Abstract
Traditionally, lactate is regarded as a byproduct of anaerobic metabolism. With the deepening of related research, the roles of lactate in cellular energy metabolism, signal transduction, and microenvironment regulation have attracted increasing attention. Against this research background, the discovery of a novel post-translational modification – lactylation modification – has further expanded its biological functions. In the context of the increasingly aging global population, neurodegenerative diseases (ND) have become a significant challenge threatening global public health. Studies have reported that lactate metabolic disorders are common metabolic characteristics in the occurrence and development of ND. In summary, this article focuses on reviewing lactate and lactylation in the brain and their roles in ND. It comprehensively outlines the process from lactate to lactylation, highlights the close connection between brain lactate metabolism and ND, and explores potential molecular mechanisms underlying disease development – providing new perspectives for understanding ND pathogenesis. Additionally, this review systematically summarizes potential therapeutic strategies for ND based on regulating lactate metabolism, aiming to offer innovative approaches for disease prevention, diagnosis, and treatment.
1 Introduction
Neurodegenerative diseases (ND) encompass Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), which are clinically characterized by cognitive impairment, motor dysfunction, and sensory deficits. The risk of developing ND is positively correlated with age (Fazio-Eynullayeva et al. 2025). According to United Nations projections, by 2050, individuals aged 60 and above will comprise 21 % of the global population (Wagner-Gutierrez et al. 2025). The accelerating global aging process has led to a sharp increase in the prevalence of ND, placing an immense burden on global public health systems. However, the etiology and pathogenesis of these diseases remain incompletely understood, and current treatment options are highly limited. Therefore, a deeper understanding of the progressive mechanisms underlying ND could contribute to the development of more targeted therapeutic strategies for patients.
Lactate has traditionally been regarded as a harmful byproduct of glycolysis. However, with advances in research and conceptual understanding, it is now recognized as a multifunctional molecule that serves as an efficient energy substrate, a signaling molecule, and an immunomodulator (Chen et al. 2022a,b). Its critical roles in maintaining cellular energy homeostasis and regulating the cellular microenvironment have become increasingly evident.
Against this research backdrop, a novel post-translational modification (PTM) derived from lactate has been identified. This modification facilitates the addition of lactyl groups to proteins, altering their structure and function and ultimately regulating key physiological processes such as gene expression, cellular metabolic pathways, and inflammatory responses (Zhang et al. 2020).
In recent years, numerous studies have demonstrated that lactate and its associated protein lactylation modifications are closely linked to neuronal injury, as well as cognitive and motor dysfunction. In ND, these pathological alterations significantly influence disease onset and progression.
In summary, by integrating previous research findings, this review focuses on lactate production and metabolism in the brain while systematically elaborating on the emerging concept of lactylation. Regarding ND pathogenesis, the review emphasizes the role of lactate and its potential underlying mechanisms. Furthermore, it explores therapeutic strategies based on lactate and lactylation, aiming to introduce novel approaches for the prevention, diagnosis, and treatment of these disorders.
2 Lactate in the brain – the energy supply system of a city
In the past, lactate was considered a byproduct of glycolysis and was often regarded as a metabolic waste product. However, increasing research evidence suggests that lactate production, metabolism, and transport mechanisms undergo significant changes in ND. This metabolic dysregulation may play a critical role in disease onset and progression. Figure 1 summarizes the key milestone events in this field.
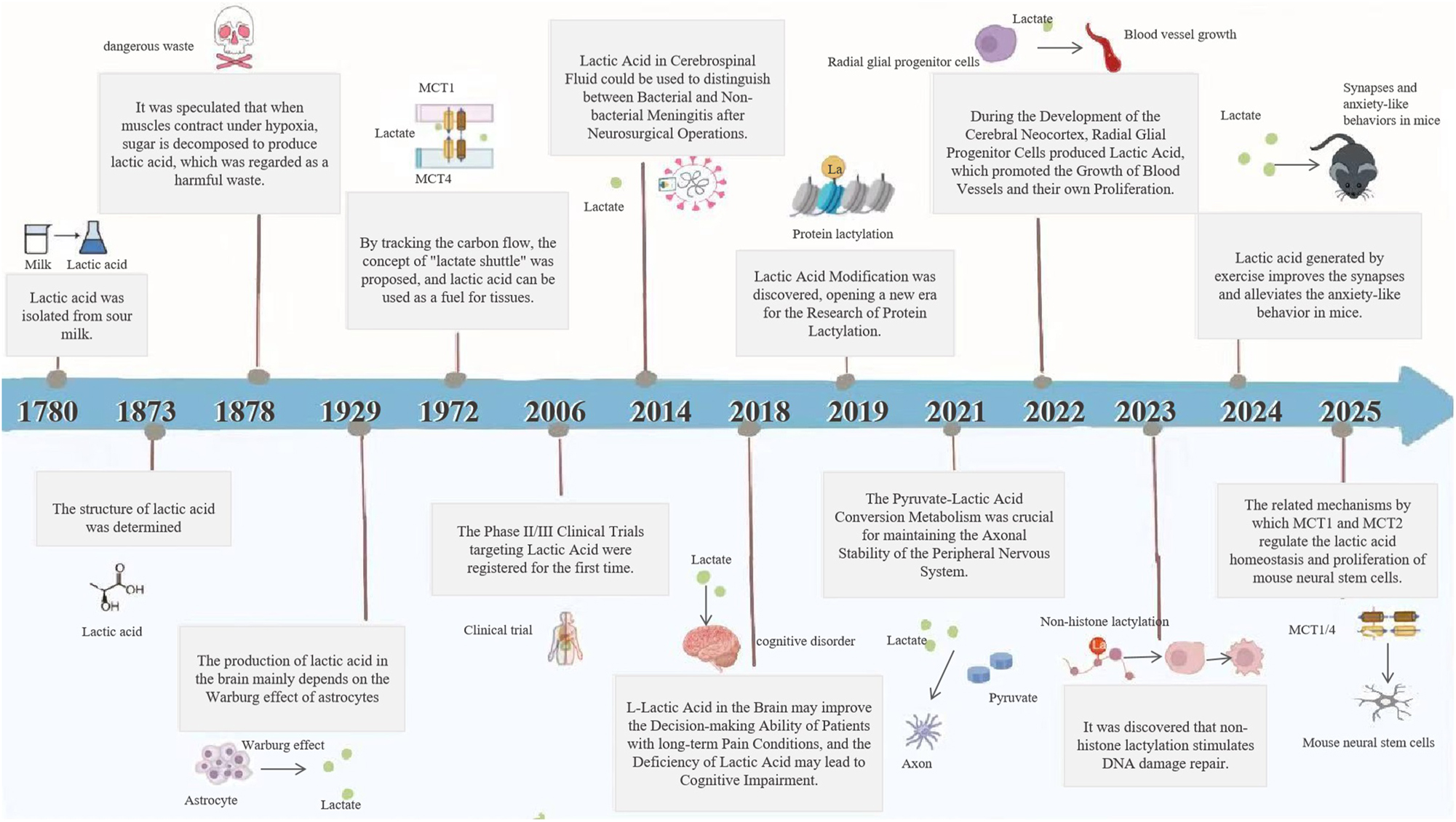
Milestone events in lactate metabolism and lactylation research.
To better understand the multifaceted functions of lactate in the brain, it can be compared to an “energy supply system of a city,” where lactate not only serves as a key energy source but also acts as a central hub for neuronal communication and functional maintenance.
2.1 Lactate production: the city’s power plant
Lactic acid is an organic acid that contains a carboxyl (–COOH) and a hydroxyl (–OH) group, with the molecular formula C3H6O3. Due to the weak acidity of the carboxyl group, lactate can release protons (H+) in aqueous solutions. Consequently, in biological systems, lactate primarily exists in its ionized form as lactate salts.
As a chiral molecule, lactate salts exist in three isomeric forms: dl-lactate, d-lactate, and l-lactate. In the brain, l-lactate is the dominant form (Saulnier-Bellemare and Patience 2024), and its production mainly relies on aerobic glycolysis in astrocytes, a distinct metabolic adaptation known as the Warburg effect (Yuan et al. 2025).
Under normal conditions, neurons primarily generate ATP via mitochondrial oxidative phosphorylation (OXPHOS). However, during periods of heightened neuronal activity and increased energy demand, astrocytes enhance glycolysis to rapidly produce lactate, even when oxygen levels are sufficient, ensuring a rapid supply of ATP (Barros et al. 2021).
This phenomenon occurs because astrocytes possess lower OXPHOS capacity compared to neurons. Additionally, they can transport energy-rich metabolites and store most of the brain’s readily accessible glycogen (Matsui et al. 2017), enabling them to sustain a high glycolytic rate and continuously supply energy as needed.
Just like power plants generate electricity, astrocytes function as the brain’s energy reservoirs. They can ramp up lactate production when necessary, playing a pivotal role in neuronal energy supply, neuroprotection, and the dynamic regulation of brain metabolism.
2.2 Lactate transport: the power transmission network
Lactate produced in the brain functions similarly to electricity generated in a power plant – it cannot remain at its production site but must be transported via a specialized “distribution network” to various regions of the brain. As lactate primarily exists as negatively charged lactate anions, it cannot directly diffuse across the lipid bilayer of cell membranes. Instead, its transmembrane transport relies on specialized transporter proteins (Drew and Boudker 2024). Monocarboxylate transporters (MCTs) play a central role in this process, ensuring efficient lactate translocation across cell membranes (Yamagata 2022), much like power lines distributing electricity to different regions. Neurons, as the ultimate “power consumers,” require these lactate molecules to support their functions.
MCT family, also known as the SLC16 gene family, consists of 14 members based on sequence homology (Perez-Escuredo et al. 2016). Their primary function is to transport monocarboxylic acids and participate in the regulation of brain acid–base balance through proton-coupled cotransport (Blaszczak et al. 2022). Among these MCTs, MCT1-4 have been identified to transport monocarboxylates in humans. Studies have shown that the substrate affinity of each member varies due to structural and functional differences, and the divergence in substrate affinity among different MCT members determines their division of labor in metabolic cooperation (Gu et al. 2025).
MCT1: Predominantly expressed in astrocytes, microvascular endothelial cells, ependymal cells, and oligodendrocytes (Wu et al. 2023), MCT1 exhibits high affinity for lactate, pyruvate, and ketone bodies. For lactate, the Michaelis constant (Km) is approximately 1–3.3 mM (Jackson et al. 2025, Liao et al. 2025a,b), a property enabling its critical role in astrocytic lactate release and ketone body transport.
MCT2: Mainly localized in neurons [3768620], MCT2 displays extremely high affinity for lactate (Km ≈ 0.7–3 mM) (Maculewicz et al. 2025) and has a preference for pyruvate, allowing it to uptake lactate via high affinity to meet the energy demands of neurons (Pierre and Pellerin 2005).
MCT3: Primarily found in retinal pigment epithelial (RPE) cells, MCT3 has low affinity for lactate (Km > 5 mM) and is involved in the regulation of local metabolites rather than bulk lactate transport (Nouri et al. 2024).
MCT4: Almost exclusively expressed in astrocytes, it is a low-affinity transporter with a Km of approximately 15–30 mM, suitable for high-lactate environments. Primarily distributed in astrocytes, it is responsible for the efficient efflux of intracellularly produced lactate (Liao et al. 2025a,b).
This process of lactate exchange between astrocytes and neurons to meet neuronal energy demands is termed the “astrocyte-neuron lactate shuttle hypothesis” (Pellerin and Magistretti 1994). Mächler et al. (2016) found a lactate gradient between astrocytes and neurons in the mouse cortex, which provides strong support for the hypothesis and highlights the critical mediating role of MCTs in monocarboxylate transport across cell membranes. Currently, drugs targeting MCTs have been developed to interfere with glycolytic metabolism (Blaszczak et al. 2022).
In addition to its critical role in neuronal energy supply, proper lactate transport serves as a key signaling mechanism for maintaining neural function (Powell et al. 2020). This is particularly evident in the metabolic regulation mediated by the dorsal vagal complex (DVC) in the hindbrain and the hypothalamus. During hypoglycemia, reduced lactate levels in the DVC activate AMPK in A2 noradrenergic neurons, initiating a neural pathway that projects to the hypothalamus and modulates the expression of multiple metabolic neuropeptides. Specifically, this pathway promotes the synthesis of neuropeptide Y in the arcuate nucleus (ARC) and orexin-A in the lateral hypothalamic area to stimulate feeding, while suppressing proopiomelanocortin (POMC) in the ARC and oxytocin in the paraventricular nucleus. Conversely, intracerebroventricular injection of lactate into the caudal fourth ventricle reverses these AMPK-dependent changes, inhibits hypoglycemia-induced feeding, and exacerbates glucose decline (Gujar et al. 2014).
Electrophysiological studies provide direct evidence for the interplay between lactate transport and neuronal activity. Using acute brain slice preparations and patch-clamp techniques, researchers demonstrated that blocking MCTs with α-cyano-4-hydroxycinnamate (4-CIN) suppresses the spontaneous firing of orexin neurons – even in the presence of extracellular glucose. This finding indicates that orexin neurons rely specifically on astrocyte-derived lactate to sustain their electrical activity during wakefulness. Furthermore, lactate modulates neuronal firing in a concentration-dependent manner, highlighting the dynamic coupling between lactate metabolism and neural excitability (Hwang et al. 2025; Parsons and Hirasawa 2010).
2.3 Lactate metabolism: energy utilization and waste disposal
Lactate is not only a source of “electricity” for neurons, but it also needs to be properly processed and cleared. Similar to how used electricity in a city must eventually be disposed of or converted into other forms of energy, lactate needs to be processed and cleared both intracellularly and extracellularly. Once lactate enters neurons, it participates in a series of metabolic processes to supply energy to the neurons.
In the brain, lactate metabolism involves two pathways: reuse and waste disposal. The lactate utilization pathway has two aspects: First, under aerobic conditions, lactate enters the mitochondria to participate in the tricarboxylic acid (TCA) cycle, generating ATP through OXPHOS. Additionally, some pyruvate can be directly converted into ATP via glycolysis, meeting the brain’s energy demands under various conditions (Sun et al. 2018). Second, lactate can serve as a precursor for the synthesis of other biomolecules, contributing to brain metabolism. For instance, during cognitive activities, lactate can be converted into acetyl-CoA, which influences the synthesis of fatty acids, cholesterol, and neurotransmitters.
However, when lactate accumulates excessively in the brain, it enters the bloodstream and is transported to the liver and kidneys. In the liver, lactate undergoes gluconeogenesis to produce glucose, while in the kidneys, it is partially oxidized or excreted in urine. Additionally, microglia and astrocytes in the brain gradually metabolize and clear lactate to maintain lactate homeostasis (Fazio-Eynullayeva et al. 2025; Jantti et al. 2022; Kann et al. 2025; Wang et al. 2022a,b).
Notably, lactate clearance also occurs via cerebrospinal fluid (CSF)-mediated glymphatic system transport. This process is state-dependent and dominated by the glymphatic system: during sleep, glymphatic activation enhances CSF flow under the regulation of astrocytic aquaporin-4 (AQP4), driving lactate transport from brain parenchyma to cervical lymph nodes for systemic metabolic clearance. In contrast, wakefulness or pathological states (e.g., sleep deprivation, AQP4 deficiency) impair CSF flow, reducing clearance efficiency and causing lactate accumulation. Future studies should further elucidate the regulatory mechanisms of the human glymphatic system and explore noninvasive interventions to optimize lactate clearance (Lundgaard et al. 2017; Liguori et al. 2016).
During these metabolic processes, there are significant differences in the expression and activity of key enzymes between neurons and astrocytes. Compared to neurons, astrocytes exhibit higher expression levels of LDHB, pyruvate dehydrogenase (PDH), and 6-phosphofructo-2-kinase (PFKFB3) (Kim et al. 2025). These differences are closely tied to lactate metabolism in the brain and align with the role of astrocytes in supporting and regulating neuronal functions.
The following is an analysis of the differences in the relevant enzymes between neurons and astrocytes:
Several studies have shown that LDH is expressed more in astrocytes than in neurons. In the human brain hippocampus and occipital cortex, immunohistochemical methods have confirmed that neurons contain only the LDH1 subunit, while astrocytes contain multiple LDH isoenzymes (Kim et al. 2025). Yao et al. (2023) also indirectly suggested this difference in their study on astrocyte regulation of depressive-like behaviors.
Under hypoxic or high-energy demand conditions, LDH is responsible for the interconversion of lactate and pyruvate in both neurons and astrocytes. It consists of five isoenzymes composed of the M subunit (encoded by LDHA) and the H subunit (encoded by LDHB), with catalytic activity proportional to LDH5/LDH1 (Mollo et al. 2018). In the brain, LDH1 is primarily present in neurons, utilizing lactate through OXPHOS, while LDH5 is mainly found in astrocytes, producing lactate via glycolysis (Zdralevic et al. 2018). With aging, changes in neuronal LDH levels affect survival rates and memory function, and changes in glial cell LDH levels are also associated with memory impairment and cell survival rates (Farmer et al. 2021).
PDH is primarily responsible for converting CoA to acetyl-CoA, which is a precursor for the TCA cycle, and its activity can be regulated by reversible phosphorylation. In astrocytes, PDH is highly phosphorylated, leading to lower activity, which helps divert pyruvate toward glycolysis and increases lactate production (Jeong et al. 2024). In neurons, PDH is primarily in an unphosphorylated, activated state, allowing pyruvate to enter the mitochondria and the TCA cycle, generating more ATP (Yang et al. 2024a,b). Defects in the PDH complex typically present as metabolic disorders, developmental delay, and dystonia, potentially exacerbating ND (Pavlu-Pereira et al. 2020).
PFKFB3 is a key enzyme regulating glucose metabolism. Under hypoxic or high-energy demand conditions, PFKFB3 is usually upregulated to increase F2,6BP levels, promoting glycolysis. In astrocytes, high levels of PFKFB3 accelerate glycolysis and generate large amounts of lactate. Additionally, the degradation rate of PFKFB3 is higher in neurons, which prevents PFKFB3 from maintaining high levels, thereby limiting glycolysis in neurons. As a result, neurons cannot acquire as much energy through rapid glycolysis as astrocytes (Wang et al. 2024a,b,c,d). In summary, PFKFB3 affects lactate production and metabolism by regulating glycolytic flux. Research has confirmed this reciprocal regulatory relationship between PFKFB3 and lactate.
Moreover, neurons and astrocytes differ not only in the expression and activity of key metabolic enzymes but also in the structure and composition of their mitochondrial complexes. In neurons, mitochondrial complexes are highly organized, which is crucial for efficient electron transfer. In contrast, in astrocytes, Complex I mostly exists in a “free” state, leading to weaker mitochondrial function and a lower respiratory rate compared to neurons. Therefore, astrocytes rely more on glycolysis to meet their energy demand (de Jager et al. 2025).
In summary, comparing lactate metabolism to a city’s energy supply system provides a clearer depiction of its crucial role in the brain. Just as the smooth operation of a city relies on a stable electricity supply, various physiological functions of the brain depend on the precise regulation of lactate. Disruptions in lactate metabolism can lead to dysfunction in this highly sophisticated organ. In the future, further exploration of the mechanisms underlying lactate function in the brain may offer new therapeutic insights for treating neurological disorders.
3 Lactylation
With the advancement of high-resolution liquid chromatography-tandem mass spectrometry (LC-MS), proteomic analysis leveraging this technology has successfully identified lactylation modifications. Lactylation is a PTM of proteins, where intracellular lactate, facilitated by enzymes or certain metabolic intermediates, attaches a lactyl group to lysine residues on proteins (Abdulrahaman 2023). This modification leads to structural and functional changes in proteins, influencing physiological processes such as gene expression and cellular metabolism. It plays a pivotal role in maintaining normal cellular functions and contributes to the onset and progression of diseases (Xu et al. 2021). The following section will discuss the mechanisms of lactylation in the brain and its regulatory aspects.
3.1 Molecular events of lactylation in the brain
The mechanisms of lactylation in the brain encompass several key aspects. At the molecular level, lactylation occurs in both histones and nonhistone proteins. In histone lactylation, lactyl groups are attached to lysine residues on histones, leading to chromatin structural modifications that play an essential role in neurodevelopment and other physiological processes in the brain. In contrast, nonhistone lactylation influences cellular signaling pathways and neurotransmitter synthesis by modifying nonhistone proteins.
3.1.1 Histone lactylation
3.1.1.1 The process of histone lactylation in the brain
The research team identified lactylation modification sites in the core histones H3 and H4 of murine bone marrow–derived macrophages and human HeLa cells using LC-MS. These sites were H3K18la and H4K5la. This study was the first to discover that lactylation of histones, derived from lactate, is a novel epigenetic alteration that can promote gene transcription (Zhang et al. 2019).
In brain ischemia and hypoxic conditions, as well as in scenarios where neuronal activity is enhanced leading to elevated lactate levels (either exogenous or endogenous), histone lactylation is triggered (Osadchiy et al. 2020). Neurons generate large amounts of l-lactate through glycolysis, and astrocytes, due to the Warburg effect, also produce significant amounts of l-lactate. The lactate is then transported into cells as a lactylation substrate through the expression of MCTs.
Once inside the cells, lactate is activated by LDH to form acetyl-CoA, which is subsequently transferred to lysine residues on histones under the catalysis of histone-modifying enzymes such as p300, completing the histone lactylation process (Wang et al. 2024a,b,c,d). This entire process is influenced by the metabolic profile, which helps maintain the dynamic balance of the body. Figure 2 shows the occurrence mechanisms of lactate and lactylation in the brain.
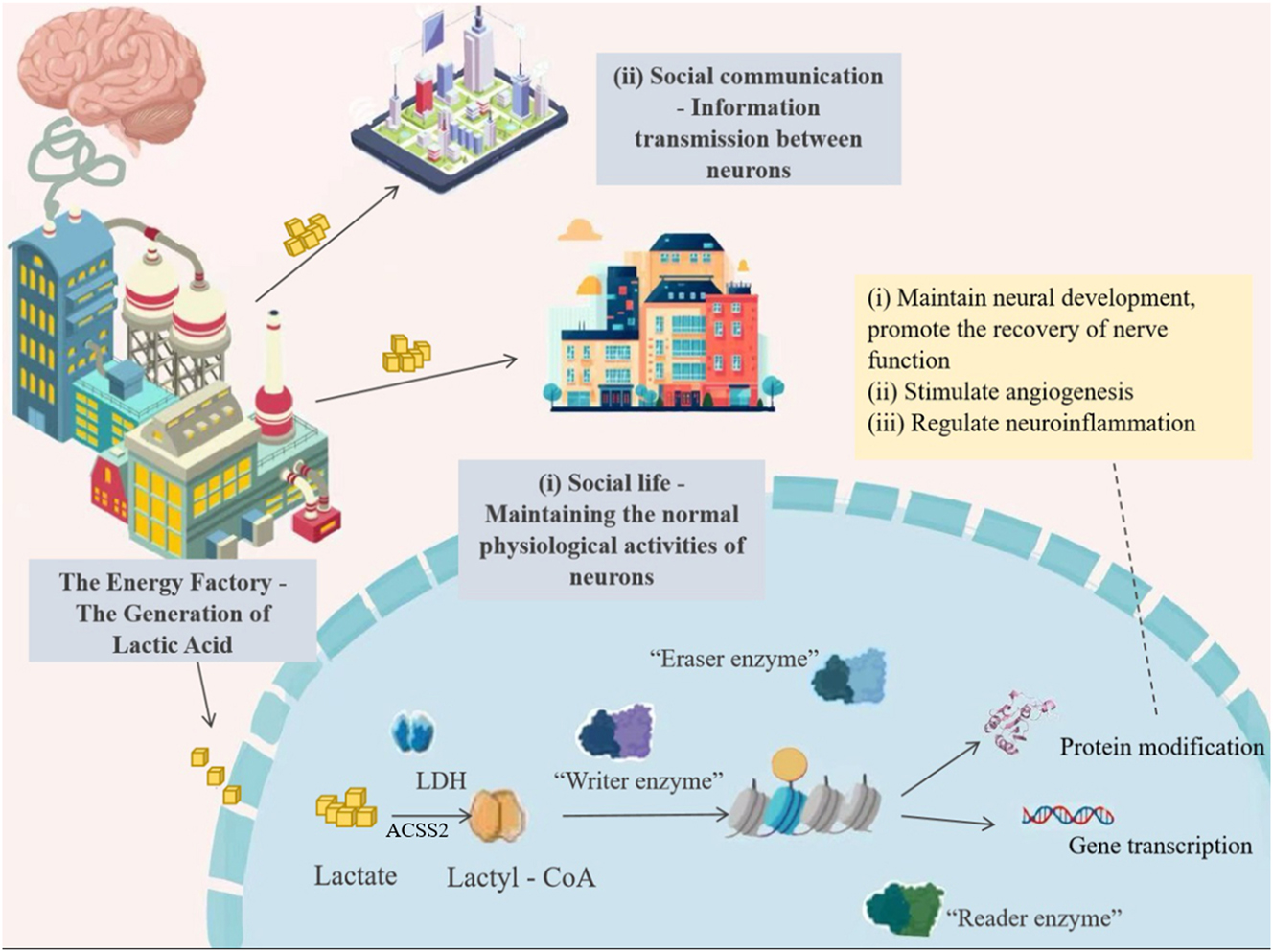
The occurrence of lactate and lactylation in the brain.
3.1.1.2 Functions of histone lactylation in the brain
Histone lactylation plays a crucial role in maintaining internal balance and regulating various pathophysiological processes. It may regulate protein function in two ways, thus participating in brain regulation. First, histone lactylation directly bind to promoter regions to promote or suppress the transcription of certain genes. Second, lactylation directly modifies proteins to regulate their biological activity (Pan et al. 2022; Wang et al. 2022a,b). The following are the functional roles of histone lactylation in the brain:
Maintaining Neurodevelopment and Promoting Neuronal Function Recovery: Recent studies have observed overall changes in histone lactylation during neurodevelopment, which are believed to play a role in this process (Dai et al. 2022; Tian and Zhou 2022; Yang et al. 2021). First, histone lactylation can regulate the differentiation of neural stem cells (NSCs) into various cell types, such as neurons, astrocytes, and oligodendrocytes. Studies have shown that Glis1 can modify chromatin by increasing H3K27Ac and H3K18la at pluripotency gene loci, keeping the chromatin regions of pluripotency-related genes like Oct4, Sox2, and Nanog in an open state, thereby promoting their expression and ensuring the self-renewal and multidirectional differentiation potential of stem cells (Dai et al. 2022; Li et al. 2020).
Moreover, the study by Li et al. (2025) revealed that lactate can target and regulate the activity of the MDM2-p53 signaling pathway via histone lactylation modification, specifically by promoting MDM2-mediated ubiquitination and degradation of p53. This process relieves p53-mediated inhibition of neural stem cell proliferation, thereby significantly promoting adult hippocampal neurogenesis. These findings strongly confirm the critical role of histone lactylation in regulating proliferation during neurodevelopment.
In neurodevelopment and memory formation in the central nervous system, BRD4 occupies a key position as an epigenetic regulator. Zhang et al. (2024a,b) discovered that BRD4 plays a decisive role in histone lactylation: targeted silencing of BRD4 in astrocytes significantly reduced H4K8la lactylation, which exacerbated A1 polarization in astrocytes, ultimately affecting the recovery and prognosis of neuronal function in mice.
Participation in Angiogenesis: In the brain, angiogenesis supports neuronal survival and function. Studies have shown that lactylation may help restore blood flow to damaged areas and promote neuronal recovery by stimulating angiogenesis (Hatakeyama et al. 2020).
In the realm of angiogenesis regulation, researchers have identified a critical regulatory loop in endothelial cells: the interplay between H3K9la and HDAC2 collaboratively modulates VEGF-induced angiogenesis. When H3K9la levels increase, they suppress HDAC2 expression, thereby epigenetically promoting the enrichment of genes such as NECTIN1, TGFBR2, ABL1, and PTGFR in pathways governing cell proliferation, migration, adhesion, and angiogenesis. These H3K9la-upregulated binding site-associated genes enhance the expression of proangiogenic protein-coding genes, contributing to VEGF-induced angiogenic processes. Conversely, elevated HDAC2 levels inhibit both H3K9la modification and angiogenesis (Fan et al. 2024).
Notably, lactylation exhibits bidirectional regulation of angiogenesis – it can exert the protective effects described above, but its aberrant activation may also drive pathological processes. Studies have shown that angiogenic abnormalities are common pathological features of various ischemic diseases. In ischemic stroke, lactylation serves as a potential bridge linking vascular pathological changes and disease progression (Zhang et al. 2025a,b). In an oxygen-induced retinopathy model, the C-terminal intrinsically disordered region of semaphorin 6A forms condensates via liquid–liquid phase separation, recruiting RHOA and P300 and activating their phosphorylation. This process promotes histone H3K9 and H3K18 lactylation in endothelial cells, further targeting PRMT5 to form a positive feedback loop of “lactate production-histone lactylation” that drives abnormal endothelial cell proliferation and pathological angiogenesis (Ma et al. 2025). In summary, lactylation regulates angiogenesis through epigenetic mechanisms and acts as a key regulatory node in pathological angiogenesis.
Based on these findings, targeting lactylation or modulating lactate levels represents a promising strategy to stimulate angiogenesis and facilitate brain tissue repair.
Regulation of Neuroinflammation: Emerging evidence highlights the critical role of lactylation in both acute and chronic inflammatory processes. As a multifunctional mediator, lactylation can either promote or inhibit inflammation, depending on the metabolic characteristics and the types of cells involved (Qin et al. 2025).
In immune cell regulation, histone lactylation in mast cells promotes the expression of IL-10, which in turn suppresses the activity of various immune cells, including macrophages and dendritic cells, reducing their secretion of proinflammatory cytokines (De Leo et al. 2024).
The functional transformation of macrophages is also closely related to histone lactylation. Notably, L-lactylation and D-lactylation exhibit different effects: L-lactylation promotes the polarization of macrophages to the anti-inflammatory M2 phenotype, while D-lactylation is associated with an increase in the production of inflammatory cytokines in macrophages (Trujillo et al. 2024). After inflammatory injury, the fragmented mitochondrial phenotype plays a crucial role in macrophage prodegradative responses. This mechanism involves histone lactylation specifically driving epigenetic changes, which lead macrophages to transition from a proinflammatory state to a proresolving state (Susser et al. 2023).
The B-cell adapter for phosphoinositide 3-kinase (BCAP) determines the transformation of reparative macrophages. In the absence of BCAP, defects in aerobic glycolysis and reduced lactate production occur, leading to a decrease in histone lactylation levels, and the gene expression of reparative macrophages is subsequently reduced (Irizarry-Caro et al. 2020).
Additionally, in a neurological study, Yao et al. (Yao and Li 2023) revealed that lactate dehydrogenase A (LDHA) mediates histone lactylation by targeting high-mobility group box 1 protein (HMGB1), thereby inducing pyroptosis – a proinflammatory programmed cell death – in neurons.
3.1.2 Nonhistone lactylation
The identification of 350 “LactoylLys”-modified proteins revealed lactate modification of lysine residues in nonhistone proteins, including metabolic enzymes, epigenetic regulators, cytoskeletal proteins, and signal transduction–related proteins. This nonhistone lactylation process uses lactate as a substrate, where the carboxyl group of lactate forms an amide bond with the amino group of specific amino acid residues in nonhistone proteins under the action of enzymes such as lactate dehydrogenase. This modification can regulate gene transcription, alter protein localization and function, participate in cell signaling, and influence brain neural activity (Gaffney et al. 2020).
Yan et al. (2024) found that lactate produced during exercise promotes lactylation of several synaptic proteins in the brain. For example, when SNAP91 in mice undergoes lactylation at the K885 site, neuronal excitability decreases, synaptic transmission function is impaired, and the mice exhibit anxiety-like behaviors. This study emphasizes the importance of nonhistone lactylation in the regulation of brain neural activity.
However, given the current insufficient research on nonhistone lactylation in the brain, further in-depth studies are needed in the future to comprehensively analyze the complex regulatory mechanisms of nonhistone lactylation in the brain, providing new theoretical insights and intervention targets for the prevention and treatment of neurological diseases.
3.2 Lactylation regulatory code in the brain
Lactylation, as a dynamic process, is finely regulated by multiple factors. The regulation of lactylation is divided into enzyme-catalyzed lactylation and nonenzymatic lactylation. The former is catalyzed by specific enzymes that attach lactyl groups to lysine residues in proteins, and this process is regulated by intracellular signaling pathways. The latter refers to the lactylation modification occurring through nonspecific chemical reactions at high concentrations of lactate. The following section will delve into the regulatory mechanisms and specific processes of lactylation in the brain environment:
3.2.1 Enzyme-catalyzed lactylation
Lactylation is an epigenetic modification regulated by lactate levels. Lactate can generate lactoyl-CoA, which then adds lactoyl groups to the lysine residues of histones. This process is regulated by various enzymes, including “writers,” “erasers,” and “readers.”
“Writers”enzymes: During the enzyme-catalyzed lactylation translation process, key enzymes act as “writers” and play a central catalytic role. These enzymes utilize amino acid residues in their active sites to form specific interactions with substrates, facilitating the transfer of the lactyl group from the donor to a specific lysine residue on the protein, thus completing the lactylation modification. This process is highly specific and influenced by intracellular environmental factors, such as metabolic status, pH, and other signaling molecules, precisely regulating gene transcription and cellular functions (Alkhammash 2025).
In the catalytic process of histone lactylation, a series of key enzymes known as “writers” exist, including p300, CBP, HBO1, KAT5, KAT8, AARS1/2, and others. Among these, p300 was the first enzyme identified with lactylation-writer activity. It participates in the lactylation modification of proteins like HMGB and plays an important role in regulating the function of related proteins (Liu et al. 2024).
Furthermore, studies have reported the unique role of HBO1 in histone lactylation. It catalyzes H3K9la-mediated gene transcription and identifies E508 as the key site for HBO1’s lactyltransferase activity. HBO1’s activity on free H3, H4, and nucleosomal H3 is regulated by BRPF2, and its short N-terminal region can bind to HBO1, enhancing its activity on H3K14. KAT5 and KAT8 primarily regulate the levels of nonhistone lactylation (Yang et al. 2022).
“Eraser” enzymes: Eraser enzymes are a class of enzymes capable of removing histone modification marks. They can specifically recognize lactylation-modified substrates and then, relying on their own catalytic domains, remove lactyl groups through hydrolysis, achieving delactylation and maintaining intracellular lactylation balance (Fan et al. 2023; Kapadia et al. 2025).
Studies have found that HDACs (histone deacetylases) function as histone lysine delactylases. They are classified into four categories based on structure and function: Class I includes HDAC1-3 and HDAC8, mainly located in the nucleus, regulating the cell cycle and gene transcription; Class II is divided into IIa (HDAC4, 5, 7, 9) and IIb (HDAC6, 10), which participate in cell differentiation and cytoskeletal regulation; Class III refers to Sirtuins (SIRT1-7), which are NAD+-dependent and associated with metabolism; Class IV contains only HDAC11, which is involved in immune regulation (King et al. 2021).
In vitro experiments have shown that HDAC1-3 can regulate histone lactylation at multiple sites, significantly reducing H3K18la and H4K5la (Zhang et al. 2019).
SIRT1 and SIRT3, as NAD+-dependent deacetylases, exhibit unique regulatory mechanisms and substrate specificity. For instance, ENO1 at K228la is regulated by SIRT1/SIRT3, while pyruvate kinase M2 (PKM2) at K207la is uniquely regulated by SIRT1. Additionally, SIRT1-3 also exert a certain degree of reduction on H3K18la and H4K5la (Du et al. 2024).
“Reader” enzymes: In lactylation modification, there also exist proteins or enzymes that function as “readers,” which specifically recognize and bind to lactylation-modified protein molecules. These readers play a crucial role in recognizing and interpreting lactylation modification signals, thereby regulating related biological processes. Recent research on reader enzymes includes the following:
Li et al. (2024) discovered that AARS1 and AARS2 can read and sense l-lactate accumulation, thereby mediating protein lactylation. Among them, AARS1 directly bind to lactate and catalyzes lactylation in an ATP-dependent manner rather than relying on lactyl-CoA, mediating lactylation modification of p53 in the DNA-binding domain. AARS2, under high lactate conditions, interacts with cyclic GMP-AMP synthase (cGAS) and directly mediates lactylation modification at the N-terminal of the cGAS protein, thereby inhibiting its ability to bind DNA and produce cGAMP (Zong et al. 2024).
Zhai et al. (2024) using CUT&Tag analysis combined with proteomics technology found that H3K14la and DPF2 are enriched at transcription start sites and adjacent regions, identifying DPF2 as a potential reader of H3K14la.
At the same time, the GAS41 protein, which contains a YEATS domain, can specifically recognize H3K27la. Abnormal recognition of H3K27la may lead to abnormal expression of related genes, thereby playing a crucial role in disease development (Wang et al. 2024a,b,c,d).
In addition, proteins such as BRD4, which contain bromodomain structures, are not only capable of recognizing acetylation modifications but may also play a role in recognizing lactylation modifications (To et al. 2023).
3.2.2 Nonenzymatic lactylation
Nonenzymatic lactylation refers to the modification of proteins through lactylation via nonspecific chemical reactions at high lactate concentrations. Unlike enzymatic reactions, it lacks strict substrate specificity and has a broader impact.
In nonenzymatic lactylation, the methylglyoxal metabolic byproduct lactoyl-glutathione can directly induce D-lactyl-lysine modification of proteins. The specific reaction mechanism involves SLG first forming a reversible S-lactylated thiol intermediate with cysteine, followed by the transfer of the lactyl moiety to proximal lysine residues, which can inhibit inflammatory responses (Zhao et al. 2025). Additionally, in neurological diseases, loss of the low-density LRP1 in astrocytes leads to increased lactate production, promoting K73 lactylation of ARF1, thereby reducing mitochondrial release in astrocytes (Zhou et al. 2024).
In summary, lactylation regulation in the brain exhibits diverse characteristics. On one hand, it relies on specific enzymes to precisely mediate lactylation modifications of biomolecules, thereby regulating gene transcription. On the other hand, changes in cellular metabolic states can alter lactate concentrations. When cellular metabolism is active and lactate production increases, even without direct enzymatic involvement, lactate can still influence metabolic regulation. The synergy between these two mechanisms maintains homeostasis and normal physiological functions in the brain, while their imbalance can lead to diseases.
4 Lactate and lactylation impact the pathogenic mechanisms of neurodegenerative diseases
In recent years, with the continuous advancement of research in the field of neuroscience, a large body of research has shown that lactate and its derivative modification, lactylation, play key roles in the pathological progression of ND. Next, we will analyze the specific roles of lactate and the imbalance of lactylation in the mechanisms of disease development. Figure 3 illustrates how lactate and lactylation impact the pathogenic mechanisms of ND.
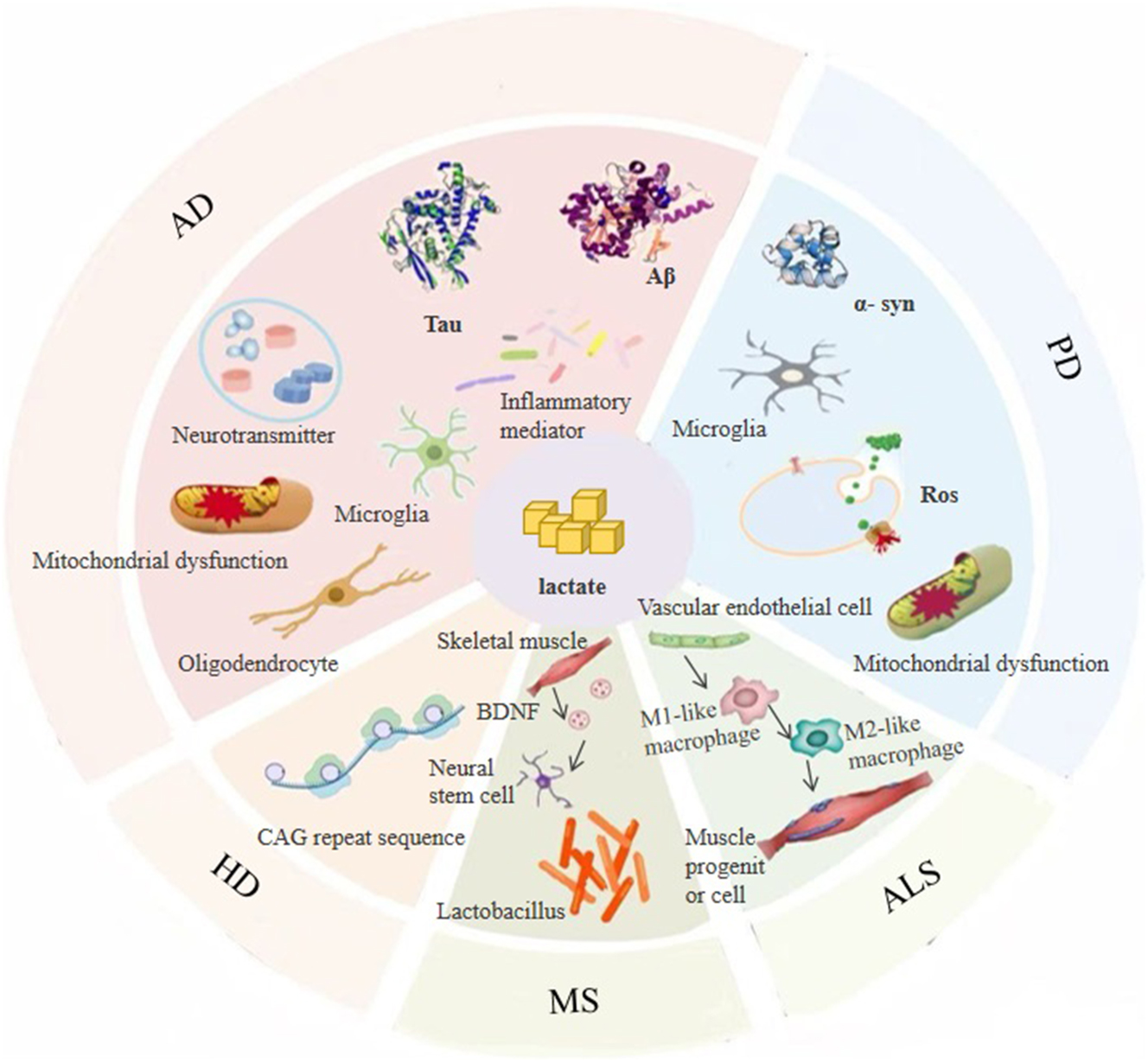
Lactic acid and lactylation impact the pathogenic mechanisms of neurodegenerative diseases.
4.1 Alzheimer’s disease (AD)
The pathogenic mechanisms of AD are complex: the abnormal accumulation of amyloid-beta (Aβ) forms plaques, tau protein is hyperphosphorylated to form neurofibrillary tangles (NFTs), and neuroinflammation, mitochondrial damage, and synaptic dysfunction all contribute to disease progression. Notably, research has found that abnormalities in lactate metabolism are closely related to these mechanisms. Imbalances in lactate levels and lactylation in the brain can disrupt energy metabolism, impair intracellular homeostasis, and affect the structural stability and function of related proteins, collectively promoting disease exacerbation. Below, we will analyze the potential pathogenic mechanisms by which lactate influences AD.
4.1.1 Aβ amyloid protein deposition
Aβ is generated by the cleavage of amyloid precursor protein (APP), and the enzyme that produces Aβ, β-secretase (BACE1), is most active under low pH conditions (Hussain et al. 2025). The accumulation of lactate within cells lowers the intracellular pH, which affects BACE1 activity and subsequently alters the processing of APP to generate Aβ.
Research by Xiang et al. (2010) demonstrated that sustained increases in lactate levels led to elevated Aβ40 and Aβ42 levels in cell culture media, while reducing APP metabolites (such as sAPPα levels). Additionally, it increased the interaction of APP with ER chaperones in the perinuclear region, promoting the aggregation of amyloid peptides and APP, which in turn induced neuronal pyroptosis. This study reveals the mechanisms by which lactate levels affect APP metabolism and Aβ production.
Furthermore, Vestigial-like family member 4 (VGLL4) has been recognized as a key regulator of the hypoxia-sensing pathway. In AD, upregulation of VGLL4 enhances the response of LDH A to hypoxia, thereby increasing the production of lactate in the brain. The use of sodium oxalate, an LDHA inhibitor, to suppress lactate production exacerbated brain energy deficits and Aβ accumulation, thereby inhibiting the neuroprotective function of VGLL4 (Tian et al. 2023).
Based on previous research, proteomics and mass spectrometry analysis identified K612 as a key site for APP lactylation. The modification of this site impacts the processing of APP amyloid proteins. In vitro experiments showed that APP-K612la could influence endosome–lysosome transport and the production of APP metabolism-related genes. In in vivo experiments, researchers observed the effects after injecting different viruses into the hippocampus of wild-type and PS45 mice. The results showed that the number of Aβ plaques and the levels of Aβ40 and Aβ42 in the APP-K612T group were lower than those in the APP-swe695 group, demonstrating that APP-K612T could reduce Aβ production in vivo. Furthermore, the APP-K612T group showed better performance in brain slices and maze tests, indicating improved synaptic and memory function. Additionally, in APP23/PS45 mice, the group treated with l-lactate showed better results on relevant indicators compared to the control group, suggesting that l-lactate could modulate APP lactylation, reduce Aβ pathology, and repair spatial learning and memory deficits (Tian et al. 2025).
4.1.2 Overphosphorylation of tau protein
Tau protein is a microtubule-associated protein in neurons that normally stabilizes microtubule structures, maintaining axonal transport and synaptic function. In AD, multiple kinases (such as CDK5, GSK-3β) are excessively activated, leading to abnormal phosphorylation of tau protein, which subsequently aggregates into NFTs, ultimately resulting in synaptic dysfunction (Kabir et al. 2022).
Isocitrate dehydrogenase (IDH3β) is one of the rate-limiting enzymes in the TCA cycle and plays a critical role in the pathological process of tau protein hyperphosphorylation. Studies have found that decreased production of IDH3β increases histone lactylation and promotes the expression of PAX6. When PAX6 expression is elevated, it binds to the binding sequence in the IDH3β gene promoter, further inhibiting the expression of IDH3β, exacerbating its reduction. The continuous decrease in IDH3β further increases histone lactylation, which in turn promotes PAX6 expression, forming a vicious cycle. This leads to excessive tau phosphorylation, synaptic damage, and learning and memory deficits, driving the progression of AD (Wang et al. 2024a,b,c,d). Therefore, upregulating IDH3β or downregulating PAX6 could break this cycle and mitigate neurodegeneration and cognitive impairment in AD. Furthermore, lactylation modifications of tau protein (e.g., tauK677la) are also associated with ferroptosis and synaptic degeneration (Kontaxi et al. 2017).
Abnormal changes in the cytoskeleton play a key role in the pathogenesis of AD, and tau protein is a critical factor causing cytoskeletal abnormalities. Overphosphorylation of tau protein leads to microtubule disassembly, disrupting the cytoskeletal structure, hindering axonal transport, and causing a reduction in dendritic spines and morphological changes, ultimately affecting neuronal signaling. Research focusing on PTMs of microtubule proteins has found that HDAC6 can catalyze α-tubulin lactylation with lactate, promoting the growth and branching of dendrites in cultured hippocampal neurons, thereby influencing the construction and function of neural circuits (Sun et al. 2024).
4.1.3 Neuroinflammation
In AD, neuronal damage and abnormal protein aggregation are critical initiating factors. These pathological changes activate astrocytes, leading to excessive lactate production. Excess lactate can bind to specific receptors or alter intracellular pH, triggering the release of inflammatory mediators, which in turn initiate an inflammatory response and recruit immune cells, further exacerbating neuroinflammation (Xiong et al. 2024).
Additionally, lactylation modifications influence histone-related inflammatory protein functions, altering chromatin conformation and thereby regulating neuroinflammatory responses (Xie et al. 2022). These effects are reflected in several key mechanisms:
Regulation of Inflammatory Mediator Release: In the brain tissue of AD patients, NF-κB signaling pathway is frequently abnormally activated. One reason is that lactylation modifications can influence NF-κB activation and nuclear translocation while also affecting other key molecules in the NF-κB signaling pathway, such as IKK and inhibitor of IκBα. This regulation ultimately modulates the expression of cytokines and inflammatory factors, promoting the release of inflammatory mediators (Tang et al. 2025).
Based on this mechanism, studies have shown that inhibitors of glycolysis and the Krebs cycle can reverse altered glycolytic parameters in the brain and mitigate neuroinflammation. Specifically, these inhibitors suppress the expression of S100B and IL-1β, inhibit microglial activation, and reduce the assembly of NLRP3 inflammasome (Vizuete et al. 2022).
During inflammation, NEK7 is a crucial component in activating the NLRP3 inflammasome. Research has confirmed that NEK7 expression is significantly increased in both the APP/PS1 transgenic mouse model and Aβ-treated BV-2 microglial cell models. Suppressing NEK7 expression enhances histone lactylation in BV-2 cells, thereby promoting pyroptosis (Cheng and Zhao 2024).
However, lactate can also inhibit inflammatory signal transduction under specific conditions. For instance, Liang et al. found that l-lactate suppresses LPS-induced changes in key inflammatory factors, including TNF-α, IL-1β, p-NF-κB, and the NLRP3 inflammasome complex (NLRP3/ASC/caspase-1) (Liang et al. 2024). This finding suggests that lactate plays a complex dual role in inflammation regulation, with its specific effects depending on varying physiological environments and conditions.
Regulation of Neuroglial cells: Lactate and lactylation not only regulate inflammation through signaling pathways such as NF-κB but also amplify neuroinflammatory processes by modulating metabolic reprogramming and proinflammatory gene expression in neuroglial cells.
Astrocytes: In the brain, lactate generated by aerobic glycolysis in astrocytes serves as a substrate for epigenetic modification, regulating gene transcription via histone lactylation and playing a critical role in neuroinflammatory progression (Yang et al. 2024a,b).
A study first elucidated the regulatory mechanism of the H3K18la/NOD2 axis in astrocyte pyroptosis and neuroinflammation. It revealed that unconjugated bilirubin stimulation enhances astrocytic glycolysis, leading to significantly elevated H3K18la levels in primary astrocytes and the hippocampus of bilirubin encephalopathy rats. H3K18la enriches in the promoter region of the NOD2 gene, promoting its transcription and activating MAPK and NF-κB signaling pathways, ultimately exacerbating neuroinflammatory responses. Additionally, glycolysis inhibition reduces H3K18la levels, alleviates astrocyte pyroptosis, and mitigates neuronal damage to improve cognitive function (Li et al. 2025).
Another study explored the association between astrocyte polarization and histone lactylation. It found that pathological conditions enhance astrocytic glycolysis and lactate production. As an epigenetic regulator, BRD4 mediates histone lactylation; when BRD4 is silenced, H4K8la decreases significantly, promoting astrocyte polarization toward the neurotoxic A1 phenotype. This triggers the release of proinflammatory factors (e.g., IL-1β, TNF-α), leading to neuronal death and impairing neurological functional recovery (Zhang et al. 2024a,b). Collectively, these findings indicate that lactate from astrocytic glycolysis precisely regulates inflammation-related gene expression via histone lactylation, driving disease progression.
Microglial Regulation: Studies have shown that microglia can polarize into proinflammatory or anti-inflammatory phenotypes in response to different stimuli. Proinflammatory microglia tend to rely on glycolysis, while anti-inflammatory microglia predominantly depend on OXPHOS and fatty acid oxidation (Fekih-Romdhane et al. 2020).
After LPS-induced neuroinflammation, astrocytic glycolytic activity is enhanced, characterized by increased glucose uptake, elevated PFK1 activity, and lactate release. Lactate produced by astrocytic glycolysis acts as a metabolic signaling molecule for microglia, functioning via activation of the GPR81 receptor. Meanwhile, it sustains proinflammatory cytokines (e.g., IL-1β, S100B) to reciprocally aggravate neuroinflammation, forming a vicious cycle of “neuroinflammation-glycolysis enhancement-inflammation maintenance” (Vizuete et al. 2022).
For instance, in brain samples from AD patients and 5xFAD transgenic mice, elevated histone lactylation levels in microglia have been observed. For example, H4K12la accumulates in microglia near Aβ plaques, activating the transcription of glycolytic genes. The resultant lactate further promotes histone lactylation, establishing a “PKM2” positive feedback loop, which exacerbates microglial dysfunction and triggers neuroinflammation (Pan et al. 2022). Notably, studies have found that activation of transient receptor potential vanilloid 1 reduces H4K12la expression in microglia, thereby alleviating disease progression (Lu et al. 2021).
However, recent research suggests that lactate does not solely play a proinflammatory role. Polymorphonuclear myeloid-derived suppressor cells generate lactate through HIF-1α-mediated glycolysis, and this lactate can reduce microglial activation and the expression of inflammatory factors. This finding implies that lactate may have anti-inflammatory effects in neuroinflammation-related diseases (Zhang et al. 2025a,b).
Oligodendrocytes: Lactate plays a crucial regulatory role in oligodendrocyte development and myelination, functioning both as an energy substrate and as a supporter of myelin and lipid synthesis (Chen and Zhu 2025).
Generally, oligodendrocytes produce lactate and transfer it to neurons via MCTs and gap junctions, where it is used for ATP synthesis, providing energy support for neurons. However, in the AD environment, inflammation may disrupt the normal function of oligodendrocytes. Studies have shown that inflammatory conditions can affect oligodendrocyte metabolism and lactate production, thereby impairing myelination, making neurons more vulnerable, and indirectly promoting inflammation (Tiwari et al. 2024).
Meanwhile, neurons regulate oligodendrocyte mitochondrial metabolism, particularly the TCA cycle and ATP production, through the derivative N-acetylaspartate. Disruption of MCT1 on oligodendrocytes leads to axonal damage and neuronal loss, exacerbating the inflammatory microenvironment in AD (Brown and Lad 2019).
Regulation of Immune Cells: Additionally, in the field of immune cell regulation, lactate and lactylation also play critical roles in inflammatory regulation.
As mononuclear phagocytes with powerful phagocytic and immunomodulatory functions, macrophages have been found to undergo lactate modification through multiple mechanisms to promote their polarization toward an anti-inflammatory phenotype:
At the metabolic transport level, MCT4 deficiency enhances p300-mediated histone lactylation (H3K18la), activating the expression of anti-inflammatory genes such as Arg1 and TCA cycle genes, driving macrophages toward a reparative phenotype. The therapeutic potential of this mechanism has been validated by the MD-43 molecule designed via PROTAC technology in in vivo models (Zhang et al. 2024a,b);
Furthermore, lactate can lactylate the K62 site of PKM2, inhibiting its dimerization, reducing nuclear distribution, and suppressing glycolytic levels, thereby promoting the transformation of proinflammatory macrophages into reparative phenotypes (Xu et al. 2024);
At the signaling pathway regulation level, high serum lactate can suppress TLR4 and NLRP2 signaling through the GPR81 pathway, interfere with the NF-κB-IL-1β-Casp1 axis, and induce YAP phosphorylation via the AMPK pathway to promote the expression of phagocytosis-related proteins (such as CD36 and MerTK), supporting macrophage polarization toward an anti-inflammatory phenotype (Llibre et al. 2025).
The above studies have revealed the epigenetic mechanism by which macrophages regulate anti-inflammatory phenotypes through lactylation modification, while the glycolytic metabolic regulation of immune cells exhibits a broader role in neuroinflammation. Kuang et al. (Kuang et al. 2024) discovered that Uncaria alkaloids (URA) inhibit glycolysis, reducing lactate production and the expression of hexokinase 2 and glucose transporter 1 in concanavalin A–induced naïve T cells. Through modulation of the PI3K/Akt/mTOR signaling pathway, URA inhibits CD4+ T-cell–mediated neuroinflammation, thereby improving AD pathology in APP/PS1 mice. This discovery provides new potential pharmacological and therapeutic approaches for the treatment of AD.
4.1.4 Mitochondrial dysfunction
As the “powerhouse” of the cell, mitochondria play a crucial role in cellular respiration. Mitochondrial dysfunction impairs aerobic respiration, reduces ATP production, disrupts cellular energy metabolism, and enhances anaerobic glycolysis, leading to lactate accumulation. Excessive lactate promotes aberrant lactylation modifications at the transcriptional level, which relax chromatin structure to activate gene transcription, thereby increasing mRNA expression of key protein-encoding genes and interfering with their normal functions (Hu et al. 2024; Zhang et al. 2019). This ultimately results in mitochondrial membrane potential imbalance and respiratory chain dysfunction. Meanwhile, this process generates more reactive ROS, exacerbating oxidative stress and further damaging mitochondria, thereby forming a vicious cycle. Under this continuous impact, cellular physiological functions deteriorate, eventually leading to cell damage or even death.
ALDH2 is an enzyme that plays an essential role within mitochondria. Studies have found that ALDH2 lactylation promotes the ubiquitination-proteasomal degradation of prohibitin 2, thereby inhibiting mitochondrial autophagy. However, SIRT3, which acts as a delactylation enzyme for ALDH2, can reduce ALDH2 lactylation levels when overexpressed, thereby alleviating mitochondrial dysfunction (Zhou et al. 2025).
Furthermore, research using Schwann cell (SC)-specific Rheb gene knockout mice has demonstrated that Rheb-regulated mitochondrial pyruvate metabolism is critical for the noncell-autonomous regulation of SC-mediated peripheral axon stability. Rheb gene deletion inhibits PDH activity, shifting pyruvate metabolism toward lactate production. The resulting increase in lactate leads to age-dependent peripheral axonal degeneration, ultimately affecting peripheral nerve function. In this study, lactate acted as both an energy substrate and a potential signaling molecule, enhancing neuronal mitochondrial metabolism and peripheral nerve energy production. However, prolonged elevation of neuronal lactate metabolism also increased ROS generation, ultimately damaging mitochondria (Jia et al. 2023).
In the brains of AD patients, Aβ deposition and excessive tau protein phosphorylation interact with mitochondrial dysfunction, collectively influencing the process of cell death.
4.1.5 Imbalance of neurotransmitter systems
In AD patients, neurotransmitter system imbalance is characterized by dysfunction of the acetylcholine system, glutamatergic system, GABAergic system, and monoamine neurotransmitter system, which significantly impairs cognition and behavior. Lactate, acting as a signaling molecule, regulates neuronal excitability. Studies have found that lactate activates hypothalamic POMC neurons. Specifically, l-lactate induces depolarization of POMC neurons via Gαi/o protein-coupled receptors. Partial depolarization is sensitive to 4-CIN (an inhibitor), suggesting that l-lactate directly depolarizes neurons intracellularly. HCAR1 is localized in astrocytes. Under high-energy conditions, lactate modulates POMC neuron activity through two pathways: (1) direct effects via MCT2-mediated neuronal entry and (2) indirect astrocytic signaling via HCAR1 binding (Ordenes et al. 2021).
Han et al. (2024a; b) further reported lactate’s role in neuropathic pain and memory deficits. In animals with sciatic nerve injury and comorbid nociceptive sensitization or memory deficits, dorsal CA1 lactate levels were reduced. Exogenous lactate supplementation or chemogenetic astrocyte activation to increase endogenous lactate release alleviated comorbidities and enhanced cellular excitability and postsynaptic potentials.
4.2 Parkinson’s disease (PD)
The pathogenesis of PD primarily involves dopaminergic neuron degeneration, resulting in reduced striatal dopamine levels and symptoms like bradykinesia and tremor. It is also linked to mitochondrial dysfunction, oxidative stress, neuroinflammation, and abnormal α-synuclein (α-syn) aggregation. Disruption of MCT1 or MCT4 impairs motor endplate innervation, a pathological hallmark in PD, suggesting lactate metabolism influences PD pathogenesis. Based on this, it is not difficult to infer that abnormal lactate metabolism affects the pathogenic mechanism of PD. The following is an analysis of relevant research.
4.2.1 Abnormal α-syn aggregation
Lactate levels significantly increased in the substantia nigra pars compacta of MPTP-induced mice and MPP+-treated SH-SY5Y cells (Ali and Dholaniya 2022, Li et al. 2022). Elevated lactate levels may have dual effects in PD: On one hand, lactate supplementation in MPP+-treated cells induces mitophagy, restoring mitochondrial activity and protecting neurons (Fedotova et al. 2022, Komilova et al. 2022). On the other hand, lactate activates AMPK, leading to persistent α-syn accumulation and α-syn in PD neurons, ultimately inducing dopaminergic neuron death (Li et al. 2022). Studies have confirmed these findings. Excessive lactate accumulation causes cellular hyperosmolarity, which specifically induces human α-syn aggregation in a cell-dependent manner compared to Tau (Fragniere et al. 2019).
Furthermore, gut microbiota are linked to lactate and α-syn aggregation in PD. PD patients exhibit increased Lactobacillus abundance, and transplantation of PD gut microbiota into mice induces gut inflammation and α-syn aggregation (Munoz-Pinto et al. 2021), suggesting a gut–brain axis–mediated effect.
Yan et al. (2024) showed that inhibiting lactylation of SNAP91 at lysine 885 via site-directed mutagenesis disrupted presynaptic vesicle density, synaptic proteins, and postsynaptic density formation, highlighting lactylation’s critical role in synaptic structure and function.
4.2.2 Neuroinflammation
Lactate plays a dual role in PD neuroinflammation. Microglial activation is a critical mechanism of dopaminergic neuron death in PD, and lactate injection inhibits neuroinflammation by suppressing classical microglial polarization, indicating neuroprotective potential of higher lactate levels. Conversely, in primary microglia, lactate may enhance proinflammatory mediator production (Han et al. 2024a; b).
SIRT1 alleviates PD neuroinflammation by inhibiting NF-κB, regulating microglial polarization, and protecting neurons, whereas PKM2 exacerbates inflammation through cytokine production, metabolic-inflammatory interactions, and immune cell modulation. The precise roles of SIRT1 and PKM2 and their interplay in PD remain unclear. Intracerebral lactate administration recapitulates PD-like phenotypes similar to SIRT1-knockdown mice, while PKM2 inhibition alleviates PD symptoms (Yan et al. 2024), suggesting SIRT1 reduces PD progression by post-translationally modifying PKM2 to inhibit lactate production and neuroinflammation.
In PD patients, worsening neuroinflammation correlates with increasing CSF lactate levels and advancing PD onset age, implying a parallel relationship between neuroinflammation and lactate.
4.2.3 Mitochondrial dysfunction
Lactate and mitochondria are critically linked, with studies across multiple dimensions revealing their interaction’s impact on disease progression. First, lactate protects mitochondrial function: in PD, lactate incubation induces mitophagy and autophagy, restoring mitochondrial activity and preventing necrosis and apoptosis (Nowag et al. 2024, Wang et al. 2018). Translocator protein (TSPO) balances mitochondrial OXPHOS and glycolysis: TSPO deficiency increases fragmentation, lactate conversion, and reduces OXPHOS (Fu et al. 2020). Circulating N-lactoyl amino acids and N-formylmethionine serve as mitochondrial dysfunction biomarkers, with N-lactoyl amino acids reflecting lactate metabolism impairment. Abnormal lipid metabolism triggers mitochondrial/endoplasmic reticulum dysfunction and ferroptosis in PD, and lactate transporter MCT modulates neuronal lipid metabolism (Liu et al. 2017), suggesting lactate transport links to mitochondria via metabolism.
Notably, lactate-mitochondria research is also advancing in other neurological diseases. In mouse traumatic brain injury models and stretch-injured neuronal cells, lactylation increases. Lactylation of Tufm protein inhibits its interaction with TOMM40, suppressing mitophagy and promoting neuronal apoptosis (Weng et al. 2025). In ischemic brain injury models, astrocytic LRP1 deletion promotes glucose uptake and lactate production, with lactate dose-dependently inhibiting astrocytic mitochondrial export. Additionally, LRP1 deletion alters ARF1 lactylation at position 73; promoting ARF1 lactylation reduces astrocyte-to-neuron mitochondrial transfer, exacerbating disease progression (Zhou et al. 2024).
In H2O2-induced oxidative stress cells and sodium iodate-induced retinal degeneration models, lactate pretreatment alleviated cell death and degeneration by activating autophagy (via LC3II/I ratio, LC3 puncta, autophagic vacuoles) and preventing mitochondrial fission. This effect is attenuated by 3-methyladenine (Zou et al. 2023).
4.2.4 Oxidative stress
In a diseased state, mitochondrial dysfunction in cells disrupts the electron transport chain and causes disorders in metal ion metabolism, leading to the generation of excessive ROS, which results in oxidative stress (Tapias et al. 2023).
Taking SH-SY5Y cells as an example, studies have found that l-lactate can promote the cell defense mechanism. For instance, it can activate the unfolded protein response and nuclear factor erythroid 2–related factor 2. Specifically, l-lactate can induce a mild ROS burst in cells, which in turn triggers the gene transcription of prosurvival pathways (such as the IGF-Akt/PI3K pathway and the endoplasmic reticulum stress pathway, etc.) (Tauffenberger et al. 2019).
On the other hand, Lu et al. (2015) discovered that the Warburg effect in cancer cells restricts the entry of pyruvate into the mitochondria for oxidative metabolism, preventing the excessive generation of ROS. This enables cancer cells to acquire the ability to resist anoikis and gain a metastatic advantage.
However, the study did not mention whether there is a similar situation between PD and the Warburg effect in cancer cells in terms of restricting the entry of pyruvate into the mitochondria for oxidative metabolism. In the current research field, the influence of lactate on the occurrence and development of diseases through oxidative stress has not been fully explored. In view of this, it is urgent to focus on this direction in the future to explore new ideas and approaches for the prevention and treatment of ND.
4.3 Amyotrophic lateral sclerosis (ALS)
ALS is a progressive and fatal ND affecting motor neurons. Aging, a key ND risk factor, disrupts fibroblast metabolism in ALS: Age-related metabolic profiles differ between ALS patients and controls, with controls showing age-dependent NADH metabolism in the presence of lactate (absent in ALS). ALS fibroblasts exhibit aging-related energy substrate metabolic defects, validated in induced neuronal progenitor-derived iAstrocytes (Gerou et al. 2021).
Multiple studies link lactate to ALS. A study of 116 patients found a potential linear relationship between serum lactate elimination rate (ER) and motor decline, with slower ER associated with faster progression (Zhang and Fan 2016). Animal experiments show that late-stage ALS mice have reduced blood lactate, increased skeletal muscle lactate, and decreased LDH activity/MCT1 levels compared to wild-type mice. Swimming training restores blood lactate, increases cytosolic MDH activity/cMDH/LDH ratio, and improves energy deficits in ALS mice, indicating lactate metabolic dysfunction (Cieminski et al. 2022).
Building on these findings, Meetha et al. confirmed that ALS pathogenesis involves an ATP-dependent muscle-neuron lactate shuttle (MNLS) at neuromuscular junctions. Respiratory chain dysfunction reduces ATP production, causing MNLS failure, lactate toxicity, nerve terminal degeneration, and junctional dysfunction. When denervation (loss of nerve-muscle connections) exceeds the critical threshold for reinnervation, remaining muscle fibers compensate by producing excess lactate, exacerbating denervation and neuronal death in a vicious cycle (Vadakkadath Meethal and Atwood 2012). This identifies lactate as a key driver of ALS neuromuscular pathology.
In superoxide dismutase 1 (SOD1)- and fused in sarcoma (FUS)-related ALS, glycolic acid (GA) and d-lactate are critical. Pal et al. (Pal et al. 2024) showed that combined GA/DL treatment restores axonal organelle phenotypes (mitochondria/lysosomes) and mitochondrial membrane potential in motor neurons from FUS- and SOD1-ALS patients. In FUS-ALS, GA/DL corrects FUS cytoplasmic mislocalization and aberrant recruitment to DNA damage sites.
4.4 Multiple sclerosis (MS)
MS is an inflammatory disease damaging myelinated axons. Lactate concentration may serve as a biomarker for MS onset/progression. A study of 118 relapsing–remitting MS (RRMS) patients and 157 controls found higher CSF lactate in RRMS, negatively correlated with disease duration, suggesting CSF lactate could predict disease severity (Klistorner et al. 2025).
MS patients also exhibit serum lactate changes: serum lactate is threefold higher than controls, linearly correlated with the Expanded Disability Status Scale, and may monitor “virtual hypoxia” and mitochondrial therapy trials. Exercise studies show MS patients have higher resting lactate, normalized by chronic exercise, but high-intensity interval training increases lactate due to hypoxia/glycolysis (Cerexhe et al. 2022; Keytsman et al. 2019). These findings highlight lactate as a marker of energy metabolism dysfunction and differential exercise effects, guiding clinical and rehabilitation strategies.
Based on these studies, lactate’s role in the muscle-brain endocrine circuit may underlie these findings. Lactate influences BDNF protein levels by prompting skeletal muscle myokine secretion (de Castro Abrantes et al. 2019), increasing glycolysis, pentose phosphate pathway activity, and TCA cycle flux in NSCs of progressive MS patients. This enhances cholesterol synthesis, leading to unsaturated fatty acid accumulation and neurotoxicity (Ionescu et al. 2024).
Additionally, lactate improves MS via the microbiota–gut–brain axis. Probiotics containing two Lactobacillus and two Bifidobacterium species modulate disease symptoms in experimental autoimmune myasthenia/encephalomyelitis models. Lactobacillus administration to MS patients improves disability scores and alleviates mood symptoms (Chen et al. 2022a,b).
4.5 Huntington’s disease (HD)
HD, also known as Huntington’s (HTT) chorea, is an autosomal-dominant caused by expanded CAG repeats in the HTT gene on chromosome 4. Multiple studies have shown elevated lactate levels in brain regions such as the occipital and frontal cortices of HD patients, with lactate concentration correlating with CAG repeat length and disease progression (Wang et al. 2024a,b,c,d).
Incremental cardiopulmonary exercise testing in HD gene-positive individuals and controls revealed reduced anaerobic thresholds during submaximal exercise in HD patients, particularly in presymptomatic mutation carriers, associated with increased plasma lactate. This confirms skeletal muscle mitochondrial dysfunction and early lactate metabolic abnormalities in HD (Steventon et al. 2018). These findings were replicated in R6/2 transgenic HD mice, which showed elevated brain lactate (Wang et al. 2024a,b,c,d).
In HD cells, despite high neuronal MCT2 catalytic efficiency, reduced GLUT3 expression impairs ascorbic acid–mediated lactate uptake and glucose transport inhibition. GLUT3 overexpression restores ascorbic acid’s stimulatory effect on lactate transport, indicating that altered GLUT3 expression may disrupt lactate utilization in HD neurons, contributing to brain energy metabolism dysfunction (Solis-Maldonado et al. 2018).
4.6 Other central nervous system diseases
Dementia with Lewy bodies (DLB), a common ND, presents with progressive dementia, parkinsonism, and psychiatric symptoms. FMRP deficiency in mice alters K-ac modifications of key enzymes, reducing ATP and increasing lactate, which links to DLB pathogenesis, although mechanisms remain unclear (Wu et al. 2022). A novel high-performance lactate imaging technology enables real-time tracking of lactate metabolism in DLB brains (Li et al. 2023a,b).
Spinocerebellar ataxia (SCA) encompasses genetically heterogeneous neurodegenerative disorders causing progressive ataxia. Mitochondrial diseases like leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL) illustrate the link between ataxia and lactate dysfunction. LBSL, caused by DARS2 mutations disrupting mitochondrial respiratory chain assembly, leads to lactate accumulation, demyelination, and cerebellar ataxia (Guang et al. 2023; Guang et al. 2023; Stellingwerff et al. 2021). While SCAs are typically caused by polyglutamine expansions, mitochondrial inheritance patterns exist, making LBSL informative for studying SCA-lactate relationships despite not being a classic SCA.
5 Therapeutic strategies targeting lactate and lactylation
A growing body of cutting-edge research indicates that modulating lactate metabolism and lactylation holds promise for opening new therapeutic avenues in disease treatment. Here, we summarize and synthesize recent advances in basic research and clinical therapeutic studies in this field, dissecting the underlying mechanisms of action and potential therapeutic strategies to provide clinicians with highly valuable reference approaches. Specific details are presented in Table 1.
Therapeutic methods and their mechanisms of action for neurodegenerative diseases in basic research.
| Disease | Treatment strategy | Specific drug/therapy | Treatment mechanism | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease | Exploration of therapeutic concentration of lactate | Intravenous infusion of 100 mM l-lactate | At this concentration, the cognitive deficits in rats are significantly reduced, and lactic acidosis is not induced. It is speculated that the effect of this concentration on improving cognitive impairment is related to ATP regeneration. | Yang et al. (2017) |
| Targeting cellular metabolic signaling pathways | mTOR inhibitor rapamycin | Inhibits astrocyte-derived l-lactate, rescues memory deficits in AD model mice, and reduces Aβ deposition. | Waetzig et al. (2021) | |
| Regulating metabolic pathways related to neuroinflammation | IDO1 small molecule inhibitor PF068 | Restores the function of astrocytes in AD mice, enhances memory ability, reduces the level of kynurenine, and restores the lactate level. | Minhas et al. (2024) | |
| Promoting the glycolytic pathway | GLP-1 (glucagon-like peptide-1) | Promotes aerobic glycolysis in astrocytes and enhances their ability to support neurons. | Zheng et al. (2021) | |
| Targeting glycolysis and immunomodulatory drugs | Uncaria rhynchophylla alkaloids (URA)) | (i) Inhibits the glycolysis of naive T cells induced by ConA and reduces lactate production. (ii) Regulates the PI3K/Akt/mTOR signaling pathway and inhibits the neuroinflammation mediated by CD4+ T cells. | Kuang et al. (2024) | |
| Regulation of APP lactylation modification | APP-K612la | Promotes the endocytosis of APP from the plasma membrane to endosomes, and then the endosome–lysosome degradation mediated by CD2AP, thus reducing the production and deposition of Aβ. | Tian et al. (2025) | |
| Advanced drug delivery technology | Nanoparticles of poly(lactic-co-glycolic acid) (PLGA) encapsulating ginsenoside Rg3 and thioflavin T | Hydrolyzes to produce lactic acid and glycolic acid. | Aalinkeel et al. (2018), Huang and Ding (2022) | |
| Advanced drug delivery technology | Nanoparticles loaded with p16(ink4a)-siRNA | Targets and delivers siRNA to senescent microglia, restores their vitality, and accelerates the clearance of Aβ amyloid. | Shin et al. (2024) | |
| Parkinson’s disease | Targeting the glycolysis-epigenetic axis | SIRT1 inhibitor + shikonin/PKM2-in-1 | Reduces lactate toxicity by blocking the occurrence of the positive feedback loop of “glycolysis/H4K12la/PKM2” in microglia and the deacetylation function of SIRT1. | Pan et al. (2022) |
| Drug intervention | LDH inhibitor RG6042 | Inhibits LDH activity, reduces lactate production, improves the abnormal energy metabolism of patients, repairs motor function, reduces neuroinflammation, and protects dopaminergic neurons. | Verma et al. (2024) | |
| Lifestyle intervention | Exercise therapy | Promotes the uptake and utilization of glucose by muscles, reduces lactate production, improves the aerobic metabolism ability of the body, and improves the motor function of PD patients. | Burtscher et al. (2024) | |
| Neuroregulation technology | Deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS) | Regulates the metabolic coupling between different regions of the brain through stimulation with specific parameters, affecting the brain metabolic network. | Honkanen et al. (2024) | |
| Cellular level | Incubating cells with lactate | Induces mitophagy and autophagy, thus repairing mitochondrial activity and preventing cell necrosis and apoptosis. | Nowag et al. (2024) | |
| Other neurodegenerative diseases | Wnt/β-catenin-glycolysis axis | Wnt pathway inhibitor XAV939 | Downregulates the abnormally activated Wnt/β-catenin pathway, inhibits the excessive enhancement of aerobic glycolysis, and delays the degeneration of motor neurons in ALS. | Afifi et al. (2014) |
| Targeting the HIF-1α-immunometabolism axis | Engineered lactate-producing probiotics | Activates the HIF-1α pathway of DC cells through intestinal d-lactate, inhibits the differentiation of Th17 cells, and reduces central nervous system inflammation. | Sanmarco et al. (2023) | |
| Personalized exercise prescription intervention | Optimizing exercise parameters (such as intensity, duration) | Improves the heterogeneity of the exercise metabolic response in HD patients, enhances energy utilization efficiency, and delays neurodegeneration. | Mueller et al. (2019) |
5.1 Basic research
5.1.1 Exploration of therapeutic strategies for AD targeting lactate and lactylation
Lactate-based therapies for AD are gaining traction. Studies show lactate intervention at specific concentrations, metabolic pathway modulation, and advanced drug delivery may improve brain function. In Morris Water Maze tests, 100 mM l-lactate treatment reduced cognitive deficits in injured rats, associated with partial ATP recovery (Yang et al. 2017). Intravenous 280 mM l-lactate was safe, supporting 100 mM as an optimal concentration. These findings drive future research toward optimizing lactate energy supply to enhance cognitive function.
In lactate metabolism, the mTOR inhibitor rapamycin suppresses astrocyte-derived l-lactate, rescuing memory deficits and reducing Aβ burden in AD mice (Waetzig et al. 2021). IDO1 mediates tryptophan catabolism, leading to suppressed glycolysis-related gene expression and impaired lactate synthesis. The IDO1 inhibitor PF068 restores astrocyte function, improves memory, dose-dependently reduces kynurenine levels, and restores lactate levels in AD mice (Minhas et al. 2024). GLP-1, an intestinal L-cell–derived incretin, enhances astrocyte support for neurons via aerobic glycolysis (Zheng et al. 2021).
In drug delivery, nanotechnology provides efficient solutions for lactate-related therapeutics, leveraging high payload, targeted delivery, and sustained release (Huang and Ding 2022). For example, p16(ink4a)-siRNA nanoparticles rejuvenate microglia and accelerate Aβ clearance in AD models (Shin et al. 2024). Aalinkeel et al. developed biodegradable PLGA nanoparticles encapsulating ginsenoside Rg3 and thioflavin T, which generate lactate/GA via hydrolysis and show AD theranostic potential. This strategy also benefits other neurologic diseases limited by solubility/pharmacokinetics (Aalinkeel et al. 2018).
5.1.2 Exploration of therapeutic strategies for PD targeting lactate and lactylation
Increased brain lactate burden promotes PD progression. Studies show that lactate perfusion in mice induces behavioral deficits, reduces TH-positive cells in the substantia nigra pars compacta, and activates microglia/astrocytes, with PKM2 and SIRT1 playing critical roles. SIRT1 inhibitors and PKM2 inhibitors (shikonin, PKM2-in-1) improve PD mouse behavior (Lian et al. 2024).
LDH inhibitors (e.g., RG6042) are promising PD therapies (Li et al. 2022; Minami et al. 2023; Verma et al. 2024), reducing lactate production to improve energy metabolism, motor function, neuroinflammation, and dopaminergic neuron protection. Ongoing trials validate their efficacy/safety.
Exercise therapy improves PD outcomes by enhancing muscle glucose utilization, reducing lactate production, and boosting aerobic metabolism (Burtscher et al. 2024). Deep brain stimulation not only alleviates motor symptoms but also modulates brain metabolic networks via regional metabolic coupling (Honkanen et al. 2024).
5.1.3 Exploration of therapeutic strategies for other ND targeting lactate and lactylation
In ALS, upregulated Wnt/β-catenin enhances aerobic glycolysis, ameliorated by XAV939 (Afifi et al. 2014). In immunology, lactate modulates DC cell mitochondrial function via HIF-1α. Engineered lactate-producing probiotics increase plasma/intestinal d-lactate, activating HIF-1α in intestinal DC cells and inhibiting Th17 cells to reduce CNS inflammation, offering a novel MS treatment strategy (Sanmarco et al. 2023; Alexander et al. 2024). Exercise testing in HD shows variable metabolic/physiological responses, suggesting optimized exercise prescriptions may improve outcomes (Mueller et al. 2019; Steventon et al. 2018).
5.2 Clinical translation
In recent years, clinical translation research for ND has made remarkable progress, expanding in depth and breadth to encompass new drug development, innovative medical device applications, and extensive clinical trial exploration. These efforts have received strong support from institutions including the U.S. Food and Drug Administration (FDA) https://www.fda.gov/, ClinicalTrials.gov https://clinicaltrials.gov/, Chinese Clinical Trial Registry (ChiCTR) https://www.chictr.org.cn/index.html, and the National Institutes of Health (NIH) https://www.nih.gov/, while fostering interdisciplinary and cross-domain collaborations.
In new drug and device development, advances in lactate research have led to the clinical application of diverse lactate-based formulations with unique roles. These formulations include various categories: for example, injectables such as HEXTEND (6 % Hydroxyethyl Starch Lactate Electrolyte Injection) replenish fluids/electrolytes and expand blood volume (https://www.fda.gov/); Lactated Ringer’s Injection contains sodium lactate to alkalize blood but requires serum lactate monitoring; Milrinone Lactate Injection serves as a specialized inotropic/vasodilator agent. Dialysis solutions like 2024 DIANEAL Low Calcium Peritoneal Dialysis Solution (Lactate-G 1.5 %, 2.5 %, 4.25 %) are critical for peritoneal dialysis. Supplements such as calcium lactate and magnesium lactate provide highly bioavailable minerals. Additionally, lactate-related reagents and other products exist. These diverse lactate-based formulations meet clinical treatment and medical research needs from multiple perspectives (https://www.fda.gov/, https://www.nih.gov/).
Currently, a number of medical studies related to lactate are underway. For example, the latest reports from the ChiCTR show that clinical experiments such as the construction of a scoring model for patients with central nervous system infections based on the lactate concentration in CSF (Clinical Trial Number: ChiCTR2500095199, Registration Date: 2025 – 01 – 03), the correlation between histone lactylation modification and cognitive impairment in diabetes (Clinical Trial Number: ChiCTR2300077072, Registration Date: 2023 – 10 – 27), and the focus on the endogenous lactate accumulation in schizophrenia and its potential association with the abnormal activation of the NLRP3/Caspase – 1/IL – 1β inflammatory signaling pathway (Clinical Trial Number: ChiCTR2400091186, Registration Date: 2024 – 10 – 22) are being carried out in an orderly manner (https://www.chictr.org.cn/index.htm).
In the field of energy and neurocognition, studies have reported that the systemic lactate concentration in the human body is related to the lactate uptake in the brain, and acute exercise can improve cognition. Through the “lactate clamp” procedure, the study compared the differences in lactate utilization between patients with AD and those with normal nervous systems, and found that AD patients have defects in lactate metabolism and utilization.
However, during the conduct of the above clinical trials, it is crucial to note that the “double-edged sword” effect of lactate under pathological conditions exhibits significant dose and time dependency.
Dose dependency: In PC12 cell and primary neuron models, the cytotoxic effects of lactate exposure demonstrate remarkable dose dependency, where low concentrations may act as energy substrates or signaling molecules, while high concentrations trigger cytotoxicity (Gong et al. 2024). When PC12 cells and primary neurons were incubated with l-lactate at concentrations ranging from 0 to 8 mM, studies showed that cytotoxicity in PC12 cells increased gradually with concentration, whereas neurons – owing to their high energy demands and low mitochondrial functional reserve – exhibited more pronounced mortality at the same concentrations, confirming the clear dose dependency of lactate toxicity in neurons and revealing cytotoxic differences across cell types (Chapp et al. 2021). Mechanistically, high-dose lactate (≥5 mM) persistently inhibits energy metabolism by competitively blocking pyruvate entry into the TCA cycle, leading to reduced ATP production and subsequent necroptosis. In contrast, low-dose lactate (≤2 mM) transiently activates protective pathways, primarily through AMPK/mTOR-mediated autophagy induction (Li et al. 2023a; b, Rauseo et al. 2024).
Time dependency: In primary neurons, the duration of lactate exposure dictates distinct pathological outcomes. Treatment with specific lactate concentrations for 1 h transiently activates the ERK/MAPK pathway, upregulating mRNA expression of early response genes (e.g., c-fos and egr1). Prolonged exposure for 24 h, however, leads to histone H3K18la enrichment at promoter regions, inducing chronic neuronal damage – manifested as severe mitochondrial structural impairment (cristae fragmentation), increased GRP78 mRNA expression (endoplasmic reticulum stress marker), and sustained activation of oxidative stress/inflammatory cascades, ultimately causing irreversible pathological changes (Gong et al. 2024; Mihaylova and Shaw 2011).
In AD models, time-dependent effects are even more pronounced: short-term low-dose lactate intervention improves cognitive function via H3K18 lactylation, as evidenced by shortened escape latency in the Morris water maze, while long-term high-dose exposure promotes Aβ deposition (increased plaque number) (Wei et al. 2023). This discrepancy is linked to lactate-mediated microglial polarization: short-term low-dose exposure induces an M2 anti-inflammatory phenotype, whereas long-term high-dose exposure drives an M1 proinflammatory state (Ye et al. 2022).
In summary, lactate exerts bidirectional regulation on neurons: low-dose/short-term exposure serves as an energy substrate or activates protective pathways, while high-dose/long-term exposure induces toxic damage. Therefore, research on promoting lactate transport to neurons should focus on precise regulation – via designing drugs that mimic physiological concentration gradients – to maximize energy supply and neuroprotection while avoiding toxicity, offering potential therapeutic strategies for ND.
Additionally, dynamic lactate metrics demonstrate unique predictive value. A 12-month study in ICU patients measured arterial blood lactate levels at specific time points for trauma, medical, and surgical patients hospitalized >24 h, comparing lactate as a mortality predictor with classic prognostic scores. Results show that standardized measurement timing/frequency, precision technologies, and combining dynamic lactate with classic scores/biomarkers improve ICU mortality prediction accuracy. However, limitations like retrospective designs necessitate future comparisons with existing models to validate its value (https://clinicaltrials.gov/).
In summary, scientific research on lactate therapy for ND remains highly complex and challenging. Ongoing advances in basic research, new drug development, medical device innovation, and clinical trials drive continuous breakthroughs. Future efforts should emphasize interdisciplinary integration and leverage modern technologies to accelerate clinical translation. With technological progress and translational advancements, this field will deliver more significant outcomes, offering improved therapeutic efficacy and broader treatment prospects for patients.
6 Limitations and prospects
Most current studies on lactylation suffer from limitations such as small sample sizes, single research indicators, and inconsistent research/detection methods. With advancements in high-throughput sequencing technology and bioinformatics, precise analysis of dynamic changes in lactate metabolism and lactylation modifications during ND pathogenesis at single-cell and subcellular levels becomes feasible. Additionally, in-depth exploration of lactate metabolism- and lactylation-related biomarkers may lead to the development of highly sensitive detection methods for early ND diagnosis. Furthermore, precision intervention strategies targeting lactate metabolic/lactylation abnormalities (e.g., small molecule inhibitors, targeted biological agents) could potentially delay or halt disease progression, improving patient outcomes.
Future research on lactate metabolism regulation as an ND treatment strategy must address key scientific challenges:
While lactate levels change significantly during ND progression, their spatiotemporal evolution across disease stages remains unclear. Understanding lactate concentration dynamics in multidimensional biological samples and regional specificity of neuron-glia lactate shuttling, including temporal/spatial heterogeneity in astrocyte-neuron lactate transport, is critical.
Consensus on lactylation-modifying enzyme/transporter expression profiles in ND and their associations with disease phenotypes is lacking. Development of microfluidic chip-based high-resolution lactate detection technologies could map dynamic lactate metabolism during ND progression. Multicenter cohort studies are needed to identify lactate metabolic phenotype differences across ND subtypes and facilitate personalized treatment.
The receptors/pathways through which lactate regulates neuroinflammation/oxidative stress networks, and how its concentration-dependent dual effects switch dynamically across disease stages, remain unknown. Humanized organoid models combined with optogenetic tools will help clarify lactate–inflammation–oxidative stress interactions. Special attention must also focus on blood–brain barrier permeability impacts on lactate intervention strategies and designing novel nanocarriers for CNS-targeted delivery.
Through integrating multidisciplinary technologies, breakthroughs in lactate metabolism regulation are expected, offering innovative strategies for ND prevention and treatment.
7 Conclusion
In ND, lactate production, clearance, and transport are altered. This review comprehensively demonstrates the close association between lactate and ND, as well as its key mechanistic roles in ND progression. The article compares brain lactate to a city’s energy supply system, using this analogy as a starting point for discussion. In the brain, lactate is primarily generated via the Warburg pathway and transported by MCTs, serving both as an energy source for neurons and a regulator of synaptic transmission. It plays critical roles in maintaining brain energy homeostasis and modulating the cellular microenvironment. Lactylation modifications, driven by excessive lactate accumulation, occur mainly in histones and nonhistone proteins. This dynamic process is finely regulated by multiple factors, including enzymatic control and metabolic etiology.
The article emphasizes in-depth analysis of lactate’s mechanistic roles in ND
(i) In AD, lactate imbalance drives cognitive decline by regulating core pathological processes such as amyloid deposition, NFTs, and neuroinflammation. (ii) In PD, lactate metabolic dysfunction contributes to pathogenesis through dual mechanisms: high concentrations exacerbate dopaminergic neuron damage, while moderate levels activate protective metabolic pathways. (iii) In ALS, dysfunction of the MNLS system leads to insufficient ATP supply at neuromuscular junctions, accelerating motor neuron degeneration. (iv) In MS, significantly elevated lactate levels in CSF and blood suggest involvement in central nervous system inflammation and myelin damage. (v) HD research reveals a positive correlation between lactate concentration and CAG repeat length, identifying lactate as a potential biomarker for disease progression.
These findings confirm that lactate dysfunction in energy metabolism, epigenetic modifications, and signaling is closely linked to ND pathogenesis. With advancing basic research and clinical translation, scientists are increasingly focusing on cellular metabolism, positioning lactate metabolism regulation as a promising novel therapeutic strategy for ND.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: BBW, CZS, and LZ participated in the conception and design of this review; SHQ, DL, TYZ, and CZ participated in the writing work; JS and YLY finalized the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Research funding: National Natural Science Foundation of China (81641048). Research Project of Yan’an University (2023JBZR-011).
-
Data availability: Not applicable.
References
Aalinkeel, R., Kutscher, H.L., Singh, A., Cwiklinski, K., Khechen, N., Schwartz, S.A., Prasad, P.N., and Mahajan, S.D. (2018). Neuroprotective effects of a biodegradable poly (lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: a potential nanotherapy for Alzheimer’s disease? J. Drug Target. 26: 182–193, https://doi.org/10.1080/1061186x.2017.1354002.Search in Google Scholar
Abdulrahaman, L.Q. (2023). Two-stage motion artifact reduction algorithm for rPPG signals obtained from facial video recordings. Arab. J. Sci. Eng. 48: 123–131, https://doi.org/10.1007/s13369-023-07845-2.Search in Google Scholar PubMed PubMed Central
Afifi, M.M., Austin, L.A., Mackey, M.A., and EL-Sayed, M.A. (2014). XAV939: from a small inhibitor to a potent drug bioconjugate when delivered by gold nanoparticles. Bioconjug. Chem. 25: 207–215, https://doi.org/10.1021/bc400271x.Search in Google Scholar PubMed PubMed Central
Alexander, M., Upadhyay, V., Rock, R., Ramirez, L., Trepka, K., Puchalska, P., Orellana, D., Ang, Q.Y., Whitty, C., Turnbaugh, J.A., et al.. (2024). A diet-dependent host metabolite shapes the gut microbiota to protect from autoimmunity. Cell Rep. 43: 114891, https://doi.org/10.1016/j.celrep.2024.114891.Search in Google Scholar PubMed PubMed Central
Ali, M.Z. and Dholaniya, P.S. (2022). Oxidative phosphorylation mediated pathogenesis of Parkinson’s disease and its implication via Akt signaling. Neurochem. Int. 157: 105344, https://doi.org/10.1016/j.neuint.2022.105344.Search in Google Scholar PubMed
Alkhammash, A. (2025). Pharmacology of epitranscriptomic modifications: decoding the therapeutic potential of RNA modifications in drug resistance. Eur. J. Pharmacol. 994: 177397, https://doi.org/10.1016/j.ejphar.2025.177397.Search in Google Scholar PubMed
Barros, L.F., Ruminot, I., San Martin, A., Lerchundi, R., Fernandez-Moncada, I., and Baeza-Lehnert, F. (2021). Aerobic glycolysis in the brain: warburg and crabtree contra pasteur. Neurochem. Res. 46: 15–22, https://doi.org/10.1007/s11064-020-02964-w.Search in Google Scholar PubMed
Blaszczak, W., Williams, H., and Swietach, P. (2022). Autoregulation of H(+)/lactate efflux prevents monocarboxylate transport (MCT) inhibitors from reducing glycolytic lactate production. Br. J. Cancer 127: 1365–1377, https://doi.org/10.1038/s41416-022-01910-7.Search in Google Scholar PubMed PubMed Central
Brown, S.D.M. and Lad, H.V. (2019). The dark genome and pleiotropy: challenges for precision medicine. Mamm. Genome. 30: 212–216, https://doi.org/10.1007/s00335-019-09813-4.Search in Google Scholar PubMed PubMed Central
Burtscher, J., Moraud, E.M., Malatesta, D., Millet, G.P., Bally, J.F., and Patoz, A. (2024). Exercise and gait/movement analyses in treatment and diagnosis of Parkinson’s disease. Ageing Res. Rev. 93: 102147, https://doi.org/10.1016/j.arr.2023.102147.Search in Google Scholar PubMed
Cerexhe, L., Easton, C., Macdonald, E., Renfrew, L., and Sculthorpe, N. (2022). Blood lactate concentrations during rest and exercise in people with multiple sclerosis: a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 57: 103454, https://doi.org/10.1016/j.msard.2021.103454.Search in Google Scholar PubMed
Chapp, A.D., Behnke, J.E., Driscoll, K.M., Hahka, T., Lalonde, Z., Shan, Z., and Chen, Q. (2021). Elevated L-lactate promotes major cellular pathologies associated with neurodegenerative diseases. Neurosci. Bull. 37: 380–384, https://doi.org/10.1007/s12264-020-00611-6.Search in Google Scholar PubMed PubMed Central
Chen, L., Huang, L., Gu, Y., Cang, W., Sun, P., and Xiang, Y. (2022a). Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int. J. Mol. Sci. 23: 11900, https://doi.org/10.3390/ijms231911943.Search in Google Scholar PubMed PubMed Central
Chen, X., Zhang, Y., Wang, H., Liu, L., Li, W., and Xie, P. (2022b). The regulatory effects of lactate on neuropsychiatric disorders. Discov. Ment. Health 2: 8, https://doi.org/10.1007/s44192-022-00011-4.Search in Google Scholar PubMed PubMed Central
Chen, X. and Zhu, X. (2025). Lactate: beyond a mere fuel in the epileptic brain. Neuropharmacology 266: 110273, https://doi.org/10.1016/j.neuropharm.2024.110273.Search in Google Scholar PubMed
Cheng, J. and Zhao, H. (2024). NEK7 induces lactylation in Alzheimer’s disease to promote pyroptosis in BV-2 cells. Mol. Brain 17: 81, https://doi.org/10.1186/s13041-024-01156-9.Search in Google Scholar PubMed PubMed Central
Cieminski, K., Flis, D.J., Dzik, K.P., Kaczor, J.J., Wieckowski, M.R., Antosiewicz, J., and Ziolkowski, W. (2022). Swim training affects on muscle lactate metabolism, nicotinamide adenine dinucleotides concentration, and the activity of NADH shuttle enzymes in a mouse model of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 23: 19, https://doi.org/10.3390/ijms231911504.Search in Google Scholar PubMed PubMed Central
Dai, S., Liu, P., Li, X., Jiao, L., Teng, Z., and Liu, C. (2022). Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development 149: 14, https://doi.org/10.1242/dev.200049.Search in Google Scholar PubMed
de Castro Abrantes, H., Briquet, M., Schmuziger, C., Restivo, L., Puyal, J., Rosenberg, N., Rocher, A., Offermanns, S., and Chatton, J. (2019). The lactate receptor HCAR1 modulates neuronal network activity through the activation of G(alpha) and G(betagamma) subunits. J. Neurosci. 39: 4422–4433, https://doi.org/10.1523/jneurosci.2092-18.2019.Search in Google Scholar PubMed PubMed Central
de Jager, C., Soliman, E., and Theus, M.H. (2025). Interrogating mediators of single-cell transcriptional changes in the acute damaged cerebral cortex: insights into endothelial-astrocyte interactions. Mol. Cell. Neurosci. 133: 104003, https://doi.org/10.1016/j.mcn.2025.104003.Search in Google Scholar PubMed PubMed Central
De Leo, A., Ugolini, A., Yu, X., Scirocchi, F., Scocozza, D., Peixoto, B., Pace, A., D’Angelo, L., Liu, J.K.C., Etame, A.B., et al.. (2024). Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity 57: 1105–1123.e8, https://doi.org/10.1016/j.immuni.2024.04.006.Search in Google Scholar PubMed PubMed Central
Drew, D. and Boudker, O. (2024). Ion and lipid orchestration of secondary active transport. Nature 626: 963–974, https://doi.org/10.1038/s41586-024-07062-3.Search in Google Scholar PubMed PubMed Central
Du, R., Gao, Y., Yan, C., Ren, X., Qi, S., Liu, G., Guo, X., Song, X., Wang, H., Rao, J., et al.. (2024). Sirtuin 1/sirtuin 3 are robust lysine delactylases and sirtuin 1-mediated delactylation regulates glycolysis. iScience 27: 110911, https://doi.org/10.1016/j.isci.2024.110911.Search in Google Scholar PubMed PubMed Central
Fan, Z., Liu, Z., Zhang, N., Wei, W., Cheng, K., Sun, H., and Hao, Q. (2023). Identification of SIRT3 as an eraser of H4K16la. iScience 26: 107757, https://doi.org/10.1016/j.isci.2023.107757.Search in Google Scholar PubMed PubMed Central
Fan, W., Zeng, S., Wang, X., Wang, G., Liao, D., Li, R., He, S., Li, W., Huang, J., Li, X., et al.. (2024). A feedback loop driven by H3K9 lactylation and HDAC2 in endothelial cells regulates VEGF-induced angiogenesis. Genome Biol. 25: 165, https://doi.org/10.1186/s13059-024-03308-5.Search in Google Scholar PubMed PubMed Central
Farmer, B.C., Williams, H.C., Devanney, N.A., Piron, M.A., Nation, G.K., Carter, D.J., Walsh, A.E., Khanal, R., Young, L.E.A., Kluemper, J.C., et al.. (2021). APOEpsilon4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis. Mol. Neurodegener. 16: 62, https://doi.org/10.1186/s13024-021-00483-y.Search in Google Scholar PubMed PubMed Central
Fazio-Eynullayeva, E., Cunnington, M., Mystkowski, P., Lv, L., Aly, A., Yee, C.W., Desai, R., Liu, C., Duh, M.S., and Mattke, S. (2025). Real-world healthcare resource utilization of Alzheimer’s disease in the early and advanced stages: a retrospective cohort study. J. Med. Econ. 28: 81–88, https://doi.org/10.1080/13696998.2024.2442240.Search in Google Scholar PubMed
Fedotova, E.I., Dolgacheva, L.P., Abramov, A.Y., and Berezhnov, A.V. (2022). Lactate and pyruvate activate autophagy and mitophagy that protect cells in toxic model of Parkinson’s disease. Mol. Neurobiol. 59: 177–190, https://doi.org/10.1007/s12035-021-02583-8.Search in Google Scholar PubMed
Fekih-Romdhane, F., Ghrissi, F., Cherif, W., and Cheour, M. (2020). Impulsivity in subjects at ultra high risk for psychosis. Encephale 46: 503–506, https://doi.org/10.1016/j.encep.2020.03.004.Search in Google Scholar PubMed
Fragniere, A.M.C., Stott, S.R.W., Fazal, S.V., Andreasen, M., Scott, K., and Barker, R.A. (2019). Hyperosmotic stress induces cell-dependent aggregation of alpha-synuclein. Sci. Rep. 9: 2288, https://doi.org/10.1038/s41598-018-38296-7.Search in Google Scholar PubMed PubMed Central
Fu, Y., Wang, D., Wang, H., Cai, M., Li, C., Zhang, X., Chen, H., Hu, Y., Zhang, X., Ying, M., et al.. (2020). TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma. Neuro. Oncol. 22: 240–252, https://doi.org/10.1093/neuonc/noz183.Search in Google Scholar PubMed PubMed Central
Gaffney, D.O., Jennings, E.Q., Anderson, C.C., Marentette, J.O., Shi, T., Schou Oxvig, A., Streeter, M.D., Johannsen, M., Spiegel, D.A., Chapman, E., et al.. (2020). Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem. Biol. 27: 206–213.e6, https://doi.org/10.1016/j.chembiol.2019.11.005.Search in Google Scholar PubMed PubMed Central
Gerou, M., Hall, B., Woof, R., Allsop, J., Kolb, S.J., Meyer, K., Shaw, P.J., and Allen, S.P. (2021). Amyotrophic lateral sclerosis alters the metabolic aging profile in patient derived fibroblasts. Neurobiol. Aging 105: 64–77, https://doi.org/10.1016/j.neurobiolaging.2021.04.013.Search in Google Scholar PubMed PubMed Central
Gong, H., Zhong, H., Cheng, L., Li, L., and Zhang, D. (2024). Post-translational protein lactylation modification in health and diseases: a double-edged sword. J. Transl. Med. 22: 41, https://doi.org/10.1186/s12967-023-04842-9.Search in Google Scholar PubMed PubMed Central
Gu, Y., Chen, K., Lei, C., Yang, X., Wang, L., Zhao, L., Jiang, W., and Deng, Q. (2025). Lactate and lactylation modifications in neurological disorders. Neural Regen. Res. 20: 123–135, https://doi.org/10.4103/NRR.NRR-D-24-01344.Search in Google Scholar PubMed
Guang, S., O’Brien, B.M., Fine, A.S., Ying, M., Fatemi, A., and Nemeth, C.L. (2023). Mutations in DARS2 result in global dysregulation of mRNA metabolism and splicing. Sci. Rep. 13: 13042, https://doi.org/10.1038/s41598-023-40107-7.Search in Google Scholar PubMed PubMed Central
Gujar, A.D., Ibrahim, B.A., Tamrakar, P., Cherian, A.K., and Briski, K.P. (2014). Hindbrain lactostasis regulates hypothalamic AMPK activity and metabolic neurotransmitter mRNA and protein responses to hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306: R457–R469, https://doi.org/10.1152/ajpregu.00151.2013.Search in Google Scholar PubMed PubMed Central
Han, S., Jiang, B., Ren, J., Gao, F., Wen, J., Zhou, T., Wang, L., and Wei, X. (2024b). Impaired lactate release in dorsal CA1 astrocytes contributed to nociceptive sensitization and comorbid memory deficits in rodents. Anesthesiology 140: 538–557, https://doi.org/10.1097/aln.0000000000004756.Search in Google Scholar
Han, R., Liu, L., Wang, Y., Wu, R., Yang, Y., Zhao, Y., Jian, L., Yuan, Y., Zhang, L., Gu, Y., et al.. (2024a). Microglial SLC25A28 deficiency ameliorates the brain injury after intracerebral hemorrhage in mice by restricting aerobic glycolysis. Inflammation 47: 591–608, https://doi.org/10.1007/s10753-023-01931-1.Search in Google Scholar PubMed
Hatakeyama, M., Ninomiya, I., and Kanazawa, M. (2020). Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 15: 16–19, https://doi.org/10.4103/1673-5374.264442.Search in Google Scholar PubMed PubMed Central
Honkanen, E.A., Ronka, J., Pekkonen, E., Aaltonen, J., Koivu, M., Eskola, O., Eldebakey, H., Volkmann, J., Kaasinen, V., Reich, M.M., et al.. (2024). GPi-DBS-induced brain metabolic activation in cervical dystonia. J. Neurol. Neurosurg. Psychiatr. 95: 300–308, https://doi.org/10.1136/jnnp-2023-331668.Search in Google Scholar PubMed
Hu, Y., He, Z., Li, Z., Wang, Y., Wu, N., Sun, H., Zhou, Z., Hu, Q., and Cong, X. (2024). Lactylation: the novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenet. 16: 72, https://doi.org/10.1186/s13148-024-01682-2.Search in Google Scholar PubMed PubMed Central
Huang, S. and Ding, X. (2022). Precise design strategies of nanotechnologies for controlled drug delivery. J. Funct. Biomater. 13: 123–135, https://doi.org/10.3390/jfb13040188.Search in Google Scholar PubMed PubMed Central
Hussain, M.K., Ahmad, M., Khatoon, S., Khan, M.V., Azmi, S., Arshad, M., Ahamad, S., and Saquib, M. (2025). Phytomolecules as Alzheimer’s therapeutics: a comprehensive review. Eur. J. Med. Chem. 288: 117401, https://doi.org/10.1016/j.ejmech.2025.117401.Search in Google Scholar PubMed
Hwang, D., Kim, T., Kyun, S., Jang, I., Kim, J., Park, H., Kim, S., and Lim, K. (2025). Exercise-induced hippocampal neurogenesis is attenuated by inhibition of monocarboxylate Transporter 2. Mol. Neurobiol. 62: 123–135, https://doi.org/10.1007/s12035-025-04986-3.Search in Google Scholar PubMed PubMed Central
Ionescu, R., Nicaise, A.M., Reisz, J.A., Williams, E.C., Prasad, P., Willis, C.M., Simoes-Abade, M.B.C., Sbarro, L., Dzieciatkowska, M., Stephenson, D., et al.. (2024). Increased cholesterol synthesis drives neurotoxicity in patient stem cell-derived model of multiple sclerosis. Cell Stem Cell 31: 1574–1590.e11, https://doi.org/10.1016/j.stem.2024.09.014.Search in Google Scholar PubMed
Irizarry-Caro, R.A., Mcdaniel, M.M., Overcast, G.R., Jain, V.G., Troutman, T.D., and Pasare, C. (2020). TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl. Acad. Sci. USA. 117: 30628–30638, https://doi.org/10.1073/pnas.2009778117.Search in Google Scholar PubMed PubMed Central
Jackson, B.T., Montero, A.M., Chakraborty, S., Brunner, J.S., Arnold, P.K., Bridgeman, A.E., Todorova, P.K., Paras, K.I., and Finley, L.W.S. (2025). Intracellular metabolic gradients dictate dependence on exogenous pyruvate. Nat. Metab. 7: 1168–1182, https://doi.org/10.1038/s42255-025-01289-8.Search in Google Scholar PubMed PubMed Central
Jantti, H., Sitnikova, V., Ishchenko, Y., Shakirzyanova, A., Giudice, L., Ugidos, I.F., Gomez-Budia, M., Korvenlaita, N., Ohtonen, S., Belaya, I., et al.. (2022). Microglial amyloid beta clearance is driven by PIEZO1 channels. J. Neuroinflamm. 19: 147, https://doi.org/10.1186/s12974-022-02486-y.Search in Google Scholar PubMed PubMed Central
Jeong, S., Park, S., Choi, J., Cho, N., Moon, J., and Gil, H. (2024). Indoxyl sulfate induces apoptotic cell death by inhibiting glycolysis in human astrocytes. Kidney Res. Clin. Pract. 43: 774–784, https://doi.org/10.23876/j.krcp.23.005.Search in Google Scholar PubMed PubMed Central
Jia, M., Yue, X., Sun, W., Zhou, Q., Chang, C., Gong, W., Feng, J., Li, X., Zhan, R., Mo, K., et al.. (2023). ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci. Adv. 9: eadg4993, https://doi.org/10.1126/sciadv.adg4993.Search in Google Scholar PubMed PubMed Central
Kabir, F., Atkinson, R., Cook, A.L., Phipps, A.J., and King, A.E. (2022). The role of altered protein acetylation in neurodegenerative disease. Front. Aging Neurosci. 14: 1025473, https://doi.org/10.3389/fnagi.2022.1025473.Search in Google Scholar PubMed PubMed Central
Kann, O., Soder, L., and Khodaie, B. (2025). Lactate is a potentially harmful substitute for brain glucose fuel: consequences for metabolic restoration of neurotransmission. Neural Regen. Res. 20: 1403–1404, https://doi.org/10.4103/nrr.nrr-d-24-00262.Search in Google Scholar PubMed PubMed Central
Kapadia, B., Roychowdhury, A., Kayastha, F., Lee, W.S., Nanaji, N., Windle, J., and Gartenhaus, R. (2025). m6A eraser ALKBH5/treRNA1/DDX46 axis regulates BCR expression. Neoplasia 62: 101144, https://doi.org/10.1016/j.neo.2025.101144.Search in Google Scholar PubMed PubMed Central
Keytsman, C., Hansen, D., Wens, I., and Eijnde, B.O. (2019). Exercise-induced lactate responses in multiple sclerosis: a retrospective analysis. NeuroRehabilitation 45: 99–106, https://doi.org/10.3233/nre-192740.Search in Google Scholar
Kim, Y., Dube, S.E., and Park, C.B. (2025). Brain energy homeostasis: the evolution of the astrocyte-neuron lactate shuttle hypothesis. Kor. J. Physiol. Pharmacol. 29: 1–8, https://doi.org/10.4196/kjpp.24.388.Search in Google Scholar PubMed PubMed Central
King, J., Patel, M., and Chandrasekaran, S. (2021). Metabolism, HDACs, and HDAC inhibitors: a systems biology perspective. Metabolites 11: 792, https://doi.org/10.3390/metabo11110792.Search in Google Scholar PubMed PubMed Central
Klistorner, S., Barnett, M., Parratt, J.D.E., Yiannikas, C., Wang, C., Wang, D., Shieh, A., and Klistorner, A. (2025). Evolution of chronic lesion tissue in relapsing-remitting patients with multiple sclerosis: an association with disease progression. Neurol. Neuroimmunol. Neuroinflamm. 12: e200377, https://doi.org/10.1212/nxi.0000000000200377.Search in Google Scholar
Komilova, N.R., Angelova, P.R., Berezhnov, A.V., Stelmashchuk, O.A., Mirkhodjaev, U.Z., Houlden, H., Gourine, A.V., Esteras, N., and Abramov, A.Y. (2022). Metabolically induced intracellular pH changes activate mitophagy, autophagy, and cell protection in familial forms of Parkinson’s disease. FEBS J. 289: 699–711, https://doi.org/10.1111/febs.16198.Search in Google Scholar PubMed
Kontaxi, C., Piccardo, P., and Gill, A.C. (2017). Lysine-directed post-translational modifications of tau protein in Alzheimer’s disease and related tauopathies. Front. Mol. Biosci. 4: 56, https://doi.org/10.3389/fmolb.2017.00056.Search in Google Scholar PubMed PubMed Central
Kuang, Y., Zhu, M., Gu, H., Tao, Y., Huang, H., and Chen, L. (2024). Alkaloids in Uncaria rhynchophylla improves AD pathology by restraining CD4(+) T cell-mediated neuroinflammation via inhibition of glycolysis in APP/PS1 mice. J. Ethnopharmacol. 331: 118273, https://doi.org/10.1016/j.jep.2024.118273.Search in Google Scholar PubMed
Li, J., Chen, L., Qin, Q., Wang, D., Zhao, J., Gao, H., Yuan, X., Zhang, J., Zou, Y., Mao, Z., et al.. (2022). Upregulated hexokinase 2 expression induces dopaminergic neuron apoptosis by promoting lactate production in Parkinson’s disease. Neurobiol. Dis. 163: 105605, https://doi.org/10.1016/j.nbd.2021.105605.Search in Google Scholar PubMed
Li, L., Chen, K., Wang, T., Wu, Y., Xing, G., Chen, M., Hao, Z., Zhang, C., Zhang, J., Ma, B., et al.. (2020). Glis1 facilitates pluripotency induction via an epigenome-metabolome-epigenome cascade. Nat. Metab. 2: 882–892, https://doi.org/10.1038/s42255-020-0267-9.Search in Google Scholar PubMed
Li, J., Li, S., Sun, Q., Li, L., Zhang, Y., and Hua, Z. (2025). H3K18 lactylation-mediated NOD2 expression promotes bilirubin-induced astrocyte pyroptosis. J. Neuroinflamm. 22: 76, https://doi.org/10.1186/s12974-025-03399-2.Search in Google Scholar PubMed PubMed Central
Li, H., Liu, C., Li, R., Zhou, L., Ran, Y., Yang, Q., Huang, H., Lu, H., Song, H., Yang, B., et al.. (2024). AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature 634: 1229–1237, https://doi.org/10.1038/s41586-024-07992-y.Search in Google Scholar PubMed
Li, L., Sun, S., Wu, Y., Lu, J., He, J., Chen, K., Chan, W., and Liu, X. (2023a). Lactate and protein lactylation: from metabolic byproduct to protein sculptor. Sci. Bull. 68: 2510–2514, https://doi.org/10.1016/j.scib.2023.09.038.Search in Google Scholar PubMed
Li, X., Zhang, Y., Xu, L., Wang, A., Zou, Y., Li, T., Huang, L., Chen, W., Liu, S., Jiang, K., et al.. (2023b). Ultrasensitive sensors reveal spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 35: 200–211.e9, https://doi.org/10.1016/j.cmet.2022.10.002.Search in Google Scholar PubMed PubMed Central
Lian, B., Zhang, J., Yin, X., Wang, J., Li, L., Ju, Q., Wang, Y., Jiang, Y., Liu, X., Chen, Y., et al.. (2024). SIRT1 regulates brain lactate homeostasis to alleviate parkinsonism via PKM2 deacetylation. Cell Rep. Med. 5: 101684, https://doi.org/10.1016/j.xcrm.2024.101684.Search in Google Scholar PubMed PubMed Central
Liang, L., Liu, P., Deng, Y., Li, J., and Zhao, S. (2024). L-lactate inhibits LPS-induced microglial inflammation in hippocampus. Int. J. Neurosci. 134: 45–52, https://doi.org/10.1080/00207454.2022.2084089.Search in Google Scholar PubMed
Liao, Y., Niu, L., Ling, J., Cui, Y., Huang, Z., Xu, J., Jiang, Y., Yu, P., and Liu, X. (2025a). Lactylation modification: a promising target for cardiovascular health. Metabolism 156: 234.10.1016/j.metabol.2025.156234Search in Google Scholar PubMed
Liao, Z., Chen, B., Yang, T., Zhang, W., and Mei, Z. (2025b). Lactylation in cardio-cerebral diseases: current advances. Ageing Res. Rev. 104: 102631, https://doi.org/10.1016/j.arr.2024.102631.Search in Google Scholar PubMed
Liguori, C., Chiaravalloti, A., Sancesario, G., Stefani, A., Sancesario, G.M., Mercuri, N.B., Schillaci, O., and Pierantozzi, M. (2016). CSF lactate correlates with [18F]FDG-PET hypometabolism in Alzheimer’s default mode network. Eur. J. Nucl. Med. Mol. Imag. 43: 2040–2049, https://doi.org/10.1007/s00259-016-3417-2.Search in Google Scholar PubMed
Liu, L., Mackenzie, K.R., Putluri, N., Maletic-Savatic, M., and Bellen, H.J. (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26: 719–737.e6, https://doi.org/10.1016/j.cmet.2017.08.024.Search in Google Scholar PubMed PubMed Central
Liu, X., Wang, J., Lao, M., Liu, F., Zhu, H., Man, K., and Zhang, J. (2024). Study on the effect of protein lysine lactylation modification in macrophages on inhibiting periodontitis in rats. J. Periodontol. 95: 50–63, https://doi.org/10.1002/jper.23-0241.Search in Google Scholar PubMed
Llibre, A., Kucuk, S., Gope, A., Certo, M., and Mauro, C. (2025). Lactate: a key regulator of the immune response. Immunity 58: 535–554, https://doi.org/10.1016/j.immuni.2025.02.008.Search in Google Scholar PubMed
Lu, J., Tan, M., and Cai, Q. (2015). The warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 356: 156–164, https://doi.org/10.1016/j.canlet.2014.04.001.Search in Google Scholar PubMed PubMed Central
Lu, J., Zhou, W., Dou, F., Wang, C., and Yu, Z. (2021). TRPV1 sustains microglial metabolic reprogramming in Alzheimer’s disease. EMBO Rep. 22: e52013, https://doi.org/10.15252/embr.202052013.Search in Google Scholar PubMed PubMed Central
Lundgaard, I., Lu, M.L., Yang, E., Peng, W., Mestre, H., Hitomi, E., Deane, R., and Nedergaard, M. (2017). Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 37: 2112–2124, https://doi.org/10.1177/0271678x16661202.Search in Google Scholar PubMed PubMed Central
Ma, Y., Zhang, Z., Cao, X., Guo, D., Huang, S., Xie, L., Wu, M., Li, J., Li, C., Chu, Y., et al.. (2025). Semaphorin 6A phase separation sustains a histone lactylation-dependent lactate buildup in pathological angiogenesis. Proc. Natl. Acad. Sci. USA 122, e2423677122, https://doi.org/10.1073/pnas.2423677122.Search in Google Scholar PubMed PubMed Central
Machler, P., Wyss, M.T., Elsayed, M., Stobart, J., Gutierrez, R., von Faber-Castell, A., Kaelin, V., Zuend, M., San Martin, A., Romero-Gomez, I., et al.. (2016). In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23: 94–102, https://doi.org/10.1016/j.cmet.2015.10.010.Search in Google Scholar PubMed
Maculewicz, E., Mastalerz, A., Garbacz, A., Mroz, A., Stastny, P., Petr, M., Kolinger, D., Vostatkova, P., and Bojarczuk, A. (2025). The interactions between monocarboxylate transporter genes MCT1, MCT2, and MCT4 and the kinetics of blood lactate production and removal after high-intensity efforts in elite males: a cross-sectional study. BMC Genom. 26: 133, https://doi.org/10.1186/s12864-025-11307-4.Search in Google Scholar PubMed PubMed Central
Matsui, T., Omuro, H., Liu, Y., Soya, M., Shima, T., Mcewen, B.S., and Soya, H. (2017). Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc. Natl. Acad. Sci. USA 114: 6358–6363, https://doi.org/10.1073/pnas.1702739114.Search in Google Scholar PubMed PubMed Central
Mihaylova, M.M. and Shaw, R.J. (2011). The AMPK signaling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13: 1016–1023, https://doi.org/10.1038/ncb2329.Search in Google Scholar PubMed PubMed Central
Minami, E., Sasa, K., Yamada, A., Kawai, R., Yoshida, H., Nakano, H., Maki, K., and Kamijo, R. (2023). Lactate-induced histone lactylation by p300 promotes osteoblast differentiation. PLoS One 18: e0293676, https://doi.org/10.1371/journal.pone.0293676.Search in Google Scholar PubMed PubMed Central
Minhas, P.S., Jones, J.R., Latif-Hernandez, A., Sugiura, Y., Durairaj, A.S., Wang, Q., Mhatre, S.D., Uenaka, T., Crapser, J., Conley, T., et al.. (2024). Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 385: eabm6131, https://doi.org/10.1126/science.abm6131.Search in Google Scholar PubMed PubMed Central
Mollo, A., Raffone, A., Travaglino, A., Di Cello, A., Saccone, G., Zullo, F., and De Placido, G. (2018). Increased LDH5/LDH1 ratio in preoperative diagnosis of uterine sarcoma with inconclusive MRI and LDH total activity but suggestive CT scan: a case report. BMC Wom. Health 18: 169, https://doi.org/10.1186/s12905-018-0662-5.Search in Google Scholar PubMed PubMed Central
Mueller, S.M., Petersen, J.A., and Jung, H.H. (2019). Exercise in Huntington’s disease: current state and clinical significance. Tremor Other Hyperkinet. Mov. (N. Y.) 9: 601, https://doi.org/10.5334/tohm.515.Search in Google Scholar
Munoz-Pinto, M.F., Empadinhas, N., and Cardoso, S.M. (2021). The neuromicrobiology of Parkinson’s disease: a unifying theory. Ageing Res. Rev. 70: 101396, https://doi.org/10.1016/j.arr.2021.101396.Search in Google Scholar PubMed
Nouri, N., Gussler, B.H., Stockwell, A., Truong, T., Kang, G.J., Browder, K.C., Malato, Y., Sene, A., Van Everen, S., Wykoff, C.C., et al.. (2024). SLC16A8 is a causal contributor to age-related macular degeneration risk. NPJ Genom. Med. 9: 50, https://doi.org/10.1038/s41525-024-00442-8.Search in Google Scholar PubMed PubMed Central
Nowag, B., Schafer, D., Hengl, T., Corduff, N., and Goldie, K. (2024). Biostimulating fillers and induction of inflammatory pathways: a preclinical investigation of macrophage response to calcium hydroxylapatite and poly-L lactate. J. Cosmet. Dermatol. 23: 99–106, https://doi.org/10.1111/jocd.15928.Search in Google Scholar PubMed
Ordenes, P., Villar, P.S., Tarifeno-Saldivia, E., Salgado, M., Elizondo-Vega, R., Araneda, R.C., and Garcia-Robles, M.A. (2021). Lactate activates hypothalamic POMC neurons by intercellular signaling. Sci. Rep. 11: 21644, https://doi.org/10.1038/s41598-021-00947-7.Search in Google Scholar PubMed PubMed Central
Osadchiy, V., Eleswarapu, S.V., Mills, S.A., Pollard, M.E., Reiter, R.E., and Mills, J.N. (2020). Efficacy of a preprostatectomy multi-modal penile rehabilitation regimen on recovery of postoperative erectile function. Int. J. Impot. Res. 32: 323–328, https://doi.org/10.1038/s41443-019-0187-y.Search in Google Scholar PubMed
Pal, A., Grossmann, D., Glass, H., Zimyanin, V., Gunther, R., Catinozzi, M., Boeckers, T.M., Sterneckert, J., Storkebaum, E., Petri, S., et al.. (2024). Glycolic acid and D-lactate-putative products of DJ-1-restore neurodegeneration in FUS- and SOD1-ALS. Life Sci. Alliance 7: e202402654.10.26508/lsa.202302535Search in Google Scholar PubMed PubMed Central
Pan, R., He, L., Zhang, J., Liu, X., Liao, Y., Gao, J., Liao, Y., Yan, Y., Li, Q., Zhou, X., et al.. (2022). Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34: 634–648.e6, https://doi.org/10.1016/j.cmet.2022.02.013.Search in Google Scholar PubMed
Parsons, M.P. and Hirasawa, M. (2010). ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J. Neurosci. 30: 8061–8070, https://doi.org/10.1523/jneurosci.5741-09.2010.Search in Google Scholar PubMed PubMed Central
Pavlu-Pereira, H., Silva, M.J., Florindo, C., Sequeira, S., Ferreira, A.C., Duarte, S., Rodrigues, A.L., Janeiro, P., Oliveira, A., Gomes, D., et al.. (2020). Pyruvate dehydrogenase complex deficiency: updating the clinical, metabolic and mutational landscapes in a cohort of Portuguese patients. Orphanet J. Rare Dis. 15: 298, https://doi.org/10.1186/s13023-020-01586-3.Search in Google Scholar PubMed PubMed Central
Pellerin, L. and Magistretti, P.J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 91: 10625–10629, https://doi.org/10.1073/pnas.91.22.10625.Search in Google Scholar PubMed PubMed Central
Perez-Escuredo, J., Van Hee, V.F., Sboarina, M., Falces, J., Payen, V.L., Pellerin, L., and Sonveaux, P. (2016). Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863: 2481–2497, https://doi.org/10.1016/j.bbamcr.2016.03.013.Search in Google Scholar PubMed PubMed Central
Pierre, K. and Pellerin, L. (2005). Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94: 1–14, https://doi.org/10.1111/j.1471-4159.2005.03168.x.Search in Google Scholar PubMed
Powell, C.L., Davidson, A.R., and Brown, A.M. (2020). Universal Glia to neuron lactate transfer in the nervous system: physiological functions and pathological consequences. Biosensors (Basel) 10: 183, https://doi.org/10.3390/bios10110183.Search in Google Scholar PubMed PubMed Central
Qin, Q., Wang, D., Qu, Y., Li, J., An, K., Mao, Z., Li, J., Xiong, Y., Min, Z., and Xue, Z. (2025). Enhanced glycolysis-derived lactate promotes microglial activation in Parkinson’s disease via histone lactylation. NPJ Parkinsons Dis. 11: 3, https://doi.org/10.1038/s41531-024-00858-0.Search in Google Scholar PubMed PubMed Central
Rauseo, D., Contreras-Baeza, Y., Faurand, H., Carcamo, N., Suarez, R., von Faber-Castell, A., Silva, F., Mora-Gonzalez, V., Wyss, M.T., Baeza-Lehnert, F., et al.. (2024). Lactate-carried mitochondrial energy overflow. bioRxiv [Preprint], https://doi.org/10.1101/2024.07.19.604361.Search in Google Scholar PubMed PubMed Central
Sanmarco, L.M., Rone, J.M., Polonio, C.M., Fernandez Lahore, G., Giovannoni, F., Ferrara, K., Gutierrez-Vazquez, C., Li, N., Sokolovska, A., Plasencia, A., et al.. (2023). Lactate limits CNS autoimmunity by stabilizing HIF-1alpha in dendritic cells. Nature 620: 881–889, https://doi.org/10.1038/s41586-023-06409-6.Search in Google Scholar PubMed PubMed Central
Saulnier-Bellemare, T. and Patience, G.S. (2024). Homogeneous and heterogeneous catalysis of glucose to lactate and lactates: a review. ACS Omega 9: 23121–23137, https://doi.org/10.1021/acsomega.3c10015.Search in Google Scholar PubMed PubMed Central
Shin, H.J., Kim, I.S., Choi, S.G., Lee, K., Park, H., Shin, J., Kim, D., Beom, J., Yi, Y.Y., Gupta, D.P., et al.. (2024). Rejuvenating aged microglia by p16(ink4a)-siRNA-loaded nanoparticles increases amyloid-beta clearance in animal models of Alzheimer’s disease. Mol. Neurodegener. 19: 25, https://doi.org/10.1186/s13024-024-00715-x.Search in Google Scholar PubMed PubMed Central
Solis-Maldonado, M., Miro, M.P., Acuna, A.I., Covarrubias-Pinto, A., Loaiza, A., Mayorga, G., Beltran, F.A., Cepeda, C., Levine, M.S., Concha, I.I., et al.. (2018). Altered lactate metabolism in Huntington’s disease is dependent on GLUT3 expression. CNS Neurosci. Ther 24: 343–352.10.1111/cns.12837Search in Google Scholar PubMed PubMed Central
Stellingwerff, M.D., Figuccia, S., Bellacchio, E., Alvarez, K., Castiglioni, C., Topaloglu, P., Stutterd, C.A., Erasmus, C.E., Sanchez-Valle, A., Lebon, S., et al.. (2021). LBSL: case series and DARS2 variant analysis in early severe forms with unexpected presentations. Neurol. Genet. 7: e559, https://doi.org/10.1212/nxg.0000000000000559.Search in Google Scholar
Steventon, J.J., Collett, J., Furby, H., Hamana, K., Foster, C., O’Callaghan, P., Dennis, A., Armstrong, R., Nemeth, A.H., Rosser, A.E., et al.. (2018). Metabolic and cardiorespiratory alterations during exercise in Huntington’s disease. Park. Relat. Disord. 54: 56–61, https://doi.org/10.1016/j.parkreldis.2018.04.014.Search in Google Scholar PubMed PubMed Central
Sun, S., Xu, Z., He, L., Shen, Y., Yan, Y., Lv, X., Zhu, X., Li, W., Tian, W., Zheng, Y., et al.. (2024). HDAC6-mediated α-tubulin lactylation regulates cytoskeletal functions. Nat. Commun. 15: 8377, https://doi.org/10.1038/s41467-024-52729-0.Search in Google Scholar PubMed PubMed Central
Sun, Y., Yang, J., Hu, X., Gao, X., Li, Y., Yu, M., Liu, S., Lu, X., Jin, C., Wu, S., et al.. (2018). Lanthanum chloride inhibits astrocytic lactate production and astrocyte-neuron lactate transport in rat cortical cultures. Arch. Toxicol. 92: 1407–1419, https://doi.org/10.1007/s00204-017-2148-x.Search in Google Scholar PubMed
Susser, L.I., Nguyen, M., Geoffrion, M., Emerton, C., Ouimet, M., Khacho, M., and Rayner, K.J. (2023). Mitochondrial fragmentation enhances macrophage inflammation resolution via histone lactylation. Mol. Cell. Biol. 43: 531–546, https://doi.org/10.1080/10985549.2023.2253131.Search in Google Scholar PubMed PubMed Central
Tang, H., Li, L., Yu, Q., Chen, L., Xu, X., Meng, Z., Zeng, Y., Chen, F., Muzaffar, H., Wang, W., et al.. (2025). Sclareol attenuates Alzheimer’s pathology through CDK9-mediated microglial anti-inflammatory effects. Phytomedicine 139: 156504, https://doi.org/10.1016/j.phymed.2025.156504.Search in Google Scholar PubMed
Tapias, V., Gonzalez-Andres, P., Pena, L.F., Barbero, A., Nunez, L., and Villalobos, C. (2023). Heterocyclic compounds targeting mitochondrial calcium in neurodegenerative diseases. Antioxidants (Basel) 12: 1190.10.3390/antiox12061282Search in Google Scholar PubMed PubMed Central
Tauffenberger, A., Fiumelli, H., Almustafa, S., and Magistretti, P.J. (2019). Lactate and pyruvate enhance oxidative stress resistance via ROS hormesis. Cell Death Dis. 10: 653, https://doi.org/10.1038/s41419-019-1877-6.Search in Google Scholar PubMed PubMed Central
Tian, Q., Li, J., Wu, B., Pang, Y., He, W., Xiao, Q., Wang, J., Yi, L., Tian, N., Shi, X., et al.. (2025). APP lysine 612 lactylation ameliorates amyloid pathology and memory decline in Alzheimer’s disease. J. Clin. Invest. 135: e168312, https://doi.org/10.1172/jci184656.Search in Google Scholar
Tian, Q., Li, J., Wu, B., Wang, J., Xiao, Q., Tian, N., Yi, L., Luo, M., Li, Z., Pang, Y., et al.. (2023). Hypoxia-sensing VGLL4 promotes LDHA-driven lactate production to improve neuronal function in Alzheimer’s disease models. FASEB J. 37: e23290, https://doi.org/10.1096/fj.202301173rrr.Search in Google Scholar PubMed
Tian, Q. and Zhou, L. (2022). Lactate induces germline and embryonic gene expression in mouse ES cells. Cells 11: 428.10.3390/cells11030548Search in Google Scholar PubMed PubMed Central
Tiwari, V., Prajapati, B., Asare, Y., Damkou, A., Ji, H., Liu, L., Naser, N., Gouna, G., Leszczynska, K.B., Mieczkowski, J., et al.. (2024). Innate immune training restores pro-reparative myeloid functions to enhance remyelination in aged CNS. Immunity 57: 2173–2190.e8, https://doi.org/10.1016/j.immuni.2024.07.001.Search in Google Scholar PubMed
To, K.K.W., Xing, E., Larue, R.C., and Li, P. (2023). BET bromodomain inhibitors: novel designs and therapeutic applications. Molecules 28: 2960.10.3390/molecules28073043Search in Google Scholar PubMed PubMed Central
Trujillo, M.N., Jennings, E.Q., Hoffman, E.A., Zhang, H., Phoebe, A.M., Mastin, G.E., Kitamura, N., Reisz, J.A., Megill, E., Kantner, D., et al.. (2024). Lactoylglutathione promotes inflammatory signaling in macrophages via histone lactoylation. Mol. Metab. 81: 101888, https://doi.org/10.1016/j.molmet.2024.101888.Search in Google Scholar PubMed PubMed Central
Vadakkadath Meethal, S. and Atwood, C.S. (2012). Lactate dyscrasia: a novel mechanism for amyotrophic lateral sclerosis. Neurobiol. Aging 33: 569–581, https://doi.org/10.1016/j.neurobiolaging.2010.04.012.Search in Google Scholar PubMed
Verma, S., Budhu, S., Serganova, I., Dong, L., Mangarin, L.M., Khan, J.F., Bah, M.A., Assouvie, A., Marouf, Y., Schulze, I., et al.. (2024). Pharmacologic LDH inhibition reprograms intratumoral glucose metabolism and enhances antitumor immunity. J. Clin. Invest. 134: e171965, https://doi.org/10.1172/jci177606.Search in Google Scholar
Vizuete, A.F.K., Froes, F., Seady, M., Zanotto, C., Bobermin, L.D., Roginski, A.C., Wajner, M., Quincozes-Santos, A., and Goncalves, C.A. (2022). LPS-induced neuroinflammation alters hippocampal glycolysis in rats. J. Neuroinflamm. 19: 255, https://doi.org/10.1186/s12974-022-02612-w.Search in Google Scholar PubMed PubMed Central
Waetzig, R., Matthes, M., Leister, J., Penkivech, G., Heise, T., Corbacioglu, S., and Sommer, G. (2021). Comparing mTOR inhibitors Rapamycin and Torin-2 in neuroblastoma targeted therapy. Int. J. Med. Sci. 18: 137–149, https://doi.org/10.7150/ijms.48393.Search in Google Scholar PubMed PubMed Central
Wagner-Gutierrez, N., Gonzalez, S.A., Rubio, M.A., Sanchez-Franco, S., Palencia-Perez, L., Blanco, M., Adlakha, D., Aguirre-Patino, J.S., Ossa, N., Vietto, G., et al.. (2025). Quality of life, mental health and social relationships in older adults participating in Recreovia physical activity program. Int. J. Equity Health 24: 145, https://doi.org/10.1186/s12939-025-02476-5.Search in Google Scholar PubMed PubMed Central
Wang, X., Gao, J., Ouyang, X., Wang, J., Sun, X., and Lv, Y. (2018). Paclitaxel-loaded PLGA nanoparticles in mesenchymal stem cells for glioma therapy. Int. J. Nanomed. 13: 5231–5248, https://doi.org/10.2147/ijn.s167142.Search in Google Scholar PubMed PubMed Central
Wang, Y., Li, P., Xu, Y., Feng, L., Fang, Y., Song, G., Xu, L., Zhu, Z., Wang, W., Mei, Q., et al.. (2024c). Lactate metabolism and histone lactylation in CNS disorders: mechanisms and impacts. J. Neuroinflamm. 21: 308, https://doi.org/10.1186/s12974-024-03303-4.Search in Google Scholar PubMed PubMed Central
Wang, X., Liu, Q., Yu, H., Xie, J., Zhao, J., Fang, Z., Qu, M., Zhang, Y., Yang, Y., and Wang, J. (2024b). IDH3β-PAX6 feedback inhibition promotes Alzheimer-like pathology. Signal Transduct. Target Ther. 9: 105, https://doi.org/10.1038/s41392-024-01812-5.Search in Google Scholar PubMed PubMed Central
Wang, M., Pan, W., Xu, Y., Zhang, J., Wan, J., and Jiang, H. (2022a). Microglia-mediated neuroinflammation: potential therapeutic target for cardiovascular diseases. J. Inflamm. Res. 15: 3083–3094, https://doi.org/10.2147/jir.s350109.Search in Google Scholar
Wang, N., Wang, W., Wang, X., Mang, G., Chen, J., Yan, X., Tong, Z., Yang, Q., Wang, M., Chen, L., et al.. (2022b). Histone lactylation enhances reparative gene activation after myocardial infarction. Circ. Res. 131: 893–908, https://doi.org/10.1161/circresaha.122.320488.Search in Google Scholar PubMed
Wang, Z., Zhao, N., Zhang, S., Wang, D., Wang, S., and Liu, N. (2024d). GAS41 regulates nuclear morphology via BRD2-mediator complex in colorectal cancer. Pharmacol. Res. 206: 107283, https://doi.org/10.1016/j.phrs.2024.107283.Search in Google Scholar PubMed
Wang, M., Zhou, Y., Li, W., Zhu, L., and Liu, D. (2024a). Lactate in neurodegenerative diseases: friend or foe? Ageing Res. Rev. 101: 102452, https://doi.org/10.1016/j.arr.2024.102452.Search in Google Scholar PubMed
Wei, L., Yang, X., Wang, J., Wang, Z., Wang, Q., Ding, Y., and Yu, A. (2023). H3K18 lactylation in senescent microglia exacerbates brain aging via NF-κB pathway. J. Neuroinflamm. 20: 208, https://doi.org/10.1186/s12974-023-02879-7.Search in Google Scholar PubMed PubMed Central
Weng, W., He, Z., Ma, Z., Huang, J., Han, Y., Feng, Q., Qi, W., Peng, Y., Wang, J., Gu, J., et al.. (2025). Tufm lactylation regulates neuronal apoptosis via mitophagy in TBI. Cell Death Differ. 32: 530–545, https://doi.org/10.1038/s41418-024-01408-0.Search in Google Scholar PubMed PubMed Central
Wu, A., Lee, D., and Xiong, W. (2023). Lactate in brain development and neurodegeneration. Int. J. Mol. Sci. 24: 13398, https://doi.org/10.3390/ijms241713398.Search in Google Scholar PubMed PubMed Central
Wu, Y., Yang, C., Yan, H., Lu, P., Zhang, L., Feng, W., and Long, Y. (2022). Hippocampal lysine acetylome reveals FMRP-linked metabolic dysregulation. J. Proteomics 269: 104720, https://doi.org/10.1016/j.jprot.2022.104720.Search in Google Scholar PubMed
Xiang, Y., Xu, G., and Weigel-van Aken, K.A.K. (2010). Lactate promotes aberrant APP processing via ER chaperones. PLoS One 5: e13820.10.1371/journal.pone.0013820Search in Google Scholar PubMed PubMed Central
Xie, Y., Hu, H., Liu, M., Zhou, T., Cheng, X., Huang, W., and Cao, L. (2022). Histone lactylation in health and disease: mechanisms and roles. Front. Genet. 13: 949252, https://doi.org/10.3389/fgene.2022.949252.Search in Google Scholar PubMed PubMed Central
Xiong, X., Pan, X., Luo, X., Wang, Y., Zhang, X., Yang, S., Zhong, Z., Liu, C., Chen, Q., Wang, P., et al.. (2024). Astrocyte-derived lactate exacerbates ischemic stroke injury via protein lactylation. Theranostics 14: 4297–4317, https://doi.org/10.7150/thno.96375.Search in Google Scholar PubMed PubMed Central
Xu, B., Liu, Y., Li, N., and Geng, Q. (2024). Lactate and lactylation in macrophage metabolic reprogramming: progress and challenges. Front. Immunol. 15: 1395786, https://doi.org/10.3389/fimmu.2024.1395786.Search in Google Scholar PubMed PubMed Central
Xu, H., Wu, M., Ma, X., Huang, W., and Xu, Y. (2021). Novel histone modifications in health and disease: functions and mechanisms. Biomed Res. Int. 2021: 6635225, https://doi.org/10.1155/2021/6635225.Search in Google Scholar PubMed PubMed Central
Yamagata, K. (2022). Astrocyte-to-neuron lactate shuttle in ischemic stroke neurodegeneration. Neuroscience 481: 219–231, https://doi.org/10.1016/j.neuroscience.2021.11.035.Search in Google Scholar PubMed
Yan, L., Wang, Y., Hu, H., Yang, D., Wang, W., Luo, Z., Wang, Y., Yang, F., So, K., and Zhang, L. (2024). Exercise-induced synaptic protein lactylation enhances stress resilience. Cell Metab. 36: 2104–2117.e4, https://doi.org/10.1016/j.cmet.2024.07.018.Search in Google Scholar PubMed
Yang, Y., Fan, C., Wang, B., Ma, Z., Wang, D., Gong, B., Di, S., Jiang, S., Li, Y., Li, T., et al.. (2017). Pterostilbene protects hippocampal neurons from high glucose-induced oxidative damage via Nrf2 activation. Biochim. Biophys. Acta Mol. Basis Dis. 1863: 827–837, https://doi.org/10.1016/j.bbadis.2017.01.005.Search in Google Scholar PubMed
Yang, Y., Kueh, A.J., Grant, Z.L., Abeysekera, W., Garnham, A.L., Wilcox, S., Hyland, C.D., Di Rago, L., Metcalf, D., Alexander, W.S., et al.. (2022). Histone acetyltransferase KAT7 regulates hematopoietic stem cell quiescence and self-renewal. Blood 139: 845–858, https://doi.org/10.1182/blood.2021013954.Search in Google Scholar PubMed
Yang, C., Pan, R., Guan, F., and Yuan, Z. (2024a). Lactate metabolism in neurodegenerative disorders. Neural Regen. Res. 19: 69–74, https://doi.org/10.4103/1673-5374.374142.Search in Google Scholar PubMed PubMed Central
Yang, W., Wang, P., Cao, P., Wang, S., Yang, Y., Su, H., and Nashun, B. (2021). Hypoxia reduces histone lactylation and impairs mouse embryonic development. Epigenet. Chromatin 14: 57, https://doi.org/10.1186/s13072-021-00431-6.Search in Google Scholar PubMed PubMed Central
Yang, D., Wang, Y., Qi, T., Zhang, X., Shen, L., Ma, J., Pang, Z., Lal, N.K., Mcclatchy, D.B., Seradj, S.H., et al.. (2024b). Pyruvate dehydrogenase phosphorylation inversely correlates with neuronal activity. Neuron 112: 959–971.e8, https://doi.org/10.1016/j.neuron.2023.12.015.Search in Google Scholar PubMed PubMed Central
Yao, X. and Li, C. (2023). LDHA-mediated histone lactylation induces pyroptosis via HMGB1. Metab. Brain Dis. 38: 1543–1553, https://doi.org/10.1007/s11011-023-01195-6.Search in Google Scholar PubMed
Yao, S., Xu, M., Wang, Y., Zhao, S., Wang, J., Chen, G., Chen, W., Liu, J., Huang, G., Sun, W., et al.. (2023). Astrocytic LDHA regulates neuronal excitability and depression through lactate homeostasis. Nat. Commun. 14: 729, https://doi.org/10.1038/s41467-023-36209-5.Search in Google Scholar PubMed PubMed Central
Ye, L., Jiang, Y., and Zhang, M. (2022). Metabolic crosstalk between glucose, lactate and immune responses. Cytokine Growth Factor Rev. 68: 81–92, https://doi.org/10.1016/j.cytogfr.2022.11.001.Search in Google Scholar PubMed
Yuan, W., Lu, G., Zhao, Y., He, X., Liao, S., Wang, Z., Lei, X., Xie, Z., Yang, X., Tang, S., et al.. (2025). Intranuclear TCA cycle and mitochondrial overload in tumor metabolism. Cancer Lett. 613: 217527, https://doi.org/10.1016/j.canlet.2025.217527.Search in Google Scholar PubMed
Zdralevic, M., Brand, A., Di Ianni, L., Dettmer, K., Reinders, J., Singer, K., Peter, K., Schnell, A., Bruss, C., Decking, S., et al.. (2018). Dual LDHA/B knockout ablates Warburg effect and restricts tumors to oxidative metabolism. J. Biol. Chem. 293: 15947–15961, https://doi.org/10.1074/jbc.RA118.004180.Search in Google Scholar PubMed PubMed Central
Zhai, G., Niu, Z., Jiang, Z., Zhao, F., Wang, S., Chen, C., Zheng, W., Wang, A., Zang, Y., Han, Y., et al.. (2024). DPF2 recognizes histone lactylation to drive oncogenic transcription. Proc. Natl. Acad. Sci. USA 121, e2421496121, https://doi.org/10.1073/pnas.2421496121.Search in Google Scholar PubMed PubMed Central
Zhang, Y. and Fan, D. (2016). Serum lactate clearance rate predicts ALS progression. Chin. Med. J. (Engl.) 129: 28–32, https://doi.org/10.4103/0366-6999.172561.Search in Google Scholar PubMed PubMed Central
Zhang, Y., Jiang, H., Dong, M., Min, J., He, X., Tan, Y., Liu, F., Chen, M., Chen, X., Yin, Q., et al.. (2024b). MCT4 inhibition promotes atherosclerosis regression via H3K18la-mediated gene repair. Cell Rep. 43: 114884, https://doi.org/10.1016/j.celrep.2024.114884.Search in Google Scholar PubMed
Zhang, J., Lin, F., Xu, Y., Sun, J., Zhang, L., and Chen, W. (2025a). Lactylation in ischemic stroke: mechanisms and therapeutic potential. Mol. Neurobiol. 62: 5359–5376, https://doi.org/10.1007/s12035-024-04624-4.Search in Google Scholar PubMed
Zhang, J., Muri, J., Fitzgerald, G., Gorski, T., Gianni-Barrera, R., Masschelein, E., D’Hulst, G., Gilardoni, P., Turiel, G., Fan, Z., et al.. (2020). Endothelial lactate promotes muscle regeneration via M2 macrophage polarization. Cell Metab. 31: 1136–1153.e7, https://doi.org/10.1016/j.cmet.2020.05.004.Search in Google Scholar PubMed PubMed Central
Zhang, X., Peng, L., Kuang, S., Wang, T., Wu, W., Zuo, S., Chen, C., Ye, J., Zheng, G., Guo, Y., et al.. (2025b). HIF-1α-mediated PMN-MDSC glycolysis limits neonatal brain injury. J. Neuroinflamm. 22: 59, https://doi.org/10.1186/s12974-025-03385-8.Search in Google Scholar PubMed PubMed Central
Zhang, D., Tang, Z., Huang, H., Zhou, G., Cui, C., Weng, Y., Liu, W., Kim, S., Lee, S., Perez-Neut, M., et al.. (2019). Histone lactylation links metabolism to gene regulation. Nature 574: 575–580, https://doi.org/10.1038/s41586-019-1678-1.Search in Google Scholar PubMed PubMed Central
Zhang, F., Zhou, J., Lu, P., Zhang, X., Yang, L., Wu, J., Zhang, L., Zhang, L., Pang, J., Xie, H., et al.. (2024a). BRD4-mediated histone lactylation regulates astrocyte polarization after subarachnoid hemorrhage. J. Neuroinflamm. 21: 186, https://doi.org/10.1186/s12974-024-03185-6.Search in Google Scholar PubMed PubMed Central
Zhao, Q., Wang, Q., Yao, Q., Yang, Z., Li, W., Cheng, X., Wen, Y., Chen, R., Xu, J., Wang, X., et al.. (2025). SLG-induced lysine D-lactylation attenuates inflammation. Cell Res. 35: 97–116, https://doi.org/10.1038/s41422-024-01060-w.Search in Google Scholar PubMed PubMed Central
Zheng, J., Xie, Y., Ren, L., Qi, L., Wu, L., Pan, X., Zhou, J., Chen, Z., and Liu, L. (2021). GLP-1 enhances astrocytic support for neurons via glycolytic activation in AD. Mol. Metab. 47: 101180, https://doi.org/10.1016/j.molmet.2021.101180.Search in Google Scholar PubMed PubMed Central
Zhou, J., Zhang, L., Peng, J., Zhang, X., Zhang, F., Wu, Y., Huang, A., Du, F., Liao, Y., He, Y., et al.. (2024). Astrocytic LRP1 facilitates mitochondrial transfer to neurons and alleviates ischemic stroke by inhibiting ARF1 lactylation. Cell Metab. 36: 2054–2068.e14, https://doi.org/10.1016/j.cmet.2024.05.016.Search in Google Scholar PubMed
Zhou, X., Zhang, N., Zhang, X., Sun, X., Ruan, Z., Yang, Q., Hu, M., and Shu, H. (2025). Acetaldehyde suppresses cGAS activity to impair antiviral and antitumor immunity. Cancer Lett. 617: 217615, https://doi.org/10.1016/j.canlet.2025.217615.Search in Google Scholar PubMed
Zong, Z., Xie, F., Wang, S., Wu, X., Zhang, Z., Yang, B., and Zhou, F. (2024). AARS1 senses lactate and lactylates p53 to promote tumorigenesis. Cell 187: 2375–2392.e33, https://doi.org/10.1016/j.cell.2024.04.002.Search in Google Scholar PubMed
Zou, G., Wang, T., Xiao, J., Wang, X., Jiang, L., Tou, F., Chen, Z., Qu, X., and Han, X. (2023). Lactate prevents oxidative stress-induced retinal degeneration via autophagy activation. Free Radic. Biol. Med. 194: 209–219, https://doi.org/10.1016/j.freeradbiomed.2022.12.004.Search in Google Scholar PubMed
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- From genetic roots to recent advancements in gene therapy targeting amyloid beta in Alzheimer’s disease
- Antimicrobial peptides and proteins in Alzheimer’s and Parkinson’s diseases: implications for biomarker exploration
- Autism spectrum disorder genetics; a comprehensive review
- Inflammation-related microRNA alterations in epilepsy: a systematic review of human and animal studies
- From lactate to lactylation: potential targets for the treatment of neurodegenerative diseases
Articles in the same Issue
- Frontmatter
- From genetic roots to recent advancements in gene therapy targeting amyloid beta in Alzheimer’s disease
- Antimicrobial peptides and proteins in Alzheimer’s and Parkinson’s diseases: implications for biomarker exploration
- Autism spectrum disorder genetics; a comprehensive review
- Inflammation-related microRNA alterations in epilepsy: a systematic review of human and animal studies
- From lactate to lactylation: potential targets for the treatment of neurodegenerative diseases


