Abstract
Ketone therapies refer to metabolic interventions aimed to elevate circulating levels of ketone bodies (KB), either through direct supplementation or stimulating endogenous production via medium-chain fatty acids intake, ketogenic diets, caloric restriction, intermittent fasting, or exercise. These strategies have gained attention as potential treatments for neurodegenerative diseases by preserving neuronal function, improving metabolic efficiency, and enhancing cellular resilience to stress. KB are taken up and metabolized by various brain cell types–including neurons, astrocytes, oligodendroglia and microglia–under basal and pathological conditions. However, their cell-type-specific effects remain incompletely understood. Notably, although astrocytes play a key role in supporting neuronal metabolism and can both produce and utilize KB, research has focused predominantly on neuronal responses, leaving the impact of ketotherapeutics on astrocytes relatively unexplored. This review aims to compile and discuss current evidence concerning astrocytes responses to both exogenous and endogenous ketotherapeutic strategies. Although still limited, available studies reveal that astrocytes undergo dynamic changes in response to these interventions, including morphological remodeling, calcium signaling modulation, transcriptional and metabolic reprogramming, regulation of transporters, and neurotransmitter uptake–a crucial process for synaptic function. Astrocytes appear to actively contribute to the neuroprotective and pro-cognitive effects of ketone therapies, particularly in the context of aging and disease. However, significant gaps still remain, concerning the stage when ketone therapy should be initiated, the underlying mechanisms, regional specificity, and long-term consequences. Future research focused on astrocyte heterogeneity, activation of intracellular pathways, and metabolic and transcriptional reprogramming will enable the translational potential of astrocyte-targeted ketone therapies for neurological disorders.
1 Introduction
Ketone bodies (KB) are used in brain as an efficient alternative energy source to glucose under nutrient limiting conditions. During development, KB blood levels are up to 10 times higher than in the adult stage (Page et al. 1971), and they are used for energy metabolism as well as precursors for lipids and myelin synthesis (DeVivo et al. 1976; Krebs et al. 1971; Reichard et al. 1974; Robinson and Williamson 1980; Yeh et al. 1977). KB are produced in liver mitochondria through the β-oxidation of fatty acids in a process known as ketogenesis. They are released to the bloodstream and uptaken in target organs, as the heart, muscle and brain, where they are converted to acetyl-CoA to generate ATP through the tricarboxylic acid cycle (TCA) (Camberos-Luna and Massieu 2020). KB include beta-hydroxybutyrate (BHB), acetoacetate (AcAc) and acetone, and among the three of them, BHB and AcAc are the most metabolically relevant since acetone, as a volatile molecule, is eliminated through exhalation. BHB is the main KB due to its higher concentration in blood.
Ketotherapeutics or ketogenic therapies refer to interventions aimed to elevate KB in blood to be used as cytoprotective molecules against diverse pathological conditions. Ketotherapeutics can be endogenous or exogenous, depending on the strategy used to increase KB levels. Endogenous ketotherapeutics involve stimulating KB production endogenously through dietary approaches such as the ketogenic diet (KD), caloric restriction (CR) and intermittent fasting (IF), or by exhaustive exercise. These approaches require chronic treatments to stimulate liver ketogenesis. Exogenous ketotherapeutics involve the external administration of KB via intravenous, enteral or oral intake of ketone salts or ketone esters (KE). These approaches lead to a rapid elevation of ketones but require repeated doses to sustain ketosis (Camberos-Luna and Massieu 2020).
Previous reviews have discussed the effectiveness of different ketotherapeutic approaches for the treatment of acute brain injury and neurodegenerative diseases, as well as the potential mechanisms involved (Camberos-Luna and Massieu 2020; Koppel and Swerdlow 2018). However, cell-specific responses to keto therapies have not been previously reviewed. KB are uptaken and metabolized by different cell types in brain, including neurons, astrocytes and oligodendrocytes (Andersen et al. 2022; Edmond et al. 1987; McKenna et al. 1993), under both basal and pathological conditions (Scafidi et al. 2022); however, KB effects in distinct cell populations have not been completely elucidated. Astrocytes play a key role in providing metabolic and structural support to neurons (Beard et al. 2022; H. G. Lee et al. 2022). They regulate energy metabolism (Attwell and Laughlin 2001; Pellerin and Magistretti 1994; A. Suzuki et al. 2011; Takano et al. 2006), participate in tissue repair after central nervous system (CNS) injury (Faulkner et al. 2004; Okada et al. 2006), participate in the control of extracellular K+ concentration (Hu et al. 2019), synaptic function (Adamsky et al. 2018; Chung et al. 2015; Martín et al. 2015; Yu et al. 2018), exert antioxidant actions through glutathione (GSH) production (Dringen and Arend 2025; Dringen and Hamprecht 1996, 1998), are responsible for lipid synthesis and metabolism (J. A. Lee et al. 2021; Nieweg et al. 2009) and synthesis and removal of neurotransmitters from the extracellular space (Daikhin and Yudkoff 1998; Danbolt 2001; Duan et al. 1999; Schousbe and Waagepetersen 2005). Additionally, astrocytes can produce KB further highlighting their relevance in brain energy metabolism. Thus, the aim of the present review is to compile and discuss the reported evidence on the effects of different keto therapies on astrocytes, expecting that this information will be useful for developing and improving future ketotherapeutic strategies.
2 Astrocytes as KB producers
KB production or ketogenesis primarily occurs in liver mitochondria, however, in the CNS, astrocytes can also produce KB, albeit at lower levels (Auestad et al. 1991; Puchalska and Crawford 2017). Besides KB metabolic role in ATP production, KB serve, though to a lesser extent, as precursors for the synthesis of fatty acids and cholesterol (Lopes-Cardozo et al. 1986; Nieweg et al. 2009). In hepatocytes, ketogenesis is activated when acetyl-CoA accumulates as a result of fatty acid β-oxidation, exceeding the capacity of the TCA cycle. The excess acetyl-CoA is then redirected toward the synthesis of KB or cholesterol. The classical ketogenesis pathway initiates with the condensation of two molecules of acetyl-CoA, catalyzed by the enzyme thiolase, to form acetoacetyl-CoA. A third acetyl-CoA molecule is then added by hydroxymethylglutaryl-CoA synthase (HMG-CoA synthase), producing β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which is the rate-limiting step of the pathway. HMG-CoA is subsequently cleaved by HMG-CoA lyase to generate AcAc and a free acetyl-CoA molecule. AcAc can be reduced to BHB by the enzyme β-hydroxybutyrate dehydrogenase 1 (BHD1) in a NADH-dependent reaction or undergo spontaneous non-enzymatic decarboxylation to form acetone, a volatile compound excreted through the lungs (Nuwayhid et al. 1988).
Astrocytes are also capable of producing KB, particularly form oxidation of fatty acids such as palmitate. In this context, approximately 90 % of metabolic products are KB, with CO2 representing only around 10 % (Blázquez et al. 1998). Notably, more than 85 % of the total KB formed in astrocytes consist of AcAc (Auestad et al. 1991). However, unlike hepatocytes, astrocytes do not appear to rely on the canonical HMG-CoA synthase pathway. Instead, research suggests that astrocytic ketogenesis proceeds through alternative mechanisms. One proposed pathway involves the enzyme succinyl-CoA:3-oxoacid-CoA transferase (SCOT), whose expression is upregulated in astrocyte cultures treated with BHB under glucose- and serum-deprived conditions (Suzuki et al. 2009). Another potential pathway involves acetoacetyl-CoA deacylase, which acts directly on acetoacetyl-CoA – an intermediate derived from the β-oxidation – to produce AcAc (Auestad et al. 1991). Further supporting the existence of a non-canonical pathway, astrocytes derived from human induced pluripotent stem cells (iPSC) have been shown to express HMG-CoA lyase but not HMG-CoA synthase (Thevenet et al. 2016). This molecular evidence reinforces the idea that astrocytes produce KB via an alternative route that bypasses the classical hepatic enzymes.
Altogether, these findings underscore the metabolic versatility of astrocytes and their potential contribution to brain energy homeostasis. The ability to produce KB suggests an important role for astrocytes in supporting neuronal function during energy stress, with possible implications for neuroprotection and metabolic adaptations.
3 Exogenous KB exposure to astrocytes
3.1 Efficiency of KB metabolism in astrocytes compared to neurons and substrate preference
During physiological conditions, both astrocytes and neurons are capable of metabolizing KB (Figure 1(1)), but their relative efficiency has been a subject of debate. Early studies suggested that astrocytes in culture metabolize AcAc at a rate four times higher than neurons after 8 h exposure to 0.5–1.0 mM, as measured by CO2 production, but AcAc oxidation is lower than glucose. Cultured neurons also metabolize AcAc, and the CO2 production rate is two times higher than that of glucose in this type of cells (Lopes-Cardozo et al. 1986). Other studies in pure cell preparations, and cell cultures from astrocytes, neurons and oligodendrocytes, reported that the ketolytic enzymes – SCOT, acetoacetyl-CoA thiolase and BHD – exhibit higher activity in astrocytes than neurons and oligodendrocytes, particularly during early stages of development as enzyme activity decreases with maturation (Chechik et al. 1987; Poduslo 1989). These results suggest that astrocytes actively metabolize KB, as previously suggested by Lopes-Cardozo et al. 1986. In astrocytes, AcAc also serves for the synthesis of lipids and cholesterol (Lopes-Cardozo et al. 1986).
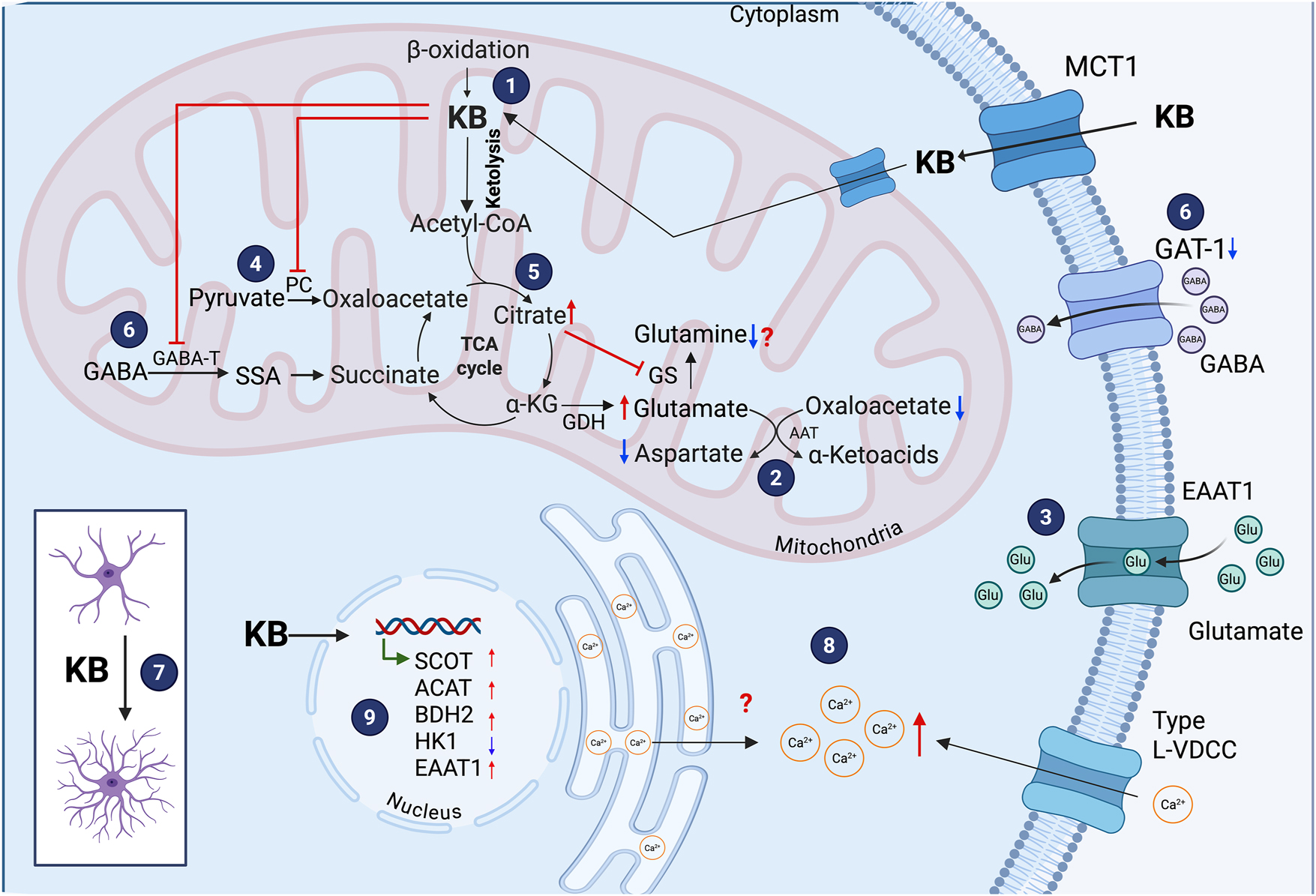
Effects of ketone bodies (KB) on astrocytes. Astrocytes can metabolize KB, although less efficiently than neurons (1). In astrocytes, β-hydroxybutyrate (BHB) decreases intracellular aspartate and increases glutamate levels due to decreased transamination reaction of aspartate (2). BHB elevates astrocytic glutamate uptake (3). KB inhibits pyruvate carboxylase (4) and reduce intracellular glutamine levels, likely due to elevated citrate concentrations (5). BHB also suppresses the expression and activity of GABA-transaminase (GABA-T), and decrease GAT-1 (6). BHB promotes a morphological transformation in astrocytes from a polygonal to a stellate shape with increased number of cellular processes (7), and elevates intracellular Ca2+ levels through Type L-VDCC (8). At the molecular level, BHB upregulates the expression of genes encoding succinyl-CoA:3-ketoacid CoA transferase (SCOT), acetoacetyl-CoA thiolase (ACAT), 3-hydroxybutyrate dehydrogenase type 2 (BDH2), and the glutamate transporter EAAT1, while downregulates hexokinase 1 (HK1) (9).
In contrast to the previously described observations, studies by Edmond et al. (1987), suggest that KB are preferentially oxidized in neurons rather than astrocytes. Using primary cultures from neurons, oligodendrocytes and astrocytes from the developing brain, their observations revealed that the three type of cells efficiently metabolize KB in the presence of glucose (incubations with 0.5 mM acetoacetate and 1.0 mM BHB for 3 h in the presence of 1.2 mM glucose), but consumption by neurons and oligodendrocytes was about three times more efficient than in astrocytes, as assessed by CO2 production. This correlated with a higher activity in neurons of the ketolytic enzyme SCOT (Edmond et al. 1987). In addition, the results from this study suggest that KB are better substrates for respiration than glucose in the three types of cells, but glucose consumption for respiration is higher in neurons and oligodendrocytes relative to astrocytes. Results also suggest that astrocytes preferentially metabolize KB rather than glucose, with no significant differences in the metabolism of AcAc and BHB. Discrepancies between these observations and those of previous studies (Chechik et al. 1987; Lopes-Cardozo et al. 1986; Poduslo 1989) might be related to differences in the preparations used and experimental conditions. In Lopes-Cardozo’s study, metabolic measurements were performed in cells attached to cultured flasks after long exposure times (8 h), while Edmond et al. (1987) used shorter incubation times (1–3 h) and measurements were performed in cell suspensions. On the other hand, the state of maturation of cells might influence their ketolytic activity as suggested by Chechik et al. (1987).
A more recent study used Föster resonance energy transfer (FRET) nanosensors for glucose and pyruvate to monitor metabolic activity in mixed neuronal/glial cultures, organotypic hippocampal slices, and acute hippocampal slices prepared from fasted ketotic mice (36 h food-deprived) (Valdebenito et al. 2016). In line with Edmond’s observations, it was reported that chronic exposure of cultured cells and brain slices to BHB (2 mM for 3 days), led to decreased glucose consumption and enhanced pyruvate oxidation in astrocytes, suggesting that KB inhibit glycolysis and stimulates mitochondrial oxidative metabolism in these cells. Furthermore, astrocytes in brain slices obtained from ketotic mice, also showed decreased glucose consumption. These results suggest that astrocytes use BHB to support mitochondrial metabolism, possibly sparing glucose for neuronal consumption. However, the effects of BHB on astrocytes were not compared to those in neurons.
A recent transcriptomic analysis showed that in human induced pluripotent stem cells (iPSCs) differentiated either to neurons or astrocytes, the mitochondrial enzymes SCOT1, SCOT2 and BDH1 are highly expressed in neurons, whereas BDH2, the cytosolic isoform, is enriched in astrocytes, suggesting that neurons possess a higher capacity for mitochondrial ketolytic metabolism (Thevenet et al. 2016). Other recent observations in mouse cortical slices using [U-13C]-BHB (1 h at 200 mM) and gas chromatography-mass spectrometry are in agreement with Edmond et al. (1987) early observations suggesting that BHB metabolism occurs preferentially in non-astrocytic cells, presumably neurons (Andersen et al. 2022). Additionally, human studies (Pan et al. 2001, 2002) previously suggested that overnight fasting resulted in increased incorporation of [2,4-13C2]-D-BHB into glutamate as compared to glutamine, suggesting a higher consumption of BHB in neurons.
A recent study by York et al. (2024) using mass spectrometry imaging (which combines chemical and spatial resolution with imaging), within the cytoarchitecture of the live hippocampal granular neuronal layer of the GD, reported that in neurons of the DG, BHB (2 mM) is more rapidly and extensively incorporated into the TCA as compared to lactate and pyruvate (2 mM each) in the presence of glucose, possibly due to its rapid conversion to Acetyl-CoA. Also, glucose and BHB are incorporated into glutamate and to a lesser extent into glutamine, although, in these experiments, the contribution of astrocytes to the changes observed could not be discarded. These results led to the conclusion that in the presence of glucose, BHB is metabolized as an energy source through the TCA and also for neurotransmitter synthesis in this hippocampal region, in agreement with the previous studies (Chowdhury et al. 2014; Jiang et al. 2011). When hippocampal slices were stimulated with a depolarizing concentration of KCl, glucose metabolism was favored relative to the other substrates, in agreement with previous studies suggesting that glucose is the main fuel under neuronal activity (Achanta et al. 2017; Chowdhury et al. 2014; Jiang et al. 2011).
Altogether, these studies suggest that KB are better substrates for respiration than glucose, and they can be used as an energy source in neurons, oligodendrocytes and astrocytes during development. Additionally, results suggest that KB are metabolized to a greater extent in neurons than in astrocytes. However, glucose is a preferred energy substrate for neurons as compared to astrocytes, while astrocytes prefer KB upon glucose relative to neurons.
The role of KB on brain metabolism has also been studied in pathological conditions, such as traumatic brain injury. It has been reported that in immature rats (21–22 days-old) BHB infusion (0.2 M) after traumatic brain injury, results in a higher KB metabolism for the synthesis of glutamate in neurons, as compared to glutamine in astrocytes, in the cortico-hippocampal formation (Scafidi et al. 2022). These results are in agreement with previous ex vivo studies suggesting that BHB is used in brain in a large proportion for acetyl-CoA production, accounting for 36–40 % of total substrate oxidation through the TCA. BHB is also used for neurotransmitter synthesis in neurons (glutamate and GABA), and to a lesser extent for glutamine synthesis in astrocytes, as measured by nuclear magnetic resonance spectrometry (NMRS) in cortical tissue from rats infused with [2,4-13C2]BHB and subjected to different protocols of activity (wakefulness, anesthetized and isoelectricity) (Chowdhury et al. 2014; Jiang et al. 2011). Results also suggested that KB do not replace glucose to sustain energy demands during increased neuronal activity (Chowdhury et al. 2014; Jiang et al. 2011). Nonetheless, during hypoglycemic conditions in cortical slices exposed to low glucose (0.4 mM [1-13C]D-glucose) and BHB (0.25–2.5 mM [U-13C]D-BHB) for 90 min, and using NMRS for metabolite determination, an increased production of neuronal neurotransmitters was observed, suggesting that KB are used in neurons to synthesize neurotransmitter during hypoglycemia (Achanta et al. 2017).
The metabolic efficiency of different energy substrates depends on their intracellular and extracellular availability, and this has been studied in competition experiments. For example, under conditions of glucose and serum deprivation, primary cultured astrocytes consume BHB as an alternative energy fuel to glucose after its chronic exposure (10 mM BHB during 5 days), preserving their viability (Y. Suzuki et al. 2009).
Under physiological conditions, when the metabolism of various substrates is evaluated individually in astrocyte primary cultures from rat brain, BHB ranked third in efficiency as compared to glucose, glutamine, lactate and malate, using CO2 production as a metabolic indicator, and a 1 mM concentration of each substrate incubated for 1 h. CO2 production from lactate and BHB was 10-11-fold higher than that of glucose, but lower than that of glutamine, which was the metabolite with the highest CO2 production in astrocytes. In contrast to astrocytes, glucose and lactate consumption was higher in synaptic terminals, and CO2 production from BHB was similar to that of glucose, but lower to glutamine and lactate (McKenna et al. 1993).
When BHB metabolism in astrocytes was assessed competitively with other substrates (glucose, glutamine, lactate and malate at 1 mM concentration each), CO2 production from labeled glutamate was the highest, suggesting its use as an energy substrate in astrocytes, and its metabolism was not altered in the presence of the other substrates. The oxidation of glutamine was higher than that of glucose, lactate, BHB and malate, and inhibited by the addition of glutamate (McKenna 2012). CO2 production from lactate and BHB was similar and substantially higher than that of glucose, and the addition of BHB did not alter CO2 production from glucose or lactate, suggesting they are metabolized in a different compartment. However, BHB consumption was substantially inhibited by glutamate and glutamine, suggesting that endogenously formed glutamate from glutamine and exogenous glutamate enter the same TCA cycle as BHB and compete (McKenna 2012). Overall, these observations suggest that astrocytes can use different substrates to produce energy through the TCA, and that glutamate and glutamine are preferred energy substrates for astrocytes over BHB and lactate.
In summary, the aforementioned observations suggest that the efficiency of KB metabolism in astrocytes is influenced by the availability of alternative substrates, their concentration and treatment duration. This flexibility highlights the metabolic adaptability of astrocytes in response to nutritional status.
On the other hand, KB influence mitochondrial function due to their role in redox balance. Since both BHB and AcAc are substrates and products of BDH, their interconversion affects the cellular NAD(P)H/NAD(P)+ ratio. In particular, it has been observed that BHB administration (at increasing concentration from 1–30 mM, during 50 min) enhances this ratio both in neurons and astrocytes derived from iPSCs, reflecting its mitochondrial oxidation to AcAc via BDH (Thevenet et al. 2016). However, the concentration of D-BHB needed to enhance NAD(P)H production was above 3 mM, which might not be reached in brain after the ketogenic diet or the intake of medium chain fatty acids (MCFA).
BHB oxidation exerts distinct – and in some cases opposite – effects on mitochondrial metabolism in astrocytes and neurons. While chronic exposure (7 days) to 4 mM BHB does not alter basal respiration, extracellular acidification rate (ECAR) or ATP production in primary cultured astrocytes, all these processes are enhanced in primary cultured neurons (Koppel et al. 2023). Additional differences are reported in mitochondrial efficiency. In astrocytes, BHB exposure reduces the proton leak and oxygen consumption, while increasing maximal respiration and the spare respiratory capacity – changes that may reflect an increase in mitochondrial mass. However, this increase does not appear to enhance energy production but may instead support anabolic functions. In contrast, neurons treated with BHB show an increased mitochondrial mass, which might enhance energy production by facilitating the throughput of the respiratory chain (Koppel et al. 2023). These findings support that energy production by BHB is more active in neurons than astrocytes, in agreement with the studies discussed above.
3.2 Astrocyte metabolic changes in response to KB exposure
3.2.1 Effect of KB on astrocytic neurotransmitter metabolism and uptake
Pioneer studies by Yudkoff et al. (1997) reported that AcAc and BHB exposure (5 mM each for 1 h) to cultured astrocytes led to a decrease in aspartate levels, an increase in glutamate content, and no modifications in glutamate uptake. These findings were attributed to a decrease in glutamate transamination, likely due to diminished oxaloacetate levels (Figure 1(2)). Hazen et al. (1997) supported this suggestion, showing that AcAc administration (5 mM) to cultured astrocytes inhibits pyruvate carboxylase (PC) (Figure 1 (4)), lowering oxaloacetate availability and thereby reducing glutamate-to-aspartate transamination (Hazen et al. 1997).
Leite et al. (2004) also found that 5 mM BHB treatment either for 1 or 24 h did not alter glutamate uptake in cultured astrocytes when it was assessed at a single time point, 7 min after BHB exposure, using a highly sensitive radiolabeled [3H] glutamate uptake assay. In contrast, Shang et al. (2024) reported increased glutamate uptake in BHB-treated primary astrocytes (2 mM for 24 h) – as indicated by lower residual glutamate levels in the culture medium at multiple time points (10, 40 and 60 min) – and using a moderately sensitive colorimetric/fluorometric assay – along with elevated mRNA and protein levels of the EAAT1/GLAST glutamate transporter (Figure 1(3, 9)). Increased expression of the glutamate transporter, EAAT1/GLAST, was confirmed in the hippocampus of mice treated with BHB (60 mg/kg for 2 days). These contrasting observations might result from differences in experimental protocols, as exposure times and detection methods. The delayed decrease in extracellular glutamate levels observed by Shang et al. (2024) suggests that BHB may induce transcriptional and translational upregulation of glutamate transporters – a response likely undetectable after 7 min BHB exposure assessed by Leite et al. (2004). However, the precise mechanism underlying the effects of BHB on astrocytic glutamate uptake remains to be elucidated.
Regarding glutamine, Yudkoff et al. (1997) reported opposite effects of AcAc and BHB (5 mM for 1 h) on glutamine levels. While AcAc led to a decrease in intracellular glutamine, attributed to elevated citrate levels, which inhibits glutamine synthetase (GS) activity, BHB incubation resulted in increased glutamine content (Figure 1 (5)).
Importantly, KB inhibit the incorporation of the GABA amino group into glutamine in astrocytes, leading to an expansion of the GABA pool (Yudkoff et al. 1997). In addition, Suzuki et al. (2009), demonstrated that BHB exposure (1–10 mM during 5 days) to astrocyte cultures (without serum and glucose) suppresses the expression and activity of GABA-transaminase (GABA-T) – the enzyme responsible for transferring the GABA amino group into glutamine (Figure 1(6)). Additionally, in the same conditions, BHB reduced the expression of the GABA transporter, GAT-1, responsible for the uptake of GABA into astrocytes and GABAergic synaptic terminals (Figure 1(6)). These findings support the hypothesis that BHB exposure, as the KD, increases GABA concentration suppressing astrocytic GABA degradation and contributing to its antiepileptic effects.
In addition to these neurotransmitter-related effects, BHB exposure may also regulate the secretion of S100β, an astrocyte-derived neurotrophic factor. Leite et al. (2004) observed an early elevation of S100β secretion 1 h after 5 mM BHB exposure to cortical primary cultures of astrocytes, which decreased below basal levels at 24 h. Given that elevated S100β abundance is associated with seizure susceptibility and neuronal damage, these changes may also contribute to the antiepileptic benefits of BHB (Leite et al. 2004).
Together, the observation described above highlight the different metabolic roles of KB in astrocytes and neurons, suggesting that BHB actions extend beyond energy production to influence neurotransmitter metabolism, mitochondrial function and bioenergetic adaptability. Further research is needed to clarify the time- and context-dependent nature of these effects, particularly in relation to neuroprotection and therapeutic applications of KB.
A limitation of the studies discussed above is the high concentrations of KB used, as in most of the studies varied between 1-5 mM or even 10 mM, which are likely not reached in the brain of individuals consuming exogenous ketones or under diet approaches to induce ketosis, as they are rapidly metabolized (Almeida-Suhett et al. 2022; Suissa et al. 2021). On the other hand, cell suspensions, primary cultured cells and cultured cells derived from human iPSCs, might exhibit metabolic differences that may account for the discrepancies observed between the different studies, and might differ from data obtained in more intact preparations as brain slices and in vivo studies.
3.3 Morphologic changes in astrocytes exposed to KB
In addition to metabolic effects, BHB can influence astrocyte morphology as it promotes the transition from a polygonal to a stellate shape characterized by the development of more cellular processes (Figure 1 (7)) (Leite et al. 2004). These changes become evident 6 h post-incubation with 2–10 mM BHB and are reversible after 24 h. This morphologic transformation is possibly mediated by the suppression of RhoA signaling, a pathway involved in cytoskeleton dynamics and the formation of stress fibers. Specifically, the small GTPase, RhoA, is inhibited by BHB (10 mM), as shown by the ability of lysophosphatidic acid (LPA) – a known RhoA activator – to block the BHB-induced morphologic changes (Leite et al. 2004). However, the mechanism involved in RhoA inhibition by BHB is unknown.
Changes in astrocyte morphology induced by BHB might reflect functional alterations, which could, in turn, influence the activity of neighboring neurons, highlighting a broader role for BHB in the regulation of neuroglial interactions. More studies are needed in order to understand the relationship between morphological changes in astrocytes and the benefits of KB in brain.
3.4 Effect of KB on astrocytic calcium concentration
Exposure of glial cells to 5 and 10 mM BHB leads to elevated cytosolic Ca2+ levels (Figure 1 (8)) (Xiao et al. 2007). This increase is observed even in Ca2+/Mg2+-free medium, indicating the contribution of intracellular Ca2+ pools to this response. However, the application of nitrendipine, an L-type voltage-dependent Ca2+ channel blocker, completely abolished the maximal calcium fluorescent signal, suggesting that BHB-induced cytosolic Ca2+ elevation is likely mediated by extracellular Ca2+ influx through these channels (Xiao et al. 2007). Further studies are required to elucidate the mechanisms and sources of intracellular Ca2+ mobilization triggered by BHB.
3.5 Gene and protein expression in astrocytes exposed to KB
There is evidence of gene and protein expression modifications in astrocytes incubated with BHB (Figure 1 (9)). In astrocyte cultures maintained under glucose- and serum-free medium, 10 mM BHB altered the expression of genes related to glycolysis and KB metabolism, decreasing hexokinase I – an enzyme that catalyzes the first irreversible reaction in glycolysis – while increasing SCOT expression (Suzuki et al. 2009). Furthermore, after a 3-h exposure to 2 mM BHB, 176 genes were differentially expressed in astrocytes, with Slc1a3 (EAAT1/GLAST) showing the most significant change. Protein analysis confirmed EAAT1/GLAST upregulation, which was also observed in vivo in BHB-treated mice (intragastric administration of 60 mg/kg for 2 days), demonstrating increased EAAT1/GLAST expression in the hippocampus (Shang et al. 2024).
Additionally, exposure of astrocytes to 2 mM BHB for 24 h was reported to support neuronal survival under conditions of glutamate toxicity in co-cultures of neurons and astrocytes (Shang et al. 2024). The upregulation of EAAT1/GLAST prompted further investigation on changes in glutamate uptake and the possible regulatory pathways. A trend toward enhanced glutamate uptake was observed, accompanied by the upregulation of EAAT1/GLAST mediated by CAMKII activation, whose phosphorylation augments after 1 h incubation with 2 mM BHB- likely through increased Ca2+ levels via L-type calcium channels. CAMKII phosphorylates EAAT1/GLAST increasing its activity (Chawla et al. 2017), thereby BHB possibly modulates both EAAT1/GLAST expression and activity (Shang et al. 2024).
3.6 Effect of KB administration on astrocytes under pathological conditions
The effect of BHB administration on astrocytes under pathologic or stressful conditions has been reported and is often related to changes in the expression of proteins involved in key astrocytic functions. In an animal model of focal ischemia, where astrogliosis is characterized by increased GFAP levels, injection of BHB (500 mg/kg i.p.) 1 h after injury reduced astrogliosis and GFAP expression in the perilesional cortex (Bazzigaluppi et al. 2018). Similarly, in a model of heat stress-induced neuroinflammation, BHB administration (200 mg/kg/day 1 h before heat stress, for 14 days) decreased the number of GFAP-positive cells in the hippocampal ventral CA1. Interestingly, BHB also increased the number of terminal astrocytic endfeet without affecting astrocyte density or area–both of which were reduced following heat stress (J. Huang et al. 2022). These observations suggest that BHB treatment might partially inhibit astrocyte reactivity under stress conditions. In another study using a rat model of painful diabetic neuropathy, BHB administration (i.p. injection of 291 mg/kg during 28 days) increased paw withdrawal, used as an index of mechanical allodynia, and restored the elevated the expression of aquaporin-4 (AQP4) in the spinal cord, a water channel protein located at astrocytes endfeet, which is involved in the glymphatic system implicated in the excretion of metabolic waste products in the nervous system (Benveniste et al. 2017). BHB treatment also restored the decreased levels of α-syntrophin (SNTA1), a protein involved in anchoring AQP4 in the plasma membrane of astrocytes facing blood vessels (Wang et al. 2022). Results suggested that BHB treatment recovered the perivascular localization of AQP4 in the spinal glymphatic system, through the upregulation of SNTA1, and the downregulation AQP4, possibly mediated by the inhibition of histone deacetylase 1 (HDAC1), as previously reported (Wang et al. 2022). These effects of BHB are suggested to be involved in the attenuation of painful diabetic neuropathy through the modulation of astrocyte function (Wang et al. 2022).
4 Endogenous ketotherapeutics in astrocytes
4.1 Effect of MCFA on astrocytes
4.1.1 Effect of MCFA on astrocytes under physiological conditions
A diet enriched in medium-chain triglycerides (MCTs) leads to the release of medium-chain fatty acids (MCFA) – such as caproic acid (hexanoate, C6), caprylic acid (octanoate, C8), capric acid (decanoate, C10), and lauric acid (dodecanoate, C12) – after enzymatic hydrolysis in the gastrointestinal tract (De Gaetano et al. 1994). These MCFAs are rapidly absorbed and transported via the portal vein to the liver (Qian et al. 1998), where they are preferentially oxidized to acetyl-CoA within mitochondria. The increased acetyl-CoA flux promotes hepatic ketogenesis, resulting in the elevated production of ketone bodies and enhanced mitochondrial energy output (Y. Cao et al. 2025; Jadhav and Annapure 2023; Kanta et al. 2025).
Beyond promoting hepatic ketogenesis, MCFAs have attracted attention for their potential to directly modulate energy metabolism in the CNS, since a fraction of the MCFAs can cross the blood–brain barrier and reach the brain, where they are rapidly metabolized (Ebert et al. 2003). Consequently, studies investigating the effects of MCFAs on astrocytes have mainly focused on their mitochondrial bioenergetic function and the oxidative preference among different MCFAs (Andersen et al. 2021, 2022; Nonaka et al. 2016; Thevenet et al. 2016).
In astrocytes derived from human iPSCs, exposure to the MCFAs, C8 and C10 (300 μM), decreases the NAD(P)H/NAD+ ratio, the mitochondrial membrane potential, and the mitochondria matrix ΔpH, but does not affect oxygen consumption or ATP synthesis (Thevenet et al. 2016). It was speculated that these effects might result from MCFAs acting as positive regulators of uncoupling proteins such as UPC-4, although this hypothesis has not been confirmed. In contrast, Andersen et al. (2021) reported that C8 increased OCR, while C10 increased the proton leak in mouse primary cultured astrocytes. These differences might result from variations in the experimental design. While Thevenet et al. (2016) used human iPSC-derived astrocytes, treated with 300 μM of C8 or C10 for 21 min before OCR measurement, Andersen et al. (2021) employed astrocyte cultures exposed to 200 μM of each fatty acid for 1 h prior to analysis. Further studies are required to clarify whether MCFAs consistently stimulate mitochondrial respiration in astrocytes in other preparations, including in vivo studies.
Concerning oxidative preference, studies have compared MCFA oxidation in astrocytes and neurons, and between individual MCFAs (C8 vs C10), addressing their metabolic fate and oxidation products. The study by Thevenet et al. (2016) showed that the oxidation products of C10 and C8 differ in human astrocytes. While C10 induced a rapid lactate production and had little effect on ketogenesis, C8 induced BHB production, although at a slower rate than lactate. From these results, it was suggested that MCFA oxidation in astrocytes might supply neighboring neurons with lactate and BHB. However, in vivo evidence of a lactate or BHB shuttle from astrocytes to neurons is still needed to confirm this suggestion.
In agreement with Thevenet et al. (2016), Nonaka et al. (2016) reported that exposing the KT-5 astrocyte cell line to MCFAs (C8, C12 and C18) for 4 h increased KB concentrations, with C8 being more rapidly oxidized and producing higher KB levels than C12 and C18, thereby supporting the ketogenic capacity of MCFAs.
A slower oxidation rate of C10 over C8 was supported by the results of Andersen et al. (2021) using 13C-labeled substrates in mouse astrocyte–neuron co-cultures. The authors suggested that the differential oxidation of C10 and C8 could underlie variations in their clinical efficacy; although, further validation is required, particularly regarding the specific glycolytic or ketogenic oxidation products (lactate and KB), which were not directly measured in this study (Andersen et al. 2021).
It has been hypothesized that the preferential oxidation of MCFA in astrocytes relative to neurons might be attributed to the distinct expression of a family of substrate-specific acyl-CoA dehydrogenases that oxidize fatty acids within a specific range of carbon chain lengths, leading to the formation of acetyl-CoA, the initial precursor of ketogenesis (Thevenet et al. 2016). This might also explain why C8 is a better substrate for ketogenesis than C10. However, this hypothesis remains to be confirmed, and further experiments are needed to elucidate the mechanisms governing the differential ketogenic potential of MCFA.
On the other hand, Andersen’s observations also suggested that C8 and C10 MCFAs are predominantly oxidized in astrocytes, leading to increased glutamine synthesis, while their oxidation in neurons enhances neuronal GABA formation, which might contribute to the anticonvulsant effects of MCT-based diets, although this mechanism has not yet been demonstrated in vivo.
Finally, competition experiments in cortical slices using radiolabeled C8, C10 and BHB, showed little antagonism for oxidation between MCFA and BHB. While C8 and C10 were oxidized to glutamine and citrate, indicating their metabolism in astrocytes, BHB was oxidized to glutamate, malate, aspartate, a-ketoglutarate, and GABA, suggesting their metabolism in neurons. These findings support a model where astrocytes actively metabolize MCFA, while BHB is primarily metabolized in neurons, with little competition with other substrates (Andersen et al. 2021, 2022).
Further investigations on MCFA-induced changes in mitochondrial oxidative metabolism in astrocytes is relevant to elucidate whether these effects contribute to the cognitive improvements reported in individuals with mild cognitive impairment and Alzheimer’s disease receiving MCT-based interventions (Fortier et al. 2019; Henderson et al. 2009; Ohnuma et al. 2016; Rebello et al. 2015).
4.1.2 MCFA effect on astrocytes under pathological conditions
The neurodevelopmental disorder tuberous sclerosis complex (TSC), represents the pathological context in which the effect of MCFAs on astrocytes have been examined (Warren et al. 2020). Specifically, Warren et al. (2020) evaluated whether C10 modulates the mTORC1 (the mechanistic target of rapamycin complex 1) signaling pathway in astrocytes derived from TSC patients, a condition resulting from mutations in TSC1 or TSC2.
Treatment with 300 μM C10 for 24 h in TSC1-mutant astrocytes reduced the phosphorylation of 4E-BP1 and S6K, two direct downstream targets of mTORC1 activity, suggesting mTORC1 inhibition. This effect was also evident in control astrocytes, but not in TSC2-mutant astrocytes, suggesting a possible heterogeneous response depending on the specific gene affected (Warren et al. 2020). Also, these findings suggest that C10 can reduce the mTORC1 signaling pathway independently of the disease. The mechanism underlying C10 inhibition of mTORC1 in human astrocytes remains unresolved. On the other hand, it remains unknown whether C10 administration increases KB levels, and C10 effects might also involve KB produced by its metabolism.
Together, these observations suggest that astrocytes may represent a cellular target of MCFAs, and that decanoic acid could modulate mTORC1 activity in the brain, possibly contributing to cognitive improvements in neurodegenerative disorders such as Alzheimer’s disease and other neurological conditions associated with mTOR dysregulation.
4.2 Effect of the KD on astrocytes
The ketogenic diet (KD), composed by 70–80 % fat, 5–20 % carbohydrates, and 10–20 % protein, has shown positive effects against neurodegeneration and cognitive impairment in rodent models (Camberos-Luna and Massieu 2020; Rubio et al. 2025). Its effects have been attributed to multiple actions of KB, including their metabolic effect to generate ATP, anti-inflammatory and antioxidant properties, epigenetic modifications, autophagy stimulation, and decreased proteotoxicity, among others (Cheng et al. 2009; Gómora-García et al. 2023; Montiel et al. 2023; Najmi et al. 2016; Zhu et al. 2022). Also, BHB can behave as a signaling molecule through its action on membrane receptors (Spigoni et al. 2022). Despite substantial evidence supporting the use of diet approaches to improve brain disease, the effects of the KD on astrocytes remain largely unknown.
4.2.1 Effect of the KD on astrocytes under physiological conditions
4.2.1.1 Astrocytic activation and morphological changes under the KD
The KD induces several adaptations in astrocytes that may contribute to its neuroprotective and antiepileptic effects. These adaptations depend largely on the astrocytic functional state, which is commonly inferred from canonical markers – glial fibrillary acidic protein (GFAP), a structural component of the reactive cytoskeleton (Eng 1985; Lee et al. 2025; Oberheim et al. 2012), S100B, an astrocyte-derived trophic cytokine measured in tissue and cerebrospinal fluid (CSF) (Emsley and Macklis 2006; Jurga et al. 2021; Lee et al. 2025), and glutamine synthetase (GS), a key enzyme of the glutamate–glutamine cycle (Anlauf and Derouiche 2013; Jurga et al. 2021; Norenberg 1979). Changes in these markers help to distinguish transient from sustained astrocytic activation (the latter often associated with inflammation) and to differentiate tissue alterations from changes in astrocytic secretion dynamics.
The effects of the ketogenic diet (KD) on the astrocytic markers GFAP and S100B appear to be transient and region-specific. In healthy Wistar rats fed a KD for 6 weeks, GFAP expression increased in the CA3 region of the hippocampus after 1 week but returned to baseline by 6 weeks (Silva et al. 2005). In contrast, S100B levels decreased only in cerebrospinal fluid (CSF) after 8 weeks of KD (Vizuete et al. 2013), while remaining unchanged in hippocampal tissue at 1, 6 and 8 weeks (Silva et al. 2005; Vizuete et al. 2013), suggesting altered secretion dynamics rather than a change in cellular content. Therefore, the mechanism underlying the reduced CSF S100B levels (whether driven by diminished secretion or enhanced clearance) remains to be clarified. Likewise, hippocampal GS activity showed no significant alterations after 1 or 6 weeks of KD (Silva et al. 2005). These patterns possibly represent an early adaptive response to KD rather than pathological activation, as they are transient and not associated with inflammation.
Modifications in these markers under KD suggest coordinated adaptations in astrocytic structure, metabolism, and intracellular signaling. Notably, S100B is functionally linked to intracellular Ca2+ dynamics, as it directly binds Ca2+, acting as both a buffer and a signaling mediator (Donato 2001), suggesting that KD effect on astrocytes may extend to Ca2+-dependent processes.
In agreement, healthy male Wistar rats aged 70 days, and fed a KD for 4 months showed increased calbindin (CB; Ca2+-binding protein)-positive cells in the hippocampus and the M1 cortical area (Gzielo et al. 2019). This increase possibly contributes to the protective effect of KD by maintaining Ca2+ homeostasis, as enhanced Ca2+ buffering through CB may help to attenuate pro-apoptotic signaling. However, further research is needed to substantiate this interpretation. In vivo Ca2+ imaging of astrocytes combined with CB expression analysis could help to determine whether elevated CB levels reduce the amplitude or frequency of astrocytic Ca2+ waves, while electrophysiological recordings could clarify whether altered Ca2+ regulation mitigates excitotoxicity or seizure propagation. The quantification of apoptotic markers in parallel with the changes in CB levels in pathological models, such as kainate-induced epilepsy or ischemia–reperfusion injury, would be helpful to stablish a possible correlation between astrocytic remodeling, increased CB and reduced apoptosis by the KD.
Regarding morphology, Gzielo et al. (2019) reported that astrocytes from KD-fed healthy animals exhibit fewer intersections, smaller cell bodies, and reduced fractal dimensions compared to those from animals fed a standard diet. These changes do not appear to reflect chronic glial activation or inflammation, as markers such as GFAP, Iba1, and other astrocyte or microglial-related markers remain unchanged, likely because the animals were in a non-pathological state. Instead, these morphological simplifications possibly reflect a metabolic shift from glucose to KB metabolism (Gzielo et al. 2019).
These morphological effects contrast with the more complex astrocytic morphology reported after acute (10 mM BHB for 6 h) BHB administration in primary astrocyte cultures from newborn Wistar rats (Leite et al. 2004). These observations underscore the complexity of KD-induced astrocytic changes and suggest that both the duration and metabolic context of KB exposure influence astrocyte structure and function. Clarifying whether these KD-induced adaptations represent beneficial neuroprotective adjustments or persistent structural changes distinct from acute BHB effects will require further study.
Future investigations incorporating female and aged cohorts, energy-matched controls, and multiple time points, will increase our understanding of the KD effects on astrocyte function. Also, high-resolution techniques (such as 3D reconstruction, light-sheet, or two-photon microscopy) could better resolve fine perisynaptic processes and clarify whether KD induces subtle structural rearrangements or broader astrocytic remodeling. Elucidating KD-induced astrocytic morphological changes is particularly important to understand their interactions with neurons and the vasculature, which might also impact brain plasticity.
4.2.1.2 Metabolic adaptations in astrocytes under the KD
The administration of the KD to rats for three weeks induces adaptive changes in astrocyte metabolism. In the cerebral cortex of adult male GAERS rats (genetic absence epilepsy rat from Strasbourg), KD feeding for three weeks decreased acetate metabolism in neurons, while astrocytic acetate metabolism increased as suggested by the glutamate, glutamine, and GABA production from acetate oxidation (Melø et al. 2006).
Pyruvate carboxylase (PC) is an astrocytic enzyme that provides oxaloacetate to the TCA, which is more active in ketotic astrocytes (from KD fed rats during 3 weeks), possibly due to its allosteric activation by acetyl-CoA derived from KB (Melø et al. 2006). Also, in these animals increased pyruvate recycling through the TCA was observed in astrocytes. However, in primary cultured rat astrocytes, 5 mM AcAc exposure for 1 h inhibited PC (Hazen et al. 1997), possibly as a result of KB limiting pyruvate availability by inhibiting glucose oxidation. Alternatively, it was suggested that the KD (as well as carbonic anhydrase (CA) inhibitors) might act in part by altering astrocytic anaplerosis.
The contrasting effects of acetoacetate (AcAc) on astrocytic PC reported by Hazen et al. (1997) and Melø et al. (2006) likely result from differences in the model and the timescale used reflecting a possible acute inhibition versus chronic adaptation. The chronic in vivo study under sustained ketosis by Melø et al., suggests a shift toward enhanced anaplerosis (higher PC/PDH ratio), whereas the acute in vitro experiments reported by Hazen et al. (1997), showed that carbonic anhydrase inhibition by short-term AcAc exposure markedly reduced PC-dependent labeling, consistent with immediate substrate limitation.
Further studies are needed to clarify whether AcAc acts as a carbonic anhydrase–like inhibitor, limiting HCO3 − generation and thereby reducing PC flux. On the other hand, the apparent rise in PC activity reported by Melø should be viewed cautiously, as the higher PC/PDH ratio may reflect PDH inhibition rather than PC upregulation – since acetyl-CoA formed during ketosis both activates PC and inhibits PDH, shifting the balance upward without necessarily increasing total PC activity. Discrepant results might also be related to differences in astrocyte metabolism in in vitro and in vivo conditions, as Hazen et al. (1997) used pure astrocyte cortical cultures while Melø et al. examined the whole cortex, where mixed neuronal–astrocytic interactions, and astrocyte-specific acetate metabolism may predominate.
Another adaptation induced by the KD in astrocytes is related to the monocarboxylate transporter system. Astrocytes take up BHB through a carrier-mediated transport system as first described by Tildon et al. (1994). Their findings showed that BHB uptake is saturable (Km = 6.06 mM, V max = 32.7 nmol/30 s/mg protein), pH-dependent (with higher uptake at pH 6.2), and inhibited by metabolic blockers, suggesting H+ co-transport (symport) (Tildon et al. 1994). A direct link between KB transport and monocarboxylate transporters (MCT) was first suggested by Poole and Halestrap (1993), and later confirmed by Halestrap and Price (1999). Their study demonstrated that MCT1, MCT2, and MCT4 mediate KB transport in various tissues, including the brain, heart and muscle (Halestrap and Price 1999). MCT belong to the SLC16A solute carrier family, which comprises proton-coupled transporters responsible for facilitating the transport of monocarboxylates such as lactate, pyruvate and KB. Among the 14 known MCT isoforms, MCT1, MCT2 and MCT4 are expressed in brain (Pierre and Pellerin 2005), whereas astrocytes express MCT4 (SLC16A3) and MCT1 (SLC16A1), while MCT2 is enriched in neurons (Bröer et al. 1997; Debernardi et al. 2003; Hanu et al. 2000; Rafiki et al. 2003; Rosafio and Pellerin 2014).
Mice subjected to KD for three weeks showed an increase in brain expression and transport activity of MCT1 in cortical astrocytes. Moreover, the frequency of Ca2+ transients induced by bicuculline (a chemical inducer of epileptiform activity) was lower in cortical astrocytes from KD-fed mice than in those from control diet-fed mice. Results suggest that the increased expression and activity of MCT1 in astrocytes, and also possibly in neurons – since MCT1 has also been reported in neurons (Pierre et al. 2007) – might play a role in attenuating epileptiform activity (Forero-Quintero et al. 2017). However, further studies are needed to fully understand their contribution.
4.2.1.3 Transcription profile modifications in astrocytes under KD
The KD induces significant transcriptional and metabolic adaptations in astrocytes that collectively favor KB metabolism over glycolytic glucose utilization. Recent transcriptomic analyses in male mice have shown that KD (3 months) downregulates astrocytic insulin-signaling genes (Koppel et al. 2021), indicating a shift away from the canonical glucose-responsive pathways. Complementary proteomic and imaging studies further suggested the upregulation of β-oxidation and ketolytic pathways protein expression, together with enhanced astrocytic-mitochondrial SCOT immunofluorescence after the KD in the cortex (Düking et al. 2022). These findings highlight substantial changes in oxidative metabolism within astrocytes from KD-fed animals, exhibiting a shift from glycolysis toward oxidative phosphorylation (OXPHOS). This metabolic reorientation suggests that astrocytes prioritize KB oxidation while potentially sparing glucose for neuronal utilization, thereby promoting greater ketone use within the brain.
Despite overall agreement regarding metabolic remodeling, Koppel et al. (2021) and Düking et al. (2022) diverge in their observations on transcriptional regulation in astrocytes. Koppel et al. (2021) reported no statistically significant differences in gene expression within astrocytes, suggesting that KD-induced adaptations might be primarily functional and metabolic rather than the consequence of an extensive transcriptional reprogramming. In contrast, Düking et al. (2022) reported a coordinated upregulation of genes related to β-oxidation, ketolysis, TCA cycle, and OXPHOS, alongside the downregulation of glycolysis and glycogen metabolism-related genes. These apparent discrepancies likely reflect differences in experimental protocols, including species (rat vs mice), dietary fat content (90 vs 75 %), exposure duration and developmental stage at KD initiation (prenatal/perinatal vs juvenile). Such factors possibly influence astrocyte maturation and MCT1 content, circulating ketone levels, and the magnitude of transcriptional responses.
Beyond metabolic reprogramming, Koppel et al. (2021) focused on the regulation of intracellular signaling, reporting that feeding the KD downregulated astrocytic glutamate receptor pathways, consistent with a reduction in Ca2+ transients in KD-fed mice. Their analysis of pathology-associated modules revealed suppression of 14 pathology-related components, including those associated with cancer, addiction, metabolic disorders, infection, and cardiovascular disease. In contrast, Düking et al. (2022) did not specifically analyze pathology-related pathways but instead emphasized the broader metabolic flexibility of astrocytes compared to oligodendrocytes and neurons under KD conditions, underscoring their central role in cerebral energy adaptation.
Taken together, these complementary findings suggest that astrocytes respond to the KD with a shift toward ketone metabolism and mitochondrial oxidative activity while attenuating glycolytic flux. Further studies should delineate how these astrocytic adaptations might influence neuronal activity, network excitability, and disease progression, and further characterize the mechanisms underlying KD-induced transcriptional plasticity.
4.2.2 Effect of the KD on astrocytes under pathological conditions
The effect of KD on astrocytes under pathological conditions has been directly examined in a few studies, focused on excitotoxic and traumatic brain injury (TBI). In the excitotoxicity model, it has been reported that 4 weeks of KD feeding, beginning at postnatal day 21 (P21) in male ICR mice, increased astrocytic CB expression in the hippocampus following excitotoxic injury induced by an intraperitoneal injection of kainic acid (KA, 25 mg/kg), and assessed 48 h later at P50 (Noh et al. 2005). From these results it was suggested that CB upregulation might contribute to the neuroprotective effects of KD through calcium buffering, thereby limiting Ca2+-dependent activation of caspase-3 and reducing apoptotic vulnerability. Further investigation is needed to support the correlation between intracellular calcium dynamics, caspase-3 activity, and CB protein levels under the KD. Studies might also include both sexes, longer survival times and functional readouts.
In the pathological context of traumatic brain injury, it was reported that a KD administered immediately after mild controlled cortical impact (CCI) in adult male ICR mice mitigated cognitive deficits and astrocytic reactivity (Har-Even et al. 2021). Mice maintained on the KD for 30 days showed improved recognition memory in the novel object recognition (NOR) test. KD treatment also reduced astrocyte reactivity – evidenced by lower GFAP immunoreactivity in the DG – while cortical astrocyte activation remained unchanged. Additionally, the authors reported that KD feeding partially restored the levels of the NAD+-dependent histone/protein deacetylase, SIRT1, which were diminished by TBI, based on measurements from bulk cortical and hippocampal tissue. Although the study did not assess SIRT1 functional activity or its downstream targets, the authors speculated that the KD might attenuate reactive gliosis and support neuronal recovery, as SIRT1 activation has been shown to regulate key neuroprotective and metabolic pathways involving p53, nuclear factor-κB (NFκB), peroxisome proliferator-activated receptors α and γ (PPARα and PPARγ), PPARγ coactivator-1α (PGC-1α), liver X receptor, and FOXO (Martin et al. 2015). Future studies should directly examine SIRT1 activity and its downstream targets in animals treated with the KD.
Overall, the limited but consistent evidence suggests that KD effects on astrocytes under pathological conditions may contribute to neuroprotection, potentially through increased CB expression and reduced astrocytic reactivity. Nevertheless, these findings remain largely correlative, highlighting the need for mechanistic studies to elucidate how KD-induced metabolic adaptations in astrocytes translate into functional protection within neural circuits.
4.3 Effect of CR on astrocytes
4.3.1 Effect of CR on astrocytes under physiological conditions
4.3.1.1 Metabolic effects induced by CR in astrocytes
Caloric restriction (CR) refers to a sustained reduction of daily caloric intake by approximately 20–40 % below the total requirements, without altering meal frequency. Beyond its systemic metabolic benefits, CR exerts profound effects on brain metabolism and glial physiology. Early studies using adult male Wistar rats subjected to progressive caloric restriction (10 %, 20 %, and 30 % reduction in daily intake during the first, second, and subsequent ten weeks, respectively) for 12 weeks, showed increased hippocampal glutamate uptake and GS activity (Ribeiro et al. 2009; Santin et al. 2011). These findings were interpreted as indicative of enhanced astrocytic regulation of extracellular glutamate, potentially supporting neurotransmitter homeostasis under reduced energy availability. However, this interpretation remains indirect, as the conversion of glutamate into glutamine for neuronal reuse was not directly demonstrated. Santin et al. (2011) further expanded this approach by examining hippocampal redox status, and reported that CR, alone or combined with moderate exercise, elevated reduced glutathione levels (GSH), decreased protein carbonyls, and increased total antioxidant response. These results suggest that CR may not only alter astrocytic glutamate metabolism but also improve redox homeostasis, contributing to the neuroprotective profile of CR. However, the functional consequences of these biochemical changes were not evaluated and redox analyses lacked cellular resolution, thereby future work might combine astrocyte-targeted metabolic tracing with functional and redox imaging, to further understand whether CR induces coordinated metabolic and antioxidant remodeling in astrocytes to support synaptic resilience.
Increased glutamate uptake might result from changes in transporter abundance, altered trafficking dynamics, or enhanced astrocytic membrane coverage of synapses. Popov et al. (2020) examined these mechanisms in detail in adult male C57BL/6 mice aged 12–18 months subjected to short-term caloric restriction (40 % reduction in daily intake for 1 month) without altering feeding frequency. It was observed that CR enhanced glutamate clearance primarily through increased astrocytic enwrapment of synapses rather than transporter upregulation, as EAAT2/GLT-1 expression remained unchanged and GS levels were even reduced. This finding differs from the increased GS activity previously observed in younger rats after longer CR protocols (Ribeiro et al. 2009; Santin et al. 2011), likely reflecting differences in species, age, and CR duration that modulate astrocytic metabolic adaptation. Based on these findings, it was suggested that enhanced astrocytic coverage may restrict glutamate spillover and thereby reduce activation of extrasynaptic NMDA receptors, contributing to more efficient synaptic glutamate control under CR conditions (Popov et al. 2020). Whether these divergent GS responses correspond to changes in enzyme activity, post-translational regulation, or regional metabolic demands remains to be determined.
Studies on maternal CR during the pregestation and gestation periods show that it significantly impacts the astrocyte function of the offspring’s hypothalamus, particularly lipid metabolism and endocannabinoid signaling. In this model, female Wistar rats were subjected to a 20 % reduction in daily caloric intake beginning 8 weeks before mating and throughout pregnancy, while control dams were fed ad libitum. Maternal CR increases astrocytic markers (GFAP, vimentin) without affecting the microglial marker Iba1, indicating a specific astrocytic response. It also upregulates genes involved in fatty acid oxidation (Cpt1, Acox1), lipogenesis (Fasn), and lipid regulation (Srebf1, Srebf2), suggesting metabolic reprogramming of hypothalamic astrocytes. In addition, maternal CR alters astrocytic endocannabinoid signaling by increasing the expression of Dagla, the enzyme responsible for the synthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG), as well as Magl and Faah, which mediate its degradation, particularly in female offspring (Tovar et al. 2021). These changes might represent a compensatory mechanism for the reduced hypothalamic 2-AG levels previously observed (Ramírez-López et al. 2017, 2016). Notably, the expression of cannabinoid receptors type 1 (CB1) and 2 (CB2) remains unchanged, suggesting that the effects of CR are more related to endocannabinoid metabolism rather than receptor-mediated signaling (Tovar et al. 2021). Together, these findings suggest a role of hypothalamic astrocytes in long-term metabolic reprogramming in the hypothalamus induced by maternal CR, with potential implications for energy balance and feeding behavior in the offspring.
4.3.1.2 Epigenetic regulation induced by CR in astrocytes
Only a few studies have investigated the epigenetic changes regulated by CR specifically in astrocytes. Wood et al. (2015) examined the epigenetic changes in the cerebral cortex of male Fischer 344 rats maintained under CR for a lifelong duration (3–28 months). They identified miR-98-3p as the only miRNA consistently upregulated in CR and lipoic acid (LA) dietary groups. Because reduced miR-98 expression has been linked to neurodegeneration, the authors suggested a neuroprotective role for this miRNA. Although the cell type was not identified, in the cortical astrocytic cell line, CTXTNA2, miR-98-3p inhibition increased HDAC and reduced HAT activity, disturbing acetylation balance, whereas its overexpression had no effect. Based on these results, it was suggested that miR-98-3p supports HAT/HDAC homeostasis in astrocytes; however, the effect of CR-induced on inhibition of HDAC activity was not directly measured. Therefore, further studies are needed in order to understand the role of miR-98-3p upregulation in different brain cell types under CR.
On the other hand, Spencer et al. (2025) performed a transcriptomic study in human astrocytes exposed to the CR mimetic, 2-deoxyglucose (2-DG) at concentrations from 10–50 mM during 24 h, in combination with IL-1b (20 ng/ml). RNA-seq analysis revealed that 2-DG attenuated the increased expression of the pro-inflammatory cytokines, IL1-6, TNFα, CXCL10, CXCL9, IFIT2 and NOS2, induced by IL-1β. According to ATAC-seq analysis, IL-1β favored a chromatin open state and accessibility to astrocytic gene promoters, and the enrichment for DNA binding motifs of AP-1 and NfkB transcription factors involved in inflammation (Spencer et al. 2025). However, although 2-DG attenuated the inflammatory response, it had a marginal effect on chromatin accessibility in astrocytes, suggesting it does not significantly alter the epigenetic mechanisms involved in the regulation of the inflammatory response induced by IL-1β.
Overall, these observations suggest that glycolysis inhibition by 2-DG reduces inflammatory gene expression, however, the mechanisms involved remain to be elucidated, and the confirmation of these findings at the protein level is still needed. Understanding the impact of these effects on neuronal function and viability will shed light about the relevance of modulating the inflammatory response as a mechanism involved in the beneficial effects of CR.
4.3.1.3 Effect of CR on Ca2+ dynamics and aging in astrocytes
CR delays brain aging and supports synaptic plasticity, and accumulating evidence suggests that astrocytes are key mediators of these effects. Studies in male C57BL/6 mice maintained on 40 % calorie reduction for at least 6 months have shown that CR preserves astrocytic function across aging (Lalo et al. 2018, 2019). In cortical astrocytes (from cortical layers 2/3) from young (2–4 months) and aged animals (14–18 months), CR increased the frequency of Ca2+ transients, an effect more pronounced in older mice, which was associated with enhanced release of ATP and D-serine via CB1 and α1-adrenergic receptor activation. These findings suggest that CR mitigates the age-related decline in astrocyte–neuron communication (Lalo et al. 2018).
CR also appears to counteract the age-related decline in astrocytic Ca2+ signaling and purinergic responsiveness, helping to preserve receptor sensitivity and regulate ATP release. Aging reduces the efficacy of astrocytic Ca2+ signaling, partly due to decreased expression or sensitivity of P2X and P2Y receptors (Lalo et al. 2019). By maintaining Ca2+-dependent and purinergic pathways, CR may help sustain the excitatory–inhibitory balance within cortical circuits and support neuronal plasticity during aging. Consistent with this, CR enhances astrocyte–neuron communication by increasing the amplitude of glial-induced currents and restoring tonic GABAergic inhibition via PAR-1 receptor activation (Lalo et al. 2019).
There is also evidence supporting a link between CR and astrocytic Ca2-dependent modulation of synaptic plasticity. In adult mice subjected to 3 months of moderate CR (30 % reduction in intake), Lalo et al. (2020) reported that enhanced long-term potentiation (LTP) may depend on the Ca2-dependent release of brain-derived neurotrophic factor (BDNF) from cortical astrocytes and the activation of neuronal MSK1 signaling. Complementary findings by Popov et al. (2020), using adult mice exposed to 1 month 30–40 % CR, showed that LTP enhancement could also result from increased hippocampal astrocytic glutamate uptake and reduced spillover, thereby limiting extrasynaptic NR2B-NMDAR activation. Although both studies were conducted in adult rather than aged animals, these findings possibly represent processes through which CR preserves or enhances astrocytic function and synaptic efficiency during aging, characterized by efficient Ca2+ signaling, balanced gliotransmission, and enhanced neurotrophic and metabolic support – thereby promoting synaptic plasticity and cognitive resilience in the aging brain.
A more detailed analysis of the effect of CR on hippocampal astrocyte Ca2+ dynamics in young mice exposed to 1 month of CR, revealed that it reduces the size, duration, and spatial spread of astrocytic Ca2+ events, likely due to a decrease in gap junction coupling, increased hemichannel activity, and changes in astrocyte morphology. Despite minimal changes in event frequency, CR enhances the amplitude and kinetics of Ca2+ transients, resulting in faster and more localized signals that may enable more precise modulation of synaptic activity and support improved neuroplasticity. Also, local Ca2+ microdomains are important for ATP synthesis, gene expression, and cell death induction in astrocytes (Denisov et al. 2021).
Additionally, CR-induced stimulation of astrocytic Ca2+ signaling has been reported to occur via inositol 1,4,5-triphosphate receptor type 2 (IP3R2), leading to ATP release and ATP neuronal signaling, which has been linked to CR antidepressant-like effects (Wang et al. 2021). This mechanism is supported by findings in IP3R2-KO mice, where exogenous ATP restored CR-induced behavioral benefits. These findings underscore the role of astrocytes in the neuroprotective and mood-stabilizing actions of CR, emphasizing astrocyte–neuron interactions as key elements in maintaining brain health and resilience to psychiatric disorders (Wang et al. 2021). Moreover, another possible mechanism underlying the antidepressant-like effects of CR could be enhanced astrocytic K+ clearance, as this process depends on K+ diffusion. This is supported by evidence showing that CR increases astrocytic K+ clearance, likely through an increased volume fraction (VF) of perisynaptic astrocytic leaflets (Popov et al. 2020).
On the other hand, the pharmacological mimetics of CR – including metformin and resveratol – were shown to produce similar enhancements in astrocytic Ca2+ signaling and synaptic modulation (Lalo and Pankratov 2021). Metformin improved mitochondrial membrane potential and adrenergic Ca2+ signaling, counteracting age-related astroglial decline, while resveratrol enhanced astrocytic Ca2+ signaling and synaptic plasticity through mechanisms requiring astroglial exocytosis and neuronal autophagy. These findings showed that CR mimetics promote astroglial remodeling and Ca2+ signaling, supporting synaptic plasticity and neuroprotection through autophagy-related pathways. These compounds promoted astrocyte function and neuron–astrocyte communication. Similarly, 2-DG, another CR mimetic, has been shown to attenuate astrocyte-mediated inflammatory response. Interleukin-1β administration increases IL-6 and other pro-inflammatory genes (IL-1β, TNFα, C3, LCN) in astrocytes, whereas 2-DG pretreatment suppresses their expression, suggesting that CR-like metabolic shifts can modulate astrocyte-driven inflammation (Vallee and Fields 2022). Collectively, both CR and CR mimetics facilitate astrocyte-driven modulation of synaptic transmission.
The effect of CR on senescence has also been addressed, specifically in senescent astrocytes from SAMP8 mice, a model of accelerated brain aging (García-Matas et al. 2015). Results showed that CR exerts cytoprotective effects by reversing age-related gene expression and restoring pathways involved in protein folding, ROS response, and ATP synthesis. While CR does not recover immune or mitotic functions, it reduces β-galactosidase levels and enhances the expression of mitochondrial, ribosomal, and antioxidant genes. It also improves mitochondrial function – elevating aconitase 2 levels, reducing the respiratory rate, and protecting against oxidative damage – though some dysfunctions, like reduced membrane potential, persist. Overall, CR partially restores a non-senescent profile (García-Matas et al. 2015).
Despite these advances, the molecular mechanisms linking CR to astrocytic Ca2+ regulation remain unclear. It is not yet known whether CR alters the expression or sensitivity of Ca2+-related receptors, or affects metabolites such as KB that could modulate astrocyte signaling. Elucidating these pathways will be essential for understanding how CR preserves glial function and synaptic homeostasis during aging.
4.3.1.4 Morphological changes induced by CR in astrocytes
CR may promote astroglial plasticity, modifying astrocyte structure and function to support synaptic activity and neuroprotection. The effects of CR on astrocyte morphology appear to be age-dependent. In aged mice (19–24 months) maintained on a long-term 60 % CR regimen that began at 14 weeks of age and was maintained until either 19 or 24 months, astrocyte size is reduced (Castiglioni et al. 1991). In contrast, in young mice (2 months old), short-term CR (1 month) increases the volume fraction (VF) of fine astrocytic processes, particularly perisynaptic leaflets, without affecting larger branches (Popov et al. 2020). This expansion might enhance astrocytic coverage of the synaptic microenvironment and improve metabolic and ionic buffering at active synapses.
Beyond these changes at the cellular level, CR also reorganizes astrocytic connectivity at the network level. It induces coordinated morphological and functional remodeling in astrocytes, enhancing network efficiency and adaptability. Popov et al. (2020) showed that CR (30 % reduction for 1 month) reduces the number of coupled astrocytes without altering gap-junction permeability, indicating preserved connexin function despite network reorganization. Specifically, C×43 expression and cluster density decrease, whereas C×30 levels remain stable with larger C×30 clusters in the soma and proximal branches, which possibly act as a reserve pool. These changes might be involved in the reorganization pattern of astrocytic interconnections and the morphological architecture of the glial network.
Further analyses are required to establish the causal relationship between astrocytic remodeling and the enhanced synaptic plasticity reported by Popov et al. (2020) and provide a mechanistic basis for these findings.
4.4 Effect of fasting on astrocytes
Intermittent fasting (IF) refers to eating patterns where the total daily caloric intake meets energy needs but is confined to specific time windows, alternating with extended periods of little or no food consumption (Johnstone 2015). Various IF protocols have been investigated and reported to increase KB production from fat, including alternate-day fasting (ADF) and time-restricted feeding (TRF), which trigger hormonal adaptations to maintain blood glucose levels and low insulin (Vasim et al. 2022). While short-term fasting increases KB blood levels from ∼0.3–1 mM, prolonged fasting can raise KB up to ∼7 mM (Camberos-Luna and Massieu 2020). The metabolic shifts induced by these interventions may shed light on our understanding of how fasting influences astrocyte function. The effects of fasting on astrocytes have been poorly explored; however, emerging studies highlight several important findings.
It has been reported that a 3 month exposure to an ADF markedly attenuates kainic acid–induced astrogliosis in rats, as evidenced by reduced GFAP levels relative to ad libitum–fed controls (Sharma and Kaur 2008). Complementary findings show that IF initiated at 24-months in rats, attenuates age-related astrocytic reactivity, as evidenced by reduced GFAP expression and diminished astrocytic hypertrophy as compared to age-matched ad libitum-fed controls. In addition, dietary restriction (DR) partially restored the age-related decline in neuronal plasticity markers, including neural cell adhesion molecule (NCAM) and its polysialylated form (PSA-NCAM). These observations suggest that DR may counteract age-associated reactive astrogliosis and help to preserve a neural environment supportive of synaptic plasticity and repair (Kaur et al. 2008).
Concerning metabolism, fasting markedly alters astrocyte energy utilization in the rat brain. It has been reported that after 36 h of fasting, BHB rapidly enters the brain and incorporates into key amino acids like glutamine, mainly synthesized by astrocytes (Jiang et al. 2011). Although neurons account for ∼70 % of BHB consumption, astrocytes contribute to about 30 %, indicating a meaningful shift in their energy utilization (Jiang et al. 2011).
On the other hand, it has been reported that overnight fasting (approximately 16 h) in mice induces morphological modifications in astrocytes within the mediobasal hypothalamus (MBH), characterized by shorter and less complex higher-order processes relative to the elongated morphology observed in the fed state (Y. Zhang et al. 2017). This remodeling was not observed in other brain regions, highlighting its importance in the hypothalamic regulation of energy balance. Although the molecular mechanism underlying fasting-induced retraction of astrocytic processes was not assessed, this structural remodeling possibly reflects a dynamic and reversible adaptation that enables the hypothalamus to respond to energy deprivation. It was suggested that such remodeling possibly involves intracellular calcium signaling or autophagy activation, both of which can influence cytoskeletal organization and cellular energy balance (Y. Zhang et al. 2017).
The molecular mechanism underlying these morphological remodeling remains unresolved. Future studies combining molecular, electrophysiological, and behavioral approaches might help to elucidate how fasting reshapes astrocyte–neuron interactions and whether similar adaptations occur under chronic or long-term fasting conditions relevant to human physiology.
4.5 Effect of exercise on astrocytes
Physical activity increases KB concentrations in blood, particularly during moderate to high-intensity exercise, regardless of whether it occurs as a single bout or as a part of long-term training regimens (Askew et al. 1975; Holcomb et al. 2021; Ohmori et al. 1990) (Table 1). However, when exercise is performed repeatedly, the magnitude of this increase appears to diminish compared to untrained individuals, who exhibit a higher rise in KB levels after exercise (Ohmori et al. 1990), suggesting that repeated exercise induces an adaptive response in KB metabolism. This adaptation possibly involves an enhanced ketolytic capacity driven by the upregulation of ketolytic enzymes, accompanied by a reduced hepatic ketogenesis (Evans et al. 2017).
Exercise protocols that increase KB levels in murine models.
| Reference | Speed description | Parameter | Ketone body blood levels (mM) | |||
|---|---|---|---|---|---|---|
| Intensity | Duration | |||||
| Per session | Frequency | Total program | ||||
| Holcomb et al. (2021) | Graded treadmill test increasing speed: 10 m/min (15 min) + 12.6 m/min (15 min) + 2.4 m/min speed increase every 15 min, until exhaustion; constant 10 % grade. | Moderate to high | Variable (typically 45–90+ min) with individual performance capacity | Single acute test | Single acute test | >1 mM |
| Askew et al. (1975) | 29.5 m/min for 120 min/day, with 60s sprints at 56.5 m/min every 10 min, constant 8 % grade | Moderate to high | Long (120 min/day) | 5 days/week | Chronic training (12 week) | >1.2 mM |
| Ohmori et al. (1990) | 15 m/min, 90 min/session; tests done at weeks 14 and 28 after 2-day rest. No grade, steady pace. | Moderate | Moderate to long (90 min) | 3 days/week | Chronic (14 and 28 weeks) | >0.5 < 1.0 mM |
The studies referred in the following sections are limited to those employing moderate- to high-intensity exercise protocols, as they have consistently shown to elevate KB levels in blood (Table 1).
4.5.1 Effect of exercise on astrocytes under physiological conditions
Due to the complexity of astrocytic responses to exercise, most studies have focused on specific functional and molecular markers of astrocytic activity. To discuss this evidence, the following section is organized according to different aspects of astrocyte function, including morphology as a key indicator of astrocytic status.
4.5.1.1 Astrocyte identity markers
The effect of exercise on GFAP gene and protein expression remains debatable. While some studies report increased GFAP levels following exercise, others report a decrease, with no clear correlation between exercise intensity and duration and the direction of changes in GFAP content (Table 2). High-intensity exercise in mice (1h/day, 5 days/week, for 1, 2 or 4 weeks on motorized treadmills) has been associated with GFAP upregulation in the prefrontal cortex (PFC) and the striatum (STR) (Lundquist et al. 2019).
Changes of GFAP and S100B in astrocytes following exercise.
| Marker | Study | Animal model | Exercise | Brain region | Measurement type | Change | |||
|---|---|---|---|---|---|---|---|---|---|
| Age | Type of rodent | Type | Duration | Protein | mRNA | ||||
| GFAP | Bernardi et al. (2013) | 90 Days old | Rats | Treadmill exercise (moderate) | 4 weeks | Hippocampus | ✓ | ↓ | |
| Pereira et al. (2015) | 8-wks-old | Mice | Treadmill exercise (high) | 8 weeks | Ventral part of spinal cord | ✓ | ↓ | ||
| Dorsal part of spinal cord | ✓ | ↑ | |||||||
| Otsuka et al. (2016) | – | Rats | Treadmill exercise (moderate to high) | 3 weeks | ✓ | ↔ | |||
| Leite et al. (2016) | 27 month-old | Rats | Swimming (moderate) | 4 weeks | Hypothalamus | ✓ | ↓ | ||
| Sun et al. (2017) | Rats | Treadmill running (high) | 7 consecutive days | Hippocampus | ✓ | ↑ | |||
| Lloyd et al. (2017) | Young (between p42-p49) | Rats | Treadmill running (low to moderate) | 6 weeks | Hippocampus dentate gyrus | ✓ | ↓ | ||
| Lundquist et al. (2019) | 8–10 week old | Mice | Treadmill running (moderate to high) | 4 weeks | Prefrontal cortex | ✓ | ✓ | ↑ | |
| Striatum | ✓ | ✓ | ↑ | ||||||
| Entorhinal cortex | ✓ | ✓ | ↑, ⌀ | ||||||
| S100B | Bernardi et al. (2013) | 90 days old | Rats | Treadmill exercise (moderate) | 4 weeks | Hippocampus | ✓ | ⌀ | |
| Borsoi et al. (2015) | Adult | Rats | Forced swimming (moderate to high) | 3 consecutive days | Frontal cortex | ✓ | ⌀ | ||
| Hippocampus | ✓ | ⌀ | |||||||
-
↑: Significant increase, ↓: Significant decrease, ⌀: No significant change.
In the spinal cord, using different overtraining protocols (OT) in mice (5 days of training followed by 2 days recovery, of downhill running/uphill running or running with no inclination), Pereira et al. (2015) reported an increase in GFAP and Iba-1 immunoreactivity in the dorsal horn of the spinal cord in OT animals running downhill. This change was not associated with apoptosis, but more likely with skeletal muscle inflammation (Table 2).
GFAP upregulation is not always beneficial. Sun et al. (2017) reported that rats subjected to high-intensity treadmill running for 7 consecutive days, exhibit GFAP overexpression in the hippocampus, accompanied by elevated proinflammatory cytokine levels and glial activation (Table 2). These changes might impair synaptic plasticity and learning, suggesting that under stress or excessive training conditions, GFAP upregulation might reflect impaired neuroinflammatory responses rather than a beneficial effect on plasticity. In contrast, Bernardi et al. (2013) reported a decrease in GFAP expression in the hippocampus of rats subjected to moderate intensity treadmill exercise during 4 weeks. This reduction was possibly mediated by elevated corticosterone levels, which modulate GFAP transcription via glucocorticoid receptors in astrocytes. Under non-pathological conditions, such downregulation might reflect enhanced neuronal plasticity rather than damage and appears to be transient.
GFAP modulation by exercise has also been investigated in the context of aging, a condition typically associated with increased GFAP due to neuroinflammation. Swimming exercise has been shown to prevent the age-related rise in GFAP within the hypothalamus, supporting that exercise exerts a protective, anti-inflammatory effect by regulating glial activation (M. R. Leite et al. 2016) (Table 2).
Regarding S100B, studies consistently report no significant changes in its abundance or release following exercise (Table 2.). For instance, hippocampal slices and cerebrospinal fluid from treadmill-trained rats showed stable S100B levels, underscoring the robustness of this marker under the examined exercise conditions (Table 2 and references therein).
4.5.1.2 Metabolic and neurotransmitter regulation
To assess exercise-induced metabolic changes in astrocytes, studies commonly measure GS activity and glutathione (GSH) content. GS supports neurotransmitter recycling and ammonia detoxification, serving as a key indicator of astrocytic metabolic function (Albrecht et al. 2010). GSH, a major antioxidant, is an index of the oxidative stress status and metabolic resilience (Averill-Bates 2023). Additionally, glutamate uptake is frequently evaluated, as it provides information not only about the astrocytic metabolic state but also about their role in regulating neurotransmitter levels (Andersen 2025).
In line with this, Bernardi et al. (2013) reported that physical exercise transiently increased brain GS activity, evident after 4 weeks of training but not sustained at 12 weeks. This upregulation might enhance glutamate turnover and ammonia detoxification, key astrocytic functions that support synaptic and metabolic homeostasis. The increase in GS activity was accompanied by reduced nitric oxide (NO) levels, suggesting a potential decrease in inflammatory signaling. Because excessive NO production, often driven by inducible nitric oxide synthase (iNOS), contributes to neuroinflammation and neurodegeneration, the exercise-induced rise in GS, together with lower NO, might represent a shift toward a neuroprotective, metabolically balanced astrocyte phenotype. Despite clear astroglial remodeling, moderate treadmill training did not alter hippocampal GSH content or glutamate uptake (Bernardi et al. 2013). In contrast, repeated forced swimming produced a transient increase in hippocampal GSH levels 48 h after the last session, which returned to baseline values by 72 h, accompanied by a transient reduction in astrocytic glutamate uptake at 48 h in hippocampal slices (Borsoi et al. 2015). These findings suggest that sustained aerobic-type exercise preserves redox homeostasis in astrocytes whereas forced swimming elicits a short-term lived, compensatory antioxidant response linked to disrupted glutamatergic uptake. Thus, GSH modulation appears to be both paradigm- and time-dependent, being stable after moderate, predictable treadmill exercise, and reactively elevated after aversive, unpredictable forced swimming, where redox pressure and impaired glutamate uptake likely trigger a transient activation of GSH antioxidant defense. Moreover, the reduction in glutamate uptake is possibly related to the redox-sensitive regulation of glutamate transporters (Trotti et al. 1997).
In the above-mentioned studies, it was not determined whether the changes in GSH originated from astrocytes rather than a mixed cell population; therefore, the contribution of neurons cannot be discarded. In addition, it is unclear whether the elevation in GSH reflects an adaptive protective mechanism or a transient state of oxidative stress. Therefore, monitoring the changes in the GSH/GSSG ratio over different times would help to elucidate this subject. Additionally, assessing EAAT1/GLAST and other glutamate transporters’ function could reveal whether restored glutamate uptake parallels GSH normalization, linking redox and glutamate homeostasis. Also, measuring the activity of glutamate-cysteine ligases would clarify whether GSH elevation results from active synthesis.
4.5.1.3 Neurotrophic and growth factors
Neurotrophic factors play essential roles in neuronal survival, proliferation, maturation, and outgrowth during brain development, and continue to provide neuroprotection in the mature brain following injury. Among them, BDNF, a member of the neurotrophin family, is well known not only for promoting neuronal survival and development but also for its crucial role in modulating synaptic plasticity (Bathina and Das 2015). Several studies have reported that the production and secretion of neurotrophic factors – particularly BDNF – are key molecules mediating the beneficial effects of physical exercise on the central nervous system (Marlatt et al. 2012; Nascimento et al. 2014; Vaynman et al. 2004). Notably, running exercise has been shown to increase BDNF mRNA expression in the hippocampus and other brain regions in intact adult rats (Neeper et al. 1996).
BDNF is synthesized de novo in neurons and can be released as mature BDNF and pro-BDNF. While mature BDNF primarily promotes synaptic strengthening, pro-BDNF is taken up and re-released by astrocytes, suggesting a role of astrocytes in modulating BDNF availability and function (Vignoli et al. 2016).
Although several studies have evaluated BDNF regulation following different exercise paradigms, it is important to note that none of them has directly assessed astrocyte-specific BDNF expression. Reported BDNF levels derive from whole-tissue homogenates or hippocampal sections, making it impossible to attribute the observed changes to astrocytes. Consequently, the discussion of astrocytic BDNF in this context remains only correlative as it is based on indirect morphological or metabolic indicators of astroglial activity.
Differences in BDNF outcomes across studies seem to reflect variations in species, exercise methods, and research techniques. Bernardi et al. (2013) showed no change in hippocampal BDNF 24 h after moderate treadmill training in rats, likely indicating a stable neurotrophic state under predictable, non-stressful conditions. Similarly, Otsuka et al. (2016) found no changes in BDNF on coronal brain sections of ischemic rats subjected to preconditioning exercise. Conversely, Borsoi et al. (2015) observed increased hippocampal – but not cortical – BDNF after repeated forced swimming, suggesting a stress-related response to glutamatergic and redox imbalance rather than a training adaptation.
Fahimi et al. (2017) also reported an elevation of hippocampal BDNF after 5 weeks of voluntary moderate exercise in mice, accompanied by astrocytic hypertrophy and enhanced TrkB expression. These findings suggest that moderate, self-paced exercise promotes astrocyte-associated BDNF signaling, particularly within the dentate gyrus (DG), a key site of neurogenesis and dendritic remodeling.
Taken together, these studies suggest that the regulation of BDNF by exercise is paradigm-, region-, and species-dependent, shaped by stress exposure and the degree of astrocytic involvement. However, since existing evidence is based on bulk-tissue analyses, the specific contribution of astrocyte-derived BDNF to exercise-induced neuroplasticity remains unresolved. Future work should employ cell-type–resolved methodologies to determine whether astrocytes act as active sources or modulators of neurotrophic signaling during physical activity.
In addition to neurotrophin signaling, recent findings have shown that exercise also modulates Flk-1 (also known as VEGFR-2, or vascular endothelial growth factor receptor 2) expression in astrocytes, particularly in the hippocampus, linking astrocytes to neurovascular remodeling (Stevenson et al. 2018). Voluntary exercise appears to enhance this response more effectively than high-intensity forced exercise, which may even suppress Flk-1 expression in brain regions like the cerebellum. As key contributors to angiogenesis and the neurovascular unit, astrocytes may regulate vascular adaptation through VEGF–Flk-1 signaling during physical activity. Together, these findings highlight the multifaceted role of astrocytes in supporting exercise-induced neuroplasticity and vascular function.
4.5.1.4 Morphological modifications
Exercise induces region- and time-specific morphological changes in astrocytes without altering their number. In mice subjected to treadmill running, astrocytes in the entorhinal cortex showed early and progressive structural remodeling, with increased process elongation and branching, and a corresponding rise in morphological complexity. The striatum exhibited delayed, localized branching changes without a global increase in complexity, while the prefrontal cortex showed only transient changes, ultimately leading to reduced complexity after 4 weeks of exercise. These findings suggest that exercise prompts functional and structural plasticity in astrocytes, depending on the brain region and exercise duration (Lundquist et al. 2019).
Similarly, in a study using double transgenic Olig2CreER/WT; ROSA26-GFAP43-EGFP mice – which harbor a population of Olig2-lineage astrocytes with a bushy morphology, predominantly in the globus pallidus (GP) – 3 weeks of voluntary wheel running induced marked structural remodeling in these cells, as shown by light and electron microscopy analyses. Olig2-lineage astrocytes displayed increased process arborization, more complex and bushier branching, and enhanced synaptic contacts, while maintaining stable cell size. These changes were reversible, returning to baseline values after a sedentary period, while perivascular endfeets remained structurally intact (Tatsumi et al. 2016).
In agreement with these findings, Fahimi et al. (2017) also reported that exercise increases the complexity of astrocytes in the hippocampal DG, by increasing the total length and surface area of astrocytic projections and their orientation, with most processes aligning toward the dentate granule cell layer. The authors proposed that these astrocytic adaptations are likely driven by elevated BDNF-TrkB signaling, which influences morphology through calcium-dependent pathways (Fahimi et al. 2017). The increase in astrocytic membrane extension likely reflects structural reorganization that changes the interactions with neurons and the surrounding vasculature.
Thus, morphological remodeling may serve as a structural basis for exercise-induced improvements in astrocyte-mediated support and regulation of neural circuits. Although Tatsumi et al. (2016); Fahimi et al. (2017), and Lundquist et al. (2019) all reported exercise-induced increases in astrocytic morphological complexity, the biological interpretation of these changes diverges substantially among studies, suggesting that similar structural outcomes may reflect distinct astrocyte responses depending on the exercise paradigm. Moderate or voluntary paradigms (Fahimi et al. 2017; Tatsumi et al. 2016) support an adaptive and reversible form of plasticity, enhancing neuroglial coupling and metabolic support. In contrast, Lundquist et al. (2019) used a forced treadmill paradigm that elicited widespread astrocytic remodeling and mild reactive gene upregulation, suggesting a regulated or “physiological” reactivity. In contrast to these adaptive effects, high-intensity forced exercise can elicit inflammatory and stress-related responses. Sun et al. (2017) demonstrated that intense treadmill training induced astrocytic hypertrophy, process swelling, and inflammatory features in the hippocampus, correlating with impaired hippocampal-dependent cognition and synaptic plasticity.
Taken together, these findings suggest that exercise-induced astrocytic remodeling is not uniformly beneficial but instead spans a spectrum from adaptive plasticity under moderate or voluntary conditions to maladaptive reactivity under excessive or stressful exercise loads. Future studies are needed to outline this threshold and clarify under which physiological or behavioral conditions astrocytic structural changes enhance, or conversely compromise, cognitive and neural function.
4.5.2 The effect of the exercise on astrocytes under pathological conditions
Studying the effects of exercise on astrocytes under pathological conditions is of particular interest, considering that physical exercise is increasingly recognized as a potent non-pharmacological (and potentially ketotherapeutic) intervention. Exercise protocols can be applied either before (pre-conditioning) or after (post-conditioning) the onset of pathological stress, and both approaches have shown promising neuroprotective effects.
In preconditioning models, exercise before ischemic stroke (MCAO) in rats has been shown to enhance neuroprotection and recovery, with notable involvement of astrocytes. Otsuka et al. (2016) showed that rats subjected to 4 weeks of treadmill exercise before MCAO exhibited increased expression of the neurotrophic factors midkine (MK), a growth factor with neurotrophic activity that has been reported to prevent neuronal death (Yoshida et al. 2001, 2014), and BDNF at the lesion site, along with elevated GFAP expression, indicating astrocyte activation. It was suggested that these activated astrocytes might contribute to MK production and angiogenesis, as indicated by increased PECAM-1 (Platelet endothelial cell adhesion molecule) levels, ultimately supporting blood-brain barrier integrity and tissue recovery.
Similarly, Lin et al. (2015) found that rats subjected to moderate treadmill running for 4 weeks before MCAO exhibited elevated heat shock protein 20 (HSP20) expression in both neurons and astrocytes. Given that HSP20 acts as a molecular chaperone with anti-apoptotic and cytoprotective functions (Edwards et al. 2011; Fan et al. 2005), its upregulation likely contributes to the reductions in infarct volume and glial and neuronal apoptosis, as well as the improvements in motor performance and neurological outcomes. In another preconditioning study, Otsuka et al. (2019) reported that treadmill running for 4 weeks before MCAO significantly increased the expression of factors with anti-apoptotic properties, involved in the regulation of cell survival, such as HIF-1α, 14-3-3γ, and p-β-catenin (Ser37) (W. D. Cao et al. 2014; Lai et al. 2014; Zhao et al. 2011). These changes were accompanied by a reduction of the infarct area and the pro-apoptotic markers Bax and caspase-3, compared to sedentary ischemic rats. HIF-1α was coexpressed with GFAP-positive and P2X7-positive astrocytes, suggesting that exercise might enable astrocytes to adopt a hypoxia-adaptive and purinergic-responsive phenotype capable of modulating inflammation and maintain metabolic stability during ischemia. Whereas, the elevation of 14-3-3γ and p-β-catenin (Ser37) might be involved in the activation of an anti-apoptotic signaling cascade to stabilize survival-related proteins, and potentially promote astrocyte proliferation. Taken together, these results likely represent a form of exercise-induced ischemic tolerance, in which astrocytes may act as key intermediaries linking metabolic adaptation, anti-apoptotic signaling, and tissue protection following stroke.
In contrast to previous reports supporting the beneficial effects of exercise preconditioning, Lovatel et al. (2014) did not find protection in rats exercised for 2 weeks (20 min/day) before global cerebral ischemia. These discrepancies likely reflect differences in ischemic model (global vs. focal), exercise duration (2 vs. 4 weeks), and brain region analyzed (DG vs. peri-infarct cortex), all of which can markedly influence glial reactivity and neuroprotective outcomes. However, when rats were subjected to exercise after ischemia, Lovatel et al. reported a partial reversal of microglial hypertrophy and an increased astrocytosis in the DG, suggesting reactive remodeling consistent with tissue repair.
Similarly, in an MCAO rat model, Q. Zhang et al. (2017) reported that two weeks of post-stroke treadmill exercise reduced the number of ramified GFAP-positive astrocytes and decreased proinflammatory cytokine expression, while improving spatial learning and memory performance in the Morris water maze, and reducing anxiety-like behavior in the elevated plus maze. These findings suggest that attenuation of astrocyte overactivation after injury may facilitate neurofunctional recovery.
On the other hand, studies in the cerebral hypoperfusion model, induced by the 2-vessel occlusion (2VO), have expanded the understanding of exercise-related effects on astrocytes. Leardini-Tristaõ et al. (2020) reported that rats subjected to treadmill exercise starting three days after 2VO and followed during 12 weeks, showed increased astrocytic vascular coverage, preserved blood–brain barrier integrity, reduced inflammation, and improved recognition memory in the novel object recognition test. Extending these findings, Cao et al. (2022) showed that post-2VO wheel running for 20 min, 2 times a day during 6 consecutive days/week for 4 weeks, reduced neurotoxic A1 astrocytes (C3/GFAP+), enhanced neuroprotective A2 astrocytes (S100A10/GFAP-positive), promoted astrocytic primary cilia formation, and modulated MAPK signaling (ERK, JNK, p38), resulting in reduced cellular stress, inflammation and improvements in spatial learning and memory, as assessed by the Morris water maze test, as well as enhanced non-spatial memory involving prefrontal and subcortical circuits.
Beyond ischemic models, post-treatment exercise has been shown to exert robust astrocyte-related effects in other neuropathological conditions, including chronic stress and cerebral hypoperfusion, as well as during normal aging. In chronic unpredictable stress (CUS) models of depression, Li et al. (2021) found that rats subjected to 6 weeks of treadmill running after 5 weeks of CUS, reversed the stress-induced reduction in hippocampal astrocyte number and stimulated astrocyte proliferation, restoring hippocampal volume and alleviating depressive behaviors, such as sucrose preference and reduced immobility in the forced swimming test. Similarly, D. Huang et al. (2022) reported that rats subjected to 20 min daily running, 5 days a week for 6 weeks after CUS exposure, restored astrocyte number and function in the medial prefrontal cortex, normalized the expression of GFAP, S100β and glutamate transporters EAAT2/GLT-1 and EAAT1/GLAST, and reversed CUS-induced anhedonia and behavioral despair. Thus, these studies showed that post-stress exercise reverses astrocytic loss and functional deficits associated with depressive phenotypes.
Finally, in a natural aging model, Latimer et al. (2011) reported that 6 weeks of voluntary wheel running in middle-aged female mice reduced hippocampal GFAP immunoreactivity, which in this context reflects decreased astrocyte reactivity and hypertrophy associated with brain aging. To better understand potential age- and sex-related differences in astrocyte responses to exercise, future studies including both sexes and multiple age groups are needed. In addition, integrative approaches linking astrocytic changes with functional and behavioral outcomes will increase our understanding of whether decreased GFAP expression reflects an adaptive normalization of glial homeostasis, or is more associated with a loss of astrocytic support during aging.
Overall, these findings highlight the versatile and critical role of astrocytes in mediating the neuroprotective, anti-inflammatory, and cognitive benefits of exercise among diverse models of brain injury, stress, and aging. They place astrocyte modulation as a promising therapeutic target and reinforce the potential of exercise, as a non-pharmacological strategy and possibly mediated in part, by increased KB or exercise-induced ketosis, to enhance brain resilience and recovery.
5 Conclusions and future directions
The data compiled in this review highlight the versatile and multifaceted role of astrocytes in response to various ketogenic interventions, including BHB exposure, MCFA, KD, CR, IF, and exercise (Figure 2). Astrocytes are not only capable of producing KB but also exhibit dynamic and adaptive responses to exogenous KB exposure, reflecting their metabolic flexibility and essential contribution to maintain cerebral energy homeostasis.
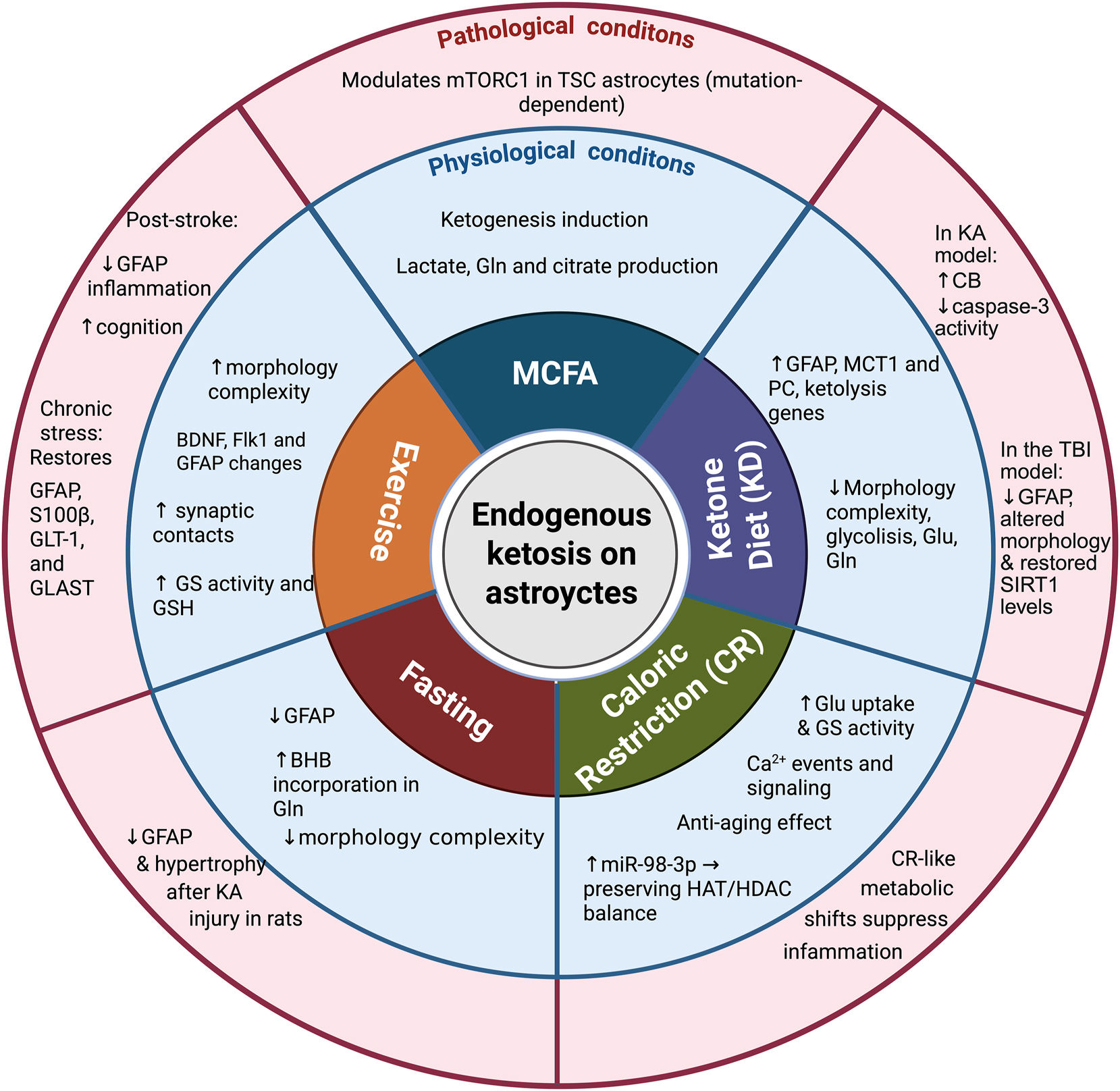
Effects of endogenous ketosis on astrocytes under physiological and pathological conditions. Schematic representation illustrating the effects of various endogenous ketotherapeutic strategies including medium-chain fatty acids (MCFAs), the ketogenic diet (KD), caloric restriction (CR), fasting, and exercise on astrocytes under both physiological (blue circle) and pathological conditions (red circle).
Ketone therapeutic strategies influence astrocytic functions at multiple levels – modulating mitochondrial metabolism, neurotransmitter recycling, calcium signaling, gene and protein expression, and structural plasticity. These changes suggest and support the idea that KB in astrocytes serve not only as an alternative energy substrates but also as a signaling molecules that drive broader cellular responses, including anti-inflammatory and neuroprotective effects. These effects may contribute to enhance cognitive resilience, particularly in the context of pathological stress and aging. Notably, astrocytes emerge not as passive responders to ketogenic signals, but as active participants in modulating energy balance, synaptic function, and neuroglial communication. Their ability to integrate and adapt to metabolic challenges underscore their pivotal role in the therapeutic effects of ketogenic strategies to promote brain health and resilience.
Despite accumulated evidence, there are still critical gaps in understanding how ketogenic interventions modulate astrocyte physiology. Due to the time- and context-dependent nature of KB effects on astrocytes, it is essential to determine how variables like the stage of treatment, duration, frequency and concentration of KB achieved, affect KB outcomes, particularly when considered as a therapy for aging and chronic disease, where outcomes might be beneficial or detrimental. On the other hand, single-cell omics and advanced imaging would help to clarify how astrocytic KB metabolism interacts with neuronal and microglial responses, including the compartmentalization of KB synthesis and use. Given astrocyte heterogeneity throughout brain regions and disease states, single-cell and spatial transcriptomics will be key for identifying subtype-specific responses to ketogenic interventions. It is essential to elucidate the intracellular signaling pathways through which ketogenic interventions, particularly BHB, drive long-term astrocytic reprogramming. For instance, while BHB influences calcium signaling, CAMKII activation, and epigenetic regulation, the precise roles of these mechanisms remain unclear. More research is needed on the long-term astrocytic changes induced by KD, CR, or exercise, whether they are sustainable and reversible, and whether they reflect protective adaptations or potentially harmful responses to chronic exposure. Understanding how KB-induced astrocytic changes translate into functional outcomes at the neural circuit level represents a promising area of research, focusing on their impact on excitatory/inhibitory balance, synaptic plasticity, and neurovascular coupling.
Finally, there is a critical need to translate mechanistic insights into clinical settings by developing astrocyte-targeted metabolic therapies for neurological conditions such as epilepsy, neurodegeneration, neuroinflammation, and age-related cognitive decline. The elucidation of the shared and distinct molecular mechanisms among these different ketogenic strategies may reveal synergistic protocols that optimize neuroprotection and cognitive resilience.
Funding source: Secreteria de Ciencia, Humanidades, Tecnologia e Innovacion (SECIHTI)
Award Identifier / Grant number: CBF-2025-I-1041
Funding source: Universidad Nacional Autonoma de Mexico
Award Identifier / Grant number: PAPIIT-UNAM IN202922 and IN215825
Acknowledgments
The authors thank the Computing Unit of IFC for computer facilities. Figures were created with BioRender.com.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Conseptualization. PC-M and LM; formal analysis PC-M; funding acquisition, LM; writing, original draft PC-M; writing and editing, LM; supervision, LM.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by Universidad Nacional Autónoma de México (PAPIIT-UNAM grants IN202922 and IN215825 to LM).
-
Data availability: Not applicable.
References
Achanta, L.B., Rowlands, B.D., Thomas, D.S., Housley, G.D., and Rae, C.D. (2017). β-hydroxybutyrate boosts mitochondrial and neuronal metabolism but is not preferred over glucose under activated conditions. Neurochem. Res. 42: 1710–1723, https://doi.org/10.1007/s11064-017-2228-6.Search in Google Scholar PubMed
Adamsky, A., Kol, A., Kreisel, T., Doron, A., Ozeri-Engelhard, N., Melcer, T., Refaeli, R., Horn, H., Regev, L., Groysman, M., et al.. (2018). Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174: 59–71, https://doi.org/10.1016/j.cell.2018.05.002.Search in Google Scholar PubMed
Albrecht, J., Sidoryk-Wȩgrzynowicz, M., Zielińska, M., and Aschner, M. (2010). Roles of glutamine in neurotransmission. Neuron Glia Biol. 6: 263–276, https://doi.org/10.1017/s1740925x11000093.Search in Google Scholar
Almeida-Suhett, C., Namboodiri, A.M., Clarke, K., and Deuster, P.A. (2022). The ketone ester, 3-hydroxybutyl-3-hydroxybutyrate, attenuates neurobehavioral deficits and improves neuropathology following controlled cortical impact in male rats. Nutr. Neurosci. 25: 1287–1299, https://doi.org/10.1080/1028415x.2020.1853414.Search in Google Scholar PubMed
Andersen, J.V. (2025). The glutamate/gaba-glutamine cycle: insights, updates, and advances. J. Neurochem. 169: 1–24, https://doi.org/10.1111/jnc.70029.Search in Google Scholar PubMed PubMed Central
Andersen, J.V., Westi, E.W., Jakobsen, E., Urruticoechea, N., Borges, K., and Aldana, B.I. (2021). Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply. Mol. Brain. 14, https://doi.org/10.1186/s13041-021-00842-2.Search in Google Scholar PubMed PubMed Central
Andersen, J.V., Westi, E.W., Neal, E.S., Aldana, B.I., and Borges, K. (2022). β-Hydroxybutyrate and medium-chain fatty acids are metabolized by different cell types in mouse cerebral cortex slices. Neurochem. Res. 48: 54–61, https://doi.org/10.1007/s11064-022-03726-6.Search in Google Scholar PubMed
Anlauf, E. and Derouiche, A. (2013). Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front. Endocrinol. 4: 1–5, https://doi.org/10.3389/fendo.2013.00144.Search in Google Scholar PubMed PubMed Central
Askew, E.W., Dohm, G.L., and Huston, R.L. (1975). Fatty acid and ketone body metabolism in the rat: response to diet and exercise. J. Nutr. 105: 1422–1432, https://doi.org/10.1093/jn/105.11.1422.Search in Google Scholar PubMed
Attwell, D. and Laughlin, S.B. (2001). An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21: 1133–1145, https://doi.org/10.1097/00004647-200110000-00001.Search in Google Scholar PubMed
Auestad, N., Korsak, R.A., Morrow, J.W., and Edmond, J. (1991). Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 56: 1376–1386, https://doi.org/10.1111/j.1471-4159.1991.tb11435.x.Search in Google Scholar PubMed
Averill-Bates, D.A. (2023). The antioxidant glutathione. In: Litwack, G. (Ed.). Vitamins and hormones. Academic Press, Toluca Lake, North Hollywood, California, pp. 109–141.Search in Google Scholar
Bathina, S. and Das, U.N. (2015). Brain-derived neurotrophic factor and its clinical Implications. Arch. Med. Sci. 11: 1164–1178, https://doi.org/10.5114/aoms.2015.56342.Search in Google Scholar PubMed PubMed Central
Bazzigaluppi, P., Lake, E.M., Beckett, T.L., Koletar, M.M., Weisspapir, I., Heinen, S., Mester, J., Lai, A., Janik, R., Dorr, A., et al.. (2018). Imaging the effects of β-hydroxybutyrate on peri-infarct neurovascular function and metabolism. Stroke 49: 2173–2181, https://doi.org/10.1161/strokeaha.118.020586.Search in Google Scholar
Beard, E., Lengacher, S., Dias, S., Magistretti, P.J., and Finsterwald, C. (2022). Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front. Physiol. 12, https://doi.org/10.3389/fphys.2021.825816.Search in Google Scholar PubMed PubMed Central
Benveniste, H., Lee, H., and Volkow, N.D. (2017). The glymphatic pathway: waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23: 454–465, https://doi.org/10.1177/1073858417691030.Search in Google Scholar PubMed PubMed Central
Bernardi, C., Tramontina, A.C., Nardin, P., Biasibetti, R., Costa, A.P., Vizueti, A.F., Batassini, C., Tortorelli, L.S., Wartchow, K.M., Dutra, M.F., et al.. (2013). Treadmill exercise induces hippocampal astroglial alterations in rats. Neural Plast. 2013, https://doi.org/10.1155/2013/709732.Search in Google Scholar PubMed PubMed Central
Blázquez, C., Sanchez, C., Velasco, G., and Guzmán, M. (1998). Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes. J. Neurochem. 71: 1597–1606, https://doi.org/10.1046/j.1471-4159.1998.71041597.x.Search in Google Scholar PubMed
Borsoi, M., Antonio, C.B., Müller, L.G., Viana, A.F., Hertzfeldt, V., Lunardi, P.S., Zanotto, C., Nardin, P., Ravazzolo, A.P., Rates, S.M.K., et al.. (2015). Repeated forced swimming impairs prepulse inhibition and alters brain-derived neurotrophic factor and astroglial parameters in rats. Pharmacol. Biochem. Behav. 128: 50–61, https://doi.org/10.1016/j.pbb.2014.11.012.Search in Google Scholar PubMed
Bröer, S., Rahman, B., Pellegri, G., Pellerin, L., Martin, J.L., Verleysdonk, S., Hamprech, B., and Magistretti, P.J. (1997). Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 272: 30096–30102.Search in Google Scholar
Camberos-Luna, L. and Massieu, L. (2020). Therapeutic strategies for ketosis induction and their potential efficacy for the treatment of acute brain injury and neurodegenerative diseases. Neurochem. Int. 133: 104614, https://doi.org/10.1016/j.neuint.2019.104614.Search in Google Scholar PubMed
Cao, Y., Kanta, J.M., Bishop, C.A., Kiens, B., Fritzen, A.M., and Kleinert, M. (2025). Dietary medium-chain triacylglycerols in metabolic regulation. TEM: 1–25, https://doi.org/10.1016/j.tem.2025.09.010.Search in Google Scholar PubMed
Cao, W.D., Kawai, N., Miyake, K., Zhang, X., Fei, Z., and Tamiya, T. (2014). Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF expression in human brain gliomas. Brain Tumor Pathol. 31: 1–10, https://doi.org/10.1007/s10014-013-0135-3.Search in Google Scholar PubMed
Cao, W., Lin, J., Xiang, W., Liu, J., Wang, B., Liao, W., and Jiang, T. (2022). Physical exercise-induced astrocytic neuroprotection and cognitive improvement through primary cilia and mitogen-activated protein kinases pathway in rats with chronic cerebral hypoperfusion. Front. Aging Neurosci. 14, https://doi.org/10.3389/fnagi.2022.866336.Search in Google Scholar PubMed PubMed Central
Castiglioni, A.J., Legare, M.E., Busbee, D.L., and Tiffany-Castiglioni, E. (1991). Morphological changes in astrocytes of aging mice fed normal or caloric restricted diets. Age 14: 102–106, https://doi.org/10.1007/bf02435015.Search in Google Scholar
Chawla, A.R., Johnson, D.E., Zybura, A.S., Leeds, B.P., Nelson, R.M., and Hudmon, A. (2017). Constitutive regulation of the glutamate/aspartate transporter EAAT1 by calcium-calmodulin-dependent protein kinase II. J. Neurochem. 140: 421–434, https://doi.org/10.1111/jnc.13913.Search in Google Scholar PubMed PubMed Central
Chechik, T., Roeder, L.M., Tildon, J.T., and Poduslo, S.E. (1987). Ketone body enzyme activities in purified neurons, astrocytes and oligodendroglia. Neurochem. Int. 10: 95–99, https://doi.org/10.1016/0197-0186(87)90179-3.Search in Google Scholar PubMed
Cheng, B., Yang, X., An, L., Gao, B., Liu, X., and Liu, S. (2009). Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 1286: 25–31, https://doi.org/10.1016/j.brainres.2009.06.060.Search in Google Scholar PubMed
Chowdhury, G.M.I., Jiang, L., Rothman, D.L., and Behar, K.L. (2014). The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow Metab. 34: 1233–1242, https://doi.org/10.1038/jcbfm.2014.77.Search in Google Scholar PubMed PubMed Central
Chung, W.S., Allen, N.J., and Eroglu, C. (2015). Astrocytes control synapse formation, function, and elimination. Cold Spring Harb. Perspect. Biol. 7, https://doi.org/10.1101/cshperspect.a020370.Search in Google Scholar PubMed PubMed Central
Daikhin, Y. and Yudkoff, M. (1998). Ketone bodies and brain glutamate and GABA metabolism. Dev Neurosci. 20: 358–364, https://doi.org/10.1159/000017331.Search in Google Scholar PubMed
Danbolt, N.C. (2001). Glutamate uptake. Prog. Neurobiol. 65: 1–105, https://doi.org/10.1016/s0301-0082(00)00067-8.Search in Google Scholar PubMed
De Gaetano, A., Castagneto, M., Gangeri, G., Mingrone, G., Sganga, G., Tataranni, P.A., Raguso, C., and Greco, A.V. (1994). Kinetics of medium-chain triglycerides and free fatty acids in healthy volunteers and surgically stressed patients. J. Parenter. Enter. Nutr. 18: 134–140, https://doi.org/10.1177/0148607194018002134.Search in Google Scholar PubMed
Debernardi, R., Pierre, K., Lengacher, S., Magistretti, P.J., and Pellerin, L. (2003). Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J. Neurosci. Res. 73: 141–155, https://doi.org/10.1002/jnr.10660.Search in Google Scholar PubMed
Denisov, P., Popov, A., Brazhe, A., Lazareva, N., Verkhratsky, A., and Semyanov, A. (2021). Caloric restriction modifies spatiotemporal calcium dynamics in mouse hippocampal astrocytes. Biochim. Biophys. Acta Mol. Cell Res. 1868, https://doi.org/10.1016/j.bbamcr.2021.119034.Search in Google Scholar PubMed
DeVivo, D.C., Fujimoto, K., Leckie, M.P., and Agrawal, H.C. (1976). Subcellular distribution of ketone body metabolizing enzymes in the rat brain. J. Neurochem. 26: 635–637, https://doi.org/10.1111/j.1471-4159.1976.tb01526.x.Search in Google Scholar PubMed
Donato, R. (2001). S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 33: 637–668, https://doi.org/10.1016/s1357-2725(01)00046-2.Search in Google Scholar PubMed
Dringen, R. and Arend, C. (2025). Glutathione metabolism of the brain – The role of astrocytes. J. Neurochem. 169, https://doi.org/10.1111/jnc.70073.Search in Google Scholar PubMed PubMed Central
Dringen, R. and Hamprecht, B. (1996). Glutathione content as an indicator for the presence of metabolic pathways of amino acids in astroglial cultures. J. Neurochem. 67: 1375–1382, https://doi.org/10.1046/j.1471-4159.1996.67041375.x.Search in Google Scholar PubMed
Dringen, R. and Hamprecht, B. (1998). Glutathione restoration as indicator for cellular metabolism of astroglial cells. Dev Neurosci. 20: 401–407, https://doi.org/10.1159/000017337.Search in Google Scholar PubMed
Duan, S., Anderson, C.M., Stein, B.A., and Swanson, R.A. (1999). Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J. Neurosci. 19: 10193–10200, https://doi.org/10.1523/jneurosci.19-23-10193.1999.Search in Google Scholar
Düking, T., Spieth, L., Berghoff, S.A., Piepkorn, L., Schmidke, A.M., Mitkovski, M., Kannaiyan, N., Hosang, L., Scholz, P., Shaib, A.H., et al.. (2022). Ketogenic diet uncovers differential metabolic plasticity of brain cells. Sci. Adv. 8: 7639, https://doi.org/10.1126/sciadv.abo7639.Search in Google Scholar PubMed PubMed Central
Ebert, D., Haller, R.G., and Walton, M.E. (2003). Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 23: 5928–5935, https://doi.org/10.1523/jneurosci.23-13-05928.2003.Search in Google Scholar PubMed PubMed Central
Edmond, J., Robbins, R.A., Bergstrom, J.D., Cole, R.A., and de Vellis, J. (1987). Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res. 18: 551–561, https://doi.org/10.1002/jnr.490180407.Search in Google Scholar PubMed
Edwards, H.V., Cameron, R.T., and Baillie, G.S. (2011). The emerging role of HSP20 as a multifunctional protective agent. Cell. Signal. 23: 1447–1454, https://doi.org/10.1016/j.cellsig.2011.05.009.Search in Google Scholar PubMed
Emsley, J.G. and Macklis, J.D. (2006). Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2: 175–186, https://doi.org/10.1017/s1740925x06000202.Search in Google Scholar
Eng, L.F. (1985). Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 8: 203–214, https://doi.org/10.1016/s0165-5728(85)80063-1.Search in Google Scholar PubMed
Evans, M., Cogan, K.E., and Egan, B. (2017). Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J. Physiol. 595: 2857–2871, https://doi.org/10.1113/jp273185.Search in Google Scholar PubMed PubMed Central
Fahimi, A., Baktir, M.A., Moghadam, S., Mojabi, F.S., Sumanth, K., McNerney, M.W., Ponnusamy, R., and Salehi, A. (2017). Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 222: 1797–1808, https://doi.org/10.1007/s00429-016-1308-8.Search in Google Scholar PubMed
Fan, G.C., Ren, X., Qian, J., Yuan, Q., Nicolaou, P., Wang, Y., Jones, W.K., Chu, G., and Kranias, E.G. (2005). Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation 111: 1792–1799, https://doi.org/10.1161/01.cir.0000160851.41872.c6.Search in Google Scholar PubMed
Faulkner, J.R., Herrmann, J.E., Woo, M.J., Tansey, K.E., Doan, N.B., and Sofroniew, M.V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24: 2143–2155, https://doi.org/10.1523/jneurosci.3547-03.2004.Search in Google Scholar
Forero-Quintero, L.S., Deitmer, J.W., and Becker, H.M. (2017). Reduction of epileptiform activity in ketogenic mice: the role of monocarboxylate transporters. Sci. Rep. 7: 1–13, https://doi.org/10.1038/s41598-017-05054-0.Search in Google Scholar PubMed PubMed Central
Fortier, M., Castellano, C.A., Croteau, E., Langlois, F., Bocti, C., St-Pierre, V., Vandenberghe, C., Bernier, M., Roy, M., Descoteaux, M., et al. (2019). A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimer’s Dementia 15: 625–634, https://doi.org/10.1016/j.jalz.2018.12.017.Search in Google Scholar PubMed
García-Matas, S., Paul, R.K., Molina-Martínez, P., Palacios, H., Gutierrez, V.M., Corpas, R., Pallas, M., Cristòfol, R., de Cabo, R., and Sanfeliu, C. (2015). In vitro caloric restriction induces protective genes and functional rejuvenation in senescent SAMP8 astrocytes. Aging Cell 14: 334–344, https://doi.org/10.1111/acel.12259.Search in Google Scholar PubMed PubMed Central
Gómora-García, J.C., Montiel, T., Hüttenrauch, M., Salcido-Gómez, A., García-Velázquez, L., Ramiro-Cortés, Y., Gomora, J.C., Castro-Obregón, S., and Massieu, L. (2023). Effect of the ketone body, D-β-Hydroxybutyrate, on Sirtuin2-mediated regulation of mitochondrial quality control and the autophagy–lysosomal pathway. Cells 12, https://doi.org/10.3390/cells12030486.Search in Google Scholar PubMed PubMed Central
Gzielo, K., Soltys, Z., Rajfur, Z., and Setkowicz, Z.K. (2019). The impact of the ketogenic diet on glial cells morphology. A quantitative morphological analysis. Neuroscience 413: 239–251, https://doi.org/10.1016/j.neuroscience.2019.06.009.Search in Google Scholar PubMed
Halestrap, A.P. and Price, N.T. (1999). The proton-linked monocarboxylate transporter (MCT) family : structure, function and regulation. Biochem. J. 343: 281–299, https://doi.org/10.1042/bj3430281.Search in Google Scholar
Hanu, R., McKenna, M., O’Nelli, A., Resneck, W.G., and Bloch, R. (2000). Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Cell Physiol. 278: C921–C930, https://doi.org/10.1152/ajpcell.2000.278.5.C921.Search in Google Scholar PubMed
Har-Even, M., Rubovitch, V., Ratliff, W.A., Richmond-Hacham, B., Citron, B.A., and Pick, C.G. (2021). Ketogenic diet as a potential treatment for traumatic brain injury in mice. Sci. Rep. 11, https://doi.org/10.1038/s41598-021-02849-0.Search in Google Scholar PubMed PubMed Central
Hazen, S.A., Waheed, Abdul, Sly William, S., LaNoue, Kathryn F., and Christopher J. Lynch. (1997). Effect of carbonic anhydrase inhibition and acetoacetate on anaplerotic pyruvate carboxylase activity in cultured rat astrocytes. Dev Neurosci. 19: 162–171, https://doi.org/10.1159/000111202.Search in Google Scholar PubMed
Henderson, S.T., Vogel, J.L., Barr, L.J., Garvin, F., Jones, J.J., and Costantini, L.C. (2009). Study of the ketogenic agent AC-1202 in mild to moderate alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr. and Metab. 6, https://doi.org/10.1186/1743-7075-6-31.Search in Google Scholar PubMed PubMed Central
Holcomb, L.E., O’Neill, C.C., De Witt, E.A., and Kolwicz, S.C. (2021). The effects of fasting or ketogenic diet on endurance exercise performance and metabolism in female mice. Metabolites 11: 1–14.Search in Google Scholar
Hu, Z.L., Sun, T., Lu, M., Ding, J.H., Du, R.H., and Hu, G. (2019). Kir6.1/K-ATP channel on astrocytes protects against dopaminergic neurodegeneration in the MPTP mouse model of Parkinson’s disease via promoting mitophagy. Brain, Behav. Immun. 81: 509–522, https://doi.org/10.1016/j.bbi.2019.07.009.Search in Google Scholar PubMed
Huang, J., Chai, X., Wu, Y., Hou, Y., Li, C., Xue, Y., Pan, J., Zhao, Y., Su, A., Zhu, X., et al.. (2022). β-Hydroxybutyric acid attenuates heat stress-induced neuroinflammation via inhibiting TLR4/p38 MAPK and NF-κB pathways in the hippocampus. FASEB J. 36, https://doi.org/10.1096/fj.202101469rr.Search in Google Scholar PubMed
Huang, D., Xiao, Q., Tang, J., Liang, X., Wang, J., Hu, M., Jiang, Y., Liu, L., Qin, L., Zhou, M., et al.. (2022). Positive effects of running exercise on astrocytes in the medial prefrontal cortex in an animal model of depression. J. Comp. Neurol. 530: 3056–3071, https://doi.org/10.1002/cne.25397.Search in Google Scholar PubMed
Jadhav, H.B. and Annapure, U.S. (2023). Triglycerides of medium-chain fatty acids: a concise review. J. Food Sci. Technol. 60: 2143–2152, https://doi.org/10.1007/s13197-022-05499-w.Search in Google Scholar PubMed PubMed Central
Jiang, L., Mason, G.F., Rothman, D.L., De Graaf, R.A., and Behar, K.L. (2011). Cortical substrate oxidation during hyperketonemia in the fasted anesthetized rat in vivo. J. Cereb. Blood Flow Metab. 31: 2313–2323, https://doi.org/10.1038/jcbfm.2011.91.Search in Google Scholar PubMed PubMed Central
Johnstone, A. (2015). Fasting for weight loss: an effective strategy or latest dieting trend? Int. J. Obes. 39: 727–733, https://doi.org/10.1038/ijo.2014.214.Search in Google Scholar PubMed
Jurga, A.M., Paleczna, M., Kadluczka, J., and Kuter, K.Z. (2021). Beyond the GFAP-astrocyte protein markers in the brain. Biomolecules 11: 1–28, https://doi.org/10.3390/biom11091361.Search in Google Scholar PubMed PubMed Central
Kanta, J.M., Lundsgaard, A.M., Havelund, J.F., Armour, S.L., Bæk, O., Nguyen, D.N., Richter, E.A., Knudsen, J.G., Kleinert, M., Færgeman, N.J., et al. (2025). Metabolic effects of medium-chain triacylglycerol consumption are preserved in obesity. Am. J. Physiol. Endocrinol. Metab. 328: E1–E20, https://doi.org/10.1152/ajpendo.00234.2024.Search in Google Scholar PubMed
Kaur, M., Sharma, S., and Kaur, G. (2008). Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 9: 441–454, https://doi.org/10.1007/s10522-008-9168-0.Search in Google Scholar PubMed
Koppel, S.J., Pei, D., Wilkins, H.M., Weidling, I.W., Wang, X., Menta, B.W., Perez-Ortiz, J., Kalani, A., Manley, S., Novikova, L., et al.. (2021). A ketogenic diet differentially affects neuron and astrocyte transcription. J. Neurochem. 157: 1930–1945, https://doi.org/10.1111/jnc.15313.Search in Google Scholar PubMed PubMed Central
Koppel, S.J. and Swerdlow, R.H. (2018). Neuroketotherapeutics: a modern review of a century-old therapy. Neurochem. Int. 117: 114–125, https://doi.org/10.1016/j.neuint.2017.05.019.Search in Google Scholar PubMed PubMed Central
Koppel, S.J., Wilkins, H.M., Weidling, I.W., Wang, X., Menta, B.W., and Swerdlow, R.H. (2023). β-Hydroxybutyrate preferentially enhances neuron over astrocyte respiration while signaling cellular quiescence. Mitochondrion 68: 125–137, https://doi.org/10.1016/j.mito.2022.12.004.Search in Google Scholar PubMed PubMed Central
Krebs, H.A., Williamson, D.H., Bates, M.W., Page, M.A., and Hawklns, R.A. (1971). The role of ketone bodies in caloric homeostasis. Adv. Enzyme Regul. 9: 387–409, https://doi.org/10.1016/s0065-2571(71)80055-9.Search in Google Scholar
Lai, X.J., Ye, S.Q., Zheng, L., Li, L., Liu, Q.R., Yu, S.B., Pang, Y., Jin, S., Li, Q., Yu, A.C.H., et al. (2014). Selective 14-3-3γ induction quenches p-β-catenin Ser37/Bax-enhanced cell death in cerebral cortical neurons during ischemia. CDD 5: 1–23, https://doi.org/10.1038/cddis.2014.152.Search in Google Scholar PubMed PubMed Central
Lalo, U., Bogdanov, A., Moss, G.W.J., Frenguelli, B.G., and Pankratov, Y. (2020). Astroglia-derived BDNF and MSK-1 mediate experience- and diet-dependent synaptic plasticity. Brain Sci. 10, https://doi.org/10.3390/brainsci10070462.Search in Google Scholar PubMed PubMed Central
Lalo, U., Bogdanov, A., and Pankratov, Y. (2018). Diversity of astroglial effects on aging- and experience-related cortical metaplasticity. Front. Mol. Neurosci. 11, https://doi.org/10.3389/fnmol.2018.00239.Search in Google Scholar PubMed PubMed Central
Lalo, U., Bogdanov, A., and Pankratov, Y. (2019). Age-and experience-related plasticity of ATP-mediated signaling in the neocortex. Front. Cell. Neurosci. 13, https://doi.org/10.3389/fncel.2019.00242.Search in Google Scholar PubMed PubMed Central
Lalo, U. and Pankratov, Y. (2021). Astrocytes as perspective targets of exercise- and caloric restriction‐mimetics. Neurochem. Res. 46: 2746–2759, https://doi.org/10.1007/s11064-021-03277-2.Search in Google Scholar PubMed PubMed Central
Latimer, C.S., Searcy, J.L., Bridges, M.T., Brewer, L.D., Popović, J., Blalock, E.M., Landfield, P.W., Thibault, O., and Porter, N.M. (2011). Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS ONE 6, https://doi.org/10.1371/journal.pone.0026812.Search in Google Scholar PubMed PubMed Central
Leardini-Tristaõ, M., Andrade, G., Garcia, C., Reis, P.A., Lourenço, M., Moreira, E.T.S., Lima, F.R.S., Castro-Faria-Neto, H.C., Tibirica, E., and Estato, V. (2020). Physical exercise promotes astrocyte coverage of microvessels in a model of chronic cerebral hypoperfusion. J. Neuroinflammation. 17, https://doi.org/10.1186/s12974-020-01771-y.Search in Google Scholar PubMed PubMed Central
Lee, J.A., Hall, B., Allsop, J., Alqarni, R., and Allen, S.P. (2021). Lipid metabolism in astrocytic structure and function. Semin. Cell Dev. Biol. 112: 123–136, https://doi.org/10.1016/j.semcdb.2020.07.017.Search in Google Scholar PubMed
Lee, B.G., Schultz, C., Zhao, A., Menon, P., Bugescu, R., and Leinninger, G.M. (2025). Glial fibrillary acidic protein vs. S100B to identify astrocytes impacted by sex and high fat diet. Physiol. Behav. 299: 1–9, https://doi.org/10.1016/j.physbeh.2025.115004.Search in Google Scholar PubMed PubMed Central
Lee, H.G., Wheeler, M.A., and Quintana, F.J. (2022). Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug. Discov. 21: 339–358, https://doi.org/10.1038/s41573-022-00390-x.Search in Google Scholar PubMed PubMed Central
Leite, M.R., Cechella, J.L., Pinton, S., Nogueira, C.W., and Zeni, G. (2016). A diphenyl diselenide-supplemented diet and swimming exercise promote neuroprotection, reduced cell apoptosis and glial cell activation in the hypothalamus of old rats. Exp. Gerontol. 82: 1–7, https://doi.org/10.1016/j.exger.2016.05.006.Search in Google Scholar PubMed
Leite, M., Frizzo, J.K., Nardin, P., Almeida, L.M.V. De, Tramontina, F., Gottfried, C., and Gonçalves, C.A. (2004). β-Hydroxy-butyrate alters the extracellular content of S100B in astrocyte cultures. Brain Res. Bull. 64: 139–143, https://doi.org/10.1016/j.brainresbull.2004.06.005.Search in Google Scholar PubMed
Li, Y., Luo, Y., Tang, J., Liang, X., Wang, J., Xiao, Q., Zhu, P., Xiao, K., Jiang, L., Dou, X., et al.. (2021). The positive effects of running exercise on hippocampal astrocytes in a rat model of depression. Transl. Psychiatry. 11, https://doi.org/10.1038/s41398-021-01216-x.Search in Google Scholar PubMed PubMed Central
Lin, C.M., Chang, C.K., Chang, C.P., Hsu, Y.C., Lin, M.T., and Lin, J.W. (2015). Protecting against ischaemic stroke in rats by heat shock protein 20-mediated exercise. European J. Clin. Invest. 45: 1297–1305, https://doi.org/10.1111/eci.12551.Search in Google Scholar PubMed
Lloyd, B.A., Hake, H.S., Ishiwata, T., Farmer, C.E., Loetz, E.C., Fleshner, M., Bland, S.T., and Greenwood, B.N. (2017). Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav. Brain Res. 323: 56–67, https://doi.org/10.1016/j.bbr.2017.01.033.Search in Google Scholar PubMed PubMed Central
Lopes-Cardozo, M., Larsson, O.M., and Schousboe, A. (1986). Acetoacetate and glucose as lipid precursors and energy substrates in primary cultures of astrocytes and neurons from mouse cerebral cortex. J. Neurochem. 46: 773–778, https://doi.org/10.1111/j.1471-4159.1986.tb13039.x.Search in Google Scholar PubMed
Lovatel, G.A., Bertoldi, K., Elsnerb, V.R., Piazza, F.V., Basso, C.G., Dos Santos Moysés, F., Worm, P.V., Netto, C.A., Marcuzzo, S., and Siqueira, I.R. (2014). Long-term effects of pre and post-ischemic exercise following global cerebral ischemia on astrocyte and microglia functions in hippocampus from Wistar rats. Brain Res. 1587: 119–126, https://doi.org/10.1016/j.brainres.2014.08.068.Search in Google Scholar PubMed
Lundquist, A.J., Parizher, J., Petzinger, G.M., and Jakowec, M.W. (2019). Exercise induces region-specific remodeling of astrocyte morphology and reactive astrocyte gene expression patterns in male mice. J. Neurosci. Res. 97: 1081–1094, https://doi.org/10.1002/jnr.24430.Search in Google Scholar PubMed
Marlatt, M.W., Potter, M.C., Lucassen, P.J., and van Praag, H. (2012). Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev. Neurobiol. 72: 943–952, https://doi.org/10.1002/dneu.22009.Search in Google Scholar PubMed PubMed Central
Martín, R., Bajo-Grañeras, R., Moratalla, R., Perea, G., and Araque, A. (2015). Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Sience 349: 730–734, https://doi.org/10.1126/science.aaa7945.Search in Google Scholar PubMed
Martin, A., Tegla, C.A., Cudrici, C.D., Kruszewski, A.M., Azimzadeh, P., Boodhoo, D., Mekala, A.P., Rus, V., and Rus, H. (2015). Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol. Res. 61: 187–197, https://doi.org/10.1007/s12026-014-8557-5.Search in Google Scholar PubMed
McKenna, M.C. (2012). Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem. Res. 37: 2613–2626, https://doi.org/10.1007/s11064-012-0901-3.Search in Google Scholar PubMed PubMed Central
McKenna, M.C., Tildon, J.T., Stevenson, J.H., Boatright, R., and Huang, S. (1993). Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 15: 320–329, https://doi.org/10.1159/000111351.Search in Google Scholar PubMed
Melø, T.M., Nehlig, A., and Sonnewald, U. (2006). Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem. Int. 48: 498–507, https://doi.org/10.1016/j.neuint.2005.12.037.Search in Google Scholar PubMed
Montiel, T., Gómora-García, J.C., Gerónimo-Olvera, C., Heras-Romero, Y., Bernal-Vicente, B.N., Pérez-Martínez, X., Tovar-y-Romo, L.B., and Massieu, L. (2023). Modulation of the autophagy-lysosomal pathway and endoplasmic reticulum stress by ketone bodies in experimental models of stroke. J. Neurochem. 166: 87–106, https://doi.org/10.1111/jnc.15852.Search in Google Scholar PubMed
Najmi, S., Shaafi, S., Aliasgharpour, H., Mahmoudi, J., Sadigh-Etemad, S., Farhoudi, M., and Baniasadi, N. (2016). The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: a rat model. Iran J Neurol. 15: 63–69.Search in Google Scholar
Nascimento, C.M.C., Pereira, J.R., Pires De Andrade, L., Garuffi, M., Ayan, C., Kerr, D.S., Talib, L.L., Cominetti, M.R., and Stella, F. (2014). Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different BDNF Val66Met genotypes. J. Alzheimer’s Disease 43: 81–91, https://doi.org/10.3233/jad-140576.Search in Google Scholar PubMed
Neeper, S.A., Gómez-Pinilla, F., Choi, J., and Cotman, C.W. (1996). Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726: 49–56.Search in Google Scholar
Nieweg, K., Schaller, H., and Pfrieger, F.W. (2009). Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem. 109: 125–134, https://doi.org/10.1111/j.1471-4159.2009.05917.x.Search in Google Scholar PubMed
Noh, H.S., Sang, S.K., Dong, W.K., Young, H.K., Chang, H.P., Jae, Y.H., Gyeong, J.C., and Choi, W.S. (2005). Ketogenic diet increases calbindin-D28k in the hippocampi of male ICR mice with kainic acid seizures. Epilepsy Res. 65: 153–159, https://doi.org/10.1016/j.eplepsyres.2005.05.008.Search in Google Scholar PubMed
Nonaka, Y., Takagi, T., Inai, M., Nishimura, S., Urashima, S., Honda, K., Aoyama, T., and Terada, S. (2016). Lauric acid stimulates ketone body production in the KT-5 astrocyte cell line. J. Oleo Sci. 65: 693–699, https://doi.org/10.5650/jos.ess16069.Search in Google Scholar PubMed
Norenberg, M.D. (1979). The distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem. 27: 756–762, https://doi.org/10.1177/27.3.39099.Search in Google Scholar PubMed
Nuwayhid, N.F., Johnson, G.F., and Feld, R.D. (1988). Kinetic measurement of the combined concentrations of acetoacetate and β-hydroxybutyrate in serum. Clin. Chem. 34: 1790–1793, https://doi.org/10.1093/clinchem/34.9.1787.Search in Google Scholar
Oberheim, N.A., Goldman, S.A., and Nedergaard, M. (2012). Heterogeneity of astrocytic form and function. In: Milner, R. (Ed.). Astrocytes: methods and protocols. Humana Press, La Jolla CA, USA, pp. 23–40.Search in Google Scholar
Ohmori, H., Kawai, K., and Yamashita, K. (1990). Enhanced ketone body uptake by perfused skeletal muscle in trained rats. Endocrinol. Japon 37: 421–429, https://doi.org/10.1507/endocrj1954.37.421.Search in Google Scholar PubMed
Ohnuma, T., Toda, A., Kimoto, A., Takebayashi, Y., Higashiyama, R., Tagata, Y., Ito, M., Ota, T., Shibata, N., and Arai, H. (2016). Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: a prospective, open-label pilot study. Clin. Interv. Aging. 11: 29–36, https://doi.org/10.2147/cia.s95362.Search in Google Scholar PubMed PubMed Central
Okada, S., Nakamura, M., Katoh, H., Miyao, T., Shimazaki, T., Ishii, K., Yamane, J., Yoshimura, A., Iwamoto, Y., Toyama, Y., et al. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12: 829–834, https://doi.org/10.1038/nm1425.Search in Google Scholar PubMed
Otsuka, S., Sakakima, H., Sumizono, M., Takada, S., Terashi, T., and Yoshida, Y. (2016). The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav. Brain Res. 303: 9–18, https://doi.org/10.1016/j.bbr.2016.01.049.Search in Google Scholar PubMed
Otsuka, S., Sakakima, H., Terashi, T., Takada, S., Nakanishi, K., and Kikuchi, K. (2019). Preconditioning exercise reduces brain damage and neuronal apoptosis through enhanced endogenous 14-3-3γ after focal brain ischemia in rats. Brain Struct. Funct. 224: 727–738, https://doi.org/10.1007/s00429-018-1800-4.Search in Google Scholar PubMed
Page, M.A., Krebs, H.A., and Williamson, D.H. (1971). Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem. J. 121: 49–53, https://doi.org/10.1042/bj1210049.Search in Google Scholar PubMed PubMed Central
Pan, J.W., De Graaf, A., Petersen, F., Shulman, I., Hetherington, H.P., and Rothman, L. (2002). [2,4-13C2]-Hydroxybutyrate metabolism in human brain. J. Cereb. Blood Flow Metab. 22: 890–898, https://doi.org/10.1097/00004647-200207000-00014.Search in Google Scholar PubMed PubMed Central
Pan, J.W., Telang, F.W., Lee, J.H., De Graaf, R.A., Rothman, D.L., Stein, D.T., and Hetherington, H.P. (2001). Measurement of β-hydroxybutyrate in acute hyperketonemia in human brain. J. Neurochem. 79: 539–544, https://doi.org/10.1046/j.1471-4159.2001.00575.x.Search in Google Scholar PubMed
Pellerin, L. and Magistretti, P.J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization (glutamate transporter/Na+/K+-ATPase/2-deoxyglucose/positron-emission tomography/magnetic resonance imaging). Proc. Natd. Acad. Sci. 91: 10625–10629, https://doi.org/10.1073/pnas.91.22.10625.Search in Google Scholar PubMed PubMed Central
Pereira, B.C., Lucas, G., Da Rocha, A.L., Pauli, J.R., Ropelle, E.R., Cintra, D., De Souza, C.T., Bueno, C.R., and Da Silva, A.S. (2015). Eccentric exercise leads to glial activation but not apoptosis in mice spinal cords. Int. J. Sports Med. 36: 378–385, https://doi.org/10.1055/s-0034-1395589.Search in Google Scholar PubMed
Pierre, K., Parent, A., Jayet, P., Halestrap, A.P., Scherrer, U., and Pellerin, L. (2007). Enhanced expression of three monocarboxylate transporter isoforms in the brain of obese mice. J. Physiol. 583: 469–486, https://doi.org/10.1113/jphysiol.2007.138594.Search in Google Scholar PubMed PubMed Central
Pierre, K. and Pellerin, L. (2005). Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 94: 1–14, https://doi.org/10.1111/j.1471-4159.2005.03168.x.Search in Google Scholar PubMed
Poduslo, S.E. (1989). Induction of ketone body enzymes in glial cells. Archives Biochem. Biophys. 272: 318–322, https://doi.org/10.1016/0003-9861(89)90225-7.Search in Google Scholar PubMed
Poole, R.C. and Halestrap, A.P. (1993). Transport of lactate and other monocarboxylates across mammalian plasma membranes. APS 264, https://doi.org/10.1152/ajpcell.1993.264.4.c761.Search in Google Scholar
Popov, A., Denisov, P., Bychkov, M., Brazhe, A., Lyukmanova, E., Shenkarev, Z., Lazareva, N., Verkhratsky, A., and Semyanov, A. (2020). Caloric restriction triggers morphofunctional remodeling of astrocytes and enhances synaptic plasticity in the mouse hippocampus. Cell Death Dis. 11, https://doi.org/10.1038/s41419-020-2406-3.Search in Google Scholar PubMed PubMed Central
Puchalska, P. and Crawford, P.A. (2017). Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25: 262–284, https://doi.org/10.1016/j.cmet.2016.12.022.Search in Google Scholar PubMed PubMed Central
Qian, Y., Ling, P.R., Qu, Z., and Bistrian, B.R. (1998). Effect of continuous enteral medium-chain fatty acid infusion on lipid metabolism in rats. Lipids 33: 261–266, https://doi.org/10.1007/s11745-998-0204-z.Search in Google Scholar PubMed
Rafiki, A., Boulland, J.L., Halestrap, A.P., Ottersen, O.P., and Bergersen, L. (2003). Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122: 677–688, https://doi.org/10.1016/j.neuroscience.2003.08.040.Search in Google Scholar PubMed
Ramírez-López, M.T., Vázquez, M., Bindila, L., Lomazzo, E., Hofmann, C., Blanco, R.N., Alén, F., Antón, M., Decara, J., Arco, R., et al. (2016). Maternal caloric restriction implemented during the preconceptional and pregnancy period alters hypothalamic and hippocampal endocannabinoid levels at birth and induces overweight and increased adiposity at adulthood in male rat offspring. Front. Behav. Neurosci. 10, https://doi.org/10.3389/fnbeh.2016.00208.Search in Google Scholar PubMed PubMed Central
Ramírez-López, M.T., Vázquez, M., Lomazzo, E., Hofmann, C., Blanco, R.N., Alén, F., Antón, M., Decara, J., Arco, R., Orio, L., et al.. (2017). A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 12, https://doi.org/10.1371/journal.pone.0174307.Search in Google Scholar PubMed PubMed Central
Rebello, C.J., Keller, J.N., Liu, A.G., Johnson, W.D., and Greenway, F.L. (2015). Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 3: 123–125, https://doi.org/10.1016/j.bbacli.2015.01.001.Search in Google Scholar PubMed PubMed Central
Reichard, G.A., Owen, O.E., Haff, A.C., Paul, P., and Bortz, W.M. (1974). Ketone body production and oxidation in fasting obese humans. J. Clin. Investig. 53: 508–515, https://doi.org/10.1172/jci107584.Search in Google Scholar
Ribeiro, L.C., Quincozes-Santos, A., Leite, M.C., Abib, R.T., Kleinkauf-Rocha, J., Biasibetti, R., Rotta, L.N., Wofchuk, S.T., Perry, M.L.S., Gonçalves, C.A., et al.. (2009). Caloric restriction increases hippocampal glutamate uptake and glutamine synthetase activity in Wistar rats. Neurosci. Res. 64: 330–334, https://doi.org/10.1016/j.neures.2009.04.004.Search in Google Scholar PubMed
Robinson, A.M. and Williamson, D.H. (1980). Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 60: 143–187, https://doi.org/10.1152/physrev.1980.60.1.143.Search in Google Scholar PubMed
Rosafio, K. and Pellerin, L. (2014). Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1α-mediated transcriptional regulation. GLIA 62: 477–490, https://doi.org/10.1002/glia.22618.Search in Google Scholar PubMed
Rubio, C., López-Landa, A., Romo-Parra, H., and Rubio-Osornio, M. (2025). Impact of the ketogenic diet on neurological diseases: a review. Life 15, https://doi.org/10.3390/life15010071.Search in Google Scholar PubMed PubMed Central
Santin, K., Da Rocha, R.F., Cechetti, F., Quincozes-Santos, A., De Souza, D.F., Nardin, P., Rodrigues, L., Leite, M.C., Moreira, J.C.F., Salbego, C.G., et al. (2011). Moderate exercise training and chronic caloric restriction modulate redox status in rat hippocampus. Brain Res. 1421: 1–10, https://doi.org/10.1016/j.brainres.2011.08.003.Search in Google Scholar PubMed
Scafidi, S., Jernberg, J., Fiskum, G., and McKenna, M.C. (2022). Metabolism of exogenous [2,4-13C]β-Hydroxybutyrate following traumatic brain injury in 21-22-Day-Old rats: an Ex vivo NMR study. Metabolites 12, https://doi.org/10.3390/metabo12080710.Search in Google Scholar PubMed PubMed Central
Schousbe, A. and Waagepetersen, H. (2005). Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox. Res. 8: 221–225, https://doi.org/10.1007/bf03033975.Search in Google Scholar
Shang, S., Wang, L., and Lu, X. (2024). β-Hydroxybutyrate enhances astrocyte glutamate uptake through EAAT1 expression regulation. Mol. Cell. Neurosci. 131, https://doi.org/10.1016/j.mcn.2024.103959.Search in Google Scholar PubMed
Sharma, S. and Kaur, G. (2008). Dietary restriction enhances kainate-induced increase in NCAM while blocking the glial activation in adult rat brain. Neurochem. Res. 33: 1178–1188, https://doi.org/10.1007/s11064-007-9503-x.Search in Google Scholar PubMed
Silva, M.C., Rocha, J., Pires, C.S., Ribeiro, L.C., Brolese, G., Leite, M.C., Almeida, L.M.V., Tramontina, F., Ziegler, D.R., and Gonçalves, C.A. (2005). Transitory gliosis in the CA3 hippocampal region in rats fed on a ketogenic diet. Nutr. Neurosci. 8: 259–264, https://doi.org/10.1080/10284150500475032.Search in Google Scholar PubMed
Spencer, M., Kulbe, J.R., Venkatesh, V., Laird, A., Ford, M., O’Brien, S., Boustani, A., Schlachetzki, J.C.M., and Fields, J.A. (2025). Caloric restriction mimetic 2-deoxyglucose alters metabolic and transcriptomic phenotype in association with changes in chromatin accessibility in human astrocytes. Sci. Rep. 15: 1–14, https://doi.org/10.1038/s41598-025-03796-w.Search in Google Scholar PubMed PubMed Central
Spigoni, V., Cinquegrani, G., Iannozzi, N.T., Frigeri, G., Maggiolo, G., Maggi, M., Parello, V., and Dei Cas, A. (2022). Activation of G protein-coupled receptors by ketone bodies: clinical implication of the ketogenic diet in metabolic disorders. Front. Endocrinol. 13, https://doi.org/10.3389/fendo.2022.972890.Search in Google Scholar PubMed PubMed Central
Stevenson, M.E., Behnke, V.K., and Swain, R.A. (2018). Exercise pattern and distance differentially affect hippocampal and cerebellar expression of FLK-1 and FLT-1 receptors in astrocytes and blood vessels. Behav. Brain Res. 337: 8–16, https://doi.org/10.1016/j.bbr.2017.09.037.Search in Google Scholar PubMed
Suissa, L., Kotchetkov, P., Guigonis, J.M., Doche, E., Osman, O., Pourcher, T., and Lindenthal, S. (2021). Ingested ketone ester leads to a rapid rise of acetyl-CoA and competes with glucose metabolism in the brain of non-fasted mice. J. Mol. Sci. 22: 1–17, https://doi.org/10.3390/ijms22020524.Search in Google Scholar PubMed PubMed Central
Sun, L.N., Li, X.L., Wang, F., Zhang, J., Wang, D.D., Yuan, L., Wu, M.N., Wang, Z.J., and Qi, J.S. (2017). High-intensity treadmill running impairs cognitive behavior and hippocampal synaptic plasticity of rats via activation of inflammatory response. J. Neurosci. Res. 95: 1611–1620, https://doi.org/10.1002/jnr.23996.Search in Google Scholar PubMed
Suzuki, A., Stern, S.A., Bozdagi, O., Huntley, G.W., Walker, R.H., Magistretti, P.J., and Alberini, C.M. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144: 810–823, https://doi.org/10.1016/j.cell.2011.02.018.Search in Google Scholar PubMed PubMed Central
Suzuki, Y., Takahashi, H., Fukuda, M., Hino, H., Kobayashi, K., Tanaka, J., and Ishii, E. (2009). β-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 1268: 17–23, https://doi.org/10.1016/j.brainres.2009.02.074.Search in Google Scholar PubMed
Takano, T., Tian, G.F., Peng, W., Lou, N., Libionka, W., Han, X., and Nedergaard, M. (2006). Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9: 260–267, https://doi.org/10.1038/nn1623.Search in Google Scholar PubMed
Tatsumi, K., Okuda, H., Morita-Takemura, S., Tanaka, T., Isonishi, A., Shinjo, T., Terada, Y., and Wanaka, A. (2016). Voluntary exercise induces astrocytic structural plasticity in the globus pallidus. Front. Cell. Neurosci. 10, https://doi.org/10.3389/fncel.2016.00165.Search in Google Scholar PubMed PubMed Central
Thevenet, J., De Marchi, U., Domingo, J.S., Christinat, N., Bultot, L., Lefebvre, G., Sakamoto, K., Descombes, P., Masoodi, M., and Wiederkehr, A. (2016). Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 30: 1913–1926, https://doi.org/10.1096/fj.201500182.Search in Google Scholar PubMed
Tildon, J.T., McKenna, M.C., and Stevenson, J.H. (1994). Transport of 3-hydroxybutyrate by cultured rat brain astrocytes. Neurochem. Res. 19: 1237–1242, https://doi.org/10.1007/bf01006812.Search in Google Scholar
Tovar, R., Vargas, A., Aranda, J., Sánchez‐salido, L., González‐gonzález, L., Chowen, J.A., de Fonseca, F.R., Suárez, J., and Rivera, P. (2021). Analysis of both lipid metabolism and endocannabinoid signaling reveals a new role for hypothalamic astrocytes in maternal caloric restriction‐induced perinatal programming. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22126292.Search in Google Scholar PubMed PubMed Central
Trotti, D., Rizzini, B.L., Rossi, D., Haugeto, O., Racagni, G., Danbolt, N.C., and Volterra, A. (1997). Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Euro. J. Neurosci. 9: 1236–1243, https://doi.org/10.1111/j.1460-9568.1997.tb01478.x.Search in Google Scholar PubMed
Valdebenito, R., Ruminot, I., Garrido-Gerter, P., Fernández-Moncada, I., Forero-Quintero, L., Alegría, K., Becker, H.M., Deitmer, J.W., and Barros, L.F. (2016). Targeting of astrocytic glucose metabolism by beta-hydroxybutyrate. J. Cereb. Blood Flow Metab. 36: 1813–1822, https://doi.org/10.1177/0271678x15613955.Search in Google Scholar PubMed PubMed Central
Vallee, K.A.J. and Fields, J.A. (2022). Caloric restriction mimetic 2-Deoxyglucose reduces inflammatory signaling in human astrocytes: implications for therapeutic strategies targeting neurodegenerative diseases. Brain Sci. 12, https://doi.org/10.3390/brainsci12030308.Search in Google Scholar PubMed PubMed Central
Vasim, I., Majeed, C.N., and DeBoer, M.D. (2022). Intermittent fasting and metabolic health. Nutrients 14: 1–15, https://doi.org/10.3390/nu14030631.Search in Google Scholar PubMed PubMed Central
Vaynman, S., Ying, Z., and Gomez-Pinilla, F. (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Euro. J. Neurosci. 20: 2580–2590, https://doi.org/10.1111/j.1460-9568.2004.03720.x.Search in Google Scholar PubMed
Vignoli, B., Battistini, G., Melani, R., Blum, R., Santi, S., Berardi, N., and Canossa, M. (2016). Peri-synaptic glia recycles brain-derived neurotrophic factor for LTP stabilization and memory retention. Neuron 92: 873–887, https://doi.org/10.1016/j.neuron.2016.09.031.Search in Google Scholar PubMed
Vizuete, A.F., De Souza, D.F., Guerra, M.C., Batassini, C., Dutra, M.F., Bernardi, C., Costa, A.P., and Gonçalves, C.A. (2013). Brain changes in BDNF and S100B induced by ketogenic diets in Wistar rats. Life Sci. 92: 923–928, https://doi.org/10.1016/j.lfs.2013.03.004.Search in Google Scholar PubMed
Wang, Q., Kong, Y., Lin, S., Wu, D.Y., Hu, J., Huang, L., Zang, W.S., Li, X.W., Yang, J.M., and Gao, T.M. (2021). The ATP level in the mPFC mediates the antidepressant effect of calorie restriction. Neurosci. Bull. 37: 1303–1313, https://doi.org/10.1007/s12264-021-00726-4.Search in Google Scholar PubMed PubMed Central
Wang, F.X., Xu, C.L., Su, C., Li, J., and Lin, J.Y. (2022). β-Hydroxybutyrate attenuates painful diabetic neuropathy via restoration of the aquaporin-4 polarity in the spinal glymphatic system. Front. Neurosci. 16, https://doi.org/10.3389/fnins.2022.926128.Search in Google Scholar PubMed PubMed Central
Warren, E.C., Dooves, S., Lugarà, E., Damstra-Oddy, J., Schaf, J., Heine, V.M., Walker, M.C., and Williams, R.S.B. (2020). Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl. Acad. Sci. 117, https://doi.org/10.1073/pnas.2008980117.Search in Google Scholar PubMed PubMed Central
Wood, S.H., Dam, S.van, Craig, T., Tacutu, R., O’Toole, A., Merry, B.J., and de Magalhães, J.P. (2015). Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging. Genome Biol. 16, https://doi.org/10.1186/s13059-015-0847-2.Search in Google Scholar PubMed PubMed Central
Xiao, X.Q., Zhao, Y., and Chen, G.Q. (2007). The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 28: 3608–3616, https://doi.org/10.1016/j.biomaterials.2007.04.046.Search in Google Scholar PubMed
Yeh, Y.-Y., Streuli, V.L., and Zee, P. (1977). Ketone bodies serve as important precursors of brain iipids in the developing rat. Lipids 12: 957–964, https://doi.org/10.1007/bf02533318.Search in Google Scholar PubMed
York, E.M., Miller, A., Stopka, S.A., Martínez-François, J.R., Hossain, M.A., Baquer, G., Regan, M.S., Agar, N.Y.R., and Yellen, G. (2024). The dentate gyrus differentially metabolizes glucose and alternative fuels during rest and stimulation. J. Neurochem. 168: 533–554, https://doi.org/10.1111/jnc.16004.Search in Google Scholar PubMed PubMed Central
Yoshida, Y., Ikematsu, S., Moritoyo, T., Goto, M., Tsutsui, J., Sakuma, S., Osame, M., and Muramatsu, T. (2001). Intraventricular administration of the neurotrophic factor midkine ameliorates hippocampal delayed neuronal death following transient forebrain ischemia in gerbils. Brain Res. 894: 46–55, https://doi.org/10.1016/s0006-8993(00)03209-1.Search in Google Scholar PubMed
Yoshida, Y., Sakakima, H., Matsuda, F., and Ikutomo, M. (2014). Midkine in repair of the injured nervous system. Br. J. Pharmacol. 171: 924–930, https://doi.org/10.1111/bph.12497.Search in Google Scholar PubMed PubMed Central
Yu, X., Taylor, A.M.W., Nagai, J., Golshani, P., Evans, C.J., Coppola, G., and Khakh, B.S. (2018). Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99: 1170–1187, https://doi.org/10.1016/j.neuron.2018.08.015.Search in Google Scholar PubMed PubMed Central
Yudkoff, M., Daikhin, Y., Nissim, I., Grunstein, R., and Nissim, J. (1997). Effects of ketone bodies on astrocyte amino acid metabolism. J. Neurochem.: 682–692, https://doi.org/10.1046/j.1471-4159.1997.69020682.x.Search in Google Scholar PubMed
Zhang, Y., Reichel, J.M., Han, C., Zuniga-Hertz, J.P., and Cai, D. (2017). Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 25: 1091–1102, https://doi.org/10.1016/j.cmet.2017.04.002.Search in Google Scholar PubMed PubMed Central
Zhang, Q., Zhang, J., Yan, Y., Zhang, P., Zhang, W., and Xia, R. (2017). Proinflammatory cytokines correlate with early exercise attenuating anxiety-like behavior after cerebral ischemia. Brain and Behav. 7, https://doi.org/10.1002/brb3.854.Search in Google Scholar PubMed PubMed Central
Zhao, J., Meyerkord, C.L., Du, Y., Khuri, F.R., and Fu, H. (2011). 14-3-3 proteins as potential therapeutic targets. Semin. Cell Dev. Biol. 22: 705–712, https://doi.org/10.1016/j.semcdb.2011.09.012.Search in Google Scholar PubMed PubMed Central
Zhu, Y., Tang, X., Cheng, Z., Dong, Q., and Ruan, G. (2022). The anti-inflammatory effect of preventive intervention with ketogenic diet mediated by the histone acetylation of mGluR5 promotor region in rat Parkinson’s disease model: a dual-tracer PET study. Parkinson’s Dis. 2022, https://doi.org/10.1155/2022/3506213.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

