Abstract
Background and purpose
In animal studies, enhanced sensitivity to painful stimuli succeeding chronic stress has been reported, while acute stress is reported to induce analgesia. Human studies on the effect of mental stress on pain are more equivocal. A disturbed stress-response resulting in an increased sensitivity to painful stimuli has also been discussed as a potential mechanism for e.g., the fibromyalgia syndrome. Endogenous analgesia may be studied in humans by measuring the analgesic effect of heterotopic noxious conditioning stimulation. In neurophysiological animal studies this phenomenon was originally denoted “diffuse noxious inhibitory controls” (DNIC), but for human studies it has been suggested to use the term conditioned pain modulation (CPM).
The clinical relevance of aberrances in CPM is not clear. Inhibitory CPM is reported as being reduced in several medically unexplained syndromes with musculoskeletal pain aggravated by mental stress. However, whether the reported reduced CPM effects are causally related to clinical pain is unknown.
In the present study the effect of a mental stressor on CPM is studied.
Methods
With tourniquet-induced pain as the conditioning stimulus we estimated the CPM effect in twenty healthy subjects. Heat pain threshold (HPT), supra-threshold heat pain level (SHPL) and pressure pain threshold (PPT) were used as test stimuli. Measurements were performed at baseline, after a stressful task and after a non-stressful task presented in a blinded cross-over design. We used repeated-measures ANOVAs in the analysis with simple contrasts for post hoc analysis.
Results
With a ANOVA repeated measures model we found a significant task effect (F = 18.5, p ≤ 0.001), indicating that CPM was successfully induced. In our ANOVA model, we found a significant effect of stress in the contrast analysis (F = 5.2, p = 0.037), indicating that CPM was affected by the stressful task. The effects on PPT could not be analyzed due to a significant carry-over effect (for PPT only).
Conclusions
In the present blinded crossover study, we found a significant small to medium inhibitory effect of mental stress upon the CPM of thermal pain.
Implications
Our results suggest that previously reported reduced inhibitory CPM in several medically unexplained syndromes with musculoskeletal pain aggravated by mental stress possibly can be related to confounding or clinically relevant stress level differences. However, the result might be modality-specific. Further studies in patients are obviously needed, and the impact of mental stress on CPM should be investigated also with other stressors.
1 Introduction
A reduced ability to engage endogenous analgesia is one potential mechanism for the fibromyalgia syndrome [1, 2, 3, 4, 5, 6]. A disturbed stress-response resulting in an increased sensitivity to painful stimuli has also been discussed as a potential mechanism [7]. Both social stress [8] and low-grade mental stress [9] increase pain in fibromyalgia patients. However, a recent paper has challenged this view by reporting that emotional distress does not predict subsequent pain in fibromyalgia, at least not in day-to-day perspective [10].
The relation between stress and pain is complicated. In animal studies, enhanced sensitivity to painful stimuli succeeding chronic stress has been reported [11, 12, 13, 14], while acute stress is reported to induce analgesia [13]. Human studies are more equivocal. Mental stress has been reported to induce increased sensitivity to painful cold stimulation [15,16]. In other studies, mental stress has led to a decreased sensation of pain using electrocutaneous [17,18] and pressure pain stimulation [18].
Heterotopic noxious conditioning stimulation (HNCS) can induce “counterirritation analgesia.” In neurophysiological animal studies this phenomenon was originally denoted “diffuse noxious inhibitory controls” (DNIC). DNIC is thought to depend on a spino-bulbo-spinal network which modulates the transmission of signals from primary to secondary afferent neurons in the spinal cord [19, 20, 21]. In order to induce “DNIC-like effects” [22] in human experimental settings, both cold-pain [23], heat-pain [24], and tourniquet-induced pain [3,25], as well as mechanical stimulation [26,27] have been used as conditioning stimuli. Moreover, it has been suggested that the term conditioned pain modulation (CPM) should be used in human experimental studies of this phenomenon [28].
The clinical relevance of inhibitory CPM is not clear [29]. This effect is reported as being reduced in patients with migraine [30], tension type headache [31], fibromyalgia [1, 2, 3], osteoarthritis [32], irritable bowel syndrome [33] and temporomandibular disorder [34], as well as in patients using oral opioids [35]. However, whether the reported reduced inhibitory CPM is causally related to their pain or not is unknown.
Furthermore, it is incompletely known if reduced inhibitory CPM in patients is caused by, or related to, a (group) difference in perceived stress or stress response magnitude. In one CPM model perceived stress during conditioning stimulation correlated with hypoalgesia in men only [36], while another study found no effect of one hour mental stress, neither in healthy subjects nor in chronic tension-type headache patients [37]. Indeed, if mental stress is able to modify CPM one should control for this in future CPM studies, especially in studies involving patients who may perceive the experimental situation as stressful.
As a first step, we aimed to study whether CPM is affected by a mental stressor in healthy subjects. We hypothesized that mental stress induced immediately before the conditioning stimuli would modulate the CPM effect.
2 Materials and methods
2.1 Subjects
Subjects were recruited by email and direct inquiry among fellow students. Written informed consent was obtained from all subjects. Exclusion criteria were: (1) any pain that had reduced the general health or the function level during the last two weeks or caused a need for analgesics in the last five days before the trials, (2) headache more than two days per month, (3) present somatic or psychiatric illness, and (4) pregnancy. None of the invited subjects were excluded and all included subjects completed the experimental procedure.
Sample size calculations revealed that (in the case of no carry-over effects) 20 subjects would be enough to detect a population mean difference of at least 70% of the standard deviation with 5% significance level and a power of 80%. Twenty subjects (ten males and ten females, age 20–28 years, median age 24.2 (SD 2.1)) years were included after written informed consent. The project was approved by the Regional Committee for Research Ethics and by the Norwegian Social Sciences Data Services. The study was conducted according to the Helsinki Declaration.
2.2 Pocedure
We used tourniquet induced pain as the conditioning stimulus and measured the inhibitory CPM with two different heat pain measures (heat pain threshold (HPT), supra-threshold heat pain level (SHPL)) and pressure pain threshold (PPT) as test stimuli, following a stressful and a non-stressful task in a blinded cross-over design.
2.2.1 Time, place and investigators
The subjects were tested on two different days at an interval of maximum three days. On day one, the procedure was explained and performed once (without the stressful and non-stressful tasks) to accustom subjects to the procedure. On day two, the order of stressand non-stressful tasks was randomized while the complete procedure (Fig. 1) was performed by another experienced technician who was blinded for the task order: (1) Recording of baseline HPT, SHPL and PPT. (2) Stressful task or non-stressful task. (3) Recording of HPT, SHPL and PPT during the painful conditioning stimulus. (4) Recording of HPT, SHPL, and PPT (“recovery-1”). (5) Non-stressful or stressful task, 5 min. (6) Recording of HPT, SHPL, and PPT during a conditioning stimulus. (7) Recording of HPT, SHPL, and PPT (“recovery-2”). The recording of test stimuli (HPT, SHPL, and PPT) lasted approximately 3 min, and were performed in the order indicated above each time and started immediately after the desired pain level of the conditioning stimulus was reached. Blood pressure and heart rate were measured before and after each session with stressful or non-stressful task, as well as before the experiment started (Fig. 1).
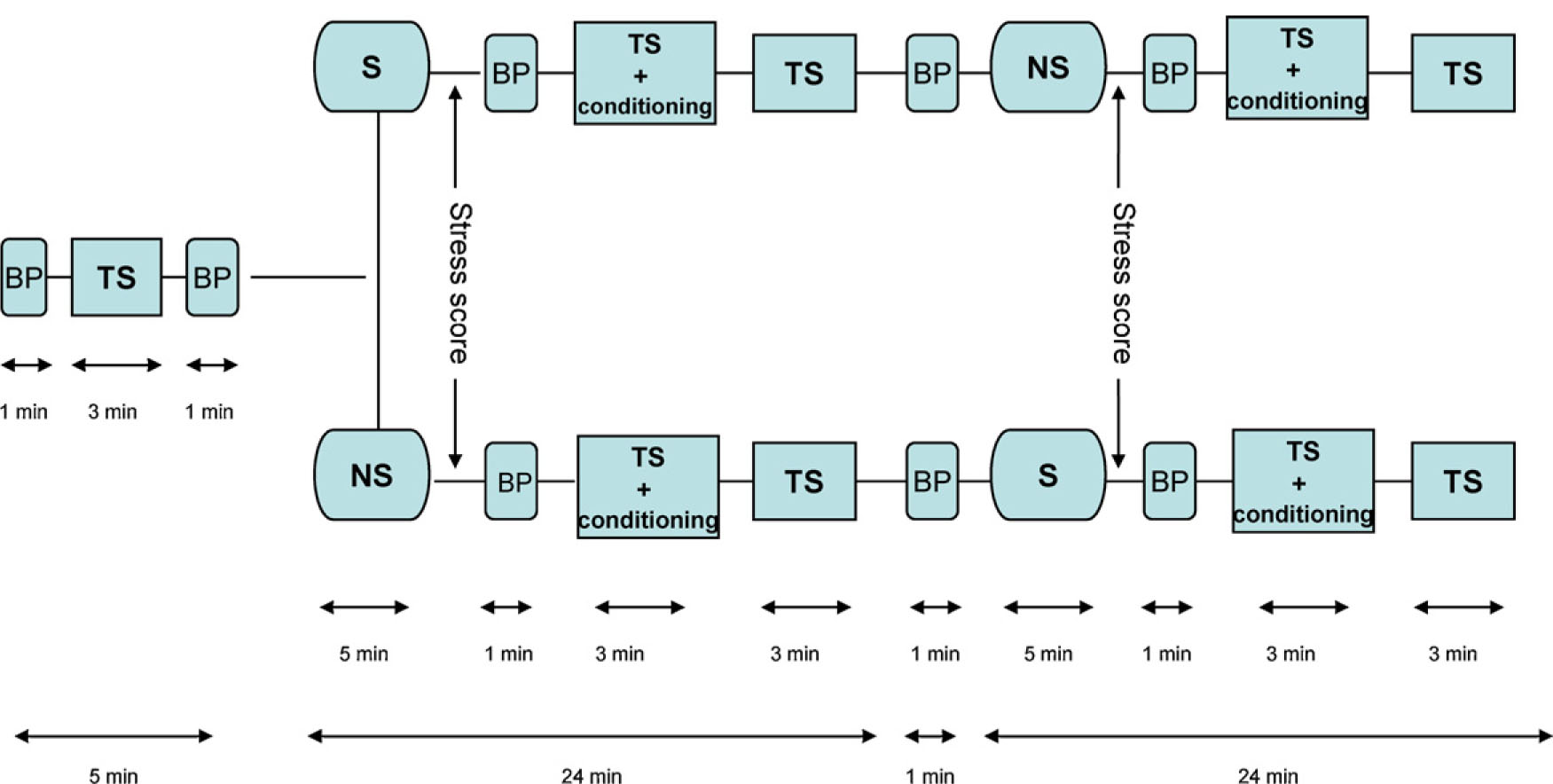
Timeline for the experiment. BP: Brachial oscillometric blood pressure recording, 30 s. TS: recording of heat pain threshold (HPT), supra-threshold heat pain level (SHPL) and pressure-pain thresholds (PPT) at baseline, approximately 3 min. S: stressful mental arithmetic task for 5min (mental arithmetic task successively subtracting 7, starting at 1000). NS: non-stressful task for 5min (listening to a CD with a well known Norwegian tale for children). TS + conditioning: conditioning stimulus for approximately 3 min; pain induced by a brachial placed blood pressure cuff inflated to 240 mmHg. Recording of HPT, SHPL and PPT during the conditioning stimulus. After TS + conditioning subjects rested 12 min before the next TS session.
2.2.2 Experimental equipment and conditions
HPT and SHPL were measured with a Somedic MSA (Sense-Lab equipment, Hörby, Sweden). The thermode was a rectangular 25 mm × 50 mm Peltier element. The baseline temperature was 32.0 °C, the maximal temperature was 55.0 °C, and the rate of change was 1 °C per second. A pressure algometer (Somedic Sales AB, Sweden) with a 1 cm2 tip was used [38] to assess PPT. The subjects were lying on a bench with the upper part of their body raised 30? during the measurements. They were sitting in an upright position in a chair during the stress-and non-stressful tasks. Blood pressure and heart rate was measured with an automatic oscillometric device (CAS 740, MAX NIBP, Bollbrügg, Germany). A cuff width of 14 cm was used, and all subjects had an arm circumference within the range specified for the use of this cuff for blood pressure measurements.
2.2.3 Pressure pain threshold measurements
Pressure pain threshold (PPT) was recorded by pushing the tip of the algometer with gradually increasing pressure (30 kPa/s) towards the belly of the temporalis muscle on the right side of the forehead. The subjects were instructed to say stop when the pain threshold level was reached. The mean value of three consecutive measurements was used for analysis.
2.2.4 Heat pain measurements
A stop button was placed in the right hand of the subjects, who were instructed to press it immediately when the desired threshold was reached. Three warm stimuli (with a random interval between 4 and 6 s) were applied to the ventral side of the right forearm. The thermode was moved proximally 5 cm after each stimulus to prevent burns. HPT and SHPL were calculated as the average of three stimuli.
For HPT subjects were instructed to press the stop button at the first moment the perception of the increasing temperature of the probe changed from “warm” to “pain.” For SHPL, the subjects were instructed to press the stop button when pain intensity was perceived as 7 on a numerical rating scale (NRS) from 0 to 10, where 0 is no pain and 10 is the worst pain imaginable. SHPL was chosen as a surrogate marker for heat pain tolerance, partly because we considered it more ethically correct, and partly because it has been successfully used by other groups studying CPM [39].
2.2.5 The conditioning stimulus
To create the painful conditioning stimulus, a blood pressure cuff (width = 14 cm) was applied proximally to the left cubital fossa and inflated to 240 mmHg to prevent blood flow. The subjects were then instructed to squeeze a soft ball with the left hand until pain was perceived as a score of seven on the NRS (recorded before the test stimuli were applied). The time before pain reached score 7 was slightly different between subjects and not recorded system-atically, but was reached within one minute for all subjects. The conditioning stimulus was chosen because of its simplicity and because it has been used in many other studies of CPM in humans [22].
2.2.6 Stressful and non-stressful task
The subjects were randomized to perform either the stressful or the non-stressful task first. Separate randomization lists were used for female and male subjects. To induce mental stress, we instructed the subjects to subtract 7 from 1000 successively for five minutes. The subjects were told that they had to have every number exactly correct to proceed, and that every mistake was recorded. They were also told that most people completed the subtractions down to approximately 100–200, and that they were expected to do so as well. For every minute during the task, it was pronounced that it was successively 4, 3, 2 and 1 min left. The number of correct subtractions was denoted as “stress task performance score.” This mental stressor was chosen because of its simplicity, and its effect was validated by BP-responses and stress scores. A well known Norwegian tale for children was used as the non-stressful task. Passive listening was chosen in an attempt to stabilize vigilance and attention levels. The subjects were instructed to relax and listen to the CD for five minutes, and they were told that they were not to be asked any questions from the story afterwards.
2.2.7 Test stimuli and stress score
First, baseline PPT, HPT, and SHPL were measured. Following the tasks, PPT, HPT, and SHPL were recorded during the painful conditioning stimulus. The subjects were subsequently asked to rate their overall perceived level of mental stress during the stress task and the non-stressful task respectively. A visual analogue scale (VAS) of 10 cm was used for this rating (endpoints were “no stress” and “worst imaginable stress”).
2.2.8 Recovery
After each testing with the conditioning stimulus, the subjects relaxed for 12 min. PPT, HPT, and SHPL were measured once more in order to estimate the rate and magnitude of the recovery process. Recovery after stress was compared with recovery after non-stress.
2.3 Data analysis and statistics
Statistical package for social sciences (SPSS) version 16 was used for statistical analysis. One-sample Kolmogorov–Smirnov test was used to assess normality of the data. We used a repeated-measures ANOVA with two within-subject factors and two between subject factors. The within-subject factors were task (three levels: baseline, stressful task and non-stressful task) and thermal pain measure (two levels: HPT and SHPL). The between-subject factors were test order (two levels: stressful or non-stressful task first) and sex (two levels: man or woman). Post hoc tests of the simple contrast between stressful and non-stressful tasks was included in the ANOVA model with both first and last task category as reference.
Heat pain recovery after stress was compared to recovery after non-stress in another repeated measure ANOVA model with three within-subject factors and two between subject factors. The within-subject factors were task (two levels: stressful task and non-stressful task), recovery (two levels: during conditioning stimulus and after recovery) and thermal pain measure (two levels: HPT and SHPL). The between-subject factors were test order (two levels: stressful or non-stressful task first) and sex (two levels: man or woman). In addition, a repeated measures ANOVA with one within-subject factor and two between subject factors was also performed for PPT. The within-subject factor was task (three levels: baseline, stressful task, and non-stressful task. The between-subject factors were test order (two levels: stressful or non-stressful task first) and sex (two levels: man or woman). Multivariate F-values are reported for ANOVAs.
The CPM effects were calculated as the difference between the threshold/level after the conditioning stimulus and the baseline threshold/level. Cohen’s effect size for the CPM effect was calculated as the difference between two effects (stressful vs. non-stressful task), divided by the pooled standard deviation. Blood pressure responses to the tasks were analyzed with Student’s t tests. Pearson analysis was used to search for correlations between blood pressure and pain variables. The variables used for the correlation analysis were: the systolic-and diastolic blood pressure levels after the tasks, blood pressure response during the tasks, HPT and SHPL following the stressful and the non-stressful tasks, as well as perceived stress score.
p-Values <0.05 were considered significant. ANOVAs with significant cross-over (order) effects were not analyzed further because the statistical power would be too low for the remaining half in the present study.
3 Results
3.1 Main analysis
All reported values were normally distributed. In our heat pain ANOVA repeated measures model we found a significant task effect (F = 18.5, p ≤ 0.001), with significant contrasts both between baseline and the stressful task (F = 18.8, p = 0.001) and between baseline and the non-stressful task (F = 38.7, p < 0.001). This proves that HPT and SHPL differed significantly between baseline, non-stress and stress conditions, indicating that CPM was successfully induced. Furthermore, we found no significant interactions with neither sex nor order, of which the last analysis indicates no carry-over effects on heat pain measures. A significant effect of the stressful task compared to the non-stressful task was found in the contrast analysis (F = 5.2, p = 0.037), indicating that the CPM effect was affected by the stressful task. As illustrated in Fig. 2, the stressful task reduced the CPM effect. Furthermore, we did not find any interaction between task and pain measure (F = 0.13, p = 0.72). The HPT and SHPL values are presented in Table 1.
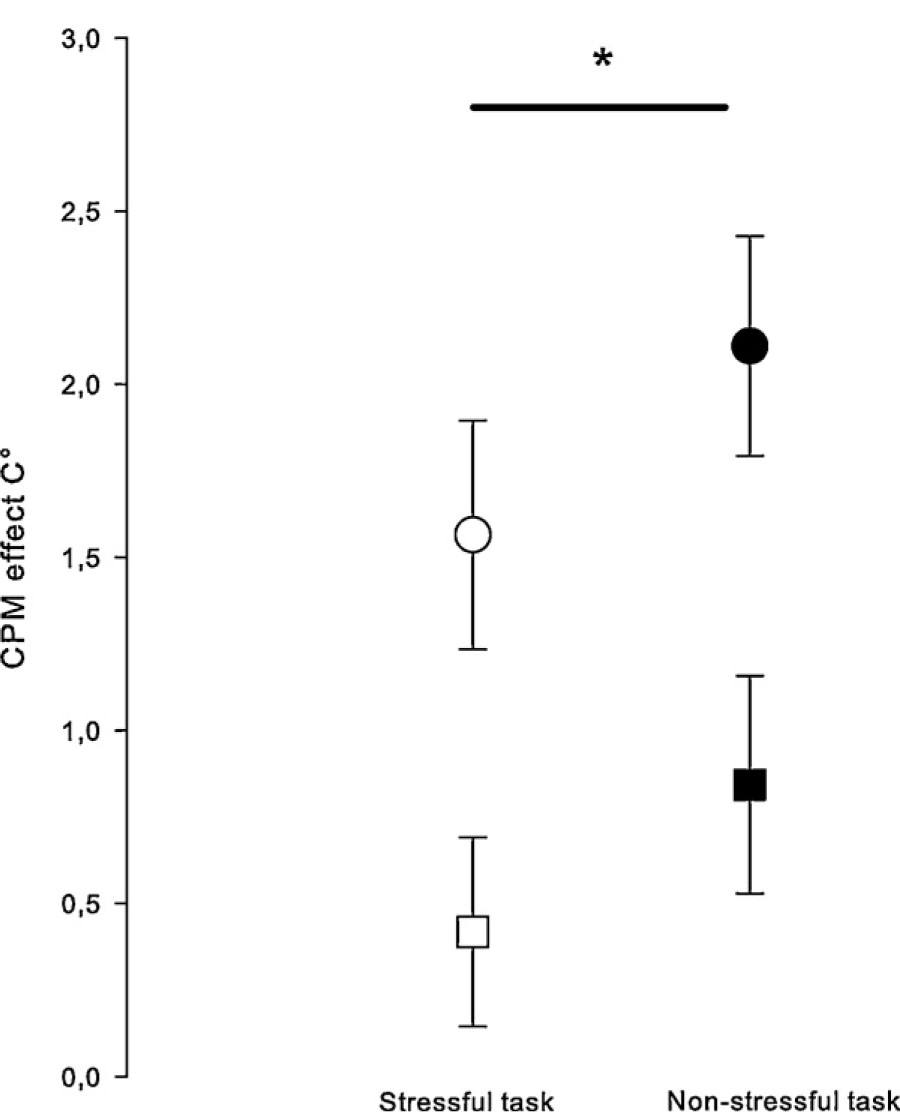
CPM effects on heat pain threshold (circles) and suprathreshold heat pain level (squares) after a stressful task (open circle/square) and after a non-stressful task (filled circle/square). Error bars indicate one standard error of the mean. *A significant effect of the stressful task was found in the ANOVA contrast analysis: p = 0.037. Thus, the stressful task reduced the CPM from tourniquet pain on heat pain.
Heat pain threshold (HPT), supra-threshold heat pain (SHPL) and temporalis pressure pain threshold (PPT) at baseline and following the stressful and non-stressful tasks in 20 healthy subjects.
| Baseline Mean ± SD | After stressful task Mean ± SD | After non-stressful task Mean ± SD | Recovery after stress | Recovery after non-stress | |
|---|---|---|---|---|---|
| HPT (°C) | 43.3 ± 2.5 | 44.9 ± 2.4[*] | 45.4 ± 2.2 | 44.2 ± 2.3 | 44.2 ± 2.4 |
| SHPL (°C) | 49.0 ± 1.9 | 49.4 ± 2.1[*] | 49.8 ± 1.4 | 48.9 ± 1.8 | 49.1 ± 1.6 |
| PPT (kPa, n = 10)[**] | 367 ± 138 | 370 ± 101 | 328 ± 110 | 360 ± 133 | 367 ± 135 |
For PPT, a significant task × order interaction was found in the ANOVA analysis (F = 3.8, p = 0.046). The task × order interaction was even more significant for the contrast between stress and non-stress (F = 7.5, p = 0.014). Hence because of this carry-over effect for PPT, PPT was not analyzed further in the present study as the remaining subgroup (n = 10, reported in Table 1) was considered too small.
3.2 Recovery
In the heat pain ANOVA recovery analysis, recovery was significant (Table 1; F = 20.3, p < 0.0005). However, no interaction was found between task type and recovery (F = 3.5, p = 0.08) or between pain type and recovery (F = 0.23, p = 0.23).
3.3 Blood pressure and stress score
Blood pressure measured after the stressful task was increased compared with level immediately before the task for both systolic (p = 0.005) and diastolic (p = 0.041) blood pressure. There was no significant change in the systolic (p = 0.37) or diastolic (p = 0.23) blood pressure during the non-stressful task (Table 2). The change in blood pressure during the tasks was significantly higher for the stressful task compared to the non-stressful task (t = 2.8, p = 0.013, t = 2.7, p = 0.014 for systolic and diastolic blood pressure respectively). There were no difference in blood pressure after the non-stressful task comparing those who performed the stressful task first with those who performed the non-stressful task first (p = 0.83).
The systolic-and diastolic blood pressure level before the stressful and non-stressful tasks and the blood pressure response during the tasks.
| Before task (absolute level) Mean ± SD | Change during the tasks Mean ± SD | Student’s t-test (before vs. after the task) | |
|---|---|---|---|
| Stressful task | |||
| Systolic (mmHg) | 116 ± 9 | 5.4 ± 7.5 | t (19) = –3.2, p = 0.01 |
| Diastolic (mmHg) | 71 ± 6 | 2.9 ± 5.8 | t (19) = –2.2, p = 0.04 |
| Non-stressful task | |||
| Systolic (mmHg) | 115 ± 7 | 1.2 ± 5.7 | t (18[a]) = 9.2, p = 0.37 |
| Diastolic (mmHg) | 71 ± 5 | –1.3 ± 4.4 | t (18) = 1.3, p = 0.23 |
The stress score was higher for the stressful task (mean ± SD: 5.1 ± SD 2.1) compared with the non-stressful task (1.1 ± 1.3), (t (19) = 11.3, p < 0.005). The group that performed the stressful task first had a significantly lower stress score after the non-stressful task than the group that did the non-stressful task first (0.2 ± 0.4) vs. (1.9 ± 1.4) respectively (t (18) = −3.75, p < 0.005). There was no difference in stress score for the stressful task between the two groups (4.4 ± 2.2 vs. 5.8 ± 1.7, t (18) = 1.59, p = 0.13).
3.4 Correlation analysis
There was no consistent general relation between blood pressure level and pain thresholds. HPT was not correlated to blood pressure. However, the blood pressure after the tasks were correlated to conditioned SHPL, most evident for the systolic blood pressure, and more consistent after the non-stressful than after the stressful task (r = 0.55–0.57, p = 0.01–0.009; Table 3).
Pearson correlations (r) between blood pressure and pain threshold/level during conditioning stimuli.
| HPT | SHPL | |||
|---|---|---|---|---|
|
|
|
|||
| After stress r (p) | After non-stress r (p) | After stress r (p) | After non-stress r (p) | |
| Baseline | ||||
| Systolic blood pressure | 0.16 (0.50) | 0.23 (0.33) | 0.41 (0.07) | 0.30 (0.20) |
| Diastolic blood pressure | 0.35 (0.13) | 0.31 (0.19) | 0.48 (0.03)[*] | 0.43 (0.06) |
| After non-stressful task | ||||
| Systolic blood pressure | 0.27 (0.25) | 0.32 (0.16) | 0.57 (0.009)[*] | 0.55 (0.01)[*] |
| Diastolic blood pressure | 0.39 (0.09) | 0.26 (0.26) | 0.41 (0.07) | 0.46 (0.04)[*] |
| After stressful task | ||||
| Systolic blood pressure | 0.15 (0.50) | 0.37 (0.11) | 0.38 (0.10) | 0.33 (0.16) |
| Diastolic blood pressure | 0.30 (0.20) | 0.36 (0.12) | 0.35 (0.13) | 0.33 (0.15) |
-
HPT, heat pain threshold; SHPL, supra-threshold heat pain level.
There were no significant correlations between the conditioned HPT and SHPL and the systolic-and diastolic blood pressure responses (p > 0.34), or between stress score and the other reported variables including the CPM effects.
4 Discussion
In the present blinded crossover study, we found a significant small to medium effect of mental stress upon inhibitory CPM on thermal pain. The effect was inhibited after completing a stressful task. Our results suggest that previously reported reduced inhibitory CPM in several medically unexplained syndromes with musculoskeletal pain aggravated by mental stress possibly can be related to confounding or clinically relevant stress level differences. While we were unable to conclude about CPM on PPT, because a carry-over order effect was found, Cathcart et al. [37] found that mental stress did not alter the CPM effect on PPT neither in healthy subjects nor in patients with tension-type headache. The different findings in the present study and the study of Cathcart may indicate that our finding is modality specific. Furthermore, one should also note that both the stressor and the test stimuli differed from those in our study while their conditioning stimulus also was considerably less painful [37].
Our stress induction was successful because both stress score and blood pressure was increased. However, we did not find a correlation between the increase in blood pressure and CPM. Hence the reduced CPM effect following stress was probably not caused by a temporarily increased blood pressure. The present study contrasts the finding of Caceres and Burns [15] who found blood pressure-related increased pain sensitivity to a cold pressor task following a mental stressor.
Altered pain sensitivity induced by CPM protocols has been demonstrated in healthy individuals for heat pain in some studies [40,41], but not in other studies [3,32,40,42]. In the present study, inhibitory CPM was observed for both HPT and SHPL in the neutral (non-stressful) condition. The post hoc analysis revealed that mental stress affected CPM more for HPT than for SHPL. This may be due to a ceiling effect, i.e., SHPL values more difficult estimate because they are closer to the maximally tolerable temperature than HPT.
We found a positive correlation between blood pressure measured after the non-stressful task and the conditioned SHPL, but not for the blood pressure measured after the stressful task. Our finding implies that the normal relationship between blood pressure and pain sensitivity (known as hypertension associated hypoalgesia) [43,44] is less evident if the blood pressure is measured after a stressful task. However, our exploratory correlation results must be interpreted with caution.
Our study had some limitations and some important strengths. A blinded crossover trial where all the subjects are exposed to both tasks and serve as their own controls will minimize variability and bias. Factors like personality and menstrual phase are less relevant in this design. However, our sample size was limited, leading to a reduced ability to detect small and moderate differences, and with low external validity of negative findings in subgroup analysis of e.g., sex. The order in which the subjects performed the stress-and non-stressful task had no impact on CPM of thermal pain. However, for another modality, deep pressure pain, a significant carry-over effect was found. Our aim was also to measure stress effects on PPT but this part could not be analyzed due to a significant carryover effect (for PPT only). PPT was also the last modality tested and we cannot exclude that the longer duration of the conditioning stimulus may have influenced the CPM effect for PPT. Moreover, the duration of the increased stress level after the stressful task could have been better monitored in the present study, e.g., with continuous measurement of blood pressure. However, no carry-over effect was found for stress scores and blood pressure.
Other studies have found duration of the CPM effect up to 10 min [45,46]. In the present study, baseline HPT was not completely reached after 12 min. However, recovery thresholds were neither used for analysis of CPM nor affected by stress in ANOVA. However, in case the CPM in the second test-sequence had been slightly affected, the randomization ensured that both tasks were equally influenced and our results are accordingly unbiased. Hence, we do not believe that the chosen recovery time influenced our results. For future studies a longer recovery period is nevertheless recommended.
5 Conclusion
Is mental stress just a confounder or is it in fact relevant for chronic pain syndromes? A substantial epidemiological literature has shown that mental and social stress is a risk factor for musculoskeletal pain [47,48,49]. However, the search for biological links between stress and pain has not been successful so far. We are not aware of other studies examining whether mental stress may affect CPM of heat pain. In this context, our study is important as it provides data to support stress-induced modulation of CPM, i.e., stress-induced suppression of endogenous pain inhibition, as a candidate mechanism for such a link. We hypothesize that this mechanism may be of relevance for several medically unexplained syndromes with musculoskeletal pain aggravated by mental stress [50]. Further studies in patients are obviously needed, and the impact of mental stress on CPM should be investigated also with other stressors.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2012.05.069.
-
Conflict of interest: The authors have no financial or other relationships to report that might lead to a conflict of interest.
Acknowledgment
We are most grateful for invaluable assistance from technician Marit Stjern in performing the experimental procedure.
References
[1] Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005;114:295–302.Suche in Google Scholar
[2] Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain 1997;13:189–96.Suche in Google Scholar
[3] Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 1997;70:41–51.Suche in Google Scholar
[4] de Souza JB, Potvin S, Goffaux P, Charest J, Marchand S. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain 2009;25:123–7.Suche in Google Scholar
[5] Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Grignon S, Marchand S. DRD3 Ser9Gly polymorphism is related to thermal pain perception and modulation in chronic widespread pain patients and healthy controls. J Pain 2009;10:969–75.Suche in Google Scholar
[6] Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Marchand S, Grignon S. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. Eur J Pain 2010;14:742–6.Suche in Google Scholar
[7] Okifuji A, Turk DC. Stress and psychophysiological dysregulation in patients with fibromyalgia syndrome. Appl Psychophysiol Biofeedback 2002;27:129–41.Suche in Google Scholar
[8] Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med 2001;23:215–26.Suche in Google Scholar
[9] Nilsen KB, Sand T, Westgaard RH, Stovner LJ, White LR, Bang Leistad R, Helde G, Ro M. Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients. Eur J Pain 2007;11:743–55.Suche in Google Scholar
[10] Okifuji A, Bradshaw DH, Donaldson GW, Turk DC. Sequential analyses of daily symptoms in women with fibromyalgia syndrome. J Pain 2011;12:84–93.Suche in Google Scholar
[11] Boccalon S, Scaggiante B, Perissin L. Anxiety stress and nociceptive responses in mice. Life Sci 2006;78:1225–30.Suche in Google Scholar
[12] da Silva Torres IL, Cucco SNS, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, Tabajara AS, Dantas G, Fontella FU, Dalmaz C, Ferreira MBC. Long-lasting delayed hyperalgesia after chronic restraint stress in rats—effect of morphine administration. Neuroscience Res 2003;45:277–83.Suche in Google Scholar
[13] Gamaro GD, Xavier MH, Denardin JD, Pilger JA, Ely DR, Ferreira MBC, Dalmaz C. The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol Behav 1998;63:693–7.Suche in Google Scholar
[14] King C, Devine D, Vierck C, Yezierski R. Effects of acute stress on two different behavioral measures of thermal nociception. J Pain 2005;6:S17.Suche in Google Scholar
[15] Caceres C, Burns JW. Cardiovascular reactivity to psychological stress may enhance subsequent pain sensitivity. Pain 1997;69:237–44.Suche in Google Scholar
[16] Dufton LM, Konik B, Colletti R, Stanger C, Boyer M, Morrow S, Compas BE. Effects of stress on pain threshold and tolerance in children with recurrent abdominal pain. Pain 2008;136:38–43.Suche in Google Scholar
[17] Fechir M, Schlereth T, Kritzmann S, Balon S, Pfeifer N, Geber C, Breimhorst M, Eberle T, Gamer M, Birklein F. Stress and thermoregulation: different sympathetic responses and different effects on experimental pain. Eur J Pain 2009;13:935–41.Suche in Google Scholar
[18] Vassend O, Knardahl S. Cardiovascular responsiveness to brief cognitive challenges and pain sensitivity in women. Eur J Pain 2004;8:315–24.Suche in Google Scholar
[19] Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Rev 2002;40:29–44.Suche in Google Scholar
[20] Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res 1995;28:113–25.Suche in Google Scholar
[21] Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I: effects on dorsal horn convergent neurones in the rat. Pain 1979;6:283–304.Suche in Google Scholar
[22] Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009;144:16–9.Suche in Google Scholar
[23] Bouhassira D, Danziger N, Attal N, Guirimand F. Comparison of the pain suppressive effects of clinical and experimental painful conditioning stimuli. Brain 2003;126:1068–78.Suche in Google Scholar
[24] Price DD, McHaffie JG. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. Pain 1988;34:245–52.Suche in Google Scholar
[25] Pertovaara A, Kemppainen P, Johansson G, Karonen S-L. Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res 1982;251: 83–92.Suche in Google Scholar
[26] Oono Y, Nie H, Matos RL, Wang K, Arendt-Nielsen L. The inter-and intra-individual variance in descending pain modulation evoked by different conditioning stimuli in healthy men. Scandinavian Journal of Pain 2011;2:162–9.Suche in Google Scholar
[27] Sowman PF, Wang K, Svensson P, Arendt-Nielsen L. Diffuse noxious inhibitory control evoked by tonic craniofacial pain in humans. Eur J Pain 2011;15:139–45.Suche in Google Scholar
[28] Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339.Suche in Google Scholar
[29] van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain 2010;11:408–19.Suche in Google Scholar
[30] de Tommaso M, Difruscolo O, Sardaro M, Libro G, Pecoraro C, Serpino C, Lamberti P, Livrea P. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J Headache Pain 2007;8:167–74.Suche in Google Scholar
[31] Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain 2005;118:215–23.Suche in Google Scholar
[32] Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000;88:69–78.Suche in Google Scholar
[33] Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601.Suche in Google Scholar
[34] King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley 3rd JL. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 2009;143:172–8.Suche in Google Scholar
[35] Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain–new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8.Suche in Google Scholar
[36] Quiton RL, Greenspan JD. Sex differences in endogenous pain modulation by distracting and painful conditioning stimulation. Pain 2007;132(Suppl. 1):S134–49.Suche in Google Scholar
[37] Cathcart S, Winefield AH, Lushington K, Rolan P. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache 2010;50:403–12.Suche in Google Scholar
[38] Jensen K, Andersen HO, Olesen J, Lindblom U. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain 1986;25:313–23.Suche in Google Scholar
[39] Leffler AS, Kosek E, Lerndal T, Nordmark B, Hansson P. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain 2002;6:161–76.Suche in Google Scholar
[40] Leffler A-S, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain 2002;6:149–59.Suche in Google Scholar
[41] Plaghki L, Delisle D, Godfraind JM. Heterotopic nociceptive conditioning stimuli and mental task modulate differently the perception and physiological corre-lates of short CO2 laser stimuli. Pain 1994;57:181–92.Suche in Google Scholar
[42] Tuveson B, Leffler A-S, Hansson P. Time dependant differences in pain sensitivity during unilateral ischemic pain provocation in healthy volunteers. Eur J Pain 2006;10:225–32.Suche in Google Scholar
[43] Ghione S. Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension 1996;28:494–504.Suche in Google Scholar
[44] Hagen K, Zwart JA, Holmen J, Svebak S, Bovim G, Stovner LJ. Does hypertension protect against chronic musculoskeletal complaints? The Nord–Trøndelag Health Study. Arch Intern Med 2005;165:916–22.Suche in Google Scholar
[45] Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain 2008;9:759–66.Suche in Google Scholar
[46] Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol 1990;182:347–55.Suche in Google Scholar
[47] Ariëns GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. Psychosocial risk factors for neck pain: a systematic review. Am J Ind Med 2001;39:180–93.Suche in Google Scholar
[48] Linton SJ. A review of psychological risk factors in back and neck pain. Spine 2000;25:1148–56.Suche in Google Scholar
[49] van der Windt DA, Thomas E, Pope DP, de Winter AF, Macfarlane GJ, Bouter LM, Silman AJ. Occupational risk factors for shoulder pain: a systematic review. Occup Environ Med 2000;57:433–42.Suche in Google Scholar
[50] Ursin H, Eriksen HR. Sensitization, subjective health complaints, and sustained arousal. Ann N Y Acad Sci 2001;933:119–29.Suche in Google Scholar
© 2012 Scandinavian Association for the Study of Pain
Artikel in diesem Heft
- Editorial comment
- Spontaneous pain is reduced by conditioning pain modulation in peripheral neuropathy but not in fibromyalgia—Implications for different pain mechanisms
- Clinical pain research
- Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia
- Editorial comment
- Pulsed radiofrequency—Time for a clinical pause and more science
- Clinical pain research
- Pulsed radiofrequency in peripheral posttraumatic neuropathic pain: A double blind sham controlled randomized clinical trial
- Editorial comment
- Phantom pains and sensations – how does it feel? Only the patient really knows
- Clinical pain research
- Phantom phenomena – Their perceived qualities and consequences from the patient’s perspective
- Editorial comment
- Impact of mental stressor on conditioned pain modulation
- Original experimental
- The effect of a mental stressor on conditioned pain modulation in healthy subjects
- Editorial comment
- Pharmacological modulation of chronic pain after whiplash injury
- Clinical pain research
- Whiplash Associated Disorders (WAD): Responses to pharmacological challenges and psychometric tests
- Editorial comment
- Why are autonomic responses to pressure pain different from those to heat pain and ischaemic pain?
- Original experimental
- Cardiovascular responses to and modulation of pressure pain sensitivity in normotensive, pain-free women
- Correspondence
- Piriformis muscle injection guided by sciatic nerve stimulation: Quick, simple, and safe technique
- Correspondence
- Musculus piriformis syndrome: Localization and injection therapy—Comment to letter from Mayo-Moldes M et al. [1]
- Abstracts
- The “pain matrix” reloaded
- Abstracts
- Endpoints in animal pain models
- Abstracts
- Evaluating pain-related behavior in spinal cord injury
- Abstracts
- The role of the amygdala in sensory and emotional-like pain behavior in neuropathic animals
- Abstracts
- Peripheral and central pain mechanisms—From animal models to clinical research
- Abstracts
- Human experimental models of central sensitization—Do they bridge the gap between animal models and clinical observations?
- Abstracts
- Assessment of central sensitization in the clinic. Is it possible?
- Abstracts
- Migraine neurobiology and treatment
- Abstracts
- Chronic headaches–Goals and obstacles
- Abstracts
- Trigeminal neuralgia and other cranial neuralgias
- Abstracts
- Temporomandibular disorders: Pathophysiology and diagnosis
- Abstracts
- HIV-associated painful polyneuropathy
- Abstracts
- Keynote: Neuronal and glial signalling in pain neuroplasticity
- Abstracts
- Neuropathic pain—From guidelines to clinical practice
- Abstracts
- Postoperative pain treatment. What’s the evidence—And how to use it?
- Abstracts
- NSAIDs in postoperative pain
- Abstracts
- How should we prevent persistent postoperative pain?
- Abstracts
- Opioids: Genetics and receptors
- Abstracts
- Chronic pain and sleep disorders
- Abstracts
- Population-based studies on chronic pain: The role of opioids
- Abstracts
- Living beyond pain: Acceptance and commitment therapy
- Abstracts
- Modality specific alterations of esophageal sensitivity caused by longstanding diabetes mellitus
- Abstracts
- Validation of a porcine behavioural model of UVB induced inflammatory pain
- Abstracts
- Recovery after a lumbar disc herniation is dependent on a gender and OPRM1 Asn40Asp genotype interaction
- Abstracts
- Pain sensitivity changes in chronic pain patients with and without spinal cord stimulation assessed by nociceptive withdrawal reflex thresholds and electrical pain thresholds
- Abstracts
- Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial
- Abstracts
- Sortilins in neuropathic pain
- Abstracts
- Systematic review of neuropathic component in persistent post-surgical pain
- Abstracts
- Pain prevalence in a university hospital in Iceland
- Abstracts
- The effect of tail-docking neonate piglets on ATF-3 and NR2B immunoreactivity in coccygeal dorsal root ganglia and spinal cord dorsal horn neurons: Preliminary data
- Abstracts
- Na+/K+-ATPase dependent regulation of astrocyte Ca2+ signalling: A novel mechanism for modulation of long-term pain?
- Abstracts
- Glutamate attenuates nitric oxide release from isolated trigeminal ganglion satellite glial cells
- Abstracts
- Acute behavioural responses to tail docking in piglets – Effects of increasing docking length?
- Abstracts
- Dose and administration-period play a key role in the effect of ceftriaxone on neuropathic pain in CCI-operated rats
- Abstracts
- Translational aspects of rectal evoked potentials: A comparative study in rats and humans
- Abstracts
- Time-course of analgesic effects of botulinum neurotoxin type A (BoNTA) on human experimental model of pain induced by injection of glutamate into temporalis muscle
- Abstracts
- The effect of nerve compression and capsaicin on contact heat evoked potentials (CHEPs) related to Aδ and C fibers
- Abstracts
- Effect of specific trapezius exercises vs. coordination training on corticomotor control of neck muscles
- Abstracts
- SNP in TNFα T308G is predictive for persistent postoperative pain following inguinal hernia surgery
- Abstracts
- Chronic pain in thoracotomy
- Abstracts
- The variability in thermal threshold-assessments in post-thoracotomy pain syndrome
- Abstracts
- Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer
- Abstracts
- Neuroplastic alterations in brain responses to painful visceral stimulations reflects individual neuropathic symptoms in diabetes mellitus patients
- Abstracts
- Exercise and conditioned pain modulation have different effects on cuff pressure pain tolerance in humans
- Abstracts
- Hyperalgesia in human skin and deep-tissues inside and outside of a UVB irradiated area
- Abstracts
- Effect of experimental jaw muscle pain on bite force during mastication
- Abstracts
- Reflex threshold assessment methodology for evaluation of central sensitisation is vulnerable to EMG crosstalk
- Abstracts
- Cognitive modulation of experimental pain at spinal and cortical levels
- Abstracts
- Influence of emotionally loaded visual and gustatory stimuli on pain perception
- Abstracts
- Modulating pain with augmented reality
- Abstracts
- Offset analgesia: A reproducibility study
- Abstracts
- Visualization of painful process in peripheral tissue using positron emission tomography and [11C]-D-deprenyl
- Abstracts
- Mirror-image sensory dysfunction in the post-thoracotomy pain syndrome
- Abstracts
- Genetic variation in opioid receptor genes and sensitivity to experimental pain in male and female healthy volunteers
- Abstracts
- Mechanical sensitivity in migraine patients during attack, remission, and pain-free periods:A preliminary study
- Abstracts
- Multivariate pattern analysis of evoked brain potentials by temporal matching pursuit and support vector machine
- Abstracts
- Pain following stroke: A prospective study
- Abstracts
- Chronic thoracic pain in children after cardiac surgery
- Abstracts
- Chronic pain after breast augmentation is associated with both signs of peripheral nerve injury and central nervous mechanisms
- Abstracts
- Sensory phenotypes in patients with peripheral neuropathic pain evaluated with quantitative sensory testing
- Abstracts
- Is health related quality of life related to the pattern of chronic pain?
- Abstracts
- Comparison between ropivacaine local infiltration analgesia with ketorolac or placebo for total knee replacement surgery
- Abstracts
- Treatment with topical capsaicin: Experience from a pain clinic
- Abstracts
- Distribution of concussion related symptoms after whiplash injury in risk strata
- Abstracts
- HIV/AIDS in different cultures
- Abstracts
- Pain perception is altered in patients with medication-overuse headache but can improve after detoxification
- Abstracts
- Detoxification in a structured programme is effective for medication-overuse headache
Artikel in diesem Heft
- Editorial comment
- Spontaneous pain is reduced by conditioning pain modulation in peripheral neuropathy but not in fibromyalgia—Implications for different pain mechanisms
- Clinical pain research
- Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia
- Editorial comment
- Pulsed radiofrequency—Time for a clinical pause and more science
- Clinical pain research
- Pulsed radiofrequency in peripheral posttraumatic neuropathic pain: A double blind sham controlled randomized clinical trial
- Editorial comment
- Phantom pains and sensations – how does it feel? Only the patient really knows
- Clinical pain research
- Phantom phenomena – Their perceived qualities and consequences from the patient’s perspective
- Editorial comment
- Impact of mental stressor on conditioned pain modulation
- Original experimental
- The effect of a mental stressor on conditioned pain modulation in healthy subjects
- Editorial comment
- Pharmacological modulation of chronic pain after whiplash injury
- Clinical pain research
- Whiplash Associated Disorders (WAD): Responses to pharmacological challenges and psychometric tests
- Editorial comment
- Why are autonomic responses to pressure pain different from those to heat pain and ischaemic pain?
- Original experimental
- Cardiovascular responses to and modulation of pressure pain sensitivity in normotensive, pain-free women
- Correspondence
- Piriformis muscle injection guided by sciatic nerve stimulation: Quick, simple, and safe technique
- Correspondence
- Musculus piriformis syndrome: Localization and injection therapy—Comment to letter from Mayo-Moldes M et al. [1]
- Abstracts
- The “pain matrix” reloaded
- Abstracts
- Endpoints in animal pain models
- Abstracts
- Evaluating pain-related behavior in spinal cord injury
- Abstracts
- The role of the amygdala in sensory and emotional-like pain behavior in neuropathic animals
- Abstracts
- Peripheral and central pain mechanisms—From animal models to clinical research
- Abstracts
- Human experimental models of central sensitization—Do they bridge the gap between animal models and clinical observations?
- Abstracts
- Assessment of central sensitization in the clinic. Is it possible?
- Abstracts
- Migraine neurobiology and treatment
- Abstracts
- Chronic headaches–Goals and obstacles
- Abstracts
- Trigeminal neuralgia and other cranial neuralgias
- Abstracts
- Temporomandibular disorders: Pathophysiology and diagnosis
- Abstracts
- HIV-associated painful polyneuropathy
- Abstracts
- Keynote: Neuronal and glial signalling in pain neuroplasticity
- Abstracts
- Neuropathic pain—From guidelines to clinical practice
- Abstracts
- Postoperative pain treatment. What’s the evidence—And how to use it?
- Abstracts
- NSAIDs in postoperative pain
- Abstracts
- How should we prevent persistent postoperative pain?
- Abstracts
- Opioids: Genetics and receptors
- Abstracts
- Chronic pain and sleep disorders
- Abstracts
- Population-based studies on chronic pain: The role of opioids
- Abstracts
- Living beyond pain: Acceptance and commitment therapy
- Abstracts
- Modality specific alterations of esophageal sensitivity caused by longstanding diabetes mellitus
- Abstracts
- Validation of a porcine behavioural model of UVB induced inflammatory pain
- Abstracts
- Recovery after a lumbar disc herniation is dependent on a gender and OPRM1 Asn40Asp genotype interaction
- Abstracts
- Pain sensitivity changes in chronic pain patients with and without spinal cord stimulation assessed by nociceptive withdrawal reflex thresholds and electrical pain thresholds
- Abstracts
- Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial
- Abstracts
- Sortilins in neuropathic pain
- Abstracts
- Systematic review of neuropathic component in persistent post-surgical pain
- Abstracts
- Pain prevalence in a university hospital in Iceland
- Abstracts
- The effect of tail-docking neonate piglets on ATF-3 and NR2B immunoreactivity in coccygeal dorsal root ganglia and spinal cord dorsal horn neurons: Preliminary data
- Abstracts
- Na+/K+-ATPase dependent regulation of astrocyte Ca2+ signalling: A novel mechanism for modulation of long-term pain?
- Abstracts
- Glutamate attenuates nitric oxide release from isolated trigeminal ganglion satellite glial cells
- Abstracts
- Acute behavioural responses to tail docking in piglets – Effects of increasing docking length?
- Abstracts
- Dose and administration-period play a key role in the effect of ceftriaxone on neuropathic pain in CCI-operated rats
- Abstracts
- Translational aspects of rectal evoked potentials: A comparative study in rats and humans
- Abstracts
- Time-course of analgesic effects of botulinum neurotoxin type A (BoNTA) on human experimental model of pain induced by injection of glutamate into temporalis muscle
- Abstracts
- The effect of nerve compression and capsaicin on contact heat evoked potentials (CHEPs) related to Aδ and C fibers
- Abstracts
- Effect of specific trapezius exercises vs. coordination training on corticomotor control of neck muscles
- Abstracts
- SNP in TNFα T308G is predictive for persistent postoperative pain following inguinal hernia surgery
- Abstracts
- Chronic pain in thoracotomy
- Abstracts
- The variability in thermal threshold-assessments in post-thoracotomy pain syndrome
- Abstracts
- Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer
- Abstracts
- Neuroplastic alterations in brain responses to painful visceral stimulations reflects individual neuropathic symptoms in diabetes mellitus patients
- Abstracts
- Exercise and conditioned pain modulation have different effects on cuff pressure pain tolerance in humans
- Abstracts
- Hyperalgesia in human skin and deep-tissues inside and outside of a UVB irradiated area
- Abstracts
- Effect of experimental jaw muscle pain on bite force during mastication
- Abstracts
- Reflex threshold assessment methodology for evaluation of central sensitisation is vulnerable to EMG crosstalk
- Abstracts
- Cognitive modulation of experimental pain at spinal and cortical levels
- Abstracts
- Influence of emotionally loaded visual and gustatory stimuli on pain perception
- Abstracts
- Modulating pain with augmented reality
- Abstracts
- Offset analgesia: A reproducibility study
- Abstracts
- Visualization of painful process in peripheral tissue using positron emission tomography and [11C]-D-deprenyl
- Abstracts
- Mirror-image sensory dysfunction in the post-thoracotomy pain syndrome
- Abstracts
- Genetic variation in opioid receptor genes and sensitivity to experimental pain in male and female healthy volunteers
- Abstracts
- Mechanical sensitivity in migraine patients during attack, remission, and pain-free periods:A preliminary study
- Abstracts
- Multivariate pattern analysis of evoked brain potentials by temporal matching pursuit and support vector machine
- Abstracts
- Pain following stroke: A prospective study
- Abstracts
- Chronic thoracic pain in children after cardiac surgery
- Abstracts
- Chronic pain after breast augmentation is associated with both signs of peripheral nerve injury and central nervous mechanisms
- Abstracts
- Sensory phenotypes in patients with peripheral neuropathic pain evaluated with quantitative sensory testing
- Abstracts
- Is health related quality of life related to the pattern of chronic pain?
- Abstracts
- Comparison between ropivacaine local infiltration analgesia with ketorolac or placebo for total knee replacement surgery
- Abstracts
- Treatment with topical capsaicin: Experience from a pain clinic
- Abstracts
- Distribution of concussion related symptoms after whiplash injury in risk strata
- Abstracts
- HIV/AIDS in different cultures
- Abstracts
- Pain perception is altered in patients with medication-overuse headache but can improve after detoxification
- Abstracts
- Detoxification in a structured programme is effective for medication-overuse headache

