Synthesis and investigation of 3,5-bis-linear and macrocyclic tripeptidopyridine candidates by using l-valine, N,N′-(3,5-pyridinediyldicarbonyl)bis-dimethyl ester as synthon
-
Abd El-Galil E. Amr
, Ahmed M. Naglah
Abstract
Interest in the synthesis of heterocyclic organic molecules with peptide moieties has gained attention due to their potential biological activities. The current work aimed at synthesizing new macrocyclic tripeptide imides and evaluating their possible antimicrobial activities. A series of 11 derivatives were prepared from dimethyl 3,5-pyridinevalinyl ester either by NaOH or NH2NH2 treatment, followed by cyclization and further reaction with NaOH or NH2NH2. The majority of synthesized derivatives showed promising antibacterial and antifungal activities in comparison to standard known antibiotics. Compounds 5a and 7b showed the most potential antibacterial against Staphylococcus aureus and antifungal activities against Candida albicans, respectively.
1 Introduction
The carboxamide group is a very interesting residue used in the synthesis of organic and bioorganic compounds [1], [2], [3], [4], [5] and synthetic drugs [6], [7], [8], [9]. In previous work, new heterocyclic compounds substituted with amide moieties were synthesized [10] and shown to have antimycobacterial [11], [12], anti-inflammatory [13], antioxidant [14] activities and transketolase inhibitors [15]. In addition, macrocyclic azacrowns and their derivatives are promising building blocks in the field of supramolecular chemistry [16], [17], [18]. In our previous work, we have studied the synthesis of some heterocyclic compounds [16], [17], [18], [19], [20] and reported their properties as antihypertensive α-blocking [19], anti-inflammatory [20], HIV-1 and HSV-1 [21] agents and reductase-2-inhibitors for chemoprevention of cancer [22] as well as 5α-reductase inhibitors [23]. Recently, compounds incorporating pyridine heterocycles and peptide moieties have been prepared [24], [25], [26] and used as antibacterial agents [27], [28], [29], [30], [31]. In this study, we report the synthesis of some new linear and macrocyclic imides and tested them as antimicrobial agents.
2 Results and discussion
A series of macrocyclic tripeptides 5a–c, 6a, b, 7a, b and 8a, b were synthesized using 2,2′-[(pyridine-3,5-dicarbonyl)bis(azanediyl)]bis(3-methylbutanoic acid) (3) and N3,N5-bis(1-hydrazinyl-3-methyl-1-oxobutan-2-yl)pyridine-3,5-dicarboxamide (4) as starting materials, which were prepared from dimethyl 2,2′-[(pyridine-3,5-dicarbonyl)bis(azanediyl)]bis(3-methylbutanoate) (2) [32], [33] according to a reported procedure [24], [25]. The reaction of dipeptides 3 and 4 with diamino alkanes or dibasic aminoacid by different methods afforded cyclic derivatives 5a–c and 6a, b, respectively. The cyclic methyl esters 6a, b were hydrolyzed (with NaOH) or hydrazinolyzed (with NH2NH2) to give cyclic tripeptide acids 7a, b and cyclic tripeptide hydrazides 8a, b, respectively (Scheme 1).
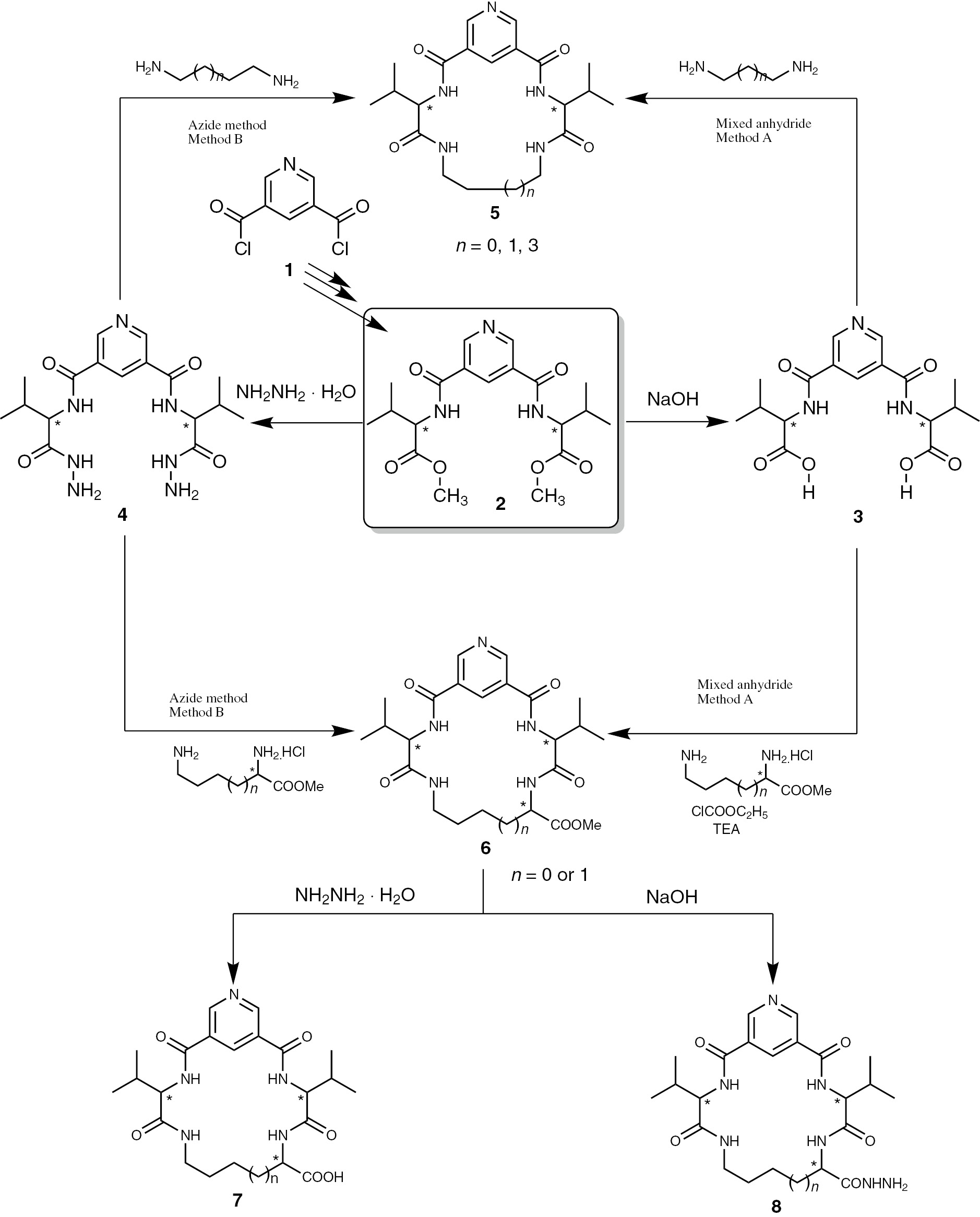
Synthetic routes for compounds 3, 4, 5a–c, 6a, b, 7a, b and 8a, b.
2.1 Antimicrobial testing
Biological activity screening of the new compounds 2, 3, 4, 5a–c, 6a, b, 7a, b and 8a, b has been performed at 50 μg mL−1 against Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis), Gram-negative bacteria (Escherichia coli) and fungi (Candida albicans), using the bioassay technique for antibiotics [34] specified in the US Pharmacopeia. Schiff’s bases have significant antimicrobial activities as compared with streptomycin and fusidic acid as antibacterial and antifungal reference drugs, respectively. The results are summarized in Table 1.
Antimicrobial activities of the new synthesized compounds 2, 3, 4, 5a–c, 6a, b, 7a, b and 8a, b.
| Comp. no. | Inhibition zones (cm) | |||
|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungi | ||
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Candida albicans | |
| 2 | 19 | 20 | 21 | 17 |
| 3 | 20 | 22 | 20 | 16 |
| 4 | 20 | 21 | 20 | 17 |
| 5a | 14 | 16 | 19 | 14 |
| 5b | 20 | 21 | 15 | 16 |
| 5c | 21 | 16 | 19 | 16 |
| 6a | 17 | 19 | 18 | 18 |
| 6b | 18 | 20 | 20 | 17 |
| 7a | 17 | 18 | 21 | 16 |
| 7b | 20 | 15 | 19 | 12 |
| 8a | 19 | 18 | 20 | 14 |
| 8b | 18 | 20 | 22 | 17 |
| Streptomycin | 21 | 22 | 22 | – |
| Fusidic acid | – | – | – | 18 |
3 Experimental section
3.1 Reagents
3,5-Pyridinedicarbonylchloride was prepared and elucidated according to literature procedures [32]. l-valine, ethanol, methanol, dichloromethane, hydrazine hydrate, 1,3-diaminopropane, 1,3-diaminobutane, 1,3-diaminohexane, ethyl chloroformate, l-lysine and sodium hydroxide were all purchased from Sigma-Aldrich (Switzerland). Melting points are uncorrected and were determined in Electro Thermal Digital melting point apparatus IA9100. Elemental microanalyses were done in National Research Centre, Cairo, Egypt, and found within the acceptable limits of the calculated values (±0.1%). IR (KBr) spectra were recorded on a Nexus 670 FTIR Fourier transform infrared spectrometer. 1H NMR and 13C NMR spectra were recorded in [D6]DMSO on a JEOL 500 MHz instrument (Tokyo, Japan). Mass spectra were run on a MAT Finnigan SSQ 7000 spectrometer (Model: QP2010 ultra; Shimadzu, Kyoto, Japan), using the electron impact technique (EI).
3.2 Syntheses
3.2.1 2,2′-[(Pyridine-3,5-dicarbonyl)bis(azanediyl)]bis(3-methylbutanoic acid) (3)
To a suspension of bis-ester 2 (1 mmol, T=−5°C) in ethanol (10 mL), NaOH (1 n, 25 mL) was added with stirring for 2 h and then for 12 h at room temperature. The mixture was acidified with 1 n HCl to pH≈3; the formed solid was filtered off, washed with water and crystallized from methanol to afford the acid derivative 3 in 80% yield; m.p. 182–184°C. –
3.2.2 N3,N5-bis(1-hydrazinyl-3-methyl-1-oxobutan-2-yl)pyridine-3,5-dicarboxamide (4)
To a solution of bis-ester 2 (1 mmol) in methanol (25 mL), hydrazine hydrate (0.8 mL, 16 mmol) was added, which was refluxed for 10 h and evaporated to dryness; the obtained residue was solidified with ether, filtered off and crystallized from ethanol to afford hydrazide 4 in 82% yield; m.p. 245–247°C. –
3.2.3 Cyclo-(Nα-dinicotinoyl)-bis[l-valinyl tetraaza] derivatives 5a–c and 6a, b
3.2.3.1 Mixed anhydride [A]
A mixture of diacid 3 (1 mmol) and ethyl chloroformate (22g, 2 mmol) in cold CH2Cl2 (25 mL, T=−20°C) was stirred at −20°C for 20 min in the presence of triethylamine (0.2g, 2 mmol); then propane-1,3-diamine, butane-1,4-diamine or hexane-1,6-diamine (1 mmol) in CH2Cl2 (5 mL) was added at −20°C with stirring for 6 h and then overnight at room temperature. The mixture was washed with H2O, 1 n HCl, 1 n NaHCO3 and H2O and then dried over anhydrous CaCl2. The solvent was evaporated to dryness, and the residue was crystallized from methanol-ether to give derivatives 5a–c, respectively.
3.2.3.2 Azide method [B]
The experimental method which was used in the preparation of 5a–c has been adopted from Azab and Amr [27].
3.2.4 4,12-Diisopropyl-3,6,10,13-tetraaza-1(3,5)-pyridinacyclotetradecaphane-2,5,11,14-tetraone (5a)
Yield 62%; m.p. 208–210°C. –
3.2.5 4,13-Diisopropyl-3,6,11,14-tetraaza-1(3,5)-pyridinacyclopentadecaphane-2,5,12,15-tetraone (5b)
Yield 65%; m.p. 216–218°C. –
3.2.6 4,15-Diisopropyl-3,6,13,16-tetraaza-1(3,5)-pyridinacycloheptadecaphane-2,5,14,17-tetraone (5c)
Yield 60%; m.p. 198–200°C. –
3.2.7 Synthesis of cyclo-(Nα-dinicotinoyl)-bis-[(l-valinyl)-l-dibasic amino acid methyl ester] (6a, b)
3.2.7.1 Mixed anhydride [A]
The same procedure as used for the synthesis of compounds 5a–c using l-ornithine methyl ester or l-lysine methyl ester (as dibasic amino acids) (1 mmol) and compound 4 (1 mmol) is followed.
3.2.7.2 Azide method [B]
The same procedure as used for the synthesis of compounds 5a–c by using l-ornithine methyl ester or l-lysine methyl ester (as dibasic amino acids) (1 mmol) and compound 4 (1 mmol) is followed.
3.2.8 Methyl 4,13-diisopropyl-2,5,12,15-tetraoxo-3,6,11, 14-tetraaza-1(3,5)-pyridinacyclopentadecaphane-7-carboxylate (6a)
Yield 60% [A], 55% [B]; m.p. 186–188°C (EtOH-n-hexane). –
3.2.9 Methyl 4,14-diisopropyl-2,5,13,16-tetraoxo-3,6,12,15-tetraaza-1(3,5)-pyridinacyclohexadecaphane-7-carboxylate (6b)
Yield 70% [A], 50% [B]; m.p. 172–174°C (EtOH-n-hexane). –
3.2.10 Cyclo (Nα-dinicotinoyl)-bis-[(l-valinyl)-l-dibasic amino carboxylic acid] (7a, b)
A cold (T=−15°C) mixture of 6a, b (1 mmol) in methanol (10 mL) and sodium hydroxide (1 n, 25 mL) was stirred at −5°C for 2 h and then at room temperature for 12 h. The reaction was acidified with 1 n HCl to pH ≈3. The obtained solid was filtered off, washed with water, dried and recrystallized from ethanol to afford the title acid derivatives 7a, b.
3.2.11 4,13-Diisopropyl-2,5,12,15-tetraoxo-3,6,11, 14-tetraaza-1(3,5)-pyridinacyclopentadecaphane- 7-carboxylic acid (7a)
Yield 72%; m.p. 202–204°C. –
3.2.12 4,14-Diisopropyl-2,5,13,16-tetraoxo-3,6,12, 15-tetraaza-1(3,5)-pyridinacyclohexadecaphane- 7-carboxylic acid (7b)
Yield 70%; m.p. 210–112°C. –
3.2.13 Synthesis of cyclo (Nα-dinicotinoyl)-bis-[(l-valinyl)-l-dibasic amino acid hydrazide] (8a, b)
A mixture of 6a, b (1 mmol) and hydrazine hydrate (0.8 mL, 16 mmol) in methanol (25 mL) was refluxed for 6 h and then evaporated under reduced pressure; the obtained residue was washed with ether several times, filtered off and recrystallized from dioxane-water to afford the corresponding cyclo 3,5-bis hydrazide derivatives 8a, b.
3.2.14 4,13-Diisopropyl-2,5,12,15-tetraoxo-3,6,11, 14-tetraaza-1(3,5)-pyridinacyclopentadecaphane-7-carbohydrazide (8a)
Yield 70%; m.p. 246–248°C. –
3.2.15 4,14-Diisopropyl-2,5,13,16-tetraoxo-3,6,12, 15-tetraaza-1(3,5)-pyridinacyclohexadecaphane-7-carbohydrazide (8b)
Yield 60%; m.p. 235–237°C. –
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this work through research group project “RGP-1435-047.”
References
[1] V. R. Pattabiraman, J. W. Bode, Nature2011, 480, 471.10.1038/nature10702Search in Google Scholar
[2] S. Mahesh, K. Tang, M. Raj, Molecules2018, 23, 2615.10.3390/molecules23102615Search in Google Scholar
[3] R. M. Figueiredo, J. Suppo, J. Campagne, Chem. Rev.2016, 116, 12029.10.1021/acs.chemrev.6b00237Search in Google Scholar
[4] P. Acosta-Guzmán, A. Mateus-Gómez, D. Gamba-Sánchez, Molecules2018, 23, 2382.10.3390/molecules23092382Search in Google Scholar
[5] E. Valeur, M. Bradley, Chem. Soc. Rev.2009, 38, 606.10.1039/B701677HSearch in Google Scholar
[6] A. Greenberg, C. M. Breneman, J. F. Liebman, The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science, Wiley-VCH, New York, NY, 2003.Search in Google Scholar
[7] L. Pauling, R. B. Corey, H. R. Branson, Proc. Natl. Acad. Sci. USA1951, 37, 205.10.1073/pnas.37.4.205Search in Google Scholar
[8] A. B. Hughes (Ed.), Amino Acids, Peptides and Proteins in Organic Chemistry, Wiley-VCH, Weinheim, 2011.10.1002/9783527631841Search in Google Scholar
[9] A. A. Kaspar, J. M. Reichert, Drug Discov. Today2013, 18, 807.10.1016/j.drudis.2013.05.011Search in Google Scholar
[10] I. Shiina, Y. Kawakita, Tetrahedron Lett.2003, 44, 1951.10.1016/S0040-4039(03)00063-7Search in Google Scholar
[11] J. Zitko, A. Mindlová, O. Valášek, O. Jand’ourek, P. Paterová, J. Janoušek, K. Konečná, M. Doležal, Molecules2018, 23, 2390.10.3390/molecules23092390Search in Google Scholar PubMed PubMed Central
[12] A. Lavanya, R. Sribalan, V. Padmini, J. Saudi Chem. Soc.2017, 21, 277.10.1016/j.jscs.2015.06.008Search in Google Scholar
[13] N. Ye, P. Hu, S. Xu, M. Chen, S. Wang, J. Hong, T. Chen, T. Cai, J. Food Qual.2018, 2018, 8579094.10.1155/2018/8579094Search in Google Scholar
[14] J. Huo, B. Zhao, Z. Zhang, J. Xing, J. Zhang, J. Dong, Z. Fan, Molecules2018, 23, 2116.10.3390/molecules23092116Search in Google Scholar PubMed PubMed Central
[15] K. E. Krakowiak, J. S. Bradshaw, J. Zamecka-Krakowiak, Chem. Rev.1989, 89, 929.10.1021/cr00094a008Search in Google Scholar
[16] R. M. Izatt, K. Pawlak, J. S. Bradshaw, R. L. Bruening, B. J. Tarbet, Chem. Rev. 1992, 92, 1261.10.1021/cr00014a005Search in Google Scholar
[17] A. H. M. Elwahy, J. Heterocycl. Chem. 2003, 40, 1.10.1002/jhet.5570400101Search in Google Scholar
[18] B. F. Abdel Wahab, S. F. Mohamed, A. E. Amr, M. M. Abdalla, Monatsh. Chem. 2008, 139, 1083.10.1007/s00706-008-0896-2Search in Google Scholar
[19] A. E. Amr, M. H. Abo-Ghalia, M. M. Abdalah, Arch. Pharm.2007, 340, 304.10.1002/ardp.200600187Search in Google Scholar PubMed
[20] N. M. Khalifa, M. A. Al-Omar, A. E. Amr, M. E. Haiba, Int. J. Biol. Macromol. 2013, 54, 51.10.1016/j.ijbiomac.2012.11.015Search in Google Scholar PubMed
[21] A. E. Amr, N. A. Abdel-Latif, M. M. Abdalla, Acta Pharm. 2006, 56, 203.Search in Google Scholar
[22] M. M. Abdalla, M. A. Al-Omar, M. A. Bhat, A. E. Amr, A. M. Al-Mohizea, Int. J. Biol. Macromol.2012, 50, 1127.10.1016/j.ijbiomac.2012.02.006Search in Google Scholar PubMed
[23] A. E. Amr, O. I. Abd El-Salam, M. A. Al-Omar, Russ. J. Gen. Chem.2015, 85, 1161.10.1134/S1070363215050278Search in Google Scholar
[24] A. A. Ibrahim, A. M. Mohamed, A. E. Amr, M. A. Al-Omar, Russ. J. Gen. Chem.2015, 85, 1506.10.1134/S1070363215060250Search in Google Scholar
[25] A. M. Naglah, A. E. Amr, N. M. Khalifa, M. A. Al-Omar, Russ. J. Gen. Chem.2015, 85, 2833.10.1134/S1070363215120324Search in Google Scholar
[26] M. E. Azab, A. E. Amr, Russ. J. Gen. Chem.2015, 85, 1513.10.1134/S1070363215050262Search in Google Scholar
[27] M. E. Azab, A. E. Amr, M. A. Al-Omar, Russ. J. Gen. Chem.2015, 85, 1952.10.1134/S1070363215080253Search in Google Scholar
[28] N. M. Khalifa, A. E. Amr, M. A. Al-Omar, E. S. Nossier, Russ. J. Gen. Chem.2016, 86, 2785.10.1134/S1070363216120409Search in Google Scholar
[29] M. E. Azab, E. M. Flefel, N. M. Sabry, A. E. Amr, Z. Naturforsch.2016, 71, 803.10.1515/znb-2016-0018Search in Google Scholar
[30] A. E. Amr, M. A. Al-Omar, Russ. J. Gen. Chem.2016, 86, 161.10.1134/S1070363216010254Search in Google Scholar
[31] A. E. Amr, M. H. Abo-Ghalia, G. O. Moustafa, M. A. Al-Omar, E. Nossir, E. A. Elsayed, Molecules, 2018, 23, 2416.10.3390/molecules23102416Search in Google Scholar PubMed PubMed Central
[32] A. G. Talma, J. Patrick, J. G. De Vries, C. B. Troostwijk, G. H. W. Buning, J. K. Waninge, J. Visscher, R. M. Kellogg, J. Am. Chem. Soc.1985, 107, 3981.10.1021/ja00299a038Search in Google Scholar
[33] J. C. Speelman, A. G. Talma, R. M. Kellogg, A. Meetsma, J. L. De Boer, P. T. Beurskens, W. P. Bosman, J. Org. Chem.1989, 54, 1055.10.1021/jo00266a012Search in Google Scholar
[34] A. A. Abou-Zeid, Y. M. Shehata, Indian J. Pharm. 1969, 31, 72.Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- The bio-relevant metals of the periodic table of the elements
- Research Articles
- Synthesis and investigation of 3,5-bis-linear and macrocyclic tripeptidopyridine candidates by using l-valine, N,N′-(3,5-pyridinediyldicarbonyl)bis-dimethyl ester as synthon
- Supramolecular assembly of two copper(II) coordination compounds of topiroxostat with dialkylformamide ligands
- Ferromagnetic interactions in a dicyanamide-bridged multinuclear metal-organic framework [Pr3NH]+ [Mn(dca)3]−·H2O
- Synthesis, structure, and properties of nickel(II) and copper(II) coordination polymers with the 5-ethyl-pyridine-2,3-dicarboxylate ligand
- Die monoklinen Seltenerdmetall(III)-Chlorid-Oxidoarsenate(III) mit der Zusammensetzung SE5Cl3[AsO3]4 (SE=La–Nd, Sm)
- Facile synthesis of model 2,4-diaryl-1,3,4-thiadiazino[5,6-h]fluoroquinolones
- The rare earth metal hydride tellurides REHTe (RE=Y, La–Nd, Gd–Er)
- Osmium and magnesium: structural segregation in the rare earth-rich intermetallics RE4OsMg (RE=La–Nd, Sm) and RE9TMg4 (RE=Gd, Tb)
- Note
- A heterometallic Cd/Ag/Se complex incorporating 3-ferrocenyl-5-(2-pyridyl)pyrazolato (fcpp) ligands: synthesis and structure of [Cd2{Ag(SePh)}2(μ3-OH2)2(μ2,η3-fcpp)4]·2C3H6O
- Book Review
- Essential Metals in Medicine. Volume 19 of the Metal Ions in Life Sciences series
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- The bio-relevant metals of the periodic table of the elements
- Research Articles
- Synthesis and investigation of 3,5-bis-linear and macrocyclic tripeptidopyridine candidates by using l-valine, N,N′-(3,5-pyridinediyldicarbonyl)bis-dimethyl ester as synthon
- Supramolecular assembly of two copper(II) coordination compounds of topiroxostat with dialkylformamide ligands
- Ferromagnetic interactions in a dicyanamide-bridged multinuclear metal-organic framework [Pr3NH]+ [Mn(dca)3]−·H2O
- Synthesis, structure, and properties of nickel(II) and copper(II) coordination polymers with the 5-ethyl-pyridine-2,3-dicarboxylate ligand
- Die monoklinen Seltenerdmetall(III)-Chlorid-Oxidoarsenate(III) mit der Zusammensetzung SE5Cl3[AsO3]4 (SE=La–Nd, Sm)
- Facile synthesis of model 2,4-diaryl-1,3,4-thiadiazino[5,6-h]fluoroquinolones
- The rare earth metal hydride tellurides REHTe (RE=Y, La–Nd, Gd–Er)
- Osmium and magnesium: structural segregation in the rare earth-rich intermetallics RE4OsMg (RE=La–Nd, Sm) and RE9TMg4 (RE=Gd, Tb)
- Note
- A heterometallic Cd/Ag/Se complex incorporating 3-ferrocenyl-5-(2-pyridyl)pyrazolato (fcpp) ligands: synthesis and structure of [Cd2{Ag(SePh)}2(μ3-OH2)2(μ2,η3-fcpp)4]·2C3H6O
- Book Review
- Essential Metals in Medicine. Volume 19 of the Metal Ions in Life Sciences series

