The need for risk stratification in type 2 diabetes and chronic kidney disease: Proposed clinical value of KidneyIntelX
-
Marina Basina
, Michael Donovan
and David Lam
Abstract
Chronic kidney disease (CKD) develops in > 40% of people living with diabetes and affects > 7 million people in the United States. Of the 15 million individuals with type 2 diabetes and CKD in the United States, > 90% are in the “early stages of CKD” (stages G1–G3). Standard risk stratification tools for progression of kidney disease have limitations, and lack precision at an individual level. Individualized risk tools, such as KidneyIntelX™, that incorporate well-validated prognostic protein biomarkers integrated with key clinical variables and are integrated into the electronic health record (EHR) can help address these challenges. KidneyIntelX can identify patients earlier in their disease course when intervention would be most impactful. Herein, 4 case studies are presented to demonstrate how 3 different physicians utilized KidneyIntelX to make clinical decisions and optimize the management of patients with type 2 diabetes and CKD.
1. Introduction
Chronic kidney disease (CKD) is a worldwide epidemic affecting > 850 million individuals and > 38 million in the United States [1]. CKD has important implications for adverse outcomes, including cardiovascular events, end-stage kidney disease (ESKD), electrolyte and bone metabolism abnormalities, and decreased quality of life [2]. Over 600,000 Americans are on dialysis, and the prevalence of ESKD is projected to increase in the next decade [2].
Diabetic kidney disease (DKD), defined by diabetes with the presence of an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2, or an urinary albumin/creatinine ratio (UACR) of ≥ 30 mg/g, or both, develops in > 40% of people living with diabetes and affects > 7 million people in the United States [3]. The prevalence of DKD has increased by > 50% in the past few decades and DKD is now the single largest cause of ESKD accounting for 44% of ESKD patients with a predicted 5-year survival of 34% [3–6]. Annual costs for CKD by Medicare are approximately $114 billion, of which at least $40–50 billion are for patients with DKD [7–9]. Thus, CKD imposes a significant burden to healthcare and financial services in the US.
Of the 15 million individuals with DKD in the United States, > 90% are in the “early stages of CKD” (stages G1–G3). Prediction of risk for kidney disease progression in the early stages is often not assessed. The Kidney Failure Risk Equation (KRFE) is a tool used to predict risk of progression in CKD stages 3–5, where clinical variables are assigned standard weights for a recursive calculated score. However, the KRFE has not been validated in individuals with relatively preserved kidney function [10]. Moreover, while KFRE is an excellent predictor for kidney failure in those with advanced CKD at a population level, use for individual risk prediction reveals significant imprecision [11]. While the Kidney Disease: Improving Global Outcomes guidelines (KDIGO) incorporates eGFR and UACR to identify risk stratification through all stages of disease including early stages [12], the classification is limited by the physiologic variability of both eGFR and UACR [11, 13, 14] as well as confounding by hyperfiltration in early stages of DKD [14–16]. As a result, primary care physicians (PCPs) and other specialists are not able to appropriately risk stratify and counsel patients on the progressive nature of DKD [17–22].
Several plasma biomarkers have been investigated to aid in the prediction of kidney disease progression. Three of the most widely studied are soluble tumor necrosis factor receptors (sTNFR) 1 and 2 and plasma kidney injury molecule-1 (KIM-1) [23–28]. Although these markers have uniformly shown independent associations with kidney function decline, they have only recently been combined into a single assay with clinical data to predict progression of kidney disease in the KidneyIntelX assay [29, 30]. Analytically and clinically validated, this assay is approved for clinical use in the state of New York along with 49 other states.
Easily integrated and accurate prognostic models that combine clinical data from patients’ electronic health records (EHR) with blood-based biomarkers such as in the KidneyIntelX assay have not been available until recently. Machine learning tools can combine biomarkers and EHR data and identify those patients at high risk of progressive decline in kidney function. An individualized, targeted approach, such as what KidneyIntelX offers, has the potential to improve patient outcomes through prioritizing the use of cardiorenal protective medications (e.g., angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blockers (ARBs), sodium glucose co-transporter 2 inhibitors (SGLT-2i), non-steroidal mineralocorticoid antagonists) [31, 32] and efficient resource allocation at the PCP level. The goals of this study are the following: (1) to understand the need for novel risk stratification tools and care management solutions in DKD; (2) to describe the deployment of KidneyIntelX and multimodal approaches to DKD treatment at large health systems; and (3) to explore 3 case studies of real-life use of KidneyIntelX and how it influenced care management decisions.
2. Identification of Issues with Diagnosis and Management of Early Stage DKD
Renalytix, NYC, NY organized a multidisciplinary clinical advisory panel through an outreach program that prioritized physician practice specialties including 3 PCPs, 2 endocrinologists, 1 cardiologist, and 3 nephrologists on 28 October 2020, titled: Type 2 diabetes and chronic kidney disease: Clinical management, issues challenges and solutions. Topics discussed include clinical management, provider awareness, barriers to care, analytical risk assessment tools, and a population health approach used to assess and treat DKD patients. Highlights include appropriateness of evidence-based clinical guidelines and the use of care navigation to mitigate risk of progression for the patient with DKD. The panel further explored the challenges of identifying patients at high risk for disease progression in the early stages of disease, intervention utilizing assessment tools, use of emerging pharmacotherapies, and finally, the importance of early specialist referral. Several themes emerged as outlined in Table 1. For example, the PCP typically represents the initial point of contact for managing the majority of the DKD population, and presents an important opportunity for patients with diabetes to learn about CKD risk and management for onset and progression of the disease. However, evidence suggests that DKD is not optimally diagnosed or managed in patients with diabetes receiving routine treatment in the primary care setting [19, 33, 34]. Thus, the themes below represent the collective ideas of the advisory panel by specialty. If appropriately addressed, these concepts could potentially lead to improvement in patient care and outcomes with thoughtful implementation of a new bioprognostic test.
Issues identified with potential mitigation strategies by the clinical advisory panel on type 2 diabetes and CKD.
| Issue | Stakeholder | Potential approach to mitigate |
|---|---|---|
| Lack of patient awareness and education regarding kidney disease | PCPs and patients | Introduce CKD risk in those with diabetes at initial point of contact (PCP). Need more educational tools |
| Anxiety about disease once diagnosed | Patients | Knowledge of organ damage sometimes motivational to patients to improve health behaviors and med compliance. Tools that indicate CKD is low risk to progress would be helpful. |
| Lack of risk assessment tools | PCPs | Risk assessment score for CKD/DKD would be very useful, especially if integrated into EMR. |
| Efficiency needed for new bioprognostic tests | PCPs, endocrinologists, and nephrologists | Population health initiatives are the most efficient manner for implementation and optimization of resource allocation; with the caveat that some initiatives can seem overbearing with excessive protocolization |
| Late referral to nephrologists | PCPs and nephrologists | Referral of higher risk patients earlier to nephrology; retain lower risk in primary care exclusively to prevent patient overload in nephrology |
2.1 KidneyIntelX overview
KidneyIntelX is a first-in-class bioprognostic test that leverages a quantitative immunoassay combined with clinical data that uses an advanced machine learning algorithm to generate a patient-specific score for assessing the 5-year risk of progressive decline in kidney function in patients with existing DKD, as defined by stages G1–G3b (eGFR ≥ 60 mL/min/1.73m2 with UACR ≥ 30 mg/g [A2–A3] or eGFR 30–59mL/min/1.73m2 with any degree of albuminuria [A1–A3]). The use of algorithms that combine both biological markers of disease along with EHR data is a novel approach to kidney disease risk stratification. Multiple inputs that result in the generation of the KidneyIntelX risk score add value to traditional measures of eGFR and UACR (Figure 1).
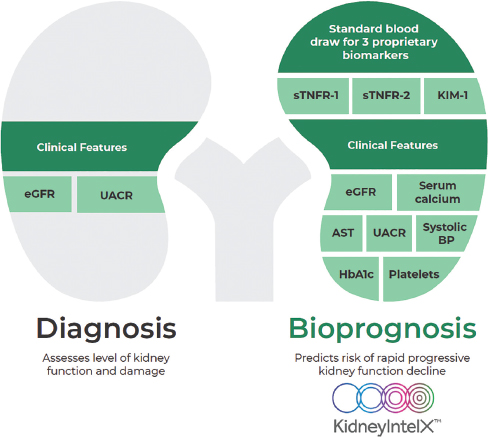
KidneyIntelX is a bioprognostic test for kidney outcomes in DKD. BP, blood pressure; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; sTNFR, soluble tumor necrosis factor receptors; UACR, urinary albumin/creatinine ratio.
2.2 KidneyIntelX robustly risk-stratifies patients with prevalent DKD stages 1–3
In a cohort study that utilized banked plasma samples linked to EHR data from the Mount Sinai BioMe Biobank and the UPenn Medicine Biobank, the random forest algorithm that KidneyIntelX uses was trained, tested, and validated to predict a composite kidney endpoint of rapid kidney function decline of ≥ 5 mL/min/1.73m2/year, a sustained 40% decline in eGFR, or kidney failure. KidneyIntelX stratified 46%, 37%, and 17% of the validation cohort into low-, intermediate-, and high-risk groups, respectively, with a PPV of 62% in the high-risk group and an NPV of 91% in the low-risk group for the composite kidney outcome [29]. In terms of time to composite event analysis, which included a sustained 40% decline in eGFR or kidney failure, patients scored high risk by KidneyIntelX had a hazard ratio of 14.7 (95% CI: 7.8–27.6) for progression than those that scored as low risk, and the hazard ratio for high vs. intermediate/low risk was 9.1 (95% CI: 5.8–14.4; Figure 2) [29].
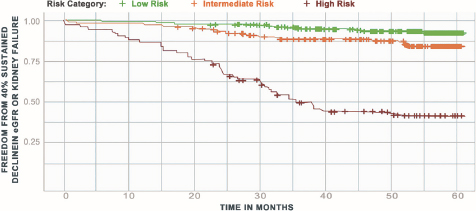
Time to kidney outcome by kidneyIntelX risk strata.
3. Integration of KidneyIntelX, Care Navigation and Real-World Evidence
The KidneyIntelX test was introduced into the Mount Sinai Health Care System in New York, NY in the fall of 2020 as part of a Real World Evidence (RWE) study (https://clinicaltrials.gov/ct2/show/NCT04802395) (Figure 3). The RWE study was approved by the Mount Sinai Program for the Protection of Human Subjects/Institutional Review Board (IRB# 21-00165). This initiative is part of a comprehensive, Renalytix-sponsored, multi-center program, which includes 2 additional prospective clinical utility studies, each designed to evaluate how the results of the KidneyIntelX impact on the clinical management of patients with DKD. The study design leverages IT-based electronic medical record (EMR) screening to proactively identify patients that meet the intended use criteria and therefore may benefit from the KidneyIntelX test. Eligible patients for testing must have documented type 2 diabetes based on ICD codes or notation in the patients “problem list,” as well as CKD stages 1–3 as defined by the last eGFR prior to testing in the range of 30 to 60 mL/min/1.73m2 or last UACR ≥ 30 mg/g if the last eGFR is ≥ 60 mL/min/1.73m2. If the last eGFR is ≥ 60 mL/min/1.73m2 and last UACR is ≤ 30 mg/g in last 2 years or is missing, then 2 historical eGFR values < 60 mL/min/1.73m2 at least 3 months apart also qualifies the patient for testing. Education is provided to the physicians on implementation and use of the test and, once education has been completed, physicians can execute a KidneyIntelX test directly in the EMR. Once initiated, an EDTA blood sample is sent to the Renalytix lab, where plasma is analyzed for biomarker levels using the multiplex assay. Biomarkers are then combined with clinical variables via the validated algorithm to determine the patient’s individual risk score for progression, and a KidneyIntelX report is returned to physicians electronically back into the EMR and to patients (via MyChart).
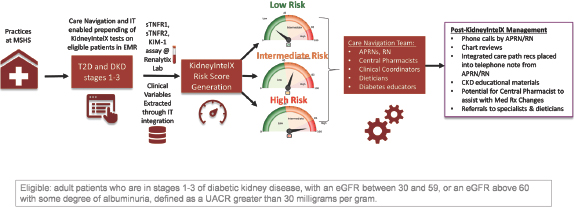
Design of real-world evidence study at Mount Sinai. CKD, chronic kidney disease; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; KIM-1, kidney injury molecule-1; UACR, urinary albumin/creatinine ratio; EMR, electronic medical record; T2D, Type 2 diabetes; MSHS, Mount Sinai Health System; APRNs, advanced practice registered nurses; RN, registered nurse.
Post-testing, the care process is supported by the Care Navigation team to facilitate physician to patient directives based on Mount Sinai Population Health recommended ambulatory care pathways, derived from professional society guidelines, based on the individual risk profile. These care pathways include blood pressure (BP) and diabetes control, lifestyle recommendations (diet and exercise), titration of ACEi/ARB to maximally tolerated doses, and initiation of SGLT2i. For patients with KidneyIntelX high and intermediate risk scores, engagement with population health pharmacists is encouraged to assist with medication management to ensure optimization of cardiorenal protective therapies (ACEi/ARBs, SGLT2i, non-steroidal mineralocorticoid receptor antagonists) to maximize kidney and cardiovascular protection. Finally, for the patients with a high-risk score, the Care Navigation team facilitates referrals to nephrologists, diabetologists, and dietitians, as clinically appropriate as determined by the ordering physician.
This unique interplay of Care Navigation personnel with physicians and patients was designed to reinforce clinical management recommendations and medication compliance with the ultimate goal of delaying and avoiding kidney disease progression. The RWE design allows the investigators to assess the real-life practice patterns and changes in care that occur with KidneyIntelX implementation at an integrated health system with less selection bias than traditional consented trials. Also, the RWE evidence design aligns with the U.S. FDA’s Center for Devices and Radiological Health (CDRH) mission to protect and promote public health by ensuring the safety and effectiveness of medical devices, while assuring patients have timely access to them, and encourages the use of RWE to support regulatory decision-making. The protocol allows for up to 10,000 patients to be studied as part of the Mount Sinai RWE program. The use of KidneyIntelX is encouraged and a recommended strategy for risk stratification for patients with DKD in both the diabetes and CKD-facing Mount Sinai guidelines (https://mshp.mountsinai.org/web/mshp/condition-management-hub).
4. Case Studies of Changes in Care in Response to KidneyIntelX Risk Scores
The following case studies illustrate real-world use of KidneyIntelX in patients with early stage DKD within physician specialties at Mount Sinai. The first three were selected patient cases that were presented at a National Kidney Foundation Renal Roundtable held on 30 March 2022.
4.1. Case 1
A 58-year-old man with a 7-year history of type 2 diabetes, hypertension, asthma, elevated body mass index (BMI), dyslipidemia, and CKD (eGFR 66 mL/min/1.73m2 and UACR 121 mg/g), was seen for routine follow-up by primary care, and referred for consultation with a nephrologist at MSHS.
HTN has been reasonably well controlled (BP 128/86 mmHg). Patient is adherent to medications but has had difficulty restricting his diet. Does not exercise regularly.
Labs: Na+ 140 meq/L, K+ 4.2 meq/L, Cl– 108 meq/L, BUN 23 mg/dL, serum creatinine 1.2 mg/dL, Ca2+ 8.6 mg/dL, phosphorus 3.6 mg/dL, hemoglobin 11.8 g/dL, platelets 427 × 103/μL, HbA1c 7.1%–7.5%, UACR 121 mg/g.
UA: no glucose, LE/nitrite, protein 100, no RBC/WBC.
Medications: Metformin 1000 mg PO BID, atorvastatin 40 mg PO daily, and albuterol MDI PRN.
KidneyIntelX score was ordered, resulted at 90 (high risk).
Nephrologist plan:
Continue with Losartan, Nifedipine, and Metformin.
Initiate the SGLT2i, Dapagliflozin, 5 mg PO daily for cardiorenal protection.
Develop plan to address difficulties in diet and lifestyle modification, referral to dietitian, and initiate discussion with patient on risk factors and long-term sequelae of CKD.
Insights from the treating nephrologist: Overall, this patient appeared well-managed based on lab results and BP readings, with no obvious signs for risk of CKD progression. The patient presented as unremarkable with stage G2A2 CKD, asthma, and slightly overweight but overall, well-managed with no parameters overtly uncontrolled. A KidneyIntelX high-risk score of 90 was surprising. If the patient had not been risk stratified, likely the provider would have continued current course of care without intervention to delay kidney disease progression. However, knowing the high-risk score allowed for more robust discussion between the provider and patient. The nephrologist learned the patient did not understand he had CKD, the cause, or the longterm consequences of sub-optimally controlled diabetes. Deficits in the patient’s knowledge were uncovered, and the need for further education was identified. Additionally, the SGLT2i, Dapagliflozin, was prescribed to reduce kidney and cardiovascular risk. Knowledge of the high-risk KidneyIntelX score provided an opportunity to deploy clinically indicated therapies in the earlier stages of disease, allowing for maximal benefit from cardiorenal medications to improve patient outcomes.
4.2. Case 2
A 52-year old male with a 11-year history of type 2 diabetes, hypertension, hyperlipidemia, obesity, NAFLD, and CKD (eGFR 75 mL/min/1.73m2 and UACR 17 mg/g) was seen by his diabetologist for diabetes follow-up in July 2021, having had an increase in HbA1C to 7.1% during the last follow-up in September 2020.
Labs: September 2020 — HbA1c 7.1%, serum creatinine 1.1 mg/dL (eGFR 74 mL/min/1.73m2), LDL 124 mg/dL, UACR 38 mg/g.
Medications: aspirin 81 mg daily, Atorvastatin 20 mg daily, Enalapril 40 mg daily, Levothyroxine 300 μg daily, Metformin 1000 mg twice daily.
In September 2020, patient was determined to re-institute lifestyle changes and Dulaglutide 0.75 mg weekly was started due to concurrent obesity.
In July 2021 — BP 114/73 mmHg, weight 134 kg (BMI 37 kg/m2), HbA1c 5.5%, serum creatinine 1.09 mg/dL (eGFR 75 mL/min/1.73m2), LDL 89 mg/dL, UACR 17 mg/g.
The diabetologist debated the best course of medical management considering the patient’s morbid obesity, long standing type 2 diabetes, and hypertension, all of which potentially position the patient for risk of kidney disease progression. Additionally, contemplating the nature of variability in UACR and following guidelines of once-a-year monitoring, the endocrinologist questioned if a SGLT2i should be initiated considering the elevated UACR from 2020.
KidneyIntelX score was ordered (simultaneous to July 2021 labs), resulted at 45 (low risk).
Diabetologist plan: Reassure patient of current regimen and continue treatment with GLP1RA, Metformin, ACEi, and routine monitoring and health maintenance.
Insights from the treating diabetologist: KidneyIntelX guided the diabetologist to appropriate therapies specific to the patient’s needs. Had the score resulted high, coupled with the patient’s risk factors and elevated UACR, the diabetologist would have been more likely to initiate a SGLT2i. However, the low-risk score provided the endocrinologist more confidence in the normal UACR and deemed a SGLT2i was not warranted at this time, providing cost savings and reduced pill burden to the patient. Additionally, the test score reassured the patient he was on a positive course in the trajectory of disease progression.
4.3. Case 3
A 69-year-old male with a 11-year history of type 2 diabetes, hypertension, depression, and CKD (eGFR 73 mL/min/1.73m2, UACR 61 mg/g). Former smoker, COPD, and high cholesterol, seen by PCP for routine follow-up.
BP 117/71 mmHg and BMI 28 kg/m2. Retired construction worker, drinks 6 standard alcoholic drinks 3–4 d/wk, quit smoking 10 years ago. Adherent to appointments and medications.
Labs: HbA1c 7.9% (previous 6.7%–6.8%), eGFR 73 mL/min/1.73m2, UACR 61 mg/g (40 mg/g 2 years ago), serum creatinine 1.0 mg/dL, total cholesterol 187 mg/dL, LDL 121 mg/dL.
Medications: Atorvastatin 20 mg daily (patient self-discontinued), Amlodipine 5 mg daily, Losartan 100 mg daily, Wellbutrin XL 150 mg daily, metformin 1000 mg BID, Sitagliptin 100 mg daily.
KidneyIntelX score was ordered, resulted at 90 (high risk).
Internist plan: Continue with ARB, discontinue Sitagliptin (DPP-4 inhibitor), initiate Empagliflozin, restart Atorvastatin, advise alcohol cessation, and referred to nutrition.
Initially, the patient did not present as high risk due to adherence to diabetes medications (metformin for 6 years and Sitagliptin past few years), BP and HbA1c historically controlled, and eGFR 73 mL/min/1.73m2. The KidneyIntelX score of 90 was surprising to the PCP. Now having a better understanding of the patient’s risk of DKD progression, the PCP changed her management approach. The patient had progressed on sitagliptin even though HbA1c had been historically well controlled with mild elevations of urine microalbumin. It is well-known that DPP-4 inhibitors do not reduce cardiovascular or kidney disease risk. Armed with this new information provided by the KidneyIntelX risk score, Sitagliptin was discontinued, and Empagliflozin, a SGLT2i with robust data on cardiorenal protection, was initiated.
Insights from the treating physician: KidneyIntelX has helped the PCP learn how to better treat her patients with DKD. In a busy primary care clinic treating chronic diseases where quality of care and measurements in patient outcomes are of top priority, the test can be a helpful tool rather than an intrusive part of patient care. KidneyIntelX helped the PCP focus on cardiorenal risk, rather than glycemic control, and resulted in the change from the DPP-4 inhibitor to an SGLT2i.
4.4. Case 4
A 69-year-old male with 15-year history of type 2 diabetes, diabetic retinopathy, heart failure with reduced ejection fraction (HFrEF), and CKD (eGFR 46 mL/min/1.73m2, UACR 1654 mg/g). Seen for follow-up visit with his nephrologist.
BP was 138/86 mmHg. HbA1c was 7.2%. His angiotensin receptor blocker was discontinued 5 months prior due to hyperkalemia (5.3 meq/L).
Medications: empagliflozin 10 mg daily, furosemide 40 mg twice daily, metoprolol, insulin glargine.
KidneyIntelX score was ordered, resulted at 100 (high risk).
Nephrologist plan: Given the high-risk KidneyIntelX score and patient HFrEF, strongly desired guideline-directed medical therapy (GDMT) for both conditions. Called patient’s cardiologist and discussed patient case and need to optimize his regimen. Jointly came up with plan to get serum potassium controlled with new K+ binders, and if comes down, start Sacubitril/Valsartan.
After his hyperkalemia was corrected into normal range over the new few weeks with the addition of sodium-zirconium cyclosilisate, he was then started on Sacubitril/Valsartan given his history of HFrEF and high-risk DKD.
Insights from treating physician: Even though this patient had high-risk CKD given diabetes and G3aA3 CKD, the high-risk KidneyIntelX test score pushed the physician to make a call to the patient’s cardiologist and come up with a plan to better mitigate his cardiorenal risk. The addition of sodium-zirconium cyclosilisate enabled treatment with 3 of the 4 pillars of GDMT for HFrEF (beta-blocker, SGLT2i, and angiotensin receptor–neprilysin inhibitor), of which the latter two agents are also protective against progression of CKD.
A side-by-side tabulated presentation of the above cases is provided below (Table 2).
Highlights from 4 cases.
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Clinician specialty | Nephrologist | Diabetologist | Internist | Nephrologist |
| Age (years) | 58 | 52 | 69 | 69 |
| eGFR (mL/min/1.73m2) | 66 | 75 | 73 | 46 |
| UACR (mg/g) | 121 | 17 | 61 | 1654 |
| BP (mmHg) | 128/86 | 114/73 | 117/71 | 138/86 |
| HbA1c (%) | 7.1–7.5 | 7.1 | 7.9 | 7.2 |
| Medications | Losartan, nifedipine, Metformin | Dulaglutide, Enalapril, Metformin | Amlodipine, Losartan, Metformin, Sitagliptin | Empagliflozin, Furosemide, Metoprolol, Insulin |
| KidneyIntelX score | 90 (high risk) | 45 (low risk) | 90 (high risk) | 100 (high risk) |
| Changes instituted | Initiate SGTL2i (dapagliflozin) | Reassure, continue with GLP-1 RA | Sitagliptin (DPP-4 inhibitor) discontinued; SGLT2i initiated | Sodium–zirconium cyclosilisate to enable correction of serum K and allow room for ARNI |
| Brief clinical reasoning | High-risk score needs SGLT2i to reduce CV risk | Don’t need to switch GLP-1 RA to SGLT2i since low risk | DPP-4 inhibitors control glycemia, but do not reduce cardiorenal risk, unlike SGLT2i. | Previous hyperkalemia had resulted in discontinuation of RAASi. Treatment with ARNI enables RAAS blockade and reduction in cardiorenal risk. |
5. Summary
Kidney disease is silent and affects millions of Americans. Estimates suggest that > 50% of American adults will develop CKD during their lifetime. The majority of care for CKD will fall on the PCP, as the current nephrology work force will lack capacity. Studies indicate that PCPs need targeted education of CKD risk factors. Determining which at-risk patients will progress to more severe disease quickly and unexpectedly is an even greater challenge in the primary care setting due to high clinical burden and the inadequacy of traditional clinical prognostic tools. Individualized risk tools, such as KidneyIntelX, that incorporate well-validated prognostic protein biomarkers integrated with key clinical variables (i.e., bioprognosticsTM) and are integrated into the EMR address these challenges. KidneyIntelX can identify patients earlier in their disease course when intervention would be most impactful. The 4 case studies presented herein demonstrate how 3 different physicians utilized the KidneyIntelX to make clinical decisions and optimize the management of patients with type 2 diabetes and CKD. RWE and clinical utility studies supporting personalized risk assessment tools, such as the KidneyIntelX test, are underway at large health systems. More evidence generation on the wider impact of KidneyIntelX implementation in health systems will be forthcoming.
Funding statement: Source of Funding Nil.
Ethics Approval and Consent to Participate: The RWE study described within was approved by the Mount Sinai Program for the Protection of Human Subjects/Institutional Review Board (IRB# 21-00165).
Conflict of Interest: G. N. Nadkarni has received fees for an advisory board role in Renalytix and owns equity in Renalytix. He has received operational funding from Goldfinch Bio and consulting fees from BioVie Inc., AstraZeneca, Reata, and GLG consulting in the past 3 years. He is supported by a career development award from the National Institutes of Health (NIH; K23DK107908) and is also supported by the following NIH grants: R01DK108803, U01HG007278, U01HG009610, and U01DK116100. S. G. Coca has received fees for advisory boards or steering committee roles for Renalytix, Nuwellis, Bayer, Boehringer Ingelheim, 3ive, Reprieve Cardiovascular, Axon Therapies, and Takeda in the past 3 years. He owns equity in Renalytix; receives salary and research support from Renalytix, ProKidney, XORTX, and the Renal Research Institute; and receives salary and research support from the following grants from the NIH: U01DK106962, R01DK115562, R01HL85757, R01DK112258, U01OH011326, and R01DK126477.
REFERENCES
[1] United States Renal Data System. 2019 USRDS Annual Data Report: epidemiology of kidney disease in the United States. 2019. Available at https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds. Accessed July 19, 2022.Search in Google Scholar
[2] United States Renal Data System. 2020 USRDS Annual Data Report: epidemiology of kidney disease in the United States. 2020. Available at https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds. Accessed July 19, 2022.Search in Google Scholar
[3] de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–9.10.1001/jama.2011.861Search in Google Scholar PubMed PubMed Central
[4] United States Renal Data System. 2018 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. 2018. Available at https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds. Accessed July 19, 2022.Search in Google Scholar
[5] Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.10.3322/caac.20073Search in Google Scholar PubMed
[6] Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31–40.10.1016/S0140-6736(11)60679-XSearch in Google Scholar PubMed
[7] Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US renal data system 2013 annual data report. Am J Kidney Dis 2014; 63: A7.10.1053/j.ajkd.2013.11.001Search in Google Scholar PubMed
[8] Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am 2013; 97: 1–8.10.1016/j.mcna.2012.10.001Search in Google Scholar PubMed
[9] Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: A report from an ADA consensus conference. Am J Kidney Dis 2014; 64: 510–33.10.1053/j.ajkd.2014.08.001Search in Google Scholar PubMed
[10] Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305: 1553–9.10.1001/jama.2011.451Search in Google Scholar PubMed
[11] Major RW, Cockwell P, Nitsch D, Tangri N. The next step in chronic kidney disease staging: individualized risk prediction. Kidney Int 2022; 102: 456–9.10.1016/j.kint.2022.06.012Search in Google Scholar PubMed
[12] Kidney Disease: Improving Global Outcomes (DDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98: S1–15.10.1016/j.kint.2020.06.019Search in Google Scholar PubMed
[13] Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 2018; 72: 538–46.10.1053/j.ajkd.2018.04.023Search in Google Scholar PubMed PubMed Central
[14] Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, et al. Trajectories of kidney function in diabetes: a clinico-pathological update. Nat Rev Nephrol 2021; 17: 740–50.10.1038/s41581-021-00462-ySearch in Google Scholar PubMed
[15] Quinn GZ, Abedini A, Liu H, Ma Z, Cucchiara A, Havasi A, et al. Renal histologic analysis provides complementary information to kidney function measurement for patients with early diabetic or hypertensive disease. J Am Soc Nephrol 2021; 32: 2863–76.10.1681/ASN.2021010044Search in Google Scholar PubMed PubMed Central
[16] Yang Y, Xu G. Update on pathogenesis of glomerular hyperfiltration in early diabetic kidney disease. Front Endocrinol (Lausanne) 2022; 13: 872918.10.3389/fendo.2022.872918Search in Google Scholar PubMed PubMed Central
[17] Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol 2009; 4: 323–8.10.2215/CJN.03510708Search in Google Scholar PubMed PubMed Central
[18] Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis 2006; 48: 192–204.10.1053/j.ajkd.2006.04.073Search in Google Scholar PubMed
[19] Duggal V, Montez-Rath ME, Thomas IC, Goldstein MK, Tamura MK. Nephrology referral based on laboratory values, kidney failure risk, or both: a study using veterans affairs health system data. Am J Kidney Dis 2022; 79: 347–53.10.1053/j.ajkd.2021.06.028Search in Google Scholar PubMed PubMed Central
[20] Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis 2006; 47: 72–7.10.1053/j.ajkd.2005.09.027Search in Google Scholar PubMed
[21] Shahinian VB, Saran R. The role of primary care in the management of the chronic kidney disease population. Adv Chronic Kidney Dis 2010; 17: 246–53.10.1053/j.ackd.2010.02.003Search in Google Scholar PubMed
[22] Datar M, Ramakrishnan S, Montgomery E, Coca SG, Vassalotti JA, Goss T. A qualitative study documenting unmet needs in the management of diabetic kidney disease (DKD) in the primary care setting. BMC Public Health 2021; 21: 930.10.1186/s12889-021-10959-7Search in Google Scholar PubMed PubMed Central
[23] Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 2017; 28: 2786–93.10.1681/ASN.2016101101Search in Google Scholar PubMed PubMed Central
[24] Gutiérrez OM, Shlipak MG, Katz R, Waikar SS, Greenberg JH, Schrauben SJ, et al. Associations of plasma biomarkers of inflammation, fibrosis, and kidney tubular injury with progression of diabetic kidney disease: a cohort study. Am J Kidney Dis 2022; 79: 849–57. e1.10.1053/j.ajkd.2021.09.018Search in Google Scholar PubMed PubMed Central
[25] Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 2012; 23: 507–15.10.1681/ASN.2011060627Search in Google Scholar PubMed PubMed Central
[26] Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019; 25: 805–13.10.1038/s41591-019-0415-5Search in Google Scholar PubMed PubMed Central
[27] Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol 2021; 32: 115–26.10.1681/ASN.2020040487Search in Google Scholar PubMed PubMed Central
[28] Sen T, Li J, Neuen BL, Neal B, Arnott C, Parikh CR, et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 2021; 64: 2147–58.10.1007/s00125-021-05512-5Search in Google Scholar PubMed PubMed Central
[29] Chan L, Nadkarni GN, Fleming F, McCullough JR, Connolly P, Mosoyan G, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia 2021; 64: 1504–15.10.1007/s00125-021-05444-0Search in Google Scholar PubMed PubMed Central
[30] Lam D, Nadkarni GN, Mosoyan G, Neal B, Mahaffey KW, Rosenthal N, et al. Clinical utility of KidneyIntelX in early stages of diabetic kidney disease in the CANVAS Trial. Am J Nephrol 2022; 53: 21–31.10.1159/000519920Search in Google Scholar PubMed
[31] Cohen S, Sternlicht H, Bakris GL. Mineralocorticoid receptor antagonists in the treatment of diabetic kidney disease: their application in the era of SGLT2 inhibitors and GLP-1 receptor agonists. Curr Diab Rep 2022; 22: 213–8.10.1007/s11892-022-01461-4Search in Google Scholar PubMed
[32] Mima A. A narrative review of diabetic kidney disease: previous and current evidence-based therapeutic approaches. Adv Ther 2022; 39: 3488–500.10.1007/s12325-022-02223-0Search in Google Scholar PubMed
[33] Tummalapalli SL, Powe NR, Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol 2019; 14: 1142–50.10.2215/CJN.00060119Search in Google Scholar PubMed PubMed Central
[34] Tuttle KR, Alicic RZ, Duru OK, Jones CR, Daratha KB, Nicholas SB, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open 2019; 2: e1918169.10.1001/jamanetworkopen.2019.18169Search in Google Scholar PubMed PubMed Central
© 2023 Marina Basina et al., published by Sciendo
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

