Abstract
Objectives
This study was aimed to investigate the diagnostic values of serum levels of asprosin and neutrophil gelatinase-associated lipocalin (NGAL) in Retinopathy of prematurity (ROP) and to assess the role of these biomarkers on the development and progression of the condition.
Methods
This study was carried out from April 2020 to February 2021 in the department of ophthalmology of a tertiary hospital in Turkey. Thirty patients diagnosed with ROP and 30 healthy newborns were included the study. Serum NGAL and asprosin levels were determined via ELISA.
Results
The median serum NGAL levels were found to be similar between the ROP group and the control group (p=0.595). Median asprosin levels were significantly higher in patients diagnosed with ROP [46.58 (12.70–142.28) ng/mL] compared to healthy subjects [13.05 (10.92–17.73) ng/mL] (p=0.001). The optimal cut-off value of asprosin by ROC analysis was 30 ng/mL (AUC: 0.754, p=0.001) for diagnosing ROP. Serum asprosin levels were positively correlated with serum ALP levels and inversely correlated with gestational week, uric acid and AST values (all, p<0.005).
Conclusions
Our results demonstrated that asprosin, but not NGAL, could be a biomarker for the diagnosis of ROP.
Introduction
Retinopathy of prematurity (ROP), also known as retrolental fibroplasia, is a vasoproliferative retinal disorder that affects prematurely born infants [1]. The prevalence of ROP varies globally, from 5 to 8% in developed countries with adequate neonatological facilities up to 30% in middle-income developing countries [2]. Despite increased ROP awareness and advances in neonatal care and management, ROP remains one of the leading causes of childhood blindness worldwide [3]. Various risk factors, including early gestational age, low birth weight, infections, and intense oxygen supplementation in the neonatal unit, have been demonstrated to contribute to ROP development [4]. However, precise pathophysiological mechanisms in ROP are yet to be identified, and there is a continuing need for novel biomarkers for the early diagnosis and management of retinal damage in order to prevent adverse outcomes.
The recently discovered asprosin protein was defined as a fasting-induced glycogenic hormone which is generated from C-terminal cleavage of profibrillin (encoded by the fibrillin-1 gene). Asprosin triggers glucose release from the liver into the bloodstream via the G-protein-cAMP-PKA pathway [5]. Studies have reported that asprosin is associated with insulin resistance, obesity, inflammation, polycystic ovarian syndrome and diabetic complications, including retinopathy and nephropathy [6]. Of note, the relationship between fibrillin-1 and TGF-beta has been demonstrated to be a factor contributing to diabetic retinopathy [7], and individuals with fibrillin-1 gene mutation exhibit Marfan syndrome, which affects skeletal, cardiovascular and ocular systems [8]. However, to date no study has examined the role of asprosin in ROP.
Neutrophil gelatinase-associated lipocalin (NGAL), also known as lipocalin-2, is a small protein that is secreted by neutrophils and epithelial cells, and is involved in metabolic homeostasis, inflammation, immunity and apoptosis [9]. Increased NGAL has been detected in various ocular diseases, including central retinal vein occlusion and idiopathic acute anterior uveitis (in aqueous humor), and in diabetic retinopathy and age-related macular degeneration (in plasma) [9, 10]. However, the role of serum NGAL levels in the pathogenesis of ROP remains unclear.
The aim of this study was to investigate the diagnostic value of serum levels of asprosin and NGAL, and to assess whether these biomarkers can be associated with the development and progression of ROP.
Materials and methods
This study was carried out from April 2020 to February 2021 in the Department of Ophthalmology, Fethi Sekin Hospital, Elazig, Turkey. Thirty patients diagnosed with ROP and 30 healthy newborns were included the study. The diagnosis of ROP was made according to the criteria put forth by the International ROP Classification Committee [11]. Briefly, after pupils were dilated at 10-min intervals with eye drops containing 1% tropicamide and 2.5% phenylephrine, ophthalmological examinations were performed with a standard binocular indirect ophthalmoscope (20 and/or 28 dioptric lenses). Ophthalmological examinations were performed by the same pediatric ophthalmologist trained specifically in the diagnosis of ROP in the neonatal unit.
Infants with a gestational age of >32 weeks, major congenital anomalies, chromosomal anomaly, congenital heart disease, mitochondrial disease, perinatal asphyxia, those with history of trauma, sepsis, or cardiac arrest, infants with metabolic disorders that may affect serum asprosin and NGAL levels, and infants with ocular conditions other than ROP were excluded from the study. The control group was selected from newborns that were evaluated as healthy after screening for prenatal retinopathy and had no disease that could affect the parameters examined in the study. Demographic and clinicopathological features, including gestational age, birth weight, APGAR score, hospital stay, presence of concomitant disorders, and need for mechanical ventilation were obtained from the hospital record system. All research procedures were evaluated and accepted by the Research Ethics Committee of Fırat University (date: 05/03/2020, decision no: 2020/05-23) and were conducted in agreement with the ethical standards specified in the Declaration of Helsinki. Written and verbal informed consent was obtained from the parents of all infants included in the study.
Venous blood samples from patients were drawn into standard serum separator tubes (1–2 days before treatment in the ROP group) and were centrifuged at 2000 g for 10 min. Since the participants were newborns and the ROP group was premature, they had to be fed frequently, so blood samples could be taken after a 2-h fasting period. Serum biochemical markers, including glucose, urea, creatinine, uric acid, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), sodium, potassium, chloride, calcium and phosphorus were measured via photometric methods with an ADVIA 2400 autoanalyzer (Siemens, Munich, Germany). Analysis of all routine biochemical markers was completed within 1 h of venipuncture. Aliquoted serum samples were stored at −80 °C until asprosin and NGAL analysis. Neonatal hyperglycemia is defined as serum glucose concentration greater than 150 mg/dL (8.3 mmol/L) [12].
Serum asprosin levels were measured by using a commercially available human enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (Sunred Bioscience, Catalog No:201-12-7193, Shanghai, China). The detection range of CgA assay was 1–300 ng/mL, with <10% intra-assay and <12% inter-assay coefficient of variations. The minimum measurable level for serum asprosin was 0.775 ng/mL.
Serum NGAL levels were also measured via an ELISA kit (Bioassay Technology Laboratory, Catalog No: E1799Hu Shanghai, China). The measurement range of the NGAL assay was 0.5–600 ng/mL with coefficient of variation values of <8% (intra-assay) and <10% (inter-assay) precision. The minimum measurable level for serum NGAL was 2.01 ng/mL.
Statistical analysis
According to descriptive statistics (effect size Cohen’s d=0.875) in the study by Wang, Wang et al. [13] sample size of 30 for each group (60 in total) achieve 90% power at the two-sided 0.05 significance level. Sample size was calculated by using two-sample t-test power analysis via PASS 11. (Hintze, J. (2011). PASS 11. NCSS, LLC. Kaysville, Utah, USA. www.ncss.com.). Other analyses were performed on SPSS v25 (SPSS Inc., Chicago, IL, USA). For the normality check, the Shapiro–Wilk test was used. Data are given as median (1st quartile – 3rd quartile) for continuous variables according to normality of distribution and as frequency percentage for categorical variables. Between groups analysis were performed with the Mann Whitney U test. Categorical variables were analyzed with the Fisher’s exact test. Diagnostic performance of the variables was assessed by Receiver Operating Characteristic (ROC) curve analysis. Spearman correlation coefficients were calculated to evaluate relationships between continuous variables. Two-tailed p-values of less than 0.05 were considered statistically significant.
Results
A total of 30 patients with ROP and 30 healthy individuals were enrolled in the study. ROP stage was 1 in 8 cases (26.7%), 2 in 19 cases (63.3%), and 3 in 3 cases (10.0%). The median gestational age was 27.5 (26–30) weeks in patients diagnosed with ROP and 39 (38–39) weeks in controls (p<0.001). There were no infants of diabetic mothers in either group. Serum glucose and ALP levels were higher in the ROP group compared to controls (p=0.006 and p<0.001, respectively). Serum levels of creatinine, uric acid, albumin, AST, ALT, sodium and chloride were found to be lower in patients with ROP compared to healthy infants (all, p<0.05). Serum NGAL levels were similar in the ROP [166.1 (124.18–244.7) ng/mL] and control groups [134.84 (114.51–300.37) ng/mL] (p=0.595). Asprosin levels were significantly higher in patients diagnosed with ROP [46.58 (12.70–142.28) ng/mL] compared to healthy infants [13.05 (10.92–17.73) ng/mL] (p=0.001) (Figure 1). The demographic and biochemical results are shown in Table 1.
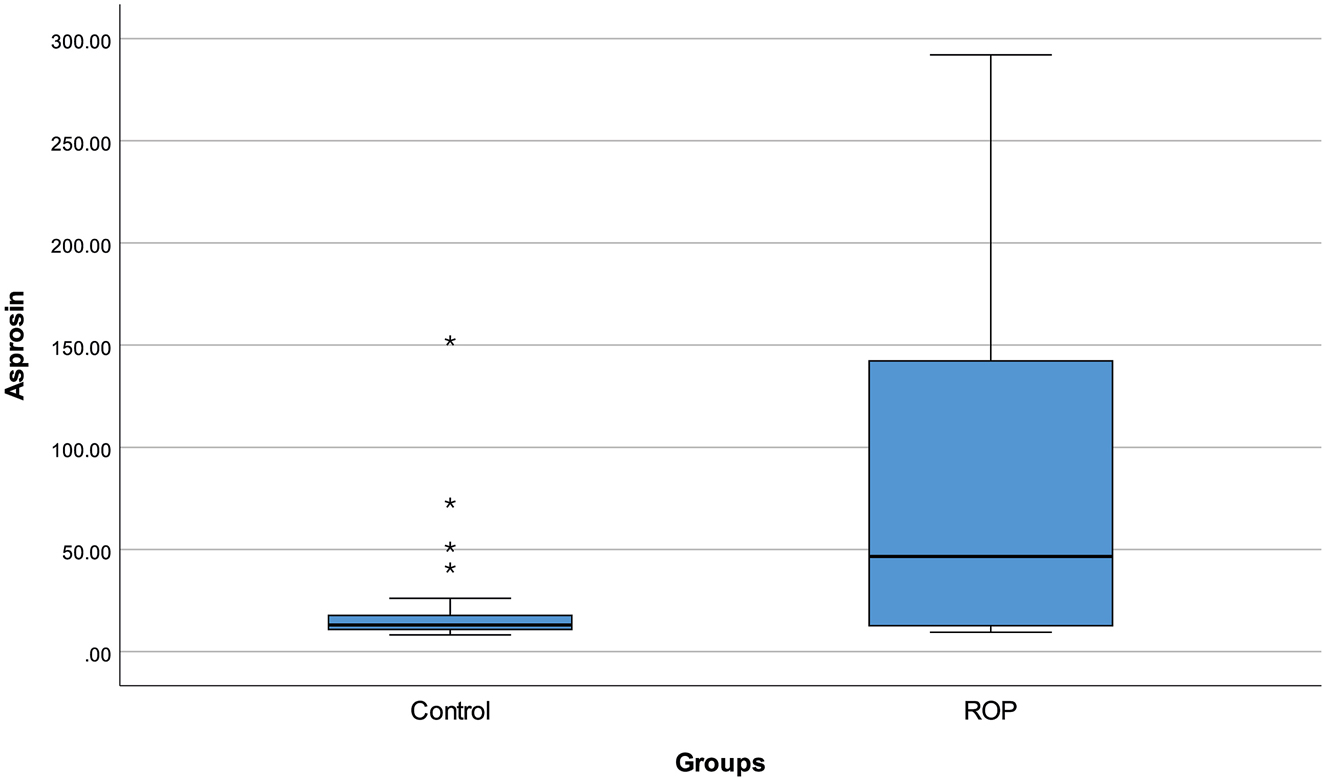
Serum asprosin level with regard to groups.
Demographics and biochemical results of participants.
| Control (n=30) | ROP (n=30) | p-Value | |
|---|---|---|---|
| Gestational age (weeks) | 39 (38–39) | 27.5 (26–30) | <0.001 |
| Glucose, mg/dL | 67 (57–85) | 83 (76–116) | 0.006 |
| Hyperglycemia (>150 mg/dL) | 1 (3.3%) | 5 (16.7%) | 0.195 |
| Urea, mg/dL | 19 (13–24) | 16.5 (7.3–27) | 0.239 |
| Creatinine, mg/dL | 0.63 (0.42–0.70) | 0.28 (0.18–0.41) | <0.001 |
| Uric acid, mg/dL | 3.78 (2.12–5.27) | 1.70 (1.36–3.12) | <0.001 |
| Albumin, g/L | 36 (33–37) | 30 (28–32) | <0.001 |
| AST, U/L | 51 (41–59) | 29.5 (26–33) | <0.001 |
| ALT, U/L | 17 (13–20) | 13 (12–14) | 0.028 |
| ALP, U/L | 168 (119–181) | 407.5 (257–467) | <0.001 |
| Sodium, mmol/L | 140 (137–140) | 137 (135–138) | 0.015 |
| Potassium, mmol/L | 4.90 (4.43–5.16) | 4.96 (4.58–5.28) | 0.284 |
| Chloride, mmol/L | 109 (107–111) | 107 (105–108) | 0.001 |
| Calcium, mg/dL | 9.2 (8.9–9.7) | 9.65 (9.2–9.9) | 0.148 |
| Phosphorus, mg/dL | 5.79 (4.95–6.33) | 6.06 (5.14–6.37) | 0.853 |
| NGAL, ng/mL | 166.10 (124.18–244.77) | 134.84 (114.51–300.37) | 0.595 |
| Asprosin, ng/mL | 13.05 (10.92–17.73) | 46.58 (12.70–142.28) | 0.001 |
-
ROP, retinopathy of prematurity; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; NGAL, neutrophil gelatinase-associated lipocalin. Data are given as median (1st quartile – 3rd quartile) for continuous variables according to normality of distribution and as frequency percentage for categorical variables. Bold values denote statistical significance at the p<0.05 level.
We performed ROC analysis to obtain optimal cut-off values of asprosin and NGAL for the diagnosis of ROP (Table 2) (Figure 2). For asprosin level, the area under the curve (AUC) was 0.754 (95% CI: 0.630–0.879; p=0.001). The >30 ng/mL cut-off value for asprosin yielded sensitivity and specificity values of 63.33 and 86.67%, respectively. There was no diagnostic role for NGAL (AUC=0.460, 95% CI: 0.309–0.611; p=0.595).
Diagnostic performance of the NGAL and asprosin levels.
| NGAL | Asprosin | |
|---|---|---|
| Cut-off | ≥200 | ≥30 |
| Sensitivity | 40.00% | 63.33% |
| Specificity | 70.00% | 86.67% |
| Accuracy | 55.00% | 75.00% |
| PPV | 57.14% | 82.61% |
| NPV | 53.85% | 70.27% |
| AUC (95.0% CI) | 0.460 (0.309–0.611) | 0.754 (0.630–0.879) |
| p-value | 0.595 | 0.001 |
-
NGAL, neutrophil gelatinase-associated lipocalin; PV, positive predictive value; NPV, negative predictive value; AUC, area under ROC curve; CI, confidence intervals. Bold value denote statistical significance at the p<0.05 level.

ROC curve of the NGAL and asprosin for ROP diagnosis.
Correlation analyses between participants’ demographic and biochemical characteristics and asprosin and NGAL levels were shown in Table 3. No significant correlations were found between NGAL and other variables (all, p>0.05) including asprosin (p=0.072). Serum asprosin levels were positively correlated with serum ALP levels (r=0.319, p=0.013) and inversely correlated with gestational age (r=−0.385, p=0.002), uric acid (r=−0.281, p=0.030) and AST (r=−0.456, p<0.001) levels.
Correlations between NGAL, asprosin and other variables.
| NGAL | Asprosin | ||
|---|---|---|---|
| NGAL | r | – | 0.234 |
| p | – | 0.072 | |
| Gestational age | r | 0.137 | −0.385 |
| p | 0.295 | 0.002 | |
| Blood glucose | r | 0.045 | 0.242 |
| p | 0.734 | 0.062 | |
| Urea | r | −0.026 | −0.077 |
| p | 0.845 | 0.561 | |
| Creatinine | r | −0.029 | −0.204 |
| p | 0.825 | 0.117 | |
| Uric acid | r | −0.074 | −0.281 |
| p | 0.572 | 0.030 | |
| Albumin | r | 0.033 | −0.162 |
| p | 0.802 | 0.217 | |
| AST | r | −0.095 | −0.456 |
| p | 0.470 | <0.001 | |
| ALT | r | 0.076 | −0.229 |
| p | 0.569 | 0.081 | |
| ALP | r | 0.011 | 0.319 |
| p | 0.935 | 0.013 | |
| Sodium | r | −0.002 | −0.052 |
| p | 0.988 | 0.693 | |
| Potassium | r | 0.115 | 0.244 |
| p | 0.383 | 0.061 | |
| Chloride | r | 0.065 | −0.200 |
| p | 0.622 | 0.125 | |
| Calcium | r | −0.082 | 0.164 |
| p | 0.535 | 0.212 | |
| Phosphorus | r | −0.039 | −0.055 |
| p | 0.767 | 0.678 |
-
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; NGAL, neutrophil gelatinase-associated lipocalin; r, spearman correlation coefficient. Bold values denote statistical significance at the p<0.05 level.
Discussion
Both asprosin and NGAL have underlying relationships with ocular structure and function; therefore, this study aimed, for the first time, to evaluate serum levels of NGAL and asprosin in patients diagnosed with ROP, compare results to controls and to evaluate whether they could be associated with ROP development and/or progression. We found increased asprosin levels in patients with ROP compared to healthy infants. Serum asprosin levels of >30 ng/mL were found to have a possible role in the diagnosis of ROP, whereas NGAL levels were similar between groups.
Embryonic retinal arteries begin to grow in the third month of pregnancy and their development is completed a few weeks before the normal time of delivery [14]. Thus, ocular development stages in premature infants are incomplete, and the growth and branching of the vessels is either lacking or abnormal; thus, available vessels may become very fragile, which can cause visual impairment and ROP. In 1942, ROP was first described in a premature baby by Terry and was quickly understood to be a significant cause of childhood visual impairment and vision loss worldwide, particularly in relation with oxygen supplementation [15]. It is crucial to identify risk factors and possible pathological mechanisms of retinal damage at an early stage in order to identify targeted management for the prevention or treatment of ROP. The key mechanism in ROP is fibrovascular proliferation [16] which is characterized by growth of new abnormal vessels. Multiple factors can influence whether the disease will progress, including gestational age, birth weight, excessive use of supplemental oxygen therapy, stage of ROP at initial diagnosis, and the presence or absence of “concomitant disorders” [17]. Studies have also reported many relevant determinants for the development of ROP, including maternal factors, environmental and nutritional factors, and inflammation. Tunay et al. demonstrated in a retrospective study that maternal diabetes mellitus was an independent risk factor for ROP in infants with a birth weight of ≥1,500 g [18]. Maternal diabetes mellitus may exert direct impact on the development of ROP through elevated retinal VEGF in association with hyperglycemia. In addition, many reports have shown that neonatal hyperglycemia was common in preterm neonates and was related with increased risk of developing ROP [19]. Hyperglycemia has been suggested to impair retinal blood flow and neovascularization during retinal development in animal models of retinopathy. Brooks et al. demonstrated in an animal model study that hyperglycemia may enhance VEGF production by retinal Müller cells under hypoxic conditions, indicating VEGF as a link between ROP and hyperglycemia [20]. Besides, Cakir et al. showed in 117 extremely preterm infants that elevated early postnatal plasma glucose levels and signs of insulin insensitivity were related with lower levels of insulin like growth factor 1 (IGF-1) and greater ROP severity [21]. They also found in a hyperglycemic retinopathy mouse model that decreased IGF-1 level induced retinal pathological neovascularization. However, the exact mechanism of the hyperglycemia–ROP relationship is still unclear, and whether hyperglycemia has a causal relationship with ROP, or whether it is a consequence of disease severity, is not understood.
Asprosin is a glucogenic adipokine mainly secreted by adipose tissue during fasting and is involved in glucose metabolism, adipogenesis, inflammation and cell apoptosis in addition to neuron-regulatory roles [22]. Studies have shown that asprosin is a key mediator for metabolic disorders, including obesity, insulin resistance, diabetes mellitus and PCOS [6, 23]. Li et al. revealed that plasma asprosin levels were increased in both type 2 diabetes mellitus and PCOS patients compared to healthy subjects, and this increase was greater in diabetes mellitus patients [6]. Romere et al. showed that a single dose of recombinant asprosin increases serum glucose and insulin levels in mice [5]. Proliferative diabetic retinopathy, like ROP, is a condition characterized by the development of new blood vessels in the retina that can extend into the vitreous of the eye [24]. In addition, retinal detachment may occur due to fibrous contractile tissue, which is similar to severe ROP. Oruc et al. recently demonstrated in a retrospective study that both serum and aqueous asprosin levels were higher in patients with diabetic retinopathy compared those without [25]. Because of the relationship between hyperglycemia and ROP, we hypothesized that asprosin may have a role in the pathogenesis of ROP. We found increased asprosin levels in ROP patients compared to controls. We also showed that serum asprosin levels of >30 ng/mL may be used as a biomarker for ROP diagnosis. This may be explained by the involvement of asprosin in glucose metabolism, and increased asprosin may have contributed to the development of ROP by inducing hyperglycemia. Asprosin may also be involved in the pathogenesis of ROP due to interactions with angiogenetic factors, such as VEGF and IGF-1, which are key regulators for retinal angiogenesis. Furthermore, asprosin may also contributed to ROP pathogenesis due to its effects on inflammatory processes triggered during development of pathological angiogenesis in the retina. In this context, possible effectors include cytokines, chemokines, hypoxia-inducible factor-1, VEGF, IGF-1, nitric oxide and inflammatory cells [26]. Li et al. demonstrated that plasma asprosin levels were positively associated with IL-6 in PCOS patients, and related with CRP in patients with type 2 diabetes mellitus [6]. In addition, studies have shown that fibrillin-1 (profibrillin → asprosin) is involved in angiogenesis through TGF-beta signaling [7], suggesting another underlying link.
Although we did not explore the link between ROP pathogenesis and asprosin elevation, our results suggest that asprosin elevation is associated with ROP development, suggesting a diagnostic role in premature infants. However, blood samples in our study were drawn after patients were diagnosed, which is a primary limitation. Further studies with larger sample sizes and temporal analysis of asprosin change in premature infants are needed to confirm these results and to explore asprosin’s role in ROP development.
NGAL is a member of the lipocalin superfamily, and increased levels have been associated with numerous pathological disorders, including inflammatory diseases, diabetes mellitus, kidney diseases, cancers, cardiovascular diseases and ocular disorders [27]. Of note, Wang et al. demonstrated a relationship between hyperglycemia and NGAL [28]. NGAL is expressed in retinal glial Müller cells and retinal pigment epithelial cells, and plays a regulatory role in ocular inflammation as well as retinal degeneration and angiogenesis [29]. Parmar et al. revealed in an experimental study that NGAL is involved in exacerbated immune activation and acute stress response of retinal pigment epithelial cells following light exposure in NGAL-knockout mice, suggesting NGAL exerts a regulatory role in retinal inflammation during retinal degeneration, including retinitis pigmentosa, age-related macular degeneration and Stargardt [29]. Increased aqueous humor NGAL levels in patients with central retinal vein occlusion can lead to intense neuroinflammatory processes, resulting in macular ischemia, macular edema, neovascularization and blindness [30]. Wang et al. showed increased vitreous fluid NGAL and VEGF in patients with proliferative diabetic retinopathy, suggesting a role for NGAL in retinal inflammation and angiogenesis [9]. Furthermore, Chung et al. found a significant relationship between serum NGAL and diabetic retinopathy [31]. In contrast, Aslanhan et al. demonstrated similar NGAL levels in diabetic patients with and without retinopathy [32]. We found similar serum NGAL levels in our ROP and control groups. This finding suggests that NGAL may not be associated with ROP development.
Limitations
The study has some limitations. First, this research was conducted in a single-center with a small sample size, which may have caused bias in the results. Second, we were unable to measure post-treatment serum levels of NGAL and asprosin, which could have been valuable to characterize their association with ROP. Third, our control group consisted of only healthy term infants who have evident differences from premature babies. Future studies utilizing preterm infants without ROP as a control group are needed.
Conclusions
This was the first study in the literature that evaluated serum NGAL and asprosin levels in patients with ROP. Our results demonstrated that asprosin, but not NGAL, could be a promising biomarker for the diagnosis of ROP, but further studies with temporal analysis of asprosin levels are needed.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Authors have no conflict of interest.
-
Ethical approval: All research procedures were evaluated and accepted by the Research Ethics Committee of Fırat University (date: 05/03/2020, decision no: 2020/05-23) and were conducted in agreement with the ethical standards specified in the Declaration of Helsinki.
References
1. Bashinsky, AL. Retinopathy of prematurity. N C Med J 2017;78:124–8. https://doi.org/10.18043/ncm.78.2.124.Search in Google Scholar PubMed
2. Yau, GS, Lee, JW, Tam, VT, Liu, CC, Yip, S, Cheng, E, et al.. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. Asia Pac J Ophthalmol (Phila) 2016;5:185–91. https://doi.org/10.1097/apo.0000000000000167.Search in Google Scholar PubMed
3. Kim, SJ, Port, AD, Swan, R, Campbell, JP, Chan, RP, Chiang, MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol 2018;63:618–37. https://doi.org/10.1016/j.survophthal.2018.04.002.Search in Google Scholar PubMed PubMed Central
4. Ali, AA, Gomaa, NA, Awadein, AR, Al-Hayouti, HH, Hegazy, AI. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr 2017;106:1919–27. https://doi.org/10.1111/apa.14019.Search in Google Scholar PubMed
5. Romere, C, Duerrschmid, C, Bournat, J, Constable, P, Jain, M, Xia, F, et al.. Asprosin, a fasting-induced glucogenic protein hormone. Cell 2016;165:566–79. https://doi.org/10.1016/j.cell.2016.02.063.Search in Google Scholar PubMed PubMed Central
6. Yuan, M, Li, W, Zhu, Y, Yu, B, Wu, J. Asprosin: a novel player in metabolic diseases. Front Endocrinol (Lausanne) 2020;11:64. https://doi.org/10.3389/fendo.2020.00064.Search in Google Scholar PubMed PubMed Central
7. Kim, SJ, Sonmez, K, Swan, R, Campbell, JP, Ostmo, S, Chan, RP, et al.. Identification of candidate genes and pathways in retinopathy of prematurity by whole exome sequencing of preterm infants enriched in phenotypic extremes. Sci Rep 2021;11:4966. https://doi.org/10.1038/s41598-021-83552-y.Search in Google Scholar PubMed PubMed Central
8. Jones, W, Rodriguez, J, Bassnett, S. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. Dis Models Mech 2019;12:dmm037283.10.1242/dmm.037283Search in Google Scholar PubMed PubMed Central
9. Wang, H, Lou, H, Li, Y, Ji, F, Chen, W, Lu, Q, et al.. Elevated vitreous lipocalin-2 levels of patients with proliferative diabetic retinopathy. BMC Ophthalmol 2020;20:260. https://doi.org/10.1186/s12886-020-01462-5.Search in Google Scholar PubMed PubMed Central
10. Matet, A, Jaworski, T, Bousquet, E, Canonica, J, Gobeaux, C, Daruich, A, et al.. Lipocalin 2 as a potential systemic biomarker for central serous chorioretinopathy. Sci Rep 2020;10:20175. https://doi.org/10.1038/s41598-020-77202-y.Search in Google Scholar PubMed PubMed Central
11. Prematurity ICFTCORO. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:991–9.10.1001/archopht.123.7.991Search in Google Scholar PubMed
12. Şimşek, DG, Ecevit, A, Hatipoğlu, N, Çoban, A, Arısoy, AE, Baş, F, et al.. Neonatal hyperglycemia, which threshold value, diagnostic approach and treatment?: Turkish neonatal and pediatric endocrinology and diabetes societies consensus report. Turk Pediatri Ars 2018;53(1 Suppl):S234–8. https://doi.org/10.5152/turkpediatriars.2018.01821.Search in Google Scholar PubMed PubMed Central
13. Wang, Y, Qu, H, Xiong, X, Qiu, Y, Liao, Y, Chen, Y, et al.. Plasma asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediat Inflamm 2018;2018:9471583. https://doi.org/10.1155/2018/9471583.Search in Google Scholar PubMed PubMed Central
14. Azami, M, Jaafari, Z, Rahmati, S, Farahani, AD, Badfar, G. Prevalence and risk factors of retinopathy of prematurity in Iran: a systematic review and meta-analysis. BMC Ophthalmol 2018;18:83. https://doi.org/10.1186/s12886-018-0732-3.Search in Google Scholar PubMed PubMed Central
15. Terry, T. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely: II. Report of cases—clinical aspects. Trans Am Ophthalmol Soc 1942;40:262.10.1016/S0002-9394(42)91858-0Search in Google Scholar
16. Ozcan, P, Çıtırk, M, Özçalışkan, S. Current epidemiology, pathophysiology and risk factors in retinopathy of prematurity. Ret Vit 2016;24:263–70.Search in Google Scholar
17. Cotruvo, J. Kinetic model for chlorophyll degradation [Ph.D. thesis]. Cambridge, MA: Massachusetts Institute of Technology; 1996.Search in Google Scholar
18. Tunay, ZÖ, Özdemir, Ö, Acar, DE, Öztuna, D, Uraş, N. Maternal diabetes as an independent risk factor for retinopathy of prematurity in infants with birth weight of 1500 g or more. Am J Ophthalmol 2016;168:201–6. https://doi.org/10.1016/j.ajo.2016.05.022.Search in Google Scholar PubMed
19. Lei, C, Duan, J, Ge, G, Zhang, M. Association between neonatal hyperglycemia and retinopathy of prematurity: a meta-analysis. Eur J Pediatr 2021;180:3433–42.10.1007/s00431-021-04140-wSearch in Google Scholar PubMed
20. Brooks, SE, Gu, X, Kaufmann, PM, Marcus, DM, Caldwell, RB. Modulation of VEGF production by pH and glucose in retinal Müller cells. Curr Eye Res 1998;17:875–82. https://doi.org/10.1076/ceyr.17.9.875.5134.Search in Google Scholar PubMed
21. Cakir, B, Hellström, W, Tomita, Y, Fu, Z, Liegl, R, Winberg, A, et al.. IGF1, serum glucose, and retinopathy of prematurity in extremely preterm infants. JCI Insight 2020;5:e140363. https://doi.org/10.1172/jci.insight.140363.Search in Google Scholar PubMed PubMed Central
22. Li, X, Liao, M, Shen, R, Zhang, L, Hu, H, Wu, J, et al.. Plasma asprosin levels are associated with glucose metabolism, lipid, and sex hormone profiles in females with metabolic-related diseases. Mediat Inflamm 2018;2018:7375294. https://doi.org/10.1155/2018/7375294.Search in Google Scholar PubMed PubMed Central
23. Luís, C, Fernandes, R, Soares, R, Von Hafe, P. A state of the art review on the novel mediator asprosin in the metabolic syndrome. Porto Biomed J 2020;5:e108. https://doi.org/10.1097/j.pbj.0000000000000108.Search in Google Scholar PubMed PubMed Central
24. Mohamed, S, Murray, JC, Dagle, JM, Colaizy, T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC Pediatr 2013;13:78. https://doi.org/10.1186/1471-2431-13-78.Search in Google Scholar PubMed PubMed Central
25. Oruc, Y, Celik, F, Ozgur, G, Beyazyildiz, E, Ugur, K, Yardim, M, et al.. Altered blood and aqueous humor levels of asprosin, 4-hydroxynonenal, and 8-hydroxy-deoxyguanosine in patients with diabetes mellitus and cataract with and without diabetic retinopathy. Retina 2020;40:2410–6. https://doi.org/10.1097/iae.0000000000002776.Search in Google Scholar PubMed
26. Rivera, JC, Holm, M, Austeng, D, Morken, TS, Zhou, TE, Beaudry-Richard, A, et al.. Retinopathy of prematurity: inflammation, choroidal degeneration, and novel promising therapeutic strategies. J Neuroinflammation 2017;14:1–14. https://doi.org/10.1186/s12974-017-0943-1.Search in Google Scholar PubMed PubMed Central
27. Batsos, G, Christodoulou, E, Vartholomatos, G, Galanis, P, Stefaniotou, M. Vitreous levels of Lipocalin-2 on patients with primary rhegmatogenous retinal detachment. PLoS One 2019;14:e0227266. https://doi.org/10.1371/journal.pone.0227266.Search in Google Scholar PubMed PubMed Central
28. Wang, Y, Lam, KS, Kraegen, EW, Sweeney, G, Zhang, J, Tso, AW, et al.. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007;53:34–41. https://doi.org/10.1373/clinchem.2006.075614.Search in Google Scholar PubMed
29. Parmar, T, Parmar, VM, Perusek, L, Georges, A, Takahashi, M, Crabb, JW, et al.. Lipocalin 2 plays an important role in regulating inflammation in retinal degeneration. J Immunol 2018;200:3128–41. https://doi.org/10.4049/jimmunol.1701573.Search in Google Scholar PubMed PubMed Central
30. Koban, Y, Sahin, S, Boy, F, Kara, F. Elevated lipocalin-2 level in aqueous humor of patients with central retinal vein occlusion. Int Ophthalmol 2019;39:981–6. https://doi.org/10.1007/s10792-018-0894-2.Search in Google Scholar PubMed
31. Chung, J, Park, S, Cho, D, Chung, D, Chung, M. Plasma neutrophil gelatinase-associated lipocalin levels are positively associated with diabetic retinopathy in patients with Type 2 diabetes. Diabet Med 2016;33:1649–54. https://doi.org/10.1111/dme.13141.Search in Google Scholar PubMed
32. Aslanhan, E, Ojalvo, D, Özsenel, EB, Basat, SU, Borlu, F. Association of neutrophil-gelatinase-associated lipocalin with microvascular complications in patients with type 2 diabetes: a cross-sectional study. Cardiovasc Endocrinol Metab 2019;8:82–7. https://doi.org/10.1097/xce.0000000000000180.Search in Google Scholar
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment

