Spanish Society of Laboratory Medicine (SEQCML). Adv Lab Med 2022;3:175–87.10.1515/almed-2021-0097Search in Google Scholar PubMed PubMed Central
23. Unger, G, Benozzi, SF, Girardi, R, Pennacchiotti, GL. Evaluation of four quality indicators of the pre-analytical phase external quality assessment subprogram of the Fundación Bioquímica Argentina. Electron J Int Fed Clinical Chem Lab Med 2023;34:203–12.Search in Google Scholar
24. Kang, F, Li, W, Xia, X, Shan, Z. Three years’ experience of quality monitoring program on pre-analytical errors in China. J Clin Lab Anal 2021;35:1–7. https://doi.org/10.1002/jcla.23699.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/almed-2025-0067).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review / Artículo de Revisión
- Implementation of circulating cell-free DNA screening for fetal aneuploidies
- Implementación del ADN libre circulante para la detección de aneuploidías fetales
- Mini Review / Mini Revisión
- Data science applied to the assessment of biological variation estimates
- Ciencia de datos aplicada a la obtención de estimados de variación biológica
- Original Article / Artículo Original
- How to handle lipemic CBC samples on Sysmex hematology analyzers?
- Cómo manejar las muestras lipémicas para realización de contaje de células sanguíneas (CBC) en los analizadores hematológicos Sysmex
- Use of artificial intelligence to assess genetic predisposition to develop critical COVID-19 disease: a comparative study of machine learning models
- Uso de inteligencia artificial en la predisposición genética a enfermedad crítica por COVID-19: evaluación comparativa de modelos de aprendizaje automático
- Use of external quality assessment in extra-analytical phases in clinical laboratories in Spain: a survey by the Spanish Society of Laboratory Medicine (SEQCML)
- Uso de la evaluación externa de la calidad en las fases extraanalíticas de los laboratorios clínicos españoles: una encuesta de la Sociedad Española de Medicina de Laboratorio (SEQCML)
- Case Report / Caso Clínico
- Diagnosis of hemogobinopathies in the clinical laboratory: an occult Hofu hemoglobin on HPLC
- Diagnóstico de hemoglobinopatías en el laboratorio clínico: hallazgo de una hemoglobina hofu oculta en HPLC
- Letter to the Editor / Carta al Editor
- Biomarker repurposing: opportunities and challenges in the evolving field of laboratory medicine
- La reutilización de biomarcadores para otros fines: oportunidades y dificultades en el cambiante campo de la medicina de laboratorio
Articles in the same Issue
- Frontmatter
- Review / Artículo de Revisión
- Implementation of circulating cell-free DNA screening for fetal aneuploidies
- Implementación del ADN libre circulante para la detección de aneuploidías fetales
- Mini Review / Mini Revisión
- Data science applied to the assessment of biological variation estimates
- Ciencia de datos aplicada a la obtención de estimados de variación biológica
- Original Article / Artículo Original
- How to handle lipemic CBC samples on Sysmex hematology analyzers?
- Cómo manejar las muestras lipémicas para realización de contaje de células sanguíneas (CBC) en los analizadores hematológicos Sysmex
- Use of artificial intelligence to assess genetic predisposition to develop critical COVID-19 disease: a comparative study of machine learning models
- Uso de inteligencia artificial en la predisposición genética a enfermedad crítica por COVID-19: evaluación comparativa de modelos de aprendizaje automático
- Use of external quality assessment in extra-analytical phases in clinical laboratories in Spain: a survey by the Spanish Society of Laboratory Medicine (SEQCML)
- Uso de la evaluación externa de la calidad en las fases extraanalíticas de los laboratorios clínicos españoles: una encuesta de la Sociedad Española de Medicina de Laboratorio (SEQCML)
- Case Report / Caso Clínico
- Diagnosis of hemogobinopathies in the clinical laboratory: an occult Hofu hemoglobin on HPLC
- Diagnóstico de hemoglobinopatías en el laboratorio clínico: hallazgo de una hemoglobina hofu oculta en HPLC
- Letter to the Editor / Carta al Editor
- Biomarker repurposing: opportunities and challenges in the evolving field of laboratory medicine
- La reutilización de biomarcadores para otros fines: oportunidades y dificultades en el cambiante campo de la medicina de laboratorio

