Abstract
Background
Bipolar disorder (BD) is a mood disorder associated with cardiovascular and metabolic diseases and premature aging. Growth differentiation factor 15 (GDF-15) has emerged as a biomarker for cardiovascular risk and aging. Our aim was to compare plasma levels of GDF-15 between BD patients and controls, and to evaluate whether they were associated with clinical parameters.
Methods
Forty-six patients with type I BD (23 in euthymia and 23 in mania) and 33 healthy controls were recruited for this study. Plasma levels of GDF-15 were measured by immunoassay.
Results
The levels of GDF-15 were significantly higher (p < 0.001) in patients with BD in comparison with controls. In patients, GDF-15 levels correlated with age (rho = 0.434; p = 0.003) and illness duration (rho = 0.502; p = 0.001).
Conclusion
Our findings corroborate the view that BD is an illness associated with accelerated aging.
1 Introduction
Bipolar disorder (BD) is a chronic and often severe mood disorder characterized by alternating episodes of depression and mania. BD affects 3-5% of adolescents and adults [1, 2, 3]. BD is associated with substantial morbidity and mortality [4; 5], resulting not only from psychiatric symptoms, but also from a wide variety of comorbid medical problems including cardiovascular diseases (CVD), diabetes mellitus, obesity and thyroid diseases [6; 7].
As a leading cause of death in BD, CVD occur five times more frequently in adults with BD than in the general population [8; 9]. Moreover, BD patients manifest CVD up to 17 years earlier than adults without BD [10; 11]. The standardized mortality ratio for CVD is high for BD patients across all age groups, but is more pronounced in adults younger than 40 years-old [12]. Similarly, metabolic complications including diabetes are significantly more frequent in patients with BD than in the general population. Therefore, early prevention and management of CVD and metabolic syndromes in BD is of paramount importance.
The progressive nature of BD has received much attention in the last decade [13, 14, 15], leading to the concept of “neuroprogression” and staging models of BD [14; 16]. However, the neurobiological evidence supporting these hypotheses is still limited. Despite great efforts, biomarkers to monitor illness progression remain elusive and so far no biomarker has been clearly linked to morbidity and mortality in BD[17]. Therefore, candidate biomarkers for molecular staging of BD need to be explored.
Recently, a divergent member of the transforming growth factor-beta family, growth differentiation factor 15 (GDF-15), also known as macrophage inhibitory cytokine 1 (MIC-1), has emerged as a relevant biomarker for CVD and diabetes [18; 19]. GDF-15 has also been investigated for its role in the aging process, including age-related changes in the brain structure and cognitive decline. The association of this molecule with aging and longevity was highlighted by animal studies in which overexpression of GDF-15 resulted in the prolongation of lifespan [20]. As BD has been associated with accelerated aging [21; 22], we hypothesized that GDF-15 is a potential biomarker of BD progression. Accordingly, the aims of this study were: i) to compare the plasma levels of GDF-15 between patients with BD and controls; ii) to evaluate whether circulating levels of GDF-15 levels were associated with clinical parameters, especially illness course.
2 Methods
2.1 Subjects and clinical evaluation
Forty-six patients with type I BD diagnosed according to the DSM-IV-TR criteria were enrolled in this study. Of these, 23 were euthymic and 23 were manic. In addition, this study included a control group consisting of 33 age- and gender-matched healthy subjects recruited from the local community. All subjects were evaluated by an experienced psychiatrist through the Mini-International Neuropsychiatric Interview [23].
Patients who met the following criteria were included: 1) aged 18-65 years; 2) DSM-IV-TR diagnosis criteria of type I BD; 3) at least one year of BD diagnosis. For each patient, the Young Mania Rating Scale (YMRS) [24] and the Hamilton Rating Scale for Depression (HRSD) [25] were used to assess the severity of manic and depressive symptoms, respectively. All patients were under treatment with mood stabilizers and/or antipsychotics.
The control group included only participants who did not have a psychiatric disorder (evaluated through Mini-International Neuropsychiatric Interview) or family history of major psychiatry disorders, suicide attempts, or completed suicides.
Subjects with dementia, infectious or autoimmune diseases, or who had used steroids, anti-inflammatory drugs, or antibiotics in the four weeks prior to the clinical evaluation were excluded from this research protocol. All subjects provided written informed consent before admission to the study. The Research Ethics Committee of the Universidade Federal de Minas Gerais, Brazil approved this study.
2.2 Sample collection and preparation
Plasma GDF-15 levels were measured in both groups. Peripheral venous blood samples were drawn by venipuncture in tubes containing heparin. Participants were in the fasting state and the blood was obtained on the same day of clinical assessment. The blood samples were centrifuged at 1,800 g for 10 min at 4°C for plasma separation. The Luminex® technique was applied to measure the plasma concentration of GDF-15 (Merck Millipore, Darmstadt, Germany). This assessment was performed blind to the clinical diagnosis.
2.3 Data analysis
The chi-square test was used to test for the difference of sex proportion between groups. The Shapiro-Wilk normality test was used to check whether the continuous variables follow a Gaussian distribution. Two groups (patients with BD vs. controls) were compared using the Mann–Whitney U test since data were determined to not follow a normal distribution. Spearman’s correlation analyses were performed to assess the correlation between GDF-15 levels and demographic and clinical variables in patients.
All data were analyzed using the SPSS for Window software (SPSS Inc.; Chicago, IL, USA). Two-tailed significance levels were set at 0.05.
3 Results
A total of 46 patients (17 males and 29 females) and 33 age- and gender-matched healthy controls (9 males and 24 females) were included in this study. Of 46 patients, 23 were euthymic and 23 were manic. The median age [interquartile range] of patients and controls were 50.5 [42.0 – 58.0] and 48.0 [38.5 – 54.0], respectively. There were no significant differences in sex and median age between patients with BD and controls as tested by Chi-square (p = 0.36) and Mann-Whitney test (p = 0.18), respectively. The median BD length [interquartile range] was 22.0 [13 - 32.25] years.
GDF-15 levels were significantly higher in BD patients (median [interquartile range] = 0.490 [0.398 – 0.875] ng/mL) than controls (0.275 [0.183 – 0.348] ng/mL) (Figure 1). There was no significant difference in GDF-15 levels between euthymic and manic patients.
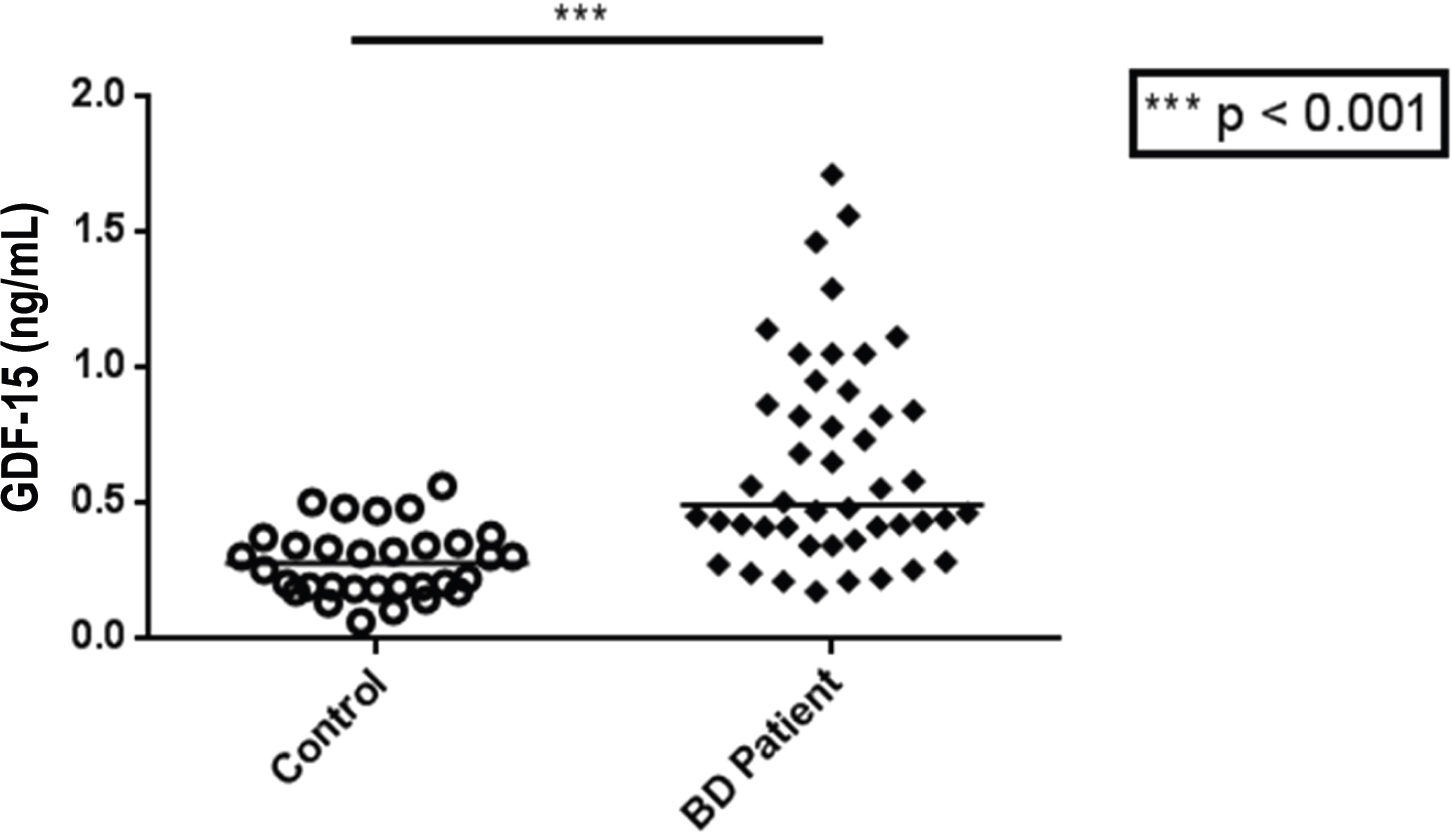
GDF-15 levels were significantly higher in BD patients than controls
A positive correlation was observed between age and GDF-15 plasma levels in patients, rho = 0.434, p = 0.003, indicating that an increase in age was moderately associated with an increase in GDF-15 levels in patients. There was also a moderate positive correlation between illness length and GDF-15 levels, rho = 0.502, p = 0.001. There was no correlation between YMRS or HRSD scores and GDF-15 levels.
4 Discussion
In the current study, we showed that patients with BD presented increased plasma levels of GDF-15 when compared with controls. To our knowledge, this was the first controlled crosssectional study that specifically evaluated GDF- 15 levels in a cohort of BD patients. Our results were consistent with two recent proteomic studies showing that GDF-15 was one of the proteins that could distinguish BD patients from healthy controls [26; 27]. We also found that the older the patients with BD, the higher the GDF- 15 levels. In addition, higher GDF-15 levels were associated with longer disease duration.
GDF-15 is primarily involved in inflammatory, apoptotic, and stress responses. It plays important roles in the development and progression of a wide variety of chronic conditions, such as coronary artery disease, atherosclerosis, obesity, insulin resistance, diabetes, and cognitive impairment [18; 19]. As shown in a cohort study of 984 patients, GDF-15 levels were elevated in the early subclinical stage of CVD and predicted adverse outcomes and mortality in these patients [28]. GDF-15 is also associated with the prognosis of type 2 diabetes mellitus [29]. Altogether, these observations supported GDF-15 as a potential diagnostic and prognostic biomarker for CVD [30], obesity [30] and diabetes [31], conditions that are frequently comorbid with BD.
In addition to its involvement in CVD, GDF- 15 was also associated with non-cardiovascular mortality [32]. Increased circulating levels of GDF-15 predicted all-cause mortality in the general population independent of multiple genetic and environmental risk factors for mortality including age, body mass index, smoking history, serum IL-6 and CRP levels, and telomere length [32]. It was hypothesized that the association between serum GDF-15 levels and all-cause mortality is related to the aging process, and accumulating evidence has supported GDF-15 as a biomarker of aging [33; 34]. In a prospective study of Uppsala seniors, plasma GDF-15 levels were also strong predictors of mortality in elderly community-dwelling individuals [35]. At the molecular level, GDF-15 influences several cellular processes implicated in aging, such as apoptosis [36], mitochondrial dysfunction [37], endoplasmic reticulum stress [38] and inflammatory response [39]. Furthermore, the expression of GDF-15 increases with age [35; 40] and is induced by many age-related stressors [40; 41]. Accordingly, there was evidence that GDF-15 levels may reflect the load of environmental toxicity [32], contributing to and/or indexing the mortality.
BD has been considered a condition of accelerated aging as a wide range of aging processes – low-grade inflammation, excessive oxidative stress, altered neurotrophic factors, mitochondrial dysfunction, and premature cellular senescence - occur in these patients [42, 43, 44]. The neuroprogression model of BD proposes that the cumulative exposure to environmental stresses and/or repeated mood episodes play a major role in the progression from early to late illness stages [16; 45, 46, 47]. We found a moderate positive correlation of GDF-15 with age and illness duration in the subjects with BD, the latter being an important parameter of illness staging. As GDF-15 circulating levels are associated with pathological aging and can be influenced by environmental factors [32], these results suggest that GDF-15 may be regarded as a candidate biomarker of neuroprogression and/or a biomarker to estimate the cumulative burden from environmental stresses and repeated mood episodes during the course of BD. The levels of GDF-15 did not show a significant difference between patients in euthymia and those in manic state, further suggesting that GDF-15 can be regarded as a marker of disease stage instead of disease state.
The role of GDF-15 in the central nervous system was largely investigated in animal models. Only a very limited studies have investigated its involvement in cognitive aging and dementia [33]. Higher concentrations of serum GDF-15 were associated with decreased grey matter volumes and white matter integrity, which were shown to mediate worse cognitive function [48; 49]. Brain structural changes in BD include enlargement of the third and lateral ventricles [50], decreased gray matter [51], and reduced volumes in certain the prefrontal cortex regions [50; 52]. It remains to be determined whether the changes in GDF-15 levels are associated with these brain structural changes in patients with BD.
Limitations of the study include the small sample size and the cross-sectional design. With larger sample size and improved statistical power in the future, we may assess the levels of GDF-15 in distinct mood states in these patients. We did not have a comparison of the levels of GDF-15 with other biomarkers implicated in BD physiopathology.
In summary, we showed that GDF-15 levels were increased in the plasma of BD patients and the levels were closely associated with illness duration, a major parameter for the staging of BD. Future investigations are needed to establish whether GDF-15 can be regarded as a biological marker for prognostic evaluation of BD. As demonstrated in animal studies [53], it is also possible that therapeutic interventions that modify GDF-15 levels might decrease mortality risk and increase longevity in BD patients.
Acknowledgements
This study was funded by the Brazilian government agencies CNPq and Fapemig. The Neuropsychiatry Program and the Immuno-Psychiatry Lab are supported by a grant from the Department of Psychiatry & Behavioral Sciences, UT Health Houston.
References
[1] Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC: Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch. Gen. Psychiatry 2007; 64:543–55210.1001/archpsyc.64.5.543Search in Google Scholar
[2] Kessler RC, Ph D, Avenevoli S, Ph D, Green JG, Ph D, Michael J, Guyer M, Ph D, He Y, Ph D, Jin R, Kaufman J, Ph D, Sampson NA, Zaslavsky AM, Ph D, Merikangas KR: The National Comorbidity Survey Adolescent Supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry 2009; 48:386–39910.1097/CHI.0b013e31819a1cbcSearch in Google Scholar
[3] Kozloff N, Cheung AH, Schaffer A, Cairney J, Dewa CS, Veldhuizen S, Kurdyak P, Levitt AJ: Bipolar disorder among adolescents and young adults: Results from an epidemiological sample [Internet]. J. Affect. Disord. 2010; 125:350–354Available from: http://dx.doi.org/10.1016/j.jad.2010.02.12010.1016/j.jad.2010.02.120Search in Google Scholar
[4] Baldessarini RJ, Tondo L: Suicide risk and treatments for patients with bipolar disorder [Internet]. JAMA 2003; 290:1517–1519Available from: http://dx.doi.org/10.1001/jama.290.11.151710.1001/jama.290.11.1517Search in Google Scholar
[5] Angst F, Stassen HH, Clayton PJ, Angst J: Mortality of patients with mood disorders: Follow-up over 34-38 years. J. Affect. Disord. 2002; 68:167–18110.1016/S0165-0327(01)00377-9Search in Google Scholar
[6] Zhao Z, Okusaga OO, Quevedo J, Soares JC, Teixeira AL: The potential association between obesity and bipolar disorder: A meta-analysis [Internet]. J. Affect. Disord. 2016; 202:120–123Available from: http://dx.doi.org/10.1016/j.jad.2016.05.05910.1016/j.jad.2016.05.059Search in Google Scholar
[7] Krishnan KRR: Psychiatric and medical comorbidities of bipolar disorder. Psychosom. Med. 2005; 67:1–810.1097/01.psy.0000151489.36347.18Search in Google Scholar
[8] Ösby U, Brandt L, Correia N, Ekbom A, Sparén P: Excess Mortality in Bipolar and Unipolar Disorder in Sweden [Internet]. Arch. Gen. Psychiatry 2001; 58:844–850Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.58.9.84410.1001/archpsyc.58.9.844Search in Google Scholar
[9] Weeke A, Juel K, Vath M: Cardiovascular death and manic-depressive psychosis. J. Affect. Disord. 1987; 13:287–29210.1016/0165-0327(87)90049-8Search in Google Scholar
[10] Goldstein BI, Fagiolini A, Houck P, Kupfer DJ: Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009; 11:657–66210.1111/j.1399-5618.2009.00735.xSearch in Google Scholar PubMed PubMed Central
[11] Goldstein B, Schaffer A, Wang S, Blanco C: Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry 2015; 76:163–910.4088/JCP.14m09300Search in Google Scholar PubMed
[12] Westman J, Hällgren J, Wahlbeck K, Erlinge D, Alfredsson L, Ösby U: Cardiovascular mortality in bipolar disorder: A population-based cohort study in Sweden. BMJ Open 2013; 3:1–810.1136/bmjopen-2012-002373Search in Google Scholar PubMed PubMed Central
[13] Berk M, Conus P, Kapczinski F, Andreazza AC, Yücel M, Wood SJ, Pantelis C, Malhi GS, Dodd S, Bechdolf A, Amminger GP, Hickie IB, McGorry PD: From neuroprogression to neuroprotection: Implications for clinical care. Med. J. Aust. 2010; 193Search in Google Scholar
[14] Fries GR, Pfaffenseller B, Stertz L, Paz AVC, Dargél AA, Kunz M, Kapczinski F: Staging and neuroprogression in bipolar disorder. Curr. Psychiatry Rep. 2012; 14:667–67510.1007/s11920-012-0319-2Search in Google Scholar PubMed
[15] Mansur RB, Cha DS, Asevedo E, McIntyre RS, Brietzke E: Selfish brain and neuroprogression in bipolar disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2013; 43:66–7110.1016/j.pnpbp.2012.12.004Search in Google Scholar PubMed
[16] Kapczinski F, Dias VV, Kauer-Sant’Anna M, Brietzke E, Vázquez GH, Vieta E, Berk M: The potential use of biomarkers as an adjunctive tool for staging bipolar disorder [Internet]. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2009; 33:1366–1371Available from: http://dx.doi.org/10.1016/j.pnpbp.2009.07.02710.1016/j.pnpbp.2009.07.027Search in Google Scholar PubMed
[17] Teixeira A, Salem H, Frey B, Barbosa I, Machado-Vieira R: Update on bipolar disorder biomarker candidates. Expert Rev Mol Diagn 2016; 16:1209–122010.1080/14737159.2016.1248413Search in Google Scholar PubMed
[18] Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Åsberg S, Granger CB, Siegbahn A: Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: Insights from the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE. Circulation 2014; 130:1847–185810.1161/CIRCULATIONAHA.114.011204Search in Google Scholar PubMed
[19] Lindahl B: The story of growth differentiation factor 15: Another piece of the puzzle. Clin. Chem. 2013; 59:1550–155210.1373/clinchem.2013.212811Search in Google Scholar PubMed
[20] Wang X, Chrysovergis K, Kosak J, Kissling G, Streicker M, Moser G, Li R, Eling TE: hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging (Albany. NY). 2014; 6:690–70410.18632/aging.100687Search in Google Scholar PubMed PubMed Central
[21] Rizzo LB, Do Prado CH, Grassi-Oliveira R, Wieck A, Correa BL, Teixeira AL, Bauer ME: Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013; 15:832–83810.1111/bdi.12121Search in Google Scholar PubMed
[22] Panizzutti B, Gubert C, Schuh AL, Ferrari P, Bristot G, Fries GR, Massuda R, Walz J, Rocha NP, Berk M, Teixeira AL, Gama CS: Increased serum levels of eotaxin/CCL11 in late-stage patients with bipolar disorder: An accelerated aging biomarker? J. Affect. Disord. 2015; 182:64–6910.1016/j.jad.2014.12.010Search in Google Scholar PubMed
[23] Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Anavs J, Weiller E, Hergueta T, Baker R, Dunbar G: The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33Search in Google Scholar
[24] Young R, Biggs J, Ziegler V, Meyer D: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–3510.1192/bjp.133.5.429Search in Google Scholar PubMed
[25] Hamilton M: A rating scale for depression. [Internet]. J. Neurol. Neurosurg. Psychiatry 1960; 23:56–62Available from: http://www.ncbi.nlm.nih.gov/pubmed/14399272%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC49533110.1136/jnnp.23.1.56Search in Google Scholar PubMed PubMed Central
[26] Frye MA, Nassan M, Jenkins GD, Kung S, Veldic M, Palmer BA, Feeder SE, Tye SJ, Choi DS, Biernacka JM: Feasibility of investigating differential proteomic expression in depression: Implications for biomarker development in mood disorders [Internet]. Transl. Psychiatry 2015; 5:e689-8Available from: http://dx.doi.org/10.1038/tp.2015.18510.1038/tp.2015.185Search in Google Scholar PubMed PubMed Central
[27] Nassan M, Jia YF, Jenkins G, Colby C, Feeder S, Choi DS, Veldic M, McElroy SL, Bond DJ, Weinshilboum R, Biernacka JM, Frye MA: Exploring hepsin functional genetic variation association with disease specific protein expression in bipolar disorder: Applications of a proteomic informed genomic approach [Internet]. J. Psychiatr. Res. 2017; 95:208–212Available from: https://doi.org/10.1016/j.jpsychires.2017.07.00510.1016/j.jpsychires.2017.07.005Search in Google Scholar PubMed
[28] Schopfer DW, Ku IA, Regan M, Whooley MA: Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study) [Internet]. Am. Heart J. 2014; 167:186–192.e1 Available from: http://dx.doi.org/10.1016/j.ahj.2013.09.01310.1016/j.ahj.2013.09.013Search in Google Scholar PubMed
[29] Berezin AE: Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15 [Internet]. Diabetes Metab. Syndr. Clin. Res. Rev. 2016; 10:S154–S157Available from: http://dx.doi.org/10.1016/j.dsx.2015.09.01610.1016/j.dsx.2015.09.016Search in Google Scholar PubMed
[30] Adela R, Banerjee SK: GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: A translational prospective. J. Diabetes Res. 2015; 201510.1155/2015/490842Search in Google Scholar PubMed PubMed Central
[31] Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, Rwitte D: Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: The Whitehall II study. Eur. J. Endocrinol. 2010; 162:913–91710.1530/EJE-09-1066Search in Google Scholar PubMed
[32] Wiklund FE, Bennet AM, Magnusson PKE, Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin P, Pedersen NL, Adami HO, Grönberg H, Breit SN, Brown DA: Macrophage inhibitory cytokine-1 (MIC-1/GDF15): A new marker of all-cause mortality. Aging Cell 2010; 9:1057–106410.1111/j.1474-9726.2010.00629.xSearch in Google Scholar PubMed PubMed Central
[33] Jiang J, Wen W, Sachdev PS: Macrophage inhibitory cytokine-1/growth differentiation factor 15 as a marker of cognitive ageing and dementia. Curr. Opin. Psychiatry 2016; 29:181–18610.1097/YCO.0000000000000225Search in Google Scholar PubMed
[34] Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M: Secreted growth differentiation factor15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int. 2016; 16:17–2910.1111/ggi.12724Search in Google Scholar PubMed
[35] Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L: Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin. Chem. 2013; 59:1091–109810.1373/clinchem.2012.201210Search in Google Scholar PubMed
[36] Jones MF, Ling Li X, Subramanian M, Shabalina SA, Hara T, Zhu Y, Huang J, Yang Y, Wakefield LM, Prasanth K V., Lal A: Growth differentiation factor-15 encodes a novel microRNA 3189 that functions as a potent regulator of cell death [Internet]. Cell Death Differ. 2015; 22:1641–1653Available from: http://dx.doi.org/10.1038/cdd.2015.910.1038/cdd.2015.9Search in Google Scholar PubMed PubMed Central
[37] Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, Kojima T, Ito M, Tanaka M, Saiki R, Koga Y: Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol. 2015; 78:814–82310.1002/ana.24506Search in Google Scholar PubMed PubMed Central
[38] Park SH, Choi HJ, Yang H, Do KH, Kim J, Kim HH, Lee H, Oh CG, Lee DW, Moon Y: Two in-and-out modulation strategies for endoplasmic reticulum stress-linked gene expression of pro-apoptotic macrophage-inhibitory cytokine 1. J. Biol. Chem. 2012; 287:19841–1985510.1074/jbc.M111.330639Search in Google Scholar PubMed PubMed Central
[39] Breit SN, Johnen H, Cook AD, Tsai VWW, Mohammad MG, Kuffner T, Zhang HP, Marquis CP, Jiang L, Lockwood G, Lee-Ng M, Husaini Y, Wu L, Hamilton JA, Brown DA: The TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011; 29:187–19510.3109/08977194.2011.607137Search in Google Scholar PubMed
[40] Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, Amin J, Jung K, Diamandis EP, Hampton GM, Russell PJ, Giles GG, Breit SN: Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin. Cancer Res. 2006; 12:89–9610.1158/1078-0432.CCR-05-1331Search in Google Scholar PubMed
[41] Bauskin AR, Brown DA, Kuffner T, Johnen H, Lou XW, Hunter M, Breit SN: Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006; 66:4983–498610.1158/0008-5472.CAN-05-4067Search in Google Scholar PubMed
[42] Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PVS, Amminger P, McGorry P, Malhi GS: Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors [Internet]. Neurosci. Biobehav. Rev. 2011; 35:804–817Available from: http://dx.doi.org/10.1016/j.neubiorev.2010.10.00110.1016/j.neubiorev.2010.10.001Search in Google Scholar PubMed
[43] Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant’Anna M, Yatham LN, Kapczinski F, Young LT: Biomarkers in bipolar disorder: A positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust. N. Z. J. Psychiatry 2013; 47:321–33210.1177/0004867413478217Search in Google Scholar
[44] Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete Santos A: Potential biomarkers of ageing. Biol. Chem. 2008; 389:257–26510.1515/BC.2008.034Search in Google Scholar
[45] McEwen BS, Wingfield JC: The concept of allostasis in biology and biomedicine. Horm. Behav. 2003; 43:2–1510.1016/S0018-506X(02)00024-7Search in Google Scholar
[46] Berk M, Hallam KT, McGorry PD: The potential utility of a staging model as a course specifier: A bipolar disorder perspective. J. Affect. Disord. 2007; 100:279–28110.1016/j.jad.2007.03.007Search in Google Scholar PubMed
[47] Berk M, Conus P, Lucas N, Hallam K, Gs M, Dodd S, Ln Y: Point of View Setting the stage : from prodrome to treatment resistance in bipolar disorder. Bipolar Disord. 2007; 9:671–67810.1111/j.1399-5618.2007.00484.xSearch in Google Scholar PubMed
[48] Jiang J, Wen W, Brown DA, Crawford J, Thalamuthu A, Smith E, Breit SN, Liu T, Zhu W, Brodaty H, Baune BT, Trollor JN, Sachdev PS: The relationship of serum macrophage inhibitory cytokine - 1 levels with gray matter volumes in community-dwelling older individuals. PLoS One 2015; 10:1–2010.1371/journal.pone.0123399Search in Google Scholar PubMed PubMed Central
[49] Jiang J, Trollor JN, Brown DA, Crawford JD, Thalamuthu A, Smith E, Breit SN, Liu T, Brodaty H, Baune BT, Sachdev PS, Wen W: An inverse relationship between serum macrophage inhibitory cytokine-1 levels and brain white matter integrity in community-dwelling older individuals [Internet]. Psychoneuroendocrinology 2015; 62:80–88Available from: http://dx.doi.org/10.1016/j.psyneuen.2015.07.61010.1016/j.psyneuen.2015.07.610Search in Google Scholar PubMed
[50] Soares JC, Kochunov P, Monkul ES, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Lancaster J, Fox P: Structural brain changes in bipolar disorder using deformation field morphometry. Neuroreport 2005; 16:541–54410.1097/00001756-200504250-00004Search in Google Scholar PubMed
[51] Moorhead TWJ, McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, McIntosh AM: Progressive Gray Matter Loss in Patients with Bipolar Disorder. Biol. Psychiatry 2007; 62:894–90010.1016/j.biopsych.2007.03.005Search in Google Scholar PubMed
[52] Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, Martin L, Gerard E, Charney DS, Peterson BS: Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: A cross-sectional study. Biol. Psychiatry 2006; 59:611–61810.1016/j.biopsych.2005.08.031Search in Google Scholar PubMed
[53] Johnen H, Lin S, Kuffner T, Brown DA, Tsai VWW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN: Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat. Med. 2007; 13:1333–134010.1038/nm1677Search in Google Scholar PubMed
© 2018 Fang Yang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- Olfactory and imaging features in atypical Alzheimer’s disease
- Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway
- Corilagin attenuates the Parkinsonismin Japanese encephalitis virus induced Parkinsonism
- Further evidence of accelerated aging in bipolar disorder: Focus on GDF-15
- The ipsilateral vestibulothalamic tract in the human brain
- Neuroprotective effect of ZnT3 knockout on subarachnoid hemorrhage
- Beneficial effect of β-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway
- Protective effects of epifriedelinol in a rat model of traumatic brain injury assessed with histological and hematological markers
- Intracerebroventricular administration of L-arginine improves spatial memory acquisition in triple transgenic mice via reduction of oxidative stress and apoptosis
- Effects of resveratrol and morin on insoluble tau in tau transgenic mice
- Neurophysiological effects of aging: A P200 ERP study
- Photobiomodulation optimization for spinal cord injury rat phantom model
- Sex differences in electrophysiology: P200 event-related potential evidence
- The schizophrenia coping oral health profile. Development and feasibility
- Baicalein inhibits neuroapoptosis via pathways in sevoflurane induced rats
- Magnesium protects in episodes of critical perfusion after aneurysmal SAH
- Evaluation of two types of drug treatment with QEEG in children with ADHD
- Fangchinoline ameliorates the expressions of angiogenic molecule in cerebral ischemia induced neuronal degeneration in neonatal rats
- The schizophrenia oral health profile: Development and feasibility
- Effect of myricetin on primary open-angle glaucoma
- 2D:4D Ratio differs in ischemic stroke: A single center experience
- P38 mitogen-activated protein kinase and Parkinson’s disease
- Tuberous sclerosis complex: Clinical spectrum and epilepsy: A retrospective chart review study
- Analysis of early stroke-induced changes in circulating leukocyte counts using transcriptomic deconvolution
- Hypoglossal-facial ‘side’-to-side neurorrhaphy combined with electrical myostimulation for facial palsy in rats
- Benificial effect of stachydrine on the traumatic brain injury induced neurodegeneration by attenuating the expressions of Akt/mTOR/PI3K and TLR4/NFκ-B pathway
- Protective effect of extract of Bletilla striata on isoflurane induced neuronal injury by altering PI3K/Akt pathway
- Age-related disturbances in DNA (hydroxy)methylation in APP/PS1 mice
- Novel homozygous mutation in the WWOX gene causes seizures and global developmental delay: Report and review
- Impaired consciousness due to injury of ascending reticular activating system
- Effect of nobiletin on experimental model of epilepsy
Articles in the same Issue
- Regular Articles
- Olfactory and imaging features in atypical Alzheimer’s disease
- Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway
- Corilagin attenuates the Parkinsonismin Japanese encephalitis virus induced Parkinsonism
- Further evidence of accelerated aging in bipolar disorder: Focus on GDF-15
- The ipsilateral vestibulothalamic tract in the human brain
- Neuroprotective effect of ZnT3 knockout on subarachnoid hemorrhage
- Beneficial effect of β-elemene alone and in combination with hyperbaric oxygen in traumatic brain injury by inflammatory pathway
- Protective effects of epifriedelinol in a rat model of traumatic brain injury assessed with histological and hematological markers
- Intracerebroventricular administration of L-arginine improves spatial memory acquisition in triple transgenic mice via reduction of oxidative stress and apoptosis
- Effects of resveratrol and morin on insoluble tau in tau transgenic mice
- Neurophysiological effects of aging: A P200 ERP study
- Photobiomodulation optimization for spinal cord injury rat phantom model
- Sex differences in electrophysiology: P200 event-related potential evidence
- The schizophrenia coping oral health profile. Development and feasibility
- Baicalein inhibits neuroapoptosis via pathways in sevoflurane induced rats
- Magnesium protects in episodes of critical perfusion after aneurysmal SAH
- Evaluation of two types of drug treatment with QEEG in children with ADHD
- Fangchinoline ameliorates the expressions of angiogenic molecule in cerebral ischemia induced neuronal degeneration in neonatal rats
- The schizophrenia oral health profile: Development and feasibility
- Effect of myricetin on primary open-angle glaucoma
- 2D:4D Ratio differs in ischemic stroke: A single center experience
- P38 mitogen-activated protein kinase and Parkinson’s disease
- Tuberous sclerosis complex: Clinical spectrum and epilepsy: A retrospective chart review study
- Analysis of early stroke-induced changes in circulating leukocyte counts using transcriptomic deconvolution
- Hypoglossal-facial ‘side’-to-side neurorrhaphy combined with electrical myostimulation for facial palsy in rats
- Benificial effect of stachydrine on the traumatic brain injury induced neurodegeneration by attenuating the expressions of Akt/mTOR/PI3K and TLR4/NFκ-B pathway
- Protective effect of extract of Bletilla striata on isoflurane induced neuronal injury by altering PI3K/Akt pathway
- Age-related disturbances in DNA (hydroxy)methylation in APP/PS1 mice
- Novel homozygous mutation in the WWOX gene causes seizures and global developmental delay: Report and review
- Impaired consciousness due to injury of ascending reticular activating system
- Effect of nobiletin on experimental model of epilepsy

