Abstract
We conducted an event-related potential (ERP) study using a 256-channel dense sensor array electroencephalography (EEG) system to examine how, and if the P200 neurophysiological signal is sexually dimorphic. We had two groups of participants: females (n= 15, mean age = 40.6 years old) and males (n = 15, mean age = 39.0 years old). ERPs from all participants were recorded while the participants performed an oddball task. Results showed that males on average had a significantly larger P200 peak amplitude and a significantly shorter P200 latency period. These results indicate that the P200 ERP is affected by sex. Therefore, suggesting that sex differences exist on an electrophysiological level, which may aid in better understanding of sex-biased biological influences, behaviors, neuropsychiatric disorders, and general brain function.
Introduction
Significant evidence exists for sex differences in behavior and neuropsychiatric disorders [1]. There are many examples of female-biased conditions that include depression, anxiety disorders, and emotional disorders. On the same note, examples of male-biased conditions include dyslexia, autism, and schizophrenia. Also, there has been identification of sex-biased differences in biological functions that influence pharmacokinetic determinations. Furthermore, there exists different vulnerability of males and females to addictive disorders [1,2,3]. However, despite the overwhelming evidence of sexbiased behavior, neuropsychiatric disorders, and biological influences, studies investigating the effects of sex on electrophysiological event-related potentials (ERPs) remain limited, and investigations into the P200 ERP are even more limited.
Although there are studies that did not show sex-related differences in the human brain [4,5], there are other studies, some of which are more recent, that provided significant evidence of the existence of sexual dimorphism in the human brain [6,7,8]. Conflicting evidence also exists with regards to event-related potential (ERP), electroencephalography (EEG), and electrophysiology in general. Some ERP studies did not show any sex-bias in ERP(9,10), while other and more recent evidence shows that sex differences in ERP exist [11,12,13].
Jiajin Yuan et al. (2008) used an oddball task in an ERP study to investigate sex-related behavioral inhibitory control. They recorded ERP signals from 15 male and 15 female participants, while the participants performed a two-choice oddball task (standard/target distinction) by pressing keys within 1000 ms. Their results indicated that men exhibited smaller amplitudes and longer latencies than women for P200 ERP. Therefore showing a general sex difference in behavioral control for adult humans [14]. In another study, Oliver-Rodriguez et al. found that the P300 ERP amplitude to be smaller among females compared to males [15]. There are also other studies (11,12) that have shown sex differences in ERP.
On the other hand, Sangal and Sangal in a 1996 publication showed no ERP difference across sex. In that study [10] they used a 32 channel EEG system to record the evoked potentials. Using a pseudo-random presentation, the participants were asked to press a button at each rare/target stimulus. The results of this study suggested that sex had no significant effect on amplitude or latency of the ERP signal. Other research work also showed no significant difference in ERP signal between males and females [9].
As portrayed above, there is overwhelming evidence on the existence of sex-biased behavior, neuropsychiatric disorders, and biological influence. Also, as portrayed above, there is still no consensus on the effects of sex on electrophysiology, more specifically event-related potentials, and relevant studies are very limited. Given all of that, we have conducted an electrophysiological ERP study to investigate the effect of sex (female vs. male) on both amplitude and latency of P200 ERP.
Methods
Participants
As volunteers, 15 females (mean age = 40.6 years old) and 15 males (mean age = 39.0 years old) were recruited. All participants were healthy, had normal or corrected-to-normal vision, and right-handed. All participants signed an informed consent form for the experiment. The experiment protocol was granted ethical approval from the Health Sciences Center Ethical Committee prior to the initiation of the study.
Experimental ERP Task
The present study used an oddball task that was programmed in EPrime 2.0 (Psychology Software Tools, Inc.) stimuli presentation software. The oddball task consisted of target (low-probability) and standard (high-probability) tasks. The ratio of targets to standards is 20:80. The target was represented by an “X” and the standard was represented by an “O”. The Xs and Os were interleaved by a fixation cross (ITI = 1000-2500 ms) and were displayed in random order for 500 ms for each appearance on the screen. Instructions were provided verbally to each respective participant before the start of the ERP recording session. The participants were instructed to sit comfortably on the chair with their right index finger situated comfortably on the response key. With the EEG electrode net on their head, each participant was respectively instructed to not respond to fixations and Os, and to only respond to Xs by pressing on the response key. Before collection of the ERP recordings, each participant had two practice runs. Then 3 experiment runs were administered for each participant.
ERP Recording
ERP was recorded using a 256-channel dense array EEG system (GES 410 by Electrical Geodesics, Inc.) in an electrically shielded and sound-attenuated room. Net Station 5.1 (Electrical Geodesics, Inc.) was used to sample the ERP at 1000 Hz. Since this EEG system uses a high impedance amplifier, the electrode impedance was kept below 50 kilo-ohms [16]. The 256 EEG electrodes were embedded in a HydroCel Geodesic Sensor Net (Electrical Geodecics, Inc.). Saline solution was used for conduction. For each participant, the net was securely situated on each respective participant’s head. The net was properly adjusted so that Cz was on the vertex and it was made sure that the Fz-Cz-Pz were on the marked midline on the scalp. The rest of the electrodes were placed in accordance with the geodesic structure of the net.
ERP Data Analysis
The recorded ERP data was pre-processed and post-processed using Net Station 5.1 (Electrical Geodesics, Inc.). Each participant’s data was first digitally filtered using a 0.1-30 Hz Bandpass filter. Then segmentation was done to segment the recorded data into epochs, which commence 100 ms before the onset of the target stimulus and end 700 ms after the onset. The offset for the segment was set at 18 ms based on a timing test done prior to the experiment runs. Then eye movements (max amplitude – min amplitude > 55 μV), eye blinks (max amplitude – min amplitude > 140 μV), bad channels (max amplitude – min amplitude > 200 μV) were removed using artifact detection and artifact removal algorithms of Net Station 5.1 (Electrical Geodesics, Inc.).
After the completion of the pre-processing steps for each participant’s data, post-processing was done. The first step in post-processing was bad channel replacement in which detected bad channels were replaced with interpolated data from remaining good channels. Followed by averaging of all segments and an average reference montage operation. Baseline correction was done with baseline beginning 100 ms before stimulus onset and lasting for 100 ms.
Careful data inspection was carried out. P200 is known to be well-defined in frontal-central scalp electrode locations. Given our interest in P200 (occurring between 150-250 ms after stimulus event) the ERP signal from the following electrode sites, and from each participant, were included for further statistical analysis for our present study: Fp1, Fz, Fp2, F3, F4, Cz.
The P200 peaks and latencies were extracted using Statistic Extraction method in Net Station 5.1 (Electrical Geodesics, Inc.). The specific time frame during which P200 occurs ( 150-250 ms after stimulus onset) was factored in the Statistic Extraction method to extract P200 peaks and latencies across the respective electrodes and in accordance with the geodesic structure of the electrode montage. The Statistic Extraction setup was used in order to derive the P200 peaks and latencies for standard and target stimuli for both males and females, using Net Station 5.1 (Electrical Geodesics, Inc.).
Then the P200 peak amplitudes from the respective electrodes were combined and further analyzed using a two-sample t test utilizing SPSS (IBM SPSS Statistics for Windows Version 25). Furthermore, the P200 latencies from the respective electrodes were, also, combined and further analyzed using a two-sample t test utilizing SPSS (IBM SPSS Statistics for Windows Version 25).
Results
The average ERP head models of both sexes were inspected by careful visualization using Net Station 5.1 (Electrical Geodesics, Inc.). Both sexes showed a clear peak in the electrophysiological signal intensity at frontalcentral scalp locations between 150-250 ms after event onset. This peak resembles the P200 ERP. The averaged female ERP head model showed a peak in ERP signal intensity around 200 ms for the standard (high-probability) stimuli at frontal-central scalp locations (Figure 1). As for the target (low-probability) stimuli, the averaged female ERP head model showed a peak in ERP signal intensity around 193 ms at frontal-central scalp locations (Figure 2). For the males, the averaged ERP head model showed a peak in signal intensity around 188 ms and 181 ms respectively for standard and target stimuli at frontal-central scalp locations (Figure 3, Figure 4). These signal intensity peaks at the 150-250 ms temporal domain are P200 ERP amplitude peaks.
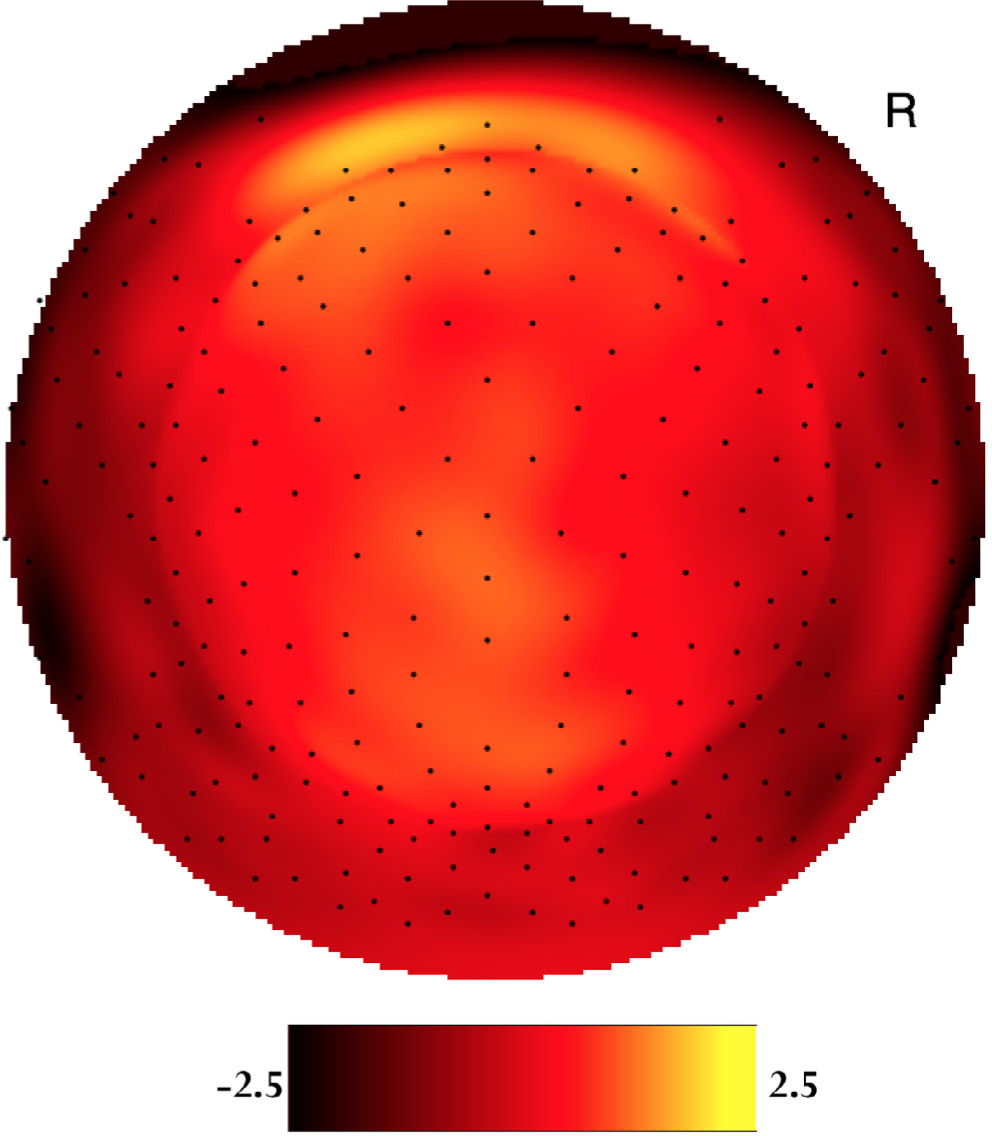
Two-dimensional voltage map of the averaged female P200 waveforms at 200 ms peak latency for the standard (high-probability) stimuli. Bright yellow is positive and dark red is negative (scaled from -2.5μV to 2.5μV). The orientation of the figure is looking down at the top of the head with the nose at the top of the figure.

Two-dimensional voltage map of the averaged female P200 waveforms at 193 ms peak latency for the target (low-probability) stimuli. Bright yellow is positive and dark red is negative (scaled from -5.3μV to 5.3μV). The orientation of the figure is looking down at the top of the head with the nose at the top of the figure.

Two-dimensional voltage map of the averaged male P200 waveforms at 188 ms peak latency for the standard (high-probability) stimuli. Bright yellow is positive and dark red is negative (scaled from -3.0μV to 3.0μV). The orientation of the figure is looking down at the top of the head with the nose at the top of the figure.

Two-dimensional voltage map of the averaged male P200 waveforms at 181 ms peak latency for the target (low-probability) stimuli. Bright yellow is positive and dark red is negative (scaled from -8.0μV to 8.0μV). The orientation of the figure is looking down at the top of the head with the nose at the top of the figure.
The results of the two-sample t test showed significant difference across sexes in both the mean P200 peak amplitude for all participants as well as the mean P200 latency for all participants. Females had a lower mean P200 peak amplitude (M = 2.06 μV, SD = 1.81) compared to males’ mean P200 peak amplitude (M = 2.79 μV, SD = 2.09) for the standard (high-probability) stimuli (p = 0.013). As for the target (low-probability) stimuli, females, also, showed lower mean P200 peak amplitude (M = 5.01 μV, SD = 3.46) compared to males’ mean P200 peak amplitude (M = 6.93 μV, SD = 3.41) at p<0.001. Furthermore, the results of the two-sample t test also showed that females had significantly longer mean latency for P200 compared to males. For the standard stimuli, females’ mean P200 latency was 200 ms (SD = 28.0) compared to 188 ms (SD = 32.5) that of males (p = 0.008). As for the target stimuli’s P200 mean latency, the females’ P200 mean latency was 193 ms (SD = 38.2) compared to 181 ms (SD = 31.8) for the males (p = 0.028). Figures 1–4 show the P200 ERP signal intensities for the respective study categories, and Figure 5 shows single-subject waveforms associated with stimuli type across sex, at the Fz electrode site.

Single-subject ERP waveforms at Fz (A: Female and standard stimuli, B: Female and target stimuli, C: Male and standard stimuli, D: Male and target stimuli).
Discussion
In the present study, participants were required to distinguish between high-probability stimuli (standard) and low-probability stimuli (target). They did so by accurately pressing a key, on which their right index finger was comfortably situated, and in response to the target stimuli. The oddball task, as the one used in this study, demonstrates adaptive reflexive processing whereby processes of inhibitory control must be recruited on the response to the standard (high-probability) stimuli during the presentation of the target (low-probability) stimuli in order for participants to provide a correct response to the target stimuli [14,17].
The P200 ERP is an attention-related component that usually peaks between 150-250 ms after the onset of a stimulus. It appears as a positive going electrical potential in the frontal-central areas of the scalp. The P200 is believed to form the basis for later cognitive processing by indexing early attentional recruitment, and is a reflection of perceptual processing [14,18,19,20]. Furthermore, a P200 peak appearing within 200 ms in the frontal scalp region is indicative of rapid detection of stimulus features [20,21].
The females showed a lower mean P200 peak compared to males. The females also had a longer mean P200 latency compared to males. Therefore, in the present study, we found that the P200 ERP is sensitive to sex in both high-probability (standard) and low-probability (target) stimuli. The present study supports the notion that sex does affect ERP.
Furthermore, our study is consistent with previous studies that did show sex sensitivity to ERP [11,12,15,22,23].
Males essentially elicited larger P200 amplitudes and shorter P200 latencies than females. Therefore, suggesting that males were faster at detecting the occurrence of the low-probability (target) stimuli and that they also directed more attentional resources to the features of the low-probability (target) stimuli compared to females. The faster detection of the low-probability (target) stimuli onset forms the bases of the succeeding recognition and resolution of response conflicts. Correspondingly, the observed sex effect on the P200 ERP during low-probability recognition further entails the probable existence of sex differences in the recognition and resolution of response conflicts subsequent phases [19,24,25].
Given that the females in the present study exhibited lower mean P200 amplitude along with longer mean P200 latency compared to males, suggests that the attentional recruitment in the brain of males may be both larger and faster than that of females. Furthermore, results of the present study suggest that low-probability or infrequent stimuli may differentially activate attentional frontal-central brain circuits and/or regions in females compared to males. This further implies a sexually dimorphic disposition that may account for various sex-specific novelties in brain function and behavior, and that can be used to aid in better understanding sex-biased biological influences and neuropsychiatric disorders.
References
[1] Bao A-M, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010:165:550–65.10.1177/1073858410377005Search in Google Scholar
[2] Thibaut F. The role of sex and gender in neuropsychiatric disorders. Dialogues Clin. Neurosci. 2016:184:351–52.10.31887/DCNS.2016.18.4/fthibautSearch in Google Scholar
[3] Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child Psychol. Psychiatry. 2003:448:1092–1115.10.1111/1469-7610.00194Search in Google Scholar
[4] Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000:2163:672–82.10.1148/radiology.216.3.r00au37672Search in Google Scholar
[5] Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR. Am. J. Neuroradiol. 1995:162:241–51.Search in Google Scholar
[6] Bourisly AK, Gejo G, Hayat AA, et al. White Matter Sexual Dimorphism of the Adult Human Brain. Transl. Neurosci. 2017:8:49–53.10.1515/tnsci-2017-0009Search in Google Scholar
[7] Allen JS, Damasio H, Grabowski TJ, et al. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003:184:880–94.10.1016/S1053-8119(03)00034-XSearch in Google Scholar
[8] Paus T, Otaky N, Caramanos Z, et al. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J. Comp. Neurol. 1996:3764:664–73.10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-MSearch in Google Scholar
[9] Polich J. Normal variation of P300 from auditory stimuli. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1986:653:236–40.10.1016/0168-5597(86)90059-6Search in Google Scholar
[10] Sangal RB, Sangal JM. Topography of Auditory and Visual P300 in Normal Adults. Clin. EEG Neurosci. 1996:273:145–50.10.1177/155005949602700307Search in Google Scholar
[11] Bourisly AK, Pothen A. Influence of sex on P300: an event-related potential electrophysiological study. Neuroreport. 2016:273:172–79.10.1097/WNR.0000000000000519Search in Google Scholar
[12] Steffensen SC, Ohran AJ, Shipp DN, et al. Gender-selective effects of the P300 and N400 components of the visual evoked potential. Vision Res. 2008:487:917–25.10.1016/j.visres.2008.01.005Search in Google Scholar
[13] Chu NS. Pattern-reversal visual evoked potentials: latency changes with gender and age. Clin. Electroencephalogr. 1987:183:159–62.Search in Google Scholar
[14] Yuan J, He Y, Qinglin Z, et al. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008:456:986–93.10.1111/j.1469-8986.2008.00693.xSearch in Google Scholar
[15] Oliver-Rodriguez JC, Guan Z, Johnston VS. Gender differences in late positive components evoked by human faces. Psychophysiology. 1999:362:176–85.10.1111/1469-8986.3620176Search in Google Scholar
[16] Ferree TC, Luu P, Russell GS, et al. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001:1123:536–44.10.1016/S1388-2457(00)00533-2Search in Google Scholar
[17] Kiehl KA, Stevens MC, Laurens KR, et al. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005:253:899–915.10.1016/j.neuroimage.2004.12.035Search in Google Scholar
[18] Carretié L, Mercado F, Tapia M, et al. Emotion, attention, and the “negativity bias”, studied through event-related potentials. Int. J. Psychophysiol. 2001:411:75–85.10.1016/S0167-8760(00)00195-1Search in Google Scholar
[19] Chen A, Xu P, Wang Q, et al. The timing of cognitive control in partially incongruent categorization. Hum. Brain Mapp. 2008:299:1028–39.10.1002/hbm.20449Search in Google Scholar PubMed PubMed Central
[20] Yuan J, Zhang Q, Chen A, et al. Are we sensitive to valence differences in emotionally negative stimuli? Electrophysiological evidence from an ERP study. Neuropsychologia. 2007:4512:2764–71.10.1016/j.neuropsychologia.2007.04.018Search in Google Scholar PubMed
[21] Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996:3816582:520–22.10.1038/381520a0Search in Google Scholar PubMed
[22] Kaneda Y, Nakayama H, Kagawa K, et al. Sex differences in visual evoked potential and electroencephalogram of healthy adults. Tokushima J. Exp. Med. 1996:433–4:143–57.Search in Google Scholar
[23] Yamamoto M, Morita K, Tomita Y, et al. Effect of Facial Affect Stimuli on Auditory and Visual P300 in Healthy Subjects. Kurume Med. J. 2000:474:285–90.10.2739/kurumemedj.47.285Search in Google Scholar PubMed
[24] Yuan J, He Y, Qinglin Z, et al. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. 2008:456:986–93.10.1111/j.1469-8986.2008.00693.xSearch in Google Scholar
[25] Nagy E, Potts GF, Loveland KA. Sex-related ERP differences in deviance detection. Int. J. Psychophysiol. 2003:483:285–92.10.1016/S0167-8760(03)00042-4Search in Google Scholar
© 2018 Ali K. Bourisly, Ali Shuaib, published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- Olfactory and imaging features in atypical Alzheimer’s disease
- Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway
- Corilagin attenuates the Parkinsonismin Japanese encephalitis virus induced Parkinsonism
- Further evidence of accelerated aging in bipolar disorder: Focus on GDF-15
- The ipsilateral vestibulothalamic tract in the human brain
- Neuroprotective effect of ZnT3 knockout on subarachnoid hemorrhage
- Beneficial effect of β-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway
- Protective effects of epifriedelinol in a rat model of traumatic brain injury assessed with histological and hematological markers
- Intracerebroventricular administration of L-arginine improves spatial memory acquisition in triple transgenic mice via reduction of oxidative stress and apoptosis
- Effects of resveratrol and morin on insoluble tau in tau transgenic mice
- Neurophysiological effects of aging: A P200 ERP study
- Photobiomodulation optimization for spinal cord injury rat phantom model
- Sex differences in electrophysiology: P200 event-related potential evidence
- The schizophrenia coping oral health profile. Development and feasibility
- Baicalein inhibits neuroapoptosis via pathways in sevoflurane induced rats
- Magnesium protects in episodes of critical perfusion after aneurysmal SAH
- Evaluation of two types of drug treatment with QEEG in children with ADHD
- Fangchinoline ameliorates the expressions of angiogenic molecule in cerebral ischemia induced neuronal degeneration in neonatal rats
- The schizophrenia oral health profile: Development and feasibility
- Effect of myricetin on primary open-angle glaucoma
- 2D:4D Ratio differs in ischemic stroke: A single center experience
- P38 mitogen-activated protein kinase and Parkinson’s disease
- Tuberous sclerosis complex: Clinical spectrum and epilepsy: A retrospective chart review study
- Analysis of early stroke-induced changes in circulating leukocyte counts using transcriptomic deconvolution
- Hypoglossal-facial ‘side’-to-side neurorrhaphy combined with electrical myostimulation for facial palsy in rats
- Benificial effect of stachydrine on the traumatic brain injury induced neurodegeneration by attenuating the expressions of Akt/mTOR/PI3K and TLR4/NFκ-B pathway
- Protective effect of extract of Bletilla striata on isoflurane induced neuronal injury by altering PI3K/Akt pathway
- Age-related disturbances in DNA (hydroxy)methylation in APP/PS1 mice
- Novel homozygous mutation in the WWOX gene causes seizures and global developmental delay: Report and review
- Impaired consciousness due to injury of ascending reticular activating system
- Effect of nobiletin on experimental model of epilepsy
Articles in the same Issue
- Regular Articles
- Olfactory and imaging features in atypical Alzheimer’s disease
- Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway
- Corilagin attenuates the Parkinsonismin Japanese encephalitis virus induced Parkinsonism
- Further evidence of accelerated aging in bipolar disorder: Focus on GDF-15
- The ipsilateral vestibulothalamic tract in the human brain
- Neuroprotective effect of ZnT3 knockout on subarachnoid hemorrhage
- Beneficial effect of β-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway
- Protective effects of epifriedelinol in a rat model of traumatic brain injury assessed with histological and hematological markers
- Intracerebroventricular administration of L-arginine improves spatial memory acquisition in triple transgenic mice via reduction of oxidative stress and apoptosis
- Effects of resveratrol and morin on insoluble tau in tau transgenic mice
- Neurophysiological effects of aging: A P200 ERP study
- Photobiomodulation optimization for spinal cord injury rat phantom model
- Sex differences in electrophysiology: P200 event-related potential evidence
- The schizophrenia coping oral health profile. Development and feasibility
- Baicalein inhibits neuroapoptosis via pathways in sevoflurane induced rats
- Magnesium protects in episodes of critical perfusion after aneurysmal SAH
- Evaluation of two types of drug treatment with QEEG in children with ADHD
- Fangchinoline ameliorates the expressions of angiogenic molecule in cerebral ischemia induced neuronal degeneration in neonatal rats
- The schizophrenia oral health profile: Development and feasibility
- Effect of myricetin on primary open-angle glaucoma
- 2D:4D Ratio differs in ischemic stroke: A single center experience
- P38 mitogen-activated protein kinase and Parkinson’s disease
- Tuberous sclerosis complex: Clinical spectrum and epilepsy: A retrospective chart review study
- Analysis of early stroke-induced changes in circulating leukocyte counts using transcriptomic deconvolution
- Hypoglossal-facial ‘side’-to-side neurorrhaphy combined with electrical myostimulation for facial palsy in rats
- Benificial effect of stachydrine on the traumatic brain injury induced neurodegeneration by attenuating the expressions of Akt/mTOR/PI3K and TLR4/NFκ-B pathway
- Protective effect of extract of Bletilla striata on isoflurane induced neuronal injury by altering PI3K/Akt pathway
- Age-related disturbances in DNA (hydroxy)methylation in APP/PS1 mice
- Novel homozygous mutation in the WWOX gene causes seizures and global developmental delay: Report and review
- Impaired consciousness due to injury of ascending reticular activating system
- Effect of nobiletin on experimental model of epilepsy

