Abstract
Objectives
Butylparaben is widely used synthetic polymer as preservative in the food, pharmaceutical and cosmetic industries. Although butylparaben is metabolized in the detoxification organs including liver and kidney, some parts of it can retain and accumulate in the body. Parabens can impair developmental and reproductive health, though there is not any published data related with the influence of the butylparaben on the oxidative stress metabolism in the detoxification organs. Therefore, we aimed to evaluate antioxidant enzyme activities in the liver, kidney and spleen of butylparaben-treated rat.
Methods
Prepubertal Wistar albino male rats were administered with 0, 100, 200, 400 mg/kg/day butylparaben for 28 days. After treatment, enzyme activities were evaluated as the biomarkers of the oxidative stress.
Results
Enzyme activities including glucose-phosphate dehydrogenase (G6PD), 6-phosphoglucanate dehydrogenase (6-PGD), glutathione reductase (GR), glutathione s-transferase (GST) and glutathione peroxidase (GPx) were impaired upon butylparaben treatment in the liver, kidney and spleen tissues.
Conclusions
Exposure to endocrine disruptors may affect enzyme activities of the detoxification organs and change the pentose phosphate glutathione (GSH) metabolisms. According to our data oxidative stress metabolism is impaired in the spleen, kidney and liver tissue upon butylparaben treatment that has been indicated first time in the literature.
Öz
Amaç
Bütilparaben yiyecek, ilaç ve kozmetik endüstrilerinde yaygın olarak kullanılan sentetik bir polimerdir. Bütilparaben kısmen karaciğer ve böbrek gibi detoksifikasyon organları tarafından metabolize edilirler, ancak bir kısım metabolize edilmeden kalır ve vücutta birikir.
Parabenlerin gelişim ve üreme sağlığına zarar verdiği bilinmektedir, ancak bütilparabenin detoksifikasyon organlarının oksidatif stres metabolizmasına etkileri henüz çalışılmamıştır. Bu nedenle çalışmamızda bütilparabene maruz kalmış sıçanların karaciğer, böbrek ve dalaklarında antioksidan enzim aktivitelerini değerlendirmeyi amaçladık.
Gereçveyöntem
Prepubertal Wistar albino erkek sıçanlara 0, 100, 200, 400 mg/kg/gün butilparaben oral gavaj ile 28 gün boyunca uygulandı. Uygulamadan sonra antioksidan enzim aktiviteleri oksidatif stres biyo-belirteçleri olarak değerlendirildi.
Bulgular
Dalak, karaciğer ve böbrek dokularında glikoz 6-fosfat dehidrogenaz, 6-fosfoglukanat dehidrogenaz, glutatyon reduktaz, glutatyon s-transferaz ve glutatyon peroksidaz enzimlerinin kontrol grubuna göre bozulduğu gözlemlenmiştir.
Sonuç
Endokrin bozuculara maruz kalmak detoksifikasyon organlarında glutatyon ve pentoz fosfat metabolizması enzim aktivitelerini bozmaktadır. Literatürde ilk defa, sonuçlarımız oksidatif stres metabolizmasının karaciğer, dalak ve böbrek dokularında bütilparaben uygulaması nedeniyle bozulduğunu göstermiştir.
Introduction
Butylparaben is an alkyl ester compound and widely used synthetic polymer as preservative in the various industrial products including food, personal care products, cosmetics and pharmaceuticals [1]. Total daily exposure to the parabens is 76 mg, although the Acceptable Daily Intake (ADI) for them is 0–10 mg/kg Bw/day for human [2], [3]. After absorption from the skin and gastrointestinal system, butylparaben is metabolized in the detoxification organs including liver and kidney. However, some part of the parabens remains and accumulates in the body over time. There is a growing concern about adverse health effects of the butylparaben, since it has been reported that parabens can impair reproductive, developmental and redox systems by acting endocrine disrupting chemical (EDC) [4], [5], [6], [7].
Liver, kidney and spleen are responsible for the detoxification of the xenobiotics including butylparaben and other EDCs which interfere with hormones. Because of the detoxification reactions, oxidative stress increases in these organs that may cause imbalance in the cellular processes leading to the tissue damage and diseases [7], [8], [9], [10], [11]. Therefore, in this study we designed our experiments to evaluate impacts of the butylparaben on the detoxification organs which have not been studied before in the literature. Our findings may reveal butylparaben-induced impairments in the oxidative branch of pentose phosphate metabolism and glutathione dependent enzyme activities which were both tightly correlated with oxidative stress metabolisms of the detoxification organs.
Material & Method
Materials
Butylparaben (Butyl 4-hydroxybenzoate, >99% purity) and all other chemicals were purchased from Sigma–Aldrich (USA).
Methods
Animals
Wistar albino male rats (Rattus norvegicus) were obtained from Hacettepe University, Ankara, Turkey. The rats were kept in a room maintained 12 h light/dark cycle and at a temperature of 22 ± 2 °C and relative humidity 50 ± 5. Standard rat diet was administered to the animals and tap water ad libitum. Ethics Committee of Hacettepe University (B.30.2.HAC.0.05.0.6.00/132) approved all experimental procedures.
Dosages and butylparaben administration
Twenty four rats were randomly divided into four groups after one week of acclimatization. Three groups served as the treatment groups (100, 200 and 400 mg/kg/day), where one group was used as the oil-control group. Butylparaben was administrated by oral gavage daily for 28 consecutive days. Corn oil was prepared weekly depending on the body weight of the rats. The rats in the oil-control group were additionally given corn oil orally in amounts equal to those used in the dosed groups. The dosages were selected by considering the toxicokinetic properties of the butylparaben and similar previous studies performed to show the degree of effect in the increasing dosages. The maximum dose was defined at as high a dose as possible, without causing any quantifiable effect at physiological effects without causing mortality. In this study, maximum tolerated dose (MTD) was estimated according to Rothfuss et al. and Garcia et al. (acute oral LD50; acute MTD) [5], [12]. Also, the investigation of estrogenic activity, highest administration dose level of butylparaben was considered as the LD50 value. After the treatment period all the tissues of all animals were immediately removed.
Evaluation of the antioxidant enzyme activities
We have used UV/visible spectrophotometry for the quantitative determination of glucose-phosphate dehydrogenase (G6PD), 6-phosphoglucanate dehydrogenase (6-PGD), glutathione reductase (GR), glutathione s-transferase (GST) and glutathione peroxidase (GPx) enzyme activities. Enzyme activities were measured two times and activities were followed for 1 min. G6PD and 6-PDG enzyme activities were evaluated as described previously by Aydemir et al. [9], [10], [11]. GR enzyme activity is evaluated by the modified Staal method as described by Aydemir et al. [9], [10], [11]. GST belongs to the family of eukaryotic and prokaryotic phase II metabolic enzyme and activity of GST was determined using reduced glutathione (GSH) and 1-chloro-2,4-dinitrobenzene (CDNB) as described by Aydemir et al. [13].
GPx activity measured at 37 °C in the 100 mmol/L potassium phosphate buffer contained GR (10 U/mL), 100 mmol/L GSH, 400 mmol/L sodium azide, 200 mmol/L EDTA, 2 mmol/L reduced NADP and 5 µL tissue supernatant [14]. Samples were incubated for 600 s then the reaction was initiated by the addition of tissue supernatant and hydrogen peroxide. This assay is based on coupled enzyme activity by GR and reduced nicotinamide adenine dinucleotide phosphate (NADPH). The pH of the buffer was 7.0 and the concentration was 0.1 mol/L.
Evaluation of protein determination
The protein concentration of liver, kidney and spleen tissue supernatant was evaluated by the bicinchoninic acid (BCA) method using Spectramax M2 microplate reader (Pierce™ BCA Protein Assay Kit).
Statistical analysis
We have analyzed our data by GraphPad Prism by one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test for multiple comparison. Our results were presented as the mean ± standard deviation (SD).
Results
Anti-oxidant enzyme activities were impaired upon butylparaben administration in the liver, kidney and spleen tissues.
GST enzyme activity meaningfully decreased in the all butylparaben administrated groups compared to the control group, however GPx activity significantly increased in the 200 mg/kg/day dose group compared to the all other groups in the liver (Figure 1). G6PD, 6PGD and GR activities were fluctuated upon butylparaben administration in the liver (Figure 1).

Anti-oxidant enzyme activities in the liver samples of male rats in control and butylparaben treatment groups. All groups are compared to CTL group and each other. All results were given as the mean ± SD of n=6 animals.
a 200 mg/kg/day butylparaben dose group is different 400 mg/kg/day dose group (p=0.0488), b 100 mg/kg/day butylparaben dose group is different from CTL group (p=0.0109), c 200 mg/kg/day butylparaben dose group is different from CTL group (p=0.0416), d 400 mg/kg/day butylparaben dose group is different from CTL group (p=0.0002), e 200 mg/kg/day butylparaben dose group is different from CTL group (p=0.0028), f 200 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0126), g 200 mg/kg/day butylparaben dose group is different from 400 mg/kg/day dose group (p=0.0002).
G6PD and GR activities significantly increased in the butylparaben treatment groups compared to the control in the kidney (Figure 2). GST activity meaningfully increased in 200 mg/kg/day butylparaben treatment group compared to the control group and other dose groups; however GPx activity significantly decreased in 200 and 400 mg/kg/day dose group compared to the other groups in the kidney samples (Figure 2).
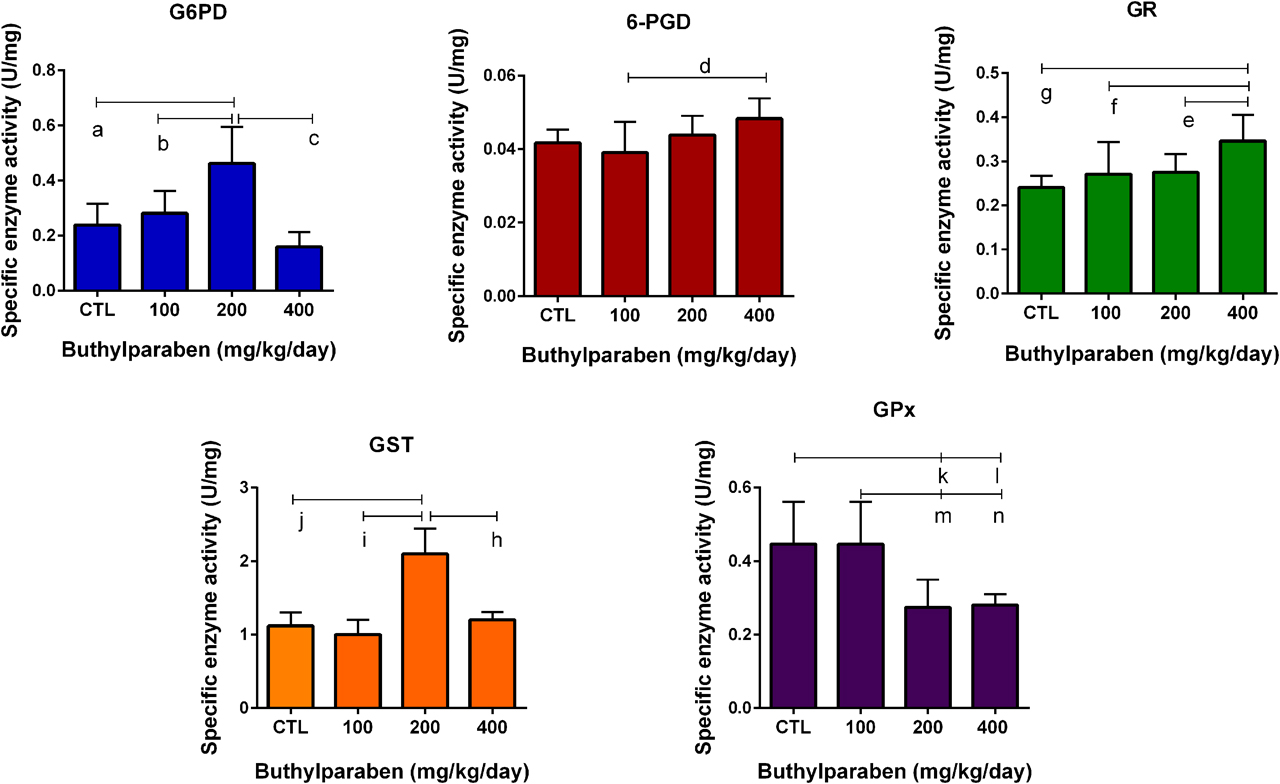
Anti-oxidant enzyme activities in the kidney samples of male rats in control and butylparaben treatment groups. All groups are compared to CTL group and each other. All results were given as the mean ± SD of n=6 animals.
a 200 mg/kg/day butylparaben dose group is different from CTL group (p=0.0028), b 200 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0126), c 200 mg/kg/day butylparaben dose group is different from 400 mg/kg/day dose group (p=0.0002), d 100 mg/kg/day butylparaben dose group is different from 400 mg/kg/day dose group (p= 0.0039), e 400 mg/kg/day butylparaben dose group is different from 200 mg/kg/day dose group (p=0.0003), f 400 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0125), g 400 mg/kg/day butylparaben dose group is different from CTL group (p=0.0187), h 200 mg/kg/day butylparaben dose group is different from 400 mg/kg/day dose group (p<00001), i 200 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p<0.0001), j 200 mg/kg/day butylparaben dose group is different from CTL group (p<00001), k 200 mg/kg/day butylparaben dose group is different from CTL group (p=0.0212), l 400 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0370), m 200 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0212), n 400 mg/kg/day butylparaben dose group is different from 100 mg/kg/day dose group (p=0.0370).
G6PD, 6-PGD, GR and GPx activities increased in butylparaben dose groups compared to the control, however this change was significant for G6PD and GR activities in the spleen tissue (Figure 3). On the other hand, GST activity significantly decreased in all dose groups, but only significant change was observed in highest butylparaben treatment groups compared to the control in the spleen tissue (Figure 3).

Anti-oxidant enzyme activities in the spleen samples of male rats in control and butylparaben treatment groups. All groups are compared to CTL group and each other. All results were given as the mean ± SD of n=6 animals.
a 100 mg/kg/day butylparaben dose group is different from CTL group (p<0.0004), b 200 mg/kg/day butylparaben dose group is different from CTL group (p<0.0001), c 400 mg/kg/day butylparaben dose group is different from CTL group (p<00001), d 100 mg/kg/day butylparaben dose group is different from CTL group (p< 00058), e 200 mg/kg/day butylparaben dose group is different from CTL group (p=0.0037), f 400 mg/kg/day butylparaben dose group is different from CTL group (p<0.0001), g 400 mg/kg/day butylparaben dose group is different from CTL group (p=0.0348).
Discussion
Effects of parabens and other EDCs have been widely studied on the reproductive and developmental systems, however there is no detailed data about the butylparaben-enhance oxidative stress in the detoxification organs including liver, kidney and spleen [1], [2], [3], [4], [5], [6], [7], [8]. Since parabens and other EDCs can induce oxidative stress resulting in the tissue damage in human and wildlife, cause formation of the various diseases [4], [9], [10], [11], [15], [16].
Pentose phosphate pathway (PPP) play vital role in xenobiotic metabolism. Endocrine disruptors or any other molecules, chemicals that affects the PPP enzyme activities especially G6PD and 6-PGD will results in fail of metabolic network of the cell and the organism [11], [].
The reduced form of GSH is one of the cell’s most significant antioxidants and reduction of this molecule is directly associated with PPP’s product NADPH and the enzyme GR [9], [11], [13], [21]. Moreover, Zhang et al. reported that GSH depletion or increased oxidized GSH concentration can increase oxidative phase of PPP and cells with low levels of GSH are susceptible hemolysis in erythrocytes [22]. Thus, we evaluated antioxidant enzyme activities including G6PD, 6-PGD, GR, GST and GPx in the detoxification after butylparaben treatment as the oxidative stress biomarkers in our study. G-6-PD and GR activities significantly increased upon butylparaben treatment in the main xenobiotic detoxification tissues (Figures 1–3). GST activity meaningfully reduced in the butylparaben treated groups compared to the control in the liver and spleen (Figures 1, 3). However, GPx activity significantly increased in the liver, where significantly decreased in the kidney tissue (Figures 1, 2).
GST, GR and GPx are involving in the GSH metabolism responsible for the detoxification and oxidative stress metabolisms of xenobiotics including EDCs such as butylparaben and phthalates. Since these enzymes keep balance of the oxidized glutathione (GSSG)/GSH ratio, impairment of this ratio causes central redox homeostasis in the organisms [9], [16], [17], [23].
G6PD and 6-PGD play key role in the PPP and responsible to produce NADPH required for the function of GR, GST and GPx. Increased levels of oxidative stress may result from impairment in the antioxidant enzyme activities such as G6PD, 6-PGD, GR, GST and GPX. Elevated levels of ROS attack lipids, proteins and DNA may end up tissue injury [4], [19], [20], [23].
Liver and kidney are the major detoxification organs, where spleen is mainly responsible for the immune defense system of the organism. Butylparaben-induced impairments have been reported previously in liver, kidney and spleen. For instance, butylparaben may cause high incidence of amyloidosis and significant atrophy in the spleen [15], [16], [17], [18], [19], [23]. Moreover, Aydemir et al. reported EDC DEHP can induce tissue injury in the liver, kidney, brain and testis via elevated levels of oxidative stress [9], [10].
Overall, our data showed that oxidative stress metabolism is activated to fight against butylparaben in the liver, kidney and spleen tissues because of fluctuated antioxidant enzyme activities. Impairment in the indicated enzyme activities may damage tissue function and cause initiation of different types of diseases.
Conclusion
There is an increasing concern and attention about adverse health effects of EDCs including butylparaben.We showed first time in the literature that oxidative stress metabolism is impaired in spleen, kidney and liver tissue upon butylparaben treatment. Our results also highlighted the importance of endocrine disruptor, butylparaben on the oxidative part of PPP enzymes as an important component in the response to xenobiotic metabolism and various anabolic reactions. The present study concludes that realistic exposure to endocrine disruptors can increase the spleen enzymes and even more hazardous and may induce immunosuppression of the target organism.
Funding source: Presidency of Turkey, Presidency of Strategy and Budget
Acknowledgments
The authors gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Research funding: This research was funded by Presidency of Turkey, Presidency of Strategy and Budget.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: All authors declare that there is no conflict of interest.
Ethical approval: The study was approved by Ethics Committee of Hacettepe University (B.30.2.HAC.0.05.0.6.00/132).
References
1. Maske, P, Dighe, V, Vanage, G. n-butylparaben exposure during perinatal period impairs fertility of the F1 generation female rats. Chemosphere 2018;213:114–23. https://doi.org/10.1016/j.chemosphere.2018.08.130.Search in Google Scholar
2. Boberg, J, Taxvig, C, Christiansen, S, Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 2010;30:301–12. https://doi.org/10.1016/j.reprotox.2010.03.011.Search in Google Scholar
3. Soni, MG, Carabin, IG, Burdock, GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005;43:985–1015. https://doi.org/10.1016/j.fct.2005.01.020.Search in Google Scholar
4. Oana, A, Eliza, D, Marina, G, Daniel, G, Rogoveanud, O, Varut, M, et al. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem Toxicol 2018;15:470–81.10.1016/j.fct.2018.03.052Search in Google Scholar
5. Garcia, T, Schreiber, E, Kumar, V, Prasad, R, Sirvent, JJ, Domingo, JL, et al. Effects on the reproductive system of young male rats of subcutaneous exposure to n-butylparaben. Food Chem Toxicol 2017;106:47–57. https://doi.org/10.1016/j.fct.2017.05.031.Search in Google Scholar
6. Yang, YJ, Hong, YP, Chae, SA. Reduction in semen quality after mixed exposure to bisphenol A and isobutylparaben in utero and during lactation periods. Hum Exp Toxicol 2016;35:902–11. https://doi.org/10.1177/0960327115608927.Search in Google Scholar
7. NIEHS - National Institute of Environmental Health Sciences. Available from: http://www.niehs.nih.gov/health/topics/agents/endocrine/ 18 February 2015.Search in Google Scholar
8. Boberg, J, Taxvig, C, Christiansen, S, Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 2010;30:301–12. https://doi.org/10.1016/j.reprotox.2010.03.011.Search in Google Scholar
9. Aydemir, D, Karabulut, G, Şimşek, G, Barlas, N, Ulusu, NN. Impact of the Di(2-Ethylhexyl) phthalate administration on trace element and mineral levels in relation of kidney and liver damage in rats. Biol Trace Elem Res 2018;186:474–88. https://doi.org/10.1007/s12011-018-1331-0.Search in Google Scholar
10. Aydemir, D, Karabulut, G, Şimşek, G, Barlas, N, Ulusu, NN. Data the DEHP induced changes on the trace element and mineral levels in the brain and testis tissues of rats. Data Brief 2019;26:104526. https://doi.org/10.1016/j.dib.2019.104526.Search in Google Scholar
11. Aydemir, D, Oztasci, B, Barlas, N, Ulusu, NN. Effects of butylparaben on antioxidant enzyme activities and histopathological changes in rat tissues. Arh Hig Rada Toksikol 2019;70:315–24. https://doi.org/10.2478/aiht-2019-70-3342.Search in Google Scholar
12. Rothfuss, M, O’Donovan, M, De Boeck, D, Brault, D, Czich, A, Custer, L, et al. Collaborative study on fifteen compounds in the rat-liver Comet assay integrated into 2- and 4-week repeat-dose studies. Mutat Res 2010;702:40–69. https://doi.org/10.1016/j.mrgentox.2010.07.006.Search in Google Scholar
13. Aydemir, D, Hashemkhani, M, Acar, HY, Ulusu, NN. In vitro interaction of glutathione S‐transferase‐pi enzyme with glutathione‐coated silver sulfide quantum dots: a novel method for biodetection of glutathione S‐transferase enzyme. Chem Biol Drug Des 2019;94:2094–102. https://doi.org/10.1111/cbdd.13614.Search in Google Scholar
14. Beutler, E. Red cell metabolism. In: A Manual of biochemical methods. New York: Grone & Stratton; 1971:66–8. pp.10.1016/S0300-9084(72)80181-0Search in Google Scholar
15. Deborah, J, Watkins, K, Ferguson, L, Anzalota Del Toro, V, Cordero, JF, Meeker, JD, et al. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health 2015;218:212–9, https://doi.org/10.1016/j.ijheh.2014.11.001.Search in Google Scholar
16. Guoyao, W, Yun-Zhong, F, Sheng, Y, Lupton, JR, Turner, ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–492 https://doi.org/10.1093/jn/134.3.489.Search in Google Scholar
17. Aydemir, D, Sarayloo, E, Ulusu, NN. Rosiglitazone-induced changes in the oxidative stress metabolism and fatty acid composition in relation with trace element status in the primary adipocytes. J Med Biochem 2019;38:1–9. https://doi.org/10.2478/jomb-2019-0041.Search in Google Scholar
18. Stincone, A, Prigione, A, Cramer, T, Wamelink, MM, Campbell, K, Cheung, E, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc 2015;90:927–63. [Epub ahead of print] 2014 Sep 22. https://doi.org/10.1111/brv.12140.Search in Google Scholar
19. Ulusu, NN. Curious cases of the enzymes. J Med Biochem 2015;34:271–81. https://doi.org/10.2478/jomb-2014-0045.Search in Google Scholar
20. Aydemir, D, Hashemkhani, M, Durmusoglu, EG, Yagci-Acar, H, Ulusu, NN. A new substrate for glutathione reductase: Glutathione coated Ag2S quantum dots. Talanta 2019;194:501–6. https://doi.org/10.1016/j.talanta.2018.10.049.Search in Google Scholar
21. Mebius, RE, Kraal, G. Structure and function of the spleen. Nat Rev Immunol 2005;5:606–16. https://doi.org/10.1038/nri1669.Search in Google Scholar
22. Zhang, ZZ, Lee, EE, Sudderth, J, Yue, AZ, Glass, D, Deberardinis, RJ, et al. Glutathione depletion, pentose phosphate pathway activation, and hemolysis in erythrocytes protecting cancer cells from vitamin C-induced oxidative stress. J Biol Chem 2016;44:22861–7. https://doi.org/10.1074/jbc.c116.748848.Search in Google Scholar
23. Aydemir, D, Ulusu, NN. Comment on the: molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes (Spectrochim Acta A Mol Biomol Spectrosc. 2019 Sep 5; 220:117104). Spectrochım Acta A 2020;229:117792. https://doi.org/10.1016/j.saa.2019.117792.Search in Google Scholar
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Articles
- Therapeutic approaches on the interaction between SARS-CoV2 and ACE2: a biochemical perspective
- Therapeutic agents currently employed against Covid-19: an effort to control the pandemic
- Association between breast milk adipokines with growth in breast feeding infants, a systematic review and meta-analysis
- Opinion Paper
- The role of biotin metabolism in the COVID-19 infection
- Value of blood IFN-I levels in COVID-19 management
- Some comments on enzyme kinetics studies
- Short Communication
- SKA3 overexpression promotes cell proliferation and migration in breast cancer cell lines
- Influence of the butylparaben administration on the oxidative stress metabolism of liver, kidney and spleen
- Probable alterations in fecal bacterial microbiota by somatostatin receptor analogs in acromegaly
- Research Articles
- A simple silica based DNA isolation method for cell-free DNA analysis from liquid biopsy
- The effects of silibinin on oxidative stress and microRNA-10b expression in animal models of breast cancer
- A novel approach for the discrimination of culture medium from Vascular Endothelial Growth Factor (VEGF) overexpressing colorectal cancer cells
- The investigation effect of weight loss on serum vaspin, apelin-13, and obestatin levels in obese individual
- Enhancer of zeste homolog 2 (EZH2) gene inhibition via 3-Deazaneplanocin A (DZNep) in human liver cells and it is relation with fibrosis
- Synthesis of 2-aminonaphthalene-1-sulfonic acid Schiff bases and their interactions with human serum albumin
- Association study of polymorphisms in ABCA7, clusterin, and MS4A6A genes with Alzheimer’s disease in the Egyptian population
- Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression
- Thiamine pyrophosphate riboswitch regulation: a new possible mechanism involved in the action of nalidixic acid
- Structural evidence for kinetic and thermal stability changes of α-amylase due to exposure to [emim][lactate] ionic liquid
- Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram
- Cytotoxic, genotoxic and apoptotic effects of Viburnum opulus on colon cancer cells: an in vitro study
- Acrylamide-encapsulated glucose oxidase inhibits breast cancer cell viability
- Explore the activation efficiency of different ligand carriers on synNotch-based contact-dependent activation system
- Expression level of miRNAS in patients with gestational diabetes
- Effect of static magnetic field with quercetin and hesperetin on MCF-7 and MDA MB-231 breast cancer cells
- In vitro antimicrobial, antioxidant, cytotoxic activities, and wound healing potential of Thymbra capitata ethanolic extract
- The association of methylene tetrahydrofolate reductase (MTHFR) A1298C gene polymorphism, homocysteine, vitamin B12, and folate with coronary artery disease (CAD) in the north of Iran
- Synthetic peptide vaccine for Foot-and-Mouth Disease: synthesis, characterization and immunogenicity
- New pathway in rheumatic mitral valve disease: cytochrome P450 and glutathione S transferase isozyme expression
- Ghrelin and orexin levels in infertile male: evaluation of effects on varicocele pathophysiology, relationship seminal and hormonal parameter
- The activities of GST isozymes in stomach tissues of female obese patients
- Analysis of blood gas beyond bicarbonate in outpatients with stage 3–5 chronic kidney disease
- Relationship between JAK2-V617F mutation and hematologic parameters in Philadelphia-negative chronic myeloproliferative neoplasms
- Case Report
- The role of the laboratory in the diagnosis process in a patient with mildly elevated hCG: a case report
- Letter to the Editor
- Hookah use and COVID-19
Articles in the same Issue
- Frontmatter
- Review Articles
- Therapeutic approaches on the interaction between SARS-CoV2 and ACE2: a biochemical perspective
- Therapeutic agents currently employed against Covid-19: an effort to control the pandemic
- Association between breast milk adipokines with growth in breast feeding infants, a systematic review and meta-analysis
- Opinion Paper
- The role of biotin metabolism in the COVID-19 infection
- Value of blood IFN-I levels in COVID-19 management
- Some comments on enzyme kinetics studies
- Short Communication
- SKA3 overexpression promotes cell proliferation and migration in breast cancer cell lines
- Influence of the butylparaben administration on the oxidative stress metabolism of liver, kidney and spleen
- Probable alterations in fecal bacterial microbiota by somatostatin receptor analogs in acromegaly
- Research Articles
- A simple silica based DNA isolation method for cell-free DNA analysis from liquid biopsy
- The effects of silibinin on oxidative stress and microRNA-10b expression in animal models of breast cancer
- A novel approach for the discrimination of culture medium from Vascular Endothelial Growth Factor (VEGF) overexpressing colorectal cancer cells
- The investigation effect of weight loss on serum vaspin, apelin-13, and obestatin levels in obese individual
- Enhancer of zeste homolog 2 (EZH2) gene inhibition via 3-Deazaneplanocin A (DZNep) in human liver cells and it is relation with fibrosis
- Synthesis of 2-aminonaphthalene-1-sulfonic acid Schiff bases and their interactions with human serum albumin
- Association study of polymorphisms in ABCA7, clusterin, and MS4A6A genes with Alzheimer’s disease in the Egyptian population
- Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression
- Thiamine pyrophosphate riboswitch regulation: a new possible mechanism involved in the action of nalidixic acid
- Structural evidence for kinetic and thermal stability changes of α-amylase due to exposure to [emim][lactate] ionic liquid
- Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram
- Cytotoxic, genotoxic and apoptotic effects of Viburnum opulus on colon cancer cells: an in vitro study
- Acrylamide-encapsulated glucose oxidase inhibits breast cancer cell viability
- Explore the activation efficiency of different ligand carriers on synNotch-based contact-dependent activation system
- Expression level of miRNAS in patients with gestational diabetes
- Effect of static magnetic field with quercetin and hesperetin on MCF-7 and MDA MB-231 breast cancer cells
- In vitro antimicrobial, antioxidant, cytotoxic activities, and wound healing potential of Thymbra capitata ethanolic extract
- The association of methylene tetrahydrofolate reductase (MTHFR) A1298C gene polymorphism, homocysteine, vitamin B12, and folate with coronary artery disease (CAD) in the north of Iran
- Synthetic peptide vaccine for Foot-and-Mouth Disease: synthesis, characterization and immunogenicity
- New pathway in rheumatic mitral valve disease: cytochrome P450 and glutathione S transferase isozyme expression
- Ghrelin and orexin levels in infertile male: evaluation of effects on varicocele pathophysiology, relationship seminal and hormonal parameter
- The activities of GST isozymes in stomach tissues of female obese patients
- Analysis of blood gas beyond bicarbonate in outpatients with stage 3–5 chronic kidney disease
- Relationship between JAK2-V617F mutation and hematologic parameters in Philadelphia-negative chronic myeloproliferative neoplasms
- Case Report
- The role of the laboratory in the diagnosis process in a patient with mildly elevated hCG: a case report
- Letter to the Editor
- Hookah use and COVID-19

