Abstract
New cyclic and spirocyclic aminosilanes were synthesised using ethylenediamine, 2-aminobenzylamine, 1,8-diaminonaphthalene, o-phenylenediamine, and trans-cyclohexane-1,2-diamine as starting material. These diamines were converted into aminosilanes using silicon tetrachloride and dimethyldichlorosilane directly and via the N,N’-bis(trimethylsilylated) amino derivatives. 15 new compounds of the type (diamino)(SiMe3)2, (diamino)2Si, (diamino)SiMe2, and (diamino)SiCl2 have been prepared. The formation of two cyclotrisilazane derivatives was observed starting from (N,N’-2-aminobenzylamino)dichlorosilane by trimerisation. All synthesised compounds have been characterised with NMR-, Raman-, or IR-spectroscopy, mass-spectrometry, and boiling or melting point. Single-crystal X-ray structure analyses of several derivatives have been performed.
The degree of substitution with trimethylsilyl groups in the final compounds depends on the ring size of the spirocycles. It was shown with quantum chemical calculations on the M062X/6-31G(d) level that trimethylsilyl groups have a stabilising effect on 5-membered ring systems and a destabilising effect on 6-membered rings in these compounds.
1 Introduction
Aminosilanes, i.e., silicon organic compounds with at least one Si–NR2 bond (R = H, hydrocarbyl), are the topic of research for several decades. They are often synthesised in non-polar aprotic solvents, such as pentane and hexane (Böhme et al., 2000, 2003a, 2003b; Herzog et al., 1998; Huber and Schmidbaur, 1999; Huber et al., 1997a, 1997b; Meinel et al., 2014, 2015; Schlosser et al., 1994; Tamao et al., 1994; Trommer et al., 1997). Up to four amino moieties can be bound to one silicon atom. Besides hydrolysis, alcoholysis and other substitution reactions, the Si–N bond can undergo insertion reactions with heteroallenes (Barluenga et al., 1989; Degl’Innocenti et al., 1992; Glidewell and Rankin, 1970; Herbig et al., 2018a; Kirilinet al., 2009; Kraushaar et al., 2017; Wolff et al., 1978). For example, CO2 can insert into the Si–N bond to form a carbamoic moiety (Kraushaar et al., 2012, 2014).
Siloxanes as derivatives of silanediols are studied for decades (e.g., Jaçoviç, 1957). The corresponding diaminosilanes (and aminosilanes with three or four Si–NR2 moieties) are less researched (Armitage, 1982). Interesting structural motifs for this class of aminosilanes are cyclic and oligocyclic structures. So far, many cyclic aminosilanes having two Si–N bonds per cycle are known (e.g., Herbig et al., 2018b). But only in a few compounds the silicon atom is part of one or two independent cyclic systems without sterically demanding substituents (Kaßner et al., 2016; Kummer and Rochow, 1963). Most of the known spirocyclic aminosilanes contain ethylene bridges like in Figure 1. The substituents R are either aliphatic (Me, Et – Yoder and Zimmermann (1964); iPr – Schlosser et al. (1994)) or feature aryl groups (benzyl – Yoder and Zimmermann (1964); p-methylphenyl – Rong et al. (1998); benzenesulfonyl, tosyl – Schlosser et al. (1994). Wannagat and Eisle (1978) have synthesised compounds with either Me or TMS moieties on each ring. Cyclic aminosilanes with exocyclic silyl moieties starting from ethylenediamine are also known (Auner et al. 1992; Böhme et al., 2007; Daniéle et al., 2001; Diedrich et al., 2002). Prout et al. (1994) reported cyclic aminosilanes starting from o-phenylenediamine. Other compounds having a Si atom as spiro atom are derivatives of o-phenylenediamine and 2-aminobenzylamine (Kaßner et al., 2016; Kummer and Rochow, 1963) or spirosilazanes (Schlingmann and Wannagat, 1976a), a series of heavier congeners of the latter of which has also been studied (Schlingmann and Wannagat, 1976b).

Generic structures of cyclic and spirocyclic aminosilanes in this work (with R1 = ethylene, o-phenylene, 1,2-cyclohexanediyl, benzyl-2-yl, 1,8-naphthylene; R2 = H, SiMe3; R3 = Me, Cl).
In this work new cyclic and spirocyclic aminosilanes according to Figure 1 are described. We set out to prepare these compounds in order to obtain an overview about the preparative accessibility to this class of compounds and to use them later for further derivatisation reactions. The amines shown in Figure 2 were used for the investigations.

Amines used as starting materials.
2 Results
The outcome of numerous reactions is summarised in Sections 2.1, 2.2, and 3.1 in order to provide a coherent overview for the reader. NMR spectroscopic data are condensed in Section 3.2. Molecular structures are discussed in detail in Section 3.3, in order to understand the diversity of the obtained structures. The results of quantum chemical calculations in Section 3.4 help to understand the formation of different products.
2.1 Syntheses of N,N’-bis(trimethylsilyl)ated aminosilanes
The N,N’-bis(trimethylsilyl)ated products (derivatives 1a–5a) were obtained in high yields by reacting the amine with chlorotrimethylsilane in the presence of triethylamine (Scheme 1). Silylation reactions of that kind are often carried out in nonpolar solvents like n-pentane or n-hexane to support the separation (i.e., precipitation) of the amine hydrochloride formed as by-product. In the current case, however, THF was used because of the better solubility of some of the diamines in this solvent.

Synthesis of derivatives a from amines and chlorotrimethylsilane in the presence of triethylamine.
2.2 Syntheses of cyclic and spirocyclic derivatives
Two different routes are pursued for the synthesis of cyclic and spirocyclic aminosilanes: The reaction of the diamines 1–5 with the chlorosilane in the presence of triethylamine as base is denoted as method A (Scheme 2). The reaction of the N,N’-bis(trimethylsilyl)ated diamine derivatives 1a–5a with the corresponding chlorosilane and triethylamine is denoted as method B (Scheme 2).

Synthesis of cyclic aminosilanes with method A and B.
Synthesised spirocyclic compounds are labelled with the number of the parent diamine and the suffix b. Two types of cyclic aminosilanes were synthesised: Derivatives with the suffix c contain two methyl groups bound to the endocyclic silicon atom. Derivatives d contain two chlorine atoms at the endocyclic silicon atom. Additional trimethylsilyl groups at the nitrogen atoms are indicated by the suffix -TMS and the number of these moieties per molecule.
2.2.1 Syntheses with 1 and 1a
Compounds 1 and 1a are colourless liquids. The reactions and experiments are shown in Scheme 3. All attempts to synthesise cyclic and spirocyclic silicon compounds starting with 1 lead to product mixtures from which no products could be isolated. Starting with the bis-trimethylsilylated derivative 1a cyclic and spirocyclic compounds could be synthesised with all trimethylsilyl moieties retained. Compound 1d-TMS2 was synthesised by our group and published earlier (Böhme et al., 2007). The substance 1b-TMS4 is also a solid while 1c-TMS2 is a colourless liquid, the structure of which is confirmed by NMR spectroscopy and the molar mass by cryoscopy.

Experiments with and reactions of ethylenediamine (1).
2.2.2 Syntheses with 2 and 2a
The attempts to synthesise aminosilanes with o-phenylenediamine 2 gave intractable product mixtures in reactions with silicon tetrachloride and dichlorodimethylsilane (see Scheme 4). In reactions with 2a half of the trimethylsilyl moieties are preserved in the cyclic and spirocyclic compounds. The compounds 2b-TMS2 and 2d-TMS were crystallised and the molecular structures were determined. Earlier, 2d-TMS2 was reported as product of the reaction of 2a with SiCl4 and triethylamine in toluene (Wagler and Roewer, 2007). Obviously, changing the solvent alters the reactivity of 2a significantly.

Experiments with and reactions of o-phenylenediamine (2).
2.2.3 Syntheses with 3 and 3a
Compound 3 gave intractable product mixtures in reactions with silicon tetrachloride and dichlorodimethylsilane. Even though silylation of 3 afforded 3a in good yield (Scheme 5), further reactions of 3a with SiCl4 and Me2SiCl2 afforded mixtures of products from which individual compounds could not be isolated or have been isolated in poor yield only. Thus, details related to the reactions of 3a can be found in Supplementary material and have not been included in the discussion.

Experiments with and reactions of 1,2-diaminocyclohexane (3).
2.2.4 Syntheses with 4 and 4a
The outcome of the reactions of 4 are different from those of 1 to 3 because the cycle to be created is a 6-membered ring unlike the 5-membered one in the previous target products (see Scheme 6). The reactions with the amine 4 gave isolable products (4b and 4c). Compound 4e is formed together with 4c. Therein, two molecules of 4c are connected via a Me2Si moiety at the aliphatic amine N atom. A changed reactivity pattern is also observed in the reaction which aimed at formation of the SiCl2 containing derivative 4d. The expected product was not obtained, but the cyclotrisilazane compounds 4f and 4g formed by subsequent substitution of Si–Cl bonds for Si–N with the aliphatic amine group. Both 4f and 4g contain 6-membered silazane rings in the centre supplemented by three 2-aminobenzylamine moieties fused to this ring. Both compounds, which are isomers, are obtained from the same reaction batch and vary only in the arrangement of the diamine moieties.

Experiments with and reactions of 2-aminobenzylamine (4).
Starting with 4a the synthesis of 4b-TMS2 is possible. The trimethylsilyl moieties on the aliphatic amine N atom are preserved. All cyclic and spirocyclic derivatives of 4 were crystallised and their molecular structures were determined (see Section 3.3).
2.2.5 Syntheses with 5 and 5a
The results of the reactions with 5 are similar to the reactions of 4. Starting with 5, the spirocyclic and cyclic products 5b and 5c are obtained (Scheme 7). The dichloro derivative 5d-TMS was synthesised, preserving one of the two trimethylsilyl groups from 5a. Further condensation reactions (as encountered with derivatives of 4) were not observed. One reason is the lower reactivity due to the absence of any aliphatic amine moiety in 5. The compounds 5b, 5c, and 5d-TMS were crystallised and their molecular structures were determined (see Section 3.3).

Experiments with and reactions of 1,8-diaminonaphthalene (5).
3 Discussion
3.1 Discussion of the outcome of the syntheses
Our current observations indicate that for the syntheses of spiro-compounds with 6-membered rings the synthetic route starting with the amine (method A) is more favourable while compounds containing 5-membered rings should be synthesised starting from the trimethylsilyl derivatives a (method B). This fact can be explained with steric aspects. If method B is applied for the synthesis of 4b, 4c, 5b, and 5c side products are observed, as outlined in Schemes 6 and 7. In fact, within the same reaction time, no indication of spirocyclic compounds b or c can be found in the NMR spectra. Nevertheless, using method A for the synthesis of 5-membered silacycles, the formation of oligo- and polymers as main products is indicated in the NMR spectra by sets of very broad signals.
It must be pointed out that the synthesised spirocyclic aminosilanes show a tendency for polymerisation. So it happened regularly, that an already isolated substance formed a gelatinous compound shortly after being dissolved in solvents like CDCl3. Most likely, ring opening reactions or further reactions with NH-functions took place. Polymerisations were not further investigated, since we were interested in the preparation of molecular compounds as precursors for insertion reactions. The results of insertion reactions will be reported in a forthcoming paper.
3.2 29Si NMR data of the synthesised compounds
All successfully synthesised and isolated compounds have been characterised in various ways such as NMR-, Raman-, and mass spectroscopy as well as melting points if possible. 29Si NMR data of the new compounds are summarised in Table 1. In the literature, many other ethylenediamine derivatives are known. Table 2 shows a compilation of their 29Si NMR shifts. The comparison of the 29Si NMR data is in support of the assignment of the silicon containing moieties in the new products.
29Si NMR data of the synthesised compounds
| Amine | Derivative a | Derivative b | Derivative c | Derivative d |
|---|---|---|---|---|
| 1 | 3.7 | 15.3 (TMS: 1.6) | 12.1 (TMS: 1.4) | −18.6 (TMS: 6.2) (Böhme et al., 2007) |
| 2 | 3.8 | −24.6 (TMS: 4.2) | 15.1 (TMS: 2.1) | −23.5 (TMS: 6.8) |
| 3 | 1.6 | exact assignment impossible | 10.6 (TMS: −1.3) | −20.7 (TMS: 3.7) |
| 4 | 1.6, 5.5 | −49.0 | −1.7 | derivative not synthesised |
| 5 | 3.6 | −59.1 | −5.6 | −37.5 |
TMS: Trimethylsilyl moieties.
29Si NMR data of compounds taken from literature
| Amine | Substituents on endocyclic silicon atom | Substituents on N | Literature | Chemical shift of endocyclic silicon atom | Chemical shift of exocyclic silicon atom |
|---|---|---|---|---|---|
| 2 | Ph, Ph | H | Prout et al., 1994 | 11.8 | -- |
| 2 | Ph, Me | H | Prout et al., 1994 | −0.7 | -- |
| 2 | Cl, Cl | TMS | Wagler and Roewer, 2007 | −18.1 | 6.5 |
| 1 | Me, CH(SiMe3)CH(Me)2 | TMS | Auner et al., 1992 | 14.0 | 1.3 (NTMS), 3.0 (CTMS) |
| 1 | Vi, Ph | TMS | Auner et al., 1992 | −9.4 | 2.8 |
| 1 | Cl, Cl | TMS | Böhme et al., 2007 | −18.6 | 6.2 |
| 1 | Cl, Cl | SiMePh2 | Wagler and Roewer, 2007 | −16.7 | −8.4 |
| 1 | Cl, Cl | SiMe2Ph | Diedrich et al., 2002 | −17.4 | −1.4 |
| 1 | Cl, Cl | SiMe2tBu | Diedrich et al., 2002 | −17.0 | 10.0 |
| 1 | Cl, H | SiMe2tBu | Diedrich et al., 2002 | −15.2 | 9.1 |
| 1 | Br, Br | SiMe2tBu | Diedrich et al., 2002 | −38.7 | 9.9 |
| 1 | iPr, iPr | TMS | Diedrich et al., 2002 | 17.7 | 0.8 |
No data for comparison are known in literature for the aminosilanes 1a, 2a, 3a, and 4a. Only for 5a the 29Si NMR shift of 3.7 ppm (Gade et al., 2002) can be found, which is close to the shift we recorded. The 29Si NMR shift for compound 4b found in literature has the value −48.9 ppm (Lange A., Cox G., Wolf H., Csihony S., Spange S., Kaßner L. et al., 2014, Stickstoffhaltige Kompositmaterialien, deren Herstellung und Verwendung. World patent, 2015086461) and is also very close to the data obtained in our experiment. The 29Si NMR shifts of derivatives b, c, and d (cyclic and spirocyclic compounds) reveal a clear difference between 6-membered and 5-membered rings. The silicon nuclei of derivatives of amines 1, 2, and 3 are less shielded while the 29Si NMR signals of derivatives of the amines 4 and 5 are high field shifted. All TMS moieties exhibit a chemical shift similar to that of hexamethyldisilazane (2.4 ppm).
3.3 Molecular structures
Compound 2b-TMS2 crystallises in the monoclinic space group C2/c with a half molecule in the asymmetric unit. Compound 2d-TMS crystallises in the orthorhombic space group P212121 with one molecule in the asymmetric unit. The molecular structures are shown in Figures 3 and 4, essential geometric parameters in Table 3. The 1,2-diaminobenzene units in both compounds carry one trimethylsilyl group each. This was already concluded from the NMR data. The bonds Si1–N1 are longer than the bonds Si1–N2. This is attributed to the substitution of N1 with the trimethylsilyl group. As a result of the five-membered ring(s), the coordination sphere about silicon atom Si1 is rather distorted tetrahedral. The intra-chelate bond angle N1–Si1–N2 (93.06(8)° in 2b-TMS2 and 95.47(9)° in 2d-TMS) is noticeably smaller than the other angles at Si1. All other bond angles are expanded to values between 115.52(8) to 121.31(12)° in 2b-TMS2 and 113.75(7)° to 115.48(6)° in 2d-TMS (see Table 3). The (N,N’-o-phenylenediamino) silicon units (involving the atoms C1 to C6, N1, N2, and Si1) are essentially planar in both molecules. The two (N,N’-o-phenylenediamino)silicon units in 2b-TMS2 intersect at an angle of 83.32(4)°. This deviation from orthogonality can be explained with the steric bulk of the two trimethylsilyl groups within the molecule. However, this value is very similar to the structural analogous thio compound Si[S2(o-C6H4)]2, where partial planarisation was already observed without additional substituents (Herzog et al., 2000). Partial planarisation of the silicon coordination sphere in spirocyclic tetrathiosilanes was explained with the high s-character of the Si–S-bonds (Wojnowski et al., 1985). The dihedral angle between the (N,N’-o-phenylenediamino)silicon unit in 2d-TMS and the plane Cl1–Si1–Cl2 is 89.89(3)°. The Si1–Cl bonds in 2d-TMS are 2.0480(7) and 2.0435(7) Å. These values are very similar to the Si–Cl bonds in analogous compounds (Böhme et al., 2007; Schlosser et al., 1994; Wagler and Roewer, 2007).

Molecular structure of 2b-TMS2 including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level.

Molecular structure of 2d-TMS including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level.
Selected geometric parameters (Å, °) of 2b-TMS2 and 2d-TMS
| 2b-TMS2 | 2d-TMS | ||
| Si(1)–N(1) | 1.7362(17) | Si(1)–N(1) | 1.7259(16) |
| Si(1)–N(2) | 1.7172(18) | Si(1)–N(2) | 1.6983(19) |
| Si(2)–N(1) | 1.7654(18) | Si(1)–Cl(1) | 2.0480(7) |
| Si(1)–Cl(2) | 2.0435(7) | ||
| Si(2)–N(1) | 1.7756(17) | ||
| N(2)–Si(1)–N(1) | 93.06(8) | N(2)–Si(1)–N(1) | 95.47(9) |
| N(2)–Si(1)–N(1A) | 115.52(8) | Cl(2)–Si(1)–Cl(1) | 103.68(3) |
| N(1A)–Si(1)–N(1) | 120.78(13) | N(1)–Si(1)–Cl(1) | 114.43(6) |
| N(2A)–Si(1)–N(2) | 115.52(8) | ||
| N(2A)–Si(1)–N(1) | |||
| N(1A)–Si(1)–N(1) | 121.31(12) | N(1)–Si(1)–Cl(2) | 115.48(6) |
| N(2A)–Si(1)–N(2) | 120.78(13) | ||
| N(1A)–Si(1)–N(1) | 121.31(12) | N(2)–Si(1)–Cl(1) | 113.75(7) |
| N(2)–Si(1)–Cl(2) | 114.54(7) | ||
The single crystal structures of four derivatives of 1d have been published previously, see Figure 5. In 2,2-dichloro-1,3-bis(trimethylsilyl)-1,3-diaza-2-silacyclopentane (Böhme et al., 2007), 1,3-dibenzyl-2,2-dichloro-1,3-diaza-2-silacyclopentane (Schlosser et al., 1994), and 2,2-dichloro-1,3-bis(methyldiphenylsilyl)-1,3-diaza-2-silacyclopentane (Wagler and Roewer, 2007) the ethylenediamine unit (N–C–C–N) adopts a torsion angle of 32° to 34°. In contrast, in 2,2-dichloro-1,3-diphenyl-1,3-diaza-2-silacyclopentane this torsion angle is 0° due to a crystallographically imposed Cs-symmetry (Schlosser et al., 1994).

Previously published derivatives of 1d.
Compound 3d-TMS2 has a similar topology like derivatives of 1d. As the bond precision of this structure suffers from disorder effects and thus does not contribute to the discussion, this structure is presented in Supplementary material only.
Compound 4b-TMS2 crystallises in the monoclinic space group C2/c with a half molecule in the asymmetric unit. The molecular structure is shown in Figure 6, essential geometric parameters in Table 4. In accord with NMR data, there is a trimethylsilyl group bound at nitrogen atom N2. The coordination geometry at silicon atom Si1 is roughly tetrahedral and, with bond angles ranging from 102.00(6)° to 116.69(7)°, less distorted than in case of compound 2b-TMS2. Unlike the latter, this molecule bears two different kinds of amine moieties: N1 bound to the aromatic ring whereas N2 is benzylic. This and the different additional substituents cause slightly different Si–N bond lengths for the central silicon atom Si1. The coordination spheres about the nitrogen atoms are noticeably planarised albeit not completely planar, as the sum of bond angles around nitrogen atoms N1 and N2 are 352.59° and 355.04°, respectively. This was rather unexpected for nitrogen atom N1, which is substituted with a phenyl group and a silicon atom, because with this substitution pattern it was expected to be planar (Meinel et al., 2014, 2015). Further steric requirements by the spirocycle and the additional trimethylsilyl groups might explain the deviation of both nitrogen atoms′ environments from planarity in order to fit into the conformation of the six-membered ring. As to the latter, ring puckering analysis reveals a boat conformation for the ring containing the atoms Si1–N1–C1–C6–C7–N2 with the parameters θ = 101.1(2)°, φ = 70.8(1)°, and a ring puckering amplitude Q = 0.549(2) Å. Ideal parameters for a boat conformation are θ = 90.0°; φ = k · 60° (Cremer and Pople, 1975), (Boeyens, 1978). The presence of the CH2-group at C7 prevents the formation of a planar 6-membered ring as it was found in 5b and 5c (see below).
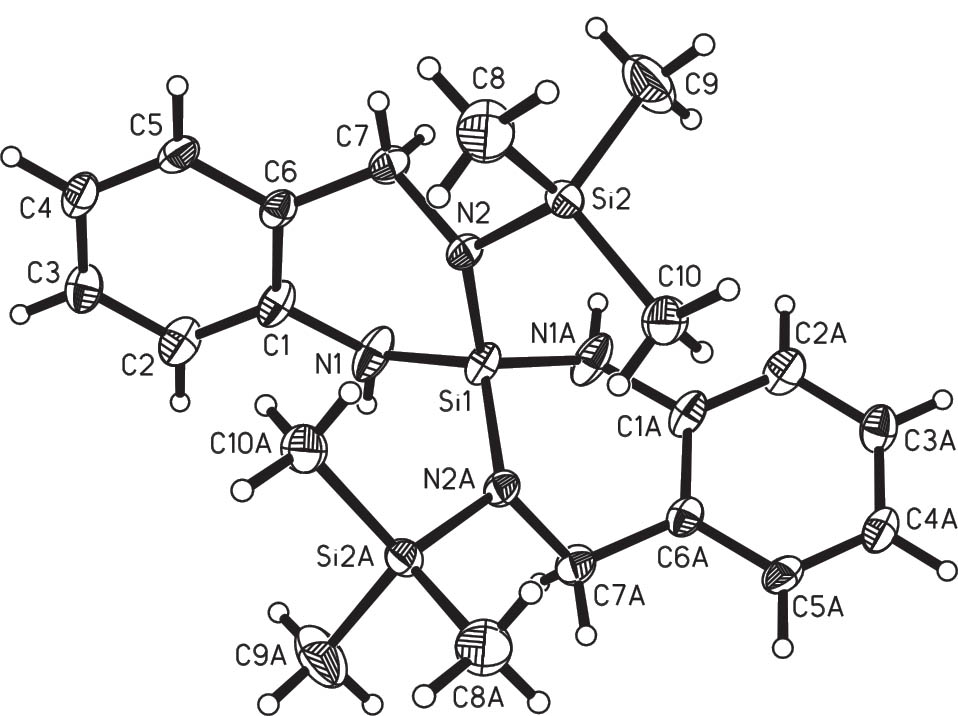
Molecular structure of 4b-TMS2 including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level.
Selected geometric parameters (Å, °) of 4b-TMS2
| Si(1)–N(2) | 1.7103(12) | ||
| Si(1)–N(1) | 1.7315(13) | ||
| N(2)–Si(2) | 1.7477(12) | ||
| N(2A)–Si(1)–N(2) | 113.64(8) | C(1)–N(1)–Si(1) | 123.29(10) |
| N(2A)–Si(1)–N(1) | 116.69(7) | C(1)–N(1)–H(1) | 114.4(15) |
| N(2)–Si(1)–N(1) | 102.00(6) | Si(1)–N(1)–H(1) | 114.9(15) |
| N(1A)–Si(1)–N(1) | 106.22(9) | C(7)–N(2)–Si(1) | 115.48(10) |
| C(7)–N(2)–Si(2) | 113.87(9) | ||
| Si(1)–N(2)–Si(2) | 125.69(7) |
Compound 5b co-crystallises with one unit of triethylammonium chloride and one molecule of chloroform per two molecules of 5b in the triclinic space group P-1. Essential intermolecular interactions between these components are summarised in the Supplementary Information. Compound 5c crystallises in the monoclinic space group P21/c. Two crystallographically independent aminosilane molecules are present in the crystal structures of both compounds. The molecular structures are shown in Figures 7 and 8, essential geometric parameters in Table 5. Since these have very similar geometric parameters, only one molecule will be discussed here as representative. No trimethylsilyl groups remained at the nitrogen atoms of both molecules (see Figures 7 and 8). The 1,8-diaminonaphthalene unit is bound to a silicon atom via both nitrogen atoms, thus forming six-membered diazasilacyclohexane rings. These heterocycles are planar in both molecules. The dihedral angle between both six-membered rings in 5b is 88.58(5)°. The Si–N bond lengths in 5b range from 1.696(2) (Si1A–N4A) to 1.712(2) Å (Si1A–N2A). This is rather short for Si–N-bonds and in accord with sp2-hybridisation at the nitrogen atoms. Mean bond lengths have been determined from X-ray structural data with 1.713 Å for Si–N(sp2)- and 1.739 Å for Si–N(sp3)-bonds (Kaftory et al., 1998). This view is supported by the presence of planar nitrogen atoms in both molecules. The silicon atoms in both molecules are located in distorted tetrahedral coordination environments. The smallest bond angles at silicon are found inside the six-membered rings, i.e., 99.75(8)° for N1A–Si1A–N2A and 100.46(8)° for N3A–Si1A–N4A of compound 5b; and 99.14(9)° for N1A–Si1A–N2A of compound 5c. All other angles at the silicon atoms in 5b and 5c range between 110° and 115°.
Selected geometric parameters (Å, °) of 5b and 5c
| 5b | 5c | ||
| Si(1A)–N(1A) | 1.7036(16) | Si(1A)–N(1A) | 1.7217(18) |
| Si(1A)–N(2A) | 1.7123(17) | Si(1A)–N(2A) | 1.7220(19) |
| Si(1A)–N(3A) | 1.7035(17) | Si(1A)–C(11A) | 1.849(3) |
| Si(1A)–N(4A) | 1.6965(16) | Si(1A)–C(12A) | 1.848(2) |
| N(1A)–Si(1A)–N(2A) | 99.75(8) | N(1A)–Si(1A)–N(2A) | 99.14(9) |
| N(3A)–Si(1A)–N(1A) | 115.48(9) | C(12A)–Si(1A)–C(11A) | 109.31(14) |
| N(3A)–Si(1A)–N(2A) | 113.65(9) | N(1A)–Si(1A)–C(11A) | 112.38(11) |
| N(4A)–Si(1A)–N(1A) | 115.46(8) | N(2A)–Si(1A)–C(11A) | 110.75(11) |
| N(4A)–Si(1A)–N(2A) | 112.74(8) | N(1A)–Si(1A)–C(12A) | 110.63(10) |
| N(4A)–Si(1A)–N(3A) | 100.46(8) | N(2A)–Si(1A)–C(12A) | 114.38(12) |

Molecular structure of 5b including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level.

Molecular structure of 5c including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level.
Compound 5d-TMS crystallises in the monoclinic space group P21/c with one molecule in the asymmetric unit. The molecular structure is shown in Figure 9, essential geometric parameters in Table 6. The 1,8-diaminonaphthalene unit is bound via both nitrogen atoms at silicon atom Si1 forming a planar six-membered diazasilacyclohexane ring. One additional trimethylsilyl group is located at nitrogen atom N1. This was already concluded from the NMR data. The Si1–Cl bond lengths are 2.0462(5) and 2.0470(5) Å. These values are similar to the Si–Cl bonds in 2d-TMS. The Si-N bond lengths in 5d-TMS are 1.6977(12) (Si1–N1) and 1.6702(13) Å (Si1–N2). This is rather short for Si–N-bonds and hints again at sp2-hybridisation at the nitrogen atoms (see discussion above). The bond Si2–N1 to the trimethylsilyl group is substantially longer with 1.7871(12) Å. The coordination geometry at silicon atom Si1 can be interpreted as distorted tetrahedral. The smallest bond angle is Cl1–Si–Cl2 with 103.21(2)°. This is a very similar value as in compound 2d-TMS. The bond angle N1–Si1–N2 within the six-membered diazasilacyclohexane ring is 107.29(6)°. This is larger than the corresponding bond angles in 5b and 5c with values at 99°. The dihedral angle between the diazasilacyclohexane ring and the plane Cl1–Si1–Cl2 is 86.613(3)°.

Molecular structure of 5d-TMS including numbering scheme. The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level. The methyl groups at C11 and C12 are rotationally disordered. Their alternative positions C11A and C12A have been omitted for clarity.
Selected geometric parameters (Å, °) of 5d-TMS
| Cl(1)–Si(1) | 2.0462(5) |
| Cl(2)–Si(1) | 2.0470(5) |
| Si(1)–N(1) | 1.6977(12) |
| Si(1)–N(2) | 1.6702(13) |
| Si(2)–N(1) | 1.7871(12) |
| N(2)–Si(1)–N(1) | 107.29(6) |
| Cl(1)–Si(1)–Cl(2) | 103.21(2) |
| N(2)–Si(1)–Cl(1) | 110.69(5) |
| N(1)–Si(1)–Cl(1) | 113.37(4) |
| N(2)–Si(1)–Cl(2) | 108.53(5) |
| N(1)–Si(1)–Cl(2) | 113.70(4) |
Compound 4e crystallises in the monoclinic space group I2/c with one molecule in the asymmetric unit. The overall composition of this molecule is surprising: Sixmembered diazasilacyclohexane rings have formed, but an additional dimethylsilyl group bridges two such units via the benzylamino nitrogen atoms N2 and N4 (see Figure 10). All three silicon atoms are situated in distorted tetrahedral coordination spheres. The smallest bond angles at silicon are found inside the diazasilacyclohexane rings with 102.6(1)° for N2–Si1–N1 and 101.5(1)° for N4–Si2–N3 (Table 7). The other bond angles at Si1 and Si2 adopt values between 106.5(2)° and 115.8(1)°. Interesting in this context is the comparison between coordination geometries of the silicon atoms Si1 and Si2, which are part of six-membered rings, and the silicon atom Si3, which is not part of a ring. The coordination sphere of silicon atom Si3 has a more “relaxed” geometry with bond angles between 108.6(1)° to 110.8(1)°, which is close to the angles of an ideal tetrahedron (109.5°). The Si–C bonds in this molecule have lengths close to 1.860 Å, which is the mean bond length for such bonds (Kaftory et al., 1998). There are two different types of nitrogen atoms in 4e: The atoms N1 and N3 are bound each to an aromatic ring, to one hydrogen atom, and to one silicon atom. The atoms N2 and N4 are bound each to two different silicon atoms and a CH2-group. This has implications for the bond lengths from these nitrogen atoms. The bonds Si1–N1, Si2–N3, Si3–N2, and Si3–N4 have lengths around 1.74 Å. This corresponds to Si-N(sp3) bonds (Kaftory et al., 1998). The bonds Si1–N2 and Si2–N4 are slightly shorter with lengths of 1.724(2) and 1.729(2) Å, respectively.

Molecular structure of 4e including numbering scheme (top). The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level. Schematic formula drawing of 4e (bottom).
Selected geometric parameters (Å, °) of 4e
| Si(1)–N(1) | 1.737(2) | Si(2)–N(3) | 1.745(2) |
| Si(1)–N(2) | 1.724(2) | Si(2)–N(4) | 1.729(2) |
| Si(1)–C(15) | 1.867(2) | Si(2)–C(17) | 1.858(2) |
| Si(1)–C(16) | 1.866(2) | Si(2)–C(18) | 1.863(2) |
| N(2)–Si(1)–N(1) | 102.58(8) | N(4)–Si(2)–N(3) | 101.52(8) |
| N(2)–Si(1)–C(15) | 111.75(9) | N(4)–Si(2)–C(18) | 113.51(9) |
| N(1)–Si(1)–C(15) | 112.01(10) | N(3)–Si(2)–C(18) | 112.83(9) |
| N(2)–Si(1)–C(16) | 115.80(9) | N(4)–Si(2)–C(17) | 113.39(10) |
| N(1)–Si(1)–C(16) | 106.88(10) | N(3)–Si(2)–C(17) | 109.10(10) |
| C(15)–Si(1)–C(16) | 107.71(10) | C(18)–Si(2)–C(17) | 106.54(11) |
| N(2)–Si(3) | 1.737(2) | N(2)–Si(3)–N(4) | 110.82(8) |
| N(4)–Si(3) | 1.740(2) | N(2)–Si(3)–C(19) | 108.57(9) |
| Si(3)–C(19) | 1.860(2) | N(4)–Si(3)–C(19) | 108.73(10) |
| Si(3)–C(20) | 1.863(2) | N(2)–Si(3)–C(20) | 110.02(10) |
| N(4)–Si(3)–C(20) | 108.61(9) | ||
| C(19)–Si(3)–C(20) | 110.09(12) | ||
The aromatic rings C1–C6 and C8–C13 are planar. The six-membered diazasilacyclohexane rings are fused to these. Conformational analysis has been performed. This reveals a boat conformation for the ring containing the atoms Si1–N1–C1–C6–C7–N2 with the parameters θ = 77.4(4)°, φ = 244.0(4)°, and a ring puckering amplitude Q = 0.430(3) Å. Ideal parameters for a boat conformation are θ = 90.0°; φ = k · 60° (Boeyens, 1978; Cremer and Pople, 1975). The six-membered ring with the atoms Si2-N3-C8-C13-C14-N4 has the parameters θ = 65.7(3)°, φ = 269.3(3)°, and a ring puckering amplitude Q = 0.595(3) Å. This corresponds to a screw-boat conformation with ideal parameters θ = 67.5° and φ = k · 60° + 30°.
On the attempt to synthesise compound 4d, two unexpected compounds crystallised: 4f and 4g. Selected geometric parameters can be found in Table 8. Both compounds are structural isomers with a six-membered silazane ring in the centre. The nitrogen atoms of the 2-aminobenzylamino units (N2, N4, N6) are integrated into the silazane ring. Isomer 4f crystallises in the monoclinic space group P21/c with one molecule in the asymmetric unit (see Figure 11). The compound co-crystallises with one molecule diethyl ether. The coordination geometries at the silicon atoms of 4f are roughly tetrahedral with bond angles between 103.84(9)° and 117.97(9)°. The bond lengths of the Si–N (about 1.70 Å) bonds and the Si–Cl bonds (about 2.06 Å) are quite similar to the corresponding bonds of the other structures discussed in this paper. Ring-puckering analysis (see Table 9) shows that the silazane ring is in twist-boat conformation while the fused non-aromatic rings are in boat or screw-boat conformation. Compound 4g crystallises in the monoclinic space group P21/n with two independent silazane molecules in the asymmetric unit (see Figure 12). Only one of the two silazane molecules in the asymmetric unit of 4g is discussed in the further course. The constitution of 4f is more symmetric than the constitution of 4g. Both substances differ in the topology of one 2-aminobenzylamino moiety. One 2-aminobenzylamino moiety is inverted with respect to the topology of 4f. This results in silicon atoms with three different coordination spheres in the structure of 4g. Si1 is bound to four nitrogen atoms (marked green in Figure 12) and has the most distorted tetrahedral coordination geometry with bond angles from 101.82(17)° to 120.72(19)°. Si2 is bound to one chlorine atom and three nitrogen atoms (marked blue in Figure 12). This atom features a more “relaxed” tetrahedral geometry with bond angles between 103.63(18)° and 115.94(18)°. The atom Si3 is bound to two chlorine and two nitrogen atoms (marked red in Figure 12). The coordination geometry of this atom is even less distorted with bond angles between 104.21(7)° and 111.77(13)°. The Si-N bond length decreases with the increasing degree of chlorine substitution. Ring puckering analyses shows that the silazane ring is in twist-boat conformation while the fused non-aromatic rings have screw-boat or boat conformation (see Table 9).

Molecular structure of 4f including numbering scheme (top). The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level. Schematic formula drawing of 4f (bottom).

Molecular structure of 4g including numbering scheme (top). The thermal displacement ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level. Schematic formula drawing of 4g (bottom). The bonding situation of the silicon atoms is colour coded (see text for explanation).
Selected geometric parameters (Å, °) of 4f and 4g
| 4f | 4g | ||
|---|---|---|---|
| Cl(1)–Si(1) | 2.0629(8) | Cl(1)–Si(2) | 2.0644(15) |
| Cl(2)–Si(2) | 2.0736(7) | Cl(2)–Si(3) | 2.0435(16) |
| Cl(3)–Si(3) | 2.0625(8) | Cl(3)–Si(3) | 2.0410(15) |
| Si(1)–N(2) | 1.6979(18) | Si(1)–N(5) | 1.706(4) |
| Si(1)–N(1) | 1.7047(18) | Si(1)–N(1) | 1.706(4) |
| Si(1)–N(6) | 1.7050(19) | Si(1)–N(2) | 1.712(4) |
| Si(2)–N(3) | 1.7000(19) | Si(1)–N(6) | 1.729(4) |
| Si(2)–N(4) | 1.7041(17) | Si(2)–N(3) | 1.684(4) |
| Si(2)–N(2) | 1.7050(17) | Si(2)–N(2) | 1.710(4) |
| Si(3)–N(4) | 1.6960(17) | Si(2)–N(4) | 1.715(4) |
| Si(3)–N(6) | 1.6994(19) | Si(3)–N(4) | 1.679(4) |
| Si(3)–N(5) | 1.7089(19) | Si(3)–N(6) | 1.699(4) |
| N(2)–Si(1)–N(1) | 104.57(9) | N(5)–Si(1)–N(1) | 108.21(19) |
| N(2)–Si(1)–N(6) | 109.02(9) | N(5)–Si(1)–N(2) | 120.72(19) |
| N(1)–Si(1)–N(6) | 117.97(9) | N(1)–Si(1)–N(2) | 101.92(17) |
| N(2)–Si(1)–Cl(1) | 112.74(7) | N(5)–Si(1)–N(6) | 101.82(17) |
| N(1)–Si(1)–Cl(1) | 106.58(7) | N(1)–Si(1)–N(6) | 120.15(19) |
| N(6)–Si(1)–Cl(1) | 106.12(7) | N(2)–Si(1)–N(6) | 105.20(17) |
| N(3)–Si(2)–N(4) | 104.72(9) | N(3)–Si(2)–N(2) | 115.94(18) |
| N(3)–Si(2)–N(2) | 115.51(9) | N(3)–Si(2)–N(4) | 103.63(18) |
| N(4)–Si(2)–N(2) | 109.43(8) | N(2)–Si(2)–N(4) | 110.75(17) |
| N(3)–Si(2)–Cl(2) | 106.45(7) | N(3)–Si(2)–Cl(1) | 108.03(14) |
| N(4)–Si(2)–Cl(2) | 112.93(6) | N(2)–Si(2)–Cl(1) | 107.51(12) |
| N(2)–Si(2)–Cl(2) | 107.89(7) | N(4)–Si(2)–Cl(1) | 110.95(13) |
| N(4)–Si(3)–N(6) | 111.35(9) | N(4)–Si(3)–N(6) | 108.23(17) |
| N(4)–Si(3)–N(5) | 117.43(9) | N(4)–Si(3)–Cl(3) | 110.72(13) |
| N(6)–Si(3)–N(5) | 103.84(9) | N(6)–Si(3)–Cl(3) | 111.77(13) |
| N(4)–Si(3)–Cl(3) | 106.05(7) | N(4)–Si(3)–Cl(2) | 110.32(14) |
| N(6)–Si(3)–Cl(3) | 113.30(7) | N(6)–Si(3)–Cl(2) | 111.59(12) |
| N(5)–Si(3)–Cl(3) | 104.94(8) | Cl(3)–Si(3)–Cl(2) | 104.21(7) |
Ring puckering analysis of structures 4f and 4g
| molecule | ring atoms | Θ [°] | φ [°] | Q [Å] | conformation |
|---|---|---|---|---|---|
| 4f | Si(1)–N(2)–Si(2)–N(4)–Si(3)–N(6) | 107.9(2) | 260.1(2) | 0.3312(13) | twist–boat |
| Si(1)–N(2)–C(7)–C(6)–C(1)–N(1) | 110.1(2) | 79.3(2) | 0.532(2) | twist–boat | |
| Si(2)–N(4)–C(14)–C(13)–C(8)–N(3) | 103.64(19) | 72.95(18) | 0.586(2) | boat | |
| Si(3)–N(6)–C(21)–C(20)–C(15)–N(5) | 70.4(2) | 260.7(2) | 0.557(2) | screw–boat | |
| 4g | Si(1)–N(2)–Si(2)–N(4)–Si(3)–N(6) | 93.4(3) | 108.6(2) | 0.644(3) | boat |
| Si(1)–N(2)–C(7)–C(6)–C(1)–N(1) | 71.8(4) | 258.5(4) | 0.545(4) | screw–boat | |
| Si(1)–N(6)–C(21)–C(20)–C(15)–N(5) | 79.0(4) | 247.4(4) | 0.578(4) | boat | |
| Si(2)–N(4)–C(14)–C(13)–C(8)–N(3) | 82.1(4) | 245.8(4) | 0.554(4) | boat |
3.4 Quantum chemical calculations
For a better understanding of the preferred number of TMS moieties remaining in the synthesised molecules (when starting from amines 1a–5a), quantum chemical calculations were performed. As example, the trimethylsilylation of compounds 2b (as a representative with 5-membered ring) and 4b (as a representative with 6-membered ring) with hexamethydisilazane (HMDS) were compared (Scheme 8). The results are shown in Table 10. This hypothetical reaction has been chosen, since it is an isodesmic reaction in which the total number of each bond type is identical in the reactants and products (Foresmann and Frisch, 2015). Isodesmic reactions are useful to predict ΔH and ΔG of chemical reactions.

The model reaction used for comparison of the relative stability of different silylation states of 2b with quantum chemical calculations. The reaction of 2b → 2b-TMS2 is shown here as an example.
Calculated Gibbs free energies and enthalpies for the silylation reactions of 2b and 4b with HMDS, calculated at the M062X/6-31G(d) level in THF solution with the PCM model
| Model reaction | Gibbs free energy (kJ/mol) | Enthalpy (kJ/mol) |
|---|---|---|
| Reactions of 2b | ||
| 2b → 2b-TMS2 | −6.2 | −15.8 |
| 2b-TMS2 → 2b-TMS4 | −21.9 | −27.1 |
| 2b→ 2b-TMS4 | −28.1 | −42.9 |
| Reactions of 4b | ||
| 4b → 4b-TMS2 (aliph.) | 15.6 | −1.7 |
| 4b → 4b-TMS2 (arom.) | 72.4 | 49.7 |
| 4b-TMS2 (aliph.) → 4b-TMS4 | 58.3 | 33.8 |
| 4b-TMS2 (arom.) → 4b-TMS4 | 1.5 | −17.5 |
| 4b → 4b-TMS4 | 73.9 | 32.1 |
It is obvious that the silylation of 2b is exergonic in all reactions. Therefore, it must be assumed, that the sterical hindrance of the TMS moieties prevents the formation of 2b-TMS4. Instead 2b-TMS2 is formed in our experiments. Additionally, all silylation reactions of 4b are endergonic. The silylation of the aliphatic nitrogen atoms is less endergonic than the silylation of the aromatic nitrogen atoms. The silylation reaction of the aliphatic nitrogen atoms is only slightly endergonic (15.6 and 1.5 kJ/mol, respectively).
Further effects might play a role in the experiments which have been performed in the laboratory. These are for instance: reaction conditions like boiling under reflux, thus fostering the formation of the volatile Me3SiCl, solvation effects, solubility of formed intermediates, or crystallisation enthalpies of products. All these effects are not taken into account in the model reaction used for the calculations. The calculated Gibbs enthalpy, which is slightly positive, could be turned to a negative value by such effects. This explains why 4b-TMS2 could be crystallised. In accord with the preferred retention of SiMe3 groups at the aliphatic amine N atoms, the silicon atom of the bridging SiMe2 moiety in 4e is also bound to the benzylamine N atoms, and cyclosilazane formation via benzylamine N atoms in 4f and 4g indicates the same trend. The results of the quantum chemical calculations of both example systems show the stabilising effect of TMS moieties to five-membered ring systems and the destabilising effect on six-membered rings. These results are in agreement with the observed preparative accessibility of spirocyclic compounds.
Furthermore, we were interested to understand the occurrence of planar and nonplanar ring conformations in case of the derivatives of 1 and 2, which feature 5-membered rings. Therefore, the energy necessary for the planarisation of 1b has been calculated with quantum chemical methods. The planar form has D2d symmetry and is 6.1 kJ/mol higher in Gibbs free energy than the same compound with bent ethylene diamine units (for details see Supplementary material). In contrast to that, the global minimum for compound 2b is planar on both (N,N’-o-phenylenamino)silicon units. The annelation of the phenylene group makes the planar form more favourable. This was indeed observed in the solid state structures of 2b-TMS2 and 2d-TMS.
Further on, the stabilities of the two isomers 4f and 4g were compared on the B3LYP/6-31G(d) level of theory. At 298 K (1 atm) the less symmetric derivative 4g is 14.2 kJ/mol lower in energy. This small energy difference can be overcome by solvation effects and crystallisation energies. In effect both isomers crystallised side by side from one reaction batch.
4 Comparative reflections and conclusions
As a result of the accomplished experiments, the aminosilanes 1a–5a, spirocyclic aminosilanes 1b–5b, and several other cyclic aminosilanes were successfully synthesised, isolated, and characterised. Spirocyclic aminosilanes with six-membered rings were obtained only from direct reactions of the amines with tetrachlorosilane (method A). Spirocyclic aminosilanes with five-membered rings are obtained from reactions of tetrachlorosilane with trimethylsilyl substituted amines (method B).
Comparison of the angles N–Si–N in the crystal structures show smaller angles in the five-membered rings than in the six-membered rings, as one would expect. Derivatives b, the spirocyclic compounds, exhibit smaller bite angles than the derivatives c and d. The TMS moieties widen that angle. This could explain why derivatives of amines 1–3 only could be synthesised by method B. The wider angle N–Si–N lowers the ring-strain in the products. Additionally, the steric hindrance caused by the TMS moieties lowers the tendency of polymerisation. Furthermore, the TMS moieties lower the nucleophilicity of the lone pair of the nitrogen atom, thus suppressing side reactions, and the cyclic products can be obtained.
It was surprising, that the degree of substitution with trimethylsilyl groups differs noticeably between the final products. Quantum chemical calculations gave insight into the thermodynamic reasons for this phenomenon. The degree of substitution with trimethylsilyl groups depends on the ring size of the spirocycles: Trimethylsilyl groups have a stabilising effect on five-membered ring systems and a destabilising effect on six-membered rings in this type of compounds.
The prepared compounds are useful as substrates for further investigations. These might be insertion reactions with carbon dioxide and with other heteroallenes into Si–N bonds, ring opening polymerisations, or the preparation of macrocyclic ligands in the coordination sphere of silicon.
Experimental
All reactions were carried out under argon using Schlenk technique (Böhme, 2020; Herzog and Dehnert, 1964). NMR spectra were recorded in CDCl3 or C6D6 with TMS as internal standard either on a BRUKER DPX 400 spectrometer at 400.13, 100.61, and 79.49 MHz for 1H, 13C, and 29Si, or on a BRUKER AVANCE III 500 MHz spectrometer at 500.13, 125.76, and 99.36 MHz for 1H, 13C, and 29Si, respectively. Raman spectra were measured with a FT-Raman spectrometer RFS/100S from BRUKER using an air-cooled Nd:YAG-laser with a wavelength of 1064 nm and a nitrogen-cooled germanium detector. Melting points were measured using a Polytherm A hot stage microscope from Wagner and Munz with an attached 52II thermometer from Fluke. The measurement of boiling points was performed with an apparatus which has been published by Herbig and Kroke (2017). Mass spectra were recorded on a expressionL CMS from Advion using ESI (sample was dissolved in acetonitrile). The setup for cryoscopy is described in the Supplementary material (section S5).
Synthesis of trimethylsilylated amines
The synthesis of the trimethylsilylated derivatives 1a–5a is shown for compound 2a as example. The other four substances are synthesised in the same manner. Variation from this general method are pointed out in the paragraph of the related compound.
In a 2-necked round-bottomed flask, 5.01 g (46 mmol) of o-phenylenediamine and 9.42 g (93 mmol) triethylamine were dissolved in about 150 mL of dry THF. The solution was stirred while cooling in an ice-water bath, and 10.00 g (92 mmol) Me3SiCl were added to the mixture dropwise. After complete addition, the suspension was heated under reflux for 2 h, followed by stirring at room temperature overnight. Thereafter, the suspension was filtered through a fritted glass filter (G4), and the solid was washed with THF (2 × 20 mL). From the combined filtrate and washings all volatile compounds were removed by distillation under reduced pressure. The residue (10.35 g, 89%) was an orange liquid which was identified as N,N’-bis(trimethylsilyl)-1,2-diaminobenzene (2a).
N,N’-Bis(trimethylsilyl)-1,2-ethylenediamine (1a)
NMR solvent: CDCl3. 1H NMR: δ [ppm] = −0.04 (s, 18 H, SiMe3), 0.58 (s, 2 H, NH), 2.59 (s, 4 H, CH2). 13C NMR: δ [ppm] = −0.1 (SiMe3), 45.7 (CH2). 29Si NMR: δ [ppm] = 3.7. bp: 35°C (0.35 mbar).
N,N’-Bis(trimethylsilyl)-1,2-diaminobenzene (2a)
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.21 (s, 18 H, SiMe3), 3.06 (s, 2 H, NH), 6.71 (m, 2 H, Ar), 6.80 (m, 2 H, Ar). 13C NMR: δ [ppm] = 0.4 (SiMe3), 119.7, 120.2, 137.6 (Ar). 29Si NMR: δ [ppm] = 3.8. Raman: ν [cm−1] = 3367 (vw), 3059 (w), 2958 (m), 2899 (vs), 1598 (w), 1499 (vw), 1410 (vw), 1300 (vw), 1250 (vw), 1189 (vw), 1156 (vw), 1044 (w), 920 (vw), 827 (vw), 780 (vw), 740 (vw), 689 (vw), 616 (m). MS: m/z = 253.2 g/mol [M+H]+. bp: 236°C (975 mbar).
N,N’-Bis(trimethylsilyl)-(±)-trans-1,2-diaminocyclohexane (3a)
The substance was synthesised like 2a using 9.51 g (83 mmol) 3, 16.95 g (167 mmol) NEt3 and, 18.10 g (167 mmol) Me3SiCl. The reaction and workup afforded a colourless liquid (14.34 g, 67%).
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.00 (s, 18 H, SiMe3), 0.67 (s, 2 H, N–H), 1.03 (m, 2 H, ring), 1.19 (m, 2 H, ring), 1.57 (m, 2 H, ring), 1.81 (m, 2 H, ring), 2.15 (d, 2 H, 3JH,H = 7.0 Hz). 13C NMR: δ [ppm] = 0.9 (SiMe3), 25.5, 36.7, 58.8 (ring). 29Si NMR: δ [ppm] = 1.6. Raman: ν [cm−1] = 2954 (m), 2939 (m), 2897 (vs), 2856 (m), 1445 (vw), 1408 (vw), 1347 (vw), 1114 (vw), 1044 (vw), 849 (vw), 784 (vw), 742 (vw), 685 (vw), 617 (m). MS: m/z = 259.23 g/mol [M+H]+. bp: 229°C (975 mbar).
N,N’-Bis(trimethylsilyl)-2-aminobenzylamine (4a)
The substance was synthesised like 2a using 5.00 g (41 mmol) 4, 8.40 g (83 mmol) NEt3, and 9.00 g (83 mmol) Me3SiCl. The reaction and workup afforded a yellow liquid (6.48 g, 59%).
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.12 (s, 9 H, SiMe3–NAr), 0.30 (s, 9 H, SiMe3-NAliph), 0.51 (s, 1H, NAliph–H), 3.84 (d, 2 H, 3JH,H = 6.0 Hz, CH2), 5.20 (s, 1 H, NAr–H), 6.65 (m, 1 H, Ar), 6.77 (m, 1 H, Ar), 7.02 (m, 1 H, Ar), 7.08 (m, 1 H, Ar). 13C NMR: δ [ppm] = −0.4 (SiMe3–NAr), 0.4 (SiMe3-NAliph), 45.5 (CH2), 115.9, 117.1, 128.1, 128.1, 129.5, 147.3 (Ar). 29Si NMR: δ [ppm] = 1.6 (SiMe3–NAliph), 5.5 (SiMe3–NAr). Raman: ν [cm−1] = 3056 (w), 2957 (m), 2898 (vs), 1606 (vw), 1583 (vw), 1496 (vw), 1458 (vw), 1410 (vw), 1296 (vw), 1248 (vw), 1187 (vw), 1158 (vw), 1045 (w), 915 (vw), 840 (vw), 776 (vw), 749 (vw), 708 (vw), 690 (vw), 626 (m), 603 (w), 553 (vw), 432 (vw). MS: m/z = 267.65 g/mol [M+H]+. bp: >300°C (974 mbar).
N,N’-Bis(trimethylsilyl)-1,8-diaminonaphthalene (5a)
The substance was synthesised like 2a using 7.30 g (46 mmol) 5, 9.33 g (92 mmol) NEt3, and 10.50 g (97 mmol) Me3SiCl. The reaction and workup yielded a dark red solid (12.52 g, 90%).
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.23 (s, 18 H, SiMe3), 5.49 (s, 2 H, N–H), 6.65 (m, 2 H, Ar), 7.14 (m, 2 H, Ar), 7.21 (m, 2 H, Ar). 13C NMR: δ [ppm] = 0.1 (SiMe3), 115.4, 120.4, 121.3, 125.7, 137.4, 144.4 (Ar). 29Si NMR: δ [ppm] = 3.6. MS: m/z = 302.40 g/mol [M]+. mp: 42–44°C.
Synthesis of cyclic and spirocyclic compounds (derivatives b, c, and d)
For the synthesis of cyclic and spirocyclic compounds, two methods are used: method A starts from the amines 1–5 and method B starts from the bis-trimethylsilylated derivatives 1a–5a. To synthesise the different derivatives, the composition of the reaction mixtures is varied. First, the preparation of 1b, 1c, and 1d are given as examples. All following syntheses differ in the workup of the products. Therefore, the workup procedures are given in the subsequent sections for each compound individually.
Synthesis of derivatives b – example 1b
In dry n-heptane (80 mL), 2.43 g (12 mmol) 1a and 2.38 g (24 mmol) triethylamine were dissolved. While cooling in an ice-water bath, a solution of 1.02 g (6 mmol) SiCl4 in about 20 mL of n-heptane was added dropwise. Thereafter, the reaction mixture was heated under reflux for 7 h, followed by stirring at room temperature overnight. The resulting suspension was filtered through a fritted glass filter (G4), and the solid was washed with dry n-heptane (2 × 20 mL). From the combined filtrate and washings all volatile compounds were removed by distillation under reduced pressure. Further purification steps are pointed out below.
Synthesis of derivatives c – example 1c
In dry THF (70 mL), 3.25 g (16 mmol) 1a and 3.7 g (36 mmol) triethylamine were dissolved. While cooling in an ice-water bath, 2.13 g (17 mmol) Me2SiCl2 was added dropwise. The reaction mixture was heated under reflux for 2 h, followed by stirring at room temperature overnight. The resulting suspension was filtered through a fritted glass filter (G4), and the solid was washed with dry THF (2 × 20 mL). From the combined filtrate and washings all volatile compounds were removed by distillation under reduced pressure. Further purification steps are pointed out below.
Synthesis of derivatives d – example 1d
In dry THF (100 mL), 3.27 g (16 mmol) 1a and 3,63 g (36 mmol) triethylamine were dissolved. While cooling in an ice-water bath, 2.9 g (17 mmol) SiCl4 was added dropwise. The reaction mixture was heated under reflux for 2 h, followed by stirring at room temperature overnight. The resulting suspension was filtered through a fritted glass filter (G4), and the solid was washed with dry THF (2 × 20 mL). From the combined filtrate and washings all volatile compounds were removed by distillation under reduced pressure. Further purification steps are pointed out below.
spiro-Bis(N,N’-1,2-ethylene-bis-(trimethylsilylamino)) silane (1b-TMS4)
This compound was synthesised by using method B: 60 mL dry n-heptane, 2.50 g (12 mmol) 1a, 2.40 g (24 mmol) NEt3, 1.00 g (6 mmol) SiCl4 were used. The silicon tetrachloride was also dissolved in dry n-heptane (20 mL). The mixture was heated for 7 h. The compound received after removing volatiles in vacuo was recrystallised from n-pentane yielding a yellow substance (2.40 g, 40%). The obtained compound contains four trimethylsilyl moieties.
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.07 (s, 36 H, SiMe3), 3.02 (s, 8 H, CH2). 13C NMR: δ [ppm] = −0.2 (SiMe3), 43.8 (CH2). 29Si NMR: δ [ppm] = 1.7 (SiMe3), 15.3 (Si). Raman: ν [cm−1] = 2956 (s), 2897 (vs), 2863 (w), 2682 (vw), 1471 (vw), 1411 (vw), 1254 (vw), 1230 (vw), 838 (vw), 750 (vw), 684 (vw), 634 (w), 460 (w). MS: m/z = 433.33 g/mol [M+H]+. mp: 131°C.
(N,N’-1,2-ethylene-bis-(trimethylsilylamino)) dimethylsilane (1c-TMS2)
This compound was synthesised by using method B: 3.25 g (16 mmol) 1a, 3.7 g (36 mmol) NEt3, 2.13 g (17 mmol) Me2SiCl2, and 70 mL THF as solvent were used. The silicon tetrachloride was used neat. The mixture was heated for 4 h. The compound received after removing volatiles in vacuo was distilled at 0.2 mbar (bp = 35°C), yielding a colourless liquid (1.794 g, 43%). The obtained compound contains two trimethylsilyl moieties.
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.08 (s, 18 H, SiMe3), 0.13 (s, 6 H, SiMe2), 3.00 (s, 4 H, CH2). 13C NMR: δ [ppm] = 0.4 (SiMe3), 3.7 (SiMe2), 46.9 (CH2). 29Si NMR: δ [ppm] = 1.4 (2 Si, SiMe3), 12.8 (1 Si, SiMe2). Raman: ν [cm−1] = 2955.0 (m), 2899.0 (vs), 2836.0 (w), 2662.0 (vw), 1464.0 (vw), 1409.0 (vw), 1351.0 (vw), 1249.0 (vw), 1222.0 (vw), 1075.0 (vw), 749.0 (vw), 703.0 (vw), 626.0 (vw), 523.0 (m), 369.0 (vw), 346.0 (vw), 327.0 (vw), 288.0 (vw), 248.0 (vw), 180.0 (vw), 120.0 (vw). MS: 261 g/mol [M+H]+. bp: 210°C (963 mbar). molecular mass: cryoscopy (see Supplementary material) calc. 206 g/mol, found 240 g/mol.
Dichloro-(N,N’-bis(trimethylsilyl)-N,N’-1,2-ethylendiamino)silane (1d-TMS2)
This compound was published earlier (Böhme et al., 2007).
spiro-Bis(N,N’-o-phenyleneamino(trimethylsilylamino)) silane (2b-TMS2)
This compound was synthesised by using method B: 5.02 g (20 mmol) 2a, 4.05 g (40 mmol) NEt3, 1.54 g (9.1 mmol) SiCl4, and THF as solvent were used. The crude product was dissolved in diethyl ether, and after addition of n-pentane and storage for several days at 4°C a light brown solid (0.55 g, 16%) was yielded. The obtained compound contains two trimethylsilyl moieties.
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.23 (s, 18 H, SiMe3), 4.28 (s, 2 H, NH), 6.75 (m, 10 H, Ar). 13C NMR: δ [ppm] = −0.06 (SiMe3), 110.8, 113.7, 118.2, 119.4, 136.2, 137.2 (Ar). 29Si NMR: δ [ppm] = −24.6 (Si), 4.2 (SiMe3). (The integral of the multiplet indicates the existence of not converted starting material or products of hydrolysis.) Raman: ν [ppm] = 3460 (vw), 3400 (vw), 3080 (w), 3059 (m), 3013 (w), 2958 (m), 2900 (vs), 1598 (m), 1586 (w), 1489 (w), 1461 (vw), 1366 (m), 1309 (vw), 1266 (m), 1222 (vw), 1200 (vw), 1156 (w), 1112 (vw), 1032 (s), 948 (vw), 925 (vw), 845 (vw), 815 (vw), 752 (vw), 616 (m), 440 (vw), 403 (w). MS: m/z = 384.11 g/mol [M]+. mp: 124°C. UV/VIS (0.00108 mol/L, d = 0.999 cm, in chloroform): [nm] 253.03 (ɛ = 2265.14 mol L−1 cm−1), 288.11 (ɛ = 2279.78 mol L−1 cm−1).
Crystals suitable for X-ray-diffraction were received from a solution in diethyl ether after addition of n-pentane and storing at 4°C for several days.
The received substance is unknown in literature, but a similar compound was synthesised by Kummer and Rochow (1963).
N,N’-(N-trimethylsilyl-o-phenylenediamino) dimethylsilane (2c-TMS)
This compound was synthesised by using method B: 4.99 g (20 mmol) 2a, 4.05 g (40 mmol) NEt3, 3.40 g (20 mmol) Me2SiCl2, and THF (80 mL) as solvent were used. A light brown compound mixture of the product and triethylammonium chloride was yielded. The obtained compound contains one trimethylsilyl moiety.
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.32 (s, 9 H, SiMe3), 0.36 (s, 6H, SiMe2), 6.51–6.73 (m, 4H, Ar). 13C NMR: δ [ppm] = 0.9 (SiMe3), 4.6 (SiMe2), 110.5, 113.4, 117.0, 118.6 (Ar), 140.0, 142.1(Aripso). 29Si NMR: δ [ppm] = 15.1 (SiMe2), 2.1 (SiMe3). Raman: ν [cm−1] = 3252 (vw), 3233 (vw), 3057 (m), 2998 (m), 2980 (s), 2901 (m), 1594 (m), 1557 (w), 1505 (w), 1480 (w), 1464 (m), 1366 (w), 1337 (w), 1277 (m), 1160 (w), 1034 (s), 905 (w), 763 (m), 705 (w), 687 (w), 614 (w), 579 (w), 550 (w), 502 (w), 464 (w), 344 (w), 331 (w), 203 (m), 128 (m), 109 (m).
Dichloro-N,N’-(N-trimethylsilyl-o-phenylenediamino) silane (2d-TMS)
This compound was synthesised by using method B: 8.45 g (33 mmol) 2a, 5.69 g (33 mmol) SiCl4, 6.68 g (66 mmol) Et3N, and THF as solvent were used. The crude product was dissolved in a mixture of CHCl3/n-hexane (1:3 volume parts, 60 mL in total) and crystallises after serval days at 4°C as a light brown solid (3.43 g, 37.5%).
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.47 (s, 9 H, SiMe3), 4.65 (br, 1 H, NH), 6.69 – 6.90 (m, 4 H, Ar). 13C NMR: δ [ppm] = 0.3 (SiMe3), 111.5, 113.9, 118.9, 120.0 (Ar), 137.2, 136.5 (Aripso). 29Si NMR: δ [ppm] = 6.8 (SiMe3), −23.5 (SiCl2). IR: ν [cm−1] = 3411, 3030, 2957, 2899, 2833, 2604, 1598, 1583, 1530, 1448, 1459, 1410, 1365, 1352, 132, 1254, 1229, 1202, 1184, 1111, 1047, 1033, 1018, 995, 901, 846, 814, 762, 742, 690, 677, 625, 576, 569, 559 527, 476, 447, 432. EA: C: 33.63% (calc. 38.98%), H: 5.92% (calc. 5.09%), N: 9.00% (calc. 10.10%). mp: 128–130°C (decomp.). UV/VIS (0.00099 mol/L, d = 0.999 cm, in CHCl3): [nm] 212.68 (ɛ = 1640.93 mol L−1 cm−1), 246.94 (ɛ = 2381.47 mol L−1 cm−1), 290.16 (ɛ = 1780.97 mol L−1 cm−1), 960.58 (ɛ = 477.65 mol L−1 cm−1).
Crystals suitable for X-ray-diffraction were received from a solution of the crude product in chloroform/n-hexane (1:3 volume parts) and storing at 4°C for several days.
spiro-Bis(N,N’-2-aminobenzylamino)silane (4b)
This compound was synthesised by using method A: 4.32 g (35 mmol) 4, 7.20 g (71 mmol) NEt3, and 2.99 g (18 mmol) SiCl4 and about 150 mL THF as solvent were used. The reaction mixture was not heated and stirred 21 h. A colourless substance (2.18 g, 46%) was received after filtration and removal of all volatiles.
NMR solvent: CDCl3. 1H NMR: δ [ppm] = 1.66 (s, 2 H, NH), 3.88 (s, 2 H, NH), 4.08 (qd, 4 H, 3JH,H = 14.8 Hz, 3JH,H = 5.5 Hz, CH2), 6.61 (d, 4 H, 3JH,H = 7.8 Hz, Ar), 6.72 (m, 2 H, Ar), 6.93 (d, 2 H, 3JH,H = 7.3 Hz, Ar), 7,04 (m, 2 H, Ar). 13C NMR: δ [ppm] = 44.5 (CH2), 117.91, 118.9, 126.5, 127.6, 127.9, 144.9 (Ar). 29Si NMR: δ [ppm] = −49.0 (−48.9; Kaßner et al., 2016). Raman: ν [cm−1] = 3057 (vs), 3046 (vs), 3032 (m), 2938 (m), 2881 (vw), 1607 (s), 1585 (w), 1455 (vw), 1300 (vw), 1279 (vw), 1257 (w), 1221 (vw), 1194 (vw), 1158 (w), 1037 (m), 760 (s), 594 (vw). MS: m/z = 310.00 g/mol [M+AcN+H]+. Decomposition: 155–160°C (Kaßner et al., 2016).
spiro-Bis(N-trimethylsilyl-N,N’-2-aminobenzylamino) silane (4b-TMS2)
This compound was synthesised by using method B: 1.34 g (11 mmol) 4, 2.30 g (23 mmol) NEt3, 1.02 g (6 mmol) SiCl4, and about 100 mL dry THF as solvent were used. The reaction mixture was heated for 8 h under reflux. From the product mixture in THF a few crystals of 4b-TMS2 were obtained after several weeks.
(N,N’-2-aminobenzylamino)dimethylsilanes (4c and 4e)
These compounds was synthesised by using method A: 4.64 g (38 mmol) 4, 7.82 g (77 mmol) NEt3, 6.464 g (38 mmol) Me2SiCl2, and about 150 mL of dry THF as solvent were used. A yellow substance was received after removing all volatiles in vacuo. After distillation under reduced pressure a residue and a distillate were obtained. The residue is 4e (0.635 g) which crystallised from the melt with small amounts of CDCl3, and the distillate is 4c (0.748 g).
4e: NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.19 (s, 6 H, Si-exocyclic), 0.30 (s, 12 H, Si-endocyclic), 3.56 (s (br), 2 H; NH), 3.93 (s, 4 H, CH2) 6.59–6.66 (m, 4 H, Ar), 6.8 (m, 2 H, Ar), 7.04–7.08 (m, 2 H, Ar). 13C NMR: δ [ppm] = 1.5 (Si-exocyclic), 2.7 (Si-endocyclic), 45.2 (CH2), 116.4, 117.6, 126.7, 127.7, 127.8, 145.9 (Ar). 29Si NMR: δ [ppm] = −2.2 (Si-endocyclic), −3.6 (Si-exocyclic). Raman: ν [cm−1] = 3358 (w), 3063 (w), 3048 (s), 2969 (s), 2903 (vs), 2834 (w), 1599 (w), 1586 (vw), 1455 (vw), 1368 (vw), 1339 (vw), 1306 (vw), 1277 (vw), 1200 (vw), 1156 (w), 1114 (vw), 1038 (w), 1009 (vw), 899 (vw), 732 (vw), 716 (w), 616 (w), 523 (w), 450 (vw), 350 (w), 311 (vw), 213 (w), 132 (m), 91 (w). After standing several days standing with small amount of CDCl3, crystals, suitable for X-Ray diffraction, were obtained. mp = 97°C. EA: C: 57.86% (calc. 58.20%), H: 7.65% (calc. 7.81%), N: 14.60% (calc. 13.57%).
4c: NMR solvent: CDCl3. 1H NMR: δ [ppm] = 0.17 (s, 6 H, SiMe2), 1.05 (s (br), 1 H; NH), 3.57 (s (br), 1 H, NH), 3.87 (s, 2 H, CH2) 6.49 (m, 1 H, Ar), 6.62 (m, 1 H, Ar), 6.85 (m, 1 H, Ar), 6.99 (m, 1 H, Ar). 13C NMR: δ [ppm] = 0.5 (SiMe2), 44.2 (CH2), 117.4, 117.5, 126.3, 127.4, 128.0, 145.5 (Ar). 29Si NMR: δ [ppm] = −1.7. bp 270°C (963 mbar). mp = 52°C. MS: m/z = 178 g/mol [M+H]+.
Silazanes 4f and 4g
These compounds was synthesised by using method A: 0.98 g (8 mmol) 4, 1.69 g (17 mmol) NEt3, 1.53 g (9 mmol) SiCl4, and 40 mL diethyl ether as solvent were used. During the addition of the silicon tetrachloride, the reaction mixture was cooled in a dry-ice/iso-propanol bath. Afterwards the mixture was allowed to attain room temperature and stirred overnight. A part of the solvent was removed in vacuo and from the resulting mixture 4f and 4g crystallised in the course of several weeks.
spiro-Bis(N,N’-1,8-diaminonaphthalenyl)silane (5b)
This compound was synthesised by using method A: 5.63 g (36 mmol) 5, 7.26 g (72 mmol) NEt3, 2.89 g (17 mmol) SiCl4, and about 200 mL of dry THF as solvent were used. After removing volatiles in vacuo a dark red compound (9.97 g) was obtained, which contained residual THF (ca. 80 mol%) according to the NMR spectra.
NMR solvent: CDCl3. 1H-NMR: δ [ppm] = 4.44 (s, 4 H, N–H), 6.22 (d, 4 H, 3JH,H = 6.1 Hz, Ar), 7.00 (s, 8 H, Ar). 13C NMR: 108.6, 115.3, 117.9, 127.0, 136.5, 143.4. 29Si NMR: δ [ppm] = −59.1. MS: m/z = 341.06 g/mol [M+H]+. Decomposition: 124°C. Raman: the Raman spectrum does not show sharp bands most likely due to luminescence effects of the compound.
Crystals suitable for X-ray-diffraction were received from the crude product with small amounts of CDCl3 and storing at room temperature for several days.
(N,N’-1,8-diaminonaphthalenyl)dimethylsilane (5c)
This compound was synthesised by using method A: 12.18 g (77 mmol) 5, 15.62 g (155 mmol) NEt3, 13.08 g (77 mmol) Me2SiCl2, and 200 mL of dry benzene as solvent were used. The crude product was dissolved in 10 mL THF and stored at 4°C. After filtration and removal of volatiles in vacuo 14.39 g (87%) of this light pink product were obtained.
NMR solvent: CDCl3. 1H-NMR: δ [ppm] = 0.35 (s, 6 H, SiMe2), 3.95 (s (br), 2 H, NH), 6.40 (d, 2 H, 3JH,H = 7.3 Hz, Ar), 7.08 (d, 2 H, 3JH,H = 8.3 Hz, Ar), 7.15 (t, 2 H, 3JH,H = 7.7 Hz, Ar). 13C NMR: 2.6 (SiMe2), 108.7 (Ar–H), 114.8 (Ar-quart), 117.6 (Ar–H), 126.9 (Ar-H), 136.7 (Ar-quart.), 142.9 (Ar-quart.). 29Si NMR: δ [ppm] = −5.6. Raman: ν [cm−1] = 3364 (vw), 3345 (vw), 3059 (m), 3023 (w), 2965 (vw), 2895 (w), 2157 (vw), 2145 (vw), 2060 (vw), 1580 (s), 1518 (vw), 1466 (m), 1445 (w), 1397 (vw), 1366 (vs), 1256 (m), 1169 (vw), 1125 (w), 1088 (w), 1067 (w), 845 (vw), 817 (w), 755 (vw), 682 (vw), 641 (w), 547 (s), 473 (w), 454 (w), 363 (w), 317 (w), 169(w), 113 (m). EA: C: 67.22% (calc. 67.24%), H: 6.68 (calc. 6.58%), N: 12.82 (calc. 13.07%). mp: 114°C.
Crystals suitable for X-ray-diffraction were obtained from the crude product with small amounts of CDCl3 upon storing at room temperature for several days.
Dichloro-(N-trimethylsilyl-N,N’-1,8-diaminonaphthalenyl) silane (5d)
To a solution of 1,8-diaminonaphthalene (5.00 g, 31.6 mmol) and triethylamine (7.98 g, 79.0 mmol) in toluene (100 mL) at room temperature a solution of chlorotrimethylsilane (7.90 g, 72.7 mmol) in toluene (10 mL) was added dropwise with stirring, whereupon the mixture was heated and stirred under reflux for 2 h. Upon cooling to room temperature the triethylamine hydrochloride precipitate was filtered off and washed with toluene (36 mL). From the combined filtrate and washings the solvent and other volatiles were removed under reduced pressure. The remaining oily residue was dissolved in toluene (50 mL), stirred at room temperature, and triethylamine (7.34 g, 72.7 mmol) as well as a solution of SiCl4 (5.64 g, 33.2 mmol) in toluene (6 mL) were added. This mixture was heated and stirred under reflux for 10 h. Upon cooling to room temperature the triethylamine hydrochloride precipitate was filtered off and washed with toluene (20 mL). From the combined filtrate and washings the solvent and other volatiles were removed under reduced pressure, and the solid residue was placed on a fritted glass filter and was extracted with n-hexane (70 mL). From the hexane extract the crude product crystallised in fine colourless needles, which, upon storage at 6°C overnight, were filtered off, washed with hexane (2 mL) and dried in vacuo. Yield: 7.46 g (22.8 mmol, 72%).
NMR solvent: CDCl3. 1H-NMR: δ [ppm] = 0.57 (s, 9 H, SiMe3), 4.69 (s (br), 1H, NH), 6,46–7,32 (m, 6H, Ar). 13C NMR: 2.7 (SiMe3), 109.7, 115.0, 117.8, 119.9, 121.5, 125.9, 126.6, 136.4, 139.3, 141.2 (Ar). 29Si NMR: δ [ppm] = 8.5 (SiMe3); −35.5 (SiCl2). EA: C: 47.73% (calc. 47.70%), N: 8.43% (calc. 8.56%), H: 5.04% (calc. 4.93%). mp = 122°C.
Crystal structure determination
Single crystal X-ray diffraction data of 2b-TMS2 and 2d-TMS were collected on a BRUKER NONIUS X8 APEX2 CCD diffractometer using graphite monochromated Mo Kα radiation (λ = 0.71073 Å) using φ- and ω-scans. Data collections for the structures 3d-TMS2, 4b-TMS2, 4e, 4f, 4g, 5b.[Et3NH]Cl·HCCl3, 5c, and 5d-TMS were performed on a STOE IPDS-2T image plate diffractometer equipped with a low-temperature device with Mo-Kα radiation (λ = 0.71073 Å) using ω-scans. Software for data collection: X-AREA, cell refinement: X-AREA and data reduction: X-RED (Stoe & Cie, 2009, X-RED and X-AREA. Stoe & Cie, Darmstadt, Germany). Preliminary structure models were derived by direct methods (Sheldrick, 2008) and the structures were refined by full-matrix least-squares calculations based on F2 for all reflections using SHELXL (Sheldrick, 2015). The positions of N-H-atoms have been refined without constraints. All other hydrogen atoms were included in the models in idealised positions and were refined as constrained to the pivot atoms. 2d-TMS crystallises in the chiral space group P212121. The molecule itself is not chiral. The absolute structure parameter was refined to a value of −0.01(2). There are rotational disorders at the methyl groups C11 and C12 in 5d-TMS. These have been refined with split atom models. Further crystallographic data are listed in Table 11.
Crystal data and structure refinement
| 2b-TMS2 | 2d-TMS | 3d-TMS2 | |
|---|---|---|---|
| Empirical formula | C18H28N4Si3 | C9H14Cl2N2Si2 | C12H28Cl2N2Si3 |
| Formula weight | 384.71 | 277.30 | 355.53 |
| T (K) | 93 | 93 | 200 |
| Crystal system, space group | Monoclinic, C2/c | Orthorhombic, P212121 | Monoclinic, P21/n |
| a (Å) | 15.0990(13) | 7.8056(12) | 9.6940(6) |
| b (Å) | 14.1234(12) | 9.0025(14) | 12.1485(7) |
| c (Å) | 10.5762(10) | 18.713(2) | 17.4157(9) |
| α (°) | 90 | 90 | 90 |
| β (°) | 114.400(4) | 90 | 105.242(4) |
| γ (°) | 90 | 90 | 90 |
| Volume (Å3) | 2053.9(3) | 1315.0(3) | 1978.9(2) |
| Z | 4 | 4 | 4 |
| ρcalc (g/cm3) | 1.244 | 1.401 | 1.193 |
| μMoKα (mm−1) | 0.240 | 0.647 | 0.501 |
| F(000) | 824 | 576 | 760 |
| Crystal size (mm) | 0.530 × 0.280 × 0.260 | 0.400 × 0.300 × 0.160 | 0.400 × 0.300 × 0.150 |
| Reflections collected / unique | 15493 / 2359 [R(int) = 0.0647] | 17564 / 3174 [R(int) = 0.0295] | 17820 / 4772 [R(int) = 0.0240] |
| Data / restraints / parameters | 2359 / 0 / 120 | 3174 / 0 / 143 | 4772 / 15 / 197 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0491, wR2 = 0.1323 | R1 = 0.0224, wR2 = 0.0574 | R1 = 0.0375, wR2 = 0.0939 |
| R indices (all data) | R1 = 0.0613, wR2 = 0.1494 | R1 = 0.0236, wR2 = 0.0579 | R1 = 0.0497, wR2 = 0.1049 |
| 4b-TMS2 | 4e | 4f · Et2O | 4g | |
|---|---|---|---|---|
| Empirical formula | C20H32N4Si3 | C20H32N4Si3 | C25H31Cl3N6OSi3 | C21H21Cl3N6Si3 |
| Formula weight | 412.76 | 412.76 | 622.18 | 548.06 |
| T (K) | 153 | 200 | 200 | 180 |
| Crystal system, space group | Monoclinic, C2/c | Monoclinic, I2/c | Monoclinic, P21/c | Monoclinic, P21/n |
| a (Å) | 16.4390(11) | 20.2108(13) | 10.4899(4) | 10.3542(7) |
| b (Å) | 14.2531(12) | 10.4857(4) | 10.2209(6) | 16.5306(8) |
| c (Å) | 9.8574(6) | 23.7581(14) | 28.1420(13) | 29.913(2) |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 94.821(5) | 113.392(5) | 91.244(4) | 99.590(5) |
| γ (°) | 90 | 90 | 90 | 90 |
| Volume (Å3) | 2301.5(3) | 4621.1(5) | 3016.6(3) | 5048.5(5) |
| Z | 4 | 8 | 4 | 8 |
| ρcalc (g/cm3) | 1.191 | 1.187 | 1.370 | 1.442 |
| μMoKα (mm−1) | 0.219 | 0.218 | 0.454 | 0.529 |
| F(000) | 888 | 1776 | 1296 | 2256 |
| Crystal size (mm) | 0.450 × 0.400 × 0.340 | 0.450 × 0.300 × 0.250 | 0.400 × 0.250 × 0.150 | 0.400 × 0.060 × 0.030 |
| Reflections collected / unique | 20017 / 2650 [R(int) = 0.0381] | 35511 / 5313 [R(int) = 0.0277] | 35175 / 6581 [R(int) = 0.0288] | 39071/8882 [R(int) = 0.1097] |
| Data / restraints / parameters | 2650 / 0 / 130 | 5313 / 0 / 258 | 6581 / 4 / 373 | 8882/0/614 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0332, wR2 = 0.0821 | R1 = 0.0430, wR2 = 0.1082 | R1 = 0.0377, wR2 = 0.0912 | R1 = 0.0498, wR2 = 0.0947 |
| R indices (all data) | R1 = 0.0404, wR2 = 0.0888 | R1 = 0.0632, wR2 = 0.1205 | R1 = 0.0555, wR2 = 0.1005 | R1 = 0.1246, wR2 = 0.1154 |
| 5b.[Et3NH]Cl·HCCl3 | 5c | 5d-TMS | |
|---|---|---|---|
| Empirical formula | C47H49Cl4N9Si2 | C12H14N2Si | C13H16Cl2N2Si2 |
| Formula weight | 937.93 | 214.34 | 327.36 |
| T (K) | 153 | 153 | 180 |
| Crystal system, space group | Triclinic, P-1 | Monoclinic, P21/c | Monoclinic, P21/c |
| a (Å) | 12.1354(6) | 14.0807(5) | 14.2310(4) |
| b (Å) | 14.1506(7) | 9.4389(2) | 9.4966(2) |
| c (Å) | 15.1245(8) | 17.4383(6) | 12.2393(4) |
| α (°) | 102.395(4) | 90 | 90 |
| β (°) | 98.013(4) | 100.415(3) | 108.065(2) |
| γ (°) | 109.180(4) | 90 | 90 |
| Volume (Å3) | 2333.0(2) | 2279.47(12) | 1572.56(8) |
| Z | 2 | 8 | 4 |
| ρcalc (g/cm3) | 1.335 | 1.249 | 1.383 |
| μMoKα (mm−1) | 0.350 | 0.174 | 0.553 |
| F(000) | 980 | 912 | 680 |
| Crystal size (mm) | 0.450 × 0.380 × 0.300 | 0.380 × 0.280 × 0.210 | 0.300 × 0.200 × 0.150 |
| Reflections collected / unique | 50078 / 10055 [R(int) = 0.0427] | 28952 / 5234 [R(int) = 0.0327] | 27189 / 3791 [R(int) = 0.0318] |
| Data / restraints / parameters | 10055 / 18 / 635 | 5234 / 0 / 291 | 3791 / 0 / 182 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0393, wR2 = 0.0902 | R1 = 0.0496, wR2 = 0.1040 | R1 = 0.0292, wR2 = 0.0740 |
| R indices (all data) | R1 = 0.0555, wR2 = 0.1016 | R1 = 0.0653, wR2 = 0.1170 | R1 = 0.0336, wR2 = 0.0762 |
CCDC-2033885 (2b-TMS2), 2033886 (2d-TMS), 2043741 (3d-TMS2), 2033889 (4b-TMS2), 2033888 and 2043739 (4e), 2043742 (4f · Et2O), 2043740 (4g), 2033887 (5b · Et3NHCl · CHCl3), 2033890 (5c), and 2043742 (5d-TMS) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crytallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Quantum chemical calculations
The quantum chemical calculations have been performed with GAUSSIAN 16 (Frisch et al., 2016). The molecules have been optimised with M062X/6-31G(d) (Francl et al., 1982; Hariharan and Pople, 1973; Zhao and Truhlar, 2008). The calculation of Hessian-matrices verified the presence of local minima on the potential energy surface with zero imaginary frequencies. The solvent THF was included into these calculations by placing the molecules in a cavity within the solvent reaction field. This method is described in the literature as Polarizable Continuum Model (PCM). See Tomasi et al. (2005) for a review about relevant literature. Enthalpy (H) and Gibbs free energy (G) data derived from the calculations correspond to 298.15 K and 1 atmosphere of pressure.
Acknowledgements
Further data can be found as electronic Supplementary material for this publication. The authors gratefully acknowledge help from Beate Kutzner (NMR), Regina Moßig (Raman), Konstantin Kraushaar (MS), and Ute Groß (EA). The authors thank the Computing Centre of the TU Bergakademie Freiberg for computing time at the high-performance computing (HPC) cluster.
Funding information: Part of this work was performed within the research group “Chemical utilization of carbon dioxide with aminosilanes (CO2-Sil)” that is financially supported by the European Union (European social fund, ESF), the Ministry of Science and Art of Saxony (SMWK), and the Sächsische Aufbaubank (SAB).
Author contributions: Marcus Herbig: conceptualization, writing – original draft, writing – review and editing, investigation, supervision; Henrik Scholz: writing – review and editing, investigation; Uwe Böhme: writing – original draft, writing – review and editing, investigation; Betty Günther: investigation; Lia Gevorgyan: formal analysis; Daniela Gerlach: investigation; Jörg Wagler: writing – review and editing, investigation; Sandra Schwarzer: funding acquisition, supervision; Edwin Kroke: funding acquisition, writing – review and editing.
Conflict of interest: One of the authors (Jörg Wagler) is a member of the Editorial Board of Main Group Metal Chemistry.
Data availability statement: Data of the crystal structures reported in this paper (in CIF format) can be obtained free of charge from The Cambridge Crytallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Further data can be found as electronic Supplementary material for this publication.
References
Auner N., Penzenstadler E., Herdtweck E., Silaheterocyclen, XX Aminosubstituierte Vinylchlorsilane und Silaethene: Bausteine zur Synthese von l,3-Diaza-2-silacyclopentanen [1]. Z. Naturforsch. B, 1992, 47(10), 1377–1385, DOI: 10.1515/znb-1992-1007Search in Google Scholar
Armitage D.A., Organosilanes. In: Wilkinson G., Stone F.G.A., Abel E.W. (Eds.), Comprehensive Organometallic Chemistry. Pergamon Press, Oxford, 1982.10.1016/B978-008046518-0.00014-3Search in Google Scholar
Barluenga J., Tomás M., Ballesteros A., López L.A., A Simple, Regioselective Synthesis of 5,6-Dihydro-1,3,5-triazine-2,4(1H,3H)-dione Derivatives from N-Trimethylsilyl Imines via 1,3-Diazabutadienes. Synthesis, 1989, 3, 228–229, DOI: 10.1055/s-1989-27212Search in Google Scholar
Boeyens J.C.A., The conformation of six-membered rings. J. Cryst. Mol. Struct., 1978, 8, 317–320, DOI: 10.1007/bf01200485Search in Google Scholar
Böhme U., Bendrath F., Günther B., 2,2-Dichloro-1,3-bis (trimethylsilyl)-1,3-diaza-2-silacyclopentane. Acta Crystallogr. C, 2007, 63, o631–o632, DOI: 10.1107/S0108270107045969Search in Google Scholar
Böhme U., Günther B., Rittmeister B., Selective Synthesis of N-Methylanilinooligosilanes. Eur. J. Inorg. Chem., 2003a, 4, 751–758, DOI: 10.1002/ejic.200390104Search in Google Scholar
Böhme U., Günther B., Rittmeister B., Synthesis of Chiral Amino-Substituted Organosilanes. In: Auner N., Weis J. (Eds.), Organosilicon Chemistry. Wiley-VCH, Weinheim, 2003b. DOI: 10.1002/9783527620777.ch88dSearch in Google Scholar
Böhme U., Günther B., Rittmeister B., Synthesis and structure determination of a novel diastereomeric diaminodichlorodisilane. Inorg. Chem. Comm., 2000, 3(8), 428–432, DOI: 10.1016/S1387-7003(00)00113-1Search in Google Scholar
Böhme U., Inertgastechnik. DeGruyter, Berlin, 2020.10.1515/9783110627046Search in Google Scholar
Cremer D., Pople J.A., General definition of ring puckering coordinates. J. Am. Chem. Soc., 1975, 97(6), 1354–1358, DOI: 10.1021/ja00839a011Search in Google Scholar
Daniéle S., Drost C., Gehrhus B., Hawkins S.M., Hitchcock P.B., Lappert M.F., et al., Synthesis and structures of crystalline dilithium diamides and aminolithium amides derived from N,N′-disubstituted 1,2-diaminobenzenes or 1,8-diaminonaphthalene. J. Chem. Soc., Dalton Trans., 2001, 3179–3188, DOI: 10.1039/B104135PSearch in Google Scholar
Degl’Innocenti A., Capperucci A., Reginato G., Mordini A., Ricci A., Reactivity of acetylenic silyl ketones: synthesis of functionalized propenoylsilanes. Tetrahedron Lett., 1992, 33, 1507–1508, DOI: 10.1016/S0040-4039(00)91660-5Search in Google Scholar
Diedrich F., Ebker C., Klingebiel U., Reiche C., Labahn T., Magull J., et al., N,N‘-Bis(silyl)ethylendiamine und 1,3-Diaza-2-silacyclopentane – Synthese, Reaktionen, Strukturen. Z. Naturforsch. B, 2002, 57, 99–106, DOI: 10.1515/znb-2002-0112Search in Google Scholar
Foresman J., Frisch Æ., Exploring Chemistry with Electronic Structure Methods (3rd ed.). Gaussian Inc., Wallingford, 2015.Search in Google Scholar
Francl M.M., Petro W.J., Hehre W.J., Binkley J.S., Gordon M.S., DeFrees D.J., et al., Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys., 1982, 77, 3654–3665, DOI: 10.1063/1.444267Search in Google Scholar
Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., et al., Gaussian 16, Revision B.01. Gaussian, Inc., Wallingford, CT, 2016.Search in Google Scholar
Gade L.H., Galka C.H., Hellmann K.W., Williams R.M., De Cola L., Scowen I.J., et al., Tetraaminoperylenes: Their Efficient Synthesis and Physical Properties. Chem. Eur. J., 2002, 8(16), 3732–3746, DOI: 10.1002/1521-3765(20020816)8:16<3732::AID-CHEM3732>3.0.CO;2-5Search in Google Scholar
Glidewell C., Rankin D.W.H., Some preparative and spectroscopic studies of silylamines. J. Chem. Soc. A, 1970, 279–286, DOI: 10.1039/J19700000279Search in Google Scholar
Hariharan P.C., Pople J.A., The influence of polarization functions on molecular orbital hydrogenation energies. Theoret. Chim. Acta, 1973, 28, 213–222, DOI: 10.1007/BF00533485Search in Google Scholar
Herbig M., Kroke E., Low cost apparatus for rapid boiling point determination of small air sensitive samples under inert atmosphere. Thermochim. Acta, 2017, 654, 81–84, DOI: 10.1016/j.tca.2017.05.005Search in Google Scholar
Herbig M., Böhme U., Kroke E., Insertion of CO2 and related heteroallenes into the Si-N-bond of methyl(N-morpholino) silanes. Inorg. Chim. Acta, 2018a, 473, 20–28, DOI: 10.1016/j.ica.2017.12.020Search in Google Scholar
Herbig M., Böhme U., Schwarzer A., Kroke E., Formation of 1-aza-2-silacyclopentanes and unexpected products from the insertion of phenylisocyanate into 2,2-dimethyl-1-(trimethylsilyl)-1-aza-2-sila-cyclo-pentane. Main Group Met. Chem., 2018b, 41(1–2), 11–19, DOI: 10.1515/mgmc-2018-0005Search in Google Scholar
Herzog S., Dehnert J., Eine rationelle anaerobe Arbeitsmethode. Z. Chem. 1964, 4(1), 1–11, DOI: 10.1002/zfch.19640040102Search in Google Scholar
Herzog U., Trommer K., Roewer G., Preparation and NMR spectroscopical investigations of (diethylamino)(methoxy)-methylchlorodi- and -trisilanes. J. Organomet. Chem., 1998, 552, 99–108, DOI: 10.1016/S0022-328X(97)00566-4Search in Google Scholar
Herzog U., Böhme U., Rheinwald G., 1,2-Dithiolate derivatives of monosilanes and disilanes. J. Organomet. Chem., 2000, 612, 133–140, DOI: 10.1016/S0022-328X(00)00432-0Search in Google Scholar
Huber G., Mitzel N. W., Schier A., Schmidbaur H., The molecular structures of triaminosilanes. Chem. Ber., 1997a, 130, 1159–1166, DOI: 10.1002/cber.19971300820Search in Google Scholar
Huber G, Schier A, Schmidbaur H., The molecular structures of tetra(amino)silanes. Chem. Ber., 1997b, 130(8), 1167–1174, DOI: 10.1002/cber.19971300821Search in Google Scholar
Huber G., Schmidbaur H., Hexa(amino)disilanes with saturated cyclic amino ligands. Monatsh. Chem., 1999, 130, 133–138, DOI: 10.1007/PL00000114Search in Google Scholar
Jaçoviç M., Kondensationsprodukte der Methylchlorsilane mit Dioxybenzolen. Z. Anorg. Allg. Chem., 1957, 288(5–6), 324–332, DOI: 10.1002/zaac.19572880510Search in Google Scholar
Kaßner L, Knoblauch A., Seifert A., Grützner E., Cox G., Lange A., et al., Nanostructured Aniline Formaldehyde Resin/Polysilazane Hybrid Materials by Twin Polymerization. Macromol. Chem. Phys., 2016, 217(22), 2462–2472, DOI: 10.1002/macp.201600152Search in Google Scholar
Kaftory M., Kapon M., Botoshansky M., The structural chemistry of organosilicon compounds. In: Rappoport Z., Apeloig Y. (Eds.), The Chemistry of Organic Silicon Compounds. J. Wiley & Sons Inc., Chichester, 1998.10.1002/0470857250.ch5Search in Google Scholar
Kirilin D., Belova L.O., Gavrilova A.V., Korobova E.A., Russ. J. Gen. Chem., 2009, 79, 2137–2141, DOI: 10.1134/S1070363209100119Search in Google Scholar
Kraushaar K., Wiltzsch C., Wagler J., Böhme U., Schwarzer A., Roewer G., et al., From CO2 to Polysiloxanes: Di(carbamoyloxy)silanes Me2Si[(OCO)NRR’]2 as Precursors for PDMS. Organometallics, 2012, 31, 4779–4785, DOI: 10.1021/om300313fSearch in Google Scholar
Kraushaar K., Schmidt D., Schwarzer A., Kroke E., Reactions of CO2 and CO2 Analogs (CXY with X, Y=O, S, NR) with Reagents Containing Si-H and Si-N Units. Adv. Inorg. Chem., 2014, 66, 117–162, DOI: 10.1016/B978-0-12-420221-4.00004-4Search in Google Scholar
Kraushaar K., Herbig M., Schmidt D., Wagler J., Böhme U., Kroke E., Insertion of phenyl isocyanate into mono- and diaminosilanes. Z. Naturforsch. B, 2017, 72, 909–921, DOI: 10.1515/znb-2017-0149Search in Google Scholar
Kummer D., Rochow E.G., 2-Silabenzimidazoline. Angew. Chem., 1963, 75(4), 207–208, DOI: 10.1002/ange.19630750409.Search in Google Scholar
Meinel B., Günther B., Schwarzer A., Böhme U., Crystalline Aminoorganosilanes – Syntheses, Structures, Spectroscopic Properties. Z. Anorg. Allg. Chem., 2014, 640(8–9), 1607–1613, DOI: 10.1002/zaac.201300646Search in Google Scholar
Meinel B., Günther B., Böhme U., Force field parameters for aminoorganosilanes. J. Mol. Struct., 2015, 1079, 363–369, DOI: 10.1016/j.molstruc.2014.08.043Search in Google Scholar
Prout T.R., Thompson M.L., Haltiwanger R.C., Schaeffer R., Norman A.D., New skeletally stabilized silazanes and siloxazanes. Inorg. Chem., 1994, 33(9), 1778–1782, DOI: 10.1021/ic00087a010Search in Google Scholar
Rong G., Keese, R., Stoeckli-Evans H., Some Silatetraazaspiroalkanes with [4 + 4] Octacoordinated Silicon. Eur. J. Inorg. Chem., 1998, 12, 1967–1973, DOI: 10.1002/(SICI)1099-0682(199812)1998:12<1967::AID-EJIC1967>3.0.CO;2-ASearch in Google Scholar
Schlingmann M., Wannagat U., Novel Inorganic Ring Systems. XXII Novel Spirosilazanes of the Si5N4, Si5N5, Si5N4O, and Si7N8 Skeleton. Z. Anorg. Allg. Chem., 1976a, 419, 108–114, DOI: 10.1002/zaac.19764190203Search in Google Scholar
Schlingmann M., Wannagat U., Novel Inorganic Ring Systems. XXIII Hetero Spirosilazanes with Ge, Sn, and Zr in the Spiral Center. Z. Anorg. Allg. Chem., 1976b, 419, 115–120, DOI: 10.1002/zaac.19764190204Search in Google Scholar
Schlosser T., Sladek A., Hiller W., Schmidbaur H., Neue mono- und bicyclische Aminosilane: Synthese, Struktur und Eigenschaften. Z. Naturforsch. B, 1994, 49, 1247–1255, DOI: 10.1515/znb-1994-0914Search in Google Scholar
Sheldrick G.M., A short history of SHELX. Acta Crystallogr. A, 2008, 64, 112–122, DOI: 10.1107/S0108767307043930Search in Google Scholar
Sheldrick G.M., Crystal structure refinement with SHELXL. Acta Crystallogr. C, 2015, 71, 3–8, DOI: 10.1107/S2053229614024218Search in Google Scholar
Spek A.L., PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C, 2015, 71, 9–18, DOI: 10.1107/S2053229614024929Search in Google Scholar
Tamao K., Kawachi A., Nakagawa Y., Ito Y., Electronic spectra of (amino)(phenyl)disilanes. J. Organomet. Chem., 1994, 473(1–2), 29–34, DOI: 10.1016/0022-328X(94)80102-9Search in Google Scholar
Tomasi J., Mennucci B., Cammi R., Quantum Mechanical Continuum Solvation Models. Chem. Rev., 2005, 105, 2999–3093, DOI: 10.1021/cr9904009Search in Google Scholar PubMed
Trommer K., Herzog U., Roewer G. Preparation and 29Si NMR spectroscopical investigations of (diethylamino)-methylchlorotetra- and pentasilanes. J. Prakt. Chem., 1997, 339(1), 637–641, DOI: 10.1002/prac.199733901113Search in Google Scholar
Wagler J., Roewer G., Intramolecular interligand charge transfer and a red hexacoordinate Si-complex with salen type ligand vs. colorless tetracoordinate salen-Si-complexes with similar substituents. Inorg. Chim. Acta, 2007, 360, 1717–1724, DOI: 10.1016/j.ica.2006.09.006Search in Google Scholar
Wannagat U., Eisle G., Neue Silaza- und Silaoxaza-spiro[4.4] nonane und [4.5]decane. Z. Naturforsch. B, 1978, 33, 471–474, DOI: 10.1515/znb-1978-0501Search in Google Scholar
Wojnowski W., Peters K., Böhm M.C., v. Schnering G.H., Die Struktur des Spiro-bis(ethylendithia)silans. Z. Anorg. Allg. Chem., 1985, 523, 169–179, DOI: 10.1002/zaac.19855230421Search in Google Scholar
Wolff G., Michalik M., Kelling H., Einschubreaktionen von Schwefelkohlenstoff in Silylamine. J. Prakt. Chem., 1978, 320(5), 807–813, DOI: 10.1002/prac.19783200514Search in Google Scholar
Yoder C., Zimmerman J., Spiro Silicon and Germanium Imidazolidines. Inorg. Chem., 1964, 3, 1329–1331, DOI: 10.1021/ic50019a034Search in Google Scholar
Zhao Y., Truhlar D.G., The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc., 2008, 120, 215–241, DOI: 10.1007/s00214-007-0310-xSearch in Google Scholar
© 2021 Marcus Herbig, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- A novel approach for the production of zinc borate (4ZnO·B2O3·H2O) using a single-step hydrothermal method
- Fabrication, characterization, and photocatalytic performance of ternary cadmium chalcogenides CdIn2S4 and Cd7.23Zn2.77S10-ZnS thin films
- New cyclic and spirocyclic aminosilanes
- Determination of essential and non-essential element contents of drinking water and baby water for infant’s nutrition
- Syntheses and molecular structures of some di(amidino)monosilanes
- New horizons for the synthesis of nanoparticles: Germanium nanoparticles from metastable GeBr-solutions
- Synthesis and antimalarial activity of some triphenyltin(IV) aminobenzoate compounds against Plasmodium falciparum
- Coordination behavior of PPh2{C6H4[CH2N(CH2CH2)2O]-2}: Case study CdCl2[PPh2{C6H4[CH2N(CH2CH2)2O]-2}]
- Rapid Communications
- Synthesis and structure of 2,8-dimethyl-10,10-dichlorophenoxatellurine
- Synthesis and crystal structure of one new Pb(II) complex constructed by 1,10-phenanthroline and two carboxylate ligands
- Organotin(IV) selenate derivatives – Crystal structure of [{(Ph3Sn)2SeO4} ⋅ CH3OH]n
- Synthesis and structural characterization of a novel 2D supramolecular lead coordination polymer with phenanthroline derivate and adipic acid
- X-ray crystal structures of carbonate and hydroxide bridged MnII/MgII heterobimetallic complexes formed by reduction of CO2 or H2O by a Mn0–MgII bonded compound
- Review Articles
- Variable combinations of organophosphines in PtP3X derivatives: Structural aspects
- Tetradentate organophosphines in Pt(η4–A4L) (A = P4, P3Si, P2X2 (X2 = N2, S2, C2), PX3 (X3 = N3, N2O)): Structural aspects
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Editorial: Topological investigations of chemical networks
- Forgotten coindex of some non-toxic dendrimers structure used in targeted drug delivery
- Eccentricity based topological indices of face centered cubic lattice FCC(n)
- Irregular topological indices of certain metal organic frameworks
- Molecular topological invariants of certain chemical networks
- Szeged-type indices of subdivision vertex-edge join (SVE-join)
- Fractional metric dimension of metal-organic frameworks
- On topological descriptors of ceria oxide and their applications
- On analysis of thermodynamic properties of cuboctahedral bi-metallic structure
- M-polynomial-based topological indices of metal-organic networks
- Two modified Zagreb indices for random structures
- Some degree-based topological indices of caboxy-terminated dendritic macromolecule
- Hosoya properties of the commuting graph associated with the group of symmetries
- On topological properties of hierarchical hypercube network based on Ve and Ev degree
- On vertex PI index of certain triangular tessellation networks
- Some topological properties of uniform subdivision of Sierpiński graphs
Articles in the same Issue
- Research Articles
- A novel approach for the production of zinc borate (4ZnO·B2O3·H2O) using a single-step hydrothermal method
- Fabrication, characterization, and photocatalytic performance of ternary cadmium chalcogenides CdIn2S4 and Cd7.23Zn2.77S10-ZnS thin films
- New cyclic and spirocyclic aminosilanes
- Determination of essential and non-essential element contents of drinking water and baby water for infant’s nutrition
- Syntheses and molecular structures of some di(amidino)monosilanes
- New horizons for the synthesis of nanoparticles: Germanium nanoparticles from metastable GeBr-solutions
- Synthesis and antimalarial activity of some triphenyltin(IV) aminobenzoate compounds against Plasmodium falciparum
- Coordination behavior of PPh2{C6H4[CH2N(CH2CH2)2O]-2}: Case study CdCl2[PPh2{C6H4[CH2N(CH2CH2)2O]-2}]
- Rapid Communications
- Synthesis and structure of 2,8-dimethyl-10,10-dichlorophenoxatellurine
- Synthesis and crystal structure of one new Pb(II) complex constructed by 1,10-phenanthroline and two carboxylate ligands
- Organotin(IV) selenate derivatives – Crystal structure of [{(Ph3Sn)2SeO4} ⋅ CH3OH]n
- Synthesis and structural characterization of a novel 2D supramolecular lead coordination polymer with phenanthroline derivate and adipic acid
- X-ray crystal structures of carbonate and hydroxide bridged MnII/MgII heterobimetallic complexes formed by reduction of CO2 or H2O by a Mn0–MgII bonded compound
- Review Articles
- Variable combinations of organophosphines in PtP3X derivatives: Structural aspects
- Tetradentate organophosphines in Pt(η4–A4L) (A = P4, P3Si, P2X2 (X2 = N2, S2, C2), PX3 (X3 = N3, N2O)): Structural aspects
- Special Issue: Topological descriptors of chemical networks: Theoretical studies (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Editorial: Topological investigations of chemical networks
- Forgotten coindex of some non-toxic dendrimers structure used in targeted drug delivery
- Eccentricity based topological indices of face centered cubic lattice FCC(n)
- Irregular topological indices of certain metal organic frameworks
- Molecular topological invariants of certain chemical networks
- Szeged-type indices of subdivision vertex-edge join (SVE-join)
- Fractional metric dimension of metal-organic frameworks
- On topological descriptors of ceria oxide and their applications
- On analysis of thermodynamic properties of cuboctahedral bi-metallic structure
- M-polynomial-based topological indices of metal-organic networks
- Two modified Zagreb indices for random structures
- Some degree-based topological indices of caboxy-terminated dendritic macromolecule
- Hosoya properties of the commuting graph associated with the group of symmetries
- On topological properties of hierarchical hypercube network based on Ve and Ev degree
- On vertex PI index of certain triangular tessellation networks
- Some topological properties of uniform subdivision of Sierpiński graphs

