Abstract
The ongoing advancement of aerospace technology globally offers technical support for human exploration of outer space. Nevertheless, astronauts encounter microgravity environments during their missions in outer space, which significantly affects the functionality of various physiological systems, including the skeletal, muscular and immune systems. Among them, microgravity-induced bone loss is particularly severe. Bone loss markedly elevates the risk of osteoporosis and fractures, presenting a substantial obstacle to astronauts’ ability to perform tasks in the space environment and maintain their physical health. Consequently, implementing scientifically grounded preventive and therapeutic measures is essential for mitigating microgravity-induced bone loss. Currently, numerous intervention strategies have been demonstrated to effectively address microgravity-induced bone loss, such as pharmacological treatment, nutritional supplementation, and exercise intervention. However, the efficacy of these interventions varies, and some may result in adverse effects. Therefore, this narrative review analyzes and summarizes the effects of various interventions on bone loss caused by microgravity, aiming to provide a scientific theoretical basis for determining the optimal intervention strategy.
Introduction
With the increasing frequency of global manned space missions, the public and various sectors have become increasingly concerned about the impact of the space environment on the health of astronauts. The space environment differs substantially from the Earth’s environment. Astronauts residing in this unique setting are exposed to adverse external factors such as radiation [1], 2], microgravity [3], and disruption of circadian rhythms [4], which pose a serious threat to their physiological functions. Notably, prolonged exposure to microgravity induces bone loss, significantly compromising the skeletal health of astronauts [5]. Recently, American female astronaut Sunita experienced a marked reduction in bone mineral density and a pronounced loss of bone mass in her distal femur following a 286-day stay in space. Research indicates that after residing on the International Space Station for 4–6 months, astronauts exhibit a 4 % decrease in tibial cortical bone thickness and an accelerated decline in bone density at a rate of 0.5–1.5 % per month. Concerningly, these effects persist even three months post-landing, with increased bone fragility detected in the radial end of the radius bone [6], 7]. This suggests that microgravity-induced bone loss not only jeopardizes astronauts’ skeletal health during space missions but also poses long-term health risks upon their return to Earth.
The microgravity-induced bone loss is closely related to the imbalance of bone homeostasis. Bone homeostasis is essential for maintaining bone health, a process primarily regulated by the balance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption [8]. Earth’s gravity and mechanical stresses are crucial for preserving the physiological function of bones, as these stresses stimulate the activities of osteoblasts and osteoclasts to maintain bone homeostasis [9]. However, the absence of mechanical stress [10] can disrupt bone homeostasis, leading to bone diseases, including bone loss and osteoporosis. Studies have demonstrated that the loss of mechanical stress in microgravity environments affects cellular function in bone tissue in multiple ways. For instance, microgravity alters hypothalamic signaling, promoting the expression of NPY and TH, which subsequently contributes to bone loss [11]. Additionally, the loss of mechanical stress in microgravity inhibits the expression of the mechanosensitive channel Piezo1, thereby weakening osteoblast differentiation and suppressing bone formation [12], 13]. Furthermore, since bone acts as an important endocrine organ, impaired bone function may exert widespread effects on multiple systems, including the musculoskeletal system [14], kidney system [15], and immune system [16]. Consequently, the development of effective preventive and therapeutic strategies is critical to safeguarding astronauts’ bone health. Collectively, the loss of mechanical stress in microgravity represents a key factor contributing to bone loss in astronauts. The progression of bone loss significantly impacts the development of skeletal diseases. Both human and animal models after spaceflight exhibit a declining trend in trabecular bone density and reduced bone mass, exposing astronauts to an elevated risk of fractures in daily life upon returning to Earth [17]. Furthermore, quantitative computed tomography (QCT) scan results reveal a marked decline in hip strength and increased expression of the bone resorption marker N-telopeptide (NTX) in astronauts exposed to microgravity. These findings indicate that spaceflight-induced bone loss significantly increases the risk of osteoporosis [17].
Therefore, the implementation of scientific preventive and therapeutic measures is crucial for safeguarding the bone health of astronauts. Current common strategies include pharmacotherapy [18], 19], nutritional supplementation [20], and exercise intervention [6], 21]. However, regarding bone loss induced by mechanical stress reduction in microgravity, the efficacy of various intervention modalities differs. This disparity may be influenced by multiple factors, such as intervention timing, dosage, and other variables. Additionally, different intervention modalities may entail certain side effects. Thus, the present study utilized databases such as PubMed, Web of Science, and searched for all relevant literature on microgravity-induced bone loss up to June 2025 using keywords like “microgravity”, “bone loss”, “Pharmacological treatment”, “Nutritional supplement”, and “Exercise”. The included literature covered both human and animal model experiments during space flights and ground-based simulation experiments, and the effects of different intervention measures on bone loss were evaluated. By analyzing the underlying mechanisms and pathways of these interventions, this review seeks to identify optimal solutions for addressing microgravity-induced bone loss, thereby providing a robust scientific foundation for the prevention and management of microgravity-related bone loss in future research and practice. The summary of this article is presented in Figure 1.

Graphical representation of this study. Key points: (1) At present, the optimal intervention strategy for preventing microgravity-induced bone loss has not yet been clearly defined. (2) Pharmacological treatment, nutritional supplementation, and exercise intervention have shown certain effects in preventing and treating microgravity-induced bone loss, but they also have certain side effects. (3) Combining exercise with pharmacological treatment or combining exercise with nutritional supplements may be a better option for preventing bone loss caused by microgravity. Figure created with BioRender.
Content
Microgravity-induced bone loss
In the microgravity environment, the absence of mechanical stress stimulation leads to a series of adverse changes in the skeletal system, including bone metabolism disorders [22], bone loss [23], and decreased bone density [24] (Table 1). Specifically, the lack of mechanical stress in microgravity accelerates bone loss. Significant cortical bone loss and trabecular bone loss were observed in the tibia during both 1 and 6 months of spaceflight, accompanied by a marked decrease in ultrasound broadband ultrasonic attenuation (BUA) of the heel bone. This declining trend became increasingly pronounced with time [25]. Similar findings were also observed in another study, where 15 astronauts spent 1, 2, and 6 months staying on the Russian space station. The results indicated that the tibia exhibited cortical and cancellous bone loss starting from the first month, which progressively worsened over time. However, the loss of cortical bone was less pronounced compared to cancellous bone. Notably, no significant loss of cancellous or cortical bone was detected at the radius, suggesting that the skeletal regions subjected to greater mechanical loads in a microgravity environment are more affected [26]. Besides the tibia, the lumbar spine and hip are also major load-bearing sites in the skeletal system [27]. After reviewing data from 35 astronauts with spaceflight durations of 120–180 days at a convened skeletal summit, the U.S. National Aeronautics and Space Administration (NASA) found that the lumbar spine experienced approximately 10 % areal bone mineral density (aBMD) loss, severely compromising skeletal integrity [28]. Additionally, 14 crewmembers who spent 4–6 months flying on the International Space Station (ISS) underwent dual-energy X-ray absorptiometry (DXA) testing combined with QCT evaluation. The results revealed that vertebral aBMD decreased at a rate of 0.9 %/month, trabecular volumetric BMD decreased at a rate of 0.7 %/month, and there was significant cortical bone loss in the hips, primarily due to cortical thinning [29]. After a 6-month space flight, astronauts exhibited a trend of decreasing vertebral trabecular density in both the upper and lower regions of the vertebrae compared to the pre-flight levels [30]. In summary, astronauts experience varying degrees of bone loss in different parts of the skeleton during space missions due to prolonged exposure to microgravity. Among these, the lumbar vertebrae and the heel bone, as the primary load-bearing bones of the human body, exhibit a much higher rate of bone loss compared to other skeletal regions. Conversely, non-weight-bearing bones such as the radius in the upper limbs show a slower rate of bone loss.
Effects of microgravity environment on the bones of different parts of humans and animals.
| Species | Duration | Body part | Effects | Change range | References | ||
|---|---|---|---|---|---|---|---|
| Human | 1 month/6 months | Tibia | Cortical bone ↓ Bone trabecular ↓ |
Cortical | Trabecular | [25] | |
| +1.01 %/−2.94 % | −2.27 %/−4.45 % | ||||||
| Human | 1 month/6 months | Calcaneus | Ultrasound BUA ↓ | −7.74 %/−13.18 % | [25] | ||
| Human | 1 month/2 months | Tibia | Cortical bone ↓ Cancellous bone ↓ |
Cortical | Cancellous | [26] | |
| 1.11 %/−1.63 % | −1.21 %/−0.42 % | ||||||
| Human | 1 month/2 months | Radius | Cortical bone BMD ↓ Cancellous bone BMD ↓ |
Cortical | Cancellous | [26] | |
| 1.48 %/−1.05 % | 1.04 %/−1.01 % | ||||||
| Human | 3–6 months | Lumbar vertebra | aBMD ↓ | −1.0 % to −1.5 % | [28] | ||
| Human | 4–12 months | Spine | aBMD ↓ Integral vBMD↓ | aBMD | vBMD | [29] | |
| −0.8 %/month | −0.9 %/month | ||||||
| Human | 4–6 months | Hip | vBMD ↓ | Femoral neck | Total femur regions | [29] | |
| −1.4 %/month | −1.5 %/month | ||||||
| Human | 6 months | L1 vertebra | BMD ↓ | Superior BMD | Transverse BMD | [30] | |
| −6.7 % | −4.3 % | ||||||
| 1.48 %/−1.05 % | 1.04 %/−1.01 % | ||||||
| SD rats | 4 weeks | Femur | Bone trabecular ↓ Cortical bone density ↓ Maximum load ↓ Stiffness ↓ IFN-γ, IL-10 ↓ |
/ | [35] | ||
| SD rats | 4 weeks | Tibia | Total bone area ↓ Total vBMD ↓ BMC ↓ |
Total bone area | Total vBMD | Total BMC of the proximal tibia | [36] |
| −8% | −9% | −17 % | |||||
| Wistar rats | 2 weeks/4 weeks/8 weeks | Hip bone | The length and width of the acetabulum ↓ Iliac wing angle ↓ Cartilage thickness ↓ |
/ | [37] | ||
| SD rats | 4 weeks | Humerus | Cancellous bone ↓ BMC↓ BMD ↑ vBMD in the proximal humerus ↑ | / | [38] | ||
Nowadays, to more comprehensively investigate the effects of microgravity on human body functions and given the high cost of conducting experiments in space, ground-based simulations such as hindlimb unloading models or bed rest experiments are frequently employed. Although hindlimb unloading cannot fully replicate the effects of microgravity, experiment results often exhibit comparable outcomes [31], 32]. Therefore, by observing skeletal changes in different parts of the hindlimb under the hindlimb unloading model, a deeper understanding and prediction of microgravity’s impact on the skeletal system can be achieved [33], 34]. Following a 4-week tail suspension in healthy SD male rats, significant trabecular bone loss in the distal femur, decreased cortical BMD, and reduced levels of bone remodeling-related cytokines, such as Interferon-γ (IFN-γ) and Interleukin-10 (IL-10), were observed compared to the control group [35]. Additionally, the three-point bending test revealed a significant reduction in maximum load and stiffness in the femoral diaphysis. Similarly, the simulated microgravity environment significantly affected the tibia. In 6-month-old SD male rats subjected to 4 weeks of tail suspension, there was an approximate 8 % decrease in total proximal tibial bone area, a 9 % decrease in volumetric bone mineral density (vBMD), a 17 % decrease in total bone mineral content, and a marked reduction in tibial diaphysis flexion force [36]. These studies demonstrated that the microgravity environment accelerates bone loss in the femur and tibia, primarily characterized by trabecular bone loss and weakened bone biomechanical properties. Beyond the femur and tibia, the hip bone is another major weight-bearing bone in the human body [39]. After 4 or 8 weeks of tail suspension in female rats, the acetabulum’s width and length decreased, and the iliac wing angle diminished over time. The cartilage thickness between the ilium and ischium gradually thinned [37]. The effects of microgravity on bones extend beyond weight-bearing bones like the femur; similar manifestations were observed in non-weight-bearing bones, such as the humerus. After 4 weeks of tail suspension in male SD rats, the vBMD of the humeral proximal end significantly decreased, and total bone mineral content markedly declined. However, the total bone density of the humerus and the vBMD of the metaphysis cortical bone increased significantly [38]. This phenomenon suggests that in a microgravity environment, cancellous bone and cortical bone exhibit differential sensitivity to mechanical changes, leading to complex alterations in the bone structure.
At present, microgravity-induced bone loss has become one of the important research projects in the field of aerospace medicine. In microgravity, the lack of mechanical stress stimulation on bones affects bone homeostasis, resulting in bone loss. This not only seriously reduces the efficiency of astronauts’ mission execution, but also poses a serious challenge to their health, and this effect has a time effect. Analyzing the effects of bone loss is conducive to the scientific prevention and treatment of microgravity-induced bone loss.
In order to further investigate the impact of the space environment on human bone health, NASA has initiated a new phase of its human research program. This project aims to determine whether space-induced bone changes alter the risk of fractures and osteoporosis. Research has demonstrated that microgravity-induced bone loss is typically characterized by the reduction of bone trabeculae [40], decreased bone mineral content [41], and deterioration of bone quality [42]. Among these factors, the loss of bone trabeculae and damage to the bone microstructure serve as significant predictors of osteoporosis [43]. In microgravity conditions, the reduction in the number of bone trabeculae and the thinning of their thickness lead to alterations in bone microstructure. Both strength and rigidity are dependent on bone mineral content. Thus, the decrease in bone mineral content under microgravity reduces bone strength and rigidity, significantly increasing the risk of osteoporosis [44] and fracture [17], 45]. These effects pose substantial challenges to astronauts’ mission performance in space and their daily activities upon returning to Earth. Although the skeletal system struggles to rapidly adapt to gravitational forces after returning to Earth, astronauts often experience difficulties in walking and instability. Over time, the bone mass may gradually recover, and improvements in the trabecular structure can occur [46], 47]. However, the recovery process is lengthy, and permanent deterioration may ensue in some cases [26], 48]. Additionally, the recovery rate varies depending on the specific bone location, and in certain instances, further deterioration may occur [7].
The microgravity environment presents a significant challenge to the maintenance of bone health, not only complicating astronaut missions, but also posing a threat to their bone health. Various interventions have been proposed and validated, offering new scientific insights into mitigating bone loss in astronauts. Such as pharmacotherapy, nutritional supplementation, and exercise interventions. The therapeutic approaches and efficacy of different intervention modalities vary considerably (Figure 2). By comprehensively evaluating the strengths and limitations of these modalities, targeted strategies can be developed to address bone loss effectively, thereby enhancing the physical health and quality of life for astronauts.
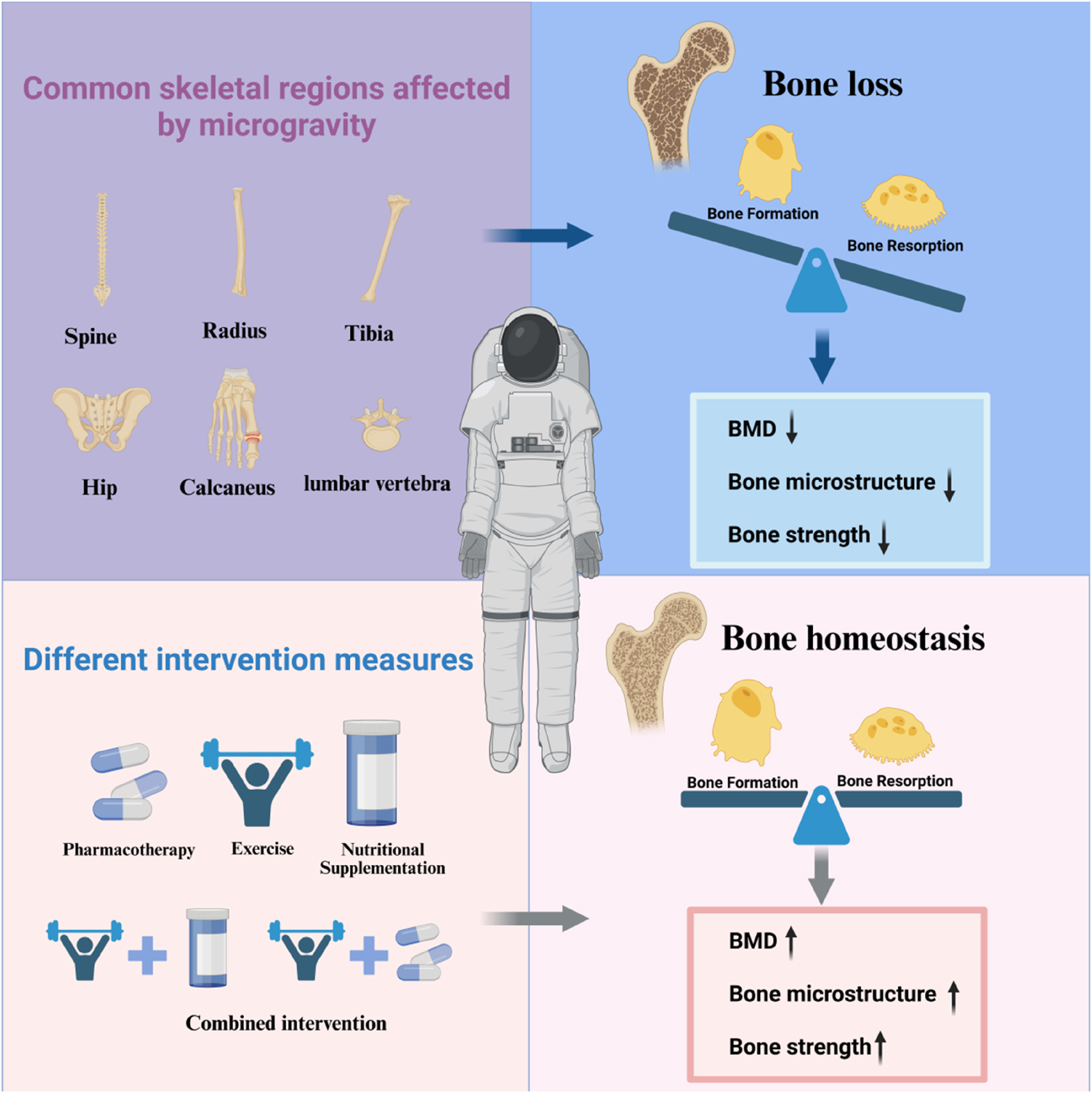
Different intervention methods attenuate microgravity-induced bone loss.
Pharmacotherapy
The absence of mechanical stress in microgravity diminishes the mechanosensitivity of osteoblasts, thereby disrupting bone homeostasis [49]. Specifically, the accelerated formation of osteoclasts and enhanced osteoclast-dominated contribute to bone loss [50], 51]. Although medications exist that can effectively suppress osteoclast-dominated bone resorption and restore bone homeostasis, there are no drugs approved for microgravity-induced bone loss so far. The most widely used drugs for bone loss include bisphosphonates, denosumab, and teriparatide [52]. Bisphosphonates have demonstrated therapeutic potential in experimental settings both in space and on Earth.
Bisphosphonates have demonstrated promising potential in inhibiting bone resorption [53]. They suppress osteoclastogenesis by targeting key regulatory enzymes within osteoclasts [54], thereby reducing the activity of these enzymes. Furthermore, bisphosphonates effectively prevent the degradation of hydroxyapatite, which plays a critical role in mitigating excessive bone resorption [18]. However, in the context of microgravity-induced bone loss, the application of bisphosphonates remains largely experimental within aerospace medicine. This is primarily due to the limited number of astronaut samples, the inability to conclusively assess the efficacy of bisphosphonates under prolonged microgravity conditions, and the associated side effects. Despite these challenges, bisphosphonates exhibit therapeutic potential. Supplementing astronauts with bisphosphonates during routine exercise regimens has been shown to significantly delay bone resorption, reduce NTX levels, and enhance hip trabecular density [19]. This regimen has demonstrated superior therapeutic potential compared to single resistance exercise [19], 55]. Furthermore, in a study involving 15 healthy participants [56], treatment with bisphosphonates following 150 days of bed rest resulted in a marked reduction in the number of osteoclasts and effectively inhibited bone resorption, further underscoring the role of bisphosphonate in mitigating significant bone resorption. While bisphosphonates have exhibited substantial efficacy in inhibiting osteoclast activity, their regulation of the bone microenvironment remains limited, such as insufficient deep regulation of the bone microenvironment by bisphosphonate drugs [57] and certain toxic side effects associated with long-term use [18]. In contrast, denosumab has shown promise in enhancing bone metabolism [58]. Denosumab is a monoclonal antibody to the nuclear factor kappa-B (NF-κB) ligand-receptor activator, which is effective in inhibiting the process of osteoclast-mediated bone resorption [59]. Although its efficacy in preventing bone loss has not been extensively reported in flight studies, bed rest experiments, or suspension models, it has demonstrated notable application value in treating postmenopausal osteoporosis [60] and other bone loss-related diseases, making it a potential drug candidate for addressing microgravity-induced bone loss. A trial involving 7,868 postmenopausal osteoporotic patients revealed that denosumab treatment significantly reduced the risk of vertebral and hip fractures compared to the placebo group, while also markedly improving BMD in the hip and lumbar spine [61]. Moreover, compared to bisphosphonate treatment, denosumab not only enhanced lumbar spine BMD but also femoral neck BMD, with even pronounced improvements observed [58], 62].
Considering the potential toxic side effects and adverse impacts of bisphosphonates and other drug treatments, it is important to investigate alternative Pharmacological strategies for the prevention and treatment of bone loss (Table 2). Recent studies have demonstrated that both synthetic drugs and traditional Chinese medicine extracts hold significant value in addressing bone loss, providing a scientific rationale for targeting and mitigating microgravity-induced bone loss. Mika Ikegame et al. utilized goldfish scales as a skeletal model coexisting with osteoclasts and osteoblasts. Their findings revealed that melatonin treatment during space flight significantly decreased osteoclast activity, reduced the number of osteoclasts, down-regulated the expression of osteoclast-related factors such as Mmp9, Ctsk, and RankL, and effectively inhibited bone resorption. Simultaneously, the expression of bone-forming factors, including Runx2, Osx, and Ocn, was up-regulated, thereby promoting bone formation and alleviating bone loss [63]. Low molecular weight chondroitin sulfate (LMWCS), an aminoglycan widely distributed in bones and cardiovascular tissues, exhibits notable antioxidant and anti-inflammatory properties [64]. In tail-suspended mice treated with LMWCS, CT imaging confirmed its efficacy in preventing tibia cortical bone loss and attenuating bone turnover. Furthermore, LMWCS treatment significantly up-regulated the expression of antioxidant enzymes such as Nrf2 mRNA while down-regulating MDA expression in the femur and serum [65], suggesting that LMWCS mitigates bone loss by reducing oxidative stress levels. Compared to synthetic drugs, herbal extracts demonstrate unique advantages in improving bone loss due to their fewer side effects. Angelica sinensis, a traditional Chinese herb rich in alkaloids and phenolic acids, possesses anti-inflammatory activity [66] and effectively inhibits osteoclast differentiation, thus suppressing bone resorption [67]. 4 weeks of tail suspension in mice fed A. sinensis extract alleviated femoral and vertebral bone loss [68] and enhanced osteoblast differentiation, promoting bone formation. Icariin, a flavonoid glucoside derived from Epimedium, exhibits potent anti-inflammatory and antioxidant effects. It has been shown to inhibit bone resorption while promoting bone formation [69], 70]. Oral administration of Epimedium glycosides to SD rats during hindlimb suspension revealed that cancellous bone loss in the distal femur was alleviated, and mRNA levels of Osteoclast-related factors such as RANKL were down-regulated, and the serum calcium ion level was restored to the normal level [71]. Compared to antiresorptive drugs such as bisphosphonates, these natural herbal compounds not only effectively inhibit the microgravity-induced increases in bone resorption but also promote osteoblast-mediated bone formation through multi-targeted regulation of bone metabolism, achieving bidirectional regulation of bone metabolism. Notably, these natural compounds exhibit significant multi-organ protective effects. For instance, Icariin improves cardiac functions [72], lung [73], skin [74], and other organ functions. The value of one-drug-multiple-action applications in aerospace medicine warrants further in-depth exploration.
The potential application of pharmacotherapy in combating microgravity-induced bone loss.
| Drug | Species | Age | Model | Treatment time | Therapeutic dose and grouping | Effects | References | |
|---|---|---|---|---|---|---|---|---|
| Bone parameters | Bone metabolism-related markers | |||||||
| Bisphosphonates | Healthy young man | 32.8 + 4.7 years | Bed rest for 120 days | / Treatment during bed rest |
Control group Treatment group (900 mg/d) Both groups consumed 2,700 calories of energy every day |
Number of osteoclasts ↓ | / | [56] |
| LMWCS (CS) | Male C57 mice | 2 months | hindlimb suspension (6 weeks) | Everyday | Control group/HLS group HLS + CS group (180 mg/kg) |
Cortical bone BMD ↑ Femurs’ mechanical strength ↑ |
GSH/SOD ↓ Nrf2/NQO1 ↓ |
[65] |
| Angelicae dahuricae radix (AR) | Male C57/BL mice | 2 months | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group HLS + AR group (2 g/kg) |
Femur and vertebrae BMD ↑ Degeneration of trabecular bone ↓ tibial biomechanical properties ↑ OB differentiation ↑ |
ALP/RUNX2/OCN↑ | [68] |
| Icariin (ICA) | Female wistar rats | 2 months | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group (0.9 % saline) HLS + ICA group (25 mg/kg) |
Femur BMD ↑ The maximal force ↑ Concentration of calcium ↑ |
ALP/GAPDH↑ BGP/GAPDH ↑ | [71] |
| Calycosin | SD male rats | / | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group (0.5 % CMC-Na) HLS + Calycosin group (30 mg/kg) |
Femur BMD ↑ Femur biomechanical properties ↑ |
RANKL/OPG ↓ NTX ↓ TNF-α/IL-6/IL-8 ↓ IL-10/IL-4 ↑ |
[35] |
| Radix dipsaci (RDE) | SD male rats | 12 weeks | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group (500 mg/kg distilled water)/HLS + RDE Group (500 mg/kg) | BMD/BMC ↑ Femur biomechanical properties ↑ |
TRAP/NTX ↓ | [83] |
| Drynariae rhizoma (DRTF) | SD male rats | 12 weeks | hindlimb suspension (4 weeks) | Everyday | Baseline group/Control group/HLS group HLS + DREF Group (75 mg/kg) |
BV/TV ↑ Tb.Th ↑ Tb.N ↑ Tb.Sp ↓ |
NTX/CTX ↓ OPG/RANKL↑ Wnt3a/β-catenin/LEF1↑ |
[84] |
| Ellagic acid (EA) | C57BL/6 mice | 2 months | hindlimb suspension (14/28 days) | Everyday | 50 mg/kg | Tb.BV/TV↑ Tb.BMD↑ Ct.Th ↑ OB differentiation ↑ |
p-RUNX2 ↓ | [85] |
| Melatonin | Goldfish scales | 14 Days | Goldfish scales were incubated for 86 h under in‐flight artificial 1 gravity (F‐1g) | 1 day/2 day/4 day | 10 nm — 1μM | / | OC reactivity ↓ MMP9/Ctsk/Trap/Rank/Rankl/Cox2a ↓ Runx2b/Osx/Col1a/Ocn ↑ |
[63] |
-
HLS, group, Hindlimb Suspension group.
The mechanism of action of antiresorptive drugs (e.g., bisphosphonates) in mitigating bone loss has been validated in space missions. However, due to the limited sample size of astronauts and the prolonged observation period, further in-depth exploration is required to clarify the profound effects of this drug class on the skeletal system. Additionally, long-term pharmacotherapy may increase the risk of certain skeletal disorders, while the microgravity environment can affect drug pharmacokinetics [75], leading to reduced stability of therapeutic efficacy. Meanwhile, real-time monitoring of bone metabolism markers and bone density changes in the space environment remains challenging, thereby hindering the accurate assessment the drug efficacy. The microgravity environment may alter the composition and functional profile of the intestinal microbiota, thereby impacting gastrointestinal digestive and absorptive functions [76]. Interindividual variations in digestion and absorption not only exacerbate difficulties in evaluating drug efficacy but also lead to discrepancies between the dosage of nutritional supplements and their actual biological effects. Notably, the improvement of microgravity-induced bone loss by Chinese herbs such as A. sinensis has been demonstrated in animal models, suggesting the development potential of natural medicines in the prevention and treatment of osteopenia. Targeted drug delivery strategies for skeletal protection therefore warrant further investigation.
Nutritional supplementation
Microgravity-induced bone loss is not only attributable to the lack of mechanical loading but is also closely associated with nutritional metabolic disorders [77]. Studies have demonstrated that astronauts’ long-term missions in space may accelerate metabolic imbalances of calcium, vitamin D, and other nutrients, potentially exacerbating the onset and progression of bone loss [78], 79]. Consequently, nutritional supplementation represents one of the strategies for preventing microgravity-induced bone loss by targeting and regulating bone metabolism signaling pathways and related mechanisms. During space flight, the absence of mechanical stress disrupts bone homeostasis, leading to increased bone resorption while bone formation remains relatively unchanged. Additionally, intestinal calcium absorption capacity decreases, resulting in reduced calcium content, which triggers substantial bone mineral loss and induces bone loss [78], 80]. Therefore, increasing calcium levels through supplementation with various nutrients can effectively mitigate bone loss. Vitamin D plays a critical role in skeletal calcium and phosphorus absorption as well as maintaining bone homeostasis [81]. However, vitamin D supplementation alone appears insufficient to alleviate microgravity-induced bone loss. The decrease in bone formation markers such as ALP and the increase in bone resorption markers observed in eight astronauts after high calcium intake combined with vitamin D supplementation during a mission suggest that this approach may be ineffective due to the limited inhibitory effect of vitamin D on bone resorption and the lack of adequate light exposure [82]. Interestingly, astronauts supplemented with vitamin K for 6 weeks during a 6-month spaceflight exhibited a significant increase in bone formation markers such as ALP and Ocn, effectively mitigating bone loss [86]. Furthermore, oral administration of vitamin K to SD rats during 4 weeks of tail suspension improved bone mineral density and maintained trabecular bone structures [87], indicating that vitamin K contributes to alleviating microgravity-induced bone loss. In addition to vitamin D and vitamin K, nutrients such as omega-3 fatty acids play a protective role in bone health. Microgravity-induced bone mineral loss was effectively mitigated, and bone microarchitecture was improved following fish oil supplementation with omega-3 fatty acids among crew members of short-duration space flights [88]. Beyond nutrient supplementation, the overall acid-base balance of the dietary regimen plays a non-negligible role in protecting bone health. Seventeen astronauts underwent 4 days of dietary control (i.e., high or low animal protein-to-potassium ratio) during their missions. The study revealed that excessive sulfur and sodium intake following high animal protein consumption may promote bone resorption by increasing the acid load. Conversely, increasing the intake of alkaline foods such as vegetables and fruits, while ensuring adequate calcium content, effectively reduces the acid load and maintains acid-base balance, thereby improving bone density [89]. This suggests that a reasonable dietary acid-base balance provides crucial support for maintaining astronauts’ bone health.
Numerous studies have now confirmed that various nutritional supplementation regimens (Table 3) exhibit potential ameliorative effects on microgravity-induced bone loss in animal models. During the 4-week period of tail suspension in SD rats, when a combined supplementation regimen of collagen peptide and calcium citrate was adopted, although it partially improved the deterioration of bone microstructure and upregulated the expression level of osteocalcin, compared with the suspension group, its effect on improving BMD was not significant [90]. This suggests that while nutrient supplementation is effective in mitigating microgravity-induced bone loss to some extent, more potent nutritional supplementation strategies may be required to further enhance bone health. Natural compounds have emerged as one of the key research focuses in aerospace medicine due to their rich nutritional value and therapeutic potential. For instance, after mice received naringenin (NAR) supplementation during a 4-week tail suspension, NAR was effective in improving the bone microstructure compared to the suspension group by promoting osteoblast differentiation through activation of the Nrf2/HO-1 signaling pathway [91]. This led to an increase in trabecular number, thickness, and overall bone biomechanical properties. Spirulina platensis, a nutrient-rich spherical microalgae, contains abundant proteins and polysaccharides that effectively alleviate bone density reduction in hindlimb-suspended mice. It inhibits FoxO3 expression and enhances Wnt signaling via activation of the FoxO3/Wnt/β-catenin pathway, thereby promoting bone formation [92]. Given its positive impact on bone health, NASA has listed it as one of the key nutritional supplements for astronauts [93].
The application of potential nutritional supplementation programs in combating microgravity-induced bone loss.
| Nutrition | Species | Age | Model | Frequency | Therapeutic dose and grouping | Effect | References | |
|---|---|---|---|---|---|---|---|---|
| Bone parameters | Bone metabolism-related markers | |||||||
| Curcumin (CUR) | Male SD rats | 2 months | hindlimb suspension (6 weeks) | Everyday | Control group/HLS group (treated with vehicle) Control + CUR group/HLS + CUR Group (40 mg/kg) |
Tibia BMD ↑ Femurs’ mechanical strength ↑ |
MDA↓ vitamin D receptor ↑ TRAP mRNA ↓ OCN ↑ |
[99] |
| Polyphenols of Pinus koraiensis (S3) | Male SD rats | 6 weeks | hindlimb suspension (30 days) | Everyday | Control group/HLS group (distilled water) HLS + S3(L) Group (15 mg/kg) HLS + S3(M) Group (37.5 mg/kg) HLS + S3(H) group (60 mg/kg) |
Femurs’ mechanical strength ↑ | Osteoblast proliferation activity ↑ ALP/BALP/PIPN ↑ β-catenin/Runx2/Osx/p-GSK3-β ↑ |
[100] |
| Collagen peptide (CP) +Calcium citrate (CC) |
Male SD rats | 3 months | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group HLS + CP group (750 mg/kg) HLS + CC group (75 mg/kg) HLS + CP/CC group (750/75 mg/kg) |
Femur BMD ↑ | Osteocalcin ↑ | [90] |
| Naringenin (NAR) | Male C57 mice | 2 months | hindlimb suspension (4 weeks) | Everyday | Control group/HLS group HLS + NAR (L) group (60 mg/kg) HLS + NAR (H) group (100 mg/kg) |
The quantity of trabecula ↑ Elastic modulus/Elastic load ↑ Maximum strain/Bending stiffness ↑ |
IL-1β/NLRP3 ↓ | [91] |
| Spirulina platensis (SP) | Male C57 mice | 2 months | hindlimb suspension (4 weeks) | Everyday | Control group/PBS group/HLS group SPP group/SPS group/SPL group/SP group/SPR group (300 mg/kg) |
BMD Tb.N ↑ Tb.Th ↑ |
MDA ↓ FoxO3a ↓ Wnt/β-catenin ↑ | [92] |
| Resveratrol (RES) | Male wistar rats | / | hindlimb suspension (2 weeks) | Everyday | Control group/HLS group Control + RES group/HLS + RES group (400 mg/kg) |
Femur BMD ↑ Femur strength ↑ |
/ | [101] |
-
SPP, Spirulina platensis proteins; SPS, polysaccharides; SPL, lipids; SPR, residue.
Nutritional supplementation represents one of the critical strategies for combating bone loss in astronauts, improving bone health by compensating for nutrient deficiencies, and targeting bone metabolic signaling pathways. Compared to single-nutrient supplementation, which mitigates bone loss to some extent but lacks significant inhibitory effects on bone resorption, natural compounds offer a superior alternative. In the future, efforts should focus on the development and application of novel nutrients, integrating them into personalized nutritional programs to provide a more comprehensive strategy for managing astronauts’ bone health.
Exercise interventions
Although pharmacological treatments and nutritional supplements effectively inhibit the increase in bone resorption through biochemical modulation, their promotion of bone formation remains limited. Given the potential toxic side effects associated with pharmacological treatments, it is imperative to actively explore sustainable interventions for improving bone health. Exercise, as a non-pharmacological treatment free of toxic side effects, has been shown to enhance bone health in terrestrial environments [94], 95]. Despite the challenges posed by microgravity in space, exercise interventions still demonstrate certain potential and advantages [96].
Studies have indicated that different exercise modes alleviate microgravity-induced bone loss by modulating distinct mechanisms. In aerospace medicine, there is a consensus that aerobic and resistance exercises are the primary interventions for maintaining an astronaut’s health [97], 98]. Astronauts from various countries performing aerobic exercises on second-generation treadmills and bicycle ergometers during missions to the ISS effectively mitigated bone loss. However, BMD at the hip and femur still tended to decrease, which was highly correlated with exercise intensity and loading conditions [102]. Additionally, Lower Body Negative Pressure Running (LBNP), a novel form of aerobic exercise, shows promise in improving bone loss by providing a low-gravity environment for the lower body via pneumatic pressure technology, thereby reducing physiological burden [103], 104]. Seven women undergoing LBNP during 30 days of bed rest found that LBNP effectively mitigated the decrease in BMD induced by prolonged bed rest [105]. Furthermore, LBNP decreased the synthesis of bone resorption markers such as NTX [106], likely due to alterations in systemic blood distribution caused by LBNP. While aerobic exercise can partially mitigate bone loss, resistance exercise often represents a superior exercise intervention in microgravity due to its greater mechanical stimulation of the skeleton [107] and the current maturity of resistance equipment technology for space applications [108]. Resistance exercise has been shown to be the primary intervention for improving bone loss in both men and women, providing stronger mechanical stimulation that not only facilitates bone mass recovery but also significantly reduces the risk of falls and fractures [109]. After six months aboard the ISS, during which 10 astronauts underwent resistance training using the Advanced Resistive Exercise Device (ARED), post-flight testing via DXA and QCT revealed some suppression of femoral neck volumetric BMD (vBMD) decline and alleviation of bone loss. However, a trend toward decreased hip trabecular vBMD persisted, and the expression of bone resorption biomarkers was up-regulated [96]. Although Resistance exercise demonstrated positive effects in improving bone loss, its inhibitory effect on bone resorption was not significant, suggesting the need for combined interventions. To better investigate the effects of microgravity on bone loss, prolonged bed rest has been used as a simulated microgravity environment [110], 111]. Nine participants performed resistance exercise on a horizontal exercise machine 6 days per week during 17 weeks of bed rest, including upper vs. lower body exercises. After 17 weeks of bed rest, the relevant data from these nine subjects were compared with those of 18 control subjects. It was found that the BMD of the lumbar spine and other parts significantly decreased, while the BMD of the exercise group significantly increased, and the levels of bone formation markers such as BSAP and ALP were elevated [111]. However, bone resorption markers such as N-terminal peptide maintained an up-regulation trend, consistent with previous findings, indicating that resistance training promoted bone formation more significantly than it inhibited bone resorption. Historically, astronauts on the ISS have typically employed a high-intensity resistance exercise combined with aerobic exercise strategy, demonstrating more significant improvements in bone health compared to single-modality exercise [112], 113]. Interestingly, the combination of resistance exercise and aerobic exercise was not effective in inhibiting bone resorption. After an exercise regimen combining LBNP with flywheel resistance exercise during 60 days of bed rest in eight women, bone resorption markers such as N-terminal cross-linked telopeptides were higher than pre-bed rest levels, and bone resorption was not inhibited. However, the regimen did promote the production of markers of bone formation such as ALP, facilitating bone formation [114].
Although aerobic exercise and resistance exercise have demonstrated some effectiveness in mitigating microgravity-induced bone loss, they are insufficient to fully address the problem. In recent years, numerous studies have indicated that vibration training may represent one of the novel interventions for combating microgravity-induced bone loss [115]. In SD female rats subjected to localized vibration training twice daily during a 21-day tail suspension, significant improvements were observed in trabecular BMD of the femur and tibia, as well as biomechanical properties such as maximum load and bending stiffness compared to the suspension group [116]. Interestingly, another study found that femoral BMD did not improve significantly when rats underwent tail suspension for 28 days followed by vibration training during their recovery period [117]. This may be related to the decreased mechanosensitivity of osteoblasts after the onset of bone loss. It is important to note that while vibration training alone has shown some efficacy in ameliorating bone wasting, its efficacy may be limited by insufficient stimulus intensity. In recent years, with advancements in research on complex mechanical stimulation interventions, resistive vibration training has emerged as a promising novel intervention for stimulating bone remodeling. In a study involving 14 men who underwent resistance vibration exercises during 60 days of bed rest, reduced bone loss was observed in the hip, lumbar spine, and Achilles tendon compared to the control group. Additionally, no significant changes were noted in urinary calcium and creatinine levels, while Ocn levels were significantly elevated [118]. This suggests that resistance vibration exercises exhibit potential for mitigating microgravity-induced bone loss. In another study, 24 male participants who performed high-load resistance exercise combined with whole-body vibration training during 60 days of bed rest demonstrated greater preservation of bone mass in the proximal femur and tibial diaphysis compared to those receiving resistance exercise alone [119]. Furthermore, exercise as a preventive modality is effective in preventing the occurrence of bone loss [120], laying a theoretical foundation for combating microgravity-induced bone loss through exercise preadaptation. However, excessive participation in high-intensity aerobic exercise prior to spaceflight may accelerate tibial trabecular bone loss during flight [121], potentially due to the relationship between exercise intensity and exercise pattern.
In conclusion, in a microgravity environment, exercise intervention can effectively slow down bone loss. It promotes bone formation through mechanical stimulation and regulates bone metabolism, as shown in Table 4. Maintaining resistance exercise during flight is more effective in improving bone health. However, engaging in intense exercise before a flight may accelerate the bone loss of astronauts in the space environment. For instance, a study by Leigh et al. found that when astronauts increased their running volume before an aircraft flight, the loss of trabecular bone in their tibia became more severe [121]. Exercise intervention is currently a common strategy for astronauts to maintain bone mass during space missions, but its application still faces challenges, such as the lack of fully defined exercise doses and parameters, as well as differing responses across skeletal regions following exercise. In the future, it will be necessary to focus on exploring personalized exercise prescriptions based on bone metabolic markers and analyzing the mechanisms of mechanical signals and bone responses using multi-omics technology to further refine the scientific strategies for combating bone loss through exercise.
The application of potential exercise intervention in combating microgravity-induced bone loss.
| Exercise | Species | Age | Frequency | Effects | References | |
|---|---|---|---|---|---|---|
| Bone parameters | Bone metabolism-related markers | |||||
| Resistance exercise | Human (astronauts) | 47 ± 5 years | / | The decrease in femoral neck bone density is inhibited Bone loss ↑ vBMD of the hip ↓ |
NTX↑ | [96] |
| Aerobic exercise | Human (astronauts) | 46.8 + 6.1 years | 5–6 days/week (within 178 + 48 days) | Bone loss↓ BMD of the hip and femur ↓ |
/ | [102] |
| Resistance exercise + Aerobic exercise | Human (astronauts) | 47 + 6 years | 6 days/week (within 160 + 36 days) | Bone mineral density is maintained | / | [112] |
| High load resistance motion + Whole-body vibration | Men (bed rest for 60 day) | 31–34 years | 3 times/week | More bone mass is preserved in the proximal femur and tibial shaft | / | [119] |
| Resistance vibration exercise | Men (bed rest for 60 day) | 25–40 years | Everyday | Hip, lumbar, and calcaneal BMD ↓ | OCN ↑ | [118] |
| LBNP + flywheel resistive exercise | Women (bed rest for 60 days) | / | LBNP (3–4 days/week) Flywheel resistive exercise (2–3 days/week) |
/ | Helical Peptide/N-terminal ↑ Procollagen type I N propeptide/ALP ↑ |
[114] |
| LBNP | Human (bed rest for 30 days) | 22–37 years | 6 days/week (within in 30 days) | Lumbar spine compressibility ↓ | / | [103], 104] |
| Resistance exercise | Human (bed rest for 17 weeks) | 23–44 years | 6 days/week | / | BMD↑ BSAP/Alp ↑ N-telopeptide ↑ |
[111] |
| Local vibration | SD rats (hindlimb unloading for 21 days) | 8 weeks | Twice a day (during hindlimb unloading) | Bone trabecular BMD of femur and tibia ↑ Biomechanical properties of femur ↑ |
/ | [116] |
| Whole-body vibration | SD rats (hindlimb unloading for 21 days) | / | / | BMD of the femur ↓ | / | [117] |
Combined intervention
Although interventions such as pharmacological treatment, nutritional supplementation, and exercise interventions have demonstrated certain application value in the prevention and treatment of microgravity-induced bone loss, their improvement effects may be limited by factors such as the singularity of the regulatory target and the unilateral regulation of bone metabolism. Considering the complex mechanisms of skeletal diseases in microgravity environments, multidimensional and synergistic interventions may present a more preferable option for achieving systematic protection of bone health.
As discussed in the preceding section, combined bisphosphonate supplementation under daily exercise in space is more effective than single bisphosphonate supplementation in ameliorating bone loss. This is primarily manifested in the fact that resistance exercise combined with bisphosphonates not only attenuates cortical bone loss but also further mitigates trabecular bone loss, thereby realizing bidirectional regulation of bone homeostasis – simultaneously promoting bone formation and inhibiting bone resorption [19]. Another ambulatory trial also demonstrated that resistance exercise combined with bisphosphonate therapy attenuated the increase in serum calcium ions and the decrease in plasma calcitonin in subjects. Additionally, this combined intervention rescued femoral neck mineral loss [122]. These findings suggest that resistance exercise combined with bisphosphonate therapy holds significant potential for improving microgravity-induced bone loss. Although antiresorptive drugs combined with exercise interventions achieve bidirectional regulation of bone metabolism and mitigate the progression of bone loss, their long-term effects may be limited by drug-induced gastrointestinal complications, which can reduce drug absorption rates [123]. Notably, nutritional supplementation combined with exercise intervention has demonstrated the applied value in maintaining bone health [124]. During 4–6 month space missions, astronauts maintained an adequate amount of resistance exercise (including ARED and iRED) and supplemented sufficient amounts of nutrients such as vitamin D, sodium, iron, and potassium through dietary intake. Upon returning to Earth, it was found that the decreasing trend of pelvic mineral content was slowed [125]. This indicates that nutritional supplementation combined with exercise intervention can effectively inhibit bone loss in microgravity environments, maintain bone density levels, and reduce the risk of osteoporosis.
Although joint interventions have shown some potential for improving microgravity-induced bone loss, there is currently insufficient clinical data to determine the effectiveness and safety of different combined intervention modes for bone protection. Further optimization of relevant protocols is therefore required. In the future, longer-term studies should be conducted, and better combinations should be selected using relevant databases and microarray technology to construct novel protection models.
A comprehensive comparison of intervention strategies
In summary, after systematically exploring the ameliorative effects of pharmacological treatments, nutritional supplementation, and exercise interventions on microgravity-induced bone loss, we further analyzed and compared these intervention modalities (Table 5). While these modalities demonstrated certain improvements, differences remained in terms of intervention timing, efficacy, and potential side effects.
Comparison of different intervention measures.
| Type of intervention | Representative measures | Efficacy | Side effects |
|---|---|---|---|
| Pharmacotherapy | Bisphosphonates [56] | Inhibit bone resorption [53], 54] Insufficient deep regulation of the bone microenvironment [57] |
Increase the likelihood of complications such as bone necrosis [134], hypocalcemia [135] and acute inflammation [136] Anti-resorptive drugs may synergistically inhibit osteoblast differentiation [128] |
| Nutritional supplementation | Omega 3/Vitamin K [86], 88] | Regulate calcium and phosphorus metabolism [78], 79] Provide bone metabolism nutrients [86] |
Excessive supplementation may cause hypercalcemia [131] There are individual differences, and it is impossible to assess the nutritional intake in real time [78], 80] Microgravity may cause a reduction in gastrointestinal peristalsis, thereby affecting the efficiency of nutrient absorption [137], 138] |
| Exercise interventions | Resistance exercise [96] Aerobic exercise [102], 103] |
Promote bone formation [107] Multi organ benefits [139], 140] The inhibition of bone resorption was not significant [96] |
The equipment has a high dependency level, and the confined space limits the diversity of movements [108] There is a potential risk of arrhythmia [141] |
| Combined intervention | Resistance exercise + Bisphosphonates [19], 122] Resistance exercise + Vitamin D [125] |
Promote bone formation [19], 122] Inhibit bone resorption [19], 122] Maintain bone density [125] |
In microgravity, bone calcium loss increases kidney stone risk; insufficient water and excessive calcium intake during exercise further strain the kidneys [142], 143] |
In terms of efficacy, pharmacological treatments for microgravity-induced bone loss have primarily focused on inhibiting bone resorption. Anti-resorptive drugs such as bisphosphonates and denosumab target and regulate osteoclast activity and the expression of osteoclast-associated factors, thereby mitigating increased bone resorption caused by stressful mechanical deficits due to microgravity. Additionally, pharmacological treatment rapidly increased BMD in the short term. For instance, bisphosphonate supplementation exhibited anti-resorptive efficacy and significantly down-regulated NTX expression over 15–30 days compared to exercise alone when daily exercise was maintained [19]. Therefore, pharmacological treatments are more suitable for addressing rapid bone loss associated with short-term space missions. However, medications may include toxic side effects. Studies have shown that long-term use of bisphosphonate medications may increase the risk of skeletal disorders such as jaw osteonecrosis [126] and atypical femur fracture [127]. Furthermore, anti-resorptive drug therapy may synergistically inhibit osteoblast differentiation, reducing the rate of bone recovery [128]. Long-term use of denosumab may induce chronic inflammation, cause pharmacologic injury [129], and rapidly lead to renewed bone loss upon discontinuation of the drug [130]. Nutritional supplementation regulates bone homeostasis by providing nutrients related to bone metabolism, such as vitamin D and vitamin K. Nutritional supplementation typically requires a longer duration and is influenced by individual metabolic and absorption capacities. Excessive intake of calcium or vitamin D may induce hypercalcemia and hypercalciuria [131], necessitating strict monitoring of nutritional intake. Compared with pharmacological treatments, exercise interventions primarily promote bone formation by applying mechanical loads to maintain bone homeostasis [96]. Exercise can also effectively improve the function of various tissues in astronauts, including the muscular system [132] and the cardiovascular system [133]. However, given the weak inhibitory effect of exercise on bone resorption and the high dependence on equipment in the space environment, joint interventions such as exercise-pharmacologic intervention or exercise-supplementation combinations may represent better options (Figure 3).
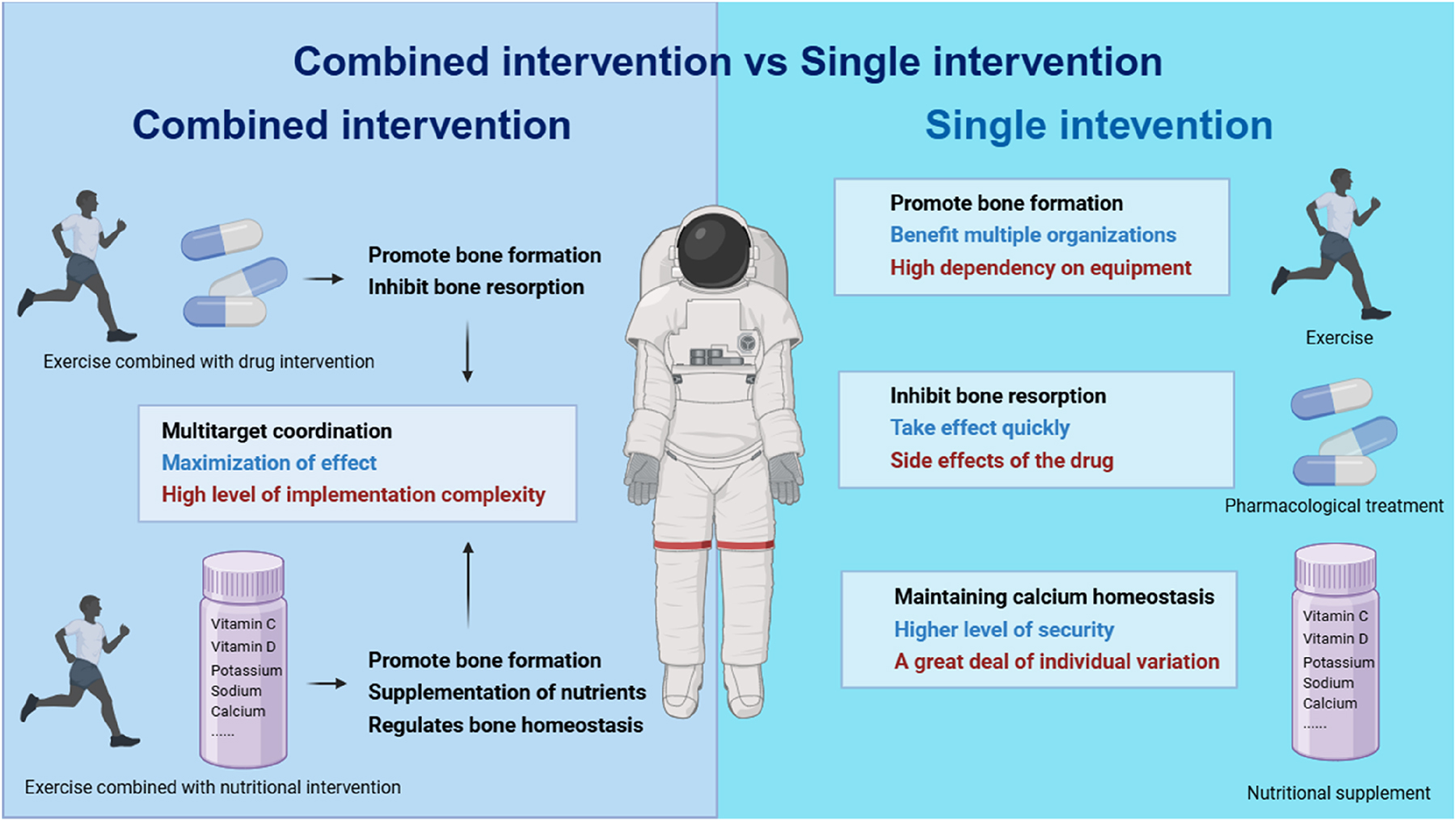
Comparison of the effects of single intervention and combined intervention on bone loss.
Summary and outlook
For microgravity-induced bone loss, different intervention strategies exhibit distinct intervention mechanisms, effects, and dosing regimens. Exercise interventions effectively promote bone formation to counteract the increased bone resorption caused by microgravity while regulating bone homeostasis. Pharmacological treatments primarily focus on inhibiting excessive bone resorption, whereas nutritional supplementation enhances the bone microenvironment by providing essential nutrients for bone metabolism. Compared with single-intervention models, combined interventions offer superior protection for bone health.
Although each of these interventions has demonstrated certain applications in mitigating bone loss, numerous challenges remain regarding their practical implementation. Exercise interventions are highly dependent on equipment, such as the AREDs currently used on the ISS. These devices occupy significant space and impose greater demands on vehicles for deep-space exploration missions. Notably, existing studies primarily based on short-duration space missions (<12 months) or ground-based experiments are insufficient to determine the potential damage to the skeletal system during long-duration space (>2 years) missions. Further research requires support from additional medical data and ground-based experiments. Implementing effective bone protection strategies for space missions necessitates the application of customized approaches across the pre-flight, in-flight, and post-flight phases. Prior to launch, adaptive training programs are employed to enhance physiological preparedness for microgravity environments. Throughout the mission, real-time monitoring systems coupled with intervention protocols are critical for mitigating progressive bone loss. Following mission completion, post-flight recovery strategies focus on gravitational re-adaptation and targeted rehabilitation therapies. However, current research is constrained by the limited availability of astronaut data and inconsistencies in evaluation methodologies, which impede direct comparisons with ground-based analog studies. Future research directions should prioritize longitudinal clinical trials designed to systematically assess the efficacy of pharmacological, nutritional, exercise-based, and multimodal countermeasures – key components in the development of personalized bone health protocols for long-duration space exploration.
To address the needs of bone health during long-term space missions, future research should focus on multiple areas. First, dynamic predictive modeling leveraging multi-omics data combined with AI can be developed to establish precise dosage regimens. Simultaneously, novel wearable devices are being designed to monitor changes in bone metabolism markers and related nutrients in real time within a microgravity environment, thereby assessing the efficacy of preventive and therapeutic measures. Additionally, exploring the discovery of novel target molecules through high-throughput databases may facilitate the bidirectional regulation of bone metabolism.
Funding source: the Guangdong Basic and Applied Basic Research Foundation
Award Identifier / Grant number: No. 2022A1515010379
Funding source: the Guangdong Province General University Innovation Team Project
Award Identifier / Grant number: 2023WCXTD011
Funding source: the Guangdong Philosophy and Social Science Foundation Regular Project
Award Identifier / Grant number: GD23YTY04
Funding source: the Special Projects in Key Fields of Ordinary Universities in Guangdong Province
Award Identifier / Grant number: No. 2024ZDZX2059
Funding source: the Guangdong Provincial Key Laboratory of Physical Activity and Health Promotion
Award Identifier / Grant number: 2021B1212040014
Award Identifier / Grant number: No. 81901430
Acknowledgment
All figures were Created in BioRender.com.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Kaihong Weng: Writing-review & editing, writing-original draft, Visualization, Conceptualization. Yuting He: Writing-review & editing. Xiquan Weng: Writing-review & editing, writing-original draft, Visualization, Conceptualization. Yu Yuan: Writing-review & editing, writing-original draft, Visualization, Conceptualization.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: the National Natural Science Foundation of China (No. 81901430), the Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010379), the Guangdong Philosophy and Social Science Foundation Regular Project (GD23YTY04), the Special Projects in Key Fields of Ordinary Universities in Guangdong Province (No. 2024ZDZX2059), the Guangdong Province General University Innovation Team Project (2023WCXTD011) and the Guangdong Provincial Key Laboratory of Physical Activity and Health Promotion (2021B1212040014).
-
Data availability: Not applicable.
References
1. Mircea, AA, Pistritu, DV, Fortner, A, Tanca, A, Liehn, EA, Bucur, O. Space travel: the radiation and microgravity effects on the cardiovascular system. Int J Mol Sci 2024;25:11812. https://doi.org/10.3390/ijms252111812.Search in Google Scholar PubMed PubMed Central
2. Beheshti, A, McDonald, JT, Hada, M, Takahashi, A, Mason, CE, Mognato, M. Genomic changes driven by radiation-induced DNA damage and microgravity in human cells. Int J Mol Sci 2021;22:10507. https://doi.org/10.3390/ijms221910507.Search in Google Scholar PubMed PubMed Central
3. Krittanawong, C, Singh, NK, Scheuring, RA, Urquieta, E, Bershad, EM, Macaulay, TR, et al.. Human health during space travel: state-of-the-art review. Cells 2022;12:40. https://doi.org/10.3390/cells12010040.Search in Google Scholar PubMed PubMed Central
4. Han, H, Jia, H, Wang, YF, Song, JP. Cardiovascular adaptations and pathological changes induced by spaceflight: from cellular mechanisms to organ-level impacts. Military Medical Research 2024;11:68. https://doi.org/10.1186/s40779-024-00570-3.Search in Google Scholar PubMed PubMed Central
5. Grimm, D, Grosse, J, Wehland, M, Mann, V, Reseland, JE, Sundaresan, A, et al.. The impact of microgravity on bone in humans. Bone 2016;87:44–56. https://doi.org/10.1016/j.bone.2015.12.057.Search in Google Scholar PubMed
6. Baran, R, Wehland, M, Schulz, H, Heer, M, Infanger, M, Grimm, D. Microgravity-related changes in bone density and treatment options: a systematic review. Int J Mol Sci 2022;23:8650. https://doi.org/10.3390/ijms23158650.Search in Google Scholar PubMed PubMed Central
7. Vico, L, van Rietbergen, B, Vilayphiou, N, Linossier, MT, Locrelle, H, Normand, M, et al.. Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following international space station missions. J Bone Miner Res 2017;32:2010–21. https://doi.org/10.1002/jbmr.3188.Search in Google Scholar PubMed
8. Kim, JM, Lin, C, Stavre, Z, Greenblatt, MB, Shim, JH. Osteoblast-osteoclast communication and bone homeostasis. Cells 2020;9. https://doi.org/10.3390/cells9092073.Search in Google Scholar PubMed PubMed Central
9. Ruggiu, A, Cancedda, R. Bone mechanobiology, gravity and tissue engineering: effects and insights. J Tissue Eng Regen Med 2015;9:1339–51. https://doi.org/10.1002/term.1942.Search in Google Scholar PubMed
10. Cheong, VS, Roberts, BC, Kadirkamanathan, V, Dall’Ara, E. Bone remodelling in the mouse tibia is spatio-temporally modulated by oestrogen deficiency and external mechanical loading: a combined in vivo/in silico study. Acta Biomater 2020;116:302–17. https://doi.org/10.1016/j.actbio.2020.09.011.Search in Google Scholar PubMed
11. Guo, Q, Chen, N, Patel, K, Wan, M, Zheng, J, Cao, X. Unloading-induced skeletal interoception alters hypothalamic signaling to promote bone loss and fat metabolism. Adv Sci (Weinh) 2023;10:e2305042. https://doi.org/10.1002/advs.202305042.Search in Google Scholar PubMed PubMed Central
12. Sun, W, Chi, S, Li, Y, Ling, S, Tan, Y, Xu, Y, et al.. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019;8:e47454. https://doi.org/10.7554/elife.47454.Search in Google Scholar
13. Wang, L, You, X, Lotinun, S, Zhang, L, Wu, N, Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun 2020;11:282. https://doi.org/10.1038/s41467-019-14146-6.Search in Google Scholar PubMed PubMed Central
14. Herrmann, M, Engelke, K, Ebert, R, Müller-Deubert, S, Rudert, M, Ziouti, F, et al.. c. Biomolecules 2020;10:432. https://doi.org/10.3390/biom10030432.Search in Google Scholar PubMed PubMed Central
15. Willey, JS, Aunon-Chancellor, S, Miles, LA, Moore, JE, Mao, XW, Wallace, RW, et al.. αKlotho decreases after reduced weight-bearing from both spaceflight and hindlimb unloading. NPJ Microgravity 2022;8:18. https://doi.org/10.1038/s41526-022-00203-w.Search in Google Scholar PubMed PubMed Central
16. Zayzafoon, M, Meyers, VE, McDonald, JM. Microgravity: the immune response and bone. Immunol Rev 2005;208:267–80. https://doi.org/10.1111/j.0105-2896.2005.00330.x.Search in Google Scholar PubMed
17. Coulombe, JC, Senwar, B, Ferguson, VL. Spaceflight-induced bone tissue changes that affect bone quality and increase fracture risk. Curr Osteoporos Rep 2020;18:1–12. https://doi.org/10.1007/s11914-019-00540-y.Search in Google Scholar PubMed
18. Drake, MT, Clarke, BL, Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83:1032–45. https://doi.org/10.4065/83.9.1032.Search in Google Scholar PubMed PubMed Central
19. Rosenthal, R, Schneider, VS, Jones, JA, Sibonga, JD. The case for bisphosphonate use in astronauts flying long-duration missions. Cells 2024;13. https://doi.org/10.3390/cells13161337.Search in Google Scholar PubMed PubMed Central
20. Costa, F, Ambesi-Impiombato, FS, Beccari, T, Conte, C, Cataldi, S, Curcio, F, et al.. Spaceflight induced disorders: potential nutritional countermeasures. Front Bioeng Biotechnol 2021;9:666683. https://doi.org/10.3389/fbioe.2021.666683.Search in Google Scholar PubMed PubMed Central
21. Lee Satcher, R, Fiedler, B, Ghali, A, Dirschl, DR. Effect of spaceflight and microgravity on the musculoskeletal system: a review. J Am Acad Orthop Surg 2024;32:535–41. https://doi.org/10.5435/jaaos-d-23-00954.Search in Google Scholar PubMed
22. Hirayama, J, Hattori, A, Takahashi, A, Furusawa, Y, Tabuchi, Y, Shibata, M, et al.. Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: implications of melatonin use and regulation as a countermeasure. J Pineal Res 2023;74:e12834. https://doi.org/10.1111/jpi.12834.Search in Google Scholar PubMed
23. Man, J, Graham, T, Squires-Donelly, G, Laslett, AL. The effects of microgravity on bone structure and function. NPJ Microgravity 2022;8:9. https://doi.org/10.1038/s41526-022-00194-8.Search in Google Scholar PubMed PubMed Central
24. Stavnichuk, M, Mikolajewicz, N, Corlett, T, Morris, M, Komarova, SV. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity 2020;6:13. https://doi.org/10.1038/s41526-020-0103-2.Search in Google Scholar PubMed PubMed Central
25. Collet, P, Uebelhart, D, Vico, L, Moro, L, Hartmann, D, Roth, M, et al.. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone 1997;20:547–51. https://doi.org/10.1016/s8756-3282(97)00052-5.Search in Google Scholar PubMed
26. Vico, L, Collet, P, Guignandon, A, Lafage-Proust, MH, Thomas, T, Rehaillia, M, et al.. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet (London, England) 2000;355:1607–11. https://doi.org/10.1016/s0140-6736(00)02217-0.Search in Google Scholar PubMed
27. Lovejoy, CO, McCollum, MA. Spinopelvic pathways to bipedality: why no hominids ever relied on a bent-hip-bent-knee gait. Philos Trans R Soc Lond B Biol Sci 2010;365:3289–99. https://doi.org/10.1098/rstb.2010.0112.Search in Google Scholar PubMed PubMed Central
28. Orwoll, ES, Adler, RA, Amin, S, Binkley, N, Lewiecki, EM, Petak, SM, et al.. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA Bone Summit. J Bone Miner Res 2013;28:1243–55. https://doi.org/10.1002/jbmr.1948.Search in Google Scholar PubMed
29. Lang, T, LeBlanc, A, Evans, H, Lu, Y, Genant, H, Yu, A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 2004;19:1006–12. https://doi.org/10.1359/jbmr.040307.Search in Google Scholar
30. Coulombe, JC, Johannesdottir, F, Burkhart, KA, Brummer, H, Allaire, BT, Bouxsein, ML. Changes in vertebral bone density and paraspinal muscle morphology following spaceflight and 1 Year readaptation on earth. JBMR Plus 2023;7:e10810. https://doi.org/10.1002/jbm4.10810.Search in Google Scholar PubMed PubMed Central
31. Morey-Holton, ER, Globus, RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 2002;92:1367–77. https://doi.org/10.1152/japplphysiol.00969.2001.Search in Google Scholar PubMed
32. Globus, RK, Morey-Holton, E. Hindlimb unloading: rodent analog for microgravity. J Appl Physiol (1985) 2016;120:1196–206. https://doi.org/10.1152/japplphysiol.00997.2015.Search in Google Scholar PubMed
33. Bloomfield, SA, Martinez, DA, Boudreaux, RD, Mantri, AV. Microgravity stress: bone and connective tissue. Compr Physiol 2016;6:645–86. https://doi.org/10.1002/cphy.c130027.Search in Google Scholar PubMed
34. Morey-Holton, ER, Globus, RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone 1998;22:83s–8s. https://doi.org/10.1016/s8756-3282(98)00019-2.Search in Google Scholar PubMed
35. Jin, X, Wang, H, Liang, X, Ru, K, Deng, X, Gao, S, et al.. Calycosin prevents bone loss induced by hindlimb unloading. NPJ Microgravity 2022;8:23. https://doi.org/10.1038/s41526-022-00210-x.Search in Google Scholar PubMed PubMed Central
36. Swift, JM, Hogan, HA, Bloomfield, SA. β-1 adrenergic agonist mitigates unloading-induced bone loss by maintaining formation. Med Sci Sports Exerc 2013;45:1665–73. https://doi.org/10.1249/mss.0b013e31828d39bc.Search in Google Scholar PubMed
37. Ezumi, S, Kaneguchi, A, Kanehara, M, Iwamoto, Y, Takahashi, M, Nishida, N, et al.. Effects of Hindlimb suspension on the development of Hip bone morphologies in growing rats. Physiol Res 2024;73:643–53. https://doi.org/10.33549/physiolres.935159.Search in Google Scholar PubMed PubMed Central
38. Metzger, CE, Anand Narayanan, S, Phan, PH, Bloomfield, SA. Hindlimb unloading causes regional loading-dependent changes in osteocyte inflammatory cytokines that are modulated by exogenous irisin treatment. NPJ Microgravity 2020;6:28. https://doi.org/10.1038/s41526-020-00118-4.Search in Google Scholar PubMed PubMed Central
39. DeSilva, JM, Rosenberg, KR. Anatomy, development, and function of the human pelvis. Anat Rec (Hoboken) 2017;300:628–32. https://doi.org/10.1002/ar.23561.Search in Google Scholar PubMed
40. Cervinka, T, Sievänen, H, Hyttinen, J, Rittweger, J. Bone loss patterns in cortical, subcortical, and trabecular compartments during simulated microgravity. J Appl Physiol (1985) 2014;117:80–8. https://doi.org/10.1152/japplphysiol.00021.2014.Search in Google Scholar PubMed
41. Rai, B, Kaur, J, Catalina, M. Bone mineral density, bone mineral content, gingival crevicular fluid (matrix metalloproteinases, cathepsin K, osteocalcin), and salivary and serum osteocalcin levels in human mandible and alveolar bone under conditions of simulated microgravity. J Oral Sci 2010;52:385–90. https://doi.org/10.2334/josnusd.52.385.Search in Google Scholar PubMed
42. Gerbaix, M, Gnyubkin, V, Farlay, D, Olivier, C, Ammann, P, Courbon, G, et al.. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci Rep 2017;7:2659. https://doi.org/10.1038/s41598-017-03014-2.Search in Google Scholar PubMed PubMed Central
43. Lewiecki, EM. Utility of trabecular bone score in the management of patients with osteoporosis. Endocrinol Metab Clin N Am 2024;53:547–57. https://doi.org/10.1016/j.ecl.2024.07.001.Search in Google Scholar PubMed
44. Sibonga, JD. Spaceflight-induced bone loss: is there an osteoporosis risk? Curr Osteoporos Rep 2013;11:92–8. https://doi.org/10.1007/s11914-013-0136-5.Search in Google Scholar PubMed
45. Currey, JD, Brear, K, Zioupos, P. Strain rate dependence of work of fracture tests on bone and similar tissues: reflections on testing methods and mineral content effects. Bone 2019;128:115038. https://doi.org/10.1016/j.bone.2019.115038.Search in Google Scholar PubMed
46. Krause, AR, Speacht, TA, Steiner, JL, Lang, CH, Donahue, HJ. Mechanical loading recovers bone but not muscle lost during unloading. NPJ Microgravity 2020;6:36. https://doi.org/10.1038/s41526-020-00126-4.Search in Google Scholar PubMed PubMed Central
47. Sibonga, JD, Evans, HJ, Sung, HG, Spector, ER, Lang, TF, Oganov, VS, et al.. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone 2007;41:973–8. https://doi.org/10.1016/j.bone.2007.08.022.Search in Google Scholar PubMed
48. Gabel, L, Liphardt, AM, Hulme, PA, Heer, M, Zwart, SR, Sibonga, JD, et al.. Incomplete recovery of bone strength and trabecular microarchitecture at the distal tibia 1 year after return from long duration spaceflight. Sci Rep 2022;12:9446. https://doi.org/10.1038/s41598-022-13461-1.Search in Google Scholar PubMed PubMed Central
49. Burger, EH, Klein-Nulend, J. Microgravity and bone cell mechanosensitivity. Bone 1998;22:127s–30s. https://doi.org/10.1016/s8756-3282(98)00010-6.Search in Google Scholar PubMed
50. Tamma, R, Colaianni, G, Camerino, C, Di Benedetto, A, Greco, G, Strippoli, M, et al.. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J 2009;23:2549–54. https://doi.org/10.1096/fj.08-127951.Search in Google Scholar PubMed
51. Klein-Nulend, J, Bacabac, RG, Veldhuijzen, JP, Van Loon, JJ. Microgravity and bone cell mechanosensitivity. Adv Space Res 2003;32:1551–9. https://doi.org/10.1016/s0273-1177(03)90395-4.Search in Google Scholar
52. Reid, IR, Billington, EO. Drug therapy for osteoporosis in older adults. Lancet (London, England) 2022;399:1080–92. https://doi.org/10.1016/s0140-6736(21)02646-5.Search in Google Scholar
53. Ganesan, K, Goyal, A, Roane, D. Bisphosphonate. StatPearls. Treasure Island (FL) ineligible companies Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies. Disclosure: Douglas Roane declares no relevant financial relationships with ineligible companies. StatPearls Publishing Copyright © 2025. Florida: StatPearls Publishing LLC; 2025.Search in Google Scholar
54. Russell, RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci 2006;1068:367–401. https://doi.org/10.1196/annals.1346.041.Search in Google Scholar PubMed
55. Smith, SM, Heer, M, Shackelford, LC, Sibonga, JD, Spatz, J, Pietrzyk, RA, et al.. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015;81:712–20. https://doi.org/10.1016/j.bone.2015.10.002.Search in Google Scholar PubMed
56. Chappard, D, Alexandre, C, Palle, S, Vico, L, Morukov, BV, Rodionova, SS, et al.. Effects of a bisphosphonate (1-hydroxy ethylidene-1,1 bisphosphonic acid) on osteoclast number during prolonged bed rest in healthy humans. Metabolism 1989;38:822–5. https://doi.org/10.1016/0026-0495(89)90226-6.Search in Google Scholar PubMed
57. Cole, LE, Vargo-Gogola, T, Roeder, RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv Drug Deliv Rev 2016;99:12–27. https://doi.org/10.1016/j.addr.2015.10.005.Search in Google Scholar PubMed
58. Brown, JP, Prince, RL, Deal, C, Recker, RR, Kiel, DP, de Gregorio, LH, et al.. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009;24:153–61. https://doi.org/10.1359/jbmr.0809010.Search in Google Scholar PubMed
59. Hofbauer, LC, Rauner, M. Denosumab-protection for bone and beyond? J Clin Endocrinol Metab 2024;109:e2159–60. https://doi.org/10.1210/clinem/dgae207.Search in Google Scholar PubMed
60. Kobayakawa, T, Miyazaki, A, Saito, M, Suzuki, T, Takahashi, J, Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep 2021;11:11801. https://doi.org/10.1038/s41598-021-91248-6.Search in Google Scholar PubMed PubMed Central
61. Cummings, SR, San Martin, J, McClung, MR, Siris, ES, Eastell, R, Reid, IR, et al.. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65. https://doi.org/10.1056/nejmoa0809493.Search in Google Scholar PubMed
62. Händel, MN, Cardoso, I, von Bülow, C, Rohde, JF, Ussing, A, Nielsen, SM, et al.. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ (Clinical Research Ed) 2023;381:e068033. https://doi.org/10.1136/bmj-2021-068033.Search in Google Scholar PubMed PubMed Central
63. Ikegame, M, Hattori, A, Tabata, MJ, Kitamura, KI, Tabuchi, Y, Furusawa, Y, et al.. Melatonin is a potential drug for the prevention of bone loss during space flight. J Pineal Res 2019;67:e12594. https://doi.org/10.1111/jpi.12594.Search in Google Scholar PubMed PubMed Central
64. Campo, GM, Avenoso, A, Campo, S, Nastasi, G, Traina, P, D’Ascola, A, et al.. The antioxidant activity of chondroitin-4-sulphate, in carbon tetrachloride-induced acute hepatitis in mice, involves NF-kappaB and caspase activation. Br J Pharmacol 2008;155:945–56. https://doi.org/10.1038/bjp.2008.338.Search in Google Scholar PubMed PubMed Central
65. Lan, R, Li, Y, Zhao, X, Shen, R, Wang, R, Mao, R, et al.. Low-molecular-weight chondroitin sulfates alleviate simulated microgravity-induced oxidative stress and bone loss in mice. Curr Issues Mol Biol 2023;45:4214–27. https://doi.org/10.3390/cimb45050268.Search in Google Scholar PubMed PubMed Central
66. Nai, J, Zhang, C, Shao, H, Li, B, Li, H, Gao, L, et al.. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int J Biol Macromol 2021;183:2337–53. https://doi.org/10.1016/j.ijbiomac.2021.05.213.Search in Google Scholar PubMed
67. Gu, DR, Yang, H, Kim, SC, Hwang, YH, Ha, H. Water extract of angelica dahurica inhibits osteoclast differentiation and bone loss. Int J Mol Sci 2023;24. https://doi.org/10.3390/ijms241914715.Search in Google Scholar PubMed PubMed Central
68. Liang, X, Jiang, S, Su, P, Yin, C, Jiang, W, Gao, J, et al.. Angelicae dahuricae radix alleviates simulated microgravity induced bone loss by promoting osteoblast differentiation. NPJ Microgravity 2024;10:91. https://doi.org/10.1038/s41526-024-00433-0.Search in Google Scholar PubMed PubMed Central
69. Li, C, Li, Q, Mei, Q, Lu, T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci 2015;126:57–68. https://doi.org/10.1016/j.lfs.2015.01.006.Search in Google Scholar PubMed
70. Huang, J, Yuan, L, Wang, X, Zhang, TL, Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci 2007;81:832–40. https://doi.org/10.1016/j.lfs.2007.07.015.Search in Google Scholar PubMed
71. He, JP, Feng, X, Wang, JF, Shi, WG, Li, H, Danilchenko, S, et al.. Icariin prevents bone loss by inhibiting bone resorption and stabilizing bone biological apatite in a hindlimb suspension rodent model. Acta Pharmacol Sin 2018;39:1760–7. https://doi.org/10.1038/s41401-018-0040-8.Search in Google Scholar PubMed PubMed Central
72. Zeng, Y, Xiong, Y, Yang, T, Wang, Y, Zeng, J, Zhou, S, et al.. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: from effects to molecular mechanisms. Biomed Pharmacother 2022;147:112642. https://doi.org/10.1016/j.biopha.2022.112642.Search in Google Scholar PubMed
73. Du, W, Tang, Z, Yang, F, Liu, X, Dong, J. Icariin attenuates bleomycin-induced pulmonary fibrosis by targeting Hippo/YAP pathway. Biomed Pharmacother 2021;143:112152. https://doi.org/10.1016/j.biopha.2021.112152.Search in Google Scholar PubMed
74. Zhao, W, Yu, HH, Meng, WW, Liu, AM, Zhang, BX, Wang, Y, et al.. Icariin restrains NLRP3 inflammasome-mediated Th2 immune responses and ameliorates atopic dermatitis through modulating a novel lncRNA MALAT1/miR-124-3p axis. Pharm Biol 2023;61:1249–59. https://doi.org/10.1080/13880209.2023.2244004.Search in Google Scholar PubMed PubMed Central
75. Simon, Á, Smarandache, A, Iancu, V, Pascu, ML. Stability of antimicrobial drug molecules in different gravitational and radiation conditions in view of applications during outer space missions. Molecules 2021;26:2221. https://doi.org/10.3390/molecules26082221.Search in Google Scholar PubMed PubMed Central
76. Siddiqui, R, Akbar, N, Khan, NA. Gut microbiome and human health under the space environment. J Appl Microbiol 2021;130:14–24. https://doi.org/10.1111/jam.14789.Search in Google Scholar PubMed
77. Dakkumadugula, A, Pankaj, L, Alqahtani, AS, Ullah, R, Ercisli, S, Murugan, R. Space nutrition and the biochemical changes caused in Astronauts Health due to space flight: a review. Food Chem X 2023;20:100875. https://doi.org/10.1016/j.fochx.2023.100875.Search in Google Scholar PubMed PubMed Central
78. Smith, SM, Wastney, ME, O’Brien, KO, Morukov, BV, Larina, IM, Abrams, SA, et al.. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res 2005;20:208–18. https://doi.org/10.1359/JBMR.041105.Search in Google Scholar PubMed
79. Iwamoto, J, Takeda, T, Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J Med 2005;54:55–9. https://doi.org/10.2302/kjm.54.55.Search in Google Scholar PubMed
80. Smith, SM, Abrams, SA, Davis-Street, JE, Heer, M, O’Brien, KO, Wastney, ME, et al.. Fifty years of human space travel: implications for bone and calcium research. Annu Rev Nutr 2014;34:377–400. https://doi.org/10.1146/annurev-nutr-071813-105440.Search in Google Scholar PubMed
81. Alshahrani, F, Aljohani, N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients 2013;5:3605–16. https://doi.org/10.3390/nu5093605.Search in Google Scholar PubMed PubMed Central
82. Heer, M. Nutritional interventions related to bone turnover in European space missions and simulation models. Nutrition (Burbank, Los Angeles County, Calif) 2002;18:853–6. https://doi.org/10.1016/s0899-9007(02)00905-x..Search in Google Scholar PubMed
83. Niu, Y, Li, C, Pan, Y, Li, Y, Kong, X, Wang, S, et al.. Treatment of Radix Dipsaci extract prevents long bone loss induced by modeled microgravity in hindlimb unloading rats. Pharm Biol 2015;53:110–6. https://doi.org/10.3109/13880209.2014.911920.Search in Google Scholar PubMed
84. Song, S, Gao, Z, Lei, X, Niu, Y, Zhang, Y, Li, C, et al.. Total flavonoids of drynariae rhizoma prevent bone loss induced by Hindlimb unloading in rats. Molecules 2017;22:1033. https://doi.org/10.3390/molecules22071033.Search in Google Scholar PubMed PubMed Central
85. Wu, Z, Hu, L, Ru, K, Zhang, W, Xu, X, Liu, S, et al.. Ellagic acid inhibits CDK12 to increase osteoblast differentiation and alleviate osteoporosis in hindlimb-unloaded and ovariectomized mice. Phytomedicine 2023;114:154745. https://doi.org/10.1016/j.phymed.2023.154745.Search in Google Scholar PubMed
86. Vermeer, C, Wolf, J, Craciun, AM, Knapen, MH. Bone markers during a 6-month space flight: effects of vitamin K supplementation. J Gravitational Physiol 1998;5:65–9.Search in Google Scholar
87. Iwasaki, Y, Yamato, H, Murayama, H, Sato, M, Takahashi, T, Ezawa, I, et al.. Maintenance of trabecular structure and bone volume by vitamin K(2) in mature rats with long-term tail suspension. J Bone Miner Metabol 2002;20:216–22. https://doi.org/10.1007/s007740200031.Search in Google Scholar PubMed
88. Zwart, SR, Pierson, D, Mehta, S, Gonda, S, Smith, SM. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-kappaB activation: from cells to bed rest to astronauts. J Bone Miner Res 2010;25:1049–57. https://doi.org/10.1359/jbmr.091041.Search in Google Scholar PubMed
89. Zwart, SR, Rice, BL, Dlouhy, H, Shackelford, LC, Heer, M, Koslovsky, MD, et al.. Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am J Clin Nutr 2018;107:834–44. https://doi.org/10.1093/ajcn/nqy029.Search in Google Scholar PubMed PubMed Central
90. Liu, J, Wang, J, Guo, Y. Effect of collagen peptide, alone and in combination with calcium citrate, on bone loss in tail-suspended rats. Molecules 2020;25:782. https://doi.org/10.3390/molecules25040782.Search in Google Scholar PubMed PubMed Central
91. Cao, S, Wang, Y, Zhang, Y, Ren, J, Fan, B, Deng, Y, et al.. Naringenin can inhibit the pyroptosis of osteoblasts by activating the Nrf2/HO-1 signaling pathway and alleviate the differentiation disorder of osteoblasts caused by microgravity. J Agric Food Chem 2024;72:25586–600. https://doi.org/10.1021/acs.jafc.4c05370.Search in Google Scholar PubMed PubMed Central
92. Zhang, J, Huang, Y, Bai, N, Sun, Y, Li, K, Ruan, H, et al.. Spirulina platensis components mitigate bone density loss induced by simulated microgravity: a mechanistic insight. Food Chem 2025;463:141361. https://doi.org/10.1016/j.foodchem.2024.141361.Search in Google Scholar PubMed
93. Maddiboyina, B, Vanamamalai, HK, Roy, H, Ramaiah, Gandhi, S, Kavisri, M, et al.. Food and drug industry applications of microalgae Spirulina platensis: a review. J Basic Microbiol 2023;63:573–83. https://doi.org/10.1002/jobm.202200704.Search in Google Scholar PubMed
94. Watson, SL, Weeks, BK, Weis, LJ, Harding, AT, Horan, SA, Beck, BR. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Miner Res 2018;33:211–20. https://doi.org/10.1002/jbmr.3284.Search in Google Scholar PubMed
95. Ott, SM. In osteoporosis or osteopenia, exercise interventions improve BMD; effects vary by exercise type and BMD site. Ann Intern Med 2022;175:Jc46. https://doi.org/10.7326/j22-0014.Search in Google Scholar PubMed
96. Sibonga, J, Matsumoto, T, Jones, J, Shapiro, J, Lang, T, Shackelford, L, et al.. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 2019;128:112037. https://doi.org/10.1016/j.bone.2019.07.013.Search in Google Scholar PubMed
97. Greenleaf, JE, Bulbulian, R, Bernauer, EM, Haskell, WL, Moore, T. Exercise-training protocols for astronauts in microgravity. J Appl Physiol (1985) 1989;67:2191–204. https://doi.org/10.1152/jappl.1989.67.6.2191.Search in Google Scholar PubMed
98. Convertino, VA, Tsiolkovsky, K. Physiological adaptations to weightlessness: effects on exercise and work performance. Exerc Sport Sci Rev 1990;18:119–66. https://doi.org/10.1249/00003677-199001000-00007.Search in Google Scholar
99. Xin, M, Yang, Y, Zhang, D, Wang, J, Chen, S, Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos Int 2015;26:2665–76. https://doi.org/10.1007/s00198-015-3153-7.Search in Google Scholar PubMed
100. Diao, Y, Chen, B, Wei, L, Wang, Z. Polyphenols (S3) Isolated from cone scales of pinus koraiensis alleviate decreased bone formation in rat under simulated microgravity. Sci Rep 2018;8:12719. https://doi.org/10.1038/s41598-018-30992-8.Search in Google Scholar PubMed PubMed Central
101. Momken, I, Stevens, L, Bergouignan, A, Desplanches, D, Rudwill, F, Chery, I, et al.. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J 2011;25:3646–60. https://doi.org/10.1096/fj.10-177295.Search in Google Scholar PubMed
102. Scott, JM, Feiveson, AH, English, KL, Spector, ER, Sibonga, JD, Dillon, EL, et al.. Effects of exercise countermeasures on multisystem function in long duration spaceflight astronauts. NPJ Microgravity 2023;9:11. https://doi.org/10.1038/s41526-023-00256-5.Search in Google Scholar PubMed PubMed Central
103. Zhang, H, Liu, HY, Zhang, CQ, Liu, ZZ, Wang, W. Simulation of the mechanical behavior of osteons using artificial gravity devices in microgravity. Comput Methods Biomech Biomed Eng 2021;24:1578–87. https://doi.org/10.1080/10255842.2021.1901086.Search in Google Scholar PubMed
104. Macias, BR, Cao, P, Watenpaugh, DE, Hargens, AR. LBNP treadmill exercise maintains spine function and muscle strength in identical twins during 28-day simulated microgravity. J Appl Physiol (1985) 2007;102:2274–8. https://doi.org/10.1152/japplphysiol.00541.2006.Search in Google Scholar PubMed
105. Zwart, SR, Hargens, AR, Lee, SM, Macias, BR, Watenpaugh, DE, Tse, K, et al.. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 2007;40:529–37. https://doi.org/10.1016/j.bone.2006.09.014.Search in Google Scholar PubMed PubMed Central
106. Smith, SM, Davis-Street, JE, Fesperman, JV, Calkins, DS, Bawa, M, Macias, BR, et al.. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J Bone Miner Res 2003;18:2223–30. https://doi.org/10.1359/jbmr.2003.18.12.2223.Search in Google Scholar PubMed
107. Benedetti, MG, Furlini, G, Zati, A, Letizia Mauro, G. The effectiveness of physical exercise on bone density in osteoporotic patients. BioMed Res Int 2018;2018:4840531. https://doi.org/10.1155/2018/4840531.Search in Google Scholar PubMed PubMed Central
108. Davis, SA, Davis, BL. Exercise equipment used in microgravity: challenges and opportunities. Curr Sports Med Rep 2012;11:142–7. https://doi.org/10.1249/jsr.0b013e3182578fc3.Search in Google Scholar
109. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95. https://doi.org/10.1001/jama.285.6.785.Search in Google Scholar PubMed
110. LeBlanc, A, Schneider, V, Shackelford, L, West, S, Oganov, V, Bakulin, A, et al.. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact 2000;1:157–60.Search in Google Scholar
111. Shackelford, LC, LeBlanc, AD, Driscoll, TB, Evans, HJ, Rianon, NJ, Smith, SM, et al.. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol (1985) 2004;97:119–29. https://doi.org/10.1152/japplphysiol.00741.2003.Search in Google Scholar PubMed
112. English, KL, Downs, M, Goetchius, E, Buxton, R, Ryder, JW, Ploutz-Snyder, R, et al.. High intensity training during spaceflight: results from the NASA Sprint Study. NPJ Microgravity 2020;6:21. https://doi.org/10.1038/s41526-020-00111-x.Search in Google Scholar PubMed PubMed Central
113. Petersen, N, Jaekel, P, Rosenberger, A, Weber, T, Scott, J, Castrucci, F, et al.. Exercise in space: the European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extreme Physiol Med 2016;5:9. https://doi.org/10.1186/s13728-016-0050-4.Search in Google Scholar PubMed PubMed Central
114. Smith, SM, Zwart, SR, Heer, M, Lee, SM, Baecker, N, Meuche, S, et al.. WISE-2005: supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone 2008;42:572–81. https://doi.org/10.1016/j.bone.2007.11.015.Search in Google Scholar PubMed
115. Zuccarelli, L, Baldassarre, G, Winnard, A, Harris, KM, Weber, T, Green, DA, et al.. Effects of whole-body vibration or resistive-vibration exercise on blood clotting and related biomarkers: a systematic review. NPJ Microgravity 2023;9:87. https://doi.org/10.1038/s41526-023-00338-4.Search in Google Scholar PubMed PubMed Central
116. Sun, LW, Luan, HQ, Huang, YF, Wang, Y, Fan, YB. Effects of local vibration on bone loss in -tail-suspended rats. Int J Sports Med 2014;35:615–24. https://doi.org/10.1055/s-0033-1358468.Search in Google Scholar PubMed
117. Yang, P, Jia, B, Ding, C, Wang, Z, Qian, A, Shang, P. Whole-body vibration effects on bone before and after hind-limb unloading in rats. Aviat Space Environ Med 2009;80:88–93. https://doi.org/10.3357/asem.2368.2009.Search in Google Scholar PubMed
118. Wang, H, Wan, Y, Tam, KF, Ling, S, Bai, Y, Deng, Y, et al.. Resistive vibration exercise retards bone loss in weight-bearing skeletons during 60 days bed rest. Osteoporos Int 2012;23:2169–78. https://doi.org/10.1007/s00198-011-1839-z.Search in Google Scholar PubMed
119. Belavý, DL, Beller, G, Armbrecht, G, Perschel, FH, Fitzner, R, Bock, O, et al.. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed rest. Osteoporos Int 2011;22:1581–91. https://doi.org/10.1007/s00198-010-1371-6.Search in Google Scholar PubMed
120. Dent, E, Daly, RM, Hoogendijk, EO, Scott, D. Exercise to prevent and manage frailty and fragility fractures. Curr Osteoporos Rep 2023;21:205–15. https://doi.org/10.1007/s11914-023-00777-8.Search in Google Scholar PubMed PubMed Central
121. Gabel, L, Liphardt, AM, Hulme, PA, Heer, M, Zwart, SR, Sibonga, JD, et al.. Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. Br J Sports Med 2022;56:196–203. https://doi.org/10.1136/bjsports-2020-103602.Search in Google Scholar PubMed PubMed Central
122. Grigoriev, AI, Morukov, BV, Oganov, VS, Rakhmanov, AS, Buravkova, LB. Effect of exercise and bisphosphonate on mineral balance and bone density during 360 day antiorthostatic hypokinesia. J Bone Miner Res 1992;7:S449–55. https://doi.org/10.1002/jbmr.5650071416.Search in Google Scholar PubMed
123. Graham, DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci 2002;47:1665–78. https://doi.org/10.1023/a:1016495221567.10.1023/A:1016495221567Search in Google Scholar
124. Daly, RM, Duckham, RL, Gianoudis, J. Evidence for an interaction between exercise and nutrition for improving bone and muscle health. Curr Osteoporos Rep 2014;12:219–26. https://doi.org/10.1007/s11914-014-0207-2.Search in Google Scholar PubMed
125. Smith, SM, Heer, MA, Shackelford, LC, Sibonga, JD, Ploutz-Snyder, L, Zwart, SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res 2012;27:1896–906. https://doi.org/10.1002/jbmr.1647.Search in Google Scholar PubMed
126. Otto, S, Pautke, C, Van den Wyngaert, T, Niepel, D, Schiødt, M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev 2018;69:177–87. https://doi.org/10.1016/j.ctrv.2018.06.007.Search in Google Scholar PubMed
127. Gehrke, B, Alves Coelho, MC, Brasil d’Alva, C, Madeira, M. Long-term consequences of osteoporosis therapy with bisphosphonates. Arch Endocrinol Metab 2023;68:e220334. https://doi.org/10.20945/2359-4292-2022-0334.Search in Google Scholar PubMed PubMed Central
128. Lloyd, SA, Travis, ND, Lu, T, Bateman, TA. Development of a low-dose anti-resorptive drug regimen reveals synergistic suppression of bone formation when coupled with disuse. J Appl Physiol (1985) 2008;104:729–38. https://doi.org/10.1152/japplphysiol.00632.2007.Search in Google Scholar PubMed
129. Ostrovsky, V, Malnick, S, Ish-Shalom, S, Ziv Sokolowskaia, N, Yosepovich, A, Neuman, M. Denosumab-induced immune hepatitis. Biomedicines 2021;9:76. https://doi.org/10.3390/biomedicines9010076.Search in Google Scholar PubMed PubMed Central
130. Ferrari, S, Langdahl, B. Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on bone. Nat Rev Rheumatol 2023;19:307–17. https://doi.org/10.1038/s41584-023-00935-3.Search in Google Scholar PubMed
131. Tebben, PJ, Singh, RJ, Kumar, R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev 2016;37:521–47. https://doi.org/10.1210/er.2016-1070.Search in Google Scholar PubMed PubMed Central
132. Murgia, M, Rittweger, J, Reggiani, C, Bottinelli, R, Mann, M, Schiaffino, S, et al.. Spaceflight on the ISS changed the skeletal muscle proteome of two astronauts. NPJ Microgravity 2024;10:60. https://doi.org/10.1038/s41526-024-00406-3.Search in Google Scholar PubMed PubMed Central
133. Shibata, S, Wakeham, DJ, Thomas, JD, Abdullah, SM, Platts, S, Bungo, MW, et al.. Cardiac effects of long-duration space flight. J Am Coll Cardiol 2023;82:674–84. https://doi.org/10.1016/j.jacc.2023.05.058.Search in Google Scholar PubMed
134. Woo, SB, Hellstein, JW, Kalmar, JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006;144:753–61. https://doi.org/10.7326/0003-4819-144-10-200605160-00009.Search in Google Scholar PubMed
135. Maalouf, NM, Heller, HJ, Odvina, CV, Kim, PJ, Sakhaee, K. Bisphosphonate-induced hypocalcemia: report of 3 cases and review of literature. Endocr Pract 2006;12:48–53. https://doi.org/10.4158/ep.12.1.48.Search in Google Scholar PubMed
136. Black, DM, Delmas, PD, Eastell, R, Reid, IR, Boonen, S, Cauley, JA, et al.. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809–22. https://doi.org/10.1056/nejmoa067312.Search in Google Scholar PubMed
137. Kast, J, Yu, Y, Seubert, CN, Wotring, VE, Derendorf, H. Drugs in space: pharmacokinetics and pharmacodynamics in astronauts. Eur J Pharmaceut Sci 2017;109s:S2–s8. https://doi.org/10.1016/j.ejps.2017.05.025.Search in Google Scholar PubMed
138. Ramos-Nascimento, A, Grenga, L, Haange, SB, Himmelmann, A, Arndt, FS, Ly, YT, et al.. Human gut microbiome and metabolite dynamics under simulated microgravity. Gut Microbes 2023;15:2259033. https://doi.org/10.1080/19490976.2023.2259033.Search in Google Scholar PubMed PubMed Central
139. Moosavi, D, Wolovsky, D, Depompeis, A, Uher, D, Lennington, D, Bodden, R, et al.. The effects of spaceflight microgravity on the musculoskeletal system of humans and animals, with an emphasis on exercise as a countermeasure: a systematic scoping review. Physiol Res 2021;70:119–51. https://doi.org/10.33549/physiolres.934550.Search in Google Scholar PubMed PubMed Central
140. Maguire, K, Wydotis, M, Bollinger, L, Caruso, J. Abating heat accrual during exercise in microgravity and implications for future long-term missions. Aerosp Med Hum Perform 2025;96:53–61. https://doi.org/10.3357/amhp.6536.2025.Search in Google Scholar
141. Grenon, SM, Xiao, X, Hurwitz, S, Ramsdell, CD, Sheynberg, N, Kim, C, et al.. Simulated microgravity induces microvolt T wave alternans. Ann Noninvasive Electrocardiol 2005;10:363–70. https://doi.org/10.1111/j.1542-474x.2005.00654.x.Search in Google Scholar
142. Buckey, JC, Thamer, S, Lan, M. Bone loss and kidney stone risk in weightlessness. Curr Opin Nephrol Hypertens 2023;32:172–6. https://doi.org/10.1097/mnh.0000000000000863.Search in Google Scholar PubMed
143. Zerwekh, JE. Nutrition and renal stone disease in space. Nutrition 2002;18:857–63. https://doi.org/10.1016/s0899-9007(02)00911-5.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- The impact of exercise on the role of lipid droplets in maintaining health: a narrative review
- Effects of different interventions on microgravity-induced bone loss
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- A therapeutic exercise program for adolescents engaged in gender diversity services: study protocol for a non-randomised clinical trial
- The role of exercise modality on psychological, behavioral, and fitness outcomes among individuals at risk of type 2 diabetes: preliminary evidence from the CHOICE pragmatic randomized trial
- Section: Physical activity/inactivity and health across the lifespan
- Serum metabolic alterations after two weeks of step-reduction and following four weeks of exercise rehabilitation in older adults: a secondary analysis of the ENDURE randomised controlled trial
- Calibration and evaluation of MET models for estimating energy expenditure using thigh and ankle-worn move 4 accelerometer
- Section: Sports medicine and movement science
- Muscular activity of hamstrings under attentional focus instructions: an electromyography based study
- Associations between anthropometric outcomes and fat percentage with physical performance in professional soccer players: a cutoff points approach
Articles in the same Issue
- Frontmatter
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- The impact of exercise on the role of lipid droplets in maintaining health: a narrative review
- Effects of different interventions on microgravity-induced bone loss
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- A therapeutic exercise program for adolescents engaged in gender diversity services: study protocol for a non-randomised clinical trial
- The role of exercise modality on psychological, behavioral, and fitness outcomes among individuals at risk of type 2 diabetes: preliminary evidence from the CHOICE pragmatic randomized trial
- Section: Physical activity/inactivity and health across the lifespan
- Serum metabolic alterations after two weeks of step-reduction and following four weeks of exercise rehabilitation in older adults: a secondary analysis of the ENDURE randomised controlled trial
- Calibration and evaluation of MET models for estimating energy expenditure using thigh and ankle-worn move 4 accelerometer
- Section: Sports medicine and movement science
- Muscular activity of hamstrings under attentional focus instructions: an electromyography based study
- Associations between anthropometric outcomes and fat percentage with physical performance in professional soccer players: a cutoff points approach

