Abstract
The prevalence of stroke and traumatic brain injury is increasing worldwide. However, current treatments do not fully cure or stop their progression, acting mostly on symptoms. Amphetamine and methylphenidate are stimulants already approved for attention deficit hyperactivity disorder and narcolepsy treatment, with neuroprotective potential and benefits when used in appropriate doses. This review aimed to summarize pre-clinical and clinical trials testing either amphetamine or methylphenidate for the treatment of stroke and traumatic brain injury. We used PubMed as a database and included the following keywords ((methylphenidate) OR (Ritalin) OR (Concerta) OR (Biphentin) OR (amphetamine) OR (Adderall)) AND ((stroke) OR (brain injury) OR (neuroplasticity)). Overall, studies provided inconsistent results regarding cognitive and motor function. Neurite outgrowth, synaptic proteins, dendritic complexity, and synaptic plasticity increases were reported in pre-clinical studies along with function improvement. Clinical trials have demonstrated that, depending on the brain region, there is an increase in motor activity, attention, and memory due to the stimulation of the functionally depressed catecholamine system and the activation of neuronal remodeling proteins. Nevertheless, more clinical trials and pre-clinical studies are needed to understand the drugs’ full potential for their use in these brain diseases namely, to ascertain the treatment time window, ideal dosage, long-term effects, and mechanisms, while avoiding their addictive potential.
Key points
Amphetamine and methylphenidate are approved for attention deficit hyperactivity disorder and narcolepsy treatment
Amphetamine and methylphenidate were tested both in preclinical and clinical trials for the treatment of stroke and traumatic brain injury.
Preclinical data revealed neurite outgrowth and synaptic plasticity along with function improvement evoked by the two drugs, meanwhile clinical trials results were mixed.
More large and robust clinical trials are needed to ascertain the treatment time window, ideal dosage, and long-term effects.
1 Introduction
Stroke and traumatic brain injury are neurological disorders with a high social impact, being stroke the most common cause of disability in the western world (Tsao et al. 2023). An increase of 3.6 % was verified in age-standardized incidence rates of traumatic brain injury between 1990 and 2016 (James et al. 2019), while stroke accounts for an increase of 70 % (Feigin et al. 2021). Despite having different etiologies, these two disorders share a comparable set of symptoms, depending on the type and location of injury. Still, in most cases there is no way to completely revert the ensuing neurological disorder, causing a decline in patients’ quality of life (Feigin et al. 2010). As a result, the discovery and development of new pharmacological treatments are a priority for millions of patients and families worldwide.
Amphetamine and methylphenidate are two psychostimulant drugs approved for attention deficit hyperactivity disorder and narcolepsy, and help many people improve focus and attention, and reduce hyperactivity and impulsivity (Cortese et al. 2021). Although used mostly in children, they are also given to adults (Faraone 2018). Mechanistically, they affect the action of dopamine and noradrenaline neurotransmitters by enhancing their activity in the brain (Faraone 2018), which will be further detailed in the manuscript.
The present review aims to give an insight into a variety of studies related to the effects of dextroamphetamine (d-amphetamine) and methylphenidate, after traumatic brain injury or stroke, revealed by pre-clinical and clinical data. A better understanding of the efficacy of these two drugs is critical to open new avenues for their reliable clinical use, including the therapeutic window, treatment time, and other key treatment features for neural function improvement.
2 Stroke
Stroke is a neurological disorder (WHO 2022) defined by the blood supply cut-off to a part of the brain. This disorder affects one in four people during their lifetime, and that number increased by 50 % in the last 17 years (Feigin et al. 2021; Owolabi et al. 2021). Statistics revealed an incidence of 12.2 million new strokes per year, and 63 % happened in people younger than 70 years, and therefore it is no longer considered an elderly disease. Worldwide 101 million people are living with stroke aftermath, a number that almost doubled over the last 30 years. With almost 143 million disability-adjusted life years, second-place cause of mortality and third-place cause of combined death and disability worldwide (Kuriakose and Xiao 2020; Owolabi et al. 2021; Virani et al. 2021).
Stroke tissue injury and repair mechanisms are complex and involve the interaction of neurons, glia, vascular cells, and matrix components (Shehjar et al. 2023). Several mechanisms have been identified ensuing brain damage including cellular excitotoxicity (cellular excitotoxicity and calcium overload in the cytoplasm and mitochondria), mitochondrial dysfunctions (ATP synthesis and energy balance are disrupted), oxidative and nitrosative stress, neuroinflammation, and blood-brain barrier impairment (Qin et al. 2022; Shehjar et al. 2023). This variety of cellular signaling cascades leads to neural death. Putative drugs that aim for clinical improvement can target one or several of these mechanisms that evoke damage.
Unfortunately, the long-term treatment of stroke remains mainly symptomatic, and supportive, and aims to prevent complications. Some new approaches have been applied to stroke patients (Hill and Hachinski 1998; Starostka-Tatar et al. 2017). Quick diagnosis and treatment are key to the patient’s prognosis (Powers et al. 2019). The treatment depends on the type and severity of the stroke. However, it begins by controlling the airway, breathing, oxygenation, blood pressure, fluids, temperature, and blood glucose (Hill and Hachinski 1998; Powers et al. 2019).
Regarding stroke rehabilitation, many gaps exist to improve patient outcomes. Several large trials targeting motor recovery reveal a similar degree of recovery in either intervention or control groups. For a review see (Stinear et al. 2020). Rehabilitative training is essential for recovery after a stroke, but its effectiveness is often insufficient. Drug therapy to support regeneration would be desirable for therapeutic success, but there is currently a lack of clinically relevant pharmacotherapy. Recovery could usually take weeks to months, or even years, depending on the disabilities and training. Rehabilitation may include speech, physical or occupational therapy, and pharmacotherapy in some cases (Stinear et al. 2020; Thieme et al. 2018). It is relevant to follow these patients closely, even after the event, through brain imaging, vascular imaging, cardiac evaluation, and glucose and cholesterol tests to prevent future strokes (Powers et al. 2019).
3 Traumatic brain injury
Traumatic brain injury is a brain function alteration or other brain pathological abnormality evidence caused by an external force (Menon et al. 2010). Each year over 54 to 60 million people are affected by traumatic brain injury. Regarding statistics, there were 27.08 million new cases in 2016, and the mortality rate reached 10.53 per 100,000 population per year, with 68 % of the individuals dying before reaching a hospital (James et al. 2019).
The mechanical harm that results from a traumatic brain injury’s primary insult cannot be repaired, but therapeutic interventions can target secondary injury mechanisms. This secondary injury defined by the tissue and cellular damage caused by the primary injury results from inflammation, the breakdown of the blood-brain barrier, excitotoxicity, oxidative stress, and cell death (Jarrahi et al. 2020). Neuroprotective drugs may target these secondary mechanisms to reach clinical improvement.
Traumatic brain injury treatment varies depending on the injury severity and affected brain area (Reis et al. 2015). Towards a high severity, the effects of the injury may be irreversible. Nevertheless, the time of treatment after injury is also a determinant of the prognosis. In addition to medication, traumatic brain injury patients may need rehabilitation therapy to regain function, relearn skills, and discover new ways of doing things, considering their new health state. These therapies may include physical therapy; occupational therapy; speech therapy; psychological counseling; and vocational counseling (Carney et al. 2017). Despite these treatments, some traumatic brain injury patients, namely moderate and severe injury patients, may have permanent disabilities influencing their daily lives, with tremendous social and economic impact.
4 Stroke and traumatic brain injury neural repair treatment
One major drawback is that current stroke and traumatic brain injury treatment mainly focuses on the symptoms and target events that underlie recovery and prevention, neglecting the improvement of the neurological function affected by injury. To enhance neurological function, neurological rehabilitation techniques include electric magnetic brain stimulation and mirror treatment (Qi et al. 2023; Thieme et al. 2018).
After an injury, the brain can change its activity and reorganize its structure, function and/or connections, in the so-called brain plasticity. However, the successful repair odds are largely reduced by a hostile microenvironment and a weak capacity of post-mitotic neurons and neural stem cells to promote healing (Huebner and Strittmatter 2009; Sanchez-Mendoza and Hermann 2016). In this sense, neural repair (regain of lost brain functions) after injury can never happen or arise spontaneously, but it may take weeks, months or years after injury (Regenhardt et al. 2020). Usually, spontaneous neural repair is limited and has a small-time window, being more prone to success when allied to a treatment from days to weeks following stroke onset. The treatment should focus on therapies depending on the gene or molecule of interest (Cassidy and Cramer 2017). Neural outgrowth may be one of the principal targets that could change the course of the disorder, promoting the recovery of brain capacities. Many therapies being tested in clinical trials emerged focusing on different targets in the last few years. Their targets include growth factors, due to their main role in normal central nervous system development and spontaneous neural repair; monoclonal antibodies, capable of neutralizing central nervous system growth inhibitory molecules, such as myelin-associated glycoprotein, oligo-myelin glycoprotein, and Nogo-A; cell-based therapies, including transformed tumor cells and adult stem cells; and drugs, mainly small nonpolar molecules with high brain access, targeting mainly a specific brain neurotransmitter system, such as monoaminergic drugs, including amphetamines, neurotransmission modulating drugs in cholinergic pathways, and drugs that modulate the GABA or glutamate receptors (Cramer 2018).
In the last years, however, several studies have emerged to identify pharmacological interventions for these patients focusing on drugs that can accelerate the recovery of neurological functions without impacting others. However, they are still insufficient and inconsistent to establish an optimal pharmacological intervention (Talsky et al. 2011). Therefore, the most studied drugs include amphetamine psychostimulants such as amphetamine and methylphenidate; selective serotonin reuptake inhibitors antidepressants, such as sertraline (Meythaler et al. 2001), fluoxetine (Chollet et al. 2011; Stengler-Wenzke and Muller 2002), paroxetine and citalopram (Müller et al. 1999); antiparkinsonian drugs, such as amantadine (Saniova et al. 2004), bromocriptine (Crismon et al. 1988), and levodopa combined with carbidopa (Haig and Ruess 1990); cholinergic drugs such as donepezil (Barrett et al. 2011); N-methyl-d-aspartate receptor antagonists have been tested in clinical trials, including memantine (Trotman et al. 2015); anticonvulsants, like valproic acid (Wroblewski et al. 1997); citicoline an exogenous form of cytidine 5ʹ-diphosphate choline, which is essential for the biosynthesis of membrane phospholipids (Davalos et al. 2012); NA-1 (Tat-NR2B9c), an inhibitor of postsynaptic density-95 protein (Hill et al. 2012); maraviroc, an oral CCR5 antagonist currently approved for human immunodeficiency virus disease (Joy et al. 2019); drugs that inhibit axonal growth, and growth factors, have also been studied in preclinical studies (Kumar and Kitago 2019). Psychostimulants were tested in the rehabilitation of both traumatic brain injury and stroke (Kakehi and Tompkins 2021), as such they will be addressed herein.
5 Amphetamines
The term amphetamines refers to a group of synthetic psychostimulant compounds (Figure 1) with stimulant, anorectic, euphoric, empathogenic, entactogenic, and hallucinogenic properties (Carvalho et al. 2012). Their clinical effects also include: increased alertness, dependence, tolerance, increased blood pressure, tachycardia, insomnia, general malaise, migraine, anorexia, constipation, irritability, and impaired sexual potency in men (Wabe 2011). Their structure derives from β-phenylethylamine, differing only on the side chain substitutions (Carvalho et al. 2012). Substitutions at the aromatic ring, at the α and β carbons of the aliphatic chain, and the amine terminal of the amphetaminic structure originated a variety of amphetamines and determined their characteristics. For example, no substitutions on the amphetamine ring are related to psychomotor stimulant, sympathomimetic, antifatigue, and strong reinforcing effects. On the other hand, side-chain substitutions tend to promote psychomotor or anorectic effects, while the terminal amine substitution derivatives have psychomotor stimulant effects at low doses and hallucinogenic activity at higher doses. Their pharmacokinetics and pharmacodynamics are fundamental characteristics that facilitate their blood-brain barrier crossing, promote brain biotransformation resistance, and affect monoamine transporters and neurotransmitters (Carvalho et al. 2012).
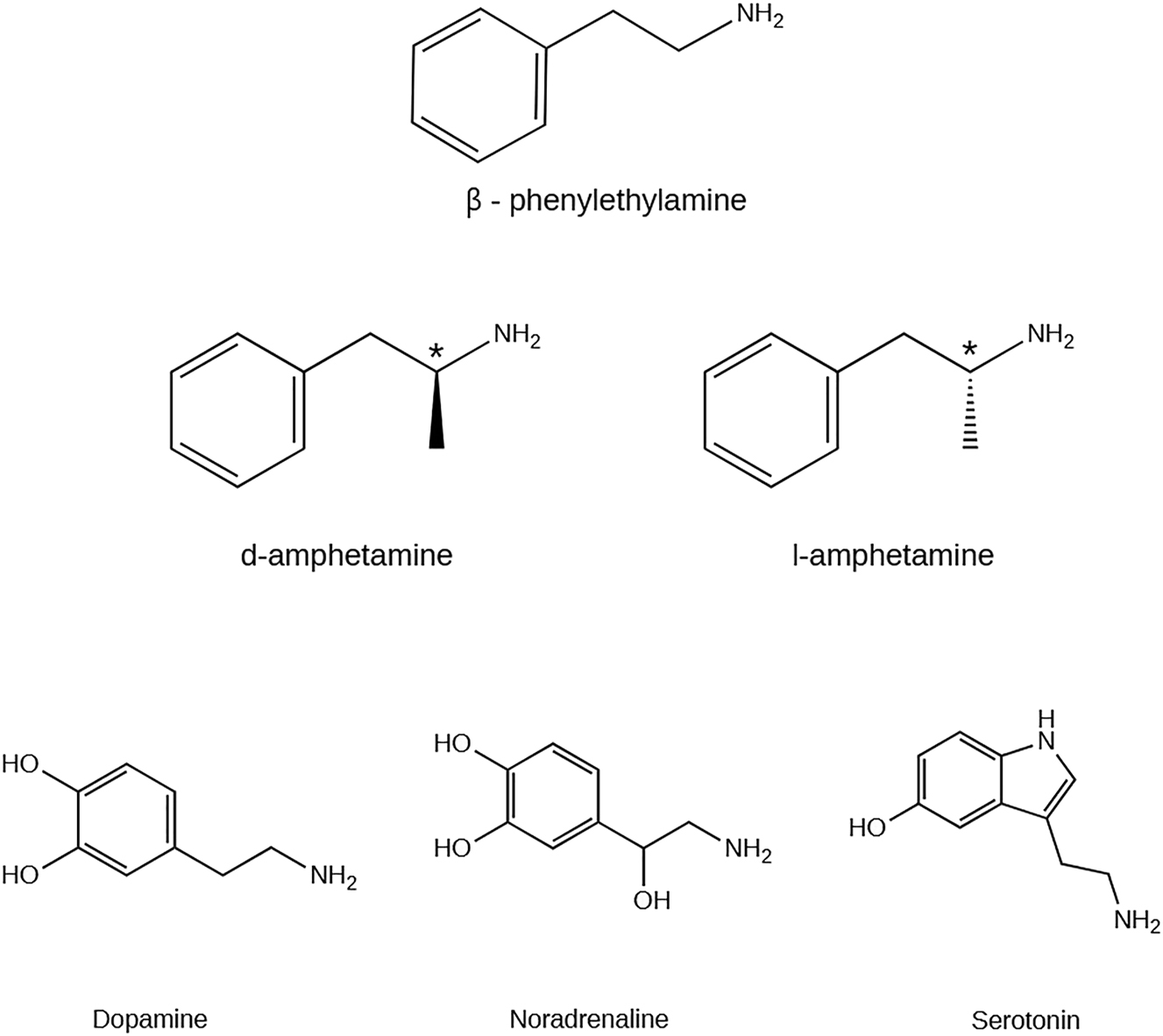
Chemical structure of β-phenylethylamine, amphetamine (AMPH) isomers (* represent the chiral carbon) and monoamines: dopamine (DA), noradrenaline (NA), and serotonin (5-HT). Created with ChemDraw version 20.0.
Over the past century, several amphetamine-like products emerged and were used to treat nasal congestion, depression, and obesity (Rasmussen 2008). However, its widespread use highlighted its harmful effects on addiction and health, and this drug was placed on the scheduled drug list (Rasmussen 2008). Considering recent events, there were 27 million global users of amphetamines, including methamphetamine and amphetamine, for non-medical use in 2019. It is also noteworthy that, in the same year, there was a record of 456 tons of global amphetamine-type stimulant seizures, which represents an increase of 64 % from the previous year (UNODC 2021). Controversially, their controlled therapeutic use has been proven to be effective, namely in diseases such as attention deficit hyperactivity disorder (Castells et al. 2018), cognitive disengagement syndrome (Wiggs et al. 2023), narcolepsy (Mitler and Hajdukovic 1991), Parkinson’s (Song et al. 2021) or even Alzheimer’s diseases (David et al. 2022).
5.1 Amphetamine
Amphetamine is known for its potent psychostimulant effect, which can increase alertness, blood pressure, self-confidence, wakefulness, sleeplessness, and vitality, thus producing arousal and insomnia. Additionally, this molecule acts as a respiratory, vasomotor system, and central nervous system stimulant, but also has an anti-spasmodic action on the smooth muscles of the gastrointestinal tract, can reverse drug-induced anesthesia, and decreases fatigue and appetite (Carvalho et al. 2012; de la Torre et al. 2004; Heal et al. 2013). Thus, it was and is still used in the treatment of nasal mucosa congestion in conditions such as coryza and vasomotor rhinitis, narcolepsy, mild depression, post-encephalitic Parkinsonism (Heal et al. 2013). The main use of amphetamine is in attention deficit hyperactivity disorder treatment (James et al. 2001), being approved by the U.S. Food and Drug Administration in 2001 (FDA 2001), as a mixture of amphetamine stereoisomer salts and inactive ingredients, known by the trade name Adderall™.
Structurally, amphetamine has an additional methyl group in the side chain of β-phenylethylamine. This methyl group has a main protector role from degradation by monoamine oxidase (MAO) and allows the entry and the persistence of amphetamine in the bloodstream (Iversen 2008). The single chiral center allows the existence of two optically active forms of amphetamine, the dextro- (d-) and levo- (l-) isomers or enantiomers, which show different pharmacological action and body distribution (Heal et al. 2013). Concerning d-amphetamine, previous research has established the main role of this isomer in dopamine activity by inducing the inhibition as well as the excitation of dopamine neurons (Shi et al. 2007).
After administration, which may be oral, intranasal, intravenous, or through smoking (Couper and Logan 2004), amphetamine is readily absorbed and distributed into most body tissues, with high concentrations occurring in the brain. d-amphetamine metabolism occurs mainly in the liver by N-deamination catalyzed by cytochrome P450 (CYP) isoenzymes of the CYP2C subfamily (Carvalho et al. 2012). Then, following oxidation into the corresponding benzoic acid derivates, they can be further conjugated with glycine and excreted as the corresponding hippuric acids (Figure 2). Another possibility is the hydroxylation in position four of the aromatic ring, and CYP2D6 is involved generating 4-hydroxyamphetamine that can be conjugated with sulfate or glucuronic acid and excreted (Carvalho et al. 2012). Roberts and coworkers described the pharmacokinetics of d-amphetamine as having an absorption rate constant of 0.527 h−1 (95 % CI 0.467–0.586), clearance of 28.7 L/h (95 % CI 27.1–30.3), and volume of distribution for the plasma compartment of 377 L (95 % CI 326–428), values that increase with the weight of the patient (Roberts et al. 2015). The half-life of d-amphetamine is 11.75 h, and a third of the drug is eliminated in the kidneys (Martinsson et al. 2003).
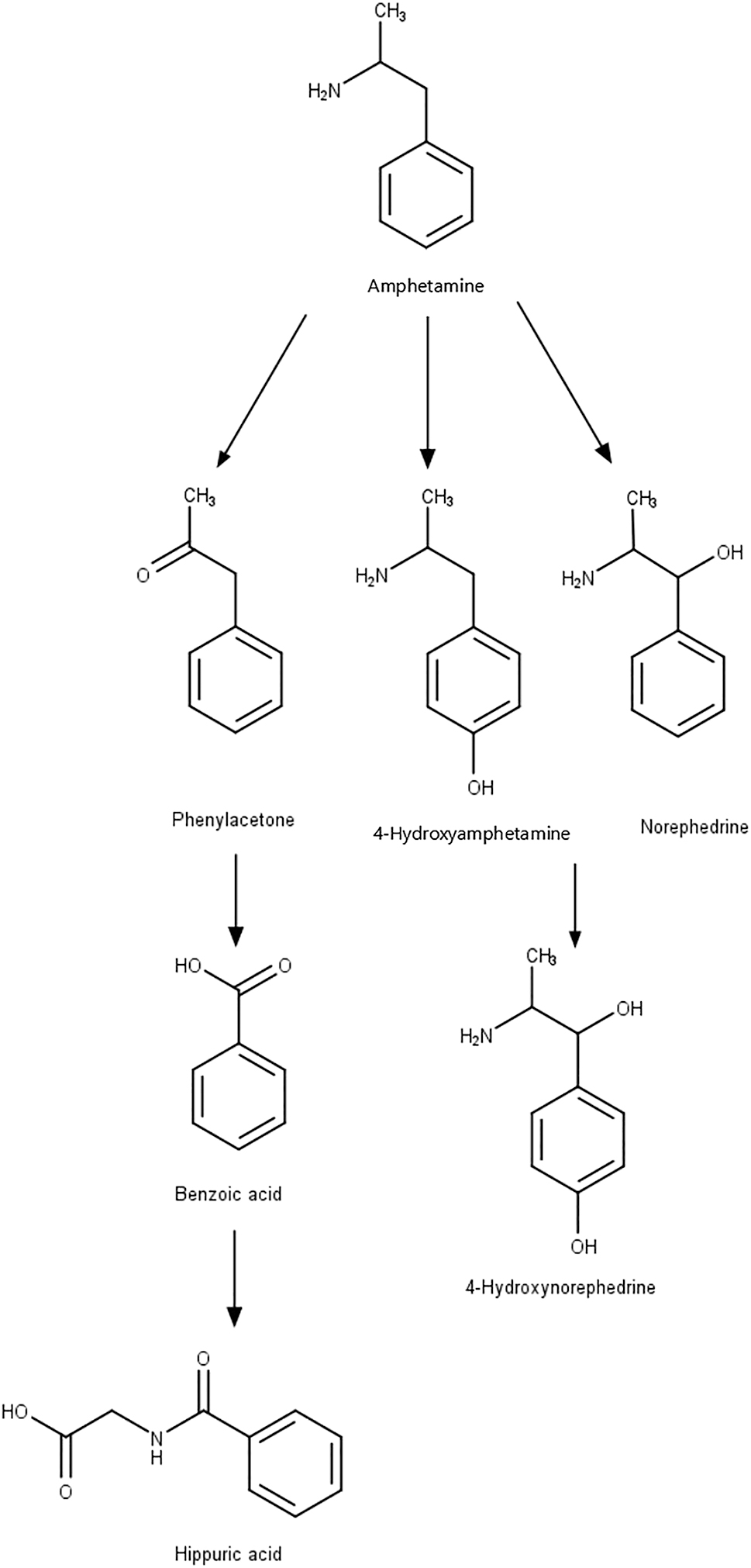
Metabolism of amphetamine (AMPH) and its main human metabolites. Created with ChemDraw version 20.0.
Nonionized and lipophilic characteristics usually facilitate the central nervous system entry by free diffusion of the compounds through the blood-brain barrier. However, around 99.7 % of the amphetamine plasma concentration is protonated and lipophobic at a pH of 7.4, due to amphetamine pKa of 9.9 (Gulaboski et al. 2007). However, Pardridge and colleagues found that d-amphetamine uptake across the blood-brain barrier occurs by a combination of two independent mechanisms, free diffusion and a carrier-mediated process, depending on saturability, pH, and competitive inhibition (Pardridge and Connor 1973).
Once in the brain, the action mechanism of d-amphetamine is still not fully understood. However, the similarity of its chemical structure with that of catecholamines and indoleamines neurotransmitters, namely noradrenaline, adrenaline, dopamine, serotonin, or tryptamine is a determining factor for its pharmacological effects (Ferrucci et al. 2019; Heal et al. 2013). Considering its’ similarity, amphetamine is a substrate of the plasma membrane transporter of the lateral column of the brainstem reticular formation, which possesses neuronal populations with specific neuronal phenotypes. There, amphetamine competes with neurotransmitters for the uptake through the noradrenaline, dopamine, or serotonin transporters into the pre-synaptic terminals (Ferrucci et al. 2019). The mechanism consists of the association of one molecule of monoamine transmitter or amphetamine with two Na+ and one Cl−, favored by the energy gradient produced by the active transport mechanism dependent on the Na+/K+ ATPase pump. The result is a molecular complex actively transported into the synaptic terminal by the respective monoamine reuptake transporter. Like monoamine regulation, the higher the concentration of amphetamine in the synapse, the higher the amount of amphetamine that is transported relative to the number of monoamines (Heal et al. 2013). Within the axoplasm, amphetamine has two possible targets. One of them is the vesicular monoamine transporter type-2 (VMAT-2), which is the monoamine transporter responsible for monoamine storage in a controlled, regulated, and concentration-dependent process. The amphetamine entry into the synaptic vesicles impairs the acidification by elevating the vesicular pH from four to 7. Since monoamines are weak bases charged at low pH values, the action of amphetamine induces their loss of charge, promoting their transport outside the vesicle and into the axoplasm, a process called reverse transport, consequently increasing their concentration in the extracellular space (Ferrucci et al. 2019; Heal et al. 2013). The other target is the mitochondrial-bound enzyme MAO, responsible for catabolizing the excess monoamines within the extracellular space by oxidative deamination. Amphetamine acts as an inhibitor of this enzyme, further increasing the concentration of neurotransmitters in the extracellular space (Ferrucci et al. 2019; Heal et al. 2013). Altogether, these mechanisms promote the amphetamine pharmacological effects, characterized by monoamine release promotion, along with inhibition of monoamine reuptake and MAO, which increase monoamine concentrations additively or synergistically (Figure 3) (Heal et al. 2013).
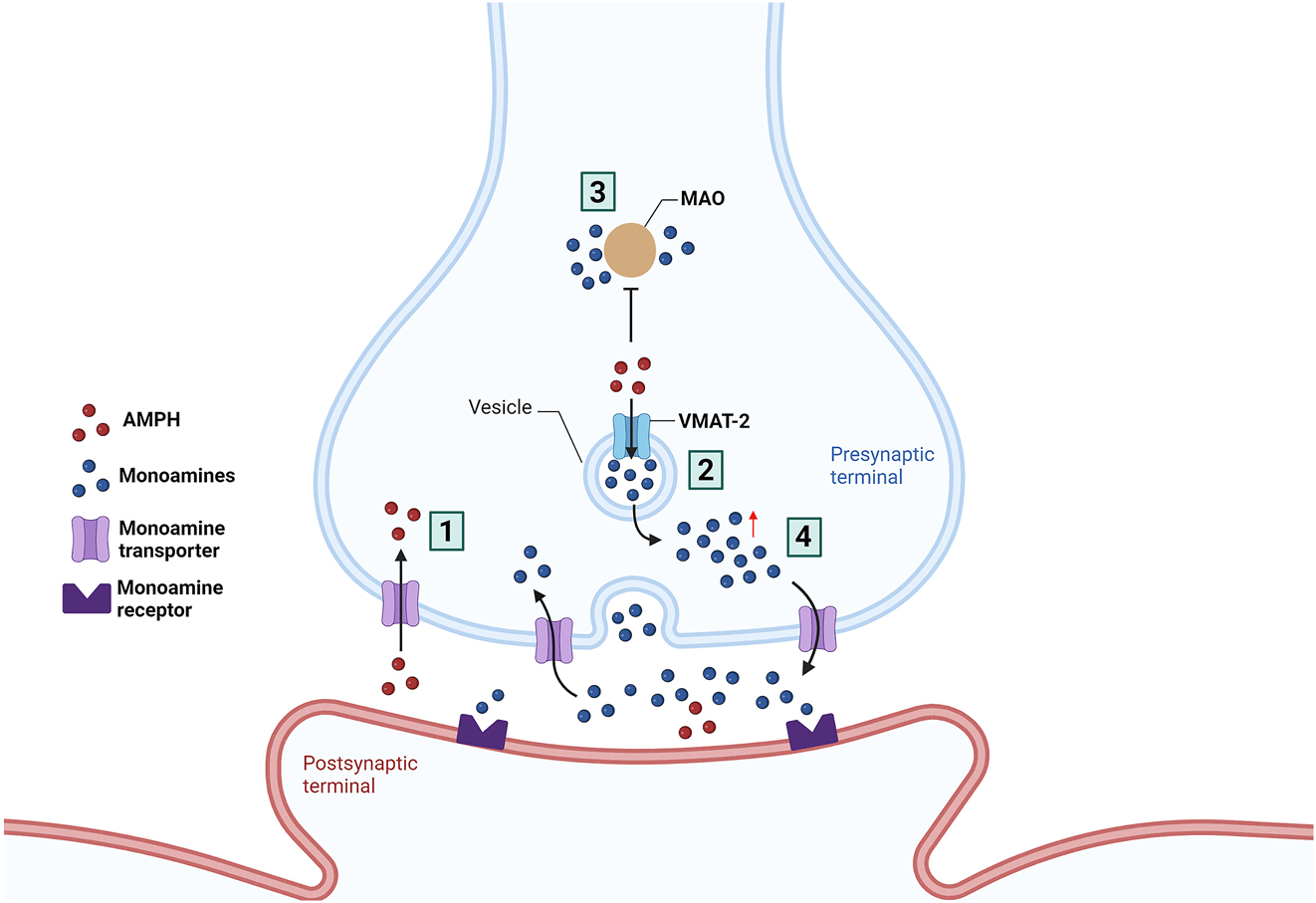
Mechanism of action of amphetamine (AMPH). AMPH inhibits: (1) monoamine transporters, transmembrane proteins that are responsible for the reuptake of dopamine, noradrenaline, and serotonin from the synaptic cleft to the presynaptic terminal and uses it to enter the neuron; (2) vesicular monoamine transporter-2 (VMAT-2), leading to increased monoamines release within synaptic vesicles increasing the cytoplasmic pool of neurotransmitters; (3) monoamine oxidase (MAO), causing an increase in presynaptic levels of monoamines. (4) Furthermore, its action leads to monoamine transporter transport reversal moving monoamines to the synaptic cleft. The significant increase in neurotransmitters in the synaptic cleft increases monoaminergic transmission. Image created using BioRender (www.biorender.com).
5.2 Methylphenidate
Methylphenidate is characterized by its stimulant effect on the central nervous system and is known for its benefits on attention deficit hyperactivity disorder. Methylphenidate promotes mainly improvements in the short term attention (Berger et al. 2018), vigilance, working memory, speed of processing, verbal and visual learning, memory, reasoning, and problem-solving (Linssen et al. 2014). Thus, Batistela and coworkers showed that these positive effects are only verified when cognitive processes are below an optimal level (Batistela et al. 2016). Due to its beneficial effects, methylphenidate is used mainly for attention deficit hyperactivity disorder (Grizenko et al. 2012; Matthijssen et al. 2019), but also for narcolepsy (Francisco and Ivanhoe 1996) since its approval by the U.S. Food and Drug Administration in 2002 (FDA 2022).
Regarding its structure, methylphenidate is a piperidine-derived with two chiral centers allowing the existence of four enantiomers (Challman and Lipsky 2000). However, previous research has established that the erythron isomers do not have any major central nervous system stimulant effect (Szporny and Görög 1961), therefore only the threo pair of enantiomers is used in clinical settings (Ferris and Tang 1979). Thus, the methylphenidate pharmaceutic form is composed of a racemic mixture of d-threo-methylphenidate and l-threo-methylphenidate, being the first enantiomer the most pharmacologically active (Patrick et al. 1987). The small size along the lipophilic characteristics of methylphenidate facilitates its direct cross through the blood-brain barrier by passive diffusion (Turowski and Kenny 2015).
The administration of methylphenidate can be oral, subcutaneous, intramuscular, or intravenous (Percheson et al. 1959). It is rapidly and almost thoroughly absorbed by the gastrointestinal tract and rapidly and gradually distributed into the kidney, lungs, brain, heart, and liver (Jaeschke et al. 2021). The pharmacokinetic characteristics of this drug depend on the pharmaceutical type used. However, pure methylphenidate peaks plasma concentrations are 10.8 and 7.8 ng/mL, reached about 2 h after administration, with an estimated half-life of 2–4.5 h (Arvidsson et al. 2020). In blood, methylphenidate and its metabolites are distributed between plasma and erythrocytes, despite the low plasma protein binding (Challman and Lipsky 2000) that together with the high solubility of methylphenidate provide a higher availability of this drug for central nervous system entrance (Kimko et al. 1999). Methylphenidate metabolism occurs by de-esterification catalyzed by carboxylesterase 1, forming the corresponding carboxylic acid metabolite, a pharmacologically inactive α-phenylpiperidine acetic acid (also known as ritalinic acid) (Kimko et al. 1999) (Figure 4). Moreover, its metabolism may also occur through aromatic hydroxylation, microsomal oxidation, or even conjugation, giving rise to the pharmacological inactive p-hydroxy-, oxo-, and conjugated metabolites, respectively (Faraj et al. 1974; Kimko et al. 1999). After metabolization, 78–97 % of methylphenidate is eliminated through urine, and the remaining in feces within 48–96 h, with a systemic clearance of 10.2 and 10.5 L/h/kg for children and adults after an oral dose, respectively, and 0.565 L/h/kg after an intravenous dose (Faraj et al. 1974; Kimko et al. 1999).
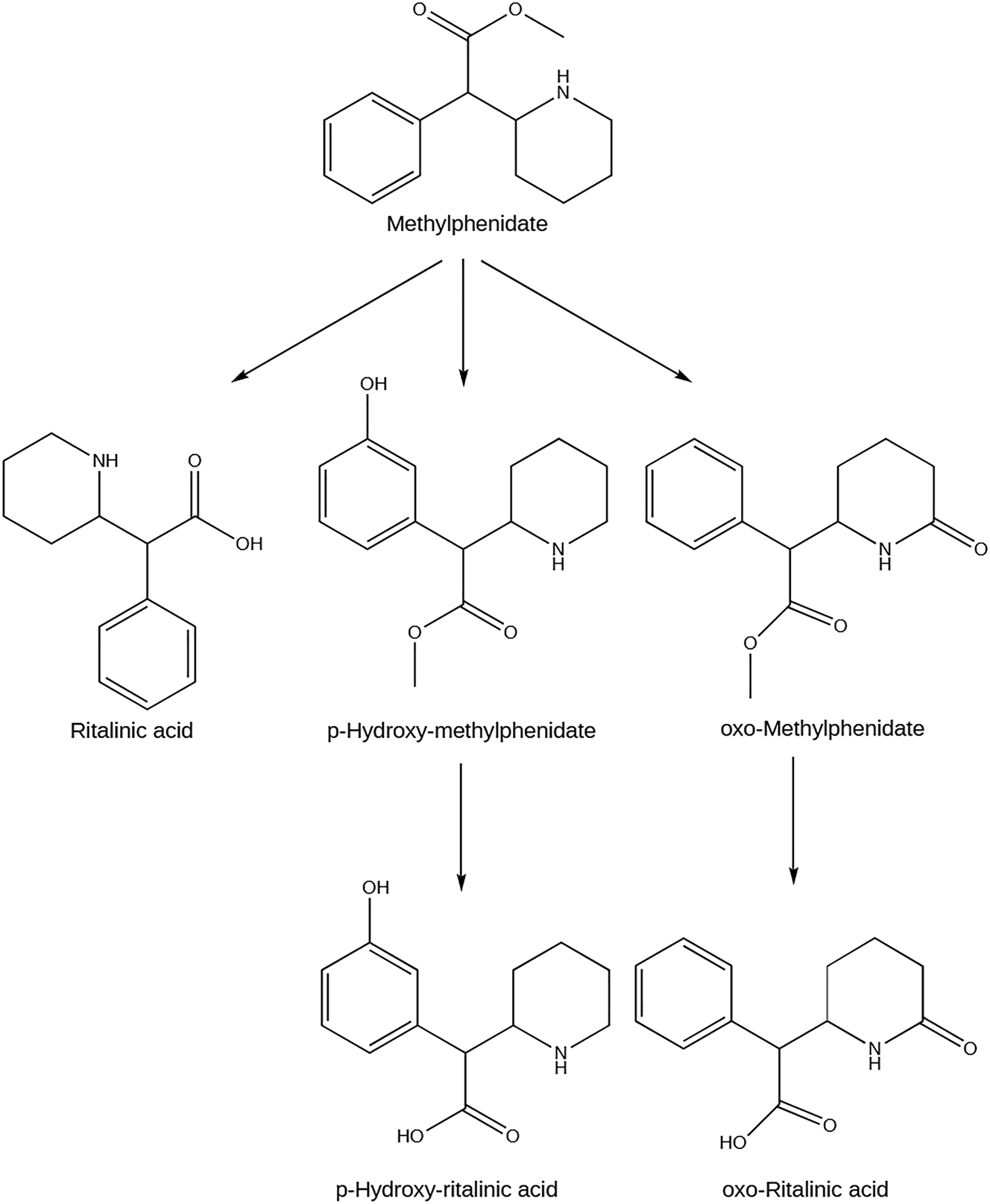
Metabolism of methylphenidate and its main human metabolites. Created with ChemDraw version 20.0.
Methylphenidate pharmacological activity focuses on the inhibition of dopamine and noradrenaline transport, namely in the prefrontal cortex and striatum, due to its partial similarity with the catecholamine structure (Jaeschke et al. 2021; Kodama et al. 2017). This inhibition can occur in two possible ways. The first relies on the methylphenidate capacity to bind and block the dopamine and noradrenaline transporters in the presynaptic cell membrane. Consequently, there is a block on the reuptake of these neurotransmitters, increasing their post-synaptic levels, and leading to a prolonged and/or intensified effect (Challman and Lipsky 2000; Jaeschke et al. 2021). The second is related to the effect of methylphenidate on VMAT-2. As previously described, VMAT-2 can move cytoplasmic dopamine into synaptic vesicles for storage and eventual exocytotic release (Harriott et al. 2018). Previous research showed that methylphenidate redistributes VMAT-2 and associated vesicles within nerve terminals away from the synaptosomal membranes and into the cytoplasm, consequently increasing the dopamine traffic, sequestration function, content, and exocytotic release function of synaptic vesicles (Riddle et al. 2007; Volz et al. 2008). The neurotransmitters increase after a therapeutic dose of methylphenidate enhances task-specific signaling, improving attention, and decreasing distractibility (Figure 5). These effects lead to improving performance (Volkow et al. 2001).
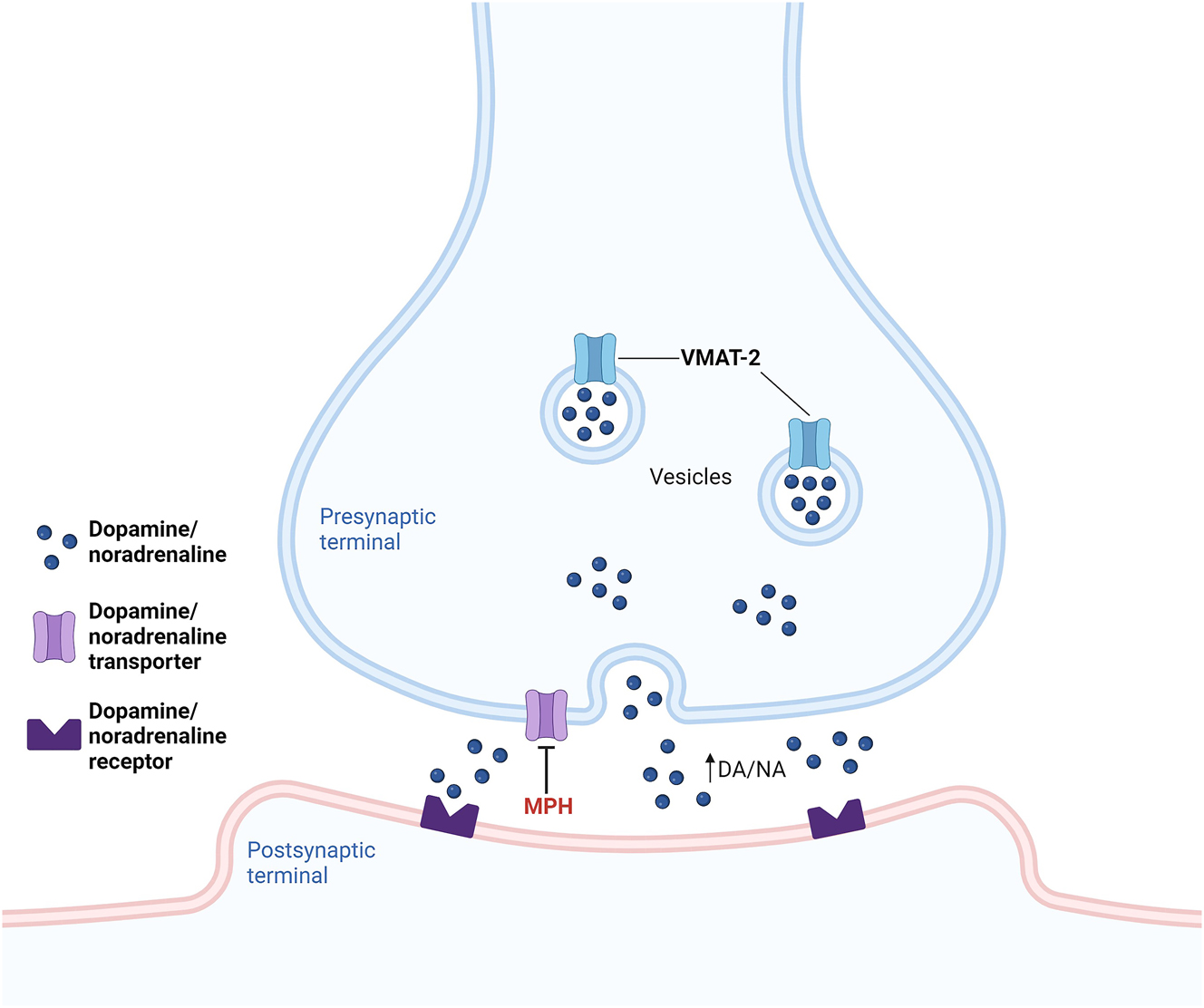
Mechanism of action of methylphenidate (MPH). MPH: (1) inhibits dopamine and noradrenaline transport by binding and blocking their transporters in the presynaptic cell membrane, blocking the reuptake of these neurotransmitters, increasing their post-synaptic levels, and leading to a prolonged and/or intensified effect; (2) redistributes vesicular monoamine transporter-2 (VMAT-2) and associated vesicles within nerve terminals away from the synaptosomal membranes and into the cytoplasm, increasing the dopamine (DA) and noradrenaline (NA) traffic, sequestration, content, and exocytotic release of synaptic vesicles. Image created using BioRender (www.biorender.com).
6 Research criteria
To address the amphetamine and methylphenidate effects after stroke or traumatic brain injury, a search on the PubMed database was performed and references were included from 1960 until November 2023 with the following keywords: ((methylphenidate) OR (Ritalin) OR (Concerta) OR (Biphentin) OR (amphetamine) OR (Adderall)) AND ((stroke) OR (brain injury) OR (neuroplasticity)). Despite neuroinflammation playing an important role in these brain diseases we excluded it as a keyword since most studies with the two drugs do not focus on inflammation or its outcomes. These keywords generated 508 articles, and not all were related to the effects of these drugs on the brain. To narrow the number of references, we excluded review and systematic review papers, getting to work with 372 articles. Not all of them were related to the topic of methylphenidate or amphetamine after a stroke or a traumatic brain injury, therefore further exclusions were taken. Thus, papers were excluded if they were about the effects of methylphenidate or amphetamine before injury or without a stroke or traumatic brain injury, also studies focusing on the evaluation of addiction to the drugs were not considered (Figure 6). A total of 91 papers on pre-clinical and clinical studies were included and their major findings will be mentioned starting with in vitro studies, then animal studies, and finally clinical trials.
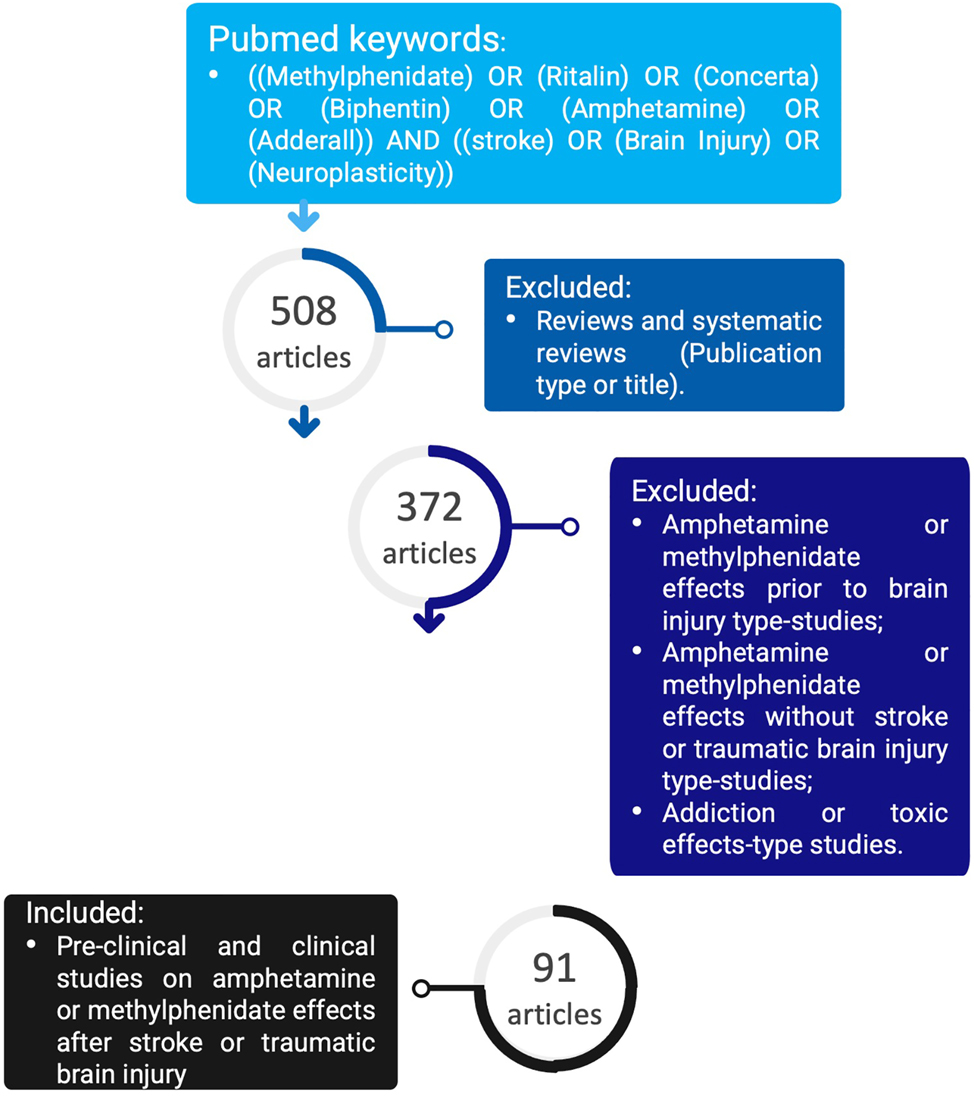
Number of articles reviewed, and the exclusion criteria taken to address the effects of methylphenidate or amphetamine after a stroke or a traumatic brain injury.
7 In vitro studies
To date, several studies have investigated the effects of amphetamine and methylphenidate after stroke or traumatic brain injury in in vivo studies, including in animals and humans. However, there is a big deficiency concerning the effect of these drugs using in vitro models, mostly due to the pathophysiological complexity of these neurological disorders that become harder to replicate in vitro. In this sense, authors use simplistic cell models, not containing the major neural cell types, and focus on some pathophysiological aspects related to these neurological disorders (Basit et al. 2022). Park and coworkers performed a study to investigate the role of protein kinases (protein kinase C, mitogen-activated protein kinase, and protein kinase A) on amphetamine-elicited neurite outgrowth and enhanced dopamine release in PC12 cells using the respective protein kinase-inhibitor. The treatment consisted of the respective kinase inhibitor administration for 30 min or 1 h previous to the administration of 1 μM of amphetamine for 5 min for 5 days, followed by 10 days of drug-free period. Cells were analyzed for neurite outgrowth and amphetamine-stimulated dopamine release. These results confirmed the initiation of the induction of these events by the activation of protein kinase C, maybe through the increase of intracellular Ca2+ that may induce its translocation and activation. Mitogen-activated protein kinase in response to amphetamine stimuli also showed to be involved in the enhancement of transporter-mediated dopamine release and neurite outgrowth, maybe by its activation following the activation of protein kinase C. Regarding protein kinase A, only dopamine release required this protein as a later effect of amphetamine action, suggesting the hypothesis that amphetamine promotes neurite outgrowth and transporter-mediated dopamine release enhancement by two different mechanisms (Park et al. 2003). Regarding methylphenidate, Grünblatt and colleagues used PC12 in a paradigm involving the exposure to methylphenidate (0.001, 0.01, 0.1, 1, 10, 100 μM) daily for 6 days followed by staining and evaluation on the seventh day. Moreover, they also used SH-SY5Y cells in a paradigm involving the exposure to methylphenidate (0.001, 0.01, 0.1, 1, 10, 100 μM) daily for 3 days followed by staining and evaluation on the fourth day. For both cell lines, methylphenidate induced neurite outgrowth and increased neurite length, related to the promotion of cell differentiation concomitantly to reduced proliferation at the cellular level (Grunblatt et al. 2018).
Although in vitro models lack the complexity of the human system, they can highlight the first paths to follow next. To sum up, our search revealed that both amphetamine and methylphenidate could promote neurite outgrowth through indirect mechanisms involving monoamine release enhancement. Still, the cellular mechanisms involved in this neurite outgrowth are not fully understood and further research is needed.
8 Animal studies
Pre-clinical studies with laboratory animals regarding the effect of amphetamine and methylphenidate after stroke or traumatic brain injury started following their introduction into the market. They were mostly concerned with promoting speech and language recovery following injury. One of these studies by Jonason and coworkers investigated the effects of amphetamine on relearning patterns and black-white discrimination following neocortical lesions in rats (Jonason et al. 1970). They started to train 33 male hooded Long-Evans rats (90–120 days old) for 4 days to perform either a pattern or black-white discrimination. On the day following training, the animals suffered bilateral posterior or bilateral anterior neocortical lesions. Three weeks following surgery, rats received six consecutive days of retraining with the administration of a daily injection of dl-amphetamine (1.0 mg/kg, i.p.) or saline from the second day of retraining. Amphetamine improved the performance of the animals that suffered injury. After the injury, the loss of the black-white discrimination habit was expected due to impaired contour vision. Knowing that a normal rat can use contour cues, flow cues, or both in learning a black-white discrimination habit, the loss and relearning of these habits after injury is likely due to cue-based learning, contour preoperatively and based on flow ranges postoperatively. In this case, amphetamine restores placement responses recovery from the use of contour cue may be the mechanism by which drug injections facilitate relearning of black-white discrimination (Jonason et al. 1970). Hovda and colleagues also studied the effect of amphetamine on locomotor function after an injury. After training on the beam, 32 mongrel cats (2.5–4.5 kg) were subjected to a unilateral frontal cortex injury followed by randomization into one of four groups. Each group received its respective treatment, which consisted of four saline injections every 4 days, starting 10 days after injury; or a single dose of amphetamine (5 mg/kg, i.p.) 10 days post-injury; or amphetamine (5 mg/kg, i.p.) injections every 4 days, starting 10 days post-injury. Amphetamine produced an immediate and enduring acceleration of beam-walking, although multiple administrations promoted a faster recovery (Hovda and Fenney 1984).
Methylphenidate was also investigated regarding neuronal injury. Kline and colleagues found a significant and facilitated improvement of spatial learning after a treatment that included training on day 0 post-injury followed by a daily injection of methylphenidate (5 mg/kg, i.p.) or saline, post-injury days 1–18, 15 min pre-testing to 24 male Sprague Dawley rats (275–325 g) which were subjected to a controlled cortical impact injury or sham surgery (Kline et al. 2000). More studies in vivo about the effects of amphetamine (Table 1) and methylphenidate (Table 2) in injured animals are presented in the tables.
Amphetamine (AMPH) effects reported in pre-clinical studies.
| Animals | Age and/or weight of animals | Injury | Extra procedures | Dose, administration route and schedule | Main results | Reference |
|---|---|---|---|---|---|---|
| 111 male albino rats. | 300–350 gr. | Unilateral motor cortex injury. | Yes. Before injury, rats received training along a narrow beam to escape white noise and bright light. | Injection of amphetamine (AMPH) (0.5 mg/kg, or 1 mg/kg, or 2 mg/kg, or 4 mg/kg, i.p.) or saline, randomly 24 h after injury. | Improvement.
|
Feeney et al. (1982) |
| 156 male Sprague-Dawley albino rats. | 60–100 days old (290–400 gr). | Right sensorimotor cortex or sham injury. | Yes. Before injury, rats received a single trial on the beam and beam-walking performance during the necessary days to achieve a pre-surgery score of 7 on 3 successive trials. | Injection of AMPH (2 mg/kg, i.p.) or saline, randomly 24 h after injury. | Improvement.
|
Sutton and Feeney (1992) |
| 21 male Long Evans rats. | 3 months old. | Bilateral frontal traumatic brain injury using controlled cortical impact or sham procedure, after reaching a stable baseline. | Yes. Before injury, rats were trained on a fixed interval 18 s schedule, following which they were placed on the peak interval procedures, with intermittent peak trials. | Injection of AMPH (1.0 mg/kg, i.p.), randomly given 8 weeks post-injury, followed by saline conditions on the following week or the inverse order. | Improvement.
|
Scott and Vonder Haar (2019) |
| 28 male Long Evans rats. | 120–180 days old. | Unilateral medial agranular cortex lesions by surgery to promote severe unilateral neglection. | No. | Injection of saline 60 minutes before injection of AMPH (2 mg/kg, i.p.) or saline treatment only, on post-injury days 0, 3 and 6. | Improvement.
|
Hylin et al. (2017) |
| Female Swiss Webster mice. | 21 days old. | Single mild traumatic brain injury or sham procedure. | No. | Injection of AMPH (2.5 mg/kg, i.p.) or saline on five consecutive days 7 weeks post-injury, and one final time 9 weeks post-injury. | Improvement.
|
Karelina et al. (2017) |
| 52 male Long Evans, black-hooded rats. | 250–300 gr. | Permanent middle cerebral artery occlusion. | Yes. After injury, rats received physical rehabilitation, represented by control environment, or enriched environment or enriched environment with additional sessions of focused activity. | Injection of AMPH (2 mg/kg, subcutaneously) or saline vehicle on post-injury days 2, 5 and 8. | Improvement.
|
Papadopoulos et al. (2009) |
| 93 male Sprague Dawley rats. | 400 gr. | Right middle cerebral artery embolization. | No. | Injection of AMPH (3.5 mg/kg, i.p.) or saline 10 min post-injury and injection of AMPH (1 mg/kg, i.p.) or saline on post-injury days 2, 5, 8 and 11. | Improvement.
|
Rasmussen et al. (2011) |
| 16 male albino Sprague Dawley rats. | 255–290 gr. | Cortex ablation or sham injury. | No. | Injection of AMPH (2 mg/kg, i.p.) or saline 24 h post-injury. | Improvement.
|
Sutton et al. (2000) |
| 48 male Sprague Dawley rats. | 285 gr. | Controlled cortical impact or sham injury. | Yes. After injury, rats were housed with or without access to a running wheel. | Injection of AMPH (1 mg/kg, via an ALZET® pump) or saline on post-injury days 0, 1, 2, 3, 4, 5 and 6. | Improvement.
|
Griesbach et al. (2008) |
| 12 male Sprague Dawley rats. | 360–400 gr. | Diffuse traumatic brain or sham injury. | No. | Injection of AMPH (5 mg/kg, subcutaneously) or saline 10 min pre-injury. | Improvement.
|
Byard et al. (2018) |
| 50 male Long Evans hooded rats. | 3–5 months old. | Left hemisphere medial agranular cortex destruction. | No. | Injection of AMPH (2 mg/kg, i.p.) or saline on post-injury days 0, 3 and 6; or post-injury days 2, 5 and 8; or post-injury days 7, 10 and 13. | Improvement.
|
Brenneman et al. (2015) |
| 45 male Long Evans black-hooded rats. | Adult. | Unilateral sensorimotor cortical aspiration lesion. | Yes. After the first injection, rats received rehabilitation or no rehabilitation. Rehabilitation was composed by enrichment environment and physiotherapy. | Injection of AMPH (2 mg/kg) or saline on post-injury days 2 and 5. | Improvement.
|
Ramic et al. (2006) |
| 22 male Lister hooded rats. | 3 months of age (230–260 gr). | Bilateral dorsal pre-frontal cortex or sham injury. | Yes. Before injury, rats received training on the combined attention-memory task. | Injection of AMPH (0.2 mg/kg, or 0.4 mg/kg, or 0.8 mg/kg, i.p.), or saline, 30 min pre-testing, on post-injury days 23, 26 and 29. | No overall improvement.
|
Chudasama et al. (2005) |
| 46 female, Long Evans hooded rats. | 112 days old. | Motor cortex stroke via pial removal. | Yes. Before injury, rats received pre-operative training. After injury, rats received daily rehabilitation in the reaching task paired with the treatment. |
AMPH (1 mg/kg, orally) added to a small amount (0.1 g) of chocolate chip cookie or pure cookie on post-injury days 1, 4, 7, 10, 13, 16, 19 and 22 (acute post-stroke phase); or AMPH (1 mg/kg, orally) added to a small amount (0.1 g) of chocolate chip cookie or pure cookie on post-injury days 42 and 45 (chronic post-stroke phase). | No significant improvement. | Alaverdashvili et al. (2007) |
| Male Sprague Dawley rats. | Adult. | Controlled distal middle cerebral artery occlusion (stroke model) or non-stroke model. | No. | Injection of AMPH (2 mg/kg, i.p.) or saline on every third post-injury day for 4 weeks. | Improvement.
|
Liu et al. (2011) |
| 74 male Sprague Dawley rats. | 2–3 months old (300–350 gr). | Right carotid embolization or sham operation. | Yes. Before injury, all rats received T-Maze and staircase training every day for 2–3 months. After injury, therapy group rats received physical therapy, including T-Maze and staircase training on post-injury days 1, 3, 5 and 7, started and finished between 1 and 2 h after drug administration. |
Injection of AMPH (2.5 mg/kg, i.p.) or saline on post-injury days 1, 3, 5 and 7. | Not overall improvement.
|
Rasmussen et al. (2006) |
| 48 male Sprague Dawley rats. | 245–320 gr. | Unilateral photochemical sensorimotor cortex or sham lesion. | Yes. Before injury, all rats received no aversive training in beam walking. After injury, the respective rats’ groups received a single session of motor training 24 h after lesion. |
Injection of AMPH (2 mg/kg, i.p.) or saline, 24 h post-injury. | No improvement.
|
Brown et al. (2004) |
| 41 male Wistar rats. | 250–400 gr. | Unilateral infarction of the left primary somatosensory cortex or sham operation. | Yes. Before injury, rats received T-maze training for a specific motor response. | Injection of AMPH (2 mg/kg, or 4 mg/kg, i.p.) or saline, 24 h pre-testing and on post-injury days 4, 6, 9 and 11. | Improvement.
|
Hurwitz et al. (1991) |
| Nonfasted male spontaneously hypertensive Wistar rats. | 260–300 gr. | Unilateral neocortical ischemia or sham injury. | No. | Injection of AMPH (2 mg/kg, i.p.) or saline vehicle on post injury days 3, 6, and 13 and every third day until day 30. | Improvement.
|
Stroemer et al. (1998) |
| 30 male Sprague Dawley rats. | 325–350 gr. | Lateral fluid percussion brain injury of moderate severity or sham injury. | No. | Injection of AMPH (4 mg/kg, i.p.) or saline 5 min post-injury. | Improvement.
|
Dhillon et al. (1998) |
| 60 female Mongolian gerbils. | 50–100 gr. | 3-min episode of forebrain ischemia or sham injury. | No. | Injection of saline right post-injury (saline group); or AMPH (1 mg/kg, or 2 mg/kg, i.p.) right post-injury to sham-operated group (sham group); or AMPH (1.0 mg/kg, i.p.) 24 h post-injury and 15 min pre-testing; or AMPH (1 mg/kg, i.p.) 24 h post-injury and post-testing. | No improvement.
|
Colbourne and Corbett (1992) |
| 48 male Sprague Dawley rats. | 325–350 gr. | Lateral fluid percussion brain injury of moderate severity or sham injury. | Yes. Before injury rats received training to perform the beam walking task. | Injection of AMPH (2 mg/kg, i.p. or 4 mg/kg, i.p.) or saline 10 min post-injury. | Improvement.
|
Prasad et al. (1995) |
| 48 male Sprague Dawley rats. | 176–200 gr. | Right cortical suction ablation lesion or sham injury. | Yes. Before injury, rats were trained to traverse the beam. | Injection of AMPH (2 mg/kg, i.p.) or saline 24 h post-injury. | Improvement.
|
Goldstein and Davis (1990) |
| 38 male Lister hooded rats. | 250–300 gr. | Focal cortical ischemic lesions or sham lesions. | No. | Injection of AMPH (2mg/kg, i.p.) or saline on post-injury day 2 and every third day thereafter until day 26. | Improvement.
|
Gilmour et al. (2005) |
| 6 male and 6 female adult squirrel monkeys. | 620–1,052 gr. | Complete ischemic infarct to the distal hand area in the primary motor cortex. | Yes. Before injury, monkeys were adapted to a reach and retrieval task requiring the skilled use of digits, wrist, and forearm. After injury, the respective monkey group received training for 14 consecutive days after drug administration. |
Injection of AMPH (0.25 mg/kg, i.p.) or saline on post-injury day 10. | Improvement.
|
Barbay et al. (2006) |
-
AMPH – amphetamine; i.p. – intraperitoneal; BDNF – brain-derived neurotrophic factor.
Methylphenidate (MPH) effects reported in pre-clinical studies.
| Animals | Age and/or weight of animals | Injury | Extra procedures | Dose, administration route and schedule | Main results | Reference |
|---|---|---|---|---|---|---|
| 48 male Sprague Dawley rats. | 300–325 gr. | Controlled cortical impact or sham injury. | Yes. Before injury, rats were subjected to a single day of beam walk training, which consisted of 3–5 trials to traverse the beam. After injury, the respective rats’ group received environmental enrichment. |
Injection of MPH (5 mg/kg, i.p.) or saline, daily on post-injury days 1–19, 15 min pre-testing. | No overall improvement.
|
Leary et al. (2017) |
| 48 male and 70 cycling female Sprague Dawley rats. | Males were 59.14 ± 3.17 days old and females were 79.8 ± 4.6 days old (302–369 gr). | Controlled cortical impact or sham injury. | No. | Injection of MPH (5 mg/kg, i.p.) or saline, daily on post-injury days 1–20, 15 min pre-testing. | Improvement.
|
Wagner et al. (2007) |
| 52 male, Sprague-Dawley rats. | Young adult. | Controlled cortical impact or sham injury. | No. | Injection of MPH (5 mg/kg, i.p.) or saline, daily on post-injury days 1–14. | Improvement.
|
Wagner et al. (2009) |
-
MPH – methylphenidate; i.p. – intraperitoneal; c-fos – striatal transcription factor.
Novel approaches designed to enhance recovery from stroke or traumatic brain injury were and still are being searched in animal models. A potential therapy may affect the levels of specific central neurotransmitters and influence the rate and the ultimate amount of functional recovery after injury. Moreover, the prolonged effect potentially provided by these drugs may be a key to the full and long-term definitive recovery.
The capacity of amphetamine to boost brain nerve growth is well-known. However, in the case of stroke and traumatic brain injury, acute or chronic amphetamine-induced recovery may be selective for some tasks, namely some behavior aspects (Alaverdashvili et al. 2007; Gilmour et al. 2005; Liu et al. 2011). Nonetheless, this dopaminergic drug has already been associated with enduring reversal of a widespread depression of glucose metabolism, probably promoted by a rapid diaschisis potentiating brain plasticity and recovery reversal (Dhillon et al. 1998; Rasmussen et al. 2011). Despite the dependency of sensitivity of subjects (Scott and Vonder Haar 2019), the lesion size (Liu et al. 2011; Papadopoulos et al. 2009), the lesion location and type (striatal vs. cortical lesion) (Brown et al. 2004; Liu et al. 2011; Rasmussen et al. 2011; Stroemer et al. 1998), but not of the blood pressure (Rasmussen et al. 2011), the accurate conditions for its clinical use on the recovery from these injuries are still under study. To identify it, a series of parameters must be established. Starting with the therapeutic window, the time course between injury and the beginning of treatment, the administration schedule, and doses. Time elapsed after injury to treatment is a dependent factor (Rasmussen et al. 2011; Scott and Vonder Haar 2019) that some researchers defend to be between 24 h and 1 week after the injury onset, whereas others extend it up until 10 days (Chudasama et al. 2005; Prasad et al. 1995), and even to 10–14 days in other studies (Gilmour et al. 2005). The administration schedule and dose are also key factors that generate discussion. Some studies argue that a multiple-schedule administration seems to generalize the improvement (Griesbach et al. 2008; Hovda and Fenney 1984; Hurwitz et al. 1991; Rasmussen et al. 2006), and Rasmussen and colleagues even defend a schedule of a high dose after acute stroke followed by a lower schedule dose (Rasmussen et al. 2011). However, Sutton and coworkers observed improvement with an acute (single) administration of amphetamine that attenuated the depression of the cerebral oxidative metabolism (Sutton et al. 2000). Regarding dose, a dose-response efficacy is suggested in the literature (Dhillon et al. 1998; Hurwitz et al. 1991), whereas the use of multiple injections of high dose amphetamine (Griesbach et al. 2008), as well as a better response from low-dose (0.2 mg/kg), when compared to a higher-dose (0.8 mg/kg), were also reported (Chudasama et al. 2005; Liu et al. 2011). Both the relevance and effectiveness of physical therapy and amphetamine treatment have been searched and shown to promote positive effects only when side by side with amphetamine administration (Ramic et al. 2006; Rasmussen et al. 2011). The combined treatment has been revealed to be even more effective than amphetamine alone, though it may have a limited effect (Colbourne and Corbett 1992; Griesbach et al. 2008; Papadopoulos et al. 2009). The final question remains regarding the duration of the treatment that influences the rehabilitation efficacy, as limited amphetamine treatment is likely to yield negative results (Papadopoulos et al. 2009). However, the amphetamine improvements seem to be prolonged in time (long-term efficacy) (Brenneman et al. 2015; Hovda and Fenney 1984), and a full recovery may be possible (Papadopoulos et al. 2009; Rasmussen et al. 2006).
As previously stated, methylphenidate is a common neurostimulator that promotes improvement of performance in cognitive processes despite scarce drug evaluation on experimental models. The few but important animal studies that evaluated methylphenidate seem to agree with the improvement promoted by the drug in these neurological disorders (Kline et al. 2000; Leary et al. 2017; Wagner et al. 2009; Wagner et al. 2007). Nonetheless, sex influenced the treatment response since female rats had increased improvement in tasks related to motor activation, whereas males had better performance in improving cognitive recovery. This difference may be related to higher dopamine levels in non-menopaused females, whose sex hormones, such as estrogens, play an important role in regulating homeostatic function in dopamine pathways, whereas males presented significant decreases in frontal cortex and striatal dopamine transporters levels. That may modulate the efficacy of methylphenidate treatment between sexes (Wagner et al. 2007). Also, drug dosage was an important factor for the injured rats’ improvement, and both methylphenidate and environmental enrichment paradigms were an asset to the improved therapy. Environmental enrichment may influence the overall effectiveness of methylphenidate treatment (Leary et al. 2017). Another significant aspect that emerged from these studies is the important role of dopamine, its pathways, and transporters in the recovery process of injured animals (Kline et al. 2000; Wagner et al. 2009). Methylphenidate was demonstrated to evoke striatal dopamine neurotransmission, with improvements in kinetic parameters. The restoration of dopamine neurotransmission induced by methylphenidate led to the alleviation of injury-related cognitive and motor deficits, maybe due to the important role of the striatum in cognition, as well as the role of dopamine in executive function, motor control, motivation, arousal, reinforcement, and reward through signaling (Wagner et al. 2009).
In general, no agreement concerning amphetamine and methylphenidate use has yet been reached in animal studies. However, all works contributed to the existing body of evidence by providing different perspectives bringing to the table new approaches, which should be considered and compared to generate a debate and further conclusions about possible clinical use. The final goal is to promote a better and improved lifestyle for stroke and traumatic brain injury patients. In this sense, the next chapter describes the clinical trials done on humans aiming to bring new insights into the therapeutic use of these drugs.
9 Clinical trials
The rationale for repurposing methylphenidate and amphetamine use after a stroke or a traumatic brain injury is, in part, attributable to their success in treating attention deficit hyperactivity disorder and narcolepsy, and also because these disorders share common cognitive, motor, and behavioral disturbances (Gillberg et al. 1997; Haertling et al. 2015). Based on the positive effects seen in animal studies, the use of psychostimulants for treating these disturbances has been investigated in clinical trials. The combined treatment with therapy pre and/or post and/or during drug administration has been evaluated in several clinical studies. To determine the effects of this type of treatment, Keser and colleagues subjected 10 chronic stroke patients (34–82 years old), 14–233 months after the injury onset, to a treatment that included the administration of amphetamine (10 mg, orally) and placebo, in a random order, on two sessions separated by 1 week of a washout, along with the simultaneous transcranial direct current stimulation and speech and language therapy, 30 min after drug administration. At the end of the treatment, which proved safe without serious adverse events, they found positive changes in speech and language performance (Keser et al. 2017). Despite this, clinical studies on the use of amphetamine and methylphenidate after traumatic brain injury and stroke are controversial. No improvement was reported by Sprigg and colleagues on a prospective, single-center, double-blind, randomized placebo-controlled phase II study with amphetamine. In detail, 33 stroke patients (33–88 years old), 3–30 days after stroke onset, received amphetamine (5 mg, initial dose, followed by 10 mg, orally) or placebo twice a week, with alternating 3/4 day apart, to a total of 11 doses for 35 days. Thirty-five and 90 days after the beginning of the treatment, the results were collected (Sprigg et al. 2007). After these two clinical studies, the differences between them enabled few conclusions including the dose of amphetamine, the treatment time, the time elapsed to treatment, the different therapies that accompanied the drug administration, and the results collection time.
Regarding methylphenidate, Johansson et al. tested its safety and its effects on mental fatigue and pain after a traumatic brain injury in a randomized, cross-over, open study. In detail, 24 subjects (18–65 years old) who suffered from moderate to mild traumatic brain injury for more than 12 months were involved. The treatment included three treatment periods of 4 weeks, in a balanced order according to the Latin square design: (a) no medication, (b) low dose scheme, (c) normal dose scheme. The low dose scheme consisted of 5 mg for the first week, 5 mg × 2 for the second, and 5 mg × 3 for the third and fourth, while the normal dose stood for 10 mg × 2 in the first week, 20 mg + 10 mg + 10 mg in the second, 20 mg + 20 mg + 10 mg in the third and 20 mg × 3 in the last week. After the outcome measures at the end of each treatment period, methylphenidate revealed safe, well-tolerated and promoted a dose-dependent improvement in mental fatigue, as well as in depression, and anxiety (Johansson et al. 2014).
In another study, the same authors reported methylphenidate safety and recommended its use starting with a low dose, which decreased mental fatigue in a dose-dependent treatment. This research corresponded to phase A of a prospective follow-up study involving 44 subjects (18–65 years) who suffered from mental fatigue and pain due to head trauma for more than 6 months. These patients were subjected to a 12 week study, including three randomized treatment periods of 4 weeks: no medication, administration of low-dose methylphenidate (up to 5 mg × 3) and normal dose methylphenidate (up to 20 mg × 3) (Johansson et al. 2015). To study the long-term effects of methylphenidate on mental fatigue, cognitive function, and safety, the same authors took a phase B study and included 30 participants who had reported positive effects with methylphenidate during phase A (Johansson et al. 2015) of this follow-up study (Johansson et al. 2017). After phase A, an individual optimal dose was defined, and most patients received 20 mg thrice daily of methylphenidate, except 7 patients who received a lower dose (one 15 mg/day, two 30 mg/day, two 40 mg/day, one 45 mg/day, and one 50 mg/day), and four patients who received a higher dose (two 70 mg/day, one 80 mg/day, and one 90 mg/day) during 6 months. Results after 6 months of methylphenidate treatment revealed significantly reduced mental fatigue, depression, and anxiety, improved attention, processing speed, and working memory, compared with the baseline, despite no differences found when compared with the final phase A results. The authors suggested that methylphenidate may be an option for long-term treatment of traumatic brain injury consequent effects (Johansson et al. 2017). 18 of the 30 patients in phase B (44.9 ± 10.4 years old) stayed to phase C of this clinical follow-up study (Johansson et al. 2018), which had an objective to evaluate the effects of a 4 week treatment break and compare both with and without the use of methylphenidate. Assessments at 22 months were undertaken 3 times for 8 weeks, starting with the first assessment during which the patient was on active treatment with methylphenidate. Then, 4 weeks of methylphenidate withdrawal, with a step-by-step reduction in doses for 4 days. After the withdrawal of 4 weeks, a second assessment was taken, followed by a new introduction of methylphenidate administration by a dose increase for 2 weeks to their original individual dose, and assessed again after 4 weeks. Compared with baseline (Johansson et al. 2015), methylphenidate decreased mental fatigue, depression, and anxiety and improved processing speed and working memory. During the 4 weeks of withdrawal, mental fatigue and cognition worsening were described, which improved after the methylphenidate treatment reintroduction. Thus, methylphenidate treatment does not seem to give rise to any long-term changes in cognitive function as the treatment effect was reversible (Johansson et al. 2018). This follow-up study continued to phase D, which intended to evaluate the long-term mental fatigue in patients with and without methylphenidate treatment (Johansson et al. 2020). Seventeen of the 18 patients of phase C (Johansson et al. 2018) continued to phase D and received methylphenidate (mean dose of 58.5 mg/day, orally) in a dose-adjusted individual for 5.5 years after baseline (Johansson et al. 2015). Their results were compared with those who had discontinued the treatment concerning baseline (Johansson et al. 2020). After 5.5 years, the group under the methylphenidate treatment showed improvements in mental fatigue, depression, and anxiety, while alterations were not verified in the group without methylphenidate regarding baseline. The results of all phases suggested that methylphenidate effects were stable and safe over the years, promoted overall improvement, and could be reversible if discontinued. Moreover, continued treatment can be a prerequisite for long-term improvement (Johansson et al. 2020).
Recent attention has been given to genetic conditions possibly related to the efficacy of these drugs. Willmott et al. investigated whether catechol-O-methyltransferase (Val158Met) allele status was associated with attentional performance and methylphenidate response following a traumatic brain injury in a randomized, cross-over, double-blind, placebo-controlled study. After a baseline assessment, 40 healthy controls and 32 moderate-severe traumatic brain injury patients were seen for six sessions over 2 weeks. The sessions were in three blocks: Days 1 and 2; Days 3 and 4; Days 5 and 6. One of each block session was assigned as methylphenidate (0.3 mg/kg, orally, twice daily) and the other as placebo. The results showed that catechol-O-methyltransferase allele status was not associated with attentional performance or response to methylphenidate in the traumatic brain injury group. The Met/Met group, whilst performing slowly, had relatively preserved strategic control of attention (Willmott et al. 2013).
To understand the best treatment conditions for possible therapy for these subjects, we compiled all clinical studies done in this research area and presented them in Tables 3 and 4. Although inconsistent, overall, these studies highlight that benefits could arise from the use of appropriate doses of amphetamine or methylphenidate for the treatment of stroke and traumatic brain injury both in the short- and long-term. However, to establish an appropriate therapy, there is a need for continuous research on patients who suffer from these disorders, knowing that even in the same injury multiple variables may influence the response. Therefore, future research should focus on phase II trials assessing data regarding the response to the treatment, including the right target population, dosages, and timing/schemes of treatment. Moreover, the continuous search for the mechanism(s) involved could help respond to these questions.
Amphetamine (AMPH) effects reported in clinical studies.
| Study design | Number of patients | Patient’s age | Drug treatment | Time interval between injury and treatment | Extra procedures | Measurement time | Main evidences | Reference |
|---|---|---|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled clinical study. | 14 stroke patients (9 male/5 female). | > 65 years. | Amphetamine (AMPH) (20 mg, orally) or placebo five times a week (every working day) for 2 weeks. | 5–10 days. | Yes. Patients received 30 min of physiotherapy 1 h after drug administration. | End of treatment and 3 months after stroke onset. | No overall improvement.
|
Sonde and Lokk (2007) |
| Chart review of AMPH treatment. | 27 brain-injured patients. | Between 15 and 75 years. | AMPH (5–30 mg/day, orally) during a 1–10 week course. | 7–99 weeks. | No. | Weekly during treatment. | Improvement.
|
Hornstein et al. (1996) |
| Randomized, placebo-controlled study. | 24 patients (19 male/5 female). | Between 45 and 67 years. | AMPH (10 mg/day, orally) or placebo every fourth day for 36 days. | < 6 weeks. | Yes. Patients received 45 min of physical therapy, five times a week during the study period of 36 days. | At days 0, 20, 36, 90 and 360 after the beginning of treatment. | No improvement.
|
Treig et al. (2003) |
| Randomized, double-blind, placebo-controlled clinical study. | 39 stroke patients with a paretic arm and/or leg (23 male/16 female). | Between 66 and 91 years. | AMPH (2 × 5 mg, orally) or placebo twice a week for 5 weeks. | 5–10 days. | Yes. Patients received training sessions, five days a week, 60 min after drug intake. | End of the treatment and 3 months after stroke. | No improvement.
|
Sonde et al. (2001) |
| Randomized, double-blind, placebo-controlled study. | 16 stroke patients (8 male/8 female). | Between 44 and 83 years. | AMPH (10 mg/day, orally) or placebo 2 days per week for 5 weeks. | 14–60 days. | Yes. Patients received physiotherapy 1 h after drug administration and once or twice a week for 30–45 min during the follow-up period. | Every week during the treatment and at follow-up 1 week, 6 months, and 12 months after treatment. | Improvement.
|
Schuster et al. (2011) |
| Randomized, double-blind pilot study | 8 cerebral infarction patients (7 male/1 female). | Between 47 and 73 years. | AMPH (10 mg, orally) or placebo. | 3–10 days. | Yes. Patients received 45 min of physical therapy, within 3 h of drug administration. | 24 h after treatment. | Improvement.
|
Crisostomo et al. (1988) |
| Randomized double-blind, placebo-controlled study. | 6 acute no-hemorrhagic stroke patients (2 male/4 female). | Between 41 and 71 years. | AMPH (10 mg, orally) or placebo, every third/fourth day for 10 sessions over 5 weeks. | 16–45 days. | Yes. Patients received 1 h of speech and language therapy, 30 min after drug administration. | 7 days after the end of the treatment and 6 months after stroke onset. | Improvement.
|
Walker-Batson et al. (1992) |
| Randomized, double-blind, placebo-controlled, repeated-measures study. | 66 acute unilateral cerebral hemispheric stroke patients (35 male/31 female). | Between 51 and 84 years. | AMPH (10 mg, orally) or placebo every third/fourth day for 10 sessions over 5 weeks. | 5–10 days. | Yes. Patients received 1 h of physiotherapy session, 90 min after drug administration. | End of each of the 10 treatment sessions, 3–4 days after the final treatment, and 3 months after the end of the treatment. | No improvement.
|
Gladstone et al. (2006) |
| Randomized, double-blind study. | 21 acute no hemorrhagic stroke patients (13 male/8 female). | Between 41 and 71 years. | AMPH (10 mg/day, orally) or placebo on every third/fourth day for 10 sessions. | 16–45 days. | Yes. Patients received 1 h of speech/language therapy, 30 min after drug administration. | 1 week after treatment and 6 months after onset. | Improvement.
|
Walker-Batson et al. (2001) |
| Randomized, double-blind, controlled dose-escalation study. | 45 acute ischemic stroke patients (26 male/19 female). | Between 18 and 85 years. | AMPH (2.5 mg twice daily, orally) or AMPH (5 mg twice daily, orally) AMPH (10 mg twice daily, orally) or placebo for 5 consecutive days. | ≤ 72 h. | No. | 7 days, 1 and 3 months after stroke. | Improvement.
|
Martinsson and Wahlgren (2003) |
| Pilot, double-blind, block-randomized clinical study. | 64 cortical or subcortical ischemic stroke patients (35 male/29 female). | Between 27 and 91 years. | AMPH (10 mg/day, orally) or placebo every fourth day for a total of 6 sessions. | 10–30 days. | Yes. Patients received 1 h of physical therapy session, 1 h after drug administration. | 3 months after stroke. | No improvement.
|
Goldstein et al. (2018) |
Methylphenidate (MPH) effects reported in clinical studies.
| Study design | Number of patients | Patient’s age | Drug treatment | Time interval between injury and treatment | Extra procedures | Measurement time | Main evidences | Reference |
|---|---|---|---|---|---|---|---|---|
| Randomized, pretest, posttest, placebo-controlled, single-blind study. | 12 traumatic brain injury patients. | Between 17 and 75 years old. | Methylphenidate (MPH) (0.3 mg/kg, orally) twice daily or placebo twice daily for 2 days. | 1–8 years | No. | Weekly during treatment. | No overall improvement.
|
Whyte et al. (1997) |
| Random, double-blind, placebo-controlled, dose-response study. | 1 traumatic brain injury patient (1 male). | 21 years old. | MPH (0.15 mg/kg, orally) and MPH (0.3 mg/kg, orally) and two placebo periods twice daily. Each treatment condition lasted 1 week in a randomized order. | 2 years | No. | Daily, 1–3 h after administration and 2 and 6 weeks after treatment. | Improvement.
|
Evans et al. (1987) |
| Double-blind, placebo-controlled experimental medicine study. | 14 healthy controls and 14 traumatic brain injury patients (11 male/3 female). | Between 19 and 58 years old. | MPH (30 mg, orally) or placebo. | > 6 months. | No. | Beginning and 75 min after treatment. | Improvement.
|
Dorer et al. (2018) |
| Randomized, placebo-controlled, double-blind, parallel pilot study. | 10 mild to severe traumatic brain injury patients with reduced processing speed and/or attention deficits (6 male/4 female). | Between 16 and 65 years. | MPH (0.6 mg/kg/day, orally) or placebo at eighth o’clock, during seven weeks. | Not mentioned. | No. | Week 7 (on-drug), week 8 (off-drug) and 9 months after treatment. | No improvement.
|
Dymowski et al. (2017) |
| Double-blind, placebo-controlled, 2 × 2 factorial design study. | 71 traumatic brain injury patients with either objective cognitive deficits or subjective cognitive complaints (49 male/22 female). | Between 18 and 65 years. | MPH (0.1 mg/kg twice daily, orally) or placebo for 2–4 days, then MPH (0.2 mg/kg twice daily, orally) or placebo for 2–4 days, then MPH (0.3 mg/kg twice daily, orally) or placebo until complete 6 weeks of treatment. | > 5 months and < 35 years. | Yes. During treatment patients received a metacognitive intervention, memory and attention adaptation training, or attention builders training. | After 6 weeks of treatment. | Improvement.
|
McDonald et al. (2017) |
| Double-blind, placebo-controlled, repeated crossover study. | 34 moderate to severe traumatic brain injury patients with attention complaints (29 male/5 female). | Between 16 and 60 years. | MPH (0.3 mg/kg/dose twice daily, orally) for 3 weeks interspersed with 1 week of placebo between each (i.e., MPH, placebo, MPH, placebo, MPH, placebo or placebo, MPH, placebo, MPH, placebo, MPH). | > 3 months. | No. | Weekly during treatment. | Improvement.
|
Whyte et al. (2004) |
| Double-blinded, randomized, crossover, placebo-controlled designed study. | 50 healthy controls (13 male/8 female) and 15 traumatic brain injury patients (12 male/5 female). | Between 18 and 60 years. | MPH (30 mg, orally) and 2–4 weeks later placebo or vice versa. | > 6 months. | No. | 75 min after treatment. | Improvement.
|
Manktelow et al. (2017) |
| Randomized, double-blind, placebo-controlled, crossover study. | 40 moderate-severe traumatic brain injury patients with cognitive impairment (34 male/6 female). | Between 20 and 65 years. | MPH (0.3 mg/kg twice daily, orally) for 2 weeks, and placebo for the following 2 weeks or vice versa. | > 3 months. | No. | Daily (between 1 and 4 h after drug administration) and at the end of each 2-week treatment period. | Not overall improvement.
|
Jenkins et al. (2019) |
| Randomized, double-masked, placebo-controlled cross-over clinical study. | 20 moderate-to-severe blunt head trauma patients (15 male/5 female). | Between 6 and 17 years. | MPH (18/27/36 mg for patients under 25 kg; 18/36/54 mg for patients above 25 kg, orally) for 4 weeks, and placebo for the following 4 weeks or vice versa. | > 6 months. | No. | Daily and at the end of each 4-week treatment period. | Improvement.
|
LeBlond et al. (2019) |
| Randomized, double-blind, placebo-controlled. | 39 ischemic stroke patients with a paretic arm and/or leg (23 male/16 female). | Not mentioned. | MPH (10 mg twice daily, orally) or placebo, 5 days a week for 3 weeks. | 15–180 days. | Yes. Patients received 45 min of physical therapy sessions, at least 60 min after drug administration. | 6 months after treatment. | Improvement.
|
Lokk et al. (2011) |
| Preliminary study. | 20 traumatic brain injury children with a clinically significant attention deficit and/or hyperactivity-impulsivity symptoms (15 male/5 female). | Between 6 and 18 years. | MPH (5 mg twice daily, orally) with a gradually increasing until achieve a maximum MPH dosage (10 mg twice daily, orally) during the first week, and MPH (10 mg thrice daily, orally) during the second week. | 1–4 years. | No. | 8 weeks after treatment. | Improvement. | Ekinci et al. (2017) |
| Randomized, double-blind, placebo-controlled trial with a 2 × 2 factorial study. | 39 ischemic stroke patients (23 male/16 female). | Between 53 and 75 years. | MPH (10 mg twice daily, orally) or placebo, 5 days a week for 3 weeks. | 15–180 days. | Yes. Patients received daily 45 min of physical therapy sessions, 60 min after drug administration. | 15, 90 and 180 days after treatment. | No improvement.
|
Delbari et al. (2011) |
| Not mentioned. | 28 stroke patients with depression (12 male/16 female). | 73.67 ± 7.44 years. | MPH (10 mg/day, orally) for at least 5 consecutive days. | 10.00 ± 6.67 months. | No. | Over 15 days after treatment. | Improvement.
|
Lazarus et al. (1994) |
| Double-blinded randomized placebo-controlled study. | 36 traumatic brain injury patients with mental sequelae, including mental fatigue and cognitive impairment (27 male/9 female). | Between 18 and 65 years. | MPH (2.5 mg twice daily, orally) with a gradual increase of 2.5 mg/day until achieving a maximum MPH dosage (10 mg twice daily, orally) or placebo for 30 weeks. | 2–52 weeks. | No. | End of the treatment. | Improvement.
|
Zhang and Wang (2017) |
| Randomized, blinded study. | 9 post-acute ischemic stroke patients (7 male/2 female). | 52.9 ± 11.9 years. | MPH (20 mg, orally) or placebo. | 1 month. | Yes. Patients received noninvasive transcranial direct current stimulation at 1 mA for 20 min over the primary motor cortex or sham transcranial direct current stimulation for 10 s, 1 h after drug administration. | End of the treatment. | Not overall improvement.
|
Wang et al. (2014) |
| Randomized, double-blinded, placebo-controlled study. | 9 mild traumatic brain injury patients with clinically significant cognitive complaints (5 male/4 female). | Between 18 and 55 years. | Placebo or MPH (5 mg twice daily, orally) at first 4 weeks, MPH (10 mg twice daily, orally) between weeks 4 and 8 of the treatment, and MPH (20 mg, twice daily, orally) between weeks 8 and 12 of the treatment. Finally, MPH (10 mg twice daily, orally) at 13 week and MPH (5 mg twice daily, orally) at week 14, and then discontinued. | Not mentioned. | No. | During treatment at weeks 4 and 8, at the end of the treatment. | Improvement.
|
McAllister et al. (2016) |
| Not mentioned. | 10 traumatic brain injury patients (7 male/3 female). | Between 3 and 16 years. | Not mentioned. | > 2 months. | No. | End of the treatment. | Improvement.
|
Hornyak et al. (1997) |
| Multicenter, randomized, double-blind, placebo-controlled study. | 21 right-handed, ischemic, or hemorrhagic right stroke patients with spatial neglect (17 male/4 female). | Between 18 and 80 years. | MPH (10 mg, twice daily, orally) or placebo between day 11 and day 15. | 1–18 months | Yes. During the treatment, patients received four sessions of prism adaptation on days 12, 13, 14 and 15. | Days 15, 22 and 45. | Improvement.
|
Luaute et al. (2018) |
| Double-blind, placebo-controlled, comparative drug study. | 20 mild to moderate traumatic brain injury patients (16 male/4 female). | Between 18 and 55 years. | Placebo or MPH (twice daily, orally) with increasing doses increasing 1.25 mg every day, during seven days. The starting dose was 2.5 mg, ending with 10 mg on the seventh day. | > 2–52 weeks. | No. | At the beginning, during and at the end of treatment. | Improvement.
|
Lee et al. (2005) |
| Randomized, double-blind crossover study. | 14 chronic traumatic brain injury patients and 20 healthy controls (10 male/4 female). | Between 22 and 53 years. | MPH (30 mg, orally) and 2–4 weeks later placebo or vice versa. | > 7 months. | No. | 1.5 h after drug administration. | Improvement.
|
Moreno-López et al. (2017) |
| Randomized. | 6 traumatic brain injury patients (4 male/2 female). | Between 18 and 47 years. | MPH (5 mg/day, orally, starting dose) with a gradual increase until achieving a maximum MPH dosage (10 mg/day, orally) over two weeks, followed by a similar two-week period of gradually diminishing dosage. | Not mentioned. | No. | 2 weeks after the beginning of the treatment, and twice after the end of treatment. | Improvement.
|
Pavlovskaya et al. (2007) |
| Double-blind, placebo-controlled design study. | 9 moderate to severe traumatic brain injury patients (8 male/1 female). | Not mentioned. | MPH (15 mg twice daily, orally) for 28 days. | 3–18 months. | No. | 4 h after treatment. | Improvement.
|
Newsome et al. (2009) |
| Randomized, crossover, double-blind, placebo-controlled study. | 40 moderate to severe traumatic brain injury patients (28 male/12 female). | Between 16 and 60 years. | MPH (0.3 mg/kg twice daily, orally) in three of the six sessions alternating with placebo in the other three sessions. | 4–133 days. | No. | 90–120 min after drug administration. | Improvement.
|
Willmott and Ponsford (2009) |
| Not mentioned. | 14 patients with an impaired consciousness after an acquired brain injury resulting from traumatic or nontraumatic causes (9 male/5 female). | Between 15 and 65 years. | MPH (0.3 mg/kg/day twice daily, orally) for 6 weeks. | 24–78 days. | No. | After 6 weeks of treatment. | Improvement.
|
Kim et al. (2009) |
| Randomized double-blind placebo-controlled study. | 18 chronic traumatic brain injury patients with cognitive impairments (16 male/2 female). | Between 18 and 49 years. | MPH (20 mg, orally) or placebo. | 1–7 years. | No. | 2 h and 2 days after treatment. | Improvement.
|
Kim et al. (2006) |
| Randomized, cross-over, double-blind, placebo-controlled study. | 8 patients with a single stroke on the corticospinal tract resulting in a pure motor hemiparesis (8 male). | Between 46 and 70 years. | MPH (20 mg, orally) on the first day and placebo on the seventh day or vice versa. | 17 days. | No. | 2 h after each drug administration. | Improvement.
|
Tardy et al. (2006) |
| Prospective multiple baseline design (A-A-B-A). | 10 acutely brain-injured patients (8 male/2 female). | Between 25 and 82 years. | MPH (2.5 mg/day twice daily, orally, starting dose) with a gradually increasing dosage of 2.5 mg until achieving a maximum MPH dosage (15 mg/day twice daily, orally) over one week. | 4–71 days. | No. | End and 7 days after treatment. | Improvement.
|
Kaelin et al. (1996) |
| Double-blind placebo-controlled with random assignment study. | 23 moderately severe traumatic brain injury patients (17 male/6 female). | Between 16 and 65 years. | MPH (0.3 mg/kg twice daily, orally) or placebo for 30 days. | Not mentioned. | No. | End and 60 days after the treatment. | Not overall improvement.
|
Plenger et al. (1996) |
| Randomized, double-blind placebo-controlled study. | 23 moderate to severe traumatic brain injury patients (18 male/5 female). | Between 16 and 60. | MPH (0.3 mg/kg, orally) on the first day of testing and placebo on the second or vice versa. | > 3 months. | No. | One hour after drug administration. | Improvement.
|
Kim et al. (2012) |
| Randomized, double-blind placebo-controlled study. | 30 traumatic brain injury patients (23 male/7 female). | Between 18 and 51 years. | MPH (2.5–5 mg, twice daily, orally) or placebo for 10 days. | Not mentioned. | No. | 24 h a day for at least 10 days during treatment. | Not overall improvement.
|
Al-Adawi et al. (2006) |
| Prospective, randomized, double-blind, placebo- controlled study. | 20 one stroke patients (11 male/10 female). | Between 66 and 77 years. | Placebo or MPH (2.5 mg twice daily, orally, starting dose) with a gradually increasing dosage at every 3 days, until achieving a maximum MPH dosage (15 mg twice daily, orally) over one-three weeks. | 14–23 days. | Yes. Patients received physical therapy during treatment. | Weekly during and thereafter treatment. | Improvement.
|
Grade et al. (1998) |
| Randomized, double-blind, placebo-controlled study. | 9 post-stroke depression patients and nine healthy controls (4 male/5 female). | Between 40 and 70 years. | MPH (20 mg/day, orally) or placebo for 3 days. | 2–19 months. | No. | 4 h after drug administration. | Improvement.
|
Ramasubbu and Goodyear (2008) |
| Placebo-controlled and double-blind, with a cross-over and random assignment study. | 15 closed head injury patients (10 male/5 female). | Between 12 and 44 years. | MPH (0.15 mg/kg twice daily, orally) and MPH (0.3 mg/kg twice daily, orally) and placebo in a randomized order during 12 days with a 2-day washout between conditions. | 5–144 months. | No. | 1 h after drug administration and 1 year after treatment. | Not overall improvement.
|
Gualtieri and Evans (1988) |
| Uncontrolled, open-label design study. | 24 traumatic brain injury patients (13 male/11 female). | Between 18 and 35 years. | MPH (5 mg/day orally, starting dose) with a gradually increasing dosage, until achieving a maximum MPH dosage (10 mg/day, orally) for 30 days. | < 2 months (9 acute traumatic brain injury patients) or ≥ 2 months (15 chronic traumatic brain injury patients). | No. | 0, 15 and 30 days after the treatment. | Improvement.
|
Al-Adawi et al. (2020) |
| Double-blind, placebo-controlled, randomized, crossover study. | 12 moderate to severe chronic closed head injury patients (5 male/7 female). | Between 21 and 43 years. | MPH (0.3 mg/kg twice daily, orally) for one week and placebo on the following week or vice versa. | 14–108 months. | No. | End of the first and the second weeks of treatment. | No improvement.
|
Speech et al. (1993) |
| Randomized, pretest, posttest, control group study. | 38 traumatic brain injury patients (38 male). | Between 18 and 50 years. | MPH (30 mg/day, orally) or placebo for 6 weeks. | > 6 months. | No. | End of the treatment. | Improvement.
|
Mooney and Haas (1993) |
| Double-blind, placebo-controlled, crossover design study. | 10 mild to severe traumatic brain injury patients (9 male/1 female). | Between 5 and 17 years. | MPH (5 mg twice daily, orally for patients under 20 kg) or MPH (7.5 mg twice daily, orally for patients between 21 and 29 kg) or MPH (10 mg twice daily, orally for patients above 30 kg) during 4 consecutive days with a 3-day interval for drug washout and placebo for the following 4 consecutive days or vice versa. | 2 months–10 years. | No. | Weekly during and at the end of the treatment. | No improvement.
|
Williams et al. (1998) |
10 Proposed mechanisms of amphetamine and methylphenidate in an injured state
Although clinical data is controversial, the positive results enable us to propose some hypotheses regarding the use of amphetamine or methylphenidate on these disorders. We summarized in Figure 7 the positive brain actions of these two drugs after a stroke or a traumatic brain injury. It is important to understand that after injury, further recovery may be due to “true improvement” or compensatory strategies in reaction to a disrupted/injured nervous system (Hornstein et al. 1996; Westmacott et al. 2010). In this sense, the pharmacological treatment should be adequate for the injury and affected section to provide a rapid and efficient recovery with further promotion of life quality to patients. As we saw before, despite different mechanisms of action, both amphetamine and methylphenidate increase the levels of monoamines in the pre-synaptic cleft of several brain regions (Leary et al. 2017; Lee et al. 2005; Moreno-López et al. 2017). In turn, traumatic brain injury and stroke are complex phenomena capable of disrupting the function of multiple neurotransmitter systems (Wagner et al. 2007). A hypothesis of a “catecholamine diaschisis” exists and consists of a functional depression of structures remote from but connected to the injury site, and the recovery occurs as the depression dissipates over time (Hovda and Fenney 1984). Interestingly, dopamine precursors have been shown protective in cerebral ischemia levodopa administration recovered the neurological function and improved the learning and memory functions in rats of the hippocampus global cerebral ischemia/reperfusion injury model (Wang et al. 2020). Furthermore, the delayed death of neurons in the hippocampus was reduced, and the number of synapses and expression of neural plasticity-related proteins were enhanced (Wang et al. 2020).
Graphical systematization of the amphetamine and methylphenidate brain’s actions. Briefly, amphetamine and methylphenidate showed the ability to stimulate the functionally depressed catecholamine system after injury, by elevating dopamine and noradrenaline neurotransmission. At the same time, functional activation of the pathways involved in neuroanatomical remodeling by the increase of density and distribution of neuronal proteins such as growth associated protein 43 (GAP43), synaptophysin, and growth factors like basic fibroblast growth factor and fibroblast growth factor 2 were reported. This increase is probably related to enhanced synaptogenesis in both neuronal and astrocyte populations, along with the growth of dendritic arbors/neurite outgrowth. The higher cerebral glucose metabolism and the decreased levels of secondary injury factors, lactic acid, and fatty free acid reported are perhaps linked to the recovery provided by amphetamine and methylphenidate actions. All these findings are ultimately linked to the patient’s recovery, leading to increased alertness, behavior, concentration, initiative, motor activity, spatial learning tasks, attention, memory, and mental processing, when compared to a nontreated patient or with the post-injury stage.
Central to a reliable treatment is the amphetamine and methylphenidate mechanisms understanding under injured conditions. Amphetamine and methylphenidate therapeutic effect is probably correlated with the stimulation of the functionally depressed catecholaminergic system (Hovda and Fenney 1984), which further promotes the alteration of synaptic connections by increasing the growth of dendritic arbors within the motor cortex, and also the number of dendritic spines within the prefrontal cortex, nucleus accumbens, caudate putamen and ventral tegmental area (Ramic et al. 2006). The increased catecholaminergic activity promoted by these drugs is correlated with increased alertness, concentration, initiative, motor activity, spatial learning tasks, attention, memory, and mental processing (Hornstein et al. 1996; Leary et al. 2017; Lee et al. 2005). In general, these effects may enhance the efficacy of rehabilitation following brain injury by improving the learning and retention of compensatory methods (Hornstein et al. 1996; Lee et al. 2005). On a preliminary investigation, Wang and colleagues speculate that methylphenidate action goes under a potentiation and strengthening mechanism of transcranial direct-current stimulation due to their synergistic effect, particularly at the subcortical level. Besides, the subcortical increase of dopamine neurotransmission by methylphenidate may affect the serotonergic pathway via the dopamine system in a “bottom-up” manner (Wang et al. 2014). Concerning amphetamine increases in density and distribution of GAP43 in the peri-infarct region and synaptophysin in the contralateral parietal cortex seen after drug treatment are possibly related to neurite growth followed by synaptogenesis in the neocortex (Stroemer et al. 1998). Controversially, Ramic and colleagues did not observe a synergistic effect of amphetamine once axonal growth was not detected between lesion-only animals and those treated with rehabilitation. However, amphetamine promoted significant neuroanatomical changes and behavioral recovery after cortical injury, when taking an amphetamine and rehabilitation treatment (Ramic et al. 2006). Although the specific mechanism is unknown, the most probable hypothesis involves the upregulation of proteins associated with neuronal remodeling due to the functional activation of pathways able to remodel the response to an active behavioral performance (Stroemer et al. 1998).
Brain metabolism during a conscious resting state may vary across different cortical regions and has already been associated with methylphenidate medication (Kim et al. 2009). Kim and coworkers reported a correlation between the cerebral glucose metabolism increase in the precorneal, posterior cingulate, retrosplenial, and parietooccipitotemporal association cortices with consciousness improvement after methylphenidate treatment to traumatic brain injury patients (Kim et al. 2009). Analysis of the amphetamine mechanism in an injured state was carried out by Dhillon and team that described an amphetamine-mediated reduction of secondary injury factors, lactic acid, and fatty free acids in the ipsilateral cortex and hippocampus, elevated after a lateral fluid percussion brain injury (Dhillon et al. 1998). The differences between cerebral areas increase the importance of understanding the target cerebral regions of each drug, as well as the mechanism (Hornstein et al. 1996) by which they act under injury (Kim et al. 2009). Stroemer and coworkers confirmed the involvement of the neocortex anteriorly, medial and lateral, and contralateral to the infarct, in the upregulation of proteins known to be involved in neuroanatomical remodeling, as a consequence of functional plasticity with resultant neural circuit and neuroanatomical reorganization (Stroemer et al. 1998). Methylphenidate promoted effects on the working memory as a possible result of dopamine effect on the prefrontal areas. The prominent effects were in the reaction time and accuracy of the working memory, and they appeared to be mediated by the dopaminergic and noradrenergic effects of this drug on specific brain areas (Kim et al. 2006). Controversially, in another study, a variable response of a catecholaminergic system to methylphenidate was reported as a potential confound factor due to an increase in the endogenous release of noradrenaline that may have opposing effects on working memory (Willmott and Ponsford 2009). The insights gained from these studies are inconsistent and vary according to the affected and evaluated brain area. Detailed examination by Moreno-Lopez and coworkers showed a disappearance of the complex relationship between the right inferior frontal gyrus and default mode network after administration of methylphenidate, when compared to placebo in traumatic brain injury patients, suggesting a normal balance between the default mode network and inhibition networks promoted by methylphenidate (Moreno-López et al. 2017). Regarding amphetamine, Papadopoulos and colleagues discovered cortico-efferent sprouting from the contra-lesional cortex to the denervated pontine nuclei associated with motor recovery after rehabilitation-combined treatment of lesioned rats. However, spontaneous redirection of major cortico-efferent pathways has not been seen (Papadopoulos et al. 2009).
Other factors involved in the putative protective effects of amphetamine and methylphenidate were suggested. For example, the up-regulation of growth factors such as basic fibroblast growth factor induced by amphetamine may enhance recovery of motor function after ischemic brain damage, presumably by contributing to synaptogenesis in both neuronal and astrocyte populations (Papadopoulos et al. 2009). The induction of the immediate early gene c-fos by amphetamine was also proposed as an involved factor. This induction may enhance the expression of proteins, including neurotrophins involved with dendritic and axonal sprouting in those pathways actively sprouting in response to the denervation, further promoting the homotypic (from the original innervation source) or heterotypic (from another innervation source) synaptogenesis (Stroemer et al. 1998). Another possible player is the fibroblast growth factor 2 described by Wolf and colleagues as being upregulated by noradrenergic activity induced by amphetamine following ischemic damage. Thus, stimulating the formation of new neural pathways in a fibroblast growth factor 2-dependent manner leads to enhanced axonal outgrowth and improved motor function (Wolf et al. 2014).
All findings reported so far are important to establish a robust theory regarding the mechanisms by which methylphenidate and amphetamine act in a resting and injured phase. However, considerably more work needs to be done, since their action mechanisms may be a key factor in searching for an effective treatment suitable for the patient.
11 Potential limitations of amphetamine and methylphenidate for treating stroke and traumatic brain injury
There are several limitations when using amphetamine and methylphenidate for treating stroke and traumatic brain injury. These drugs can be responsible for several adverse effects and their long-term safety in these patients still needs to be evaluated.
Cardiovascular events following amphetamine administration are a main concern. Cardiotoxicity (manifested as cardiomyopathy, acute myocardial infarction/necrosis, heart failure, or arrhythmia) after therapeutic use of amphetamine has been documented (Carvalho et al. 2012; Cooper et al. 2011; Sprigg et al. 2007). Central effects of amphetamine use include headache, nervousness, insomnia, dizziness, anxiety, psychosis, addiction, appetite decrease (Biederman et al. 2005), and growth attenuation in children (Richardson et al. 2017). Other adverse events reported in adults taking amphetamine were accidental injury, abdominal pain, and weight loss (Biederman et al. 2005).
On the other hand, methylphenidate might evoke adverse effects such as hypertension, tachycardia, increased blood pressure and pulse rate, angina, dysrhythmias, anorexia, nausea, weight loss, rash, and urticaria (Challman and Lipsky 2000). Even though methylphenidate is usually linked to well-being and cognitive function improvement, it may cause adverse effects related to the central nervous system, namely insomnia, anxiety headache, dizziness, dyskinesia, changes in mood or personality, hallucinations, and facial tics (Challman and Lipsky 2000).
Cardiovascular changes evoked by these two drugs are among the most prevalent and feared adverse effects found in patients. Increasing blood pressure might be contradictive in stroke or traumatic brain injury patients since it can increase the risk of cardiovascular events or even predispose to brain diseases. The cardiovascular effects of drugs were monitored in trials, and in patients after ischemic stroke amphetamine significantly increased blood pressure and heart rate without altering cerebral hemodynamics, or changing the course of motor recovery (Sprigg et al. 2007). However, as a matter of concern amphetamine abuse in humans has been linked to an increased risk of stroke (Westover et al. 2007). Arguing for the safety of therapeutic doses of this group of drugs are the results of a large retrospective cohort study, which found no evidence that the current use of an attention deficit-hyperactivity disorder drug could be associated with an increased risk of serious cardiovascular events in children and young adults between the ages of 2 and 24 years (Cooper et al. 2011). In this cohort study patients were included if they would take any stimulant used to treat attention deficit-hyperactivity disorder (methylphenidate, dexmethylphenidate, dextroamphetamines, amphetamine salts, atomoxetine, and pemoline).
In studies conducted with animals amphetamine and methylphenidate could evoke neurotoxicity, accompanied by neuronal death and neuroinflammation (Loureiro-Vieira et al. 2017; Serra et al. 2024; Teixeira-Gomes et al. 2015). It must be emphasized that these pre-clinical studies showing neurotoxicity evoked by these drugs administered much higher doses than the therapeutic dosages used in humans and are not comparable to clinical settings.
Even so, these adverse effects highlight that amphetamine and methylphenidate must be administered according to carefully controlled regimens to improve stroke or traumatic brain injury and avoid the worsening of neurological or cardiovascular outcomes in these patients.
12 Conclusions
There is an urgent need for a forceful search for treatments against stroke or traumatic brain injury, due to the increase in their prevalence and their impact on the quality of life of patients. Amphetamine and methylphenidate are first-line therapies for attention deficit hyperactivity disorder and narcolepsy treatment. Moreover, their use extends to other diseases because of their ability to interact with the monoaminergic system, being repurposed to other therapies and costing less than finding new ones. Their effects have rendered them possible therapeutic tools reasons are summarized in Table 5.
Summary of points establishing amphetamine and methylphenidate as promising drug candidates for therapeutical use following stroke or traumatic brain injury.
| Reasons for amphetamine and methylphenidate being drug candidates following stroke or traumatic brain injury |
|---|
|
Pre-clinical studies with amphetamine showed significant results in central nervous system injury when paired with rehabilitation, with enhanced neuronal plasticity, lower oxidative stress, and motor and cognitive function improvements. Several clinical trials have proven that in stroke and traumatic brain injury, amphetamine and methylphenidate can be valuable treatment options, improving motor and cognitive functions and activities of daily life, consequently ameliorating the patient’s morbidity. However, these results face up to negative findings after amphetamine and methylphenidate treatment in animals and humans, with no improvement in the same motor and cognitive functions. Possible reasons for these inconsistent effects are several limitations, including a low number of treated patients and short-term treatment, not completely blind studies, the different treatment variables (drug dose, treatment initiation time and their relation to the injury onset, additional treatment as physical therapy, among others), and the occurrence of adverse effects, complicating the assignment of effects to drug treatment or other factors.
More research is needed to counteract difficulties and establish the value of these drugs for these neurological problems. Further studies should approach the key research questions summarized in Table 6. Future research and clinical trials should investigate these topics, with the goal of identifying optimal treatment conditions and enhancing patients’ quality of life.
Key questions that future studies should focus.
| Key research questions |
|---|
|
Funding source: Fundação para a Ciência e a Tecnologia (FCT) I.P.
Award Identifier / Grant number: EXPL/MED-FAR/0203/2021
Funding source: Fundação para a Ciência e a Tecnologia (FCT) I.P.
Award Identifier / Grant number: UIDP/04378/2020 and UIDB/04378/2020
Funding source: Fundação para a Ciência e a Tecnologia (FCT) I.P.
Award Identifier / Grant number: LA/P/0140/2020
-
Research ethics: Not applicable.
-
Author contributions: João Paulo Capela conceptualized the manuscript. Mariana Ferreira and Patrícia Carneiro prepared the main body text and figures. Vera Marisa Costa, Félix Carvalho and Andreas Meisel review and edited the manuscript. All authors have read and approved the final version of the article.
-
Competing interests: The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
-
Research funding: This work was supported by national funds through from Fundação para a Ciência e a Tecnologia (FCT), I.P., in the scope of the project “EXPL/MED-FAR/0203/2021”, and also in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences (UCIBIO), and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
-
Data availability: This review article does not have applicable data availability, and it should be specified in each corresponding original article published on this topic.
References
Al-Adawi, S., Al-Naamani, A., Jaju, S., Al-Farsi, Y.M., Dorvlo, A.S.S., Al-Maashani, A., Al-Adawi, S.S.H., Moustafa, A.A., Al-Sibani, N., Essa, M.M., et al.. (2020). Methylphenidate improves executive functions in patients with traumatic brain injuries: a feasibility trial via the idiographic approach. BMC Neurol. 20: 103, https://doi.org/10.1186/s12883-020-01663-x.Suche in Google Scholar PubMed PubMed Central
Al-Adawi, S., Burke, D.T., and Dorvlo, A.S. (2006). The effect of methylphenidate on the sleep-wake cycle of brain-injured patients undergoing rehabilitation. Sleep Med. 7: 287–291, https://doi.org/10.1016/j.sleep.2005.11.008.Suche in Google Scholar PubMed
Alaverdashvili, M., Lim, D.H., and Whishaw, I.Q. (2007). No improvement by amphetamine on learned non-use, attempts, success or movement in skilled reaching by the rat after motor cortex stroke. Eur. J. Neurosci. 25: 3442–3452, https://doi.org/10.1111/j.1460-9568.2007.05594.x.Suche in Google Scholar PubMed
Arvidsson, M., Dahl, M.-L., Beck, O., Ackehed, G., Nordin, K., and Rosenborg, S. (2020). Pharmacokinetics of methylphenidate and ritalinic acid in plasma correlations with exhaled breath and oral fluid in healthy volunteers. Eur. J. Clin. Pharmacol. 76: 229–237, https://doi.org/10.1007/s00228-019-02787-x.Suche in Google Scholar PubMed
Barbay, S., Zoubina, E.V., Dancause, N., Frost, S.B., Eisner-Janowicz, I., Stowe, A.M., Plautz, E.J., and Nudo, R.J. (2006). A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil. Neural Repair 20: 455–458, https://doi.org/10.1177/1545968306290773.Suche in Google Scholar PubMed
Barrett, K.M., Brott, T.G., Brown, R.D.Jr., Carter, R.E., Geske, J.R., Graff-Radford, N.R., McNeil, R.B., and Meschia, J.F., and Mayo Acute Stroke Trial for Enhancing Recovery Study, G. (2011). Enhancing recovery after acute ischemic stroke with donepezil as an adjuvant therapy to standard medical care: results of a phase IIA clinical trial. J. Stroke Cerebrovasc. Dis. 20: 177–182, https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.12.009.Suche in Google Scholar PubMed
Basit, R.H., Wiseman, J., Chowdhury, F., and Chari, D.M. (2022). Simulating traumatic brain injury in vitro: developing high throughput models to test biomaterial based therapies. Neural Regen. Res. 18: 289–292, https://doi.org/10.4103/1673-5374.346465.Suche in Google Scholar PubMed PubMed Central
Batistela, S., Bueno, O.F.A., Vaz, L.J., and Galduróz, J.C.F. (2016). Methylphenidate as a cognitive enhancer in healthy young people. Dement. Neuropsychol. 10: 134–142, https://doi.org/10.1590/s1980-5764-2016dn1002009.Suche in Google Scholar PubMed PubMed Central
Berger, C., Müller-Godeffroy, J., Marx, I., Reis, O., Buchmann, J., and Dück, A. (2018). Methylphenidate promotes the interaction between motor cortex facilitation and attention in healthy adults: a combined study using event-related potentials and transcranial magnetic stimulation. Brain Behav. 8: e01155, https://doi.org/10.1002/brb3.1155.Suche in Google Scholar PubMed PubMed Central
Biederman, J., Spencer, T.J., Wilens, T.E., Weisler, R.H., Read, S.C., and Tulloch, S.J. (2005). Long-term safety and effectiveness of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 10: 16–25, https://doi.org/10.1017/s1092852900002406.Suche in Google Scholar PubMed
Brenneman, M.M., Hylin, M.J., and Corwin, J.V. (2015). The time-dependent and persistent effects of amphetamine treatment upon recovery from hemispatial neglect in rats. Behav. Brain Res. 293: 153–161, https://doi.org/10.1016/j.bbr.2015.07.032.Suche in Google Scholar PubMed
Brown, A.W., Bjelke, B., and Fuxe, K. (2004). Motor response to amphetamine treatment, task-specific training, and limited motor experience in a postacute animal stroke model. Exp. Neurol. 190: 102–108, https://doi.org/10.1016/j.expneurol.2004.07.005.Suche in Google Scholar PubMed
Byard, R.W., Donkin, J., and Vink, R. (2018). The forensic implications of amphetamine intoxication in cases of inflicted blunt craniocerebral trauma. J. Forensic. Sci. 63: 151–153, https://doi.org/10.1111/1556-4029.13509.Suche in Google Scholar PubMed
Carney, N., Totten, A.M., O’Reilly, C., Ullman, J.S., Hawryluk, G.W., Bell, M.J., Bratton, S.L., Chesnut, R., Harris, O.A., Kissoon, N., et al.. (2017). Guidelines for the management of severe traumatic brain injury. Neurosurgery 80: 6–15, https://doi.org/10.1227/neu.0000000000001432.Suche in Google Scholar
Carvalho, M., Carmo, H., Costa, V.M., Capela, J.P., Pontes, H., Remião, F., Carvalho, F., and Bastos Mde, L. (2012). Toxicity of amphetamines: an update. Arch. Toxicol. 86: 1167–1231, https://doi.org/10.1007/s00204-012-0815-5.Suche in Google Scholar PubMed
Cassidy, J.M. and Cramer, S.C. (2017). Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl. Stroke Res. 8: 33–46, https://doi.org/10.1007/s12975-016-0467-5.Suche in Google Scholar PubMed PubMed Central
Castells, X., Blanco-Silvente, L., and Cunill, R. (2018). Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 8: CD007813, https://doi.org/10.1002/14651858.cd007813.pub3.Suche in Google Scholar
Challman, T.D. and Lipsky, J.J. (2000). Methylphenidate: its pharmacology and uses. Mayo Clin. Proc. 75: 711–721, https://doi.org/10.1016/s0025-6196(11)64618-1.Suche in Google Scholar
Chollet, F., Tardy, J., Albucher, J.F., Thalamas, C., Berard, E., Lamy, C., Bejot, Y., Deltour, S., Jaillard, A., Niclot, P., et al.. (2011). Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 10: 123–130, https://doi.org/10.1016/s1474-4422(10)70314-8.Suche in Google Scholar
Chudasama, Y., Nathwani, F., and Robbins, T.W. (2005). D-Amphetamine remediates attentional performance in rats with dorsal prefrontal lesions. Behav. Brain Res. 158: 97–107, https://doi.org/10.1016/j.bbr.2004.08.011.Suche in Google Scholar PubMed
Colbourne, F. and Corbett, D. (1992). Effects of d-amphetamine on the recovery of function following cerebral ischemic injury. Pharmacol. Biochem. Behav. 42: 705–710, https://doi.org/10.1016/0091-3057(92)90018-b.Suche in Google Scholar PubMed
Cooper, W.O., Habel, L.A., Sox, C.M., Chan, K.A., Arbogast, P.G., Cheetham, T.C., Murray, K.T., Quinn, V.P., Stein, C.M., Callahan, S.T., et al.. (2011). ADHD drugs and serious cardiovascular events in children and young adults. N. Engl. J. Med. 365: 1896–1904, https://doi.org/10.1056/nejmoa1110212.Suche in Google Scholar
Cortese, S., Newcorn, J.H., and Coghill, D. (2021). A practical, evidence-informed approach to managing stimulant-refractory attention deficit hyperactivity disorder (ADHD). CNS Drugs 35: 1035–1051, https://doi.org/10.1007/s40263-021-00848-3.Suche in Google Scholar PubMed
Couper, F.J. and Logan, B.K. (2004). Drugs and human performance fact sheets, United States. Natl. Highw. Traffic Saf. Adm.Suche in Google Scholar
Cramer, S.C. (2018). Treatments to promote neural repair after stroke. J. Stroke 20: 57–70, https://doi.org/10.5853/jos.2017.02796.Suche in Google Scholar PubMed PubMed Central
Crismon, M.L., Childs, A., Wilcox, R.E., and Barrow, N. (1988). The effect of bromocriptine on speech dysfunction in patients with diffuse brain injury (akinetic mutism). Clin. Neuropharmacol. 11: 462–466, https://doi.org/10.1097/00002826-198810000-00007.Suche in Google Scholar PubMed
Crisostomo, E.A., Duncan, P.W., Propst, M., Dawson, D.V., and Davis, J.N. (1988). Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann. Neurol. 23: 94–97, https://doi.org/10.1002/ana.410230117.Suche in Google Scholar PubMed
Davalos, A., Alvarez-Sabin, J., Castillo, J., Diez-Tejedor, E., Ferro, J., Martinez-Vila, E., Serena, J., Segura, T., Cruz, V.T., Masjuan, J., et al.. (2012). Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 380: 349–357, https://doi.org/10.1016/s0140-6736(12)60813-7.Suche in Google Scholar PubMed
David, M.C.B., Del Giovane, M., Liu, K.Y., Gostick, B., Rowe, J.B., Oboh, I., Howard, R., and Malhotra, P.A. (2022). Cognitive and neuropsychiatric effects of noradrenergic treatment in Alzheimer’s disease: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 93: 1080–1090, https://doi.org/10.1136/jnnp-2022-329136.Suche in Google Scholar PubMed PubMed Central
de la Torre, R., Farre, M., Navarro, M., Pacifici, R., Zuccaro, P., and Pichini, S. (2004). Clinical pharmacokinetics of amfetamine and related substances: monitoring in conventional and non-conventional matrices. Clin. Pharmacokinet. 43: 157–185, https://doi.org/10.2165/00003088-200443030-00002.Suche in Google Scholar PubMed
Delbari, A., Salman-Roghani, R., and Lokk, J. (2011). Effect of methylphenidate and/or levodopa combined with physiotherapy on mood and cognition after stroke: a randomized, double-blind, placebo-controlled trial. Eur. Neurol. 66: 7–13, https://doi.org/10.1159/000329275.Suche in Google Scholar PubMed
Dhillon, H.S., Dose, J.M., and Prasad, R.M. (1998). Amphetamine administration improves neurochemical outcome of lateral fluid percussion brain injury in the rat. Brain Res. 804: 231–237, https://doi.org/10.1016/s0006-8993(98)00639-8.Suche in Google Scholar PubMed
Dorer, C.L., Manktelow, A.E., Allanson, J., Sahakian, B.J., Pickard, J.D., Bateman, A., Menon, D.K., and Stamatakis, E.A. (2018). Methylphenidate-mediated motor control network enhancement in patients with traumatic brain injury. Brain Inj. 32: 1040–1049, https://doi.org/10.1080/02699052.2018.1469166.Suche in Google Scholar PubMed
Dymowski, A.R., Ponsford, J.L., Owens, J.A., Olver, J.H., Ponsford, M., and Willmott, C. (2017). The efficacy and safety of extended-release methylphenidate following traumatic brain injury: a randomised controlled pilot study. Clin. Rehabil. 31: 733–741, https://doi.org/10.1177/0269215516655590.Suche in Google Scholar PubMed
Ekinci, O., Direk, M.C., Gunes, S., Teke, H., Ekinci, N., Yildirim, F., and Okuyaz, C. (2017). Short-term efficacy and tolerability of methylphenidate in children with traumatic brain injury and attention problems. Brain Dev. 39: 327–336, https://doi.org/10.1016/j.braindev.2016.11.005.Suche in Google Scholar PubMed
Evans, R.W., Gualtieri, C.T., and Patterson, D. (1987). Treatment of chronic closed head injury with psychostimulant drugs: a controlled case study and an appropriate evaluation procedure. J. Nerv. Ment. Dis. 175: 106–110, https://doi.org/10.1097/00005053-198702000-00007.Suche in Google Scholar PubMed
Faraj, B.A., Israili, Z.H., Perel, J.M., Jenkins, M.L., Holtzman, S.G., Cucinell, S.A., and Dayton, P.G. (1974). Metabolism and disposition of methylphenidate-14C: studies in man and animals. J. Pharmacol. Exp. Ther. 191: 535–547.Suche in Google Scholar
Faraone, S.V. (2018). The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 87: 255–270, https://doi.org/10.1016/j.neubiorev.2018.02.001.Suche in Google Scholar PubMed PubMed Central
FDA (2001). FDA drug approval Package for dextroamphetamine. U.S. Food and Drug Administration, Silver Spring.Suche in Google Scholar
FDA (2022). FDA drug approval Packtage for ritalin LA (Methylphenidate Hydrochloride). U.S. Food and Drug Administration, Silver Spring.Suche in Google Scholar
Feeney, D.M., Gonzalez, A., and Law, W.A. (1982). Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217: 855–857, https://doi.org/10.1126/science.7100929.Suche in Google Scholar PubMed
Feigin, V.L., Barker-Collo, S., Krishnamurthi, R., Theadom, A., and Starkey, N. (2010). Epidemiology of ischaemic stroke and traumatic brain injury. Best. Pract. Res. Clin. Anaesthesiol. 24: 485–494, https://doi.org/10.1016/j.bpa.2010.10.006.Suche in Google Scholar PubMed
Feigin, V.L., Stark, B.A., Johnson, C.O., Roth, G.A., Bisignano, C., Abady, G.G., Abbasifard, M., Abbasi-Kangevari, M., Abd-Allah, F., Abedi, V., et al.. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20: 795–820, https://doi.org/10.1016/s1474-4422(21)00252-0.Suche in Google Scholar
Ferris, R.M. and Tang, F.L. (1979). Comparison of the effects of the isomers of amphetamine, methylphenidate and deoxypipradrol on the uptake of l-[3H]norepinephrine and [3H]dopamine by synaptic vesicles from rat whole brain, striatum and hypothalamus. J. Pharmacol. Exp. Ther. 210: 422–428.Suche in Google Scholar
Ferrucci, M., Limanaqi, F., Ryskalin, L., Biagioni, F., Busceti, C.L., and Fornai, F. (2019). The effects of amphetamine and methamphetamine on the release of norepinephrine, dopamine and acetylcholine from the brainstem reticular formation. Front. Neuroanat. 13: 48, https://doi.org/10.3389/fnana.2019.00048.Suche in Google Scholar PubMed PubMed Central
Francisco, G.E. and Ivanhoe, C.B. (1996). Successful treatment of post-traumatic narcolepsy with methylphenidate: a case report. Am. J. Phys. Med. Rehabil. 75: 63–65, https://doi.org/10.1097/00002060-199601000-00016.Suche in Google Scholar PubMed
Gillberg, C., Melander, H., von Knorring, A.L., Janols, L.O., Thernlund, G., Hagglof, B., Eidevall-Wallin, L., Gustafsson, P., and Kopp, S. (1997). Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatr. 54: 857–864, https://doi.org/10.1001/archpsyc.1997.01830210105014.Suche in Google Scholar PubMed
Gilmour, G., Iversen, S.D., O’Neill, M.F., O’Neill, M.J., Ward, M.A., and Bannerman, D.M. (2005). Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav. Brain Res. 165: 98–109, https://doi.org/10.1016/j.bbr.2005.06.027.Suche in Google Scholar PubMed
Gladstone, D.J., Danells, C.J., Armesto, A., McIlroy, W.E., Staines, W.R., Graham, S.J., Herrmann, N., Szalai, J.P., Black, S.E., Subacute Therapy with, A., et al.. (2006). Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke 37: 179–185, https://doi.org/10.1161/01.str.0000195169.42447.78.Suche in Google Scholar
Goldstein, L.B. and Davis, J.N. (1990). Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury. J. Neurosci. Method. 31: 101–107, https://doi.org/10.1016/0165-0270(90)90154-8.Suche in Google Scholar PubMed
Goldstein, L.B., Lennihan, L., Rabadi, M.J., Good, D.C., Reding, M.J., Dromerick, A.W., Samsa, G.P., and Pura, J. (2018). Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol. 75: 1494–1501, https://doi.org/10.1001/jamaneurol.2018.2338.Suche in Google Scholar PubMed PubMed Central
Grade, C., Redford, B., Chrostowski, J., Toussaint, L., and Blackwell, B. (1998). Methylphenidate in early poststroke recovery: a double-blind, placebo-controlled study. Arch. Phys. Med. Rehabil. 79: 1047–1050, https://doi.org/10.1016/s0003-9993(98)90169-1.Suche in Google Scholar PubMed
Griesbach, G.S., Hovda, D.A., Gomez-Pinilla, F., and Sutton, R.L. (2008). Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience 154: 530–540, https://doi.org/10.1016/j.neuroscience.2008.04.003.Suche in Google Scholar PubMed PubMed Central
Grizenko, N., Qi Zhang, D.D., Polotskaia, A., and Joober, R. (2012). Efficacy of methylphenidate in ADHD children across the normal and the gifted intellectual spectrum. J. Am. Acad. Child. Adolesc. Psychiatr. 21: 282–288.Suche in Google Scholar
Grunblatt, E., Bartl, J., and Walitza, S. (2018). Methylphenidate enhances neuronal differentiation and reduces proliferation concomitant to activation of Wnt signal transduction pathways. Transl. Psychiatr. 8: 51, https://doi.org/10.1038/s41398-018-0096-8.Suche in Google Scholar PubMed PubMed Central
Gualtieri, C.T. and Evans, R.W. (1988). Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj. 2: 273–290, https://doi.org/10.3109/02699058809150898.Suche in Google Scholar PubMed
Gulaboski, R., Cordeiro, M.N., Milhazes, N., Garrido, J., Borges, F., Jorge, M., Pereira, C.M., Bogeski, I., Morales, A.H., Naumoski, B., et al.. (2007). Evaluation of the lipophilic properties of opioids, amphetamine-like drugs, and metabolites through electrochemical studies at the interface between two immiscible solutions. Anal. Biochem. 361: 236–243, https://doi.org/10.1016/j.ab.2006.11.006.Suche in Google Scholar PubMed
Haertling, F., Mueller, B., and Bilke-Hentsch, O. (2015). Effectiveness and safety of a long-acting, once-daily, two-phase release formulation of methylphenidate (Ritalin (R) LA) in school children under daily practice conditions. Atten. Defic. Hyperact. Disord. 7: 157–164, https://doi.org/10.1007/s12402-014-0154-x.Suche in Google Scholar PubMed PubMed Central
Haig, A.J. and Ruess, J.M. (1990). Recovery from vegetative state of six months’ duration associated with Sinemet (levodopa/carbidopa). Arch. Phys. Med. Rehabil. 71: 1081–1083.Suche in Google Scholar
Harriott, N.D., Williams, J.P., Smith, E.B., Bozigian, H.P., and Grigoriadis, D.E. (2018). VMAT2 inhibitors and the path to ingrezza (valbenazine). In: Witty, D.R. and Cox, B. (Eds.), Progress in medicinal chemistry. Elsevier, Amesterdam, pp. 87–111.10.1016/bs.pmch.2017.12.002Suche in Google Scholar PubMed
Heal, D.J., Smith, S.L., Gosden, J., and Nutt, D.J. (2013). Amphetamine, past and present--a pharmacological and clinical perspective. J. Psychopharmacol. 27: 479–496, https://doi.org/10.1177/0269881113482532.Suche in Google Scholar PubMed PubMed Central
Hill, M.D. and Hachinski, V. (1998). Stroke treatment: time is brain. Lancet 352(Suppl. 3): SIII10–14, https://doi.org/10.1016/s0140-6736(98)90088-5.Suche in Google Scholar PubMed
Hill, M.D., Martin, R.H., Mikulis, D., Wong, J.H., Silver, F.L., Terbrugge, K.G., Milot, G., Clark, W.M., Macdonald, R.L., Kelly, M.E., et al.. (2012). Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 11: 942–950, https://doi.org/10.1016/s1474-4422(12)70225-9.Suche in Google Scholar
Hornstein, A., Lennihan, L., Seliger, G., Lichtman, S., and Schroeder, K. (1996). Amphetamine in recovery from brain injury. Brain Inj. 10: 145–148, https://doi.org/10.1080/026990596124647.Suche in Google Scholar PubMed
Hornyak, J.E., Nelson, V.S., and Hurvitz, E.A. (1997). The use of methylphenidate in paediatric traumatic brain injury. Pediatr. Rehabil. 1: 15–17, https://doi.org/10.3109/17518429709060937.Suche in Google Scholar PubMed
Hovda, D.A. and Fenney, D.M. (1984). Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 298: 358–361, https://doi.org/10.1016/0006-8993(84)91437-9.Suche in Google Scholar PubMed
Huebner, E.A. and Strittmatter, S.M. (2009). Axon regeneration in the peripheral and central nervous systems. Results Probl. Cell Differ. 48: 339–351, https://doi.org/10.1007/400_2009_19.Suche in Google Scholar PubMed PubMed Central
Hurwitz, B.E., Dietrich, W.D., McCabe, P.M., Alonso, O., Watson, B.D., Ginsberg, M.D., and Schneiderman, N. (1991). Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke 22: 648–654, https://doi.org/10.1161/01.str.22.5.648.Suche in Google Scholar PubMed
Hylin, M.J., Brenneman, M.M., and Corwin, J.V. (2017). Noradrenergic antagonists mitigate amphetamine-induced recovery. Behav. Brain Res. 334: 61–71, https://doi.org/10.1016/j.bbr.2017.07.035.Suche in Google Scholar PubMed
Iversen, L. (2008). Speed, ecstasy, ritalin: the science of amphetamines. OUP, Oxford, pp. 1–120.10.1093/acprof:oso/9780198530909.003.0001Suche in Google Scholar
Jaeschke, R.R., Sujkowska, E., and Sowa-Kucma, M. (2021). Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review. Psychopharmacology 238: 2667–2691, https://doi.org/10.1007/s00213-021-05946-0.Suche in Google Scholar PubMed PubMed Central
James, R.S., Sharp, W.S., Bastain, T.M., Lee, P.P., Walter, J.M., Czarnolewski, M., and Castellanos, F.X. (2001). Double-blind, placebo-controlled study of single-dose amphetamine formulations in ADHD. J. Am. Acad. Child. Adolesc. Psychiatr. 40: 1268–1276, https://doi.org/10.1097/00004583-200111000-00006.Suche in Google Scholar PubMed
James, S.L., Theadom, A., Ellenbogen, R.G., Bannick, M.S., Montjoy-Venning, W., Lucchesi, L.R., Abbasi, N., Abdulkader, R., Abraha, H.N., Adsuar, J.C., et al.. (2019). Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18: 56–87, https://doi.org/10.1016/s1474-4422(18)30415-0.Suche in Google Scholar
Jarrahi, A., Braun, M., Ahluwalia, M., Gupta, R.V., Wilson, M., Munie, S., Ahluwalia, P., Vender, J.R., Vale, F.L., Dhandapani, K.M., et al.. (2020). Revisiting traumatic brain injury: from molecular mechanisms to therapeutic interventions. Biomedicines 8: 389, https://doi.org/10.3390/biomedicines8100389.Suche in Google Scholar PubMed PubMed Central
Jenkins, P.O., De Simoni, S., Bourke, N.J., Fleminger, J., Scott, G., Towey, D.J., Svensson, W., Khan, S., Patel, M.C., Greenwood, R., et al.. (2019). Stratifying drug treatment of cognitive impairments after traumatic brain injury using neuroimaging. Brain 142: 2367–2379, https://doi.org/10.1093/brain/awz149.Suche in Google Scholar PubMed
Johansson, B., Andrell, P., Ronnback, L., and Mannheimer, C. (2020). Follow-up after 5.5 years of treatment with methylphenidate for mental fatigue and cognitive function after a mild traumatic brain injury. Brain Inj. 34: 229–235, https://doi.org/10.1080/02699052.2019.1683898.Suche in Google Scholar PubMed
Johansson, B., Wentzel, A.P., Andrell, P., Mannheimer, C., and Ronnback, L. (2015). Methylphenidate reduces mental fatigue and improves processing speed in persons suffered a traumatic brain injury. Brain Inj. 29: 758–765, https://doi.org/10.3109/02699052.2015.1004747.Suche in Google Scholar PubMed
Johansson, B., Wentzel, A.P., Andrell, P., Odenstedt, J., Mannheimer, C., and Ronnback, L. (2014). Evaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and pain. Brain Inj. 28: 304–310, https://doi.org/10.3109/02699052.2013.865267.Suche in Google Scholar PubMed
Johansson, B., Wentzel, A.P., Andrell, P., Ronnback, L., and Mannheimer, C. (2017). Long-term treatment with methylphenidate for fatigue after traumatic brain injury. Acta Neurol. Scand. 135: 100–107, https://doi.org/10.1111/ane.12587.Suche in Google Scholar PubMed
Johansson, B., Wentzel, A.P., Andrell, P., Ronnback, L., and Mannheimer, C. (2018). Two-year methylphenidate treatment of mental fatigue and cognitive function after a traumatic brain injury: a clinical prospective study. J. Clin. Psychopharmacol. 38: 164–165, https://doi.org/10.1097/jcp.0000000000000854.Suche in Google Scholar
Jonason, K.R., Lauber, S.M., Robbins, M.J., Meyer, P.M., and Meyer, D.R. (1970). Effects of amphetamine upon relearning pattern and black-white discriminations following neocortical lesions in rats. J. Comp. Physiol. Psychol. 73: 47–55, https://doi.org/10.1037/h0030013.Suche in Google Scholar PubMed
Joy, M.T., Ben Assayag, E., Shabashov-Stone, D., Liraz-Zaltsman, S., Mazzitelli, J., Arenas, M., Abduljawad, N., Kliper, E., Korczyn, A.D., Thareja, N.S., et al.. (2019). CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 176: 1143–1157.e1113, https://doi.org/10.1016/j.cell.2019.01.044.Suche in Google Scholar PubMed PubMed Central
Kaelin, D.L., Cifu, D.X., and Matthies, B. (1996). Methylphenidate effect on attention deficit in the acutely brain-injured adult. Arch. Phys. Med. Rehabil. 77: 6–9, https://doi.org/10.1016/s0003-9993(96)90211-7.Suche in Google Scholar PubMed
Kakehi, S. and Tompkins, D.M. (2021). A review of pharmacologic neurostimulant use during rehabilitation and recovery after brain injury. Ann. Pharmacother. 55: 1254–1266, https://doi.org/10.1177/1060028020983607.Suche in Google Scholar PubMed
Karelina, K., Gaier, K.R., and Weil, Z.M. (2017). Traumatic brain injuries during development disrupt dopaminergic signaling. Exp. Neurol. 297: 110–117, https://doi.org/10.1016/j.expneurol.2017.08.003.Suche in Google Scholar PubMed PubMed Central
Keser, Z., Dehgan, M.W., Shadravan, S., Yozbatiran, N., Maher, L.M., and Francisco, G.E. (2017). Combined dextroamphetamine and transcranial direct current stimulation in poststroke aphasia. Am. J. Phys. Med. Rehabil. 96: S141–S145, https://doi.org/10.1097/phm.0000000000000780.Suche in Google Scholar PubMed
Kim, J., Whyte, J., Patel, S., Europa, E., Wang, J., Coslett, H.B., and Detre, J.A. (2012). Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: a perfusion fMRI study. Psychopharmacology 222: 47–57, https://doi.org/10.1007/s00213-011-2622-8.Suche in Google Scholar PubMed PubMed Central
Kim, Y.H., Ko, M.H., Na, S.Y., Park, S.H., and Kim, K.W. (2006). Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: a double-blind placebo-controlled study. Clin. Rehabil. 20: 24–30, https://doi.org/10.1191/0269215506cr927oa.Suche in Google Scholar PubMed
Kim, Y.W., Shin, J.C., and An, Y.S. (2009). Effects of methylphenidate on cerebral glucose metabolism in patients with impaired consciousness after acquired brain injury. Clin. Neuropharmacol. 32: 335–339, https://doi.org/10.1097/wnf.0b013e3181b40678.Suche in Google Scholar PubMed
Kimko, H.C., Cross, J.T., and Abernethy, D.R. (1999). Pharmacokinetics and clinical effectiveness of methylphenidate. Clin. Pharmacokinet. 37: 457–470, https://doi.org/10.2165/00003088-199937060-00002.Suche in Google Scholar PubMed
Kline, A.E., Yan, H.Q., Bao, J., Marion, D.W., and Dixon, C.E. (2000). Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 280: 163–166, https://doi.org/10.1016/s0304-3940(00)00797-7.Suche in Google Scholar PubMed
Kodama, T., Kojima, T., Honda, Y., Hosokawa, T., Tsutsui, K.-i., and Watanabe, M. (2017). Oral administration of methylphenidate (ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: a microdialysis study in the monkey. J. Neurosci. 37: 2387–2394, https://doi.org/10.1523/jneurosci.2155-16.2017.Suche in Google Scholar
Kumar, A. and Kitago, T. (2019). Pharmacological enhancement of stroke recovery. Curr. Neurol. Neurosci. Rep. 19: 43, https://doi.org/10.1007/s11910-019-0959-2.Suche in Google Scholar PubMed
Kuriakose, D. and Xiao, Z. (2020). Pathophysiology and treatment of stroke: present status and future perspectives. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21207609.Suche in Google Scholar PubMed PubMed Central
Lazarus, L.W., Moberg, P.J., Langsley, P.R., and Lingam, V.R. (1994). Methylphenidate and nortriptyline in the treatment of poststroke depression: a retrospective comparison. Arch. Phys. Med. Rehabil. 75: 403–406, https://doi.org/10.1016/0003-9993(94)90163-5.Suche in Google Scholar PubMed
Leary, J.B., Bondi, C.O., LaPorte, M.J., Carlson, L.J., Radabaugh, H.L., Cheng, J.P., and Kline, A.E. (2017). The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J. Neurotrauma 34: 444–450, https://doi.org/10.1089/neu.2016.4438.Suche in Google Scholar PubMed PubMed Central
LeBlond, E., Smith-Paine, J., Riemersma, J.J., Horn, P.S., Wade, S.L., and Kurowski, B.G. (2019). Influence of methylphenidate on long-term neuropsychological and everyday executive functioning after traumatic brain injury in children with secondary attention problems. J. Int. Neuropsychol. Soc. 25: 740–749, https://doi.org/10.1017/s1355617719000444.Suche in Google Scholar
Lee, H., Kim, S.W., Kim, J.M., Shin, I.S., Yang, S.J., and Yoon, J.S. (2005). Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum. Psychopharmacol. 20: 97–104, https://doi.org/10.1002/hup.668.Suche in Google Scholar PubMed
Linssen, A.M., Sambeth, A., Vuurman, E.F., and Riedel, W.J. (2014). Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int. J. Neuropsychopharmacol. 17: 961–977, https://doi.org/10.1017/s1461145713001594.Suche in Google Scholar
Liu, H.S., Shen, H., Harvey, B.K., Castillo, P., Lu, H., Yang, Y., and Wang, Y. (2011). Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage 56: 280–289, https://doi.org/10.1016/j.neuroimage.2011.02.049.Suche in Google Scholar PubMed PubMed Central
Lokk, J., Salman Roghani, R., and Delbari, A. (2011). Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke--a randomized, double-blind, placebo-controlled trial. Acta Neurol. Scand. 123: 266–273, https://doi.org/10.1111/j.1600-0404.2010.01395.x.Suche in Google Scholar PubMed
Loureiro-Vieira, S., Costa, V.M., de Lourdes Bastos, M., Carvalho, F., and Capela, J.P. (2017). Methylphenidate effects in the young brain: friend or foe? Int. J. Dev. Neurosci. 60: 34–47, https://doi.org/10.1016/j.ijdevneu.2017.04.002.Suche in Google Scholar PubMed
Luaute, J., Villeneuve, L., Roux, A., Nash, S., Bar, J.Y., Chabanat, E., Cotton, F., Ciancia, S., Sancho, P.O., Hovantruc, P., et al.. (2018). Adding methylphenidate to prism-adaptation improves outcome in neglect patients. A randomized clinical trial. Cortex 106: 288–298, https://doi.org/10.1016/j.cortex.2018.03.028.Suche in Google Scholar PubMed
Manktelow, A.E., Menon, D.K., Sahakian, B.J., and Stamatakis, E.A. (2017). Working memory after traumatic brain injury: the neural basis of improved performance with methylphenidate. Front. Behav. Neurosci. 11: 58, https://doi.org/10.3389/fnbeh.2017.00058.Suche in Google Scholar PubMed PubMed Central
Martinsson, L. and Wahlgren, N.G. (2003). Safety of dexamphetamine in acute ischemic stroke: a randomized, double-blind, controlled dose-escalation trial. Stroke 34: 475–481, https://doi.org/10.1161/01.str.0000050161.38263.ae.Suche in Google Scholar PubMed
Martinsson, L., Yang, X., Beck, O., Wahlgren, N.G., and Eksborg, S. (2003). Pharmacokinetics of dexamphetamine in acute stroke. Clin. Neuropharmacol. 26: 270–276, https://doi.org/10.1097/00002826-200309000-00012.Suche in Google Scholar PubMed
Matthijssen, A.M., Dietrich, A., Bierens, M., Kleine Deters, R., van de Loo-Neus, G.H.H., van den Hoofdakker, B.J., Buitelaar, J.K., and Hoekstra, P.J. (2019). Continued benefits of methylphenidate in ADHD after 2 Years in clinical practice: a randomized placebo-controlled discontinuation study. Am. J. Psychiatr. 176: 754–762, https://doi.org/10.1176/appi.ajp.2019.18111296.Suche in Google Scholar PubMed
McAllister, T.W., Zafonte, R., Jain, S., Flashman, L.A., George, M.S., Grant, G.A., He, F., Lohr, J.B., Andaluz, N., Summerall, L., et al.. (2016). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 41: 1191–1198, https://doi.org/10.1038/npp.2015.282.Suche in Google Scholar PubMed PubMed Central
McDonald, B.C., Flashman, L.A., Arciniegas, D.B., Ferguson, R.J., Xing, L., Harezlak, J., Sprehn, G.C., Hammond, F.M., Maerlender, A.C., Kruck, C.L., et al.. (2017). Methylphenidate and memory and attention adaptation training for persistent cognitive symptoms after traumatic brain injury: a randomized, placebo-controlled trial. Neuropsychopharmacology 42: 1766–1775, https://doi.org/10.1038/npp.2016.261.Suche in Google Scholar PubMed PubMed Central
Menon, D.K., Schwab, K., Wright, D.W., and Maas, A.I. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91: 1637–1640, https://doi.org/10.1016/j.apmr.2010.05.017.Suche in Google Scholar PubMed
Meythaler, J.M., Depalma, L., Devivo, M.J., Guin-Renfroe, S., and Novack, T.A. (2001). Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Inj. 15: 321–331, https://doi.org/10.1080/026990501750111274.Suche in Google Scholar PubMed
Mitler, M.M. and Hajdukovic, R. (1991). Relative efficacy of drugs for the treatment of sleepiness in narcolepsy. Sleep 14: 218–220, https://doi.org/10.1093/sleep/14.3.218.Suche in Google Scholar PubMed PubMed Central
Mooney, G.F. and Haas, L.J. (1993). Effect of methylphenidate on brain injury-related anger. Arch. Phys. Med. Rehabil. 74: 153–160.Suche in Google Scholar
Moreno-López, L., Manktelow, A.E., Sahakian, B.J., Menon, D.K., and Stamatakis, E.A. (2017). Anything goes? Regulation of the neural processes underlying response inhibition in TBI patients. Eur. Neuropsychopharmacol. 27: 159–169, https://doi.org/10.1016/j.euroneuro.2016.12.002.Suche in Google Scholar PubMed
Müller, U., Murai, T., Bauer-Wittmund, T., and von Cramon, D.Y. (1999). Paroxetine versus citalopram treatment of pathological crying after brain injury. Brain Inj. 13: 805–811, https://doi.org/10.1080/026990599121197.Suche in Google Scholar PubMed
Newsome, M.R., Scheibel, R.S., Seignourel, P.J., Steinberg, J.L., Troyanskaya, M., Li, X., and Levin, H.S. (2009). Effects of methylphenidate on working memory in traumatic brain injury: a preliminary FMRI investigation. Brain Imag. Behav. 3: 298–305, https://doi.org/10.1007/s11682-009-9072-5.Suche in Google Scholar PubMed
Owolabi, M.O., Thrift, A.G., Martins, S., Johnson, W., Pandian, J., Abd-Allah, F., Varghese, C., Mahal, A., Yaria, J., Phan, H.T., et al.. (2021). The state of stroke services across the globe: report of world stroke organization-world health organization surveys. Int. J. Stroke 16: 889–901, https://doi.org/10.1177/17474930211019568.Suche in Google Scholar PubMed PubMed Central
Papadopoulos, C.M., Tsai, S.Y., Guillen, V., Ortega, J., Kartje, G.L., and Wolf, W.A. (2009). Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke 40: 294–302, https://doi.org/10.1161/strokeaha.108.519769.Suche in Google Scholar
Pardridge, W.M. and Connor, J.D. (1973). Saturable transport of amphetamine across the blood-brain barrier. Experientia 29: 302–304, https://doi.org/10.1007/bf01926490.Suche in Google Scholar
Park, Y.H., Kantor, L., Guptaroy, B., Zhang, M., Wang, K.K., and Gnegy, M.E. (2003). Repeated amphetamine treatment induces neurite outgrowth and enhanced amphetamine-stimulated dopamine release in rat pheochromocytoma cells (PC12 cells) via a protein kinase C- and mitogen activated protein kinase-dependent mechanism. J. Neurochem. 87: 1546–1557, https://doi.org/10.1046/j.1471-4159.2003.02127.x.Suche in Google Scholar PubMed
Patrick, K.S., Caldwell, R.W., Ferris, R.M., and Breese, G.R. (1987). Pharmacology of the enantiomers of threo-methylphenidate. J. Pharmacol. Exp. Ther. 241: 152–158.Suche in Google Scholar
Pavlovskaya, M., Hochstein, S., Keren, O., Mordvinov, E., and Groswasser, Z. (2007). Methylphenidate effect on hemispheric attentional imbalance in patients with traumatic brain injury: a psychophysical study. Brain Inj. 21: 489–497, https://doi.org/10.1080/02699050701311117.Suche in Google Scholar PubMed
Percheson, P.B., Carroll, J.J., and Screech, G. (1959). Ritalin (methylphenidate): clinical experiences. Can. Anaesth. Soc. J. 6: 277–282, https://doi.org/10.1007/bf03014251.Suche in Google Scholar PubMed
Plenger, P.M., Dixon, C.E., Castillo, R.M., Frankowski, R.F., Yablon, S.A., and Levin, H.S. (1996). Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch. Phys. Med. Rehabil. 77: 536–540, https://doi.org/10.1016/s0003-9993(96)90291-9.Suche in Google Scholar PubMed
Powers, W.J., Rabinstein, A.A., Ackerson, T., Adeoye, O.M., Bambakidis, N.C., Becker, K., Biller, J., Brown, M., Demaerschalk, B.M., Hoh, B., et al.. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50: e344–e418, https://doi.org/10.1161/str.0000000000000211.Suche in Google Scholar PubMed
Prasad, R.M., Dose, J.M., Dhillon, H.S., Carbary, T., and Kraemer, P.J. (1995). Amphetamine affects the behavioral outcome of lateral fluid percussion brain injury in the rat. Restor. Neurol. Neurosci. 9: 65–75, https://doi.org/10.3233/rnn-1995-9201.Suche in Google Scholar
Qi, F., Nitsche, M.A., Ren, X., Wang, D., and Wang, L. (2023). Top-down and bottom-up stimulation techniques combined with action observation treatment in stroke rehabilitation: a perspective. Front. Neurol. 14: 1156987, https://doi.org/10.3389/fneur.2023.1156987.Suche in Google Scholar PubMed PubMed Central
Qin, C., Yang, S., Chu, Y.H., Zhang, H., Pang, X.W., Chen, L., Zhou, L.Q., Chen, M., Tian, D.S., and Wang, W. (2022). Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct. Target Ther. 7: 215, https://doi.org/10.1038/s41392-022-01064-1.Suche in Google Scholar PubMed PubMed Central
Ramasubbu, R. and Goodyear, B.G. (2008). Methylphenidate modulates activity within cognitive neural networks of patients with post-stroke major depression: a placebo-controlled fMRI study. Neuropsychiatr. Dis. Treat. 4: 1251–1266, https://doi.org/10.2147/ndt.s4246.Suche in Google Scholar PubMed PubMed Central
Ramic, M., Emerick, A.J., Bollnow, M.R., O’Brien, T.E., Tsai, S.Y., and Kartje, G.L. (2006). Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 1111: 176–186, https://doi.org/10.1016/j.brainres.2006.06.063.Suche in Google Scholar PubMed
Rasmussen, N. (2008). America’s first amphetamine epidemic 1929-1971: a quantitative and qualitative retrospective with implications for the present. Am. J. Public Health 98: 974–985, https://doi.org/10.2105/ajph.2007.110593.Suche in Google Scholar
Rasmussen, R.S., Overgaard, K., Hildebrandt-Eriksen, E.S., and Boysen, G. (2006). D-amphetamine improves cognitive deficits and physical therapy promotes fine motor rehabilitation in a rat embolic stroke model. Acta Neurol. Scand. 113: 189–198, https://doi.org/10.1111/j.1600-0404.2005.00547.x.Suche in Google Scholar PubMed
Rasmussen, R.S., Overgaard, K., Kristiansen, U., and Johansen, F.F. (2011). Acute but not delayed amphetamine treatment improves behavioral outcome in a rat embolic stroke model. Neurol. Res. 33: 774–782, https://doi.org/10.1179/1743132811y.0000000009.Suche in Google Scholar
Regenhardt, R.W., Takase, H., Lo, E.H., and Lin, D.J. (2020). Translating concepts of neural repair after stroke: structural and functional targets for recovery. Restor. Neurol. Neurosci. 38: 67–92, https://doi.org/10.3233/rnn-190978.Suche in Google Scholar
Reis, C., Wang, Y., Akyol, O., Ho, W.M., Ii, R.A., Stier, G., Martin, R., and Zhang, J.H. (2015). What’s new in traumatic brain injury: update on tracking, monitoring and treatment. Int. J. Mol. Sci. 16: 11903–11965, https://doi.org/10.3390/ijms160611903.Suche in Google Scholar PubMed PubMed Central
Richardson, E., Seibert, T., and Uli, N.K. (2017). Growth perturbations from stimulant medications and inhaled corticosteroids. Transl. Pediatr. 6: 237–247, https://doi.org/10.21037/tp.2017.09.14.Suche in Google Scholar PubMed PubMed Central
Riddle, E.L., Hanson, G.R., and Fleckenstein, A.E. (2007). Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur. J. Pharmacol. 571: 25–28, https://doi.org/10.1016/j.ejphar.2007.05.044.Suche in Google Scholar PubMed PubMed Central
Roberts, J.K., Cook, S.F., Stockmann, C., Rollins, D.E., Wilkins, D.G., and Sherwin, C.M. (2015). A population pharmacokinetic analysis of dextroamphetamine in the plasma and hair of healthy adults. Clin. Drug Investig. 35: 633–643, https://doi.org/10.1007/s40261-015-0323-5.Suche in Google Scholar PubMed PubMed Central
Sanchez-Mendoza, E.H. and Hermann, D.M. (2016). Correlates of post-stroke brain plasticity, relationship to pathophysiological settings and implications for human proof-of-concept studies. Front. Cell. Neurosci. 10: 196, https://doi.org/10.3389/fncel.2016.00196.Suche in Google Scholar PubMed PubMed Central
Saniova, B., Drobny, M., Kneslova, L., and Minarik, M. (2004). The outcome of patients with severe head injuries treated with amantadine sulphate. J. Neural. Transm. 111: 511–514, https://doi.org/10.1007/s00702-004-0112-4.Suche in Google Scholar PubMed
Schuster, C., Maunz, G., Lutz, K., Kischka, U., Sturzenegger, R., and Ettlin, T. (2011). Dexamphetamine improves upper extremity outcome during rehabilitation after stroke: a pilot randomized controlled trial. Neurorehabil. Neural Repair 25: 749–755, https://doi.org/10.1177/1545968311405674.Suche in Google Scholar PubMed
Scott, T.L. and Vonder Haar, C. (2019). Frontal brain injury chronically impairs timing behavior in rats. Behav. Brain Res. 356: 408–414, https://doi.org/10.1016/j.bbr.2018.09.004.Suche in Google Scholar PubMed PubMed Central
Serra, M., Simola, N., Pollack, A.E., and Costa, G. (2024). Brain dysfunctions and neurotoxicity induced by psychostimulants in experimental models and humans: an overview of recent findings. Neural Regen. Res. 19: 1908–1918, https://doi.org/10.4103/1673-5374.390971.Suche in Google Scholar PubMed PubMed Central
Shehjar, F., Maktabi, B., Rahman, Z.A., Bahader, G.A., James, A.W., Naqvi, A., Mahajan, R., and Shah, Z.A. (2023). Stroke: molecular mechanisms and therapies: update on recent developments. Neurochem. Int. 162: 105458, https://doi.org/10.1016/j.neuint.2022.105458.Suche in Google Scholar PubMed PubMed Central
Shi, W.X., Zhang, X.Y., Pun, C.L., and Bunney, B.S. (2007). Clozapine blocks D-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology 32: 1922–1928, https://doi.org/10.1038/sj.npp.1301334.Suche in Google Scholar PubMed
Sonde, L. and Lokk, J. (2007). Effects of amphetamine and/or L-dopa and physiotherapy after stroke - a blinded randomized study. Acta Neurol. Scand. 115: 55–59, https://doi.org/10.1111/j.1600-0404.2006.00728.x.Suche in Google Scholar PubMed
Sonde, L., Nordstrom, M., Nilsson, C.G., Lokk, J., and Viitanen, M. (2001). A double-blind placebo-controlled study of the effects of amphetamine and physiotherapy after stroke. Cerebrovasc. Dis. 12: 253–257, https://doi.org/10.1159/000047712.Suche in Google Scholar PubMed
Song, A.K., Hay, K.R., Trujillo, P., Aumann, M., Stark, A.J., Yan, Y., Kang, H., Donahue, M.J., Zald, D.H., and Claassen, D.O. (2021). Amphetamine-induced dopamine release and impulsivity in Parkinson disease. Brain 145: 3488–3499, https://doi.org/10.1093/brain/awab487.Suche in Google Scholar PubMed PubMed Central
Speech, T.J., Rao, S.M., Osmon, D.C., and Sperry, L.T. (1993). A double-blind controlled study of methylphenidate treatment in closed head injury. Brain Inj. 7: 333–338, https://doi.org/10.3109/02699059309034959.Suche in Google Scholar PubMed
Sprigg, N., Willmot, M.R., Gray, L.J., Sunderland, A., Pomeroy, V., Walker, M., and Bath, P.M. (2007). Amphetamine increases blood pressure and heart rate but has no effect on motor recovery or cerebral haemodynamics in ischaemic stroke: a randomized controlled trial (ISRCTN 36285333). J. Hum. Hypertens. 21: 616–624, https://doi.org/10.1038/sj.jhh.1002205.Suche in Google Scholar PubMed
Starostka-Tatar, A., Labuz-Roszak, B., Skrzypek, M., Gasior, M., and Gierlotka, M. (2017). [Definition and treatment of stroke over the centuries]. Wiad Lek 70: 982–987.Suche in Google Scholar
Stengler-Wenzke, K. and Muller, U. (2002). Fluoxetine for OCD after brain injury. Am. J. Psychiatr. 159: 872, https://doi.org/10.1176/appi.ajp.159.5.872.Suche in Google Scholar PubMed
Stinear, C.M., Lang, C.E., Zeiler, S., and Byblow, W.D. (2020). Advances and challenges in stroke rehabilitation. Lancet Neurol. 19: 348–360, https://doi.org/10.1016/s1474-4422(19)30415-6.Suche in Google Scholar
Stroemer, R.P., Kent, T.A., and Hulsebosch, C.E. (1998). Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29: 2381–2393, discussion 2393-2385, https://doi.org/10.1161/01.str.29.11.2381.Suche in Google Scholar PubMed
Sutton, R.L. and Feeney, D.M. (1992). α-Noradrenergic agonists and antagonists affect recovery and maintenance of beam-walking ability after sensorimotor cortex ablation in the rat. Restor. Neurol. Neurosci. 4: 1–11, https://doi.org/10.3233/rnn-1992-4101.Suche in Google Scholar
Sutton, R.L., Hovda, D.A., Chen, M.J., and Feeney, D.M. (2000). Alleviation of brain injury-induced cerebral metabolic depression by amphetamine: a cytochrome oxidase histochemistry study. Neural. Plast. 7: 109–125, https://doi.org/10.1155/np.2000.109.Suche in Google Scholar PubMed PubMed Central
Szporny, L. and Görög, P. (1961). Investigations into the correlations between monoamine oxidase inhibition and other effects due to methylphenydate and its stereoisomers. Biochem. Pharmacol. 8: 263–268, https://doi.org/10.1016/0006-2952(61)90100-9.Suche in Google Scholar
Talsky, A., Pacione, L.R., Shaw, T., Wasserman, L., Lerry, A., Verma, A., Hurwitz, G., Waxman, R., Morgan, A., and Bhalerao, S. (2011). Pharmacological interventions for traumatic brain injury. BC. Med. J. 53: 26–31, Clinical Articles.Suche in Google Scholar
Tardy, J., Pariente, J., Leger, A., Dechaumont-Palacin, S., Gerdelat, A., Guiraud, V., Conchou, F., Albucher, J.F., Marque, P., Franceries, X., et al.. (2006). Methylphenidate modulates cerebral post-stroke reorganization. Neuroimage 33: 913–922, https://doi.org/10.1016/j.neuroimage.2006.07.014.Suche in Google Scholar PubMed
Teixeira-Gomes, A., Costa, V.M., Feio-Azevedo, R., Bastos Mde, L., Carvalho, F., and Capela, J.P. (2015). The neurotoxicity of amphetamines during the adolescent period. Int. J. Dev. Neurosci. 41: 44–62, https://doi.org/10.1016/j.ijdevneu.2014.12.001.Suche in Google Scholar PubMed
Thieme, H., Morkisch, N., Mehrholz, J., Pohl, M., Behrens, J., Borgetto, B., and Dohle, C. (2018). Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 7: CD008449, https://doi.org/10.1002/14651858.cd008449.pub3.Suche in Google Scholar PubMed PubMed Central
Treig, T., Werner, C., Sachse, M., and Hesse, S. (2003). No benefit from D-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin. Rehabil. 17: 590–599, https://doi.org/10.1191/0269215503cr653oa.Suche in Google Scholar PubMed
Trotman, M., Vermehren, P., Gibson, C.L., and Fern, R. (2015). The dichotomy of memantine treatment for ischemic stroke: dose-dependent protective and detrimental effects. J. Cereb. Blood. Flow Metab. 35: 230–239, https://doi.org/10.1038/jcbfm.2014.188.Suche in Google Scholar PubMed PubMed Central
Tsao, C.W., Aday, A.W., Almarzooq, Z.I., Anderson, C.A.M., Arora, P., Avery, C.L., Baker-Smith, C.M., Beaton, A.Z., Boehme, A.K., Buxton, A.E., et al.. (2023). Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation 147: e93–e621, https://doi.org/10.1161/cir.0000000000001123.Suche in Google Scholar
Turowski, P. and Kenny, B.A. (2015). The blood-brain barrier and methamphetamine: open sesame? Front. Neurosci. 9: 156, https://doi.org/10.3389/fnins.2015.00156.Suche in Google Scholar PubMed PubMed Central
UNODC (2021). Drug market trends: cocaine amphetamine-type stimulants in world drug report 2021. United Nations, Vienna, pp. 47–72.10.18356/9789210058032c031Suche in Google Scholar
Virani, S.S., Alonso, A., Aparicio, H.J., Benjamin, E.J., Bittencourt, M.S., Callaway, C.W., Carson, A.P., Chamberlain, A.M., Cheng, S., Delling, F.N., et al.. (2021). Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation 143: e254–e743, https://doi.org/10.1161/cir.0000000000000950.Suche in Google Scholar
Volkow, N.D., Wang, G., Fowler, J.S., Logan, J., Gerasimov, M., Maynard, L., Ding, Y., Gatley, S.J., Gifford, A., and Franceschi, D. (2001). Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 21: Rc121, https://doi.org/10.1523/jneurosci.21-02-j0001.2001.Suche in Google Scholar
Volz, T.J., Farnsworth, S.J., Hanson, G.R., and Fleckenstein, A.E. (2008). Methylphenidate-induced alterations in synaptic vesicle trafficking and activity. Ann. N. Y. Acad. Sci. 1139: 285–290, https://doi.org/10.1196/annals.1432.012.Suche in Google Scholar PubMed PubMed Central
Wabe, N.T. (2011). Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): a review. Addict. Health 3: 137–149.Suche in Google Scholar
Wagner, A.K., Drewencki, L.L., Chen, X., Santos, F.R., Khan, A.S., Harun, R., Torres, G.E., Michael, A.C., and Dixon, C.E. (2009). Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 108: 986–997, https://doi.org/10.1111/j.1471-4159.2008.05840.x.Suche in Google Scholar PubMed PubMed Central
Wagner, A.K., Kline, A.E., Ren, D., Willard, L.A., Wenger, M.K., Zafonte, R.D., and Dixon, C.E. (2007). Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav. Brain Res. 181: 200–209, https://doi.org/10.1016/j.bbr.2007.04.006.Suche in Google Scholar PubMed PubMed Central
Walker-Batson, D., Curtis, S., Natarajan, R., Ford, J., Dronkers, N., Salmeron, E., Lai, J., and Unwin, D.H. (2001). A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke 32: 2093–2098, https://doi.org/10.1161/hs0901.095720.Suche in Google Scholar PubMed
Walker-Batson, D., Unwin, H., Curtis, S., Allen, E., Wood, M., Smith, P., Devous, M.D., Reynolds, S., and Greenlee, R.G. (1992). Use of amphetamine in the treatment of aphasia. Restor. Neurol. Neurosci. 4: 47–50, https://doi.org/10.3233/rnn-1992-4106.Suche in Google Scholar PubMed
Wang, Q.M., Cui, H., Han, S.J., Black-Schaffer, R., Volz, M.S., Lee, Y.T., Herman, S., Latif, L.A., Zafonte, R., and Fregni, F. (2014). Combination of transcranial direct current stimulation and methylphenidate in subacute stroke. Neurosci. Lett. 569: 6–11, https://doi.org/10.1016/j.neulet.2014.03.011.Suche in Google Scholar PubMed
Wang, W., Liu, X., Yang, Z., Shen, H., Liu, L., Yu, Y., and Zhang, T. (2020). Levodopa improves cognitive function and the deficits of structural synaptic plasticity in Hippocampus induced by global cerebral ischemia/reperfusion injury in rats. Front. Neurosci. 14: 586321, https://doi.org/10.3389/fnins.2020.586321.Suche in Google Scholar PubMed PubMed Central
Westmacott, R., Askalan, R., MacGregor, D., Anderson, P., and Deveber, G. (2010). Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev. Med. Child Neurol. 52: 386–393, https://doi.org/10.1111/j.1469-8749.2009.03403.x.Suche in Google Scholar PubMed
Westover, A.N., McBride, S., and Haley, R.W. (2007). Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch. Gen. Psychiatr. 64: 495–502, https://doi.org/10.1001/archpsyc.64.4.495.Suche in Google Scholar PubMed
WHO (2022). ICD-11: International classification of diseases, 11th revision. World Health Organization, Geneva.Suche in Google Scholar
Whyte, J., Hart, T., Schuster, K., Fleming, M., Polansky, M., and Coslett, H.B. (1997). Effects of methylphenidate on attentional function after traumatic brain injury. A randomized, placebo-controlled trial. Am. J. Phys. Med. Rehabil. 76: 440–450, https://doi.org/10.1097/00002060-199711000-00002.Suche in Google Scholar PubMed
Whyte, J., Hart, T., Vaccaro, M., Grieb-Neff, P., Risser, A., Polansky, M., and Coslett, H.B. (2004). Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 83: 401–420, https://doi.org/10.1097/01.phm.0000128789.75375.d3.Suche in Google Scholar PubMed
Wiggs, K.K., Froehlich, T.E., and Becker, S.P. (2023). Pharmacologic management of cognitive disengagement syndrome (CDS) and implications for attention-deficit/hyperactivity disorder (ADHD) treatment: emerging treatments and recommendations for future research. CNS Drugs 37: 293–304, https://doi.org/10.1007/s40263-023-00999-5.Suche in Google Scholar PubMed
Williams, S.E., Ris, M.D., Ayyangar, R., Schefft, B.K., and Berch, D. (1998). Recovery in pediatric brain injury: is psychostimulant medication beneficial? J. Head Trauma Rehabil. 13: 73–81, https://doi.org/10.1097/00001199-199806000-00007.Suche in Google Scholar PubMed
Willmott, C. and Ponsford, J. (2009). Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: a randomised, crossover, double blind, placebo controlled inpatient trial. J. Neurol. Neurosurg. Psychiatr. 80: 552–557, https://doi.org/10.1136/jnnp.2008.159632.Suche in Google Scholar PubMed
Willmott, C., Ponsford, J., McAllister, T.W., and Burke, R. (2013). Effect of COMT Val158Met genotype on attention and response to methylphenidate following traumatic brain injury. Brain Inj. 27: 1281–1286, https://doi.org/10.3109/02699052.2013.809553.Suche in Google Scholar PubMed
Wolf, W.A., Martin, J.L., Kartje, G.L., and Farrer, R.G. (2014). Evidence for fibroblast growth factor-2 as a mediator of amphetamine-enhanced motor improvement following stroke. PLoS One 9: e108031, https://doi.org/10.1371/journal.pone.0108031.Suche in Google Scholar PubMed PubMed Central
Wroblewski, B.A., Joseph, A.B., Kupfer, J., and Kalliel, K. (1997). Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj. 11: 37–47, https://doi.org/10.1080/026990597123791.Suche in Google Scholar PubMed
Zhang, W.T. and Wang, Y.F. (2017). Efficacy of methylphenidate for the treatment of mental sequelae after traumatic brain injury. Medicine 96: e6960, https://doi.org/10.1097/md.0000000000006960.Suche in Google Scholar
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Amphetamine and methylphenidate potential on the recovery from stroke and traumatic brain injury: a review
- Insights on cognitive reorganization after hemispherectomy in Rasmussen’s encephalitis. A narrative review
- Task-based EEG and fMRI paradigms in a multimodal clinical diagnostic framework for disorders of consciousness
- The impact of genetic factors on the response to migraine therapy
- Metabolic dysregulation of tricarboxylic acid cycle and oxidative phosphorylation in glioblastoma
Artikel in diesem Heft
- Frontmatter
- Amphetamine and methylphenidate potential on the recovery from stroke and traumatic brain injury: a review
- Insights on cognitive reorganization after hemispherectomy in Rasmussen’s encephalitis. A narrative review
- Task-based EEG and fMRI paradigms in a multimodal clinical diagnostic framework for disorders of consciousness
- The impact of genetic factors on the response to migraine therapy
- Metabolic dysregulation of tricarboxylic acid cycle and oxidative phosphorylation in glioblastoma

