Abstract
Objectives
The role of vitamin D in primary osteoarthritis (OA) has not been clarified yet. vitamin D receptor (VDR) and fibroblast growth factor-23 (FGF-23) are proteins that play an important role in the metabolism of vitamin D. In this preliminary study, we aimed to examine serum 25-(OH) vitamin D3, VDR, and FGF-23 levels in primary knee OA patients.
Methods
This study includes 60 post-menopausal women who were enrolled into two groups with primary knee OA (n=30, age range between 59.6 ± 5.7 years) and control (n=30, age range between 61.7 ± 6.3 years). Serum levels of 25-(OH) vitamin D3 were measured by chemiluminescence and serum VDR, and FGF-23 were measured by enzyme-linked immunosorbent assay methods.
Results
In knee OA group, serum levels of 25-(OH) vitamin D3 were significantly lower (p=0.033), and serum VDR and FGF-23 levels were significantly higher than those of the control group respectively (p=0.000 and p=0.006). Also, FGF-23 levels showed correlations with 25-(OH) vitamin D3, VDR, and calcium levels (p<0.05).
Conclusions
This is the first study showing a relationship between serum VDR and FGF-23 in knee OA patients. Extensive clinical studies are required to assess the applicability of these parameters in clinical practice.
Introduction
Primary osteoarthritis (OA) is a musculoskeletal disease that causes chronic pain and disability [1]. It includes all elements of the joint and is characterised by the degeneration of the articular cartilage and subchondral bone [2]. Advancing age, previous history of injury, and obesity are shown among the most significant risk factors [3].
Mechanical, inflammatory, and metabolic factors play a role in its pathogenesis. In addition, it is a dynamic pathology caused by the imbalance between damage and repair processes in the joint rather than a passive degeneration [4]. Vitamin D has pivotal roles in bone and cartilage metabolism [5]. Vitamin D deficiency (VDD) is a worldwide health concern that can cause musculoskeletal problems [6]. Previously, many trials have been made to reveal the outcomes of VDD on the pathogenesis of OA. Although several studies show that VDD augments the incidence of OA [7, 8] and accelerates its progression [8], [9], [10], some studies do not support these findings [11, 12].
Vitamin D shows its effects on target tissues through vitamin D receptor (VDR). After calcitriol 1,25 (OH)2 vitamin D3 binds to VDR, it triggers two different cellular effects; genomic and non-genomic. In genomic (classical) effect calcitriol mostly binds to cytosolic VDR and then together translocate to the nucleus to exert its genomic effects. On the other hand in non-genomic effect, calcitriol binds to VDR which is associated with the plasma membrane caveolae to initiate cellular responses via rapid response pathways [13]. The active presence of VDR is shown in many tissues, involving bone, intestine, and kidney [14], as well as the cartilage tissue [15].
Studies have shown that the structure and content of matrix vesicles released from hypertrophic chondrocytes, which play an essential role in cartilage degeneration, are regulated by vitamin D [16, 17]. Clinical and experimental studies demonstrated that vitamin D may be responsible for altered mineral ion hemostasis and increased matrix degradation observed in articular cartilage tissue with OA. These trials have substantiated that both VDR expression and the amount of protein increase in the joint cartilage tissue with OA [18, 19].
Fibroblast growth factor-23 (FGF-23) is a hormone that is secreted from osteocytes, primarily inhibits inorganic phosphate reabsorption in renal tubular epithelial cells and thus increases phosphate excretion [20]. FGF-23 also inhibits renal 1-α hydroxylase and activates 24-α hydroxylase, reducing the levels of calcitriol [21]. In turn, calcitriol stimulates FGF-23, a cartilage mineralization regulator, through VDR [19] and involves in endochondral ossification and chondrocyte hypertrophy in OA [22].
Thus, we hypothesized that both the VDR and the FGF-23 might have some regulatory roles in the pathogenesis of OA. This preliminary study examines both serum VDR and FGF-23 levels in individuals with or without knee OA.
Materials and methods
Patients and study design
This cross-sectional trial was undertaken in Antalya Research and Training Hospital between January and July 2018. The case group (Group I) consisted of subjects with primary knee OA, and the control group (Group II) encompasses patients who were selected among subjects without knee pain and OA. All participants were women, and Group I consisted of 30 outpatients (age range between 59.6 ± 5.7 years) who presented with daily knee pain for at least 3 months, the control group consisted of 30 outpatients (age range between 61.3 ± 6.3 years) with no complaints in the knee joint. Knee OA was evaluated regarding to the criteria established by the American College of Rheumatology (ACR) [23]. Kellgren–Lawrence radiological scoring system was applied to classify the severity of knee OA [24]. Patients with normal knee joint examination and no complaints of knee pain were included in the control group.
All participants in our study were postmenopausal and >50 years of age. The menopausal condition was characterized as the absence of menses for ≥1 year. Subjects with diseases that lead to secondary osteoporosis (Cushing’s syndrome, hyperthyroidism, hypogonadism, osteogenesis imperfecta, cirrhosis), inflammatory joint diseases, and those who had used vitamin D supplements in the last 6 months prior to blood collection were excluded from the study. Also, chronic diseases such as hypothyroidism, diabetes, chronic kidney or liver diseases, etc., were eliminated from the trial. All subjects were notified about the trial and informed written consent was received following the Declaration of Helsinki. The study protocol was approved by the Local Ethics Committee (Approval Number: 1/8, ID: 2018-007).
Biochemical parameters
Fasting blood samples were used to assess all biochemical parameters and erythrocyte sedimentation rate (ESR). Venous blood specimens to be used to analyze biochemical parameters were collected (between January and February 2018) in evacuated serum separator tubes (BD Vacutainer® SST™ II). These specimens were centrifuged at 1150×g for 15 min at 4 °C for the analysis of ESR venous blood samples were collected in tubes with EDTA-K2 (Isotherm green vac-tube®, Uni Lab, İstanbul, Türkiye). To analyze the serum samples in bulk for FGF-23 and VDR, we kept them frozen at −80 °C. Other biochemical tests were assayed on the day of blood sampling. Blood urea nitrogen (enzymatic method), calcium (arsenazo III method), inorganic phosphorus (molybdenum blue method), magnesium (colorimetric, xylidyl blue assay), anti-streptolysin O (ASO) (immunoturbidimetric), C-reactive protein (CRP) (immunoturbidimetric), rheumatoid factor (RF) (immunoturbidimetric) levels were examined with an analyzer (Beckman AU5800; Beckman Coulter Diagnostics, USA). Serum urea levels were calculated according to formula: Urea=BUN × 2.14. Serum 25-(OH) vitamin D3 and parathyroid hormone (PTH) concentrations were assayed by the chemiluminescence method (Access® DxI800; Beckman Coulter, Inc., CA, USA). The intra- and interassay coefficients of variation (CVs) for 25-(OH) vitamin D3 assay were 1.5–3.8% and 6.8–7.7% respectively. ESRs were assayed on VISION-C (Shenzhen YHLO Biotech Co., Shenzhen, China) ESR analyzer. Serum VDR and FGF-23 concentrations were analyzed by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Sunred Biological Technology, Shanghai, China). The detection limits of the VDR and FGF-23 ELISA kits were 0.38 ng/mL and 9.38 pg/mL respectively. The intra- and interassay CVs for the VDR ELISA kit were 3.66–5.44% and 4.68–5.63% and for the FGF-23 ELISA kit were 4.56–6.51% and 4.36–5.62% respectively.
Statistical analysis
SPSS 18.0 (SPSS for Windows, Chicago, IL, USA) was used for all statistical analyses. The normality of data distribution was analyzed by the Kolmogorov–Smirnov test. Comparisons between groups were established by the Student’s t-test for normally distributed data (presented as mean ± standard deviation) and by the Mann–Whitney U-test for the non-normally distributed data (presented as medians [minimum–maximum]). The Chi-squared tests were used for the comparison of categorical variables in independent groups. We specified the Pearson or Spearman coefficients by correlation analysis. FGF-23 was used as a dependent variable for multiple linear regression analysis, and 25-(OH) vitamin D3, VDR, and calcium levels were used as independent variables. A value of p<0.05 was recognized as statistically significant.
Results
The mean age of the groups was 59.6 ± 5.7 years and 61.7 ± 6.3 years, respectively, for control and knee OA groups (p=0.276). Twenty-one of the OA group patients were classified as Grade III according to the Kellgren–Lawrence radiological scoring system classification and 9 as Grade IV. The demographic and biochemical characteristics of the participants were shown in Table 1. 25-(OH) vitamin D3 levels (ng/mL) were significantly lower in knee OA group than in control group (17.1±11.2 vs. 22.8±8.7, p=0.033) and also VDD prevalence were significantly higher in knee OA group than control group (p<0.018). There were no remarkable differences between the knee OA and control groups for age, phosphorus, parathormone, calcium, CRP, urea, magnesium, ASO, RF, and ESR levels.
Demographic and biochemical characteristics of the knee OA and control groups.
| Controls (n=30) | Knee OA (n=30) | p-Value | |
|---|---|---|---|
| Age, years (SD) | 59.6 (±5.7) | 61.7 (±6.3) | 0.276 |
| 25-(OH) vitamin D3, ng/mL (SD) | 22.8 (±8.7) | 17.1 (±11.2) | 0.033a |
| VDD prevalence, n (%) | 13 (43.3) | 22 (73.3) | 0.018a |
| VDR, ng/mL median (IQR) | 0.47 (0.31–0.68) | 1.23 (1.13–1.73) | 0.001b |
| FGF-23, pg/mL (SD) | 85.4 (±41.9) | 125.4 (±66.0) | 0.007b |
| Urea, mmol/L (SD) | 5.33 (±1.44) | 5.04 (±1.26) | 0.451 |
| Creatinine, mg/dL (SD) | 0.82 (±0.11) | 0.80 (±0.18) | 0.536 |
| Calcium, mg/dL (SD) | 9.76 (±0.38) | 9.88 (±0.61) | 0.123 |
| Magnesium, mg/dL median (IQR) | 2.1 (2–2.2) | 2.1 (2–2.2) | 0.593 |
| Phosphorus, mg/dL (SD) | 3.8 (±0.4) | 3.7 (±0.3) | 0.317 |
| ASO, IU (SD) | 79.5 (±59.9) | 77.9 (±67.3) | 0.253 |
| CRP, mg/L (SD) | 4.12 (±3.43) | 5.54 (±3.97) | 0.282 |
| RF, U/mL median (IQR) | 6 (6–10) | 7 (6–8) | 0.669 |
| PTH, pg/mL (SD) | 48.6 (±16.2) | 58.8 (±25.8) | 0.074 |
| ESR, mm/h (SD) | 27.9 (±13.6) | 21.3 (±9.6) | 0.145 |
-
OA, osteoarthritis; VDD, vitamin D deficiency; VDR, vitamin D receptor; FGF-23, fibroblast growth factor-23; ASO, anti-streptolysin O; CRP, C-reactive protein; RF, rheumatoid factor; PTH, parathyroid hormone; ESR, erythrocyte sedimentation rate. Data are presented as n (%), mean (± standard deviation), median (IQR, interquartile range, 25th −75th percentile). aStatistically significant difference (p<0.05), bStatistically significant difference (p<0.01).
In knee OA group serum VDR concentrations (ng/mL) were remarkably higher than those of control group [1.23 (1.13–1.73) vs. 0.47 (0.31–0.68), p<0.001] as shown in Figure 1. Also, in the knee OA group, serum FGF-23 levels (pg/mL) were significantly higher than in the control group (125.4±66.0 vs. 85.4±41.9, p=0.007) as shown in Figure 2.
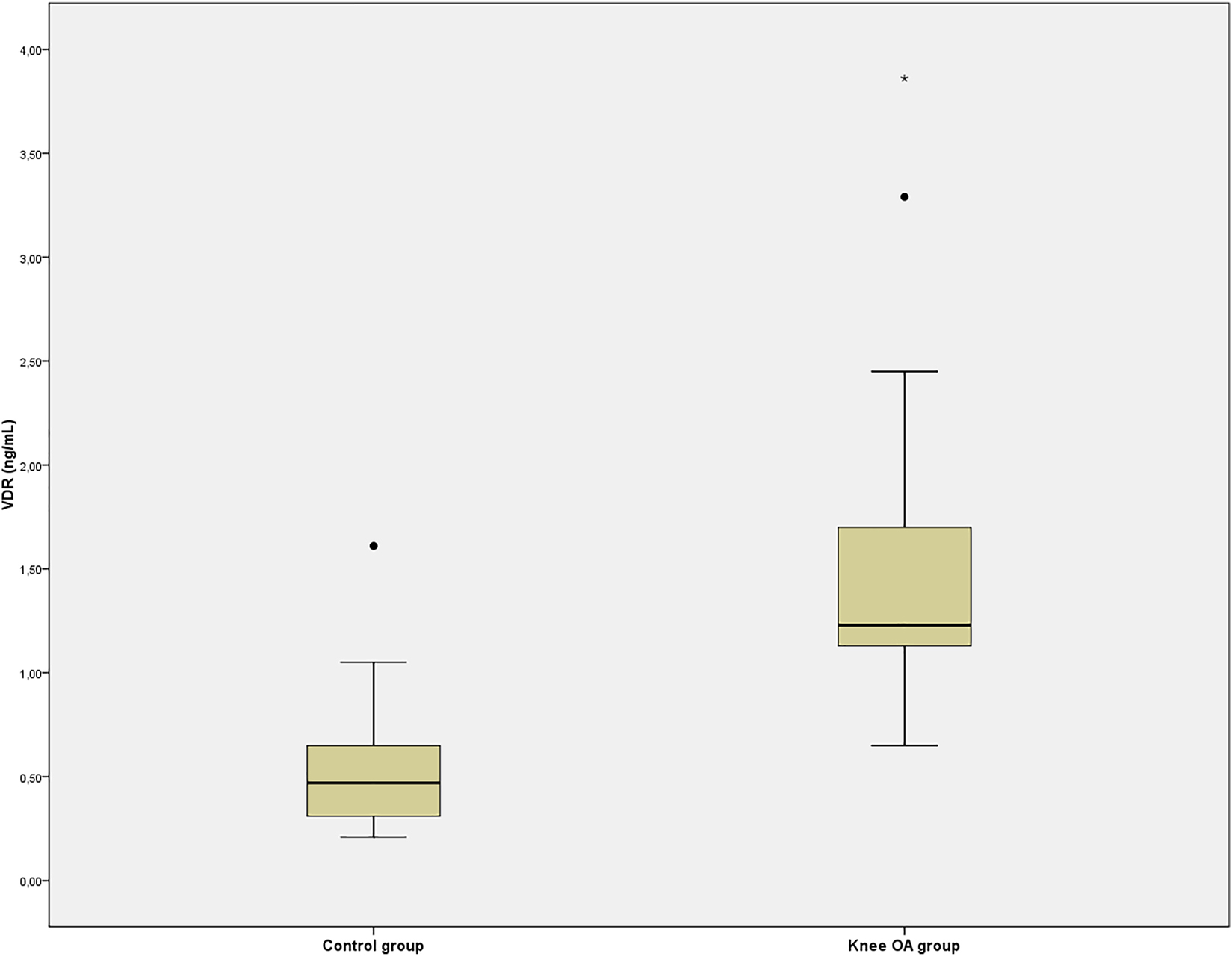
Serum vitamin D receptor (VDR) concentrations of the control and knee osteoarthritis (OA) groups.
**p<0.01 vs. control group.

Serum fibroblast growth factor-23 (FGF-23) levels of the knee osteoarthritis (OA) and control groups.
**p<0.01 vs. control group.
Significant positive correlations were observed between serum FGF-23 levels with serum VDR and calcium levels (r=0.315, p<0.05; r=0.258, p<0.05 respectively). Serum FGF-23 levels were negatively correlated with 25-(OH) vitamin D3 levels (r=−0.139, p<0.05) and display no significant correlation with serum phosphorus levels. Moreover, the positive correlation between serum FGF-23 and PTH levels in control group (r=0.464, p<0.05) was lost in patient group and in both groups together (r=−0.059, p=0.767; r=0.153, p=0.26 respectively).
Multiple linear regression analysis showed that serum 25-(OH) vitamin D3 levels were negatively and independently, calcium and VDR levels were positively and independently associated with serum FGF-23 levels after adjusting for age (Table 2). This model predicted 21.9% of the variance of serum FGF-23 levels, with serum 25-(OH) vitamin D3 levels being the strongest predictor.
Multiple linear regression analysis model to assess the independent factors affecting FGF-23 (pg/mL).
| Beta (95% CI) | SE | p-Value | |
|---|---|---|---|
| VDR, ng/mL | 14.98 (0.78–29.17) | 7.08 | 0.039a |
| 25-(OH) vitamin D3, ng/mL | −1.51 (−2.88−0.23) | 0.68 | 0.030a |
| Calcium, mg/dL | 30.14 (2.38–57.9) | 13.85 | 0.034a |
-
Dependent variable: FGF-23, Independent variables: VDR, 25-(OH) vitamin D3, calcium. Adjusting age, R2=0.219. p<0.05 was considered as statistically significant. FGF-23, fibroblast growth factor 23; VDR, vitamin D receptor; SE, standard error; IQR, interquartile range; CI, confidence interval. aStatistically significant difference (p<0.01).
Discussion
To the best of our knowledge, this inductive study is the first in the literature to show the serum concentrations of both FGF-23 and VDR in knee OA patients. Our results illustrated that 25-(OH) vitamin D3 levels were markedly lower, serum FGF-23 and VDR concentrations were markedly higher in knee OA group than those of the control group.
Many clinical studies have been undertaken to survey the association between knee OA and vitamin D in the literature. However, these studies revealed conflicting results which were interpreted in different ways. The Framingham Heart Study, which was performed on 556 participants over 4 years, showed that low 25-(OH) vitamin D3 did not affect knee OA incidence but related to an increased risk of progression of knee OA [10]. McAlindon et al. interpreted these results as that vitamin D does not play a remarkable role at the onset of knee OA pathogenesis; however, the bone tissue’s healthy response to damage to articular cartilage is an important predictor of OA progression [25].
Furthermore, in the Rotterdam study, 1,248 subjects were monitored throughout six and a half years for knee OA incidence and progression, and it was shown that low vitamin D levels increased knee OA incidence and accelerated its progression, especially in elderly patients with low bone mineral density [9]. In addition, it was also reported that improving the vitamin D status could prevent the development and progression of knee OA, particularly in the elderly.
In the study of Felson et al., where the results of two longitudinal studies were presented, it was reported that low suboptimal vitamin D levels did not affect knee OA incidence and progression [11]. In a cross-sectional study of 153 patients undergoing arthroplasty for knee OA, VDD prevalence was higher in the radiographic knee OA group [26]. In parallel with the findings of this study, the prevalence of VDD was observed to be higher in knee OA group in our study.
Joint is an organ that should be evaluated not only with cartilage, synovium, and subchondral bone but also with related ligaments, meniscus, periarticular muscles, and nerves (neuromuscular control system). Although OA is a pathology that is limited basically to the cartilage, synovium, and subchondral bone, other joint structures also play role in its etiopathogenesis. Factors, such as obesity, aging, and previous trauma, that disrupt the harmony of functional structures of an especially weight-bearing joint (e.g., obesity, aging, trauma etc.) lead to altered joint biomechanics and abnormal joint loading. In this manner, as a result of following microtraumas and inflammation, the process of OA begins [4, 27]. At this stage, we can mention the protective role of vitamin D [25]. But in later stages, it seems that as a consequence of repetitive microtraumas and inflammation local and uncontrolled production of 1,25 (OH)2 vitamin D3 (will be discussed in the following paragraphs) leads to behavioral changes in chondrocytes; upregulation of matrix metalloproteinase production, reduction in proteoglycan synthesis [18, 19]. This means that imbalance towards the degradation of cartilage tissue. Studies have postulated that the release, structure, and content of matrix vesicles liberated from hypertrophic chondrocytes play crucial roles in the course of endochondral ossification, and all are regulated by vitamin D [16, 17]. Accordingly, it would be wiser to implement the higher prevalence of VDD in our patient group, as a tendency for knee OA in the past.
FGF-23 is one of the modulators of chondrocyte function, especially in maintenance of the specialized extracellular matrix [28, 29]. But in osteoarthritic cartilage increased FGF-23 makes its own contribution by causing chondrocyte hypertrophy and impaired mineralization [19, 22]. In our study, we have confirmed the findings of two previous studies that serum FGF-23 levels were increased in knee OA patients [30, 31]. FGF-23 is a phosphaturic hormone released from osteocytes in response to physiologically increased phosphorus, PTH and calcitriol levels [32, 33]. We did not find significant differences in serum PTH and phosphorus levels between knee OA and patient groups. Also, the positive correlation between serum FGF-23 and PTH levels in control group was lost in patient group and in both groups together. According to these results, it is reasonable to assume that 1,25 (OH)2 vitamin D3 is responsible for the increment of FGF-23 levels in knee OA patients. 1,25 (OH)2 vitamin D3 is one of the main regulators of FGF-23 expression and exerts its action on FGF-23 via binding to VDR [19]. Increased levels of the 1,25 (OH)2 vitamin D3 have been demonstrated in synovial fluids and cartilage tissues of patients with inflammatory arthritis. And it is believed that 1,25 (OH)2 vitamin D3 is synthesized in osteoarthritic synovial macrophages and hypertrophic chondrocytes by 1 alpha-hydroxylase rather than synthesized systemically [15, 18, 34, 35]. Animal and human chondrocyte studies showed that calcitriol triggered the release of FGF-23 [33, 34], and FGF-23 decreased calcitriol (by suppressing 25-hydroxyvitamin D-1α-hydroxylase and increasing 25-hydroxyvitamin D-24-hydroxylase) in a negative feedback loop [21]. Since 1,25 (OH)2 vitamin D3 synthesis in synovial fluid macrophages cannot be downregulated by FGF-23, cartilage tissue 1,25 (OH)2 vitamin D3 levels increase, and by this way vitamin D signaling pathway is upregulated [36].
Besides FGF-23 we also have shown that serum VDR levels were higher in knee OA patients which may be attributed to increased vitamin D signalization. Orfanidou et al. showed that calcitriol increases FGF-23 levels through VDR [19]. The increase in VDR expression and VDR amount was also shown in tissue samples collected from knee OA joint cartilage and chondrocyte cultures [18]. However, it is pretty remarkable that VDR protein can be detected from simple blood samples taken from patients. In spite of there were very few studies that reported serum VDR levels in the literature [37, 38], apparently, VDR molecules can be detected in human serum samples and can show concentration differences between patient and control groups. From this fact, it can be said that VDR, associated with the plasma membrane caveolae, one that can be shed in blood like other measurable transmembrane proteins [39].
The regression model that we employed with significant independent variables (serum calcium, VDR, and 25-(OH) vitamin D3 levels) predicted only 21.9% variance of serum FGF-23. We believe that if we could add synovial fluid 1,25 (OH)2 vitamin D3 levels to our regression model as an independent variable, we might have a much stronger prediction. This was a limitation of our study. Furthermore, there are some other limitations of our study. This study included a small number of subjects, limiting the study’s statistical power. However, all patients were strictly selected according to ACR criteria. Another limitation of our study is that the control group does not have knee radiographs. However, as the researchers, we consider it ethically inappropriate for the control group who do not have any clinical findings in terms of knee OA to undergo knee radiography and be exposed to radiation.
The effect of vitamin D on target tissue depends on various factors such as, but not only, local calcitriol concentrations and VDR expression levels. For this reason, it would not be sufficient to investigate the effect of vitamin D on knee OA by only measuring 25-(OH) vitamin D3 levels, particularly in large prospective cohort studies. We need parameters that can be measurable in blood and reflect vitamin D signalization sensitively and specifically. Our work can be described as a step taken in this direction.
Funding source: the Scientific Research Projects Coordination Unit of Antalya Education and Research Hospital
Award Identifier / Grant number: 81266704-869
-
Research funding: The present study was supported by the Scientific Research Projects Coordination Unit of Antalya Education and Research Hospital (Grant no: 81266704-869).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The research related to human use has complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ Institutional Review Board or equivalent committee (Antalya Research and Training Hospital Ethics Committee, 2018-007 1/8).
References
1. Altman, RD, Bloch, DA, Bole, GGJr, Brandt, KD, Cooke, DV, Greenwald, RA, et al.. Development of clinical criteria for osteoarthritis. J Rheumatol 1987;14. Spec No: 3–6.Search in Google Scholar
2. Felson, DT, Zhang, Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41:1343–55, https://doi.org/10.1002/1529-0131(199808)41:8<1343::aid-art3>3.0.co;2-9.10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9Search in Google Scholar
3. Prieto-Alhambra, D, Judge, A, Javaid, MK, Cooper, C, Diez-Perez, A, Arden, NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659–64, https://doi.org/10.1136/annrheumdis-2013-203355.Search in Google Scholar
4. Fu, K, Robbins, SR, McDougall, JJ. Osteoarthritis: the genesis of pain. Rheumatology 2018;57:S43–50, https://doi.org/10.1093/rheumatology/kex419.Search in Google Scholar
5. Bikle, DD. Vitamin D and bone. Curr Osteoporos Rep 2012;10:151–9, https://doi.org/10.1007/s11914-012-0098-z.Search in Google Scholar
6. Holick, MF, Chen, TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:S1080–6, https://doi.org/10.1093/ajcn/87.4.1080s.Search in Google Scholar
7. Lane, NE, Gore, LR, Cummings, SR, Hochberg, MC, Scott, JC, Williams, EN, et al.. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum 1999;42:854–60, https://doi.org/10.1002/1529-0131(199905)42:5<854::aid-anr3>3.0.co;2-i.10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-ISearch in Google Scholar
8. Ding, C. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum 2009;60:1381–9, https://doi.org/10.1002/art.24486.Search in Google Scholar
9. Bergink, AP, Uitterlinden, AG, Van Leeuwen, JP, Buurman, CJ, Hofman, A, Verhaar, JA, et al.. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam Study. J Clin Rheumatol 2009;15:230–7, https://doi.org/10.1097/rhu.0b013e3181b08f20.Search in Google Scholar
10. McAlindon, TE, Felson, DT, Zhang, Y, Hannan, MT, Aliabadi, P, Weissman, B, et al.. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med 1996;125:353–9, https://doi.org/10.7326/0003-4819-125-5-199609010-00001.Search in Google Scholar
11. Felson, DT, Niu, J, Clancy, M, Aliabadi, P, Sack, B, Guermazi, A, et al.. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum 2007;56:129–36, https://doi.org/10.1002/art.22292.Search in Google Scholar
12. Konstari, S, Paananen, M, Heliövaara, M, Knekt, P, Marniemi, J, Impivaara, O, et al.. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: a 22-year follow-up study. Scand J Rheumatol 2012;41:124–31, https://doi.org/10.3109/03009742.2011.617314.Search in Google Scholar PubMed
13. Haussler, MR, Jurutka, PW, Mizwicki, M, Norman, AW. Vitamin D receptor (VDR)-mediated actions of 1α, 25(OH)₂vitamin D₃: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metabol 2011;25:543–59, https://doi.org/10.1016/j.beem.2011.05.010.Search in Google Scholar PubMed
14. Wang, Y, Zhu, J, DeLuca, HF. Where is the vitamin D receptor? Arch Biochem Biophys 2012;523:123–33, https://doi.org/10.1016/j.abb.2012.04.001.Search in Google Scholar PubMed
15. Hansen, AK, Figenschau, Y, Zubiaurre-Martinez, I. Co-expression of 1α-hydroxylase and vitamin D receptor in human articular chondrocytes. BMC Muscoskel Disord 2017;18:432, https://doi.org/10.1186/s12891-017-1791-y.Search in Google Scholar PubMed PubMed Central
16. Denison, TA, Koch, CF, Shapiro, IM, Schwartz, Z, Boyan, BD. Inorganic phosphate modulates responsiveness to 24, 25(OH)2D3 in chondrogenic ATDC5 cells. J Cell Biochem 2009;107:155–62, https://doi.org/10.1002/jcb.22111.Search in Google Scholar PubMed
17. Dean, DD, Boyan, BD, Schwart, Z, Muniz, OE, Carreno, MR, Maeda, S, et al.. Effect of 1alpha, 25-dihydroxyvitamin D3 and 24R, 25-dihydroxyvitamin D3 on metalloproteinase activity and cell maturation in growth plate cartilage in vivo. Endocrine 2001;14:311–23, https://doi.org/10.1385/endo:14:3:311.10.1385/ENDO:14:3:311Search in Google Scholar
18. Tetlow, LC, Woolley, DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage 2001;9:423–31, https://doi.org/10.1053/joca.2000.0408.Search in Google Scholar PubMed
19. Orfanidou, T, Malizos, KN, Varitimidis, S, Tsezou, A. 1, 25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp Biol Med (Maywood, NJ, U S) 2012;237:241–53, https://doi.org/10.1258/ebm.2011.011301.Search in Google Scholar PubMed
20. Komaba, H, Fukagawa, M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int 2010;77:292–8, https://doi.org/10.1038/ki.2009.466.Search in Google Scholar PubMed
21. Shimada, T, Hasegawa, H, Yamazaki, Y, Muto, T, Hino, R, Takeuchi, Y, et al.. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429–35. https://doi.org/10.1359/jbmr.0301264.Search in Google Scholar PubMed
22. Orfanidou, T, Iliopoulos, D, Malizos, KN, Tsezou, A. Involvement of SOX-9 and FGF-23 in RUNX-2 regulation in osteoarthritic chondrocytes. J Cell Mol Med 2009;13:3186–94, https://doi.org/10.1111/j.1582-4934.2008.00678.x.Search in Google Scholar
23. Altman, R, Asch, E, Bloch, D, Bole, G, Borenstein, D, Brandt, K, et al.. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49, https://doi.org/10.1002/art.1780290816.Search in Google Scholar PubMed
24. Kellgren, JH, Lawrence, JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502, https://doi.org/10.1136/ard.16.4.494.Search in Google Scholar PubMed PubMed Central
25. Radin, EL, Rose, RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop 1986;213:34–40, https://doi.org/10.1097/00003086-198612000-00005.Search in Google Scholar
26. Heidari, B, Heidari, P, Hajian-Tilaki, K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop 2011;35:1627–31, https://doi.org/10.1007/s00264-010-1186-2.Search in Google Scholar PubMed PubMed Central
27. Brandt, KD, Radin, EL, Dieppe, PA, van de Putte, L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis 2006;65:1261–4, https://doi.org/10.1136/ard.2006.058347.Search in Google Scholar PubMed PubMed Central
28. Sitara, D, Razzaque, MS, Hesse, M, Yoganathan, S, Taguchi, T, Erben, RG, et al.. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 2004;23:421–32, https://doi.org/10.1016/j.matbio.2004.09.007.Search in Google Scholar PubMed PubMed Central
29. Raimann, A, Ertl, DA, Helmreich, M, Sagmeister, S, Egerbacher, M, Haeusler, G. Fibroblast growth factor 23 and Klotho are present in the growth plate. Connect Tissue Res 2013;54:108–17, https://doi.org/10.3109/03008207.2012.753879.Search in Google Scholar PubMed
30. Mohammed, MA, Rady, SAK, Mohammed, RA, Fadda, SMH. Relation of plasma fibroblast growth factor-23 (FGF-23) to radiographic severity in primary knee osteoarthritis patients. Egypt Rheum 2018;40:261–4, https://doi.org/10.1016/j.ejr.2018.01.007.Search in Google Scholar
31. Zhou, W, Liu, G-H, Yang, S-H, Ye, S-N, Wang, J, Liu, X-Z. Increased serum fibroblast growth factor-23 (FGF-23) and bone turnover in patients with osteoarthritis of knee. Int J Clin Exp Med 2016;9:1630–8.Search in Google Scholar
32. Liu, S, Tang, W, Zhou, J, Stubbs, JR, Luo, Q, Pi, M, et al.. Fibroblast growth factor 23 is a counterregulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 2006;17:1305–15, https://doi.org/10.1681/asn.2005111185.Search in Google Scholar
33. Saito, H, Maeda, A, Ohtomo, S, Hirata, M, Kusano, K, Kato, S, et al.. Circulating FGF-23 is regulated by 1alpha, 25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 2005;280:2543–9, https://doi.org/10.1074/jbc.m408903200.Search in Google Scholar
34. Hayes, ME, Denton, J, Freemont, AJ, Mawer, EB. Synthesis of the active metabolite of vitamin D, 1, 25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis 1989;48:723–9, https://doi.org/10.1136/ard.48.9.723.Search in Google Scholar PubMed PubMed Central
35. Liu, B, Zhang, M, Zhao, J, Zheng, M, Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med 2018;16:5009–14, https://doi.org/10.3892/etm.2018.6852.Search in Google Scholar PubMed PubMed Central
36. Davies, M, Mawer, EB, Hayes, ME, Lumb, GA. Abnormal vitamin D metabolism in Hodgkin’s lymphoma. Lancet 1985;1:1186–8, https://doi.org/10.1016/s0140-6736(85)92864-8.Search in Google Scholar PubMed
37. Al-Ghafari, AB, Balamash, KS, Al Doghaither, HA. Serum vitamin D receptor (VDR) levels as a potential diagnostic marker for colorectal cancer. Saudi J Biol Sci 2020;27:827–32, https://doi.org/10.1016/j.sjbs.2020.01.006.Search in Google Scholar PubMed PubMed Central
38. Tekeli, SÖ, Tekeli, FY, Erol, O, Ellidag, HY, Eren, E, Yılmaz, N. Serum vitamin D receptor levels in gestational diabetes mellitus. J Lab Med 2018;42:149–54, https://doi.org/10.1515/labmed-2017-0149.Search in Google Scholar
39. Moss, ML, Lambert, MH. Shedding of membrane proteins by ADAM family proteases. Essays Biochem 2002;38:141–53, https://doi.org/10.1042/bse0380141.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment
Articles in the same Issue
- Frontmatter
- Review Article
- The implication of molecular markers in the early stage diagnosis of colorectal cancers and precancerous lesions
- Research Articles
- What are the predominant parameters for Down syndrome risk estimation in first-trimester screening: a data mining study
- Comparison between liquid chromatography-tandem mass spectrometry and immunoassay methods for measurement of plasma 25 (OH) vitamin D
- Comparison of Barricor tube and serum separator tube in outpatients
- Effect of hemoglobin, triglyceride, and urea in different concentrations on compatibility between methods used in HbA1c measurement
- Evaluation of hemolysis interference and possible protective effect of N-phenyl maleimide on the measurement of small peptides
- Neuroprotective and metabotropic effect of aerobic exercise training in female patients with type 2 diabetes mellitus
- Serum asprosin levels in patients with retinopathy of prematurity
- Neutrophil/lymphocyte and platelet/lymphocyte ratios as a biomarker in postoperative wound infections
- Relationship between dental caries and saliva’s visfatin levels, total antioxidant capacity (TAC) and total oxidant status (TOS)
- Might periostin serve as a marker of bone marrow involvement in patients with diffuse large B-cell lymphoma?
- A new approach for the pleiotropic effect of metformin use in type 2 diabetes mellitus
- IGFBP7 is a predictor of diuretic-induced acute kidney injury in the patients with acute decompensated heart failure
- Serum vitamin D receptor and fibroblast growth factor-23 levels in postmenopausal primary knee osteoarthritis patients
- Antioxidant activity of ethanol extract and fractions of Piper crocatum with Rancimat and cuprac methods
- Lycorine impedes 7,12-dimethylbenz(a)anthracene exposed hamster oral carcinogenesis through P13K/Akt and NF-κB inhibition
- Sublethal effects of acrylamide on thyroid hormones, complete blood count and micronucleus frequency of vertebrate model organism (Cyprinus carpio)
- Acknowledgment
- Acknowledgment

