Abstract
Oxide solubility in chloride melts depends on temperature and composition of molten solvent. The solubility of barium oxide in the solvents with barium chloride content is essentially higher than that in molten alkali chlorides. Spectral data demonstrate the existence of oxychloride ionic groupings in such melts. This work presents the results of the BaO solubility in two molten BaCl2–NaCl systems with different barium chloride content. The received data together with earlier published results revealed the main regularities of BaO solubility in molten BaO–BaCl2–MCl systems.
1 Introduction
Oxide admixtures in molten chlorides essentially affect properties of electrolyte such as liquids temperatures, viscosity, and electroconductivity [1], [2], [3], [4], [5]. Mixtures of molten alkali metal chlorides and barium chloride can be used as electrolytes in process of barium and its alloys production [6]. The motivation of this article was to establish the BaO solubility and the mechanism of oxide dissolution in molten solvents of interest for the synthesis of nano powders of the barium and rare-earth metal complex oxides [7].
It is well known that oxide solubility in chloride melts depends on temperature and composition of molten solvent. It reaches maximum when the ionic potential of the oxide cation coincides with that of the solvent-salt cation [8]. That is why the solubility of barium oxide in the solvents containing barium chloride is essentially higher than that in molten alkali chlorides [6], [7], [8], [9], [10], [11], [12]. Spectral data [12], [13] demonstrate the existence of oxychloride ionic groupings in such melts.
We have previously studied the BaO dissolution process in molten 0.27BaCl2–0.73KCl [7] and 0.27BaCl2–0.36NaCl–0.37KCl [12] eutectics. The type, structure, and composition of oxychloride groupings were determined in situ in molten BaO–BaCl2–NaCl–KCl system by Raman spectroscopy [12]. The oxide dissolution was found to proceed according to the chemical mechanism:
where M – Na or K.
In this regard, the increasing in the barium oxide solubility with the growth of barium chloride concentration in the melt is clarified: the larger content of barium-containing chloride ionic groupings [BaCl6] results in larger content of “construction material” for oxychloride groupings [Ba2OCl6]. In addition, the ionic potential of alkali metal in BaCl2–MCl molten solvent should affect the stability of the initial chloride and generated oxychloride ionic groupings and, respectively, the BaO solubility.
This work presents the results of the BaO solubility in molten BaCl2–NaCl systems with different barium chloride content. The received data together with our earlier published results [7], [12] revealed the main regularities of BaO solubility in molten BaO–BaCl2–MCl systems.
2 Experimental Section
Chemically pure grade NaCl (99.9%) and BaCl2 (99.5%) (“Vekton,” St. Petersburg, Russian Federation) were used. Barium oxide was prepared before each experiment from barium nitrate (99.5%) (“Vekton,” St. Petersburg, Russian Federation) using the well-known technique [14].
The DSC studies were carried out using the STA 449C Jupiter® thermal analyser produced by NETZSCH Company (Germany). The Raman spectra were recorded using the optic fiber spectrometric Ava-Raman complex (Avantes, The Netherlands), which includes the monochromatic laser (λ=532 nm, radiation power of 50 mW). The methods of salt preparation, liquidus temperature measuring, and the high-temperature Raman spectra analysis were described in detail in our previous works [7], [12].
3 Results and Discussion
The melting temperature of the 0.40BaCl2–0.60NaCl eutectic (926±5 K) defined by the cooling curve analysis agrees with literature data (927 K) [15]. The measurements carried out by the DSC method showed that the eutectic melting temperature was 928±1 K. The liquidus temperature of the double system 0.27BaCl2–0.73NaCl was found to be equal 1003±5 K.
The dependence of liquidus temperatures on the added barium oxide concentration is shown in Figure 1. At first, the BaO additions decrease the temperature of initial crystallisation and then increase it. Experimental results for earlier studied barium-containing oxide-chloride melts [7], [12] were the similar. The change of the dependence flow may be due to the new solid phase which was the first to crystallise during the melt cooling. The increasing portion of liquidus temperature curve in Figure 1 corresponds to the barium oxide solubility (S).
![Figure 1: Liquidus temperatures of molten systems: BaO–[0.27BaCl2–0.73NaCl] (1); BaO–[0.40BaCl2–0.60NaCl] (2).](/document/doi/10.1515/zna-2016-0163/asset/graphic/j_zna-2016-0163_fig_001.jpg)
Liquidus temperatures of molten systems: BaO–[0.27BaCl2–0.73NaCl] (1); BaO–[0.40BaCl2–0.60NaCl] (2).
The solubility values thus obtained may be approximated by the (2) in the temperature range under study:
where A and B are the empirical coefficients. Table 1 shows the A and B values for BaCl2–NaCl systems under study together with earlier published results [7], [12]. The BaO solubility in molten 0.40BaCl2–0.60NaCl eutectic is larger than that in molten 0.27BaCl2–0.73NaCl system at the same temperature. This fact agrees well with the literature data. The BaO solubility in the molten NaCl–KCl–BaCl2 mixtures increases at 973 K from 0.77 to 10.94 mol.% with increasing of BaCl2 content from 5 to 55 mol.% [11]. The similar tendency was observed by Boghosian et al. [10] in the NaCl–BaCl2 melts.
Coefficients of (2) for molten BaCl2–MCl systems.
| Solvent | Temperature interval (K) | A±ΔA | –(B±ΔB)·10−3 | Δ(lnS) | R2 | S at 973 K (m.f) |
|---|---|---|---|---|---|---|
| 0.27BaCl2–0.73NaCl | 978–1083 | 2.35±0.7 | 6.0±0.7 | 0.07 | 0.98 | 0.021 |
| 0.40BaCl2–0.60NaCl | 910–1068 | 2.55±0.2 | 5.20±0.2 | 0.03 | 0.99 | 0.061 |
| 0.27BaCl2–0.36NaCl–0.37KCl | 799–1058 | 1.27±0.1 | 4.38±0.1 | 0.02 | 0.99 | 0.040 |
| 0.27BaCl2–0.73KCl | 963–1033 | 0.77±0.7 | 3.86±0.8 | 0.07 | 0.98 | 0.064 |
Figure 2 illustrates the temperature dependence of BaO solubility in BaCl2–MCl solvents with the same (0.27 mole fraction) barium chloride concentration. It is obvious that substitution of potassium chloride on sodium chloride results in slightly reduction of the barium oxide solubility. The solubility values in molten BaCl2–NaCl–KCl [12] eutectic agree well with data [11] determined by isothermal saturation method (Fig. 2, curve 4).
![Figure 2: Temperature dependence of BaO solubility in molten chloride systems: 0.27BaCl2–0.73NaCl (1); 0.27BaCl2–0.36NaCl–0.37KCl (2); 0.27BaCl2–0.73KCl (3); and 0.27BaCl2–0.36NaCl–0.37KCl [11] (4).](/document/doi/10.1515/zna-2016-0163/asset/graphic/j_zna-2016-0163_fig_002.jpg)
Temperature dependence of BaO solubility in molten chloride systems: 0.27BaCl2–0.73NaCl (1); 0.27BaCl2–0.36NaCl–0.37KCl (2); 0.27BaCl2–0.73KCl (3); and 0.27BaCl2–0.36NaCl–0.37KCl [11] (4).
The Gibbs energy changes during the BaO dissolution can be expressed by (3).
where k* is the conditional equilibrium constant of the reaction: BaO(solid)↔BaO(solution), with the assumption that the barium activity coefficients in chloride and oxychloride groupings are unchangeable in the concentration range under study.
Substitution of (2) to (3) gives the following equation for temperature dependence of the Gibbs energy changes:
The conditional enthalpy (ΔH*=–RB) and entropy changes (ΔS*=RA) during the BaO dissolution are presented in Table 2.
Thermodynamic characteristics of BaO solubility in molten chlorides.
| Solvent | ΔH* (kJ/mol) | ΔS* (J/(mol·K)) |
|---|---|---|
| 0.27BaCl2–0.73NaCl | 49.9±5.8 | 19.5±5.8 |
| 0.27BaCl2–0.36NaCl–0.37KCl | 43.2±0.8 | 21.2±0.8 |
| 0.27BaCl2–0.73KCl | 36.3±5.8 | 10.5±6.6 |
| 0.40BaCl2–0.60NaCl | 32.0±1.7 | 10.0±1.7 |
One can see that substitution of sodium chloride on potassium chloride at the same BaCl2 concentration reduces the endothermic effect of the BaO dissolution. For all solvents under study, the enthalpy changes during the BaO dissolution are smaller than the barium oxide melting enthalpy (58.47 kJ/mol [16]). This fact denotes the exothermal interaction between barium oxide and solvent particles at their mixing.
According to literature data [17], the solubility of such oxides as CaO and SrO in molten MCl–CaCl2 and MCl–SrCl2 increases with the reduction of the alkali metal cation radius (M+). While the enthalpy of dissolution increases [17] indicating the same tendency as for barium containing molten systems (Tab. 2). In this connection, the decreasing of the lead oxide (II) solubility in molten MCl–PbCl2 with substitution of cesium chloride on potassium chloride should be pointed out. As it was shown in [5], the solubility of lead oxide in molten 0.5KCl–0.5PbCl2 system at 806 K was about 8 mol.%, whereas in molten 0.713CsCl–0.287PbCl2 (with smaller content of lead chloride), 12 mol.% of oxide dissolved.
Probably the reduction of the radius of the alkali metal cation which located in the second coordinating area results in the decrease in the oxychloride ionic groupings stability.
For all the solvents under study, the XRD data of frozen fusion after the experiment show the presence of Ba4OCl6. For example, Figure 3 shows the XRD data for frozen [NaCl–BaCl2 eutectic+BaO (8 mol.%)] melt. The composition of molten solvent should change during the Ba4OCl6 solid-phase formation according to (5):
![Figure 3: XRD data for frozen [NaCl–BaCl2 eutectic+BaO (8 mol.%)] melt.](/document/doi/10.1515/zna-2016-0163/asset/graphic/j_zna-2016-0163_fig_003.jpg)
XRD data for frozen [NaCl–BaCl2 eutectic+BaO (8 mol.%)] melt.
To confirm flowing reactions (1) and (5), the presence of proper compounds and ionic groupings in the melt should be certificated by Raman spectroscopy.
The Raman spectrum of NaCl–BaCl2 eutectic melt is shows in Figure 4. In addition to the intense Rayleigh wing scattering, the band of small intensity in the area of 210 cm−1 is observed. This band we attributed to a symmetric valence vibration of the [BaCl6] ionic groupings [12]. The location of the band in molten NaCl–BaCl2 shifts toward larger frequencies as compared with that in molten NaCl–KCl–BaCl2 [12]. It can be due to the strengthening of the force constant of Ba–Cl bond in [BaCl6] ionic grouping when substituting of potassium cation on sodium cation. The small intensity of the vibration band may be the evidence of the mainly ionic type of interaction in this grouping.
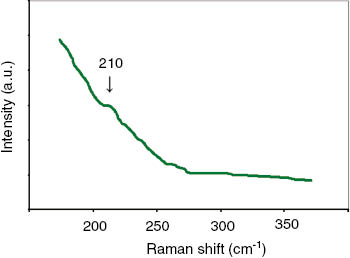
Raman spectra of molten NaCl–BaCl2 eutectic, 943 K.
Figure 5 demonstrates the Raman spectrum of NaCl–BaCl2 eutectic melt after the considerable BaO addition exceeding its solubility at this temperature. The vibration band at 210 cm−1, corresponding to the [BaCl6] ionic grouping vibration is not observed but new vibration bands that can be attributed to [Ba2OCl6] grouping [12] appear at the 300 cm−1 area. The disappearance of the vibration band at 210 cm−1 as barium oxide is added can be connected with the [BaCl6] chloride groupings destruction in the presence of O2− anions with a greater ionic potential as compared to the Cl− anions. The analysis of the obtained spectroscopy results directly demonstrates the chemical mechanism of BaO dissolution in barium-containing chloride melts according to the (1). The vibration band at 220 cm−1 corresponds to the residue solid BaO [18] after interaction with the melt.

Raman spectra of molten NaCl–BaCl2 eutectic with barium oxide addition (20 mol.%), 983 K.
Figure 6 shows the Raman spectra of solidified oxide-chloride mixture NaCl–BaCl2–BaO after the high-temperature experiment. The intensive vibration bands related to Ba4OCl6 [12] phase are observed. The disappearance of the BaCl2 phase and the occurrence of the oxychloride Ba4OCl6 one confirm the chemical mechanism of oxide dissolution in molten NaCl–BaCl2 eutectic.

Raman spectra of solidified oxide-chloride mixture NaCl–BaCl2–BaO after the high-temperature experiment.
4 Conclusion
The liquidus temperatures of (NaCl–BaCl2)–BaO systems with different barium chloride concentration were determined by the thermal analysis methods. The barium oxide solubility in molten NaCl-BaCl2 was determined as a function of temperature.
The same regularities of barium oxide dissolution in molten NaCl–BaCl2, NaCl–KCl–BaCl2, and KCl–BaCl2 systems were revealed. The substitution of potassium chloride on sodium chloride was shown to result in slightly reduction of the barium oxide solubility in the melts with the same barium chloride content.
In situ Raman spectroscopic analysis provided direct evidence of chemical interaction between BaO and barium containing chloride melts.
Acknowledgments
The XRD-analysis was performed using the facilities of the Shared Access Centre “Composition of Compounds” (Institute of High Temperature Electrochemistry, Ural Branch of RAS).
References
[1] V. L. Cherginets, Oxoacidity: Reactions of Oxo-Compounds in Ionic Solvents, Elsevier Science, Amsterdam 2005.Search in Google Scholar
[2] S.-L. Wang, W. Wang, S.-C. Li, and S.-H. Cao, Int. J. Miner. Metall. Mater. 17, 791 (2010).10.1007/s12613-010-0391-8Search in Google Scholar
[3] P. S. Pershin, V. P. Batukhtin, N. I. Shurov, P. A. Arkhipov, and Y. P. Zaikov. J. Chem. Eng. Data 57, 2811 (2012).10.1021/je300649fSearch in Google Scholar
[4] Y. P. Zaykov, A. V. Isakov, I. D. Zakiryanova, O. G. Reznitskikh, O. V. Chemezov, et al. J. Phys. Chem. B 118, 1584 (2014).10.1021/jp4086816Search in Google Scholar PubMed
[5] P. A. Arkhipov, I. D. Zakiryanova, A. S. Kholkina, A. V. Bausheva, and A. O. Khudorozhkova, Z. Naturforsch. 70, 851 (2015).10.1515/zna-2015-0273Search in Google Scholar
[6] A. V. Volkovich, Melts 4, 280 (1991).Search in Google Scholar
[7] E. V. Nikolaeva, A. L. Bovet, I. V. Korzun, and I. D. Zakiryanova, Rasplavy 1, 78 (2014) [In Russian].Search in Google Scholar
[8] N. M. Barbin, A. P. Pekar, V. N. Nekrasov, and L. Ye. Ivanovskii, Melts 2, 87 (1992).Search in Google Scholar
[9] B. Neumann, C. Kröger, and H. Jütter, Z. Elektrochem. Angew. Phys. Chem. 41, 725 (1935).Search in Google Scholar
[10] S. Boghosian, A. Godo, H. Mediaas, W. Ravlo, and T. Osvold, Acta Chem. Scand. 45, 145 (1991).10.3891/acta.chem.scand.45-0145Search in Google Scholar
[11] M. V. Solodkova, V. I. Zhuravlev, A. B. Volkovich, and D. S. Yermakov, Rasplavy 4, 69 (2002) [In Russian].Search in Google Scholar
[12] E. V. Nikolaeva, I. D. Zakiryanova, I. V. Korzun, A. L. Bovet, and B. D. Antonov, Z. Naturforsch. 70, 325 (2015).10.1515/zna-2014-0370Search in Google Scholar
[13] A. Khokhryakov and A. M. Khokhlova, Melts 5, 408 (1989).Search in Google Scholar
[14] G. Brauer, Handbuch der Praparativen Anorganischen, Ferdinand Enke Verlag, Stuttgart 1975.Search in Google Scholar
[15] E. Vortisch, Neues Jahrb. Mineral. Geol. Palaeontol. Beilageband 38, 182 (1914).Search in Google Scholar
[16] I. Barin, Thermochemical Data of Pure Substances, Part I; VCH Verlags Gesellschaft, Weinheim 1993.Search in Google Scholar
[17] D. S. Yermakov, V. I. Zhuravlev, and A. B. Volkovich, Rasplavy 2, 35 (2000) [In Russian].Search in Google Scholar
[18] B. M. Weckhuysen, G. Mestl, M. P. Rosynek, T. R. Krawietz, J. F. Haw, et al. J. Phys. Chem. 102, 3773 (1998).10.1021/jp980185kSearch in Google Scholar
©2016 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Numerical Investigation of Electromagnetic Scattering Problems Based on the Compactly Supported Radial Basis Functions
- Reliable Sampled-Data Control of Fuzzy Markovian Systems with Partly Known Transition Probabilities
- Method of Multiple Scales and Travelling Wave Solutions for (2+1)-Dimensional KdV Type Nonlinear Evolution Equations
- Nonlinear Dynamic Characteristics of Oil-in-Water Emulsions
- On Barium Oxide Solubility in Barium-Containing Chloride Melts
- Nonlocal Symmetries, Explicit Solutions, and Wave Structures for the Korteweg–de Vries Equation
- On Symmetry Analysis and Conservation Laws of the AKNS System
- Unexpected Behavior on Nonlinear Tunneling of Chirped Ultrashort Soliton Pulse in Non-Kerr Media with Raman Effect
- First-Principles Study of Lattice Dynamics, Structural Phase Transition, and Thermodynamic Properties of Barium Titanate
- Theoretical Studies of the Spin Hamiltonian Parameters and Local Distortions for Cu2+ in Alkaline Earth Lead Zinc Phosphate Glasses
Articles in the same Issue
- Frontmatter
- Numerical Investigation of Electromagnetic Scattering Problems Based on the Compactly Supported Radial Basis Functions
- Reliable Sampled-Data Control of Fuzzy Markovian Systems with Partly Known Transition Probabilities
- Method of Multiple Scales and Travelling Wave Solutions for (2+1)-Dimensional KdV Type Nonlinear Evolution Equations
- Nonlinear Dynamic Characteristics of Oil-in-Water Emulsions
- On Barium Oxide Solubility in Barium-Containing Chloride Melts
- Nonlocal Symmetries, Explicit Solutions, and Wave Structures for the Korteweg–de Vries Equation
- On Symmetry Analysis and Conservation Laws of the AKNS System
- Unexpected Behavior on Nonlinear Tunneling of Chirped Ultrashort Soliton Pulse in Non-Kerr Media with Raman Effect
- First-Principles Study of Lattice Dynamics, Structural Phase Transition, and Thermodynamic Properties of Barium Titanate
- Theoretical Studies of the Spin Hamiltonian Parameters and Local Distortions for Cu2+ in Alkaline Earth Lead Zinc Phosphate Glasses

