Abstract
Background and aims
Among the factors associated with cancer are the oxidative stress and increased expression of some microRNA (miRs). Silibinin has an anti-tumor effect. Therefore, this study evaluates the effects of silibinin on oxidative stress indices and miR-10b expression in the animal models of breast cancer.
Material and methods
In this study, 48 Balb/c mice were divided into six groups (each group contains eight mice): the healthy control, the cancer control, the healthy group receiving 20 mg of silibinin, the cancer group receiving 20 mg of silibinin, the cancer group receiving 40 mg of silibinin and the cancer group receiving 80 mg of silibinin for three weeks. In order to induce cancer, 4T1 cell line was used. After obtaining breast tumor samples, the levels of Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPX) and miR-10b expression in breast tumor biopsy were evaluated. Data were analyzed using one-way ANOVA, Kruskal–Wallis, Mann–Whitney and t-test (p<0.05).
Results
The use of silibinin at different doses increased the activity of SOD and GPX (significantly) and the level of TAC (significantly) in the treatment group compared to untreated cancerous mice, but mir-10b and MDA were decreased non-significant and significantly respectively.
Conclusion
Silibinin led to a non-significant reduction of miR-10b in the treatment group compared to untreated cancerous mice. Silibinin has been shown to improve oxidative stress in breast cancer mice.
Öz.
Giriş ve Amaç
Oksidatif stres ve bazı artmış microRNA (miR’lerin) ekspresyonu kanserle ilişkili faktörler arasındadır. Silibininin bir anti-tümör etkisi vardır. Bu nedenle bu çalışmanın amacı, meme kanserinin hayvan modellerinde silibininin oksidatif stres indeksleri ve miR-10b ekspresyonu üzerindeki etkilerini değerlendirmektedir.
Gereç ve Yöntemler
Bu çalışmada 48 Balb / c fare 6 gruba ayrıldı (her grup 8 fare içerir): sağlıklı kontrol, kanser kontrolü, 20 mg silibinin alan sağlıklı grup, 20 mg alan kanser grubu 40 mg silibinin alan kanser grubu ve 3 hafta boyunca 80 mg silibinin alan kanser grubu. Kanseri indüklemek için 4T1 hücre hattı kullanıldı. Meme tümör örnekleri elde edildikten sonra, göğüs tümör biyopsisinde Malondialdehid (MDA), süperoksit dismutaz (SOD), glutatyon peroksidaz (GPX) ve miR-10b ekspresyon seviyeleri değerlendirildi. Veriler tek yönlü ANOVA, Kruskal–Wallis, Mann–Whitney ve T testi kullanılarak analiz edildi (p<0.05).
Bulgular
Farklı dozlarda silibinin kullanımı, tedavi edilmemiş kanserli farelere kıyasla tedavi grubunda SOD ve GPX aktivitesinde artışa neden oldu, ancak TAC’yi önemli ölçüde artırdı ve MDA’yı azalttı.
Sonuç
Silibinin tedavi edilmemiş kanserli farelere kıyasla tedavi grubunda önemli olmayan miR-10b azalmasına yol açmıştır. Silibininin meme kanseri farelerinde oksidatif stresi azalttığı gösterilmiştir.
Introduction
The prevalence of breast cancer in most countries increases one to two percent annually, and an additional one million new cases are added each year [1]. According to studies, the association of oxidative stress with cancer has been confirmed [2]. In other words, oxidative stress is created as a result of disturbance between the production of free radicals (active oxygen and nitrogen species) and the antioxidant defense system [3]. In aerobic biological systems, superoxide dismutase enzymes (SOD), glutathione peroxidase (GPX), catalase and vitamins are important in controlling free radicals [4]. Superoxide dismutase exists in forms of Zn/Cu-SOD and Mn-SOD in cytoplasm, lysozymes and mitochondria, and it catalyzes the dismutation of anion superoxide into H2O2 [5]. Moreover, Glutathione peroxidase via glutathione reduces hydrogen peroxide and lipid hydroperoxides into the water and related alcohols. In this process, glutathione is oxidized to glutathione disulfide, which finally turns into a reduced form by glutathione reductase enzyme [6]. The result of lipid peroxidation followed by oxidative stress is metabolites such as malondialdehyde (MDA) which is considered as an index of lipid peroxidation and a major oxidative stress biomarker, and it increases in a variety of cancers, such as breast cancer [7]. In the process of apoptosis and cancer, other molecules are involved among which miRNAs can be mentioned. It seems that up to 50% of all genes are regulated by miRNAs [8]. MicroRNA-10b (miR-10b) extensively increases in metastatic breast cancer cells and stimulates migration and invasion [9]. This molecule has implication in cancer progression, particularly metastatic progression of breast cancer and beside another miRNA such as miR-21, miR-155, and Let-7a is the potential biomarker for the monitoring of breast cancer patients [10].
Lots of studies show that silibinin has an anti-tumor effect on breast cancer, and its toxic effect has been supported in several breast cancer cell lines, and it inhibits their growth [11]. Silibinin antioxidant is a natural polyphenol and a flavonoid which is widely used as a nutritional supplement and forms a major biological component of the active protein derived from Silibum marianum (milk thistle) [12]. Therefore, this study investigated the effect of silibinin on oxidative stress indices and mir-10b expression in the animal models of breast cancer.
Material and methods
This study was an experimental one with 48 Balb/c mice (6–7 weeks old) with a weight of 19–24 g. The 4T1 metastatic breast cancer cell line was injected subcutaneously adjacent to the lower left mammary tumor after purchasing and culturing the tissue on 1st day of the experiment, and the treatment was started after two weeks of the tumor appearance [13]. The sample volume was calculated using n=2 + C(s/d)2 formula [14] and previous study [15]. Animals were divided into six groups of eight mice: the first group was healthy and intact mice without disease, the second group was mice with breast tumors without treatment that received distilled water, the third group included healthy mice receiving 20 mg/kg of silibinin, the fourth group was cancerous mice receiving 20 mg/kg silibinin, the fifth group was cancerous mice receiving 40 mg/kg silibinin, and the sixth group was cancerous mice which received 80 mg/kg of silibinin. It has been shown that a dose of 120 mg of silymarin whose main component is silibinin is lethal, so the lower doses of silibinin were used [16]. The treatments were administered intraperitoneally and the control group was administered with a normal diet. Recipient groups of silibinin, from the 14th day of cell line injection (the time for tumor appearance) received silibinin for 3 weeks. Intraperitoneal injection is the most common and crucial method for injecting medications in rodents because the peritoneum has plenty of veins in besides having a large size; therefore, solutions that are injected in relatively high volumes enter the general blood circulation shortly after injection [17]. On the other hand, since the solubility of silibinin in the lipids is low, its transport from the membrane of the intestinal tract is limited and, as a result, it has less intestinal absorption; consequently, it was intraperitoneally injected [18]. In the 15th day, 100 mg of tumor tissue was removed to evaluate oxidative stress indices and mir-10b expression.
Cell culture
4T1 cell line was obtained from Pasteur Institute of Iran and cultured in RPMI including 10% fetal bovine serum, 1% penicillin and streptomycin antibiotics at a 4.5 g/L of glucose and oxygen concentration. The CO2 level of cell culture incubator was set at 5% and its temperature was set at 37 °C. The cell culture flasks were examined microscopically to reach about 80–90% confluency, and no bacterial or fungal infections were observed. The previous cell culture of each flask was discarded and after washing twice with PBS, the trypsin/EDTA solution was added and the flasks were transferred to the incubator at 37 and 25 °C for about 3–5 min. After examining with inverted microscope, we added 2 mL of FBS containing medium to each flask in order to inactivate trypsin. Five-hundred micro liter of this solution was taken to count the cells and the rest of the solution was transferred to 25 cm square flasks and transferred to the incubator after microscopic examination as before [18]. A cell suspension with a density of 10 million per mL was prepared in PBS buffer in order to be injected. Then, one million cells were injected adjacent to the lower left mammary gland of each Balb/c female mouse. At the end of the 14th day, blood sampling from the heart of the mice were performed, the mice were sacrificed, and the tumor tissue was removed and stored in a nitrogen tanks at a temperature of −70 °C. One-hundred milligram of tumor tissue was placed in a homogenizer containing lysate solution to completely lysis the tissue. Then, the suspension was collected and, after centrifugation, supernatant was used to evaluate the activity of the SOD, GPX, MDA and TAC enzymes.
Tumor volume calculations
The volume of the tumor was calculated using the following equation: where V=(L × W × W)/2, V=the volume, L=the length and W=the width of the tumor [19].
Assessing the antioxidant indices
Total antioxidant capacity assessment using FRAP method (ferric reducing ability of plasma)
This method relies on the ability of the compound to reduce ferric ions in the presence of TPTZ (Tripyridyl-S-Triazine). The Fe2+-TPTZ complex is a purple colored one whose maximum absorption is at 519 nm. The reduction capacity of the compound was measured by the spectrophotometer at 593 wavelengths by increasing the concentration of the complex, which was accompanied by an increase in the absorbance of the solution [20].
Assessing the superoxide dismutase activity
The activity of the superoxide dismutase enzyme was assessed using the kit (MANUAL/RX MONZA-RANSOD-SD 125, RANDOX Laboratories Ltd. Co. Antrim, UK). In this method, xanthine and xanthine oxidase were used to produce superoxide radicals reacted with phenyltiazolium chloride and formed a red formazan complex, which is measurable by absorbance readings at 505 nm. Superoxide dismutase enzyme inhibits the formation of formazan by converting superoxide radicals into H2O2 and molecular oxygen [21].
Assessing the GPX activity
The GPX enzyme activity was measured using GPX MANUAL/Ransel kit, RANDOX Laboratories Ltd. Co. Antrim UK. The GPX catalyzes the reaction of glutathione oxidation (GSH) by cumene hydroperoxide (Cumene Hyroperoxide). In the presence of glutathione reductase NADPH, the oxidized glutathione (GSSG) is converted again to glutathione reduction, which is accompanied by the simultaneous oxidation of the NADPH to NADP+ In this reaction, a decrease in light absorption was measured at 340 nm [22].
Measurement of MDA
The level of lipid peroxidation was assessed with thiobarbituric acid (TBA) method. Oxidative stress increases the lipid peroxidation of unsaturated fatty acids and, by free radicals’ attacking to lipids, various aldehydes, such as MDA, are produced which react with aqueous TBA at acidic pH and high temperature. Lipid peroxidation was evaluated by increasing the concentration of MDA with spectrophotometer at 523 nm wavelengths [23].
miR-10b expression assessment
microRNA extraction
Extraction of total RNA containing micro RNA was performed by the miRCURY™ RNA Isolation-Biofluids-Exiqon kit according to manufacturer’s instructions. After extracting the RNA, its concentration should be measured, and the required RNA at the cDNA synthesis step was determined for uniformity. To make sure about the quantity of extracted RNA, its concentration was assessed by the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The extracted RNA quality was determined using 1.5% agarose gel electrophoresis. The ratio of OD 260–280 nm extracted RNA was considered as the RNA purity index. To monitor the expression of miRNA precursors, the (Exiqon, Denmark) cDNA kit and the Real Time PCR method were employed. This method is based on reverse transcription followed by real-time PCR and LNA™ enhanced primers. Reverse transcription was fulfilled in a 20 µL reaction including 2 µL total RNA, 1 µL of 10 µM RT primer, 8 µL of 100 mM dNTPs, 2 µL 10X PrimeScript buffer and 200 units of PrimeScript RTase. Due to the small size of miRNAs, it is hard to detect these small pieces by common Q-PCR protocols. To prolong the short chain of miRNAs, all miRNAs were initially added a poly-A tail on its 3′ side by E. coli poly A polymerase (PAP). The kit provides all the materials needed for the polyadenylation reaction. The sequence of primers is presented in Table 1 which was in conformity with primers sequences in reference [24] and were made by Sina-Ghan Co., Iran. All experiments were repeated twice, and the temperature condition included: 95 °C for 15 s, 60–55 °C for 60 s, and 72 °C for 30 s. The relative expression of these genes was performed using 2−ΔΔCT method. The miR-6U gene was used as an internal control.
Primer sequence of used genes.
| Gene | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|
| miR-10b | CCCUGUAACCGAAUUUGUGUAA | CCAGTGAGCAGAGTGACG |
| U6 RNA | CTCGCTTCGGCAGCACA | CCAGTGAGCAGAGTGACG |
Statistical data analysis
The effect of silibinin on MDA and TAC levels, GPX and SOD activity, tumor volume and miR-10b expression in 21st day were analyzed using SPSS software version 18. Dara were compared using one-way ANOVA (to compare the means of the groups), and the t-test method (to compare the mean between the two groups when data has normal distribution). For miR-10b gene expressions, CTs were first calculated for each gene by 2−ΔΔCT formula. Because the investigated groups were independent, the normal distribution of the results of the groups was assessed by Kruskal–Wallis test and results between two groups were assessed by Mann–Whitney test to compare median between two groups (because the data were not normally distributed). The significant level was at p<0.05.
Results
The results of antioxidants indices in the related groups
As Figure 1 and Table 2 show, there is a significant difference between cancer and healthy groups in regard to MDA levels (p=0.00) and the use of silibinin (40 mg dose) caused a significant reduction of MDA in breast tumor tissue compared with untreated cancerous mice, but this effect was not significant with the other two doses.
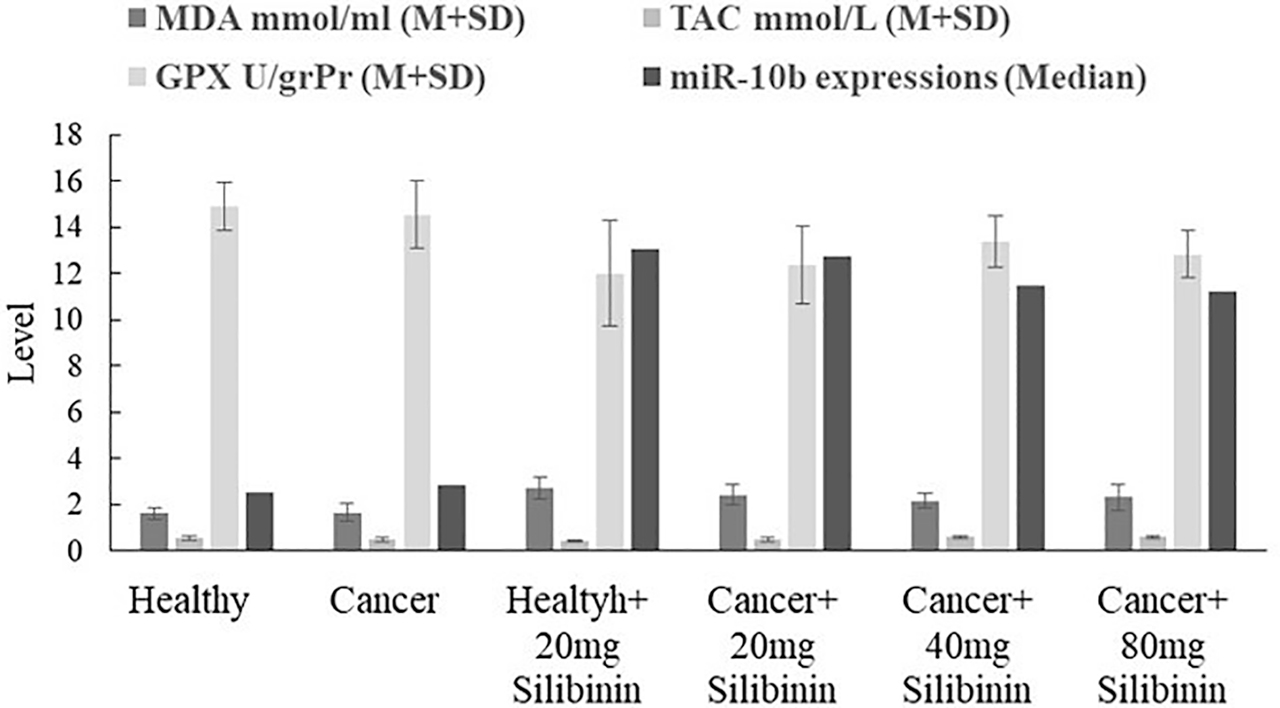
Comparison of TAC, MDA, GPX and miR-10b levels in the groups in 21st day (p between groups were 0.009, 0.001, 0.01 and 0.01 respectively). Use of 40 and 80 mg silibinin increased significantly the TAC in the tumor tissue compared with untreated cancerous mice (both of the p were 0.00). As Figure shows, only the use 40 mg dose of silibinin resulted in a significant reduction of MDA in breast tumor tissue compared with untreated cancerous mice (p=0.04). The use of all dosage of silibinin increased non-significantly the GPX activity and miR-10b expression of the tumor tissue compared with the cancerous untreated group.
Results for tumor volume, stress oxidative indice and miR-10b expression measurement in the related groups.
| Groups | MDA mmoL/mL (M + SD) | TAC mmoL/L (M + SD) | GPX U/grPr (M + SD) | SOD U/grPr (M + SD) | miR-10b expressions (median) | Tumor volume (mm3) |
|---|---|---|---|---|---|---|
| Healthy | 1.61 ± 0.26 | 0.54 ± 0.10 | 14.89 ± 1.05 | 1208.33 ± 127.65 | 2.52 | |
| Cancer | 1.65 ± 0.38 | 0.49 ± 0.08 | 14.56 ± 1.45 | 1236.67 ± 128.32 | 2.87 | 137.50 ± 24.85 |
| Healthy + 20 mg silibinin | 2.71 ± 0.49 | 0.42 ± 0.05 | 12.02 ± 2.26 | 1071.67 ± 111.43 | 13.09 | |
| Cancer + 20 mg silibinin | 2.42 ± 0.45 | 0.49 ± 0.08 | 12.37 ± 1.71 | 1140.0 ± 133.56 | 12.77 | 108.33 ± 14.37 |
| Cancer + 40 mg silibinin | 2.17 ± 0.32 | 0.59 ± 0.07 | 13.41 ± 1.11 | 1211.67 ± 105.53 | 11.45 | 92.50 ± 14.05 |
| Cancer + 80 mg silibinin | 2.30 ± 0.56 | 0.59 ± 0.06 | 12.84 ± 1.01 | 1140.00 ± 136.23 | 11.24 | 83.83 ± 10.77 |
As shown in Figure 1 and Table 2, there is a significant difference between cancer and healthy groups in regard to TAC levels (p=0.03) and the use of silibinin increased the TAC in the tumor tissue compared with untreated cancerous mice, and this effect was significant for all doses except for 20 mg. The most significant effect was observed in the concentration of 80 mg silibinin.
Figure 1 and Table 2 show there is a significant difference between cancer and healthy groups in regard to GPX activity (p=0.01) and GPX activity was decreased significantly in the cancer group compared with the healthy control group and the use of silibinin increased the GPX activity of the tumor tissue compared with untreated mice, but effect was not significant in all doses.
Figure 2 and Table 2 show there is a non-significant difference between cancer and healthy groups in regard to SOD activity (p=0.15) and reveal that SOD activity was decreased in the cancer group in compared with the healthy control group and the use of silibinin increased the activity of SOD non-significantly in the breast tumor tissue compared with untreated cancerous mice.

Comparison of SOD activity and tumor volume in the groups 21st day (p between groups was 0.22 and 0.00 respectively). SOD activity was decreased non-significantly in the all treatment groups in compared with the untreated cancerous mice and 40 and 80 mg silibinin dosages decreased tumor volume significiantaly in 21st day (p=0.009 and 0.004 respectively).
The results for tumor volume measurements
Table 2 and Figure 2 show the results for tumor volume measured in the related groups. As the table and figure show, all dosages of silibinin decreased tumor volume in 21st day that this effect was maximum and significant in cases of 40 and 80 mg silibinin. Eighty milligram dose has a greater inhibitory effect on tumor growth.
Comparison of miR-10b gene expressions in the groups
Figure 1 and Table 2 show there is a significant difference between cancer and healthy groups in regard to miR-10b expression (p=0.00) and reveal that miR-10b expression was increased in the cancer group compared with the healthy control group and silibinin reduced the miR-10b expression non-significantly in breast tumor tissue compared with untreated cancerous mice.
Discussion and conclusion
Silibinin has a considerable antioxidant, anti-cancer and anti-inflammatory effects, and it is widely used because it is safe and does not have any side effects [25]. By collecting free radicals, particularly through dual bonds in its structure, silibinin increases the activity of antioxidant enzymes such as GPX and SOD, stimulates the expression of antioxidant transcription factors, inhibits free radical releasing enzymes and reduces inflammatory responses with inhibition of NF-κB pathways ,and thereby it increases the antioxidant capacity of the body under oxidative stress conditions [26], [27]. It seems that Silibinin is an important protective factor in repairing damage caused by free radicals in various pathological conditions [27]. Kalemci et al. [28] have reported that silibinin improves the methotrexate-induced lung injury by reducing the oxidative stress. Prabu et al. [29] also have found out that silibinin improves arsenic-induced nephrotoxicity by reducing oxidative stress and by inhibiting inflammation in the kidney. Silibinin protects the heart cells from phenylephrine toxicity through antioxidant mechanisms, which mainly involves inhibition of intracellular signals [30]. Kan et al. [31] have reported that silibinin injections inhibit tumorgenesis in skin and reduces oxidative stress and inflammation by reducing NO and IL-6. In this study, silibinin administration resulted in a significant decrease in the lipid peroxidation index (MDA) and a significant increase in TAC as well. Furthermore, this antioxidant increased the activity of the antioxidant enzymes SOD and GPX. All in all, the activities of this compound reduced the oxidative stress in the breast tumor tissue. Küçükdurmaz et al. [32] have reported that silibinin injection significantly increased SOD and GPX activity and decreased MDA level in ischemic priapism mice. Moreover, Haddad and colleagues have showed that Lipid peroxidation and MDA production were efficiently inhibited by silibinin in a rat model of nonalcoholic steatohepatitis [17]. In addition, their study showed that silibinin was able to significantly reduce O2− in these animals [17]. Vessale and colleagues proved that Milk thistle extract with less extent silymarin can increase GPX activity and decrease lipid peroxidation in renal tissue of diabetic rats [33]. One of the recent discovries about this molecule is its ability to change the expression of different miRNAs of various cells. That’s why silibinin has significant beneficial effects, particularly in the cancer field. It has been shown that silibinin can induce apoptosis and stop the cell division cycle in breast cancer cells by increasing PTEN (an apoptotic and cell cycle stop stimulator) and by decreasing the anti-apoptotic Bcl-2 protein and miR-21 [34]. Ma et al. [9] demonstrated that miR-10b expression was increased in early stage of cancers in metastatic breast tumors, and excessive expression of miR-10b triggers invasion and metastasis in breast cancer models and its expression is asscociated with clinical progression in primary cancer carcinoma. The effect of silibinin on other miRs has been shown that 100 μg/mL of silibinin does reduced significantly miR-21 and miR-155 expression MCM-7 breast cancer cells [10]. Silibinin inhibits growth and cell proliferation through inhibition of oncomers expression. It has been revealed that silibinin in both free and nano forms decreases miR-21 in T47D cell line, which is associated with an increase in apoptosis and a decrease in the cancer cell viability [35].
Conclusion
This study showed that silibinin improves oxidative stress in low doses via increasing antioxidant capacity and decreasing MDA in breast tumors of cancerious mice compared with the control cancerious mice. This effect was not meaningful with regard to miR-10b, SOD and GPX.
Research funding: This study was supported by the Department of Biology, Islamic Azad University of Hamedan, Iran.
Author contributions: All the authors of this study (Farhad Soleimani, Rasoul Sharifi, Minoo Mahmoodi & Seyed Mehrdad Kassaee) declare that they do not have any conflicts of interest with regards to authorship and/or publication of this article.
Conflict of interest: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: This article does not contain any studies with human participants. All experiments were performed in compliance with the relevant laws and institutional guidelines of Faculty of Veterinary Medicine of Islamic Azad University, Tabriz branch, Tabriz, Iran.
References
1. Nafissi, N, Khayamzadeh, M, Zeinali, Z, Pazooki, D, Hosseini, M, Akbari, ME. Epidemiology and histopathology of breast cancer in Iran vs. other Middle Eastern countries. Middle East J Cancer 2015;6:243–51, https://doi.org/10.30476/mejc.2018.42130.Search in Google Scholar
2. Nourazarian, AR, Kangari, P, Salmaninejad, A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev 2014;15:4745–51, https://doi.org/10.7314/apjcp.2014.15.12.4745.Search in Google Scholar
3. Sharifi, R, Nouri, M, Eidi, A, Noormohammadi, Z, Dolatkhah, H, Shirmohammadi, M. Dietary PUFA increase apoptosis in stomach of patients with dyspeptic symptoms and infected with H. pylori. Lipids 2017;52:549–58, https://doi.org/10.1007/s11745-017-4257-y.Search in Google Scholar
4. Bjelakovic, G, Nikolova, D, Gluud, C. Antioxidant supplements and mortality. Curr Opin Clin Nutr Metab Care 2014;17:40–4, https://doi.org/10.1097/MCO.0000000000000009.Search in Google Scholar
5. Wang, Y, Branicky, R, Noë, A, Hekimi, S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 2018 Jun 4;217:1915–28, https://doi.org/10.1083/jcb.201708007.Search in Google Scholar
6. Tan, BL, Norhaizan, ME, Liew, W-P-P, Sulaiman Rahman, H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol 2018;9:1–28, https://doi.org/10.3389/fphar.2018.01162.Search in Google Scholar
7. Rao, S, Kumari, S. Changes in plasma lipid peroxidation and the antioxidant system in women with breast cancer. IJBAS 2012;1:429–38, https://doi.org/10.14419/ijbas.v1i4.267.Search in Google Scholar
8. Zhang, Y, Chan, HF, Leong, KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev 2013;65:104–20, https://doi.org/10.1016/j.addr.2012.10.003.Search in Google Scholar
9. Sheedy, P, Medarova, Z. The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res 2018;8:1674–88. PMID: 30323962.Search in Google Scholar
10. Zadeh, MM, Motamed, N, Ranji, N, Majidi, M, Falahi, F. Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J Breast Cancer 2016;19:45–52, https://doi.org/10.4048/jbc.2016.19.1.45.Search in Google Scholar
11. Pashaei-Asl, F, Pashaei-Asl, R, Khodadadi, K, Akbarzadeh, A, Ebrahimie, E, Pashaiasl, M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif Cells Nanomed Biotechnol 2018 Nov;46:1483–7, https://doi.org/10.1080/21691401.2017.1374281.Search in Google Scholar
12. Loguercio, C, Festi, D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol 2011;17:2288–301, https://doi.org/10.3748/wjg.v17.i18.2288.Search in Google Scholar
13. Soleimani, N, Farhangi, B, Mohabati, MA, Etyabi, F. VEGF and MMP-9 gene expression caused by treatment with helicobacter pylori neutrophilactivating recombinant protein in a breast cancer model. JBUMS 2015;17:13–9 (persian), https://doi.org/10.22088/jbums.17.3.13.Search in Google Scholar
14. Khan, AQ, Khan, R, Tahir, M, Rehman, MU, Lateef, A, Ali, F, et al. Silibinin inhibits tumor promotional triggers and tumorigenesis against chemically induced two-stage skin carcinogenesis in Swiss albino mice: possible role of oxidative stress and inflammation. Nutr Canc 66 2014:1–10. https://doi.org/10.1080/01635581.2014.863365.Search in Google Scholar
15. Dell, RB, Holleran, S, Ramakrishnan, R. Sample size determination. ILAR J 2002;43:207–13, https://doi.org/10.1093/ilar.43.4.207.Search in Google Scholar
16. Jahromi, H. The Investigation of silymarin effect on colon ulcer induced acetic acid in mice. JCT 2011;1:21–8.Search in Google Scholar
17. Haddad, Y, Vallerand, D, Brault, AS, Haddad, P. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid-Based Complement Altern Med 2009;2011:1–10, https://doi.org/10.1093/ecam/nep164.Search in Google Scholar
18. Wu, JW, Lin, LC, Hung, SC, Chi, CW, Tsai, TH. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J Pharm Biomed Anal 2007;45:635–41, https://doi.org/10.1016/j.jpba.2007.06.026.Search in Google Scholar
19. Jones, LW, Viglianti, BL, Tashjian, JA, Kothadia, SM, Keir, ST, Freedland, SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol 2010;108:343–8, https://doi.org/10.1152/japplphysiol.00424.2009.Search in Google Scholar
20. Benzie, IF, Strain, J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 1999;299:15–27, https://doi.org/10.1016/s0076-6879(99)99005-5.Search in Google Scholar
21. L’Abbé, MR, Fischer, PW. Automated assay of superoxide dismutase in blood. Methods Enzymol 1990;186:232–7, https://doi.org/10.1016/0076-6879(90)86113-a.Search in Google Scholar
22. Paglia, DE, Valentine, WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–69, https://doi.org/10.5555/uri:pii:0022214367900765.Search in Google Scholar
23. Sabarimuthu, D, Pamakanthan, SR. Effects of epicatechin, a flavonoid on lipid peroxidation and antioxidant in STZ induced diabetic rats liver, kidney and heart. Pharmacol Rep 2005;57:610–15. PMID: 16227644.Search in Google Scholar
24. Mohammadipoor-ghasemabad, L, Sangtarash, MH, Esmaeili-Mahani, S, Sheibani, V, Sasan, HA. The effect of regular treadmill exercise on miR-10b and brain-derived neurotrophic factor (BDNF) expression in the hippocampus of female rats. J Birjand Univ Med Sci 2018;25:276–85.Search in Google Scholar
25. Surai, PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants 2015;4:204–47, https://doi.org/10.3390/antiox4010204.Search in Google Scholar
26. Prabu, SM, Muthumani, M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 2012;39:11201–16, https://doi.org/10.1007/s11033-012-2029-6.Search in Google Scholar
27. Kim, BR, Seo, HS, Ku, JM, Kim, GJ, Jeon, CY, Park, JH, et al. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells. Inflamm Res 2013;62:941–50, https://doi.org/10.1007/s00011-013-0640-1.Search in Google Scholar
28. Prabu, SM, Muthumani, M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 2012;39:11201–16, https://doi.org/10.1007/s11033-012-2029-6.Search in Google Scholar
29. Kalemci, S, Topal, Y, Celik, SY, Yilmaz, N, Beydilli, H, Kosar, MI, et al. Silibinin attenuates methotrexate-induced pulmonary injury by targeting oxidative stress. Exp Ther Med 2015;10:503–7, https://doi.org/10.3892/etm.2015.2542.Search in Google Scholar
30. Anestopoulos, I, Kavo, A, Tentes, I, Kortsaris, A, Panayiotidis, M, Lazou, A, et al. Silibinin protects H9c2 cardiac cells from oxidative stress and inhibits phenylephrine-induced hypertrophy: potential mechanisms. J Nutr Biochem 2013;24:586–94, https://doi.org/10.1016/j.jnutbio.2012.02.009.Search in Google Scholar
31. Khan, AQ, Khan, R, Tahir, M, Rehman, MU, Lateef, A, Ali, F, et al. Silibinin inhibits tumor promotional triggers and tumorigenesis against chemically induced twostage skin carcinogenesis in Swiss albino mice: possible role of oxidative stress and inflammation. Nutr Cancer 2014;66:249–58, https://doi.org/10.1080/01635581.2014.863365.Search in Google Scholar
32. Küçükdurmaz, F, Efe, E, Ergün, Y, Kılıç, M, Resim, S. The effects of silibinin on corporal oxidative stress and antioxidant enzymes in ischemic priapism. J Clin Anal Med 8 (suppl 4) 2017. https://doi.org/10.4328/JCAM.4977.Search in Google Scholar
33. Vessal, G, Akmali, M, Najafi, P, Reza Moein, M, Mahdi Sagheb, M. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren Fail 2010;32:733–9, https://doi.org/10.3109/0886022x.2010.486488.Search in Google Scholar
34. Jahanafrooz, Z, Motamed, N, Bakhshandeh, B. Effects of miR-21 downregulation and silibinin treatment in breast cancer cell lines. Cytotechnology 2017;69:667–80, https://doi.org/10.1007/s10616-017-0076-5.Search in Google Scholar
35. Yazdi Rouholamini, SE, Moghassemi, S, Maharat, Z, Hakamivala, A, Kashanian, S, Omidfar, K. Effect of silibinin-loaded nanoniosomal coated with trimethyl chitosan on miRNAs expression in 2D and 3D models of T47D breast cancer cell line. Artif Cells Nanomed Biotechnol 2018;46:524–35, https://doi.org/10.1080/21691401.2017.1326928.Search in Google Scholar
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Review Articles
- Therapeutic approaches on the interaction between SARS-CoV2 and ACE2: a biochemical perspective
- Therapeutic agents currently employed against Covid-19: an effort to control the pandemic
- Association between breast milk adipokines with growth in breast feeding infants, a systematic review and meta-analysis
- Opinion Paper
- The role of biotin metabolism in the COVID-19 infection
- Value of blood IFN-I levels in COVID-19 management
- Some comments on enzyme kinetics studies
- Short Communication
- SKA3 overexpression promotes cell proliferation and migration in breast cancer cell lines
- Influence of the butylparaben administration on the oxidative stress metabolism of liver, kidney and spleen
- Probable alterations in fecal bacterial microbiota by somatostatin receptor analogs in acromegaly
- Research Articles
- A simple silica based DNA isolation method for cell-free DNA analysis from liquid biopsy
- The effects of silibinin on oxidative stress and microRNA-10b expression in animal models of breast cancer
- A novel approach for the discrimination of culture medium from Vascular Endothelial Growth Factor (VEGF) overexpressing colorectal cancer cells
- The investigation effect of weight loss on serum vaspin, apelin-13, and obestatin levels in obese individual
- Enhancer of zeste homolog 2 (EZH2) gene inhibition via 3-Deazaneplanocin A (DZNep) in human liver cells and it is relation with fibrosis
- Synthesis of 2-aminonaphthalene-1-sulfonic acid Schiff bases and their interactions with human serum albumin
- Association study of polymorphisms in ABCA7, clusterin, and MS4A6A genes with Alzheimer’s disease in the Egyptian population
- Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression
- Thiamine pyrophosphate riboswitch regulation: a new possible mechanism involved in the action of nalidixic acid
- Structural evidence for kinetic and thermal stability changes of α-amylase due to exposure to [emim][lactate] ionic liquid
- Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram
- Cytotoxic, genotoxic and apoptotic effects of Viburnum opulus on colon cancer cells: an in vitro study
- Acrylamide-encapsulated glucose oxidase inhibits breast cancer cell viability
- Explore the activation efficiency of different ligand carriers on synNotch-based contact-dependent activation system
- Expression level of miRNAS in patients with gestational diabetes
- Effect of static magnetic field with quercetin and hesperetin on MCF-7 and MDA MB-231 breast cancer cells
- In vitro antimicrobial, antioxidant, cytotoxic activities, and wound healing potential of Thymbra capitata ethanolic extract
- The association of methylene tetrahydrofolate reductase (MTHFR) A1298C gene polymorphism, homocysteine, vitamin B12, and folate with coronary artery disease (CAD) in the north of Iran
- Synthetic peptide vaccine for Foot-and-Mouth Disease: synthesis, characterization and immunogenicity
- New pathway in rheumatic mitral valve disease: cytochrome P450 and glutathione S transferase isozyme expression
- Ghrelin and orexin levels in infertile male: evaluation of effects on varicocele pathophysiology, relationship seminal and hormonal parameter
- The activities of GST isozymes in stomach tissues of female obese patients
- Analysis of blood gas beyond bicarbonate in outpatients with stage 3–5 chronic kidney disease
- Relationship between JAK2-V617F mutation and hematologic parameters in Philadelphia-negative chronic myeloproliferative neoplasms
- Case Report
- The role of the laboratory in the diagnosis process in a patient with mildly elevated hCG: a case report
- Letter to the Editor
- Hookah use and COVID-19
Articles in the same Issue
- Frontmatter
- Review Articles
- Therapeutic approaches on the interaction between SARS-CoV2 and ACE2: a biochemical perspective
- Therapeutic agents currently employed against Covid-19: an effort to control the pandemic
- Association between breast milk adipokines with growth in breast feeding infants, a systematic review and meta-analysis
- Opinion Paper
- The role of biotin metabolism in the COVID-19 infection
- Value of blood IFN-I levels in COVID-19 management
- Some comments on enzyme kinetics studies
- Short Communication
- SKA3 overexpression promotes cell proliferation and migration in breast cancer cell lines
- Influence of the butylparaben administration on the oxidative stress metabolism of liver, kidney and spleen
- Probable alterations in fecal bacterial microbiota by somatostatin receptor analogs in acromegaly
- Research Articles
- A simple silica based DNA isolation method for cell-free DNA analysis from liquid biopsy
- The effects of silibinin on oxidative stress and microRNA-10b expression in animal models of breast cancer
- A novel approach for the discrimination of culture medium from Vascular Endothelial Growth Factor (VEGF) overexpressing colorectal cancer cells
- The investigation effect of weight loss on serum vaspin, apelin-13, and obestatin levels in obese individual
- Enhancer of zeste homolog 2 (EZH2) gene inhibition via 3-Deazaneplanocin A (DZNep) in human liver cells and it is relation with fibrosis
- Synthesis of 2-aminonaphthalene-1-sulfonic acid Schiff bases and their interactions with human serum albumin
- Association study of polymorphisms in ABCA7, clusterin, and MS4A6A genes with Alzheimer’s disease in the Egyptian population
- Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression
- Thiamine pyrophosphate riboswitch regulation: a new possible mechanism involved in the action of nalidixic acid
- Structural evidence for kinetic and thermal stability changes of α-amylase due to exposure to [emim][lactate] ionic liquid
- Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram
- Cytotoxic, genotoxic and apoptotic effects of Viburnum opulus on colon cancer cells: an in vitro study
- Acrylamide-encapsulated glucose oxidase inhibits breast cancer cell viability
- Explore the activation efficiency of different ligand carriers on synNotch-based contact-dependent activation system
- Expression level of miRNAS in patients with gestational diabetes
- Effect of static magnetic field with quercetin and hesperetin on MCF-7 and MDA MB-231 breast cancer cells
- In vitro antimicrobial, antioxidant, cytotoxic activities, and wound healing potential of Thymbra capitata ethanolic extract
- The association of methylene tetrahydrofolate reductase (MTHFR) A1298C gene polymorphism, homocysteine, vitamin B12, and folate with coronary artery disease (CAD) in the north of Iran
- Synthetic peptide vaccine for Foot-and-Mouth Disease: synthesis, characterization and immunogenicity
- New pathway in rheumatic mitral valve disease: cytochrome P450 and glutathione S transferase isozyme expression
- Ghrelin and orexin levels in infertile male: evaluation of effects on varicocele pathophysiology, relationship seminal and hormonal parameter
- The activities of GST isozymes in stomach tissues of female obese patients
- Analysis of blood gas beyond bicarbonate in outpatients with stage 3–5 chronic kidney disease
- Relationship between JAK2-V617F mutation and hematologic parameters in Philadelphia-negative chronic myeloproliferative neoplasms
- Case Report
- The role of the laboratory in the diagnosis process in a patient with mildly elevated hCG: a case report
- Letter to the Editor
- Hookah use and COVID-19

