Abstract
Food consumption during the rest phase promotes circadian desynchrony, which is corrected with harmful physiological and mental disorders. Previously, we found that circadian desynchrony was involved in isoflurane-induced cognitive impairment. Here, we scheduled food access to modulate daily rhythm to examine its impact on isoflurane-induced cognitive impairments. Mice were randomly transferred to restricted feeding (RF) time groups: Control group (Zeitgeber time (ZT) 0–ZT24, ad libitum feeding), Day-Feeding group (ZT0–ZT12, misaligned feeding), and Night-Feeding group (ZT12–ZT24, aligned feeding). Then, some of them were subjected to 5 h of 1.3% isoflurane anaesthesia from ZT14 to ZT19 and were divided into the Control + Anes group, the Day-Feeding + Anes group, and the Night-Feeding + Anes group. Mini-Mitter was used to monitor the daily rhythm. Fear conditioning system was conducted to assess cognition of mice. We observed that the Night-Feeding group adapted to RF gradually, whereas the Day-Feeding group exhibited a disturbed daily rhythm. The Night-Feeding + Anes group exhibited a partially enhanced daily rhythm, whereas the Day-Feeding + Anes group exhibited sustained phase advances and diurnality score increase 7 days after isoflurane anaesthesia. Notably, in tests of hippocampus-dependent contextual memory, the Night-Feeding + Anes group demonstrated decreased deficits; the Day-Feeding + Anes group showed prolonged post-anaesthetic deficits 14 days after isoflurane anaesthesia. However, amygdala-dependent cued-fear conditioning post-anaesthesia was not altered by the RF schedule. In conclusion, we demonstrated that misaligned feeding disturbed the daily rhythm and led to persistent post-anaesthetic cognitive dysfunction. Aligned feeding enhanced the daily rhythm partially and improved post-anaesthetic cognitive dysfunction.
1 Introduction
Synchronizing internal rhythmicity with environmental cues is essential for the optimal adjustment of the body to predictable daily challenges. It has long been recognized that light is the primary zeitgeber for the central clock in the suprachiasmatic nucleus (SCN) of the brain [1]. Evidence is accumulating that the timing of food availability is an important entrainment signal for circadian clocks outside the SCN [2]. A restricted feeding (RF) schedule has been well studied in rodent models [3,4], in which the food availability time is restricted for several hours per day. Food consumption during the rest phase can deliver contradicting inputs to the circadian timing system [5].
Circadian rhythm disruption is emerging as a new risk factor for cognitive dysfunction in clinical and rodent investigations. Clinical investigations have found significant cognitive impairments in long-time shift workers [6]. Sprecher and Videnovic demonstrated that circadian oscillation is disturbed in neurodegenerative diseases and that chronobiological interventions can be used therapeutically [7,8]. Desynchronization or misalignment of circadian oscillators causes decrements in cognitive function, as observed in animal models. It was reported that experimental jet-lag conditions caused acute and persistent spatial memory deficits in hamsters [9]. The use of chronobiological manipulation of scheduled feedings as reinforcements of circadian rhythms has been shown to alleviate cognitive impairments in Alzheimer’s, Parkinson’s, and Huntington’s models [10,11,12].
In our previous study, we found that the daily rhythm was involved in isoflurane-induced cognitive impairment in mice [13]. Therefore, we hoped to find a manoeuvrable procedure without genetic, pharmacological, or surgical interventions to modulate the daily rhythm and thereby relieve memory decline. We hypothesized that misaligned/aligned feeding might play a significant role in post-anaesthetic cognitive modifications by modulating the daily rhythm.
2 Materials and methods
2.1 Experimental animals
Two-month-old male C57BL/6J mice (Model Animal Research Center of Nanjing University, China) were housed in the same room, with a constant temperature (22 ± 2°C) and humidity (55% ± 5%). The light intensity in the housing room was about 150–200 lux. There were six mice in one cage during the adaptation period. The mice were acclimatized to a 12 h:12 h light:dark (LD) cycle for 3 weeks before any interventions were undertaken. The objective time was defined as the zeitgeber time (ZT) rather than external time. Lights on at 8:00 was defined as ZT 0, and lights off at 20:00 was defined as ZT 12. Food was available ad libitum during the adaptation period and was scheduled in the experimental period. Water was available ad libitum.
2.2 Ethical agreement
All procedures were conducted in accordance with the applicable laws and guidelines regarding animal care and were approved by the Nanjing Drum Tower Hospital Experimental Animals Welfare and Ethical Committee (No. 20171102). All processing methods were conducted in accordance with Directive 2010/63/EU. This manuscript adheres to the applicable EQUATOR guidelines.
2.3 Scheduled food access
All mice were fed freely for 3 weeks and then transferred into different restricted time feeding groups. Mice in the Control group were given access to the food chamber from ZT 0 to 24; then some of them received isoflurane anaesthesia and were placed in the Control + Anes group randomly. Mice in the Night-Feeding group were given access to the food chamber during the dark phase from ZT12 to 24, which aligned with their active phase, and then some of them received isoflurane anaesthesia and were placed in the Night-Feeding + Anes group. Mice in the Day-Feeding group were given access to the food chamber during the light phase from ZT0 to 12, which misaligned with their active phase, and then some of them received isoflurane anaesthesia randomly and were placed in the Day-Feeding + Anes group.
2.4 Anaesthesia protocol
Mice were placed in plexiglass chambers, exposed to a mixture of 1.3% isoflurane (Lunan Better Pharmaceutical Co., Ltd, 64150704) in 100% oxygen at a flow rate of 2–3 L/min for 5 h from ZT14 to ZT19; thus, we manipulated the mice anaesthesia during the activity period. The concentrations of isoflurane were continuously monitored with an anaesthesia gas monitor.
2.5 Fear conditioning system (FCS)
We used a FCS (Panlab, Harvard Apparatus, Spain) to test hippocampus-dependent contextual memory and amygdala-dependent cue memory. Mice were trained and tested only once to avoid the effects of prior manipulation. To avoid daily rhythm in performance on this test, we conducted FCS at the same time of day (from ZT0 to ZT2) in all groups. Each mouse was allowed to explore the FCS chamber for 5 min before presentation of a 2 Hz pulsating tone (80 dB, 4,000 Hz), which persisted for 60 s. The tone was immediately followed by a mild foot shock (0.8 mA for 0.5 s). Twenty-four hours later, each mouse was allowed to stay in the same chamber for a total of 300 s. The tone test was performed 60 min after the end of the contextual test. Each mouse was allowed to stay in a different chamber for a total of 360 s. The same tone was presented for the second 180 s without the foot shock. Cognitive function during the test was assessed by measuring the amount of time the mouse demonstrated “freezing behaviour” (freezing time), which was defined as a completely immobile posture (except for respiratory efforts), using the PACKWIN software.
2.6 Measurements of daily rhythm
As previously described [13], a radio transmitter (G2 E-Mitter; Mini Mitter Co. Inc., A Respironics Company, Bend, OR, USA) was planted into the abdomen of each mouse to record the rest-activity and core body temperatures of the mice. Each mouse was housed in an individual cage on the receiver board (ER-4000 receiver). All receivers were connected to a computer with the VitalView Data Acquisition System (version 4.2; Respironics, Inc.). The data were recorded in 6 min intervals. After at least a 2 week recovery from surgery, the mice were then used in the experiments.
2.7 Experimental design
2.7.1 Experiment 1
To examine the effect of RF on the isoflurane anaesthesia-induced locomotor activity and core body temperature rhythms, 40 mice were randomly divided into five groups, and each group was treated, as described previously in Section 2.2 Scheduled food access (Figure 1a). All mice were put into cages to monitor the gross motor activity using a Mini-Mitter from the third week after the wireless telemetry was implanted. The data recording ended 1 week after isoflurane anaesthesia. To minimize the usage of mice, we did not use a Control group in this experiment; instead, we recorded 7 days before the RF as the Control baseline (D-pre).
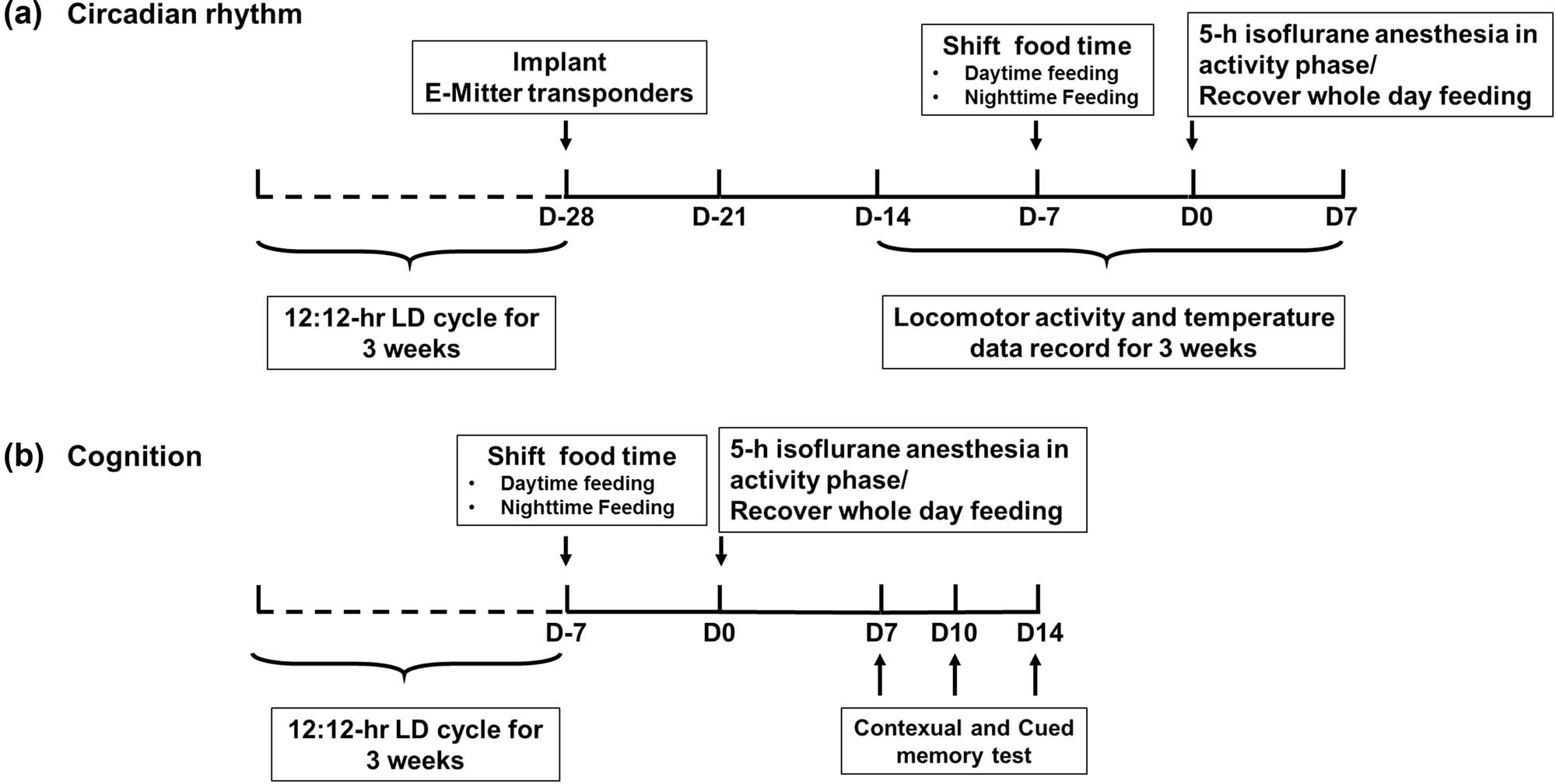
(a) The effect of feeding schedule on daily rhythm before and after isoflurane anaesthesia. After manipulating RF, mice were divided into five groups: control group (feeding throughout the entire day, from ZT0 to ZT24, n = 8), Night-Feeding group (feeding during nighttime, from ZT12 to ZT24, n = 8), Day-Feeding group (feeding during daytime, from ZT0 to ZT12, n = 8), Night-Feeding + Anes group (feeding during nighttime from ZT12 to ZT24 and receive isoflurane on D0, n = 8), and Day-Feeding + Anes group (feeding during daytime from ZT0 to ZT12 and receive isoflurane on D0, n = 8). (b) The effect of feeding schedule on post-anaesthetic fear memory at D7, D10, D14. After manipulating RF, mice were divided into six groups: Control group (feeding throughout the entire day, from ZT0 to ZT24, n = 8), Control + Anes group (feeding throughout the entire day, from ZT0 to ZT24 and receive isoflurane on D0, n = 8), Night-Feeding group (feeding during nighttime, from ZT12 to ZT24, n = 8), and Day-Feeding group (feeding during daytime, from ZT0 to ZT12, n = 8), Night-Feeding + Anes group (feeding during nighttime from ZT12 to ZT24 and receive isoflurane on D0, n = 8), Day-Feeding + Anes group (feeding during daytime from ZT0 to ZT12 and receive isoflurane on D0, n = 8).
2.7.2 Experiment 2
To examine the effect of RF on isoflurane anaesthesia-induced memory deficits, 144 mice were divided randomly into six groups, and each group was treated, as described previously in Section 4.2 Scheduled food access (Figure 1b). We used an FCS to test the contextual and cued memory on D7, D10, and D14 after isoflurane anaesthesia.
2.8 Statistical analyses
All data were expressed as the mean ± SD (standard deviation). In the FCS test, one-way ANOVA, followed by Bonferroni’s multiple comparisons test, was applied at each time point. Daily rhythm analysis was performed by fitting a cosinor function to the data using the Chronos-Fit program for data analysis. This program fits a cosine curve to the measured data points and calculates the following statistics of rhythm: acrophase (peak time of the rhythm), amplitude (half of the peak-to-trough of the rhythmic change), MESOR (a rhythm-adjusted 24 h mean), and diurnality score (light/dark ratio). For clarity, amplitude and MESOR were calculated as the percentage from the baseline and phase shift for acrophase. For each row parameter examined, two-way ANOVAs (Feeding conditions × Anaesthesia) with repeated measures were conducted, followed by Bonferroni post hoc analysis. The statistical analysis was performed using the SPSS 20.0 software (IBM Corporation, Armonk, NY). The differences were considered statistically significant at the level of P < 0.05.
3 Results
3.1 RF and isoflurane anaesthesia did not change the weight of mice
We measured body weight from the first day after shifting the feeding protocol until 7 days after isoflurane anaesthesia (Table 1). The body weights did not significantly differ between the treatment groups throughout the duration of scheduled feedings and 7 days after anaesthesia.
Body weight during the 7-day scheduled feeding period and 7 days after anaesthesia
| Control | Control + Anes | Day-Feeding | Day-Feeding + Anes | Night-Feeding | Night-Feeding + Anes | |
|---|---|---|---|---|---|---|
| D-7 | 22.40 ± 0.51 | 22.90 ± 1.11 | 22.41 ± 0.82 | 22.51 ± 0.91 | 22.51 ± 1.42 | 22.78 ± 1.31 |
| D-6 | 22.10 ± 1.42 | 22.91 ± 1.10 | 22.61 ± 1.20 | 22.82 ± 1.02 | 22.91 ± 1.12 | 22.69 ± 1.21 |
| D-5 | 22.11 ± 1.21 | 22.92 ± 1.09 | 22.51 ± 1.22 | 22.71 ± 1.22 | 22.61 ± 1.11 | 22.79 ± 1.01 |
| D-4 | 22.32 ± 1.11 | 22.92 ± 1.39 | 22.71 ± 1.23 | 22.82 ± 1.22 | 22.61 ± 0.81 | 22.89 ± 1.51 |
| D-3 | 22.61 ± 1.31 | 23.12 ± 1.15 | 22.91 ± 1.12 | 22.82 ± 1.31 | 23.21 ± 0.72 | 22.79 ± 1.21 |
| D-2 | 22.92 ± 1.20 | 22.89 ± 0.89 | 22.69 ± 1.29 | 23.42 ± 1.02 | 22.42 ± 0.91 | 23.81 ± 0.81 |
| D-1 | 23.12 ± 1.29 | 22.91 ± 1.01 | 22.82 ± 1.28 | 23.43 ± 1.12 | 22.19 ± 0.81 | 23.32 ± 1.21 |
| D1 | 23.09 ± 1.22 | 22.92 ± 1.02 | 22.12 ± 1.31 | 23.33 ± 1.21 | 22.22 ± 0.92 | 23.43 ± 1.01 |
| D2 | 23.54 ± 1.31 | 23.23 ± 1.03 | 23.13 ± 1.32 | 23.63 ± 0.91 | 22.52 ± 0.92 | 23.32 ± 0.92 |
| D3 | 23.52 ± 1.31 | 23.32 ± 0.89 | 23.21 ± 1.22 | 23.63 ± 0.92 | 22.69 ± 1.11 | 23.61 ± 0.89 |
| D4 | 23.51 ± 1.31 | 23.71 ± 0.89 | 23.51 ± 1.21 | 23.63 ± 0.92 | 22.89 ± 1.23 | 23.09 ± 1.82 |
| D5 | 23.71 ± 1.41 | 23.75 ± 0.81 | 23.52 ± 1.11 | 23.81 ± 0.91 | 23.09 ± 1.01 | 23.59 ± 0.89 |
| D6 | 23.92 ± 1.51 | 23.72 ± 0.71 | 23.61 ± 1.21 | 23.62 ± 1.12 | 22.72 ± 1.18 | 23.79 ± 0.88 |
| D7 | 24.09 ± 1.61 | 23.81 ± 0.41 | 23.71 ± 1.52 | 23.81 ± 1.13 | 23.41 ± 1.02 | 23.59 ± 0.92 |
3.2 Misaligned feeding disrupted pre-anaesthetic daily rhythm levels
3.2.1 Misaligned feeding disrupted pre-anaesthetic rest-activity rhythm
The activity patterns of the mice were analysed with Chronos-Fit and displayed as double-plotted actograms (Figure 2a and b). The black sections in the actograms represent a greater amount of activity than the average value over 48 h, while the white sections represent less activity. The rhythm of locomotor activity exhibited different trends of change between the Day-Feeding and the Night-Feeding mice. After several days of fluctuation, the Night-Feeding group adapted to the shift in food access gradually. The Day-Feeding group showed increased activity durations during the daytime and decreased activity durations at night, suggesting that their nocturnality was disrupted. We used four parameters to represent the daily rhythm: acrophase, amplitude, MESOR, and diurnality score. The Night-Feeding group exhibited a phase delay on D-7 (18.29 ± 0.36, P < 0.001), which reversed on D-6. The Day-Feeding group demonstrated a phase advance to phase-inverted over time, compared with the group’s own baseline (D-6, 10.07 ± 1.46, P < 0.001; D-5, 10.08 ± 3.31, P < 0.001; D-4, 5.34 ± 1.53, P < 0.001; D-3, 7.07 ± 2.14, P < 0.001; D-2, 6.56 ± 2.78, P < 0.001; and D-1, 7.19 ± 2.43, P < 0.001) (Table 2). The Night-Feeding group demonstrated a decreased amplitude on D-6 (37.94 ± 4.06, P < 0.001) and D-5 (40.25 ± 4.93, P = 0.001), which reversed on D-4. The Day-Feeding group exhibited a decreased amplitude of the rest-activity rhythm on D-7–D-5 (D-7, 33.39 ± 3.34, P < 0.001; D-6, 40.97 ± 5.63, P = 0.005; D-5, 44.96 ± 5.04, P = 0.024), which then reversed on D-4. The Night-Feeding group exhibited a decreased MESOR of the rest-activity rhythm on D-7 (73.85 ± 6.19; P < 0.001) and D-6 (78.7 ± 6.27; P < 0.001), which then reversed to baseline on D-5. The Day-Feeding group demonstrated a significantly decreased MESOR of the rest-activity rhythm on D-7 (62.72 ± 4.84; P < 0.001), which increased on D-5–D-1 (D-5, 130.75 ± 10.44, P < 0.001; D-4, 128.54 ± 11.81, P < 0.001; D-3, 152.59 ± 4.85, P < 0.001; D-2, 141.41 ± 7.02, P < 0.001; and D-1, 145.69 ± 4.89, P < 0.001). The Night-Feeding group displayed a decreased diurnality score on D-3 and D-1. The diurnality score of the Day-Feeding group increased over the 7 days.
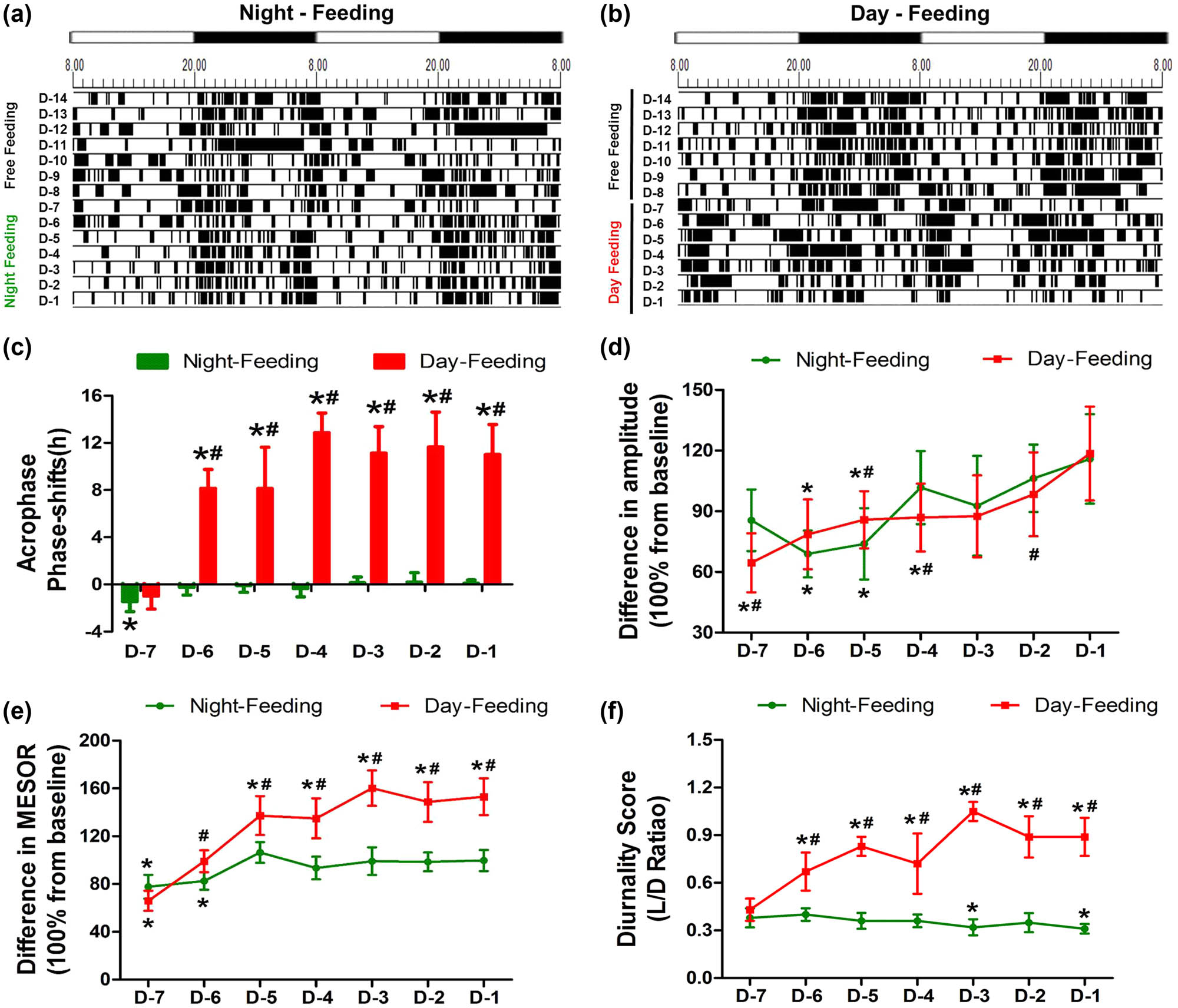
The effect of feeding schedule on rest-activity rhythm during 7 days between the Night-Feeding group and the Day-Feeding group. (a and b) Representative double-plotted actograms show the activity patterns after feeding schedule in a 12 h:12 h light:dark cycle. The black sections in the actograms represent a greater amount of activity than the average value in 48 h, while the white ones represent less activity, the abscissa 8.00 means ZT0; 20.00 means ZT12. (c) Phase shift of acrophase. (d) Amplitude. (e) MESOR. (f) Diurnality Score. *P < 0.05 vs mean values in 7-day pre-feeding period; #P < 0.05 vs Night-Feeding group. Positive values represent phase advance, n = 8.
Results of pre-anaesthetic cosinor analysis after RF
| Locomotor activity | Temperature | |||
|---|---|---|---|---|
| Night-Feeding | Day-Feeding | Night-Feeding | Day-Feeding | |
| Acrophase | ||||
| D-Pre | 18.29 ± 0.36 | 18.21 ± 0.53 | 18.36 ± 0.25 | 18.31 ± 0.54 |
| D-7 | 19.72 ± 0.67 | 19.20 ± 0.84 | 20.69 ± 0.61 | 20.91 ± 0.81 |
| D-6 | 18.53 ± 0.53 | 10.07 ± 1.46 | 20.23 ± 0.19 | 7.85 ± 1.27 |
| D-5 | 18.38 ± 0.55 | 10.08 ± 3.31 | 18.49 ± 0.26 | 9.33 ± 1.26 |
| D-4 | 18.64 ± 0.71 | 5.34 ± 1.53 | 18.43 ± 0.34 | 5.45 ± 1.62 |
| D-3 | 18.15 ± 0.45 | 7.07 ± 2.14 | 18.33 ± 0.28 | 8.75 ± 1.80 |
| D-2 | 18.11 ± 0.73 | 6.56 ± 2.78 | 18.31 ± 0.21 | 7.13 ± 1.37 |
| D-1 | 18.19 ± 0.28 | 7.19 ± 2.43 | 18.16 ± 0.41 | 7.92 ± 1.39 |
| Amplitude | ||||
| D-Pre | 56.13 ± 8.68 | 53.28 ± 7.85 | 0.79 ± 0.08 | 0.79 ± 0.08 |
| D-7 | 46.9 ± 4.30 | 33.39 ± 3.34 | 0.61 ± 0.08 | 0.58 ± 0.13 |
| D-6 | 37.94 ± 4.06 | 40.97 ± 5.63 | 0.51 ± 0.08 | 0.63 ± 0.13 |
| D-5 | 40.25 ± 4.93 | 44.96 ± 5.04 | 0.51 ± 0.08 | 0.61 ± 0.14 |
| D-4 | 55.69 ± 2.63 | 45.38 ± 5.56 | 0.74 ± 0.09 | 0.77 ± 0.14 |
| D-3 | 50.24 ± 5.96 | 45.62 ± 7.85 | 0.71 ± 0.10 | 0.77 ± 0.15 |
| D-2 | 58.65 ± 6.51 | 51.33 ± 7.08 | 0.71 ± 0.09 | 0.70 ± 0.14 |
| D-1 | 63.31 ± 3.55 | 61.56 ± 5.00 | 0.71 ± 0.08 | 0.71 ± 0.14 |
| MESOR | ||||
| D-Pre | 95.57 ± 5.92 | 95.79 ± 7.27 | 36.22 ± 0.14 | 36.20 ± 0.24 |
| D-7 | 73.85 ± 6.19 | 62.72 ± 4.84 | 36.13 ± 0.21 | 35.92 ± 0.61 |
| D-6 | 78.70 ± 6.27 | 94.40 ± 4.94 | 35.90 ± 0.35 | 36.05 ± 0.30 |
| D-5 | 101.30 ± 5.30 | 130.75 ± 10.44 | 36.15 ± 0.19 | 36.21 ± 0.41 |
| D-4 | 88.89 ± 6.03 | 128.54 ± 11.81 | 36.17 ± 0.26 | 36.17 ± 0.25 |
| D-3 | 94.18 ± 6.26 | 152.59 ± 4.85 | 36.02 ± 0.39 | 36.01 ± 0.21 |
| D-2 | 93.85 ± 4.65 | 141.41 ± 7.02 | 36.08 ± 0.25 | 36.18 ± 0.39 |
| D-1 | 95.01 ± 7.07 | 145.69 ± 4.89 | 36.28 ± 0.23 | 36.01 ± 0.49 |
| Diurnality score | ||||
| D-Pre | 0.38 ± 0.03 | 0.38 ± 0.03 | ||
| D-7 | 0.38 ± 0.06 | 0.43 ± 0.07 | ||
| D-6 | 0.40 ± 0.04 | 0.67 ± 0.12 | ||
| D-5 | 0.36 ± 0.05 | 0.83 ± 0.06 | ||
| D-4 | 0.36 ± 0.04 | 0.72 ± 0.19 | ||
| D-3 | 0.32 ± 0.05 | 1.05 ± 0.06 | ||
| D-2 | 0.35 ± 0.06 | 0.89 ± 0.13 | ||
| D-1 | 0.31 ± 0.03 | 0.89 ± 0.12 | ||
3.2.2 Misaligned feeding disrupted pre-anaesthetic core body temperature rhythm
The core body temperature patterns of the mice were analysed with Chronos-Fit and were displayed as double-plotted actograms (Figure 3a and b). The black sections of the actograms represent higher temperatures than the average value over 48 h, while the white sections represent lower temperatures. The rhythm of the core body temperatures had different trends of change between the Day-Feeding and the Night-Feeding mice. After several days of fluctuations, the Night-Feeding group adapted to the shift in food access gradually. The Day-Feeding group showed increased durations of higher temperatures in the daytime and decreased durations of higher temperatures at nighttime, suggesting that their nocturnality was disrupted. The temperature phase shift from baseline differed significantly between the Day-Feeding and the Night-Feeding groups. The Night-Feeding group showed phase delays on D-7 (20.69 ± 0.61, P < 0.001), which then reversed on D-6. The Day-Feeding group showed phase delays on D-7 (20.91 ± 0.81, P < 0.001), which then phase-inverted gradually over the subsequent days (D-6, 7.85 ± 1.27, P < 0.001; D-5, 9.33 ± 1.26, P < 0.001; D-4, 5.45 ± 1.62, P < 0.001; D-3, 8.75 ± 1.8, P < 0.001; D-2, 7.13 ± 1.37, P < 0.001; and D-1, 7.92 ± 1.39, P < 0.001) (Table 2). The Night-Feeding group exhibited a decreased amplitude on D-7–D-5 (D-7, 0.61 ± 0.08, P < 0.001; D-6, 0.51 ± 0.08, P < 0.001; and D-5, 0.51 ± 0.08, P < 0.001), an effect that reversed on D-4. The Day-Feeding group demonstrated a decreased amplitude of the temperature rhythm on D-7–D-5 (D-7, 0.58 ± 0.13, P = 0.007; D-6, 0.63 ± 0.13, P = 0.001; D-5, 0.61 ± 0.14, P = 0.002), which then reversed on D-4. For both the Night-Feeding and the Day-Feeding groups, the food schedule had no significant effect on the MESOR of body temperature rhythm.
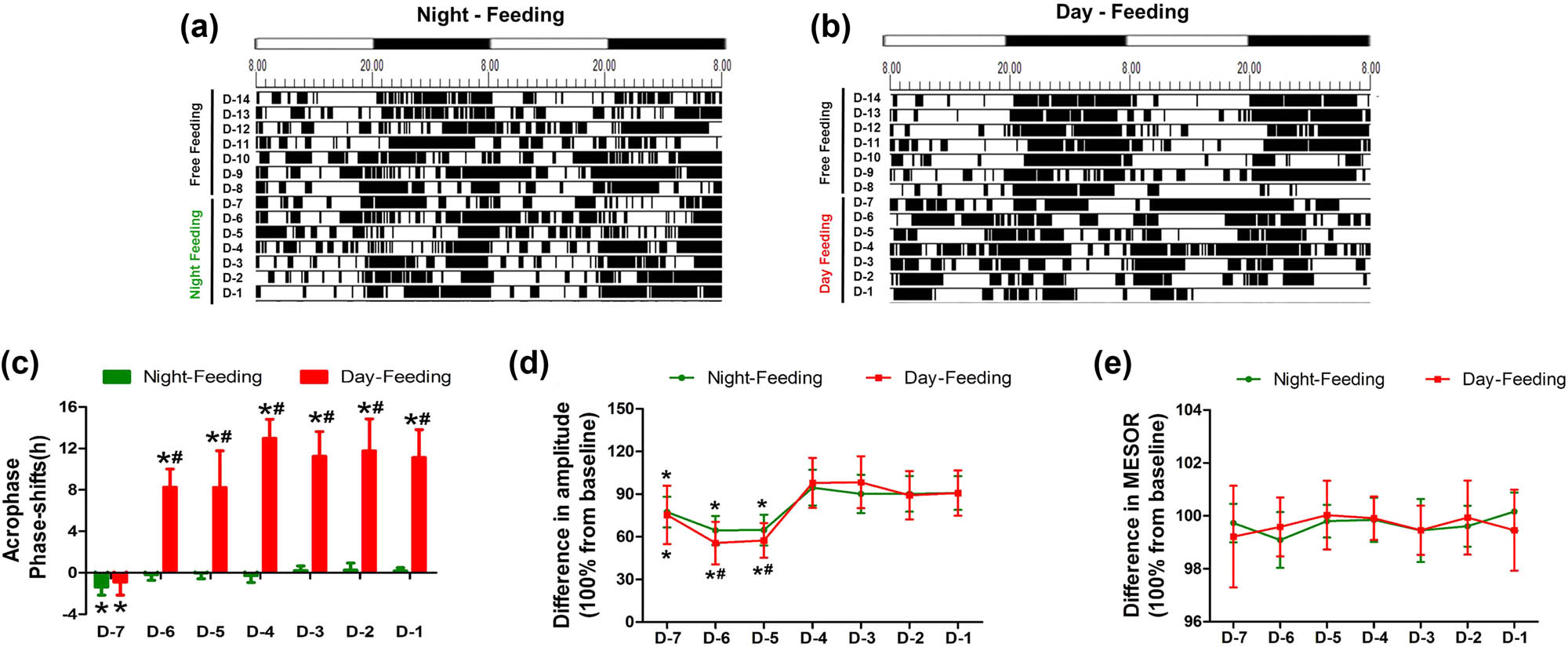
The effect of feeding schedule on temperature rhythm during 7 days. (a and b) Representative double-plotted actograms show the temperature patterns after feeding schedule in a 12 h:12 h light:dark cycle. The black sections in the actograms represent higher temperature than the average value in 48 h, while the white ones represent lower temperature, the abscissa 8.00 means ZT0; 20.00 means ZT12. (c) Phase shift of acrophase. (d) Amplitude. (e) MESOR. *P < 0.05 vs mean values in 7-day pre-feeding period; #P < 0.05 vs Night-Feeding group, n = 8.
3.3 RF altered post-anaesthetic daily rhythm levels
3.3.1 RF altered the post-anaesthetic rest-activity rhythm
From the actograms, the Night-Feeding group and the Night-Feeding + Anes group showed transient rhythm fluctuations, while the Day-Feeding group gradually normalized their rhythm in the subsequent days; the Day-Feeding + Anes group showed persistently increased activity durations in the daytime and decreased activity durations at nighttime. After the RF protocol was omitted, the Night-Feeding group exhibited no rhythm parameter changes; the Day-Feeding group advanced to the acrophase on D1–D3, their amplitude decreased on D1 and D2, and MESOR decreased on D1–D4 and then recovered during the subsequent days.
Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on rest-activity rhythm acrophase showed significant effects of days (F7,38 = 12.73, P < 0.001), with significant interaction (days × food: F14,78 = 5.89, P < 0.001 and days × anaesthesia: F7,38 = 3.8, P = 0.03). After isoflurane anaesthesia, the Control + Anes group exhibited a delayed acrophase on D1 (21.58 ± 1.02, P < 0.001), a decreased amplitude on D1 (32.27 ± 4.18, P < 0.001), and an increased diurnality score on D1 (0.67 ± 0.1, P < 0.05), which then recovered on D2. The acrophase of the Night-Feeding + Anes group was also delayed on D1 compared to the baseline but restored approximately 1 h relative to that of the Anes group (20.39 ± 0.57, P < 0.001). The Day-Feeding + Anes group showed fluctuating phase advances from baseline on D1–D7 (D1, 14.36 ± 1.38, P < 0.001; D2, 13.07 ± 1.3, P < 0.001; D3, 8.62 ± 1.35, P < 0.001; D4, 16.08 ± 0.82, P < 0.001; D5, 10.15 ± 1.89, P < 0.001; D6, 17.40 ± 1.14, P < 0.01; and D7, 16.74 ± 1.55, P = 0.026), which was more difficult to reverse (Figure 4 and Table 3). Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on rest-activity rhythm amplitude showed significant effects of days (F7,38 = 43.61, P < 0.001), food (F16,76 = 6.75, P < 0.001), and anaesthesia (F8,37 = 14.18, P < 0.001) with significant interaction (days × food: F14,78 = 4.20, P < 0.001 and days × anaesthesia: F7,38 = 10.66, P < 0.001). The amplitude of the Night-Feeding + Anes group decreased on D1 but recovered by approximately 11% compared with that of the Control + Anes group (38.5 ± 4.26, P = 0.003 vs. Baseline, P = 0.026 vs. Control + Anes) and reversed on D2. The amplitude of the Day-Feeding + Anes group decreased on D1–D3 (D1, 24.97 ± 3.7, P < 0.001; D2, 37.61 ± 2.66, P < 0.001; D3, 28.51 ± 2.86, P < 0.001), which then reversed on D4. Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on rest-activity rhythm MESOR showed significant effects of days (F7,38 = 12.71, P < 0.001), food (F16,76 = 51.89, P < 0.001), and anaesthesia (F8,37 = 19.58, P < 0.001) with significant interaction (days × food: F14,78 = 3.28, P < 0.001). The MESOR of the Night-Feeding + Anes group did not change after isoflurane anaesthesia. The MESOR of the Day-Feeding + Anes group significantly decreased on D1–D7 (D-7, 62.12 ± 10.78, P < 0.001; D-6, 67.51 ± 5.02, P < 0.001; D-5, 61.95 ± 4.51, P < 0.001; D-4, 61.59 ± 5.06, P < 0.001; D-3, 69.91 ± 6.16, P < 0.001; D-2, 69.35 ± 7.79, P < 0.001; and D-1, 68.78 ± 5.67, P < 0.001). Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on rest-activity rhythm diurnality score showed significant effects of days (F7,38 = 12.73, P < 0.001), food (F16,76 = 82.66, P < 0.001), and anaesthesia (F8,37 = 8.21, P < 0.001) with significant interaction (days × food: F14,78 = 5.89, P < 0.001 and days × anaesthesia: F7,38 = 3.8, P = 0.003). The diurnality score of the Day-Feeding + Anes group increased progressively on D1–D7.
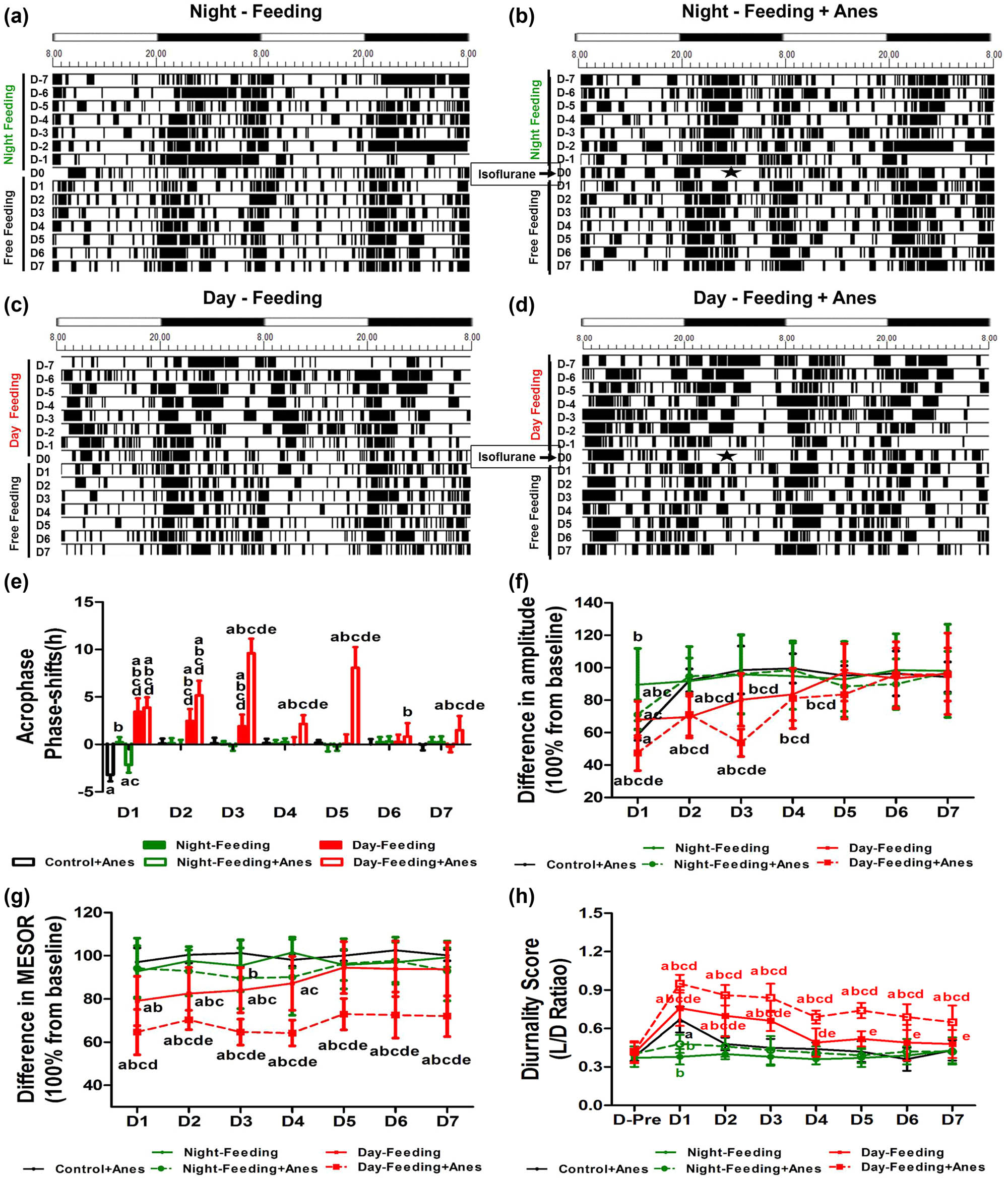
The effect of feeding schedule and isoflurane anaesthesia on rest-activity rhythm during 7 days (D1, D2, D3, D4, D5, D6, D7). (a–d) Representative double-plotted actograms show the activity patterns after feeding schedule and isoflurane anaesthesia in a 12 h:12 h light:dark cycle. The black sections in the actograms represent a greater amount of activity than the average value in 48 h, while the white ones represent less activity, the abscissa 8.00 means ZT0; 20.00 means ZT12. Pentagram represents anaesthesia administration from ZT14 to ZT19. (e) Phase shift of acrophase. (f) Amplitude. (g) MESOR. (h) Diurnality score. aP < 0.05 vs mean values in 7-day pre-administration period; bP < 0.05 vs Control + Anes group; cP < 0.05 vs Night-Feeding group; dP < 0.05 vs Night-Feeding + Anes group; eP < 0.05 vs Day-Feeding group, n = 8.
Results of post-anaesthetic locomotor activity cosinor analysis after RF
| Locomotor activity | |||||
|---|---|---|---|---|---|
| Control + Anes | Night-Feeding + Anes | Night-Feeding | Day-Feeding + Anes | Day-Feeding | |
| Acrophase | |||||
| D-Pre | 18.36 ± 0.41 | 18.36 ± 0.41 | 18.23 ± 0.31 | 18.18 ± 0.63 | 18.23 ± 0.44 |
| D1 | 21.58 ± 1.02 | 18.13 ± 0.31 | 20.39 ± 0.57 | 14.74 ± 1.53 | 14.36 ± 1.38 |
| D2 | 18.26 ± 0.42 | 18.23 ± 0.47 | 18.23 ± 0.45 | 15.71 ± 1.35 | 13.07 ± 1.30 |
| D3 | 18.23 ± 0.44 | 18.35 ± 0.33 | 18.40 ± 0.70 | 16.27 ± 0.80 | 8.62 ± 1.35 |
| D4 | 18.23 ± 0.31 | 18.31 ± 0.36 | 17.98 ± 0.27 | 18.19 ± 0.40 | 16.08 ± 0.82 |
| D5 | 18.18 ± 0.63 | 18.38 ± 0.56 | 18.42 ± 0.24 | 18.14 ± 0.75 | 10.15 ± 1.89 |
| D6 | 18.36 ± 0.29 | 18.13 ± 0.33 | 17.92 ± 0.57 | 17.93 ± 0.34 | 17.40 ± 1.14 |
| D7 | 18.37 ± 0.44 | 18.13 ± 0.42 | 17.98 ± 0.46 | 18.41 ± 0.52 | 16.74 ± 1.55 |
| Amplitude | |||||
| D-Pre | 55.16 ± 7.52 | 57.66 ± 11.11 | 54.59 ± 5.72 | 52.49 ± 7.75 | 54.07 ± 8.41 |
| D1 | 32.27 ± 4.18 | 49.62 ± 4.47 | 38.50 ± 4.26 | 34.96 ± 2.02 | 24.97 ± 3.70 |
| D2 | 50.66 ± 6.38 | 51.3 ± 6.81 | 51.10 ± 1.54 | 35.82 ± 3.56 | 37.61 ± 2.66 |
| D3 | 54.07 ± 8.41 | 52.99 ± 2.71 | 51.52 ± 9.92 | 41.34 ± 5.78 | 28.51 ± 2.86 |
| D4 | 54.59 ± 5.72 | 52.99 ± 5.16 | 53.22 ± 6.84 | 43.26 ± 7.47 | 42.96 ± 6.83 |
| D5 | 52.49 ± 7.75 | 51.48 ± 5.33 | 47.96 ± 7.89 | 49.95 ± 5.39 | 44.18 ± 4.08 |
| D6 | 52.49 ± 4.54 | 55.13 ± 7.17 | 48.88 ± 9.27 | 47.80 ± 2.86 | 50.28 ± 4.52 |
| D7 | 51.57 ± 5.38 | 54.2 ± 8.95 | 52.66 ± 6.74 | 49.10 ± 9.10 | 51.18 ± 7.82 |
| MESOR | |||||
| D-Pre | 95.29 ± 7.32 | 96.37 ± 4.97 | 94.77 ± 6.70 | 95.40 ± 8.53 | 96.19 ± 6.33 |
| D1 | 92.14 ± 5.54 | 88.93 ± 8 | 88.95 ± 12.66 | 75.05 ± 10.11 | 62.12 ± 10.78 |
| D2 | 95.76 ± 9.27 | 93.85 ± 2.49 | 87.37 ± 6.55 | 78.12 ± 9.23 | 67.51 ± 5.02 |
| D3 | 96.19 ± 6.33 | 91.45 ± 7.91 | 84.14 ± 9.22 | 79.64 ± 8.01 | 61.95 ± 4.51 |
| D4 | 93.52 ± 8.70 | 97.68 ± 7.25 | 84.55 ± 13.26 | 82.54 ± 9.38 | 61.59 ± 5.06 |
| D5 | 95.40 ± 8.53 | 92.03 ± 4.93 | 90.82 ± 9.52 | 89.43 ± 7.63 | 69.91 ± 6.16 |
| D6 | 97.44 ± 5.20 | 93.2 ± 7.43 | 91.95 ± 6.23 | 88.80 ± 7.80 | 69.35 ± 7.79 |
| D7 | 95.4 ± 6.57 | 95.42 ± 3.52 | 87.33 ± 8.26 | 88.76 ± 9.06 | 68.78 ± 5.67 |
| Diurnality score | |||||
| D-Pre | 0.38 ± 0.03 | 0.40 ± 0.06 | 0.37 ± 0.07 | 0.42 ± 0.08 | 0.41 ± 0.08 |
| D1 | 0.67 ± 0.10 | 0.48 ± 0.07 | 0.38 ± 0.06 | 0.95 ± 0.07 | 0.76 ± 0.14 |
| D2 | 0.48 ± 0.05 | 0.46 ± 0.08 | 0.40 ± 0.04 | 0.86 ± 0.08 | 0.70 ± 0.16 |
| D3 | 0.45 ± 0.07 | 0.43 ± 0.11 | 0.38 ± 0.07 | 0.84 ± 0.11 | 0.66 ± 0.08 |
| D4 | 0.44 ± 0.05 | 0.41 ± 0.05 | 0.36 ± 0.04 | 0.69 ± 0.05 | 0.49 ± 0.11 |
| D5 | 0.42 ± 0.08 | 0.39 ± 0.06 | 0.37 ± 0.07 | 0.74 ± 0.06 | 0.52 ± 0.06 |
| D6 | 0.46 ± 0.09 | 0.42 ± 0.10 | 0.39 ± 0.07 | 0.69 ± 0.1 | 0.49 ± 0.14 |
| D7 | 0.43 ± 0.08 | 0.42 ± 0.10 | 0.43 ± 0.07 | 0.65 ± 0.13 | 0.48 ± 0.11 |
3.3.2 RF altered post-anaesthetic core body temperature rhythm
From the actograms, we found that the core body temperatures of the different groups had similar trends of change in terms of locomotor activity. The acrophase of the Day-Feeding group advanced on D1–D3, the amplitude decreased on D1–D5, which then recovered over the subsequent days. The MESOR of the Day-Feeding showed no change.
Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on temperature acrophase showed significant effects of days (F7,38 = 31.17, P < 0.001), food (F16,76 = 48.73, P < 0.001), and anaesthesia (F8,37 = 13.17, P = 0.001) with significant interaction (days × food: F14,78 = 6.37, P < 0.001 and days × anaesthesia: F7,38= 15.65, P < 0.001). After isoflurane anaesthesia, the Control + Anes group exhibited a delayed acrophase on D1 (20.21 ± 0.74, P < 0.001), a decreased amplitude on D1 (0.50 ± 0.08, P < 0.001), which then recovered on D2. The Night-Feeding + Anes group acrophase was delayed on D1 (20.46 ± 0.91, P < 0.001), compared with the baseline. The Day-Feeding + Anes group showed persistent phase advances from baseline on D1–D7 (D1, 7.47 ± 1.69, P < 0.001; D2, 12.78 ± 1.42, P < 0.001; D3, 12.15 ± 1.15, P < 0.001; D4, 12.92 ± 1.58, P < 0.001; D5, 9.99 ± 1.69, P < 0.001; D6, 12.33 ± 2.32, P < 0.001; and D7, 14.05 ± 1.26, P< 0.001), an effect that was difficult to reverse (Figure 5 and Table 4). Multivariate repeated-measure analysis (between factors: food and anaesthesia); within factor: days on temperature amplitude showed significant effects of days (F7,38 = 40.21, P < 0.001), food (F16,76 = 143.18, P < 0.001) with significant interaction (days × food: F14,78 = 5.32, P < 0.001 and days × anaesthesia: F7,38 = 16.90, P < 0.001). The Night-Feeding + Anes group’s amplitude decreased on D1 (0.46 ± 0.03, P < 0.001), an effect that was reversed on D2. The Day-Feeding + Anes group exhibited a decreased amplitude in the temperature rhythm on D1–D5 (D1, 0.36 ± 0.04, P < 0.001; D2, 0.38 ± 0.04, P < 0.001; D3, 0.41 ± 0.04, P < 0.001; D4, 0.56 ± 0.06, P = 0.001; and D5, 0.54 ± 0.04, P < 0.001), an effect that was reversed on D6. For both the Night-Feeding + Anes and the Day-Feeding + Anes groups, the food schedule had no significant effect on the MESOR of the body temperature rhythm on D1–D7.
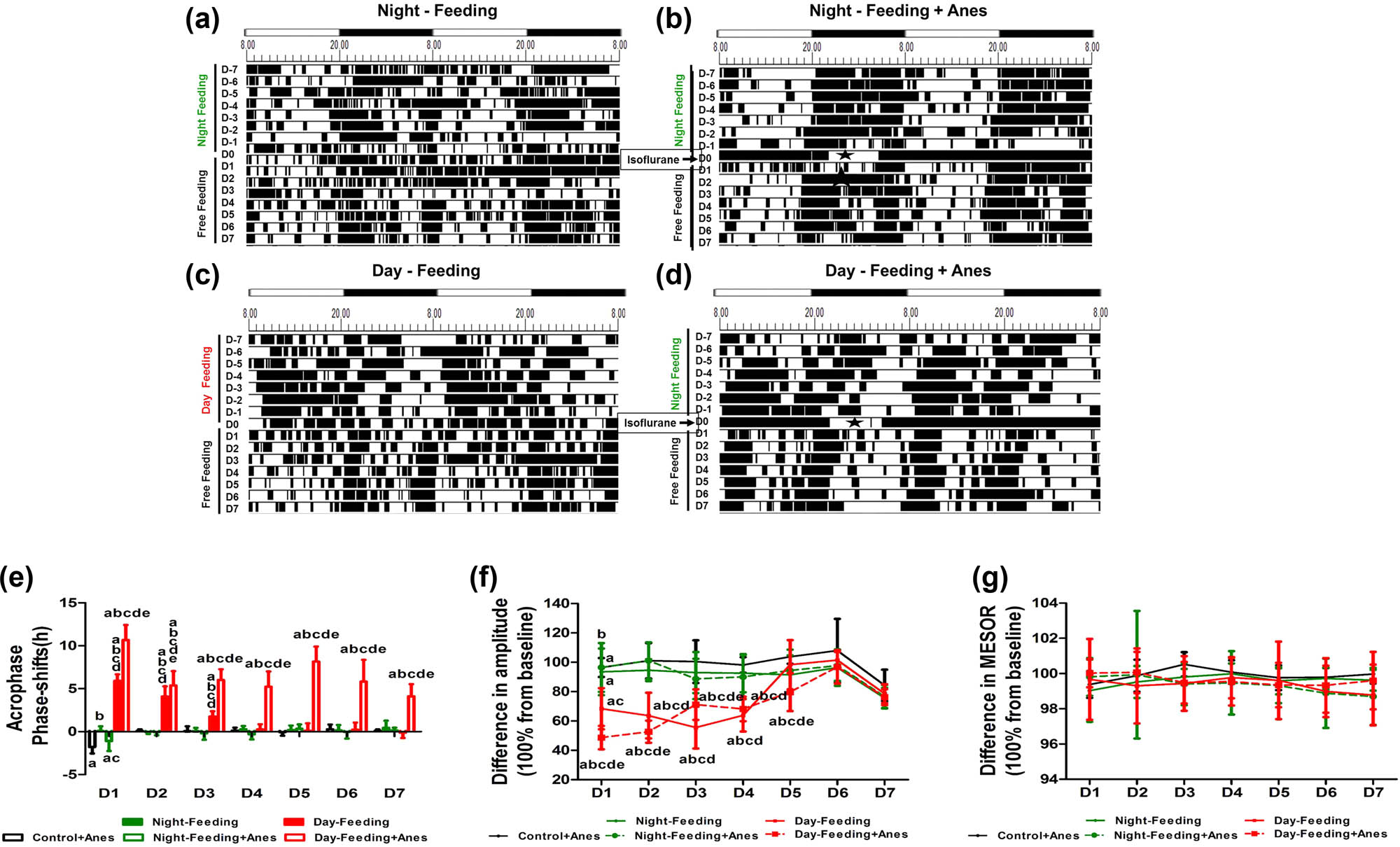
The effect of feeding schedule and isoflurane anaesthesia on temperature rhythm during 7 days (D1, D2, D3, D4, D5, D6, D7). (a–d) Representative double-plotted actograms show the temperature patterns after feeding schedule and isoflurane anaesthesia in a 12 h:12 h light:dark cycle. The black sections in the actograms represent higher temperature than the average value in 48 h, while the white ones represent lower temperature, the abscissa 8.00 means ZT0; 20.00 means ZT12. Pentagram represents anaesthesia administration from ZT14 to ZT19. (e) Phase shift of acrophase. (f) Amplitude. (g) MESOR. aP < 0.05 vs Mean values in 7-day pre-administration period; bP < 0.05 vs Control + Anes group; cP < 0.05 vs Night-Feeding group; dP < 0.05 vs Night-Feeding + Anes group; eP < 0.05 vs Day-Feeding group, n = 8.
Results of post-anaesthetic body core temperature cosinor analysis after RF
| Temperature | |||||
|---|---|---|---|---|---|
| Control + Anes | Night-Feeding + Anes | Night-Feeding | Day-Feeding + Anes | Day-Feeding | |
| Acrophase | |||||
| D-Pre | 18.42 ± 0.25 | 18.42 ± 0.25 | 18.31 ± 0.24 | 18.48 ± 0.62 | 18.15 ± 0.41 |
| D1 | 20.21 ± 0.74 | 18.32 ± 0.42 | 20.46 ± 0.91 | 12.57 ± 0.52 | 7.47 ± 1.69 |
| D2 | 18.33 ± 0.39 | 18.45 ± 0.34 | 18.35 ± 0.26 | 14.39 ± 1.09 | 12.78 ± 1.42 |
| D3 | 18.40 ± 0.56 | 18.39 ± 0.39 | 18.50 ± 0.72 | 16.73 ± 0.19 | 12.15 ± 1.15 |
| D4 | 18.31 ± 0.24 | 18.15 ± 0.30 | 18.61 ± 0.54 | 18.22 ± 0.45 | 12.92 ± 1.58 |
| D5 | 18.48 ± 0.62 | 18.22 ± 0.40 | 17.96 ± 0.56 | 18.36 ± 0.46 | 9.99 ± 1.69 |
| D6 | 18.15 ± 0.42 | 18.27 ± 0.53 | 18.33 ± 0.75 | 18.25 ± 0.54 | 12.33 ± 2.32 |
| D7 | 18.33 ± 0.17 | 17.97 ± 0.73 | 18.14 ± 0.27 | 18.61 ± 0.42 | 14.05 ± 1.26 |
| Amplitude | |||||
| D-Pre | 0.79 ± 0.08 | 0.79 ± 0.08 | 0.79 ± 0.08 | 0.78 ± 0.09 | 0.80 ± 0.08 |
| D1 | 0.50 ± 0.08 | 0.73 ± 0.08 | 0.46 ± 0.03 | 0.50 ± 0.05 | 0.36 ± 0.04 |
| D2 | 0.76 ± 0.08 | 0.73 ± 0.09 | 0.75 ± 0.07 | 0.52 ± 0.06 | 0.38 ± 0.04 |
| D3 | 0.80 ± 0.08 | 0.75 ± 0.05 | 0.79 ± 0.05 | 0.49 ± 0.08 | 0.41 ± 0.04 |
| D4 | 0.79 ± 0.08 | 0.73 ± 0.08 | 0.69 ± 0.06 | 0.42 ± 0.08 | 0.56 ± 0.06 |
| D5 | 0.78 ± 0.09 | 0.73 ± 0.06 | 0.70 ± 0.08 | 0.49 ± 0.06 | 0.54 ± 0.04 |
| D6 | 0.82 ± 0.10 | 0.71 ± 0.07 | 0.73 ± 0.07 | 0.75 ± 0.06 | 0.63 ± 0.07 |
| D7 | 0.84 ± 0.11 | 0.76 ± 0.07 | 0.77 ± 0.08 | 0.79 ± 0.06 | 0.76 ± 0.05 |
| MESOR | |||||
| D-Pre | 36.21 ± 0.14 | 36.21 ± 0.14 | 36.24 ± 0.14 | 36.21 ± 0.33 | 36.20 ± 0.14 |
| D1 | 35.98 ± 0.17 | 35.86 ± 0.68 | 36.17 ± 0.38 | 36.08 ± 0.62 | 36.20 ± 0.26 |
| D2 | 36.17 ± 0.28 | 36.03 ± 0.32 | 36.21 ± 1.24 | 35.95 ± 0.63 | 36.22 ± 0.35 |
| D3 | 36.40 ± 0.14 | 36.14 ± 0.15 | 36.02 ± 0.41 | 36.00 ± 0.38 | 36.01 ± 0.37 |
| D4 | 36.24 ± 0.14 | 36.20 ± 0.16 | 36.04 ± 0.61 | 36.12 ± 0.19 | 36.03 ± 0.44 |
| D5 | 36.12 ± 0.19 | 36.06 ± 0.27 | 35.99 ± 0.29 | 36.06 ± 0.55 | 35.96 ± 0.40 |
| D6 | 36.13 ± 0.13 | 36.11 ± 0.19 | 35.83 ± 0.63 | 35.84 ± 0.41 | 35.95 ± 0.50 |
| D7 | 36.20 ± 0.08 | 36.07 ± 0.16 | 35.76 ± 0.49 | 35.77 ± 0.47 | 36.05 ± 0.54 |
3.4 Misaligned feeding prolonged post-anaesthetic hippocampus-independent learning and memory deficits
Compared with the Control group, the Control + Anes group showed a contextual memory decrease on D7 (53.43 ± 4.73%, P < 0.001) and then recovered on D10 (70.18 ± 2.82%, P > 0.05). The Day-Feeding group exhibited a reduction in contextual fear-conditioned behaviour, which lasted until D10 (D7, 63.34 ± 2.47%, P < 0.001; and D10, 61.58 ± 2.81%, P < 0.001) and recovered on D14 (70.22 ± 3.17%, P > 0.05), indicating that the circadian misalignment damaged hippocampus-dependent memory. There was no difference between the Night-Feeding group and the Control group. Compared to the Control + Anes group, the Night-Feeding + Anes group showed an increase in contextual fear memory on D7 (62.86 ± 3.24%, P < 0.001), though this increase was still lower than that of the Control group (P < 0.001). The Day-Feeding group did not exhibit aggravated memory deficits on D14, compared with the Control group (P = 0.647). However, compared with the Control group, the Day-Feeding + Anes group showed a prolonged memory deficit, lasting even 2 weeks post-anaesthesia (D7, 49.49 ± 4.95%, P < 0.001; D10, 46.16 ± 5.46%, P < 0.001; and D14, 58.72 ± 3.97%, P < 0.001), with the Control + Anes group recovering on D10 and the Day-Feeding group recovering on D14. The Day-Feeding + Anes group showed reduced contextual memory compared to the Day-Feeding group on D7 (P < 0.001), D10 (P < 0.001), and D14 (P < 0.001); the Night-Feeding + Anes group also showed reduced contextual memory compared to the Night-Feeding group on D7 (P = 0.007), which suggested that isoflurane anaesthesia aggravated the misaligned feeding-induced hippocampus-dependent memory dysfunction. Amygdala-dependent cued-fear conditioning was not altered by the alignment/misalignment of feeding (Figure 6b). Isoflurane induced cued memory deficits on D7 (P < 0.001).
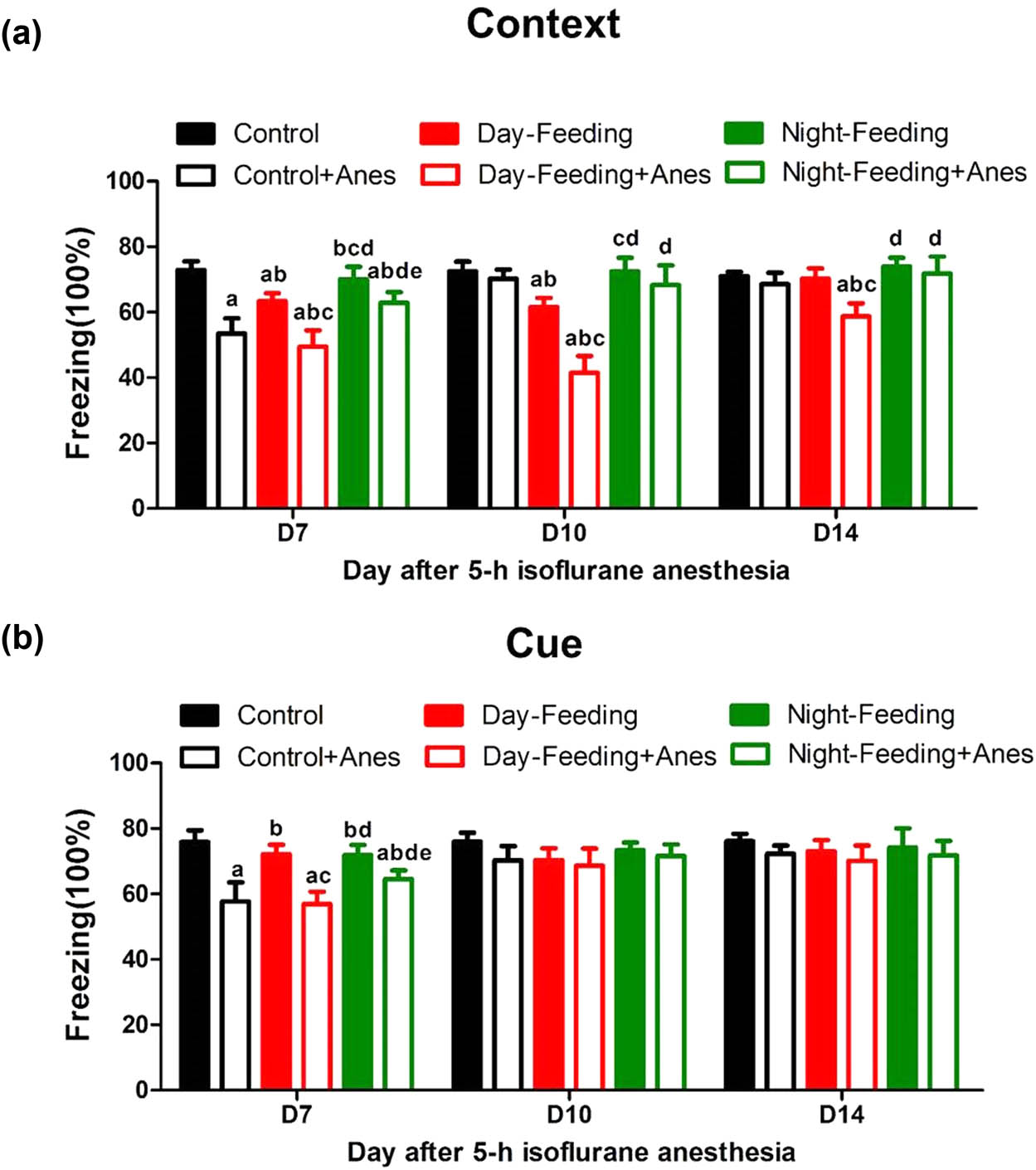
The effect of 7-day feeding schedule on post-anaesthetic fear memory at D7, D10, D14. (a) Contextual fear conditioning. Daytime feeding and isoflurane induced persistent contextual fear memory decline on D7, D10, D14. (b) Cued-fear conditioning. Isoflurane induced cued-fear memory decline on D7. aP < 0.05 vs Control; bP < 0.05 vs Control + Anes group; cP < 0.05 vs Day-Feeding group; dP < 0.05 vs Day-Feeding + Anes group; eP < 0.05 vs Night-Feeding + Anes group; n = 8.
4 Discussion
In this study, we sought to determine if temporally RF schedules in mice could impact post-anaesthetic cognition through daily rhythm modulation. We proved that a 1-week short period of misaligned food before anaesthesia was sufficient to lead to sustained post-anaesthetic daily rhythm disruption; meanwhile, aligned feeding partially restored post-anaesthetic daily rhythm parameters. One-week aligned feeding attenuated and even reversed post-anaesthetic memory deficits, while 1 week misaligned feeding led to substantial post-anaesthetic hippocampus-dependent contextual fear conditioning impairment, even when the mice were switched back to ad libitum feeding.
In our experiments, the mice did not lose or gain weight. Therefore, the present study emphasized how short-term disrupted feeding schedules induced daily rhythm disturbance, instead of metabolic effects. Animal studies [14,15] have shown that food intake can serve as a major time cue for peripheral tissue clocks, such as the liver, but not for the SCN. Conflicting zeitgeber timings can lead to internal desynchrony, a state in which different clocks throughout the body are out of sync [16]. In this experiment, we quantitated several parameters that characterize daily rhythm: the acrophase, amplitude, and MESOR, along with diurnality score. Alterations in these measures indicate impairments in the regulation of the circadian system [17]. The daily rhythms of locomotor activity and body temperature have been simultaneously monitored in many studies conducted on laboratory animals [18,19]. Our results showed a daily nocturnal rhythm of body temperature and locomotor activity: longer duration of higher values during the night and shorter duration of higher values during the day; the rhythm of body temperature proceeding closely to the rhythm of locomotor activity, except in regard to the MESOR parameter. The MESOR of the different rhythms could not be compared because they refer to distinct physical quantities. The robustness of rhythm should be taken into consideration.
Loh reported that 2 weeks of ZT3 to ZT9 food access alters diurnal rhythms of activity and sleep [20]. In our experiments, before anaesthesia, with 7 days of feeding during the subjective rest time, the acrophase of locomotor activity converted 18.21 to 7.19, and the diurnality score converted 0.38–0.89, which suggested that we successfully modify the basic daily rhythm. Others have suggested that feeding-entrained rhythms persist for several days, if feedings are omitted [21]. Consistently, we confirmed that after 1 week of restricted food, the locomotor activity and temperature rhythm parameters mainly returned to baseline (Figures 4 and 5).
Post-operative patients usually experience various sleep disturbances, including sleep-wake cycle disturbances [22]. There have been an increasing number of studies that have demonstrated that anaesthetics can disrupt the daily rhythm [23,24,25]. We also proved in this experiment that long-term isoflurane anaesthesia caused mice with ad libitum feeding an approximately 3 h acrophase delay and 40% amplitude decrease, and 76% diurnality score increase on D1 and returned back to baseline on D2. Nowadays, many people live in an environment where their circadian system is challenged by inappropriate work-times associated with dietary intake. In this study, we manipulated feeding time shifts to explore their effects on post-anaesthetic daily rhythm. Daytime feeding for 1 week caused aggravated post-anaesthetic daily rhythm parameter disruption, which lasted for approximately 5–7 days; nighttime feeding partially recovered post-anaesthetic acrophase shift, the amplitude decrease, and diurnality score increase. Therefore, we demonstrated that 7 days of misaligned feeding was sufficient to induce basic circadian dysrhythmia, which then aggravated and prolonged the post-anaesthetic circadian rhythmicity disruption. Meanwhile, aligned feeding partially enhanced post-anaesthetic daily rhythm.
Circadian rhythms modulate many physiological behaviours, including memory processes [26]. Long-term exposure to internal desynchrony is thought to contribute to the adverse effects of circadian misalignment, including the increased risk for cognitive dysfunction [27,28]. Some pieces of clinical evidence have suggested that mealtime interventions improve behavioural symptoms in elderly people with dementia [29,30]. In our previous work, we also found that melatonin pre-treatment prevented isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythms in mice. Isoflurane anaesthesia induced contextual and cue memory dysfunction on D7 and then recovered on D10 in mice. Therefore, we tried to explore whether a 1 week aligned feeding schedule was sufficient to enhance post-anaesthetic daily rhythm, which could ameliorate post-anaesthetic memory deficits. Then, we proved that feeding at activity time for 1 week improved post-anaesthetic memory on D7. We did not see aggravated post-anaesthetic memory deficits after daytime feeding. A reason for this may have been a “floor effect”, as misaligned feeding could not aggravate the sharp memory decrease induced by isoflurane anaesthesia. Another explanation may be that FCS was not sufficient for the detection of precise differences between the two groups. Automatically, we lengthened the observation time and sought to determine whether pre-anaesthetic daily rhythm disturbance could prolong post-anaesthetic cognitive dysfunction. We proved that 1 week of feeding during rest time prolonged post-anaesthetic contextual memory deficits, which lasted 2 weeks, with feeding being omitted after anaesthesia, whereas the memory of the wild-type recovered 10 days after isoflurane anaesthesia. Compared with the contextual memory test, RF did not change amygdala-dependent cued-fear conditioning. This implied that some learned behaviours are more vulnerable to the impact of misaligned feeding. Potential mechanisms should be considered in the future study. A possible mechanism may be 5-HT7 receptor, a subtype of the 5-HT receptor family, and is highly expressed in the hippocampus and SCN, where it is strongly involved in cognition and diurnal–nocturnal rhythm of animal’s activity.
Our study demonstrated that an incorrect time of food intake and the subsequent circadian disruption might aggravate and prolong post-anaesthetic cognitive dysfunction. Meanwhile, aligned feeding induced robust rhythmicity and could improve post-anaesthetic memory deficits. Meal timing can be under individual control and thus represents a potentially complementary approach for future postoperative cognitive dysfunction prevention strategies.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81730033, 81701371, 81801380), Natural Science Foundation of Jiangsu Province (BK20170654, BK20170129), and the Key Talent's of 13th Five-Year Plan for Strengthening Health of Jiangsu Province (ZDRCA2016069).
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121, 121–5, 281–4.10.1002/0470090839.ch9Search in Google Scholar
[2] Sato M, Murakami M, Node K, Matsumura R, Akashi M. The role of the endocrine system in feeding-induced tissue-specific circadian entrainment. Cell Rep. 2014;8:393–401.10.1016/j.celrep.2014.06.015Search in Google Scholar PubMed
[3] Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3:694–704.10.1016/j.molmet.2014.07.002Search in Google Scholar PubMed PubMed Central
[4] Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–53.10.1016/j.cell.2010.08.016Search in Google Scholar PubMed
[5] Wang D, Opperhuizen AL, Reznick J, Turner N, Su Y, Cooney GJ, et al. Effects of feeding time on daily rhythms of neuropeptide and clock gene expression in the rat hypothalamus. Brain Res. 2017;1671:93–101.10.1016/j.brainres.2017.07.006Search in Google Scholar PubMed
[6] Marquie JC, Tucker P, Folkard S, Gentil C, Ansiau D. Chronic effects of shift work on cognition: findings from the. VISAT longitudinal study. Occup Environ Med. 2015;72:258–64.10.1136/oemed-2013-101993Search in Google Scholar PubMed
[7] Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89:445–53.10.1212/WNL.0000000000004171Search in Google Scholar PubMed PubMed Central
[8] Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74:411–8.10.1001/jamaneurol.2016.5192Search in Google Scholar PubMed PubMed Central
[9] Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267.10.1371/journal.pone.0015267Search in Google Scholar PubMed PubMed Central
[10] Kent BA. Synchronizing an aging brain: can entraining circadian clocks by food slow Alzheimer's disease? Front Aging Neurosci. 2014;6:234.10.3389/fnagi.2014.00234Search in Google Scholar PubMed PubMed Central
[11] Willis GL, Freelance CB. Emerging preclinical interest concerning the role of circadian function in Parkinson's disease. Brain Res. 2018;1678:203–13.10.1016/j.brainres.2017.09.027Search in Google Scholar PubMed
[12] Cuesta M, Aungier J, Morton AJ. Behavioral therapy reverses circadian deficits in a transgenic mouse model of Huntington's disease. Neurobiol Dis. 2014;63:85–91.10.1016/j.nbd.2013.11.008Search in Google Scholar PubMed
[13] Xia T, Cui Y, Chu S, Song J, Qian Y, Ma Z, et al. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain Res. 2016;1634:12–20.10.1016/j.brainres.2015.10.036Search in Google Scholar PubMed
[14] Pendergast JS, Oda GA, Niswender KD, Yamazaki S. Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s). Proc Natl Acad Sci USA. 2012;109:14218–23.10.1073/pnas.1206213109Search in Google Scholar PubMed PubMed Central
[15] Sheward WJ, Maywood ES, French KL, Horn JM, Hastings MH, Seckl JR, et al. Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice. J Neurosci. 2007;27:4351–8.10.1523/JNEUROSCI.4843-06.2007Search in Google Scholar PubMed PubMed Central
[16] Foster RG, Kreitzman L. The rhythms of life: what your body clock means to you! Exp Physiol. 2014;99:599–606.10.1113/expphysiol.2012.071118Search in Google Scholar PubMed
[17] Huitron-Resendiz S, Marcondes MC, Flynn CT, Lanigan CM, Fox HS. Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc Natl Acad Sci USA. 2007;104:15138–43.10.1073/pnas.0707171104Search in Google Scholar PubMed PubMed Central
[18] Refinetti R, Kenagy GJ. Circadian rhythms of body temperature and locomotor activity in the antelope ground squirrel, Ammospermophilus leucurus. J Therm Biol. 2018;72: 67–72.10.1016/j.jtherbio.2018.01.001Search in Google Scholar PubMed
[19] Schreiber S, Bader M, Lenchinski T, Meningher I, Rubovitch V, Katz Y, et al. Functional effects of synthetic cannabinoids versus Delta(9)-THC in mice on body temperature, nociceptive threshold, anxiety, cognition, locomotor/exploratory parameters and depression. Addict Biol. 2019;24:414–25.10.1111/adb.12606Search in Google Scholar PubMed
[20] Loh DH, Jami SA, Flores RE, Truong D, Ghiani CA, O'Dell TJ, et al. Misaligned feeding impairs memories. Elife. 2015;4:e09460.10.7554/eLife.09460Search in Google Scholar PubMed PubMed Central
[21] Szentirmai E, Kapas L, Sun Y, Smith RG, Krueger JM. Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2010;298:R467–77.10.1152/ajpregu.00557.2009Search in Google Scholar PubMed PubMed Central
[22] Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109:769–75.10.1093/bja/aes252Search in Google Scholar PubMed
[23] Touitou Y, Mauvieux B, Reinberg A, Dispersyn G. Disruption of the circadian period of body temperature by the anesthetic propofol. Chronobiol Int. 2016;33:1247–54.10.1080/07420528.2016.1208664Search in Google Scholar PubMed
[24] Cheeseman JF, Winnebeck EC, Millar CD, Kirkland LS, Sleigh J, Goodwin M, et al. General anesthesia alters time perception by phase shifting the circadian clock. Proc Natl Acad Sci USA. 2012;109:7061–6.10.1073/pnas.1201734109Search in Google Scholar PubMed PubMed Central
[25] Matsuo I, Iijima N, Takumi K, Higo S, Aikawa S, Anzai M, et al. Characterization of sevoflurane effects on Per2 expression using ex vivo bioluminescence imaging of the suprachiasmatic nucleus in transgenic rats. Neurosci Res. 2016;107:30–7.10.1016/j.neures.2015.11.010Search in Google Scholar PubMed
[26] Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–88.10.1038/nrn2881Search in Google Scholar PubMed PubMed Central
[27] Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50.10.3389/fnmol.2012.00050Search in Google Scholar PubMed PubMed Central
[28] Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology – a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9.10.1038/nrneurol.2013.269Search in Google Scholar PubMed PubMed Central
[29] Whear R, Abbott R, Thompson-Coon J, Bethel A, Rogers M, Hemsley A, et al. Effectiveness of mealtime interventions on behavior symptoms of people with dementia living in care homes: a systematic review. J Am Med Dir Assoc. 2014;15:185–93.10.1016/j.jamda.2013.10.016Search in Google Scholar PubMed
[30] Wong F, Keller HH, Schindel ML, Sutherland O. A recipe for mealtime resilience for families living with dementia. Scand J Caring Sci. 2015;29:486–94.10.1111/scs.12176Search in Google Scholar PubMed
© 2020 Jia Song et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Changing the tone of clinical study design in the cannabis industry
- Integrated Chinese and western medicine for acute guillain-barré syndrome treatment
- Hydrochloride fasudil attenuates brain injury in ICH rats
- Protective role of maize purple plant pigment against oxidativestress in fluorosis rat brain
- A novel low-cost electrode for recording the local field potential of freely moving rat’s brain
- Neuroprotective mechanisms of erythropoietin in a rat stroke model
- Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia
- Dysbiosis characteristics of gut microbiota in cerebral infarction patients
- miR-142-3p suppresses apoptosis in spinal cord-injured rats
- Decreased MiR-30a promotes TGF-β1-mediated arachnoid fibrosis in post-hemorrhagic hydrocephalus
- Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease
- EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats
- Efficacy of Novavit in ameliorating the neurotoxicity of propionic acid
- High-fat diet-induced obesity and impairment of brain neurotransmitter pool
- Erratum to “Changing the tone of clinical study design in the cannabis industry”
- Development of a trunk motor paradigm for use in neuroimaging
- Notoginsenoside R1 attenuates sevoflurane-induced neurotoxicity
- Saikosaponin A attenuates neural injury caused by ischemia/reperfusion
- Safety profile of the transcription factor EB (TFEB)-based gene therapy through intracranial injection in mice
- Partial enzyme digestion facilitates regeneration of crushed nerve in rat
- Correlation between calcium, water contents and ultrasonographic appearance of atherosclerotic lesions of carotid artery lesions
- Diabetes does not affect motor recovery after intracerebral hemorrhage
- IRF8 is crucial for the nicotine withdrawal-induced hyperalgesia in mice
- Prediction of muscle loss after stroke by analysis of corticospinal tract
- Interleukin-27 levels in patients with myasthenia gravis
- Artesunate attenuates traumatic brain injury-induced impairments in rats
- BDMC protects AD in vitro via AMPK and SIRT1
- Injury of the optic radiation in patients with mild TBI: A DTT study
- Time-restricted feeding alters isoflurane-induced memory deficits
- Video-based education improves the image quality of diagnostic percutaneous cerebral angiography among elderly patients
- The effect of rTMS in the management of pain associated with CRPS
- EGCG treats ICH via up-regulating miR-137-3p and inhibiting Parthanatos
- Modified hemispherectomy for infantile hemiparesis and epilepsy
- Isovitexin modulates autophagy in Alzheimer’s disease via miR-107 signalling
- Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model
- Role of prefrontal cortex during Sudoku task: fNIRS study
- Tongxinluo promotes axonal plasticity and functional recovery after stroke
- Eriodictyol corrects functional recovery and myelin loss in SCI rats
- Letter to the Editor
- Ataxic hemiparesis after corona radiata infarct: Diffusion tensor imaging correlation of corticoponto-cerebellar tract injury
- Compression of the lateral antebrachial cutaneous nerve by a traumatic arteriovenous fistula
- Rapid Communication
- Anticholinergic drugs and oral health-related quality of life in patients with schizophrenia: a pilot study
- Deviant cortical sulcation related to schizophrenia and cognitive deficits in the second trimester
- Case Report
- A case of primary central nervous system lymphoma mimic neuromyelitis optica
- A Moving Residual Limb: Botulinum Toxin to the Rescue
- Clinical and imaging features of reversible splenial lesion syndrome with language disorder
- Impaired consciousness due to injury of the ascending reticular activating system in a patient with bilateral pontine infarction: A case report
- Commentary
- A comment on Morey et al. (2020)
- Review Articles
- Advances in transcription factors related to neuroglial cell reprogramming
- The “authentic subjective experience” of memory in Alzheimer’s disease
- Chronic neurological diseases and COVID-19: Associations and considerations
- Special Issue "Neuroinflammation: from basic to clinical perspectives"
- Ormosanine improves neuronal functions in spinal cord-injured rats by blocking peroxynitrite/calpain activity
- Retraction
- Retraction of: Identification of biological markers for better characterization of older subjects with physical frailty and sarcopenia
Articles in the same Issue
- Research Articles
- Changing the tone of clinical study design in the cannabis industry
- Integrated Chinese and western medicine for acute guillain-barré syndrome treatment
- Hydrochloride fasudil attenuates brain injury in ICH rats
- Protective role of maize purple plant pigment against oxidativestress in fluorosis rat brain
- A novel low-cost electrode for recording the local field potential of freely moving rat’s brain
- Neuroprotective mechanisms of erythropoietin in a rat stroke model
- Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia
- Dysbiosis characteristics of gut microbiota in cerebral infarction patients
- miR-142-3p suppresses apoptosis in spinal cord-injured rats
- Decreased MiR-30a promotes TGF-β1-mediated arachnoid fibrosis in post-hemorrhagic hydrocephalus
- Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease
- EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats
- Efficacy of Novavit in ameliorating the neurotoxicity of propionic acid
- High-fat diet-induced obesity and impairment of brain neurotransmitter pool
- Erratum to “Changing the tone of clinical study design in the cannabis industry”
- Development of a trunk motor paradigm for use in neuroimaging
- Notoginsenoside R1 attenuates sevoflurane-induced neurotoxicity
- Saikosaponin A attenuates neural injury caused by ischemia/reperfusion
- Safety profile of the transcription factor EB (TFEB)-based gene therapy through intracranial injection in mice
- Partial enzyme digestion facilitates regeneration of crushed nerve in rat
- Correlation between calcium, water contents and ultrasonographic appearance of atherosclerotic lesions of carotid artery lesions
- Diabetes does not affect motor recovery after intracerebral hemorrhage
- IRF8 is crucial for the nicotine withdrawal-induced hyperalgesia in mice
- Prediction of muscle loss after stroke by analysis of corticospinal tract
- Interleukin-27 levels in patients with myasthenia gravis
- Artesunate attenuates traumatic brain injury-induced impairments in rats
- BDMC protects AD in vitro via AMPK and SIRT1
- Injury of the optic radiation in patients with mild TBI: A DTT study
- Time-restricted feeding alters isoflurane-induced memory deficits
- Video-based education improves the image quality of diagnostic percutaneous cerebral angiography among elderly patients
- The effect of rTMS in the management of pain associated with CRPS
- EGCG treats ICH via up-regulating miR-137-3p and inhibiting Parthanatos
- Modified hemispherectomy for infantile hemiparesis and epilepsy
- Isovitexin modulates autophagy in Alzheimer’s disease via miR-107 signalling
- Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model
- Role of prefrontal cortex during Sudoku task: fNIRS study
- Tongxinluo promotes axonal plasticity and functional recovery after stroke
- Eriodictyol corrects functional recovery and myelin loss in SCI rats
- Letter to the Editor
- Ataxic hemiparesis after corona radiata infarct: Diffusion tensor imaging correlation of corticoponto-cerebellar tract injury
- Compression of the lateral antebrachial cutaneous nerve by a traumatic arteriovenous fistula
- Rapid Communication
- Anticholinergic drugs and oral health-related quality of life in patients with schizophrenia: a pilot study
- Deviant cortical sulcation related to schizophrenia and cognitive deficits in the second trimester
- Case Report
- A case of primary central nervous system lymphoma mimic neuromyelitis optica
- A Moving Residual Limb: Botulinum Toxin to the Rescue
- Clinical and imaging features of reversible splenial lesion syndrome with language disorder
- Impaired consciousness due to injury of the ascending reticular activating system in a patient with bilateral pontine infarction: A case report
- Commentary
- A comment on Morey et al. (2020)
- Review Articles
- Advances in transcription factors related to neuroglial cell reprogramming
- The “authentic subjective experience” of memory in Alzheimer’s disease
- Chronic neurological diseases and COVID-19: Associations and considerations
- Special Issue "Neuroinflammation: from basic to clinical perspectives"
- Ormosanine improves neuronal functions in spinal cord-injured rats by blocking peroxynitrite/calpain activity
- Retraction
- Retraction of: Identification of biological markers for better characterization of older subjects with physical frailty and sarcopenia

