One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones
Abstract
New 2-(1,2,3-triazol-1-yl)pyrimidines were synthesized in good yields by the three-component reaction of pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones in the presence of K2CO3 at room temperature. This procedure eliminates the need to handle organic azides in the synthesis of analogous compounds.
Introduction
Since the copper(I)-catalyzed azide-alkyne cycloaddition was reported independently by the groups of Sharpless and Meldal, the synthesis of 1,4- and 1,5-disubstituted 1,2,3-triazoles have attracted a great deal of attention due to their potential biological activities [1]. In recent years, 1,4,5-trisubstituted 1,2,3-triazoles have found important applications in medicinal chemistry [2–5]. A few methods for the synthesis of fully substituted 1,2,3-triazoles have been described [6]. One of the most attractive approaches to the synthesis of these compounds is the direct Pd- or Cu-catalyzed arylation of 1,4-disubstituted triazoles with aryl halides [7–12]. Transition metal-catalyzed reactions for the chemoselective and regioselective synthesis of 1,4,5-trisubstituted 1,2,3-triazoles from organic azides and alkyl iodides or bromides have also been developed [13–16]. Another access to these compounds is the highly regioselective 1,3-dipolar cycloaddition between an azide and an enamine [17]. 1,4,5-Trisubstituted 1,2,3-triazoles can also be obtained by treatment of aryl azides with ketones in the presence of amines [18–20].
In our previous reports [21, 22], we have described an efficient protocol for the synthesis of C2-substituted pyrimidines by cross-coupling reaction of readily available pyrimidin-2-yl sulfonates with N-, S-, O- and C-nucleophiles. In this work, the synthesis of polysubstituted 2-(1,2,3-triazol-1-yl)pyrimidines by the reaction of pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones is described.
Conditions for the three-component reaction were explored using pyrimidin-2-yl sulfonate (1a), NaN3 and ethyl acetoacetate (2a) as the substrates (Scheme 1) and various base catalysts. In the presence of Et3N and Et2NH in acetone, product 3a was obtained in only 10% and 17% yield, respectively. By contrast, in the presence of inorganic bases, namely K2CO3, K3PO4, NaOH and KOH in acetone product 3a was obtained in the respective yields of 81%, 53%, 19% and 54%. The yields were also significantly affected by solvents. For example, the reactions conducted in acetonitrile, ethanol, DMF and DMSO in the presence of K2CO3 delivered 3a in the respective yields of 54%, 15%, 60% and 66%. Traces of 3a were obtained for the reaction conducted in dichloromethane. The best result was obtained when the reaction was conducted in acetone in the presence of 1.2 equivalent amounts of K2CO3.
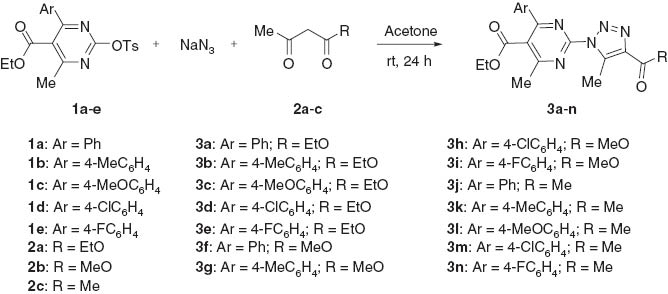
Various pyrimidin-2-yl sulfonates (1) were allowed to react with NaN3 and ethyl acetoacetate (2a) under optimized conditions (Scheme 1). In general, good yields were obtained under the standard reaction conditions. Methyl acetoacetate (2b) and pentane-2,4-dione (2c) were also used to further explore the scope of the synthesis of the triazolylpyrimidines. As expected, the desired products 3f–n were obtained smoothly in good to excellent yields (Scheme 1; see Supplementary material online). The structures of all products are fully consistent with their spectral data and that for 3j was unambiguously assigned by using X-ray diffraction analysis (Figure 1). Crystallographic data for the structure analysis have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication, CCDC No. CCDC 944956 for 3j. Copies of this information can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
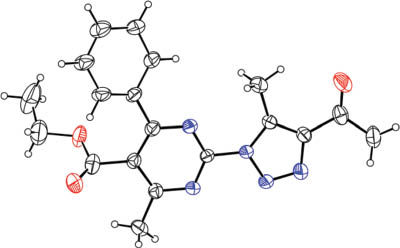
The single crystal X-ray crystallographic structure of product 3j.
Experimental details
Melting points were determined on an XT-4 electrothermal micromelting point apparatus and are uncorrected. 1H NMR (400 MHz) spectra and 13C NMR (100 MHz) spectra were recorded in CDCl3 on a Varian Mercury plus-400 instrument. IR spectra were recorded using KBr pellets on Nicolet Avatar 360 spectrophotometer. Electron-impact mass spectra were recorded at 70 eV on a TRACE DSQ instrument. Elemental analyses were performed on a Carlo-Erba 1106 Elemental Analysis instrument. X-Ray diffraction data were recorded using a Rigaku Mercury CCDC area detector with graphite monochromated Mo Kα radiation. Column chromatography was performed on silica gel (300–400 mesh). Commercially available reagents were used without further purification.
General procedure for the synthesis of 2-(1,2,3-triazol-1-yl)pyrimidines 3a–n
A mixture of a pyrimidin-2-yl sulfonate (1, 0.5 mmol), NaN3 (1.0 mmol), a methylene ketone (2, 1.0 mmol), K2CO3 (1.2 mmol) and acetone (5 mL) was stirred at room temperature for 24 h. After complete consumption of the starting material, as evidenced by TLC analysis, the mixture was quenched with saturated NH4Cl aqueous solution (5 mL) and extracted with diethyl ether (2 × 10 mL). The combined extracts were washed with brine, dried over MgSO4 and concentrated. The residue was purified by flash chromatography on silica gel eluting with petroleum ether/ethyl acetate (5:1) to provide product 3.
Ethyl 2-[4-(ethoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]-4-methyl-6-phenylpyrimidine-5-carboxylate (3a)
Yellow oil; yield 81%; 1H NMR: δ 7.74 (q, J = 8.0 Hz, 2H, ArH), 7.55–7.50 (m, 3H, ArH), 4.48 (q, J = 8.0 Hz, 2H, CH2), 4.28 (q, J = 8.0 Hz, 2H, CH2), 2.98 (s, 3H, CH3), 2.77 (s, 3H, CH3), 1.45 (t, J = 8.0 Hz, 3H, CH3), 1.13 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.8, 166.9, 165.6, 161.6, 154.2, 140.6, 137.3, 136.2, 131.1, 128.8, 128.5, 125.4, 62.4, 61.2, 22.8, 14.4, 13.7, 11.5; IR: 2980, 1722, 1542, 1428, 1251, 1089 cm-1; EI-MS: m/z 395 (M+). Anal. Calcd for C20H21N5O4: C, 60.75; H, 5.35; N, 17.71. Found: C, 60.90; H, 5.40; N, 17.62.
Ethyl 2-[4-(ethoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]-4-methyl-6-p-tolylpyrimidine-5-carboxylate (3b)
White solid; mp 36–37°C; yield 70%; 1H NMR: δ 7.65 (d, J = 8.0 Hz, 2H, ArH), 7.30 (d, J = 8.0 Hz, 2H, ArH), 4.48 (q, J = 8.0 Hz, 2H, CH2), 4.31 (q, J = 8.0 Hz, 2H, CH2), 2.97 (s, 3H, CH3), 2.75 (s, 3H, CH3), 2.43 (s, 3H, CH3), 1.45 (t, J = 8.0 Hz, 3H, CH3), 1.19 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.6, 167.2, 165.4, 161.6, 154.2, 141.7, 140.5, 137.2, 133.3, 129.7, 128.5, 125.2, 62.4, 61.2, 22.8, 21.5, 14.4, 13.7, 11.5; IR: 1722, 1578, 1542, 1425, 1251, 1089 cm-1; EI-MS: m/z 409 (M+). Anal. Calcd for C21H23N5O4: C, 61.60; H, 5.66; N, 17.10. Found: C, 61.72; H, 5.60; N, 17.19.
Ethyl 2-[4-(ethoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]-4-(4-methoxyphenyl)-6-methylpyrimidine-5-carboxylate (3c)
Yellow solid; mp 85–86°C; yield 78%; 1H NMR: δ 7.75 (d, J = 8.0 Hz, 2H, ArH), 7.01 (d, J = 8.0 Hz, 2H, ArH), 4.49 (q, J = 8.0 Hz, 2H, CH2), 4.34 (q, J = 8.0 Hz, 2H, CH2), 3.89 (s, 3H, CH3), 2.98 (s, 3H, CH3), 2.74 (s, 3H, CH3), 1.46 (t, J = 8.0 Hz, 3H, CH3), 1.23 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.4, 167.4, 164.7, 162.2, 161.7, 154.1, 140.5, 137.2, 130.4, 128.4, 124.7, 114.3, 62.4, 61.2, 55.5, 22.8, 14.4, 13.8, 11.5; IR: 2980, 1722, 1542, 1428, 1254, 1089 cm-1; EI-MS: m/z 425 (M+). Anal. Calcd for C21H23N5O5: C, 59.29; H, 5.45; N, 16.46. Found: C, 59.17; H, 5.51; N, 16.54.
Ethyl 4-(4-chlorophenyl)-2-(4-(ethoxycarbonyl)-5-methyl-1H- 1,2,3-triazol-1-yl)-6-methylpyrimidine-5-carboxylate (3d)
Yellow oil; yield 76%; 1H NMR: δ 7.68 (t, J = 8.0 Hz, 2H, ArH), 7.47 (t, J = 8.0 Hz, 2H, ArH), 4.45 (q, J = 8.0 Hz, 2H, CH2), 4.29 (q, J = 8.0 Hz, 2H, CH2), 2.95 (s, 3H, CH3), 2.74 (s, 3H, CH3), 1.42 (t, J = 8.0 Hz, 3H, CH3), 1.17 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 169.0, 166.7, 164.3, 161.5, 154.2, 140.5, 137.6, 137.3, 134.6, 129.9, 129.1, 125.3, 62.6, 61.2, 22.9, 14.3, 13.7, 11.5; IR: 2980, 1722, 1542, 1428, 1248, 1089 cm-1; EI-MS: m/z 429 (M+). Anal. Calcd for C20H20ClN5O4: C, 55.88; H, 4.69; N, 16.29. Found: C, 55.61; H, 4.74; N, 16.20.
Ethyl 2-[4-(ethoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]-4-(4-fluorophenyl)-6-methylpyrimidine-5-carboxylate (3e)
Yellow oil; yield 78%; 1H NMR: δ 7.73–7.70 (m, 2H, ArH), 7.17–7.13 (m, 2H, ArH), 4.43 (q, J = 8.0 Hz, 2H, CH2), 4.26 (q, J = 8.0 Hz, 2H, CH2), 2.93 (s, 3H, CH3), 2.71 (s, 3H, CH3), 1.39 (t, J = 8.0 Hz, 3H, CH3), 1.14 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.8, 166.8, 165.7, 164.3, 163.2, 161.5, 154.1, 140.5, 137.2, 132.3, 130.8, 125.2, 116.1, 115.9, 62.5, 61.2, 14.3, 13.7, 11.4; IR: 2980, 1722, 1542, 1431, 1251, 1092 cm-1; EI-MS: m/z 413 (M+). Anal. Calcd for C20H20FN5O4: C, 58.11; H, 4.88; N, 16.94. Found: C, 58.24; H, 4.83; N, 17.03.
Ethyl 2-(4-(methoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl)-4-methyl-6-phenylpyrimidine-5-carboxylate (3f)
Yellow oil; yield 73%; 1H NMR: δ 7.73–7.71 (m, 2H, ArH), 7.53–7.49 (m, 3H, ArH), 4.26 (q, J = 8.0 Hz, 2H, CH2), 3.98 (s, 3H, CH3), 2.98 (s, 3H, CH3), 2.75 (s, 3H, CH3), 1.11 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.8, 166.9, 165.6, 161.9, 154.2, 140.8, 137.0, 136.2, 131.1, 128.8, 128.5, 125.4, 62.4, 52.1, 22.8, 13.6, 11.5; IR: 2950, 1722, 1578, 1542, 1437, 1251, 1092 cm-1; EI-MS: m/z 381 (M+). Anal. Calcd for C19H19N5O: C, 59.84; H, 5.02; N, 18.36. Found: C, 59.99; H, 4.96; N, 18.51.
Ethyl 2-[4-(methoxycarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]-4-methyl-6-p-tolylpyrimidine-5-carboxylate (3g)
Yellow solid; mp 88–90°C; yield 90%; 1H NMR: δ 7.63 (d, J = 8.0 Hz, 2H, ArH), 7.28 (d, J = 8.0 Hz, 2H, ArH), 4.28 (q, J = 8.0 Hz, 2H, CH2), 3.97 (s, 3H, CH3), 2.96 (s, 3H, CH3), 2.72 (s, 3H, CH3), 2.41 (s, 3H, CH3), 1.16 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 168.6, 167.1, 165.4, 161.9, 154.1, 141.7, 140.7, 136.9, 133.3, 129.6, 128.5, 125.2, 62.4, 52.1, 22.8, 21.5, 13.7, 11.5; IR: 2980, 1722, 1578, 1542, 1437, 1251, 1092 cm-1; EI-MS: m/z 395 (M+). Anal. Calcd for C20H21N5O: C, 60.75; H, 5.35; N, 17.71. Found: C, 60.87; H, 5.31; N, 17.79.
Ethyl 4-(4-chlorophenyl)-2-(4-(methoxycarbonyl)-5-methyl-1H- 1,2,3-triazol-1-yl)-6-methylpyrimidine-5-carboxylate (3h)
Colorless oil; yield 62%; 1H NMR: δ 7.69 (d, J = 8.0 Hz, 2H, ArH), 7.48 (d, J = 8.0 Hz, 2H, ArH), 4.30 (q, J = 8.0 Hz, 2H, CH2), 3.99 (s, 3H, CH3), 2.97 (s, 3H, CH3), 2.75 (s, 3H, CH3), 1.19 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 169.0, 166.7, 164.3, 161.9, 154.2, 140.7, 137.6, 137.1, 134.6, 129.9, 129.2, 125.3, 62.6, 52.1, 22.9, 13.7, 11.5; IR: 2974, 1725, 1578, 1542, 1434, 1251, 1092 cm-1; EI-MS: m/z 415 (M+). Anal. Calcd for C19H18ClN5O4: C, 54.88; H, 4.36; N, 16.84. Found: C, 54.75; H, 4.41; N, 16.92.
Ethyl 4-(4-fluorophenyl)-2-[4-(methoxycarbonyl)-5-methyl-1H- 1,2,3-triazol-1-yl]-6-methylpyrimidine-5-carboxylate (3i)
Yellow oil; yield 77%; 1H NMR: δ 7.75–7.72 (m, 2H, ArH), 7.19–7.15 (m, 2H, ArH), 4.28 (q, J = 8.0 Hz, 2H, CH2), 3.97 (s, 3H, CH3), 2.95 (s, 3H, CH3), 2.73 (s, 3H, CH3), 1.15 (t, J = 8.0, 3H, CH3); 13C NMR: δ 168.9, 166.8, 165.8, 164.4, 163.2, 161.9, 154.1, 140.7, 137.0, 132.3 (d), 130.8 (d), 125.2, 116.2, 115.9, 62.5, 52.1, 22.8, 13.7, 11.4; IR: 2956, 1722, 1542, 1434, 1251, 1089 cm-1; EI-MS: m/z 399 (M+). Anal. Calcd for C19H18FN5O4: C, 57.14; H, 4.54; N, 17.54. Found: C, 57.26; H, 4.50; N, 17.44.
Ethyl 2-[4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl]-4-methyl-6-phenylpyrimidine-5-carboxylate (3j)
Yellow solid; mp 94–96°C; yield 83%; 1H NMR: δ 7.75–7.73 (m, 2H, ArH), 7.55–7.50 (m, 3H, ArH), 4.28 (q, J = 8.0 Hz, 2H, CH2), 2.97 (s, 3H, CH3), 2.78 (s, 6H, CH3), 1.13 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 194.5, 168.8, 166.9, 165.6, 154.2, 143.8, 139.1, 136.2, 131.1, 128.8, 128.5, 125.4, 62.5, 28.4, 22.8, 13.7, 11.5; IR: 2980, 1725, 1686, 1569, 1542, 1425, 1248, 1083 cm-1; EI-MS: m/z 365 (M+). Anal. Calcd for C19H19N5O3: C, 62.46; H, 5.24; N, 19.17. Found: C, 62.32; H, 5.27; N, 19.26.
Ethyl 2-(4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl)-4-methyl-6-p- tolylpyrimidine-5-carboxylate (3k)
Yellow oil; yield 68%; 1H NMR: δ 7.64 (d, J = 8.0 Hz, 2H, ArH), 7.28 (d, J = 8.0 Hz, 2H, ArH), 4.29 (q, J = 8.0 Hz, 2H, CH2), 2.95 (s, 3H, CH3), 2.74 (d, J = 8.0 Hz, 6H, CH3), 2.41 (s, 3H, CH3), 1.17 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 194.4, 168.5, 167.1, 165.4, 154.2, 143.8, 141.7, 139.0, 133.3, 129.5, 128.5, 125.1, 62.4, 28.3, 22.8, 21.4, 13.7, 11.4; IR: 2980, 1725, 1686, 1569, 1542, 1425, 1248, 1080 cm-1; EI-MS: m/z 379 (M+). Anal. Calcd for C20H21N5O3: C, 63.31; H, 5.58; N, 18.46. Found: C, 63.20; H, 5.64; N, 18.61.
Ethyl 2-(4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl)-4-(4-methoxyphenyl)-6-methylpyrimidine-5-carboxylate (3l)
Yellow solid; mp 128–130°C; yield 70%; 1H NMR: δ 7.74 (d, J = 8.0 Hz, 2H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 4.32 (q, J = 8.0 Hz, 2H, CH2), 3.87 (s, 3H, CH3), 2.95 (s, 3H, CH3), 2.76 (s, 3H, CH3), 2.72 (s, 3H, CH3), 1.21 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 194.5, 168.4, 167.4, 164.7, 162.2, 154.1, 143.8, 138.9, 130.4, 128.4, 124.6, 114.3, 62.4, 55.5, 28.3, 22.7, 13.8, 11.4; IR: 2974, 1722, 1686, 1569, 1542, 1425, 1251, 1080 cm-1; EI-MS: m/z 395 (M+). Anal. Calcd for C20H21N5O: C, 60.75; H, 5.35; N, 17.71. Found: C, 60.90; H, 5.41; N, 17.63.
Ethyl 2-(4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl)-4-(4-chlorophenyl)-6-methylpyrimidine-5-carboxylate (3m)
Yellow oil; yield 65%; 1H NMR: δ 7.69 (d, J = 8.0 Hz, 2H, ArH), 7.48 (d, J = 8.0 Hz, 2H, ArH), 4.31 (q, J = 8.0 Hz, 2H, CH2), 2.95 (s, 3H, CH3), 2.73 (t, J = 8.0 Hz, 6H, CH3), 1.20 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 194.4, 168.9, 166.7, 164.3, 154.2, 143.9, 139.0, 137.6, 134.6, 129.9, 129.1, 125.3, 62.59, 28.3, 22.8, 13.7, 11.4; IR: 2974, 1725, 1686, 1542, 1428, 1245, 1083 cm-1; EI-MS: m/z 399 (M+). Anal. Calcd for C19H18ClN5O3: C, 57.07; H, 4.54; N, 17.52. Found: C, 57.18; H, 4.58; N, 17.59.
Ethyl 2-(4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl)-4-(4-fluorophenyl)-6-methylpyrimidine-5-carboxylate (3n)
Yellow oil; yield 72%; 1H NMR: δ 7.77 (q, J = 8.0 Hz, 2H, ArH), 7.20 (t, J = 8.0 Hz, 2H, ArH), 4.31 (q, J = 8.0 Hz, 2H, CH2), 2.96 (s, 3H, CH3), 2.77 (d, J = 8.0 Hz, 6H, CH3), 1.19 (t, J = 8.0 Hz, 3H, CH3); 13C NMR: δ 194.5, 168.9, 166.9, 165.8, 164.4, 163.3, 154.2, 143.9, 139.0, 132.3 (d), 130.8 (d), 125.2, 116.2, 115.9, 62.6, 22.9, 13.8, 11.5; IR: 2980, 1722, 1686, 1542, 1425, 1245, 1083 cm-1; EI-MS: m/z 383 (M+). Anal. Calcd for C19H18FN5O3: C, 59.52; H, 4.73; N, 18.27. Found: C, 59.41; H, 4.79; N, 18.35.
We are thankful for the financial support from the NSFC (Nos. 21362032, 21362031 and 21062017), the NSF of Gansu Province (No. 1208RJYA083), and the Scientific and Technological Innovation Engineering program of NWNU (Nos. nwnu-kjcxgc-03-64, nwnulkqn-10-15).
References
[1] Hein, J. E.; Fokin, V. V. Copper-catalyzed dipolar cycloaddition and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315.Search in Google Scholar
[2] Agalave, S. G.; Maujan, S. R.; Pore, V. S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718.Search in Google Scholar
[3] Calderone, V.; Fiamingo, F. L.; Amato, G.; Giorgi, I.; Livi, O.; Martelli, A.; Martinotti, E. 1,2,3-Triazol-carboxanilides and 1,2,3-triazol-(N-benzyl)-carboxamides as BK-potassium channel activators. XII. Eur. J. Med. Chem. 2008, 43, 2618–2626.Search in Google Scholar
[4] Shu, H.; Izenwasser, S.; Wade, D.; Stevens, E. D.; Trudell, M. L. Synthesis and CB1 cannabinoid receptor affinity of 4-alkoxycarbonyl-1,5-diaryl-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2009, 19, 891–893.Search in Google Scholar
[5] Bromidge, S. M.; Arban, R.; Bertani, B.; Bison, S.; Borriello, M.; Cavanni, P.; Forno, G. D.; Di-Fabio, R.; Donati, D.; Fontana, S.; et al. Design and synthesis of novel tricyclic benzoxazines as potent 5-HT1A/B/D receptor antagonists leading to the discovery of 6-{2-[4-(2-methyl-5-quinolinyl)-1-piperazinyl]ethyl}-4H-imidazo[5,1-c][1,4]benzoxazine-3-carboxamide (GSK588045). J. Med. Chem. 2010, 53, 5827–5843.Search in Google Scholar
[6] Jin, G.; Zhang, J.; Fu, D.; Wu, J.; Cao, S. One-pot, three-component synthesis of 1,4,5-trisubstituted 1,2,3-triazoles starting from primary alcohols. Eur. J. Org. Chem. 2012, 5446–5449.10.1002/ejoc.201200830Search in Google Scholar
[7] Ackermann, L.; Vicente, R. Catalytic direct arylations in polyethylene glycol (PEG): recyclable palladium(0) catalyst for C-H bond cleavages in the presence of air. Org. Lett. 2009, 11, 4922–4925.Search in Google Scholar
[8] Fukuzawa, S. I.; Oki, H.; Hosaka, M.; Sugasawa, J.; Kikuchi, S. Click ferrophos: new chiral ferrocenyl phosphine ligands synthesized by click chemistry and the use of their metal complexes as catalysts for asymmetric hydrogenation and allylic substitution. Org. Lett. 2007, 9, 5557–5560.Search in Google Scholar
[9] Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Copper-catalyzed “click” reaction/direct arylation sequence: modular syntheses of 1,2,3-triazoles. Org. Lett. 2008, 10, 3081–3084.Search in Google Scholar
[10] Chuprakov, S.; Chernyak, N.; Dudnik, A. S.; Gevorgyan, V. Direct Pd-catalyzed arylation of 1,2,3-triazoles. Org. Lett. 2007, 9, 2333–2336.Search in Google Scholar
[11] He, T.; Wang, M.; Li, P.; Wang, L. Pd-NHC-catalyzed direct arylation of 1,4-disubstituted 1,2,3-triazoles with aryl halides. Chin. J. Chem. 2012, 30, 979–984.Search in Google Scholar
[12] Fukuzawa, S. I.; Shimizu, E.; Ogata, K. Copper(I)-catalyzed direct arylation of 1,4-disubstituted 1,2,3-triazoles with aryl iodides. Heterocycles 2009, 78, 645–656.Search in Google Scholar
[13] Hein, J. E.; Tripp, J. C.; Krasnova, L. B.; Sharpless, K. B.; Fokin, V. V. Copper(I)-catalyzed cycloaddition of organic azides and 1-iodoalkynes. Angew. Chem. Int. Ed. 2009, 48, 8018–8021.Search in Google Scholar
[14] Spiteri, C.; Moses, J. E. Copper-catalyzed azide-alkyne cycloaddition: regioselective synthesis of 1,4,5-trisubstituted 1,2,3-triazoles. Angew. Chem. Int. Ed. 2010, 49, 31–33.Search in Google Scholar
[15] Bogdan, A. R.; James, K. Synthesis of 5-iodo-1,2,3-triazole-containing macrocycles using copper flow reactor technology. Org. Lett. 2011, 13, 4060–4063.Search in Google Scholar
[16] Krasinski, A.; Fokin, V. V.; Sharpless, K. B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles. Org. Lett. 2004, 6, 1237–1240.Search in Google Scholar
[17] Bianchetti, G.; Dalla Croce, P.; Pocar, D. Synthesis and reactivity of dienamines. Tetrahedron Lett. 1965, 6, 2039–2041.10.1016/S0040-4039(00)90149-7Search in Google Scholar
[18] Danence, L. J. T.; Gao, Y.; Li, M.; Huang, Y.; Wang, J. Organocatalytic enamide-azide cycloaddition reactions: regiospecific synthesis of 1,4,5-trisubstituted-1,2,3-triazoles. Chem. Eur. J. 2011, 17, 3584–3587.Search in Google Scholar
[19] Wang, L.; Peng, S.; Danence, L. J. T.; Gao, Y.; Wang, J. Amine-catalyzed [3+2] Huisgen cycloaddition strategy for the efficient assembly of highly substituted 1,2,3-triazoles. Chem. Eur. J. 2012, 18, 6088–6093.Search in Google Scholar
[20] Belkheira, M.; El Abed, D.; Pons J.-M.; Bressy, C. Organocatalytic synthesis of 1,2,3-triazoles from unactivated ketones and aryl azides. Chem. Eur. J. 2011, 17, 12917–12921.Search in Google Scholar
[21] Wang, X.-C.; Yang, G.-J.; Quan, Z.-J.; Ji, P.-Y.; Liang, J.-L.; Ren, R.-G. Synthesis of 2-substituted pyrimidines via cross-coupling reaction of pyrimidin-2-yl sulfonates with nucleophiles in polyethylene glycol 400. Synlett 2010, 1657–1660.10.1055/s-0030-1258080Search in Google Scholar
[22] Quan, Z.-J.; Jing, F.-Q.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Palladium(II) catalyzed Suzuki/Sonogashira cross-coupling reactions of sulfonates: an efficient approach to C2-functionalized pyrimidines and pyridines. Eur. J. Org. Chem. 2013, 7175–7183.10.1002/ejoc.201300592Search in Google Scholar
©2014 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones
- Research Articles
- A convenient synthesis of 5,5′-bi-1,2,4-triazines via direct S-arylation and its application in the synthesis of 2,2′-bipyridines
- An efficient approach to the key intermediate of rosuvastatin
- Synthesis and properties of multifunctional hindered amine light stabilizers
- Tertiary formylated amines by microwave irradiation of N,N-dimethyl-N′-(2-pyridyl)formamidines with methyl vinyl ketone
- Synthesis and antimicrobial activity of some novel 2-thienyl substituted heterocycles
- Synthesis of 2-amino-5-mercapto-1,3,4-thiadiazole derivatives
- An easy and efficient protocol for the condensation reaction of isatin and N-substituted isatins with 1,2-diaminobenzene using low cost reusable clay catalyst
- Synthesis and antimicrobial activities of novel 6-(1,3-thiazol-4-yl)-1,3-benzoxazol-2(3H)-one derivatives
- A concise and efficient synthesis of (+)-preussin
- Synthesis, X-ray structural characterization, NLO, MEP, NBO and HOMO-LUMO analysis using DFT study of Zn(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate
Articles in the same Issue
- Frontmatter
- Preliminary Communication
- One-pot three-component synthesis of substituted 2-(1,2,3-triazol-1-yl)pyrimidines from pyrimidin-2-yl sulfonates, sodium azide and active methylene ketones
- Research Articles
- A convenient synthesis of 5,5′-bi-1,2,4-triazines via direct S-arylation and its application in the synthesis of 2,2′-bipyridines
- An efficient approach to the key intermediate of rosuvastatin
- Synthesis and properties of multifunctional hindered amine light stabilizers
- Tertiary formylated amines by microwave irradiation of N,N-dimethyl-N′-(2-pyridyl)formamidines with methyl vinyl ketone
- Synthesis and antimicrobial activity of some novel 2-thienyl substituted heterocycles
- Synthesis of 2-amino-5-mercapto-1,3,4-thiadiazole derivatives
- An easy and efficient protocol for the condensation reaction of isatin and N-substituted isatins with 1,2-diaminobenzene using low cost reusable clay catalyst
- Synthesis and antimicrobial activities of novel 6-(1,3-thiazol-4-yl)-1,3-benzoxazol-2(3H)-one derivatives
- A concise and efficient synthesis of (+)-preussin
- Synthesis, X-ray structural characterization, NLO, MEP, NBO and HOMO-LUMO analysis using DFT study of Zn(II)bis(3,4 dimethoxybenzoate)bis(nicotinamide) dihydrate


