Abstract
Objectives
The prevalence of osteoarthritis (OA) is rising, and pain is the hallmark symptom of OA. Pain in OA is complicated and can be influenced by multiple joint-related factors and factors related to, e.g., physiological, epigenetic, and pain sensory profiles. Increasing evidence suggests that a subset of patients with OA are pain sensitive. This can be assessed using quantitative sensory testing (QST). Common treatments of OA are total knee arthroplasty (TKA) and administration of 3-weeks of non-steroidal anti-inflammatory drugs (NSAIDs), which provide pain relief to many patients with OA. However, approx. 20% of patients experience chronic postoperative pain after TKA, whereas NSAIDs provide an average pain relief of approx. 25%. The current topical review focuses on the emerging evidence linking pretreatment QST to the treatment response of TKA and NSAID treatments.
Content
MEDLINE was systematically searched for all studies from 2000 to 2022 on pretreatment QST, TKA, and NSAIDs. Pre-clinical studies, reviews, and meta-analyses were excluded.
Summary
Currently, 14 studies on TKA and four studies on NSAIDs have been published with the aim to attempt prediction of the treatment response. The QST methodologies in the studies are inconsistent, but 11/14 (79%) studies on TKA and 4/4 (100%) studies on NSAIDs report statistically significant associations between pretreatment QST and chronic postoperative pain after TKA or analgesic effect after NSAID treatment. The strength of the associations remains low-to-moderate. The most consistent pretreatment QST predictors are pressure pain thresholds, temporal summation of pain, and conditioned pain modulation.
Outlook
The use of QST as predictors of standard OA treatment is interesting, but the predictive strength remains low-to-moderate. A transition of QST from a research-based setting and into the clinic is not advised until the predictive strength has been improved and the methodology has been standardized.
Introduction
Osteoarthritis (OA) is characterized by cartilage degeneration and associated with decreased function, lowered quality of life, and pain. Studies have found that the assessment of cartilage degeneration does not explain the pain reported in OA [1], [2], [3], suggesting that other factors must be responsible for the pain in OA. Pain in OA is complex and it has been shown that cognitive factors [4], inflammation [5, 6], and pain sensitivity [7] are among the factors being associated with clinical pain in OA, see Figure 1.
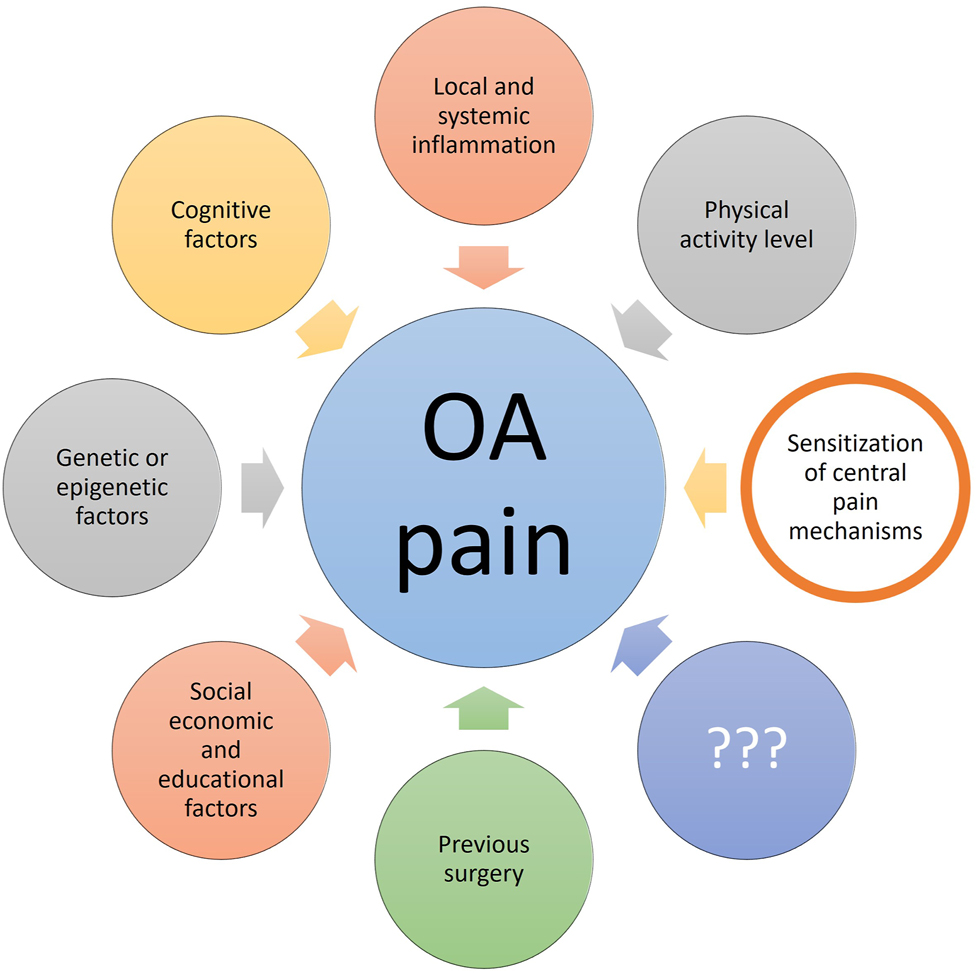
Factors associated with pain in osteoarthritis (OA). The current review focuses on the contribution of sensitization of central pain mechanisms, and it is important to mention that this is one of many factors associated with OA pain. The figure has been copied with permission from the doctoral thesis by Kristian K. Petersen, 2021, Aalborg University, Denmark (link: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://vbn.aau.dk/ws/portalfiles/portal/450213713/1061952_thesis_mechanistic_pain_profiling_of_patients_with_osteoarthritis_kkp.pdf).
The Osteoarthritis Research International (OARSI) organization provides clinical guidelines for the treatment of pain in OA. Some of the most utilized treatments are non-steroidal anti-inflammatory drugs (NSAIDs) plus paracetamol and total knee arthroplasty (TKA) [8, 9].
Standard pain treatment for osteoarthritis
Treatment of OA is focused on alleviating pain, improving joint function, and increasing quality of life. Based on this, the OARSI provides recommendations for the treatment of OA, which are divided into non-surgical [8] and surgical [9] therapies.
Pharmacological and non-pharmacological options are available [8, 10, 11]. Hence, the 2019 OARSI guidelines strongly endorse the use of topical NSAIDs and conditionally (with high consensus) recommend oral non-selective NSAIDs and COX-2 inhibitors [8]. The analgesic effect of NSAIDs has been widely documented [12, 13], but the underlying pain-relieving mechanisms remain largely unclear [14]. NSAIDs (and paracetamol) inhibit the synthesis of prostaglandins through modulation of cyclooxygenase (COX). Animal studies document that non-selective NSAIDs and paracetamol increase the activity of the cannabinoid system [15] and that the effect of NSAIDs and selective COX-2 inhibitors depends on an intact serotonin system [14]. Clinical studies suggest that COX-2 inhibitors can modulate widespread hyperalgesia [16], [17], [18], which further suggests that COX-2 inhibitors act on the central pain mechanisms. The long-term use of NSAIDs is harmful [17], and a study has found that 1 g of paracetamol and 400 mg of Ibuprofen three times daily (a total of 3 g of paracetamol and 1.2 g of ibuprofen daily) for a 3-week period provide analgesia with limited side-effects [19]. The analgesic effect of topical and oral NSAIDs is similar [20], and the average analgesic effect is approx. 20–35% [19].
Surgical treatment options for OA include arthroscopic surgery and total knee arthroplasty (TKA) [9], with TKA focusing on pain relief. TKA is considered the end-stage treatment of OA and is frequently performed [21]. After TKA, the majority of patients will improve in pain, function, and quality of life when comparing with non-surgical treatments [22]. However, it is evident that 10–20% of the patients experience chronic postoperative pain after TKA [23], which is a major issue since the number of TKAs is increasing world-wide [24], [25], [26], [27], [28], [29].
It is evident that both NSAIDs and TKA provide pain relief to patients with OA. However, some patients might respond better than others to these treatments and identifying these patients prior to treatment is needed to advance the concept of “personalized pain medicine”.
Pain profiling of patients with osteoarthritis using quantitative sensory testing
“Personalized medicine” aims to provide the right treatment to the right patients, and this concept is being developed in, e.g., cancer medicine [30], [31], [32], [33]. The concept may also be applied to pain medicine in future. Thus, the current research is focusing on (1) identifying the underlying mechanisms of pain, (2) identifying parameters which might predict treatment outcomes, and (3) developing new treatments or utilizing available treatments for new indications to target the underlying mechanisms of pain.
Quantitative sensory testing (QST) aims to assess nerve function, and QST assessments are often used to asses pain sensitivity in patients with OA [7]. Pressure pain thresholds (PPTs) assessed over the OA-affected joint mainly reflect localized pressure hyperalgesia, whereas PPTs assessed outside of the OA-affected joint indicate widespread pressure hyperalgesia [34, 35]. Both localized and widespread pressure hyperalgesia are found in patients with severe OA compared with pain-free individuals [36, 37], see Figure 2A. The wind-up process of dorsal horn neurons reflects the excitability of dorsal horn neurons [38], and temporal summation of pain (TSP), the human surrogate model for the wind-up process [34], is facilitated in patients with severe OA compared with pain-free individuals [7], see Figure 2B. The descending pain inhibitory system originates from the brain stem and projects towards the dorsal horn. One human surrogate assessment for these systems is conditioned pain modulation (CPM) [39], which has been found impaired in patients with severe knee OA compared with healthy individuals [7], see Figure 2C. It seems evident that a certain subset of patients with OA are more pain sensitive than other patients [40, 41]. Studies suggest that sleep deprivation [42, 43], pain catastrophizing thoughts [44], and low-grade inflammation [45] could be factors that impact the degree of pain sensitivity.
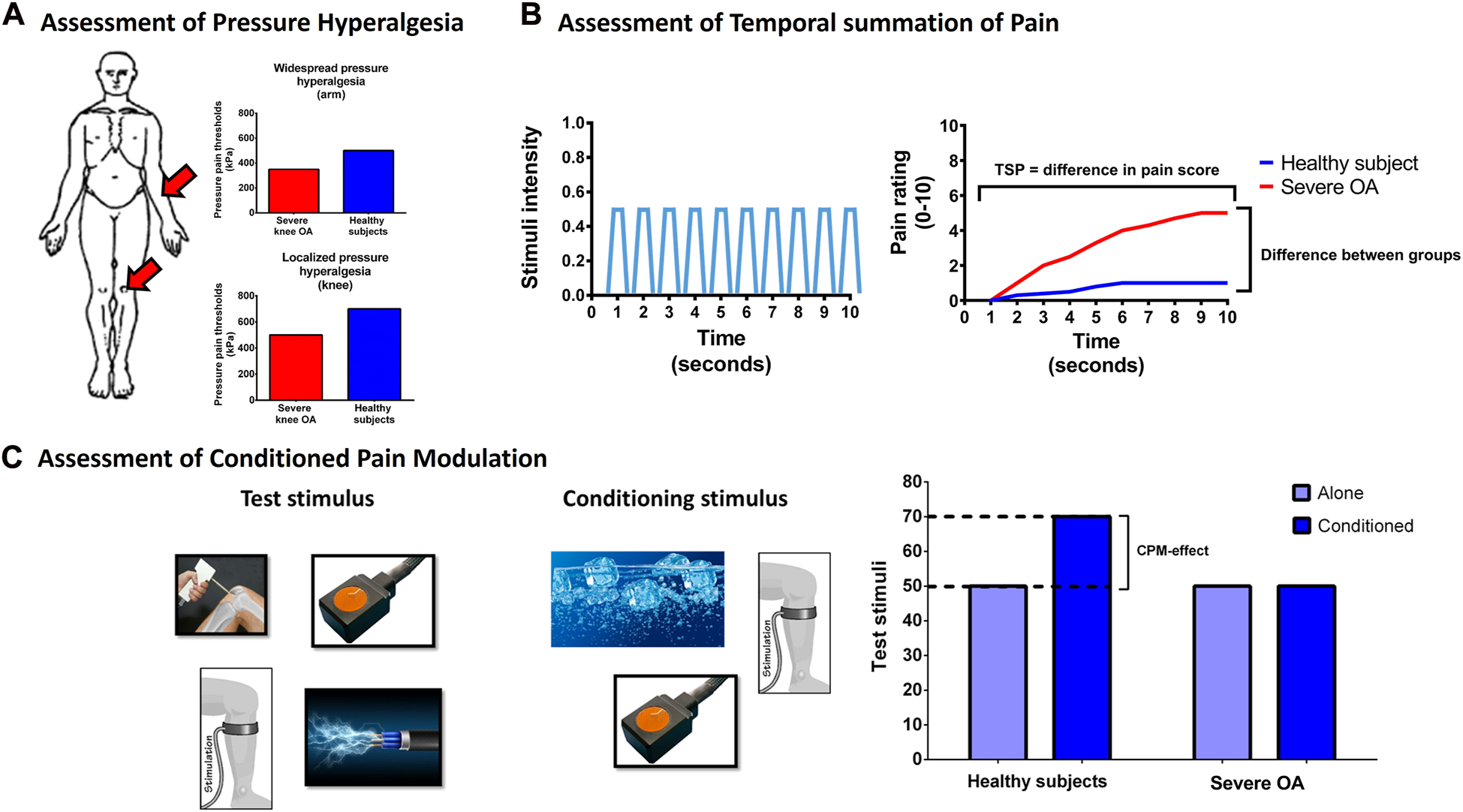
Illustrations of assessments and outcomes of (A) pressure hyperalgesia, (B) temporal summation of pain (TSP), and (C) conditioned pain modulation (CPM) of patients with severe knee osteoarthritis (OA) when compared with healthy pain-free individuals.
(A) Assessments of pressure pain thresholds (PPTs) at the knee (assessment of localized hyperalgesia) and at the arm (assessment of widespread hyperalgesia). (B) TSP relies on continuously fast stimuli with the same intensity and is calculated as the pain score to these painful stimuli over time. (C) Assessment of CPM requires a test stimulus and a conditioning stimulus, and multiple paradigms of test and conditioning stimuli have been utilized. In general, patients with severe OA demonstrate localized and widespread hyperalgesia, facilitated TSP and impaired CPM when compared with pain-free healthy individuals. The figure has been copied and modified with permission from the doctoral thesis by Kristian K. Petersen, 2021, Aalborg University, Denmark (link: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://vbn.aau.dk/ws/portalfiles/portal/450213713/1061952_thesis_mechanistic_pain_profiling_of_patients_with_osteoarthritis_kkp.pdf).
Studies on patients undergoing abdominal [46] and thorax [47] surgery have indicated an association between preoperative pain sensitivity and chronic postoperative pain. Further, a recent systematic review found that this might apply to a range of surgical procedures [48]. Similarly, pretreatment QST has been associated with analgesic effects of opioid pain relief [49], duloxetine [50], or pregabalin [51]. The current topical review will present the current evidence for QST as a predictor of NSAID and TKA treatment of pain in OA.
Methods
This topical review is based on a systematic literature search, which was conducted in May 2022 in the MEDLINE database. Studies focusing on patients with knee OA, pre-treatment QST, and treatment responses to NSAIDs and TKA surgery were included. Reviews, meta-analyses, and preclinical studies were excluded.
Mechanistic pain profiling using quantitative sensory testing of patients with osteoarthritis
Currently, 14 studies on TKA [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65] and four studies on NSAIDs [18, 66], [67], [68] have been published utilizing pre-treatment QST aiming to predict the treatment response.
The methodologies in the TKA and NSAID studies differ with, e.g., sample size ranging from n=14 [58] to n=288 patients [62], the number of QST modalities tested ranging from one [56, 60, 65] to six [63], and outcome measures ranging from the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [53, 56, 59, 62], visual analogue scale (VAS) scores [18, 52, 55, 57, 60, 61, 63, 64, 66, 67], numerical rating scale (NRS) scores [54, 58, 65] to change in average daily pain intensity [68].
A total of eleven (out of fourteen) studies (79%) found associations between preoperative QST and chronic postoperative pain. Likewise, four (out of four) studies (100%) found associations between pretreatment QST and analgesic response to NSAIDs. A full overview of the studies can be found in the supplementary material (https://vbn.aau.dk/da/publications/mechanistic-pain-profiling-of-patients-with-osteoarthritis-curren).
Stimulation modalities for the prediction models
Multiple modalities were utilized to assess QST prior to TKA and NSAID therapies. The predictive strength of the models varied from low to moderate and the stimuli modalities might impact the predictive strength.
Preoperative assessment prior to total knee arthroplasty
A single study [52] utilized electrical stimuli and found lower electrical detection and pain thresholds to predict higher chronic postoperative pain after TKA (100%). Pressure stimuli were assessed in 10 studies [53], [54], [55], [56], [57], [58, 60, 62], [63], [64], and lower pressure pain and tolerance thresholds were found to predict higher chronic postoperative pain after TKA in four studies (40%) [53, 57, 60, 63]. Thermal stimuli were assessed in three studies [53, 54, 61], and lower warm detection thresholds and lower heat pain thresholds were found predict higher chronic postoperative pain in one study (33%) [61]. TSP was assessed in six studies [55, 57, 59, 61], [62], [63], and facilitated TSP was found to predict higher chronic postoperative pain in four studies (67%) [55, 61], [62], [63]. CPM was assessed in eight studies [55, 57], [58], [59, 62], [63], [64], [65], and impaired CPM was found to predict higher chronic postoperative pain in three studies (38%) [58, 64, 65]. Finally, one study [58] assessed exercise-induced hypoalgesia and found that lower exercise-induced hypoalgesia predicted higher chronic postoperative pain (100%).
Pre-treatment assessments prior to non-steroidal anti-inflammatory drugs treatments
Pressure stimuli were reported in three studies [18, 67, 68] and were found predictive for analgesic effect of NSAIDs in none of the studies (0%). TSP was reported in three studies [18, 67, 68], and facilitated TSP was found to be predictive of a poor analgesic effect of NSAIDs in two studies (67%) [18, 67]. CPM was reported in two studies [66, 68], and impaired CPM was found to be predictive of a poor analgesic effect of NSAIDs in both studies (100%). Offset analgesia was assessed in one study [66] and not found predictive of the analgesic effect of NSAIDs (0%).
Discussion
The current topical review highlights that the majority of studies demonstrate that pain sensitive patients with knee osteoarthritis are more likely to report chronic postoperative pain after total knee arthroplasty. Further, they also report a poor analgesic response to non-steroidal anti-inflammatory drugs. Pressure pain thresholds, temporal summation of pain, and conditioned pain modulation are the most frequently assessed modalities and are most often found as pre-treatment predictors. The strength of the associations between pretreatment pain sensory profiles and treatment responses to total knee arthroplasty and non-steroidal anti-inflammatory drugs is low-to-moderate.
Predicting treatment responses in patients with osteoarthritis
The current topical review focuses on the predictive value of QST for treatment responses to TKA and NSAIDs in patients with knee OA. The results highlight that the predictive value is low-to-moderate. It is well-known that factors such as psychological factors [44], inflammation [69], and sleep [42, 70] can influence QST parameters. Studies have found that high levels of preoperative pain catastrophizing [4], high levels of preoperative inflammation, dysregulated epigenetic profiles [5, 71, 72], and poor preoperative sleep quality [73] are directly or indirectly associated with chronic postoperative pain after TKA. Larsen et al. 2021 [64] found that the combination of preoperative CPM and pain catastrophizing predicted chronic postoperative pain after TKA better than each of the factors alone, suggesting that combining known pretreatment assessments is likely to improve future predictive models.
The current review focuses on TKA and NSAID treatment of pain in OA, but OARSI also recommends exercise-based therapy as a treatment of pain in OA [8]. Studies have found that long-term exercise programs provide approx. 25% pain relief in patients with OA [74, 75]. Four studies utilized QST to predict the pain relief following long-term exercise programs in OA with two studies finding associations [76, 77] and two studies finding no associations [60, 78] between pre-treatment QST and pain-relief following exercise-based therapy. The studies which did find associations between pre-treatment QST and treatment response utilized assessments of central pain mechanisms (i.e., TSP, exercise-induced hypoalgesia, widespread pressure hyperalgesia) [76, 77], whereas the studies which did not find associations mainly used pressure pain thresholds [60, 78]. Future large-scale studies should be conducted utilizing assessments of central pain mechanisms to understand if QST can predict the response to exercise-based therapy.
Finally, the 2019 OARSI recommendations added duloxetine as a treatment option for patients with knee OA, depression, and/or widespread pain [8]. Studies have found that duloxetine provides an analgesic effect when compared with placebo [79, 80]. In a placebo-controlled trial, Petersen et al. 2022 [81] demonstrated that a combination of pre-treatment QST, psychological factors, and clinical pain could predict the analgesic effect of 14 weeks’ treatment of duloxetine and that the strength was much stronger for the prediction of duloxetine than for the prediction of the placebo response.
Methodological considerations
QST as an assessment tool is associated with substantial inter-person variability and CPM has been discussed in recent years [82]. CPM can be assessed using a range of different tools in combination, and it is evident that combining these assessment tools will affect the reliability [83]. In 2015, Yarnitsky and colleagues [84] published the first CPM recommendations aiming to standardize the assessment of CPM, but it is still evident that CPM assessments are different across studies [82] which limits the generalizability of the results.
This topical review highlights associations between pre-treatment QST and treatment responses to TKA and NSAIDs but also highlights that there is inconsistency of QST assessments which are found as predictors. Pre-treatment PPTs were found as a predictor in 40% of TKA [53, 57, 60, 63] and 0% of NSAID studies, TSP was found as a predictor in 67% of TKA [55, 61], [62], [63] and 67% of NSAID studies [18, 67], and CPM was found as a predictor in 38% of TKA [58, 64, 65] and 100% of NSAID studies [66, 68]. Based on this, one could argue that TSP seems to be the most consistent predictor of OA treatment responses. However, large-scale studies specifically designed to investigate the predictive value of TSP are needed to confirm this hypothesis.
Targeting pain sensitivity
It is still widely unknown why some patients are more pain sensitive than others. It seems evident that some healthy individuals are more pain sensitive than others [85, 86]. A recent study on young pain-free individuals demonstrated that sleep deprivation could lead to an impairment of CPM and facilitation of TSP [87]. Sleep deprivation has also been linked to increased pain sensitivity in patients with OA [43], but studies aiming to improve sleep quality and the potential effect on QST is currently lacking.
Serotonin and noradrenaline are important neurotransmitters for the descending pain inhibitory systems [88, 89], and the balance of the descending pain inhibitory system is assessed by CPM in humans [39, 90, 91]. Duloxetine is an anti-depressant serotonin-noradrenalin re-uptake inhibitory and studies on patients with diabetic neuropathy [50] and migraine [92] suggest that duloxetine might provide an analgesic effect in pain sensitive patients. A recent small study on OA demonstrated that pain sensitive patients are more likely to gain an analgesic effect of duloxetine than non-pain sensitive patients, but the study also found many adverse events to the duloxetine treatment [81]. Pre- and postoperative administration of duloxetine to pain sensitive patients scheduled for TKA seems to decrease postoperative pain [93] although contradicting results exist [94]. These findings may suggest that duloxetine could potentially be a treatment option for pain sensitive OA patients and may be used as an add-on treatment prior to TKA. However, larger trials need to confirm this hypothesis. Additionally, studies should investigate if short-term administration of duloxetine is sufficient to provide analgesic effects in pain sensitive patients since a life-long administration of duloxetine is unwanted due to adverse events.
The N-methyl-D-aspartate (NMDA) receptors are involved in TSP [38], and a study on patients with fibromyalgia found that ketamine (an NMDA-antagonist) attenuates TSP when compared with placebo [95]. Other studies suggest that perioperative administration of ketamine reduces acute postoperative pain after TKA [96, 97]. However, a small study investigating the effect of 48-h postoperative infusion of ketamine or placebo did not find any differences in pain scores 12 months after TKA when comparing ketamine with placebo [98]. The direct use of ketamine in the treatment of OA pain and especially as an add-on treatment in relation to TKA is interesting but needs further investigations.
Conclusions
This topical review highlights that pretreatment assessments of quantitative sensory testing can be linked with treatment responses to total knee arthroplasty and NSAIDs in patients with osteoarthritis. The predictive value of quantitative sensory testing for treatment responses remains low-to-moderate. The use of quantitative sensory testing as a prognostic tool for patients with osteoarthritis is promising, but a transition from a research-based setting and into the clinic is not advised before the predictive strength has been improved and the methodology has been standardized.
-
Side note: This work is based on the doctoral thesis by Kristian K. Petersen to obtain the higher doctorate degree of Doctor Medicinae (D.M.Sc.). The thesis was defended on September 10th, 2021, at Aalborg University, Denmark. See supplementary material for the full thesis work.
-
Research funding: KKP acknowledges support from The Aalborg University Talent Management Programme (j.no. 771126) providing the funding to initiate this work. Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121). The Center for Mathematical Modeling of Knee Osteoarthritis (MathKOA) is funded by the Novo Nordisk Foundation (NNF21OC0065373).
-
Author contributions: KKP conceptualized the idea, KKP conducted the review, and KKP wrote the first draft and KKP submitted the manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interests: No conflict of interest.
-
Informed consent: Not relevant due to the nature of the review.
-
Ethical approval: Not relevant due to the nature of the review.
References
1. Dieppe, P, Lohmander, L. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965–73. https://doi.org/10.1016/s0140-6736(05)71086-2.Search in Google Scholar PubMed
2. Felson, DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol 2005;17:624–8. https://doi.org/10.1097/01.bor.0000172800.49120.97.Search in Google Scholar PubMed
3. Hannan, MT, Felson, DT, Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 2000;27:1513–7.Search in Google Scholar
4. Edwards, RR, Cahalan, C, Mensing, G, Smith, M, Haythornthwaite, JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7:216–24. https://doi.org/10.1038/nrrheum.2011.2.Search in Google Scholar PubMed
5. Giordano, R, Petersen, KK, Andersen, HH, Simonsen, O, Arendt-Nielsen, L. Serum inflammatory markers in patients with knee osteoarthritis: a proteomic approach. Clin J Pain 2020;36:229–37. https://doi.org/10.1097/ajp.0000000000000804.Search in Google Scholar PubMed
6. Eitner, A, Pester, J, Vogel, F, Marintschev, I, Lehmann, T, Hofmann, GO, et al.. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain 2017;158:1743–53. https://doi.org/10.1097/j.pain.0000000000000972.Search in Google Scholar PubMed
7. Arendt-Nielsen, L, Skou, ST, Nielsen, TA, Petersen, KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep 2015;13:225–34. https://doi.org/10.1007/s11914-015-0276-x.Search in Google Scholar PubMed
8. Bannuru, RR, Osani, MC, Vaysbrot, EE, Arden, NK, Bennell, K, Bierma-Zeinstra, SMA, et al.. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 2019;27:1578–89. https://doi.org/10.1016/j.joca.2019.06.011.Search in Google Scholar PubMed
9. Zhang, W, Moskowitz, RW, Nuki, G, Abramson, S, Altman, RD, Arden, N, et al.. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartil 2008;16:137–62. https://doi.org/10.1016/j.joca.2007.12.013.Search in Google Scholar PubMed
10. Wallis, JA, Taylor, NF. Pre-operative interventions (non-surgical and non-pharmacological) for patients with hip or knee osteoarthritis awaiting joint replacement surgery-a systematic review and meta-analysis. Osteoarthr Cartil 2011;19:1381–95. https://doi.org/10.1016/j.joca.2011.09.001.Search in Google Scholar PubMed
11. Skou, ST, Roos, EM. Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol 2019;37(120 Suppl):112–7.Search in Google Scholar
12. Jordan, KM, Arden, NK, Doherty, M, Bannwarth, B, Bijlsma, JWJ, Dieppe, P, et al.. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. https://doi.org/10.1136/ard.2003.011742.Search in Google Scholar PubMed PubMed Central
13. Hochberg, MC, Altman, RD, April, KT, Benkhalti, M, Guyatt, G, McGowan, J, et al.. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. https://doi.org/10.1002/acr.21596.Search in Google Scholar PubMed
14. Graham, GG, Davies, MJ, Day, RO, Mohamudally, A, Scott, KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013;21:201–32. https://doi.org/10.1007/s10787-013-0172-x.Search in Google Scholar PubMed
15. Ahn, DK, Choi, HS, Yeo, SP, Woo, YW, Lee, MK, Yang, GY, et al.. Blockade of central cyclooxygenase (COX) pathways enhances the cannabinoid-induced antinociceptive effects on inflammatory temporomandibular joint (TMJ) nociception. Pain 2007;132:23–32. https://doi.org/10.1016/j.pain.2007.01.015.Search in Google Scholar PubMed
16. Reinold, H, Ahmadi, S, Depner, UB, Layh, B, Heindl, C, Hamza, M, et al.. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest 2005;115:673–9. https://doi.org/10.1172/jci23618.Search in Google Scholar PubMed PubMed Central
17. Malfait, AM, Schnitzer, TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol 2013;9:654–64. https://doi.org/10.1038/nrrheum.2013.138.Search in Google Scholar PubMed PubMed Central
18. Arendt-Nielsen, L, Egsgaard, LL, Petersen, KK. Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain 2016;157:1634–44. https://doi.org/10.1097/j.pain.0000000000000562.Search in Google Scholar PubMed
19. Doherty, M, Hawkey, C, Goulder, M, Gibb, I, Hill, N, Aspley, S, et al.. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee pain. Ann Rheum Dis 2011;70:1534–41. https://doi.org/10.1136/ard.2011.154047.Search in Google Scholar PubMed
20. Zeng, C, Doherty, M, Persson, MSM, Yang, Z, Sarmanova, A, Zhang, Y, et al.. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr Cartil 2021;29:1242–51. https://doi.org/10.1016/j.joca.2021.06.004.Search in Google Scholar PubMed
21. Carr, AJ, Robertsson, O, Graves, S, Price, AJ, Arden, NK, Judge, A, et al.. Knee replacement. Lancet 2012;379:1331–40. https://doi.org/10.1016/s0140-6736(11)60752-6.Search in Google Scholar
22. Skou, ST, Roos, EM, Laursen, MB, Rathleff, MS, Arendt-Nielsen, L, Simonsen, O. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597–606. https://doi.org/10.1056/nejmoa1505467.Search in Google Scholar PubMed
23. Beswick, AD, Wylde, V, Gooberman-Hill, R, Blom, A, Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of Prospective studies in unselected patients. BMJ Open 2012;2:1–12. https://doi.org/10.1136/bmjopen-2011-000435.Search in Google Scholar PubMed PubMed Central
24. Kim, HA, Kim, S, Seo, YI, Choi, HJ, Seong, SC, Song, YW, et al.. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology 2008;47:88–91. https://doi.org/10.1093/rheumatology/kem308.Search in Google Scholar PubMed
25. Singh, JA, Vessely, MB, Harmsen, WS, Schleck, CD, Melton, LJ, Kurland, RL, et al.. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc 2010;85:898–904. https://doi.org/10.4065/mcp.2010.0115.Search in Google Scholar PubMed PubMed Central
26. Culliford, DJ, Maskell, J, Beard, DJ, Murray, DW, Price, AJ, Arden, NK. Temporal trends in hip and knee replacement in the United Kingdom: 1991 to 2006. J Bone Joint Surg Br 2010;92:130–5. https://doi.org/10.1302/0301-620x.92b1.22654.Search in Google Scholar PubMed
27. Kurtz, S, Ong, K, Lau, E, Mowat, F, Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J bone Jt Surg 2007;89:780–5. https://doi.org/10.2106/jbjs.f.00222.Search in Google Scholar
28. Singh, JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J 2011;5:80–5. https://doi.org/10.2174/1874325001105010080.Search in Google Scholar PubMed PubMed Central
29. Ackerman, IN, Bohensky, MA, de Steiger, R, Brand, CA, Eskelinen, A, Fenstad, AM, et al.. Substantial rise in the lifetime risk of primary total knee replacement surgery for osteoarthritis from 2003 to 2013: an international, population-level analysis. Osteoarthr Cartil 2017;25:455–61. https://doi.org/10.1016/j.joca.2016.11.005.Search in Google Scholar PubMed
30. Li, Q, Shao, Y, Zhang, X, Zheng, T, Miao, M, Qin, L, et al.. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol 2015;36:2007–12. https://doi.org/10.1007/s13277-014-2807-y.Search in Google Scholar PubMed
31. Duan, W, Du, L, Jiang, X, Wang, R, Yan, S, Xie, Y, et al.. Identification of a serum circulating lncRNA panel for the diagnosis and recurrence prediction of bladder cancer. Oncotarget 2016;7:78850–8. https://doi.org/10.18632/oncotarget.12880.Search in Google Scholar PubMed PubMed Central
32. Crea, F, Watahiki, A, Quagliata, L, Xue, H, Pikor, L, Parolia, A, et al.. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget 2014;5:764–74. https://doi.org/10.18632/oncotarget.1769.Search in Google Scholar PubMed PubMed Central
33. Longo, DL. Personalized medicine for primary treatment of serous ovarian cancer. N Engl J Med 2019;381:2471–4. https://doi.org/10.1056/nejme1914488.Search in Google Scholar
34. Arendt-Nielsen, L, Graven-Nielsen, T. Translational musculoskeletal pain research. Best Pract Res Rheumatol 2011;25:209–26. https://doi.org/10.1016/j.berh.2010.01.013.Search in Google Scholar PubMed
35. Graven-Nielsen, T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl 2006;122(122 Suppl):1–43. https://doi.org/10.1080/03009740600865980.Search in Google Scholar PubMed
36. Graven-Nielsen, T, Wodehouse, T, Langford, RM, Arendt-Nielsen, L, Kidd, BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012;64:2907–16. https://doi.org/10.1002/art.34466.Search in Google Scholar PubMed
37. Arendt-Nielsen, L, Nie, H, Laursen, MB, Laursen, BS, Madeleine, P, Simonsen, OH, et al.. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81. https://doi.org/10.1016/j.pain.2010.04.003.Search in Google Scholar PubMed
38. Price, DD, Mao, J, Frenk, H, Mayer, DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain 1994;59:165–74. https://doi.org/10.1016/0304-3959(94)90069-8.Search in Google Scholar PubMed
39. Cummins, TM, Kucharczyk, M, Graven-Nielsen, T, Bannister, K. Activation of the descending pain modulatory system using cuff pressure algometry: back translation from man to rat. Eur J Pain 2020;24:1330–8. https://doi.org/10.1002/ejp.1580.Search in Google Scholar PubMed
40. Finan, PH, Buenaver, LF, Bounds, SC, Hussain, S, Park, RJ, Haque, UJ, et al.. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 2013;65:363–72. https://doi.org/10.1002/art.34646.Search in Google Scholar PubMed PubMed Central
41. Arendt-Nielsen, L, Egsgaard, LL, Petersen, KK, Eskehave, TN, Graven- Nielsen, T, Hoeck, HC, et al.. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain 2015;19:1406–17. https://doi.org/10.1002/ejp.651.Search in Google Scholar PubMed
42. Staffe, AT, Bech, MW, Clemmensen, SLK, Nielsen, HT, Larsen, DB, Petersen, KK. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One 2019;14. https://doi.org/10.1371/journal.pone.0225849.Search in Google Scholar PubMed PubMed Central
43. Campbell, CM, Buenaver, LF, Finan, P, Bounds, SC, Redding, M, McCauley, L, et al.. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res 2015;67:1387–96. https://doi.org/10.1002/acr.22609.Search in Google Scholar PubMed PubMed Central
44. Christensen, KS, O’Sullivan, K, Palsson, TS. Conditioned pain modulation efficiency is associated with pain catastrophizing in patients with chronic low back pain. Clin J Pain 2020;36:825–32. https://doi.org/10.1097/ajp.0000000000000878.Search in Google Scholar
45. Schaible, HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 2014;16:470.10.1186/s13075-014-0470-8Search in Google Scholar PubMed PubMed Central
46. Wilder-Smith, OH, Schreyer, T, Scheffer, GJ, Arendt-Nielsen, L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119–28. https://doi.org/10.3109/15360281003706069.Search in Google Scholar PubMed
47. Yarnitsky, D, Crispel, Y, Eisenberg, E, Granovsky, Y, Ben-Nun, A, Sprecher, E, et al.. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–8. https://doi.org/10.1016/j.pain.2007.10.033.Search in Google Scholar PubMed
48. Petersen, KK, Vaegter, HB, Stubhaug, A, Wolff, A, Scammell, BE, Arendt-Nielsen, L, et al.. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain 2021;162:31–44. https://doi.org/10.1097/j.pain.0000000000002019.Search in Google Scholar PubMed
49. Edwards, RR, Smith, MT, Kudel, I, Haythornthwaite, J. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain 2006;126:272–9. https://doi.org/10.1016/j.pain.2006.07.004.Search in Google Scholar PubMed
50. Yarnitsky, D, Granot, M, Nahman-Averbuch, H, Khamaisi, M, Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–8. https://doi.org/10.1016/j.pain.2012.02.021.Search in Google Scholar PubMed
51. Olesen, SS, Graversen, C, Bouwense, SAW, van Goor, H, Wilder-Smith, OHG, Drewes, AM. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One 2013;8:e57963. https://doi.org/10.1371/journal.pone.0057963.Search in Google Scholar PubMed PubMed Central
52. Lundblad, H, Kreicbergs, A, Jansson, K-å. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Jt Surg 2008;90-B:166–71. https://doi.org/10.1302/0301-620x.90b2.19640.Search in Google Scholar PubMed
53. Wylde, V, Palmer, S, Learmonth, ID, Dieppe, P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthr Cartil 2013;21:1253–6. https://doi.org/10.1016/j.joca.2013.05.008.Search in Google Scholar PubMed
54. Noiseux, NO, Callaghan, JJ, Clark, CR, Zimmerman, MB, Sluka, KA, Rakel, BA. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty 2014;29:1383–7. https://doi.org/10.1016/j.arth.2014.01.034.Search in Google Scholar PubMed PubMed Central
55. Petersen, KK, Arendt-Nielsen, L, Simonsen, O, Wilder-Smith, O, Laursen, MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156:55–61. https://doi.org/10.1016/j.pain.0000000000000022.Search in Google Scholar PubMed
56. Wylde, V, Sayers, A, Lenguerrand, E, Gooberman-Hill, R, Pyke, M, Beswick, AD, et al.. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement. Pain 2015;156:47–54. https://doi.org/10.1016/j.pain.0000000000000002.Search in Google Scholar PubMed PubMed Central
57. Petersen, KK, Graven-Nielsen, T, Simonsen, O, Laursen, MBMB, Arendt-Nielsen, L. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain 2016;157:1400–6. https://doi.org/10.1097/j.pain.0000000000000531.Search in Google Scholar PubMed
58. Vaegter, HB, Handberg, G, Emmeluth, C, Graven-Nielsen, T. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 Months after total knee replacement. Clin J Pain 2017;33:475–84. https://doi.org/10.1097/ajp.0000000000000428.Search in Google Scholar
59. Bossmann, T, Brauner, T, Wearing, S, Horstmann, T. Predictors of chronic pain following total knee replacement in females and males: an exploratory study. Pain Manag 2017;7:391–403. https://doi.org/10.2217/pmt-2017-0023.Search in Google Scholar PubMed
60. Arendt-Nielsen, L, Simonsen, O, Laursen, MB, Roos, EM, Rathleff, MS, Rasmussen, S, et al.. Pain and sensitization after total knee replacement or nonsurgical treatment in patients with knee osteoarthritis: identifying potential predictors of outcome at 12 months. Eur J Pain 2018;22:1088–102. https://doi.org/10.1002/ejp.1193.Search in Google Scholar PubMed
61. Petersen, KK, Simonsen, O, Laursen, MB, Arendt-Nielsen, L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;34:193–7. https://doi.org/10.1097/ajp.0000000000000528.Search in Google Scholar
62. Rice, DA, Kluger, MT, McNair, PJ, Lewis, GN, Somogyi, AA, Borotkanics, R, et al.. Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors. Br J Anaesth 2018;121:804–12. https://doi.org/10.1016/j.bja.2018.05.070.Search in Google Scholar PubMed
63. Kurien, T, Arendt-Nielsen, L, Petersen, KK, Graven-Nielsen, T, Scammell, BE. Preoperative neuropathic pain-like symptoms and central pain mechanisms in knee osteoarthritis predicts poor outcome 6 Months after total knee replacement surgery. J Pain 2018;19:1329–41. https://doi.org/10.1016/j.jpain.2018.05.011.Search in Google Scholar PubMed
64. Larsen, DB, Laursen, M, Edwards, RR, Simonsen, O, Arendt-Nielsen, L, Petersen, KK. The combination of preoperative pain, conditioned pain modulation, and pain catastrophizing predicts postoperative pain 12 months after total knee arthroplasty. Pain Med 2021;22:1583–90. https://doi.org/10.1093/pm/pnaa402.Search in Google Scholar PubMed
65. Dürsteler, C, Salazar, Y, Rodriguez, U, Pelfort, X, Verdié, LP. Conditioned pain modulation predicts persistent pain after knee replacement surgery. Pain reports 2021;6:e910. https://doi.org/10.1097/pr9.0000000000000910.Search in Google Scholar
66. Petersen, KK, Simonsen, O, Olesen, AE, Mørch, CD, Arendt-Nielsen, L. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain 2019;23:1904–12. https://doi.org/10.1002/ejp.1465.Search in Google Scholar PubMed
67. Petersen, KK, Olesen, AE, Simonsen, O, Arendt-Nielsen, L. Mechanistic pain profiling as a tool to predict the efficacy of 3-week nonsteroidal anti-inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain 2019;160:486–92. https://doi.org/10.1097/j.pain.0000000000001427.Search in Google Scholar PubMed
68. Edwards, RR, Dolman, AJ, Martel, MO, Finan, PH, Lazaridou, A, Cornelius, M, et al.. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord 2016;17:284–92. https://doi.org/10.1186/s12891-016-1124-6.Search in Google Scholar PubMed PubMed Central
69. Schaible, H-G. Spinal mechanisms contributing to joint pain. Novartis Found Symp 2004;260:4–22. discussion 22-7, 100–4, 277–9.10.1002/0470867639.ch2Search in Google Scholar
70. Edwards, RR, Grace, E, Peterson, S, Klick, B, Haythornthwaite, JA, Smith, MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain 2009;13:1043–7. https://doi.org/10.1016/j.ejpain.2008.12.007.Search in Google Scholar PubMed PubMed Central
71. Gandhi, R, Santone, D, Takahashi, M, Dessouki, O, Mahomed, NN. Inflammatory predictors of ongoing pain 2 years following knee replacement surgery. Knee 2013;20:316–8. https://doi.org/10.1016/j.knee.2012.10.015.Search in Google Scholar PubMed
72. Giordano, R, Petersen, KK, Andersen, HH, Lichota, J, Valeriani, M, Simonsen, O, et al.. Preoperative serum circulating microRNAs as potential biomarkers for chronic postoperative pain after total knee replacement. Mol Pain 2020;16:174480692096292. https://doi.org/10.1177/1744806920962925.Search in Google Scholar PubMed PubMed Central
73. Larsen, DB, Laursen, MB, Simonsen, OH, Arendt-Nielsen, L, Petersen, KK. The association between sleep quality, preoperative risk factors for chronic postoperative pain, and postoperative pain intensity 12 months after knee and hip arthroplasty. Br J Pain 2021;15:486–96. https://doi.org/10.1177/20494637211005803.Search in Google Scholar PubMed PubMed Central
74. Skou, ST, Bricca, A, Roos, EM. The impact of physical activity level on the short- and long-term pain relief from supervised exercise therapy and education: a study of 12, 796 Danish patients with knee osteoarthritis. Osteoarthr Cartil 2018;26:1474–8. https://doi.org/10.1016/j.joca.2018.07.010.Search in Google Scholar PubMed
75. Roos, EM, Grønne, DT, Skou, ST, Zywiel, MG, McGlasson, R, Barton, CJ, et al.. Immediate outcomes following the GLA:D® program in Denmark, Canada and Australia. A longitudinal analysis including 28, 370 patients with symptomatic knee or hip osteoarthritis. Osteoarthr Cartil 2021;29:502–6. https://doi.org/10.1016/j.joca.2020.12.024.Search in Google Scholar PubMed
76. OʼLeary, H, Smart, KM, Moloney, NA, Blake, C, Doody, CM. Pain sensitization associated with nonresponse after physiotherapy in people with knee osteoarthritis. Pain 2018;159:1877–86. https://doi.org/10.1097/j.pain.0000000000001288.Search in Google Scholar PubMed
77. Hansen, S, Vaegter, HB, Petersen, KK. Pretreatment exercise-induced hypoalgesia is associated with change in pain and function after standardized exercise therapy in painful knee osteoarthritis. Clin J Pain 2020;36:16–24. https://doi.org/10.1097/ajp.0000000000000771.Search in Google Scholar PubMed
78. Henriksen, M, Klokker, L, Graven-Nielsen, T, Bartholdy, C, Schjødt Jørgensen, T, Bandak, E, et al.. Association of exercise therapy and reduction of pain sensitivity in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res 2014;66:1836–43. https://doi.org/10.1002/acr.22375.Search in Google Scholar PubMed
79. Chen, B, Duan, J, Wen, S, Pang, J, Zhang, M, Zhan, H, et al.. An updated systematic review and meta-analysis of duloxetine for knee osteoarthritis pain. Clin J Pain 2021;37:852–62. https://doi.org/10.1097/ajp.0000000000000975.Search in Google Scholar
80. Blikman, T, Rienstra, W, van Raaij, TM, ten Hagen, AJ, Dijkstra, B, Zijlstra, WP, et al.. Duloxetine in OsteoArthritis (DOA) study: effects of duloxetine on pain and function in end-stage hip and knee OA – a pragmatic enriched randomized controlled trial. BMC Musculoskelet Disord 2022;23:115. https://doi.org/10.1186/s12891-022-05034-0.Search in Google Scholar PubMed PubMed Central
81. Petersen, KKS, Drewes, AM, Olesen, AE, Ammitzbøll, N, Bertoli, D, Brock, C, et al.. The effect of duloxetine on mechanistic pain profiles, cognitive factors and clinical pain in patients with painful knee osteoarthritis-A randomized, double-blind, placebo-controlled, crossover study. Eur J pain 2022. [Epub ahead of print].10.1002/ejp.1988Search in Google Scholar PubMed PubMed Central
82. Kennedy, DL, Kemp, HI, Ridout, D, Yarnitsky, D, Rice, AS. Reliability of conditioned pain modulation. Pain 2016;157:2410–9. https://doi.org/10.1097/j.pain.0000000000000689.Search in Google Scholar PubMed PubMed Central
83. Imai, Y, Petersen, KK, Mørch, CD, Arendt Nielsen, L. Comparing test–retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somatosens Mot Res 2016;33:169–77. https://doi.org/10.1080/08990220.2016.1229178.Search in Google Scholar PubMed
84. Yarnitsky, D, Bouhassira, D, Drewes, AM, Fillingim, RB, Granot, M, Hansson, P, et al.. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. https://doi.org/10.1002/ejp.605.Search in Google Scholar PubMed
85. Kristensen, NS, Hertel, E, Skadhauge, CH, Kronborg, SH, Petersen, KK, McPhee, ME. Psychophysical predictors of experimental muscle pain intensity following fatiguing calf exercise. PLoS One 2021;16:e0253945. https://doi.org/10.1371/journal.pone.0253945.Search in Google Scholar PubMed PubMed Central
86. Izumi, M, Hayashi, Y, Saito, R, Oda, S, Petersen, KK, Arendt-Nielsen, L, et al.. Detection of altered pain facilitatory and inhibitory mechanisms in patients with knee osteoarthritis by using a simple bedside tool kit (QuantiPain). PAIN Reports 2022;7:e998. https://doi.org/10.1097/pr9.0000000000000998.Search in Google Scholar
87. Staffe, AT, Bech, MW, Clemmensen, SLK, Nielsen, HT, Larsen, DB, Petersen, KK. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One 2019;14:e0225849. https://doi.org/10.1371/journal.pone.0225849.Search in Google Scholar PubMed PubMed Central
88. Lockwood, SM, Bannister, K, Dickenson, AH. An investigation into the noradrenergic and serotonergic contributions of diffuse noxious inhibitory controls in a monoiodoacetate model of osteoarthritis. J Neurophysiol 2019;121:96–104. https://doi.org/10.1152/jn.00613.2018.Search in Google Scholar PubMed PubMed Central
89. Bannister, K, Patel, R, Goncalves, L, Townson, L, Dickenson, AH. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015;156:1803–11. https://doi.org/10.1097/j.pain.0000000000000240.Search in Google Scholar PubMed
90. Petersen, KK, McPhee, ME, Hoegh, MS, Graven-Nielsen, T. Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: manifestations and implications for pain progression. Curr Opin Support Palliat Care 2019;13:99–106. https://doi.org/10.1097/spc.0000000000000419.Search in Google Scholar PubMed
91. Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–5. https://doi.org/10.1097/aco.0b013e32833c348b.Search in Google Scholar PubMed
92. Kisler, LB, Weissman-Fogel, I, Coghill, RC, Sprecher, E, Yarnitsky, D, Granovsky, Y. Individualization of migraine prevention. Clin J Pain 2019;35:753–65. https://doi.org/10.1097/ajp.0000000000000739.Search in Google Scholar PubMed
93. Koh, IJ, Kim, MS, Sohn, S, Song, KY, Choi, NY, In, Y. Duloxetine reduces pain and improves quality of recovery following total knee arthroplasty in centrally sensitized patients. J Bone Jt Surg 2019;101:64–73. https://doi.org/10.2106/jbjs.18.00347.Search in Google Scholar PubMed
94. Rienstra, W, Blikman, T, Dijkstra, B, Stewart, R, Zijlstra, W, van Raaij, T, et al.. Effect of preoperative duloxetine treatment on postoperative chronic residual pain after total hip or knee arthroplasty: a randomised controlled trial. BMJ Open 2021;11:e052944. https://doi.org/10.1136/bmjopen-2021-052944.Search in Google Scholar PubMed PubMed Central
95. Graven-Nielsen, T, Kendall, SA, Henriksson, KG, Bengtsson, M, Èrensen, JS, Johnson, A, et al.. Ketamine reduces muscle pain, temporal summation, and referred pain in ®bromyalgia patients. Pain 2000;85:483–91. https://doi.org/10.1016/S0304-3959(99)00308-5.Search in Google Scholar PubMed
96. Himmelseher, S, Ziegler-Pithamitsis, D, Argiriadou, H, Martin, J, Jelen-Esselborn, S, Kochs, E. Small-dose S(+)-ketamine reduces postoperative pain when applied with ropivacaine in epidural anesthesia for total knee arthroplasty. Anesth Analg 2001;92:1290–5. https://doi.org/10.1097/00000539-200105000-00040.Search in Google Scholar PubMed
97. Wang, P, Yang, Z, Shan, S, Cao, Z, Wang, Z. Analgesic effect of perioperative ketamine for total hip arthroplasties and total knee arthroplasties: a PRISMA-compliant meta-analysis. Medicine 2020;99:e22809. https://doi.org/10.1097/md.0000000000022809.Search in Google Scholar
98. Aveline, C, Roux, AL, Hetet, HL, Gautier, JF, Vautier, P, Cognet, F, et al.. Pain and recovery after total knee arthroplasty: a 12-month follow-up after a prospective randomized study evaluating Nefopam and Ketamine for early rehabilitation. Clin J Pain 2014;30:749–54. https://doi.org/10.1097/ajp.0000000000000033.Search in Google Scholar PubMed
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial Comment
- What do we mean by “mechanism” in pain medicine?
- Topical Reviews
- Topical review – salivary biomarkers in chronic muscle pain
- Tendon pain – what are the mechanisms behind it?
- Systematic Review
- Psychological management of patients with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review
- Topical Review
- Predicting pain after standard pain therapy for knee osteoarthritis – the first steps towards personalized mechanistic-based pain medicine in osteoarthritis
- Clinical Pain Researches
- Neuropathy and pain after breast cancer treatment: a prospective observational study
- Neuropeptide Y and measures of stress in a longitudinal study of women with the fibromyalgia syndrome
- Nociceptive two-point discrimination acuity and body representation failure in polyneuropathy
- Pain sensitivity in relation to frequency of migraine and tension-type headache with or without coexistent neck pain: an exploratory secondary analysis of the population study
- Clinician experience of metaphor in chronic pain communication
- Observational studies
- Chronic vulvar pain in gynecological outpatients
- Male pelvic pain: the role of psychological factors and sexual dysfunction in a young sample
- A bidirectional study of the association between insomnia, high-sensitivity C-reactive protein, and comorbid low back pain and lower limb pain
- Burden of disease and management of osteoarthritis and chronic low back pain: healthcare utilization and sick leave in Sweden, Norway, Finland and Denmark (BISCUITS): study design and patient characteristics of a real world data study
- Factors influencing quality of life in patients with osteoarthritis: analyses from the BISCUITS study
- Prescription patterns and predictors of unmet pain relief in patients with difficult-to-treat osteoarthritis in the Nordics: analyses from the BISCUITS study
- Lifestyle factors, mental health, and incident and persistent intrusive pain among ageing adults in South Africa
- Inequalities and inequities in the types of chronic pain services available in areas of differing deprivation across England
- Original Experimentals
- Conditioned pain modulation is not associated with thermal pain illusion
- Association between systemic inflammation and experimental pain sensitivity in subjects with pain and painless neuropathy after traumatic nerve injuries
- Endometriosis diagnosis buffers reciprocal effects of emotional distress on pain experience
- Educational Case Reports
- Intermediate cervical plexus block in the management of treatment resistant chronic cluster headache following whiplash trauma in three patients: a case series
- Trigeminal neuralgia in patients with cerebellopontine angle tumors: should we always blame the tumor? A case report and review of literature
- Short Communication
- Less is more: reliability and measurement error for three versions of the Tampa Scale of Kinesiophobia (TSK-11, TSK-13, and TSK-17) in patients with high-impact chronic pain
Articles in the same Issue
- Frontmatter
- Editorial Comment
- What do we mean by “mechanism” in pain medicine?
- Topical Reviews
- Topical review – salivary biomarkers in chronic muscle pain
- Tendon pain – what are the mechanisms behind it?
- Systematic Review
- Psychological management of patients with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review
- Topical Review
- Predicting pain after standard pain therapy for knee osteoarthritis – the first steps towards personalized mechanistic-based pain medicine in osteoarthritis
- Clinical Pain Researches
- Neuropathy and pain after breast cancer treatment: a prospective observational study
- Neuropeptide Y and measures of stress in a longitudinal study of women with the fibromyalgia syndrome
- Nociceptive two-point discrimination acuity and body representation failure in polyneuropathy
- Pain sensitivity in relation to frequency of migraine and tension-type headache with or without coexistent neck pain: an exploratory secondary analysis of the population study
- Clinician experience of metaphor in chronic pain communication
- Observational studies
- Chronic vulvar pain in gynecological outpatients
- Male pelvic pain: the role of psychological factors and sexual dysfunction in a young sample
- A bidirectional study of the association between insomnia, high-sensitivity C-reactive protein, and comorbid low back pain and lower limb pain
- Burden of disease and management of osteoarthritis and chronic low back pain: healthcare utilization and sick leave in Sweden, Norway, Finland and Denmark (BISCUITS): study design and patient characteristics of a real world data study
- Factors influencing quality of life in patients with osteoarthritis: analyses from the BISCUITS study
- Prescription patterns and predictors of unmet pain relief in patients with difficult-to-treat osteoarthritis in the Nordics: analyses from the BISCUITS study
- Lifestyle factors, mental health, and incident and persistent intrusive pain among ageing adults in South Africa
- Inequalities and inequities in the types of chronic pain services available in areas of differing deprivation across England
- Original Experimentals
- Conditioned pain modulation is not associated with thermal pain illusion
- Association between systemic inflammation and experimental pain sensitivity in subjects with pain and painless neuropathy after traumatic nerve injuries
- Endometriosis diagnosis buffers reciprocal effects of emotional distress on pain experience
- Educational Case Reports
- Intermediate cervical plexus block in the management of treatment resistant chronic cluster headache following whiplash trauma in three patients: a case series
- Trigeminal neuralgia in patients with cerebellopontine angle tumors: should we always blame the tumor? A case report and review of literature
- Short Communication
- Less is more: reliability and measurement error for three versions of the Tampa Scale of Kinesiophobia (TSK-11, TSK-13, and TSK-17) in patients with high-impact chronic pain

