The impact of exercise on the role of lipid droplets in maintaining health: a narrative review
-
Chunlu Fang
, Yuan Wei
, Guohua Zheng
and Liangming Li
Abstract
Introduction
Lipid droplets (LDs) are omnipresent intracellular organelles. Their number, size, and composition exhibit considerable heterogeneity within cells. Dysregulated lipid accumulation or depletion is not only a hallmark but also a potential contributor to various human pathologies. This narrative review aims to elucidate the impact of excessive LDs on health in various tissues, summarize the potential mechanisms by which exercise-induced amelioration of related diseases through the regulation of LDs homeostasis, and provide important reference evidence for the prevention and treatment of related diseases.
Content
This review elucidates the life cycle, tissue specificity and functions of LDs. Furthermore, it examines the regulatory effects of exercise on LDs metabolism, focusing on mechanisms involving inflammatory factors, liposomes, LDs-associated proteins and immune function, glomerular filtration rate, and insulin sensitivity. Additionally, it highlights the activation of key signaling pathways, including IL-15, AMPK and CaMKII, as well as the enhancement of lysosomal activity in diseases characterized by excessive LDs accumulation and aberrant distribution.
Summary and outlook
LDs are formed in the endoplasmic reticulum and are primarily degraded through lipolysis and lipophagy, exhibiting distinct tissue specificity and multifunctionality. While moderate levels of lipid droplets can protect against lipotoxicity, excessive accumulation may trigger various diseases by promoting inflammation, viral infection, and tumor cell proliferation. Exercise has a significant ameliorative effect on these related diseases. Future research will focus on leveraging lipid droplets and their derivatives as signaling nodes to gain deeper insights into cellular signal transduction and the regulatory role of exercise in these processes.
Introduction
Lipid droplets (LDs) are ubiquitous organelles throughout evolutionary history. These organelles dynamically stockpile lipids and are primarily found within eukaryotic cells, as well as in certain prokaryotic organisms. While LDs are typically localized in the cytoplasm, specific cell types may as well contain them in the nucleus [1]. LDs share a common monolayer, which is integrated with specific proteins and surrounds the neutral lipids (see Figure 2) [2], 3]. Structurally, LDs feature a central reservoir of neutral lipids, also referred to as the oil phase [3]. For decades, these organelles were considered merely inert cytoplasmic lipid inclusions. However, in the last decade, LDs have been recognized as dynamic organelles and biological macromolecules [4]. LDs exhibit significant fluctuations between periods of dynamic synthesis, growth, and breakdown, processes that closely reflect cellular needs and environmental signals [5]. Although the dynamic properties of LDs have been extensively investigated in the existing literature, the heterogeneity of LDs across different tissues and organs remains poorly characterized.
In the 5th century BC, Hippocrates, often referred to as the father of medicine in ancient Greece, stated: “The various parts of the body, if used moderately and through habitual bouts of exercise, become healthy, well-developed, and delay aging; however, if they are not used or remain idle, they become susceptible to disease, growth defects, and accelerated aging [6].” This statement not only laid the theoretical foundation for considering exercise as a therapeutic method (exercise is medicine), but also revealed the ancient wisdom that exercise maintains an organism’s homeostasis through mechanical biological effects. Physical activity involves complex biological processes, activating multigenic interactions among cells, tissues, organs, and systems, with significant cross-talk occurring among them. Given the systemic and health-promoting nature of exercise, the concept of “exercise as medicine” continues to be integrated into clinical practice. Conversely, physical inactivity is a significant contributor to chronic illness and mortality. But, by the 21st century, public belief in the value of exercise for health has diminished significantly, rendering physical inactivity a major public health challenge [7]. Exercise has garnered considerable research attention due to its multisystem responses [8]. However, the mechanisms by which exercise influences LDs in various tissues to promote health remain poorly understood. In this article, we summarize existing research findings and propose potential mechanisms of action.
The summary of this article is presented in Figure 1.

Graphical representation of this study. Key points: (1) lipid droplets (LDs), synthesized on the endoplasmic reticulum and degraded via lipolysis and lipophagy, display tissue-specific distributions and play key roles in cellular homeostasis. (2) while moderate lipid droplet (LD) levels confer protection against lipotoxicity, their aberrant accumulation promotes pathogenesis across tissues by exacerbating inflammation and fibrosis, facilitating viral infection, and fostering oncogenesis. Physical exercise constitutes an effective non-pharmacological intervention that mitigates these pathological processes through pleiotropic mechanisms. (3) future studies should prioritize lipid droplets and their derivatives as signaling hubs to decipher their role in cellular signal transduction and exercise-mediated regulation. Figure created with BioRender.
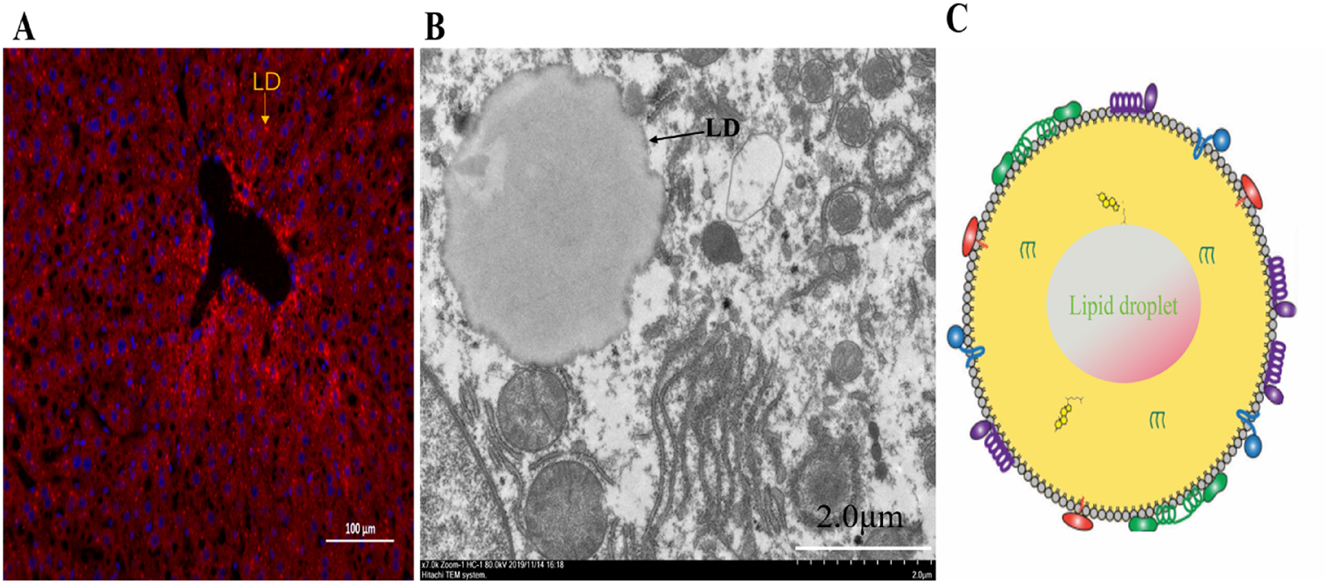
Lipid droplets (LDs). (A) Fluorescence microscopy image illustrates LDs stained red with Nile red inside the hepatic tissue of a C57BL/6 mouse (scale bar: 100 μm). (B) Transmission electron microscope showing a single LD inside the hepatic tissue of a C57BL/6 mouse (scale bar: 2.0 μm). Arrow heads: LDs (C) schematic diagram depicts the structural makeup of a LD, where the colored shapes denote proteins attached to the LD surface, situated within the phospholipid monolayer. The neutral lipid core comprises TAGs and SE.
Study selection and search strategy
The current body of literature on LDs primarily focuses on their involvement in obesity, cardiovascular diseases, metabolic syndrome, and insulin resistance, as well as their regulatory dynamics in response to exercise. While studies consistently conclude that excessive ectopic lipid deposition impacts health, findings on the impact of exercise on such lipid accumulation in different tissues, along with the properties of LDs in various tissue sites, are not universally consistent. Indeed, the effects of ectopic lipid deposition on health in diverse tissues remain contentious. Studies have reported that LDs as a buffering mechanism for lipids, mitigating lipotoxic cellular damage [2], whereas other studies indicate that excessive ectopic lipid deposition can lead to inflammation and the onset of various diseases [9], 10]. To clarify the confusion surrounding this significant subject, we performed a narrative review, incorporating studies of varying complexity and design across a broad range of topics. A literature search covered publications from 1894 to January 2025 in PubMed, Google Scholar, and Web of Science. The inclusion criteria comprised English-language empirical studies and review articles that: (I) Investigated LDs in different tissues/organs and elucidated the physiological mechanisms by which exercise influences LDs to prevent or treat lipid overload-induced diseases; or (II) Demonstrated that exercise significantly ameliorates disease phenotypes induced by excessive lipid accumulation, while suggesting that the protective effects may be mediated, at least in part, through exercise-induced modulation of LDs dynamics (e.g., turnover, lipolysis, lipid partitioning, or protein composition). Two investigators independently performed the initial screening of search results following the predefined eligibility criteria. The remaining articles were further examined by reviewing their titles and abstracts to identify and eliminate duplicate publications. Any disagreements regarding article were first discussed to reach consensus; unresolved discrepancies were then adjudicated by an independent third reviewer. we screened all available results, including studies related to LDs characteristics and the effects of exercise and LDs on health in different tissues (see Figure 3). Notably, we did not employ biased selection to exclude unfavorable data. This review explores and analyzes the effects of LDs on health across different tissues and evaluates exercise implications, and provides insights into potential future research directions.
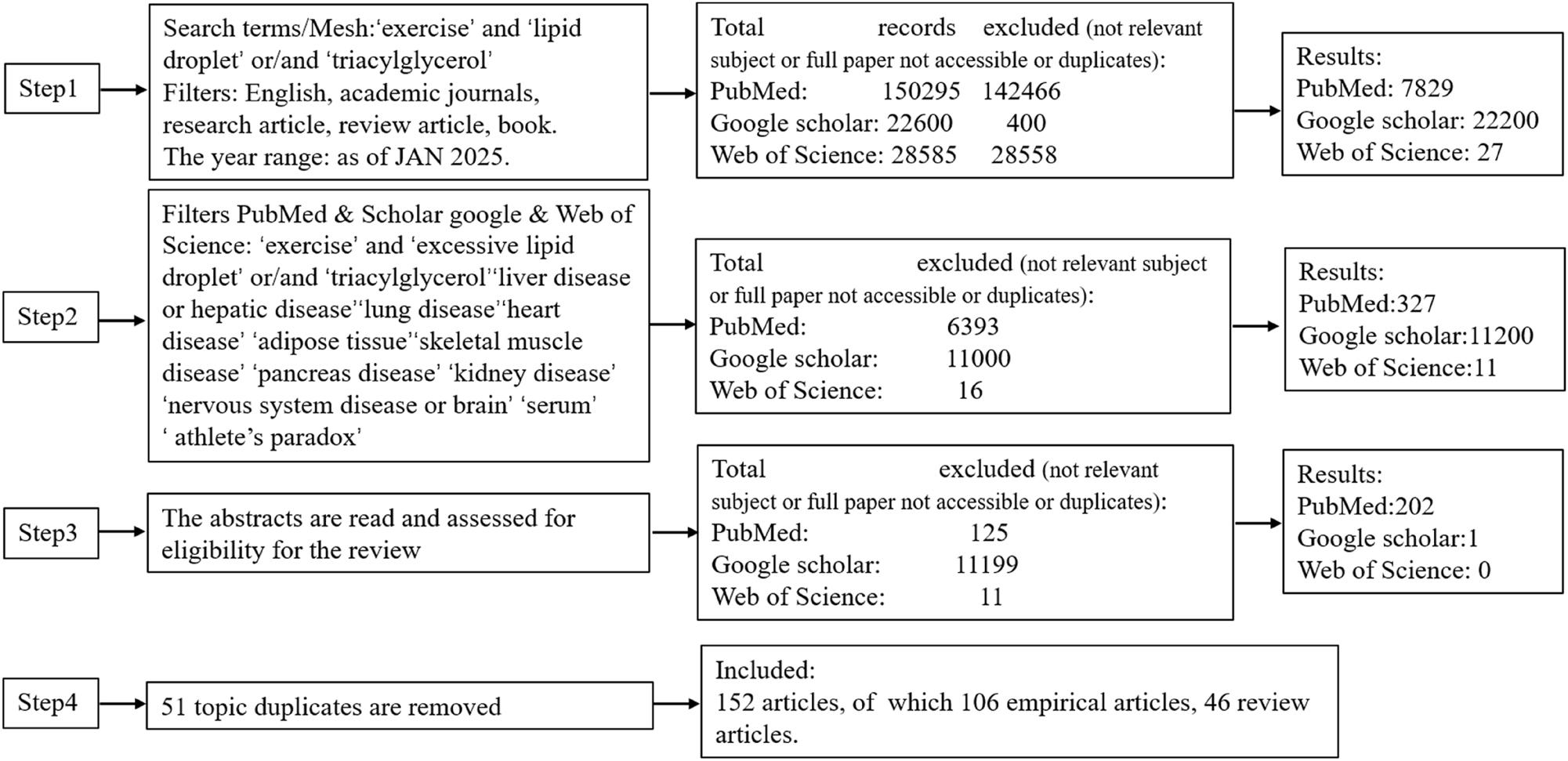
Flowchart of literature source selection.
Content
The origin and breakdown of LDs
The origin of LDs
In recent years, the origin of LDs has been gradually revealed. It is generally accepted that LDs biogenesis involves multiple steps, and is intimately related to the Endoplasmic Reticulum (ER). ER budding theory is a relatively mainstream theory.
First, the synthesis of triacylglycerol occurs in the endoplasmic reticulum.
Triacylglycerols (TAGs) were initially discovered in the early 1800s, with Richard Altmann’s early observation of triglyceride storage in cells dating back to the 1890s [11]. Yet, it wasn’t until around 1960 that Kennedy and his team clarified the biochemical mechanism responsible for the production of triglycerides [12]. This pathway utilizes glycerol phosphate and fatty acyl-CoA to produce glycerol lipids, including glycerophospholipids and TAGs. Additionally, another pathway involving monoacylglycerol is utilized for synthesizing triglycerides in both intestinal enterocytes and adipocytes. This pathway is essential for the reclamation of monoacylglycerols and diacylglycerols produced during triglyceride hydrolysis, assisting in their transformation back into triglycerides through a reesterification mechanism [13]. It is important to highlight that sterol esters are also generated within the endoplasmic reticulum. The majority of LDs comprise a combination of triglycerides and sterol esters. Nonetheless, genetic alterations in yeast facilitate the production of LDs that predominantly consist of either type of neutral lipid [14]. In comparison to research focused on TAG-rich LDs, there has been a relative paucity of exploration regarding the formation mechanisms of SE (sterol esters)-rich LDs.
Secondly, triacylglycerols phase separation and oil droplet biogenesis in the endoplasmic reticulum.
The biogenesis of LDs has been investigated through multiple experimental models, yielding several distinct mechanistic theories: (I) the membrane contacts site (MCS)-dependent formation theory [15]; (II) the de novo self-assembly hypothesis [16]; (III) the phospholipid remodeling-mediated mechanism [17]; (IV) the protein-nucleated assembly model [18]. Up to now, however, the mainstream theory posits that triglycerides could start an oil phase, thereby decreasing the entropy penalties linked to the disruption of the endoplasmic reticulum bilayer leaflets [19]. The emergence of an oil lens in the endoplasmic reticulum enables the accommodation of newly synthesized triglyceride, which consequently results in a relative reduction of triglycerides in other regions of the bilayer leaflet [20]. This hypothesis was informed by early experimental observations that model bilayers can only hold a finite amount of triglyceride in a dispersed state before the formation of oil droplets [21].
Finally, this is followed by slow formation of budding and emerging LDs, and growth and expansion of LDs via the incorporation of particular proteins. Although the origin theory of LDs provides a compelling framework, its limitations in elucidating the physiological functions of LD-associated proteins underscore the necessity for further investigation. The effects of exercise on the biogenesis of lipid droplets remain poorly characterized, highlighting a critical gap in current understanding.
The impact of exercise on LDs degradation
LDs can undergo degradation through two distinct pathways: (I) enzymatic lipolysis, mediated by enzymes like adipose triacylglycerol lipase and hormone-sensitive lipase, and (II) autophagic degradation, involving trafficking to autolysosomes and subsequent breakdown by lysosomal acid lipases [22]. During starvation, the hydrolysis of triacylglycerol is enhanced to provide sufficient free fatty acids for β-oxidation needed to satisfy the energy demands of cells. Moreover, lipophagy serves as an alternative energy source. PLIN2 (perilipin 2) is a critical regulator for lipophagy, and its expression is significantly downregulated by aerobic exercise via AMPK signaling pathway activation, this downregulation enhances lysosomal acid lipase (LAL) activity, promotes autophagosome biogenesis and autolysosome formation, and thereby augments lipophagic flux. Consequently, liberated free fatty acids are mobilized for β-oxidation to meet cellular energy demands [23]. Moreover, research has found that elevated levels of cellular triacylglycerol and cholesterol levels, alongside LDs accumulation, have been linked to the inhibition of lysosomal function [24]. While current research explores the effects of exercise on LD-associated proteins, autophagic lysosomes, and lipases, the impact on lipase substrates remains underexplored. Overall, exercise has been shown to facilitate LDs degradation, highlighting its potential role in lipid metabolism regulation. Exercise may further regulate the activity of lipolytic enzymes and the autophagosome-lysosome system through AMPKα-dependent PLIN2–LIPA axis, facilitating lipolytic processes [23].
The tissue specificity and functions of LDs
Recent research reveals that LDs are highly dynamic organelles with intricate functional interconnections, extending beyond their conventional role as mere fat reserves. Cells primarily defend against lipotoxicity by removing damaged or excess lipids. In this process, LDs play a crucial role as specialized organelles that regulate lipid uptake, storage, trafficking, and oxidation. Beyond their metabolic functions, LDs serve as dynamic signaling platforms that influence various cellular processes, including nuclear function and gene expression. Additionally, LDs facilitate intercellular communication by releasing bioactive lipid mediators. Furthermore, LDs serve as immune-responsive organelles that dynamically engage with the endoplasmic reticulum and mitochondria through membrane contact sites. These interactions position LDs as pivotal regulators of immunometabolism and inflammatory signaling, enabling their modulation of immune cell activity [25]. Further physiological roles may yet to be uncovered through systematic investigation.
Structurally, LDs are closely associated with multiple organelles. Notably, a 10–15 nm neck connects LDs to the endoplasmic reticulum, enabling lipid exchange [26]. Proteins associated with LDs (LDAPs) are critical regulators of LDs turnover, significantly impacting lipid homeostasis [27]. An alternative possibility is that LDs may form intimate associations with other organelles, although such interactions remain to be identified.
Lipid intermediates, including diacylglycerol, ceramide, and fatty acyl-CoA, contribute to lipid-induced insulin resistance. Dysregulation of lipid storage, lipolysis, and fatty acid oxidation, along with alterations in LDAPs, may contribute to insulin resistance [28]. Even though our research found that exercise has an impact on ceramides, the molecular diversity and physiological roles of ceramides remain incompletely characterized.
In summary, these diverse functions underscore the versatility of LDs across different biological contexts. Notably, LDs exhibit distinct morphological and physiological properties depending on their tissue-specific localization (See Table 1), adipose tissue contains the largest LDs, with diameters exceeding 100 μm. In contrast, other organ tissues demonstrate a broad distribution of LD diameters ranging from submicron scale (<1 μm) to several tens of micrometers. Furthermore, LD abundance varies substantially across tissues, with some exhibiting high LD density while others contain relatively few. This size and distribution variation reflect specialized functional adaptations in lipid storage and metabolism across different tissues.
Tissue specificity of LDs.
| Tissue | Morphological characteristics of LDs | Physicochemical properties of LDs |
|---|---|---|
| Adipose tissue | Nearing 100 μm, one or a few droplets filling almost the entire cell. As pre-adipocytes transition into mature adipocytes, gradually gather small LDs [29]. | Early-stage adipocytes exhibit elevated de novo lipogenesis and LD formation, which decline at later differentiation stages [29]. Enhanced pyruvate oxidation in peridroplet mitochondria increases malonyl-CoA production, promoting lipid synthesis while inhibiting lipolysis and lipid oxidation [30]. |
| Heart | Small LDs, the diameter ranges from 0.3 to 1.5 μm [31]. | Obtain fatty acids through lipoprotein uptake or endogenous triglyceride hydrolysis [32]. Low TAG levels and rapid LDs turnover [33]. |
| Skeletal muscle | In healthy individuals, LDs range from 0.3 to 1.5 μm, while genetic modifications increase the minimum size to 3 μm. Type 1 muscle fibers exhibit larger LDs than type 2 fibers [31]. | Intramyocellular LDs exhibit heterogeneity in size, localization, lipid composition, and protein associations. They serve as energy reserves, complementing glycogen and supplying fuel via the bloodstream [31]. |
| liver | LDs range from 1 μm to several tens of micrometers, with uneven distribution influenced by portal and hepatic vein blood flow. Periportal hepatocytes initially contain fewer LDs [34]. | Excessive lipid accumulation in hepatocytes under pathological conditions [35] is driven by mitochondrial expansion and oxidative stress [36]. |
| Kidney | LDs are scarce and primarily localized in proximal tubule cells, with minimal presence in glomeruli [37]. | Total ceramide levels inversely correlate with triglyceride quantities. Moderate triglyceride storage in LDs protects cells from harmful metabolite accumulation [37]. |
| Lung | Small LDs within cytoplasm in blood vessels and lung tissue, exhibiting a consistent structural composition in blood vessels [38]. | LDs exhibit significant mechanical stability in lung cells, alveolar walls, and blood vessels [38]. |
| Pancreas | Size of LDs (≤3 μm) and supersized LDs (≥3 μm) [39]. | LDs influence beta-cell sensitivity to free fatty acids, while aberrant cholesteryl ester and LDs accumulation impact pancreatic cell function [39]. |
| Nervous system | Small LDs form and accumulate in abundance within the glial cells of the cortex [40]. | Hypoxia and metabolic disturbances enhance LD formation in astrocytes [36]. Blood-brain barrier permeability allows triglyceride-rich lipoproteins to enter the brain, further promoting LD accumulation [41]. |
The role of exercise in LDs-associated diseases
LDs serve as reservoirs for various bioactive molecules, including vitamins, signaling precursors, and proteins, along with their role in membrane synthesis, is what LDs engage in. Additionally, LDs are key players in cellular signaling, protein management, and the response to cellular stress [42]. Despite these insights, the interplay between excessive LDs accumulation, exercise, and disease pathophysiology remains incompletely understood across different tissues.
Exercise training has been shown to reduce lipid intermediates, such as short-chain ceramides in liver, in obese mice [43], even without a concurrent reduction in ectopic lipid storage. Regular physical activity provides widespread health benefits, potentially by mitigating lipotoxicity through enhanced lipid turnover and improved LD quality [44]. Additionally, exercise stimulates the secretion of myokines from skeletal muscle, which enter circulation and modulate LD dynamics in non-exercising organs. However, the tissue-specific effects of these adaptations remain insufficiently characterized, limiting their broader applicability. Moreover, the precise mechanisms underlying these effects have yet to be systematically summarized, leaving significant research gaps to be addressed. The review synthesizes the latest findings on the role of LDs in both health and disease, emphasizing their function in mitigating cellular stress. We present current knowledge on LD contact sites and their associations with genetic, metabolic, and infectious disorders (see Table 2). Furthermore, we discuss emerging therapeutic strategies targeting LD formation, expansion, and degradation, with potential applications in conditions such as steatosis, viral infections, inflammation, insulin resistance and cancer.
The contact site protein of LDs in diseases.
| Organelles/organism | Contact site protein | Disease | Description | Refs |
|---|---|---|---|---|
| Endoplasmic reticulum | Seipin/SNX14/VPS13A、VPS13C/Rab18 | Berardinelli-Seip congenital generalized lipodystrophy type 2, neurological seipinopathies, progressive encephalopathy with or without lipodystrophy, autosomal recessive spinocerebellar ataxia 20, Chorea acanthocytosis, McLeod syndrome, early onset Parkinson’s disease, Warburg Micro syndrome, hereditary spastic paraplegia | Genetic diseases | [15], [45], [46], [47] |
| Peroxisome | Spastin/ABCD1 | Hereditary spastic paraplegia, adrenoleukodystrophy | Infectious diseases | [48], 49] |
| Replication organelle | Poliovirus proteins 2B and 2C | Poliomyelitis | Infectious diseases | [50], 51] |
| Autophagosome | DENV protein NS4A | Dengue fever | Infectious diseases | [52] |
| The endoplasmic reticulum includes locations for viral replication and assembly. | NS5A | Hepatitis C | Infectious diseases | [53], 54] |
| Phagosome | LAM and PIM | Tuberculosis | Infectious diseases | [55] |
| Bacterial inclusions | Chlamydia protein Lda3 | Chlamydia infection | Infectious diseases | [56] |
| Parasitophorous vacuole | Rab7 | Toxoplasmosis | Infectious diseases | [56] |
| Mitochondria, plasma membrane, and endoplasmic reticulum, and fragmentation of the Golgi | Extended synaptotagmin 2, VPS13A, VPS13D | Non-alcoholic fatty liver disease | Metabolic liver disease | [57] |
Excess LDs promote pathogen infection, while exercise mitigates the risk
Numerous intracellular pathogens, which encompass a range of viruses, bacteria, and parasites, exhibit a specific affinity for host LDs throughout their life cycle. For instance, certain viruses, including the hepatitis C virus (HCV), dengue virus, and rotaviruses, exploit LDs as critical sites for assembling viral components. This strategic utilization allows these viruses to enhance their replication and viability within the host. In addition to viruses, various bacteria, such as mycobacteria and chlamydia, along with parasites like trypanosomes, also take advantage of host LDs, primarily for nutritional gain. By utilizing these lipid reserves, they can efficiently fulfill their metabolic needs, which aids in their survival and proliferation within the host organism. Furthermore, the engagement of LDs by these intracellular pathogens may signify a sophisticated anti-immunity strategy, enabling them to avoid the host’s immune defenses and establish a successful infection [58].
HCV is the most recognized pathogen closely associated with LDs. LDs are believed to be potential sites for the assembly of the virus during the replication of HCV [59]. The process of assembling infectious HCV particles consists of multiple phases, such as the formation of the nucleocapsid, budding into the endoplasmic reticulum, and the maturation of virions. The capsid’s core protein forms a robust association with LDs and attracts nonstructural proteins to their vicinity to facilitate virus production [59]. It is likely that the assembly of HCV occurs in locations that require the interaction of the endoplasmic reticulum with LDs [60]. Recent high-resolution imaging research conducted in cell culture systems reveals the targeted recruitment of endoplasmic reticulum membranes that encase LDs, consequently forming a membranous structure that connects the replication and assembly of the HCV [53]. Additionally, HCV infection can induce endoplasmic reticulum stress, resulting in the Ca2+ release, causing mitochondrial impairment. This sequence of occurrences initiates oxidative stress, enhances lipogenesis [61]. The Core protein of HCV can also be effectively directed to LDs, independent of virion assembly, resulting in a redistribution of LDs and the progress of hepatic steatosis [62]. This occurrence partially clarifies the reason metabolic dysfunction-associated fatty liver disease (MDAFLD) is a prominent feature in individuals suffering from chronic hepatitis C, and research has shown that antiviral therapy, which eliminates the HCV, results in a marked decrease in liver steatosis [63]. A key feature of hepatocellular carcinoma is their increased lipogenesis, alongside altered lipid metabolism [64]. Liver lesions that contain fat are frequently found in patients diagnosed with hepatocellular carcinoma [65], the lipogenesis pathway experiences heightened activation, while the process of fatty acid oxidation is concurrently reduced [66]. Due to the rapid tumor growth associated with hepatocellular carcinoma, these cancer cells have an elevated demand for fatty acids to facilitate their proliferation [67]. When exposed to hypoxic environments, the catabolism of LDs is restricted by ATGL, which is the key lipase for lipolysis, resulting in the buildup of LDs and consequently altering cellular metabolism, thereby inducing resistance to apoptosis [68]. HCV patients generally receive biological therapy or antiviral treatment. However, the human studies have shown that aerobic exercise can reduce body fat and improve lipid metabolism in patients with Hepatitis C virus infection, and the clinical course of the disease demonstrated significant amelioration, accompanied by marked improvement in hepatic functional parameters [69]. Nevertheless, there are few studies investigating the impact of resistance exercise or forms of exercise in HCV infection. The exact molecular mechanisms that drive this effect require further empirical validation. Exercise-induced LDs degradation provides novels insight into reducing the risk of HCV infection.
Most pathogen research has focused on identifying specific species that may contribute to the development of human diseases. However, given that bacterial functionality is not only based on their own genetic information but also on interactions with other microorganisms, the use of microbial consortia can be more effective in treating pathogen-induced infectious diseases. Existing studies have shown that exercise can regulate the structure and interactions of pathogens by improving host lipid metabolism, thereby reducing their pathogenicity [70]. This mechanism may partially explain the preventive effect of exercise intervention on infectious diseases. Additionally, existing literature indicates that the moderate intensity and duration of exercise, along with scientific and healthy diet, significantly inhibit both the onset and progression of pathogen infections. Notably, current studies endorse a “J” curve relationship that links the risk of pathogen infection with escalating levels of exercise workloads. The alterations in immune function triggered by exercise may offer a physiological explanation for the “J” curve phenomenon [71]. However, the molecular mechanisms by which exercise, including aerobic exercise and resistance training, prevents and combats pathogen infections through LDs degradation remain inadequately understood, highlighting a critical research gap that warrants further investigation.
Exercise regulates lipid metabolism to reduce LDs-related skeletal muscle disease risk
Previous studies employing transmission electron microscopy have identified two separate reservoirs of LDs within skeletal muscle cells: one situated beneath the sarcolemma and another found between the myofibrils. The skeletal muscle of chronically trained elite athletes demonstrates significant ectopic lipid accumulation, manifesting as a marked increase in intramyocellular lipid (IMCL) droplet content. The paradoxical coexistence of high IMCL accumulation and improved insulin sensitivity is referred to as the “athlete’s paradox.” Despite elevated IMCL accumulation, athletes demonstrate superior insulin sensitivity relative to two distinct groups: (I) insulin-resistant individuals with similar IMCL deposition, and (II) lean, sedentary individuals with reduced IMCL content [72]. In the past few years, significant research efforts have been directed toward elucidating the paradoxical metabolic effects associated with IMCL accumulation. The prevailing hypothesis posits that IMCL itself is not intrinsically lipotoxic. However, existing studies remain inconclusive. For instance, while one investigation reported significantly lower intramuscular saturated diacylglycerol (DAG) levels in athletes compared to a lean sedentary control group [73], another study observed elevated DAG concentrations in trained individuals [74]. These conflicting findings suggest that the athletes’ paradox may involve differential regulation of specific lipid intermediates. As an alternative mechanistic explanation, it has been proposed that the dynamic turnover of intramuscular lipid stores, rather than their absolute content, serves as a critical determinant of lipotoxicity [75]. Furthermore, excessive IMCL deposition disrupts mitochondrial bioenergetics and activates pro-inflammatory signaling, collectively promoting the development and progression of skeletal muscle disease [25], 76].
Diseases linked to elevated intramyocellular lipids levels include age-related sarcopenia [77], spastic paraplegia [77], and related disorders. In these cases, the increase in LDs accumulation within skeletal muscle may primarily result from the associated muscle fiber atrophy involving diminished cross-sectional area and fiber quantity and insufficient mechanical stimuli. Variations in muscle mass are determined by the net balance of protein, organelle, and cytoplasmic constituent content. Dysferlinopathy, a variant of muscular dystrophy, is characterized by intramyocellular lipids accumulation and an early increase in the expression of the lipogenic transcription factor C/EBP-δ before related pathological symptoms [78], indicating a premature disturbance in lipid metabolism within this particular condition. Moreover, emerging evidence indicates that LDs dysfunction may contribute to hereditary spastic paraplegia. Research has demonstrated that spastin can interact with LDs and influence lipid metabolism in skeletal muscle tissues [79]. Despite limited research on the exercise-C/EBP-δ/spastin association, exercise is strongly rationalized as a potent modulator of skeletal muscle mass and function and a regulator of lipid metabolic pathways [80]. Current evidence substantiates that different types of exercise modalities effectively reduce IMCL accumulation in skeletal muscle, such as, endurance training significantly reduced the numerical density of LDs in both central and peripheral regions of type I and IIα muscle fibers, a process mediated through AMP-activated protein kinase (AMPK) and calcium/calmodulin-dependent protein kinase II (CaMKII) signaling pathways in skeletal muscle tissue [81]. While, aerobic exercise [82] and chronic high-intensity interval training (HIIT) [83] have been demonstrated to regulate the secretion of myokines (e.g., irisin) [82] and adipokines (e.g., leptin and adiponectin) [83], ameliorate myosteatosis and degenerative changes, and enhance muscle mass and functional capacity, thereby attenuating sarcopenia progression. Furthermore, acute exercise enhances LD-mitochondria contacts, facilitating lipid substrate channeling to augment lipid oxidative lipid metabolism and improve cellular function in skeletal muscle, and reducing the risk of skeletal muscle diseases [84].
Exercise attenuates LDs-associated kidney disease risk
Previous research has demonstrated ectopic lipid deposition in various renal diseases, underscoring the pivotal role of renal lipid metabolism in disease pathogenesis [85]. The primary avenue for decreasing lipid levels in the kidney is through fatty acid oxidation [86]. Ectopic lipid accumulation within renal compartments induces structural and functional alterations in mesangial cells, podocytes, and proximal tubular epithelium – key cell types governing nephron integrity. This lipid overload triggers ER stress, resulting in upregulation of TNF-α and IL-6, which potentiates reactive oxygen species overproduction, culminating in renal cytotoxicity, inflammatory cascades, and progressive fibrotic remodeling [86], 87]. An ever-growing body of clinical research highlights the positive impact of physical activity across all stages of chronic renal disease. A 12-week high-intensity interval training program (4 intervals of 4 min at 80–95 % of peak heart rate) has been proven to be a practical, safe choice and has shown positive effects on 14 individuals suffering from chronic kidney disease in stages 3 and 4 [88]. A meta-analysis of 11 randomized controlled trials included 362 individuals with stage 3 or 4 chronic kidney disease, Vanden Wyngaert et al. [89] demonstrated benefits for renal function that an 8-month aerobic exercise program, compared to standard therapy, improved estimated glomerular filtration rate by +2.16 mL/min/1.73 m2. Previous studies have demonstrated that both endurance exercise and resistance training can significantly reduce serum sphingolipids (e.g., ceramides) [43]. Evidence indicates that sphingolipid-mediated perturbations in podocyte function represent a key mechanism altering glomerular filtration barrier permeability [90]. Although this highlights the role of exercise in regulating lipid and enhancing kidney function, the signaling pathways through which exercise modulates renal LDs remain incompletely elucidated in kidney disease model, whether in animal models or human populations. Further investigation is needed to establish whether exercise-mediated reductions in cyclic and renal sphingolipids (e.g., ceramides, Sphingosine-1-phosphate) confer protective effects on podocyte function and glomerular filtration rate. Moreover, critical knowledge gaps remain regarding the temporal patterns of exercise-induced benefits, differential effects across exercise modalities, and the precise optimization of exercise load/intensity for renal protection.
Anti-inflammation serves as a potential mechanism by which exercise alleviates LDs-associated lung disease
Lipid depositions were initially characterized morphologically in pneumonia related to tuberculosis near the area of caseous necrosis in the early 20th century, and later referenced in studies detailing unusual lung morphology [91]. In particular, within inflamed lung tissue, we observed small LDs situated in the cytoplasm of cells, as well as large, homogeneous LDs within blood vessels that lacked surrounding membranes. These latter droplets could potentially obstruct blood flow and disrupt gas exchange, which is critical to various forms of pneumonia [92], thereby playing a significant role in disease progression. Abnormalities in lipid metabolism might represent an early occurrence in lung inflammation, with tissue in the upper lung regions, while still appearing macroscopically normal, showing the initial morphological changes induced by pneumonia. This is supported by the morphological and physiological evidence indicating that abnormal lipid deposition could significantly influence the pathological processes associated with pneumonia [38].
LDs are essential for nutrient storage, but play a vital role in tumor growth and proliferation. The frequently elevated expression of the LDs shell protein PLIN3 within the lungs is linked to the pathological characteristics of lung cancer. This highlights the significance of LDs in relation to lung cancer. A more comprehensive understanding of lipid biology related to lung cancer could enhance the creation of novel therapeutic approaches, such insights can contribute to the formulation of new therapeutic approaches [93]. For patients with pneumonia or lung cancer, engaging in exercise serve as effective nonpharmacological approaches. Exercise may help mitigate pneumonia and other lung diseases by reducing LDs-driven inflammation, which is supported by empirical evidence. Aerobic exercise notably reduced the total cell count, including neutrophils and lymphocytes, in bronchoalveolar lavage fluid. Furthermore, aerobic exercise reduced inflammatory cell infiltration and significantly lowered pro-inflammatory cytokines (IL-1β, IL-6, CXCL1, and TNF-α) while enhancing IL-10 (anti-inflammatory cytokine) expression [94]. Combining moderate intensity aerobic with resistance training partially restored the expression of interleukin-10 in airway epithelium of rats with pulmonary inflammation, suggesting an anti-inflammatory mechanism underlying the observed therapeutic effects [95]. The evidence suggests that investigating exercise-induced benefits will advance the development of exercise-based therapeutics for lipid disorder-associated pulmonary diseases, potentially stimulating broader scientific engagement in this field.
Exercise lowers MDAFLD risk by modulating LDs autophagy
The liver stands as one of the most critical and multifunctional metabolic organs within the human body. Lipid metabolism, including lipid droplets, is a highly orchestrated process requiring coordinated interorganellar cross talk to maintain cellular homeostasis. Disruption of this equilibrium may induce organellar damage and promote the occurrence and development of diseases (e.g., MDAFLD, hepatitis, liver fibrosis, liver cancer). MDAFLD is characterized by hepatic steatosis accompanied by at least one of three factors: obesity/overweight, type 2 diabetes mellitus, or metabolic dysregulation [96]. As a newly defined condition, its precise epidemiology remains unclear, but it is estimated to affect about 25 % of the global population [97]. Wei Zhu identified exercise and dietary intervention as the most effective approaches to improving hepatic lipid metabolism [98]. Additionally, Chunlu Fang et al. revealed that 3-month aerobic exercise intervention reduced hepatic LDs accumulation and ameliorated non-alcoholic fatty liver disease (NAFLD was redefined as MDAFLD by international expert consensus) in a high-fat diet-induced mouse model. This beneficial effect was mediated through PLIN2-LIPA axis-regulated lipophagy) [23]. This article elucidated the mechanisms by which aerobic exercise promoted lipophagy but did not assess the effects of resistance training or compare the effectiveness of different exercise types. Future studies should explore the comparative effectiveness of various exercise modalities to better understand their effectiveness in improving MDAFLD.
Anti-inflammatory pathways or enhanced islet cell function: mechanisms underlying exercise-induced reduction of pancreatic cancer risk
Pancreatic cancer is a highly fatal malignancy and is expected to rank as the second leading cause of cancer-related mortality by 2030 [99]. LDs are pivotal in tumor cell metabolic reprogramming, promoting pancreatic cancer cell invasion and migration [100]. Although the precise mechanisms by which exercise reduces pancreatic cancer risk through modulation of pancreatic lipid droplets remain incompletely elucidated, it has been confirmed that exercise induces the alleviation of toxicity and apoptosis in pancreatic islet cells through transcriptional regulation in pancreatic islets, increases energy expenditure, thereby disrupting the carcinogenic process and reducing the incidence of pancreatic cancer [101]. Physical activity slows early obesity-related pancreatic ductal adenocarcinoma progression, highlighting its preventive potential. Its antitumor effects involve reduced tumor-associated inflammation and fibrosis, lower circulating inflammatory cytokines induced by a high-fat diet, and IL-15 pathway activation in white adipose tissue [102]. The findings provide evidence supporting the anti-carcinogenic potential of exercise, but the underlying mechanistic pathways of different forms of exercise require systematic elucidation through rigorously designed in vivo studies and complementary in vitro experiments.
Exercise reduces the risk of LDs-associated brain diseases
LDs are closely associated with various diseases of the nervous system, such as Alzheimer’s disease, hereditary spastic paraplegia, Parkinson’s disease, Huntington’s disease, multiple sclerosis, stroke and glioblastomas (see Table 3). The excessive accumulation of intracellular lipids induces an inflammatory phenotype, which ultimately impairs the repair processes within the central nervous system. This inflammatory condition can lead to prolonged damage and reduced efficacy in recovery and regeneration efforts following injury or disease. The LD-associated proteins PLIN2 is essential for protecting LDs from degradation via lipolysis, thereby maintaining the phagocytes’ capacity to effectively process lipids derived from myelin. In the absence of PLIN2, there is an increase in LDs turnover within foamy phagocytes, facilitating a shift toward a less inflammatory phenotype. This transition back to a more anti-inflammatory state could enhance the functionality of phagocytes and their overall capacity to support central nervous system repair mechanisms. Consequently, modulating PLIN2 levels may offer a potentially effective treatment approach for alleviating inflammation and promoting recovery in central nervous system injuries [127]. Studies have indicated that PLIN2 is implicated in mediating the exercise-induced lipid metabolic improvements in the hypothalamus of Goto-Kakizaki rats [128]. While exercise-induced cognitive improvements are well-documented, including mechanisms such as APOEε4 modulation or LD-targeted therapies, the specific LD-related signaling pathways underlying exercise’s neuroprotective effects in neurological disorders (e.g., Alzheimer’s disease, Parkinson’s disease) require further investigation (see Table 3), its intermediate pathway remains largely unexplored.
Exercise improves brain-related diseases closely associated with LDs.
| Diseases | Lesion characteristics | The association with LDs | The association with exercise |
|---|---|---|---|
| Alzheimer’s disease | Impacts cognitive functions that influenced by neurogenesis: Hippocampus-dependent learning and memory [103]. | In the subventricular zone niche in the 3xTg mouse: a Significant reduction in subependymal cells and the accumulation of large LDs in the ependyma, alterations in metabolism [103]. | Aerobic exercise and Apolipoprotein E (APOE)ε4 interact to promote synaptic/neuronal health, thereby preventing Alzheimer’s disease [104]. |
| Hereditary spastic paraplegia | A range of inherited neurological conditions marked by significant lower-extremity spasticity, largely stemming from the degeneration of corticospinal neurons [105]. | Endoplasmic reticulum-resident proteins promote LDs formation. Impaired fatty acid transport from LDs promotes hereditary spastic paraplegia [106]. | Physical activity (squatting exercises, kneeling position exercises, movements involved in bathing) is beneficial in halting further deterioration of lower limb function, and in maintaining the individual’s capacity to carry out daily living activities [106]. Yet, the intermediate pathway involved in this process requires further investigation. |
| Parkinson’s disease | The second most prevalent neurodegenerative condition, motor symptoms: Tremors, stiffness, and slowness of movement. Non-motor symptoms: Sleep issues and a decline in cognitive functions [107]. | A lipidopathy lipid dysfunction is a contributing factor [108]. drugs that target lipid-related mechanisms demonstrate significant potential for mitigating toxicity associated with Parkinson’s disease [109]. | Six months of aerobic exercise (3 × /week, 30–45 min) may slow corticostriatal network degeneration and enhance cognitive function, thereby improving Parkinson’s disease [110]. However, whether the intermediate pathway modulates lipid metabolism and subsequently mitigates Parkinson’s disease through this metabolic regulation remains to be elucidated. |
| Huntington’s disease | A neurodegenerative disorder: Difficulties in exercise skills and cognitive functions. A dominant trinucleotide repeat expansion in the huntingtin gene leads to an excessive accumulation of polyglutamine residues in the huntingtin protein [111]. | LDs accumulation in most cases of neuronal loss [112]. This disease driven by the LDs dysfunction [113]. | A 12-week progressive exercise (3 × /week) has a therapeutic effect [114].The specific molecular mechanisms require further investigation. |
| Multiple sclerosis | A persistent condition marked by neuroinflammation and neurodegeneration, featuring the axonal demyelination and neuronal loss in the central nervous system [115]. | Myelin consists of ∼80 % lipids, with LDs enriched in active demyelination sites. During demyelination, LDs accumulate in infiltrating macrophages and microglia [116]. Elevated triglyceride and LD levels in motor neurons [117]. | Exercise-induced brain adaptations: Connectivity, neuroprotection, and remyelination [118]. The mechanistic details of its intermediate pathway have not been fully elucidated. |
| Stroke | Ischemic stroke, caused by reduced blood and oxygen supply, leads to cell death primarily through glutamate-induced excitotoxicity [119]. | Blood-brain barrier disruption during stroke increases lipoprotein influx, promoting LD formation [120]. Lipid accumulation in microglia worsens ischemic damage and impairs recovery [121]. | High-intensity interval exercise promotes LD degradation in microglia, while lactate from aerobic exercise may aid in restoring inhibitory control post-stroke [122]. |
| Glioblastomas | Highly aggressive glial-derived central nervous system tumors: Glioblastoma and oligodendroglioma [123]. | LDs drive aggressive brain tumor progression [124]. Glioblastomas upregulate DGAT1, increasing neutral lipid production and tumor progression [125]. | LD-targeted therapy and a 6-min walk exercise in glioblastoma treatment and support neural regeneration [126]. |
Summary: LDs in disease pathogenesis
In summary, excess LDs accumulation is implicated in the pathogenesis of multiple diseases, by facilitating viral infection, inflammation, and fibrosis. To date, although no specific exercise guidelines for patients with LDs-associated diseases are available, certain forms of exercise have been proven to have ameliorative effects. Even if certain molecular mechanisms have yet to be fully validated, extensive research has established the positive impact of exercise on inflammatory and fibrosis regulation, lipid metabolism, LD-associated proteins, immune function, glomerular filtration rate, insulin sensitivity, and the activation of key signaling pathways, including IL-15, interleukin-10, AMPK, and CaMKII. These findings underscore its therapeutic potential for disorders involving aberrant LD accumulation (see Figure 4).2
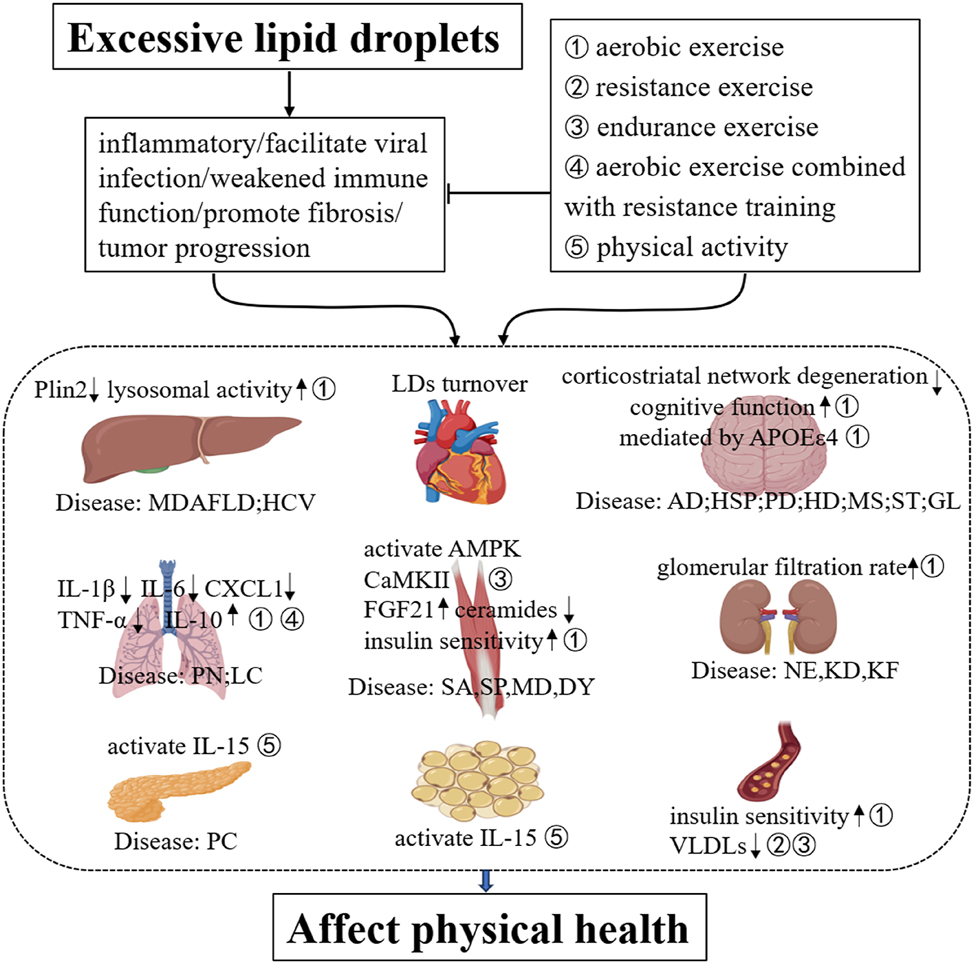
Postulated chronology of excessive LDs and exercise in health. (1) ①②③④⑤corresponding to different exercise modalities; (2) diseases: (Liver) MDAFLD (metabolic dysfunction-associated fatty liver disease), HCV (hepatitis C virus); (lung) PN: pneumonia, LC: lung cancer; (skeletal muscle) SA: sarcopenia, SP: Spastic paraplegia, MD: muscular dystrophy, DY: dysferlinopathy; (kidney) NE: nephritis, KD: kidney disease, KF: Kidney failure; (pancreas) PC: Pancreatic cancer; (brain) AD: Alzheimer’s disease, HSP: Hereditary spastic paraplegia, PD: Parkinson’s disease, HD: Huntington’s disease, MS: multiple sclerosis, ST: stroke, GL: glioblastomas.
The response of lipid to exercise
Regular, suitable aerobic exercise can effectively limit the buildup of surplus lipids in different tissues and cells while ameliorating lipid metabolic disorders. The proposed mechanisms involve increased energy expenditure, enhanced lipid oxidation, reversal of lipid deposits, and improve insulin sensitivity [73], 129]. Empirical evidence includes: (I) a 12-week exercise intervention significantly remodeled skeletal muscle lipid partitioning, as evidenced by elevated IMCL content coinciding with reduced DAG and ceramide concentrations. Crucially, the morphology and spatial distribution of LDs serve as key determinants of skeletal muscle insulin sensitivity. Specifically, the characteristic storage pattern in untrained individuals – featuring fewer, larger subsarcolemmal LDs in type II fibers-is associated with insulin resistance. In contrast, the trained phenotype demonstrates a protective lipid distribution pattern, with numerous smaller LDs localized in the intramyofibrillar region of type I fibers, which maintains insulin sensitivity [130]; (II) the benefits of exercise in improving lysosomal activity – particularly regarding the fusion of lysosomes and autophagosomes, acidification processes, and levels of acid lipase – thereby improving lipid degradation during the final phases of lipophagy [131]; (III) exercise stimulates the production of fibroblast growth factor 21 (FGF21) in muscle tissue, which in turn promotes hepatic lipophagy, and this process facilitates lipid clearance by activating the AMPK signaling pathway [132]; and (IV) conversely, sustained elevated plasma insulin levels, lack of exercise promotes LDs accumulation in hepatocytes and striated muscle. This accumulation hinders the translocation of glucose transporters, leading to heightened insulin resistance linked to obesity, potentially further facilitating the buildup of LDs [133].
The intramyocellular neutral lipids and several proteins essential for developing the ability to undergo lipid remodeling are affected by acute training, resistance exercise, short-term sprint interval workouts, and moderate-intensity continuous training (see Table 4). Different types of exercise exhibit distinct physiological effects.
Impact of various exercise modalities on intracellular lipid dynamics.
| Subjects | Research design | Primary result | Refs |
|---|---|---|---|
| Acute exercise (AE) effects on IMCL | |||
| Sixteen sedentary males | Muscle biopsies pre and post 60-min cycling at 65 % VO2max both pre- and post- a 6-week intervention of either sprint interval training or endurance exercise. | (i) Resting IMTG increased post- 6-week training; (ii) pre-training AE reduced IMCL by 15–17 % in type 1 fibers; (iii) post-training AE further decreased IMCL by 27–43 % in type 1 fibers. | [134] |
| Ten individuals | Muscle biopsy (triceps brachii/vastus lateralis) pre- and post-training 20-km cross-country skiing (∼57 min). | (i) AE reduced LD density but not size; (ii) IMCL decreased by 53 % in arms but remained unchanged in legs. | [135] |
| Twenty-one male participants | Muscle biopsies pre- and post-60-min cycling at 50 % VO2max. | (i) AE decreased IMCL by 20–30 %; (ii) In older overweight individuals, exercise increased LD size by ∼ 25 % in the sub-sarcolemma and by ∼7 % in the intramyofibrillar region. | [136] |
| 44 individuals (n=14 obese, sedentary controls, n=15 type 2 diabetes mellitus (T2DM), n=15 endurance-trained) | Muscle biopsies pre- and post-90-min cycling at 50 % VO2max. | (i) AE decreased IMCL exclusively in the trained participants (approximately 25 %). (ii) AE had no significant impact on IMCL in obese or T2DM groups. (iii) the fractional synthesis rate of IMCL increased during AE, but notably dropped in recovery for T2DM and obese individuals. | [137] |
| Short-term sprint interval training and moderate-intensity continuous training effect on IMCL (training adaptation) | |||
| Sixteen sedentary obese males | Muscle biopsies pre- and post-4 weeks of either short-term sprint interval training exercise (4–7 × 30 s sprints,3 × /week) or moderate-intensity continuous training (60 min≥time≥40 cycling at<65 % VO2peak, 5 × /week). | (i) PLIN2 and PLIN5 increased in type I fibers, PLIN3 increased in type I, II fibers. (ii) Unchanged total LDs, increased LD-mitochondria association. (iii) ceramide decreased, diacylglycerol levels remained stable. | [138] |
| Resistance training effect on IMCL (training adaptation) | |||
| Thirteen sedentary males | Muscle biopsies pre- and post-6 weeks resistance training. | Post-resistance training: Increased LDs and PLIN2, PLIN5 in type I, II fibers. | [139] |
Table 4 is shown here. Table 4 Impact of various exercise modalities on intracellular lipid dynamics.
The lipid and lipoprotein dynamics in response to acute exercise
The acute effects of endurance exercise temporarily lower triglyceride levels while increasing HDL-C (high-density lipoprotein cholesterol) levels. These changes are likely associated with total energy expenditure; however, the existing evidence remains inadequate to establish whether these effects are primarily driven by caloric expenditure, exercise intensity, or a combination of both factors. Studies examining exercise cessation suggest that the elevated HDL levels seen in highly active individuals cannot be solely attributed to acute exercise effects. Conversely, fluctuations in triglyceride and HDL-C levels resulting from short-term exercise training may be predominantly, if not entirely, attributable to the immediate physiological response to acute exercise [140].
The highest rate of fat oxidation occurs during moderate-intensity endurance exercises (especially exceeds 1 h), peaking at approximately 65 % of a person’s peak oxygen uptake [141]. Acute endurance exercise reduced LDs size and showed a trend toward decreasing total intramyocellular lipid content. Moreover, the density of smaller LDs in the peripheral sarcoplasmic region significantly increased, while larger LDs significantly declined [142]. Additionally, fat oxidation increases immediately following exercise [143]. The requirement for fatty acids is partially fulfilled by plasma lipids originating from adipose tissue, the intestine, and the liver. While both VLDLs (very-low-density lipoproteins) and chylomicrons contribute to supplying substrates for muscle metabolism [144], there is no indication that acute exercise enhances hepatic VLDL production [145], implying that acute exercise may instead facilitate the clearance of VLDLs and chylomicrons from circulation.
The adaptations of lipid to chronic exercise
Long-term exercise training enhances lipid utilization for energy, promotes overall reductions in lipid levels, and induces adaptive changes in skeletal muscle lipid metabolism. These adaptations lead to increased lipid oxidation, as well as improved storage and metabolism of neutral lipids. Endurance training has been demonstrated to improve fat oxidation capacity [146], which is accompanied by an increased accumulation of intramyocellular lipids (IMCLs) [147]. Interestingly, lipid accumulation within skeletal muscle is paradoxically associated with insulin resistance [28]. A phenomenon known as the athlete’s paradox [72], 148]. Despite their high sensitivity to insulin, endurance athletes exhibit higher amounts of intramyocellular lipids [149], Moreover, exercise interventions designed to reduce IR in obese patients with type 2 diabetes have been observed to increase IMCL content instead [150]. The paradox faced by athletes arises from the distinct impacts of training and diabetes on muscle lipid turnover, lipid intermediate accumulation, and oxidative capacity [28]. Notably, research by Bergman et al. [73] indicated that the incorporation of palmitate into triacylglycerol within skeletal muscle is more pronounced in athletes who are endurance-trained than in sedentary individuals. This evidence indicates that long-term exercise enhances the diversion of fatty acids towards triacylglycerol synthesis, which may reduce the build-up of lipotoxic intermediates linked to insulin resistance. Consequently, this metabolic adaptation contributes to an improved lipid profile and enhanced metabolic efficiency in trained individuals.
Summary and outlook
LDs have garnered significant interest in recent years, leading to numerous important and exciting discoveries across various domains. Significant advancements have been achieved in clarifying the mechanisms governing LDs formation and degradation. Furthermore, excess LDs are implicated in disease progression, including cancer, by facilitating viral infections, promoting inflammation and fibrosis. Importantly, exercise has been found to regulate inflammatory cytokines, lipid intermediates, and LD-associated proteins, as well as to activate the IL-15, AMPK, and CaMKII signaling pathways. Additionally, exercise enhances lysosomal activity, improves glomerular filtration rate, boosts insulin sensitivity, and strengthens immune function, mitigating these detrimental processes. Elucidating the characteristics of LD-associated diseases, and the dynamic regulation of exercise represents a significant advancement in this field of research. A key challenge lies in deciphering the signaling pathways associated with each disease, including the roles of relevant signaling cytokines and their regulation by cellular physiology. A detailed exploration of LDs tissue specificity, function, and exercise-induced modulation, may ultimately deepen our knowledge of the interplay between LDs and exercise in maintaining metabolic health. However, exercise has several limitations, such as, more prolonged exercise can cause lipid oxidation, and promote inflammation. Marathon runners are prone to developing myocarditis, minor hepatic injury and temporary decline in kidney function [151], 152].
Future research will focus on establishing lipid droplets (LDs) as central signaling hubs, with an emphasis on their interactions with other organelles and their roles in cellular signaling pathways. Elucidating the relationship between LDs and the epigenome may yield critical insights into the mechanisms underlying LD-nuclear signaling. A key research priority will be defining the role of fatty acids derived from LDs in nuclear signaling cascades. Currently, our understanding of the regulatory mechanisms governing LD-associated proteins, their interactions with organelles, and the influence of exercise remains limited. The crosstalk between exercise and LD-associated proteins represents another pivotal area of investigation. To date, few studies have examined the effects of exercise on the LD-mitochondria axis in the heart, a topic that warrants further exploration. Moreover, future empirical studies should investigate – in greater depth – the tissue-specific heterogeneity of LDs and their adaptive responses to exercise.
Funding source: Hunan Provincial Children’s Health Promotion Science and Technology Innovation Team
Funding source: the Special projects in key fields and colleges and universities of Guangdong Province of China
Award Identifier / Grant number: 2021ZDZX2054
Funding source: the Education Science Planning Project of Guangdong Province in 2022 and Higher Education Special fund
Award Identifier / Grant number: 2022GXJK241
Acknowledgments
We thank Dr. Liangming Li and the members of our groups for constructive discussions during the writing of the manuscript.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: L.L., S.P., G.Z., and W.Y. performed the study concept and design. C.F., L.Z., S.L., W.Y., X.G., T.C., F.W., C.F., and F.Z. performed the literature search and provided analysis, and interpretation of the material. C.F., L.Z., S.P., and L.L. prepared the manuscript. All authors read and approved the final version of this manuscript to be published.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors declare no competing interests.
-
Research funding: This work was supported by grants from the Education Science Planning Project of Guangdong Province in 2022 and Higher Education Special fund (2022GXJK241 to F.C.), the National Natural Science foundation of China (32100920 to L.S.), Hunan Provincial Children’s Health Promotion Science and Technology Innovation Team (to Z.L.),the Guangdong Basic and Applied Basic Research Foundation (2021A1515010740 to S.P.) and Science and Technology Program of Guangzhou, China (202102080176 to S.P.), the Guangdong Basic and Applied Basic Research Foundation (2019A1515012204 to L.L.), the Special projects in key fields and colleges and universities of Guangdong Province of China (2021ZDZX2054 to L.L.).
-
Data availability: Not applicable.
References
1. Ohsaki, Y, Kawai, T, Yoshikawa, Y, Cheng, J, Jokitalo, E, Fujimoto, T. PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol 2016;212:29–38. https://doi.org/10.1083/jcb.201507122.Search in Google Scholar PubMed PubMed Central
2. Listenberger, LL, Han, X, Lewis, SE, Cases, S, Farese, RVJr, Ory, DS, et al.. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003;100:3077–82. https://doi.org/10.1073/pnas.0630588100.Search in Google Scholar PubMed PubMed Central
3. Ohsaki, Y, Suzuki, M, Fujimoto, T. Open questions in lipid droplet biology. Chem Biol 2014;21:86–96. https://doi.org/10.1016/j.chembiol.2013.08.009.Search in Google Scholar PubMed
4. Olzmann, JA, Carvalho, P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 2019;20:137–55. https://doi.org/10.1038/s41580-018-0085-z.Search in Google Scholar PubMed PubMed Central
5. Walther, TC, Farese, RV. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 2012;81:687–714. https://doi.org/10.1146/annurev-biochem-061009-102430.Search in Google Scholar PubMed PubMed Central
6. Kokkinos, P, Myers, J. Exercise and physical activity: clinical outcomes and applications. Circulation 2010;122:1637–48. https://doi.org/10.1161/circulationaha.110.948349.Search in Google Scholar PubMed
7. Booth, FW, Roberts, CK, Laye, MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012;2:1143–211. https://doi.org/10.1002/cphy.c110025.Search in Google Scholar PubMed PubMed Central
8. Ruegsegger, GN, Booth, FW. Health benefits of exercise. Cold Spring Harb Perspect Med 2018;8:a029694. https://doi.org/10.1101/cshperspect.a029694.Search in Google Scholar PubMed PubMed Central
9. Pereira-Dutra, FS, Teixeira, L, de Souza Costa, MF, Bozza, PT. Fat, fight, and beyond: the multiple roles of lipid droplets in infections and inflammation. J Leukoc Biol 2019;106:563–80. https://doi.org/10.1002/jlb.4mr0119-035r.Search in Google Scholar PubMed
10. Eseiwi, O, Daniel, A, Sumit, B, Hanaa, H. Lipid droplets and fatty acid‐induced lipotoxicity: in a nutshell. FEBS Lett 2024;598:1207–14. https://doi.org/10.1002/1873-3468.14808.Search in Google Scholar PubMed PubMed Central
11. Altmann, R. Die Elementarorganismen und ihre Beziehungen zu den Zellen. Berlin: Walter de Gruyter; 1894:10–20 pp.10.1515/9783112366967Search in Google Scholar
12. Weiss, SB, Kennedy, EP, Kiyasu, JY. The enzymatic synthesis of triglycerides. J Biol Chem 1960;235:40–4. https://doi.org/10.1016/s0021-9258(18)69581-x.Search in Google Scholar
13. Buhman, KK, Chen, HC, Farese, RVJr. The enzymes of neutral lipid synthesis. J Biol Chem 2001;276:40369–72. https://doi.org/10.1074/jbc.r100050200.Search in Google Scholar PubMed
14. Fu, D, Yu, Y, Folick, A, Currie, E, Farese, RV, Tsai, TH, et al.. In vivo metabolic fingerprinting of neutral lipids with hyperspectral stimulated Raman scattering microscopy. J Am Chem Soc 2014;136:8820–8. https://doi.org/10.1021/ja504199s.Search in Google Scholar PubMed PubMed Central
15. Kumar, N, Leonzino, M, Hancock-Cerutti, W, Horenkamp, FA, Li, P, Lees, JA, et al.. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol 2018;217:3625–39. https://doi.org/10.1083/jcb.201807019.Search in Google Scholar PubMed PubMed Central
16. Thiam, AR, Antonny, B, Wang, J, Delacotte, J, Wilfling, F, Walther, TC, et al.. COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc. Natl. Acad. Sci. U.S.A 2013;110:13244–9. https://doi.org/10.1073/pnas.1307685110.Search in Google Scholar PubMed PubMed Central
17. Dorighello, G, McPhee, M, Halliday, K, Dellaire, G, Ridgway, ND. Differential contributions of phosphotransferases CEPT1 and CHPT1 to phosphatidylcholine homeostasis and lipid droplet biogenesis. J Biol Chem 2023;299:104578. https://doi.org/10.1016/j.jbc.2023.104578.Search in Google Scholar PubMed PubMed Central
18. Zoni, V, Khaddaj, R, Lukmantara, I, Shinoda, W, Yang, H, Schneiter, R, et al.. Seipin accumulates and traps diacylglycerols and triglycerides in its ring-like structure. Proc Natl Acad Sci USA 2021;118:e2017205118. https://doi.org/10.1073/pnas.2017205118.Search in Google Scholar PubMed PubMed Central
19. Walther, TC, Chung, J, Farese, RVJr. Lipid droplet biogenesis. Annu Rev Cell Dev Biol 2017;33:491–510. https://doi.org/10.1146/annurev-cellbio-100616-060608.Search in Google Scholar PubMed PubMed Central
20. Choudhary, V, Ojha, N, Golden, A, Prinz, WA. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J Cell Biol 2015;211:261–71. https://doi.org/10.1083/jcb.201505067.Search in Google Scholar PubMed PubMed Central
21. Hamilton, JA, Miller, KW, Small, DM. Solubilization of triolein and cholesteryl oleate in egg phosphatidylcholine vesicles. J Biol Chem 1983;258:12821–6. https://doi.org/10.1016/s0021-9258(17)44044-0.Search in Google Scholar
22. Xu, S, Zhang, X, Liu, P. Lipid droplet proteins and metabolic diseases. Biochim Biophys Acta Mol Basis Dis 2018;1864:1968–83. https://doi.org/10.1016/j.bbadis.2017.07.019.Search in Google Scholar PubMed
23. Fang, C, Liu, S, Yang, W, Zheng, G, Zhou, F, Gao, X, et al.. Exercise ameliorates lipid droplet metabolism disorder by the PLIN2-LIPA axis-mediated lipophagy in mouse model of non-alcoholic fatty liver disease. Biochim Biophys Acta Mol Basis Dis 2024;1870:167045. https://doi.org/10.1016/j.bbadis.2024.167045.Search in Google Scholar PubMed
24. Singh, R, Kaushik, S, Wang, Y, Xiang, Y, Novak, I, Komatsu, M, et al.. Autophagy regulates lipid metabolism. Nature 2009;458:1131–5. https://doi.org/10.1038/nature07976.Search in Google Scholar PubMed PubMed Central
25. Jarc, E, Petan, T. Lipid droplets and the management of cellular stress. Yale J Biol Med 2019;92:435–52.Search in Google Scholar
26. Salo, VT, Belevich, I, Li, S, Karhinen, L, Vihinen, H, Vigouroux, C, et al.. Seipin regulates ER-lipid droplet contacts and cargo delivery. EMBO J 2016;35:2699–716. https://doi.org/10.15252/embj.201695170.Search in Google Scholar PubMed PubMed Central
27. Goldberg, IJ, Trent, CM, Schulze, PC. Lipid metabolism and toxicity in the heart. Cell Metab 2012;15:805–12. https://doi.org/10.1016/j.cmet.2012.04.006.Search in Google Scholar PubMed PubMed Central
28. Bosma, M, Kersten, S, Hesselink, MK, Schrauwen, P. Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res 2012;51:36–49. https://doi.org/10.1016/j.plipres.2011.11.003.Search in Google Scholar PubMed
29. Goldman, SJ, Zhang, Y, Jin, S. Autophagic degradation of mitochondria in white adipose tissue Differentiation. Antioxidants Redox Signal 2011;14:1971–8. https://doi.org/10.1089/ars.2010.3777.Search in Google Scholar PubMed PubMed Central
30. Benador, IY, Veliova, M, Mahdaviani, K, Petcherski, A, Wikstrom, JD, Assali, EA, et al.. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab 2018;27:869–85.e6. https://doi.org/10.1016/j.cmet.2018.03.003.Search in Google Scholar PubMed PubMed Central
31. He, J, Goodpaster, BH, Kelley, DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res 2004;12:761–9. https://doi.org/10.1038/oby.2004.92.Search in Google Scholar PubMed
32. Pulinilkunnil, T, Rodrigues, B. Cardiac lipoprotein lipase: metabolic basis for diabetic heart disease. Cardiovasc Res 2006;69:329–40. https://doi.org/10.1016/j.cardiores.2005.09.017.Search in Google Scholar PubMed
33. Paul, A, Chan, L, Bickel, PE. The PAT family of lipid droplet proteins in heart and vascular cells. Curr Hypertens Rep 2008;10:461–6. https://doi.org/10.1007/s11906-008-0086-y.Search in Google Scholar PubMed PubMed Central
34. Kochan, K, Maslak, E, Krafft, C, Kostogrys, R, Chlopicki, S, Baranska, M. Raman spectroscopy analysis of lipid droplets content, distribution and saturation level in non‐alcoholic fatty liver disease in mice. J Biophot 2015;8:597–609. https://doi.org/10.1002/jbio.201400077.Search in Google Scholar PubMed
35. Schulze, RJ, McNiven, MA. Lipid droplet formation and lipophagy in fatty liver disease. Semin Liver Dis 2019;39:283–90. https://doi.org/10.1055/s-0039-1685524.Search in Google Scholar PubMed PubMed Central
36. Natarajan, SK, Eapen, CE, Pullimood, AB, Balasubramanian, KA. Oxidative stress in experimental liver microvesicular steatosis: role of mitochondria and peroxisomes. J Gastroenterol Hepatol 2006;21:1240–9. https://doi.org/10.1111/j.1440-1746.2006.04313.x.Search in Google Scholar PubMed
37. Bobulescu, IA, Yair, L, Jianning, Z, Tara, RR, James, VR, Beverley, AH, et al.. Triglycerides in the human kidney cortex: relationship with body size. PLoS One 2014;9:e101285. https://doi.org/10.1371/journal.pone.0101285.Search in Google Scholar PubMed PubMed Central
38. DM, P, AV, T, AA, S, TN, S, EA, A, TV, L, et al.. Lung inflammation is associated with lipid deposition. bioRxiv [Preprint] 2023;30:522299.Search in Google Scholar
39. Janikiewicz, J, Dobosz, AM, Majzner, K, Bernas, T, Dobrzyn, A. Stearoyl-CoA desaturase 1 deficiency exacerbates palmitate-induced lipotoxicity by the formation of small lipid droplets in pancreatic β-cells. Biochim Biophys Acta Mol Basis Dis 2023;1869:166711. https://doi.org/10.1016/j.bbadis.2023.166711.Search in Google Scholar PubMed
40. Tina, S, Petra, T, Anemari, H, Urška, Č, Ana Halužan, V, Larisa, T, et al.. Astrocytes in stress accumulate lipid droplets. Glia 2021;69:1540–62. https://doi.org/10.1002/glia.23978.Search in Google Scholar PubMed PubMed Central
41. Lee, LL, Aung, HH, Wilson, DW, Anderson, SE, Rutledge, JC, Rutkowsky, JM. Triglyceride-rich lipoprotein lipolysis products increase blood-brain barrier transfer coefficient and induce astrocyte lipid droplets and cell stress. Am J Physiol Cell Physiol 2017;312:C500–16. https://doi.org/10.1152/ajpcell.00120.2016.Search in Google Scholar PubMed PubMed Central
42. Welte, MA, Gould, AP. Lipid droplet functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862:1260–72. https://doi.org/10.1016/j.bbalip.2017.07.006.Search in Google Scholar PubMed PubMed Central
43. Cornelia, M, Karsten, K, Gerhard, L, Michael, S, Aline, C, Robert, R, et al.. Endurance and resistance training affect high fat diet-induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J Diabetes Res 2016;2016:4536470. https://doi.org/10.1155/2016/4536470.Search in Google Scholar PubMed PubMed Central
44. John, JD, Francesca, A, Maja, S-R, Frederico, GST, Sarah, ES, Bret, HG. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 2008;294:E882–8. https://doi.org/10.1152/ajpendo.00769.2007.Search in Google Scholar PubMed PubMed Central
45. Lesage, S, Drouet, V, Majounie, E, Deramecourt, V, Jacoupy, M, Nicolas, A, et al.. Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin-dependent mitophagy. Am J Hum Genet 2016;98:500–13. https://doi.org/10.1016/j.ajhg.2016.01.014.Search in Google Scholar PubMed PubMed Central
46. Cai, S, Wu, Y, Guillén-Samander, A, Hancock-Cerutti, W, Liu, J, De Camilli, P. In situ architecture of the lipid transport protein VPS13C at ER-lysosome membrane contacts. Proc Natl Acad Sci U S A 2022;119:e2203769119. https://doi.org/10.1073/pnas.2203769119.Search in Google Scholar PubMed PubMed Central
47. Yeshaw, WM, van der Zwaag, M, Pinto, F, Lahaye, LL, Faber, AI, Gómez-Sánchez, R, et al.. Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. eLife 2019;8:e43561. https://doi.org/10.7554/elife.43561.Search in Google Scholar
48. Hazan, J, Fonknechten, N, Mavel, D, Paternotte, C, Samson, D, Artiguenave, F, et al.. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet 1999;23:296–303. https://doi.org/10.1038/15472.Search in Google Scholar PubMed
49. Mosser, J, Douar, AM, Sarde, CO, Kioschis, P, Feil, R, Moser, H, et al.. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993;361:726–30. https://doi.org/10.1038/361726a0.Search in Google Scholar PubMed
50. Laufman, O, Perrino, J, Andino, R. Viral generated inter-organelle contacts redirect lipid flux for genome replication. Cell 2019;178:275–89.e16. https://doi.org/10.1016/j.cell.2019.05.030.Search in Google Scholar PubMed PubMed Central
51. Melia, CE, Peddie, CJ, de Jong, AWM, Snijder, EJ, Collinson, LM, Koster, AJ, et al.. Origins of enterovirus replication organelles established by whole-cell electron microscopy. mBio 2019;10:e00951-19. https://doi.org/10.1128/mbio.00951-19.Search in Google Scholar PubMed PubMed Central
52. Tang, WC, Lin, RJ, Liao, CL, Lin, YL. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol 2014;88:6793–804. https://doi.org/10.1128/jvi.00045-14.Search in Google Scholar PubMed PubMed Central
53. Lee, JY, Cortese, M, Haselmann, U, Tabata, K, Romero-Brey, I, Funaya, C, et al.. Spatiotemporal coupling of the hepatitis C virus replication cycle by creating a lipid DropletProximal membranous replication compartment. Cell Rep 2019;27:3602–17.e5. https://doi.org/10.1016/j.celrep.2019.05.063.Search in Google Scholar PubMed
54. Salloum, S, Wang, H, Ferguson, C, Parton, RG, Tai, AW. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog 2013;9:e1003513. https://doi.org/10.1371/journal.ppat.1003513.Search in Google Scholar PubMed PubMed Central
55. Roque, NR, Lage, SL, Navarro, R, Fazolini, N, Maya-Monteiro, CM, Rietdorf, J, et al.. Rab7 controls lipid droplet-phagosome association during mycobacterial infection. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158703. https://doi.org/10.1016/j.bbalip.2020.158703.Search in Google Scholar PubMed
56. Cocchiaro, JL, Kumar, Y, Fischer, ER, Hackstadt, T, Valdivia, RH. Cytoplasmic lipid droplets are translocated into the lumen of the chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A 2008;105:9379–84. https://doi.org/10.1073/pnas.0712241105.Search in Google Scholar PubMed PubMed Central
57. Krahmer, N, Najafi, B, Schueder, F, Quagliarini, F, Steger, M, Seitz, S, et al.. Organellar proteomics and phospho-proteomics reveal subcellular reorganization in diet-induced hepatic steatosis. Dev Cell 2018;47:205–21.e7. https://doi.org/10.1016/j.devcel.2018.09.017.Search in Google Scholar PubMed
58. Roingeard, P, Melo, RC. Lipid droplet hijacking by intracellular pathogens. Cell Microbiol 2017;19:e12688. https://doi.org/10.1111/cmi.12688.Search in Google Scholar PubMed
59. Miyanari, Y, Atsuzawa, K, Usuda, N, Watashi, K, Hishiki, T, Zayas, M, et al.. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 2007;9:1089–97. https://doi.org/10.1038/ncb1631.Search in Google Scholar PubMed
60. Filipe, A, McLauchlan, J. Hepatitis C virus and lipid droplets: finding a niche. Trends Mol Med 2015;21:34–42. https://doi.org/10.1016/j.molmed.2014.11.003.Search in Google Scholar PubMed
61. Scrima, R, Piccoli, C, Moradpour, D, Capitanio, N. Targeting endoplasmic reticulum and/or mitochondrial Ca 2+ fluxes as therapeutic strategy for HCV infection. Front Chem 2018;6:73. https://doi.org/10.3389/fchem.2018.00073.Search in Google Scholar PubMed PubMed Central
62. Camus, G, Schweiger, M, Herker, E, Harris, C, Kondratowicz, AS, Tsou, CL, et al.. The hepatitis C virus core protein inhibits adipose triglyceride lipase (ATGL)-mediated lipid mobilization and enhances the ATGL interaction with comparative gene identification 58 (CGI-58) and lipid droplets. J Biol Chem 2014;289:35770–80. https://doi.org/10.1074/jbc.m114.587816.Search in Google Scholar PubMed PubMed Central
63. Tada, T, Kumada, T, Toyoda, H, Sone, Y, Takeshima, K, Ogawa, S, et al.. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment Pharmacol Ther 2018;47:1012–22. https://doi.org/10.1111/apt.14554.Search in Google Scholar PubMed
64. Hu, B, Lin, JZ, Yang, XB, Sang, XT. Aberrant lipid metabolism in hepatocellular carcinoma cells as well as immune microenvironment: a review. Cell Prolif 2020;53:e12772. https://doi.org/10.1111/cpr.12772.Search in Google Scholar PubMed PubMed Central
65. Balci, NC, Befeler, AS, Bieneman, BK, Fattahi, R, Saglam, S, Havlioglu, N. Fat containing HCC: findings on CT and MRI including serial contrast-enhanced imaging. Acad Radiol 2009;16:963–8. https://doi.org/10.1016/j.acra.2009.02.010.Search in Google Scholar PubMed
66. Yamashita, T, Honda, M, Takatori, H, Nishino, R, Minato, H, Takamura, H, et al.. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol 2009;50:100–10. https://doi.org/10.1016/j.jhep.2008.07.036.Search in Google Scholar PubMed
67. Menendez, JA, Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007;7:763–77. https://doi.org/10.1038/nrc2222.Search in Google Scholar PubMed
68. Zhang, X, Saarinen, AM, Hitosugi, T, Wang, Z, Wang, L, Ho, TH, et al.. Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. eLife 2017;6:e31132. https://doi.org/10.7554/elife.31132.Search in Google Scholar PubMed PubMed Central
69. Ichiro, K, Yoichi, H, Yoshio, T, Masanori, A, Shinya, F, Kumiko, T, et al.. Aerobic exercise improves insulin resistance and decreases body fat and serum levels of leptin in patients with hepatitis C virus. Hepatol Res 2011;41:928–35. https://doi.org/10.1111/j.1872-034x.2011.00833.x.Search in Google Scholar
70. Susanne, C, Xiuqiang, C, Howell, L, Ville, M, Heikki, P, Milla-Maria, T, et al.. Gut ecological networks reveal associations between bacteria, exercise, and clinical profile in non-alcoholic fatty liver disease patients. mSystems 2023;8:e0022423. https://doi.org/10.1128/msystems.00224-23.Search in Google Scholar PubMed PubMed Central
71. Nieman, DC. Exercise, infection, and immunity. Int J Sports Med 1994;15:S131–41. https://doi.org/10.1055/s-2007-1021128.Search in Google Scholar PubMed
72. Goodpaster, BH, He, J, Watkins, S, Kelley, DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–61. https://doi.org/10.1210/jcem.86.12.8075.Search in Google Scholar PubMed
73. Bergman, BC, Perreault, L, Hunerdosse, DM, Koehler, MC, Samek, AM, Eckel, RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol 1985)2010;108:1134–41. https://doi.org/10.1152/japplphysiol.00684.2009.Search in Google Scholar PubMed PubMed Central
74. Amati, F, Dubé, JJ, Alvarez-Carnero, E, Edreira, MM, Chomentowski, P, Coen, PM, et al.. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–97. https://doi.org/10.2337/db10-1221.Search in Google Scholar PubMed PubMed Central
75. Thrush, AB, Brindley, DN, Chabowski, A, Heigenhauser, GJ, Dyck, DJ. Skeletal muscle lipogenic protein expression is not different between lean and obese individuals: a potential factor in ceramide accumulation. J Clin Endocrinol Metab 2009;94:5053–61. https://doi.org/10.1210/jc.2008-2565.Search in Google Scholar PubMed
76. Guillaume, V, Cécile, CG, Alexandre, P, Fabien, W, Carole, C, Daniel, B, et al.. Lipid accumulation and mitochondrial abnormalities are associated with fiber atrophy in the skeletal muscle of rats with collagen-induced arthritis. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158574. https://doi.org/10.1016/j.bbalip.2019.158574.Search in Google Scholar PubMed
77. Gueugneau, M, Coudy-Gandilhon, C, Théron, L, Meunier, B, Barboiron, C, Combaret, L, et al.. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci 2015;70:566–76. https://doi.org/10.1093/gerona/glu086.Search in Google Scholar PubMed
78. Grounds, MD, Terrill, JR, Radley-Crabb, HG, Robertson, T, Papadimitriou, J, Spuler, S, et al.. Lipid accumulation in dysferlin-deficient muscles. Am J Pathol 2014;184:1668–76. https://doi.org/10.1016/j.ajpath.2014.02.005.Search in Google Scholar PubMed
79. Papadopoulos, C, Orso, G, Mancuso, G, Herholz, M, Gumeni, S, Tadepalle, N, et al.. Spastin binds to lipid droplets and affects lipid metabolism. PLoS Genet 2015;11:e1005149. https://doi.org/10.1371/journal.pgen.1005149.Search in Google Scholar PubMed PubMed Central
80. Juha, JH, Juha, PA, Tuomas, K, Eija, P, Keijo, H, Markku, A, et al.. Postexercise myostatin and activin IIb mRNA levels: effects of strength training. Med Sci Sports Exerc 2007;39:289–97. https://doi.org/10.1249/01.mss.0000241650.15006.6e.Search in Google Scholar PubMed
81. Fell, JM, Hearris, MA, Ellis, DG, Moran, JEP, Jevons, EFP, Owens, DJ, et al.. Carbohydrate improves exercise capacity but does not affect subcellular lipid droplet morphology, AMPK and p53 signalling in human skeletal muscle. J Physiol 2021;599:2823–49. https://doi.org/10.1113/jp281127.Search in Google Scholar
82. Sılasu, A, Nuray, A, Dilek, Ö, Merve Açıkel, E, Serap, A, Guldal Gulec, S. Effects of moderate aerobic exercise, low-level laser therapy, or their combination on muscles pathology, oxidative stress and irisin levels in the mdx mouse model of Duchenne muscular dystrophy. Laser Med Sci 2022;37:2925–36. https://doi.org/10.1007/s10103-022-03562-8.Search in Google Scholar PubMed
83. Fanghui, L, Lei, S, Mingyan, Z, Tao, L, Hao-En, G, Da-Shuai, W, et al.. Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model. Exp Gerontol 2018;113:150–62. https://doi.org/10.1016/j.exger.2018.10.006.Search in Google Scholar PubMed
84. De Almeida, ME, Ørtenblad, N, Petersen, MH, Schjerning, AN, Wentorf, EK, Jensen, K, et al.. Acute exercise increases the contact between lipid droplets and mitochondria independently of obesity and type 2 diabetes. J Physiol 2023;601:1797–815. https://doi.org/10.1113/JP284386.Search in Google Scholar PubMed
85. Ping, Y, Yayun, X, Xuan, L, Yunfei, Z, Lei, Z, Yan, W, et al.. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J Lipid Res 2017;58:1417–27. https://doi.org/10.1194/jlr.m076216.Search in Google Scholar
86. Han, YZ, Du, BX, Zhu, XY, Wang, YZ, Zheng, HJ, Liu, WJ. Lipid metabolism disorder in diabetic kidney disease. Front Endocrinol 2024;15:1336402. https://doi.org/10.3389/fendo.2024.1336402.Search in Google Scholar PubMed PubMed Central
87. Reidy, K, Kang, HM, Hostetter, T, Susztak, K. Molecular mechanisms of diabetic kidney disease. J Clin Investig 2014;124:2333–40. https://doi.org/10.1172/jci72271.Search in Google Scholar PubMed PubMed Central
88. Wilkinson, TJ, McAdams-DeMarco, M, Bennett, PN, Wilund, K. Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens 2020;29:471–9. https://doi.org/10.1097/mnh.0000000000000627.Search in Google Scholar
89. Karsten, VW, Amaryllis, HVC, Wim, VB, Annemieke, D, Anouk, T, Ans, VG, et al.. The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3-4: a systematic review and meta-analysis. PLoS One 2018;13:e0203662. https://doi.org/10.1371/journal.pone.0203662.Search in Google Scholar PubMed PubMed Central
90. Shamroop, KM, Sandra, M, Alessia, F. Implications of sphingolipid metabolites in kidney diseases. Int J Mol Sci 2022;23:4244. https://doi.org/10.3390/ijms23084244.Search in Google Scholar PubMed PubMed Central
91. Grootemaat, AE, van der Niet, S, Scholl, ER, Roos, E, Schurink, B, Bugiani, M, et al.. Lipid and nucleocapsid N-protein accumulation in COVID-19 patient lung and infected cells. Microbiol Spectr 2022;10:e0127121. https://doi.org/10.1128/spectrum.01271-21.Search in Google Scholar PubMed PubMed Central
92. Gattinoni, L, Gattarello, S, Steinberg, I, Busana, M, Palermo, P, Lazzari, S, et al.. COVID-19 pneumonia: pathophysiology and management. Eur Respir Rev 2021;30:210138. https://doi.org/10.1183/16000617.0138-2021.Search in Google Scholar PubMed PubMed Central
93. Lung, J, Hung, MS, Wang, TY, Chen, KL, Luo, CW, Jiang, YY, et al.. Lipid droplets in lung cancers are crucial for the cell growth and starvation survival. Int J Mol Sci 2022;23:12533. https://doi.org/10.3390/ijms232012533.Search in Google Scholar PubMed PubMed Central
94. Stravinskas, DT, MacKenzie, B, Carneiro Oliveira-Junior, M, Santos-Dias, A, De, AK, Malfitano, C, et al.. Aerobic exercise protects from Pseudomonas aeruginosa-induced pneumonia in elderly mice. J Innate Immun 2018;10:279–90. https://doi.org/10.1159/000488953.Search in Google Scholar PubMed PubMed Central
95. Nascimento, ESP, Nunes, WMC, Guerra, EM, da Roza, MR, Silva-Costa, S, Machado-Silva, W, et al.. Combined exercise training improved exercise capacity and lung inflammation in rats with hepatopulmonary syndrome. Life Sci 2021;287:120112. https://doi.org/10.1016/j.lfs.2021.120112.Search in Google Scholar PubMed
96. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al.. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. https://doi.org/10.1016/j.jhep.2020.03.039.Search in Google Scholar PubMed
97. Younossi, ZM, Koenig, AB, Abdelatif, D, Fazel, Y, Henry, L, Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. https://doi.org/10.1002/hep.28431.Search in Google Scholar PubMed
98. Zhu, W. Effective roles of exercise and diet adherence in non-alcoholic fatty liver disease. World J Gastroenterol 2024;30:3456–60. https://doi.org/10.3748/wjg.v30.i29.3456.Search in Google Scholar PubMed PubMed Central
99. Gentiluomo, M, Dixon-Suen, SC, Farinella, R, Peduzzi, G, Canzian, F, Milne, RL, et al.. Physical activity, sedentary behavior, and pancreatic cancer risk: a mendelian randomization study. J Endocr Soc 2024;8:bvae017. https://doi.org/10.1210/jendso/bvae017.Search in Google Scholar PubMed PubMed Central
100. Sunami, Y, Rebelo, A, Kleeff, J. Lipid metabolism and lipid droplets in pancreatic cancer and stellate cells. Cancers (Basel) 2017;10:3. https://doi.org/10.3390/cancers10010003.Search in Google Scholar PubMed PubMed Central
101. Michele, C, Søren, G, Mogens, K, Andreas, A, Jianzhong, X, Per, BJ, et al.. Prevention of hyperglycemia in Zucker diabetic fatty rats by exercise training: effects on gene expression in insulin-sensitive tissues determined by high-density oligonucleotide microarray analysis. Metabolism 2005;54:1571–81. https://doi.org/10.1016/j.metabol.2005.06.003.Search in Google Scholar PubMed
102. Sogunro, A, Muzumdar, MD. Keep it moving: physical activity in the prevention of obesity-driven pancreatic cancer. Cancer Res 2024;84:2935–7. https://doi.org/10.1158/0008-5472.can-24-1474.Search in Google Scholar PubMed
103. Hamilton, LK, Aumont, A, Julien, C, Vadnais, A, Calon, F, Fernandes, KJ. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer’s disease. Eur J Neurosci 2010;32:905–20. https://doi.org/10.1111/j.1460-9568.2010.07379.x.Search in Google Scholar PubMed
104. Kate, EF, Cory, D, Amanda, EH, Dylan, G, Michael, S, Gareth, RH. APOEε4 and exercise interact in a sex‐specific manner to modulate dementia risk factors. Alzheimer’s Dement 2022;8:e12308. https://doi.org/10.1002/trc2.12308.Search in Google Scholar PubMed PubMed Central
105. Meyyazhagan, A, Orlacchio, A. Hereditary spastic paraplegia: an update. Int J Mol Sci 2022;23:1697. https://doi.org/10.3390/ijms23031697.Search in Google Scholar PubMed PubMed Central
106. Sato, M, Kannari, K, Tomari, M, Kawaguchi, T. Physical therapy intervention with a low frequency of exercise for a patient with a complicated form of hereditary spastic paraplegia: a case report. J Phys Ther Sci 2019;31:545–9. https://doi.org/10.1589/jpts.31.545.Search in Google Scholar PubMed PubMed Central
107. Beitz, JM. Parkinson s disease a review. Front Biosci (Schol Ed) 2014;6:65–74. https://doi.org/10.2741/s415.Search in Google Scholar PubMed
108. Fanning, S, Selkoe, D, Dettmer, U. Parkinson’s disease: proteinopathy or lipidopathy? Npj Parkinson’s Dis 2020;6:3. https://doi.org/10.1038/s41531-019-0103-7.Search in Google Scholar PubMed PubMed Central
109. Fanning, S, Selkoe, D, Dettmer, U. Vesicle trafficking and lipid metabolism in synucleinopathy. Acta Neuropathol 2021;141:491–510. https://doi.org/10.1007/s00401-020-02177-z.Search in Google Scholar PubMed PubMed Central
110. Johansson, ME, Cameron, IGM, Van der Kolk, NM, de Vries, NM, Klimars, E, Toni, I, et al.. Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Ann Neurol 2022;91:203–16. https://doi.org/10.1002/ana.26291.Search in Google Scholar PubMed PubMed Central
111. Ha, AD, Fung, VS. Huntington’s disease. Curr Opin Neurol 2012;25:491–8. https://doi.org/10.1097/wco.0b013e3283550c97.Search in Google Scholar
112. Marasigan, SM, Sato, M, Miyoshi, K. Experimental striatal degeneration induced by kainic acid administration: relevance to morphological changes in huntington’s disease. Jpn J Psychiatry Neurol 1986;40:113–21. https://doi.org/10.1111/j.1440-1819.1986.tb01618.x.Search in Google Scholar PubMed
113. Gruber, A, Hornburg, D, Antonin, M, Krahmer, N, Collado, J, Schaffer, M, et al.. Molecular and structural architecture of polyQ aggregates in yeast. Proc Natl Acad Sci U S A 2018;115:E3446–53. https://doi.org/10.1073/pnas.1717978115.Search in Google Scholar PubMed PubMed Central
114. Quinn, L, Hamana, K, Kelson, M, Dawes, H, Collett, J, Townson, J, et al.. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat Disord 2016;31:46–52. https://doi.org/10.1016/j.parkreldis.2016.06.023.Search in Google Scholar PubMed
115. Grajchen, E, Hendriks, JJA, Bogie, JFJ. The physiology of foamy phagocytes in multiple sclerosis. Acta Neuropathol Commun 2018;6:124. https://doi.org/10.1186/s40478-018-0628-8.Search in Google Scholar PubMed PubMed Central
116. Voss, EV, Škuljec, J, Gudi, V, Skripuletz, T, Pul, R, Trebst, C, et al.. Characterisation of microglia during de- and remyelination: can they create a repair promoting environment? Neurobiol Dis 2012;45:519–28. https://doi.org/10.1016/j.nbd.2011.09.008.Search in Google Scholar PubMed
117. Fourcade, S, Goicoechea, L, Parameswaran, J, Schlüter, A, Launay, N, Ruiz, M, et al.. High‐dose biotin restores redox balance, energy and lipid homeostasis, and axonal health in a model of adrenoleukodystrophy. Brain Pathol 2020;30:945–63. https://doi.org/10.1111/bpa.12869.Search in Google Scholar PubMed PubMed Central
118. Lozinski, BM, Yong, VW. Exercise and the brain in multiple sclerosis. Mult Scler 2022;28:1167–72. https://doi.org/10.1177/1352458520969099.Search in Google Scholar PubMed
119. Belov Kirdajova, D, Kriska, J, Tureckova, J, Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front Cell Neurosci 2020;14:51. https://doi.org/10.3389/fncel.2020.00051.Search in Google Scholar PubMed PubMed Central
120. Danielle, ND, Heng, H, Jiahong, S, Sara, EL, James, WS, Xuefang, R. Mitochondrial crisis in cerebrovascular endothelial cells opens the blood–brain barrier. Stroke 2015;46:1681–9. https://doi.org/10.1161/strokeaha.115.009099.Search in Google Scholar PubMed PubMed Central
121. Maria, A-R, Jordi, P, Juan José, L, Carme, C, Albert, P, Mattia, G, et al.. Aged lipid‐laden microglia display impaired responses to stroke. EMBO Mol Med 2023;15:e17175. https://doi.org/10.15252/emmm.202217175.Search in Google Scholar PubMed PubMed Central
122. Palmer, JA, Whitaker, AA, Payne, AM, Bartsch, BL, Reisman, DS, Boyne, PE, et al.. Aerobic exercise improves cortical inhibitory function after stroke: a preliminary investigation. J Neurol Phys Ther 2024;48:83–93. https://doi.org/10.1097/npt.0000000000000453.Search in Google Scholar
123. Hans-Georg, W, Evanthia, G, Michael, W. Glioblastoma. Handb Clin Neurol 2016;134:381–97.10.1016/B978-0-12-802997-8.00023-2Search in Google Scholar PubMed
124. Feng, G, Xiang, C, Xiao, NW, Ji, YY, Chun, MC, Jeffrey, YHG, et al.. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1–mediated lipogenesis. Clin Cancer Res 2016;22:5337–48. https://doi.org/10.1158/1078-0432.ccr-15-2973.Search in Google Scholar
125. Xiang, C, Feng, G, Mei, XP, Xiao, NW, Yao, GZ, Chun, YW, et al.. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab 2020;32:229–42.e8. https://doi.org/10.1016/j.cmet.2020.06.002.Search in Google Scholar PubMed PubMed Central
126. Emily, R, David, AR, April, C, James, EH, Whitney, EH, Mike, W, et al.. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol 2011;29:2918–23. https://doi.org/10.1200/jco.2011.34.9852.Search in Google Scholar
127. Loix, M, Wouters, E, Vanherle, S, Dehairs, J, McManaman, JL, Kemps, H, et al.. Perilipin-2 limits remyelination by preventing lipid droplet degradation. Cell Mol Life Sci 2022;79:515. https://doi.org/10.1007/s00018-022-04547-0.Search in Google Scholar PubMed PubMed Central
128. Shuang, F, Yuhuan, M, Shudai, L, Wenlu, Z, Yuting, H, Lizhen, H, et al.. Transcriptomic responses of hypothalamus to acute exercise in type 2 diabetic Goto-Kakizaki rats. PeerJ 2019;7:e7743. https://doi.org/10.7717/peerj.7743.Search in Google Scholar PubMed PubMed Central
129. Johnson, NA, Sachinwalla, T, Walton, DW, Smith, K, Armstrong, A, Thompson, MW, et al.. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. https://doi.org/10.1002/hep.23129.Search in Google Scholar PubMed
130. Sabine, D, Anne, G, Bram, B, Ruth, CRM, Peter, H, Gert, S, et al.. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol Metab 2018;17:71–81. https://doi.org/10.1016/j.molmet.2018.08.004.Search in Google Scholar PubMed PubMed Central
131. Yang, Y, Li, X, Liu, Z, Ruan, X, Wang, H, Zhang, Q, et al.. Moderate treadmill exercise alleviates NAFLD by regulating the biogenesis and autophagy of lipid droplet. Nutrients 2022;14:4910. https://doi.org/10.3390/nu14224910.Search in Google Scholar PubMed PubMed Central
132. Gao, Y, Zhang, W, Zeng, LQ, Bai, H, Li, J, Zhou, J, et al.. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol 2020;36:101635. https://doi.org/10.1016/j.redox.2020.101635.Search in Google Scholar PubMed PubMed Central
133. Sarwar, H, Rafiqi, SI, Ahmad, S, Jinna, S, Khan, SA, Karim, T, et al.. Hyperinsulinemia associated depression. Clin Med Insights Endocrinol Diabetes 2022;15:11795514221090244. https://doi.org/10.1177/11795514221090244.Search in Google Scholar PubMed PubMed Central
134. Shepherd, SO, Cocks, M, Tipton, KD, Ranasinghe, AM, Barker, TA, Burniston, JG, et al.. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol 2013;591:657–75. https://doi.org/10.1113/jphysiol.2012.240952.Search in Google Scholar PubMed PubMed Central
135. Koh, HE, Nielsen, J, Saltin, B, Holmberg, HC, Ørtenblad, N. Pronounced limb and fibre type differences in subcellular lipid droplet content and distribution in elite skiers before and after exhaustive exercise. J Physiol 2017;595:5781–95. https://doi.org/10.1113/jp274462.Search in Google Scholar
136. Chee, C, Shannon, CE, Burns, A, Selby, AL, Wilkinson, D, Smith, K, et al.. Relative contribution of intramyocellular lipid to whole-body fat oxidation is reduced with age but subsarcolemmal lipid accumulation and insulin resistance are only associated with overweight individuals. Diabetes 2016;65:840–50. https://doi.org/10.2337/db15-1383.Search in Google Scholar PubMed PubMed Central
137. Bergman, BC, Perreault, L, Strauss, A, Bacon, S, Kerege, A, Harrison, K, et al.. Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. Am J Physiol Endocrinol Metab 2018;314:E152–64. https://doi.org/10.1152/ajpendo.00142.2017.Search in Google Scholar PubMed PubMed Central
138. Shepherd, SO, Cocks, M, Meikle, PJ, Mellett, NA, Ranasinghe, AM, Barker, TA, et al.. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int J Obes 2017;41:1745–54. https://doi.org/10.1038/ijo.2017.170.Search in Google Scholar PubMed
139. Shepherd, SO, Cocks, M, Tipton, KD, Witard, OC, Ranasinghe, AM, Barker, TA, et al.. Resistance training increases skeletal muscle oxidative capacity and net intramuscular triglyceride breakdown in type I and II fibres of sedentary males. Exp Physiol 2014;99:894–908. https://doi.org/10.1113/expphysiol.2014.078014.Search in Google Scholar PubMed
140. Thompson, PD, Crouse, SF, Goodpaster, B, Kelley, D, Moyna, N, Pescatello, L. The acute versus the chronic response to exercise. Med Sci Sports Exerc 2001;33:S438–45. https://doi.org/10.1097/00005768-200106001-00012.Search in Google Scholar PubMed
141. van Loon, LJ, Greenhaff, PL, Constantin-Teodosiu, D, Saris, WH, Wagenmakers, AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 2001;536:295–304. https://doi.org/10.1111/j.1469-7793.2001.00295.x.Search in Google Scholar PubMed PubMed Central
142. Bajpeyi, S, Apaflo, JN, Rosas, V, Sepulveda-Rivera, K, Varela-Ramirez, A, Covington, JD, et al.. Effect of an acute long-duration exercise bout on skeletal muscle lipid droplet morphology, GLUT 4 protein, and perilipin protein expression. Eur J Appl Physiol 2023;123:2771–8. https://doi.org/10.1007/s00421-023-05266-5.Search in Google Scholar PubMed PubMed Central
143. Chan, HH, Burns, SF. Oxygen consumption, substrate oxidation, and blood pressure following sprint interval exercise. Appl Physiol Nutr Metab 2013;38:182–7. https://doi.org/10.1139/apnm-2012-0136.Search in Google Scholar PubMed
144. Magkos, F. Basal very low-density lipoprotein metabolism in response to exercise: mechanisms of hypotriacylglycerolemia. Prog Lipid Res 2009;48:171–90. https://doi.org/10.1016/j.plipres.2009.02.003.Search in Google Scholar PubMed
145. Magkos, F, Tsekouras, YE, Prentzas, KI, Basioukas, KN, Matsama, SG, Yanni, AE, et al.. Acute exercise-induced changes in basal VLDL-triglyceride kinetics leading to hypotriglyceridemia manifest more readily after resistance than endurance exercise. J Appl Physiol 2008;105:1228–36. https://doi.org/10.1152/japplphysiol.90761.2008.Search in Google Scholar PubMed
146. Sparks, LM, Johannsen, NM, Church, TS, Earnest, CP, Moonen-Kornips, E, Moro, C, et al.. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab 2013;98:1694–702. https://doi.org/10.1210/jc.2012-3874.Search in Google Scholar PubMed PubMed Central
147. McCormack, SE, McCarthy, MA, Harrington, SG, Farilla, L, Hrovat, MI, Systrom, DM, et al.. Effects of exercise and lifestyle modification on fitness, insulin resistance, skeletal muscle oxidative phosphorylation and intramyocellular lipid content in obese children and adolescents. Pediatr Obes 2014;9:281–91. https://doi.org/10.1111/j.2047-6310.2013.00180.x.Search in Google Scholar PubMed PubMed Central
148. Devries, MC, Samjoo, IA, Hamadeh, MJ, McCready, C, Raha, S, Watt, MJ, et al.. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J Clin Endocrinol Metab 2013;98:4852–62. https://doi.org/10.1210/jc.2013-2044.Search in Google Scholar PubMed
149. van Loon, LJ, Goodpaster, BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch 2006;451:606–16. https://doi.org/10.1007/s00424-005-1509-0.Search in Google Scholar PubMed
150. Shaw, CS, Shepherd, SO, Wagenmakers, AJ, Hansen, D, Dendale, P, van Loon, LJ. Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2012;303:E1158–65. https://doi.org/10.1152/ajpendo.00272.2012.Search in Google Scholar PubMed PubMed Central
151. Traiperm, N, Gatterer, H, Pariwat, P, Burtscher, M. Energy metabolism, liver and kidney function in adolescent marathon runners. Eur J Clin Invest 2016;46:27–33. https://doi.org/10.1111/eci.12561.Search in Google Scholar PubMed
152. Ragab, H, Lund, GK, Breitsprecher, L, Sinn, MR, Muellerleile, K, Cavus, E, et al.. Prevalence and pattern of focal and potential diffuse myocardial fibrosis in male and female marathon runners using contrast-enhanced cardiac magnetic resonance. Eur Radiol 2023;33:4648–56. https://doi.org/10.1007/s00330-023-09416-3.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.

