Abstract
Background
The “gold standard” for pain relief after thoracotomy has been thoracic epidural analgesia (TEA). The studies comparing TEA with paravertebral block (PVB) and recent reviews recommend PVB as a novel, safer method than TEA.
Methods
A systematic search of the Cochrane and PubMed databases for prospective, randomized trials (RCTs) comparing TEA and PVB for post-thoracotomy analgesia was done. We assessed how TEA and PVB were performed, methods of randomization, assessment of pain relief, and complications. Abstracts only were excluded.
Results
Ten studies were included, comprising 224 patients randomized to TEA, 243 to PVB. The studies were heterogeneous. Therefore, a systematic narrative review with our evaluations is presented.
Only 3/10 trials reported the method of randomization. Pain during coughing was reported in only 5/10, pain assessment not specified in 5/10. Only 1/10 trials found PVB superior to TEA, but placed TEA catheters too low (<T7). TEA was superior to PVB in 1/10, during first 1.5 days. PVB and TEA were equally effective in 8/10. 5/10 trials found PVB had less hypotension or urinary retention. None of the studies used appropriate and optimal TEA: TEA was started after end of surgery in half, catheters placed too low (2/10), too high (1/10), not reported in (1/10). 7/10 infused local anaesthetic only, 2/10 added fentanyl, 1/10 added morphine, and none added adrenaline. PVB infusions had higher concentration of bupivacaine (5 mg/ml) in 2/10, 1/10 added fentanyl, 1/10 added ornipressin. Loading doses were higher in 5/10, and with more concentrated solutions in 5/10 of PVB than in the TEA group.
Conclusions
10 heterogeneous, mostly small, studies comparing TEA and PVB for post-thoracotomy analgesia do not allow conclusions on which method has superior analgesic efficacy and safety. The main methodological problem was that none of the studies use optimal thoracic epidural analgesia, with siting of catheters inappropriate in some and the epidural infusion containing too concentrated local anaesthetic because opioid and adrenaline were not added. Anatomical considerations (the paravertebral space comprises parts of the epidural space and contains spinal cord arteries) and personally experienced complications with PVB (paraplegia) convince us that PVB must have higher risk of, infrequent but serious, spinal cord complications than TEA. Percutaneous PVB may puncture pleura and lung.
Some surgeons expressed satisfaction with PVB because the method omits costly acute pain services for monitoring on surgical wards and saves time in the operating room. They are, however, bound to experience serious complications from PVB, sooner or later.
To our knowledge, optimally conducted epidural analgesia has not been compared with PVB. Current literature and our experience with both techniques for up to four decades, indicate that PVB may be an alternative for post-thoracotomy pain when TEA is infeasible for various patient-related reasons (Breivik et al., 2009). Severely disturbed haemostasis is a contraindication for PVB and TEA. Higher concentrations of local anaesthetics are needed to obtain intercostal nerve blocks and epidural analgesia with PVB, risking local anaesthetic intoxication. Robust monitoring regimen for effects and adverse effects is as important for PVB as for TEA.
Contents
3.1. Definitions of the paravertebral space (Fig. 1)
3.2. Techniques of paravertebral block (PVB)
3.4. Techniques of thoracic epidural analgesia, TEA
3.5. Primary and secondary outcome variables
3.6. Heterogenicity of the ten studies (Tables 1 and 2)
3.7. Evaluation of ten RCTs comparing PVB and TEA for pain after thoracotomy
3.7.1. Matthews and Govenden (1989)
3.7.2. Pertunnen et al. (1995)
3.7.4. Richardson et al. (1999)
3.7.10. Gulbahar et al. (in press)
4.1. Techniques of PVB and TEA
4.2. Outcome variables for pain relief
4.3. PVB is also an epidural approach
4.4. Comparing PVB and TEA is comparing two of the same kind; two extremes of a continuum
4.5. Prevalence of complications
1 Introduction
Paravertebral block (PVB) was a frequently used technique for analgesia and anaesthesia in the 20th century. The method was introduced by Hugo Sellheim in 1905. He used bilateral PVB for abdominal analgesia (Karmakar, 2001). The method was subsequently refined to produce surgical anaesthesia. In 1919 a technique for PVB similar to one presently used technique (Eason and Wyatt, 1979) was described (Kappis, 1919). Bilateral PVBs at thoracic segments T11 and T12 was used for labour analgesia in the 1930s. PVB, which was called “posterior intercostal nerve block”, was routinely used by anaesthesiologists in Norway in the 1950s for the surgical technique of thoracoplasty in patients with resistant tuberculosis of the lungs, i.e. fracturing the upper ribs and compressing and collapsing the tuberculosis caverns in the apex of the lungs (Bjørn Lind, personal communication). During the 1960s, PVB gradually lost popularity. Authors argued the technique should be left out of the anaesthesiologists’ armamentarium (Atkinson and Rushman, 1977). Thoracic epidural analgesia (TEA) became the “gold standard” for postoperative analgesia in major thoracic and abdominal surgery (Bromage, 1978; Wildsmith, 1989).
In 1979 Eason and Wyatt reintroduced thoracic PVB by presenting a catheter technique, allowing repeated injections into the paravertebral space (PVS) (Eason and Wyatt, 1979). Referred to as a safe, effective, technically simple, easy-to-learn technique with few contraindications (Lönnqvist et al., 1995; Karmakar, 2001), PVB has aroused great enthusiasm, and is about to have its renaissance and possibly regain its former strong position as a technique for regional analgesia.
PVB has been reported to be superior to thoracic epidural blocks for thoracotomy patients (Richardson et al., 1999). A more widespread use has been advocated (Lönnqvist, 2005). PVB is now considered advantageous compared with thoracic epidural analgesia (TEA) and recommendable for thoracotomy by some (e.g. (Davies et al., 2006)). In a recent publication from the PROSPECT working group, both thoracic epidural and PVB are recommended as the primary approach for post-thoracotomy analgesia (Joshi et al., 2008). It has also been suggested that thoracic PVB will replace TEA (Conlon et al., 2008).
It is important to emphasize that the paravertebral space and the epidural space inside the vertebral column communicate. Spread of a solution placed in the paravertebral space into the epidural space has been demonstrated very convincingly and occurs in 3/4 of cases (Conacher and Kokri, 1987; Purcell-Jones et al., 1989). This fact raises the question of epidural and spinal cord contribution to the effects attributed paravertebral spinal nerve blocks. In this article, we review all published studies comparing PVB and TEA for thoracotomy pain and address clinical implications and concerns as a consequence of thoracic PVB and TEA representing a continuum rather than two completely different techniques for post-thoracotomy analgesia in adults.
2 Methods
The Cochrane database was searched using the terms “paravertebral and thoracotomy and epidural/injections, epidural/analgesia, epidural/anaesthesia, epidural/injections, spinal”. The PubMed database was searched using the terms “paravertebral thoracotomy epidural”. Reviews were searched for references. The searches were completed on September 3, 2009.
3 Results
We identified 15 prospective, randomized clinical trials (RCTs) comparing PVB and TEA, or PVB, TEA and intercostal nerve blocks for pain during and after thoracic surgery in adults (Matthews and Govenden, 1989; Pertunnen et al., 1995; Kaiser et al., 1998; Bimston et al., 1999; Richardson et al., 1999; Dhole et al., 2001; De Cosmo et al., 2002; Wedad et al., 2004; Luketich et al., 2005; Leaver et al., 2006; Hemmati et al., 2006; Casati et al., 2006; Mehta et al., 2008; Szebla and Machala, 2008; Gulbahar et al., in press).
Main aspects of paravertebral comparisons with thoracic epidural analgesia after thoracotomies in ten randomized studies.
| Study | Paravertebral blocks | Epidural blocks | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| N/days | LA | Opioid | VP | Continuous infusion | Loading dose | Segm. level | N/days | LA | Opioid | VP | Continuous infusion | Loading dose | Segm. level | |
| Matthews and Govenden (1989) | 10[a]/0.5–1 | 0.25% B | – | – | 3–10 ml/h | 0.25% B, 10 ml | T4–T5 | 9[a]/1 | 0.25% B | – | – | 3–10 ml/h | 0.25% B, 10 ml | T4–T5 |
| Pertunnen et al. (1995) | 15[a]/2 | 0.25% B | – | – | 4–8 ml/h[c] | 0.25% B, 8–12 ml[c] | T2–T3 | 15[a]/2 | 0.25% B | – | – | 4–8 ml/h[c] | 0.25% B, 8–12 ml[c] | T5–T7 |
| Kaiser et al. (1998) | 13[a]/6.6! | 0.5% B | – | 0.05 U/ml OP | 0.1 ml/kg/h | 0.5% B, 20 ml[d] | ?[e] | 13[a]/2.7! | 0.25–0.375% B | 2 μg/ml F | – | 4–8 ml/h | ?[f] | T5–T6 |
| Richardson et al. (1999) | 46[b]/2 | 0.5% B | – | – | 0.1 ml/kg/h | 0.5% B, 20 ml[g] | T6–T8 | 49[b]/2 | 0.25% B | – | – | 0.1 ml/kg/h | 0.25% B, 10–15 ml[h] | T7–T10 |
| Bimston et al. (1999) | 29[b],[i]/4?[j]/4?[j] | 0.1% B | 10 μg/ml F | – | 10–15 ml/h initially, titrated down to 5–10 ml/h from 48 [h][k] | 0.5% B + 5 μg/ml A, 18 ml + 100 μg F | ?[k] | 21[b], [i]/4?[j] | 0.1% B | 10 μg/ml F | – | 10–15 ml/h initially, titrated, down to 5–10 ml/h from 48 h[k] | ?[k] | ?[k] |
| Dhole et al. (2001) | 20[a]/0.5 | 0.25% B | – | – | 6 ml/h | 0.5% B, 8 ml | T4–T5 | 20[a] 0.5 | 0.25% B | – | – | 6 ml/h | 0.5% B, 8 ml | T4–T5 |
| Luketich et al. (2005) | 47[a]/3–4 | 0.25% B | – | – | 0.1 ml/kg/h | 0.25% B, 10 ml + 0.5% B,10 ml[l] | T8–T9 | 44[a] /4 | 0.125% B | 50 μg/ml M | – | 4–8 ml/h | ?[m] | T3–T6 |
| Casati et al. (2006) | 21[b]/2 | 0.2% R | – | – | 5–10 ml/h | 0.75% R, 15 ml | T4 | 21[b]/2 | 0.2% R | – | – | 5–10 ml/h | 0.75% R, 5 ml | T5–T7 |
| Mehta et al. (2008) | 17[a]/1 | 0.25% B | – | – | 0.1 ml/kg/h | 0.5% B, 8 ml | T4–T5 | 19[a] /1 | 0.25% B | – | – | 0.1 ml/kg/h | 0.5% B, 8 ml | C7–T1 |
| Gulbahar et al. (in press) | 25[a],[n]/3 | 0.25% B | – | – | 0.1 ml/kg/h (+patient controlled bolus of 2 ml q. 1 h) | ?[o] | ?[p] | 13[a],[n]/3 | 0.25% B | – | – | 0.1 ml/kg/h (+patient controlled bolus of 2 ml q. 1 h) | 0.25% B, 5 ml | T7–T10 |
-
N, number of patients; LA, local anaesthetic; VP, vasopressor; B, bupivacaine; R, ropivacaine; OP, ornipressin; F, fentanyl; A, adrenaline; M, morphine.
Method of randomization, pain relief out-come, original authors’ conclusions, and reviewers’ comments.
| Study | PVB (N) | TEA (N) | Method of randomization | Pain at rest | Pain on cough | Reported pain not specified | Original authors’ conclusions | Reviewers’ comments | Surgery performed |
|---|---|---|---|---|---|---|---|---|---|
| Matthews and Govenden (1989) | 10 | 9 | − | − | − | + | TEA and PVB equally good analgesia. Hypotension and urine retention more frequent with TEA. | Small study, for only 12–24 h after surgery. 30% of PVB patients dropped out at 12 h. Same solution (only bupivacaine 2.5 mg/ml) and regimen used for PVB and TEA[a]. Very high dose of bupivacaine (17.5 mg/h/70kg). Rescue analgesics not reported. Avoidable side effects from TEA if opioid and adrenaline had been added to a much lower concentration of bupivacaine. | Pulmonary |
| Pertunnen et al. (1995) | 15 | 15 | − | + | + | − | TEA and PVB equally effective. No statistically difference in rescue PCA morphine consumption, respiratory function, or adverse advents. | Good study for 48 h after surgery! Unfortunately TEA was with bupivacaine only, without opioid or adrenaline, started after end of surgery. Rescue morphine consumption high, causing most of the adverse effects. Plasma concentrations tended to be higher and forced expiratory volume lower in PVB compared with TEA. | Pulmonary |
| Kaiser et al. (1998) | 13 | 13 | − | − | − | + | TEA and PVB equally effective for analgesia. PVB alternative to TEA, especially for patients not qualified for TEA. | Twice as high bupivacaine, with vasopressor, in PVB for almost 7 days on surgical wards. TEA catheter removed after 2.7 days in the ICU. Bupivacaine serum concentrations into toxic levels after 3 days of PVB. Authors not aware of importance of adrenaline in TEA infusion and proven safety of “optimal” TEA on surgical wards. Patients “not qualified” for TEA, will often not be qualified for PVB either. | Pulmonary |
| Richardson et al. (1999) | 46 | 49 | + | + | + | − | PVB provided better analgesia than TEA at rest and on coughing, preservation of pulmonary function and glycemic control. Nausea, vomiting and postoperative morbidity[b] more frequent in TEA group. | Different local anaesthetic concentration and volume given in the two groups, near double dose in PVB group. Confusion possibly caused by bupivacaine accumulation reported[c]. The study has one possibly fatal flaw in that TEA catheters were sited much too caudally (T7–T10): advancing catheters 5 cm may have caused even lower final catheter tip site. This could easily explain the findings. | Pulmonary and oesophagal |
| Bimston et al. (1999) | 29 | 21 | + | − | − | + | TEA more effective than PVB during first 1.5 days, thereafter equally effective for postoperative analgesia. Urinary retention[d] more frequent in TEA group. PVB saves costs of TEA for patient. | Catheter medication (composition, volume) is reported in detail for PVB group only, but it appears to be similar for the TEA group. Method of randomization is faulty. Urinary catheter remained in place as long as the TEA catheter was used! Pneumonectomy in 28% of TEA group, only 7% of PVB group. | Pulmonary |
| Dhole et al. (2001) | 20 | 20 | − | + | + | − | PVB and TEA equally effective for postoperative analgesia. PVB technically easier and possibly safer than TEA as “PVB carries no risk of spinal haematoma”. Postoperative cardiac index higher in TEA. | Same solution and regimen used for PVB and TEA, both placed percutaneously at T4–T5, with blinded observers. Combination of heparin for left internal mammary artery (LIMA) bypassing and ketorolac rescue analgesic potentially increases risk of spinal bleeding. PVB also connected with epidural space, making the infrequent, but serious bleeding and infectious complications in the epidural space a (remote?) possibility during/after PVB as well as during/after TEA. | Minimally invasive CABG through anterolateral thoracotomy |
| Luketich et al. (2005) | 47 | 44 | – | – | – | + | PVB combined with PCA morphine i.v. as effective as TEA for postoperative analgesia. Total urine catheter days[e] were lower for PVB group. Surgeons believe PVB may save costs for patient (in US health care system), as acute pain service is not involved. | Different composition of PVB (bupivacaine 25 mg/ml 0.1 ml/kg/h) and TEA (Bup 25 mg/ml–0.1 ml/kg/h) catheter infusion. Intraoperative loading dose only in PVB group. Composite pain score not helpful. To save money, the acute pain expert service not involved in monitoring of PVB patients: increased risk of detecting infrequent but serious complications too late? One such complication will increase cost of postoperative pain tremendously. | Pulmonary |
| Casati et al. (2006) | 21 | 21 | + | + | + | – | PVB and TEA with ropivacaine 2 mg/ml equally effective for postoperative analgesia. PVB associated with less hypotension. | “Blinded” observers. Same solution and regimen used for PVB and TEA postoperatively, intraoperative loading dose for PVB group three times loading dose for TEA. No difference in procedure time for percutaneous PVB catheter and TEA catheter placement. TEA with too high local anaesthetic, no opioid, no adrenaline. No monitoring ofsegmental hypoaesthetic area. | Pulmonary |
| Mehta et al.(2008) | 17 | 19 | – | + | + | – | PVB and TEA with bupivacaine 2.5 mg/ml equally effective for postoperative analgesia. PVB "may be used safely” despite recent anticoagulation. | Same solution and regimen used for PVB and TEA, both placed percutaneously, with blinded observers. TEA catheter placed much too high (at C7–T1), causing numbness of arms. PVB at T4–T5. Combination of heparin for LIMA and diclofenac rescue analgesic potentially increases risk of spinal bleeding – in PVB as well as TEA! | Robotic– assisted CABG with thoracotomy |
| Gulbahar et al. (in press) | 25[f] | 13[f] | – | – | – | + | PVB and TEA equally effective for postoperative analgesia. Side effects[g] were seen only in TEA group. PVB placement required less time, and might be preferred over TEA. | Same solution and regimen used for PVB and TEA postoperatively, intraoperative loading dose only in TEA group. TEA catheter placed too low (T7–T10). TEA side effects related to too low catheter placement, too high bupivacaine concentration, and no opioid, or adrenergic agonist in epidural infusion. | Pulmonary |
-
N, number of patients.
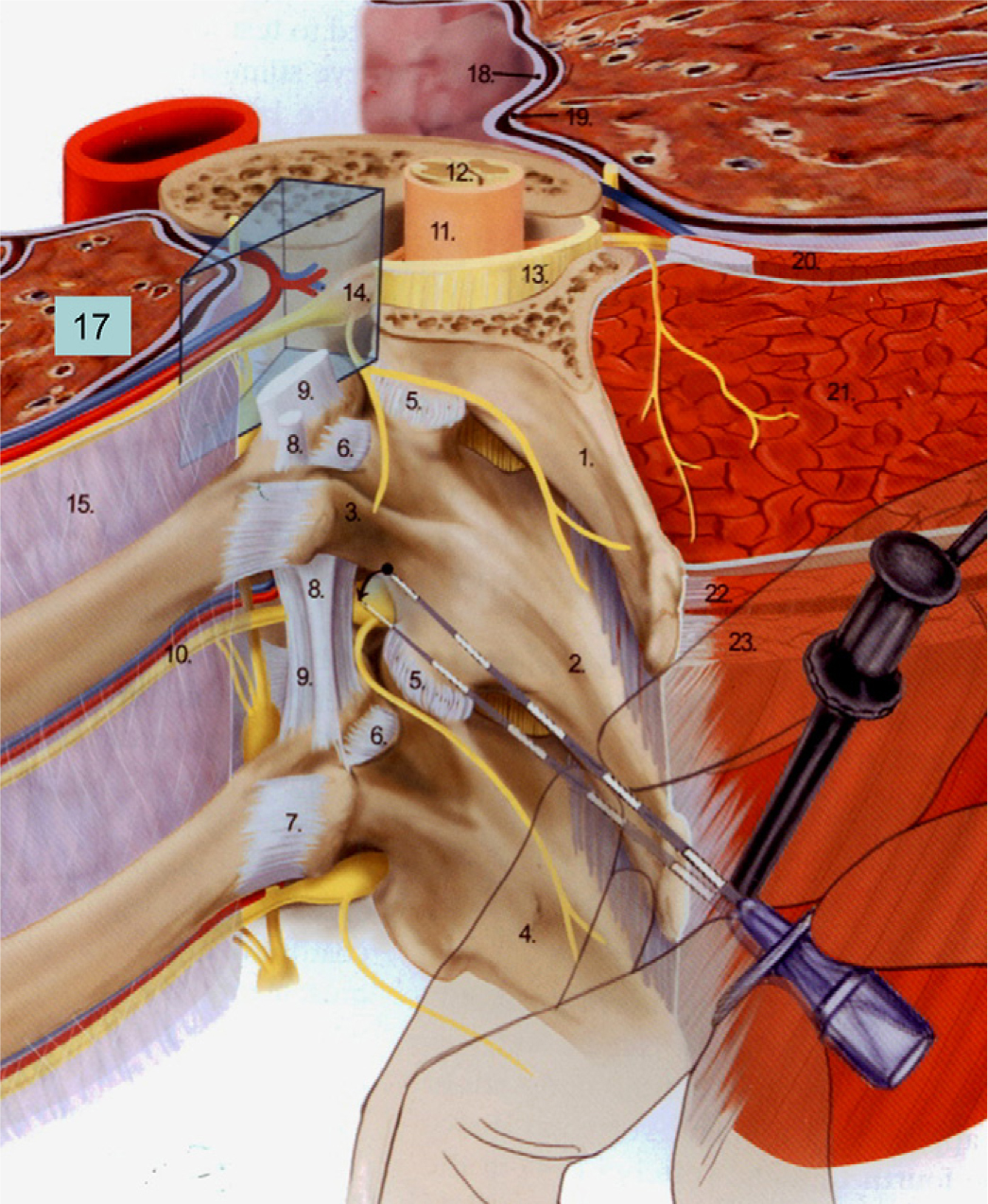
Reproduced from Boezaart (2009, Fig. 18-4) with permission. The wedge-shaped paravertebral space is clearly indicated: comprises vessels to spinal cord, spinal nerve root and intercostal nerve, chain of sympathetic ganglia and its rami communicantes, and paravertebral part of the epidural space. 1. Transverse process of T3; 2. transverse process of T4; 5. Facet-joint; 6. costotransverse ligament; 7. lateral costotransverse ligament; 8. intertransverse ligament; 9. superior costotransverse ligament; 10. intercostal vessels and nerve; 11. dura mater; 12. Spinal cord; 13. ligamentum flavum; 14. nerve root; 15. internal intercostal membrane; 17. left lung; 18. parietal pleura; 19. visceral pleura; 21. erector spina muscle; 23. trapezius muscle.
From the 15 identified trials five were excluded: Two trials were excluded because of languages we could not interpret (De Cosmo et al., 2002; Szebla and Machala, 2008). One was published in abstract form only (Hemmati et al., 2006). One trial (Leaver et al., 2006) included in a recent review (Davies et al., 2006) was excluded because it is still unpublished. One trial (Wedad et al., 2004) was excluded since full text proved unobtainable from our university library and its international collaborators.
Aspects of PVB and TEA as described in the PVB- and TEA- literature and in the 10 included RCTs, as well as their results and conclusions, are described below and summarized in Tables 1 and 2.
3.1 Definitions of the paravertebral space (Fig. 1)
The paravertebral space (PVS) is wedge-shaped and lateral to the vertebral column. Each PVS is bounded anteriolaterally by the parietal pleura and posteriorly by the superior costotransverse ligament. The body of the vertebra, the intervertebral foramen and its contents (spinal nerve, artery and vein to/from the spinal cord) form the medial boundary (Eason and Wyatt, 1979; Boezaart, 2008). Heads and necks of adjoining ribs bound the PVS superiorly and inferiorly. Each PVS communicates with the intercostal space laterally, the epidural space medially, the contralateral PVS via the prevertebral (Tenicela and Pollan, 1990) and epidural space (Karmakar, 2001), and with the PVS above and below (Eason and Wyatt, 1979; Boezaart, 2008). The neural content of the thoracic PVS comprises the sympathetic chain, the intercostal nerve, the spinal nerve and its dorsal ramus, and the rami communicantes of the sympathetic nervous system (Eason and Wyatt, 1979).
3.2 Techniques of paravertebral block (PVB)
Paravertebral blocks are performed with several approaches, varying from a percutaneous needle below or above the transverse vertebral process for single shot or prolonged catheter infusion to visually guided placement of a catheter during surgery. All techniques aim at depositing local anaesthetic in the PVS, causing unilateral somatic and sympathetic blocks (Richardson and Lönnqvist, 1998). The traditional percutaneous route (Kappis, 1919; Eason and Wyatt, 1979) has been modified with the use of a nerve stimulator (Lang, 2002) and ultrasound guidance (Hara et al., 2009). During thoracotomy a catheter may be placed by the surgeon under direct vision intraoperatively (Sabanathan et al., 1988; Berrisford and Sabanathan, 1990). This was done in six of the studies included in our review (Pertunnen et al., 1995; Kaiser et al., 1998; Richardson et al., 1999; Bimston et al., 1999; Luketich et al., 2005; Gulbahar et al., in press). The remaining studies placed catheters percutaneously before (Dhole et al., 2001; Casati et al., 2006; Mehta et al., 2008) or during (Matthews and Govenden, 1989) surgery.
3.3 Agents for PVB
Various local anaesthetic solutions, often highly concentrated, were used for PVB in the ten included studies (Table 1). One study added fentanyl and adrenaline (Bimston et al., 1999), another added ornipressin (Kaiser et al., 1998). Only three of the studies included in our review measured vascular concentrations of local anaesthetics (Pertunnen et al., 1995; Kaiser et al., 1998; Bimston et al., 1999). Pertunnen et al. found a tendency towards higher concentration, and more individual variations, in arterial bupivacaine concentrations in the PVB patients, some of whom had concentrations in the CNS-toxic range (Rosenberg et al., 2004). With prolonged PVB catheter infusions, the risk of local anaesthetic toxic reaction from the cardiovascular and the central nervous system must be clinically significant (Pertunnen et al., 1995; Richardson et al., 1999).
3.4 Techniques of thoracic epidural analgesia, TEA
In the ten studies comparing PVB and TEA, it is a striking finding that most studies use less than optimal TEA, as local anaesthetic solutions alone were used. Only 3 of the 10 studies added an opioid; fentanyl 10 μg/ml (Bimston et al., 1999), fentanyl 2 μg/ml (Kaiser et al., 1998), morphine 50 μg/ml (Luketich et al., 2005). None of the investigators added vasopressor or α2-agonist in the TEA infusion. For thoracotomy, the segmental level of TEA catheter insertion was too low (too caudad) in two trials (Richardson et al., 1999; Gulbahar et al., in press), too cranial in one (Mehta et al., 2008), and not reported in one (Bimston et al., 1999). Thus, only 6 of the 10 trials had appropriate and optimal segmental placement of the TEA epidural catheter (Table 1) between T4 and T7 (Breivik, 1995; Niemi, 2004). None of the studies had what we consider optimal composition of the epidural infusion with bupivacaine, fentanyl, and adrenaline (Breivik, 1995; Niemi and Breivik, 2003; Niemi, 2004). Seven of the ten studies report the volume and composition of the initial bolus dose (Table 1).
3.5 Primary and secondary outcome variables
In studies on pain relief after thoracotomy the primary outcome variable should ideally be pain intensity during deep inspiration and forceful coughing (Niemi and Breivik, 2003; Niemi, 2004; Stubhaug and Breivik, 2008). Only 5 of the 10 studies report pain on coughing (Table 2), whereas 4 of the remaining 5 do not specify at all how they measured postoperative pain as an outcome (Table 2).
3.6 Heterogenicity of the ten studies (Tables 1 and 2)
The ten included studies in this review vary in techniques and measurements of outcome variables too much to make systematic meta-analysis meaningful.
3.7 Evaluation of ten RCTs comparing PVB and TEA for pain after thoracotomy
3.7.1 Matthews and Govenden (1989)
Matthews and Govenden compared the analgesic efficacy of continuous postoperative infusion of bupivacaine 2.5 mg/ml (0.25%) paravertebrally and epidurally. They aimed for similar loading doses and infusion rates in the two groups. Data from 19 thoracotomy patients for lung surgery are reported. At the T4/T5 level, 10 patients (PVB group) had a paravertebral catheter placed percutaneously during thoracotomy. Nine patients (TEA group) received an epidural catheter after wound closure. Both groups received an infusion of 3–10 ml/h of bupivacaine 2.5 mg/ml, preceded by a 10 ml bolus. Mean infusion rates were similar in the groups, as were pain scores, which they describe as a linear analogue pain scores, probably a visual analogue scale (VAS). Unfortunately they do not give results as pain at rest or pain on deep breathing and coughing, but they report that co-operation with physiotherapy and expectoration was adequate. Neither do they report any rescue analgesic medication, nor any method of randomization, nor how the patients were allocated to the PVB or TEA group. The authors concluded that both techniques provide good analgesia, although 30% of the PVB patients appeared to have lost their catheter already after 12 h of infusion. Hypotension and urinary retention were significantly more frequent in the TEA group.
Comments: This is a small study and any differences in efficacy would have to be large to be detected. Patients were followed only up to 24 h. The epidural infusion of bupivacaine 2.5 mg/ml up to 10 ml/h (average=7 ml/h/70 kg, or 17.5 mg/h/70 kg). This is overdosing, explaining the unnecessary haemodynamic and urinary side effects during TEA. We use about 5 mg/h/70 kg when fentanyl and adrenaline, 2 μg/ml each, are added to bupivacaine 1 mg/ml, and we almost never see hypotension or urinary retention caused by the TEA (Breivik, 1995; Niemi and Breivik, 2003; Niemi, 2004). Placing an epidural catheter after wound closure (with the patient still under general anaesthesia and curarized) is not optimal and it is not safe: The analgesic effect from TEA infusion during surgery, reduces the need for anaesthetic and opioid analgesics and gives the patient a “flying start” of postoperative analgesia after waking up after general anaesthesia. Siting an epidural catheter in a patient curarized under general anaesthesia has an unnecessary risk of spinal cord damage because the warning clinical symptoms of erroneous placement of needle and catheter are lost (Moen et al., 2004; Breivik et al., 2009).
3.7.2 Pertunnen et al. (1995)
Pertunnen et al. studied the analgesic effect of epidural (TEA), intercostal (IC), and paravertebral (PVB) block on post-thoracotomy pain, pulmonary functions, plasma concentration of bupivacaine, and adverse effects after lung surgery. Data from 15 patients in each of the three groups were compared. Before wound closure, the IC group was injected a total of 16 ml bupivacaine 5 mg/ml (0.5%) for unilateral T3–T7 intercostal nerve blocks, but not repeated thereafter. In the PVB group, a T2–T3 level catheter was placed intraoperatively under direct vision. The TEA group had their T5–T7 level catheter inserted preoperatively and tested with 4ml lidocaine 20 mg/ml (2%). Before wound closure, both catheter groups received a bolus of 8–12 ml bupivacaine 2.5 mg/ml (0.25%) followed by a 4–8 ml/h bupivacaine 2.5 mg/ml (0.25%) infusion, bolus volume and infusion rate defined by patient height. Rescue analgesic was provided as i.v. patient controlled analgesia (PCA) with boluses of morphine 30μg/kg. Pain at rest and pain during coughing were scored using VAS and verbal rating scale (VRS). Pain scores, morphine consumption and arterial plasma bupivacaine concentration were similar between the PVB and TEA groups. For the IC group, pain score during coughing immediately postoperatively was significantly lower compared with the TEA group. Also, for the IC group, significantly higher arterial plasma bupivacaine concentration compared with the other two groups was found immediately postoperatively. The concentration was significantly lower after 6 h. For plasma bupivacaine concentrations, great interindividual variations were observed, especially in the PVB group. Method of randomization was not reported. The authors conclude that neither method produced good post-thoracotomy analgesia.
Comments: A well-designed study. Unfortunately, less than optimal TEA is used, omitting opioid and α2 -agonist. Also, TEA was started after completing surgery, instead of exploiting the epidural analgesia during surgery. Pulmonary functions tended to be lower in the PVB group and plasma concentrations tended to be higher in the PVB group compared with TEA. Patients in all three groups had high morphine PCA-consumption causing many of the observed adverse effects, especially nausea, vomiting, pruritus, drowsiness, confusion, hallucinations, and difficulties in breathing.
3.7.3 Kaiser et al. (1998)
Kaiser et al. evaluated PVB, which they call extrapleural intercostal analgesia and post-thoracotomy TEA after lung surgery. They randomized 30 patients to two groups, but had 13% catheter failure in each group. The TEA group (13 patients) received a T5–T6 level catheter preoperatively and bupivacaine 5 mg/ml (0.5%) 4–6 ml/h intraoperatively, followed by 4–8 ml/h of bupivacaine 2.5–3.75 mg/ml (0.25–0.375%) plus fentanyl 2 μg/ml postoperatively. The PVB group (13 patients) had a catheter placed paravertebrally at incision level under direct vision before wound closure and received 0.1 ml/kg/h bupivacaine 5 mg/ml (0.5%) plus ornipressin 0.05 U/ml postoperatively, preceded by 20 ml bupivacaine 5 mg/ml (0.5%) over 20 min towards end of surgery. Both groups got meflumenaminic acid 500 mg every 6 h and were provided additional rescue analgesic as s.c. nicomorphine 0.1 mg/kg every 4–6 h. Unspecified (rest or coughing?) pain intensity was scored on a 5 categories verbal rating scale. TEA was only provided during ICU-stay and was discontinued after 2.7 days (mean). PVB catheter infusion on surgical wards following 2 days in the ICU was continued for an average of 6.6 days. Pain scores were similar in the two groups until the TEA was discontinued on the 2nd or 3rd post-operative day when the patient left the ICU. The TEA patients then reported more pain. Nicomorphine consumption was higher in the TEA group throughout the study. There was no statistical difference in complications between the groups, although one patient in the TEA group, after discontinuation of the TEA, developed atelectasis, pneumonia, sepsis, multiorgan failure and died.
Bolus dose for the TEA was not reported, neither was total volume of infused bupivacaine solution, nor whether pain scores represented pain at rest or pain during coughing. Serum bupivacaine concentrations were similar on the first postoperative day, on the 3rd postoperative day measured only in the PVB group, on average = 1.3 mg/l but up to 3.5 mg/l in one patient. Toxic concentration is 2.6–4.6 mg/l (Rosenberg et al., 2004). Method of randomization was not reported. The authors conclude that TEA and PVB are equipotent and safe methods for post-thoracotomy analgesia, and that PVB should be the preferred analgesic approach for patients who do not qualify for or do not accept TEA. They presume PVB to be safe enough for use on surgical wards for up to a week, whereas their TEA method required ICU-monitoring, they kept their TEA patients in the ICU and therefore TEA was a more costly method than PVB in their hospital. They therefore suggested that PVB should be considered as the method of choice in all patients.
Comments: This is a study highly biased toward PVB, using bupivacaine 5 mg/ml with a vasopressor for almost 7 days on surgical wards, and 2.5–3.75 mg/ml without a vasopressor but with fentanyl 2 μg/ml for less than 3 days of TEA in the ICU. They were convinced that PVB was more effective and safer than TEA. Their TEA infusions have a too high bupivacaine and too low fentanyl concentration, not adding adrenaline. Thus, their TEA infusion was neither effective nor safe. They especially recommended PVB when TEA is contraindicated when haemostasis is disturbed. However, PVB infusions also enter the epidural space in most cases (Conacher and Kokri, 1987; Purcell-Jones et al., 1989). The risk of bleeding and infections in the spinal canal must be equal in the two catheter methods.
3.7.4 Richardson et al. (1999)
Richardson et al. compared the effects of preoperative and continuous paravertebral (PVB) and epidural (TEA) bupivacaine on pain, pulmonary functions, and stress responses after thoracotomy. Mostly lung surgery and a few oesophagastrectomy procedures were performed. Randomization was by sequential allocation of eligible patients via computer-generated random numbers. In the TEA group (49 patients), an epidural catheter was placed preoperatively at T7–T10 and 3 ml bupivacaine 5 mg/ml (0.5%) followed by a bolus of 10–15 ml bupivacaine 2.5 mg/ml (0.25%) before and up to 10 ml at chest closure. The PVB group (46 patients) preoperatively received a bolus of up to 20 ml bupivacaine 5 mg/ml (0.5%) at T6–T8 level, had a catheter placed under direct vision intraoperatively, through which a bolus of up to 20 ml bupivacaine 2.5 mg/ml (0.25%) was injected at chest closure. Postoperatively, bupivacaine 2.5 mg/ml (0.25%) for TEA and bupivacaine 5 mg/ml (0.5%) for PVB were infused at a rate of 0.1 ml/kg/h. All patients received diclofenac 50 mg every 8 h, orally or rectally, and i.v. PCA morphine 1 mg boluses with 5 min lockout time. Outcome variables were documented for 2 days: Pain was scored using VAS at rest and during coughing. Pain scores were low in both groups, but pain scores and cumulative morphine consumption were statistically lower in the PVB group. Peak expiratory flow rate was somewhat better preserved in the PVB group, and increases in plasma cortisol and glucose were less in the PVB group. Incidence of nausea, vomiting and postoperative respiratory morbidity was lower in the PVB group. Compared with the TEA group, the PVB group received about twice as much bupivacaine. The authors suggested that the observed difference in pulmonary function and nausea and vomiting might have been related to the higher consumption of rescue morphine in the TEA group. Three PVB patients developed temporary confusion, possibly due to bupivacaine accumulation. The authors concluded that TEA and PVB are effective for post-thoracotomy analgesia, and that PVB is superior for analgesia, pulmonary function, neuroendocrine stress responses, side effects, and postoperative respiratory morbidity.
Comments: This is a well-done study with a good number of patients. However, in this study as in all the other 9 studies, TEA is far from “optimal” thoracic epidural analgesia. Epidural catheters were sited below T7, and although they advanced the catheter 5 cm, one never knows in which direction the epidural catheter goes: It may curl up or turn down (caudad) as often as cephalad. Extent of segmental sensory losses to ice were checked, but not documented. The study may therefore have had a major, fatal flaw, in favour of PVB. The PVB catheters were always placed in segmentally optimal position, whereas the TEA catheters may have been far too caudad. This could explain the differences between the PVB and TEA in this study. Generalization to thoracic epidural analgesia as less effective and with more pulmonary complications than PVB cannot be made from this study.
3.7.5 Bimston et al. (1999)
Bimston et al. compared the efficacy and complications of post-thoracotomy PVB and TEA after lung surgery. 30 patients intraoperatively received a paravertebral catheter under direct vision with a loading dose of 18 ml bupivacaine 5 mg/ml (0.5%) with adrenaline 5 g/ml plus 2 ml fentanyl 50 μg/ml. 20 patients had a thoracic epidural catheter placed preoperatively. However, neither test dose nor loading dose nor level of insertion of the catheter is reported. One PVB patient, who had the catheter accidentally removed at the end of surgery, received an epidural catheter and was transferred to the TEA group. Both catheter groups received a solution of bupivacaine 1 mg/ml (0.1%) plus fentanyl 10 g/ml postoperatively, titrated to provide adequate analgesia, the infusion rates are not reported in detail but were 10–15 ml/h initially (although they write 10–15 ml/min, which must be an error, unless this was intended as a very rapid bolus!), gradually reduced to 5–10 ml/h after 48 h. They do not report figures, but it appears that the infusion rates were similar in the two groups. Additional analgesics were provided, the frequency of requests, which was equal between the groups, but not the actual drugs and their amounts are reported. Pain was measured with a VAS. Immediately postoperatively and at 40 h and later VAS-values were similar for the groups. Between 8 and 32 h after surgery the TEA group had a significantly lower VAS than the PVB group. Whether pain while coughing or pain at rest was evaluated is not reported. Less urinary retention, defined as Foley catheter replacement after initial discontinuation, was seen in the PVB group. However, they report that urinary catheters were removed on the 2nd postoperative day in the PVB group, whereas not until the epidural catheter was removed in the TEA group! In our studies, using optimal TEA, we routinely remove (successfully!) urinary catheters in the morning of the 1st postoperative day (Niemi and Breivik, 2003; Niemi, 2004). Other side effects, including hypotension, nausea, vomiting and pulmonary complications were equally distributed between the groups.
Randomization was “blindly, by random units table” method, but this resulted in 30 patients in the PVB group and only 20 in the TEA group. The authors conclude that both methods provide excellent pain control. However, they save US$ 500 on the patient’s bill by avoiding TEA, which is managed by the Acute Pain Service of the anaesthesia-department. The corresponding author, a surgeon, prefers to be independent of the pain service and manage post-thoracotomy pain on surgical wards by using PVB.
Comments: A correct method of randomization should always give an equal number of patients in treatment and control group, unless otherwise planned, e.g. a placebo-group can be smaller than an active treatment group, for ethical reasons. Their method of randomization is therefore suspect. They do not report segmental site of the TEA catheter, or any details of the infusion rates. They used a very large amount of fentanyl in both groups, and many of the observed side effects were undoubtedly due to systemic fentanyl-effects, not due to the block techniques. It is not a laudatory illustration of the US health care system, that the surgeons prefer the least effective analgesic technique because the anaesthesia department’s Acute Pain Service charges US$ 500 for providing safe epidural analgesia.
3.7.6 Dhole et al. (2001)
Dhole et al. compared TEA and PVB for postoperative analgesia after minimally invasive direct coronary artery bypass (MIDCAB) performed through a minithoracotomy. Data from 20 patients in each group are reported. An epidural catheter was placed at T4–T5 level in the TEA group, the same level was used for percutaneous placement of catheter in the PVB group. Both groups received a 3 ml test dose of lidocaine 20 mg/ml (2%), followed by a bolus of 8 ml bupivacaine 5 mg/ml (0.5%) and a 6 ml/h infusion of bupivacaine 2.5 mg/ml (0.25%). Segmental spread of needlestick-hypoaesthesia confirmed correct catheter placement. Rescue analgesia was i.m. ketorolac 30 mg. Respiratory rate was significantly lower in the PVB group. VAS at rest and during coughing was not different. No difference in consumption of rescue analgesia with ketorolac was observed. No complications were seen in the PVB group, in the TEA group, one patient suffered transient hypotension, another pain at the site of catheter insertion. Cardiac index was higher in the TEA group. Method of randomization is not reported. The authors concluded that PVB is as effective as TEA for post-thoracotomy analgesia in MIDCAB patients, and that PVB may be safer than TEA.
Comments: Although no bleeding in the spinal canal or paravertebral space was observed, the heparin regimen during CABG and NSAID for rescue analgesia may disturb primary and secondary haemostasis enough so that TEA is unsafe in these patients. However, the paravertebral space is part of the epidural space, making the infrequent, but serious bleeding and infectious complications in the epidural space a possibility during and after PVB, as with traditional TEA.
3.7.7 Luketich et al. (2005)
Luketich et al. studied the data from 91 lung surgery patients to compare post-thoracotomy TEA with paravertebral analgesia. Preoperatively, the TEA group (44 patients) had a catheter inserted at the T3–T6 level, which was tested with a 3 ml lidocaine 15 mg/ml (1.5%) plus adrenaline 5 μg/ml, followed by an infusion of bupivacaine 1.25 mg/ml (0.125%) plus morphine 50 g/ml, 4–8 ml/h. No TEA loading dose is reported. The paravertebral group (47 patients) received 10 ml bupivacaine 2.5 mg/ml (0.25%) percutaneously through a needle before thoracotomy, followed by an under direct vision placement of a paravertebral catheter at T8–T9 level by the surgeon. Another loading dose—10 ml of bupivacaine 5.0 mg/ml (0.5%) was given before chest closure. Postoperatively the PVB group received bupivacaine 2.5 mg/ml (0.25%) 0.1 ml/kg/h plus i.v. morphine patient controlled analgesia (PCA) for 4–6 h. Pain was reported by a composite score and no difference between the groups was identified. Whether dynamic pain or pain at rest was assessed was not specified. Additional analgesics were provided; the exact amounts and drugs are not reported. Complications, including pneumonia, were similar between the groups. Foley catheter days were significantly fewer in the PVB group, definition of need for Foley catheter is not reported. Method of randomization is not reported. The authors concluded that satisfactory pain control was achieved with both regimens, and they recommended PVB plus i.v. PCA for patients in whom epidural catheter placement is not feasible.
Comments: The PVB group received about twice as much bupivacaine as the TEA group, but no opioid in their postoperative infusion. Their “composite pain score” is unfortunately only an average of three measurements of pain intensity scores and therefore not a composite score—at all. A meaningful composite pain score must comprise more variables than merely pain intensity scores, e.g. a pain intensity score and the amount of rescue analgesic consumed (Silverman et al., 1993; Romundstad et al., 2006). The surgeons express preference for PVB because “daily consulting fees to the Acute Pain Team” are avoided and no time is lost in the operating room.
3.7.8 Casati et al. (2006)
Casati et al. compared the analgesic efficacy of continuous PVB and TEA for post-thoracotomy pain for 2 days after lung surgery with “blinded” observers. Each group included 21 patients. The TEA group received a T5–T7 level catheter and a loading dose of 5 ml ropivacaine 7.5 mg/ml (0.75%). As loading, the PVB group had three paravertebral injections, each 5 ml ropivacaine 7.5 mg/ml (0.75%), into T4, T5 and T6 level. Thereafter, a PVB catheter was percutaneously inserted at the T4 level. Postoperatively, both groups received paracetamol 1 g every 8 h plus ropivacaine 2 mg/ml (0.2%) continuously. Infusion rate was 5–10 ml/h, adjusted to maintain pain score on a visual analogue scale (VAS) ≤4/10. Also, i.v. morphine 5 mg was provided as rescue analgesic to maintain VAS ≤4/10. VAS was scored at rest and during coughing. For total postoperative ropivacaine volume, amount of rescue analgesicand VAS at rest and when coughing, no difference was found between the groups. Hypotension, defined as >30% reduction in systolic blood pressure, was more frequent in the TEA group. No severe complications occurred in either group. For randomization, computer-generated sequence and sealed envelopes were used. The authors concluded that PVB is as effective as TEA for analgesia, but has less haemodynamic side effects and is an alternative to TEA for post-thoracotomy pain control.
Comments: This study is one of 3 of the 10 included studies that placed the PVB-catheter percutaneously, 2.5 cm lateral to the spinous process, below the transverse process. A Tuohy needle was advanced 1 cm deeper than first contact with the transverse process and catheter inserted 2–3 cm beyond the tip of the needle. This allowed blinding of the outcome variableobservers, as type of analgesic block could not be identified. However, in our setting bilateral upper and lower segmental levels (hypoaesthetic areas) are documented regularly, thus any “blinding” of the postoperative observers of effects would be impossible. They have randomized properly, and observed dynamic pain during coughing. We regret the use of a too concentrated local anaesthetic only, without opioid and adrenaline, for the TEA. An “optimal” three component epidural mixture would have avoided hypotension and urinary retention (Niemi and Breivik, 2002, 2003).
3.7.9 Mehta et al. (2008)
Mehta et al. compared continuous TEA and PVB for postoperative analgesia after robotic-assisted coronary artery bypass surgery accompanied by minithoracotomy. The TEA group (19 patients) had an epidural catheter inserted at C7–T1 level. The PVB group (17 patients) had a catheter inserted percutaneously at T4–T5 level into to the left PVS. Both groups received a catheter test dose of 3 ml lidocaine 20 mg/ml (2%), followed by a bolus 8 ml bupivacaine 5 mg/ml (0.5%) and an infusion of bupivacaine 2.5 mg/ml (0.25%) 0.1 ml/kg/h. VAS was scored at rest and during coughing, and diclofenac sodium 75 mg i.m. was given on patient demand or at VAS >5/10 at rest. No significant differences in pain scores, consumption of rescue analgesics, mean time to extubation, haemodynamic or respiratory variables were found. Two patients in the TEA group experienced upper limb numbness, which recovered after stopping the infusion of local anaesthetic. Method of randomization is not reported. The authors concluded that PVB appears to be safe, effective and comparable to TEA for postoperative analgesia after robotic-assisted coronary artery bypass surgery. They consider PVB safe despite recent anticoagulation.
Comments: Same solution and regimen used for PVB and TEA, both placed percutaneously, with blinded observers. TEA catheter placed much too high (at C7–T1), causing numbness of arms in 10% of patients. PVB-catheters were placed at appropriate level T4–T5. Combination of heparin for LIMA and diclofenac rescue analgesic potentially increases risk of spinal bleeding—in PVB as well as TEA. Again we register that TEA would have performed better with lower concentration of bupivacaine (1 mg/ml instead of 2.5 mg/ml) with fentanyl (2 g/ml), and adrenaline (2 g/ml) (Niemi and Breivik, 2002, 2003).
3.7.1 Gulbahar et al. (in press)
Gulbahar et al. compared TEA (19 patients) and PVB (25 patients) for post-thoracotomy analgesia after lung surgery. Data from 13 patients in the TEA group are reported. Six were excluded due to catheter misplacement or too early removal. TEA catheter at T7–T10 level was inserted preoperatively and 5 ml bupivacaine 2.5 mg/ml (0.25%) injected at chest closure. The PVB group included 25 patients who had their catheter placed at thoracotomy level under direct visual guidance prior to chest closure. Postoperatively, both groups received 0.10 ml/kg/h bupivacaine 2.5 mg/ml (0.25%) plus patient controlled bolus of 2 ml with a lock out time of 1 h. Morphine was provided for rescue analgesia. No significant differences between the groups were found for VAS, morphine consumption, attempted PCA boluses, or respiratory variables. Urinary retention, nausea, vomiting, and hypotension were significantly more frequent in the TEA group, the authors suggested this could be attributed to the epidural administration of too concentrated local anaesthetic. No side effects were noted in the PVB group. Method of randomization is not reported. The authors concluded that the methods are equally effective for post-thoracotomy analgesia. They concluded that PVB is preferable as catheter placement procedure was quicker and PVB had fewer adverse effects postoperatively.
Comments: The TEA catheters were sited much too low (below T7) for relieving pain after thoracotomy, explaining part of the problems with patients randomized to TEA. We are not informed of whether pain scores were assessed at rest or when coughing. The epidural infusion contained too high concentration of bupivacaine, no fentanyl, and no adrenaline. Therefore this was not a fair comparison of thoracic epidural analgesia with paravertebral block. Still they did not find any difference in effect on pain during 3 days following thoracotomy. We agree with the authors that the higher incidence of adverse effects in the TEA group was due to too high concentrations of local anaesthetic, as well as too low segmental placement of the epidural catheters. Unequal numbers of patients in the two groups indicate that the, unreported, method of randomization was not appropriate.
4 Discussion
Ten prospective trials comparing TEA (N = 224) and PVB (N = 243) for post-thoracotomy analgesia were evaluated. The trials were small, reporting data from 9 to 49 patients per study group. This causes a large type II error, i.e. with small numbers of patients in each group, a finding of no statistically significant difference, may not be true. All the ten studies reported having randomized patients to either TEA or PVB, but only three described the method of randomization (Richardson et al., 1999; Bimston et al., 1999; Casati et al., 2006) one of which describes a dubious way of randomizing.
Because many aspects of the ten studies varied, the heterogeneity made a formal meta-analyses difficult so that we chose to comment on each trial in a narrative style, drawing on our extensive experience with both PVB and TEA for up to four decades. Below are further discussions of important aspects of the ten included studies and our over all conclusions.
4.1 Techniques of PVB and TEA
The various techniques and regimens for PVB provided post-thoracotomy analgesia similar to TEA. Only one study concluded that PVB was significantly superior to TEA, but the TEA catheters were placed below T7 (Richardson et al., 1999). Whereas the PVB- catheters were placed at optimal segmental positions in all the ten studies, this was not the case in the TEA groups in three of the studies, and was not reported in another two. In all the ten trials the TEA solutions were suboptimal, opioid added in only three, adrenaline in none, all over-administering local anaesthetics, causing more sympathetic blocks, hypotension, and urinary retention. Still, one study found TEA to be significantly superior to PVB during the first 1.5 days after thoracotomy, excluding the immediate postoperative hours (Bimston et al., 1999).
4.2 Outcome variables for pain relief
Pain at rest and during quiet, superficial breathing is less intense and not difficult to relieve, even after thoracotomy. Effects on the intense dynamic pain during deep inspiration and coughing is a much more sensitive variable to distinguish effective from less effective analgesic techniques (Niemi and Breivik, 2002, 2003; Niemi, 2004). Only 5 of the 10 studies reported pain during coughing, one of which concluded that PVB was superior to TEA (Richardson et al., 1999). However, this study was one of the two siting the TEA catheters below T7. The one study that found TEA to be significantly superior to PVB during the first 1.5 days after thoracotomy do not report whether they observed pain during coughing (Bimston et al., 1999). This is unfortunate, as dynamic post-thoracotomy pain is at its most intense during the day of surgery and the first postoperative day.
4.3 PVB is also an epidural approach
In a recent editorial Boezaart (Boezaart, 2009) reminded the readers of the anatomical basis for paravertebral blocks (PVB). The nerves in the paravertebral space (PVS) are nerve roots surrounded by cerebrospinal fluid (CSF), covered with arachnoidea and dura. The PVS is part of the paraspinal epidural space, a continuation of the spinal epidural space. Injections into the PVS are epidural injections (Boezaart et al., 2009). We agree completely with Boezaart (Boezaart, 2009) who emphasizes the need for the same respect for PVB as for any epidural block concerning indications, technique and potential complications (Breivik et al., 2009).
Spread of solution from a paravertebral injection has been demonstrated to be unpredictable (Conacher and Kokri, 1987; Purcell-Jones et al., 1989). For injections of 5 ml radiocontrast through a needle radiologically verified to be in the PVS, spread was epidural in 70% (exclusively epidural spread in 31%) and paravertebral only in 18%. Repeated injections in the same patient did not necessarily give the same pattern of distribution. These conclusions were supported in a recent cadaver study (Luyet et al., 2009), describing an ultrasound-guided technique for accurate puncture of the PVS. Catheters were inserted through a Tuohy needle verified to have its tip in the PVS. Injection of 10 ml contrast through the catheter resulted in 55% paravertebral spread, 30% exclusively epidural spread, 10% mediastinal, and 5% pleural spread. Also, prevertebral spread may occur (Tenicela and Pollan, 1990).
4.4 Comparing PVB and TEA is comparing two of the same kind;two extremes of a continuum
Although PVB is in part a spinal nerve block, requiring a higher concentration of local anaesthetics, all the reviewed studies comparing thoracic PVB and TEA could be regarded as trials comparing epidural blocks from two different approaches. The one trial that found PVB to be superior to TEA for post-thoracotomy analgesia (Richardson et al., 1999; Sharrock, 1980), sited the epidural catheter too low for post-thoracotomy pain, although a T7–T10 TEA may be appropriate for oesophagastrectomy or anti-reflux procedures, which were also included in their study. Moreover, local anaesthetic only was administered; neither opioid, nor adrenaline was added to the TEA infusion. Importantly, the PVB group received about twice the bupivacaine dose, as higher volumes and concentrations than in the TEA group were used.
PVB has been described as a posterior intercostal nerve block (Nunn and Slavin, 1980). Interestingly, a study comparing TEA with and without intercostal nerve block could not document any additional analgesia from the intercostal nerve block to that obtained by TEA alone (TEA with local anaesthetic and opioid) (Allen et al., 2009).
4.5 Prevalence of complications
Eight of the ten trials found the two blocks to be equally effective. For the PVB group in several of the studies, this effect was reached by the administration of larger doses of local anaesthetics (Table 1) (Kaiser et al., 1998; Richardson et al., 1999; Luketich et al., 2005; Casati et al., 2006). For TEA, none of the trials administered an optimal, balanced solution for TEA. A combination of local anaestethic, opioid, and adrenergic agonist may deliver pain relief with a minimum of side effects (Breivik, 1995; Niemi and Breivik, 2003; Niemi, 2004). The unusually high prevalence of urine retention and hypotension in the TEA groups of several of the RCTs reviewed (e.g. (Matthews and Govenden, 1989; Casati et al., 2006)), is almost nonexistent when a triple component, low concentrations, balanced epidural infusion is administered (Niemi and Breivik, 2003; Niemi, 2004).
Thoracic epidural analgesia carries risks of infrequent but serious neurological complications from epidural haematoma and abscesses, or meningitis (Moen et al., 2004). Hypotension, brady-cardia may occur, especially in hypovolaemic patients. Dural perforation or unrecognised dural penetration of an epidural catheter may lead to epiarachnoid or even intrathecal administration and high or total spinal anaesthesia, which has happened (Chaudri et al., 2009; Breivik, 1998).
4.6 Specific complications from PVB
As paravertebral block represents an epidural block, the risk exists that PVB will prove to cause the same complications as those above described for TEA (Boezaart et al., 2009). In addition, the paravertebral technique has its own list of complications, including pleural puncture (1.1%), pneumothorax (0.5%), vascular puncture (3.8%) (Lönnqvist et al., 1995). Incidence of pneumothorax associated with bilateral PVB has been reported to be eight times the incidence seen in unilateral PVB (Naja and Lönnqvist, 2001). Systemic absorption of local anaesthetic occurs, causing confusion as an early sign of central nervous system intoxication (Richardson et al., 1999), which may progress to fulminant grand mal seizures. With continuous infusion of a local anaesthetic, accumulation over days may lead to increasing plasma local anaesthetic levels (Pertunnen et al., 1995). When bupivacaine is used for prolonged PVB, we fear that the previously well-known cardiotoxic effects of this local anaesthetic are bound to resurface—sooner or later.
Independent of technique, paravertebral injections are done in close proximity to the intervertebral foramen and its contents (Fig. 1). Spread through the intervertebral foramen after unilateral paravertebral approach may result in contralateral epidural distribution of local anaesthetic and bilateral anaesthesia (Frohm et al., 2006). Also, bilateral Horner’s syndrome after unilateral paravertebral injection has been described (Purcell-Jones et al., 1989). Purcell-Jones et al. (Purcell-Jones et al., 1989) documented radio-logically that 33% of (5 ml only!) PVB injections spread to both sides of the spinal epidural space. Thus, bilateral epidural anaesthesia and side effects may be caused by unilateral PVB.
Paraplegia and serious paraparesesis have been reported after radiologically guided lumbosacral nerve root blocks (Houten and Errico, 2002). The mechanism is most likely undetected penetration of a low originating artery of Adamkiewicz, the major supply to the anterior spinal artery below T8. Damage to, or injection into this or any of the thoracic radiculomedullary arteries, all running through the intervertebral foramen (Fig. 1), may induce spinal cord infarction and devastating complications. The senior author of the present review experienced exactly this complication some years ago in a neighbouring hospital. The permanent paraplegia that followed a thoracic PVB in that case was not published, unfortunately.
5 Conclusions
The publications comparing TEA and PVB for post-thoracotomy pain relief do not allow conclusions on which method has superior analgesic efficacy and safety. PVB represents unpredictable epidural analgesia from a paravertebral approach, plus spinal nerve or nerve root blockade. The ten comparison studies reviewed comprise much too few patients to indicate anything about occurrence of serious neurological complications from PVB or TEA. However, anatomical considerations and previously known complications have convinced us that PVB must have at least the same risks of serious complications as TEA. Maybe even more, because a PVB causes epidural injection in most cases, and in addition PVB risks damaging the pleura and important radicular supply-arteries to the spinal cord (Fig. 1). Respect for technical details, indications and concerns of complications should be at least the same for PVB as for any epidural technique.
In some of the papers, surgeons are pleased with PVB because, in their health care setting, they do not depend on costly acute pain services when using PVB for pain relief after thoracotomies. They also claim that the PVB saves precious time in the operating room and does not delay surgery, as TEA seems to do in their hospital. However, sooner or later, they are bound to experience one or more of the infrequent, but serious complications to PVB.
So far, to our knowledge, optimally conducted epidural analgesia has not been compared with PVB in a well-designed RCT. But current literature and our experience with both techniques for up to four decades, indicate that PVB may be an alternative for post-thoracotomy pain when TEA is not feasible for various patient-related reasons (Breivik et al., 2009). Severely disturbed haemostasis is a contraindication to PVB as well as TEA.
DOI of refers to article: 10.1016/j.sjpain.2009.11.002.
References
Allen MS, Halgren L, Nichols III FC, Cassivi SD, Harmsen WS, Wigle DA, et al. A randomized controlled trial of bupivacaine through intracostal catheters for pain management after thoracotomy. Ann Thorac Surg 2009;88:903–10.Search in Google Scholar
Atkinson RS, Rushman GB. A synopsis of anaesthesia. 8th ed. Bristol: Wright; 1977.Search in Google Scholar
Berrisford RG, Sabanathan SS. Direct access to the paravertebral space at thoracotomy. Ann Thorac Surg 1990;49:584.Search in Google Scholar
Bimston DN, McGee JP, Liptay MJ, Fry WA. Continuous paravertebral extrapleural infusion for post-thoracotomy pain management. Surgery 1999;126:650–6.Search in Google Scholar
Boezaart AP. Atlas of Peripheral Nerve Blocks and Anatomy for Orthopaedic Anesthesia. Philadelphia: Saunders/Elsevier; 2008.Search in Google Scholar
Boezaart AP. That which we call a rose by any other name would smell as sweet-and its thorns would hurt as much. Reg Anesth Pain Med 2009;34:3–7.Search in Google Scholar
Boezaart AP, Lucas SD, Elliott CE. Paravertebral block: cervical, thoracic, lumbar, and sacral. Curr Opin Anaesthesiol 2009;22:637–43.Search in Google Scholar
Breivik H. Safe perioperative spinal and epidural analgesia: importance of drug combinations, segmental site of injection, training and monitoring. Acta Anaesthesiol Scand 1995;39:869–71.Search in Google Scholar
Breivik H. Komplikasjoner under og etter operasjoner og fødsler utført i spinaleller epidural bedøvelse. Råd om trygg praksis [Complications under and after operations and vaginal deliveries preformed under spinalor epidural anaesthesia]. Tidskr Nor Lægeforen 1998;118:1708–16.Search in Google Scholar
Breivik H, Bang U, Jalonen J, Vigfússon G, Alahuhta S, Lagerkranser M. Nordic guidelines for neuraxial blockade in patients with disturbed haemostasis proposed by the Scandinavian Society of Anaesthesiology and Intensive Care Medicine taskforce. Acta Anaesthesiol Scand 2009;53 [E-pub ahead of print].Search in Google Scholar
Bromage PR. Epidural Analgesia. Philadelphia: Saunders; 1978.Search in Google Scholar
Casati A, Alessandrini P, Nuzzi M, Tosi M, Iotti E, Ampollini L, et al. A prospective, randomized blinded comparison between continuous thoracic paravertebral and epidural infusion of 0.2% ropivacaine after lung resection surgery. Eur J Anaesth 2006;23:999–1004.Search in Google Scholar
Chaudri BB, Macfie A, Kirk AJ. Inadvertent total spinal anesthesia after intercostal nerve block placement during lung resection. Ann Thorac Surg 2009;88:283–4.Search in Google Scholar
Conacher ID, Kokri M. Postoperative paravertebral blocks for thoracic surgery. A radiological appraisal. Br J Anaesth 1987;59:155–61.Search in Google Scholar
Conlon NP, Shaw AD, Grichnik KP. Postthoracotomy paravertebral analgesia: will it replace epidural analgesia? Anesthesiol Clin 2008;26:369–80.Search in Google Scholar
Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418–26.Search in Google Scholar
De Cosmo G, Aceto P, Campanale E, Congedo E, Clemente A, Mascia A, et al. Comparison between epidural and paravertebral intercostal nerve block with ropivacaine after thoracotomy: effects on pain relief, pulmonary function and patient satisfaction. Acta Med Romana 2002;40:340–7.Search in Google Scholar
Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2001;15:288–92.Search in Google Scholar
Eason MJ, Wyatt R. Paravertebral thoracic block—a reappraisal. Anaesthesia 1979;34:638–42.Search in Google Scholar
Frohm RM, Raw MR, Haider N, Boezaart AP. Epidural spread after continuous cervical paravertebral block: a case report. Reg Anesth Pain Med 2006;31:279–81.Search in Google Scholar
Gulbahar G, Kocer B, Muratli SN, Yildirim E, Gulbahar O, Dural K, Sakinci U. A comparison of epidural and paravertebral catheterisation techniques in post-thoracotomy pain management. Eur J Cardiothorac Surg. 2009 Aug 24 [E-pub ahead of print].Search in Google Scholar
Hara K, Sakura S, Nomura T, Saito Y. Ultrasound guided thoracic paravertebral block in breast surgery. Anaesthesia 2009;64:216–29.Search in Google Scholar
Hemmati H, Rahim MB, Emami SA. Comparison of the efficacy of extraplural paravertebral catheter analgesia with thoracic epidural analgesia and systemic administration of morphine in postthoracotomy pain. Chest (Suppl) 2006;130:S271.Search in Google Scholar
Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine 2002; 2:70–5.Search in Google Scholar
Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026–40.Search in Google Scholar
Kaiser AM, Zollinger A, De Lorenzi D, Largiadèr F, Weder W. Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg 1998;66:367–72.Search in Google Scholar
Kappis M. Sensilität und lokale Anästhesiegebiet der Bauchhöhle mit besonderer Berücksichtigung der Splanchnicus Anästhesie. Beitr Klin Chir 1919;115:161–75.Search in Google Scholar
Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771–80.Search in Google Scholar
Lang SA. The use of a nerve stimulator for thoracic paravertebral block. Anesthesiology 2002;97:521.Search in Google Scholar
Leaver A, Yeomans M, Shelton A, Opie J, Graham J. A randomised trial comparing thoracic epidural with paravertebral blocks for postoperative analgesia after pneumonectomy; 2006 (see Davies et al., 2006).Search in Google Scholar
Lönnqvist PA. Pre-emptive analgesia with thoracic paravertebral blockade? Br J Anaesth 2005;95:727–8.Search in Google Scholar
Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia 1995;50:813–5.Search in Google Scholar
Luketich JD, Land SR, Sullivan EA, Alvelo-Rivera M, Ward J, Buenaventura PO, et al. Thoracic epidural versus intercostal nerve catheter plus patient controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845–9.Search in Google Scholar
Luyet C, Eichenberger U, Greif R, Vogt A, Szücs Farkas, Moriggl B. Ultrasound-guided paravertebral puncture and placement in human cadavers: an imaging study. Br J Anaesth 2009;102:534–9.Search in Google Scholar
Matthews PJ, Govenden V. Comparison of continuous paravertebral and extradural infusions of bupivacaine for pain relief after thoracotomy. Br J Anaesth 1989;62:204–5.Search in Google Scholar
Mehta Y, Arora D, Sharma KS, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Cardiol Anaesth 2008;11:91–6.Search in Google Scholar
Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–99. Anesthesiology 2004;101:950–9.Search in Google Scholar
Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia 2001;56:1184–8.Search in Google Scholar
Niemi G. Optimizing postoperative epidural analgesia. Doctoral thesis submitted to Faculty of Medicine, University of Oslo. Oslo: Unipub; 2004.Search in Google Scholar
Niemi G, Breivik H. Epinephrine markedly improves thoracic epidural analgesia produced by a small-dose infusion of ropivacaine, fentanyl, and epinephrine after major thoracic or abdominal surgery: a randomized, double-blinded crossover study with and without epinephrine. Anesth Analg 2002;94:1598–605.Search in Google Scholar
Niemi G, Breivik H. Minimally effective concentration of adrenaline in a low-concentration thoracic epidural analgesic infusion of bupivacaine, fentanyl and adrenaline after major surgery. Acta Anaesthesiol Scand 2003;47:439–50.Search in Google Scholar
Nunn JF, Slavin G. Posterior intercostal nerve block for pain relief after cholecystectomy: anatomical basis and efficacy. Br J Anaesth 1980;52:253–60.Search in Google Scholar
Pertunnen K, Nilsson E, Heinonen J, Hirvisalo E-L, Salo JA, Kalso E. Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth 1995;75:541–7.Search in Google Scholar
Purcell-Jones G, Pither CE, Justins DM. Paravertebral somatic nerve block: a clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg 1989;68:32–9.Search in Google Scholar
Richardson J, Lönnqvist PA. Thoracic paravertebral block. Br J Anaesth 1998;81:230–8.Search in Google Scholar
Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, Merans AJ. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth 1999;83:387–92.Search in Google Scholar
Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, et al. Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomised, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo,. Anesth Analg 2006;102:418–25.Search in Google Scholar
Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med 2004;29:564–75.Search in Google Scholar
Sabanathan S, Bickford-Smith PJ, Pradhan GN, Hashimi H, Eng J-B, Merans AJ. Continuous intercostal nerve block for pain relief after thoracotomy. Ann Thorac Surg 1988;46:425–6.Search in Google Scholar
Sharrock NE. Postural headache following thoracic somatic paravertebral block. Anesthesiology 1980;52:360–2.Search in Google Scholar
Silverman DG, O’Connor TZ, Brull SJ. Integrated assessment of pain scores and rescue morphine use during studies of analgesic efficacy. Anesth Analg 1993;77:168–70.Search in Google Scholar
Stubhaug A, Breivik H. Clinical trials: acute and chronic pain. In: Breivik H, Campbell WI, Nicholas MK, editors. Clinical pain management—practice and procedures. 2nd ed. London: Hodder Arnold; 2008. p. 514–28.Search in Google Scholar
Szebla R, Machala W. Continuous epidural anaesthesia vs paravertebral block for lung surgery—a comparative study. Anestezjol Intens Ter 2008;40:152–5.Search in Google Scholar
Tenicela R, Pollan SB. Paravertebral–peridural block technique: a unilateral thoracic block. Clin J Pain 1990;6:227–34.Search in Google Scholar
Wedad M, Zaki MK, Haleem M. The effect of addition of wound infiltration with local anaesthetics to interpleural block on post-thoracotomy pain, pulmonary function and stress response in comparison to thoracic epidural and paravertebral block. Egypt J Anaesth 2004;20:67–72.Search in Google Scholar
Wildsmith JA. Developments in local anaesthetic drugs and techniques for pain relief. Br J Anaesth 1989;63:159–64.Search in Google Scholar
© 2009 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Editorial
- Scandinavian Journal of Pain: A networking and publishing tool for pain researchers and pain clinicians in the Nordic countries
- Editorial comments
- Pain relief with paravertebral blocks or epidural analgesia? Those who do not know the history of paravertebral blocks are condemned to rediscover the complications
- Editorial comments
- Investigation of drug–drug interactions and pain—From volunteer studies to randomized controlled trials in patients with chronic pain
- Editorial comments
- Those who do not know their pain-history will repeat previous errors in pain management
- Editorial comments
- Fear and catastrophizing thoughts aggravate risks of chronic pain after a fracture
- Editorial comments
- Important knowledge of pain and phantom experiences after breast surgery and leg- or arm-amputation: Value of qualitative pain research
- Editorial comments
- Dialectical behavioural therapy for complex chronic pain conditions
- Editorial comments
- Chronic pain conditions after herniorrhaphy decrease with time, but slowly
- Editorial comments
- Norwegian patients with chronic pain conditions that can be managed with reasonable cost/benefit now have a legally binding right to treatment in Norway
- Review
- A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy
- Original articles
- Does co-administration of paroxetine change oxycodone analgesia: An interaction study in chronic pain patients
- Original articles
- A personal experience learning from two pain pioneers, J.J. Bonica and W. Fordyce: Lessons surviving four decades of pain practice
- Original articles
- Pain-related fear, catastrophizing and pain in the recovery from a fracture
- Original articles
- Adult limb and breast amputees’ experience and descriptions of phantom phenomena—A qualitative study
- Original articles
- Applying dialectical behavior therapy to chronic pain: A case study
- Original articles
- Natural course of long-term postherniorrhaphy pain in a population-based cohort
- Original articles
- National guidelines for evaluating pain—Patients’ legal right to prioritised health care at multidisciplinary pain clinics in Norway implemented 2009
Articles in the same Issue
- Editorial
- Scandinavian Journal of Pain: A networking and publishing tool for pain researchers and pain clinicians in the Nordic countries
- Editorial comments
- Pain relief with paravertebral blocks or epidural analgesia? Those who do not know the history of paravertebral blocks are condemned to rediscover the complications
- Editorial comments
- Investigation of drug–drug interactions and pain—From volunteer studies to randomized controlled trials in patients with chronic pain
- Editorial comments
- Those who do not know their pain-history will repeat previous errors in pain management
- Editorial comments
- Fear and catastrophizing thoughts aggravate risks of chronic pain after a fracture
- Editorial comments
- Important knowledge of pain and phantom experiences after breast surgery and leg- or arm-amputation: Value of qualitative pain research
- Editorial comments
- Dialectical behavioural therapy for complex chronic pain conditions
- Editorial comments
- Chronic pain conditions after herniorrhaphy decrease with time, but slowly
- Editorial comments
- Norwegian patients with chronic pain conditions that can be managed with reasonable cost/benefit now have a legally binding right to treatment in Norway
- Review
- A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy
- Original articles
- Does co-administration of paroxetine change oxycodone analgesia: An interaction study in chronic pain patients
- Original articles
- A personal experience learning from two pain pioneers, J.J. Bonica and W. Fordyce: Lessons surviving four decades of pain practice
- Original articles
- Pain-related fear, catastrophizing and pain in the recovery from a fracture
- Original articles
- Adult limb and breast amputees’ experience and descriptions of phantom phenomena—A qualitative study
- Original articles
- Applying dialectical behavior therapy to chronic pain: A case study
- Original articles
- Natural course of long-term postherniorrhaphy pain in a population-based cohort
- Original articles
- National guidelines for evaluating pain—Patients’ legal right to prioritised health care at multidisciplinary pain clinics in Norway implemented 2009

