Associations of gallbladder and gallstone parameters with clinical outcomes in patients with cirrhosis
-
Min Ding
Abstract
Background
Morphologic changes in the gallbladder and gallstones are common in cirrhotic patients, but their associations with outcomes of cirrhotic patients are unclear.
Methods
We retrospectively enrolled 206 cirrhotic patients and measured their gallbladder length and width, gallbladder wall thickness, presence of gallstones, and gallstones’ length and width in axial contrast-enhanced computed tomography (CT) images. X-tile software was utilized to calculate the optimal cutoff values of these parameters for evaluating survival and hepatic decompensation events in the cirrhosis group. Their associations with survival were explored by Cox regression analyses and Kaplan–Meier curve analyses. Their associations with hepatic decompensation events were evaluated by competing risk analyses and Nelson-Aalen cumulative risk curve analyses where death was a competing event.
Results
Cirrhotic patients with gallbladder length < 72 mm had a significantly higher cumulative survival rate than those with a length of ≥ 72 mm (P = 0.049 by log-rank test), but gallbladder width, gallbladder wall thickness, presence of gallstones, and gallstones’ length and width were not significantly associated with survival (P = 0.10, P = 0.14, P = 0.97, P = 0.73, and P = 0.73 by log-rank tests, respectively). Cirrhotic patients with gallbladder wall thickness < 3.4 mm had a significantly lower cumulative rate of hepatic decompensation events than those with a wall thickness of ≥ 3.4 mm (P = 0.02 by Gray’s test), but gallbladder length and width, presence of gallstones, and gallstones’ length and width were not significantly associated with hepatic decompensation events (P = 0.15, P = 0.15, P = 0.54, P = 0.76, and P = 0.54 by Gray’s tests, respectively).
Conclusion
Changes in gallbladder length and gallbladder wall thickness, rather than gallstone parameters, may be in parallel with the long-term outcomes of cirrhotic patients.
Introduction
Liver cirrhosis, the end stage of chronic liver diseases, carries a high morbidity and mortality.[1, 2, 3] It can lead to many lethal complications, including gastroesophageal variceal bleeding, ascites, hepatic encephalopathy, and jaundice.[4] According to the Global Burden of Disease Study 2017, liver cirrhosis caused more than 1.32 million deaths, which constituted 2.4% of total deaths globally.[5]
Gallbladder, a pear-shaped organ, stores and concentrates bile between meals.[6] Hemodynamically, gallbladder venous drainage is through the portal venous system, which subsequently flows into the inferior vena cava.[7, 8] In liver cirrhosis, cholecystic venous outflow tract can be impaired due to increased portal venous pressure, resulting in gallbladder congestion manifesting as changes in gallbladder length and width and gallbladder wall thickness in the axial images.[9, 10] On the other hand, gallbladder motility may be reduced due to portal hypertension in liver cirrhosis.[11] Consequently, liver cirrhosis is associated with a high risk of gallstones, and the prevalence of gallstones is twice higher in patients with liver cirrhosis than in the general population.[12, 13, 14] However, the associations of gallbladder morphologic changes and gallstones with the outcomes of patients with cirrhosis remain unclear. For this reason, the present study mainly aimed to investigate the associations of various gallbladder and gallstone parameters, including gallbladder length and width, gallbladder wall thickness, presence of gallstones, and gallstones’ length and width, with the longterm survival and development of hepatic decompensation events in patients with cirrhosis. Besides, their correlations with the Child-Pugh score and the model for end-stage liver disease (MELD) score were also evaluated.
Methods
This study was carried out following the rules of the 1975 Declaration of Helsinki and was approved by the Medical Ethical Committee of our hospital (Approval No. Y [2022] 071). Patients’ written informed consents had been waived by the Medical Ethical Committee of our hospital due to the retrospective nature of this study.
Study design
This retrospective study was performed on the basis of our prospective database in which a total of 570 patients with cirrhosis were consecutively admitted to the Department of Gastroenterology of our hospital and underwent abdominal contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans from December 2014 to November 2021. A diagnosis of liver cirrhosis was mainly based on clinical manifestations including gastrointestinal bleeding, ascites, and hepatic encephalopathy, laboratory tests including liver dysfunction, decreased serum albumin, and coagulation dysfunction, imaging features including liver morphology changes with splenomegaly on abdominal CT scans, and liver histological features including pseudolobule, if necessary.
Inclusion criteria were as follows: (1) patients who were diagnosed with liver cirrhosis and (2) patients who performed abdominal contrast-enhanced CT scans during hospitalization. Exclusion criteria were as follows: (1) a history of malignancy; (2) gallbladder and gallstone parameters could not be sufficiently evaluated on CT images; (3) a history of cholecystectomy; and (4) acute or chronic cholecystitis was diagnosed based on disease history, clinical presentations, and/or biochemical signs. Notably, patients with asymptomatic gallstones were not excluded.
Additionally, by reviewing both electronic medical records and imaging reports system, all patients without a history of chronic liver disease and hepatocellular carcinoma or other malignancies who were consecutively admitted to the Department of Gastroenterology of our hospital and underwent abdominal contrast-enhanced CT between January 2020 and October 2021 were selected as the control group.
Clinical data collection
All patients were subjected to complete clinical evaluation. Clinical data at admission were collected as follows: age, gender, red blood cell (RBC), white blood cell (WBC), platelets count (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), γ-glutamine transferase (GGT), serum creatinine (Scr), serum sodium (Na), prothrombin time (PT), and international normalized ratio (INR). The Child-Pugh score and the MELD score were calculated.
CT images
Two investigators independently reviewed all available CT images of each included patient, selected the specific layers, where the maximum length and width of gallbladder, the maximum thickness of the gallbladder wall, and the maximum length and width of gallstones were obtained, and then measured these parameters according to the standard methods as shown in Figure 1. A disagreement between them was resolved by discussing with another investigator to determine the most appropriate layer where the value measured should be maximal as the final value. Notably, all patients are routinely requested to be fasting before undergoing abdominal contrast-enhanced CT or MRI scan.
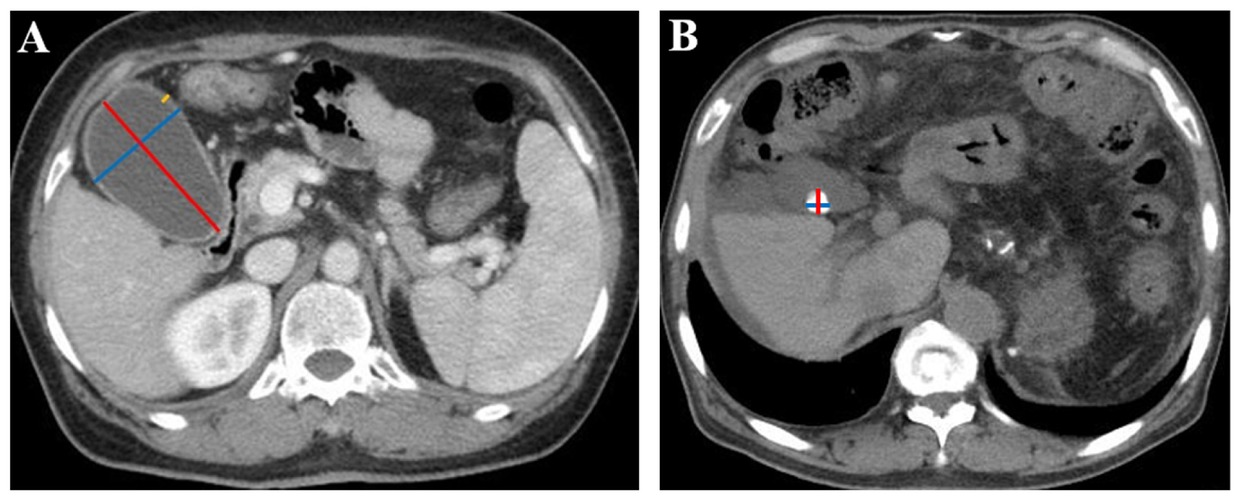
Gallbladder and gallstone parameters measured in CT images. (A) Measurement of gallbladder parameters in a 63-year-old woman with liver cirrhosis. The red line represents the maximum gallbladder length, the blue line represents the maximum gallbladder width, and the yellow line represents the maximum gallbladder wall thickness. The maximum gallbladder length was 77.6 mm, the maximum gallbladder width was 49.0 mm, and the maximum gallbladder wall thickness was 3.5 mm. (B) Measurement of gallstone parameters in a 65-year-old man with liver cirrhosis. The red line represents the maximum gallstone length, and the blue line represents the maximum gallstone width. The maximum gallstone length was 12.7 mm, and the maximum gallstone width was 12.4 mm. CT: computed tomography.
Follow-up
All enrolled patients were followed by reviewing inpatient and outpatient medical records and telephone visits. The last follow-up date was December 9, 2021. We recorded the dates of hepatic decompensation events and deaths during follow-up. Hepatic decompensation events evaluated in this study included gastrointestinal bleeding, ascites, hepatic encephalopathy, and jaundice.[15, 16, 17, 18]
Statistical analyses
First, continuous variables were expressed as the mean ± standard deviation or median (range) and compared using nonparametric Mann-Whitney U test. Categorical variables were expressed as frequency (percentage) and compared using the χ2 test. Second, the correlations of various gallbladder and gallstone parameters with the Child-Pugh score and the MELD score were analyzed by Spearman’s rank correlation tests, and the Spearman’s rank correlation coefficients (rs) were calculated. Third, X-tile software was utilized to calculate the optimal cutoff values of gallbladder and gallstone parameters for evaluating the long-term overall survival and hepatic decompensation events. Fourth, univariate Cox regression analyses were performed to explore the associations of gallbladder and gallstone parameters with survival and multivariate Cox regression analyses were performed by adjusting for age, sex, and the Child-Pugh score to identify which parameter was an independent predictor of survival. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. Cumulative survival rates were evaluated by Kaplan-Meier curve analyses and compared by log-rank tests. Fifth, competing risk analyses were performed to analyze the associations of these parameters with hepatic decompensation events during follow-up, where death was considered a competing event. Sub-distribution hazard ratios (sHRs) and their 95% CIs were calculated. Cumulative rates of hepatic decompensation events were evaluated by Nelson-Aalen cumulative risk curve analyses and compared by Gray’s tests. All statistical analyses were performed on IBM Statistical Package for the Social Sciences (SPSS) version 25.0 (IBM Corp, Armonk, NY, USA), X-tile version 3.6.1 (Yale University, New Haven, CT, USA), and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) with the packages ggplot2, survival, survminer, and cmprsk. A two-tailed P value < 0.05 was considered statistically significant.
Results
Study population
Overall, 206 patients with cirrhosis were included (Figure 2). Median age was 56 years (28–89) and 142 (69%) patients were male (Table 1). During a median follow-up duration of 2.25 years (0.09–6.03), 54 patients developed gastrointestinal bleeding, 42 developed ascites, 10 developed hepatic encephalopathy, 29 died, and none underwent liver transplantation. Causes of death were related to liver diseases (n = 23), non-liver diseases (n = 1), and were unknown (n = 5).
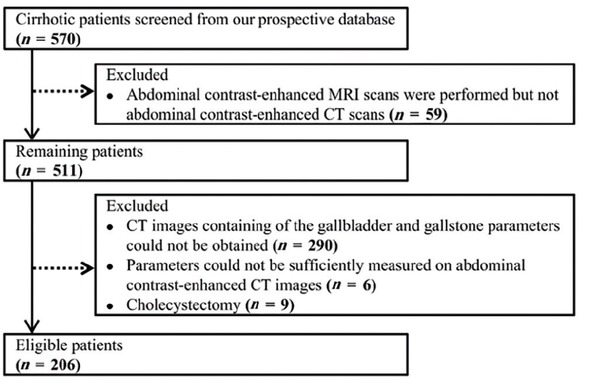
Flowchart of cirrhotic patients’ selection. MRI: magnetic resonance imaging.
Differences between cirrhosis group and non-cirrhosis group
| Variables | No. pts | Cirrhosis group | No. pts | Non-cirrhosis group | P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yr | 206 | 56 (28–89) | 104 | 58 (19–81) | 0.22 |
| Male, n (%) | 206 | 142 (69) | 104 | 67 (64) | 0.42 |
| Laboratory tests | |||||
| RBC, ×1012/L | 206 | 3.48 (1.24–5.20) | 99 | 4.06 ± 0.68 | < 0.001 |
| WBC, ×109/L | 206 | 3.20 (0.70–19.60) | 99 | 6.00 (2.20–15.30) | < 0.001 |
| PLT, ×109/L | 206 | 80 (24–646) | 99 | 233 (25–759) | < 0.001 |
| TBIL, μmol/L | 206 | 19.40 (5.70–216.50) | 99 | 10.10 (3.10–300.70) | < 0.001 |
| ALB, g/L | 205 | 33.95 ± 6.72 | 99 | 37.96 ± 4.66 | < 0.001 |
| ALT, U/L | 206 | 24.52 (4.23–1465.50) | 99 | 15.42 (2.68–215.29) | < 0.001 |
| GGT, U/L | 206 | 36.35 (8.23–1283.02) | 99 | 22.50 (5.97–651.14) | < 0.001 |
| Scr, μmol/L | 203 | 63.43 (14.80–130.40) | 99 | 68.17 (22.22–436.80) | 0.03 |
| Na, mmol/L | 206 | 138.90 (118.00–151.00) | 98 | 140.05 (123.80–147.00) | < 0.001 |
| PT, s | 205 | 15.70 (12.60–28.00) | 99 | 13.52 ± 0.84 | < 0.001 |
| INR | 205 | 1.26 (0.99–2.77) | 99 | 1.02 (0.84–1.27) | < 0.001 |
| Gallbladder and gallstone parameters | |||||
| Gallbladder length, mm | 206 | 61.07 ± 19.24 | 104 | 56.71 ± 18.40 | 0.06 |
| Gallbladder width, mm | 206 | 32.07 ± 9.21 | 104 | 29.62 ± 7.29 | 0.03 |
| Gallbladder wall thickness, mm | 201 | 3.30 (1.44–7.30) | 99 | 2.50 (1.31–6.28) | < 0.001 |
| Gallstones, n (%) | 206 | 39 (19) | 104 | 3 (3) | < 0.001 |
| Gallstones’ length, mm | 39 | 6.90 (1.80–22.40) | 3 | 11.17 (1.79–30.34) | < 0.001 |
| Gallbladders’ width, mm | 39 | 8.30 (1.70–29.78) | 3 | 11.31 (4.02–13.26) | < 0.001 |
Continuous data that were normally distributed were expressed as mean ± standard deviation, and those that were skewed were expressed as median (range). pts: numbers of patients; RBC: red blood cell; WBC: white blood cell; PLT: platelets count; TBIL: total bilirubin; ALB: albumin; ALT: alanine aminotransferase; GGT: γ-glutamine transferase; Scr: serum creatinine; Na: serum sodium; PT: prothrombin time; INR: international normalized ratio.
Cirrhosis versus non-cirrhosis
Overall, 104 patients with non-cirrhosis were selected as the control group (Supplementary Figure 1). Patients with cirrhosis had longer gallbladder and wider gallbladder, thicker gallbladder wall, and higher prevalence of gallstones than those with non-cirrhosis (Table 1).
Correlations of gallbladder and gallstone parameters with the Child-Pugh score and the MELD score in cirrhosis
Spearman’s rank correlation tests demonstrated that gallbladder width significantly correlated with the MELD score (P = 0.03, rs = 0.148) and gallbladder wall thickness significantly correlated with the Child-Pugh score (P = 0.005, rs = 0.196) and the MELD score (P = 0.002, rs = 0.212). However, gallbladder length, presence of gallstones, gallstones’ length, and gallstones’ width did not significantly correlate with the Child-Pugh score or the MELD score (Table 2).
Correlations of gallbladder and gallstone parameters with the Child-Pugh score and the MELD score in cirrhosis
| Variables | Child-Pugh score | MELD score | ||
|---|---|---|---|---|
| rs | P value | rs | P value | |
| Gallbladder length | –0.020 | 0.77 | 0.012 | 0.87 |
| Gallbladder width | 0.021 | 0.76 | 0.148 | 0.03 |
| Gallbladder wall thickness | 0.196 | < 0.01 | 0.212 | < 0.01 |
| Gallstones | 0.018 | 0.80 | 0.080 | 0.26 |
| Gallstones’ length | 0.025 | 0.88 | 0.066 | 0.35 |
| Gallstones’ width | 0.104 | 0.53 | 0.077 | 0.27 |
MELD: model for end-stage liver disease.
Associations of gallbladder and gallstone parameters with survival in cirrhosis
The optimal cutoff values of gallbladder length, gallbladder width, gallbladder wall thickness, gallstones’ length, and gallstones’ width for predicting survival were 72.0, 38.6, 3.6, 1.9, and 2.0 mm, respectively.
Univariate Cox regression analyses demonstrated that gallbladder length < 72.0 mm, gallbladder width < 38.6 mm, gallbladder wall thickness < 3.6 mm, absence of gallstones, gallstones’ length < 1.9 mm, and gallstones’ width < 2.0 mm were not significant predictors of survival in cirrhosis. However, after adjusting for age, sex, and the Child-Pugh score, multivariate Cox regression analyses demonstrated that gallbladder width < 38.6 mm was an independent predictor of survival (HR = 3.01, 95% CI: 1.28–7.06; P = 0.01), but not gallbladder length < 72.0 mm, gallbladder wall thickness < 3.6 mm, absence of gallstones, gallstones’ length < 1.9 mm, or gallstones’ width < 2.0 mm (Table 3).
Cox regression analyses regarding the associations of gallbladder and gallstone parameters with survival in cirrhosis
| Variables | Univariate analyses | Multivariate analysesa | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gallbladder length < 72.0 versus ≥ 72.0 mm | 2.07 | 0.99–4.34 | 0.05 | 1.99 | 0.94–4.22 | 0.07 |
| Gallbladder width < 38.6 versus ≥ 38.6 mm | 1.93 | 0.88–4.25 | 0.10 | 3.01 | 1.28–7.06 | 0.01 |
| Gallbladder wall thickness < 3.6 versus ≥ 3.6 mm | 1.72 | 0.83–3.56 | 0.15 | 1.09 | 0.49–2.42 | 0.84 |
| Without gallstones versus with gallstones | 0.98 | 0.40–2.41 | 0.97 | 0.63 | 0.24–1.63 | 0.34 |
| Gallstones’ length < 1.9 versus ≥ 1.9 mm | 0.85 | 0.32–2.22 | 0.74 | 0.51 | 0.18–1.43 | 0.20 |
| Gallstones’ width < 2.0 versus ≥ 2.0 mm | 0.85 | 0.32–2.22 | 0.74 | 0.51 | 0.18–1.43 | 0.20 |
aMultivariate analyses by adjusting for age, sex, and the Child-Pugh score. HR: hazard ratio; CI: confidence interval.
Kaplan-Meier curve analysis demonstrated that patients with gallbladder length < 72.0 mm had a significantly higher cumulative survival rate than those with a length of ≥ 72.0 mm (P = 0.049 by log-rank test) (Figure 3). But there was no significant difference in the cumulative survival rate between patients with gallbladder width < 38.6 mm versus those with a width of ≥ 38.6 mm (P = 0.10 by log-rank test), those with gallbladder wall thickness < 3.6 mm versus patients with a wall thickness of ≥ 3.6 mm (P = 0.14 by log-rank test), those without gallstones versus those with gallstones (P = 0.97 by log-rank test), those with gallstones’ length < 1.9 mm versus those with a length of ≥ 1.9 mm (P = 0.73 by log-rank test), or those with gallstones’ width < 2.0 mm versus those with a width of ≥ 2.0 mm (P = 0.73 by log-rank test).
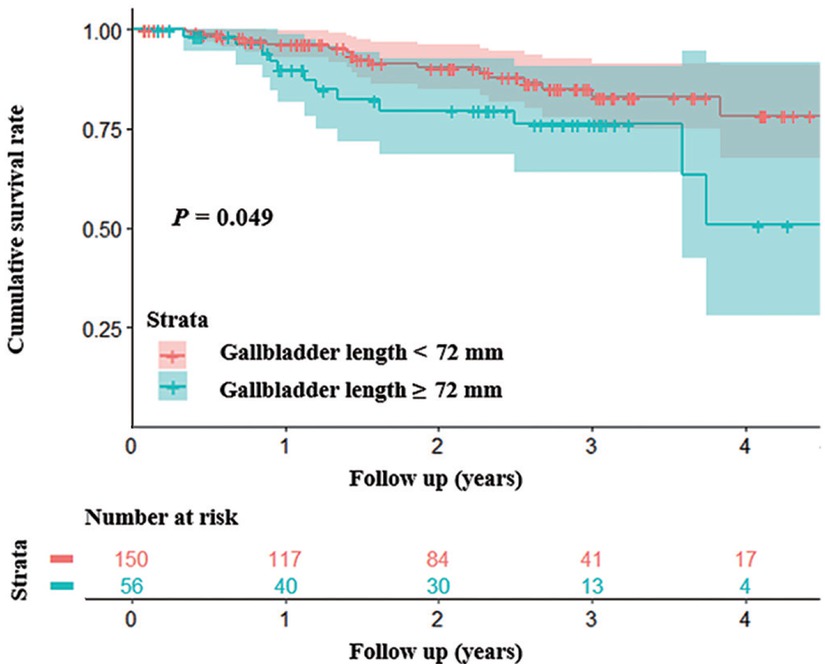
Kaplan-Meier curve analysis demonstrating that patients with gallbladder length < 72 mm had a significantly higher cumulative survival rate than those with a length of ≥ 72 mm (P = 0.049 by log-rank test).
Associations of gallbladder and gallstone parameters with hepatic decompensation events in cirrhosis
The optimal cutoff values of gallbladder length, gallbladder width, gallbladder wall thickness, gallstones’ length, and gallstones’ width for predicting hepatic decompensation events were 55.8, 31.1, 3.4, 1.9, and 1.7 mm, respectively. Competing risk analyses demonstrated that gallbladder wall thickness < 3.4 mm was significantly associated with decreased risk of hepatic decompensation events (sHR = 1.62, 95% CI: 1.09–2.41; P = 0.02), but not gallbladder length < 55.8 mm, gallbladder width < 31.1 mm, absence of gallstones, gallstones’ length < 1.9 mm, or gallstones’ width < 1.7 mm (Table 4).
Competing risk analyses regarding the associations of gallbladder and gallstone parameters with hepatic decompensation events in cirrhosis
| Variables | sHR | 95% CI | P value |
|---|---|---|---|
| Gallbladder length < 55.8 versus ≥ 55.8 mm | 0.75 | 0.50–1.11 | 0.15 |
| Gallbladder width < 31.1 versus ≥ 31.1 mm | 1.35 | 0.91–2.01 | 0.14 |
| Gallbladder wall thickness < 3.4 versus ≥ 3.4 mm | 1.62 | 1.09–2.41 | 0.02 |
| Without gallstones versus with gallstones | 1.16 | 0.75–1.81 | 0.51 |
| Gallstones’ length < 1.9 versus ≥ 1.9 mm | 1.08 | 0.68–1.72 | 0.74 |
| Gallstones’ width < 1.7 versus ≥ 1.7 mm | 1.16 | 0.75–1.81 | 0.51 |
sHR: sub-distribution hazard ratio; CI: confidence interval.
Nelson–Aalen cumulative risk curve analysis demonstrated that patients with gallbladder wall thickness < 3.4 mm had a significantly lower cumulative rate of hepatic decompensation events than those with a wall thickness of ≥ 3.4 mm (P = 0.02 by Gray’s test) (Figure 4). But there was no significant difference in the cumulative rate of hepatic decompensation events between patients with gallbladder length < 55.8 mm versus those with a length of ≥ 55.8 mm (P = 0.15 by Gray’s test), those with gallbladder width < 31.1 mm versus those with a width of ≥ 31.1 mm (P = 0.15 by Gray’s test), those without gallstones versus those with gallstones (P = 0.54 by Gray’s test), those with gallstones’ length < 1.9 mm versus those with a length of ≥ 1.9 mm (P = 0.76 by Gray’s test), or those with gallstones’ width < 1.7 mm versus those with a width of ≥ 1.7 mm (P = 0.54 by Gray’s test).
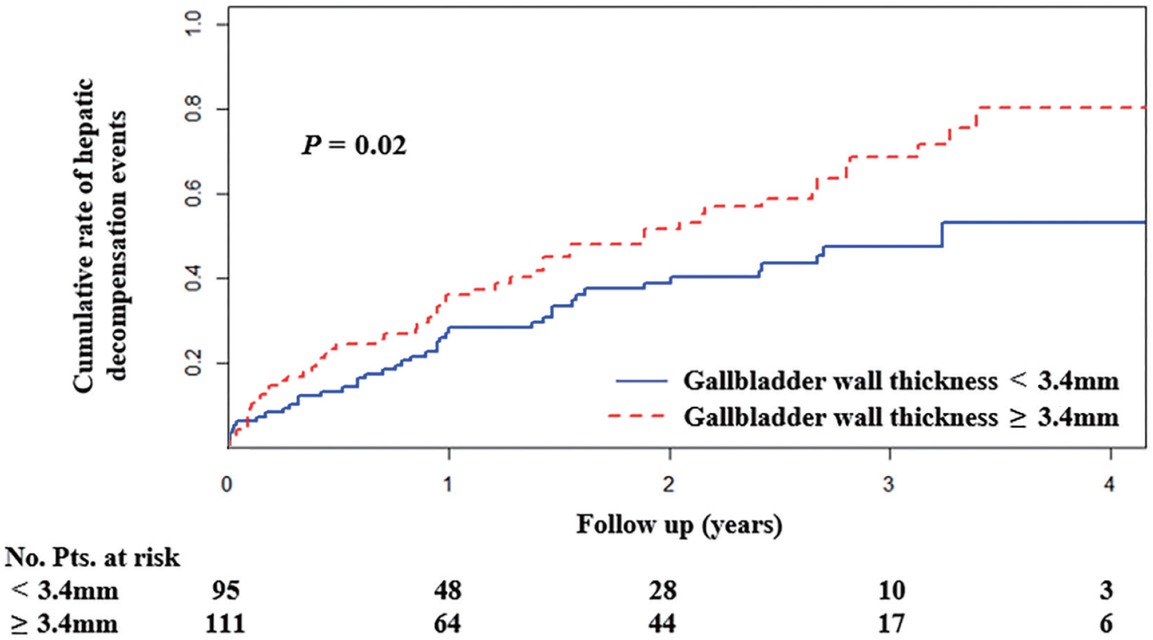
Nelson-Aalen cumulative risk curve analysis demonstrating that patients with gallbladder wall thickness < 3.4 mm had a significantly lower cumulative rate of hepatic decompensation events than those with a thickness of ≥ 3.4 mm (P = 0.02 by Gray’s test). Pts: patients.
Discussion
Our study found that patients with cirrhosis had longer and wider gallbladder and thicker gallbladder wall than those without, after excluding the possibilities of gallbladder morphologic changes caused by acute or chronic cholecystitis. Abnormal gallbladder morphology is related to hypoproteinemia caused by reduced liver synthetic function in cirrhotic patients.[19] Hypoproteinemia can lead to a decrease of colloid osmotic pressure, and then the formation of ascites, in which the gallbladder remains immersed for a long time, enlarging the gallbladder size and thickening the gallbladder wall.[20] More importantly, this might be primarily due to gallbladder congestion caused by portal hypertension in liver cirrhosis. Indeed, as the portal pressure increases, multiple hepatic decompensation events will develop. For example, esophageal varices develop and progress, thereby causing variceal rupture and massive gastrointestinal bleeding,[21,22] and the vascular hydrostatic pressure in the abdominal viscera increases, thereby decreasing tissue fluid reabsorption and then aggravating the occurrence and grade of ascites.[23] Recently, the association of gallbladder wall thickness with hepatic decompensation has been explored in some cross-sectional studies.[7,24, 25, 26] Elkerdawy et al.[7] found that gallbladder wall thickness was associated with the presence of esophageal varices in patients with cirrhosis. Mohammadi et al.[25] demonstrated that thickened gallbladder wall was highly predictive for the presence of ascites in liver cirrhosis. Our cross-sectional data supported that gallbladder wall thickness significantly correlated with the Child-Pugh and MELD scores, which are well-known prognostic factors in patients with cirrhosis,[27,28] and that gallbladder width also significantly correlated with the MELD score. Notably, all previous studies reported only cross-sectional data without long-term outcomes, and therefore, they could not provide any evidence regarding the associations of gallbladder wall thickness with the development and progression of decompensation events and death during follow-up. Moreover, they employed ultrasound to measure gallbladder changes. By comparison, our study employed abdominal contrast-enhanced CT scans, which could provide more accurate and reproducible data, followed patients with cirrhosis for a median duration of 2.25 years, and further demonstrated that gallbladder wall thickness and gallbladder length were positively associated with the risk of hepatic decompensation events and death, respectively. Additionally, the normal gallbladder length ranges 80–120 mm and the upper limit of normal of gallbladder wall thickness is 3 mm.[29] We also identified that the optimal cutoff values of gallbladder length and width for predicting the risk of death should be ≥ 72.0 and ≥ 38.6 mm, respectively. The optimal cutoff value of gallbladder wall thickness for predicting the risk of hepatic decompensation events should be ≥ 3.4 mm. The changes in gallbladder morphology in cirrhosis may reflect the severity of liver cirrhosis itself to a certain extent and can provide observable indicators for the outcomes of cirrhotic patients.
Our study also found a significantly higher prevalence of gallstones in patients with cirrhosis than in those without. This might be because cirrhotic patients with portal hypertension often have reduced gallbladder motility,[30,31] patients with advanced liver cirrhosis may present with autonomic neuropathy leading to sphincter of Oddi dysfunction and gallbladder emptying impairment,[32,33] and those with hypersplenism develop chronic hemolysis resulting in the formation of black pigment gallstones.[34] Previous studies have shown that gallstone disease was associated with high risk of all-cause death and disease-specific death, including cardiovascular disease and cancer-related mortality.[35] However, Ruhl and Everhart[36] reported that gallstone disease was not related to the mortality from digestive diseases, in which chronic liver disease accounted for nearly half of all deaths from digestive diseases. Despite a higher prevalence of gallstones in patients with cirrhosis than in those without,[37, 38, 39] our study further showed no significant association between the presence and size of gallstones and the outcomes of patients with cirrhosis. This may be due to a small proportion of gallbladder stones in our patients. Notably, we should acknowledge that some gallstones could not be clearly observed on abdominal CT images, and transabdominal ultrasonography should be the first-line approach for diagnosing gallstones.[40]
Our study had several features. First, the study population was from our prospectively established database of liver cirrhosis and was regularly followed. Second, the data measured by CT were more objective and reproducible. Third, various gallbladder and gallstone parameters measured by abdominal contrast-enhanced CT scans were carefully collected to identify the differences between patients with and without cirrhosis, evaluate their correlations with the severity of liver dysfunction in patients with cirrhosis, and predict hepatic decompensation events and long-term survival. Our study also had several limitations. First, this retrospective study had a potential bias in patient selection. Second, not all our patients had well-preserved contrast-enhanced CT images to measure gallbladder and gallstone parameters. Third, only the gallbladder length and width obtained in the axial images were employed in our study. By comparison, the gallbladder volume should be an optimal indicator of gallbladder morphology. Fourth, the cutoff values of gallbladder and gallstone parameters calculated in our study might not be readily extrapolated to other patients. Fifth, the rate of agreement in the measurement of gallbladder and gallstone parameters among investigators had not been designed.
In summary, patients with liver cirrhosis often develop changes in gallbladder morphology and gallstones. Importantly, thickened gallbladder wall and increased gallbladder length, but not gallstones, predict their worse outcomes. Further studies should elucidate the impact of dynamic changes of gallbladder-related parameters on the prognosis of patients with cirrhosis.
Author Contributions
Conceptualization: Xingshun Qi; data collection and revision: Min Ding, Yue Yin, Xueying Wang, and Xingshun Qi; data analysis and revision: Min Ding, Yue Yin, Menghua Zhu, Fangfang Yi, and Xingshun Qi; methodology and writing: Min Ding, Yue Yin, and Xingshun Qi; critical comments and revision: Min Ding, Yue Yin, Xueying Wang, Menghua Zhu, Shixue Xu, Le Wang, Fangfang Yi, Cyriac Abby Philips, Fernando Gomes Romeiro, and Xingshun Qi; and supervision: Xingshun Qi.
Acknowledgments
The authors are indebted to their study team, including Han Deng, Ran Wang, Jing Li, Yingying Li, Xiangbo Xu, Zhaohui Bai, Qianqian Li, Kexin Zheng, Le Wang, Fangfang Yi, Yanyan Wu, Li Luo, Yue Yin, Shixue Xu, Mengyuan Peng, Weiwei Wang, Xueying Wang, Yiyan Zhang, and Xiaojie Zheng, for their efforts in establishing and updating their prospective database of liver cirrhosis.
-
Source of Funding
This work was partially supported by the Young and Middle-aged Scientific and Technological Innovation Talents Support Plan Project of Shenyang (RC210011).
-
Ethical Approval and Informed Consent
This study was carried out following the rules of the 1975 Declaration of Helsinki and was approved by the Medical Ethical Committee of our hospital (Approval No. Y [2022] 071). Patients’ written informed consents had been waived by the Medical Ethical Committee of our hospital due to the retrospective nature of this study.
-
Conflict of Interest
Xingshun Qi is an Editorial Board member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this editor and his research group.
References
1 Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61.10.1016/S0140-6736(14)60121-5Search in Google Scholar PubMed
2 Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650–66.10.1016/j.cgh.2019.07.060Search in Google Scholar PubMed PubMed Central
3 Liu YB, Chen MK. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J Gastroenterol 2022;28:5910–30.10.3748/wjg.v28.i41.5910Search in Google Scholar PubMed PubMed Central
4 Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet 2021;398:1359–76.10.1016/S0140-6736(21)01374-XSearch in Google Scholar PubMed
5 GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245–66.10.1016/S2468-1253(19)30349-8Search in Google Scholar PubMed PubMed Central
6 Housset C, Chrétien Y, Debray D, Chignard N. Functions of the Gallbladder. Compr Physiol 2016;6:1549–77.10.1002/cphy.c150050Search in Google Scholar PubMed
7 Elkerdawy MA, Ahmed MH, Zaghloul MS, Haseeb MT, Emara MH. Does gallbladder wall thickness measurement predict esophageal varices in cirrhotic patients with portal hypertension? Eur J Gastroenterol Hepatol 2021;33:917–25.10.1097/MEG.0000000000002024Search in Google Scholar PubMed
8 Hui CL, Loo ZY. Vascular disorders of the gallbladder and bile ducts: Imaging findings. J Hepatobiliary Pancreat Sci 2021;28:825–36.10.1002/jhbp.930Search in Google Scholar PubMed
9 Son JY, Kim YJ, Park HS, Yu NC, Ko SM, Jung SI, et al. Diffuse gallbladder wall thickening on computed tomography in patients with liver cirrhosis: correlation with clinical and laboratory variables. J Comput Assist Tomogr 2011;35:535–8.10.1097/RCT.0b013e31822d2adeSearch in Google Scholar PubMed
10 Bremer SCB, Knoop RF, Porsche M, Amanzada A, Ellenrieder V, Neesse A, et al. Pathological gallbladder wall thickening is associated with advanced chronic liver disease and independent of serum albumin. J Clin Ultrasound 2022;50:367–74.10.1002/jcu.23077Search in Google Scholar PubMed
11 Acalovschi, M., D.L. Dumitrascu, and C.D. Nicoara, Gallbladder contractility in liver cirrhosis: comparative study in patients with and without gallbladder stones. Dig Dis Sci 2004;49:17–24.10.1023/B:DDAS.0000011596.33237.5cSearch in Google Scholar
12 Bouchier IA. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut 1969;10:705–10.10.1136/gut.10.9.705Search in Google Scholar PubMed PubMed Central
13 Mallick B, Anand AC. Gallstone Disease in Cirrhosis-Pathogenesis and Managemen t. J Clin Exp Hepatol 2022;12:551–9.10.1016/j.jceh.2021.09.011Search in Google Scholar PubMed PubMed Central
14 Hussain A, Nadeem MA, Nisar S, Tauseef HA. Frequency of gallstones in patients with liver cirrhosis. J Ayub Med Coll Abbottabad 2014;26:341–3.Search in Google Scholar
15 Bai Z, Li B, Lin S, Liu B, Li Y, Zhu Q, et al. Development and Validation of CAGIB Score for Evaluating the Prognosis of Cirrhosis with Acute Gastrointestinal Bleeding: A Retrospective Multicenter Study. Adv Ther 2019;36:3211–20.10.1007/s12325-019-01083-5Search in Google Scholar PubMed PubMed Central
16 Gallo A, Dedionigi C, Civitelli C, Panzeri A, Corradi C, Squizzato A. Optimal Management of Cirrhotic Ascites: A Review for Internal Medicine Physicians. J Transl Int Med 2020;8:220–36.10.2478/jtim-2020-0035Search in Google Scholar PubMed PubMed Central
17 Ridola L, Faccioli J, Nardelli S, Gioia S, Riggio O. Hepatic Encephalopathy: Diagnosis and Management. J Transl Int Med 2020;8:210–9.10.2478/jtim-2020-0034Search in Google Scholar PubMed PubMed Central
18 Fargo MV, Grogan SP, Saguil A. Evaluation of Jaundice in Adults. Am Fam Physician 2017;95:164–8.Search in Google Scholar
19 Buob S, Johnston AN, Webster CR. Portal hypertension: pathophysiology, diagnosis, and treatment. J Vet Intern Med 2011;25:169–86.10.1111/j.1939-1676.2011.00691.xSearch in Google Scholar PubMed
20 Risson JR, Macovei I, Loock M, Paquette B, Martin M, Delabrousse E. Cirrhotic and malignant ascites: differential CT diagnosis. Diagn Interv Imaging 2012;93:365–70.10.1016/j.diii.2012.02.008Search in Google Scholar PubMed
21 Fullwood D. Portal hypertension and varices in patients with liver cirrhosis. Nurs Stand 2012;26:52–8.10.7748/ns2012.08.26.48.52.c9230Search in Google Scholar PubMed
22 Zimmermann HW, Trautwein C, Bruns T. Aktuelle Diagnostik und Therapie der portalen Hypertension [Current diagnostics and treatment of portal hypertension]. Inn Med (Heidelb). 2022;63:1257–67.10.1007/s00108-022-01427-4Search in Google Scholar PubMed
23 Simonetto DA, Liu M, Kamath PS. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin Proc 2019;94:714–26.10.1016/j.mayocp.2018.12.020Search in Google Scholar PubMed
24 Xu Y, Yuan X, Zhang X, Hu W, Wang Z, Yao L, et al. Prognostic value of inflammatory and nutritional markers for hepatocellular carcinoma. Medicine (Baltimore) 2021;100:e26506.10.1097/MD.0000000000026506Search in Google Scholar PubMed PubMed Central
25 Mohammadi A, Ghasemi-Rad M, Mohammadifar M. Differentiation of benign from malignant induced ascites by measuring gallbladder wall thickness. Maedica (Bucur) 2011;6:282–6.Search in Google Scholar
26 Tsaknakis B, Masri R, Amanzada A, Petzold G, Ellenrieder V, Neesse A, et al. Gall bladder wall thickening as non-invasive screening parameter for esophageal varices - a comparative endoscopic - sonographic study. BMC Gastroenterol 2018;18:123.10.1186/s12876-018-0852-5Search in Google Scholar PubMed PubMed Central
27 Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore) 2016;95:e2877.10.1097/MD.0000000000002877Search in Google Scholar PubMed PubMed Central
28 Jamil Z, Perveen S, Khalid S, Aljuaid M, Shahzad M, Ahmad B, et al. Child-Pugh Score, MELD Score and Glasgow Blatchford Score to Predict the In-Hospital Outcome of Portal Hypertensive Patients Presenting with Upper Gastrointestinal Bleeding: An Experience from Tertiary Healthcare System. J Clin Med 2022;11:6654.10.3390/jcm11226654Search in Google Scholar PubMed PubMed Central
29 Khammas ASA, Mahmud R. Ultrasonographic Measurements of the Liver, Gallbladder Wall Thickness, Inferior Vena Cava, Portal Vein and Pancreas in an Urban Region, Malaysia. J Med Ultrasound 2020;29:26–31.10.4103/JMU.JMU_53_20Search in Google Scholar PubMed PubMed Central
30 Kassem MI, Hassouna EM. Short-term outcome of total clipless laparoscopic cholecystectomy for complicated gallbladder stones in cirrhotic patients. ANZ J Surg 2018;88:E152–E6.10.1111/ans.13855Search in Google Scholar PubMed
31 Buzaş C, Chira O, Mocan T, Acalovschi M. Comparative study of gallbladder motility in patients with chronic HCV hepatitis and with HCV cirrhosis. Rom J Intern Med 2011;49:37–44.Search in Google Scholar
32 Kul K, Serin E, Yakar T, Coşar AM, Özer B. Autonomic neuropathy and gallbladder motility in patients with liver cirrhosis. Turk J Gastroenterol 2015;26:254–8.10.5152/tjg.2015.4469Search in Google Scholar PubMed
33 Li CP, Hwang SJ, Lee FY, Chang FY, Lin HC, Lu RH, et al. Evaluation of gallbladder motility in patients with liver cirrhosis: relationship to gallstone formation. Dig Dis Sci 2000;45:1109–14.10.1023/A:1005537632665Search in Google Scholar PubMed
34 Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6:172–87.10.5009/gnl.2012.6.2.172Search in Google Scholar PubMed PubMed Central
35 Zheng Y, Xu M, Heianza Y, Ma W, Wang T, Sun D, et al. Gallstone disease and increased risk of mortality: Two large prospective studies in US men and women. J Gastroenterol Hepatol 2018;33:1925–31.10.1111/jgh.14264Search in Google Scholar PubMed PubMed Central
36 Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology 2011;140:508–16.10.1053/j.gastro.2010.10.060Search in Google Scholar PubMed PubMed Central
37 Acalovschi M. Gallstones in patients with liver cirrhosis: incidence, etiology, clinical and therapeutical aspects. World J Gastroenterol 2014;20:7277–85.10.3748/wjg.v20.i23.7277Search in Google Scholar PubMed PubMed Central
38 Del Olmo JA, García F, Serra MA, Maldonado L, Rodrigo JM. Prevalence and incidence of gallstones in liver cirrhosis. Scand J Gastroenterol 1997;32:1061–5.10.3109/00365529709011225Search in Google Scholar PubMed
39 Cremer A, Arvanitakis M. Diagnosis and management of bile stone disease and its complications. Minerva Gastroenterol Dietol 2016;62:103– 29.Search in Google Scholar
40 Shen HJ, Hsu CT, Tung TH. Economic and medical benefits of ultrasound screenings for gallstone disease. World J Gastroenterol 2015;21:3337–43.10.3748/wjg.v21.i11.3337Search in Google Scholar PubMed PubMed Central
© 2023 Min Ding, Yue Yin, Xueying Wang, Menghua Zhu, Shixue Xu, Le Wang, Fangfang Yi, Cyriac Abby Philips, Fernando Gomes Romeiro, Xingshun Qi, published by De Gruyter on behalf of Scholar Media Publishing
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review Article
- Single photon emission computed tomography/computed tomography imaging of gouty arthritis: A new voice
- Original Article
- A bibliometric analysis of immune-related adverse events in cancer patients and a meta-analysis of immune-related adverse events in patients with hepatocellular carcinoma
- Nomogram for predicting risk of mild renal dysfunction among general residents from rural Northeast China
- Effects of metaraminol and norepinephrine on hemodynamics and kidney function in a miniature pig model of septic shock
- Analysis and prediction of research hotspots and trends in heart failure research
- Methylation of FAM110C is a synthetic lethal marker for ATR/CHK1 inhibitors in pancreatic cancer
- RN486, a Bruton's Tyrosine Kinase inhibitor, antagonizes multidrug resistance in ABCG2-overexpressing cancer cells
- Distribution of the causes of fever of unknown origin in China, 2013-2022
- Associations of gallbladder and gallstone parameters with clinical outcomes in patients with cirrhosis
- Letter to Editor
- Treatment strategies with combined agency against severe viral pneumonia in patients with advanced cancer
- Application of body area network wearable smart bracelet in epidemic isolation scenario
Articles in the same Issue
- Review Article
- Single photon emission computed tomography/computed tomography imaging of gouty arthritis: A new voice
- Original Article
- A bibliometric analysis of immune-related adverse events in cancer patients and a meta-analysis of immune-related adverse events in patients with hepatocellular carcinoma
- Nomogram for predicting risk of mild renal dysfunction among general residents from rural Northeast China
- Effects of metaraminol and norepinephrine on hemodynamics and kidney function in a miniature pig model of septic shock
- Analysis and prediction of research hotspots and trends in heart failure research
- Methylation of FAM110C is a synthetic lethal marker for ATR/CHK1 inhibitors in pancreatic cancer
- RN486, a Bruton's Tyrosine Kinase inhibitor, antagonizes multidrug resistance in ABCG2-overexpressing cancer cells
- Distribution of the causes of fever of unknown origin in China, 2013-2022
- Associations of gallbladder and gallstone parameters with clinical outcomes in patients with cirrhosis
- Letter to Editor
- Treatment strategies with combined agency against severe viral pneumonia in patients with advanced cancer
- Application of body area network wearable smart bracelet in epidemic isolation scenario


