Abstract
Objectives
The consumption of popular tobacco products has adverse effects on public health. Early diagnosis and treatment practices are essential based on the disease-symptom relationship in public health problems. There is a need to evaluate biochemical parameters to elucidate the pathophysiological mechanisms of these problems with experimental studies. We aimed to investigate the biochemical and physiological effects of cigarettes, hookahs, and electronic cigarettes (e-cigarettes) that people commonly use.
Methods
We have used Wistar albino rats, and the rats were exposed to cigarette smoke, e-cigarette smoke, and hookah smoke for 60 min/day for seven days. We detected malondialdehyde (MDA), nitric oxide (NOx), protein carbonyl oxidation (PCO), 8-hydroxy-2′-deoxyguanosine (8-OHdG), total antioxidant status (TAS), total oxidant status (TOS), reduced glutathione (GSH), and superoxide dismutase (SOD) levels in blood samples taken after the experiment.
Results
We observed that the redox balance was disturbed in all groups. E-cigarettes mainly triggered lipid peroxidation; only hookah activated the intracellular antioxidant system.
Conclusions
Cigarette, hookah, and e-cigarette smoking should be considered high-risk factors for individual and public health. The use of tobacco products adversely affects public health.
Öz
Amaç
Popüler tütün ürünlerinin tüketiminin halk sağlığı üzerinde olumsuz etkileri bulunmaktadır. Halk sağlığı sorunlarında hastalık-semptom ilişkisine dayalı erken tanı ve tedavi uygulamaları esastır. Bu problemlerin patofizyolojik mekanizmalarını aydınlatabilmek için biyokimyasal parametrelerin deneysel çalışmalarla değerlendirilmesine ihtiyaç bulunmaktadır. Bu çalışmada insanların yaygın olarak kullandığı sigara, nargile ve elektronik sigaraların (e-sigara) biyokimyasal ve fizyolojik etkilerini araştırmayı amaçladık.
Gereç ve yöntemler
Bu çalışmada Wistar albino sıçanlar kullanıldı ve sıçanlar yedi gün boyunca 60 dakika/gün olacak şekilde sigara dumanına, e-sigara dumanına ve nargile dumanına maruz bırakıldı. Deneyden sonra alınan kan örneklerinde malondialdehit (MDA), nitrik oksit (NOx), protein karbonil oksidasyonu (PCO), 8-hidroksi-2′-deoksiguanozin (8-OHdG), total antioksidan statü (TAS), total oksidan statü (TOS), redükte glutatyon ve süperoksit dismutaz (SOD) düzeylerini tespit ettik.
Bulgular
Tüm gruplarda redoks dengesinin bozulduğunu gözlemledik. E-sigara esas olarak lipid peroksidasyonunu tetikledi; yalnızca nargile hücre içi antioksidan sistemi harekete geçirdi.
Sonuç
Sigara, nargile ve e-sigara kullanımı birey ve toplum sağlığı için yüksek risk faktörleri olarak düşünülmelidir. Tütün ürünlerinin kullanımı halk sağlığını olumsuz etkilemektedir.
Introduction
Presently, tobacco is widely used in several ways, such as cigarette smoking, tobacco chewing, or sniffing, named snuff. Active cigarette smoking leads to chronic obstructive pulmonary disease, respiratory tract diseases causing emphysema, acute and chronic myocardial spasm, cardiovascular, cerebral, and peripheral vascular diseases accompanied by atherosclerosis. At the same time, lung cancer is a significant medical issue among cigarette-associated death causes [1]. World Health Organization has reported that more than eight million people die from the diseases due to cigarette use and that this number will exponentially increase until 2025 [2].
The increasing use of electronic cigarettes (e-cigarette) and hookah smoking is also noticeable in recent years. Particularly hookah smoking is preferred for the addibility of the desired aromatic chemicals besides tobacco [3]. Hookah is a system such that tobacco and flavoring chemicals are used together, and the smoke released by burning this mixture using hookah charcoal is drawn through the water in a closed system and inhaled by inspiration. The studies have demonstrated that hookah smoking is significantly associated with lung cancer, respiratory tract diseases, low birth weight, and periodontal diseases. That passage of the hookah smoke through water has no filtering impact regarding exposure to the inhaled chemicals. Despite the perception that hookah smoking is less harmful than cigarette smoking stands as an essential factor in the increasingly widespread use of hookah, there is no scientific evidence for this consideration [4]. The e-cigarette is a nicotine-delivery device with the appearance of conventional cigarettes developed to help addicted persons who ostensibly wanted to quit smoking. A liquid containing propylene glycol or glycerol is present in a refillable cartridge producing an inhalable aerosol by adding nicotine and various flavors to the vaping liquid in this device. Since e-cigarette does not involve the burning process of tobacco, inhaled aerosol does not contain CO, tar, and most of the other toxic ingredients of the conventional cigarette. However, e-cigarette cartridges include varying amounts of nicotine (0–26 mg) [5, 6]. The studies have reported that exposure to cigarette smoke deteriorates oxidative stress parameters at the level of tissues [7] and causes structural changes in the genetic material [8]. Similar risks and findings are also valid for e-cigarette [9] and hookah [10].
In the present study, we have aimed to suggest the effects of cigarette and hookah as the tobacco products with globally widespread consumption and e-cigarette composed of different flavors intended to be used to prevent the harms of these products and as assumed to be harmless with a progressively increasing prevalence concerning redox parameters and DNA damage.
Materials and methods
Animals
We have used 40 Wistar albino male rats for the study. The rats were fed by ad libitum method using standard rat pellet and drinking water for 10 days in Experimental Animal Research and Application Center. Then they were randomly distributed to four groups as control (C), e-cigarette smoke (ECS), cigarette smoke (CS), and Hookah Smoke (HS) groups, and these rat groups were taken to separate sheltering cages. We applied the experimental procedure to all groups in isolated environments.
Experimental protocol
The rats in the CS, ECS, and HS groups were exposed to 60 min/day cigarette smoke, e-cigarette smoke, and hookah smoke, respectively. On the other hand, we applied conditions such as motor noise to which the other groups were exposed to rats in group C and transportation from the cage to the experimental cabin. The experiment duration was seven days to observe the biochemical changes described by Alomari et al. [11].
Cabinets
For inhalation of cigarette, e-cigarette, and hookah smoke by the subjects; cabinets made of polycarbonate material in the dimensions of 38 × 25 × 25 cm (length × width × height) were used. We drilled a hole with a diameter of 0.5 cm from the bottom middle point of the cabinet, fixed a pipe to this part, and ensured its sealing by applying cold silicone sealant. Using this pipe, the smoke flow coming through the smoke transport pipe could fill into the cabinet. The gaps of this material except the fan ventilation hole were isolated using cold silicone sealant to prevent smoke leakage. Separate cabinets with the exact dimensions were used for each group to avoid the contamination (tar, smoke, aerosol) occurring due to cigarette, e-cigarette, or hookah in the inner surface of the cage. The cabinet settings and the materials used for the setting are in Figure 1.
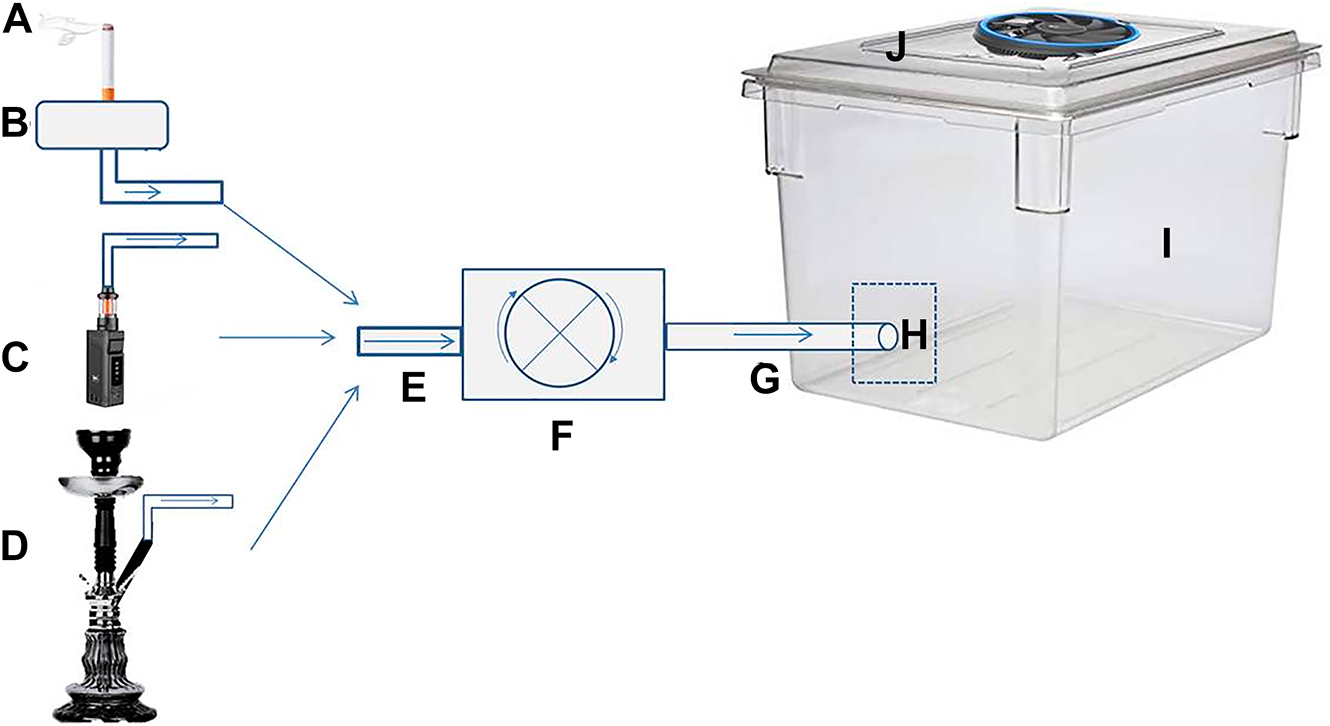
Cabinet settings and the materials used for setting.
A: cigarette; B: smoke chamber; C: e-cigarette; D: hookah; E: flexible plastic pipe (inlet); F: engine (electric motor); G: flexible plastic pipe (outlet); H: plastic fitting pipe; I: polycarbonate cabinet; J: ventilation fan.
Engine, annexes, and operating principles
Rats were exposed to smoke using a negative pressure engine (micro diaphragm pump, DC12 V, 80 W, 0.9 Mpa) used in ornamental aquariums. The peristaltic pump of the engine allowed a unidirectional flow of the smoke by generating negative pressure. As seen in Figure 1, we mounted flexible plastic aquarium pipes on the motor’s inlet and outlet ends. Thanks to negative pressure, the chamber’s smoke sucked towards the engine through the entrance pipe. That smoke pushed through the entrance towards the exit pipe can fill into the cabinet thanks to the created connection [7].
The experimental setting applied for cigarette
The previous studies have reported that a complete burning of a cigarette takes 15–20 min. However, under the influence of negative pressure, we observed that this was 6 min, so we spent 60 min/day on 10 cigarettes and replaced the finished cigarette with a new one [7]. We prevented the rats from suffocating by running the ventilation fan for 1 min.
The experimental setting applied for e-cigarette
For the ECS group, we have applied the application method in the CS group. The consumed e-cigarette cartridges were replaced with a new one to assure continuity of the experiment for 60 min. The Watt values and vaporization time (10 W and 10 s) were adjusted as recommended by the manufacturer [12]. We started the engine simultaneously with the vaporization so that the smoke filled the cabin. We applied this procedure for 6 min to fill the cabin with smoke then applied the ventilation process.
The experimental setting applied for hookah
For the HS group, we have selected the type of hookah that is common to use. We placed the two hookah charcoal on top of the aluminum foil and ignited, so the 10 g of tobacco created smoke. The hookah bottle was half full of water. We operated the engine for 6 min, filled the cabin with smoke, and applied the ventilation process.
Obtaining blood samples
At the end of the 7-day administration, we administered anesthesia (10 mg/kg Xylazine HCl (i.m.) and 50 mg/kg Ketamine HCl (i.m.) to the fasted rats the night before the experiment and obtained 5–7 mL of blood by cardiac puncture into heparinized tubes. We reserved some of the blood samples for malondialdehyde (MDA) and reduced glutathione (GSH) measurement, and the remainder centrifuged at 3,000 rpm (2,054×g) for 10 min to obtain the blood plasma. The plasma samples were taken to 1.5 mL Eppendorf tubes and stored at −80 °C until other biochemical analyses.
Analysis of the redox parameters
The level of MDA as the indicator of lipid peroxidation was measured by the double heating method described previously [13]. According to this method, lipid peroxides react with thiobarbituric acid and give an absorbance at 532 nm. We calculated the amount in nmol/mL. According to the Griess method NOx as a free radical compound that shows a deviation from its physiological state in the inflammatory pathologies was measured spectrophotometrically [14]. This method is based on reduced nitrogen compounds with N-(1-naphthyl) ethylenediamine dihydrochloride forming colorful complexes and measuring their absorbances at 545 nm. We calculated the amount in μmol/L. According to Ellman’s method, we measured GSH spectrophotometrically at 412 nm and calculated the GSH amount in mg/dL. The enzymatic activity determination of SOD, an enzymatic antioxidant and scavenger of superoxide radicals, is based on preventing nitroblue tetrazolium reduction. We measured the color intensity spectrophotometrically at 560 nm and calculated the results in U/gHb [15].
We used a colorimetric test kit (Relassay Diagnostics, Gaziantep, Turkey) to measure total antioxidant status (TAS). According to the operating principle of the assay kit, oxidized radical ABTS is reduced by the antioxidant compounds in the samples and causes color changes. We measured the color intensity spectrophotometrically at 660 nm and calculated the results in mmol/L. We used a colorimetric test kit (Relassay Diagnostics, Gaziantep, Turkey) to measure total oxidant status (TOS). The oxidation of reduced Fe+2 to Fe+3 by the oxidant compounds was determined spectrophotometrically at 660 nm and calculated in μmol/L. We measured the parameters of protein carbonyl oxidation (PCO) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) with ELISA kits (Cayman Chemical, Michigan, USA) and calculated the results in ng/mL.
Statistical analysis
The results were expressed as mean ± SD The normality assumption test was carried out in the analysis of the samples, and the ANOVA test was performed for the variance analysis. The Posthoc Bonferroni test was used in the absence of differences between the groups.
Results
As expressed in Table 1 and Figure 2, it was detected that the levels of MDA, PCO, and 8-OHdG statistically significantly increased in the ECS group, whereas GSH decreased compared with the control group. In the CS group, only the levels of PCO and 8-OHdG were found to be statistically significantly increased, whereas the changes in the levels of the other parameters were statistically insignificant. TOS, PCO, 8-OHdG, and GSH were determined to increase in the HS group significantly.
Some oxidant-antioxidant parameters measured in all groups (X ± SEM, n=10).
| Control | E-cigarette | Cigarette | Hookah | p | |
|---|---|---|---|---|---|
| TAS, mmol/L | 0.91 ± 0.16 | 0.88 ± 0.11 | 0.87 ± 0.09 | 0.94 ± 0.092 | 0.521 |
| NOx, μmol/L | 39.57 ± 5.15 | 40.81 ± 6.40 | 46.79 ± 14.50 | 46.15 ± 9.48 | 0.24 |
| SOD, U/ gHb | 57.01 ± 5.05 | 59.59 ± 3.04 | 55.47 ± 13.76 | 57.53 ± 5.35 | 0.713 |
-
TAS, total antioxidant status; NOx, nitric oxide; SOD, superoxide dismutase. The statistical significance level between the groups is *p<0.05
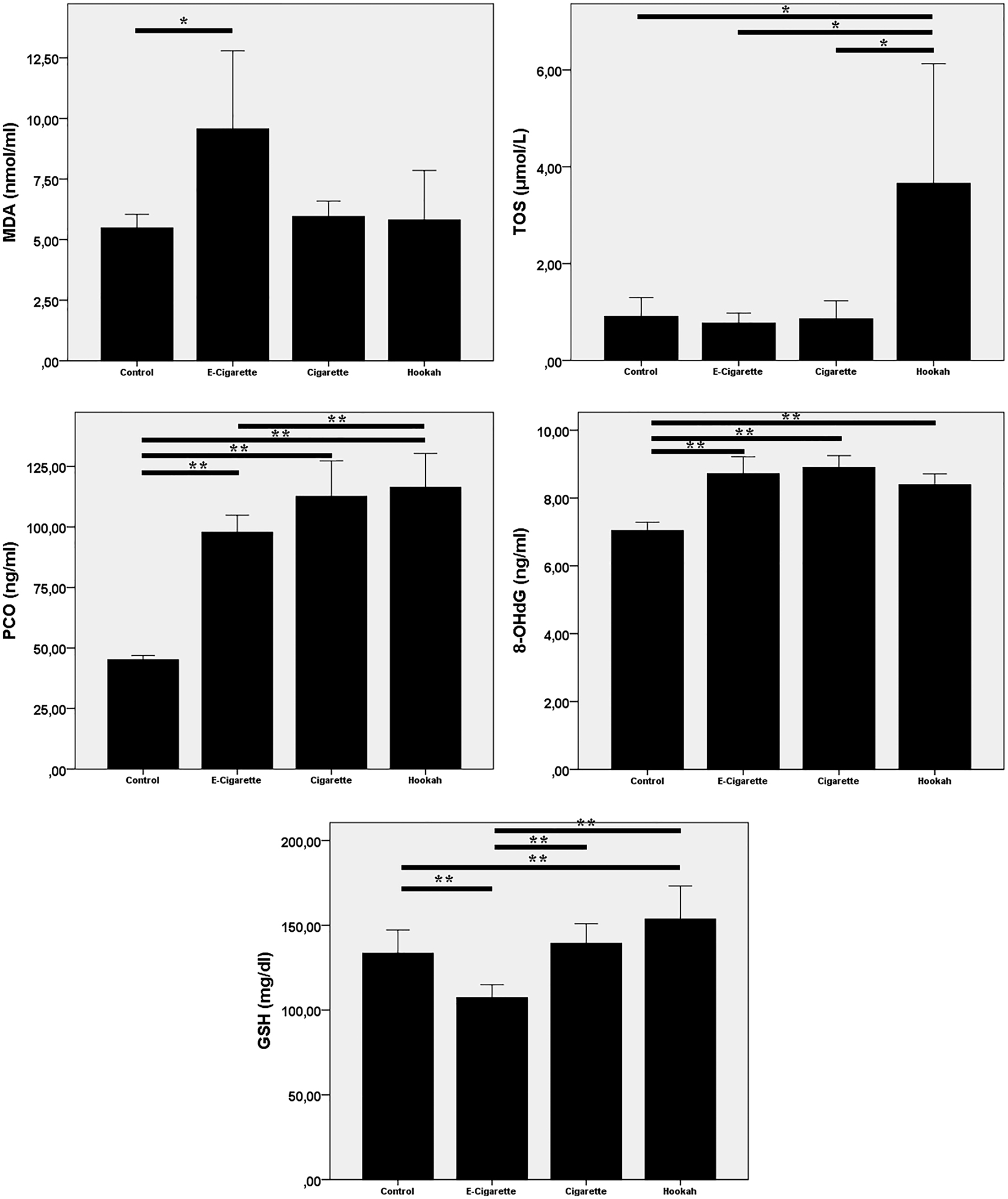
Redox parameter graphs
Bar graphs showing the quantification in various units ± SD of malondialdehyde (MDA), total oxidant status (TOS), protein carbonyl (PCO), DNA damage marker (8-OHdG), and reduced glutathione (GSH) parameters measured in the study groups (ANOVA, posthoc Bonferroni test, *p<0.05; **p<0.0001).
Discussion
People inhale more than 4,000 irritant substances such as CO, nicotine, hydrogen cyanide, butane, methanol by smoking [16]. These chemicals may cause acute and chronic damage by spreading in the whole body via their local effects entering the blood circulation. The rate of cigarette smoking-related deaths is 30% among all cancer-related deaths, while lung and laryngeal cancer types are the most common type among those [1]. Smoking-related free radicals can increase the levels of reactive oxygen species (ROS) produced by phagocytes, causing them to enter the bloodstream, alter antioxidant activities in the blood, and develop various complications.
The ROS produced endogenously in mitochondria, leukocytes, and peroxisomes under physiological circumstances in the organism may also increase due to cigarette smoke, inflammation, anesthetics, pesticides, alcohol, UV radiation, and environmental contaminants [17, 18]. Increased ROS level induces oxidative stress in the structure of lipid, protein, and DNA via oxidation. Lipid peroxidation is a chain reaction starting with oxidation of polyunsaturated fatty acids in the cell membrane by ROS, continuing with the formation of lipid hydroperoxides, and ending with many by-products [18]. Malondialdehyde is one of those final products and a generally accepted indicator of oxidative stress [19]. As a result of ROS-induced oxidation of proteins, oxidative damage occurs in amino acids such as histidine, proline, arginine, and lysine or the peptide structure [20]. PCO is a sensitive indicator used in the determination of oxidative protein damage [21]. Guanosine is the most susceptible to oxidation among purine and pyrimidine bases and is converted to 8-OHdG, an essential indicator of DNA damage in diabetes, cancer, and atherosclerosis [22]. In the present study, the PCO and 8-OHdG out of the parameters that indicate protein and DNA oxidation statistically significantly increased in all three groups compared with the control group, while the highest TOS and MDA levels were encountered in the HS and ECS groups, respectively. We did not observe a statistically significant change in NOx levels, which is one of these indicators. According to the obtained data, detection of increased levels of plasma 8-OHdG and PCO in all three groups supported the reports stating that exposure to cigarette smoke leads to oxidative stress in the rats [7] and causes structural changes in the genetic material [8]. In this context, also the reported conclusion that ROS such as superoxide anion (O2 •), hydrogen peroxide, hydroxyl radical (OH•), MDA, and NOx continuously damage DNA and directly participate in the carcinogenesis process [23] has supported our results. It has been reported that superoxide and nitrogen oxide radicals formed in the gas phase of the cigarette smoke convert guanine to 8-OHdG by transforming to peroxynitrites while hydroquinone and hydrogen peroxide form in the tar phase causes oxidative damage in the cellular DNA at the same time [18]. It has also been reported that the same condition is valid for both e-cigarette [9] and hookah [24].
Additionally, an increase in MDA levels in the ECS group is consistent with the report suggesting that ROS in the smoke of the e-cigarette causes an increased level of MDA in the lung homogenates of the mice [25]. A similar study has reported that, i.p. injection of nicotine causes elevation of MDA level in the renal and hepatic tissues of the rats, whereas e-cigarette liquid containing nicotine does not cause a similar elevation [26]. Nevertheless, it has also been reported that flavoring and sweetening substances in the e-cigarette liquid change the production amount of ROS in the aerosol and that added ethyl vanillin decreases free radicals. In contrast, linalool, piperonal, and citrulline increase the lipid peroxidation products [3]. According to these reports, the high level of MDA detected in the ECS group may result from the natural and artificial sweeteners and mixed flavoring substances in the content of the e-cigarette liquid used in our study. Toxic substances found in hookah smoke originate from tobacco and flavorings [27]. Our observation that TOS is higher in the HS group can be attributed to the long-term burning of hookah coal and the higher amount of heavy metal in hookah users than smokers [10, 24].
The antioxidant defense system performs the cellular inactivation or removal of ROS [17, 28]. GSH is an essential intracellular antioxidant and is primarily found in reduced form. It participates in protecting cells against oxidative damage, toxic compounds, and radiation [29] and preventing DNA synthesis, and repairing broken DNA fragments [30].
We observed that the changes in TAS and SOD levels we measured were not statistically significant compared to the control group. GSH level decreased in the ECS group but increased in the HS group. How and to what extent smoking affects antioxidant enzyme activities is a controversial issue [31]. Pittilo [32] has reported that cigarette smoking impairs the antioxidant activity in the blood serum, whereas Strain et al. [33] have noted that the levels of hemoglobin and ceruloplasmin increase in smokers. However, antioxidant enzymatic activities in the blood exhibit no change [17] and have notified that cigarette smoking increases plasma ferritin level and elevates MDA in the erythrocytes and plasma. Also, Kıral et al. [34] have denoted that MDA levels are high, whereas SOD activity does not change in the rats. In our study, decreased level of GSH despite increased levels of MDA, PCO, and 8-OHdG in the ECS group support the outcomes of the studies that have reported increased oxidative stress and the report of Dinçer et al. [35] stating that there is a negative correlation between the level of GSH and DNA fractures in the smoking males. Besides, an increased level of GSH in parallel with the levels of MDA, PCO, 8-OHdG and TOS in the HS group is a noticeable result of our study and different from the results of the other studies. This significant increase in the level of GSH may be resulting from the interruption of the cycle that GSH is used as a substrate as well as it may be caused by the use of aromatic compounds, sugar molasses, honey, menthol and other various fruit flavors used in the hookah.
It can be suggested that cigarette, hookah, and e-cigarette smoking cause DNA damage and protein oxidation. The GSH levels decrease due to the increased lipid peroxidation by e-cigarette smoking, whereas TOS increases due to hookah smoking. From this aspect, the approach that “e-cigarette is a helping or harmless product used for cessation of smoking” and the perception that “the water of the hookah filters tobacco smoke. Therefore, hookah smoking is harmless” is incorrect. There is a need to investigate the effects of various sweeteners used in e-cigarettes and hookahs, the effects of varying test times, and passive smoking.
Funding source: Afyon Kocatepe University
-
Research funding: This work was supported by Afyon Kocatepe University Scientific Research Projects Commission (No. 18.KARIYER.281).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. B.D.: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing Original Draft, Visualization, Formal analysis. G.A.: Formal analysis, Methodology, Validation, Writing-Review & Editing. B.A.: Operations assistance. A.F.F.: Conceptualization, Formal analysis, Resources, Data curation, Writing-Review & Editing. R.A.: Supervision, Project administration, Funding acquisition.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: The study was approved by Afyon Kocatepe University Animal Experiments Local Ethics Committee (Ref. No. 49533702/128; Reg.No. 114).
References
1. Bartal, M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis 2001;56:545–54.Search in Google Scholar
2. WHO. WHO global report on trends in prevalence of tobacco use 2000–2025, 3rd ed. University of Newcastle, Australia: World Health Organization; 2019.Search in Google Scholar
3. Bitzer, ZT, Goel, R, Reilly, SM, Elias, RJ, Silakov, A, Foulds, J, et al.. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. J Free Radic Biol Med 2018;120:72–9. https://doi.org/10.1016/j.freeradbiomed.2018.03.020.Search in Google Scholar
4. Akl, EA, Gaddam, S, Gunukula, SK, Honeine, R, Jaoude, PA, Irani, J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol 2010;39:834–57. https://doi.org/10.1093/ije/dyq002.Search in Google Scholar
5. Cahn, Z, Siegel, M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Publ Health Pol 2011;32:16–31. https://doi.org/10.1057/jphp.2010.41.Search in Google Scholar
6. Odum, LE, O’Dell, KA, Schepers, JS. Electronic cigarettes: do they have a role in smoking cessation? J Pharm Pract 2012;25:611–4. https://doi.org/10.1177/0897190012451909.Search in Google Scholar
7. Park, E-M, Park, Y-M, Gwak, Y-S. Oxidative damage in tissues of rats exposed to cigarette smoke. J Free Radic Biol Med 1998;25:79–86. https://doi.org/10.1016/s0891-5849(98)00041-0.Search in Google Scholar
8. Izzotti, A, Izzotti, A, Calin, GA, Arrigo, P, Steele, VE, Croce, CM, et al.. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806–12. https://doi.org/10.1096/fj.08-121384.Search in Google Scholar PubMed PubMed Central
9. Dinakar, C, O’Connor, GT. The health effects of electronic cigarettes. N Engl J Med 2016;375:1372–81. https://doi.org/10.1056/nejmra1502466.Search in Google Scholar
10. Maziak, W, Rastam, S, Ibrahim, I, Ward, KD, Shihadeh, A, Eissenberg, T. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob Res 2009;11:806–11. https://doi.org/10.1093/ntr/ntp066.Search in Google Scholar PubMed PubMed Central
11. Alomari, MA, Al-Sheyab, NA, Khabour, OF, Alzoubi, KH. Brain-derived neutrophic factor in adolescents smoking waterpipe: the Irbid TRY. Int J Dev Neurosci 2018;67:14–8. https://doi.org/10.1016/j.ijdevneu.2018.03.007.Search in Google Scholar PubMed
12. Floyd, EL, Queimado, L, Wang, J, Regens, JL, Johnson, DL. Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS One 2018;13:e0210147. https://doi.org/10.1371/journal.pone.0210147.Search in Google Scholar PubMed PubMed Central
13. Draper, HH, Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990;186:421–31. https://doi.org/10.1016/0076-6879(90)86135-i.Search in Google Scholar
14. Miranda, KM, Espey, MG, Yamada, K, Krishna, M, Ludwick, N, Kim, S, et al.. Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem 2001;276:1720–7. https://doi.org/10.1074/jbc.m006174200.Search in Google Scholar
15. Sun, Y, Oberley, LW, Li, Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34:497–500. https://doi.org/10.1093/clinchem/34.3.497.Search in Google Scholar
16. Das, PK, Zahan, T, Rakib, A, Khanam, JA, Pillai, S, Islam, F. Natural compounds targeting cancer stem cells: a promising resource for chemotherapy. Curr Med Chem Anti Cancer Agents 2019;19:1796–808. https://doi.org/10.2174/1871520619666190704111714.Search in Google Scholar
17. Şekeroğlu, M, Aslan, R, Tarakçıoğlu, M, Algün, E, Kara, M. Sigara kullananlarda lipid peroksidasyonu ve antioksidan aktivite. Tuberk Toraks 1997;45:105.Search in Google Scholar
18. Van Der Vliet, A, Eiserich, JP, Halliwell, B, Cross, CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: a potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 1997;272:7617–25. https://doi.org/10.1074/jbc.272.12.7617.Search in Google Scholar
19. Bandeira Junior, G, Baldisserotto, B. Fish infections associated with the genus Aeromonas: a review of the effects on oxidative status. J Appl Microbiol 2020;131:1083–101. https://doi.org/10.1111/jam.14986.Search in Google Scholar
20. Stadtman, E, Levine, R. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003;25:207–18. https://doi.org/10.1007/s00726-003-0011-2.Search in Google Scholar
21. Dalle-Donne, I, Rossi, R, Giustarini, D, Milzani, A, Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329:23–38. https://doi.org/10.1016/s0009-8981(03)00003-2.Search in Google Scholar
22. Wu, LL, Chiou, C-C, Chang, P-Y, Wu, JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 2004;339:1–9. https://doi.org/10.1016/j.cccn.2003.09.010.Search in Google Scholar PubMed
23. Patel, BP, Rawal, UM, Shah, PM, Prajapati, JA, Rawal, RM, Dave, TK, et al.. Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology 2005;68:511–9. https://doi.org/10.1159/000086995.Search in Google Scholar PubMed
24. Maziak, W, Jawad, M, Jawad, S, Ward, KD, Eissenberg, T, Asfar, T. Interventions for waterpipe smoking cessation. Cochrane Database Syst Rev 2015;Art. No.: CD005549. https://doi.org/10.1002/14651858.CD005549.pub3.Search in Google Scholar PubMed PubMed Central
25. Sussan, TE, Gajghate, S, Thimmulappa, RK, Ma, J, Kim, J-H, Sudini, K, et al.. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015;10:e0116861. https://doi.org/10.1371/journal.pone.0116861.Search in Google Scholar PubMed PubMed Central
26. Golli, NE, Jrad-Lamine, A, Neffati, H, Dkhili, H, Rahali, D, Dallagi, Y, et al.. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol 2016;77:109–16. https://doi.org/10.1016/j.yrtph.2016.02.012.Search in Google Scholar PubMed
27. Sepetdjian, E, Saliba, N, Shihadeh, A. Carcinogenic PAH in waterpipe charcoal products. Food Chem Toxicol 2010;48:3242–5. https://doi.org/10.1016/j.fct.2010.08.033.Search in Google Scholar PubMed PubMed Central
28. Żukowski, P, Maciejczyk, M, Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch Oral Biol 2018;92:8–17. https://doi.org/10.1016/j.archoralbio.2018.04.018.Search in Google Scholar PubMed
29. Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev 2005;4:288–314. https://doi.org/10.1016/j.arr.2005.02.005.Search in Google Scholar PubMed
30. Chavan, S, Sava, L, Saxena, V, Pillai, S, Sontakke, A, Ingole, D. Reduced glutathione: importance of specimen collection. Indian J Clin Biochem 2005;20:150. https://doi.org/10.1007/bf02893062.Search in Google Scholar PubMed PubMed Central
31. Taati, B, Arazi, H, Suzuki, K. Oxidative stress and inflammation induced by waterpipe tobacco smoking despite possible protective effects of exercise training: a review of the literature. Antioxidants 2020;9:777. https://doi.org/10.3390/antiox9090777.Search in Google Scholar PubMed PubMed Central
32. Pittilo, M. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Clin Exp Pathol 2000;81:219–30. https://doi.org/10.1046/j.1365-2613.2000.00162.x.Search in Google Scholar PubMed PubMed Central
33. Strain, JJ, Carville, DG, Barker, ME, Thompson, KA, Welch, RW, Young, P, et al.. Smoking and blood antioxidant enzyme activities. Biochem Soc Trans 1989;17:497–8. https://doi.org/10.1042/bst0170497.Search in Google Scholar
34. Kiral, F, Ulutaş, PA, Fidanci, UR. Lipid peroxidation and antioxidant enzymes in rats exposed to cigarette smoke. Ankara Univ Vet Fak Derg 2008;55:145–8.Search in Google Scholar
35. Dinçer, Y, Saygılı, E, Akçay, T. Sigaranın DNA Hasarı Ve Kan Glutatyon Düzeyi Üzerine Etkisi. Turk Klin Tıp Bilim Derg 2003;23:108–11.Search in Google Scholar
© 2022 Barış Denk et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Opinion Paper
- The impact of visual thinking in medical education
- Research Articles
- Early creatinine and e-GFR changes as prognostic predictors of COVID-19 patients
- Matrix Gla-protein expression in peripheral blood mononuclear cells is related to risk factors in cardiovascular diseased patients
- Thiol/disulfide homeostasis and oxidant status in children with congenital heart disease
- The predictive value of serum SCUBE-1 level for left ventricular thrombus in patients with post myocardial infarction heart failure
- Comparison of the modified polyacrylamide gradient gel electrophoresis and high-performance liquid chromatography methods in determining LDL size
- Determination of reference ranges for automated erythrocyte and reticulocyte parameters in healthy adults
- Distribution of HLA epitope frequencies in Turkish population
- Clusterin mRNA silencing reduces cell proliferation, inhibits cell migration, and increases CCL5 expression in SW480, SW620, and Caco2 cells
- miR-22-3p relieves the osteoarthritis by targeting to inflammasome in vivo and in vitro
- The evaluation of PIK3CA gene variation and serum PI3K level in breast cancer risk and prognosis in Turkish population
- The composition of the gut microbiome in patients with sarcopenia
- Sugammadex affects GH/GHR’s signaling transduction on muscle cells by regulating the membrane-localized GHR level
- Redox-changing effects of popular tobacco products in rats
- Kefir protects the liver against high fructose corn syrup induced phosphodiesterase hyperactivity
- Investigation of anti-cholinesterase and anti-amyloidogenic activities of β-lactam antibiotics
- Education Section
- Development and usability testing of an educational mobile learning app for climate change and health impacts
- Medical students and habits of access to information
Articles in the same Issue
- Frontmatter
- Opinion Paper
- The impact of visual thinking in medical education
- Research Articles
- Early creatinine and e-GFR changes as prognostic predictors of COVID-19 patients
- Matrix Gla-protein expression in peripheral blood mononuclear cells is related to risk factors in cardiovascular diseased patients
- Thiol/disulfide homeostasis and oxidant status in children with congenital heart disease
- The predictive value of serum SCUBE-1 level for left ventricular thrombus in patients with post myocardial infarction heart failure
- Comparison of the modified polyacrylamide gradient gel electrophoresis and high-performance liquid chromatography methods in determining LDL size
- Determination of reference ranges for automated erythrocyte and reticulocyte parameters in healthy adults
- Distribution of HLA epitope frequencies in Turkish population
- Clusterin mRNA silencing reduces cell proliferation, inhibits cell migration, and increases CCL5 expression in SW480, SW620, and Caco2 cells
- miR-22-3p relieves the osteoarthritis by targeting to inflammasome in vivo and in vitro
- The evaluation of PIK3CA gene variation and serum PI3K level in breast cancer risk and prognosis in Turkish population
- The composition of the gut microbiome in patients with sarcopenia
- Sugammadex affects GH/GHR’s signaling transduction on muscle cells by regulating the membrane-localized GHR level
- Redox-changing effects of popular tobacco products in rats
- Kefir protects the liver against high fructose corn syrup induced phosphodiesterase hyperactivity
- Investigation of anti-cholinesterase and anti-amyloidogenic activities of β-lactam antibiotics
- Education Section
- Development and usability testing of an educational mobile learning app for climate change and health impacts
- Medical students and habits of access to information

