Tetrakis(2-fluoro-2,2-dinitroethyl) ortho-carbonate (1) and tris(2-fluoro-2,2-dinitroethyl) orthoformate (2) were synthesized by the reaction of carbon tetrachloride, respectively chloroform, with 2-fluoro-2,2-dinitroethanol and catalytic amounts of anhydrous iron(III) chloride. The compounds were characterized by single-crystal X-ray diffraction, vibrational spectroscopy (IR and Raman), multinuclear NMR spectroscopy, elemental analysis, and multi-temperature DSC measurements. The suitability of the compounds as potential oxidizers in energetic formulations has been investigated and discussed. The heats of formation of the products were determined experimentally using bomb calorimetric methods. With this value and the experimental (X-ray) density, several detonation parameters such as the detonation pressure, velocity, energy, and temperature were computed using the EXPLO5 code. The sensitivity towards impact, friction and electrostatic discharge was tested using the BAM drop hammer, a friction tester and a small-scale electrostatic discharge device.
Graphical Abstract
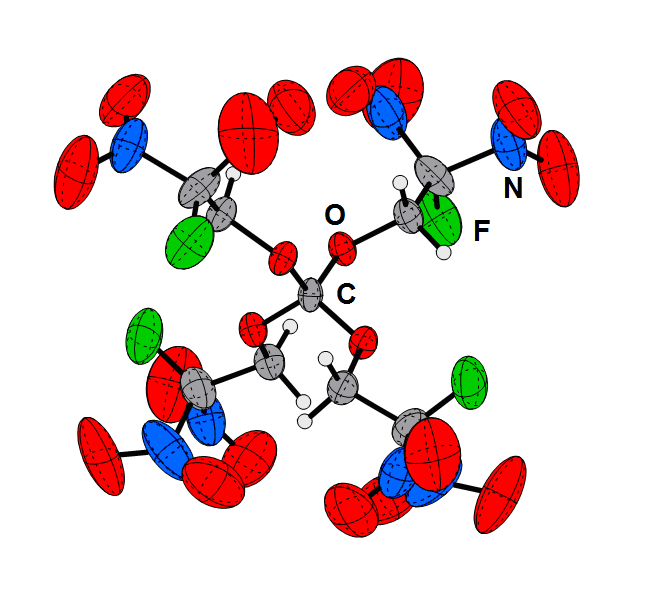
Fluorodinitroethyl Ortho-carbonate and -formate as Potential High Energy Dense Oxidizers
© 1946 – 2014: Verlag der Zeitschrift für Naturforschung
Articles in the same Issue
- The Hexaammine Copper(II) Fluoride Monohydrate [Cu(NH3)6][F(H2O)F]: Synthesis and Crystal Structure
- Fluorodinitroethyl Ortho-carbonate and -formate as Potential High Energy Dense Oxidizers
- Single-Crystal Structures and Vibrational Spectra of Li[SCN] and Li[SCN] · 2H2O
- Phosphanchalkogenide und ihre Metallkomplexe. II. Komplexe einiger Gold(I)-Halogenide mit Diphosphanmonochalkogeniden [1] / Phosphane Chalcogenides and their Metal Complexes. II. Gold(I) Halide Complexes of some Diphosphane Monochalcogenides
- Synthesis, Crystal Structures and Properties of Two Heterometallic Complexes [Cu2M] (M = Cd, Mn) with a Novel Oxamato-bridged Ligand
- In Search for Novel Sn2Co3S2-based Half-metal Ferromagnets
- Syntheses, Crystal Structures and Magnetic Properties of Two Nickel(II) Compounds with 4,4´- Bipyridine Ligands
- 2,3-Dihydro-1,3,4,5-tetraisopropylimidazol-2-yliden / 2,3-Dihydro-1,3,4,5-tetraisopropylimidazol-2-ylidene
- Improved Synthesis and Characterization of 2-(Dinitromethylene)-1- nitro-1,3-diazacyclopentane
- Functionalized Pyrazoles as Agents in C–C Cross-Coupling Reactions
- Monosaccharidic Push-pull Butadienes: Versatile Synthetic Intermediates
- Synthesis, Characterization and Antifungal Evaluation of Novel 2H-1,4-Benzoxazin-3(4H)-one Derivatives Linked with a 1,2,3-Triazole Moiety
- Ab-initio Studies of the Electronic Structures of the Hexavalent Uranium Compounds K2UO4 and Na4UO5
- Two New Diterpenoids from the Beibu Gulf Gorgonian Anthogorgia caerulea
- Sr2Au6Al3 and Eu2Au6Al3 – First Representatives of the Sr2Au6Zn3 Type with Aluminum Triangles
- Reaction of Copper(I) Nitrotetrazolate (DBX-1) with Sodium m-Periodate
Articles in the same Issue
- The Hexaammine Copper(II) Fluoride Monohydrate [Cu(NH3)6][F(H2O)F]: Synthesis and Crystal Structure
- Fluorodinitroethyl Ortho-carbonate and -formate as Potential High Energy Dense Oxidizers
- Single-Crystal Structures and Vibrational Spectra of Li[SCN] and Li[SCN] · 2H2O
- Phosphanchalkogenide und ihre Metallkomplexe. II. Komplexe einiger Gold(I)-Halogenide mit Diphosphanmonochalkogeniden [1] / Phosphane Chalcogenides and their Metal Complexes. II. Gold(I) Halide Complexes of some Diphosphane Monochalcogenides
- Synthesis, Crystal Structures and Properties of Two Heterometallic Complexes [Cu2M] (M = Cd, Mn) with a Novel Oxamato-bridged Ligand
- In Search for Novel Sn2Co3S2-based Half-metal Ferromagnets
- Syntheses, Crystal Structures and Magnetic Properties of Two Nickel(II) Compounds with 4,4´- Bipyridine Ligands
- 2,3-Dihydro-1,3,4,5-tetraisopropylimidazol-2-yliden / 2,3-Dihydro-1,3,4,5-tetraisopropylimidazol-2-ylidene
- Improved Synthesis and Characterization of 2-(Dinitromethylene)-1- nitro-1,3-diazacyclopentane
- Functionalized Pyrazoles as Agents in C–C Cross-Coupling Reactions
- Monosaccharidic Push-pull Butadienes: Versatile Synthetic Intermediates
- Synthesis, Characterization and Antifungal Evaluation of Novel 2H-1,4-Benzoxazin-3(4H)-one Derivatives Linked with a 1,2,3-Triazole Moiety
- Ab-initio Studies of the Electronic Structures of the Hexavalent Uranium Compounds K2UO4 and Na4UO5
- Two New Diterpenoids from the Beibu Gulf Gorgonian Anthogorgia caerulea
- Sr2Au6Al3 and Eu2Au6Al3 – First Representatives of the Sr2Au6Zn3 Type with Aluminum Triangles
- Reaction of Copper(I) Nitrotetrazolate (DBX-1) with Sodium m-Periodate

