Abstract
Objectives
To investigate the effects of glucagon-like peptide-1 (GLP-1) for the apoptosis of human umbilical vein endothelial cells (HUVECs) in high glucose and the related mechanisms.
Material and methods
HUVECs were cultured under different conditions for 48 h. The apoptosis rate of cells was detected by flow cytometry, the expression of p-Akt and p-eNOS was measured by Western blot, and the production of nitric oxide (NO) was detected by NO assay kit.
Results
HUVECs were incubated in high glucose, the apoptosis rate of cells increased, the expression of p-Akt and p-eNOS reduced, and the production of NO decreased. After GLP-1 was added into the high glucose, the apoptosis rate of cells significantly reduced, the expression of p-Akt and p-eNOS and the production of NO obviously increased. After exendin, wortmannine and L-NAME were added into high glucose and GLP-1, respectively, exendin, wortmannine and L-NAME increased the cell apoptosis, down-regulated the expression of p-Akt and p-eNOS and dropped the production of NO, except that L-NAME made no difference on the expression of p-Akt.
Conclusion
GLP-1 increased the expression of Akt and endothelial nitric oxide synthase (eNOS) in HUVECs via the up-regulation of PI3K/Akt/eNOS pathway and decreased the apoptosis rate of HUVECs in high glucose.
Özet
Amaç
Glukagon benzeri peptit-1‘in (GLP-1)Y yüksek glukoz varlığında insan göbek bağı ven endotel hücrelerinin (HUVEC‘ler) apoptozu üzerine etkilerini ve ilgili mekanizmaları araştırmak.
Gereç ve Yöntem
HUVEC‘ler 48 saat boyunca farklı koşullar altında büyütüldü. Hücrelerin apoptoz oranı flow sitometri ile tespit edildi, p-Akt‘in ve p-eNOS ekspresyonu, Western blot ile ölçüldü ve NO üretimi, NO assay kiti ile tespit edildi.
Bulgular
HUVEC‘ler yüksek glukozda inkübe edildi, hücrelerin apoptoz oranı arttı, p-Akt ve p-eNOS ifadesi azaldı ve NO üretimi azaldı. Yüksek glukoza GLP-1 ilave edildikten sonra, hücrelerin apoptoz oranı, p-Akt ve p-eNOS‘un ekspresyonunu önemli ölçüde düştü ve NO üretimi belirgin bir şekilde arttı. Exendini takiben, yüksek glukozlu hücrelere wortmannine ve GLP-1 eklenenhücrelere de L-NAME eklendi, ekstendin, wortmannine ve L-NAME, hücre apoptozunu arttıran, p-Akt ve p-eNOS ekspresyonunu aşağı regüle ederek, NO üretimini düşürdü. Ancak L-NAME, p-Akt‘in ekspresyonu üzerinde herhangi bir fark yaratmadı.
Sonuç
GLP-1, PI3K/Akt/eNOS yolağının yukarı regülasyonu yoluyla HUVEC‘lerde Akt ve eNOS ekspresyonunu arttırdı ve yüksek glukozda HUVEC‘lerin apoptoz oranını düşürdü.
Introduction
Glucagon-like peptide-1 (GLP-1), one of incretin peptides, is secreted by enteroendocrine L cells mainly located in the mucosa of the distal part of the small intestine and colon after ingesting food [1]. GLP-1 can stimulate β-cells in the pancreas to secrete insulin and play the role of lowering blood sugar [2]. Meanwhile, GLP-1 inhibits glucagon secretion, suppresses gastrointestinal peristalsis, increases satiety and reduces appetite and food intake [3]. Thus, GLP-1 is widely used to treat patients with type 2 diabetes.
Vascular complications of diabetes are the leading cause of death and disability in diabetic patients [4]. Chronic hyperglycemia causing endothelial cell apoptosis is the main factor of cardiovascular disease patients with type 2 diabetes mellitus [5]. Therefore, preventing the endothelial cell apoptosis and protecting the endothelial function can control the diabetic vascular complications and improve the prognosis of patients with diabetes.
These studies showed that PI3K/Akt/endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signaling pathways play an important role in preventing endothelial cell apoptosis that hyperglycemia results in Ref. [6]. Phosphatidylinositol 3-kinase (PI3K) activates downstream Akt to prevent cell apoptosis death stimulating factors causing [7]. Akt, also known as protein kinase B, is a serine/threonine kinase family which is an important anti-apoptosis protein in body [8]. Activation of Akt requires the phosphorylation of Thr308 in the activation loop by the phosphoinositide-dependent kinase 1 (PDK1) and Ser473 within the carboxyl-terminal hydrophobic motif by an unknown kinase [9]. The activated Akt in endothelial cells can activate eNOS which plays an important role in regulating a broad spectrum of functions in the cardiovascular system, including vasorelaxation, the inhibition of leukocyte-endothelial adhesion, vascular smooth muscle cell (SMC) migration and proliferation, and platelet aggregation [10]. The eNOS synthesizes NO from the substrate L-arginine in endothelial cells after activated. NO served as super oxygen anion scavenger in endothelial cells reacts with super oxygen anion, prevents it from creating hydrogen peroxide (H2O2) and preserve endothelial cells from apoptosis [11].
Recent studies have found GLP-1 also has protective effect on endothelial cell [12], while the functional mechanism is still unclear. In order to better understand the mechanism by which GLP-1 may protect endothelial cell, we investigated whether the mechanism is related with up-regulation of PI3K/Akt/eNOS/NO signaling pathways in the present study.
Materials and methods
Reagents
GLP-1, exendin, wortmannin and L-NAME were purchased from Sigma (USA). P-Akt antibody [phospho-Akt (Ser473) antibody: #9271] and p-eNOS antibody [phospho-eNOS (Ser1177) antibody: #9571] were purchased from Cell Signaling Technology, Inc. (USA). Annexin-V/PI double staining kits and NO assay kit were obtained from Beyotime (China). Exendin, wortmannine and L-NAME were purchased from ApexBio (USA).
Phospho-Akt (Ser473) antibody, a mouse polyclonal antibody, can detect endogenous levels of Akt1 only when phosphorylated at Ser473 of Akt from human by Western blot. Phospho-eNOS (Ser1177) antibody, a rabbit polyclonal antibody, can detect endogenous levels of eNOS only when phosphorylated at Ser1177 of Akt from human by Western blot.
Cell culture
Primary human umbilical vein endothelial cells (HUVECs, Catalog Number: C-12200) from normal human placenta umbilical cord tissues were obtained from Promo Cell GmbH (Heidelberg, Germany) as cryopreserved primary cultures. After purchased, HUVECs were recovered and sub-cultured in endothelial cell medium containing 5% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., USA) and 1% endothelial cell growth supplement in a humidified atmosphere of 95% air and 5% CO2 at 37°C. After added different experimental factors, HUVECs in logarithmic growth phase were divided into six groups. It is as follows: physiological concentration of glucose (control: 5 mmol/L of glucose), high glucose (HG: 33 mmol/L of glucose), high glucose and GLP-1 (HG+G: 33 mmol/L of glucose and 10 nmol/L of GLP-1), physiological concentration of glucose and GLP-1 (control+G: 5 mmol/L of glucose and 10 nmol/L of GLP-1), high glucose, GLP-1and exendin (HG+G+E: 33 mmol/L of glucose, 10 nmol/L of GLP-1 and 5 nmol/L of exendin), high glucose, GLP-1 and wortmannine (HG+G+W: 33 mmol/L of glucose, 10 nmol/L of GLP-1 and 100 nmol/L of wortmannine), and high glucose, GLP-1 and L-NAME (HG+G+L: 33 mmol/L of glucose, 10 nmol/L of GLP-1 and 100 μmol/L of L-NAME). Exendin, wortmannine and L-NAME were added in 3 min ago.
The detection of cell apoptosis rate
The flow cytometry was used to detect the cell apoptosis rate. The HUVECs in logarithmic growth phase were inoculated into six-well plates, and a suspension of HUVECs at 4×105 cells/mL in a 2-mL volume of culture medium was incubated at 37°C. The experimental groups were based on the above method, and every group was in triplicate. After HUVECs incubated for 48 h, the culture solution was discarded. Next, after washed with D-Hank’s and digested with pancreatic enzymes successively, the cells were gathered. After the cell solution was centrifuged (10,000×g, 10 min, 4°C), the supernatant was removed. After washed with phosphate buffer saline (PBS) precooling at 4°C, the cell solution was centrifuged (10,000×g, 10 min, 4°C) again, and then the supernatant PBS was removed. The washing was repeated twice. According to the operating instructions of Annexin-V/PI double staining kits, the HUVECs were stained and quantitatively analyzed by the flow cytometry (FACS Calibur 95, BD Biosciences). The data were presented as dot plots showing fluorescence intensity of Annexin-V and PI.
The detection of p-Akt and p-eNOS expression
The Western blot was used to detect the expression of p-Akt and p-eNOS. The HUVECs in logarithmic growth phase were inoculated into 25 cm3 of culture bottle, and a suspension of HUVECs at 1×106 cells/mL in a 5-mL volume of culture medium was incubated at 37°C. The experimental groups were based on the above method, and every group was in triplicate. After HUVECs incubated for 48 h, the HUVECs was washed twice with PBS precooling at 4°C and collected by cell scraper. Next, according to the operating instructions of protein extraction kit, the total protein of each group was extracted and measured by Bradford method. After 40 μg of the total protein in each group was denatured at 100°C for 5 min, the protein was separated by 12% SDS-PAGE and transferred to PVDE membrane, and then sealed by 5% skimmed milk for 2 h. After the membrane was washed, p-Akt antibody (1:1000) and p-eNOS antibody (1:1000) were added and incubated overnight. After that, horseradish peroxidase labeled second antibody was added and incubated at room temperature for 1 h. After washed, the membrane dried, and the target protein was detected by chemiluminescence method. β-Aatin was internal reference and used to calculate the relative content of the target protein. Signal intensity of the bands was analyzed to use ImagePro Plus Software of version 6.0 (Media Cybernetics, Inc., MD, USA).
The detection of NO concentration
The HUVECs in logarithmic growth phase were inoculated into six-well plates, and a suspension of HUVECs at 4×105 cells/mL in a 2-mL volume of culture medium was incubated at 37°C. The experimental groups were based on the above method, and every group was in triplicate. After incubated for 48 h, 0.1 mL of supernatant was taken out. According to the operating instructions of NO assay kit, the absorbance of the supernatant was determined by ELISA at 550 nm to calculate the concentration of NO.
Statistical analysis
The statistical analyses in this work were carried out by SPSS version 17.0. All data were presented as the mean±SD. Comparisons between two groups were performed using student’s t-test. Values of p<0.05 were considered to indicate a statistically significant difference.
Results
The effect of GLP-1 and high glucose for the apoptosis rate of HUVECs
The percentage of apoptotic cells was examined by Annexin-V/PI double staining. The apoptosis rate of HUVECs in HG was 23.8% and significantly higher than 4.8% of control group (p<0.05). The apoptosis rate of HUVECs in HG+G was 4.5% and significantly reduced in comparison with HG. The apoptosis rate of HUVECs in HG+G+E (23.1%), HG+G+W (24.7%) and HG+G+L (21.8%) was markedly higher than HG+G. The all above results were showed in Figure 1.
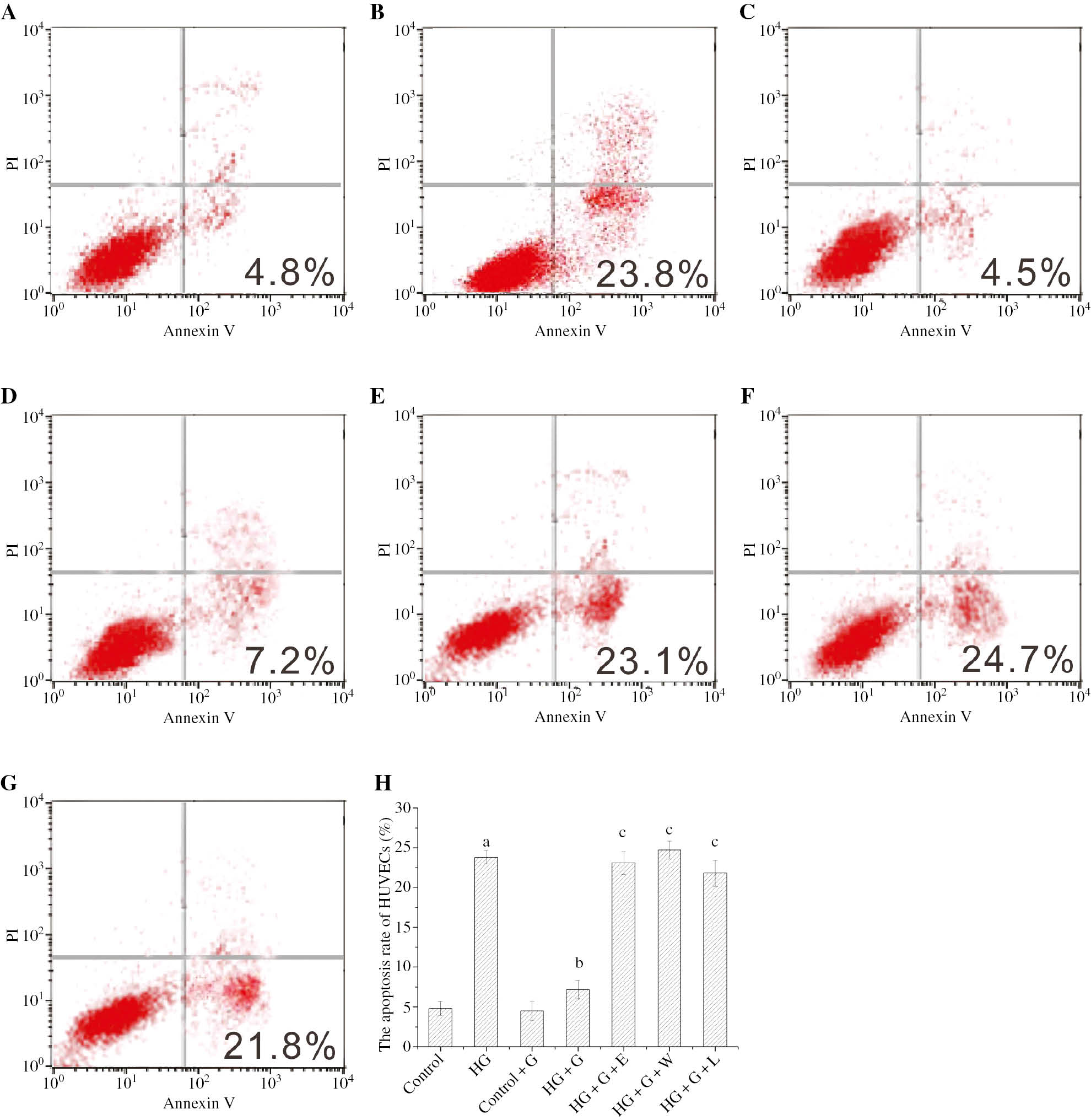
The apoptosis rate of HUVECs in the different conditions was labeled with Annexin V/PI and detected by the flow cytometry.
ap<0.05 compared to control; bp<0.05 compared to HG; cp<0.05 compared to HG+G. (A) control, (B) HG, (C) control+G, (D) HG+G; (E) HG+G+E; (F) HG+G+W, (G) HG+G+L and (H) a histogram of the apoptosis rate from A to G.
The effect of GLP-1 and high glucose for the p-Akt and p-eNOS expression of HUVECs
The expression of p-Akt and p-eNOS in HG reduced (p<0.05) in comparison with control group, but the expressions in HG+G significantly increased (p<0.05), and the expression in HG+G+E with exendin and HG+G+W with wortmannine also decreased in comparison with HG+G, as showed in Figure 2. The expression of p-Akt in HG+G+L with L-NAME were not significantly different from HG+G (p>0.05), as shown in Figure 2A and B. However, there was a significant difference between HG+G+L and HG+G in the expression of p-eNOS (p<0.05), as shown in Figure 2C and D.
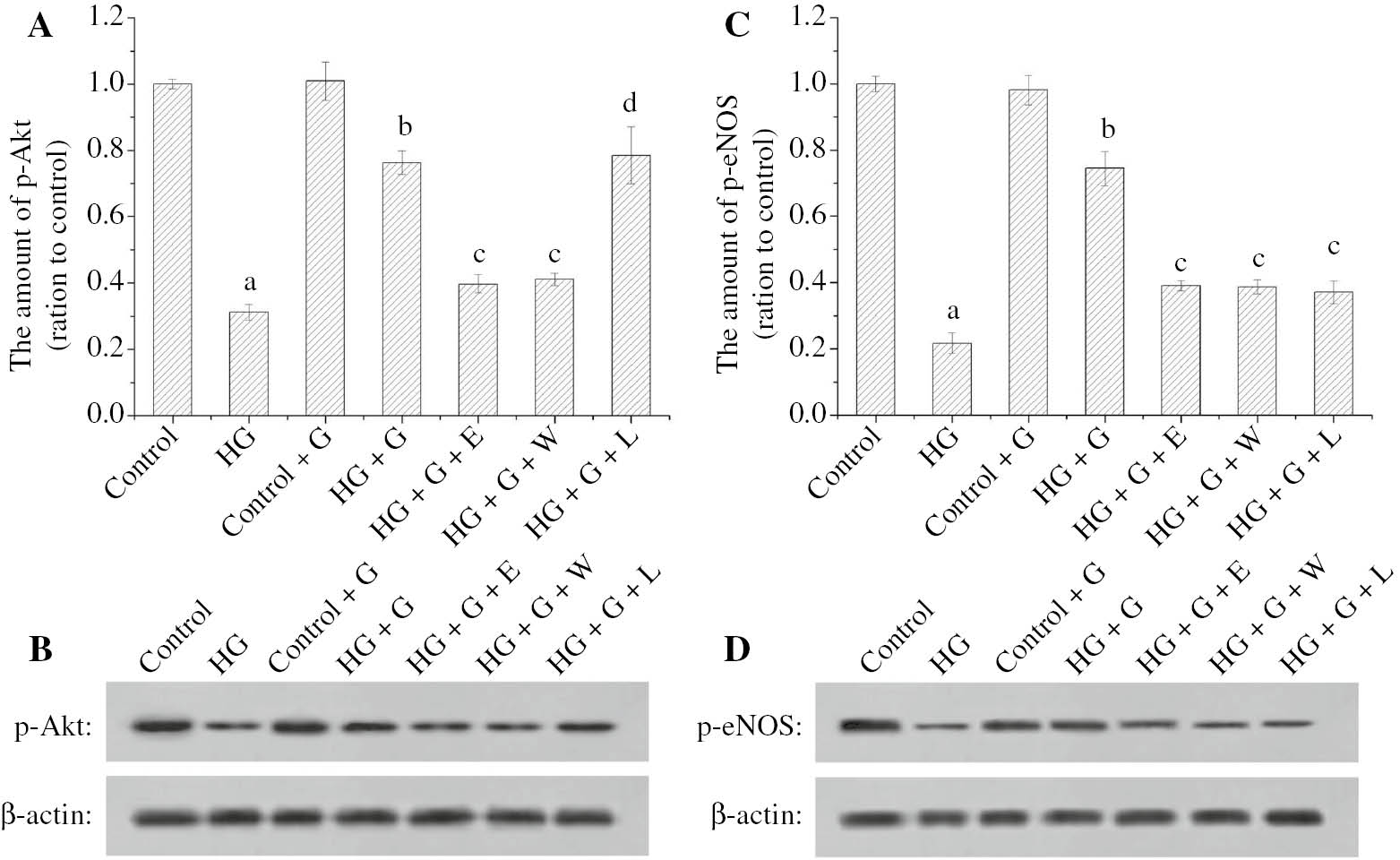
The effect of GLP-1 and high glucose for the p-Akt and p-eNOS expression of HUVECs.
The histogram (A) showed the relative amount of p-Akt in HUVECs. The images (B) showed the analyzed result of p-Akt in HUVECs by Western blot. The histogram (C) showed the relative amount of p-eNOS in HUVECs. The images (D) showed the analysis result of p-eNOS in HUVECs by Western blot. ap<0.05 compared to control; bp<0.05 compared to HG; cp<0.05 compared to HG+G; dp>0.05 compared to HG+G.
The effect of GLP-1 and high glucose for the NO production of HUVECs
The NO production of HG was obviously less than the control group (p<0.05), but the HG+G produced more NO than HG (p<0.05). The NO production of HG+G+E with exendin, HG+G+W with wortmannine or HG+G+L with L-NAME obviously reduced (p<0.05). The all above results were showed in Figure 3.
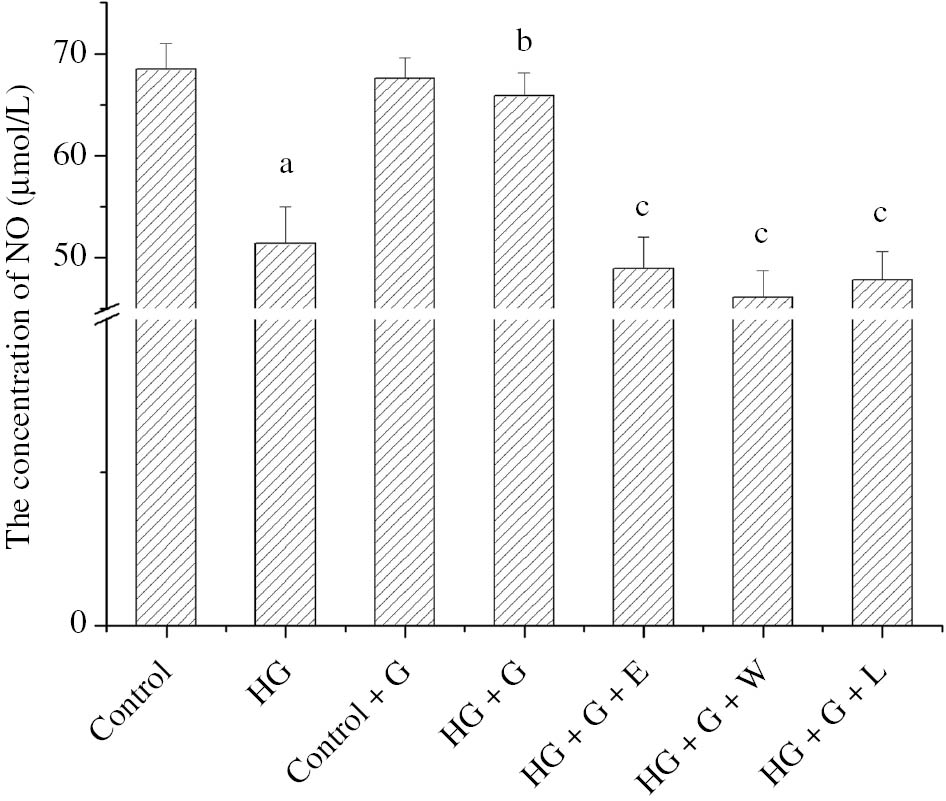
The histogram showed the effect of GLP-1 and high glucose for the NO concentration of HUVECs in the different conditions.
ap<0.05 compared to control; bp<0.05 compared to HG; cp<0.05 compared to HG+G.
Discussion
GLP-1, a brain-gut insulinotropic peptide, plays an important role in regulating glucose homeostasis, and protecting pancreatic β cell and cardiovascular system [13]. The function of GLP-1 protecting vascular endothelial cells attracts extensive attention, and GLP-1 is applied in clinical treatment of diabetes.
The results of the flow cytometry in Figure 1 showed that GLP-1 could obviously reduce the apoptosis rate of HUVECs, so we further investigated the pathways that GLP-1 protects vascular endothelial cells under hyperglycemia. The results of Figure 2 displayed that the expression of p-Akt and p-eNOS in HG was lower than the control group, meanwhile the production of NO in HG was obviously less than the control group, as shown in Figure 3. The p-Akt is one of the downstream effector molecules of PI3K signal pathway and an important anti-apoptosis protein in the body. The excitation of p-Akt plays a significant role in protecting endothelial cells and inhibiting the cells apoptosis [14]. The p-eNOS plays an important role in maintaining the normal function of endothelial cells, and its dysfunction may be significantly associated with the cardiovascular complications in patients with type 2 diabetes mellitus [15]. After GLP-1 was added into the HG, the expression of p-Akt and p-eNOS in high blood glucose was markedly improved and the production of NO in high blood glucose markedly increased. In order to make certain the relative mechanism, we did the further research. Exendin, a potent GLP-1 receptor antagonist, is capable of competing with GLP-1 for receptor binding [16]. After exendin was added into the HG+G, the apoptosis rate of HUVECs was significantly higher than HG+G in Figure 1, the expression of p-Akt and p-eNOS was significantly lower than HG+G in Figure 2, and the production of NO was observably less than HG+G in Figure 3. Wortmannine, a PI3K inhibitor, specifically inhibits PI3K and represses the PI3K/Akt signal pathway [17]. The experimental results wortmannine produced were similar to the exendin. L-NAME is an eNOS inhibitor. L-NAME caused the increasing of HUVECs apoptosis rate (Figure 1), the down-regulation of p-eNOS expression (Figure 2C and D) and the reduction of NO production (Figure 3), in comparison with HG+G. However, L-NAME had no effect on the expression of p-Akt. This is because p-Akt is the upstream molecular of p-eNOS and L-NAME only inhibit the activity of p-eNOS.
According to the above experimental results, we speculate that the mechanism is as follows: GLP-1 activated GLP-1R of HUVECs and further increased the expression of PI3K; PI3K activated the downstream Akt, and then activated Akt activated and increased eNOS, increased the production of NO and released NO into the HUVECs; the NO reacted with superoxide anion in HUVECs, avoided the production of H2O2 and prevented HUVECs from the apoptosis of HUVECs.
In conclusion, GLP-1 can protect the HUVECs through the GLP-1R-dependent and GLP-1 related pathways. In addition, we will focus far more on the protected effects of GLP-1 does factor for high glucose inducing apoptosis of HUVECs.
Acknowledgments
We gratefully acknowledge the financial support of the Science and Technology Project of Education Department of Fujian Province (JA11117).
Conflict of interest: All authors declared that there were no any potential conflicts of interest.
Compliance with ethical standards: This article does not contain any studies with human subjects or animals performed by any of the authors.
References
1. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–39.10.1152/physrev.00034.2006Search in Google Scholar PubMed
2. Cho YM, Wideman RD, Kieffer TJ. Clinical application of GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. Endocrinol Metab 2013;28:262–74.10.3803/EnM.2013.28.4.262Search in Google Scholar PubMed PubMed Central
3. Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol 2014;76:535–59.10.1146/annurev-physiol-021113-170315Search in Google Scholar PubMed
4. Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res 2016;118:1771–85.10.1161/CIRCRESAHA.115.306884Search in Google Scholar PubMed
5. Ding L, Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 2012;33:75–81.10.1038/aps.2011.149Search in Google Scholar PubMed PubMed Central
6. Wu J, Lei MX, Xie XY, Liu L, She YM, Mo J, et al. Rosiglitazone inhibits high glucose-induced apoptosis in human umbilical vein endothelial cells through the PI3K/Akt/eNOS pathway. Can J Physiol Pharm 2009;87:549–55.10.1139/Y09-040Search in Google Scholar
7. Liu CL, Xie LX, Li M, Durairajan SS, Goto S, Huang JD. Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt Signaling. PLoS One 2007;12:e1321.10.1371/journal.pone.0001321Search in Google Scholar PubMed PubMed Central
8. Xiao M, Men LN, Xu MG, Wang GB, Lv HT, Liu C. Berberine protects endothelial progenitor cell from damage of TNF-α via the PI3K/AKT/eNOS signaling pathway. Eur J Pharmacol 2014;743:11–6.10.1016/j.ejphar.2014.09.024Search in Google Scholar PubMed
9. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307:1098–101.10.1126/science.1106148Search in Google Scholar PubMed
10. King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci 2014;111:3182–7.10.1073/pnas.1321871111Search in Google Scholar PubMed PubMed Central
11. Wang BH, Luo T, Chen D, Ansley DM. Propofol reduces apoptosis and up-regulates endothelial nitric oxide synthase protein expression in hydrogen peroxide-stimulated human umbilical vein endothelial cells. Anesth Analg 2007;105:1027–33.10.1213/01.ane.0000281046.77228.91Search in Google Scholar PubMed
12. Liu MM, Shen YF, Chen C, Lai XY, Zhang MY, Yu R. Protective effect of glucagon-like peptide-1 analogue on cardiomyocytes injury induced by hypoxia/reoxygenation. Chin J Intern Med 2016;55:311–6.Search in Google Scholar
13. Aronisa KN, Chamberlanda JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism 2013;62:1279–86.10.1016/j.metabol.2013.04.010Search in Google Scholar PubMed PubMed Central
14. Tran J, Magenau A, Rodriguez M, Rentero C, Royo T, Enrich C, et al. Activation of endothelial nitric oxide (eNOS) occurs through different membrane domains in endothelial cells. PLoS One 2016;11:e0151556.10.1371/journal.pone.0151556Search in Google Scholar PubMed PubMed Central
15. Chan SW, Lu ZB, Lin G, Yew DT, Yeung CK, Rudd JA. The differential antiemetic properties of GLP-1 receptor antagonist, exendin (9–39) in Suncus murinus (house musk shrew). Neuropharmacology 2014;83:71–8.10.1016/j.neuropharm.2014.03.016Search in Google Scholar PubMed
16. Leiria LO, Sollon C, Báu FR, Mónica FZ, D’Ancona CL, De Nucci G, et al. Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J Physiol 2013;591:2259–73.10.1113/jphysiol.2013.251843Search in Google Scholar PubMed PubMed Central
17. Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J 2005;19:1755–7.10.1096/fj.05-4008fjeSearch in Google Scholar PubMed
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- A Src/Abl kinase inhibitor, bosutinib, downregulates and inhibits PARP enzyme and sensitizes cells to the DNA damaging agents
- Effects of chromium picolinate on the parameters of oxidative and chromosomal DNA damage in rabbits
- Glucagon-like peptide-1 affects human umbilical vein endothelial cells in high glucose by the PI3K/Akt/eNOS signaling pathway
- Evaluation of apoptotic caspase levels in estimation of the wound age
- Use of immunohistochemical versus microsatellite analyses as markers for colorectal cancer
- Estimation of the Y-chromosomal short tandem repeat (Y-STR) mutation rates in Turkey
- Synthesis and evaluation of new benzimidazole derivatives with hydrazone moiety as anticancer agents
- Effects of chemoradiotherapy on acute-phase protein levels in glioblastoma multiforme and locally advanced non-small cell lung cancer
- Association between polymorphisms of DNA repair genes and risk of type 2 diabetes mellitus in the Turkish population
- Letter to the Editor
- Association of CTLA-4 polymorphisms and autoimmune type-1 diabetes mellitus susceptibility in Pakistani population
- Research Articles
- The effects of royal jelly on the oxidant-antioxidant system in rats with N-methyl-N-nitrosourea-induced breast cancer
- Meta-analysis of the relationship between MnSOD polymorphism and cancer in the Turkish and Cypriot population
- Resveratrol induces cell cycle arrest and apoptosis in epithelioid malignant pleural mesothelioma cells
Articles in the same Issue
- Frontmatter
- Research Articles
- A Src/Abl kinase inhibitor, bosutinib, downregulates and inhibits PARP enzyme and sensitizes cells to the DNA damaging agents
- Effects of chromium picolinate on the parameters of oxidative and chromosomal DNA damage in rabbits
- Glucagon-like peptide-1 affects human umbilical vein endothelial cells in high glucose by the PI3K/Akt/eNOS signaling pathway
- Evaluation of apoptotic caspase levels in estimation of the wound age
- Use of immunohistochemical versus microsatellite analyses as markers for colorectal cancer
- Estimation of the Y-chromosomal short tandem repeat (Y-STR) mutation rates in Turkey
- Synthesis and evaluation of new benzimidazole derivatives with hydrazone moiety as anticancer agents
- Effects of chemoradiotherapy on acute-phase protein levels in glioblastoma multiforme and locally advanced non-small cell lung cancer
- Association between polymorphisms of DNA repair genes and risk of type 2 diabetes mellitus in the Turkish population
- Letter to the Editor
- Association of CTLA-4 polymorphisms and autoimmune type-1 diabetes mellitus susceptibility in Pakistani population
- Research Articles
- The effects of royal jelly on the oxidant-antioxidant system in rats with N-methyl-N-nitrosourea-induced breast cancer
- Meta-analysis of the relationship between MnSOD polymorphism and cancer in the Turkish and Cypriot population
- Resveratrol induces cell cycle arrest and apoptosis in epithelioid malignant pleural mesothelioma cells

