Impaired recognition memory and cognitive flexibility in the ratL5–L6 spinal nerve ligation model of neuropathic pain
-
Orla Moriarty
Abstract
Background and aims
Although neuropathic pain is known to negatively affect cognition, the neural mechanisms involved are poorly understood. Chronic pain is associated with changes in synaptic plasticity in the brain which may impact on cognitive functioning. The aim of this study was to model neuropathic pain in mid-aged rats using spinal nerve ligation (SNL). Following establishment of allodynia and hyperalgesia, behaviour was assessed in a battery of cognitive tests. Expression of the presynaptic protein, synaptophysin, and its colocalisation with the vesicular GABA and glutamate transporters (vGAT and vGLUT, respectively), was investigated in the medial prefrontal cortex (mPFC) and hippocampus.
Methods
Nine month old male Sprague Dawley rats underwent L5-L6 spinal nerve ligation or a sham procedure. Mechanical and cold allodynia and thermal hyperalgesia were assessed using von Frey, acetone and Hargreaves tests, respectively. Cognition was assessed in the novel-object recognition, air-puff passive avoidance and Morris water maze behavioural tasks. Immunohistochemistry was used to examine the expression of synaptophysin in the mPFC and CA1 region of the hippocampus and double labelling of synaptophysin and the vesicular transporters vGAT and vGlut was used to investigate the distribution of synaptophysin on GABAergic and glutamatergic neurons.
Results
SNL rats displayed impaired performance in the novel-object recognition task. Passive-avoidance responding, and spatial learning and memory in the Morris water maze, were unaffected by SNL surgery. However, in the water maze reversal task, pain-related impairments were evident during training and probe trials. SNL surgery was not associated with any differences in the expression of synaptophysin or its colocalisation with vGAT or vGLUT in the mPFC or the hippocampal CA1 region.
Conclusions
These results suggest that the SNL model of neuropathic pain is associated with deficits in recognition memory and cognitive flexibility, but these deficits are not associated with altered synaptophysin expression or distribution in the mPFC and CA1.
Implications
Cognitive complaints are common amongst chronic pain patients. Here we modelled cognitive impairment in a well-established animal model of neuropathic pain and investigated the neural mechanisms involved. A better understanding of this phenomenon is an important prerequisite for the development of improved treatment of patients affected.
1 Introduction
Chronic pain impacts negatively on both physical and psychological functioning. Impairments in cognitive domains including attention and working memory have been demonstrated in chronic pain patients using both subjective reports and objective neuropsy-chological testing (for review see [1, 2, 3]). However, the mechanisms by which pain affects cognition are still poorly understood. Recent research has demonstrated that cognitive deficits associated with chronic pain can be modelled in rodents [4, 5, 6, 7, 8, 9,10, 11, 12, 13, 14], thus allowing for the neurobiological mechanisms underpinning this association to be investigated.
Age is thought to have an important influence on the relationship between pain and cognitive function. This is not surprising, given that ageing is associated with alterations in both pain sensitivity [15, 16, 17] and cognition [18,19]. While most studies suggest a direct negative effect of pain on cognition, that worsens with increasing age, a positive correlation between reported pain severity and executive function in healthy older adults and Alzheimer’s patients has also been observed [20,21], suggesting that the relationship can be complex. Recently, the effect of the pain-age interaction on cognitive performance was investigated further in human subjects, with one study showing no additive effect of age [22] while another suggested that age moderates the relationship between pain and cognition [23]. Preclinical studies also illustrate the importance of the pain-age interaction in relation to cognition. Leite-Almeida et al. [8] demonstrated age-dependent effects of pain on cognition in a rat model of neuropathic pain; in this study, mid-aged (but not young or old) rats that had undergone spared nerve-injury (SNI) to model neuropathic pain, showed impaired function in the Morris water maze reversal task, which assesses cognitive flexibility. Cognitive flexibility in rodents is believed to be analogous to executive function in humans, and clinical research has shown that chronic pain patients are impaired on tasks measuring executive function, including attentional interference, switching and process-dissociation tasks [24, 25, 26, 27, 28, 29].
Alterations in synaptic plasticity play a key role in both pain and cognition, and it has been suggested that neuroplas-tic changes occur in chronic pain, resulting in neural rewiring, which in turn affects cognition [3,30]. Synaptic connectivity is altered in cognitive-associated brain regions such as the hippocampus [11,14,31,32], amygdala [33, 3435] and anterior cingulate cortex [36,37] in models of chronic pain. Synaptophysin, a presynaptic protein, is commonly used as a marker of presynaptic terminals due to its abundance and localisation to synaptic vesicles [38,39]. Changes in the expression of synaptophysin have been reported in both age-related [38,40] and disease-related [41,42] cognitive impairment, and synaptophysin knockdown is associated with impaired learning and memory in mice [43]. Synaptophysin expression is also altered in the periphery and spinal cord in animal models of chronic pain [14,44, 45, 46,47] and recently pain-related cognitive impairment in the rat SNI model of neuropathic pain was shown to be associated with a decrease in the number of synaptophysin-positive boutons in the CA1 region of the hippocampus [14].
The aims of the present study were (1) to investigate cognitive function in the rat spinal nerve ligation (SNL) model of neuropathic pain using a battery of cognitive tests, (2) to quantify the expression of synaptophysin in two cognition-associated brain regions, the medial prefrontal cortex (mPFC) and CA1 region of the hippocampus and (3) to investigate the distribution of synaptophysin on excitatory (glutamatergic) and inhibitory (GABAergic) terminals.
2 Materials and methods
2.1 Animals
Mid-aged male Sprague-Dawley rats (OFA sub-strain, Charles River, L′Arbresle, France) weighing 465–600 g (41 weeks old) on arrival were used in this experiment. Rats of this age were used because previous work has shown impaired cognitive performance in mid-aged rats, but not young or old rats, in another model of neuropathic pain, the SNI model [8]. Rats were singly housed in plastic-bottomed cages, 40 cm (l) × 25 cm (w) × 20 cm (h), containing wood-shavings for bedding. Rats were maintained under standard laboratory conditions of temperature (20 ± 2 °C), humidity (40–60%) and lighting (12:12 h light/dark cycle, lights on at 08:00 h). Food and water were available ad libitum. Animals were regularly weighed and handled, and health status monitored. No major adverse events were observed. Rats were habituated to the facility for a period of one week prior to baseline testing and all procedures were carried out during the light phase. Experimental procedures were approved by the Animal Care and Research Ethics Committee of the National University of Ireland, Galway, and the work has carried out under licence from the Irish Department for Health and Children and in compliance with European Communities Council Directives 86/609/EEC and 2010/63/EU. All sections of the study adhered to the ARRIVE Guidelines for reporting in animal research [48], and to the guidelines of the Committee for Research and Ethical Issues of the International Association for the study of Pain (IASP).
2.2 Experimental protocol
The experimental timeline is illustrated in Fig. 1. Prior to L5-L6 spinal nerve ligation (SNL) surgery, rats were randomly assigned to SNL(n = 11)or sham groups (n = 12). von Frey testing for mechanical allodynia was carried out 2 or 4 days before surgery (baseline), and at day 1 post surgery and every second day thereafter until day 13 post surgery. Hargreaves testing for thermal hyperalgesia was carried out 1 or 3 days before surgery and on days 8 and 16 post surgery. Testing for cold allodynia was carried out 5 or 3 days prior to surgery and on days 4 and 12 post surgery. Mechanical allodynia, thermal hyperalgesia and cold allodynia were also reassessed at the end of the experimental period on days 61–65 post surgery.
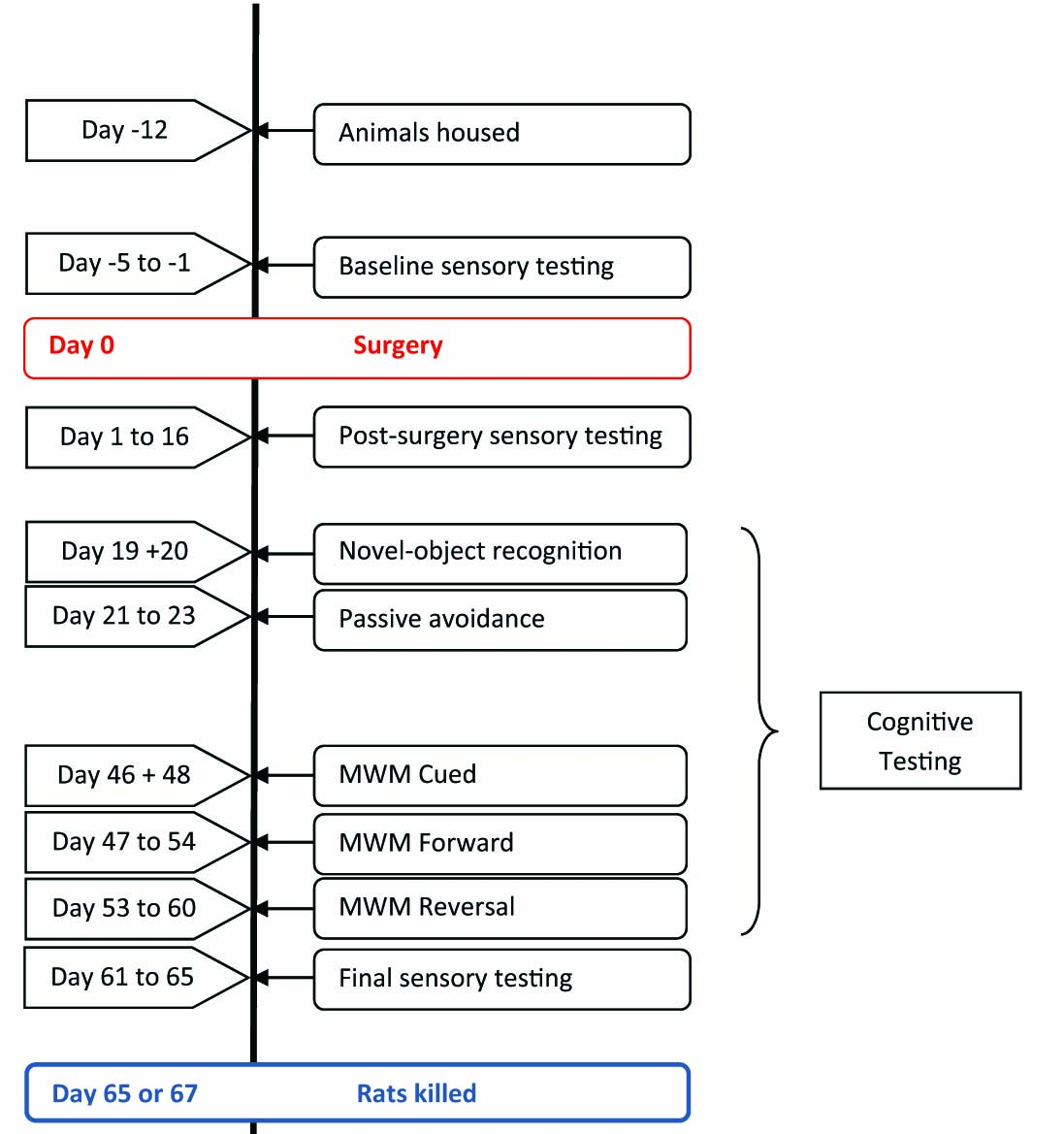
Experimental timeline.
Cognitive testing began on day 19 post surgery. Rats were habituated to the novel-object recognition (NOR) test arena, and recognition memory was assessed the following day. Aver-sive memory was measured using the air-puff passive-avoidance paradigm on days 21–23 post surgery. Morris water maze testing was carried out between days 46 and 60 post surgery. A one-day cued test was followed by 5 days of acquisition training and a one-day forward probe trial. Rats then underwent 5 days of reversal training and a one-day reversal probe trial. One rat did not complete the full schedule of Hargreaves testing due to treatment for an infection of the surgical wound and so was excluded from analyses for this test. Three rats in the SNL group and one rat in the sham group were excluded from the novel-object analyses as they failed to explore both objects during the familiarisation period. Three rats from the SNL group and two rats from the sham control group did not enter the dark compartment of the passive-avoidance arena within 300 s on the acquisition day. These rats were excluded from subsequent testing in this paradigm and were not included in the passive-avoidance analyses. Animals were sacrificed on day 65 or 67 post surgery, and immunohistochemistry was carried out thereafter. All behavioural testing, tissue staining and image processing was carried out by experimenters blind to the surgical treatment of the animal and each animal was considered an experimental unit.
2.3 Spinal nerve ligation surgery
L5-L6 spinal nerve ligation (SNL) surgery was carried out as described previously [49,50,51, 52, 53, 54]. Briefly, the rats were anaesthetised with isoflurane (Isoflo®, Abbott Laboratories, Berkshire, UK, 2.5% in 0.6l/min oxygen), the fur lateral to the midline on the left-hand side at the lower lumbar and sacral regions was clipped closely, and an incision was made through the skin between the spinal column and the left iliac crest. Paraspinal muscles were removed using a toothed forceps to visualise the L6 transverse process. This bone was then removed using a small rongeur to expose the L4 and L5 spinal nerves. The L5 nerve was tightly ligated using 6–0 silk suture. The L6 nerve, located underneath the sacrum, was also ligated. In the sham-operated rats, the L5 and L6 nerves were exposed but were not ligated. The duration of anaesthesia was approximately 40 min. Post surgery, rats were allowed to recover from anaesthesia in recovery cages maintained at a constant temperature on a heating pad and then re-housed singly with fresh bedding in their home cages.
2.4 Behavioural measurement of sensory responses
2.4.1 von Frey test for mechanical allodynia
von Frey testing was carried out as described previously [49, 50,51, 52,54,55]. The arena used for von Frey testing consisted of six adjoining chambers with dimensions 25 cm (l) × 20 cm (w) × 14 cm (h). The sides were made of clear Perspex and the partitions between chambers from white melamine-coated chipboard. The arena was placed on a raised wire-mesh floor so that the experimenter could access the rats’ hind-paws from below. Rats received an initial habituation period of 30 min during which they were placed in individual chambers of the arena and no testing was performed. On subsequent test days, the rats were habituated to the arena for 20 min prior to assessment. von Frey filaments (Touch-Test® Sensory Evaluators, North Coast Medical, Inc., CA, USA) of different weights (0.16–180 g), were applied perpendicular to the plantar surface of the hind-paw, with sufficient force to cause slight buckling of the filament, up to a maximum of 6 s or until a positive result (flinching, licking or withdrawal of the paw) was observed. Filaments were applied to both left and right hind-paws five times (alternating between paws) in order of increasing weight until a 100% positive response (5 positive responses to 5 applications) was observed. The filament weight eliciting 50% response was calculated by plotting the % response versus filament weight for each rat. A mild detergent was used to clean the arena between each group of 6 rats.
2.4.2 Hargreaves test for thermal hyperalgesia
A commercial apparatus (IITC Life Science Inc., Woodland Hills, CA, USA) was used for Hargreaves testing as previously described [49,50,52,54]. The apparatus consisted of a three-chambered Perspex arena (chamber dimensions 22 cm × 20 cm × 15 cm, l×w×h) placed on top of a glass plate heated to 30 ± 1 °C. Rats were habituated to the arena for 30 min on the day before baseline testing began, and on test days were habituated to the arena for 20 min prior to testing. A moveable radiant heat source was positioned underneath the glass and could be focused on the rat’s hind-paw. The heat source was set to an active intensity of 30% and focused from below on the plantar surface of the rat’s hind-paw. The thermal stimulus was applied until a positive response (criteria similar to those used for von Frey testing) was recorded or until a cutoff time of 20 s was reached. Right and left hind-paws were tested 4 times, alternating between paws, and the average withdrawal latency for each paw was calculated. The arena was cleaned with mild detergent between testing each group of animals.
2.4.3 Acetone drop test for cold allodynia
The acetone-drop test was used to measure responding to an innocuous cold stimulus as previously described [49, 52, 53,54]. The arena used for this test was identical to that used for von Frey testing. A 1 ml syringe with a short length of polyethylene Portex® tubing (1 mm internal diameter) attached was used to apply approximately 0.2 ml of acetone (Sigma, Ireland) to the plantar surface of the hind-paw, without mechanically stimulating the paw. Each hind-paw was tested 3 times, alternating between paws, the number of positive responses (flinch, lick or withdrawal of the hind-paw) within 60 s of acetone application for each trial was recorded, and the total across trials calculated. The arena was cleaned with mild detergent between testing of each group of animals.
2.5 Cognitive testing
2.5.1 Novel-object recognition test
The novel-object recognition test procedure used herein was based on a number of protocols described previously [56,57] with some modifications. Testing was carried out in a circular arena with a wooden base and metal sides (both painted black), with dimensions 75 cm (d) × 38 cm (h). The arena was illuminated by four 60 W bulbs which provided constant light intensity of 100lx. A video camera positioned above the arena was used to record behaviour during testing for subsequent analysis. The objects used were plastic Coca-Cola® bottles (filled with water) with a base diameter of 4.5 cm and 23.5 cm height and a plastic structure constructed from green and blue toy blocks with dimensions base area 5 cm2 and height 16 cm. The objects had no apparent natural significance to the rats, and were secured to the base of the arena with white tack such that they were difficult to displace. Animals were habituated to the arena in the absence of objects for 20 min on the day before the test day. The test day comprised of three stages: (i) habituation, (ii) exposure 1 and (iii) exposure 2. Rats were introduced to the arena for a 3 min habituation period and then returned to their home cage for 7 min. During exposure 1, two identical objects (Coca-Cola® bottles) were placed in opposite quadrants of the arena, 16 cm from the perimeter. The rat was allowed to freely explore the arena and objects for a period of 3 min, after which the animal was removed from the arena and returned to its home cage for an interval of 5 min. Prior to exposure 2, one of the bottles was replaced with a novel object (plastic structure made from interlocking toy blocks). The animal was again allowed to freely explore the arena and objects for a period of 3 min and then returned to its home cage. The arena was cleaned with a mild detergent between rats to remove odours and olfactory cues, and faecal pellets were removed between exposures. Exploration of an object was defined as sniffing the object, rearing against the object or having the head directed towards the object within a 2 cm annulus of the object. Exploratory and general behaviours were manually rated from the DVD recordings of each of the three test stages, with the aid of Ethovision® behavioural tracking software (Noldus, The Netherlands). The software was also used to track the distance moved (in cm) by the animal during testing. The proportion of time spent exploring the object was assessed by calculating a discrimination ratio as follows:
2.5.2 Air-puff-induced passive avoidance
The procedure was similar to that described by Moriarty et al. [54]. Passive-avoidance testing was carried out in a specially constructed light/dark arena. The light compartment was made of white melamine-coated chipboard with dimensions of 30 cm (l) × 30 cm (w) × 40 cm (h) and was lit from above using a standard 60 W bulb, such that the compartment was maintained at a constant light intensity of 100l×. The dark compartment was made of dark grey Perspex with a black wooden lid, and its dimensions were identical to those of the light compartment. Light intensity in the dark compartment was negligible. The two compartments were separated by a manually controlled guillotine door. For delivery of air-puff directed at the rat’s face, an air-duster canister (Electrolube, GDP, UK) was mounted to the outside of the dark compartment, such that a nozzle could protrude up to 3.5 cm into the dark compartment through a 5 mm diameter hole in the compartment wall opposite the guillotine door. The nozzle was positioned 3 cm from the floor of the arena (approximately nose-height of the rat). Rats were habituated to the arena for 5 min 24 h prior to testing. During this period, rats were placed in the light compartment of the arena and allowed to freely explore both the light and dark compartments of the arena and then returned to their home cages. In the acquisition trial, the rat was placed in the light compartment of the arena, facing away from the guillotine door and the latency to enter the dark compartment was recorded up to a maximum of 300 s. Once the rat had entered the dark compartment, the guillotine door was lowered by the experimenter. Once the rat was facing the wall opposite the guillotine door, a single, brief puff of air (duration of ~1 s) was administered to the face. The rat was confined to the dark compartment for a further 90s post-air-puff, after which it was removed from the arena and returned to its home cage. If the rat did not enter the dark chamber within the 300-s period it was removed from the arena, returned to its home cage and excluded from further testing. For the retention trial (carried out 24 h post acquisition) the rat was again placed back into the light compartment of the arena, facing away from the guillotine door and the step-through latency to enter the dark compartment was recorded. If the animal did not enter the dark compartment within 300 s they were removed from the arena and returned to their home cage, and latency was recorded as 300 s. No air puff was administered during the retention trial and the arena was cleaned with mild detergent between rats to remove olfactory cues.
2.5.3 Morris water maze
Procedures for water maze cued test, acquisition training, forward probe trial, reversal training and reversal probe trials were similar to those described previously [58]. The Morris water maze (MWM) apparatus consisted of a circular white plastic pool, 2 m in diameter, and a platform made of clear Perspex (10 cm × 30 cm, d × h). The lighting of the maze was kept constant at 100lx (at water level) and the water temperature was maintained at 25 ± 3 °C throughout the testing. A video camera located above the apparatus was connected to a DVD recorder for subsequent behavioural tracking using Ethovision® software (Noldus, The Netherlands). The maze was surrounded by curtains, on which visual cues were hung during acquisition and reversal training and during probe trials. The cues consisted of geometric shapes (a filled circle, an open square and a series of wavy lines) printed in black on white A3 paper.
The cued test was used as a control procedure to assess rats’ vision, ability to swim and ability to identify the platform as an escape route from the maze. The pool was filled to 2 cm below the height of the platform and the top of the platform was covered with black plastic so that it was clearly visible. Rats underwent 4 trials each and both the start position and the platform position were quasi-randomised. Rats were given 120 s to locate the visible platform. Once the animal located the platform successfully, they were allowed to remain there for 10 s. If the animal did not locate the platform within 120 s, it was guided towards it by the experimenter and placed onto the platform for 10 s. The animal was then removed from the pool, dried off with a cotton towel and returned to a heated recovery cage for an inter-trial period (approximately 5–15 min). For acquisition training, the pool was filled to 2 cm above the level of the platform such that the platform was hidden below the surface of the water. The cues were hung on the curtains surrounding the pool and were visible at water level. The training consisted of four trials per day over five consecutive days, throughout which the platform was positioned in the southwest quadrant of the pool. For each trial, the animal was released from one of four release points in a quasi-random order, with its head facing towards the wall of the pool and away from the platform. Rats were given 120 s to locate the hidden platform. Once the animal located the platform successfully, they were allowed to remain there for 10 s. If the animal did not locate the platform within 120 s, it was guided towards it by the experimenter and placed onto the platform for 10 s. The animal was then removed from the pool, dried off with a cotton towel and returned to a heated recovery cage for an inter-trial period (approximately 5–15 min). The distance moved to get onto the platform (path length) was the primary outcome measure and was determined with the aid of Ethovision® software. The order in which the rats were tested was randomised every day to avoid any confounding effects of time of day. A probe trial was carried out the day after acquisition training was completed. The platform was removed from the maze, and rats were released from the northeast quadrant. The probe trial consisted of a single 120-s trail for each rat. The proportion of time spent and distance moved in each quadrant was determined with the aid of Ethovision® software. For reversal training, the protocol was the same as for acquisition training except that the platform was positioned in the opposite quadrant of the pool (northeast). Reversal training was performed over five consecutive days. The parameters tested were: proportion of time spent and proportion of distance moved in the “old” location or area where the platform had been located (southwest quadrant), and the proportion of time spent and proportion of distance moved in the “new” platform location (northeast quadrant). At the end of reversal training, a reversal probe trial was carried out. This was similar to the initial forward probe trial except the rats were all released from the southwest quadrant.
2.6 Transcardial perfusion
At the end of the experimental period (day 65 or 67 post-surgery), rats were terminally anaesthetised with pentobarbital (100mg/0.5ml i.p.) and transcardially perfused with heparinised saline followed by 4% paraformaldehyde prepared in 0.1 M phosphate buffer (pH 7.4). Brains were removed and post-fixed overnight in 4% paraformaldehyde then stored in cryoprotective solution (25% sucrose-azide in PBS) until sectioning.
2.7 Immunohistochemistry
Whole, perfused brains were removed from the cryoprotective sucrose solution, snap-frozen by immersion in isopentane on dry ice and cut into 10μm coronal sections on a cryostat. Sequential sections were taken from approximately Bregma 3.70 mm so as to include the prelimbic region of the PFC, and from approximately Bregma –3.14 mm so as to include the CA1 region of the hippocampal formation. For PFC sections, a series of six slides was collected per brain, with six sections per slide, and for hippocampal sections a series of nine slides per brain, with four sections per slide, were collected. The sections were mounted directly onto Superfrost® charged microscope slides which were washed (3× 10 min in phosphate buffered saline (PBS), 0.1 M, pH 7.4, or PBS-Tween 20 (PBS-T), 0.5 μl/ml) and incubated at room temperature for 2h with a blocking solution (for single label synaptophysin immunohistochemistry: 3% normal rabbit serum (Sigma, Ireland) and 0.2% Triton-X (Sigma, Ireland) in PBS; for double-label synpato-physin and vGlut/vGAT staining: 5% normal goat serum (Sigma, Ireland) and 0.2% Triton-X in PBS). Sections were then incubated overnight in a humidity chamber with the appropriate primary antibody diluted in blocking solution. The primary antibodies used were: mouse monoclonal anti-synaptophysin (1:1000, Millipore, Ireland), guinea-pig polyclonal anti-vGLUT1 (1:1000, Synaptic Systems, Germany) and rabbit polyclonal anti-vGAT (1:500, Synaptic Systems, Germany). Sections were again washed three times in PBS or PBS-T before being incubated for 3 h with the appropriate secondary antibody. Alexa Fluor® (AF) 488-conjugated rabbit anti-mouse IgG (1:100, Invitrogen, supplied by Biosciences, Ireland) was used for single-label synaptophysin staining, and AF488-conjugated goat anti-mouse (1:200, Invitrogen, supplied by Biosciences, Ireland) and cyanine (CY)3-conjgated goat anti-guinea pig or CY3-conjugated goat anti-rabbit (both 1:200, Jackson ImmunoResearch Europe Ltd., UK) were used for double labelling. Sections were washed a further three times (PBS-T, PBS and phosphate buffer, 1 × 10 min in each) and then coverslipped with the fluorescent mounting medium, FluoromountTM (Sigma Ireland). No staining was observed in sections where the primary antibody was omitted (negative control).
For synaptophysin single staining, sections from four to six rats per group were collected and six to seven sections from each rat were imaged (randomly selected, non-sequential sections from multiple slides). Three to four images were obtained for each of the regions of interest (PFC and hippocampus) for each hemisphere. The images were adjacent, but not overlapping, and the average of these images was used to calculate the density of synaptophysin in the region of interest for that section. For double labelling, sections from 4 rats per group were collected. Three sections per rat were analysed for double labelling of synaptophysin and vGLUT, and three sections per rat for synaptophysin and vGAT. For each section, 2 images were captured from one hemisphere for each of the regions of interest and the hemisphere imaged was alternated between sections.
Images were obtained using an Olympus Fluoview 300 laser scanning confocal microscope or an Andor Olympus spinning disc microscope with a 60× objective oil-immersion lens (NA 1.42). For each image, serial z-sectioning was performed, yielding 13–20 optical sections, each of thickness 0.5 μm. Single-labelled optical sections were combined through the z-axis into a compressed single z-stack image and compressed images were analysed with the aid of McMaster Biophotonics Facility (MBF) ImageJ software to determine the density of synaptophysin as a percentage of the tissue area. Double-labelled serial z-stacks were imaged (using a red laser λ561 nm excitation, followed by green laser 488nm) and complete stacks were analysed using the MBF ImageJ colocalisa-tion analysis plugin. Pearson’s coefficient was used as a measure of colocalisation (+1 total colocalisation, –1 total exclusion). Representative double-labelled images were generated by compressing the serial z-stacks through the z-axis.
2.8 Statistical analysis
The experimental outcomes assessed were behavioural and immunohistochemical measures. All data were tested for normality and homogeneity of variance using Shapiro-Wilk and Levene’s tests, respectively. Parametric data were analysed using two-way repeated measures analysis of variance (ANOVA) or by a one-way ANOVA. Fisher’s LSD tests were used to make pairwise post hoc comparisons, as appropriate. For simple sham vs. SNL group comparisons, Student’s unpaired two-tailed t-test was used. Where possible, non-parametric data were log-transformed, and analysed similarly to parametric data. If non-parametric data could not be transformed, they were analysed using Friedman’s two-way ANOVA by ranks, followed by Mann-Whitney U or Wilcoxon signed-ranks tests. p ≤ 0.05 was considered statistically significant. Data were analysed using SPSS software for Windows and results were depicted graphically with the aid of GraphPad Prism software. For clarity of presentation, all data are expressed as means ± SEM.
3 Results
3.1 Sensory testing
3.1.1 von Frey test
The 50% mechanical response threshold of the ipsilateral hind-paw was decreased in the SNL group compared with the sham control group on days 3–13 post-surgery and at the final time-point day 62/64 post surgery, suggesting persistent expression of mechanical allodynia in SNL rats (Fig. 2). The response threshold was also significantly decreased post-surgery in both sham and SNL groups compared with their respective baselines. Full details of the outcome of statistical analyses are described in the figure legends.
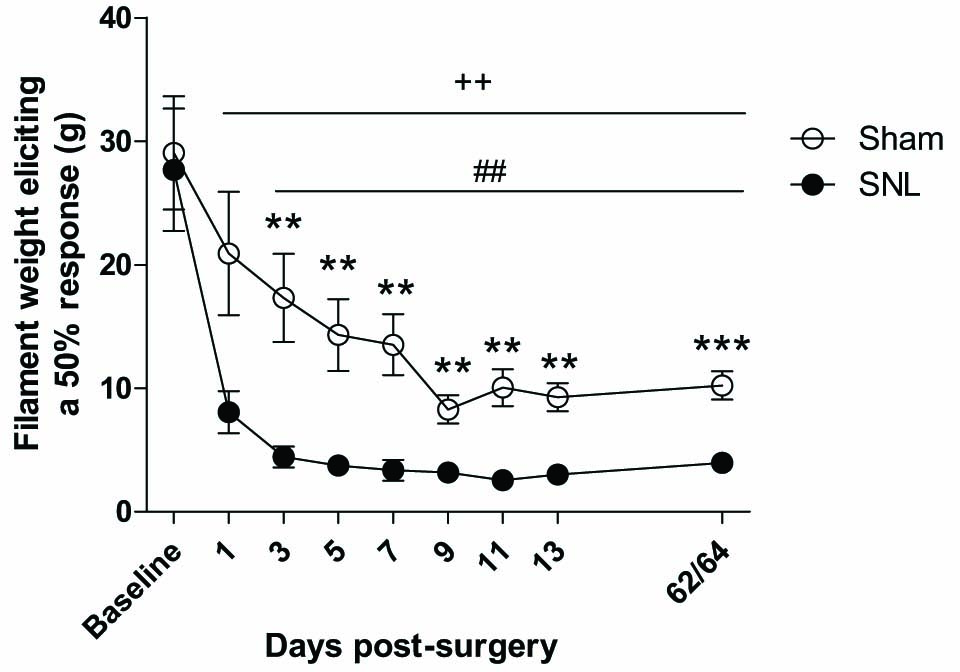
Effect of SNL surgery on the 50% mechanical withdrawal threshold of the ipsilateral hind-paw. An overall Friedman’s ANOVA by ranks produced a significant result (χ2 = 113.39, p<0.001). Friedman’s ANOVA by ranks on individual groups revealed significant effects of time in both sham (χ2 =36.06, p<0.001) and SNL groups (χ2 =43.15, p<0.001). SNL rats expressed mechanical allodynia from day 3 post surgery onwards compared with sham controls (Mann-Whitney Ö tests; *p<0.05, **p<0.01, ***p<0.001) and with pre-surgery baseline (Wilcoxon signed-ranks test; ++p< 0.001). There was also a decrease in threshold in the sham controls post-surgery compared with baseline (Wilcoxon signed-ranks test; ##p<0.01). n= 11–12
3.1.2 Hargreaves test
Paw withdrawal latency was decreased in the ipsilateral hind-paw of SNL rats compared with sham rats on days 8,16 and 63/65 post-surgery (Fig. 3). Latency was also decreased in both the sham (days 8 and 16) and SNL group (days 8, 16 and 63/65) compared with their respective baselines. These results suggest expression of thermal hyperalgesia in the SNL group which was still evident 63–65 days post-surgery.

Effect of SNL surgery on ipsilateral hind-paw withdrawal latency in the Har-greaves test. The overall Friedman’s ANOVA by ranks was significant (χ2 =37.10, p< 0.001). Friedman’s ANOVA by ranks for the SNL group indicated a significant effect of time in that group (χ2 = 16.56, p = 0.001). Paw withdrawal latency was decreased in the SNL group compared with the sham control group at all post-surgery time points (Mann-Whitney U tests; **p<0.01, *p<0.05). Withdrawal latency decreased over time in the sham group (Wilcoxon signed-ranks tests; #p<0.05 days 8 and 16 vs. baseline) and in the SNLgroup (+p<0.01 days 8,16 and 63/65 vs. baseline). n= 10–12.
3.1.3 Acetone-drop test
The number of responses to acetone application was significantly greater in the SNL group than in the control group on days 12 and 61/63 post-surgery (Fig. 4). There was a strong trend for a similar increase on day 4, that just failed to reach the level of statistical significance (p = 0.059). The number of responses was also increased post-surgery compared with baseline in both sham and SNL groups compared with their respective baselines. These data suggest persistent expression of cold allodynia in the nerve-ligated rats.

Effect of SNL surgery on the total number of hind-paw withdrawal responses following acetone application to the ipsilateral hind-paw. The overall Friedman’s ANOVA by ranks was significant (χ2 =57.54, p<0.001) and Friedman’s ANOVA by ranks showed effects of time in both the sham (χ2 =26.04, p< 0.001) and the SNL (χ2 = 26.06, p< 0.001) groups. The number of ipsilateral hind-paw withdrawal responses to acetone was significantly greater in SNL rats compared with sham controls on days 12 and 61/63 post-surgery (Mann-Whitney U tests; **p<0.01) and a similar trend was observed on day 4 (p = 0.056) There was an increase in the number of responses post-surgery compared with baseline in both the sham (Wilcoxon signed-ranks tests; #p<0.05) and the SNL groups (++p<0.01) compared with their respective baselines. n= 11–12.
3.2 Cognitive testing
3.2.1 Novel-object recognition
Discrimination ratios for the identical objects in exposure 1, and familiar and novel objects in exposure 2 were calculated according to the formula given in Section 2.5.1 There were no differences in object exploration (object 1 vs. object 2) in either group or between groups in exposure 1 (Fig. 5). In exposure 2, only the sham group displayed a significant preference for the novel object as shown by a greater discrimination ratio for the novel-object than for the familiar object. Familiar object exploration was significantly lower and novel-object exploration was significantly higher in the sham group than in the SNL group, suggesting there was a deficit in recognition memory in the nerve-injured rats (Fig. 5). SNL surgery was not associated with alterations in locomotor activity as measured by the total distance moved during the 5 min novel-object habituation trial (sham 1402.9 ±172.2 cm vs. SNL 1284.2 ± 132.7 cm, p = 0.62, Student’s unpaired two-tailed t-test), suggesting that the reduced novel-object discrimination was not due to surgery-related impairment of movement.

Novel-object recognition in sham and SNL rats. Each exposure was analysed by one-way ANOVA (exposure 1: F(3,37) = 0.049, p = 0.985; exposure 2:F(3,37) = 3.534, p = 0.025). There were no differences in object discrimination in exposure 1. In exposure 2, sham rats only expressed a preference for the novel object (Fisher’s LSD post hoc test; **p<0.01 vs. sham-familiar). The exploration of the familiar object was higher in the in the SNL group than in the sham group and exploration of the novel object was lower in the SNL group than in the sham group (#p<0.05). n = 8–ll.
3.2.2 Air-puff passive avoidance
Exposure to air-puff produced a significant passive-avoidance response in both sham and SNL groups, as indicated by the increase in retention step-through latency compared with acquisition but there was no significant difference in the response between the sham and the SNL groups (Fig. 6). These results suggest that SNL surgery did not affect passive-avoidance responding in this paradigm.

Passive-avoidance responding following air-puff exposure in sham and SNL rats. An overall Friedman’s ANOVA including both groups and both time points was significant (χ2 = 18.52, p< 0.001). Both the sham (##p < 0.01) and SNL (#p< 0.05) groups showed an increase in step-through latency in the retention trial compared with the acquisition trial (Wilcoxon signed-ranks tests) but there was no difference in the retention step-through latency between sham and SNL groups. n = 8–10.
3.2.3 Morris water maze
3.2.3.1 Acquisition training and forward probe trial
In the acquisition training phase, the path length decreased over time in both the sham and the SNL groups, which suggests that rats successfully learned to locate the platform (Fig. 7A). There were no between-group differences, indicating that both groups learned equally well, and therefore SNL surgery did not affect the acquisition of spatial reference memory. A similar pattern of results was observed for the latency to get onto the platform (data not shown). In the forward probe trial, the percentage distance moved in each of the quadrant zones, and the platform and annulus zone, by sham and SNL groups, was compared by Student’s unpaired two-tailed r-tests. There were no differences in the percentage distance moved (Fig. 7B) or the percentage time spent (data not shown) in any of the zones, suggesting there was no effect of SNL surgery on spatial memory as assessed by the paradigm. As expected, the percentage distance moved in the SW quadrant, where the platform had been located, was above the level of chance (25%, Fig. 7B).

(A) Path length to locate the platform in sham and SNL rats in the acquisition phase of the Morris water maze. Two-way repeated measures ANOVA of log transformed data revealed an overall effect of time (F(4,84) = 21.97, p<0.001). The path length decreased over days in both the sham (##p<0.01 days 2–5 vs. day 1) and the SNL groups (+++p< 0.001 days 3–5 vs. day 1; Fisher’s LSD post hoc tests). There were no between-group differences at anytime point. (B) Percentage distance moved in arena zones during the probe trial in sham and SNL groups. The dashed line represents the % distance animals would move in each quadrant by chance (25%). Distance moved by both sham and SNL groups in the SW quadrant, where the platform had been located, was above the level of chance. There were no between-group differences (Student’s unpaired r-tests). n= 11–12.
3.2.3.2 Reversal training and reversal probe trial
The percentage distance moved in the southwest quadrant decreased over time in both sham and SNL groups. The proportion of distance moved in the SW quadrant, i.e. the old platform location, was significantly greater in the SNL rats compared with the sham rats on day 2 (Fig. 8A). A similar result was observed in assessing the percentage time spent in the old quadrant location, with SNL rats spending a significantly greater proportion of time in the old quadrant location than the sham controls on day 2 of reversal training (sham 16.9± 1.6% vs. SNL 24.2 ±2.8%; p<0.05, data not shown). These results suggest that the SNL rats adapted more slowly to the change in platform location compared with sham controls and tended to revert to the old location more frequently. The percentage distance moved in the new platform location increased over time in both the sham and SNL groups. There were no significant differences between the groups at any of the time points (data not shown). In the reversal probe trial, the percentage distance moved by sham and SNL rats in each of the defined zones was calculated. The percentage distance moved in the NE quadrant was, as expected, greater than the level of chance as this is where the platform had most recently been located. Student’s unpaired two-tailed t-tests were used to compare the percentage distance moved in each of the four quadrant zones and the platform and annulus zone in sham and SNL groups. The sham group spent a significantly greater proportion of their total distance moved in the NE quadrant (new platform location) than the SNL group in the reversal probe trial (Fig. 8B). These data indicate that despite reversal training over 5 days, learning and memory of the new platform location was impaired in the SNL group compared with the sham controls. Taken together, the results of the reversal training and reversal probe tasks suggest a pattern of impaired cognitive flexibility, with SNL rats tending to return to the old platform location more than sham controls, and to spend less time in the new platform location. This was also evident from the tracking of the paths of individual rats in the reversal trials, which was done with the aid of Ethovision® software (see Fig. 8C).

(A) Percentage distance moved in the SW quadrant (old platform location) by sham and SNL rats (data averaged over 4 trials per day). Two-way repeated measures ANOVA revealed a significant effect of time (F(4,84) – 20.43, p< 0.001) and a significant interaction of time and surgery (F(4,84) – 2.76, p – 0.033). The % distance moved in the old location decreased overtime in both groups (Fisher’s LSD post hoc tests; ##p<0.01 sham days 2–5 vs. day 1, ++p<0.01, SNL days 3–5 vs. day 1). The % distance moved in the old quadrant was significantly greater in the SNL group than the sham control group on day 2 (Fisher’s LSD post hoc test; *p<0.05). (B) Percentage distance moved in arena zones during the reversal probe trial in sham and SNL groups. The dashed line represents the % distance animals would move in each quadrant by chance (25%). The % distance moved in the NE quadrant was above the level of chance for both sham and SNL groups. The sham rats’ distance moved in the NE quadrant as a % of their total distance moved during the trial was greater than that of the SNL rats (Student’s unpaired t-test; *p<0.05). n= 11–12. (C) Representative images of individual Ethovision® tracks for an individual (i) sham or (ii) SNL rat. Images indicate that the proportion of time and movement in the old location was greater in the SNL rats than in the sham controls.
3.3 Immunohistochemistry
The synaptophysin density, or the area of positive synapto-physin staining as a percentage of the total tissue area, was calculated for all CA1 and mPFC (prelimbic area) images. No significant hemispheric differences were observed and therefore images from the right and left sides of the brain were pooled. The results from each rat were used to calculate an average density per rat and the data presented shows the average per group, taking each rat as an experimental unit (n = 4–6, see Figs. 9D and 10D). There were no significant differences in synaptophysin density between sham and SNL groups in either region of interest. Representative 60 × images from sham and SNL rats from both regions are presented in Figs. 9 and 10 (B and C). To investigate whether SNL surgery was associated with differences in the distribution of synaptophysin, double-labelling immunohistochemistry for synaptophysin and the vesicular GABA transporter vGAT, and synaptophysin and the vesicular glutamate transporter vGLUT was performed. Pearson’s coefficient was used as a measure of colocalisation. Similar to single-labelled images, an average Pearson’s value per rat was calculated and then used to calculate an average per group. Results are presented in Table 1 and representative 60× double stained images are presented in Fig. 11. There were no between-group differences in the colocalistaion of synaptophysin and either vGLUT or vGAT in the regions of interest, the mPFC and the CA1.
Pearson’s coefficients for colocalisation of synaptophysin and vGAT/vGLUT. n = 4 per group.
| CA1 | mPFC | |||
|---|---|---|---|---|
|
|
|
|||
| Sham | SNL | Sham | SNL | |
| Immunostain | ||||
| Synaptophysin+vGAT | 0.328 ± 0.027 | 0.373 ± 0.026 | 0.372 ± 0.040 | 0.414 ± 0.033 |
| Synaptophysin+vGLUT | 0.365 ± 0.022 | 0.342 ± 0.008 | 0.428 ± 0.063 | 0.467 ± 0.060 |
![Fig. 9
(A) CA1 region of the hippocampus adapted from the Rat Brain Atlas [59]. The black squares indicate the area from which the images were obtained. (B) 60x representative image of synaptophysin immunostained section from the CA1 of a sham-operated and a (C) SNL-operated rat. Scale bar–30 μm. (D) Quantification of synaptophysin staining density in the CA1 of sham and SNL rats. There was no difference in the average % area of synaptophysin staining between groups (Student’s unpaired t–test, p > 0.05).](/document/doi/10.1016/j.sjpain.2015.09.008/asset/graphic/j_j.sjpain.2015.09.008_fig_010.jpg)
(A) CA1 region of the hippocampus adapted from the Rat Brain Atlas [59]. The black squares indicate the area from which the images were obtained. (B) 60x representative image of synaptophysin immunostained section from the CA1 of a sham-operated and a (C) SNL-operated rat. Scale bar–30 μm. (D) Quantification of synaptophysin staining density in the CA1 of sham and SNL rats. There was no difference in the average % area of synaptophysin staining between groups (Student’s unpaired t–test, p > 0.05).
![Fig. 10
(A) Medial prefrontal cortex (mPFC, prelimbic area) adapted from the Rat Brain Atlas [59]. The black squares indicate the area from which the images were obtained. (B) 60 × representative image of synaptophysin immunostained section from the mPFC of a sham-operated and a(C) SNL-operated rat. Scale bar–30μm. (D) Quantification of synaptophysin staining density in the mPFC of sham and SNL rats. There was no difference in the average % area of synaptophysin staining between groups (Student’s unpaired t–test, p > 0.05).](/document/doi/10.1016/j.sjpain.2015.09.008/asset/graphic/j_j.sjpain.2015.09.008_fig_011.jpg)
(A) Medial prefrontal cortex (mPFC, prelimbic area) adapted from the Rat Brain Atlas [59]. The black squares indicate the area from which the images were obtained. (B) 60 × representative image of synaptophysin immunostained section from the mPFC of a sham-operated and a(C) SNL-operated rat. Scale bar–30μm. (D) Quantification of synaptophysin staining density in the mPFC of sham and SNL rats. There was no difference in the average % area of synaptophysin staining between groups (Student’s unpaired t–test, p > 0.05).
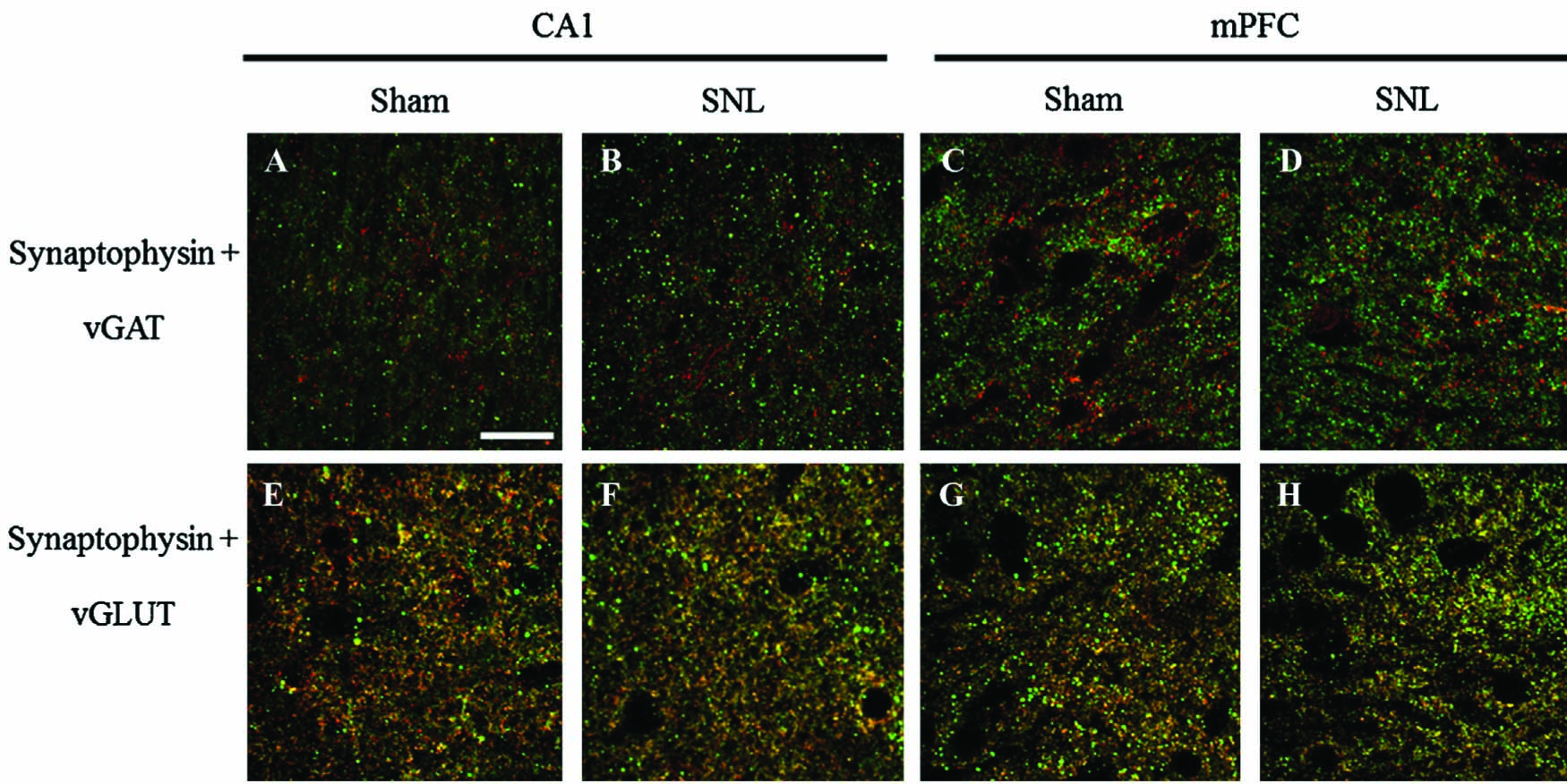
Representative images of double immunostaining for synaptophysin and vGAT (A-D) and synaptophysin and vGLUT (E-H) in the CA1 region of the hippocampus and the mPFC (prelimbic area). Scale bar–30 μm.
4 Discussion
SNL surgery in mid-aged rats resulted in mechanical and cold allodynia and thermal hyperalgesia. In addition, SNL-operated rats showed impairments in novel-object recognition and MWM reversal tasks. These results represent the first report that the L5-L6 SNL model of neuropathic pain in rats is associated with deficits in recognition memory and cognitive flexibility. These behavioural effects were not accompanied by any significant changes in expression of the synaptic vesicle protein synaptophysin in the CA1 region of the hippocampus or in the prelimbic region of the mPFC. Furthermore, there were no surgery-related changes in colocalisation of synaptophysin with either vGLUT or vGAT, suggesting that distribution of synaptic connections on GABAergic and glutamatergic neurons was not altered by SNL.
Significant mechanical and cold allodynia, and thermal hyper-algesia, were observed in the ipsilateral hind-paw of SNL rats compared with sham controls. Changes were also observed in sham sensitivity to mechanical, heat and cold stimuli compared with baseline. Significant decreases in sham withdrawal thresholds relative to baseline have been reported previously in the SNL model [60] and other nerve-injury models [61,62], up to 40 days post-surgery. It is possible that these changes are due to sensitisation of the hind-paws over time. This is consistent with the observation by Chaplan at al. [63] that repetitive low-intensity stimulation with von Frey filaments of the hind-paw of naïve unoperated rats was associated with a gradual decrease in mechanical response threshold. This is presumed to be adaption of behaviour in response to “annoyance or nuisance in the absence of nociception”. Nevertheless, sensitivity to mechanical and thermal stimuli was significantly greater in SNL rats compared with the sham controls, confirming that nerve injury produced the expected pain-related phenotype.
In the novel-object recognition task, mid-aged SNL rats displayed no preference for the novel object compared with the familiar object, and their exploration of the novel object was reduced compared with that of the sham control group. This indicates a specific deficit in recognition memory, which was not explained by altered locomotor activity in the nerve-injured rats. Deficits in novel-object recognition associated with pain have been demonstrated previously in younger rats in an inflammatory pain model [10] and in a mouse neuropathic pain model [64].
SNL surgery did not affect the performance of mid-aged rats in the air-puff passive-avoidance paradigm. This finding corroborates our previous results [54], and indicates that air-puff-induced passive-avoidance performance is not a reliable predictor of pain-related cognitive impairment in the SNL model. It is also possible that aversive memory specifically, as opposed to other types of cognition, is not affected in chronic pain. A recent study by Mutso et al. [11] found that nerve injury was associated with impaired extinction of context-conditioned fear in rats, an effect accompanied by abnormal hippocampal functioning. Thus, failure to detect effects of SNL surgery on passive avoidance in the present study could relate to a deficit in aversive-memory extinction in SNL rats, though this hypothesis requires further investigation.
There were no effects of SNL surgery on spatial learning or memory in the traditional water-maze task. A study by Hu et al. [6] found impairments in a similar task in an L5-transection model of neuropathic pain. It is possible, therefore, that pain-related impairment of spatial memory may depend on the specific neuropathic pain model used. However, our finding of impaired spatial reversal in the water maze in mid-aged rats supports and extends the work of Leite-Almeida et al. [8], who showed a similar effect in mid-aged rats in the SNI model. The fact that this deficit was preserved across different models strengthens the case for a link between neuropathic pain and cognitive impairment. Furthermore, these results suggest that mid-age may be the optimum point at which to detect pain-related differences in cognition, possibly due to an age-specific neurochemical, neuroendocrine and neuroplastic state. The observations also suggest that cognitive flexibility may be particularly sensitive to the effects of pain. Cognitive flexibility is analogous to executive functioning in humans, and impaired executive functioning has been observed in chronic pain patients [24, 25, 26,27, 2829]. Executive functions are thought to be heavily dependent on the PFC, and this region has consistently shown structural and functional alteration in pain conditions in humans [65, 66, 67, 68, 69, 70, 71,72] and rodents [73,74]. Interestingly, a more recent study by Leite-Almeida et al. [75] found differential effects of lateralised nerve injury, with left SNI resulting in increased anxiety-like behaviour and right SNI selectively resulting in cognitive dysfunction. Right SNI rats performed poorly compared with left SNI and sham control rats on PFC-dependent water maze working memory, attentional set-shifting and variable delay-to-signal impulse control tasks. This finding appears to contradict both those authors’ previous study showing impaired cognitive flexibility in mid-aged rats following left SNI, and the present results demonstrating cognitive impairment following left SNL. However, it was noted that lateralisation of task-related activity in the PFC decreased with increasing age, i.e., tasks associated with unilateral activation in young subjects are associated with bilateral activation in older subjects [76,77].
It has been hypothesised that pain-related changes in synaptic plasticity may, in part, be responsible for cognitive deficits in chronic pain. The number of synapses (and the strength of synaptic connections) is believed to be the cellular mechanism by which memory is encoded in the brain [78]. We found expression of the synaptic-vesicle protein synaptophysin to be unaltered in mid-aged SNL rats compared with sham controls, both in the prelimbic mPFC and in the hippocampal CA1 region. The regions were investigated based on their relevance to the cognitive deficits observed. Changes in synaptophysin expression have been shown previously in the hippocampus [14] and spinal cord in rodent models of neuropathic pain [44, 45, 46, 47,79]. Lesions of the prelimbic mPFC sub-region are associated with deficits in both delayed-non-matching-to-sample tasks (similar to the novel-object recognition paradigm used in the present study) and reversal learning [80]. Structural and functional alterations in the PFC have been shown previously in pain patients and in animal models of neuropathic pain, and dysfunction of amygdala-PFC circuitry has been proposed as a mechanism for pain-related cognitive impairment [7]. However, expression of synaptophysin in the mPFC in relation to pain and cognition has not been investigated previously. The hippocampus is known to be involved in learning and memory and spatial navigation. There has been some debate as to the involvement of the CA1 region in novel-object recognition [81]; however, it has been shown to be important for working memory and spatial novelty, but not detection of novel objects [82,83]. The novel-object paradigm used in this study did not involve the use of intra- or extra-maze reference cues and, therefore, likely assessed recognition of both spatial and object novelty. Lesion of the CA1 has been shown to impair memory acquisition in the water maze [84]. The hippocampus is activated by the experience of pain [85,86], and there is some evidence for a decrease in total hippocampal volume in older adults with chronic pain [87]. Furthermore, pain is associated with alterations in hippocampal synaptic plasticity [11,31,32], and recently a reduction in synaptophysin in the CA1 has been demonstrated in a model of pain-related cognitive impairment [14]. Ren et al. showed that impairments in short-term and working memory in the rat SNI model of neuropathic pain were associated with a reduced number of synaptophysin-positive terminals in the CA1 [14]. Therefore, reduced synaptophysin expression in the CA1 (and possibly in the mPFC) was anticipated in the present study. Thus, the lack of pain-related effects on synpatophysin expression is surprising, though there are a number of possible explanations. The supraspinal representation of peripheral models of neuropathic pain has not been studied extensively, and synaptophysin expression in discrete brain regions may differ depending on the model used (SNI vs. SNL). The age discrepancy between the rats in the two studies may also account for differences in the results. The CA1 and mPFC are implicated in normal age-related cognitive decline, and as such the effects of pain in these regions may be expressed differently in mid-aged rats. Furthermore, the lack of disparity in synpatophysin expression between left and right hemispheres may relate to the age of the animals used. Despite the methodological differences, the behavioural outcomes here, and in a previous study [14] appear to imply an overall inhibition of hippocampal and prefrontal activity in neuropathic pain models. Therefore, the possibility that synaptophysin-positive immunoreactivity was differentially distributed following SNL surgery was also investigated by double-labelling with vGLUT and vGAT. We hypothesised that altered distribution of synpatophysin, favouring expression on inhibitory terminals (without an overall change in expression), could result in the behavioural inhibition of cognitive performance we observed. However, colocalisation of synaptophysin and vGLUT appeared to be greater than that of synaptophysin and vGAT, and no pain-related differences between colocalisation of either transporter and synpatophysin were observed.
In conclusion, the current study has provided the first evidence for impaired reversal learning and novel-object recognition in mid-aged SNL (L5-L6) rats. These results were not explained by differences in synaptophysin expression or distribution in the CA1 region of the hippocampus or mPFC. Therefore, the mechanisms underlying the pain-related cognitive deficits observed require further investigation.
Highlights
L5-L6 spinal nerve ligation in rats was associated with allodynia and hyperalgesia.
Nerve-injured rats exhibited impaired recognition memory.
Nerve-injured rats exhibited impaired cognitive flexibility.
Nerve injury did not affect synaptophysin expression in the medial prefrontal cortex or hippocampal CA1
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2015.11.006.
-
Disclosures: This work was supported through the National Biophotonics and Imaging Platform, Ireland, and funded by the Irish Government’s Programme for Research in Third Level Institutions, Cycle 4, Ireland’s EU Structural Funds Programmes 2007–2013. The funding agency played no part in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
-
Conflicts of interest: The authors have no conflicts of interest to declare.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Dr. Daniel Kerr, Mr. Ambrose O’ Halloran, Ms. Elizabeth Rutledge and Dr. Elizabeth Bate.
References
[1] Berryman C, Stanton TR, Bowering JK, Tabor A, McFarlane A, Moseley LG. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain 2013;154:1181–96.Search in Google Scholar
[2] Moriarty O, Finn DP. Cognition and pain. Curr Opin Support Palliat Care 2014;8:130–6.Search in Google Scholar
[3] Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 2011;93:385–404.Search in Google Scholar
[4] Boyette-Davis JA, Thompson CD, Fuchs PN. Alterations in attentional mechanisms in response to acute inflammatory pain and morphine administration. Neuroscience 2008;151:558–63.Search in Google Scholar
[5] Cain CK, Francis JM, Plone MA, Emerich DF, Lindner MD. Pain-related disability and effects of chronic morphine in the adjuvant-induced arthritis model of chronic pain. Physiol Behav 1997;62:199–205.Search in Google Scholar
[6] Hu Y, Yang J, Hu Y, Wang Y, Li W. Amitriptyline rather than lornoxicam ameliorates neuropathic pain-induced deficits in abilities of spatial learning and memory. EurJ Anaesthesiol 2010;27:162–8.Search in Google Scholar
[7] Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 2010;30:5451–64.Search in Google Scholar
[8] Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain 2009;144:57–65.Search in Google Scholar
[9] Lindner MD, Plone MA, Francis JM, Cain CK. Chronic morphine reduces painrelated disability in a rodent model of chronic, inflammatory pain. Exp Clin Psychopharmacol 1999;7:187–97.Search in Google Scholar
[10] Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain 2004;109:214–24.Search in Google Scholar
[11] Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 2012;32:5747–56.Search in Google Scholar
[12] Pais-Vieira M, Lima D, Galhardo V. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci Lett 2009;463:98–102.Search in Google Scholar
[13] Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 2009;161:671–9.Search in Google Scholar
[14] Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology 2011;36:979–92.Search in Google Scholar
[15] Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain 2009;10:343–53.Search in Google Scholar
[16] Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev 2000;24:843–54.Search in Google Scholar
[17] Gagliese L, Melzack R. Age-relateddifferences in the qualitiesbut notthe intensity of chronic pain. Pain 2003;104:597–608.Search in Google Scholar
[18] Salthouse TA. The processing-speed theory of adult age differences incognition. Psychol Rev 1996;103:403–28.Search in Google Scholar
[19] Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychol Aging 1998;13:445–61.Search in Google Scholar
[20] Oosterman JM, de Vries K, Dijkerman HC, de Haan EH, Scherder EJ. Exploringthe relationship between cognition and self-reported pain in residents of homes forthe elderly. Int Psychogeriatr 2009;21:157–63.Search in Google Scholar
[21] Scherder EJ, Eggermont L, Plooij B, Oudshoorn J, Vuijk PJ, Pickering G, Lautenbacher S, Achterberg W, Oosterman J. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with Alzheimer’s disease. The need to control for mood. Gerontology 2009;54:50–8.Search in Google Scholar
[22] Oosterman JM, Derksen LC, van Wijck AJ, Veldhuijzen DS, Kessels RP. Memory functions in chronic pain: examining contributions of attention and age to test performance. ClinJ Pain 2011;27:70–5.Search in Google Scholar
[23] Oosterman JM, Gibson SJ, Pulles WL, Veldhuijzen DS. On the moderating role of age in the relationship between pain and cognition. Eur J Pain 2013;17:73–41.Search in Google Scholar
[24] Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. Eur J Pain 1999;3:325–33.Search in Google Scholar
[25] Grisart JM, Van der Linden M. Conscious and automatic uses of memory in chronic pain patients. Pain 2011;94:305–13.Search in Google Scholar
[26] Ryan CM, Williams TM, Finegold DN, Orchard TJ. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 1993;36:329–34.Search in Google Scholar
[27] Verdejo-Garcia A, Lopez-Torrecillas F, Calandre EP, Delgado-Rodriguez A, Bechara A. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol 2009;24:113–22.Search in Google Scholar
[28] Karp JF, Reynolds 3rd CF, Butters MA, Dew MA, Mazumdar S, Begley AE, Lenze E, Weiner DK. The relationship between pain and mental flexibility in olderadult pain clinic patients. Pain Med 2006;7:444–52.Search in Google Scholar
[29] Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling olderadults with chronic low back pain. Pain Med 2006;7:60–70.Search in Google Scholar
[30] Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev 2000;10:131–49.Search in Google Scholar
[31] Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol 2007;574:127–32.Search in Google Scholar
[32] Zhao XY, Liu MG, Yuan DL, Wang Y, He Y, Wang DD, Chen XF, Zhang FK, Li H, He XS, Chen J. Nociception-induced spatial and temporal plasticity of synaptic connection and function in the hippocampal formation of rats: a multi-electrode array recording. Mol Pain 2009;5:55.Search in Google Scholar
[33] Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol 2005;564:907–21.Search in Google Scholar
[34] Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain 2008;4:26.Search in Google Scholar
[35] Neugebauer V, Li W, Bird GC, Bhave G. Gereau RWt Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 2003;23:52–63.Search in Google Scholar
[36] Zhuo M. Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 2006;84:927–33.Search in Google Scholar
[37] Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 2007;23:259–71.Search in Google Scholar
[38] Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging 1998;19:599–606.Search in Google Scholar
[39] Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 2011;70:847–54.Search in Google Scholar
[40] Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006;137:413–23.Search in Google Scholar
[41] King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW)transgenic mouse model for Alzheimer’s disease. Behav Brain Res 1999;103:145–62.Search in Google Scholar
[42] Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 1999;38:349–59.Search in Google Scholar
[43] Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 2009;162:234–43.Search in Google Scholar
[44] Chou AK, Muhammad R, Huang SM, Chen JT, Wu CL, Lin CR, Lee TH, Lin SH, Lu CY, Yang LC. Altered synaptophysin expression in the rat spinal cord after chronic constriction injur of sciatic nerve. Neurosci Lett 2002;333:155–8.Search in Google Scholar
[45] Jaken RJ, Joosten EA, Knuwer M, Miller R, van der Meulen I, Marcus MA, Deumens R. Synaptic plasticity in the substantia gelatinosa in a model of chronic neuropathic pain. Neurosci Lett 2009;469:30–3.Search in Google Scholar
[46] Lin JY, Peng B, Yang ZW, Min S. Number of synapses increased in the rat spinal dorsal horn after sciatic nerve transection: a stereological study. Brain Res Bull 2011;84:430–3.Search in Google Scholar
[47] Peng B, Lin JY, Shang Y, Yang ZW, Wang YP. Plasticity in the synaptic number associated with neuropathic pain in the rat spinal dorsal horn: a stereological study. Neurosci Lett 2010;486:24–8.Search in Google Scholar
[48] Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412.Search in Google Scholar
[49] Burke NN, Finn DP, Roche M. Chronic administration of amitriptyline differentially alters neuropathic pain-related behaviour in the presence and absence of a depressive-like phenotype. Behav Brain Res 2015;278:193–201.Search in Google Scholar
[50] Burke NN, Geoghegan E, Kerr DM, Moriarty O, Finn DP, Roche M. Altered neuropathic pain behaviour in a rat model of depression is associated with changes in inflammatory gene expression in the amygdala. Genes Brain Behav 2013;12:705–13.Search in Google Scholar
[51] Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun 2014;42:147–56.Search in Google Scholar
[52] Burke NN, Llorente R, Marco EM, Tong K, Finn DP, Viveros MP, Roche M. Maternal deprivation is associated with sex-dependent alterations in nociceptive behavior and neuroinflammatory mediators in the rat following peripheral nerve injury. J Pain 2013;14:1173–84.Search in Google Scholar
[53] Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992;50:355–63.Search in Google Scholar
[54] Moriarty O, Roche M, McGuire BE, Finn DP. Validation of an air-puff passiveavoidance paradigm for assessment of aversive learning and memory in rat models of chronic pain. J Neurosci Methods 2012;204:1–8.Search in Google Scholar
[55] Burke NN, Hayes E, Calpin P, Kerr DM, Moriarty O, Finn DP, Roche M. Enhanced nociceptive responding in two rat models of depression is associated with alterations in monoamine levels in discrete brain regions. Neuroscience 2010;171:1300–13.Search in Google Scholar
[56] Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial nonmatching-to-sample learning task to study ‘recognition memory’. Nat Protoc 2006;1:1306–11.Search in Google Scholar
[57] King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC. 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation - an effect sensitive to NMDA receptor antagonism. Neuropharmacology 2004;47:195–204.Search in Google Scholar
[58] Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006;1:848–58.Search in Google Scholar
[59] Paxinos G, Watson C. The rat brain in stereotactic coordinates. 4th ed. San Diego: Elsevier; 1998.Search in Google Scholar
[60] Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 2004;101:476–87.Search in Google Scholar
[61] Blenk KH, Habler HJ, Janig W. Neomycin and gadolinium applied to an L5 spinal nerve lesion prevent mechanical allodynia-like behaviour in rats. Pain 1997;70:155–65.Search in Google Scholar
[62] Pitcher GM, Ritchie J, Henry JL. Nerve constriction in the rat: model of neuropathic, surgical and central pain. Pain 1999;83:37–46.Search in Google Scholar
[63] Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63.Search in Google Scholar
[64] Kodama D, Ono H, Tanabe M. Increased hippocampal glycine uptake and cognitive dysfunction after peripheral nerve injury. Pain 2011;152:809–17.Search in Google Scholar
[65] Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelma DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410–5.Search in Google Scholar
[66] DaSilva AF, Becerr L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One 2008;3:e3396.Search in Google Scholar
[67] Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 2008;60:570–81.Search in Google Scholar
[68] Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain. J Neurosci 2007;27:4004–7.Search in Google Scholar
[69] Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlate with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain 2008;131:3222–31.Search in Google Scholar
[70] May A. Chronic pain may change the structure of the brain. Pain 2008;137:15.Search in Google Scholar
[71] Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia - a voxel-based morphometry study. Pain 2007;132(Suppl. 1): S109–16.Search in Google Scholar
[72] Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 2011;31:7540–50.Search in Google Scholar
[73] Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA 2009;106:2423–8.Search in Google Scholar
[74] Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage 2009;47: 1007–14.Search in Google Scholar
[75] Leite-Almeida H, Cerqueira JJ, Wei H, Ribeiro-Costa N, Anjos-Martins H, Sousa N, Pertovaara A, Almeida A. Differential effects of left/right neuropathy on rats’ anxiety and cognitive behavior. Pain 2012;153:2218–25.Search in Google Scholar
[76] Bergerbest D, Gabrieli JD, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage 2009;45:237–46.Search in Google Scholar
[77] Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci 2004;24:7939–44.Search in Google Scholar
[78] Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 2000;23:649–711.Search in Google Scholar
[79] Sun T, Xiao HS, Zhou PB, Lu YJ, Bao L, Zhang X. Differential expression of synaptoporin and synaptophysin in primary sensory neurons and up-regulation of synaptoporin after peripheral nerve injury. Neuroscience 2006;141:1233–45.Search in Google Scholar
[80] Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 2004;28:771–84.Search in Google Scholar
[81] Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem 2002;9:49–57.Search in Google Scholar
[82] Steckler T, Drinkenburg WH, Sahgal A, Aggleton JP. Recognition memory in rats - II. Neuroanatomical substrates. Prog Neurobiol 1998;54: 313–32.Search in Google Scholar
[83] Vago DR, Kesner RP. Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res 2008;189:273–83.Search in Google Scholar
[84] Stubley-Weatherly L, Harding JW, Wright JW. Effects of discret kainic acidinduced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res 1996;716:29–38.Search in Google Scholar
[85] Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–84.Search in Google Scholar
[86] Shih YY, Chen YY, Chen CC, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res 2008;86:1801–11.Search in Google Scholar
[87] Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: apilot study. Neurology 2009;73:1567–70.Search in Google Scholar
© 2015 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial comment
- Plasma pro-inflammatory markers in chronic neuropathic pain: Why elevated levels may be relevant for diagnosis and treatment of patients suffering chronic pain
- Original experimental
- Plasma pro-inflammatory markers in chronic neuropathic pain: A multivariate, comparative, cross-sectional pilot study
- Editorial comment
- Genetic variability of pain – A patient focused end-point
- Observational study
- COMT and OPRM1 genotype associations with daily knee pain variability and activity induced pain
- Editorial comment
- Complex Regional Pain Syndrome (CRPS) after viper-bite in a pregnant young woman: Pathophysiology and treatment options
- Clinical pain research
- Complex regional pain syndrome following viper-bite
- Editorial comment
- An investigation into enlarging and reducing the size of mirror reflections of the hand on experimentally induced cold-pressor pain in healthy volunteers
- Original experimental
- An investigation into enlarging and reducing the size of mirror reflections of the hand on experimentally-induced cold-pressor pain in healthy human participants
- Editorial comment
- Multimodal Rehabilitation Programs (MMRP) for patients with longstanding complex pain conditions – The need for quality control with follow-up studies of patient outcomes
- Observational study
- Patients with chronic pain: One-year follow-up of a multimodal rehabilitation programme at a pain clinic
- Editorial comment
- Advancing methods for characterizing structure and functions of small nerve fibres in neuropathic conditions
- Clinical pain research
- Structural and functional characterization of nerve fibres in polyneuropathy and healthy subjects
- Editorial comment
- Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy
- Original experimental
- Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy
- Editorial comment
- Targeting glial dysfunction to treat post-surgical neuropathic pain
- Topical review
- Glial dysfunction and persistent neuropathic postsurgical pain
- Editorial comment
- Mechanisms of cognitive impairment in chronic pain patients can now be studied preclinically by inducing cognitive deficits with an experimental animal model of chronic neuropathic pain
- Original experimental
- Impaired recognition memory and cognitive flexibility in the ratL5–L6 spinal nerve ligation model of neuropathic pain
- Editorial comment
- Pain treatment with intrathecal corticosteroids: Much ado about nothing? But epidural corticosteroids for radicular pain is still an option
- Original experimental
- Analgesic properties of intrathecal glucocorticoids in three well established preclinical pain models
- Editorial comment
- The obesity epidemic makes life difficult for patients with herniated lumbar discs – and for back-surgeons: Increased risk of complications
- Observational study
- Obesity has an impact on outcome in lumbar disc surgery
- Editorial comment
- Finnish version of the fear-avoidance-beliefs questionnaire (FABQ) and the importance of validated questionnaires on FAB in clinical praxis and in research on low-back pain
- Clinical pain research
- Translation and validation of the Finnish version of the Fear-Avoidance Beliefs Questionnaire (FABQ)
- Editorial comment
- Pain, sleep and catastrophizing: The conceptualization matters Comment on Wilt JA et al. “A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients”
- Clinical pain research
- A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients
Articles in the same Issue
- Editorial comment
- Plasma pro-inflammatory markers in chronic neuropathic pain: Why elevated levels may be relevant for diagnosis and treatment of patients suffering chronic pain
- Original experimental
- Plasma pro-inflammatory markers in chronic neuropathic pain: A multivariate, comparative, cross-sectional pilot study
- Editorial comment
- Genetic variability of pain – A patient focused end-point
- Observational study
- COMT and OPRM1 genotype associations with daily knee pain variability and activity induced pain
- Editorial comment
- Complex Regional Pain Syndrome (CRPS) after viper-bite in a pregnant young woman: Pathophysiology and treatment options
- Clinical pain research
- Complex regional pain syndrome following viper-bite
- Editorial comment
- An investigation into enlarging and reducing the size of mirror reflections of the hand on experimentally induced cold-pressor pain in healthy volunteers
- Original experimental
- An investigation into enlarging and reducing the size of mirror reflections of the hand on experimentally-induced cold-pressor pain in healthy human participants
- Editorial comment
- Multimodal Rehabilitation Programs (MMRP) for patients with longstanding complex pain conditions – The need for quality control with follow-up studies of patient outcomes
- Observational study
- Patients with chronic pain: One-year follow-up of a multimodal rehabilitation programme at a pain clinic
- Editorial comment
- Advancing methods for characterizing structure and functions of small nerve fibres in neuropathic conditions
- Clinical pain research
- Structural and functional characterization of nerve fibres in polyneuropathy and healthy subjects
- Editorial comment
- Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy
- Original experimental
- Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy
- Editorial comment
- Targeting glial dysfunction to treat post-surgical neuropathic pain
- Topical review
- Glial dysfunction and persistent neuropathic postsurgical pain
- Editorial comment
- Mechanisms of cognitive impairment in chronic pain patients can now be studied preclinically by inducing cognitive deficits with an experimental animal model of chronic neuropathic pain
- Original experimental
- Impaired recognition memory and cognitive flexibility in the ratL5–L6 spinal nerve ligation model of neuropathic pain
- Editorial comment
- Pain treatment with intrathecal corticosteroids: Much ado about nothing? But epidural corticosteroids for radicular pain is still an option
- Original experimental
- Analgesic properties of intrathecal glucocorticoids in three well established preclinical pain models
- Editorial comment
- The obesity epidemic makes life difficult for patients with herniated lumbar discs – and for back-surgeons: Increased risk of complications
- Observational study
- Obesity has an impact on outcome in lumbar disc surgery
- Editorial comment
- Finnish version of the fear-avoidance-beliefs questionnaire (FABQ) and the importance of validated questionnaires on FAB in clinical praxis and in research on low-back pain
- Clinical pain research
- Translation and validation of the Finnish version of the Fear-Avoidance Beliefs Questionnaire (FABQ)
- Editorial comment
- Pain, sleep and catastrophizing: The conceptualization matters Comment on Wilt JA et al. “A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients”
- Clinical pain research
- A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients

