One-pot hydrothermal synthesis of H3PW12O40 supported on zeolite imidazolate frameworks (ZIF-8): a highly efficient heterogeneous catalyst for oxidation of sulfides to sulfoxides and sulfones
Abstract
The microporous, sodalite-like zeolite imidazolate framework ZIF-8 [Zn(MeIM); MeIM = 2-methylimidazole] has been selected as a host matrix for stabilization of guest H3PW12O40 (PW12) in this study. To modify ZIF-8 by H3PW12O40 (PW12), a one-pot hydrothermal synthesis strategy has been developed and used in the chemoselective oxidation of sulfides to the corresponding sulfoxides (and sulfones) in the presence of 30 % H2O2 in ethanol (and CH3CN) as solvent. Some of the advantages of our method include excellent yields, heterogeneous conditions, simplicity, compatibility with a variety of functionalities, and ease of isolation of the products. X-ray diffraction, scanning electron microscopy, energy-dispersive analysis X-ray spectroscopy, ultraviolet/visible rays, and Brunauer–Emmett–Teller have been used to characterize the samples prepared. Large-sized pores and cavities of ZIF-8 become occupied by PW12 units on modification with PW12, as shown by analysis results. This method might bring light to new opportunities in the development of high-performance polyoxometalate catalysts using rapidly growing ZIFs as supports.
1 Introduction
Rapidly emerging as an important family of crystalline materials for application as catalysts in organic reactions, metal organic frameworks (MOFs) consist of metal ions or metal ion clusters occupying nodal framework positions coordinated with di- or multi-modal organic ligands [1, 2]. In comparison with other porous matrices, MOFs have large surface areas, low framework densities, and high pore volumes, which make them potentially significant for a wide range of applications ranging from gas storage to heterogeneous catalysis [3–5]. A new subclass of MOFs, zeolite imidazolate frameworks (ZIFs), has emerged as a novel type of highly porous materials, combining advantages of both zeolites and conventional MOFs [6, 7]. Some zeolitic architectures, such as zeolitic imidazole frameworks (ZIFs), have been successfully synthesized as hybrid frameworks. ZIFs adopt a crystalline architecture, in which Zn2+ ions typically play the role of silicon and the imidazolate anions form bridges mimicking zeolite oxygens [8–10].
ZIF-8, which is an important MOF, is prepared by coordination of Zn(II) with 2-methylimidazolate. ZIF-8 possesses a sodalite, zeolite-type structure, with large cavities (11.6 Å) and small apertures (3.4 Å). The bridging unit between the Zn(II) centers is the five-membered imidazolate ring imparting an angle of 145° throughout the framework by coordination of N atoms in the 1,3-positions of the ring, thus forming small pore apertures of 3.4 Å, which is particularly selective to smaller molecules.
Ru [11], Fe3O4 [12], Pd/SiO2 [13], graphene oxide [14], polyoxometalate (POM) anions [15], GaN@ZIF-8 [16], and polystyren@ZIF-8 [17] can be used to functionalize ZIF-8. The composite materials thus obtained are effective in organic reactions as oxidation catalysts. Phosphotungstic acid (PW12), the strongest heteropoly acid (HPA) known, with a good thermal stability in the solid state, is another POM used in the functionalization of ZIF-8 frameworks [18].
The increasing amount of interest directed toward sulfoxides and sulfones has stimulated research on their efficient synthesis because of their extensive applications as synthetic intermediates for the construction of various chemically and biologically active molecules [19, 20]. Owing to the great interest in these compounds, different synthetic methods have been developed for chemoselective oxidation of sulfides. However, many of these procedures utilize environmentally unfavorable reagents, solvents, or catalysts that give rise to concern regarding their eco-efficiency in our environmentally conscious times. These include, for example, the use of Na2WO4 phase-transfer catalysts [21], the ternary catalyst systems Na2WO4-phosphonic acid-Q+HSO4− [22], Mg3Al-P2W17X [23], PW12/Al-MCF [24], PW12-APTES@KIT-6 [25], V/MIL-101 [26], fluorous thiourea [27], BisILs-C8H17-W2 [28], or multi-walled carbon nanotubes (MWCNTs-COOH) [29].
Capturing and retaining PW12 in zeolitic imidazolate frameworks (PW12@ZIF-8, hereafter) through a one-pot hydrothermal synthesis and its catalytic activity in the oxidation of sulfides and sulfoxides with H2O2 have been the strategy in this work (Scheme 1).

Oxidation of sulfides (1) to sulfoxides (2) and sulfones (3) in the presence of PW12@ZIF-8 as catalyst.
2 Results and discussion
2.1 Characterization of catalyst
Figure 1 shows the X-ray diffraction (XRD) patterns of a ZIF-8 and a PW12@ZIF-8 sample. Both samples exhibit good crystallinity. An XRD pattern for the bare ZIF-8 is shown in Fig. 1a. The sharp main diffraction peak appears at 2θ = 7.2°, and relative diffraction intensities of the prepared samples are found to be the same as those of the standard data for ZIF-8 [30, 31].

XRD patterns for (a) ZIF-8 and (b) PW12/ZIF-8.
The crystallite size was calculated from the 110 reflection, using the Scherrer equation [32]. The average crystallite size is 44 and 46 nm for sample ZIF-8 and PW12@ZIF-8, respectively (see Table 1). The XRD intensity ratios provide information about the degree of relatively preferred orientations and crystal structure of ZIF-8 and PW12@ZIF-8 (Table 1).
The texture parameters of bare ZIF-8 and PW12@ZIF-8 in comparison with the bulk PW12 materials.
| Entry | Sample | BET, surface area, m2/g | Pore volume, cm3/g | Pore diameter, nm | I(110)/[I(110) + I(200) + I(211)] | Crystallite size, nm |
|---|---|---|---|---|---|---|
| 1 | ZIF-8 | 1025.25±1.39 | 0.45±0.01 | 1.18±0.07 | 0.65 | 44.2 |
| 2 | PW12 | 6 | – | – | – | – |
| 3 | PW12@ZIF-8 | 595.48±1.17 | 0.15±0.01 | 2.50±0.06 | 0.56 | 46.0 |
The ZIF-8 crystal structure is completely maintained, and ZnO is not formed as the final calcination product, as indicated by the XRD pattern of the PW12@ZIF-8 sample.
Figure 2 shows the scanning electron microscopy (SEM) images of the PW12@ZIF-8 sample at different magnifications. The crystals are of skeleton fish-like shape in PW12@ZIF-8, as observed, and sample dimensions are below 100 nm. PW12 is indeed well admixed with ZIF-8, as revealed by SEM images. However, the characterization of the interface region of PW12@ZIF-8 in the framework is difficult using available methods.
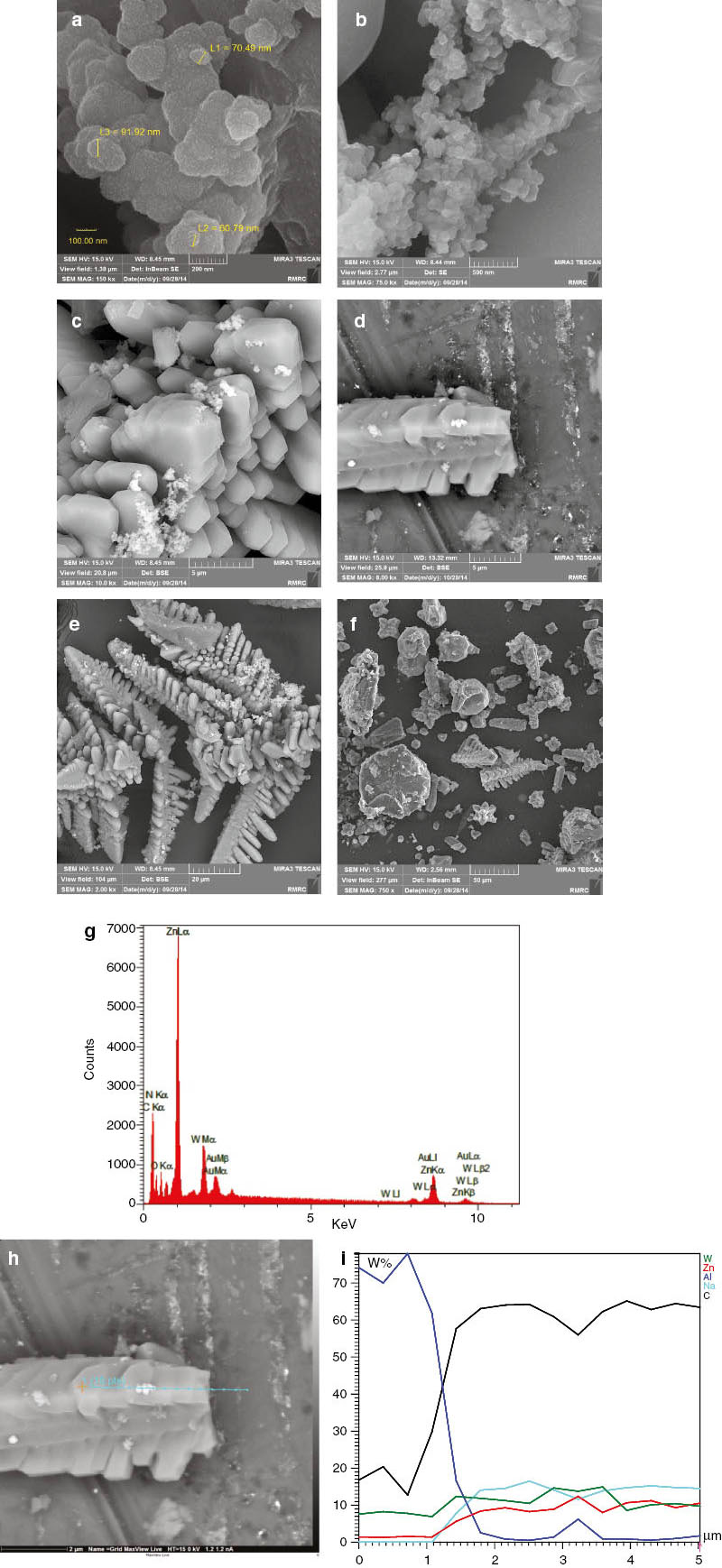
(a–f) SEM images; (g) EDX; (h, i) SEM image, and EDS linear scanning along PW12/ZIF-8.
Figure 2g shows the SEM-EDX (energy-dispersive analysis X-ray spectroscopy) spectra of the PW12@ZIF-8 sample, indicating the presence of O, Zn, and W. The presence of tungsten confirms the loading of PW12 onto the ZIF-8 surface or into the pores. EDS linear scanning has been performed to check the distribution throughout the PW12@ZIF-8 sample (Fig. 2h and i). The components are homogeneously distributed throughout the ZIF-8, as is shown by EDS linear scanning.
Clear evidence for the presence of PW12 is also given by ultraviolet/visible ray (UV/Vis) spectroscopy. The UV/Vis spectrum of the PW12@ZIF-8 sample is shown in Fig. 3. Two main absorptions appear in the PW12 spectrum: one is centered at 255 nm and is attributed to the O–W charge transfer absorption band for the Keggin unit at the W–O–W bond [33]. The PW12@ZIF-8 sample clearly shows this transition, although the intensity is weaker. This fact suggests that at least a part of the Keggin molecules remains intact after direct inclusion in the porous structure. The second broad absorption in the PW12 is centered at 360 nm, which can be related to Zn–O–W interactions, analogous to Fe- [34] and Cr-containing POMs [25, 35].

Diffuse reflectance UV visible (DRUV/Vis) spectra of PW12@ZIF-8 samples.
The adsorption-desorption isotherm of N2 at 77 K for PW12@ZIF-8 sample is shown in Fig. 4. The PW12@ZIF-8 shows the typical type IV adsorption isotherm with a noticeable hysteresis loop, as observed, indicating that the pore structure of the PW12@ZIF-8 contains micropores. A hysteresis profile is shown by the isotherms in Fig. 4, which can be interpreted as an H1 type according to the IUPAC classification [36, 37]. The adsorption-desorption branches of the isotherms of the PW12@ZIF-8 samples were used to obtain the pore size distribution curves (Fig. 4, inset). The obtained pore size distribution is multi-modal with two maxima for PW12@ZIF-8 (Fig. 4, inset). The smaller pores may correspond to the pores inside the ZIF-8, whereas the larger ones (20–100 nm diameters) can be attributed to the voids in the aggregation of the nanotubes, according to other published reports [36, 38, 39].

N2 adsorption-desorption isotherms and (inset) pore size distributions calculated by the Barrett-Joyner-Halenda (BJH) method of PW12/ZIF-8.
The structural parameters of the PW12 and ZIF-8 samples are listed in Table 1. Hydrogen forms (or free acids) of HPAs usually have low surface areas (the drawback to the H3PW12O40 (PW12) is its low surface area, 1–6 m2/g, and low porosity, <0.1 cm3/g). Supported HPA catalysts have much greater surface areas [18]. The PW12@ZIF-8 Brunauer–Emmett–Teller (BET) surface area appears to be lower than that of ZIF-8, which has been reported to be between 1000 and 1700 m2/g, and is therefore highly dependent on the preparation methods employed. The pore volume of all PW12@ZIF-8 samples decreases, confirming that PW12 has occupied the pores in ZIF-8. The incorporation of PW12 in the pores of ZIF-8 also leads to reduced surface area.
The arrangement of the ZIF-8 frameworks is still retained well after incorporation of PW12 in a PW12@ZIF-8 sample, as observed in the SEM and N2 adsorption data.
2.2 Catalytic activity
2.2.1 Oxidation of sulfides to sulfoxides and sulfones
The oxidation was carried out in methanol, n-hexane, dichloromethane (DCM), acetonitrile, ethanol, acetone, and H2O to check the feasibility of the PW12@ZIF-8 system in an organic medium. Solvents of high hydrogen bonding capacity, such as ethanol and water, favor the formation of sulfide with high chemoselectivity, as is clearly indicated in Table 2, and in agreement with our studies [40]. Furthermore, the yield of sulfoxide was low (34 or 23 %) when only PW12 or ZIF-8 was added as catalyst, respectively.
Effect of different conditions in the oxidation of diphenyl sulfides to sulfoxides after 0.5 h at reflux conditions.
| Entry | Catalyst | mol% | Solvent | H2O2/sulfide ratio | Yield,a % |
|---|---|---|---|---|---|
| 1 | – | – | Ethanol | 8:1 | 3 |
| 2 | PW12 | 3 | Ethanol | 8:1 | 34 |
| 3 | ZIF-8 | 3 | Ethanol | 8:1 | 23 |
| 4 | 25 % PW12@ZIF-8 | 3 | Ethanol | 5:1 | 65 |
| 5 | 25 % PW12 @ZIF-8 | 3 | Ethanol | 8:1 | 98 |
| 6 | 25 % PW12@ZIF-8 | 3 | Ethanol | 15:1 | 98 |
| 7 | 25 % PW12@ZIF-8 | 4 | Ethanol | 8:1 | 85 |
| 8 | 25 % PW12@ZIF-8 | 3 | Methanol | 8:1 | 85 |
| 9 | 25 % PW12@ZIF-8 | 3 | Acetonitrile | 8:1 | 55 |
| 10 | 25 % PW12@ZIF-8 | 3 | n-Hexane | 8:1 | 23 |
| 11 | 25 % PW12@ZIF-8 | 3 | DCM | 8:1 | 27 |
| 12 | 25 % PW12@ZIF-8 | 3 | Acetone | 8:1 | 43 |
| 13 | 25 % PW12@ZIF-8 | 3 | H2O | 8:1 | 85 |
| 14 | 35 % PW12@ZIF-8 | 3 | Ethanol | 8:1 | 85 |
| 15 | 15 % PW12@ZIF-8 | 3 | Ethanol | 8:1 | 65 |
aIsolated yields.
A set of preliminary experiments on diphenyl sulfide were next performed to examine the effects of different amounts of PW12@ZIF-8 in H2O at 25 °C (Table 2). No significant oxidation was observed in the absence of PW12@ZIF-8 in a blank experiment (Table 2, entry 1) and only a low yield of sulfoxide was obtained in the presence of H2O2 after 0.5 h. The optimal ratio of sulfide/H2O2/PW12@ZIF-8 was found to be 1:8:3 for complete conversion of sulfides to sulfoxides. The results are presented in Table 2.
To examine the scope and limitations of this procedure, several types of sulfides were selected (Table 3). Dialkyl (1a), aryl alkyl (1b), and diaryl (1c) sulfides could be oxidized to the corresponding sulfoxides in excellent yields. Sulfides with electron-withdrawing (1d, e) or donating (1f) substituents gave the corresponding sulfides in excellent yields with high purity. Importantly, this method was compatible with functional groups such as ester (1g). Oxidations could typically be stopped at the sulfoxide stage without over-oxidation to the sulfone.
Oxidation of sulfides to sulfoxides and sulfones using the PW12@ZIF-8/H2O2 system.
| Sulfide | Yield,a %, and time, h | TONb | TOFc | |||
|---|---|---|---|---|---|---|
| Sulfoxided 2 | Sulfonee 3 | Sulfoxided 2 | Sulfonee 3 | Sulfoxided 2 | Sulfonee 3 | |
| 1a | 98, 0.5 | 95, 1.0 | 3.26 | 1.90 | 6.25 | 1.90 |
| 1b | 95, 0.5 | 90, 1.5 | 3.16 | 1.80 | 6.32 | 1.20 |
| 1c | 95, 1.3 | 95, 2.5 | 3.16 | 1.90 | 2.43 | 0.76 |
| 1d | 95, 1.0 | 92, 1.8 | 3.16 | 1.84 | 3.16 | 1.02 |
| 1e | 90, 2.2 | 90, 4.5 | 3.00 | 1.80 | 1.36 | 0.40 |
| 1f | 92, 3.3 | 85, 5.5 | 3.06 | 1.70 | 0.93 | 0.31 |
| 1g | 90, 4.5 | 78, 7.0 | 3.00 | 1.56 | 0.67 | 0.22 |
aIsolated yields. b
The chemoselective oxidation of sulfides to sulfones was also investigated to further demonstrate the efficiency and applicability of the H2O2-PW12@ZIF-8 system. The optimal conditions for the reaction specified in Table 3 were determined using CH3CN solvent at ambient temperature (ratio of sulfide/H2O2/PW12@ZIF-8 = 1:13:5). Application of a smaller amount of oxidizing agent gave a mixture of sulfoxide and sulfone. It was found that a wide variety of diaryl, dialkyl, and arylalkyl sulfides were oxidized to their corresponding sulfones in excellent yields in CH3CN at room temperature, as shown in Table 3. The recovery and reusability of the catalyst have been studied. It was observed that the catalyst can be easily recovered quantitatively by a simple filtration after the addition of CHCl3 to the reaction mixture. The wet catalyst was recycled and characterized by NAA, XRD, and FT-IR spectra. No appreciable change in activity was observed after three cycles.
3 Experimental section
Reagent-type chemicals were used in this work. Infrared spectra (400–4000 cm−1) were obtained using a Perkin Elmer Spectrum 65 spectrophotometer from KBr pellets. XRD diffraction patterns were obtained by CuKα (λ = 1.54056 Å) radiation with automatic control on an XRD, Bruker D8 ADVANCE and PW1830 instrument. Step scanning was performed for all samples from 0.5 to 10° at the rate of 0.05°/s in 2θ. BET-specific surface areas and pore volumes of the catalysts were determined by adsorption-desorption of nitrogen at liquid nitrogen temperature using a Micromeritics Bel sorp mini II instrument. Peak positions of the distribution curves, determined by the adsorption branches of the isotherms, were used to obtain catalyst pore sizes. A Philips XL30 SEM [accelerating voltage, 0.5–30 kV (100 V steps), and 3–30 kV (1 kV steps); SEM resolution, 2.0 nm at 30 kV and 5.0 nm at 1 kV], equipped with an EDX, was used to study the surface morphology and particle sizes of the samples, respectively.
3.1 Preparation of PW12@ZIF-8
In a typical synthesis, a solid mixture of zinc nitrate hexahydrate (Zn(NO3)2·6H2O) (1.88 g, 6.33 mmol) and 2-methylimidazole (H-MeIM) (0.43 g, 5.82 mmol) and H3PW12O40 (0.05 g, 0.017 mmol) was dissolved in 130 mL of N,N′-dimethylformamide (DMF). The obtained solution was then transferred in equal portions into 20-mL vials, which were tightly capped and heated at a rate of 5–140 °C in a temperature-programmable oven. The solution temperature was kept constant for 24 h, after which cooling to room temperature was carried out at a rate of 0.4 °C/min. After removal of the mother liquor from the mixture, chloroform (20 mL) was added to each vial. Colorless polyhedral crystals were collected from the upper chloroform layer, stirred in DMF (3 × 15 mL) for 3 days. DMF was then replaced by DCM (3 × 15 mL), and stirring was continued for 3 days [19]. The residual solvents were removed at reduced pressure at 200 °C for 6 h to yield 0.26 g of white polyhedral crystals (23 % based on 2-methylimidazole).
3.2 Synthesis of sulfoxides and sulfones
H2O2 (30 %, 8 mmol, 0.8 mL) was added in one portion to a stirred suspension of the selected sulfide (1 mmol) and 25 % PW12@ZIF-8 (3 mol%) heterogeneous catalyst in ethanol (5 mL). The resulting slurry was then stirred at ambient temperature for the time indicated in Table 1. Ethanol (10 mL) was used to filter off and wash the catalyst. Ethyl acetate (10 mL) was added, and the resulting solution was dried over anhydrous sodium sulfate, and the solvents were removed in vacuo to afford the crude product, which was purified by column chromatography using silica gel and 10 % EtOAc in hexane as the eluent to afford pure sulfoxide. An identical procedure was employed using 30 % H2O2 (13 mmol, 1.3 mL) and 25 % PW12@ZIF-8 (5 mol%) in CH3CN (5 mL) for the oxidation of sulfides to sulfones.
4 Conclusion
In summary, a one-pot hydrothermal synthesis has been used in the preparation of H3PW12O40 enclosed in the ZIF-8 nanoparticles without the need for an inert atmosphere. XRD, SEM, EDS, SEM-Line, DRUV, and BET have been used to characterize the structure, composition, and morphology of the PW12@ZIF-8 sample. PW12@ZIF-8 is an excellent heterogeneous system for promotion of the highly chemoselective and rapid oxidation of functionalized sulfides with H2O2 at room temperature in H2O. It is noteworthy that the reaction tolerates oxidatively sensitive functional groups and the sulfur atom is selectively oxidized.
Acknowledgments
We thank the Shahreza Branch of the Islamic Azad University for financial support.
References
[1] A. Corma, H. Garcia, F. X. Llabresi, I. Xamena, Chem. Rev.2010, 110, 4606.10.1021/cr9003924Search in Google Scholar
[2] D. Farrusseng, S. Aguado, C. Pinel, Angew. Chem. Int. Ed.2009, 48, 7502.10.1002/anie.200806063Search in Google Scholar
[3] J. R. Long, O. M. Yaghi, Chem. Soc. Rev.2009, 38, 1213.10.1039/b903811fSearch in Google Scholar
[4] G. K. H. Shimizu, R. Vaidhyanathan, J. M. Taylor, Chem. Soc. Rev. 2009, 38, 1430.10.1039/b802423pSearch in Google Scholar
[5] M. D. Allendorf, C. Bauer, R. K. Bhakta, R. J. T. Houk, Chem. Soc. Rev.2009, 38, 1330–1352.10.1039/b802352mSearch in Google Scholar
[6] A. Phan, C. J. Doonan, F. J. Uribe-Romo, B. Knobler, M. O’Keeffe, O. M. Yaghi, Acc. Chem. Res.2010, 43, 58.10.1021/ar900116gSearch in Google Scholar
[7] S. R. Venna, M. A. Carreon, J. Am. Chem. Soc.2010, 132, 76.10.1021/ja909263xSearch in Google Scholar
[8] Y. L. Liu, V. C. Kravtsov, R. Larsen, M. Eddaoudi, Chem. Commun.2006, 1488.10.1039/b600188mSearch in Google Scholar
[9] X. C. Huang, Y. Y. Lin, J. P. Zhang, X. M. Chen, Angew. Chem. Int. Ed.2006, 45, 1557.Search in Google Scholar
[10] Y. Q. Tian, C. X. Cai, X. M. Ren, C. Y. Duan, Y. Xu, S. Gao, X. Z. You, Chem. Eur. J.2003, 9, 5673.10.1002/chem.200304957Search in Google Scholar
[11] M. Liu, B. Fan, X. Shi, R. Li, Catal. Commun.2013, 42, 20.10.1016/j.catcom.2013.07.038Search in Google Scholar
[12] T. Zhang, X. Zhang, X. Yan, L. Kong, G. Zhang, H. Liu, J. Qiu, K. L. Yeung, Chem. Eng. J.2013, 228, 398.10.1016/j.cej.2013.05.020Search in Google Scholar
[13] L. Lin, T. Zhang, X. Zhang, H. Liu, K. L. Yeung, J. Qiu, Ind. Eng. Chem. Res.2014, 53, 10906.10.1021/ie5013695Search in Google Scholar
[14] A. Huang, Q. Liu, N. Wang, Y. Zhu, J. Caro, J. Am. Chem. Soc. 2014, 136, 14686.10.1021/ja5083602Search in Google Scholar
[15] R. Li, X. Ren. J. Zhao, X. Feng, X. Jiang, X. Fan, Z. Lin, X. Li, C. Hu, B. Wang, J. Mater. Chem. A.2014, 2, 2168.10.1039/C3TA14267ASearch in Google Scholar
[16] D. Esken, S. Turner, C. Wiktor, S. Babu Kalidindi, G. Van Tendeloo, R. A. Fischer, J. Am. Chem. Soc.2011, 133, 16370.10.1021/ja207077uSearch in Google Scholar
[17] H. J. Lee, W. Cho, M. Oh, Chem. Commun.2012, 48, 221.10.1039/C1CC16213FSearch in Google Scholar
[18] I. V. Kozhevnikov, in Catalysis by Polyoxometalates in Catalysts for Fine Chemical Synthesis, Vol. 2 (Eds.: S. N. Roberts, I. V. Kozhevnikov, E. Derouane), Wiley, New York, 2002.Search in Google Scholar
[19] I. Fernandez, N. Khiar, Chem. Rev. 2003, 103, 3651.10.1021/cr990372uSearch in Google Scholar
[20] P. Kowalski, K. Mitka, K. Ossowska, Z. Kolarska, Tetrahedron2005, 61, 1933.10.1016/j.tet.2004.11.041Search in Google Scholar
[21] K. Sato, M. Hyodo, M. Aoki, X.-Q. Zheng, R. Noyori, Tetrahedron2001, 57, 2469.10.1016/S0040-4020(01)00068-0Search in Google Scholar
[22] R. Noyori, M. Aoki, K. Sato, Chem. Commun.2003, 1977.10.1039/b303160hSearch in Google Scholar
[23] K. Liu, Z. Yao, Y.-F. Song, Ind. Eng. Chem. Res. 2015, 54, 9133.10.1021/acs.iecr.5b02298Search in Google Scholar
[24] R. Fazaeli, H. Aliyan, M. A. Ahmadi, S. Hashemian, Catal. Commun. 2012, 29, 48.10.1016/j.catcom.2012.09.018Search in Google Scholar
[25] R. Fazaeli, H. Aliyan, S. Parishani Foroushani, Z. Mohagheghian, Z. Heidari, Iran. J. Catal.2013, 3, 129.Search in Google Scholar
[26] R. Fazaeli, H. Aliyan, M. Moghadam, M. Masoudinia, J. Mol. Catal. A: Chem. 2013, 374–375, 46.10.1016/j.molcata.2013.03.020Search in Google Scholar
[27] Y. B. Huang, W. B. Yi, C. Cai, J. Fluor. Chem. 2011, 132, 554.10.1016/j.jfluchem.2011.05.026Search in Google Scholar
[28] X. Shi, X. Hana, W. Ma, J. Weia, J. Li, Q. Zhang, Z. Chen, J. Mol. Catal. A: Chem. 2011, 341, 57.10.1016/j.molcata.2011.03.024Search in Google Scholar
[29] H. Veisi, F. Hosseini Eshbala, S. Hemmati, M. Baghayeri, RSC Adv. 2015, 5, 10152.10.1039/C4RA14964ESearch in Google Scholar
[30] M. J. Janik, K. A. Campbell, B. B. Bardin, R. J. Davis, M. Neurock, Appl. Catal. A: Gen.2013, 256, 51.10.1016/S0926-860X(03)00388-0Search in Google Scholar
[31] R. Fazaeli, H. Aliyan, Appl. Catal. A: Gen.2007, 331, 78.10.1016/j.apcata.2007.07.030Search in Google Scholar
[32] L. J. Meng, M. P. Dos Santos, Thin Solid Films1993, 226, 22.10.1016/0040-6090(93)90200-9Search in Google Scholar
[33] M. H. Youn, H. Kim, J. C. Jung, I. K. Song, K. P. Barteau, M. A. Barteau, J. Mol. Catal. A: Chem.2005, 241, 227.10.1016/j.molcata.2005.07.023Search in Google Scholar
[34] K. Nowinska, M. Sopa, A. Waclaw, D. Szuba, Appl. Catal. A: Gen.2002, 225, 141.10.1016/S0926-860X(01)00851-1Search in Google Scholar
[35] J. Juan-Alcaniz, E.V. Ramos-Fernandez, U. Lafont, J. Gascon, F. J. Kapteijn, J. Catal.2010, 269, 229.10.1016/j.jcat.2009.11.011Search in Google Scholar
[36] E. Morgado Jr, M. A. S. De Abreu, G. T. Moure, B. A. Marinkovic, P. M. Jardim, A. S. Araujo, Chem. Mater.2007, 19, 665.10.1021/cm061294bSearch in Google Scholar
[37] G. Leofanti, M. Padovan, G. Tozzola, B. Venturelli, Catal. Today1998, 41, 207.10.1016/S0920-5861(98)00050-9Search in Google Scholar
[38] T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino, K. Niihara, Langmuir, 1998, 14, 3160.10.1021/la9713816Search in Google Scholar
[39] T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino, K. Niihara, Adv. Mater. 1999, 11, 1307.10.1002/(SICI)1521-4095(199910)11:15<1307::AID-ADMA1307>3.0.CO;2-HSearch in Google Scholar
[40] M. V. Gomez, R. Caballero, E. Vazquez, A. Moreno, A. De la Hoz, A. Diaz-Ortiz, Green Chem.2007, 9, 331.10.1039/B614847FSearch in Google Scholar
©2016 by De Gruyter
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics CeTX – review III
- Fusiformines A and B: new indole alkaloids from Melodinus fusiformis
- Preparation, crystal structure, thermal behavior, and theoretical studies of N,N′-dinitro-4, 4′-azo-bis(1,2,4-triazolone) (DNZTO)
- Design, synthesis, and biological evaluation of new series of 2-amido-1,3,4-thiadiazole derivatives as cytotoxic agents
- One-pot hydrothermal synthesis of H3PW12O40 supported on zeolite imidazolate frameworks (ZIF-8): a highly efficient heterogeneous catalyst for oxidation of sulfides to sulfoxides and sulfones
- Catalytic performance of a Keplerate-type, giant-ball nanoporous isopolyoxomolybdate as a highly efficient recyclable catalyst for the synthesis of biscoumarins
- Thermal behavior of benzobis(tetraethyldisilacyclobutene)
- Synthesis and crystal structures of three novel benzimidazole/benzoindolizine hybrids
- NQR and X-ray crystal structure studies of cadmium halide complexes: [C(NH2)3]CdI3 and [4-ClC6H5NH3]3CdBr5
- Phosphanchalkogenide und ihre Metallkomplexe. IV. Halogenierungsprodukte der Gold(I)halogenidkomplexe einiger Diphosphanmonochalkogenide
- Note
- Synthesis of aminomethyl derivatives of 5-substituted-3-(prop-2-ynyl)dihydrofuran-2(3H)-ones
Articles in the same Issue
- Frontmatter
- In this Issue
- Review
- Cerium intermetallics CeTX – review III
- Fusiformines A and B: new indole alkaloids from Melodinus fusiformis
- Preparation, crystal structure, thermal behavior, and theoretical studies of N,N′-dinitro-4, 4′-azo-bis(1,2,4-triazolone) (DNZTO)
- Design, synthesis, and biological evaluation of new series of 2-amido-1,3,4-thiadiazole derivatives as cytotoxic agents
- One-pot hydrothermal synthesis of H3PW12O40 supported on zeolite imidazolate frameworks (ZIF-8): a highly efficient heterogeneous catalyst for oxidation of sulfides to sulfoxides and sulfones
- Catalytic performance of a Keplerate-type, giant-ball nanoporous isopolyoxomolybdate as a highly efficient recyclable catalyst for the synthesis of biscoumarins
- Thermal behavior of benzobis(tetraethyldisilacyclobutene)
- Synthesis and crystal structures of three novel benzimidazole/benzoindolizine hybrids
- NQR and X-ray crystal structure studies of cadmium halide complexes: [C(NH2)3]CdI3 and [4-ClC6H5NH3]3CdBr5
- Phosphanchalkogenide und ihre Metallkomplexe. IV. Halogenierungsprodukte der Gold(I)halogenidkomplexe einiger Diphosphanmonochalkogenide
- Note
- Synthesis of aminomethyl derivatives of 5-substituted-3-(prop-2-ynyl)dihydrofuran-2(3H)-ones

