How exercise shapes the anti-inflammatory environment in multiple sclerosis – a conceptual framework focusing on tryptophan-derived molecules in T cell differentiation
Abstract
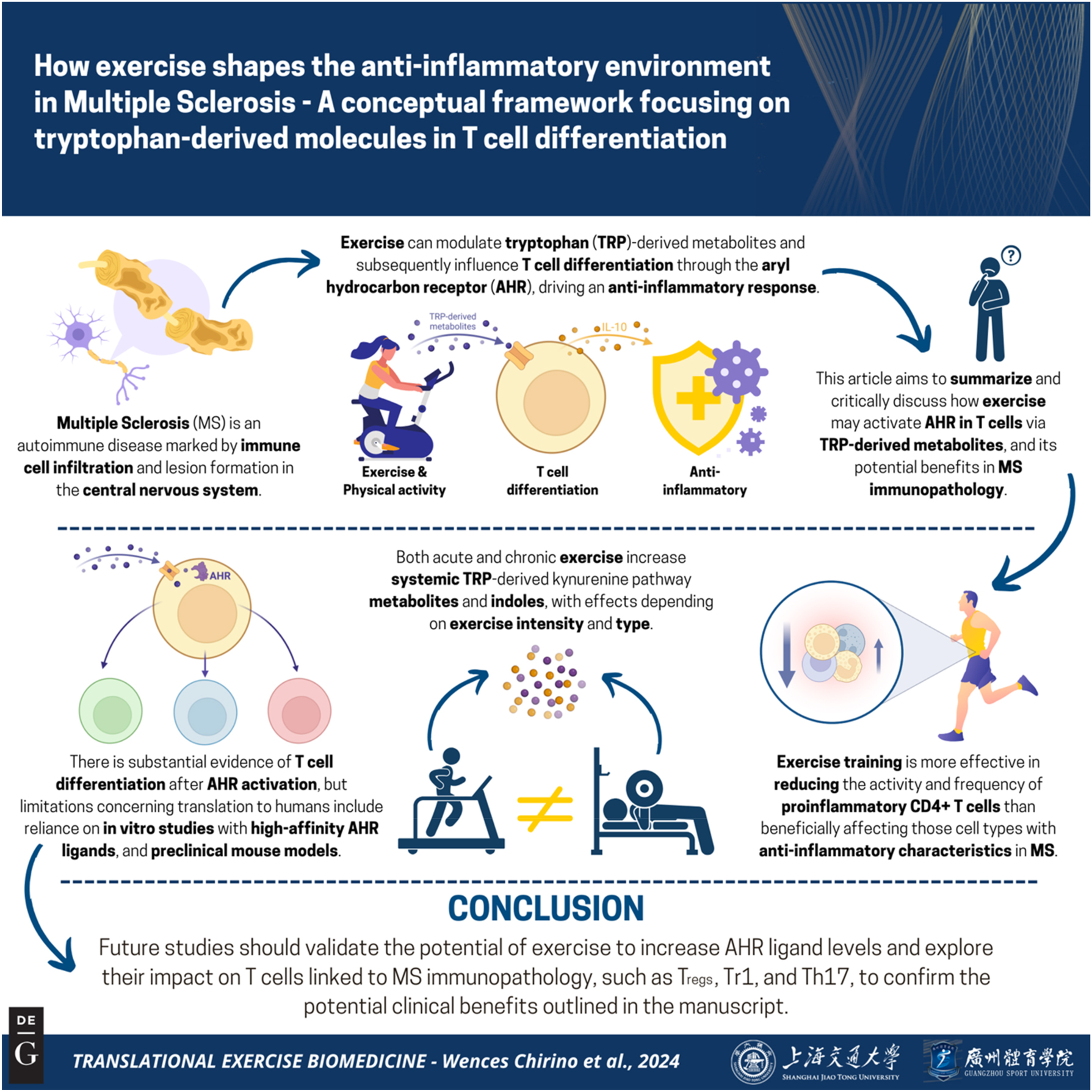
Multiple Sclerosis (MS) is a chronic neuroinflammatory autoimmune characterized by inflammation-induced lesion formation after immune cell infiltration into the central nervous system. T cells play an intriguing role in MS immunopathology and research over the past decade has shown that tryptophan (TRP)-derived metabolites are crucial molecules affecting T cell differentiation, also in MS, and are modulated by exercise. The aryl hydrocarbon receptor (AHR), for which TRP metabolites are well-known ligands, has been elucidated as main driver of T cell differentiation and an enhanced anti-inflammatory cellular milieu in human MS and preclinical mouse models. By integrating evidence from different research fields, the aim of this article is to summarize and critically discuss the potential of exercise to activate the AHR in T cells by modulating circulating TRP-derived metabolites and to provide a conceptual framework on potential benefits in MS immunopathology.
Introduction
Multiple Sclerosis (MS) is a chronic neuroinflammatory autoimmune disease affecting roughly three million people worldwide [1]. The disease typically manifests during young adulthood and is characterized by inflammation-induced lesion formation after immune cell infiltration into the central nervous system (CNS) [2]. The loss of neuronal signaling due to progressing neurodegeneration leads to significant clinical symptoms depending on the location which include motor impairments, cognitive decline, fatigue, and reduced quality of life [3].
Several immune cell types both from the myeloid and lymphoid lineage have been linked to MS immunopathology [4]. Although a complex interplay between subsets from both lineages is suggested, it is well known that T cells play a critical role. An imbalance within the T helper (Th) compartment, typically characterized by a predominance of Th1 and Th17 cells at an expanse of anti-inflammatory regulatory cells, represents a hallmark in MS 5], [6], [7], [8], [9. Although the proinflammatory activity of cytotoxic CD8+ T cells has been acknowledged for a long time, their role in MS is sometimes underappreciated, since most preclinical experimental autoimmune encephalomyelitis (EAE) models of MS are exclusively driven by CD4+ T cells [4, 10]. Within the last years, however, emerging evidence substantiates their role in MS immunopathology 11], [12], [13.
In general, T cells are well known for their phenotypic plasticity, including the differentiation from naïve cells as well as the transdifferentiation from one subset into another 14], [15], [16. The transcriptional rearrangements leading to (trans-)differentiation are mainly dictated by proteins, peptides, or metabolites from the cell´s microenvironment via receptor-mediated signaling. Research over the past decade has shown that tryptophan (TRP)-derived metabolites are crucial molecules affecting phenotype switches in T cells. On the molecular level, the metabolites converge on the cytosolic aryl hydrocarbon receptor (AHR) and induce downstream signaling cascades leading to metabolic and phenotypic alterations 17], [18], [19], [20. In fact, some TRP-derived metabolites are recognized as physiological AHR ligands, namely kynurenine (KYN), kynurenic acid (KA), xanthurenic acid (XA), indole-3-acetate (IAA), indole-3-lactate (ILA), indole-3-aldehyde (IAld), and indole-3-propionate (IPA) [21]. In MS, inflammatory episodes lead to increased degradation of TRP and generation of neuroactive and immunomodulatory kynurenine pathway (KP) metabolites, as previously reviewed by Lovelace et al. [22]. Currently, modulating the kynurenine pathway as a treatment for MS is actively being pursued in clinical trials, primarily through the use of Laquinimod [23]. This includes the manipulation of the KP, including indoleamine (IDO) boosting strategies and manipulation of the KP downstream of IDO in EAE mice [24, 25].
Physical exercise has the capacity to exert an acute influence on the immune system in an intensity-dependent manner [26], modulating the degradation of TRP and increasing the systemic concentrations of KA, a well know potent endogenous AHR ligand [27]. Exercise training in the rehabilitative context has been proven an effective approach for improving functional outcomes (i.e., muscle strength, aerobic capacity, balance, mobility), reducing fatigue, and enhancing quality of life in persons with MS (pwMS) 28], [29], [30. Indeed, regular exercise is considered a behavioral strategy inducing anti-inflammatory effects on the humoral and cellular level which is (sub-)clinically advantageous in multiple chronic diseases, including MS 31], [32], [33. Further, it has been repetitively shown that mice with induced EAE had a delayed disease onset and a lower clinical score when exercise training was applied [34]. This has been explained by favorable effects on the immune system in the periphery and CNS, respectively 35], [36], [37. The evidence is less clear in humans, but some studies observed significant effects especially on Th17-associated outcomes after exercise training [38, 39]. Despite this evidence, the underlying molecular mechanisms responsible for the advantageous immunological changes are still unknown.
In this regard, KP-derived endogenous ligands for the cytosolic AHR are increasingly discussed [40]. AHR activation has been shown to induce Foxp3+ regulatory (Treg) and Type 1 regulatory T cell (Tr1) differentiation from naïve T cells [19, 41], [42], [43, transdifferentiation from Th17 to Tr1 cells [17], but also a transition from an activated to a more exhausted phenotype in CD8+ T cells [18, 20, 44]. Further, Laquinimod, an approved oral drug for the treatment of MS, promote immune homeostasis and ameliorate EAE in an AHR-dependent manner [45, 46].
In this article, we will critically discuss the potential of exercise-induced modulation of circulating TRP degradation products with proven AHR-activating characteristics to build a conceptual framework how this may contribute to an increased anti-inflammatory milieu in MS through AHR-mediated T cell differentiation. A brief overview on exercise-induced effects on T cells relevant in MS (Section 2) as well as TRP-derived molecules (i.e., KP metabolites and indoles) (Section 3) will be provided before discussing molecular mechanisms that underlie the proposed framework (Section 4). The summary of this article is presented in Figure 1.
Graphical representation of this study. Key points: (1) modulation of the TRP-derived metabolites in MS is actively being pursued in clinical trials (e.g., Laquinimod). Furthermore, serum/plasma concentrations of these metabolites are affected especially by acute exercise. (2) The activation of the AHR has been shown to promote T cell differentiation, resulting in an enhanced anti-inflammatory environment, in both human MS cases and preclinical models. As some TRP-derived metabolites are well-known AHR ligands, AHR activation via these metabolites could mediate beneficial effect of exercise training on disease-related outcomes in MS. (3) Whether exercise training influences MS immunopathology by promoting peripheral immune homeostasis through modulation of TRP-derived metabolites should be further investigated. Figure created with BioRender.
Exercise training modulates circulating T cell subsets relevant in MS
Although it has been shown in preclinical models that exercise training beneficially affects MS severity and progression through modulation of CD4+ T cell subsets in peripheral organs and the CNS 34], [35], [36], [37, evidence from human studies on T cell biology in MS is limited. However, it has been shown in multiple population cohorts that the frequency of circulating Tregs is positively associated with cardiorespiratory fitness [47], that only after one week of intense exercise Treg frequency further increases, and that the overall Treg-mediated immunosuppressive potential is higher in athletes compared to healthy age- and gender-matched controls [48]. Since regular endurance exercise increases cardiorespiratory fitness [49], i.e. in populations with relatively low levels such as in pwMS 50], [51], [52, it can be speculated that Tregs are increased concomitantly. Another important regulatory T cell subset being dysregulated in MS are Tr1 cells [9, 53], characterized by the production of significant amounts of interleukin (IL)-10, expression of AHR, but the absence of the Treg lineage marker Foxp3 [54]. So far, only a few clinical trials have investigated exercise-induced effects on both Tregs and Tr1 cells in MS, with Mähler [38] and Deckx et al. [55] revealing no significant effects after 4 and 12 weeks, respectively.
On the other hand, evidence for beneficial effects on proinflammatory Th17 cells is more robust, evidenced by a decline in IL17A-producing CD4+ T cells [38] or reduced plasma levels and peripheral blood mononuclear cells (PBMC)-derived secretion of IL-17 [39]. Interestingly, the transdifferentiation potential between Th17 cells and Tregs is well known and could be harnessed therapeutically by cell-specific targeting to re-balance the increased Th17/Treg ratio in MS [17, 56, 57]. By changing the concentration of circulating proteins (e.g., decreasing IL-6, increasing IL-2, TGF-β, IL-10, and IL-27) or metabolites (increasing KYN and KA) towards a more anti-inflammatory state through exercise training [32, 33], the transdifferentiation from Th17 cells into Tregs or Tr1 cells may be enhanced. Indeed, serum IL-6 levels are negatively associated with physical activity and decrease following exercise training 58], [59], [60, thereby diminishing the potential for Th17 differentiation.
In contrast to the well-established diverse contributions of the CD4+ T cell compartment in MS immunopathology, the role of cytotoxic CD8+ T cells is currently under intensive investigation. They represent the main T cell type within CNS lesions, and an increase in subsets harboring a phenotype of enhanced migration, proinflammation, and activation is associated with MS 11], [12], [13, 61]. Although it is well known that single bouts of exercise significantly increase the number of circulating CD8+ T cell and elicit shifts within the T cell compartment 62], [63], [64, effects of exercise training on CD8+ T cells have not been investigated so far in pwMS or any other chronic inflammatory diseases, resulting in a significant knowledge gap.
Taken together, current evidence suggests that exercise training is more effective in reducing the activity and frequency of proinflammatory CD4+ T cells than beneficially affecting those cell types with anti-inflammatory characteristics, whereas a significant knowledge gap exists for exercise-induced changes in CD8+ T cells in MS.
Acute exercise and exercise training modulate circulating levels of TRP-derived metabolites
Intracellular levels of KP metabolites in T cells in response to exercise have not been addressed, but uptake of TRP and KYN with subsequent degradation along the pathway is mainly dependent on SLC7A5 expression [65]. Nevertheless, circulating levels of KYN-derived metabolites measured in plasma or serum, as well as transcription of key enzymes, provide indirect evidence of transient upregulation of the KP.
TRP is initially catabolized to KYN and downstream metabolites with tissue-specific enzymes IDO or tryptophan-2,3-dioxygenase (TDO). The enzyme TDO2 is primarily responsible for TRP conversion in the liver while IDO activity is stimulated by cytokines and proinflammatory mediators in extrahepatic organs and this is considered a pivotal step in T cell immunity [66], although the role of TDO in this context should not be fully disregarded [67]. KYN can later be catabolized in an enzyme-dependent manner to produce KA via the kynurenine aminotransferases (KATs); towards the generation of anthranilic acid; or primarily to produce NAD+ with 3-hydroxykynurenine, 3-hydroxyanthranillic acid, and quinolinic acid (QA) as intermediates [68] (Figure 2).

The breakdown of tryptophan to form metabolites of the kynurenine pathway (green) or indoles (orange). After ingestion, tryptophan is absorbed in the small intestine toward the liver, where it can be degraded via the kynurenine pathway or released directly into the circulation. The remaining tryptophan is directed to the colon, where it can be degraded by the host microbiota to produce indoles. These metabolites can then enter the circulation. Acute or chronic exercise affects the circulatory levels or some of these metabolites, as measured in serum or plasma levels. TDO-2, Tryptophan-2,3-dioxygenase; IDO-1/2, indolamin-2,3-dioxygenase 1/2; KATs, kynurenine aminotransferases; KMO, kynurenin-3-monooxygenase; KYNU, kynureninase; AMO, anthranilate 3-monooxygenase; HAAO, 3-hydroxyanthranilate 3,4-dioxygenase; ACMSD, aminocarboxymuconate semialdehyde decarboxylase; QPRT, quinolinate phosphoribosyltransferase; TNA, trytophanase; TMO, tryptophan monooxygenase; CYP2E1, cytochrome P450 2E1; SULT, sulfotransferase; ArAT, aromatic amino acid aminotransferase; IL4i1, interleukin-4-induced-1; IaaH, indoleacetamide hydrolase; ID, indolepyruvate decarboxylase; IaaDH, indoleaetaldehyde dehydrogenase; ILDH, indolelactate dehydrogenase; ILD, indolelactate dehydratase; ACD, acyl-CoA dehydrogenase.
Changes in circulating levels of KP metabolites in response to endurance exercise have been reviewed previously [69] and indicate that QA, KA and AA are acutely increased while TRP decreases. The direction of change in circulating KYN levels is less clear and varies with different modalities, intensity and/or duration of exercise, in particular endurance types and High Intensity Interval Training (HIIT) [70]. In contrast, in healthy persons, exercise training elicits no changes in the resting levels of circulating metabolites (see Figure 2).
In MS, exercise training is thought to be an effective way to rebalance the dysregulated KP, particularly during highly inflammatory phases of MS (early stages, relapses) [71]. The implementation of this strategy as a preventive measure and the potential impact on the duration or severity of symptoms related to inflammatory processes in pwMS are complex to assess, but certainly worth evaluating. Acutely, HIIT activates the KP in relapsing-remitting and secondary progressive MS subtypes, reducing TRP and increasing KA levels immediately after exercise cessation [72, 73]. KP activation has also been shown in a 3-week HIIT exercise training intervention, where HIIT promoted KP activation (as reflected by an upregulation of the KYN/TRP index) compared to continuous moderate intensity training [73]. Regarding key enzymes, the expression of IDO1 is increased in vitro in PBMCs in response to interferon gamma treatment, mimicking inflammatory conditions [66], while an acute bout of moderate continuous exercise increased the transcription of IDO1, IDO2, KAT3, KAT4, and an acute bout of HIIT increased IDO1 and KAT3 [26].
In the colon, TRP can be converted by the microbiota into indole compounds in three main pathways in an enzyme-dependent manner. The first pathway is Tryptophanase which generates 3-indoxyl sulfate, while the second generates IPA, with ILA as an intermediate. The third strain generates IAld, with IAA as an intermediate [74]. Moreover, these metabolites can enter the bloodstream and can therefore be quantified in diverse bodily fluids [75] (Figure 2).
Untargeted metabolomics in serum samples revealed higher levels of TRP and ILA and associations with a lower risk of developing pediatric MS, while IPA levels were inversely associated with the Expanded Disability Status Scale (EDSS) score [76]. In adults with MS, IPA and ILA levels were found to be lower than in comparison to individuals without MS [77]. This evidence, although limited, suggests that in people with MS, the degradation of TRP to form IPA, including its intermediate metabolites, is worth investigating.
The abundance of enzymes that mediate TRP-derived indole production is directly related to microorganism diversity. In this sense, MS microbiome profiles have found to differ from that of healthy persons [77]. Additionally, the functional abundance of microorganisms is highly modified by lifestyle changes including exercise both in mice [75] and previously sedentary humans [78]. In general, this is reflected by a decrease in Bacteroides species and an increase in Firmicutes strains. This, in turn, increases the functional abundance of enzymes that promote the catabolism of indoles, as revealed in fecal samples from mice undergoing voluntary wheel exercise [75].
Acute exercise has not yet been shown to influence circulating (serum or plasma) levels of indole metabolites, however, exercise training does. In this sense, 216 obese individuals, 24 weeks of various forms of exercise increased circulating levels of 3-indoxyl sulfate and ILA compared to non-exercise controls. ILA also increased when high-volume, high-intensity exercise was performed [79]. Increased levels of plasmatic IPA were also found after 80 days of aerobic and resistance training in young adult males [80] (Figure 2). From our perspective, there is still a lack of sufficient evidence of changes in indole metabolites in the MS population in response to specific (mainly chronic) interventions, and changes in enzymatic abundance and activity, related to the composition of the microbiota, should be considered in this regard.
As exercise training interventions are most probably exerting changes in circulating indole concentrations, it should also be noted that disease-modifying treatments (e.g. dimethyl fumarate) may additionally modulate the gut microbiota and influence the production of metabolites [81]. Therefore, we consider that treatment-specific analysis of TRP-derived metabolites would improve the understanding of these metabolomic changes. At the same time, it reinforces the need to include treatment as a cofactor in the assessment of these metabolites.
Additionally, it is important to emphasize the importance of compensatory mechanisms that restore TRP metabolites to physiological levels [82] after acute inflammatory insults. As such, the question remains whether these exercise-induced transient changes are intense and/or long enough to attain biologically relevant concentrations to promote AHR activation.
In summary, both acute and exercise training influence TRP degradation in pwMS, resulting in an increase of systemic KP metabolites and indoles, respectively. This effect is dependent on exercise intensity and modality, mainly occurring during endurance exercise. Further evidence of treatment-specific differences in these metabolites in response to exercise would strengthen the translation to the MS population.
The AHR-a potential link between exercise-induced changes in KP metabolism and T cell differentiation in MS
The AHR is a cytosolic receptor that integrates environmental, dietary, microbial, and metabolic information to control transcription directly and indirectly through interaction with other transcription factors such as nuclear factor kappa B (NF-κB) [83]. It is predominantly expressed in the lung and liver tissue as primary organs exposed to environmental signals, but is also expressed in many other cell types including immune cells [84]. Besides polycyclic aromatic hydrocarbons, dietary carotenoids, flavonoids, and tetrapyrrole indoles, the AHR can be activated by TRP derivatives such as indoles and some KP metabolites [85]. The structural aromatic ring in TRP derivatives, namely KYN, KA, XA, IAA, ILA, IAld, and IPA [21], enable them to function as endogenous AHR ligands (see Figure 2) if present in adequate concentrations [65, 86]. So far, the available evidence in the exercise context is restricted to healthy individuals engaged in an acute bout of high-intensity interval exercise or moderate continuous intensity exercise [26].
AHR signaling induces phenotypic changes in T cells
The role of AHR-activating KP-derivatives in re-establishing immune homeostasis in chronic inflammatory diseases as well as their modulatory role in T cell biology has been recently reviewed in detail [87, 88]. Since IDO1 activity plays a significant role through the metabolization from TRP to KYN [89, 90], but is not constitutively expressed in T cells, they are mostly reliant on the uptake of KYN via members of the solute carrier (SLC) family, including SLC7A5, SLC7A8, or SLC7A11 [18, 65, 91]. After uptake, KYN functions intracellularly as AHR ligand or can be further metabolized into the more potent AHR-ligands KA and XA. Although acute exercise increases SLC7A5 mRNA expression within the muscle [92, 93], it is unknown if the expression is also increased in T cells during exercise. It has been shown that KA and XA serum concentrations are lower in patients with inflammatory bowel disease and that oral supplementation of both metabolites in mice with induced colitis activate AHR signaling and ameliorate intestinal inflammation through modulation of epithelial and T cells [94]. Interestingly, the supplementation increased Th17 differentiation within the gut due to glycolysis-promoting effects. This is in contrast to results showing a preferential differentiation into Tregs by KYN or KYN-derived metabolites through enhanced gene expression of Foxp3, whereas transcription of the Th17-inducing factor RORγt is inhibited [19, 88]. Similar findings have been revealed in preclinical MS models as a consequence of IDO1-mediated TRP degradation [90, 95]. Furthermore, an increased transdifferentiation from Th17 cells to Tr1 cells was observed in the presence of the highly potent AHR agonist 6-formylindolo(3,2-b)carbazole [17]. However, an AHR-dependent differentiation into Th17 cells with subsequent detrimental effects in identical MS models has also been observed [43, 96]. Indeed, induction of both Th17 and Tregs have been reported after AHR activation [43, 96], [97], [98 which, at least partly, depend either on the type of agonist that binds to the AHR [43] or on available proteins in the cellular environment during AHR signaling [98]. This renders the AHR a versatile transcription factor in immunity, inducing both favorable and unfavorable effects. These partly contradictive effects of AHR-mediated T cell differentiation add complexity to the picture and stress the role of context-dependent environmental factors, i.e., circulating and tissue-derived molecules, in contributing to the outcome of AHR-dependent differentiation processes. Other aspects such as extracellular concentration, exposure time, and cellular uptake of AHR ligands play a significant role in T cell differentiation processes which vary considerably between in-vitro experiments of published studies. Whereas cultured cells are commonly exposed to the metabolites for several hours, the exposure of increased concentrations of some KP derivatives in the exercise context is simply the exercise duration, since the levels commonly approximate baseline values within 1 h after exercise cessation. However, especially for pwMS of a moderate to high disability, it might be hard to keep up exercising for a longer time at an intensity sufficient to increase levels of AHR ligands which could limit the effectiveness.
Although a KYN-dependent differentiation of naïve CD4+ T cells towards Tregs were shown in initial experiments [19], a more recent paper assessing KYN uptake on a single cell level identified that naïve T cells take up significantly lower amounts compared to antigen-experienced cells [65]. This can be explained by the observation that naïve human T cells express an almost undetectable amount of SLC7A5, whereas TCR-activated cells markedly induce SLC7A5 expression [99] which is in line with the observed upregulation of the AHR after T cell activation [100]. Therefore, it can be suggested that in peripheral tissues, where the relative amount of naïve T cells is low, the competition for available KYN is too high to take up enough KYN for activating the AHR and inducing cell differentiation. Which implications these results have for circulating naïve T cells is hard to answer, since their relative amount depend on factors such as infection history, age, and morbidity [101, 102] and decrease during acute exercise in an intensity-dependent manner [63, 64]. However, immune cells and AHR ligands in the blood are of major importance when assessing exercise-induced effects, since it represents the easiest and usually the only option for acquiring biospecimen due to methodological and ethical restrictions. Furthermore, data from different cell types show that KYN levels of 10–13 µM are necessary to induce AHR activation [65, 103, 104]. Although the peripheral KYN/TRP ratio is elevated in MS compared to healthy controls [105], plasma/serum levels of KYN typically found in the healthy population and pwMS are ∼2 µM [26, 69, 72, 73, 105] so that the plausible question “Do microenvironments exist where kynurenine could function as an AHR ligand?” [65] arises, where ideally also competitive metabolites for the SLC7A5 with a 2-10-fold lower Km (i.e., leucine, tryptophan or methionine) are present in low concentrations. Although this might be the case in some tumors [103], it is unknown whether this can be expected either in a healthy physiological state or in other chronic disease states such as MS. However, Borges et al. [106] revealed recently that an HIIT session did not significantly increase amino acids commonly transported via SLC7A5, thereby not enhancing competition during exercise.
While serum KYN levels do not commonly change in response to acute exercise, KA levels consistently increase significantly in both pwMS and healthy subjects [26, 73]. For healthy recreational runners [26], the concentration reached roughly 100 nM after exercise which has been reported to be sufficient to induce AHR activation [27]. However, it should be mentioned that DiNatale et al. [27] cultured MCF-7 breast cancer cells for 3 h with 100 nM KA, whereas in the exercise condition KA levels dropped almost back to baseline 1 h after exercise, thereby resulting only in a transient exposure of physiologically relevant concentrations for AHR activation. Whether this concentration and exposure time is enough to induce robust AHR signaling in immune cells has not been investigated. However, an exercise-induced activation in gene expression of the AHR signaling pathways was observed immediately and 1 h after exercise in PBMCs, whereas downstream target gene expression of CYP1A1, CYP1B1, AHRR and TIPARP reveal partly heterogeneous kinetics [26]. This already gives valuable information of exercise-induced changes on the transcription level in lymphocytes, but as highlighted by the authors, focusing on concrete subsets such as CD4+ and CD8+ T cells in future experiments will be important to deconvolute the heterogeneity within results from complex PBMC samples. Interestingly, IDO1 mRNA levels were increased intracellularly immediately and 1 h after exercise, indicating that available TRP can be degraded to AHR ligands (Figure 2) which in turn commence downstream signaling.
Since the role of CD8+ T cells is increasingly considered in human MS immunopathology, an AHR-mediated downregulation of cytotoxic activity via upregulation of inhibitory molecules, i.e., programmed cell death protein 1 (PD-1), or reduced proliferation might have advantageous implications. Although this has been shown for KYN and other TRP-derived molecules [18, 20, 44, 107], either in cell culture experiments with isolated primary T cells or in-vivo within tumor tissue, Dean et al. [108] observed an increased granzyme B production in intestinal CD8+ T cells as well as an essential role in the differentiation into resident memory cells subsequent to AHR activation by binding directly to the key genes Cd69 and Itgae. Similar to experiments on AHR-mediated changes in CD4+ T cell phenotypes, the effects of AHR signaling on CD8+ T cell phenotype and function seem to be context-specific [109]. In the context of acute exercise, it has been shown that both resistance and endurance exercise decrease cytosolic AHR protein levels, which has been argued as an increased translocation to the nucleus, whereas decreased protein levels of PD-1 were found 1 h after endurance exercise [110].
Compared to resistance exercise, endurance exercise has superior effects to increase circulating KA levels in healthy subjects [26, 69, 111], whereas in pwMS intense endurance exercise is more effective to raise KA concentrations compared to moderately intense endurance exercise [73]. Although not associated with KP-metabolites, preclinical animal studies show that intense endurance exercise is more effective compared to strength and moderately intense endurance in regard to clinical symptoms and immune-related outcomes [35, 36]. This further stresses the relevance of intense endurance exercise to beneficially modulate factors clearly associated with MS disease progression and (neuro-)pathology. However, exercise training in different cohorts (including MS) usually do not significantly change systemic levels of AHR ligands, which mainly constitute KYN or KA [69, 73]. This suggests that potential AHR-mediated T cell differentiation or phenotypic switches within the CD4+ and CD8+ T cell compartment takes place primarily during or within the early recovery period after exercise when levels of KA are increased to bioactive concentrations. However, it has not been investigated whether acute exercise increases AHR ligand concentrations within tissues and if so, whether increased levels persists for a longer time period which could induce beneficial T cell differentiation locally.
Taken together, substantial evidence is available that proves (trans-)differentiation in T cells after AHR activation (Figure 3A). However, limitations that hampers the transferability to the human immune system mainly consist of in-vitro experiments using exogenous AHR-ligands with very high affinities to the AHR, KYN metabolites of supra-physiological concentrations, non-immune cells as responsive cellular systems, or preclinical in-vivo experiments in mice. Despite acknowledgeable differences in the immune system between mice and men [112], these experiments show multifaceted mechanistic insights on the immune cell level to reveal AHR-mediated amelioration of disease severity in models of MS. Whether similar AhR-mediated effect on cellular and clinical outcomes can be achieved by exposing mice to exercise training should be investigated in future studies. Of utmost relevance, however, are future studies that aim to validate AHR-mediated changes in peripheral T cells, induced by TRP-derived metabolites, following exercise in pwMS and whether this is associated with long-term clinical benefits (Figure 3C). The lack of studies in pwMS for both settings (acute exercise; exercise training) underlines the need to assess effects of different exercise conditions (i.e., endurance-related) such as intensity, duration, modality, and frequency on alterations in circulating levels of AHR-ligands and associated changes on T cells in pwMS.
![Figure 3:
Depiction of the hypothesized conceptual framework by integration of AHR- and HIF-1α-mediated signaling on CD4+ and CD8+ T cell phenotypes (A), suggested exercise-induced shifts in AHR/HIF-1α pathway activity (B), and potential downstream immunoregulatory effects in MS (C). (A) KYN is taken up in T cells mainly via SLC7A5 transporters and induces AHR signaling either directly or via generating (more potent) AHR agonists KA, I3A, and potentially TEACOP270 [104]. AHR can also be activated by circulating indoles derivatives from TRP metabolism of intestinal bacteria, although the precise uptake mechanism into T cells is unclear. After activation, the AHR translocates to the nucleus and dimerizes with ARNT at the XRE, which subsequently leads to differentiation into Tregs or Tr1 cells through enhanced Foxp3 or AHR transcription, respectively, including proteins relevant for the functioning of the respective subset. Hypoxia or circulating proinflammatory cytokines initiate HIF-1α signaling through inhibiting its proteasomal degradation. After nuclear translocation and binding to ARNT at the HRE, key genes determining Th17 fate are transcribed. Similar signaling events occur in CD8+ T cells, leading to an exhausted state (AHR signaling) or increased effector functions (HIF-1α signaling). (B) A default higher priority of HIF-1α in binding ARNT compared to AHR favors transcription of HIF-1α-induced genes when both transcription factors are present in similar concentrations, favoring the generation and stability of activated CD8+ T cells and Th17 cells. Hypothesized exercise-induced effects of a shift towards enhanced AHR signaling by well-known anti-inflammatory effects (reduction of proinflammatory cytokines, increase in anti-inflammatory cytokines) and repetitively increased AHR ligands following acute exercise, leading to increased differentiation of Tregs and Tr1 cells. (C) Combined effects of acute exercise (increased AHR ligands) and exercise training (increased anti-inflammatory cytokines), including the resulting shift to an increased amount of circulating anti-inflammatory cells, in MS. The current evidence suggests more profound effects on endurance exercise on circulating molecules and T cells compared to resistance exercise. It is hypothesized that these exercise-induced alterations ultimately translate to beneficial MS-related clinical outcomes (e.g., reduced neuroinflammation and lesion formation). AHR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; CCR4/6, C-C chemokine receptor type 4/6; GPR15/35, G protein-coupled receptor 15/35; HIF-1α, hypoxia-inducible factor-1; HRE, hypoxia response element; I3A, indole-3-aldehyde; KA, kynurenic acid; KYN, kynurenine; SLC, solute carrier; TEACOP270, trace-extended aromatic condensation product, ion 270; TGF-β, transforming growth factor β; Th17 cell, T helper 17 cell; TNF-α, tumor necrosis factor alpha; Treg, Foxp3+ regulatory T cell; Tr1, Type 1 regulatory T cell; XRE, xenobiotic response element; XA, xanthurenic acid.](/document/doi/10.1515/teb-2024-0037/asset/graphic/j_teb-2024-0037_fig_003.jpg)
Depiction of the hypothesized conceptual framework by integration of AHR- and HIF-1α-mediated signaling on CD4+ and CD8+ T cell phenotypes (A), suggested exercise-induced shifts in AHR/HIF-1α pathway activity (B), and potential downstream immunoregulatory effects in MS (C). (A) KYN is taken up in T cells mainly via SLC7A5 transporters and induces AHR signaling either directly or via generating (more potent) AHR agonists KA, I3A, and potentially TEACOP270 [104]. AHR can also be activated by circulating indoles derivatives from TRP metabolism of intestinal bacteria, although the precise uptake mechanism into T cells is unclear. After activation, the AHR translocates to the nucleus and dimerizes with ARNT at the XRE, which subsequently leads to differentiation into Tregs or Tr1 cells through enhanced Foxp3 or AHR transcription, respectively, including proteins relevant for the functioning of the respective subset. Hypoxia or circulating proinflammatory cytokines initiate HIF-1α signaling through inhibiting its proteasomal degradation. After nuclear translocation and binding to ARNT at the HRE, key genes determining Th17 fate are transcribed. Similar signaling events occur in CD8+ T cells, leading to an exhausted state (AHR signaling) or increased effector functions (HIF-1α signaling). (B) A default higher priority of HIF-1α in binding ARNT compared to AHR favors transcription of HIF-1α-induced genes when both transcription factors are present in similar concentrations, favoring the generation and stability of activated CD8+ T cells and Th17 cells. Hypothesized exercise-induced effects of a shift towards enhanced AHR signaling by well-known anti-inflammatory effects (reduction of proinflammatory cytokines, increase in anti-inflammatory cytokines) and repetitively increased AHR ligands following acute exercise, leading to increased differentiation of Tregs and Tr1 cells. (C) Combined effects of acute exercise (increased AHR ligands) and exercise training (increased anti-inflammatory cytokines), including the resulting shift to an increased amount of circulating anti-inflammatory cells, in MS. The current evidence suggests more profound effects on endurance exercise on circulating molecules and T cells compared to resistance exercise. It is hypothesized that these exercise-induced alterations ultimately translate to beneficial MS-related clinical outcomes (e.g., reduced neuroinflammation and lesion formation). AHR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; CCR4/6, C-C chemokine receptor type 4/6; GPR15/35, G protein-coupled receptor 15/35; HIF-1α, hypoxia-inducible factor-1; HRE, hypoxia response element; I3A, indole-3-aldehyde; KA, kynurenic acid; KYN, kynurenine; SLC, solute carrier; TEACOP270, trace-extended aromatic condensation product, ion 270; TGF-β, transforming growth factor β; Th17 cell, T helper 17 cell; TNF-α, tumor necrosis factor alpha; Treg, Foxp3+ regulatory T cell; Tr1, Type 1 regulatory T cell; XRE, xenobiotic response element; XA, xanthurenic acid.
AHR competition and unconventional signaling
The nuclear target of AHR for transcription initiation is the aryl hydrocarbon receptor nuclear translocator (ARNT), also known as hypoxia-inducible-factor (HIF)-1β. As the name suggests, HIF-1β interacts also with the well know transcription factor HIF-1α which is evolutionary conserved across metazoans, closely linked to mTOR activation, constitutively produced at a high level, but rapidly degraded under normoxic conditions [113]. The AHR and HIF-1α are both members of the class I bHLH/PAS (basic helix-loop-helix/PER-ARNT-SIM) protein family. As a central sensor of extracellular oxygen, HIF-1α induces a genetic program prioritizing glycolysis over oxidative phosphorylation, which has been further shown to exert profound effects in innate and adaptive immunity [114]. Of note, HIF-1α can be induced by proinflammatory cytokines (i.e., IL-1β and TNF-α), lipopolysaccharides and reactive oxygen species independent of oxygen depletion [115, 116]. HIF-1α signaling determines T cell fate and function, similar to AHR activation, by dimerizing with the same nuclear target ARNT. However, multifaceted in-vitro and in-vivo experiments revealed that HIF-1α activation primarily favors induction of Th17 cells, i.e. by direct induction of RORγt and IL-17 transcription, while Foxp3 is ubiquitinated and degraded 117], [118], [119], [120. A detailed overview on AHR- and HIF-1α-mediated effects on the T cell phenotype are depicted in Figure 3A. It has been further shown that HIF-1α activity hampers immunosuppressive activity of Tregs [121, 122]. Importantly, it has been shown by two independent groups that EAE-induced mice harboring T cells that lack HIF-1α have significantly diminished numbers of Th17 cells, whereas Tregs were increased and thereby protected from autoimmune neuroinflammation [117, 118]. A similar picture that is contrary to AHR-dependent signaling can be observed for HIF-1α-induced changes in the CD8+ T cell phenotype and function, namely enhanced glycolysis, increased expression of proteolytic molecules (perforin, granzymes), inflammatory mediators (IFNγ, TNF-α), and migratory capacity 123], [124], [125. Although it is known that exercise, especially under hypoxic conditions and conducted as HIIT, increases activity of oxidative enzymes, mitochondrial density, and capillary-to-fiber ratio within muscles [126], it also enhances perfusion of “ectopic” organs such as the brain or tumor tissue 127], [128], [129], [130. These responses are not exclusively, but primarily mediated via the vascular endothelial growth factor A (VEGF-A), a main target gene of HIF-1α. However, it is yet unknown if the transient exercise-induced hypoxemia, leading to muscle deoxygenation [131], exerts hypoxia responses also in circulating or resident immune cells, thereby affecting their metabolism and ultimately their phenotype. Taken together, balancing the substantial differences in AHR and HIF-1α downstream signaling effects on T cell metabolism and phenotype through targeting the same nuclear target, namely ARNT, appears to be critical for eliciting beneficial effects in MS. There has some effort being done to elucidate the crosstalk between AHR and HIF-1α and their competition for ARNT by using various AHR ligands, different conditions to induce or mimic hypoxia, and via oxygen-independent mechanisms involving cytokines or immunogenic substances [116, 132, 133]. A recent study clearly demonstrated that AHR signaling does not inhibit the HIF-1α signaling pathway, whereas the activation of the HIF-1α pathway inhibit AHR signaling [134], but the current available literature reveals rather mixed and inconclusive results [132]. Theoretical considerations on exercise-induced alterations regarding the preference of utilizing the HIF-1α or AHR signaling routes, mediated by either acute exercise or exercise training, are depicted in Figure 3B. Interestingly, Mascanfroni et al. [135] revealed that HIF-1α controls the early metabolic reprograming of Tr1 cells, an important regulatory cell type dysregulated in MS [9], while AHR promotes HIF-1α degradation and determines mature Tr1 cell metabolism.
In addition to the crosstalk between AHR and HIF-1α pathways, conventional AHR/ARNT signaling can be impeded by the interaction of AHR with other molecules such as NRF2 or NF-κB, thereby affecting different pathways and gene programs. This is clearly beyond the scope of this article and the reader is referred to a recently published comprehensive review on this topic [133].
Summary and outlook
Due to its profound role in affecting fate and functional characteristics in T cells, activation of the AHR represents a promising target especially in chronic inflammatory diseases such as MS. The consistent finding of transiently increased concentrations of potent endogenous AHR ligands, i.e., KA, after acute endurance exercise may contribute to subsequent AHR signaling in T cells, leading to the well-known anti-inflammatory effects of exercise training. However, more research is needed to provide substantial evidence by addressing open questions to prove this theoretical framework. Fundamental to this are experiments involving humans (ideally pwMS) or human samples, respectively, as well as experimental in-vitro conditions that accurately resemble physiological in-vivo conditions. Major open questions essential to confirm the proposed conceptual framework are listed below.
Are the increased concentrations of extracellular AHR-ligands, i.e. KYN and KA, during/following acute exercise high enough and elevated for a sufficient amount of time to enhance AHR-signaling in T cells? Specifically, do intracellular concentrations of endogenous AHR-ligands increase to levels being physiologically relevant to activate the AHR?
Does AHR-mediated signaling in human Th17 cells promote transdifferentiation into regulatory cell types similar to findings observed in murine Th17 cells? And are TRP-derived metabolites potent enough to induce this phenotype switch?
Since immune cells can be extracted almost exclusively from peripheral blood in humans in the exercise context, especially in clinical cohorts such as pwMS, it is currently unknown whether acute exercise or exercise training increases AHR ligand levels within peripheral tissues to induce T cell differentiation and promote immune homeostasis locally.
Assuming that cellular adaption following AHR-signaling takes place during and shortly after acute exercise when levels are increased, it is unknown (1) for how long a single exercise session or (2) for how many weeks/months an exercise intervention should be conducted to induce the postulated changes on a systemic level.
In preclinical in-vitro and in-vivo experiments, TRP-derivates KYN, KYNA and XA have been shown (with or without reported AHR-activation) to induce differentiation into either anti-inflammatory Tregs and Tr1 cells or proinflammatory Th17 cells, with former cell types being the best described ones. Especially in the context of MS, where Tregs and Tr1 cells are dysregulated and Th17 bear substantial pathogenic potential, it is of utmost importance that future studies prove that exercise-induced AHR activation in pwMS favors Treg/Tr1 generation and significantly outweighs Th17 differentiation.
How do changes in these metabolic pathways translate to clinical benefits in MS (i.e., imaging-based measures of disease activity and progression [T2 hyperintense and gadolinium-enhancing T1 lesion load, brain volume], relapse rate, scores of physical/cognitive dysfunctions [EDSS, Multiple Sclerosis Functional Composite], neuropsychological and physical performance)?
Lastly, it has to be investigated whether pwMS of a higher disability (e.g., EDSS≥4.5) similarly benefit from exercise training by the proposed molecular and cellular changes, since the execution of such intense (endurance) exercise programs might be difficult for them to conduct.
Since current evidence further suggests that increasing circulating levels of potent AHR ligands by exercise training is more effective in clinical populations [69, 136, 137], future studies are needed to validate those findings in pwMS and to integrate in depth analysis of T cells that are associated with MS immunopathology and sensitive to AHR activation, namely Tregs, Tr1, and Th17 cells. Whether the postulated effects discussed in this article translate into clinical benefits as outlined in Figure 3C need to be confirmed in the future.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: S.P. received funding from the Hans-Böckler-Stiftung. T.Y.W.C. received funding from the German Academic Exchange Service (DAAD).
-
Data availability: Not applicable.
References
1. Walton, C, King, R, Rechtman, L, Kaye, W, Leray, E, Marrie, RA, et al.. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler J 2020;26:1816–21. https://doi.org/10.1177/1352458520970841.Search in Google Scholar PubMed PubMed Central
2. Dendrou, CA, Fugger, L, Friese, MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015;15:545–58. https://doi.org/10.1038/nri3871.Search in Google Scholar PubMed
3. Woo, MS, Engler, JB, Friese, MA. The neuropathobiology of multiple sclerosis. Nat Rev Neurosci 2024:1–21. https://doi.org/10.1038/s41583-024-00823-z.Search in Google Scholar PubMed
4. Murúa, SR, Farez, MF, Quintana, FJ. The immune response in multiple sclerosis. Annu Rev Pathol 2024;17:39.Search in Google Scholar
5. Hedegaard, CJ, Krakauer, M, Bendtzen, K, Lund, H, Sellebjerg, F, Nielsen, CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 2008;125:161–9. https://doi.org/10.1111/j.1365-2567.2008.02837.x.Search in Google Scholar PubMed PubMed Central
6. Moser, T, Akgün, K, Proschmann, U, Sellner, J, Ziemssen, T. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev 2020;19:102647. https://doi.org/10.1016/j.autrev.2020.102647.Search in Google Scholar PubMed
7. Tischner, D, Weishaupt, A, Van Den Brandt, J, Müller, N, Beyersdorf, N, Ip, CW, et al.. Polyclonal expansion of regulatory T cells interferes with effector cell migration in a model of multiple sclerosis. Brain 2006;129:2635–47. https://doi.org/10.1093/brain/awl213.Search in Google Scholar PubMed
8. Venken, K, Hellings, N, Liblau, R, Stinissen, P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med 2010;16:58–68. https://doi.org/10.1016/j.molmed.2009.12.003.Search in Google Scholar PubMed
9. Astier, AL, Meiffren, G, Freeman, S, Hafler, DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest 2006;116:3252–7. https://doi.org/10.1172/jci29251.Search in Google Scholar PubMed PubMed Central
10. Friese, MA, Fugger, L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain 2005;128:1747–63. https://doi.org/10.1093/brain/awh578.Search in Google Scholar PubMed
11. Kavaka, V, Mutschler, L, De La Rosa Del Val, C, Eglseer, K, Gómez Martínez, AM, Flierl-Hecht, A, et al.. Twin study identifies early immunological and metabolic dysregulation of CD8 + T cells in multiple sclerosis. Sci Immunol 2024;9:8094. https://doi.org/10.1126/sciimmunol.adj8094.Search in Google Scholar PubMed
12. Annibali, V, Ristori, G, Angelini, DF, Serafini, B, Mechelli, R, Cannoni, S, et al.. CD161highCD8+T cells bear pathogenetic potential in multiple sclerosis. Brain 2011;134:542–54. https://doi.org/10.1093/brain/awq354.Search in Google Scholar PubMed
13. Sabatino, JJ, Wilson, MR, Calabresi, PA, Hauser, SL, Schneck, JP, Zamvil, SS. Anti-CD20 therapy depletes activated myelin-specific CD8+ T cells in multiple sclerosis. Proc Natl Acad Sci USA 2020;116:25800–7. https://doi.org/10.1073/pnas.1915309116.Search in Google Scholar PubMed PubMed Central
14. Nakayamada, S, Takahashi, H, Kanno, Y, O’Shea, JJ. Helper T cell diversity and plasticity. Curr Opin Immunol 2012;24:297–302. https://doi.org/10.1016/j.coi.2012.01.014.Search in Google Scholar PubMed PubMed Central
15. Fonseca, R, Beura, LK, Quarnstrom, CF, Ghoneim, HE, Fan, Y, Zebley, CC, et al.. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol 2020;21:412–21. https://doi.org/10.1038/s41590-020-0607-7.Search in Google Scholar PubMed PubMed Central
16. Dupage, M, Bluestone, JA. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat Rev Immunol 2016;16:149–63. https://doi.org/10.1038/nri.2015.18.Search in Google Scholar PubMed
17. Gagliani, N, Amezcua Vesely, MC, Iseppon, A, Brockmann, L, Xu, H, Palm, NW, et al.. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015;523:221–5. https://doi.org/10.1038/nature14452.Search in Google Scholar PubMed PubMed Central
18. Liu, Y, Liang, X, Dong, W, Fang, Y, Lv, J, Zhang, T, et al.. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell 2018;33:480–94.e7. https://doi.org/10.1016/j.ccell.2018.02.005.Search in Google Scholar PubMed
19. Mezrich, JD, Fechner, JH, Zhang, X, Johnson, BP, Burlingham, WJ, Bradfield, CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010;185:3190–8. https://doi.org/10.4049/jimmunol.0903670.Search in Google Scholar PubMed PubMed Central
20. Amobi-McCloud, A, Muthuswamy, R, Battaglia, S, Yu, H, Liu, T, Wang, J, et al.. Ido1 expression in ovarian cancer induces PD-1 in T cells via aryl hydrocarbon receptor activation. Front Immunol 2021;12. https://doi.org/10.3389/fimmu.2021.678999.Search in Google Scholar PubMed PubMed Central
21. Gutiérrez-Vázquez, C, Quintana, FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018;48:19–33. https://doi.org/10.1016/j.immuni.2017.12.012.Search in Google Scholar PubMed PubMed Central
22. Lovelace, MD, Varney, B, Sundaram, G, Franco, NF, Ng, ML, Pai, S, et al.. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol 2016;7:1–22. https://doi.org/10.3389/fimmu.2016.00246.Search in Google Scholar PubMed PubMed Central
23. Pires, AS, Sundaram, G, Heng, B, Krishnamurthy, S, Brew, BJ, Guillemin, GJ. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol Ther 2022;236.10.1016/j.pharmthera.2021.108055Search in Google Scholar PubMed
24. Platten, M, Ho, PP, Youssef, S, Fontoura, P, Garren, H, Hur, EM, et al.. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 2005;310:850–5. https://doi.org/10.1126/science.1117634.Search in Google Scholar PubMed
25. Lemos, H, Mohamed, E, Ou, R, McCardle, C, Zheng, X, McGuire, K, et al.. Co-Treatments to boost Ido activity and inhibit production of downstream catabolites induce durable suppression of experimental autoimmune encephalomyelitis. Front Immunol 2020;11. https://doi.org/10.3389/fimmu.2020.01256.Search in Google Scholar PubMed PubMed Central
26. Joisten, N, Walzik, D, Schenk, A, Metcalfe, AJ, Belen, S, Schaaf, K, et al.. Acute exercise activates the AHR in peripheral blood mononuclear cells in an intensity-dependent manner. Am J Physiol Cell Physiol 2024;327:C438–45. https://doi.org/10.1152/ajpcell.00282.2024.Search in Google Scholar PubMed
27. DiNatale, BC, Murray, IA, Schroeder, JC, Flaveny, CA, Lahoti, TS, Laurenzana, EM, et al.. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 2010;115:89–97. https://doi.org/10.1093/toxsci/kfq024.Search in Google Scholar PubMed PubMed Central
28. Khan, F, Amatya, B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil 2017;98:353–67. https://doi.org/10.1016/j.apmr.2016.04.016.Search in Google Scholar PubMed
29. Motl, RW, Sandroff, BM, Kwakkel, G, Dalgas, U, Feinstein, A, Heesen, C, et al.. Exercise in patients with multiple sclerosis. Lancet Neurol 2017;16:848–56. https://doi.org/10.1016/s1474-4422(17)30281-8.Search in Google Scholar
30. Halabchi, F, Alizadeh, Z, Sahraian, MA, Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol 2017;17:1–11. https://doi.org/10.1186/s12883-017-0960-9.Search in Google Scholar PubMed PubMed Central
31. Neufer, PD, Bamman, MM, Muoio, DM, Bouchard, C, Cooper, D, Goodpaster, B, et al.. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabol 2015;22:4–11. https://doi.org/10.1016/j.cmet.2015.05.011.Search in Google Scholar PubMed
32. Pedersen, BK, Saltin, B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1–72. https://doi.org/10.1111/sms.12581.Search in Google Scholar PubMed
33. Gleeson, M, Bishop, NC, Stensel, DJ, Lindley, MR, Mastana, SS, Nimmo, MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–10. https://doi.org/10.1038/nri3041.Search in Google Scholar PubMed
34. Einstein, O, Katz, A, Ben-Hur, T. Physical exercise therapy for autoimmune neuroinflammation: application of knowledge from animal models to patient care. Autoimmun Rev 2022;21:103033. https://doi.org/10.1016/j.autrev.2022.103033.Search in Google Scholar PubMed
35. Xie, Y, Li, Z, Wang, Y, Xue, X, Ma, W, Zhang, Y, et al.. Effects of moderate- versus high- intensity swimming training on inflammatory and CD4+ T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol 2019;328:60–7. https://doi.org/10.1016/j.jneuroim.2018.12.005.Search in Google Scholar PubMed
36. Souza, PS, Gonçalves, ED, Pedroso, GS, Farias, HR, Junqueira, SC, Marcon, R, et al.. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol Neurobiol 2017;54:4723–37. https://doi.org/10.1007/s12035-016-0014-0.Search in Google Scholar PubMed
37. Einstein, O, Fainstein, N, Touloumi, O, Lagoudaki, R, Hanya, E, Grigoriadis, N, et al.. Exercise training attenuates experimental autoimmune encephalomyelitis by peripheral immunomodulation rather than direct neuroprotection. Exp Neurol 2018;299:56–64. https://doi.org/10.1016/j.expneurol.2017.10.008.Search in Google Scholar PubMed
38. Mähler, A, Balogh, A, Csizmadia, I, Klug, L, Kleinewietfeld, M, Steiniger, J, et al.. Metabolic, mental and immunological effects of normoxic and hypoxic training in multiple sclerosis patients: a pilot study. Front Immunol 2018;9:2819. https://doi.org/10.3389/fimmu.2018.02819.Search in Google Scholar PubMed PubMed Central
39. Golzari, Z, Shabkhiz, F, Soudi, S, Kordi, MR, Hashemi, SM. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int Immunopharmacol 2010;10:1415–9. https://doi.org/10.1016/j.intimp.2010.08.008.Search in Google Scholar PubMed
40. Joisten, N, Walzik, D, Metcalfe, AJ, Bloch, W, Zimmer, P. Physical exercise as kynurenine pathway modulator in chronic diseases: implications for immune and energy homeostasis. Int J Tryptophan Res 2020;13. https://doi.org/10.1177/1178646920938688.Search in Google Scholar PubMed PubMed Central
41. Gandhi, R, Kumar, D, Burns, EJ, Nadeau, M, Dake, B, Laroni, A, et al.. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat Immunol 2010;11:846–53. https://doi.org/10.1038/ni.1915.Search in Google Scholar PubMed PubMed Central
42. Apetoh, L, Quintana, FJ, Pot, C, Joller, N, Xiao, S, Kumar, D, et al.. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 2010;11:854–61. https://doi.org/10.1038/ni.1912.Search in Google Scholar PubMed PubMed Central
43. Quintana, FJ, Basso, AS, Iglesias, AH, Korn, T, Farez, MF, Bettelli, E, et al.. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453:65–71. https://doi.org/10.1038/nature06880.Search in Google Scholar PubMed
44. Liu, Y, Zhou, N, Zhou, L, Wang, J, Zhou, Y, Zhang, T, et al.. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol 2021;22:358–69. https://doi.org/10.1038/s41590-020-00850-9.Search in Google Scholar PubMed
45. Rothhammer, V, Kenison, JE, Li, Z, Tjon, E, Takenaka, MC, Chao, CC, et al.. Aryl hydrocarbon receptor activation in astrocytes by Laquinimod ameliorates autoimmune inflammation in the CNS. Neurol Neuroimmunol Neuroinflamm 2021;8. https://doi.org/10.1212/nxi.0000000000000946.Search in Google Scholar
46. Kaye, J, Piryatinsky, V, Birnberg, T, Hingaly, T, Raymond, E, Kashi, R, et al.. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 2016;113:E6145–52. https://doi.org/10.1073/pnas.1607843113.Search in Google Scholar PubMed PubMed Central
47. Proschinger, S, Winker, M, Joisten, N, Bloch, W, Palmowski, J, Zimmer, P. The effect of exercise on regulatory T cells: a systematic review of human and animal studies with future perspectives and methodological recommendations. Exerc Immunol Rev 2021;27.Search in Google Scholar
48. Weinhold, M, Shimabukuro-Vornhagen, A, Franke, A, Theurich, S, Wahl, P, Hallek, M, et al.. Physical exercise modulates the homeostasis of human regulatory T cells. J Allergy Clin Immunol 2016;137:1607–10. https://doi.org/10.1016/j.jaci.2015.10.035.Search in Google Scholar PubMed
49. Milanović, Z, Sporiš, G, Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 2015;45:1469–81. https://doi.org/10.1007/s40279-015-0365-0.Search in Google Scholar PubMed
50. Klaren, RE, Motl, RW, Dlugonski, D, Sandroff, BM, Pilutti, LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2013;94:2342–8. https://doi.org/10.1016/j.apmr.2013.07.011.Search in Google Scholar PubMed
51. Schlagheck, ML, Bansi, J, Langeskov-Christensen, M, Zimmer, P, Hvid, LG. Cardiorespiratory fitness (V̇O2peak) across the adult lifespan in persons with multiple sclerosis and matched healthy controls. J Sci Med Sport 2024;27:10–5. https://doi.org/10.1016/j.jsams.2023.10.009.Search in Google Scholar PubMed
52. Youssef, H, Gönül, MN, Sobeeh, MG, Akar, K, Feys, P, Cuypers, K, et al.. Is high-intensity interval training more effective than moderate continuous training in rehabilitation of multiple sclerosis: a comprehensive systematic review and meta-analysis. Arch Phys Med Rehabil 2024;105:1545–58. https://doi.org/10.1016/j.apmr.2023.12.012.Search in Google Scholar PubMed
53. Martinez-Forero, I, Garcia-Munoz, R, Martinez-Pasamar, S, Inoges, S, Lopez-Diaz de Cerio, A, Palacios, R, et al.. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol 2008;38:576–86. https://doi.org/10.1002/eji.200737271.Search in Google Scholar PubMed
54. Roncarolo, MG, Gregori, S, Bacchetta, R, Battaglia, M, Gagliani, N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 2018;49:1004–19. https://doi.org/10.1016/j.immuni.2018.12.001.Search in Google Scholar PubMed
55. Deckx, N, Wens, I, Nuyts, AH, Hens, N, De Winter, BY, Koppen, G, et al.. 12 Weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediators Inflamm 2016;2016:6789276. https://doi.org/10.1155/2016/6789276.Search in Google Scholar PubMed PubMed Central
56. Shi, C, Zhang, J, Wang, H, Chen, C, Han, M, Gao, L, et al.. Trojan horse nanocapsule enabled in situ modulation of the phenotypic conversion of Th17 cells to Treg cells for the treatment of multiple sclerosis in mice. Adv Mater 2023;35:2210262. https://doi.org/10.1002/adma.202210262.Search in Google Scholar PubMed
57. Kleinewietfeld, M, Hafler, DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 2013;25:305–12. https://doi.org/10.1016/j.smim.2013.10.009.Search in Google Scholar PubMed PubMed Central
58. Hamer, M, Sabia, S, Batty, GD, Shipley, MJ, Tabák, AG, Singh-Manoux, A, et al.. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the whitehall II cohort study. Circulation 2012;126:928–33. https://doi.org/10.1161/circulationaha.112.103879.Search in Google Scholar
59. Zheng, G, Qiu, P, Xia, R, Lin, H, Ye, B, Tao, J, et al.. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci 2019;11. https://doi.org/10.3389/fnagi.2019.00098.Search in Google Scholar PubMed PubMed Central
60. Vella, CA, Allison, MA, Cushman, M, Jenny, NS, Miles, MP, Larsen, B, et al.. Physical activity and adiposity-related inflammation: the MESA. Med Sci Sports Exerc 2017;49:915–21. https://doi.org/10.1249/mss.0000000000001179.Search in Google Scholar
61. Lückel, C, Picard, F, Raifer, H, Campos Carrascosa, L, Guralnik, A, Zhang, Y, et al.. IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat Commun 2019;10:5722. https://doi.org/10.1038/s41467-019-13731-z.Search in Google Scholar PubMed PubMed Central
62. Graff, RM, Kunz, HE, Agha, NH, Baker, FL, Laughlin, M, Bigley, AB, et al.. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun 2018;74:143–53. https://doi.org/10.1016/j.bbi.2018.08.017.Search in Google Scholar PubMed
63. Campbell, JP, Riddell, NE, Burns, VE, Turner, M, van Zanten, JJV, Drayson, MT, et al.. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun 2009;23:767–75. https://doi.org/10.1016/j.bbi.2009.02.011.Search in Google Scholar PubMed
64. Proschinger, S, Schenk, A, Metcalfe, AJ, Zimmer, P. HIIT induces stronger shifts within the peripheral T cell compartment independent of sex. Int J Sports Med 2023;45:211–21. https://doi.org/10.1055/a-2197-0882.Search in Google Scholar PubMed
65. Sinclair, LV, Neyens, D, Ramsay, G, Taylor, PM, Cantrell, DA. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat Commun 2018;9. https://doi.org/10.1038/s41467-018-04366-7.Search in Google Scholar PubMed PubMed Central
66. Jones, SP, Franco, NF, Varney, B, Sundaram, G, Brown, DA, de Bie, J, et al.. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS One 2015;10. https://doi.org/10.1371/journal.pone.0131389.Search in Google Scholar PubMed PubMed Central
67. Lanz, TV, Williams, SK, Stojic, A, Iwantscheff, S, Sonner, JK, Grabitz, C, et al.. Tryptophan-2,3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci Rep 2017;7. https://doi.org/10.1038/srep41271.Search in Google Scholar PubMed PubMed Central
68. Badawy, AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res 2017;10. https://doi.org/10.1177/1178646917691938.Search in Google Scholar PubMed PubMed Central
69. Joisten, N, Kummerhoff, F, Koliamitra, C, Schenk, A, Walzik, D, Hardt, L, et al.. Exercise and the Kynurenine pathway: current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc Immunol Rev 2020;26:24–42.Search in Google Scholar
70. Metcalfe, AJ, Koliamitra, C, Javelle, F, Bloch, W, Zimmer, P. Acute and chronic effects of exercise on the kynurenine pathway in humans – a brief review and future perspectives. Physiol Behav 2018;194:583–7. https://doi.org/10.1016/j.physbeh.2018.07.015.Search in Google Scholar PubMed
71. Watzlawik, JO, Wootla, B, Rodriguez, M. Tryptophan catabolites and their impact on multiple sclerosis progression. Curr Pharm Des 2016;22:1049–59.10.2174/1381612822666151215095940Search in Google Scholar
72. Bansi, J, Koliamitra, C, Bloch, W, Joisten, N, Schenk, A, Watson, M, et al.. Persons with secondary progressive and relapsing remitting multiple sclerosis reveal different responses of tryptophan metabolism to acute endurance exercise and training. J Neuroimmunol 2018;314:101–5. https://doi.org/10.1016/j.jneuroim.2017.12.001.Search in Google Scholar PubMed
73. Joisten, N, Rademacher, A, Warnke, C, Proschinger, S, Schenk, A, Walzik, D, et al.. Exercise diminishes plasma neurofilament light chain and reroutes the kynurenine pathway in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021;8:1–11. https://doi.org/10.1212/nxi.0000000000000982.Search in Google Scholar PubMed PubMed Central
74. Gao, J, Xu, K, Liu, H, Liu, G, Bai, M, Peng, C, et al.. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 2018;8. https://doi.org/10.3389/fcimb.2018.00013.Search in Google Scholar PubMed PubMed Central
75. Anderson, GM. The quantitative determination of indolic microbial tryptophan metabolites in human and rodent samples: a systematic review. J Chromatogr, B: Anal Technol Biomed Life Sci 2021;1186. https://doi.org/10.1016/j.jchromb.2021.123008.Search in Google Scholar PubMed
76. Nourbakhsh, B, Bhargava, P, Tremlett, H, Hart, J, Graves, J, Waubant, E. Altered tryptophan metabolism is associated with pediatric multiple sclerosis risk and course. Ann Clin Transl Neurol 2018;5:1211–21. https://doi.org/10.1002/acn3.637.Search in Google Scholar PubMed PubMed Central
77. Levi, I, Gurevich, M, Perlman, G, Magalashvili, D, Menascu, S, Bar, N, et al.. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep Med 2021;2. https://doi.org/10.1016/j.xcrm.2021.100246.Search in Google Scholar PubMed PubMed Central
78. Allen, JM, Mailing, LJ, Niemiro, GM, Moore, R, Cook, MD, White, BA, et al.. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018;50:747–57. https://doi.org/10.1249/mss.0000000000001495.Search in Google Scholar
79. Brennan, AM, Benson, M, Morningstar, J, Herzig, M, Robbins, J, Gerszten, RE, et al.. Plasma metabolite profiles in response to chronic exercise. Med Sci Sports Exerc 2018;50:1480–6. https://doi.org/10.1249/mss.0000000000001594.Search in Google Scholar
80. Koay, YC, Stanton, K, Kienzle, V, Li, M, Yang, J, Celermajer, DS, et al.. Effect of chronic exercise in healthy young male adults: a metabolomic analysis. Cardiovasc Res 2021;117:613–22. https://doi.org/10.1093/cvr/cvaa051.Search in Google Scholar PubMed
81. Ntranos, A, Park, HJ, Wentling, M, Tolstikov, V, Amatruda, M, Inbar, B, et al.. Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain 2022;145:569–83. https://doi.org/10.1093/brain/awab320.Search in Google Scholar PubMed PubMed Central
82. Vazquez-Medina, A, Rodriguez-Trujillo, N, Ayuso-Rodriguez, K, Marini-Martinez, F, Angeli-Morales, R, Caussade-Silvestrini, G, et al.. Exploring the interplay between running exercises, microbial diversity, and tryptophan metabolism along the microbiota-gut-brain axis. Front Microbiol 2024;15. https://doi.org/10.3389/fmicb.2024.1326584.Search in Google Scholar PubMed PubMed Central
83. Rothhammer, V, Quintana, FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 2019;19:184–97. https://doi.org/10.1038/s41577-019-0125-8.Search in Google Scholar PubMed
84. Stockinger, B, Meglio, PD, Gialitakis, M, Duarte, JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 2014;32:403–32. https://doi.org/10.1146/annurev-immunol-032713-120245.Search in Google Scholar PubMed
85. Jaronen, M, Quintana, FJ. Immunological relevance of the coevolution of Ido1 and AHR. Front Immunol 2014;5. https://doi.org/10.3389/fimmu.2014.00521.Search in Google Scholar PubMed PubMed Central
86. Nguyen, LP, Bradfield, CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 2008;21:102–16. https://doi.org/10.1021/tx7001965.Search in Google Scholar PubMed PubMed Central
87. Joisten, N, Ruas, JL, Braidy, N, Guillemin, GJ, Zimmer, P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol Med 2021;27:946–54. https://doi.org/10.1016/j.molmed.2021.07.006.Search in Google Scholar PubMed
88. Stone, TW, Williams, RO. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol Sci 2023;44:442–56. https://doi.org/10.1016/j.tips.2023.04.006.Search in Google Scholar PubMed
89. Baban, B, Chandler, PR, Sharma, MD, Pihkala, J, Koni, PA, Munn, DH, et al.. Ido activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009;183:2475–83. https://doi.org/10.4049/jimmunol.0900986.Search in Google Scholar PubMed PubMed Central
90. Kenney, LL, Chiu, RSY, Dutra, MN, Wactor, A, Honan, C, Shelerud, L, et al.. mRNA-delivery of Ido1 suppresses T cell-mediated autoimmunity. Cell Rep Med 2024:101717. https://doi.org/10.1016/j.xcrm.2024.101717.Search in Google Scholar PubMed PubMed Central
91. Fiore, A, Zeitler, L, Russier, M, Groß, A, Hiller, MK, Parker, JL, et al.. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell 2022;82:920–32.e7. https://doi.org/10.1016/j.molcel.2022.02.007.Search in Google Scholar PubMed
92. Drummond, MJ, Fry, CS, Glynn, EL, Timmerman, KL, Dickinson, JM, Walker, DK, et al.. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–42. https://doi.org/10.1152/japplphysiol.01408.2010.Search in Google Scholar PubMed PubMed Central
93. Pillon, NJ, Gabriel, BM, Dollet, L, Smith, JAB, Sardón Puig, L, Botella, J, et al.. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun 2020;11. https://doi.org/10.1038/s41467-019-13869-w.Search in Google Scholar PubMed PubMed Central
94. Michaudel, C, Danne, C, Agus, A, Magniez, A, Aucouturier, A, Spatz, M, et al.. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2023;72:1296–307. https://doi.org/10.1136/gutjnl-2022-327337.Search in Google Scholar PubMed PubMed Central
95. Yan, Y, Zhang, GX, Gran, B, Fallarino, F, Yu, S, Li, H, et al.. Ido upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol 2010;185:5953–61. https://doi.org/10.4049/jimmunol.1001628.Search in Google Scholar PubMed PubMed Central
96. Veldhoen, M, Hirota, K, Christensen, J, O’Garra, A, Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med 2009;206:43–9. https://doi.org/10.1084/jem.20081438.Search in Google Scholar PubMed PubMed Central
97. Veldhoen, M, Hirota, K, Westendorf, AM, Buer, J, Dumoutier, L, Renauld, JC, et al.. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008;453:106–9. https://doi.org/10.1038/nature06881.Search in Google Scholar PubMed
98. Kimura, A, Naka, T, Nohara, K, Fujii-Kuriyama, Y, Kishimoto, T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA 2008;105:9721–6. https://doi.org/10.1073/pnas.0804231105.Search in Google Scholar PubMed PubMed Central
99. Hayashi, K, Jutabha, P, Endou, H, Sagara, H, Anzai, N. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol 2013;191:4080–5. https://doi.org/10.4049/jimmunol.1300923.Search in Google Scholar PubMed
100. Prigent, L, Robineau, M, Jouneau, S, Morzadec, C, Louarn, L, Vernhet, L, et al.. The aryl hydrocarbon receptor is functionally upregulated early in the course of human T-cell activation. Eur J Immunol 2014;44:1330–40. https://doi.org/10.1002/eji.201343920.Search in Google Scholar PubMed
101. Farber, DL, Yudanin, NA, Restifo, NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 2014;14:24–35. https://doi.org/10.1038/nri3567.Search in Google Scholar PubMed PubMed Central
102. Van Den Broek, T, Borghans, JAM, Van Wijk, F. The full spectrum of human naive T cells. Nat Rev Immunol 2018;18:363–73. https://doi.org/10.1038/s41577-018-0001-y.Search in Google Scholar PubMed
103. Opitz, CA, Litzenburger, UM, Sahm, F, Ott, M, Tritschler, I, Trump, S, et al.. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478:197–203. https://doi.org/10.1038/nature10491.Search in Google Scholar PubMed
104. Seok, SH, Ma, ZX, Feltenberger, JB, Chen, H, Chen, H, Scarlett, C, et al.. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J Biol Chem 2018;293:1994–2005. https://doi.org/10.1074/jbc.ra117.000631.Search in Google Scholar
105. Lim, CK, Bilgin, A, Lovejoy, DB, Tan, V, Bustamante, S, Taylor, BV, et al.. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep 2017;7:1–9. https://doi.org/10.1038/srep41473.Search in Google Scholar PubMed PubMed Central
106. Borges, N, Doering, TM, Murphy, G, Macdonald, M, Dunstan, RH. Amino acid distribution in blood following high-intensity interval exercise: a preliminary study. Amino Acids 2024;56. https://doi.org/10.1007/s00726-023-03378-y.Search in Google Scholar PubMed PubMed Central
107. Sadik, A, Somarribas Patterson, LF, Öztürk, S, Mohapatra, SR, Panitz, V, Secker, PF, et al.. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020;182:1252–70.e34. https://doi.org/10.1016/j.cell.2020.07.038.Search in Google Scholar PubMed
108. Dean, JW, Helm, EY, Fu, Z, Xiong, L, Sun, N, Oliff, KN, et al.. The aryl hydrocarbon receptor cell intrinsically promotes resident memory CD8+ T cell differentiation and function. Cell Rep 2023;42. https://doi.org/10.1016/j.celrep.2022.111963.Search in Google Scholar PubMed PubMed Central
109. Fransen, NL, Hsiao, CC, Van Der Poel, M, Engelenburg, HJ, Verdaasdonk, K, Vincenten, MCJ, et al.. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 2020;143:1714–30. https://doi.org/10.1093/brain/awaa117.Search in Google Scholar PubMed
110. Schenk, A, Joisten, N, Walzik, D, Koliamitra, C, Schoser, D, Bloch, W, et al.. Acute exercise impacts AhR and PD-1 levels of CD8+ T-cells—exploratory results from a randomized cross-over trial comparing endurance versus resistance exercise. Eur J Appl Physiol 2020;0123456789.10.1007/s00421-020-04552-wSearch in Google Scholar PubMed PubMed Central
111. Joisten, N, Schumann, M, Schenk, A, Walzik, D, Freitag, N, Knoop, A, et al.. Acute hypertrophic but not maximal strength loading transiently enhances the kynurenine pathway towards kynurenic acid. Eur J Appl Physiol 2020;120:1429–36. https://doi.org/10.1007/s00421-020-04375-9.Search in Google Scholar PubMed PubMed Central
112. Masopust, D, Sivula, CP, Jameson, SC. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol 2017;199:383–8. https://doi.org/10.4049/jimmunol.1700453.Search in Google Scholar PubMed PubMed Central
113. Kaelin, WG, Ratcliffe, PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393–402. https://doi.org/10.1016/j.molcel.2008.04.009.Search in Google Scholar PubMed
114. McGettrick, AF, O’Neill, LAJ. The role of HIF in immunity and inflammation. Cell Metabol 2020;32:524–36. https://doi.org/10.1016/j.cmet.2020.08.002.Search in Google Scholar PubMed
115. Malkov, MI, Lee, CT, Taylor, CT. Regulation of the hypoxia-inducible factor (HIF) by pro-inflammatory cytokines. Cells 2021;10. https://doi.org/10.3390/cells10092340.Search in Google Scholar PubMed PubMed Central
116. Taylor, CT, Scholz, CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol 2022;18:573–87. https://doi.org/10.1038/s41581-022-00587-8.Search in Google Scholar PubMed PubMed Central
117. Shi, LZ, Wang, R, Huang, G, Vogel, P, Neale, G, Green, DR, et al.. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011;208:1367–76. https://doi.org/10.1084/jem.20110278.Search in Google Scholar PubMed PubMed Central
118. Dang, EV, Barbi, J, Yang, HY, Jinasena, D, Yu, H, Zheng, Y, et al.. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 2011;146:772–84. https://doi.org/10.1016/j.cell.2011.07.033.Search in Google Scholar PubMed PubMed Central
119. Shehade, H, Acolty, V, Moser, M, Oldenhove, G. Cutting edge: hypoxia-inducible factor 1 negatively regulates Th1 function. J Immunol 2015;195:1372–6. https://doi.org/10.4049/jimmunol.1402552.Search in Google Scholar PubMed
120. Clever, D, Roychoudhuri, R, Constantinides, MG, Askenase, MH, Sukumar, M, Klebanoff, CA, et al.. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 2016;166:1117–31.e14. https://doi.org/10.1016/j.cell.2016.07.032.Search in Google Scholar PubMed PubMed Central
121. Lee, JH, Elly, C, Park, Y, Liu, YC. E3Ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to maintain regulatory T cell stability and suppressive capacity. Immunity 2015;42:1062–74. https://doi.org/10.1016/j.immuni.2015.05.016.Search in Google Scholar PubMed PubMed Central
122. Miska, J, Lee-Chang, C, Rashidi, A, Muroski, ME, Chang, AL, Lopez-Rosas, A, et al.. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of Tregs in glioblastoma. Cell Rep 2019;27:226–37.e4. https://doi.org/10.1016/j.celrep.2019.03.029.Search in Google Scholar PubMed PubMed Central
123. Finlay, DK, Rosenzweig, E, Sinclair, LV, Feijoo-Carnero, C, Hukelmann, JL, Rolf, J, et al.. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 2012;209:2441–53. https://doi.org/10.1084/jem.20112607.Search in Google Scholar PubMed PubMed Central
124. Doedens, AL, Phan, AT, Stradner, MH, Fujimoto, JK, Nguyen, JV, Yang, E, et al.. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat Immunol 2013;14:1173–82. https://doi.org/10.1038/ni.2714.Search in Google Scholar PubMed PubMed Central
125. Palazon, A, Tyrakis, PA, Macias, D, Veliça, P, Rundqvist, H, Fitzpatrick, S, et al.. An HIF-1α/VEGF-A Axis in cytotoxic T cells regulates tumor progression. Cancer Cell 2017;32:669–83.e5. https://doi.org/10.1016/j.ccell.2017.10.003.Search in Google Scholar PubMed PubMed Central
126. Millet, GP, Debevec, T, Brocherie, F, Malatesta, D, Girard, O. Therapeutic use of exercising in hypoxia: promises and limitations. Front Physiol 2016;7. https://doi.org/10.3389/fphys.2016.00224.Search in Google Scholar PubMed PubMed Central
127. Betof, AS, Lascola, CD, Weitzel, D, Landon, C, Scarbrough, PM, Devi, GR, et al.. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J Natl Cancer Inst 2015;107. https://doi.org/10.1093/jnci/djv040.Search in Google Scholar PubMed PubMed Central
128. McCullough, DJ, Stabley, JN, Siemann, DW, Behnke, BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst 2014;106. https://doi.org/10.1093/jnci/dju036.Search in Google Scholar PubMed PubMed Central
129. Schumacher, O, Galvão, DA, Taaffe, DR, Chee, R, Spry, N, Newton, RU. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis 2021;24. https://doi.org/10.1038/s41391-020-0245-z.Search in Google Scholar PubMed PubMed Central
130. Morland, C, Andersson, KA, Haugen, ØP, Hadzic, A, Kleppa, L, Gille, A, et al.. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun 2017;8. https://doi.org/10.1038/ncomms15557.Search in Google Scholar PubMed PubMed Central
131. Brocherie, F, Millet, GP. Hypoxic exercise as an effective nonpharmacological therapeutic intervention. Exp Mol Med 2020;52:529–30. https://doi.org/10.1038/s12276-020-0400-6.Search in Google Scholar PubMed PubMed Central
132. Vorrink, SU, Domann, FE. Regulatory crosstalk and interference between the and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem Biol Interact 2014;218:82–8. https://doi.org/10.1016/j.cbi.2014.05.001.Search in Google Scholar PubMed PubMed Central
133. Sondermann, NC, Faßbender, S, Hartung, F, Hätälä, AM, Rolfes, KM, Vogel, CFA, et al.. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem Pharmacol 2023;208.10.1016/j.bcp.2022.115371Search in Google Scholar PubMed PubMed Central
134. Zhang, M, Hu, Y, Yang, F, Zhang, J, Zhang, J, Yu, W, et al.. Interaction between AhR and HIF-1 signaling pathways mediated by ARNT/HIF-1β. BMC Pharmacol Toxicol 2022;23. https://doi.org/10.1186/s40360-022-00564-8.Search in Google Scholar PubMed PubMed Central
135. Mascanfroni, ID, Takenaka, MC, Yeste, A, Patel, B, Wu, Y, Kenison, JE, et al.. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med 2015;21:638–46. https://doi.org/10.1038/nm.3868.Search in Google Scholar PubMed PubMed Central
136. Javelle, F, Bloch, W, Knoop, A, Guillemin, GJ, Zimmer, P. Toward a neuroprotective shift: eight weeks of high intensity interval training reduces the neurotoxic kynurenine activity concurrently to impulsivity in emotionally impulsive humans – a randomized controlled trial. Brain Behav Immun 2021;96:7–17. https://doi.org/10.1016/j.bbi.2021.04.020.Search in Google Scholar PubMed
137. Zimmer, P, Schmidt, ME, Prentzell, MT, Berdel, B, Wiskemann, J, Kellner, KH, et al.. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front Oncol 2019;9. https://doi.org/10.3389/fonc.2019.00962.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter on behalf of Shangai Jiao Tong University and Guangzhou Sport University
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Issue 3: Skeletal muscle, exercise, aging and chronic disease
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Impact of exercise and fasting on mitochondrial regulators in human muscle
- Effectiveness of aerobic exercise interventions on balance, gait, functional mobility and quality of life in Parkinson’s disease: an umbrella review
- Creatine and strength training in older adults: an update
- Creatine supplementation strategies aimed at acutely increasing and maintaining skeletal muscle total creatine content in healthy, young volunteers
- Section: Physical activity/inactivity and health across the lifespan
- Independent mobility and physical activity among children residing in an ultra-dense metropolis
- Physical activity and cardiometabolic risk factors in sprint and jump-trained masters athletes, young athletes and non-physically active men
- Cross-sectional analysis of blood leukocyte responsiveness to interleukin-10 and interleukin-6 across age and physical activity level
- Section: Exercise and E-health, M-health, AI and technology
- Assessing core body temperature in a cool marathon using two pill ingestion strategies
- Issue 4: Preclinical and clinical approaches to translational exercise biomedicine
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Nicotinic acid improves mitochondrial function and associated transcriptional pathways in older inactive males
- Exogenous Beta-guanidinopropionic acid administration enhances electromyostimulation-induced mitochondrial biogenesis in rat skeletal muscle
- How exercise shapes the anti-inflammatory environment in multiple sclerosis – a conceptual framework focusing on tryptophan-derived molecules in T cell differentiation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Acute effects of high-intensity interval training on microvascular circulation: a case control study in uveal melanoma
- Discrepancies in walking speed measurements post-bed-rest: a comparative analysis of real-world vs. laboratory assessments
- Section: Sports medicine and movement science
- Lower-body strength, power and sprint front crawl performance
- Section: Letter to the editor
- Comment on: “A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist”
- Author’s response to “letter to the editor comment on: ‘A unique pseudo-eligibility analysis of longitudinal laboratory performance Data from a transgender female competitive cyclist’” by Lundberg, O’Connor, Kirk, Pollock, and Brown
Articles in the same Issue
- Frontmatter
- Issue 3: Skeletal muscle, exercise, aging and chronic disease
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Impact of exercise and fasting on mitochondrial regulators in human muscle
- Effectiveness of aerobic exercise interventions on balance, gait, functional mobility and quality of life in Parkinson’s disease: an umbrella review
- Creatine and strength training in older adults: an update
- Creatine supplementation strategies aimed at acutely increasing and maintaining skeletal muscle total creatine content in healthy, young volunteers
- Section: Physical activity/inactivity and health across the lifespan
- Independent mobility and physical activity among children residing in an ultra-dense metropolis
- Physical activity and cardiometabolic risk factors in sprint and jump-trained masters athletes, young athletes and non-physically active men
- Cross-sectional analysis of blood leukocyte responsiveness to interleukin-10 and interleukin-6 across age and physical activity level
- Section: Exercise and E-health, M-health, AI and technology
- Assessing core body temperature in a cool marathon using two pill ingestion strategies
- Issue 4: Preclinical and clinical approaches to translational exercise biomedicine
- Section: Integrated exercise physiology, biology, and pathophysiology in health and disease
- Nicotinic acid improves mitochondrial function and associated transcriptional pathways in older inactive males
- Exogenous Beta-guanidinopropionic acid administration enhances electromyostimulation-induced mitochondrial biogenesis in rat skeletal muscle
- How exercise shapes the anti-inflammatory environment in multiple sclerosis – a conceptual framework focusing on tryptophan-derived molecules in T cell differentiation
- Section: Personalized and advanced exercise prescription for health and chronic diseases
- Acute effects of high-intensity interval training on microvascular circulation: a case control study in uveal melanoma
- Discrepancies in walking speed measurements post-bed-rest: a comparative analysis of real-world vs. laboratory assessments
- Section: Sports medicine and movement science
- Lower-body strength, power and sprint front crawl performance
- Section: Letter to the editor
- Comment on: “A unique pseudo-eligibility analysis of longitudinal laboratory performance data from a transgender female competitive cyclist”
- Author’s response to “letter to the editor comment on: ‘A unique pseudo-eligibility analysis of longitudinal laboratory performance Data from a transgender female competitive cyclist’” by Lundberg, O’Connor, Kirk, Pollock, and Brown


