Abstract
In this study, changes in chemical composition of aged and unaged Scots pine and beech wood decayed by brown-rot fungi Coniophora puteana and Poria placenta were presented by Fourier transform infrared (FT-IR) spectra. Samples were exposed to six complete cycles of accelerated aging for 12 days and then subjected to brown-rot fungi attack for 8 weeks. Weight loss of samples was found to be 25–46% depending on wood and fungi species and being aged ones. Accelerated aging treatments seemed to have a slight role on chemical composition of Scots pine and beech samples while they increased biodegradation of samples. FT-IR spectra showed degradation of wood carbohydrates revealed by reduction of the peaks responsible for hemicellulose and cellulose at 1730, 1370, 1150, and 897 cm-1 in pine and 1730, 1370, 1027, and 897 cm-1 in beech. Stronger lignin peaks at 1650, 1593, 1506, 1455, 1422, 1261, and 1230 cm-1 in pine and 1650, 1506, 1455, and 1422 cm-1 in beech were obtained after decay test. FT-IR spectra of samples were consistent with the degradation mechanism of brown-rot fungi. In general, changes in the carbohydrate and lignin peaks were greater in samples exposed to accelerated aging treatments for both wood species.
1 Introduction
Wood as a natural polymer is biodegradable by fungal action under some use conditions. It may decay or discolor and require replacement after its short service life unless it is not protected with suitable wood preservatives. Fungal decay in wood is caused by brown-rot, white-rot, and soft-rot fungi. Brown-rot fungi cause a rapid destructive decay that is the main reason of failure in wooden structures and buildings [1] and selectively decay the cell-wall carbohydrates, leaving behind a modified brown-colored, demethoxylated lignin residuum by using Fenton chemistry [1–4]. The decay with brown-rot fungi is based on both nonenzymatic and enzymatic systems [5]. Cleavage of glycosidic bonds, depolymerization of wood polysaccharides, and degradation of pectic substances occur during the incipient decay. Accordingly, intramolecular hydrogen bonding in cellulose is reduced, while the phenolic groups in wood are increased [6]. In the early stages of brown-rot decay, hemicelluloses are removed more rapidly than cellulose [1, 7, 8]. Mechanical properties decrease dramatically during the attack of brown-rot fungi. Weight losses of 1–18% caused by brown-rot fungi attack were linearly related to strength reductions of 5–70%, because strength reductions of wood were closely related to degradation of hemicelluloses [9]. Fungi in wood chips also cause some important changes in the chemical composition [10, 11], and as a result of this, properties of decayed chips mainly differ in industrial products. Besides fungal decay, outdoor conditions such as rain, snow, wind, and UV, degrade wood, chips, lignocellulosic materials, and wood-based composites. When wood is exposed to outdoor conditions, gray color is observed first on wood surface and then surface fibers are loosen and erode upon extended exposure. In addition to gray-color changes, mildew growth, checking, splitting, and warping occur [12]. Change in wood components also causes some decreases on physical, mechanical, and biological properties of wood and wood products and cause a short-term failure in service [12–14].
Durability of wood and wood-based composites is very important in structural applications. For such applications, information on long-term durability of wood and wood-based composites under realistic environmental conditions is gaining an importance. Long-term tests known as outdoor exposure tests take long time for evaluation and are difficult to carry out in many times [15]. Short-term test generally known as laboratory accelerated aging offers some advantages, as it is easy to perform and more standardized than long-term tests [16]. Reduction in strength properties of wood-based composites [15–18] and reduction in biological resistance of wood-based composites and solid wood [19] were reported after accelerated aging treatments such as water immersion, boiling, steaming, freezing, and drying.
Infrared spectroscopy provides reliable information of the changes in wood composition during decay with basidiomycetes [7, 20]. Likewise, Fourier transform infrared (FT-IR) analysis on decayed wood by brown-rot fungi [3, 5–7, 21–23], white-rot fungi [10, 24–27], both brown and white-rot fungi [8, 20, 28–30], and soft-rot fungi [31, 32] were extensively studied. Because outdoor conditions influence service life of wood in many applications, studying effect of both biological agents and environmental conditions on wood properties is gaining an importance. Studies carried out to investigate the effect of accelerated aging treatments on wood decay and chemical composition are very rare. It is believed that there is a need on the studies dealing with the changes in chemical composition of aged samples after decay test. Environmental conditions before the decay test can be simulated with the accelerated aging treatments such as water immersion, boiling, steaming, freezing, and drying.
In this study, FT-IR attenuated total reflection (ATR) analysis of the chemical changes in Scots pine and beech wood decayed by brown-rot fungi Coniophora puteana and Poria placenta were investigated in comparison with the chemical composition of undegraded wood. Furthermore, the effect of accelerated aging treatments on decay and chemical composition of wood was studied.
2 Materials and methods
2.1 Wood samples and accelerated aging procedure
Sapwood of Scots pine (Pinus sylvestris L.) and beech (Fagus orientalis L.) 10×5×20 mm (radial×tangential×longitudinal) were machined from the logs obtained from Gumushane and Macka located in the northeast Black Sea region of Turkey. Ten replicate samples for each group were conditioned in a conditioning room at 22°C and 65% relative humidity (RH) for 4 weeks before accelerated aging procedure. Accelerated aging procedure was performed according to ASTM D1037-89 [33] standard. Samples exposed to total six cycles for 12 days. One cycle consisted of six treatment steps: (1) immersion in water at 49°C for 1 h, (2) steaming at 93°C for 3 h, (3) freezing at -12°C for 20 h, (4) drying at 99°C for 3 h, (5) steaming at 93°C for 3 h, and (6) drying at 99°C for 18 h.
Samples were conditioned at 22% and 65% RH for 6 weeks after accelerated aging procedure.
2.2 Decay test
Decay test was performed according to principles of EN 113 [34], with some modifications on sample dimensions, Kolle flasks, and total test period. Instead of Kolle flasks, plastic sterile Petri dishes (∅, 9 cm) were used. Malt extract agar of 4.8% concentration and samples were sterilized in an autoclave at pressure of about 0.1 MPa at 120°C for 25 and 45 min, respectively. Two brown-rot fungi, namely, C. puteana (Mad-515) and P. placenta (Mad-698-R), were inoculated to sterile malt extract agar medium in the Petri dishes. Two samples (unaged and aged) were placed on the growing mycelium in each Petri dish and then were incubated at 20°C and 65% RH for 8 weeks. At the end of the test, samples were removed from the Petri dishes, cleaned, and dried at a temperature of 103±2°C. Weight loss was calculated on the basis of oven-dry weight before and after decay test. Brown-rot fungi were chosen in the study because brown-rot wood decay represents a major problem in the storage and preservation of wooden structures [35] and causes a rapid destructive decay, which is the main reason of failure in wooden structures and buildings [1].
2.3 FT-IR ATR measurements
Scots pine and beech samples were ground in a Wiley mill with a mesh size of 0.5 mm (IKA MF10, IKA-Werke, Staufen, Germany) for the FT-IR ATR analysis. Finely ground wood flour was pressed into pellets with a diameter of 10 mm. FT-IR spectra were obtained from pellets using a Perkin-Elmer Spectrum 100 with a Universal ATR sampling accessory. Four accumulated spectra were collected in the wave number region of 650–4000 cm-1, with a spectral resolution of 4 cm-1. In this study, fingerprint region (650–1800 cm-1), where the main structural changes occurred, was studied. The assignments of the peaks to structural components [25, 29, 36, 37] are shown in Table 1.
Assignments of absorption infrared spectra bands in wood [25, 29, 36, 37]
| Peak number | Wave number (cm-1) Scots pine | Wave number (cm-1) Beech | Assignments and remarks |
|---|---|---|---|
| 1 | 3337–3342 | 3336–3342 | Bonded O-H stretching |
| 2 | 2902–2923 | 2899–2907 | C-H stretching |
| 3 | 1726–1731 | 1732–1733 | Unconjugated C=O in xylans |
| 4 | 1652–1656 | 1646–1656 | Keto-carbonyl conjugated with benzene ring |
| 5 | 1593–1606 | 1593–1594 | C=C in aromatic ring in lignin |
| 6 | 1506–1508 | 1504–1506 | C=C in aromatic ring in lignin |
| 7 | 1452–1454 | 1453–1456 | C-H deformation in lignin and carbohydrates |
| 8 | 1421–1423 | 1422 | C-H deformation in lignin and carbohydrates |
| 9 | 1368–1370 | 1369–1371 | CH2 bending in cellulose and hemicellulose |
| 10 | 1316–1318 | 1322–1324 | CH2 wagging vibration in cellulose |
| 11 | 1261–1264 | – | Guaiacyl nuclei in lignin |
| 12 | 1226–1231 | 1228–1234 | Syringyl nuclei in lignin and C-O in xylan |
| 13 | 1150–1153 | 1153–1155 | C-O-C asymmetric band in cellulose and hemicellulose |
| 14 | 1097–1102 | 1097–1105 | O-H association band in cellulose and hemicellulose |
| 15 | 1024–1026 | 1027–1029 | C-O stretching in cellulose and hemicellulose |
| 16 | 892–897 | 895–897 | C-H deformation in cellulose |
| 17 | 808–811 | 834 | 1,3,4-subsituated benzene ring in softwood lignin |
| 18 | 664 | 664 | COH out-of-plane bending in cellulose |
3 Results and discussion
3.1 Weight loss of samples
Weight loss of samples after decay test is shown in Table 2. Weight loss was found to be 25.2–45% for Scots pine samples and 29.1–46.4% for beech samples depending on fungi and being aged and unaged samples, respectively.
Weight loss of samples due to decay
| Groups | Weight loss (%) | |
|---|---|---|
| C. puteana | P. placenta | |
| Scots pine/unaged | 34.2±9.3 | 25.2±5.35 |
| Scots pine/aged | 30.3±5.0 | 45.0±10.8 |
| Beech/unaged | 35.3±12.1 | 29.1±6.2 |
| Beech/aged | 38.4±11.7 | 46.4±8.6 |
Accelerated aging treatments generally increased weight loss of samples for both wood species, probably affected wood structure and natural durability of wood negatively, and made the wood more susceptible to decay. Kartal and Green [19] reported that accelerated aging treatments caused more weight loss in MDF and solid wood samples in comparison with the controls and found that the weight loss of aged pine and beech wood samples was 30% greater than controls after P. placenta attack for 12 weeks. Results were in accordance with the findings in this study. Accelerated aging treatments such as water immersion, boiling, steaming, freezing, and drying may result in leaching of the water-soluble substances and hence reduce decay resistance [19]. Poria placenta caused more weight loss in aged samples than C. puteana did. Probably, the degradation of the cell wood attacked by C. puteana occurs firstly on the wall surface, but in the case of P. placenta the destruction of cellulose proceeds deep inside the wall even after 1 month of fungal attack [38]. Aging treatments accelerated biodegradation of wood with P. placenta in comparison with C. puteana. This assumption is also confirmed by FT-IR spectra (Figures 1 and 2). In contrast to findings in aged samples, C. puteana caused more weight losses in unaged samples in comparison with the weight losses observed by P. placenta attack. This could be related to nature of enzymes. It is reported that P. placenta tends to degrade amorphous regions of cellulose more readily, while the crystalline regions remain less damaged due to P. placenta containing endoglucanases and glycosidases but not exoglucanases. Coniophora puteana is able to degrade both amorphous and crystalline regions due to Coniophoroid brown-rot fungi that produce the full enzyme complement [38]. Surprisingly, beech samples showed greater weight loss than Scots pine samples. It was reported that brown-rot fungi, Gloeophyllum trabeum and Laetiporus sulphureus, could equally decay softwood and hardwood species [23].
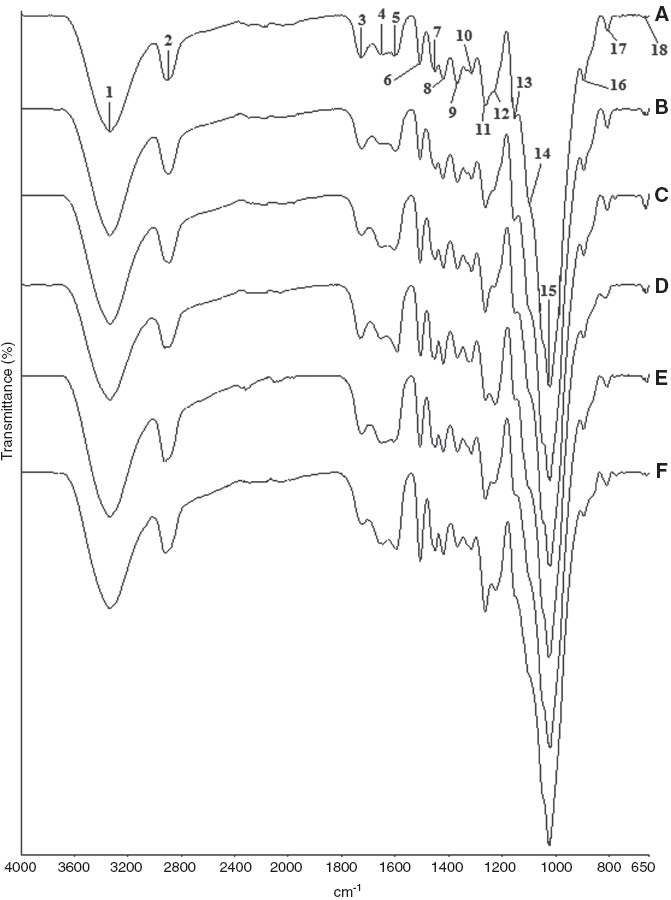
FT-IR spectral changes of Scots pine samples: (A) unaged samples, (B) aged samples, (C) unaged samples degraded by C. puteana, (D) aged samples degraded by C. puteana, (E) unaged samples degraded by P. placenta, and (F) aged samples degraded by P. placenta.
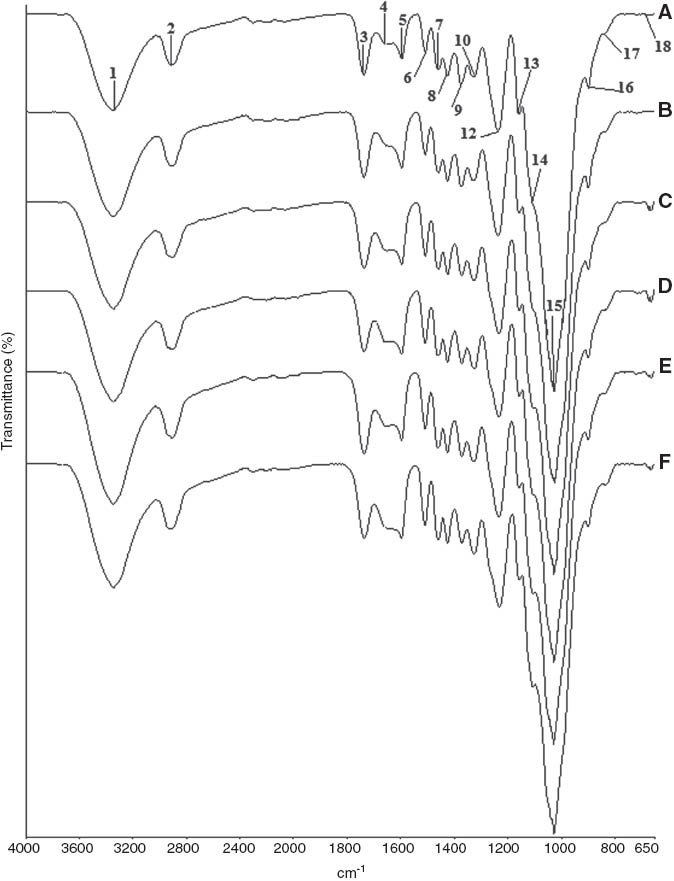
FT-IR spectral changes of beech samples: (A) unaged samples, (B) aged samples, (C) unaged samples degraded by C. puteana, (D) aged samples degraded by C. puteana, (E) unaged samples degraded by P. placenta, and (F) aged samples degraded by P. placenta.
3.2 Changes in chemical composition of wood samples due to aging treatments
FT-IR ATR spectra show the changes in chemical composition of aged and unaged samples exposed to brown-rot fungi in Figure 1 for Scots pine and Figure 2 for beech, respectively. The intensity of peaks at around 1730 cm-1 (3), 1593 cm-1 (5), 1455 cm-1 (7), 1422 cm-1 (8), 1370 cm-1 (9), 1323 cm-1 (10), and 1230 cm-1 (12) were higher in control beech samples, while the peaks at around 1506 cm-1 (6), 1261 cm-1 (11), and 808 cm-1 (17) were higher in control Scots pine samples. Beech as a hardwood species has higher xylan (1730 cm-1) and carbohydrate content than pine, and Scots pine as a softwood species has higher lignin content (1506 cm-1) than beech [29]. It was shown that guaiacyl-type lignin (softwood lignin) developed the peak at around 1261 cm-1 (11) and 1230 cm-1 (12) for Scots pine and that the syringyl-type lignin (hardwood lignin) developed the peak at around 1230 cm-1 (12) for beech similar to findings in a study carried out by Pandey and Pitman [29].
Accelerated aging treatments seemed to have a slight role on chemical composition of Scots pine and beech samples. No marked changes were observed at the intensity of peaks, except for some slight changes in the following peaks responsible for lignin, cellulose, and hemicellulose. Peaks of aged pine samples at around 1730 cm-1 (3), 1650 cm-1 (4), 1506 cm-1 (6), and 1370 cm-1 (9) slightly decreased, while the peaks at around 1593 cm-1 (5), 1422 cm-1 (8), 808 cm-1 (17), and 664 cm-1 (18) slightly increased compared to peaks of unaged samples. Peaks of aged beech samples at around 1027 cm-1 (15) slightly decreased, while the peaks at around 1730 cm-1 (3), 1593 cm-1 (5), 1506 cm-1 (6), and 1422 cm-1 (8) slightly increased compared to peaks of unaged samples. As can be seen from Table 1, changes in peak intensities of aged Scots pine were supposed to be related to some modifications in hemicellulose and cellulose after aging treatments. Heat applications and weathering factors (UV and rain) generally cause a decrease at the intensity of 1730 cm-1 peak probably due to cleavage of acetyl groups [39–41] and leaching of functional groups [42, 43]. The small decrease of the water peak at 1650 cm-1 for pine samples might be related to the decrease of the carbonyl peak [40]. The small decrease at 1370 cm-1 peak for aged Scots pine and at 1027 cm-1 peak of aged beech samples was related to the CH2 bending in cellulose and hemicellulose and C-O stretching in cellulose and hemicellulose, respectively. The increase at the intensity of 1422 cm-1 peak showed C-H deformation in lignin and carbohydrates after aging treatments for aged Scots pine and beech samples, and the increase at the intensity of 664 cm-1 peak showed some modifications in cellulose for aged Scots pine samples. The lignin peak at 1506 cm-1 decreased for aged Scots pine samples, while it increased for aged beech samples probably due to composition of lignin types in softwoods and hardwoods. Slight or negligible changes in lignin component of wood might be related to its greater resistant against heat applications compared to other wood components [41]. Lignin component of wood samples seemed to be increased after aging. This might be related to the degradation of wood carbohydrates relative to lignin component of wood. Changes in beech samples were considerably less than changes in Scots pine samples due to beech having greater density than Scots pine samples, which improved resistance against aging treatments. Heavy and strong woods are more resistant to weathering factors than softwoods [43].
3.3 Changes in chemical composition of wood samples due to decay
A broad band attributed to hydroxyl groups or absorbed water was seen at 3336–3342 cm-1 (1) for both wood species, and following C-H stretching, absorption band (2) was observed. The carbonyl peak at 1730 cm-1 (3) stayed nearly unchanged after C. puteana attack in aged Scots pine samples; however, the peak in aged samples decreased after P. placenta attack. Decrease in the peak intensity of unaged pine samples was recorded after both fungi attack. In the case of beech samples, intensity of carbonyl peak at 1730 cm-1 (3) decreased compared to the initial peak before decay test, except for unaged samples that were exposed to P. placenta. The reduction on this peak related to the degradation of glucurono-xylanes connected with splitting of acetyl groups and 4-O-methyl glucuronic acids side units [21]. The presence of the carbonyl peak is due to residual xylan that still remains in decayed samples [29, 31]. Decrease in carbonyl absorption peak was in accordance with the increase in weight losses for both wood and fungi species.
The peaks responsible for wood carbohydrates at 1370 cm-1 (9), 1150 cm-1 (13), and 897 cm-1 (16) decreased for both aged and unaged pine samples after fungi attack. The reduction at 1150 and 897 cm-1 was greater in pine samples after P. placenta attack than C. puteana attack. The peak intensities at 1316 cm-1 (10) and 664 cm-1 (18) decreased in aged samples after P. placenta attack; however, those peaks showed an increasing tendency in unaged samples. In the case of beech samples, the peaks responsible for wood carbohydrates at 1370 cm-1 (9), 1027 cm-1 (15), and 897 cm-1 (16) decreased for both aged and unaged samples after fungi attack. The reduction at 1370 and 897 cm-1 was greater in aged beech samples after P. placenta attack than C. puteana attack. The peak at 1322 cm-1 (10), 1100 cm-1 (14), and 664 cm-1 (18) increased in beech samples after fungi attack. The small increase in the peak intensity of samples at 1200–1000 cm-1 after decay could be supposed to be related to higher glucan content compared to control samples [28]. In beech samples, the peak intensity at 664 cm-1 (18) was greater after C. puteana attack than the peak intensity of samples after P. placenta attack. Peak intensities responsible for wood carbohydrates decrease with the increase in weight loss caused by brown-rot fungi attack [8, 29, 30]. In general, changes in the carbohydrate peaks were greater in pine and beech samples degraded by P. placenta and in samples exposed to accelerated aging treatments.
Changes in lignin shown at the peaks of 1650 cm-1 (4), 1593 cm-1 (5), 1506 cm-1 (6), 1455 cm-1 (7), 1422 cm-1 (8), 1261 cm-1 (11), and 1230 cm-1 (12) resulted in an increase in the intensities and changes in lignin at the peak of 808 cm-1 (17) resulted in a reduction in the intensity after fungi attack for both aged and unaged pine samples. Coniophora puteana caused more increase in the peak intensity at 1593 cm-1 (5) and 1422 cm-1 (8) but more reduction at 808 cm-1 (17) than P. placenta did. The increase at the peak intensities of 1506 cm-1 (6) and 1455 cm-1 (7) was greater in pine samples exposed to P. placenta attack than C. puteana attack. The peak at 1261 cm-1 (11) increased more in aged pine samples after P. placenta attack, while the peak increased more in unaged samples after C. puteana attack. The intensity at 1230 cm-1 (12) increased in aged samples after fungi attack. In the case of beech samples, changes in lignin shown at the peaks of 1650 cm-1 (4), 1506 cm-1 (6), 1455 cm-1 (7), and 1422 cm-1 (8) resulted in an increase in the intensities, but changes in lignin at the peak of 1593 cm-1 (5) resulted in a slight reduction in the intensity after fungi attack for both aged and unaged samples. The increase at the peak intensities of 1506 cm-1 (6) and 1455 cm-1 (7) was greater in aged beech samples exposed to P. placenta attack than C. puteana attack. Lignin and carbohydrate peaks in aged beech samples behaved well-adjusted with the weight loss of samples. Intensity increase at 1660 cm-1 peak supports the proposed oxidative degradation [5] and suggested that brown-rot fungi promote the formation of new conjugated and unconjugated acid substructures in the side chain of the lignin [28]. The peaks at 1455, 1422, and 1230 cm-1 have some contribution from carbohydrates. In general, changes in lignin peaks were greater in aged pine and beech samples than unaged samples, and P. placenta seemed to cause more changes in lignin for aged samples than C. puteana did.
Intensity of the carbohydrate peaks decreased, whereas the intensity of the lignin peaks increased as the brown-rot decay progressed [5, 6, 8, 22, 29, 30] and elevated levels of the syringyl moiety in beech and guaiacyl moiety in pine left [29]. An increase in the lignin absorption peaks in decayed wood was attributed to its oxidative modification by Fenton reaction-derived reactive oxygen species [5].
4 Conclusion
Some cellulose and hemicellulose modifications were found in aged samples, and increased intensity of lignin peak was supposed to be related to the degradation of wood carbohydrates after accelerated aging treatments. Changes in beech samples due to aging treatments were considerably less than changes in Scots pine samples. Weight loss caused by brown-rot fungi attack increased in Scots pine and beech samples after accelerated aging treatments. Intensity of the carbohydrate peaks decreased, whereas intensity of the lignin peaks increased and modified lignin was supposed to be left in the samples after C. puteana and P. placenta attack. Decrease in carbonyl absorption peak was in accordance with the increase in weight losses for both wood and fungi species. In general, the changes in the carbohydrate and lignin peaks were greater in samples degraded by P. placenta and samples exposed to accelerated aging treatments.
Acknowledgments
The author would like to thank research assistant Elif Topaloglu for her help in decay testing and sample preparation at the Department of Forest Industry Engineering Department, KTU Turkey, and research assistant Kaan Karaoglu for FT-IR ATR measurements at the Department of Chemistry, Recep Tayyip Erdogan University Turkey.
References
[1] Zabel RA, Morrell JJ. Wood Microbiology Decay and Its Prevention, Academic Press: San Diego, 1992.Search in Google Scholar
[2] Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. Appl. Environ. Microbiol. 2001, 67, 2705–2711.Search in Google Scholar
[3] Sun QN, Qin TF, Li GY. Int. J. Polym. Anal. Chem. 2009, 14, 19–33.Search in Google Scholar
[4] Eriksson KEL, Blanchette RA, Ander P. Microbial and Enzymatic Degradation of Wood and Wood Components, Springer: Berlin, 1990.10.1007/978-3-642-46687-8Search in Google Scholar
[5] Irbe I, Andersone I, Andersons B, Noldt G, Dizhbite T, Kurnosova N, Nuopponen M, Stewart D. Biodegradation 2011, 22, 719–728.10.1007/s10532-010-9449-6Search in Google Scholar
[6] Fackler K, Stevanic JS, Ters T, Hinterstoisser B, Schwanninger M, Salmén L. Enzyme Microb. Technol. 2010, 47, 257–267.Search in Google Scholar
[7] Pandey KK, Pitman AJ. J. Polym. Sci. A Polym. Chem. 2004, 42, 2340–2346.Search in Google Scholar
[8] Pandey KK, Nagveni HC. Eur. J. Wood Wood Prod. 2007, 65, 477–481.Search in Google Scholar
[9] Winandy JE, Morrell JJ. Wood Fiber Sci. 1993, 25, 278–288.Search in Google Scholar
[10] Ferraz A, Parra C, Freer J, Baeza J, Rodríguez J. World J. Microb. Biot. 2000, 16, 641–645.Search in Google Scholar
[11] Talaiepour M, Hemmasi AH, Kasmani JE, Mirshokraie SA, Khademieslam H. BioResources 2010, 5, 477–487.Search in Google Scholar
[12] Williams RS. Weathering of Wood, CRC Press: Florida, 2005.Search in Google Scholar
[13] Zhang J, Kamdem PD. The International Research Group on Wood Protection 2000, IRG/WP 00-40155.Search in Google Scholar
[14] Evans P, Chowdhury JM, Mathews B, Schmalzl K, Ayer S, Kiguchi M, Kataoka Y. Handbook of Environmental Degradation of Materials, William Andrew, Inc.: New York, 2005.Search in Google Scholar
[15] Kojima Y, Suzuki S. J. Wood Sci. 2011, 57, 7–13.Search in Google Scholar
[16] Kojima Y, Suzuki S. J. Wood Sci. 2011, 57, 126–133.Search in Google Scholar
[17] Kajita H, Mukudai J, Yano H. Wood Sci. Technol. 1991, 25, 239–249.Search in Google Scholar
[18] Okkonen EA, River BH. For. Prod. J. 1996, 46, 68–74.Search in Google Scholar
[19] Kartal SN, Green F. Int. Biodeter. Biodegr. 2003, 5, 29–35.Search in Google Scholar
[20] Fackler K, Schwanninger M, Gradinger C, Hinterstoisser B, Messner K. FEMS Microbiol. Lett. 2007, 271, 162–169.Search in Google Scholar
[21] Solár R, Kurjatko S, Mamon M, Kosikova B, Neuschlová E, Výbohová E, Hudec J. Drvna Ind. 2007, 58, 3–11.Search in Google Scholar
[22] Li GY, Huang LH, Hse CY, Qin TF. Carbohydr. Polym. 2011, 85, 560–564.Search in Google Scholar
[23] Monrroy M, Ortega I, Ramírez M, Baeza J, Freer J. Enzyme Microb. Technol. 2011, 49, 472–477.Search in Google Scholar
[24] Guerra A, Mendonça R, Ferraz A, Lu F, Ralph J. Appl. Environ. Microbiol. 2004, 70, 4073–4078.Search in Google Scholar
[25] Mohebby B. Int. Biodeter. Biodegr. 2005, 55, 247–251.Search in Google Scholar
[26] Naumann A, Navarro-González M, Peddireddi S, Kües U, Polle A. Fungal Genet. Biol. 2005, 42, 829–835.Search in Google Scholar
[27] Mahajan S, Jeremic D, Goacher RE, Master ER. Appl. Environ. Microbiol. 2012, 94, 1303–1311.Search in Google Scholar
[28] Ferraz A, Baeza J, Rodriguez J, Freer J. Bioresour. Technol. 2000, 74, 201–212.Search in Google Scholar
[29] Pandey KK, Pitman AJ. Int. Biodeter. Biodegr. 2003, 52, 151–160.Search in Google Scholar
[30] Huang Z, Maher K, Amartey S. The International Research Group on Wood Protection 2004, IRG/WP 04-10542.Search in Google Scholar
[31] Popescu CM, Popescu MC, Vasile C. Carbohydr. Polym. 2010, 79, 362–372.Search in Google Scholar
[32] Popescu CM, Larsson PT, Vasile C. Carbohydr. Polym. 2011, 83, 808–812.Search in Google Scholar
[33] American Society for Testing and Materials (ASTM), 1989, ASTM D1037-89.Search in Google Scholar
[34] European Committee for Standardization (EN), 1997, EN 113-97.10.1016/S0338-9898(97)80129-1Search in Google Scholar
[35] Voda K, Boh B, Vrtačnik M, Pohleven F. Int. Biodeter. Biodegr. 2003, 51, 51–59.Search in Google Scholar
[36] Temiz A. Ph.D. Thesis, Karadeniz Technical University, 2005.Search in Google Scholar
[37] Ximene FA, Evans PD. For. Prod. J. 2006, 56, 116–122.Search in Google Scholar
[38] Irbe I, Andersons B, Chirkova J, Kallavus U, Andersone I, Faix O. Int. Biodeter. Biodegr. 2006, 57, 99–106.Search in Google Scholar
[39] Nuopponen M, Vuorinen T, Jamsa S, Viitaniemi P. J. Wood Chem. Technol. 2004, 24, 13–26.Search in Google Scholar
[40] Miklecic J, Rajkovic VJ, Antonovic A, Spanic N. BioResources 2011, 6, 434–446.10.15376/biores.6.1.434-446Search in Google Scholar
[41] Yildiz S, Tomak ED, Yildiz UC, Ustaomer D. Polym. Degrad. Stabil. 2013, 98, 1419–1427.Search in Google Scholar
[42] Anderson EL, Pawlak Z, Owen NL, Feist WC. Appl. Spectrosc. 1991, 45, 641–647.Search in Google Scholar
[43] Anderson EL, Pawlak Z, Owen NL, Feist WC. Appl. Spectrosc. 1991, 45, 648–652.Search in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Preparation and mechanical properties of nano-silica/UPR polymer composite
- Synthesis, characterization, and microwave properties of polypyrrole/molybdenum trioxide composites
- On the use of lock-in thermography to monitor delamination growth in composite panels under compression
- Experimental and numerical investigation in failure of cracked aluminum plates repaired with bonded FML composite patch, under impact loading
- Physical and mechanical properties of stir-casting processed AA2024/B4Cp composites
- Synthesis and properties of 0.3%Y2O3/0.3%La2O3/0.3%Al2O3/Cu composite
- Performance properties of vacuum insulation panels produced with various filling materials
- Multiobjective optimization of composite cylindrical shells for strength and frequency using genetic algorithm and neural networks
- Flexural-torsional buckling of FRP thin-walled composite with various sections
- Finite element studies on buckling of laminated cylindrical skew panels
- Harmonic response analysis of symmetric laminated composite beams with different boundary conditions
- A semi-analytical three-dimensional free vibration analysis of functionally graded curved panels integrated with piezoelectric layers
- Changes in chemical composition of decayed Scots pine and beech wood
- Combined effect of nano-SiO2 particles and steel fibers on flexural properties of concrete composite containing fly ash
- Investigation of CFRP- and GFRP-confined concrete cylinders under monotonic and cyclic loading
Articles in the same Issue
- Frontmatter
- Original articles
- Preparation and mechanical properties of nano-silica/UPR polymer composite
- Synthesis, characterization, and microwave properties of polypyrrole/molybdenum trioxide composites
- On the use of lock-in thermography to monitor delamination growth in composite panels under compression
- Experimental and numerical investigation in failure of cracked aluminum plates repaired with bonded FML composite patch, under impact loading
- Physical and mechanical properties of stir-casting processed AA2024/B4Cp composites
- Synthesis and properties of 0.3%Y2O3/0.3%La2O3/0.3%Al2O3/Cu composite
- Performance properties of vacuum insulation panels produced with various filling materials
- Multiobjective optimization of composite cylindrical shells for strength and frequency using genetic algorithm and neural networks
- Flexural-torsional buckling of FRP thin-walled composite with various sections
- Finite element studies on buckling of laminated cylindrical skew panels
- Harmonic response analysis of symmetric laminated composite beams with different boundary conditions
- A semi-analytical three-dimensional free vibration analysis of functionally graded curved panels integrated with piezoelectric layers
- Changes in chemical composition of decayed Scots pine and beech wood
- Combined effect of nano-SiO2 particles and steel fibers on flexural properties of concrete composite containing fly ash
- Investigation of CFRP- and GFRP-confined concrete cylinders under monotonic and cyclic loading

