Abstract
A series of core-shell magnetic nanomaterials have been synthesized with the intent of applying them for metal ion capture in a newly designed pipeline reactor. The synthetic chemistry is an extension of a previously developed family of materials based on amorphous silica gel, which has been used in the mining and remediation industries. The nanoparticles were characterized by infrared spectroscopy and TEM and SEM techniques. The size of the starting magnetite core was critical to the behavior of the particles under metal sequestering conditions. The capture kinetics of the resulting nanoagregates is 10 times faster than related micro composites. All of the tests performed point to the future successful development of a technology that circumvents the disadvantages associated with the use of column based microparticles.
Introduction
The use of ion exchange technologies in the mining and remediation industries has experienced significant growth in recent years as the ability to modify support surfaces such as polystyrene has advanced considerably [1]. In addition, hybrid materials consisting of an organic polymer and the more rigid and porous amorphous silica gel have gained considerable popularity. These materials offer less sensitivity to swings to temperature and pH and in general have faster capture kinetics compared to their polystyrene analogs [2], [3]. A fairly recent example of this type of material is silica polyamine composites (SPC) (Fig. 1) [3]. This patented and licensed technology has been widely used in the mining and remediation industry [2], [3]. These materials are synthesized by a stepwise process, which has been found to be compatible with standard techniques for modifying silica gel surfaces [2], [3].
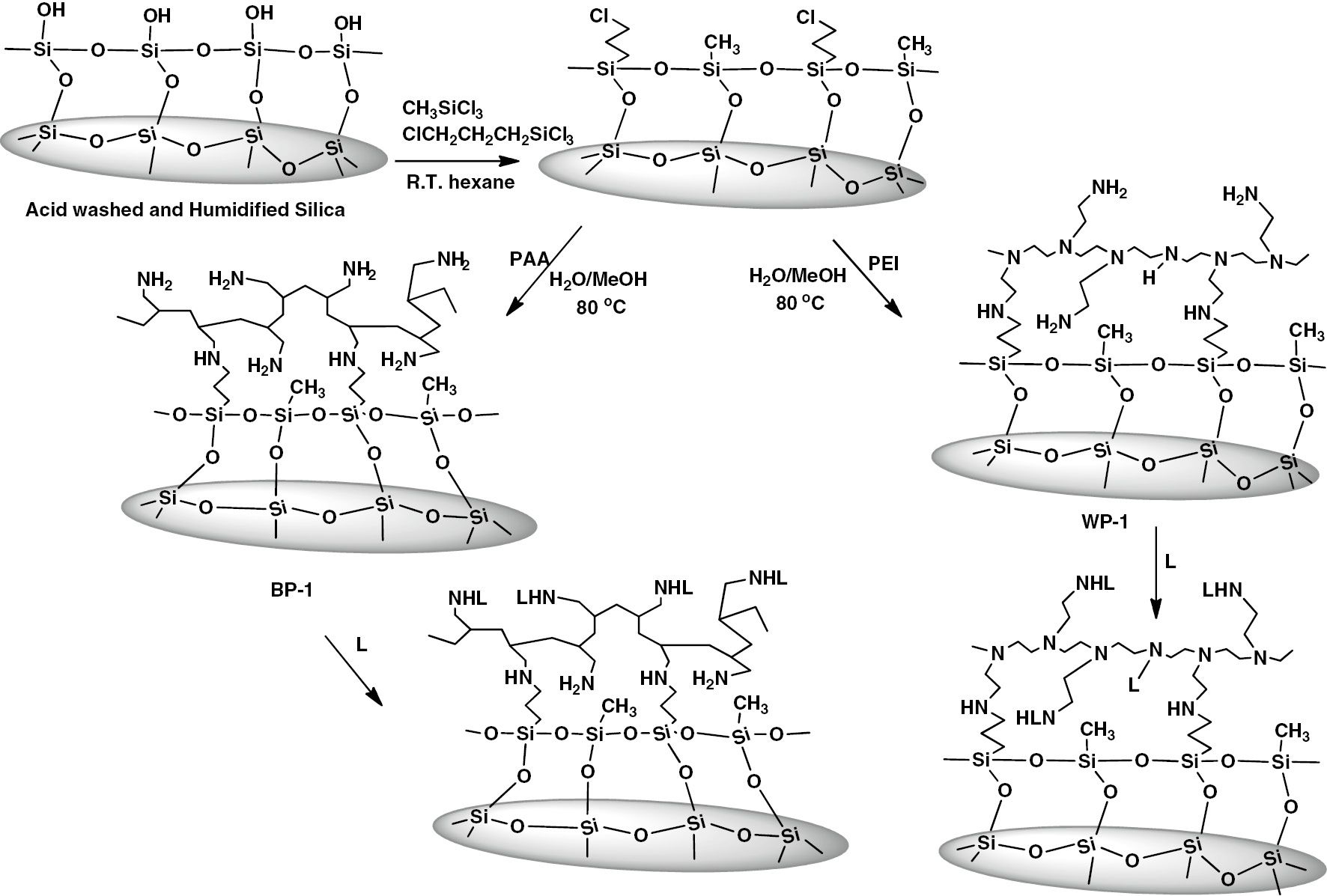
Stepwise synthesis of SPC.
These hybrid materials are made with two polyamines: poly(ethyleneimine) (PEI), a branched polymer with a distribution of different amines (35% 2°, 35% 1°, 30% 3°) that comes in a wide variety of molecular weights (300–23 K); poly(allylamine) (PAA) that contains only primary amines and comes in two molecular weights (15 K and 60 K). The PAA offers more sites for ligand modification and potentially higher metal loading while the branched polymer offers local higher coordination sites providing selectivity with just the various types of amines and is cheaper to produce. The applications of these materials in industry involve the use of ion exchange columns. The design of these columns is complex and must take into account the nature of the ions to be captured, their concentration and the flow requirements of the process to name a few of the system parameters that must be considered. Once designed and built the column is difficult to adapt to the system to process changes or to changes in the nature of the feed. In addition, the system is very sensitive to the presence of suspended solids that can clog fluid flow leading to increased backpressure and eventually deconstruction or at least extensive back flushing. These drawbacks to fixed bed ion exchange systems can lead to considerable process costs and will interfere with continuous process operation [1].
An alterative to fixed bed columns is the use of suspended magnetic particles that can selectively capture desired ions while leaving unwanted suspended solids in the feed solution. The loaded particles are then captured by an electromagnet passing the depleted stream and then released to a stripping regeneration circuit for reuse. A proposed system of this type is shown in Fig. 2.
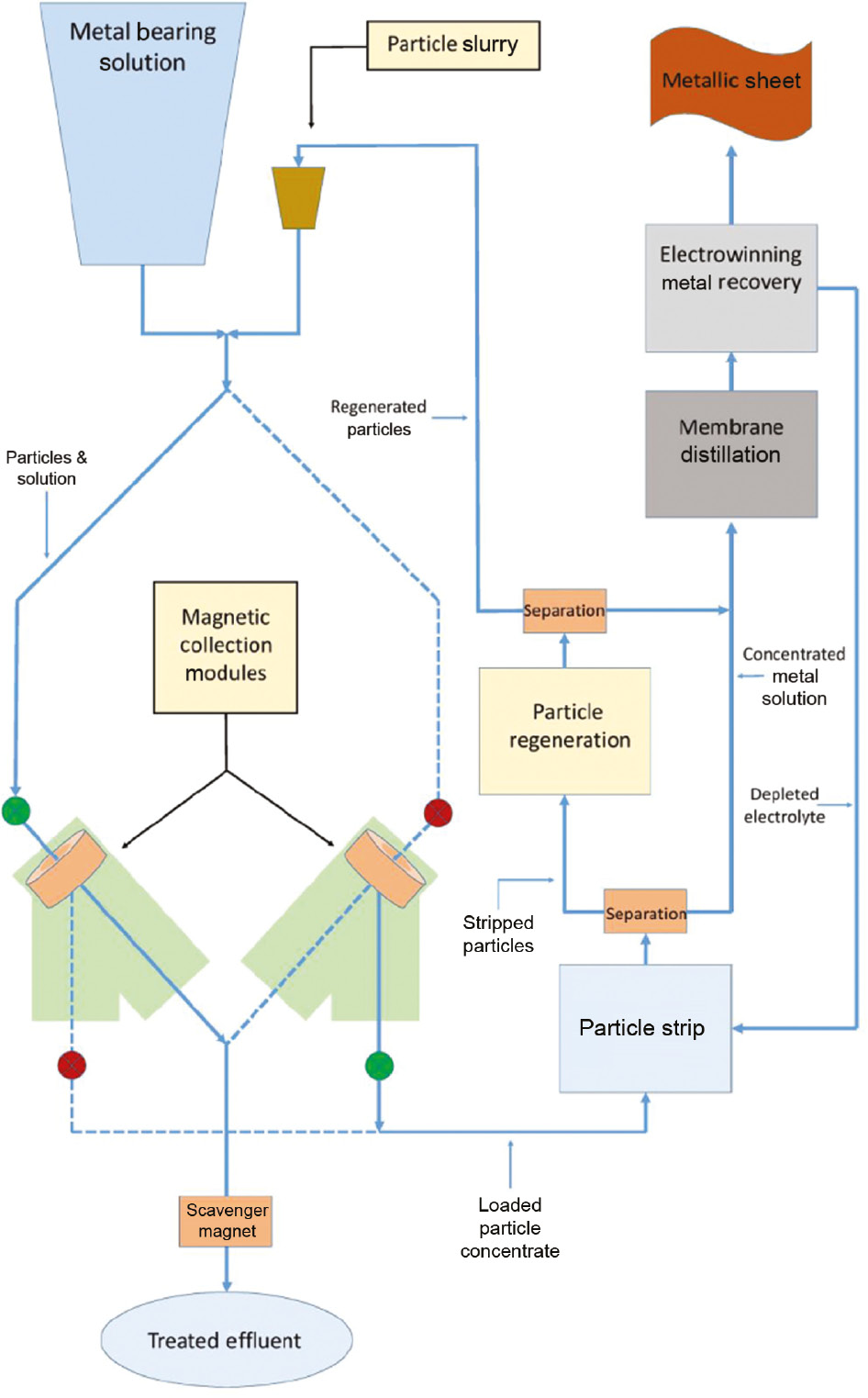
Proposed ion exchange system utilizing core-shell magnetic nanoparticles in a fluidized reactor.
The system would consist of one or more tubes connected to a reservoir of the metal bearing solution and a smaller reservoir containing a slurry of the metal capturing core-shell magnetic nanoparticles. After passing through the tubes that contain a static mixer, at a controlled rate the suspended particles are captured by an electromagnet. The loaded particles are then released into the stripping circuit while the depleted feed solution is sent to storage or released into the environment after final scrubbing. Stripping of the loaded particles is accomplished with dilute acid (0.1 M) and separated with an electromagnet, regenerated (dilute base) and then returned to the particle storage as a slurry. The concentrated strip solution is then sent to the electrowhinning circuit where metal sheets are produced using a set of low volume electroplating cells. In some cases a membrane concentration step may be necessary if the strip solution contains <5 g/L in case of copper. Given a sufficient inventory of particles and computer controlled pumping and valving the process could be made continuous. In this report we focus on the synthesis, characterization and testing at both the bench and pilot scale of the core-shell magnetic nanoparticles as this is an important part of the system described in Fig. 2. There have been many reports on the use of core-shell magnetic nanoparticles [4], but none used the pipeline reactor or the stepwise SPC modification methods reported here.
Results and discussion
Construction of the core-shell magnetic nanoparticles starts with the magnetite core, which can be synthesized by coprecipitation of ferrous and ferric salts under basic conditions (Eq. 1) [4]. This method gives superparamagnetic magnetite particles in good yield with 5–15 nm average diameters. TEM and SEM micrographs showed well-formed rectangular crystals. These nanoparticles were then subjected to two different procedures for producing the required silica shell. One procedure utilized silicic acid directly on the magnetite nanoparticles made by removal of sodium from sodium silicate by ion exchange (Eq. 2) [4].
The other involved base promoted hydrolysis of tetraethylorthosilicate (TEOS) in the presence of the magnetite nanoparticles (Eq. 3) [5].
Both methods provided silicate shells suitable for further modification (vide infra). The silicic acid method gave a slightly thicker silicate shell and the reagents were less expensive. However, the TEOS method was much simpler to implement and it was decided to use that method throughout the remainder of the project. TEM and SEM micrographs of the resulting silica coated nanoparticles showed well-defined particles and DLS measurements gave an average hydrodynamic radius range of 200–300 nm. The silica layer thickness is estimated to be 2–4 nm from the TEM image (Fig. 3). Coating of Superparamagnetic particles with silica is a well-known process [6], [7], [8], [9], [10], [11]. However, there have been few studies on reacting a polyamine with the silica coated nanoparticles [10]. No studies on further functionalization of the tethered polyamine have been reported. SEM of these particles showed well-formed rectangular particles as small aggregates (Fig. 4).
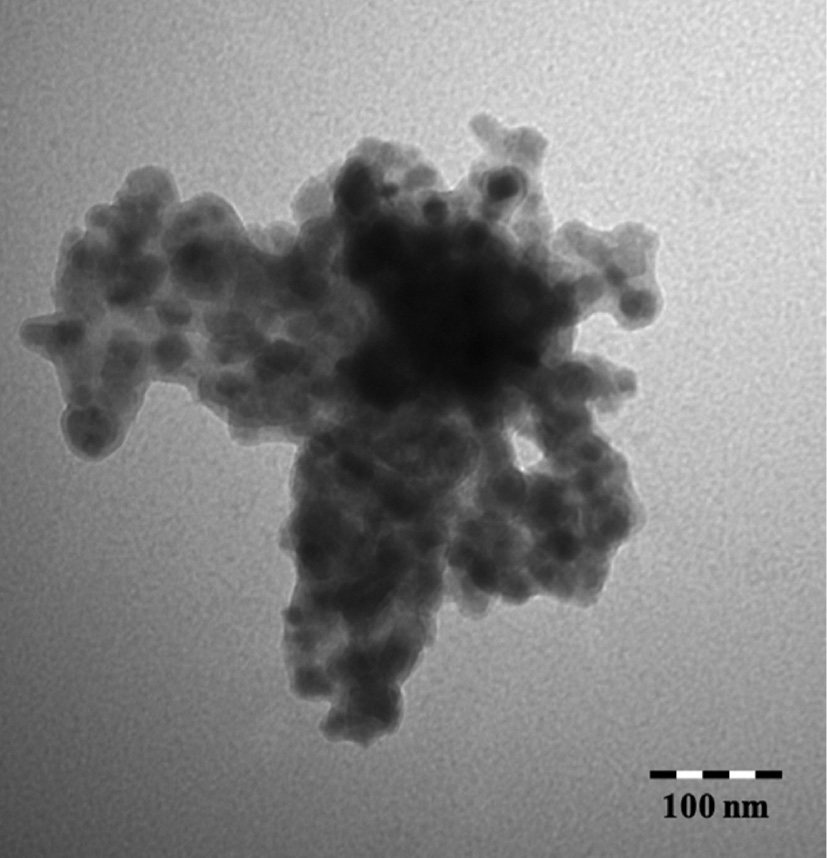
TEM micrograph of TEOS coated magnetite.
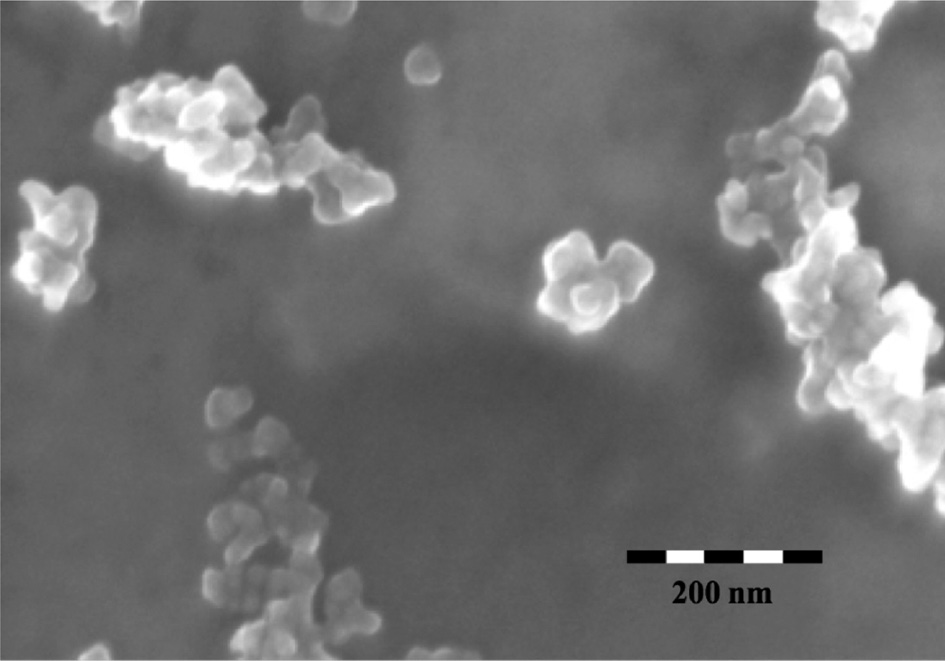
SEM of TEOS coated magnetite particles.
The next step in the synthesis is to react the TEOS coated magnetite particles directly with a trimethoxysilyl substituted amine. For this purpose we chose the commercially available ethylenediaminetrimethoxysilane (EDTMS) and aminopropyltrimethoxysilane (APTMS). The polymeric amines PEI (MW=23000) and PAA (MW=15000 or 60000) require reaction with a 7.5:1 mixture of trimethoxymethylsilane (TMMS) and 3-chloropropyltrimethoxysilane (CPTMS). This step provides the chloropropyl silane linker that will react with the amine molecules used in the next step. The TMMS provides a spacer, which was found to be necessary to maximize metal capacity for the previously studied micro particles [12]. All these reactions were done in toluene for 1 h in the case of the monomeric amines and 24 h for the polyamines. The equilibrium copper batch capacities were determined for all of these composites and it was found that the capacities were similar to those obtained for the amorphous silica micro particles and the silica nanoparticles (Fig. 5) [12]. As with these other silica surfaces we found that PAA gave significantly higher capacities than the other amines. The EDTMS, The APTMS and the PEI composites all gave copper capacities in the range of 0.5–0.6 mmol/g while the PAA gave a capacity of 0.8 mmol/g. Most of our subsequent testing was done with this composite.
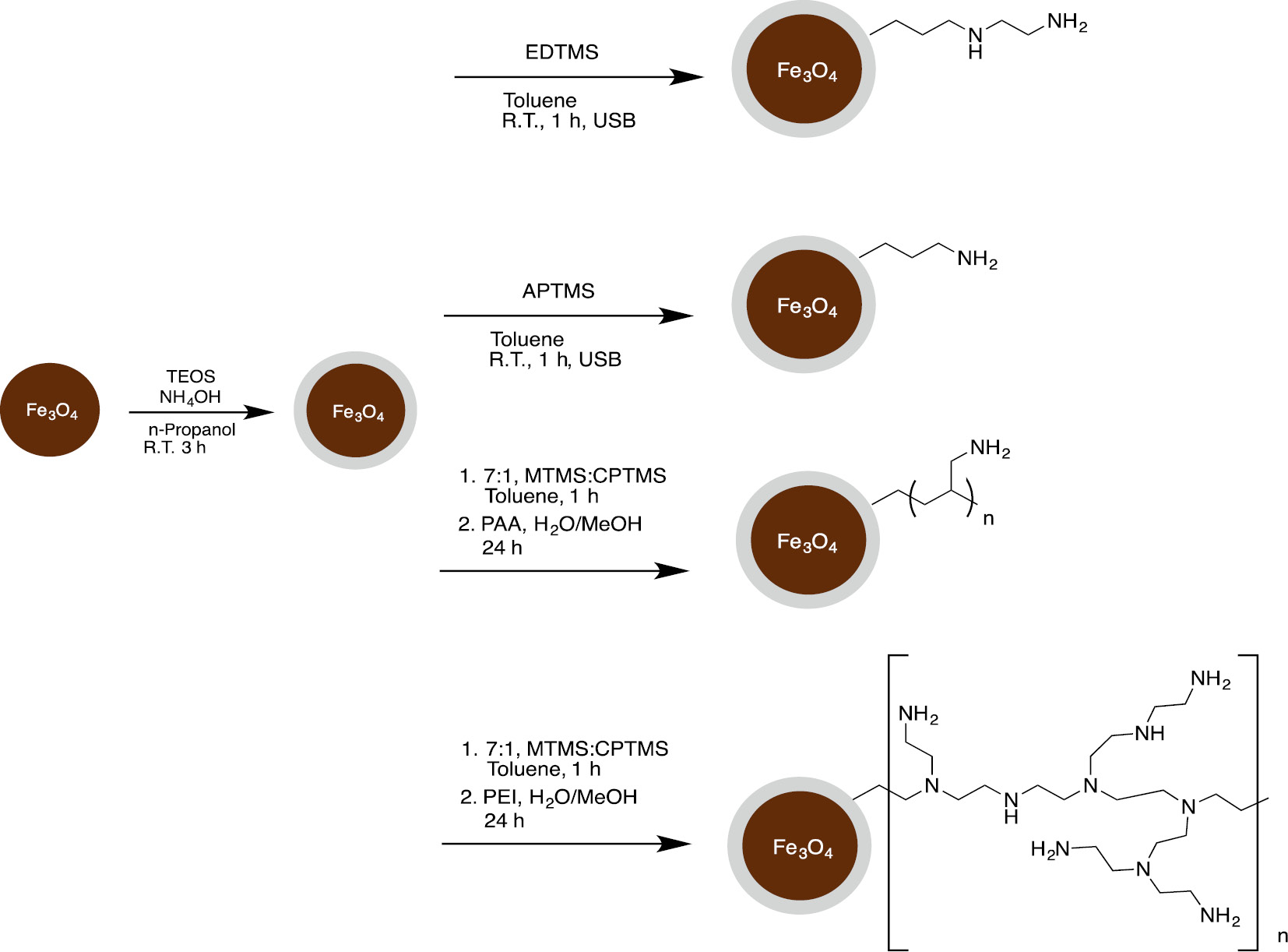
Amine modified core-shell silica magnetite nanoparticles.
For these nanoparticles to be useful in the proposed system they must be reusable. To evaluate this we subjected the PAA composite to repeated load-strip-regenerate cycles. The results of these initial studies are summarized in Fig. 6.
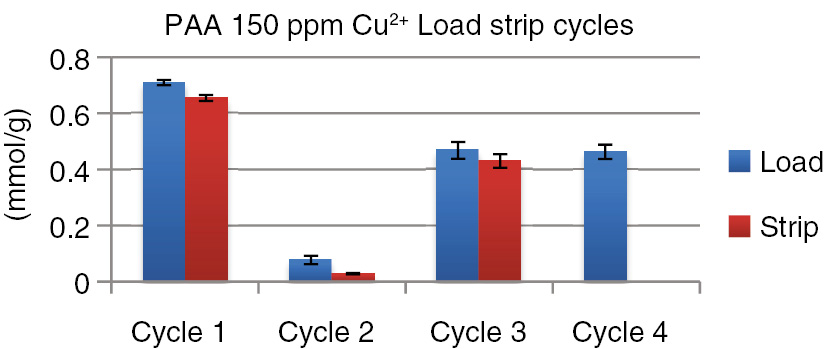
Initial cycle testing for PAA composite.
In Cycle 1, after loading to equilibrium capacity with a 150 ppm solution at pH=3.5 of copper sulfate, a strip solution of 0.1 M citric acid was applied for 1 h. The pH of 3.5 is the intrinsic pH of copper sulfate solutions at ambient temperatures. These materials have been tested in the pH range of 2–8 for the related micro particles [12], [13], [14], [15], [16], [17], [18]. Recovery of >95% was realized even with the weak citric acid solution used. In Cycle 2 we attempted regeneration with water and it can be seen that very little copper was captured or stripped. This is in sharp contrast to the results obtained with the amorphous micro silica gel where the higher porosity allowed more efficient reloading and stripping with just a water wash. Regeneration with 1 M ammonium hydroxide however, restored capacity in Cycle 3 and stripping was >95% with the same citric acid solution. In Cycle 4, stripping was the same as in Cycle 3 after regeneration. More extensive recycling will be reported for the pilot scale discussed below. These initial results do demonstrate reusability and point to the first significant difference between the previously reported micro particles and the nanoparticles presented here. Given that these particles will be used in both acid and basic environments a primary concern is the leaching of iron from the magnetite core. To test for iron leaching, samples of PAA core-shell nanoparticles were soaked in 0.1 M citric acid for 1, 2 and 3 h, respectively. Figure 7 shows that only 0.8–0.9 mass % of iron was lost from the core based on the calculated initial mass of iron in the nanoparticles. Leached iron in the citric acid was measured by atomic absorption spectroscopy.
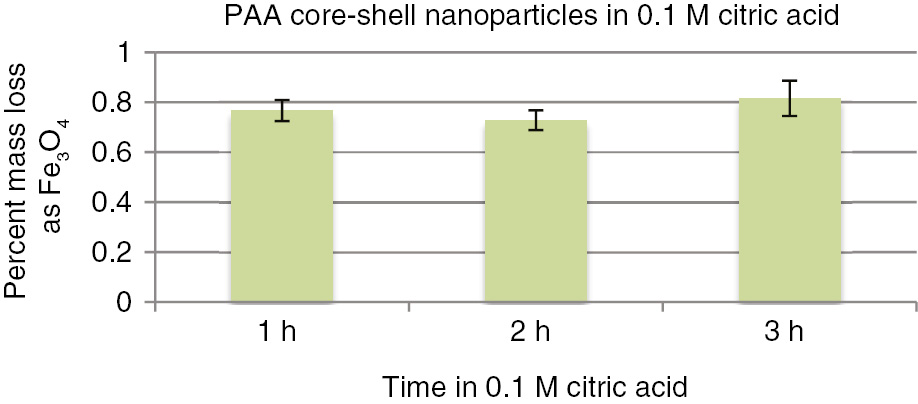
Iron leaching from magnetite core of PAA nanoparticles.
Thus the core-shell magnetite-silica-PAA nanoparticles show good capacity, reusability and low leaching of the iron core. The particles suspended easily and remained suspended in water in the absence of a magnetic field. However, when the magnetic filed was applied to these suspensions on the pilot-scale (vide infra) it was found that a significant portion (~20%) passes the inline electromagnet. Running particles that broke through the magnet again led to further capture showing that all the particles were paramagnetic. From a practical point of view this is a serious drawback for the magnetite cores made by coprecipitation. It is not deemed feasible to increase the strength of the electromagnet given the design of the pilot-scale pipeline reactor where pipeline diameter and field strength need to be well matched.
It was therefore decided to go to a larger diameter magnetite core that is available from SkySpring™ Nanomaterials. These magnetite particles have average core diameters of 20–30 nm rather than the 5–15 nm particles made by coprecipitation. Attempts to make larger magnetite cores by coprecipitation on a preparative scale failed due to incomplete conversion of the starting iron salts. The commercially available SkySpring magnetite particles proved more difficult to disperse but it was possible to modify them as above and they proved to be very efficiently captured by the in-line electromagnets used in the pilot scale reactor (vide infra). TEM showed that the particles were less individualized and DLS measurements showed a broad distribution of particle sizes with hydrodynamic radii of 1000–3000 nm (Fig. 8). However, copper batch capacities were similar to the particles made with the smaller magnetite cores, 0.5 mmol/g, and could be stripped and regenerated for 10 cycles without loss of capacity. Thus the core-shell particles made with the larger core are actually nano-aggregates. These nano-aggregates with diameters of about 1 μm showed properties that were better suited to the pilot-scale pipeline reactor. They were efficiently captured by the electromagnets, they settled more quickly and could be safely stripped in repeated cycles with 0.1 M sulfuric acid. These nano-aggregates show the right balance between the strength of the magnetic core and their ability to capture metal ions while maintaining matrix stability (low iron leaching).
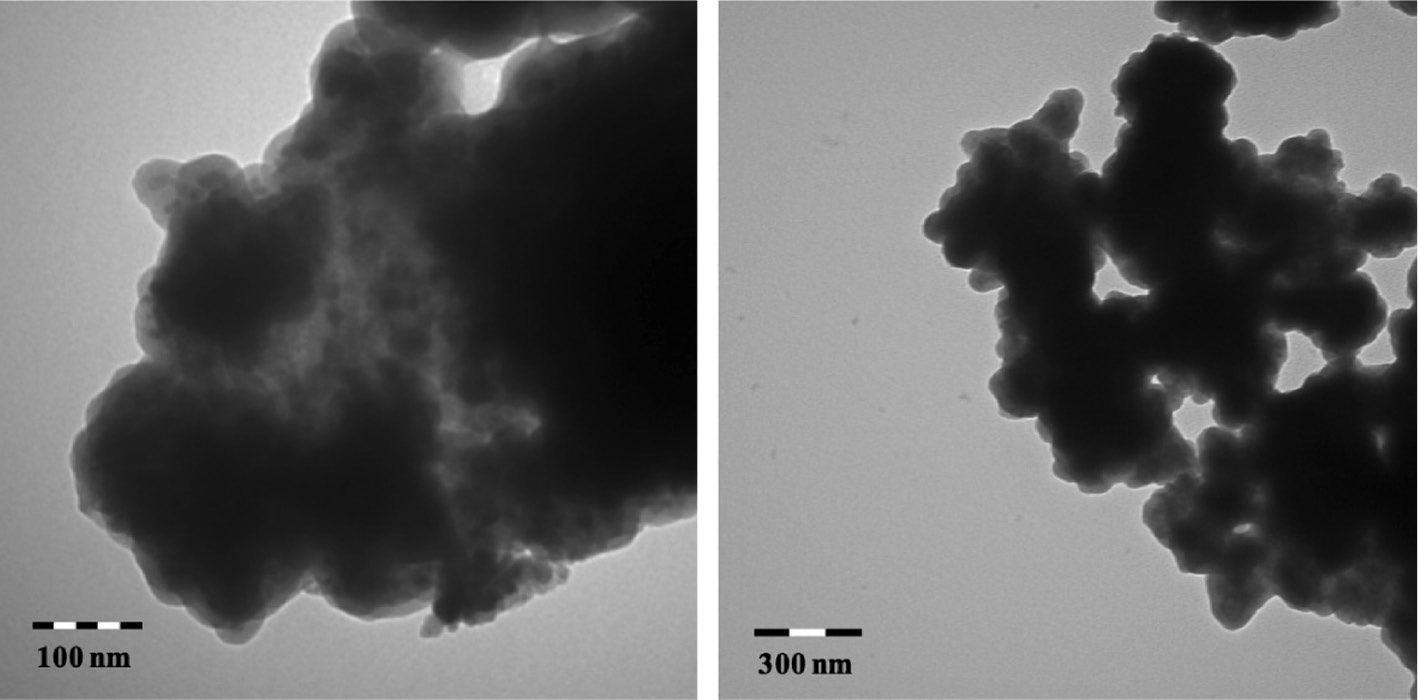
TEM micrographs of nano-aggregates made from the SkySpring™ magnetite cores on a 50 g scale and having an equilibrium batch capacity of 0.5 mmol/g.
Kinetic measurements on solutions containing 150 ppm copper sulfate at pH=3.5 showed that equilibrium was reached within 2–3 min (Fig. 9). This is more than 10 times faster than the related amorphous silica micro-particles. This much faster capture kinetics is a property critical to the successful use of the pipeline reactor and will make this configuration more efficient than the column configurations currently employed with the micro particles.
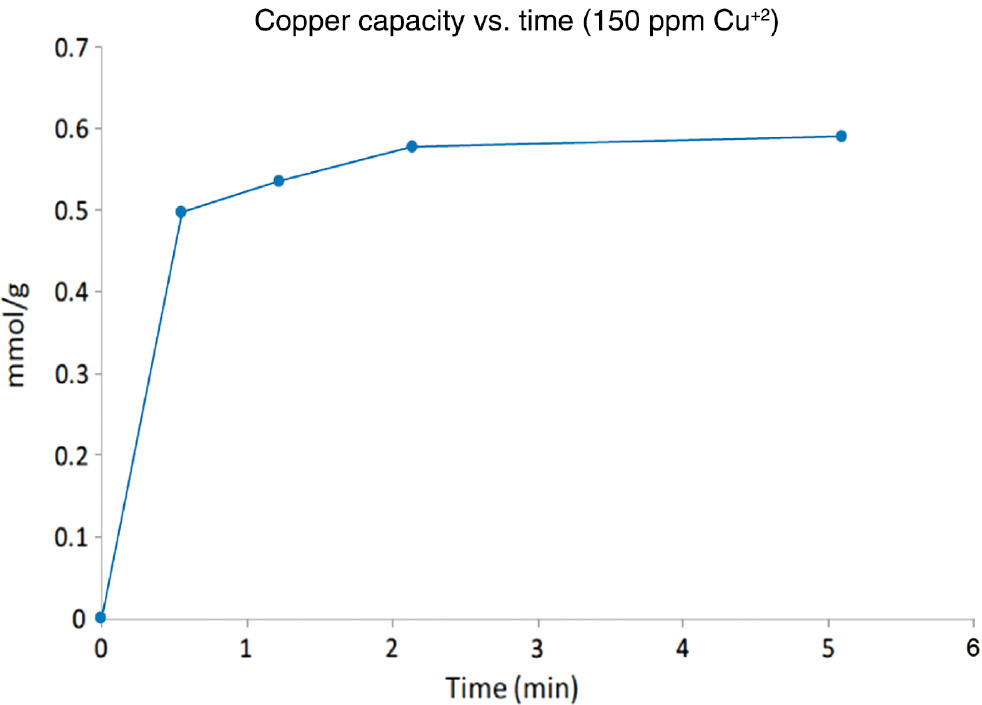
Kinetic plot of adsorption of Cu2+ using SkySpring-TEOS-PAA core-shell nano-aggregates.
Although these nano-aggregates are not individualized particles they behave as single site solid phase adsorbents. This is supported by the results of their adsorption isotherms. Thus they give linear plots with both the Langmuir and Freundlich models, which gave correlation coefficients of 0.97 and 0.96 (Fig. 10). This provides evidence that these materials active as single site non-cooperative solid phase adsorbents.
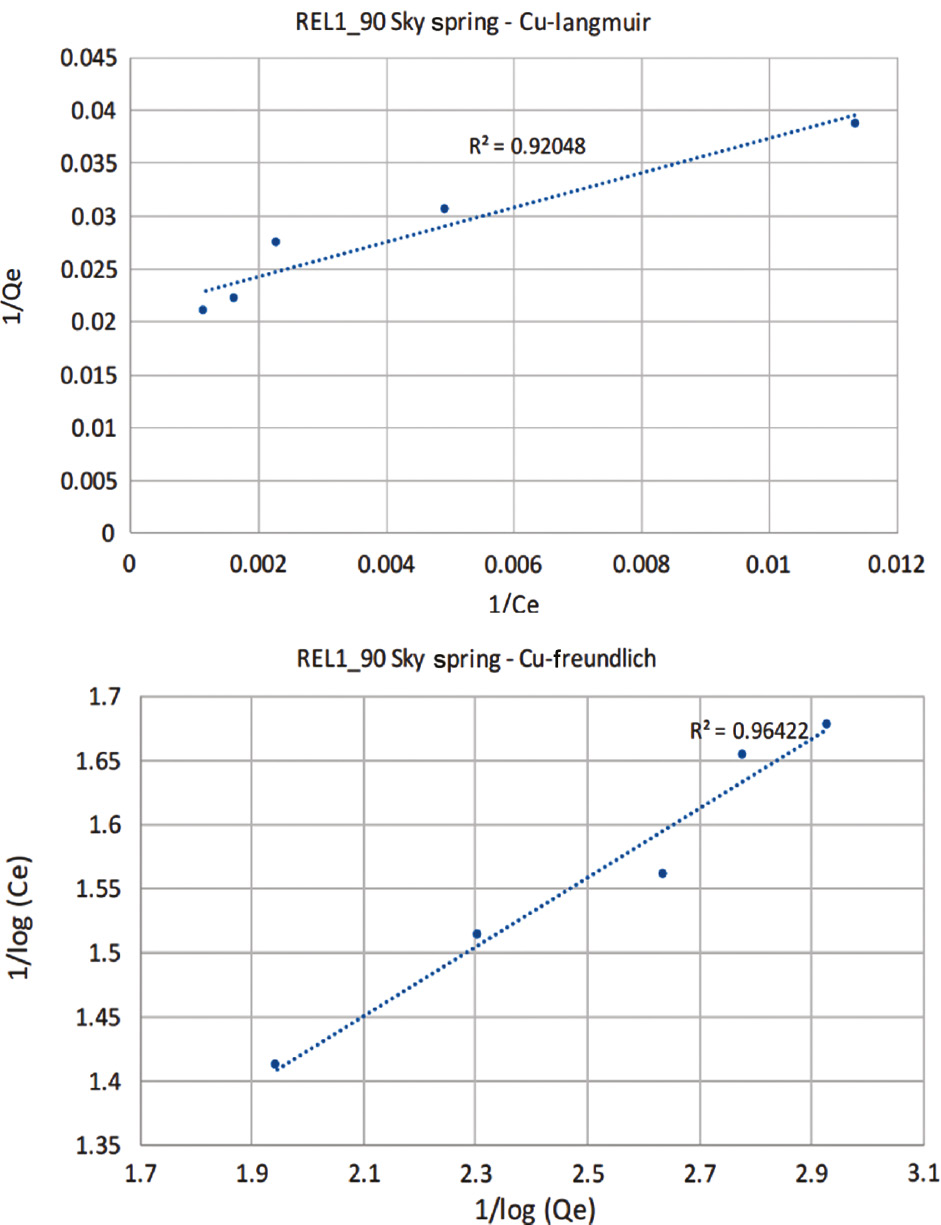
Langmuir (top) and Fruendlich (bottom) plots of the adsorption isotherms for SkySpring-TEOS-PAA core-shell nano-aggregates.
Our engineering collaborators at Montana Tech have completed construction of the adsorption-stripping and regeneration side of the pipeline reactor. A photograph of the adsorption pipeline with the inline electromagnet, the wastewater reservoir and the nano-aggregate feed container is shown in Fig. 11.
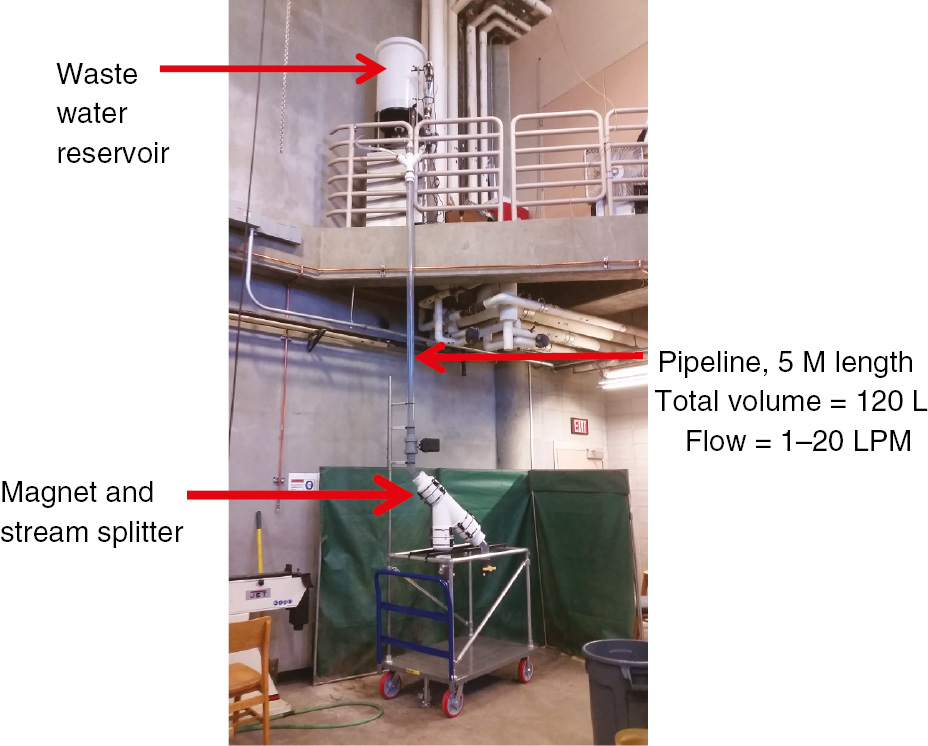
Photograph of the pipeline reactor adsorption circuit.
Experiments were conducted by mixing 18 L of copper sulfate solution with 40–50 g of nano-aggregate. The mixture is then released into the 120 L pipeline that contains a static mixer to keep the particles suspended at a flow rate of 1–20 LPM. At 20 LPM the residence time of the slurry is about 2 min. The electromagnet is on during this period and captures 100% of the suspended particles. The captured-loaded particles are then released into a container of 0.1 M sulfuric acid by turning off the magnetic field. The strip solution is then measured for copper content by atomic absorption spectroscopy. The particles are then regenerated with ammonium hydroxide, rinsed and reused. To date we have run 15 cycles like this with less than 10% loss in copper capacity from the original 0.5 mmol/g composite. The rapid capture and efficient recovery of copper in these preliminary tests bodes well for the further development of the rest of the circuit.
Conclusions and future work
The fluidized pipeline reactor is compatible with the Sky Spring™ magnetite core, modified with SPC chemistry. Capacities on the pilot scale were about the same as in the lab studies. The captured metals can be efficiently recovered and reused. Very short residence times (2 min) in the pipeline are realized owing to the fast capture kinetics of the materials. The size of the magnetite core relative to the silica coating is critical to the performance of the material. The composite that worked best is a nano-aggregate, not a collection of nanoparticles. The next steps in the development of this process are to design and implement the stripping – regeneration circuits. We plan to continue development of metal selective functional groups as has already been done with the amorphous silica gels [13], [14], [15], [16], [17], [18]. Once we can apply metal selectivity, testing of actual waste streams will begin followed by a study of system scalability and economics. The work done so far demonstrates the feasibility of designing particles that can be efficiently captured by an electromagnet after suitable modification of the surface using technology developed for amorphous silica.
Experimental
The synthetic procedures reported here have not yet been published as applied to the core-shell magnetic particles. Experimental details for the amorphous silica micro particles have been extensively described in the literature [13], [14], [15], [16], [17], [18]. For the sake of brevity we summarize below only the protocols that were used for making the particles employed in the pipeline reactor.
Materials and methods
TEOS, CPTMS and MTMS were all purchased from Sigma Aldrich and used as received. PAA hydrochloride (MW=60 K) and PEI (MW=25 K) were purchased from Sigma-Aldrich and used as received. Solvents were reagent grade and Toluene was dried over 4a molecular sieves before use.
IR spectra were taken on a Thermo-Nicolet 633 FT-IR spectrometer as KBr pellets. Transmission Electron Microscopy (TEM) was performed on a Hitachi H-7100. Samples were prepared by incorporating the nanoparticles in an epoxy plug, after curing, thin slices were shaved off and the TEM taken on a selection of the slices.
Atomic Absorption (AA) analyses were done on an S series Thermo Electron corporation AA spectrometer. Metal ion solutions were run in a 2% nitric acid solution and were diluted to give approximately 0.1–0.2 absorbance units. AA analysis was used to determine the metal ion remaining bound on the surface of composites by measurement of the difference between the total metal ion in the initial solution and the total metal ion in the filtrate and rinses.
The dynamic light scattering measurements were made on a Malvern Zetasizer NS. The samples were under sonication until ~30 s before measurements began. Each sample underwent three measurements, consisting of between 12 and 15 s scans. The data for each measurement was then compiled using the Malver Zetasizer software and presented as graphs.
Synthesis
Silica coating of Fe3O4 nanoparticles
Eighty gram of Fe3O4 nanoparticles (NPs) were suspended in one liter of 2-propanol in a beaker and sonicated in a bath sonicator for 15 min while occasionally stirring before being placed in a 12 L three-neck round-bottom flask. While stirring (overhead stirrer, 285 rpm) 3 L of 2-propanol and 1 L of deionized water were added, followed by 200 mL of 28% ammonium hydroxide solution. After stirring for 5 min, 70 mL of tetraethyl orthosilicate (TEOS) was added. The suspension was stirred at room temperature for 3 h under nitrogen atmosphere. A permanent magnet was carefully placed on the bottom side of the flask to collect the NPs and the supernatant was removed using an aspirator vacuum. Multiple portions (~800 total volume) of 2-propanol were used to remove the NPs from the flask and then collected in a beaker. The NPs were washed with 2×300 mL of 2-propanol and 2×250 mL of acetone by collecting the NPs on the bottom of a beaker with a permanent magnet and discarding the supernatant. A stream of air was used to evaporate most of the residual acetone before drying the NPs in a vacuum chamber. Typical isolated yield: 90–100 g.
Surface functionalization with 3-chloropropyltrimethoxysilane (CPTMS) and methyltrimethoxysilane (MTMS)
One hundred gram of silica-coated Fe3O4 nanoparticles were suspended in 800 mL of toluene and stirred (overhead, 200 rpm) and sonicated in a bath sonicator for 20 min in a 3 L three-neck round bottom flask under ambient conditions. Three hundred and fifty milliliter of MTMS and 50 mL of CPTMS (7:1 volume/volume ratio) were dissolved in 400 mL of toluene before adding to the nanoparticle suspension. After stirring for 3 h, the NPs were washed with 2×300 mL of toluene and 2×250 mL of acetone by collecting the NPs on the bottom of a beaker with a permanent magnet and discarding the supernatant. A stream of air was used to evaporate most of the residual acetone before drying the NPs in a vacuum chamber. Typical isolated yield was ~100 g, depending on the amount of NPs that were lost during wash steps.
Surface functionalization with poly(allylamine) polymer
One hundred gram of MTMS/CPTMS-functionalized NPs were added suspended in 100 mL of methanol followed by the addition of 350 mL of aqueous 15% wt/wt poly(allylamine) solution. NPs were stirred (overhead, 200 RPM) and sonicated in a bath sonicator under ambient conditions overnight using the same configuration as shown in Fig. 2. The NPs were washed with 4×400 mL of deionized water and 2×250 mL of acetone by collecting the NPs on the bottom of a beaker with a permanent magnet and discarding the supernatant. A stream of air was used to evaporate most of the residual acetone before drying the NPs in a vacuum chamber. Typical isolated yields were 95–97 g depending on the amount of NPs that were lost during wash steps.
Preparation of aqueous 15% wt/wt poly(allylamine) solution from PAA-HCl salt
Twenty five gram of poly(allylamine) hydrochloride (17500 average MW, Sigma-Aldrich) was dissolved in 50 mL of deionized water in a tarred beaker containing a magnetic stir bar. The pH of the solution was adjusted to 12.35 by the addition of 4 M sodium hydroxide solution (~50 mL). Enough deionized water was added to achieve a total mass of 166.50 g.
Article note:
A collection of invited papers based on presentations at 8th International IUPAC Symposium on Macro- and Supramolecular Architectures and Materials: Multifunctional Materials and Structures (MAM-17) held in Sochi, Russia, 6–10 June 2017.
Acknowledgements
We gratefully acknowledge the support of the Montana Research and Economic Development Initiative for their support of this research. We also acknowledge our collaborators at Montana Tech, Professor Jerome Downey and Graduate Student David Hutchins for their design and construction of the pipeline reactor depicted here in Figure 11 in which the nano-aggregates were used.
References
[1] A. A. Zagorodni. Ion Exchange Materials, Properties and Applications, Elsevier, Amsterdam (2007).10.1016/B978-008044552-6/50006-XSearch in Google Scholar
[2] E. Rosenberg. in Macromolecules Containing Metal and Metal Like Elements, Volume 4, C. E. Carraher, C. U. Pittman, A. S. Abd-El-Aziz, M. Zeldin, J. E. Sheats (Eds.), pp. 51–78, J. Wiley & Sons, New York (2005).Search in Google Scholar
[3] M. Hughes, P. Miranda, D. Nielsen, E. Rosenberg, R. Gobetto, A. Viale, S. Burton. in Recent Advances and Novel Approaches in Macromolecule-Metal Complexes, (R. Barbucci, F. Ciardelli, G. Ruggeri (Eds.), pp. 161–178, Wiley-VCH (Macromolecular Symposia 235), Weinheim (2006).10.1002/masy.200650320Search in Google Scholar
[4] M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi, J. I. Zink. ACS Nano2, 889 (2008).10.1021/nn800072tSearch in Google Scholar PubMed PubMed Central
[5] G. Abbott, R. Brooks, E. Rosenberg. Appl. Poly. Sci. 132, 42271 (2015).10.1002/app.42271Search in Google Scholar
[6] D. Dupont, W. Brullot, M. Bloemen, T. Verbiest, K. Binnemans. ACS Appl. Mater. Interfaces6, 4980 (2014).10.1021/am406027ySearch in Google Scholar PubMed
[7] D. Dupont, J. Luyten, M. Bloemen, T. Verbiest, K. Bineman. Ind. Eng. Chem. Res. 53, 15222 (2014).10.1021/ie502546cSearch in Google Scholar
[8] Y. Huang, A. A. Keller. Water Res.80, 159 (2015).10.1016/j.watres.2015.05.011Search in Google Scholar PubMed
[9] W. Wu, Q. He, C. Jiang. Nanoscale Res Lett.3, 397 (2008).10.1007/s11671-008-9174-9Search in Google Scholar PubMed PubMed Central
[10] L. Zhong, Q. Zhang, M. Sun, Y. Zhang, H. Jiang, H. Lian. Desal. and Water Treat. 1 (2015).Search in Google Scholar
[11] Y. Lu, Y. Yin, B. T. Mayers, Y. Xia. Nano Lett.2, 183 (2002).; M. Ma, Y. Zhang, W. Yu, H. Shen, H. Hang, N. Gu. Colloids and Surfaces A: Physicochem. Eng. Aspects212, 219 (2003).Search in Google Scholar
[12] M. Hughes, D. Nielsen, E. Rosenberg, R. Gobetto, A. Viale, S. D. Burton. Ind. Eng. Chem. Res.45, 6538 (2006).10.1021/ie0601448Search in Google Scholar
[13] S. T. Beatty, R. J. Fischer, D. L. Hagers, E. Rosenberg. Ind. Eng. Chem. Res. 38, 4402 (1999).10.1021/ie9903386Search in Google Scholar
[14] Y. O. Wong, P. Miranda, E. Rosenberg. J. Appl. Poly. Sci. 115, 2855 (2010).10.1002/app.31229Search in Google Scholar
[15] G. Abbott, R. Brooks, E. Rosenberg, M. Terwilliger, J. B. Alexander Ross, O. O. L. Ichire. Organometallics33, 2467 (2014).10.1021/om401153xSearch in Google Scholar PubMed PubMed Central
[16] Md A. Goni, E. Rosenberg, S. Meregude, G. Abbott, J. Organometall. Chem.807, 1 (2016).10.1016/j.jorganchem.2016.01.032Search in Google Scholar
[17] E. Karakhanov, A. Maximov, Y. Kardasheva, V. Semernina, A. Zolotukhina, A. Ivanov, G. Abbott, E. Rosenberg. ACS Appl. Mat. Interf.6, 8807 (2014).10.1021/am501528aSearch in Google Scholar PubMed
[18] V. Kailasam, E. Rosenberg, D. Nielsen. Ind. Eng. Chem. Res.48, 3991 (2009).10.1021/ie8016362Search in Google Scholar
©2018 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Core-shell Fe-SiO2-polyamine magnetic nanoparticles for metal recovery using a continuous flow pipeline reactor
- Characterizations of radicals formed in radical polymerizations and transfer reactions by electron spin resonance spectroscopy
- IUPAC Technical Reports
- The ongoing challenge of novel psychoactive drugs of abuse. Part I. Synthetic cannabinoids (IUPAC Technical Report)
- Engineered nanomaterials and human health: Part 1. Preparation, functionalization and characterization (IUPAC Technical Report)
- Engineered nanomaterials and human health: Part 2. Applications and nanotoxicology (IUPAC Technical Report)
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Core-shell Fe-SiO2-polyamine magnetic nanoparticles for metal recovery using a continuous flow pipeline reactor
- Characterizations of radicals formed in radical polymerizations and transfer reactions by electron spin resonance spectroscopy
- IUPAC Technical Reports
- The ongoing challenge of novel psychoactive drugs of abuse. Part I. Synthetic cannabinoids (IUPAC Technical Report)
- Engineered nanomaterials and human health: Part 1. Preparation, functionalization and characterization (IUPAC Technical Report)
- Engineered nanomaterials and human health: Part 2. Applications and nanotoxicology (IUPAC Technical Report)

