EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats
-
Jianjun Wang
Abstract
Background
Spinal cord injury (SCI) causes devastating loss of function and neuronal death without effective treatment. (−)-Epigallocatechin-3-gallate (EGCG) has antioxidant properties and plays an essential role in the nervous system. However, the underlying mechanism by which EGCG promotes neuronal survival and functional recovery in complete spinal cord transection (ST) remains unclear.
Methods
In the present study, we established primary cerebellar granule neurons (CGNs) and a T10 ST rat model to investigate the antioxidant effects of EGCG via its modulation of protein kinase D1 (PKD1) phosphorylation and inhibition of ferroptosis.
Results
We revealed that EGCG significantly increased the cell survival rate of CGNs and PKD1 phosphorylation levels in comparison to the vehicle control, with a maximal effect observed at 50 µM. EGCG upregulated PKD1 phosphorylation levels and inhibited ferroptosis to reduce the cell death of CGNs under oxidative stress and to promote functional recovery and ERK phosphorylation in rats following complete ST.
Conclusion
Together, these results lay the foundation for EGCG as a novel strategy for the treatment of SCI related to PKD1 phosphorylation and ferroptosis.
1 Background
Many studies have reported that severe spinal cord injury (SCI) leads to neuronal death and axonal loss in the local microenvironment, eventually resulting in a devastating loss of function [1,2]. Various intrinsic and extrinsic mechanisms have been investigated to promote developmentally related and injury-induced changes that limit neuronal survival in the adult central nervous system [3,4].
Many of the beneficial effects of green tea on the nervous system were attributed to its abundant catechin. (−)-Epigallocatechin-3-gallate (EGCG), the major catechin found in green tea (Camellia sinensis), has been extensively investigated as the predominant active polyphenol and a promising therapeutic agent for the treatment of chronic inflammation and oxidative damage-related diseases [5,6,7]. EGCG also inhibits the TNF-α-activated nuclear factor-kappa B (NF-kB) pathway and enhances the nuclear factor E2-related factor 2 protein levels in macrophages [8]. EGCG was verified to protect against liver injury via its antioxidant effects [9]. EGCG targeting HO-1 reduces contrast-induced kidney damage through antioxidative stress pathways [10]. Several experimental studies have shown that EGCG can provide neuroprotection against brain injury, SCI, and sciatic nerve injury [11,12]. These benefits are mainly due to free radical scavenging or the antioxidant and anti-apoptotic properties of EGCG [13,14]. However, the underlying mechanisms remain to be investigated.
Protein kinase D1 (PKD1), also called protein kinase Cµ [15], is a serine/threonine protein kinase that is different from PKC family members owing to its special structural, regulatory, and enzymatic properties [16]. Moreover, PKD1 can function not only in a normal state but also in a diseased state [15,17,18]. PKD1 is increasingly implicated in modulating a diverse range of functions within the cell, including signal transduction pathways, cell proliferation, angiogenesis, invasion, motility, survival, and apoptosis [17,19,20,21,22,23]. It has been shown that activation of PKD1 induced by oxidative stress can, in turn, activate the transcription factor NF-κB to protect injured cells from cell death following oxidative stress-induced lesions [24]. Interestingly, the neuronal damage caused by oxidative stress has been shown to be involved in SCI [25,26].
Ferroptosis was recently identified as an iron-dependent novel cell death mechanism [27,28,29] with morphological, genetic, and mechanistic differences from traditional regulated cell death pathways, including necrosis, apoptosis, and autophagy [27,28,30]. Apoptosis is a noninflammatory process, and the process of iron-induced cell death is often accompanied by inflammatory manifestations. Ferroptosis is a result of failure of membrane lipid repair, leading to the accumulation of reactive oxygen species (ROS) on membrane lipids [31,32,33] and eventually leading to cell death. Ferroptosis requires the simultaneous depletion of glutathione (GSH) or inactivation of GSH-dependent antioxidant enzyme glutathione peroxidase 4 (GPX4) and the incorporation of oxidizable polyunsaturated fatty acids into phospholipids [34], and the process of iron-dependent cell death is accompanied by inflammatory manifestations.
Therefore, the aim of this study was to investigate the antioxidant effects of EGCG on promoting neuronal survival and functional recovery after spinal cord transection (ST), focusing on its anti-ferroptotic properties and its modulatory effect on PKD1 phosphorylation.
1.1 Primary culture of cerebellar granule neurons (CGNs) and treatments
The primary culture of CGNs was performed according to previous studies with minor modifications [35,36,37]. Briefly, cerebellum from P7 rats was dissected. The tissues were kept on ice in Ca2+/Mg2+-free Hank’s balanced salt solution, followed by digestion with 0.125% trypsin at 37°C in a humidified 5% CO2 atmosphere for 30 min followed by trituration and seeding into culture wells. After 4 h in culture with complete Dulbecco's modification of Eagle's medium (DMEM), the medium was replaced with Neurobasal-A (Life Technologies) culture medium supplemented with 2% B27 (Life Technologies) and 1% penicillin/streptomycin mixture (Solarbio Biotech Corp).
To test the effect of EGCG on the CGNs, cells were subjected to two different treatments: (i) CGNs were treated with Neurobasal-A medium containing 0, 0.5, 5, 50, or 500 µM EGCG for 48 h, followed by MTT assay; (ii) CGNs were exposed to 20 µM H2O2 for 2 h and then maintained in Neurobasal-A medium containing 50 µM EGCG with or without PKD1 inhibitor (CID755673)/ferroptosis inducer (erastin) for 48 h. Finally, cell viability, western blot, and ELISA assays were performed.
-
Ethical approval:: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals.
1.2 Cell viability assay
Cell viability assays were performed according to previous studies with minor modifications [38,39]. The Cell Counting Kit 8 (CCK-8; HY-K0301, MedChem Express, China) assay was used to evaluate cell viability. At the indicated time points, 10 µL of CCK-8 solution was added to each well in a 96-well culture plate, following a 2-h incubation at 37°C. Then, absorbance was measured in a multiwell plate reader (Tecan Infinite® M1000 Pro) at 490 nm.
1.3 Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed according to previous studies with minor modifications [40]. ELISA kits manufactured by Hanhong Biochemical Company (Sino Biological, China) were used to measure PKD1 phosphorylation and related ferroptosis marker activities. Absorbance at 450 nm was measured by an ELISA reader (Tecan Infinite® M1000 Pro).
1.4 Surgical procedures for complete ST
The surgical procedures were performed on female rats according to previous studies with minor modifications [39]. Briefly, the T9 segment of the spinal cord was completely removed using angled microscissors. After confirming the completed transection by lifting the cut ends of the cord, the surgical incisions were sutured. Following surgery, animals were placed on a heating pad to facilitate their recovery from anesthesia and surgery and then housed in individual cages. All animals survived for 2 months after the surgery.
To investigate the neuroprotective role of EGCG after complete ST, eight rats per group were randomly divided into four groups: (A) ST, (B) ST + EGCG, (C) ST + EGCG + CID755673 (PKD1 inhibitor), and (D) ST + EGCG + erastin (ferroptosis inducer). The treatment groups were intraperitoneally injected with 100 µL of phosphate-buffered saline or EGCG (the final concentration diluted in blood was 50 µM) with or without CID755673/erastin once daily for 7 days after the surgery. The rats without ST or EGCG treatment were used as the Sham control. No animals died during the 12-week survival study, after which the animals were sacrificed.
1.5 Behavioral tests
Hindlimb locomotor function was investigated by the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale, inclined grid climbing assessment and rotarod test.
BBB scoring was performed as previously described [39,41], and the rats were placed in an open field (70 cm × 70 cm × 50 cm) to move freely.
The grid climbing test was performed as previously described [39,42]. Animals had to move from the bottom to the top of a grid placed 45° from the horizontal plane. Videos were recorded for more than 5 min each. The knee joint angle of animals climbing the grid and the number of animals whose hindlimbs gripped the grid during climbing were calculated.
The rotarod test was performed as previously described [43]. The Rotamex Rotarod system (UGO Basile Rat&Mouse Rota-Rod 47700/600, Italy) contained a rotating rod that was divided into five parts by a splitter plate to permit the testing of five animals at one time. The test was performed in the same mode, and the speed when the rat fell off was recorded. Each animal was tested three times to obtain the average value.
1.6 Tissue processing
At 8 weeks after the surgery, the animals were euthanized by deep anesthesia with isoflurane and transcardially perfused with saline. Then, the L3 segment of the spinal cord and biceps femoris muscle was dissected and harvested for further analyses.
1.7 Western blot analysis
Equivalent quantities of cell lysates were combined with 20% loading buffer (LB) (0.125 mol/L Tris–HCl, pH 6.8, 20% glycerol, 10% sodium dodecyl sulfate, 0.1% bromophenol blue and 5% β-mercaptoethanol) and heated at 95°C for 15 min. Samples were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Nonspecific protein binding sites were blocked with 5% nonfat milk or 5% bovine serum albumin (BSA) diluted in Tris–HCl saline buffer containing 0.1% Tween-20 (TBST, pH 7.4). Membranes were incubated with the specific antibodies rabbit anti-pErk1/2 (1:1,000, ab4370; Abcam), rabbit anti-Erk1/2 (1:1,000, ab4695; Abcam), and anti-GAPDH (1:1,000; Beyotime Biotechnology) overnight at 4°C. After three washes with 0.1% TBST for 5 min, a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:1,000; Boster) diluted in TBST was added, followed by three washes with 0.1% TBST for 5 min each at room temperature. Antigens were visualized using enhanced chemiluminescence (Beyotime Biotechnology). The intensity of immunostaining was measured with ImageJ 6.0 software.
1.8 Statistics
All statistical analyses were performed using GraphPad Prism 6 software. Data are reported as mean ± standard deviation and were analyzed using analysis of variance followed by the post hoc Bonferroni test. p < 0.05 was considered to be statistically significant.
2 Results
2.1 EGCG increases the survival rate and upregulates PKD1 phosphorylation in a dose-dependent manner
To evaluate the effect of EGCG on CGNs, cell viability and ELISA assays were performed after the CGNs were treated with various concentrations of EGCG (0, 0.5, 5, 50, and 500 µM) for 48 h.
We observed that the survival rate of CGNs was increased in response to EGCG treatment in a dose-dependent manner, with a peak level observed at a concentration of 50 µM (Figure 1a). A similar pattern for PKD1 phosphorylation levels was also observed (Figure 1b).
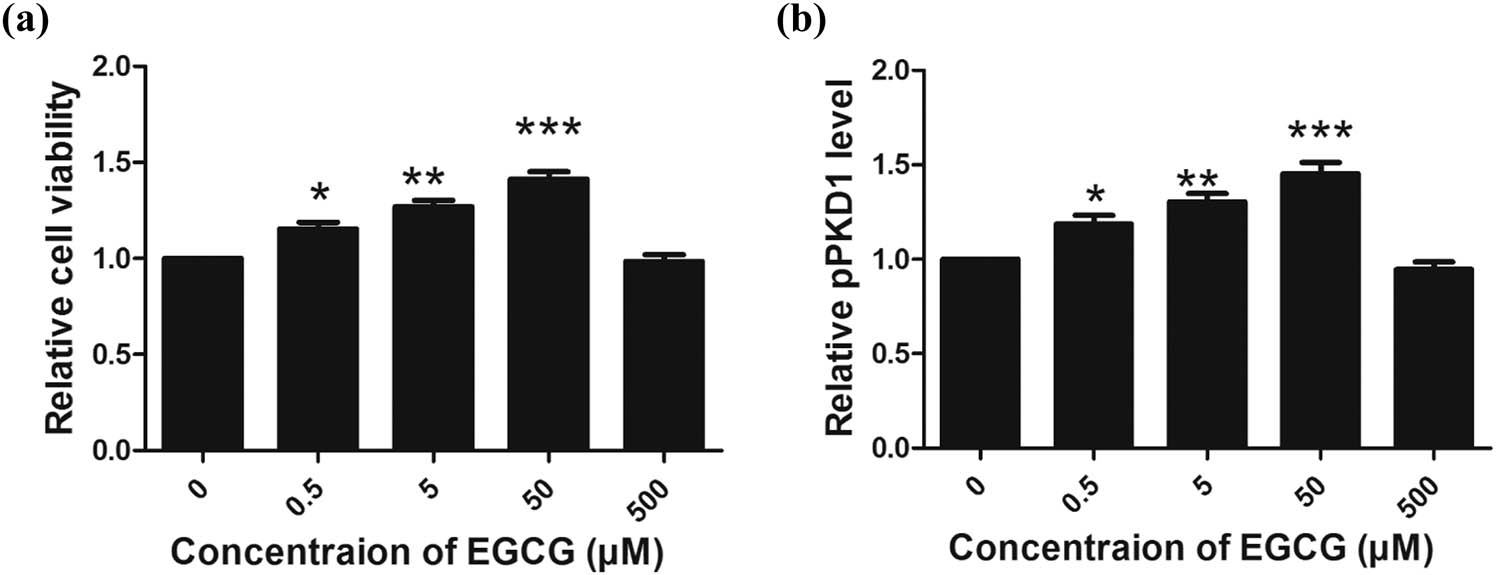
Effect of EGCG on cell viability and PKD1 phosphorylation in CGNs in vitro. (a) EGCG increased the peak levels of CGN survival and PKD1 phosphorylation at a concentration of 50 µM (a and b). (*p < 0.05, **p < 0.01, ***p < 0.0001, five independent experiments.)
These results revealed that EGCG treatment at a concentration of 50 µM can increase the CGN cell survival rate with a maximum effect. As a consequence, 50 µM, the maximum protective concentration of EGCG (also observed in our previous study [44]) was selected to investigate the neuroprotective role of EGCG in the following in vitro and in vivo models.
2.2 EGCG upregulates PKD1 phosphorylation to inhibit ferroptosis and increase the survival rate of CGNs under oxidative stress
To evaluate the effect of EGCG on the survival of CGNs under oxidative stress, cell viability and western blotting were performed after CGNs were treated with 20 µM H2O2 for 2 h and following treatment with 50 µM EGCG or CID755673 for 48 h.
We found that treatment with H2O2 decreased the cell survival rates of CGNs and PKD1 phosphorylation and promoted ferroptosis, whereas EGCG increased the cell survival of CGNs and PKD1 phosphorylation and inhibited ferroptosis induced by H2O2. When the cells were treated with CID755673, EGCG did not increase the cell survival of CGNs or inhibit ferroptosis induced by H2O2. After treating the cells with erastin, EGCG did not increase the cell survival of CGNs exposed to H2O2 (Figure 2a–h).

Effect of EGCG on CGN survival under oxidative stress in vitro. (a) EGCG protected against the cell death of CGNs induced by H2O2 by modulating PKD1 and ferroptosis. (b) PKD1 phosphorylation levels were increased in response to EGCG treatment in CGNs under oxidative stress. (c–h) Ferroptosis was inhibited in response to EGCG treatment in CGNs under oxidative stress, as indicated by the downregulation of ACSL4, COX2, NOX1, and PTGS2 and the upregulation of FTH1 and GPX4. (*p < 0.05, **p < 0.01, ***p < 0.0001, five independent experiments.)
2.3 EGCG upregulates PKD1 phosphorylation to inhibit ferroptosis and promote the functional recovery of rats following ST
To investigate the effect of EGCG on the promotion of functional recovery in rats after complete ST, BBB scoring and grid climbing tests were performed.
We observed that complete ST decreased the BBB score, whereas EGCG increased the BBB score after injury. In rats treated with CID755673, EGCG did not increase the BBB score after injury. After treating the rats with erastin, EGCG did not increase the BBB score (Figure 3a). Similar patterns for the knee joint angle and speed were also observed (Figure 3b and c).
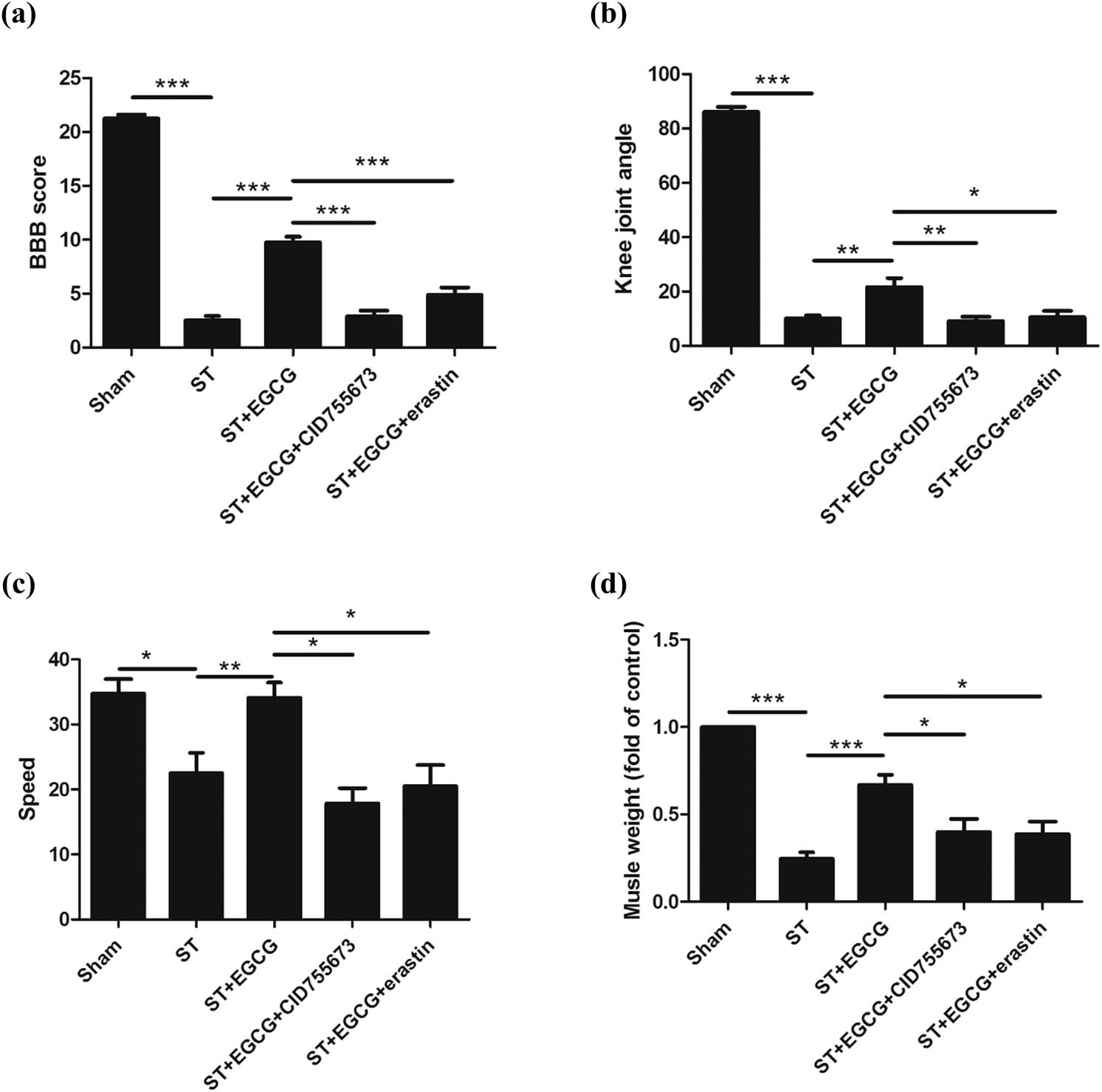
Effect of EGCG on CGNs and functional recovery in rats after complete ST. EGCG increased PKD1 phosphorylation and inhibited ferroptosis to promote functional recovery in rats, as indicated by (a) increased BBB score, (b) knee joint angle, (c) speed in the rotarod test, and (d) biceps femoris muscle weight. (*p < 0.05, **p < 0.01, ***p < 0.0001, n = 8.)
We also observed that complete ST decreased the weight of the biceps femoris muscle, whereas EGCG increased the weight of the biceps femoris muscle. In rats treated with CID755673, EGCG did not increase the weight of the biceps femoris muscle after injury. After treating the rats with erastin, EGCG did not increase the weight of the biceps femoris muscle (Figure 3d).
2.4 EGCG upregulates PKD1 phosphorylation to inhibit ferroptosis and promote the survival of neurons in the spinal cord of rats following ST
To evaluate the effect of EGCG on the survival of neurons after complete ST, ELISA and western blotting were performed.
We found that ferroptosis was activated in response to complete ST, whereas EGCG inhibited ferroptosis in the spinal cord; moreover, EGCG could not inhibit ferroptosis when rats were treated with CID755673 and erastin (Figure 4a–f).

Effect of EGCG on neuronal survival in the spinal cord in rats after complete ST. (a–f) Ferroptosis was inhibited in response to EGCG treatment in the spinal cord, as indicated by the downregulation of ACSL4, COX2, NOX1, and PTGS2 and upregulation of FTH1 and GPX4. (g) PKD1 phosphorylation levels were increased in response to EGCG treatment. (h and i) ERK phosphorylation levels were increased in response to EGCG treatment. (*p < 0.05, **p < 0.01, ***p < 0.0001, n = 5.)
We found that PKD1 phosphorylation levels were downregulated in response to complete ST, whereas EGCG increased PKD1 phosphorylation levels in the spinal cord; moreover, EGCG did not increase PKD1 phosphorylation levels when CID755673 and erastin were used (Figure 4g).
We found that ERK phosphorylation levels were downregulated in response to complete ST, whereas EGCG increased ERK phosphorylation levels; moreover, EGCG could not upregulate pERK under injury in response to CID755673 and erastin (Figure 4h and i).
3 Discussion
Previous studies, including ours, have indicated the neuroprotective roles of EGCG in the nervous system [11,12,44]. In the present study, we revealed that EGCG increases neuronal survival and promotes functional recovery by modulating PKD1 phosphorylation and inhibiting ferroptosis under complete ST.
After SCI, the local microenvironment critically influences neuronal survival, axonal regeneration, and functional recovery [45,46]. Assessment of neurological function is a commonly used measure to evaluate the degree of injury and the therapeutic effect of medications [39]. In the present study, we observed that EGCG can promote functional recovery after injury.
Functional recovery can be spontaneously regained in SCI due to the survival of neurons in the spinal cord that control hindlimb locomotor activity below the lesion [47]. PKD1 has been reported to prevent neuronal cell death [24]. Moreover, PKD1 may function by regulating the ERK signaling pathway [36]. Previous studies have also shown that Erk1/2 signaling cascades exert a key role in the regulation of gene expression and prevention of apoptosis [48]. It has also been reported that the activation of MAPK/Erk can promote the differentiation and survival of neurons [49,50]. In the present study, we indicate that EGCG can increase PKD1 and ERK phosphorylation levels to promote neuronal survival after injury.
Therefore, about the question remains about what are the underlying mechanisms of EGCG to exert its neuroprotective effects. We then focused on the newly discovered type of cell death, ferroptosis, which requires simultaneous depletion of GSH or inactivation of GSH-dependent antioxidant enzyme GPX4 and incorporation of oxidizable polyunsaturated fatty acids into phospholipids [34]. GPX4, a lipid repair enzyme, is the central regulator of ferroptosis [32,33,51,52]. In the present study, we observed that EGCG can inhibit ferroptosis to promote neuronal survival after injury.
Taken together, these results indicate that treatment with EGCG partially accelerates functional recovery after complete ST by affecting PKD1 phosphorylation and inhibiting ferroptosis.
Abbreviations
- BBB
-
Basso, Beattie, and Bresnahan
- CGNs
-
Cerebellar granule neurons
- EGCG
-
(−)-Epigallocatechin-3-gallate
- ELISA
-
Enzyme-linked immunosorbent assay
- GPX4
-
Glutathione peroxidase 4
- GSH
-
Glutathione
- LB
-
Loading buffer
- NF-κB
-
Nuclear factor-kappa B
- PKD1
-
Protein kinase D1
- ROS
-
Reactive oxygen species
- SCI
-
Spinal cord injury
- ST
-
Spinal cord transection.
-
Funding: The Research Foundation of Education Department of Hunan Province, China (No. 16B245), Scientific research projects of Chenzhou science and Technology Bureau (No. CZ-XNXY201509), Natural Science Foundation of Hunan Province in China (No. 2017JJ3273), and Key scientific research project of Hunan Health Committee (No. 20201963). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
-
Author contributions: JW and YT conceived, designed, and wrote the manuscript. YC, LC, YD, YL, YX, HJ, QT, SZ, and BZ performed the experiments and data analysis. All authors approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72:235–7.10.1001/jamaneurol.2014.2927Search in Google Scholar PubMed
[2] Lai BQ, Che MT, Du BL, Zeng X, Ma YH, Feng B, et al. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials. 2016;109:40–54.10.1016/j.biomaterials.2016.08.005Search in Google Scholar PubMed
[3] Xia X, Qu B, Ma Y, Yang LB, Huang HD, Cheng JM, et al. Analyzing time-series microarray data reveals key genes in spinal cord injury. Mol Biol Rep. 2014;41:6827–35.10.1007/s11033-014-3568-9Search in Google Scholar PubMed
[4] Gwak SJ, Macks C, Jeong DU, Kindy M, Lynn M, Webb K, et al. RhoA knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model. Biomaterials. 2017;121:155–66.10.1016/j.biomaterials.2017.01.003Search in Google Scholar PubMed PubMed Central
[5] Min SY, Yan M, Kim SB, Ravikumar S, Kwon SR, Vanarsa K, et al. Green tea epigallocatechin-3-gallate suppresses autoimmune arthritis through indoleamine-2,3-dioxygenase expressing dendritic cells and the nuclear factor, erythroid 2-like 2 antioxidant pathway. J Inflamm (Lond). 2015;12:53.10.1186/s12950-015-0097-9Search in Google Scholar PubMed PubMed Central
[6] Shanmugam T, Selvaraj M, Poomalai S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: an in-vivo and in-silico study. Int Immunopharmacol. 2016;39:128–39.10.1016/j.intimp.2016.07.022Search in Google Scholar PubMed
[7] Alvarez E, Leiro J, Orallo F. Effect of (−)-epigallocatechin-3-gallate on respiratory burst of rat macrophages. Int Immunopharmacol. 2002;2:849–55.10.1016/S1567-5769(02)00032-2Search in Google Scholar
[8] Jiang J, Mo ZC, Yin K, Zhao GJ, Lv YC, Ouyang XP, et al. Epigallocatechin-3-gallate prevents TNF-alpha-induced NF-kappaB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int J Mol Med. 2012;29:946–56.10.3892/ijmm.2012.924Search in Google Scholar
[9] Liu D, Zhang X, Jiang L, Guo Y, Zheng C. Epigallocatechin-3-gallate (EGCG) attenuates concanavalin A-induced hepatic injury in mice. Acta Histochem. 2014;116:654–62.10.1016/j.acthis.2013.12.002Search in Google Scholar PubMed
[10] Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X, et al. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS One. 2016;11:e0149032.10.1371/journal.pone.0149032Search in Google Scholar PubMed PubMed Central
[11] Machova Urdzikova L, Ruzicka J, Karova K, Kloudova A, Svobodova B, Amin A, et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology. 2017;126:213–23.10.1016/j.neuropharm.2017.09.006Search in Google Scholar PubMed
[12] Renno WM, Benov L, Khan KM. Possible role of antioxidative capacity of (−)-epigallocatechin-3-gallate treatment in morphological and neurobehavioral recovery after sciatic nerve crush injury. J Neurosurg Spine. 2017;27:593–613.10.3171/2016.10.SPINE16218Search in Google Scholar PubMed
[13] Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-inflammatory action of green tea. Antiinflamm Antiallergy Agents Med Chem. 2016;15:74–90.10.2174/1871523015666160915154443Search in Google Scholar PubMed
[14] Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol Res. 2016;104:70–85.10.1016/j.phrs.2015.12.027Search in Google Scholar PubMed
[15] Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A. 1994;91:8572–6.10.1073/pnas.91.18.8572Search in Google Scholar PubMed PubMed Central
[16] Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiol (Bethesda). 2011;26:23–33.10.1152/physiol.00037.2010Search in Google Scholar PubMed PubMed Central
[17] Jaggi M, Du C, Zhang W, Balaji KC. Protein kinase D1: a protein of emerging translational interest. Front Biosci. 2007;12:3757–67.10.2741/2349Search in Google Scholar PubMed
[18] Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–8.10.1016/S0021-9258(17)37580-4Search in Google Scholar
[19] Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–8.10.1074/jbc.R500002200Search in Google Scholar PubMed
[20] Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, et al. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200.10.1016/S0962-8924(02)02262-6Search in Google Scholar
[21] Rykx A, De Kimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, et al. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–6.10.1016/S0014-5793(03)00487-3Search in Google Scholar PubMed
[22] Guha S, Tanasanvimon S, Sinnett-Smith J, Rozengurt E. Role of protein kinase D signaling in pancreatic cancer. Biochem Pharmacol. 2010;80:1946–54.10.1016/j.bcp.2010.07.002Search in Google Scholar PubMed PubMed Central
[23] LaValle CR, George KM, Sharlow ER, Lazo JS, Wipf P, Wang QJ. Protein kinase D as a potential new target for cancer therapy. Biochim Biophys Acta. 2010;1806:183–92.10.1016/j.bbcan.2010.05.003Search in Google Scholar PubMed PubMed Central
[24] Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–30.10.1128/MCB.25.19.8520-8530.2005Search in Google Scholar PubMed PubMed Central
[25] Visavadiya NP, Patel SP, VanRooyen JL, Sullivan PG, Rabchevsky AG. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016;8:59–67.10.1016/j.redox.2015.12.011Search in Google Scholar PubMed PubMed Central
[26] Kong G, Huang Z, Ji W, Wang X, Liu J, Wu X, et al. The ketone metabolite beta-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class i histone deacetylases. J Neurotrauma. 2017;34:2645–55.10.1089/neu.2017.5192Search in Google Scholar PubMed
[27] Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.10.1016/j.cell.2012.03.042Search in Google Scholar PubMed PubMed Central
[28] Lachaier E, Louandre C, Ezzoukhry Z, Godin C, Maziere JC, Chauffert B, et al. Ferroptosis, a new form of cell death relevant to the medical treatment of cancer. Med Sci (Paris). 2014;30:779–83.10.1051/medsci/20143008016Search in Google Scholar PubMed
[29] Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Maziere JC, Chauffert B, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–42.10.1002/ijc.28159Search in Google Scholar PubMed
[30] Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.10.1016/j.cell.2017.09.021Search in Google Scholar PubMed PubMed Central
[31] Cardoso BR, Hare DJ, Bush AI, Roberts BR. Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry. 2017;22:328–35.10.1038/mp.2016.196Search in Google Scholar PubMed
[32] Conrad M, Friedmann Angeli JP. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what's so special about it? Mol Cell Oncol. 2015;2:e995047.10.4161/23723556.2014.995047Search in Google Scholar PubMed PubMed Central
[33] Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–70.10.1007/82_2016_508Search in Google Scholar PubMed
[34] Dixon SJ. Ferroptosis: bug or feature? Immunol Rev. 2017;277:150–7.10.1111/imr.12533Search in Google Scholar PubMed
[35] Xu J, Hu C, Chen S, Shen H, Jiang Q, Huang P, et al. Neuregulin-1 protects mouse cerebellum against oxidative stress and neuroinflammation. Brain Res. 2017;1670:32–43.10.1016/j.brainres.2017.06.012Search in Google Scholar PubMed
[36] Chen SX, Hu CL, Liao YH, Zhao WJ. L1 modulates PKD1 phosphorylation in cerebellar granule neurons. Neurosci Lett. 2015;584:331–6.10.1016/j.neulet.2014.11.012Search in Google Scholar PubMed
[37] Jiang Q, Chen S, Hu C, Huang P, Shen H, Zhao W. Neuregulin-1 (Nrg1) signaling has a preventive role and is altered in the frontal cortex under the pathological conditions of Alzheimer's disease. Mol Med Rep. 2016;14:2614–24.10.3892/mmr.2016.5542Search in Google Scholar PubMed PubMed Central
[38] Chen S, Hou Y, Zhao Z, Luo Y, Lv S, Wang Q, et al. Neuregulin-1 accelerates functional motor recovery by improving motoneuron survival after brachial plexus root avulsion in mice. Neuroscience. 2019;404:510–8.10.1016/j.neuroscience.2019.01.054Search in Google Scholar PubMed
[39] Li J, Chen S, Zhao Z, Luo Y, Hou Y, Li H, et al. Effect of VEGF on inflammatory regulation, neural survival, and functional improvement in rats following a complete spinal cord transection. Front Cell Neurosci. 2017;11:381.10.3389/fncel.2017.00381Search in Google Scholar PubMed PubMed Central
[40] Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22:3826–36.Search in Google Scholar
[41] Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251–60.10.1089/08977150260338038Search in Google Scholar PubMed
[42] Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–35.10.1016/S0896-6273(00)80905-8Search in Google Scholar PubMed
[43] Zhang N, Luo Y, He L, Zhou L, Wu W. A self-assembly peptide nanofibrous scaffold reduces inflammatory response and promotes functional recovery in a mouse model of intracerebral hemorrhage. Nanomedicine. 2016;12:1205–17.10.1016/j.nano.2015.12.387Search in Google Scholar PubMed
[44] Tang Y, Wang J, Wan S, Luo L, Qiu Y, Jiang S, et al. Epigallocatechin gallate enhances the motor neuron survival and functional recovery after brachial plexus root avulsion by regulating FIG4. Folia Neuropathologica. 2019;57:340–7.10.5114/fn.2019.90819Search in Google Scholar PubMed
[45] Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–52.10.1146/annurev-neuro-061010-113723Search in Google Scholar PubMed
[46] Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323–37.10.1038/s41583-018-0001-8Search in Google Scholar PubMed PubMed Central
[47] Tillakaratne NJ, Guu JJ, de Leon RD, Bigbee AJ, London NJ, Zhong H, et al. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience. 2010;166:23–33.10.1016/j.neuroscience.2009.12.010Search in Google Scholar PubMed PubMed Central
[48] McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79.10.1016/j.advenzreg.2006.01.004Search in Google Scholar PubMed
[49] Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83.10.1210/edrv.22.2.0428Search in Google Scholar PubMed
[50] Yuan Q, Su H, Chiu K, Wu W, Lin ZX. Contrasting neuropathology and functional recovery after spinal cord injury in developing and adult rats. Neurosci Bull. 2013;29:509–16.10.1007/s12264-013-1356-5Search in Google Scholar PubMed PubMed Central
[51] Sakai O, Yasuzawa T, Sumikawa Y, Ueta T, Imai H, Sawabe A, et al. Role of GPx4 in human vascular endothelial cells, and the compensatory activity of brown rice on GPx4 ablation condition. Pathophysiology. 2017;24:9–15.10.1016/j.pathophys.2016.11.002Search in Google Scholar PubMed
[52] Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.10.1016/j.cell.2013.12.010Search in Google Scholar PubMed PubMed Central
© 2020 Jianjun Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Changing the tone of clinical study design in the cannabis industry
- Integrated Chinese and western medicine for acute guillain-barré syndrome treatment
- Hydrochloride fasudil attenuates brain injury in ICH rats
- Protective role of maize purple plant pigment against oxidativestress in fluorosis rat brain
- A novel low-cost electrode for recording the local field potential of freely moving rat’s brain
- Neuroprotective mechanisms of erythropoietin in a rat stroke model
- Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia
- Dysbiosis characteristics of gut microbiota in cerebral infarction patients
- miR-142-3p suppresses apoptosis in spinal cord-injured rats
- Decreased MiR-30a promotes TGF-β1-mediated arachnoid fibrosis in post-hemorrhagic hydrocephalus
- Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease
- EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats
- Efficacy of Novavit in ameliorating the neurotoxicity of propionic acid
- High-fat diet-induced obesity and impairment of brain neurotransmitter pool
- Erratum to “Changing the tone of clinical study design in the cannabis industry”
- Development of a trunk motor paradigm for use in neuroimaging
- Notoginsenoside R1 attenuates sevoflurane-induced neurotoxicity
- Saikosaponin A attenuates neural injury caused by ischemia/reperfusion
- Safety profile of the transcription factor EB (TFEB)-based gene therapy through intracranial injection in mice
- Partial enzyme digestion facilitates regeneration of crushed nerve in rat
- Correlation between calcium, water contents and ultrasonographic appearance of atherosclerotic lesions of carotid artery lesions
- Diabetes does not affect motor recovery after intracerebral hemorrhage
- IRF8 is crucial for the nicotine withdrawal-induced hyperalgesia in mice
- Prediction of muscle loss after stroke by analysis of corticospinal tract
- Interleukin-27 levels in patients with myasthenia gravis
- Artesunate attenuates traumatic brain injury-induced impairments in rats
- BDMC protects AD in vitro via AMPK and SIRT1
- Injury of the optic radiation in patients with mild TBI: A DTT study
- Time-restricted feeding alters isoflurane-induced memory deficits
- Video-based education improves the image quality of diagnostic percutaneous cerebral angiography among elderly patients
- The effect of rTMS in the management of pain associated with CRPS
- EGCG treats ICH via up-regulating miR-137-3p and inhibiting Parthanatos
- Modified hemispherectomy for infantile hemiparesis and epilepsy
- Isovitexin modulates autophagy in Alzheimer’s disease via miR-107 signalling
- Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model
- Role of prefrontal cortex during Sudoku task: fNIRS study
- Tongxinluo promotes axonal plasticity and functional recovery after stroke
- Eriodictyol corrects functional recovery and myelin loss in SCI rats
- Letter to the Editor
- Ataxic hemiparesis after corona radiata infarct: Diffusion tensor imaging correlation of corticoponto-cerebellar tract injury
- Compression of the lateral antebrachial cutaneous nerve by a traumatic arteriovenous fistula
- Rapid Communication
- Anticholinergic drugs and oral health-related quality of life in patients with schizophrenia: a pilot study
- Deviant cortical sulcation related to schizophrenia and cognitive deficits in the second trimester
- Case Report
- A case of primary central nervous system lymphoma mimic neuromyelitis optica
- A Moving Residual Limb: Botulinum Toxin to the Rescue
- Clinical and imaging features of reversible splenial lesion syndrome with language disorder
- Impaired consciousness due to injury of the ascending reticular activating system in a patient with bilateral pontine infarction: A case report
- Commentary
- A comment on Morey et al. (2020)
- Review Articles
- Advances in transcription factors related to neuroglial cell reprogramming
- The “authentic subjective experience” of memory in Alzheimer’s disease
- Chronic neurological diseases and COVID-19: Associations and considerations
- Special Issue "Neuroinflammation: from basic to clinical perspectives"
- Ormosanine improves neuronal functions in spinal cord-injured rats by blocking peroxynitrite/calpain activity
- Retraction
- Retraction of: Identification of biological markers for better characterization of older subjects with physical frailty and sarcopenia
Articles in the same Issue
- Research Articles
- Changing the tone of clinical study design in the cannabis industry
- Integrated Chinese and western medicine for acute guillain-barré syndrome treatment
- Hydrochloride fasudil attenuates brain injury in ICH rats
- Protective role of maize purple plant pigment against oxidativestress in fluorosis rat brain
- A novel low-cost electrode for recording the local field potential of freely moving rat’s brain
- Neuroprotective mechanisms of erythropoietin in a rat stroke model
- Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia
- Dysbiosis characteristics of gut microbiota in cerebral infarction patients
- miR-142-3p suppresses apoptosis in spinal cord-injured rats
- Decreased MiR-30a promotes TGF-β1-mediated arachnoid fibrosis in post-hemorrhagic hydrocephalus
- Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease
- EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats
- Efficacy of Novavit in ameliorating the neurotoxicity of propionic acid
- High-fat diet-induced obesity and impairment of brain neurotransmitter pool
- Erratum to “Changing the tone of clinical study design in the cannabis industry”
- Development of a trunk motor paradigm for use in neuroimaging
- Notoginsenoside R1 attenuates sevoflurane-induced neurotoxicity
- Saikosaponin A attenuates neural injury caused by ischemia/reperfusion
- Safety profile of the transcription factor EB (TFEB)-based gene therapy through intracranial injection in mice
- Partial enzyme digestion facilitates regeneration of crushed nerve in rat
- Correlation between calcium, water contents and ultrasonographic appearance of atherosclerotic lesions of carotid artery lesions
- Diabetes does not affect motor recovery after intracerebral hemorrhage
- IRF8 is crucial for the nicotine withdrawal-induced hyperalgesia in mice
- Prediction of muscle loss after stroke by analysis of corticospinal tract
- Interleukin-27 levels in patients with myasthenia gravis
- Artesunate attenuates traumatic brain injury-induced impairments in rats
- BDMC protects AD in vitro via AMPK and SIRT1
- Injury of the optic radiation in patients with mild TBI: A DTT study
- Time-restricted feeding alters isoflurane-induced memory deficits
- Video-based education improves the image quality of diagnostic percutaneous cerebral angiography among elderly patients
- The effect of rTMS in the management of pain associated with CRPS
- EGCG treats ICH via up-regulating miR-137-3p and inhibiting Parthanatos
- Modified hemispherectomy for infantile hemiparesis and epilepsy
- Isovitexin modulates autophagy in Alzheimer’s disease via miR-107 signalling
- Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model
- Role of prefrontal cortex during Sudoku task: fNIRS study
- Tongxinluo promotes axonal plasticity and functional recovery after stroke
- Eriodictyol corrects functional recovery and myelin loss in SCI rats
- Letter to the Editor
- Ataxic hemiparesis after corona radiata infarct: Diffusion tensor imaging correlation of corticoponto-cerebellar tract injury
- Compression of the lateral antebrachial cutaneous nerve by a traumatic arteriovenous fistula
- Rapid Communication
- Anticholinergic drugs and oral health-related quality of life in patients with schizophrenia: a pilot study
- Deviant cortical sulcation related to schizophrenia and cognitive deficits in the second trimester
- Case Report
- A case of primary central nervous system lymphoma mimic neuromyelitis optica
- A Moving Residual Limb: Botulinum Toxin to the Rescue
- Clinical and imaging features of reversible splenial lesion syndrome with language disorder
- Impaired consciousness due to injury of the ascending reticular activating system in a patient with bilateral pontine infarction: A case report
- Commentary
- A comment on Morey et al. (2020)
- Review Articles
- Advances in transcription factors related to neuroglial cell reprogramming
- The “authentic subjective experience” of memory in Alzheimer’s disease
- Chronic neurological diseases and COVID-19: Associations and considerations
- Special Issue "Neuroinflammation: from basic to clinical perspectives"
- Ormosanine improves neuronal functions in spinal cord-injured rats by blocking peroxynitrite/calpain activity
- Retraction
- Retraction of: Identification of biological markers for better characterization of older subjects with physical frailty and sarcopenia

