Abstract
Objectives
To explore serum vascular endothelial growth factor (VEGF), β-subunit human chorionic gonadotropin (β-hCG), and soluble Fms-like tyrosine kinasereceptor 1 (sFlt-1) levels in pregnant women with placenta accreta spectrum (PAS) and their prognostic implications.
Methods
Serum levels were measured in PAS patients and non-PAS controls. Depending on the depth of placental penetration into the uterine wall, PAS patients were further classified into placenta accreta, placenta increta and placenta percreta subgroups. Diagnostic efficacy of individual biomarkers and combined indices was evaluated using receiver operating characteristic curves. Correlations between biomarker levels, disease severity, and prognosis were analyzed.
Results
Serum levels of VEGF and β-hCG showed significant positive correlations with the extent of PAS invasion, whereas sFlt-1 levels were inversely associated with disease progression. Combined pregnancy complications, elevated serum VEGF levels and decreased serum sFlt-1 levels were risk factors for poor prognosis in patients with PAS. The AUC values of the indicators combined to predict the diagnosis and prognosis of patients with PAS were greater than serum VEGF, hCG, and sFlt-1 levels alone.
Conclusions
Serum levels of VEGF, β-hCG, and sFlt-1 demonstrate the potential to differentiate between women with and without PAS, and further exhibit a correlation with the depth of myometrial invasion in PAS cases. The combined use of these serum markers enhances both the sensitivity and specificity of prenatal diagnosis and prognostic assessment for PAS compared to individual markers, thereby offering valuable guidance for clinical diagnosis and management of PAS.
Introduction
Placenta accreta spectrum (PAS) is a severe obstetric complication characterized by abnormal invasion of cytotrophoblast cells at the endometrial-myometrial junction. This condition is classified into three categories: placenta accreta, increta, and percreta, based on the extent of placental infiltration into the myometrial layer of the uterus [1]. Under normal circumstances, the placenta can detach from the uterine wall after delivery, but in the presence of PAS, some or all of the placenta remains attached to the uterine wall after delivery, which can lead to severe postpartum hemorrhage and shock [2]. PAS is a contributor to poor maternal and infant outcomes, and it is critical to find reliable indicators early on to diagnose PAS and assess patient prognosis. Clinical data show that abnormal placental vascular growth and development and associated vascular growth factor abnormalities are the main pathogenesis of PAS [3]. Vascular endothelial growth factor (VEGF) can directly stimulate the proliferation and differentiation of vascular endothelial cells and promote neoangiogenesis [4]. Soluble Fms-like tyrosine kinasereceptor 1 (sFlt-1) is a soluble isoform of VEGF receptor-1 (VEGFR-1) generated through alternative mRNA splicing. This secreted glycoprotein retains the extracellular ligand-binding domain of VEGFR-1 while lacking transmembrane and intracellular kinase domains. Functionally, sFlt-1 binds and antagonizes VEGF to inhibit its angiogenic function [5], 6]. Measurement of serum VEGF and sFlt-1 levels is gaining recognition as a diagnostic adjunct for suspected PAS disorders, with emerging prognostic value in predicting disease progression severity [7], 8]. Human chorionic gonadotropin (hCG), a glycoprotein hormone composed of α and β subunits, is primarily secreted by trophoblasts. This hormone holds critical implications for confirming normal gestation, diagnosing gestational-specific disorders, and screening fetal congenital anomalies or developmental pathologies [9], 10]. β-subunit hCG (β-hCG) is synthesized by trophoblastic cells and predominantly secreted into maternal circulation, while placental tissue concentrations remain comparatively low. During the second trimester, this biomarker exhibits high detectability and stability, serving as a reliable indicator of trophoblastic activity. Studies demonstrate elevated β-hCG levels in PAS patients compared to non-PAS controls, with concentrations progressively increasing across PAS severity [11]. However, there is no clear report about the relationship between each index and the prognosis of patients with PAS.
Therefore, the aim of this study was to investigate the changes in serum VEGF, β-hCG, and sFlt-1 levels in women with PAS and their effects on prognosis, so as to provide a reference for the early assessment of the prognosis of patients with this disease.
Materials and methods
Research object
A retrospective analysis was conducted on the clinical data of 120 pregnant women giving birth at the Jiangsu Taizhou People’s Hospital from January 2017 to December 2020. The study group comprised 68 pregnant women diagnosed with PAS, while the control group consisted of 52 women with uncomplicated physiological pregnancies (non-PAS).
Inclusion criteria: ① underwent routine antenatal ultrasonography prior to delivery; ② antenatal PAS high-risk factors such as history of uterine incision, cesarean section, multiple deliveries, induced abortion, advanced maternal age; ③ gestational weeks ≥ 32 weeks; ④ single pregnancy; ⑤ complete antenatal examination data.
Exclusion criteria: ① hepatic and renal insufficiency; ② incomplete clinical data; gynecological malignant tumors; ③ fetal anomalies; ④ fetal chromosomal abnormalities; ⑤ multiple pregnancies.
Diagnostic criteria and classification of PAS
The prenatal diagnosis of PAS was primarily based on ultrasonography [12], [13], [14]. The following sonographic features, in accordance with the International Federation of Gynecology and Obstetrics (FIGO) recommendations [15], were utilized for the evaluation of PAS: the presence of placental lacunae, absence of the retroplacental clear zone (also known as the hypoechoic space), abnormalities at the utero-bladder interface, and color Doppler findings such as hypervascularity and bridging vessels. The diagnosis was further confirmed either by intraoperative identification of a “glued” appearance indicating placental adherence to the uterine wall in hysterectomy specimens, or by postoperative histopathological examination of focally excised tissue.
The grading of PAS into accreta, increta, and percreta was validated by postoperative pathological examination. According to the FIGO classification [15], placenta accreta was characterized by placental villi attached to the myometrium; placenta increta was defined by villi invading into the myometrial layer; and placenta percreta was confirmed by villi penetrating through or breaching the uterine serosa.
Study design and methods
Maternal clinical data were collected and analyzed, including age, gestational age, area of PAS, parity, history of cesarean section, placental morphology, and the presence of adverse pregnancy outcomes.
Enzyme-linked immunosorbent assay was conducted to detect serum VEGF and sFlt-1 levels in patients (kits provided by Shanghai Bogu Biological Technology Co., Ltd., Shanghai, China). Serum β-hCG level was detected by magnetic particle chemiluminescence method, and the kit was provided by Xiamen Innodx BIOTECH Co., Ltd. (Fujian, China).
Serum levels of VEGF, β-hCG, and sFlt-1 were measured and compared between the PAS group and the non-PAS group, as well as among the placenta accreta (n = 25), placenta increta (n = 38), and placenta percreta (n = 5) subgroups. The diagnostic value of each index for PAS was evaluated. Correlation analyses were performed to assess the relationship between each index and the depth of invasion in PAS (accreta, increta, and percreta). All patients underwent surgical treatment, and based on the occurrence of postoperative adverse pregnancy outcomes, the study cohort was categorized into a group with better prognosis (n = 51) and a group with poor prognosis (n = 17). The impact of serum VEGF, β-hCG, and sFlt-1 levels on patient prognosis was subsequently analyzed.
Statistical analysis
SPSS 22.0 software was taken to process the data. Enumeration data were expressed as % and compared by χ2 test. Measurement data were expressed as (±s) after the normal test, and comparatively assessed using t-test between two groups or one-way ANOVA between multiple groups. The diagnostic value of PAS was analyzed by the receiver operating characteristic (ROC) curve using the levels of serum VEGF, β-hCG, and sFlt-1. The correlation between serum VEGF, β-hCG and sFlt-1 levels and the severity of PAS was examined by spearman test, and the influencing factors of the prognosis of patients with PAS were analyzed by multivariate logistic regression. Differences were statistically significant at p < 0.05.
Results
Serum VEGF, β-hCG and sFlt-1 levels in the PAS group and non-PAS group
The serum VEGF and β-hCG levels in the PAS group (n = 68) were higher than those in the non-PAS group (n = 52), and the sFlt-1 levels were lower (p < 0.05, Figure 1).
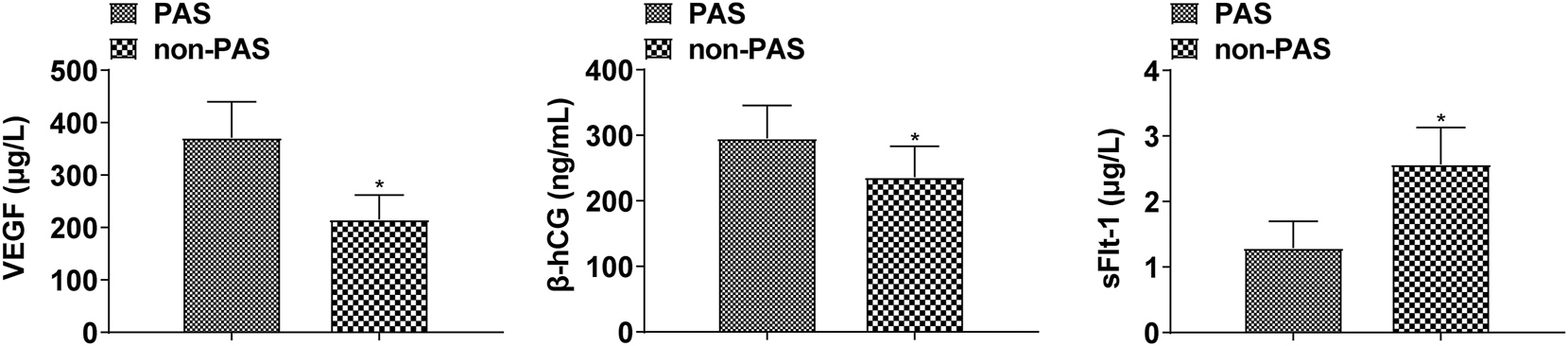
Comparison of serum VEGF, β-hCG, and sFlt-1 levels in the PAS group and the non-PAS group(*p < 0.05 compared with the PAS group).
Diagnostic value of serum VEGF, β-hCG, and sFlt-1 levels for PAS
The AUC value of the combined test of each index for the diagnosis of PAS was greater than that of serum VEGF, β-hCG, and sFlt-1 levels alone (p < 0.05), as shown in Table 1 and Figure 2.
Diagnostic value of serum VEGF, β-hCG, and sFlt-1 levels for PAS.
| Indicators | Cut-off value | AUC | SE | 95%CI |
|---|---|---|---|---|
| VEGF | 312.73 µg/L | 0.737a | 0.046 | 0.647∼0.827 |
| β-hCG | 276.51 ng/mL | 0.818a | 0.04 | 0.740∼0.897 |
| sFlt-1 | 1.89 µg/L | 0.716a | 0.047 | 0.624∼0.808 |
| Combined | 0.881 | 0.031 | 0.821∼0.941 |
-
Compared with the combined, aP < 0.05.

ROC curves of serum VEGF, β-hCG, and sFlt-1 levels for the diagnosis of PAS.
Serum VEGF, β-hCG, and sFlt-1 levels in patients with different severity of PAS
Based on the depth of villous invasion into the myometrium, 68 cases with PAS were classified into three subgroups: placenta accreta (n = 25), placenta increta (n = 38), and placenta percreta (n = 5). Comparing serum VEGF and β-hCG levels, the placenta percreta group was the highest, followed by the placenta increta group, and then the placenta accreta group. sFlt-1 levels were highest in the placenta accreta group, followed by the placenta increta group, and then the placenta percreta group (p < 0.05, Figure 3).

Comparison of serum VEGF, β-hCG and sFlt-1 levels in patients with different severity of PAS (*p < 0.05 compared with the placenta accreta group; #p < 0.05 compared with the placenta increta group).
Correlation analysis of serum VEGF, β-hCG, and sFlt-1 levels with the severity of PAS
Serum VEGF and β-hCG levels were positively correlated with the severity of PAS, and sFlt-1 levels were negatively correlated with the severity of PAS (p < 0.05, Table 2).
Correlation analysis of serum VEGF, β-hCG and sFlt-1 levels with the severity of PAS.
| Indicators | r | p-Value |
|---|---|---|
| VEGF | 0.407 | < 0.001 |
| β-hCG | 0.546 | < 0.001 |
| sFlt-1 | −0.371 | < 0.001 |
Univariate analysis of prognosis of patients with PAS
The area of PAS in the poor prognosis group was larger than that in the better prognosis group. The proportion of pregnant women with abnormal placental morphology and combined pregnancy complications was higher, VEGF and β-hCG were higher, and sFlt-1 was lower than those in the better prognosis group (p<0.05, Table 3).
Univariate analysis of prognosis of patients with PAS.
| Factors | Good prognosis group (n = 51) | Poor prognosis group (n = 17) | χ2/t | p-Value |
|---|---|---|---|---|
| Age | 28.96 ± 4.05 | 30.28 ± 3.63 | 1.193 | 0.237 |
| Gestational age | 35.01 ± 2.74 | 35.09 ± 2.88 | 0.103 | 0.918 |
| Area of PAS | 22.63 ± 3.16 | 26.18 ± 4.21 | 3.681 | < 0.001 |
| Multipara | 30 | 8 | 0.716 | 0.398 |
| Previous history of cesarean section | 31 | 9 | 0.324 | 0.569 |
| Abnormal placental morphology | 20 | 12 | 5.037 | 0.025 |
| Adverse pregnancy history | 12 | 8 | 3.4 | 0.065 |
| Combined pregnancy complications | 19 | 13 | 7.87 | 0.005 |
| VEGF (µg/L) | 346.89 ± 50.61 | 445.41 ± 43.09 | 7.195 | < 0.001 |
| β-hCG (ng/mL) | 279.03 ± 47.62 | 344.43 ± 31.38 | 5.279 | < 0.001 |
| sFlt-1 (µg/L) | 1.38 ± 0.26 | 1.02 ± 0.21 | 5.167 | < 0.001 |
Multifactorial analysis of the prognosis of patients with PAS
Combined pregnancy complications, elevated serum VEGF levels and decreased serum sFlt-1 levels were risk factors for poor prognosis in patients with PAS (p < 0.05, Table 4).
Multifactorial analysis of prognosis of patients with PAS.
| Factors | β | SE | wald χ2 | OR | 95%CI | p-Value |
|---|---|---|---|---|---|---|
| Abnormal placental morphology | 0.583 | 0.306 | 3.63 | 1.791 | 0.983∼3.263 | 0.057 |
| Combined pregnancy complications | 0.923 | 0.218 | 17.926 | 2.517 | 1.642∼3.859 | < 0.001 |
| VEGF | 0.764 | 0.25 | 9.339 | 2.147 | 1.315∼3.504 | 0.002 |
| β-hCG | 0.396 | 0.207 | 3.66 | 1.486 | 0.990∼2.229 | 0.056 |
| sFlt-1 | −0.913 | 0.345 | 7.003 | 0.401 | 0.204∼0.789 | 0.008 |
Predictive value of serum VEGF, β-hCG, and sFlt-1 levels on the prognosis of patients with PAS
The AUC value of the combined test of each index for predicting the prognosis of patients with PAS was greater than that of serum VEGF, β-hCG, and sFlt-1 levels tested individually (p < 0.05, Table 5 and Figure 4).
Predictive value of serum VEGF, β-hCG, and sFlt-1 levels on the prognosis of patients with PAS.
| Indicators | Cut-off value | AUC | SE | 95%CI |
|---|---|---|---|---|
| VEGF | 397.28 µg/L | 0.724a | 0.072 | 0.582∼0.866 |
| β-hCG | 308.94 ng/mL | 0.755a | 0.072 | 0.614∼0.897 |
| sFlt-1 | 1.15 µg/L | 0.684a | 0.07 | 0.547∼0.821 |
| Combined | 0.872 | 0.052 | 0.770∼0.974 |
-
Compared with the combined, aP < 0.05.

ROC curves of serum VEGF, β-hCG, and sFlt-1 levels to predict prognosis in patients with PAS.
Discussion
The etiology of PAS is still unclear, and it is currently believed that it is mainly caused by uterine dysplasia or injury, interruption and partial loss of the decidua, and invasion of trophoblasts in the chorionic villi of placenta into the myometrium, or even breakthrough of the plasma membrane layer. PAS is one of the serious complications in obstetrics, which can lead to maternal hemorrhage, shock, uterine perforation, secondary infection, and even death [16], 17]. The development of the placenta throughout pregnancy is associated with multiple modulations of placental vascular growth and differentiation, and these changes occur simultaneously on both the maternal and placental surfaces, including recasting of the uterine spiral arteries as well as vascularization of the placenta [18], 19].
It was found in this work that the serum VEGF and β-hCG levels of the PAS group were higher than those of the non-PAS group, and the sFlt-1 level was lower. In addition, ROC curve analysis demonstrated that the AUC values for diagnosing PAS using VEGF, β-hCG, and sFlt-1 individually were 0.737, 0.818, and 0.716, respectively, while the combination of all three markers achieved an AUC of 0.881. These results indicate that each marker alone has substantial diagnostic value, and that the combined panel performs better than any single biomarker. Furthermore, the optimal cut-off values for VEGF, β-hCG, and sFlt-1 were determined to be 312.73 µg/L, 276.51 ng/mL, and 1.89 µg/L, respectively. Each of these values represents the threshold at which the optimal balance between sensitivity and specificity was achieved for distinguishing PAS from non-PAS cases.
Abnormal angiogenesis can lead to dysplasia of the decidua and excessive invasion of the trophoblastic layer, resulting in PAS. Various vascular growth factors are involved in placental angiogenesis, among which VEGF is the mitogen with the highest specificity for endothelial cells and the strongest pro-angiogenic effect, which can directly stimulate vascular endothelial cells to move, proliferate, and divide, increase microvessel permeability, and promote neovascularization [20]. sFlt-1 has been identified as an antagonist of VEGF, and may exert regulatory influence on trophoblast hyperinvasion and pathological vascular remodeling in PAS. hCG plays a well-characterized role in implantation and placental development. Current evidence recognizes five distinct hCG isoforms: regular hCG (r-hCG), hyperglycosylated hCG (H-hCG), pituitary hCG (p-hCG), free β-hCG, and hyperglycosylated free β-hCG (H-β-hCG), each demonstrating unique biological activities [21]. Notably, the free β-subunit and its hyperglycosylated variant exhibit specific associations with malignant pathologies [9]. Emerging clinical data demonstrate elevated β-hCG concentrations in PAS cases compared to uncomplicated pregnancies [22], 23]. Pathophysiologically, impaired placental villous vascular exchange in PAS may induce hypoxia, triggering compensatory β-hCG hypersecretion [24], [25], [26].
In placenta accreta, there is no obvious abnormality in the attachment position of the placenta in the uterine wall, and there is usually no obvious bleeding during pregnancy, nor does it significantly affect the physiological function of the placenta, and the development of the fetus is largely unaffected. Once placenta percreta occurs, it may lead to uterine rupture, causing severe abdominal pain and serious bleeding, endangering the lives of mother and baby [27], 28]. The present study demonstrated that as the degree of PAS increased, the serum VEGF level also elevated. Meanwhile, the serum sFlt-1 level exhibited a negative correlation with the degree of PAS. Homeostatic regulation of placental angiogenesis requires precise dynamic equilibrium between VEGF-mediated vascular proliferation (pro-angiogenic) and sFlt-1-induced signaling suppression (anti-angiogenic). This molecular interplay governs physiological placentation and controlled trophoblast invasion through coordinated modulation of endothelial permeability and vascular remodeling processes [29]. Elevated VEGF promotes placental neovascularization and trophoblast invasiveness, potentiating PAS pathogenesis. Conversely, sFlt-1 binds irreversibly to VEGF, neutralizing its biological activity while independently inducing endothelial dysfunction through vascular hyperpermeability and structural destabilization [30], 31]. Previous studies substantiate our observations, documenting reduced sFlt-1 expression in PAS compared to normal controls [32], 33]. Complementary evidence confirms VEGF-A’s critical role in modulating trophoblast invasion [34], with signaling blockade effectively inhibiting cytotrophoblast penetration [29].
PAS is prone to cause maternal hemorrhage, uterine perforation, secondary infections, death, and may also cause hemorrhagic shock, infections, and other complications, so it is crucial to analyze the risk factors affecting the prognosis of women with PAS [35]. The univariate analysis and multifactorial analysis conducted in our study identify concurrent pregnancy complications, elevated serum VEGF, and reduced sFlt-1 as prognostic markers for adverse outcomes in PAS. Mechanistically, VEGF-driven angiogenesis facilitates placental neovascularization through endothelial cell proliferation, while sFlt-1 competitively inhibits VEGF bioactivity, inducing endothelial dysfunction and vascular malformation. In cases of endometrial injury or congenital uterine anomalies, impaired spiral artery remodeling creates a hypoxic placental microenvironment. This hypoxia suppresses sFlt-1 expression while upregulating VEGF. The resultant angiogenic imbalance drives pathological trophoblast invasion into the myometrium to compensate for perfusion deficits, ultimately exacerbating clinical outcomes [36]. Further ROC analysis revealed that serum levels of VEGF, β-hCG, and sFlt-1 exhibited AUC values of 0.724, 0.755, and 0.684, respectively, for predicting the prognosis of patients with PAS. The combination of all three biomarkers achieved a superior AUC of 0.872, demonstrating enhanced predictive performance. The optimal cut-off values for VEGF, β-hCG, and sFlt-1 were identified as 397.28 µg/L, 308.94 ng/mL, and 1.15 µg/L, respectively, with each value representing the threshold that best balances sensitivity and specificity for predicting PAS prognosis. This robust analysis reinforces the credibility of serum VEGF, β-hCG, and sFlt-1 as significant prognostic predictors in PAS.
Our study acknowledges several limitations that warrant consideration. First, the cross-sectional design and relatively limited sample size may compromise the generalizability of our findings. Future studies should prioritize longitudinal designs with larger cohort sizes to rigorously validate the diagnostic and prognostic utility of biomarkers in PAS. Single time-point measurements, while informative, are insufficient for analyzing the temporal trajectory of pathophysiological progression. Second, although our univariate analysis did not reveal significant prognostic associations between some maternal characteristics and outcomes, multivariate analyses incorporating additional variables such as body mass index (BMI), hypertension status, and parity are necessary to account for potential confounding factors. Lastly, the nonsignificant correlation of β-hCG with prognosis may be attributable to both the limited sample size and inherent measurement variability, including cross-reactivity risks, analytical discrepancies across commercial platforms, and biological fluctuations dependent on gestational age, despite adherence to standardized calibration protocols.
Conclusions
In conclusion, changes in serum VEGF, β-hCG, and sFlt-1 levels in women with PAS are related to the severity of the disease, and the combination of these indicators has predictive value for the prognosis of patients with PAS.
-
Research ethics: All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All subjects was approved by Jiangsu Taizhou People’s Hospital (No. 20160718TZPH).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: P. S designed the research study. D.M. P. and Y.L. Z. performed the research. D.M. P. and Y.L. Z. provided help and advice on the experiments. P.S. analyzed the data. P.S. wrote the manuscript. Y.L. Z. reviewed and edited the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
1. Einerson, BD, Gilner, JB, Zuckerwise, LC. Placenta accreta spectrum. Obstet Gynecol 2023;142:31–50. https://doi.org/10.1097/aog.0000000000005229.Search in Google Scholar PubMed PubMed Central
2. Adu-Bredu, TK, Collins, SL, Nieto-Calvache, AJ. Ultrasound discrimination between placenta accreta spectrum and urinary bladder varices. Aust N Z J Obstet Gynaecol 2023;63:725–7. https://doi.org/10.1111/ajo.13703.Search in Google Scholar PubMed
3. Hawkins, R, Evans, M, Hammond, S, Hartopp, R, Evans, E. Placenta accreta spectrum disorders - peri-operative management: the role of the anaesthetist. Best Pract Res Clin Obstet Gynaecol 2021;72:38–51. https://doi.org/10.1016/j.bpobgyn.2020.08.003.Search in Google Scholar PubMed
4. Melincovici, CS, Boşca, AB, Şuşman, S, Mărginean, M, Mihu, C, Istrate, M, et al.. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018;59:455–67.Search in Google Scholar
5. Dighe, M. Intraplacental fetal vessels: an additional sign for placenta accreta spectrum. Radiology 2021;298:413–4. https://doi.org/10.1148/radiol.2020204118.Search in Google Scholar PubMed
6. Trottmann, F, Raio, L, Amylidi-Mohr, S, Mosimann, B, Jarquin Campos, A, Messerli, FH, et al.. Soluble fms-like tyrosine kinase 1 (sFlt-1): a novel biochemical marker for acute fatty liver of pregnancy. Acta Obstet Gynecol Scand 2021;100:1876–84. https://doi.org/10.1111/aogs.14218.Search in Google Scholar PubMed
7. Uyanıkoğlu, H, İncebıyık, A, Turp, AB, Çakmak, G, Sak, S, Hilali, NG. Serum angiogenic and anti-angiogenic markers in pregnant women with placenta percreta. Balkan Med J 2018;35:55–60. https://doi.org/10.4274/balkanmedj.2016.1890.Search in Google Scholar PubMed PubMed Central
8. Lizárraga-Verdugo, E, Beltrán-Ontiveros, SA, Gutiérrez-Grijalva, EP, Montoya-Moreno, M, Gutiérrez-Arzapalo, PY, Avendaño-Félix, M, et al.. The underlying molecular mechanisms of the aaccreta spectrum: a narrative review. Int J Mol Sci. 2024;25:9722. https://doi.org/10.3390/ijms25179722.Search in Google Scholar PubMed PubMed Central
9. Evans, J. Hyperglycosylated HhCG: a unique human implantation and invasion factor. Am J Reprod Immunol 2016;75:333–40. https://doi.org/10.1111/aji.12459.Search in Google Scholar PubMed
10. Makrigiannakis, A, Vrekoussis, T, Zoumakis, E, Kalantaridou, SN, Jeschke, U. The role of HCG in implantation: a mini-review of molecular and clinical evidence. Int J Mol Sci 2017;18:1305. https://doi.org/10.3390/ijms18061305.Search in Google Scholar PubMed PubMed Central
11. Cai, SN, Wu, YT, Zeng, L, Ding, YQ. Value of 3D ultrasound flow imaging combined with serum AFP, β-hCG, sFlt-1 and CK in the diagnosis of placenta accreta. BMC Womens Health 2022;22:556. https://doi.org/10.1186/s12905-022-02107-z.Search in Google Scholar PubMed PubMed Central
12. Bayramoğlu Tepe, N, Gelebek Yilmaz, F, Bozdag, Z, Uğur, MG. Subgroup analysis of accreta, increta and percreta cases using acoustic radiation force impulse elastography. J Obstet Gynaecol Res 2020;46:699–706. https://doi.org/10.1111/jog.14229.Search in Google Scholar PubMed
13. Yang, X, Zheng, W, Yan, J, Yang, H. Comparison between placenta accreta scoring system, ultrasound staging, and clinical classification. Medicine (Baltim) 2022;101:e31622. https://doi.org/10.1097/md.0000000000031622.Search in Google Scholar PubMed PubMed Central
14. Young, D, Khan, N, Hobson, SR, Sussman, D. Diagnosis of placenta accreta spectrum using ultrasound texture feature fusion and machine learning. Comput Biol Med 2024;178:108757. https://doi.org/10.1016/j.compbiomed.2024.108757.Search in Google Scholar PubMed
15. Jauniaux, E, Ayres-de-Campos, D, Langhoff-Roos, J, Fox, KA, Collins, S, FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet 2019;146:20–4. https://doi.org/10.1002/ijgo.12761.Search in Google Scholar PubMed
16. Jauniaux, E, Collins, S, Burton, GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol 2018;218:75–87. https://doi.org/10.1016/j.ajog.2017.05.067.Search in Google Scholar PubMed
17. Afshar, Y, Kashani Ligumsky, L, Bartels, HC, Krakow, D. Biology and pathophysiology of placenta accreta spectrum disorder. Obstet Gynecol 2025;145:611–20 https://doi.org/10.1097/aog.0000000000005903.Search in Google Scholar
18. Segal, TR, Amini, P, Wang, J, Peters, G, Skomorovska-Prokvolit, Y, Mainigi, MA, et al.. Superovulation with human chorionic gonadotropin (hCG) trigger and gonadotropin releasing hormone agonist (GnRHa) trigger differentially alter essential angiogenic factors in the endometrium in a mouse ART model. Biol Reprod. 2020;102:1122–33. https://doi.org/10.1093/biolre/ioaa014.Search in Google Scholar PubMed
19. Matsuzaki, S, Nagase, Y, Takiuchi, T, Kakigano, A, Mimura, K, Lee, M, et al.. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: a systematic review and meta-analysis. Sci Rep 2021;11:9205. https://doi.org/10.1038/s41598-021-88551-7.Search in Google Scholar PubMed PubMed Central
20. Svanvik, T, Jacobsson, AK, Carlsson, Y. Prenatal detection of placenta previa and placenta accreta spectrum: evaluation of the routine mid-pregnancy obstetric ultrasound screening between 2013 and 2017. Int J Gynaecol Obstet 2022;157:647–53. https://doi.org/10.1002/ijgo.13876.Search in Google Scholar PubMed
21. Schumacher, A, Zenclussen, AC. Human chorionic gonadotropin-mediated immune responses that facilitate embryo implantation and placentation. Front Immunol 2019;10:2896. https://doi.org/10.3389/fimmu.2019.02896.Search in Google Scholar PubMed PubMed Central
22. Büke, B, Akkaya, H, Demir, S, Sağol, S, Şimşek, D, Başol, G, et al.. Relationship between first trimester aneuploidy screening test serum analytes and placenta accreta. J Matern Fetal Neonatal Med 2018;31:59–62. https://doi.org/10.1080/14767058.2016.1275546.Search in Google Scholar PubMed
23. Berezowsky, A, Pardo, J, Ben-Zion, M, Wiznitzer, A, Aviram, A. Second trimester biochemical markers as possible predictors of pathological placentation: a retrospective case-control study. Fetal Diagn Ther 2019;46:187–92. https://doi.org/10.1159/000492829.Search in Google Scholar PubMed
24. Chau, K, Xu, B, Hennessy, A, Makris, A. Effect of placental growth factor on trophoblast-endothelial cell interactions in vitro. Reprod Sci 2020;27:1285–92. https://doi.org/10.1007/s43032-019-00103-7.Search in Google Scholar PubMed
25. Valent, AM, Choi, H, Kolahi, KS, Thornburg, KL. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J 2021;35:e21423. https://doi.org/10.1096/fj.202000326rr.Search in Google Scholar PubMed PubMed Central
26. Silasi, M, You, Y, Simpson, SJ, Kaislasuo, J, Pal, L, Guller, S, et al.. Human chorionic Kaislasuo gonadotropin modulates CXCL10 expression through histone methylation in human decidua. Sci Rep 2020;10:5785. https://doi.org/10.1038/s41598-020-62593-9.Search in Google Scholar PubMed PubMed Central
27. Bachmann, C, Abele, H, Hoopmann, M. Placenta previa et percreta: a potentially life-threatening condition. Diagnostics 2023;13:539. https://doi.org/10.3390/diagnostics13030539.Search in Google Scholar PubMed PubMed Central
28. Tîrnovanu, MC, Tîrnovanu, VG, Toma, B, Toma, L, Țarcă, E, Stătescu, L, et al.. Unexpected dramatic evolution of placenta increta: case report and literature review. J Personalized Med 2023;13:1563. https://doi.org/10.3390/jpm13111563.Search in Google Scholar PubMed PubMed Central
29. El-Badawy, O, Abbas, AM, Radwan, E, Makboul, R, Khamis, AA, Ali, M, et al.. Cross-talk between mucosal-associated invariant T, natural killer, and natural killer T cell populations is implicated in the pathogenesis of placenta accreta spectrum. Inflammation 2023;46:1192–208. https://doi.org/10.1007/s10753-023-01799-1.Search in Google Scholar PubMed PubMed Central
30. Ji, Y, Zhou, L, Wang, G, Qiao, Y, Tian, Y, Feng, Y. Role of LAMA4 gene in regulating extravillous trophoblasts in pathogenesis of preeclampsia. Med Sci Monit 2019;25:9630–6. https://doi.org/10.12659/msm.917402.Search in Google Scholar
31. Maurea, S, Verde, F, Romeo, V, Stanzione, A, Mainenti, PP, Raia, G, et al.. Prediction of placenta accreta spectrum in patients with placenta previa using a clinical, US and MRI combined model: a retrospective study with external validation. Eur J Radiol 2023;168:111116. https://doi.org/10.1016/j.ejrad.2023.111116.Search in Google Scholar PubMed
32. Lumbanraja, S, Yaznil, MR, Siahaan, AM, Berry Eka Parda, B. Soluble FMS-like tyrosine kinase-1: role in placenta accreta spectrum disorder. F1000Res 2021;10:618. https://doi.org/10.12688/f1000research.54719.1.Search in Google Scholar
33. Zhang, F, Gu, M, Chen, P, Wan, S, Zhou, Q, Lu, Y, et al.. Distinguishing placenta accreta from placenta previa via maternal plasma levels of sFlt-1 and PLGF and the sFlt-1/PLGF ratio. Placenta 2022;124:48–54. https://doi.org/10.1016/j.placenta.2022.05.009.Search in Google Scholar PubMed
34. Schwickert, A, Chantraine, F, Ehrlich, L, Henrich, W, Muallem, MZ, Nonnenmacher, A, et al.. Maternal serum VEGF predicts abnormally invasive placenta better than NT-proBNP: a multicenter case-control study. Reprod Sci 2021;28:361–70. https://doi.org/10.1007/s43032-020-00319-y.Search in Google Scholar PubMed PubMed Central
35. Holmes, VJ, Skinner, S, Silagy, M, Rolnik, DL, Mol, BW, Kroushev, A. Changes in practice and management of placenta accreta spectrum disorder: a 20-year retrospective cohort study. Aust N Z J Obstet Gynaecol 2023;63:786–91. https://doi.org/10.1111/ajo.13724.Search in Google Scholar PubMed
36. Trapiella-Alfonso, L, Alexandre, L, Fraichard, C, Pons, K, Dumas, S, Huart, L, et al.. VEGF (vascular endothelial growth factor) functionalized magnetic beads in a microfluidic device to improve the angiogenic balance in preeclampsia. Hypertension 2019;74:145–53. https://doi.org/10.1161/hypertensionaha.118.12380.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


