Cracking the Shell on Egg-Hypersensitive Patients and Egg-Containing Vaccines
-
Leah R. Chernin
Abstract
Hens' eggs are a common food in the American diet. They are consumed as a primary food source and added as an ingredient to other foods. In individuals who are hypersensitive to eggs, egg-containing foods can cause mild to severe allergic reactions if ingested. These individuals may also have adverse reactions to vaccines produced on egg media. Vaccines that are created on egg media include those for measles, mumps, and rubella; rabies; yellow fever; and influenza. The authors discuss recent developments in the use of egg-containing vaccines in hypersensitive patients.
In vaccine manufacturing, enriched media must be used to grow the virus that will be used in the vaccine. A hen's egg or its components are typically used as the enriched medium for growing the viruses that cause measles, mumps, and rubella (MMR); rabies; yellow fever; and influenza. Each virus grows best in a certain area of the egg or in an embryonic culture. When the virus is injected into the egg, the subsequent hole is sealed with gelatin. By growing this infectious pathogen in an egg, the virus becomes less pathogenic to humans, and its replication rate slows—resulting in a decrease in disease manifestation in the host but still allowing for a host immunogenic response.1
When the virus is collected from the egg or culture, picogram to microgram amounts of the egg are collected as well. During this process, egg allergen gets introduced into the vaccine, making the vaccine capable of causing allergic reactions, which may be as subtle as a mild local reaction requiring only a topical or oral antihistamine or as severe as anaphylaxis requiring intramuscular epinephrine.2 In the present article, we describe the recently updated recommendations for using some of these vaccines in egg-hypersensitive patients.
Measles, Mumps, and Rubella
The MMR vaccine contains attenuated virus that has been grown in chick embryonic fibroblast cell cultures. This vaccine contains picograms to nanograms of egg. Most sources agree that the amount of egg in the MMR vaccine is not enough to cause allergic reactions in egg-hypersensitive patients.3
There is the potential for allergic reactions to the MMR vaccine caused by other contents of the vaccine—in particular, gelatin and neomycin. Gelatin may be present in this vaccine at concentrations of 14,500 μg per 0.5 mL of vaccine.4 Physicians administering this vaccine should have the best available knowledge, medication, and equipment to manage any allergic reactions that develop in patients.
Rabies
The rabies vaccine contains attenuated virus that has also been grown in chick embryonic fibroblast cell cultures. The vaccine contains micrograms of egg. Although we know of no reliable safety data on the use of the purified chick embryo cell (PCEC) rabies vaccine in egg-hypersensitive patients, this vaccine is contraindicated in patients who are hypersensitive to eggs.5
If a rabies vaccine is needed for an egg-hypersensitive patient, alternative vaccines—human diploid cell vaccine (HDCV) or purified Vero cell rabies vaccine (PVRV)—that contain no egg components may be administered.
A patient may be tested for egg hypersensitivity by means of a prick test to observe if there is a cutaneous response. If the test has negative results, the PCEC rabies vaccine may be administered in a graded manner in a setting in which anaphylaxis can be readily recognized and managed. It should be kept in mind that all rabies vaccine formulations may contain as much as 12,000 μg of gelatin per 1 mL of vaccine.4 Thus, patients with a history of gelatin hypersensitivity should be evaluated by an allergist or immunologist before administration of this vaccine.
Yellow Fever
The yellow fever vaccine contains a live attenuated yellow fever virus. The virus is grown in chick embryos, and the vaccine may contain micrograms of egg. This vaccine is contraindicated in patients with egg hypersensitivity.
Testing for a cutaneous response may be performed with the yellow fever vaccine, and the vaccine may be administered in a graded manner. Physicians should be aware of the risk of anaphylaxis and the treatment for this condition (eg, epinephrine should be available). Another concern is the amount of gelatin present in this vaccine—7500 μg per 0.5 mL of vaccine.4 As with the rabies vaccine, the yellow fever vaccine requires evaluation for allergic reactions before administration in gelatin-hypersensitive patients.
Influenza
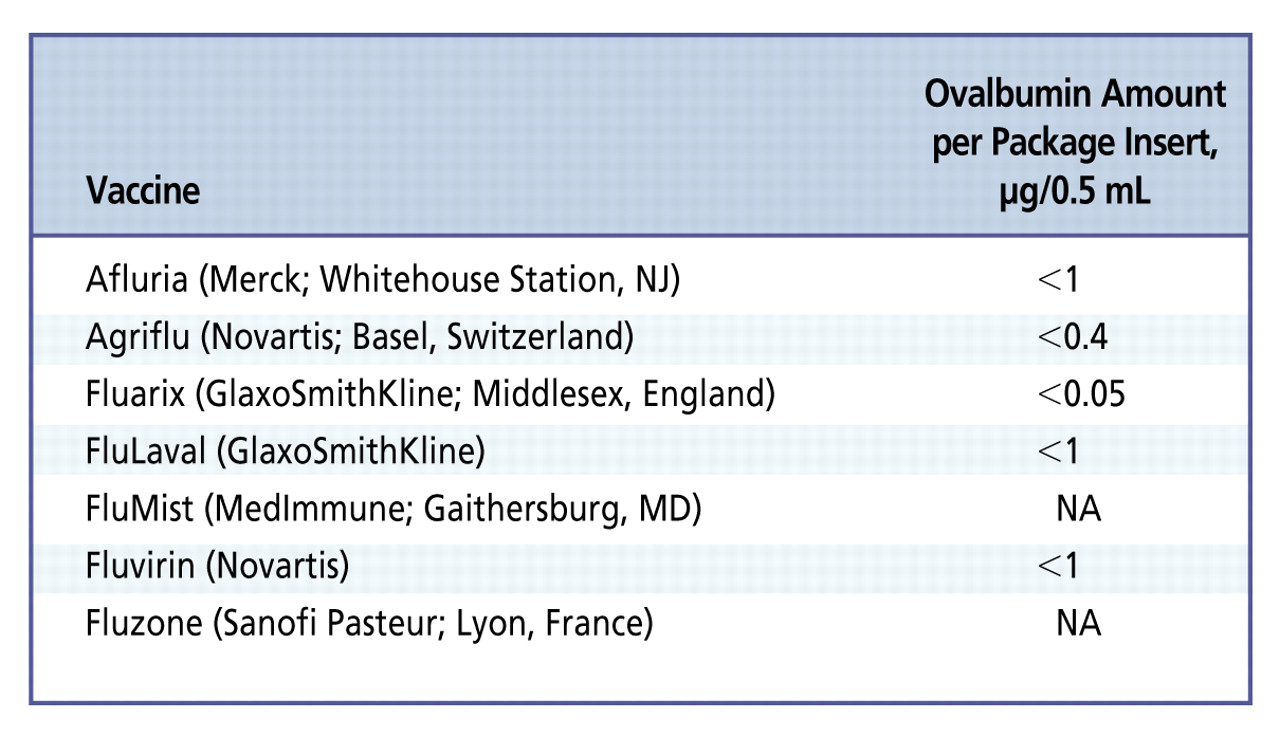
The influenza vaccine contains an inactivated live influenza virus. The virus is grown in chick extraembryonic allantoic fluid. The greatest amount of egg measured in the influenza vaccine is 0.7 μg per 0.5 mL of vaccine.6 Most manufacturers list the content of ovalbumin in the package insert. However, the concentration of ovalbumin may differ from year to year and from 1 brand of vaccine to another (Figure).6,7 On the basis of results in previous studies, vaccine lots containing less than 1.2 μg/mL of oval-bumin have been tolerated well by egg-hyper sensitive individuals. Data regarding the least concentration of egg in the influenza vaccine that will cause an adverse reaction remain unclear.
Recent recommendations by the US Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) state that the influenza vaccine can be administered to egg-hypersensitive patients without any precautions.8 If the patient can ingest lightly cooked eggs (eg, scrambled eggs) without reaction, he or she may receive the vaccine per usual protocol. If the patient develops hives only after ingesting eggs or egg-containing products, they may receive the vaccine but with a 30-minute observation period after the immunization. If any systemic events occur after the ingestion of egg, the the patient should be referred to a physician with expertise in managing allergic conditions for further evaluation before immunization. The ACIP recommendations differ from those made by other organizations—including the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; the Joint Council of Allergy, Asthma, and Immunology; and the National Institute of Allergy and Infectious Diseases—which recommend using split dosing in patients who have had anaphylactic events after ingesting eggs.8,9
The influenza vaccine administered nasally contains 2000 μg of gelatin per 0.2-mL dose, compared to 250 μg of gelatin per 0.5-mL dose when administered intramuscularly.4 Physicians administering this vaccine should be aware of the signs and symptoms of anaphylaxis and have appropriate medications available for treatment.
General Precautions
Regardless of recommendations, the potential of any vaccine antigen to trigger anaphylaxis must be respected in susceptible patients. The information on package inserts must be read carefully to determine the amount of ovalbumin present in a vaccine. In patients who are egg-hypersensitive, the vaccine containing the least amount of ovalbumin is recommended.
Anaphylaxis may occur with any antigen. Reaction severity can be influenced by the patient's sensitivity to the allergen and the amount of allergen administered. When any antigen is administered subcutaneously or intramuscularly in the office, the patient should be kept in the office for at least 30 minutes for observation.9 Medical offices and clinics should develop protocols for handling an anaphylactic event. Epinephrine should be readily available.
-
Financial Disclosures: None reported.
References
1 Smith KA Colvin CJ Weber PS Spatz SJ Coussens PM . High titer growth of human and avian influenza viruses in an immortalized chick embryo cell line without the need for exogenous proteases[published online ahead of print May 8, 2008]. Vaccine. 2008:26(29-30):3778-3782.10.1016/j.vaccine.2008.04.048Search in Google Scholar
2 Offit PA Jew RK . Addressing parents' concerns: do vaccines contain harmful preservatives, adjuvants, additives, or residuals?Pediatrics. 2003;112(6 pt 1):1394-1397.10.1542/peds.112.6.1394Search in Google Scholar
3 Freigang B Jadavji TP Freigang DW . Lack of adverse reactions to measles, mumps, and rubella vaccine in egg-allergic children. Ann Allergy. 1994;73(6):486-488.Search in Google Scholar
4 Kelso JM Li JT Nicklas RA et al. Joint Task Force on Practice Parameters Joint Task Force on Practice Parameters for Allergy & Immunology . Adverse reactions to vaccines. Ann Allergy Asthma Immunol.2009;103(4 suppl 2):S1-S14.10.1016/S1081-1206(10)60350-XSearch in Google Scholar
5 Dobardzic A Izurieta H Woo EJ et al. . Safety review of the purified chick embryo cell rabies vaccine: data from the Vaccine Adverse Event Reporting System (VAERS), 1997-2005. Vaccine. 2007;25(21):4244-4251.10.1016/j.vaccine.2007.02.075Search in Google Scholar PubMed
6 Li JT Rank MA Squillace DL Kita H . Ovalbumin content of influenza vaccines[published online ahead of print May 7, 2010]. J Allergy Clin Immunol.2010;125(6):1412-1413.10.1016/j.jaci.2010.03.009Search in Google Scholar PubMed PubMed Central
7 Waibel KH Gomez R . Ovalbumin content in 2009 to 2010 seasonal and H1N1 monovalent influenza vaccines[published online ahead of print January 8, 2010]. J Allergy Clin Immunol.2010;125(3):749-751.10.1016/j.jaci.2009.12.015Search in Google Scholar PubMed
8 Howe LE Conlon AS Greenhawt MJ Sanders GM . Safe administration of seasonal influenza vaccine to children with egg allergy of all severities[published online ahead of print February 24, 2011]. Ann Allergy Asthma Immunol.2011;106(5):446-447.10.1016/j.anai.2011.01.024Search in Google Scholar PubMed
9 Greenhawt MJ Li JT Bernstein DI et al. . Administering influenza vaccine to egg allergic recipients: a focused practice parameter update. Ann Allergy Asthma Immunol.2011;106(1):11-16. http://www.vaccines.mil/documents/library/Influenza-vaccine-and-egg-allergic-patients-2010.pdf. Accessed August 21, 2011.10.1016/j.anai.2010.11.015Search in Google Scholar PubMed
© 2011 The American Osteopathic Association
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Editor's Message
- Promoting Safe and Efficacious Vaccines for Adults
- ARTICLES
- Revisiting the Influenza Vaccine
- Cracking the Shell on Egg-Hypersensitive Patients and Egg-Containing Vaccines
- Tdap Vaccine: Current Indications for Adolescent and Adult Patients in the United States
- Vaccines for Measles, Mumps, Rubella, Varicella, and Herpes Zoster: Immunization Guidelines for Adults
- A Shot at Hepatitis Prevention
- Protecting the World Against Meningitis: New Recommendations From the CDC's Advisory Committee on Immunization Practices
- Time for Changes in Pneumococcal Vaccination of Adults?
- Vaccinations Recommended During Pregnancy and Breastfeeding
- Update on the Advisory Committee on Immunization Practices' Recommendations for Use of Herpes Zoster Vaccine
Articles in the same Issue
- Editor's Message
- Promoting Safe and Efficacious Vaccines for Adults
- ARTICLES
- Revisiting the Influenza Vaccine
- Cracking the Shell on Egg-Hypersensitive Patients and Egg-Containing Vaccines
- Tdap Vaccine: Current Indications for Adolescent and Adult Patients in the United States
- Vaccines for Measles, Mumps, Rubella, Varicella, and Herpes Zoster: Immunization Guidelines for Adults
- A Shot at Hepatitis Prevention
- Protecting the World Against Meningitis: New Recommendations From the CDC's Advisory Committee on Immunization Practices
- Time for Changes in Pneumococcal Vaccination of Adults?
- Vaccinations Recommended During Pregnancy and Breastfeeding
- Update on the Advisory Committee on Immunization Practices' Recommendations for Use of Herpes Zoster Vaccine