Abstract
Over the past few decades, biomaterials have been used extensively in medical science. These biomaterials have effectively replaced a variety of bodily tissues found in the human body, including teeth, ligaments, bones, tendons, and others. The primary barrier to the adoption of biomaterials is immune rejection, as bone replacement and lifetime implants currently require biocompatibility in addition to the mechanical and biological properties of the biomaterial. Numerous materials are presently being researched and commercially accessible to preserve and restore physiological functioning; they are widely used in medical science and biotherapy. Novel biomaterials were developed in response to emerging therapeutic needs, and recently discovered biomaterials made it possible to undertake novel clinical applications. When it was recognized that biomaterials must have an essential quality biocompatibility, the term “biomaterials” was coined to refer to materials specifically designed for biomedical applications. Presently, biological tissues and materials generated from biology are also included. The current review looks at the scientific literature published on the subject while exploring the application of biomaterials. Lastly, a brief overview of some recent uses for biomaterials is given, along with predictions about their potential future use.
Highlights
The basic requirement of implantable biomaterials such as mechanical strength, biocompatibility, and corrosion characteristics are deeply explained.
Surface modifications such as coatings and laser treatments are suggested to improve the chemical bonding ability with natural bone and anti-corrosion property.
Different types of coatings used by several researchers are compared, the advantages and disadvantages of each coating methods are briefly explained.
The interfacial interactions between the bioimplant-tissues with respect to the applications in both hard and soft tissue are explained.
1 Introduction
Biomaterials that interact with biological systems are used in the engineering of functional extra-organ tissues, the prevention, diagnosis, and treatment of disease, the safe and efficient delivery of drugs, and the encouragement of the body’s natural healing process. With an emphasis on features that allowed for function restoration and acute pathology mitigation, the field has developed beyond choosing materials that were initially intended for different uses. For instance, biomaterials are used in the development of stents to open blocked blood vessels or in the creation of artificial skin for burn victims. In order to integrate with biological complexity and carry out specific, high-level functions in the body, biomaterials are now logically constructed with regulated structure and dynamic functionality. Biomaterials is a scientific field that integrates biology, physics, chemistry, medicine, and, more recently, materials science and tissue engineering. The field of research has expanded dramatically over the last ten years as a result of advancements in regenerative medicine, tissue engineering, medical contrast imaging agents, gene therapy, controlled drug delivery systems, artificial organs, immobilized enzyme bioreactors, biosensors, and materials for minimally invasive surgery. 1 , 2
Biomaterials can be made from metals, ceramics, glass, plastic, and biological tissue and cells. For usage in biomedical devices and goods, they can be reengineered into coatings, fibers, films, foams, textiles, and molded or machined fragments. Contact lenses, hip joint replacements, dental implants, and heart valves are a few examples. They are frequently biodegradable, and some are bio-absorbable, which means that after serving a purpose, they are progressively removed from the body. Applications for biomaterials can be found in immunology, histology, neurology, cardiovascular surgery, dentistry, orthopaedics, ophthalmology, and veterinary medicine. The essential features of biomaterials are corrosion and wear resistance, which should provide fracture strength, low elastic modulus, high strength, excellent biocompatibility, adhesion, bio functionality and non-toxicity. A necessity of biomaterial is biocompatibility: It is the ability of the materials to perform effectively with appropriate host response in a specific situation for suitable applications. 3 , 4
According to a few authors, biomaterials are defined as: Buchaman 5 defined “Biomaterials as a non-viable material used in medical devices intended to interact with the biological system”. They can be distinguished from other materials, which possess a combination of properties including chemical, mechanical, physical and biological properties. Sharma et al., defined “Biomaterials as any systemically, pharmacologically inert substances or combination of substances utilized for implantation within or incorporation with a living system to supplement”. 6 To achieve that purpose, biomaterial must be in contact with living tissues, resulting in an interface between living and nonliving substances. According to Hench and Ethridge, “Biomaterial is a bioactive material that elicits a specific biological response at the interface of the materials, which results in the formation between the tissue and the materials”. 7
Biomaterials are used to mimic the tissues by a combination of physical and chemical properties with minimum foreign body host response. In the field of biomaterials, a new technique known as “biomimetic biomaterials” has emerged, integrating system analysis for the treatment of patients with disabilities and in surgery to assist the elderly and promote their rehabilitation. Furthermore, the goal of replacing injured or sick human body parts has been sought after for generations. Gaspare and Zimbler, and other early plastic surgeons were able to repair lost noses with autogenous skin flaps in the sixteenth century. Since there was no knowledge of sterilization, immunological reaction, inflammation, and biodegradation at the time, all of these initial surgical procedures had been carried out without any awareness of the issues and constraints pertaining to material science and biological phenomena. Their “unconscious” success, however, amply illustrates how remarkable the human body is in adapting to and incorporating foreign substances. This made it possible to proceed with the development of biomaterials before considering the basic interactions between the body and the materials that were implanted; the systematic investigation of these interactions only started around 150 years ago, when researchers and medical professionals began to assess the body’s response to exogenous materials. 8
The most recent applications of different biomaterials (natural and synthetic) as well as the usage of different bioactive mineral fillers for tissue engineering and biomedicine will be discussed in this article. The materials, mechanical properties, and structure of natural bone are then covered, along with bone tissue engineering. The current knowledge of biomaterials, including their types, characteristics, and uses, that are employed in the development of scaffolds for more biological applications is the special emphasis of this paper. By emphasizing the potential of biomaterials and their prospects for use in biomedical applications in the future, the review also offers insight into the most recent strategies and applications in the field of biomedical applications.
2 Progress of biomaterials
Biomaterials have been developed based on their intended usage for biomedical purposes over the last 60 years and have evolved through three different generations as the first, second and third generations of biomaterials. These general classifications are given in Table 1, along with the important properties with applications of biomaterial.
Progress of biomaterials and its applications.
| S. No. | Periods | Category of biomaterials | Applications | Examples | References |
|---|---|---|---|---|---|
| 1 | 1950–1969 | First generation ( Bioinert ) | – Fracture fixation – Bone and joint replacement – Dentals implants – Surgical instruments |
– Metallic alloys (316L SS, Ti alloys, Co–Cr alloys) – Ceramic materials (Al 2 O 3 , ZrO 2 and porous materials, etc.,) – Polymers (Silicone rubber, polyurethanes, polypropylene and polymethyl methacrylate) |
9 , 10 |
| 2 | 1970–2000 | Second generation ( Bioactive and biodegradable ) | – Coatings of tissues in growth – Dental implants – Temporary bone space fillers – Percutaneous access devices – Artificial tendon and ligaments – Biodegradable polymers and hydro gel |
– Ceramics (Calcium phosphates, glass ceramics and bioactive glasses) – Metals (Bioactive coated and chemically modified surfaces of metallic alloys) – Biodegradable polymers (Polylactide-PLA, Polydioxanone-PDS, Poly(3-caprolactone)-PCL, Polyhydroxybutyrate-PHB) |
11 , 12 |
| 3 | 2000–till date | Third generation ( Materials designed to stimulate specific for cellular responses at the molecular level ) | MEMS and NEMS devices – Blood pressure sensors – DNA array systems – Tissue engineering – Tissue repair and regeneration – Cell encapsulation – Bioartificial skin and liver construction |
– Three dimensional Scaffolds (Bioactive ceramics and biodegradable polymers) – Hydrogels – Nanofibers |
13 , 14 |
| Nanostructured biological system – Nanoparticles-based scaffolds encapsulation on pharmaceutical |
2.1 First generation biomaterials
The first-generation biomaterials were developed for wound curing, as well as orthopaedic, dental, and cardiovascular treatment applications during the 1950s and 1960s. Generally, in this generation, naturally available materials such as polymers, proteins and polysaccharide-based materials are considered for implantation into the human body due to their functionality, biocompatibility and practicability. When first-generation materials are inserted into the human body, they should exhibit adequate physio-mechanical properties to enhance the growth or substitute the body tissues. The practicability is the amenability of the biomaterial to the machine or the ability to form different shapes for ready usage at a low cost. 15 , 16
To treat skeletal defects, synthetic metallic material is used for fracture fixation and to replace tissue that has lost its activity due to damage or disease. 17 According to Rodriguez et al., a 2000 BC skull was discovered in Peru that had a bone defect repaired with a thin gold plate. Abundant classes of biomaterials (metallic and polymeric materials) have been approved since the twentieth century. 18 In an in vivo investigation using a feline model, Bothe, showed that titanium was readily absorbed by the surrounding tissues while showing development of bone in contact with titanium. It should be emphasized that inventions extended beyond advanced metal applications. 19 The main goal of first-generation materials was the creation of bioinert material with mechanical properties, corrosion resistance, biocompatibility and non-toxicity.
2.2 Second-generation materials
The second generation of biomaterials was produced between 1970 and 2000 and retained the biocompatibility property from the first generation. However, in this generation of biomaterials, the bioinert behaviour was replaced by the development of bioactive, bioresorbable and biodegradable materials. The paramount importance of second-generation biomaterials is that they can act as bioactive by combining biodegradable and bioresorbable behaviour. Bioactive materials are widely used in clinical applications and are in enormous demand. When placed in the human body, these bioactive materials can form a stable chemical bond with natural bone and no response for the inert materials. Hence, these second-generation bioactive materials are mostly used in dentistry and orthopaedic fields. 20
Second-generation materials are developed for a novel biomaterial with specific tissue response. e.g., Hydroxyapatite is a bioactive ceramic with similar bone mineral composition. Duchene 1994 stated bioactive ceramics hydroxyapatite promotes bone ingrowth with excellent biocompatibility. Hydroxyapatite is coated on the maxillofacial orthopaedic and dental implant for bone cell attachment and proliferation. 21 According to Hassan, bioresorbable polymer is the second class of second-generation material used in drug-elating stents to maintain the patency of coronary arteries. 22
2.3 Third-generation biomaterials
The biomaterials developed from 2000 are the third generation, which have excellent properties. Third-generation biomaterials are used in clinical applications to heal severe problems and enhance the quality of human health. In this generation, biomaterials possess the characteristics of the second generation, such as bioactivity, biosorption, and biodegradability. Micro and nanofabrication techniques are embraced to develop the third generation of biomaterials for usage in the advanced tissue engineering field. The primary motivation for producing the third generation is to recreate the human tissue rather than repair or replace the diseased part of the human body. 23 , 24
Third-generation material is developed to promote cell-specific activities. Williams, 1999 defined third-generation material as a suitable host response for a specified application. 25 Material with suitable microstructure is developed for medical applications by novel processing and biological interaction, such as antigen-antibody and protein signal transductions. 26 , 27 Tissue engineering is the use of knowledge and skills from a variety of disciplines to create and produce therapeutic products that repair, restore, or regenerate damaged cells or tissues caused by disease or injury by combining matrix scaffolds with viable human cell systems or cell-responsive biomolecules derived from such cells. It is beneficial to replicate the natural extracellular matrix’s (ECM) nano topography in order to successfully regenerate injured tissues or organs. For tissue engineering applications, synthetic and natural polymer scaffolds based on electrospun nanofibers are being investigated that resembles natural extracellular matrix. 28 Figure 1 Denotes evolution of biomaterials and its examples.
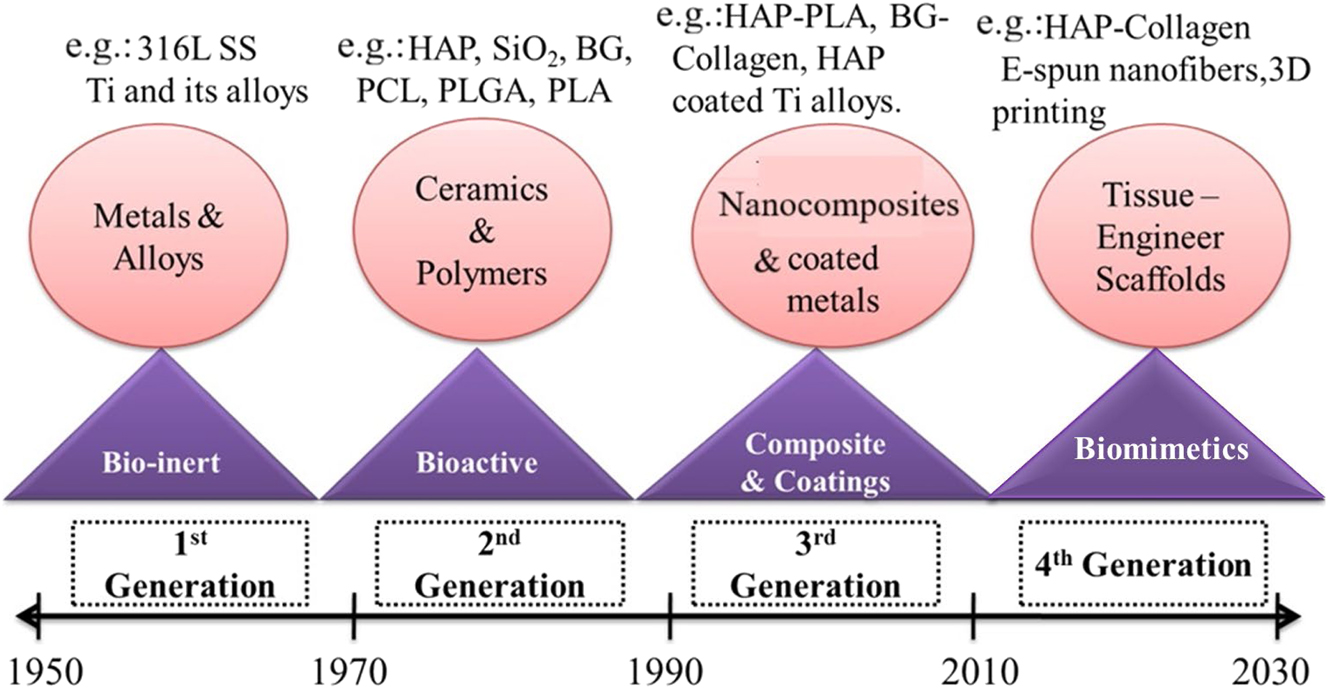
Evolution of biomaterials and examples.
3 Types of biomaterials
Biomaterials are mainly classified into the two types such as naturally derived and synthetic biomaterials.
3.1 Natural biomaterials
Natural biomaterials are highly recommended for surgical treatments than synthetic biomaterials due to their higher biocompatibility, bone remodeling and biodegradation rate in biological systems. 29 Therefore, these natural biomaterials are used to repair or replace damaged parts of human tissues and organs. Natural biomaterials are categorized into various types based on their occurrence, such as protein, polysaccharide, and decellularized tissue-based natural biomaterials. Natural biomaterials promote cell attachment, migration, proliferation, and differentiation when placed in bone-defective areas. They also promote faster bone regeneration, which leads to the formation of an extracellular matrix for bone development. 30 , 31 , 32 The varieties of natural biomaterials are shown in Figure 2.

Classification of natural biomaterials.
3.2 Synthetic biomaterials
Synthetic biomaterials are classified into different types such as polymer, ceramics, composites and metals and its alloys based on their uses in orthopaedic and dental field. The synthetic biomaterials are commercialized and widely applied in clinical applications. Classification of synthetic biomaterials are given in Figure 3.
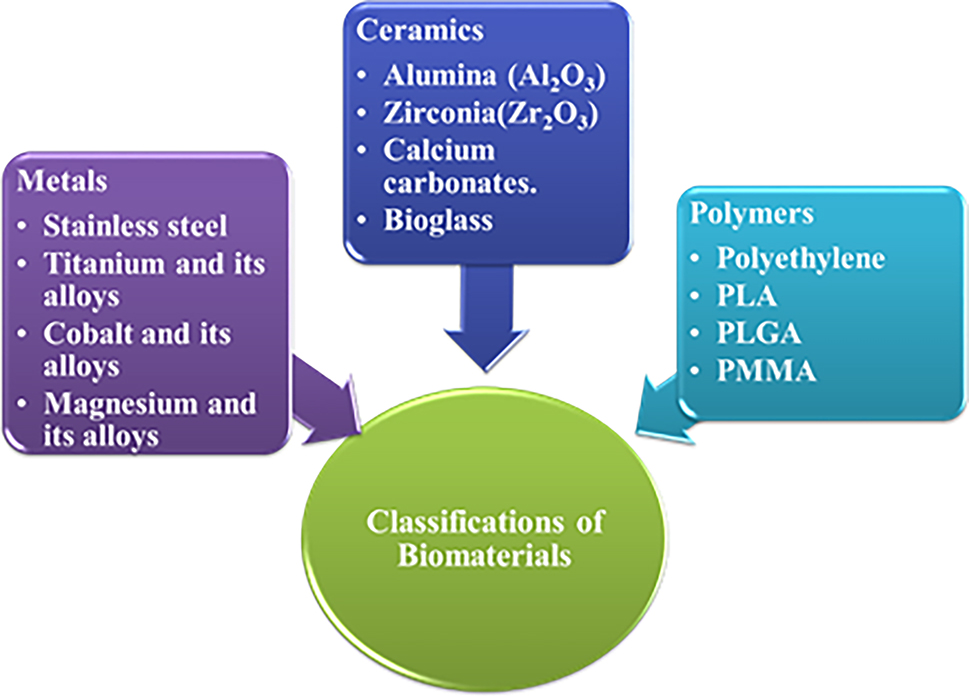
Classification of biomaterials.
3.2.1 Bioceramics
A broad category of specifically engineered crystalline, semicrystalline, or amorphous materials known as “bioceramics” are utilized in the restoration and replacement of diseased or damaged body components. 33 The long and noble history of employing ceramics in medicine began in the 10th century AD when it was found that calcium sulfate could heal the broken bones of cadavers and mummies. 34 In 1892, surviving patients with tuberculous osteomyelitis had the first implant of calcium sulfate, commonly referred to as “plaster of Paris,” as a bone void filler (Dreesmann, 1892, cited in 35]). More recently, in 1959, Peltier brought it back in the form of high-purity pellets as a replacement for bone. 35 Bioceramics are polycrystalline in nature with adequate properties like chemical stability, high elastic modulus, high strength, hardness, brittleness, wear resistance and low density.
It is useful to note that, after blood, bone is currently the tissue that needs repair the most. 36 Bone reconstruction can now be carried out with artificial implants or transplanted tissues. Numerous ceramic compositions, including hydroxyapatite (HA), bioactive glasses, and inert ceramics like alumina and zirconia, have been tested as bone grafting materials throughout the years. 37 Currently, the major applications for bone repair are mouldable pastes (e.g., injectable cements for spine surgery), porous granules and stiff scaffolds for filling bone gaps, and monolithic devices (e.g., employed in the rebuilding of tiny bones in the middle ear).
The capacity to establish a stable connection with host tissue is crucial in the selection of ceramics for bone substitution; in this context, bioactive and bioresorbable ceramics offer a significant answer. 38 , 39 Moreover, bioresorbable ceramics have the added benefit of progressively disintegrating over time as natural bone replaces them; after they have served their purpose of serving as templates for new tissue, they vanish. 40 The subsequent segments concentrate on the primary categories of ceramic materials that are presently obtainable for therapeutic purposes; they also offer a brief outlook for enhancements and forthcoming investigations.
There are three classes of bioceramics
Bioinert ceramics (e.g., alumina, zirconia, titania)
Bioresorbable ceramics (e.g., calcium phosphate (HAP, TCP))
Bioactive ceramics (e.g., bioglass and glass ceramics)
3.2.1.1 Bioinert ceramics
When ceramic materials are placed in a human body as an implant, the interactions between the implant and the human tissue are minimal as well, and the interaction between the implant and the host tissue is chemically inactive. As a result, the ceramic material acts as an inert material in the human environment. Examples of bio-inert materials are Al2O3, ZrO2, TiO2, metallic alloys and polythene. However, bioinert ceramics are commonly used as fixation screws and wires in orthopaedic applications. 41
3.2.1.2 Bioresorbable ceramics
Bioresorbable ceramics are recommended for use in the drug delivery system and medical industry. Bioresorbable ceramics start slowly to dissolve in the human system. Hence, bioresorbable materials’ degradation or dissolution rate is necessary for the formation or regeneration rate of the general tissues in the body. Examples of bioresorbable ceramics are low-temperature heated hydroxyapatite and tricalcium phosphate. 42
3.2.1.3 Bioactive ceramics
The bioceramics materials, which initiate the proper interaction with the biological system, are called bioactive ceramics. The bioactive ceramic materials help in the formation of specific chemical bonds between the host tissues with the active interface of the material. Bioactive ceramics mainly have time-dependent activation surfaces with the exchange of ions between the ceramics and physiological fluids, which leads to the construction of bioactive layers on their surfaces. The rate of apatite layer formation on bioactive ceramics can enhance the cell interaction that promotes bone remodeling for a faster bone healing process. Examples of bioactive ceramics are hydroxyapatite, glass ceramics and bioactive glasses.
Ceramics are used in various fields like dentistry (e.g., crowns, bridges, denture teeth, dental root implants), orthopaedic, and medical sensors. It is used as an alternate natural tissue to fill the gap, reconstitute the function of the surrounding tissues, physical interaction with body fluids, stimulate osteogenesis, stress-bearing skeletal recovery, etc.; an important characteristic feature of ceramics is surface reactivity. It has the ability to bond with bones and enhance tissue formation. 43 Inert bioceramics elicit minimal tissue response and lead to a thin layer of fibrous immediately adjacent to the surface. Surface-active ceramics are partially soluble, resulting in ion exchange and potentially leading to direct chemical bonds with the bone. Resorbable bio-ceramics have greater solubility than surface-active ceramics. 44
3.2.2 Alumina (Al2O3)
Over the past decades, alumina ceramics have been found to be bioinert, have high hardness with increased abrasion, and have chemical inertness that found applications in load bearing, hip prosthesis, and the dental field. It has excellent wear and corrosion resistance. 45 Noiri et al., studied the histopathology of alumina with highly biocompatible material in socket implantation. 46 As a result, there was no damage or rejection of ceramics even after four weeks of implantations, with enhanced tissue growth with the proliferation of fibroblast cells and vascular invasion observed. Moreover, knee joints made up of alumina ceramics were found to induce weak tissue reactions when it is inserted into a Japanese white rabbit. An in vivo biocompatibility study of an alumina-coated implant caused an alteration in the platelets and was found to interact with the blood. Yuhta et al., studied the blood compatibility of alumina ceramics coated on the titanium implant by sputter deposition. The carcinogenic reaction was observed in the long-term application of alumina ceramics and proved non-toxic to the bone marrow upon implantation. 47
3.2.3 Zirconia (ZrO2)
Zirconia is widely used in prosthetic devices due to its excellent chemical properties, dimension stability, high mechanical strength, toughness and high Young’s modulus (210 GPa). 48 High mechanical strength and fracture toughness of zirconia have been found to have various applications in the biomedical field. It has the ability to undergo transformation toughening mechanism that is advantageous over the ceramics. It is mainly used in total hip replacement. Recently, zirconia ceramics were used in head balls for total hip replacements. The addition of stabilizing oxides like CaO, MgO, CeO2 and Y2O3 to zirconia ceramics results in multiphase ceramics material known as “partially stabilized zirconia”. It was found to have improved mechanical properties over other ceramics. 49
Lilley, studied the degradation strength of zirconia in wet environments and its corrosion resistance behaviour. He reported that the zirconia-coated material implanted in the paraspinal muscles of rabbits has shown excellent corrosion resistance behaviour in both wet and hot environments. 50 Biocompatibility of partially stabilized zirconia was observed in osteoblastic cell cultivation. By means of histomorphometries, the osteointegration of zirconia was examined in rats. The data confirmed the biocompatibility of zirconia with high bone mineral density over the orthopaedic implants. Since 1980, zirconia has been found to consist of highly radioactive elements that could cause adverse effects in tissue and organs. A study by Piconi and Maccauro and Chevalier et al., have proved that zirconia is biologically the safest ceramics, and its in-vivo cytotoxicity and cytocompatibility tests have proved that zirconia is highly biocompatible with fibroblast, osteoblast, cells, human lymphocytes, human cytoplast, macrophages, etc. 51 , 52
3.2.4 Titania (TiO2)
Titanium oxide and titanium alloys are the most commonly used biomaterials, and they are superior to other materials. It has excellent mechanical properties and biocompatibility and is non-toxic with multi-faced bioceramics. TiO2 (Anatase), TiO2 (Rutite), and Ti2O3 (Brookite) are the three types of oxides that exist at different sintering temperatures. The most commonly formed stable oxide is Anatase form. It has an elastic modulus of 110 GPa and is used to help in uniform stress distribution, 53 which is suitable for load-bearing applications. Various coating deposition techniques are used to deposit TiO2 coatings. Verket et al., have deposited three different coatings, TiO2, SiO2, and calcium phosphates, and compared the release of protein using normal human osteoblast cell lines. 54 From the observation, they found that TiO2 coatings support osteoblast growth and bone remodeling more than the other coatings. Vijayalakshmi et al., electrophoretically deposited titania and its coatings on Ti–6Al–4V and evaluated the corrosion resistance behavior in the ringer’s solution. They found that all the coatings exhibited good corrosion resistance and antibacterial activity with better surface uniformity on the implant. 55
3.2.5 Calcium phosphate
Bone is a ceramic that consists of 20 % collagen, 69 % calcium phosphate, 9 % water. The inorganic phase of bone is made of calcium phosphate. Since 1920, these materials have been used as a powder for filling new bone tissue formations. Saenz et al., 1999, reported that Ca/P ratio, porosity and crystalline structure are important factors for the success of calcium phosphate coatings. 56 They evaluated its biocompatibility and non-toxicity nature by in-vivo studies. Hydroxyapatite-based materials are most widely used in biomedical devices due to their osteoconductivity, crystallographic structure and chemical composition, which is similar to that of bone tissues. Calcium phosphate was found to interact with living cells and form excellent chemical bonding with bone. The use of different precursor materials will lead to different phase structures along with different morphology 57 , 58 and calcium fluorapatite is extensively used in dental applications. 59
For over twenty years, calcium phosphate ceramics were used to replace periodontal defects, augmentation of alveolar bone, sinus lifts, tooth replacements, and repair of large bone defects. Hydroxyapatite (HAP) and tricalcium phosphate are the most widely used calcium phosphates. Bio-resorption and bioactivity are the important properties of calcium phosphates. The biocompatibility of calcium phosphate was studied by Ruan and Grant, and according to him, HAP and TCP stimulate cell proliferation. 60 Yuan et al., evaluated the osteointegration property of calcium phosphate ceramics with different doping materials on HAP. 61
3.2.6 Hydroxyapatite
Hydroxyapatite is the most suitable material for doping different functional groups as it has a similar calcium-to-phosphate ratio with human bone minerals. Hydroxyapatite Ca10(PO4)6(OH)2 with Ca/p ratio of 1.67 with composition 39.68 wt% Ca, 18.45 wt% p wt. ratio-2.15 % hexagonal structure used as a model for inorganic components of bone and teeth. The lattice parameters are a = b = 9.432 Å, c = 6.881 Å, and z = 1. HAP is more stable, non-decomposable, and bioactive among calcium phosphates due to its resorbable behaviour. It is used as coatings on implants to repair and reconstruct diseased or damaged tissue. 62 Figure 4 represents Crystal Structure of Hydroxyapatite (Ca10(PO4)6(OH)2).

Crystal structure of hydroxyapatite (Ca10(PO4)6(OH)2).
It can be synthesized in two ways:
Solid-state reaction (an alternative to sol-gel), flux method, electro crystallization, spray pyrolysis, freeze-drying, etc.
Wet chemical method or co-precipitation methods.
HAP is widely used in the medical field as implants, coatings, and prostheses. It has excellent osteoconductivity, non-toxicity and non-inflammatory in nature. Its functions are to promote osteoblast adhesion, migration, differentiation, and proliferation, which are used in bone regeneration applications. It also has the ability to bond directly to bone. HAP nanoparticles were found to induce cell apoptosis, and their bioactivity properties made HAP ceramics a favorable material for implant applications. 63 , 64 , 65 The crystalline form of HAP exhibits bio-integration, and there is no formation of fibrous tissue. HAP can be synthesized in the form of dense ceramics, powder, ceramic coating or porous ceramics. Sadat Shojdi et al. reported different synthesis methods, such as the dry method, wet method and high-temperature processes. 66 They have synthesized different morphology and crystallography with various mechanical properties. Along with the pure HAP, carbonated and fluorapatite materials with similar apatite structures with low solubility can be used for biomedical purposes. 67 , 68 , 69 HAP can also be prepared using waste materials such as eggshell and snail-shell, and they exhibit excellent biocompatibility with different osteoblast and fibroblast cell lines. 70 , 71 The advantages and disadvantages of biomaterials are presented in Table 2.
Advantages and disadvantages of biomaterials.
| Biomaterials | Advantages | Disadvantages | References |
|---|---|---|---|
| (1) Natural materials: Collagen, human tissues, hyaluronic acid, grafts | Availability in the human body, biocompatibility | Host rejection | 72 |
| (2) Metals: 316L SS, titanium and its alloys, Co–Cr, Co–Cr–Mo, silver | Ductility, high mechanical resistances to wear and shock | Low biocompatibility, corrosion mechanical properties | 73 , 74 |
| (3) Ceramics: Alumina, titanium oxides, zirconia, bioglass | High biocompatibility, corrosion resistance, bioinert, low thermal and electrical conductivity | Low impact resistance, processing difficulties, fabrication and properties | 75 , 76 |
| (4) Composites: Alumina-HA, alumina-zirconia-HA, HA-silica | High biocompatibility, bioactivity and bio inertness | Low stability and mechanical strength | 77 , 78 , 79 |
3.2.7 Tricalcium phosphate
Tri-calcium phosphate exhibits different polymorphic forms (α, β, γ and super-α) and α and β TCP are the most preferred materials for biomedical devices. Tri-calcium phosphate has been used as a long-term material for bone replacement. Tri-calcium phosphate acts as an osteoconductive material and promotes bone growth on the surface of the implants. Applications were found in dental implants (capping agents, cleft palate), vertical bone defects and implant coatings. It has poor mechanical strength and low crack resistance. Levin et al., first reported the use of TCP as dental ceramics, in the periodontal defects of dogs. 80 TCP can be used as bone graft material, and α- can be stabilized by doping with dopants such as Si, Zn and Mg, which promotes excellent bioactivity, Clarke et al., reported. 81
β-TCP has chemical formula Ca3(PO4)2, Ca/P ratio of 1.5 with hexagonal crystal structure at 1,200 °C temperature. The conversion of β-TCP into α-TCP happened at the range of 700–1200 °C. In 1978, Nery et al., reported the first preclinical application of BCP in dog using a calcium phosphate they referred to as “tricalcium phosphate.” However, X-ray diffraction analysis revealed the mixture to be HA and β-TCP, and as the consequence, the mixture is known as a biphasic calcium phosphate, or BCP. This results in higher resorbability and biphasic ceramics with superior stability that can be employed for biomedical purposes. 82
3.2.8 Bioactive glass
First, Hench, 1998 developed bioactive glass, and he synthesized different glasses containing a mixture of silica, phosphate, calcium and soda. These materials are used as bulk implants on metallic implants and used as scaffolds. 83 The degree of biological activity and physiological properties depend on the chemical composition of bioglass 84 . Kokubu et al., used different glass compositions to study the formation of apatite in simulated body fluid. It was observed that apatite ion activity is higher and disrupts the silicon network, which acts as a place for apatite nucleation. Therefore, in SBF solution, the growth of apatite is based on the utilization of calcium and phosphate ions. The chemical and physical properties of glass ceramics interact with physiological solutions and impart biological activity on SBF immersion in the biological environment. 85
Ceramics are susceptible to surface changes in the human body fluid environment due to their resorbable nature. The reactions at surfaces and grain boundaries result in ion exchange, depending on PH and exhibit different bioactivity. 86 In a physiological environment, bioglass may leach out various metal ions such as Na+, K+, Ca2+, P5+, Si4+ through ion exchange reaction and these ions are replaced with H2O− ions, which produce a SiOH ions on the surface of silica-gel layer. Silica-based ceramics are considered as bioactive material in biomedical applications. Bioactivity is the ability of the material with definite chemical compositions and structure in a biological fluid, leading to the nucleation process. Further research was conducted with mesoporous materials for biomedical applications such as drug delivery systems, bone tissue regenerations and corrosion resistivity over metallic implants. 87 , 88 Peltola et al., suggested that bioglass prepared by the sol-gel process has increased bioactivity. In an aqueous solution, bioactive glasses react with it and lead to changes in the structure and chemical composition with the formation of HCAP. 89
The formation of bioglass with SiO2–CaO–P2O5 was demonstrated by Rounan and Hench, using the sol-gel method, and it was observed that glasses made from sol-gel techniques require low sintering temperatures. SiO2, CaO and P2O5 are bioglass that are widely used as bone substitution materials. 90 Silicate ceramics are the most commonly used materials with abundant raw materials, and they provide adequate mechanical, thermal and optical properties. They developed bioglass with increased use of nanocrystalline silicate in biomedical applications. Based on the composition of bioglass, 45S5 (45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O and 6 wt% P2O5) have been used in various dental and medical applications such as ear bones, surgery, denture weakness, maintenance of jaw bone, bone restoration. Apart from silica glass materials, calcium pyrophosphate materials have been widely used as coating materials on implants. A study was conducted on 316L SS to predict the corrosion resistance behavior in ringer’s solution. 91 Litkowski et al., conducted an in-vitro study and used 45S5 bioglass on the dental surface of teeth and demonstrated that 45S5 bioglass has increased occlusion of dentinal tubules than non-45S5 compounds, whereas a decrease in dentine hypersensitivity in vivo studies. 92
Even though ceramics are bioactive and have biocompatibility, the major disadvantage of ceramics is their brittleness and poor tensile properties. Ceramics and glass materials fail in high-stress and load-bearing applications. To overcome the disadvantages of ceramics, composite materials with excellent load-bearing properties can be used in bone replacement applications. 93
3.2.9 Cerium
The most abundant rare earth metal oxide of the lanthanide series that contains two oxidation states such as 3+/4+. It has an entrenched role in biomedical and industrial applications. Cerium in biomedical applications is expanding from protection against cellular damages caused by toxicants, pathological damages such as brain, cardiac ischemia, neurological disorders, neuro-regeneration of retina, reduces chronic infection, toxicity towards cancer cells and radiation. Due to its unique properties (shifting of oxidation states), it found applications in UV-absorber, catalyst, O2 permeation membranes, and polishing agents. It is used as an electrode in gas sensors and solid oxide fuel cells owing to its excellent ion conduction. 94 , 95
Cerium has excellent anti-bacterial activity due to its ROS (reactive oxygen species) that cleaves the cell wall and damages the cells, ultimately leading to cellular death. Many types of research were carried out to test the antibacterial activity of cerium against pseudomonas aeruginosa. Nano ceria plays a significant role in self-regenerating anti-oxidant properties by interaction with superoxide radicals, hydroxyl radicals and hydrogen peroxide. Through oxidative stress, it restricts cell death. Several reviews have shown the anti-oxidant capacity of cerium in relation to untreatable oxidative stress-related diseases. 96 , 97
Cerium nanoparticles provide biological activity through Ce3+/4+ redox coupling on the surface of the nanoparticles. They mimic the superoxide dismutase activity (Ce3+ oxidize into Ce4+ ions) by reducing superoxide into hydrogen peroxide. The catalytic activity of cerium induces hydrogen peroxide oxidation to molecular oxygen that oxidizes Ce3+ to Ce4+. Nanoparticle interaction with cells was crucial in tissue engineering and regenerative applications. Due to the existence of Ce3+/4+ oxidation states, cerium induces cell proliferation and protects normal cells. Cerium-based scaffolds promoted osteogenic differentiation and proliferation of bone marrow-derived mesenchyme cells. 98 , 99 , 100 Li et al., manipulated the valance state of cerium oxide NPs on titanium substrates to modulate the cell fate of bone formation. 101 The researchers elicited the beneficial outcome of cerium for new bone formation and osteointegration. The biomedical applications of cerium are displayed in Figure 5.

Biomedical applications of cerium.
Cerium was used as coatings on metal implants to prevent corrosion and to improve mechanical properties. Cerium oxide plays a vital role as a corrosion inhibitor by forming a thin protective layer of cerium hydroxide. Ce3+ ions oxidize to Ce4+ ions, and with a mixed oxidation state, it forms a self-healing, stable and insoluble layer on the surface. 102 Lin et al., immersed AZ31 substrates into a cerium nitrate solution to achieve cerium conversion coating. 103
3.2.10 Magnetic nanoparticles
‘Nanomedicine’ is an emerging field in nanotechnology with increased applications due to its nanostructure in medical areas such as analytical tools, Nanoimaging, nanomaterials, modern clinical therapeutics, drug delivery and toxicological studies. 104
In recent years, iron oxide nanoparticles have various resources in the biomedical field due to their significant role in MRI, gene therapy, drug delivery, hyperthermia treatment, coating on the metal implant to enhance the interaction between the biological tissues, cell separation and detection. 105 , 106 , 107 The physicochemical and electrochemical properties of magnetite nanoparticles have several multifunctional applications that are used in multi-disciplinary sources like nanotechnology, molecular biology, chemistry and material sciences. Clinical trials with the IONps specify them as image probes for locating and diagnosing cancer through targeted drug delivery and MRI imaging. 108 It also found clinical applications in the case of musculoskeletal abnormalities such as joints, bone ligaments, removal of cancer cells, diagnosis and treatment. 109 The biocompatible natures of the nanoparticles were modified with ceramics or polymers. One of the global problems was cancer therapy; research should be carried out for conventional applications of magnetic nanoparticles in cancer treatment, such as chemotherapy, radiotherapy and hyperthermia, which kills the cancer cells from the normal cells. Hyperthermia treatment includes various methods: localized surface, deep body, cavity tumor, and whole-body therapy. 110 , 111 Despite multiple applications, MNP is used to control/prevent the growth of microorganisms at lower concentrations. Various biomedical applications of iron oxide nanoparticles are given in Figure 6.

Biomedical applications of magnetic nanoparticles.
3.3 Composite materials
It is a type of material consisting of two or more fundamentally different compounds. Composites can be made using bioinert and bioactive ceramics, which have excellent biocompatibility and mechanical strength. Alumina ceramics composite was prepared using hydroxyapatite ceramic and found to be well bioactive with high adhesion strength. The animal experiment was conducted on HAP/Alumina composite and found that they have excellent osteointegration with bone. 112 Konduk and Ahmet, investigated the biocompatible nature of alumina/zirconia. An animal experiment was performed with this composite material on rats for a period of 2 months. The results of the animal studies revealed that the ceramic composites do not produce any adverse effects on tissues during implantation. 113
Zeng et al., selected ZrO2 as substrate and Na2O, SiO2, –B2O3–CaO glass as a medium for ZrO2 and HAP bonding, and they implanted on dogs legs. After three months of implantation, the gradient bone implant formed a bond with bone and increased bond strength between the bioactive material implant and bone. 114 Further research by Chellappa and Vijayalakshmi, demonstrated that the ZrO2 coating on Ti–6Al–4V exhibits good corrosion resistance in a simulated body fluid environment. 115 Rogojan, have prepared composite materials using Alumina-Zirconia-HAP using the sol-gel method. The single advantage of each material, like high biocompatibility and bioactivity with excellent chemical inertness and mechanical properties, was highlighted. 116 In vitro studies of these materials were tested in a medium containing human osteoblastic cell, and in vivo studies were carried out by implanting ceramic composites in the parietal bones of Wistar rats. These results have shown excellent bioactive properties and bone repair properties. Bellucci et al., obtained high HAP-Silica glass with excellent bioactive and biocompatible composite materials for bone tissue engineering. 117 An in vitro study was carried out in SBF solutions, and in vivo tests were done using calvarias-derived pre-osteoblastic cell line MC3T3, where the cell line mimics the osteoblast progenitors. The result showed that HAP-Silica enhances cell adhesion, proliferation, and differentiation towards osteoblast phenotypes. Although composites have excellent biocompatibility and mechanical strength, low fracture toughness is a disadvantage; hence, they cannot be used in long-term applications like permanent fracture fixation devices.
3.4 Polymers
Polymeric-based biomaterials are broadly used as implants in orthopaedic and tissue engineering applications. Many polymers exist, such as polysiloxanes, polyethene, polyamides, polyurethane, etc. These polymers play a vital role in tissue regeneration, such as polyvinyl siloxane, polyglycolic acid, polyglycolic-co-lactic acid, and polymethyl methacrylate act as dental impression, degradable structures, bone screws and bone cement, respectively. 89 Co-polymers of ethylene-vinyl acetate membranes were used for controlled drug delivery systems. The polymers are used for kidney, liver, bladder, artificial heart, soft tissue replacement, catheters, pacemakers, contact lenses, encapsulations, ear repairs, implantable pumps, artificial blood vessels, artificial skin and sutures. 118 , 119 The main drawbacks of polymers are higher degradation rates and low mechanical strength; these could be overcome by developing composites with metals or ceramic materials for advanced biomedical applications. 120
Biological materials including collagen, proteoglycans, alginate, fibrin, chitosan, gelatine, and agarose were employed in the production of scaffolds to get around these limitations. 121 , 122 , 123 Natural polymers are physiologically active and can encourage superior cell adhesion and development. Despite this, their inherent biological variability makes them difficult to manufacture in homogeneous batches with repeatable features and frequently results in poor mechanical qualities, particularly in load-bearing applications.
Hydrogels are commonly developed for tissue engineering using both natural and synthetic polymers. 124 , 125 , 126 , 127 Chains of hydrophilic polymers interconnected by covalent or non-covalent bonds constitute hydrogels. Its physicochemical and mechanical qualities are akin to many soft tissues because of their capacity to absorb enormous quantities of water and their soft mechanical properties. Hydrogels can be classified as conventional or smart hydrogels depending on the method they respond to external stimuli. The latter type can alter their structure or swelling behaviour in response to various stimuli, such as temperature, pressure, light, ionic strength, electric or magnetic field, and others. Since they can have their mechanical properties tailored, the chemical composition of the hydrogel modified, and crosslinking facilitated, they are suitable materials for scaffolds. 128 Hydrogels provide immuno-isolation while simultaneously permitting gaseous exchange and nutrient diffusion, which makes them appropriate for cell seeding. 129 Currently, the majority of applications for hydrogels include wound dressing and scaffolds for bone or soft tissue regeneration, particularly in cartilage healing.
Biomaterials produced through conventional 3D printing were typically positioned layer by layer in vitro before being surgically implanted in vivo. However, Elvassore et al.’s recent study 130 used photo-active polymers injected into living animals to create in situ 3D constructions without the need for surgery, achieving intravital 3D bioprinting. Many coumarin derivatives were examined, and the raw material for their 3D bioprinting was ultimately determined to be 7-hydroxycoumarin-3-carboxylate-poly (ethylene glycol) conjugates. Xu et al., controlled the stiffness for bone regeneration by filling a 3D-printed polycaprolactone scaffold with laponite. 131 To enhance the mechanical capabilities, Liu et al., created a composite scaffold by enclosing nanohydroxyapatite within a collagen hydrogel. 132
4 Basic requirement of implantable biomaterials
For biomedical applications, biomaterials implanted in the body take on a specific functional role that is dictated by the surgical technique, the host’s features, the biomaterial’s physical-chemical characteristics, and any metabolites. Extensive preclinical research may not ensure the implanted biomaterial’s intended use and effectiveness. The characteristics of the implant, its function, or its performance in vivo may be changed by the immediate inflammatory response that invariably follows the surgery. 133 , 134 , 135 In order to lessen the potential for tissue loss and inflammatory reactions that come with more extensive surgical exposures and tissue dissections, so-called minimally invasive surgical treatments have been investigated more recently. In fact, persistent fibrosis and inflammation may encase the biomaterial, isolating it and unintentionally changing its intended use or performance. The latest advancements in biomaterials specifically made for bone cell interaction necessitate implants with bioactive surfaces that can promote bone integration and inhibit atypical fibrosis brought on by the foreign body reaction (FBR). 136 , 137 , 138 Figure 7 illustrates the properties of bone for biomedical applications.

The properties of bone for biomedical applications.
4.1 Properties of metallic biomaterials
Applications for surgical implant devices have traditionally included metallic biomaterials, such as those based on iron, cobalt, and titanium. These alloys’ mechanical qualities – modulus, strength, and ductility – have been utilized to create long-lasting in vivo stabilizers for devices that replace skeletal structures. Furthermore, in biological contexts, the passive surface oxide layers have offered chemical inertness. 139 Recent efforts to create porous metallic environments for fixation and biologic ingrowth have raised concerns about the relative strength and biodegradation characteristics. The criticality of design is highlighted by the fact that some biomaterial strengths have been diminished to magnitudes less than 50 % of the nonporous alloys. Increases in surface area of three to ten times have highlighted the extent of biocorrosion, the substances released into the tissues, and the physiological effects of these products. 140 Figure 8 shows the schematic representation of factors affecting the biomaterials implants.
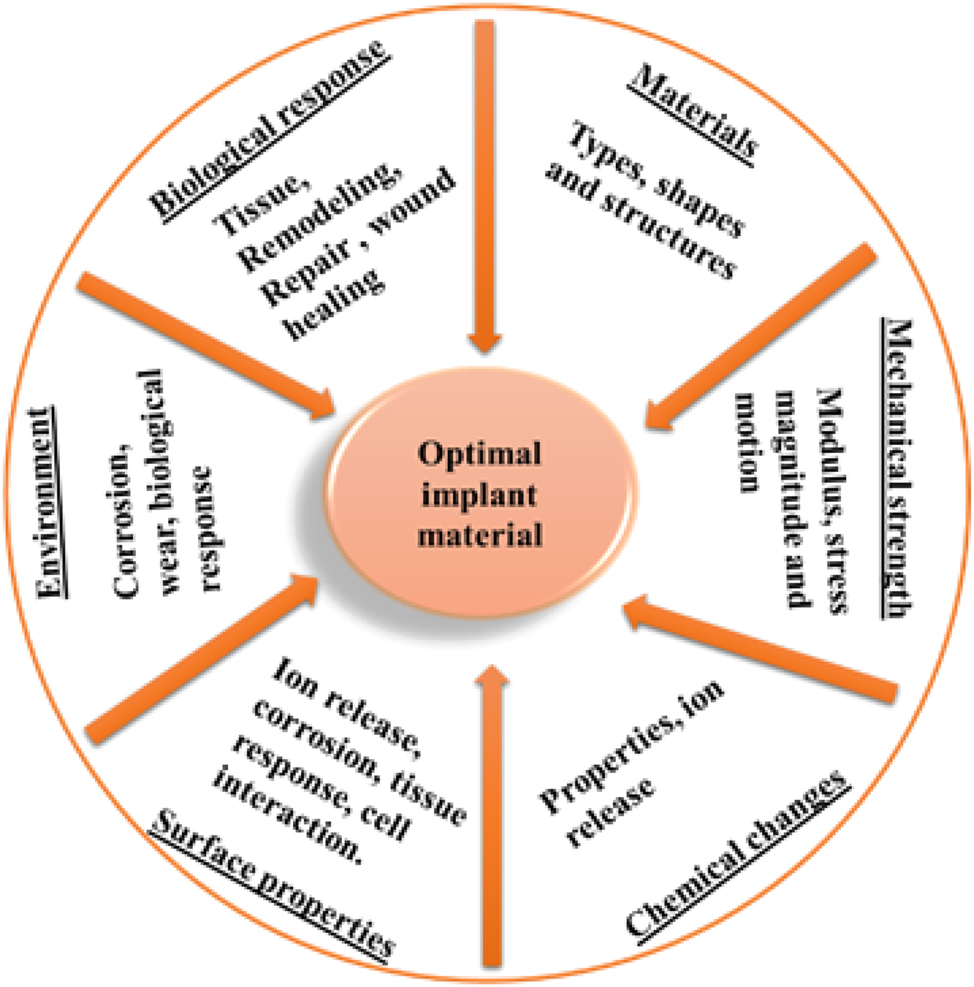
Schematic representation of factors affecting the biomaterials implants.
4.2 Mechanical strength of metallic implants
The biomaterials can be decided by their properties to use them in particular applications such as maxillofacial and hard tissue repairs. The bioimplant response to high loads or strains has been identified by fatigue strength; this property of fatigue strength verifies the long-term stability of implantation. When an implant undergoes cracks owing to poor mechanical strength between the natural bone and biomaterials, it is called biomechanical incompatibility. In major bone fractures, the bone is probably replaced by the biomaterial with an equivalent modulus of the natural bone. The modulus of bone is from 4 to 30 GPa and varies for different bones depending on the direction. However, few of the implants have a higher modulus, which shifts the stress to the nearby bones and makes that adjacent bone to resorption. This incompatibility behaviour of implants leads to bone cell death, and this is referred to as the stress shielding effect. For an implant in the application of total joint replacement, higher yield strength is required with lower modulus. Young’s modulus is considered an important factor for total knee joint replacement. 141 , 142 , 143
4.3 Requirements for bone scaffolds
Further research on appropriate design and material properties – which should be customized in the scaffolds according to the many kinds of bone deformities and fracture sites is necessary to create more therapeutically feasible scaffolds. Moreover, it is ideal for a bone scaffold material to be biodegradable, which means that it may gradually decompose into harmless substances that the body can metabolize and eliminate. 144 , 145 , 146 At the same time, the new tissue develops and fills the deficiency over time. The strength and modulus of elasticity/stiffness of a bone scaffold material are particularly significant for mechanical properties. 147 , 148 As bone scaffolds deteriorate over time, they must have short-term mechanical stability and be able to tolerate early biomechanical pressures such body loads and wound contraction forces. In clinical practice, the bone may occasionally be stabilized using a short-term external fixation. The material and manufacturing process have an impact on the mechanical strength. For instance, Peters et al. created unique porous scaffolds from β-tricalcium phosphate (β-TCP) using two distinct methods: inkjet 3D printing (IJP) and traditional shaping (milling). 149 The 3D-printed scaffolds’ compressive strength was significantly lower than that of cortical bone (100–150 MPa), 150 but it was within the range of trabecular compressive strength (1.5–38 MPa), 151 according to the authors. The bone scaffold’s stiffness should be neither too high nor too low for the tissue’s healing in order to offer mechanical stability and stress-shielding, respectively. Human cancellous and cortical bone tissues have elastic moduli between 10 and 1,570 MPa and 14.9 and 35.3 GPa, respectively. 152 For the bone to naturally rebuild, it must be sufficiently stiff, near the bone’s density, at the defect site.
Alterations in physical characteristics, such elastic moduli, are proportional to the load-carrying section’s relative areal fractions (AA). For the majority of the porous systems that are now on the market, there are a few theoretical computations and measurements of these kinds of characteristics. The size, shape, distribution, and connectivity of the pores are among the physical characteristics of porosity that are known to be important for both short- and long-term biologic interactions. The pore size features span a large range; the mean values (l) are typically between 100 and 250 μm, while the intercept (l) measurement values range from 10 to 500 μm. 153 , 154 The majority of systems exhibit surface-interconnected porosity, while connectivity measures occasionally reveal solitary pores. Using the selective laser melting (SLM) technology, a 3D printing approach, numerous researchers have examined various porous Ti–6Al–4V scaffolds and were able to generate low elastic moduli that were comparable to those of the cancellous or cortical bone. 155 , 156 , 157 , 158 The majority of porous Ti–6Al–4V structures have ultimate compressive strength values that are somewhat lower than those of cortical bone, hence achieving high compressive strength is a difficulty.
4.4 Biocompatibility
Biocompatibility testing measures the effects of materials on body tissue and organs. Surface contamination affects the biocompatibility of the materials, resulting in surface modification of the material and toxicity. It should not adversely affect the host environment (bone, soft tissue, ionic composition of plasma and intracellular and extracellular fluids). Material should be non-carcinogenic, non-pyrogenic, non-toxic, non-allergic, blood-compatible, and non-inflammatory. William defined the biocompatibility of the biomaterials for total joint replacement, and it should reduce corrosion rate, tissue response to the corrosion products, and decreased wear resistance. Titanium and its alloys have excellent biocompatibility. They are inert and form an oxide layer on the surface that protects them from corrosion products in a biological environment. Titanium adsorbs proteins such as albumins, laminin, glycosaminoglycan, collagenase, fibronectins and complement proteins and fibrogenesis on the implant surface. It enhances cell growth and differentiation. Once the material is implanted into the body, neutrophils and macrophages bond to the surface, leading to the formation of foreign cells and differentiation of osteoblasts cells (bone cells). 159 , 160 Various cell viability assays to study biocompatibility are shown in Figure 9.

Various cell viability assays to study biocompatibility.
4.5 Osteointegration
Osteointegration is a fundamental process of new bone formation and bone healing in orthopaedic applications. The important properties of osteointegration are surface topography, roughness and toughness. If the implant surface is non-capable to joint with adjacent tissue, it results in fibrous tissue formations and prosthesis loosening. Hence, the surface properties of the implants are necessary for osteointegration. 161
4.6 Surface wettability
Surface properties play a vital role in the persistence of cell-cell interaction, protein adsorption, etc. Topography chemical composition wettability is mentioned in terms of contact angle that influences the biological response hydrophilic surface, which is an important surface property of biomaterials in the determination of direct cellular contact. When a liquid is placed on the implant or solid surface, it forms a dewdrop (hydrophobic) or feast on the complete surface (hydrophilic), which depends on the surface properties such as interfacial free energy. The contact angle (θ) measurement for the liquid droplet is illustrated in Figure 10.

Contact angle.
Hydrophilic surfaces facilitate the proliferation of a material in contact with blood cells’ adhesion: platelets activation and inflow of proteins. Hydrophilicity has reduced interfacial free energy with improved hemocompatibility. Hydrophilic surface can be increased by surface treatment such as chemical treatment (acid or alkali treatment) and radiation grafting plasma discharge.
4.7 Corrosion resistance
For an implant, it should be highly corrosion resistant because the metallic implant is placed in a corrosive environment (human body fluid). 162 Corrosion decreases the life of implant devices, and a decrease in corrosion products may accumulate in tissues, leading to a decrease in the human life span. 163
5 Implant corrosion
Corrosion is the major problem associated with metals and their alloys. 164 It is a gradual degradation of the biomaterial when it is placed in an electrolytic environment by electrochemical compounds like water, sodium, chlorine, proteins, plasma, amino acids, and saliva. The human body consists of aqueous environments such as Na+, K+, Ca2+, and Mg2+ of cation groups and anions like chloride, phosphate and bicarbonates, and it is important to understand the principle of corrosion environment. 165
Biological molecules influence the electrochemical behaviour of biomaterial implants. Proteins are absorbed onto the surface of the implants and can interface with hydrogen and oxygen redox reactions. Bacteria and other microbes induce corrosion by absorbing hydrogen present in surrounding areas of implants. Changes in the pH of the human body also influence corrosion. Typically, pH is maintained at 7.0, and this value changes from 3 to 9 due to disease, accidents, and infections. 166
The tolerance corrosion rate for biomaterial implants is 2.5 × 10−4 mm/year. Metallic implants form a passive oxide film on the surface, which inhibits corrosion environments in which the materials undergo corrosion. 167 Only noble metals like gold, platinum, titanium or chromium have an acceptable level of corrosion rate. The most common forms of corrosion are pitting, crevice, galvanic, intergranular, stress corrosion, cracking and fretting corrosion. The types of corrosion and its prevention methods are mentioned in Table 3.
Types of corrosion and its prevention methods.
| Types of corrosions | Preventions | References |
|---|---|---|
| Crevice corrosion | Crevice corrosion is improved in stagnant or slowly flowing solutions by properly designing connections and joints. | 176 |
| Galvanic corrosion | Appropriate metal and alloy determinations, as well as a good cathode to anode ratio. Insulating coatings for dissimilar joints. | 177 |
| Corrosion fatigue | Elimination of the corrosive environment and cyclic stress. | 178 |
| Stress corrosion cracking | Three elements work together to cause SCC: a material that is vulnerable, exposure to a corrosive environment, and tensile stresses that are higher than a threshold. | 179 |
| Eliminating any one of these factors prevents SCC. | ||
| Hydrogen embrittlement | Diminished cathodic protection and less vulnerable alloy finish. | 180 |
| Erosion corrosion cavitation | Using corrosion inhibitors or cathodic protection, more resilient materials, and streamlined pipework can reduce turbulence and regulate fluid velocity. | 181 |
5.1 Pitting corrosion
Pitting results from extensive damage and release of metal ions and is a form of localized corrosion. The formation of small cavities on the implant surface is referred to as pitting, which is protected from the passive oxide film, which is adherent. Generally, pitting is non-visible and causes stress corrosion cracking (SCC) and fatigue cracks, which results in the failure of the implant. Pitting occurs when there is breakage of the passive oxide layer; incomplete coatings occur when anodic ions flow is fixed on a smart area of implants and scaling. This results in an increased corrosion rate.
Fontana, studied the movement of hydrogen ions and the mechanism of pitting corrosion. 168 Kamachi Mudali et al., revealed the importance of pitting corrosion resistance, which is determined by the pit propagation rate (PPR). Pitting most commonly occurs in the underside of the screw head of the implant. Most frequently, it occurs in a media containing chloride ions. Pitting corrosion can be decreased by the addition of molybdenum in a saline environment. 169
5.2 Crevice corrosion
It occurs in the screw heads and plates, hip nails etc. Crevice corrosion occurs when metal and its alloy surface are partially shielded in the electrochemical environment. Bates, reported that 316LSS metal is most commonly subjected to this corrosion. It can be eliminated by modifying the choice of materials. 170
5.3 Galvanic corrosion
It occurs when two dissimilar metals are in contact in an electrolytic solution such as serum or interstitial fluids. It occurs when bone plates and bone screws are made up of different metals. Hip prostheses made up of 316 L stainless steel and sockets made up of Ti–Al–4V alloys are subjected to galvanic corrosion. Factors involved in galvanic corrosion: potentials, polarization, electrode areas, resistance and galvanic current, electrolyte medium, aeration and agitation. 171 , 172
5.4 Corrosion fatigue
Combined interactions of electrochemical reactions and cyclic loading lead to fracture failure and result in corrosion fatigue. In load-bearing implants, corrosion and fatigue resistance are important in cyclic motion applications. Usually, there is no implant failure, but cracks, surface damage, minute flow and chemical attack were observed. Fatigue strength is reduced by changes in body fluid, such as pH, O2 content, and temperature. Hu et al., studied the failure mechanism of implants, and they reported that failure was due to cracks, which could be initiated by fretting and propagated by stress-corrosion cracking (SCC). 173
5.5 Fretting corrosion
It occurs when two opposing surfaces rub each other continuously in an oscillating way in the fluid body environment (e.g., bone plates and screws). It appears even in the corrosion-free environment. The clinical significance of fretting lies in the intensity of increased corrosion products that result in the initiation of cracks and fractures in implants. Weight loss of implants due to corrosion was directly proportional to the load transmission across the surface. Mudali et al., reported that implant weight loss is inversely proportional to the hardness and frequency of stroke. In a few cases, implants fail due to accelerated wear and fretting, not corrosion. To protect implants or to limit oxidation, initially formed passive film must have certain characteristics like a non-porous, atomic structure that limits ion migration and high abrasion resistance. 174
Hence, the biomaterial for implant application should be subjected to corrosion tests, wear, fretting, stress, etc. Dearnley, determined the corrosion behaviour of the scratched-coated implants to examine the wear-accelerated corrosion behaviour of coating and to measure corrosion and wear methodology. 175
6 Surface modification of the implant
In selecting a suitable surface modification technique with non-toxicity for specific biomedical applications, corrosion resistance, modulus of elasticity, fatigue strength and controlled degradability have been recognized as basic properties. The rationale behind the surface modification of an implant is that the surface of a material determines the response of the biological environment to implanted materials. Surface modification of implants is broadly classified into two approaches: accelerated bone healing and enhanced bone bonding of an implant.
In the enhanced bone-bonding approach, the surface topography of an implant material is modified using a suitable approach that enables the modified surface to increase the mechanical interlocking of the bone with the implanted material. The modified surface automatically increases the surface area and surface energy of the material, which leads to enhanced matrix protein absorption, cell adhesion and proliferation, and finally, to better osteointegration of the implant with the bone. Mechanical processing is the simple physical treatment and shaping of a material surface by cutting, blasting, grinding and polishing to produce an improved surface topography and roughness, remove contaminated materials and reinforce its bonding strength through increased adhesion.
In accelerated bone healing, inorganic or composite bone materials are incorporated into the bone’s surface to enhance the bone-forming capability of the cells and cause biochemical interlocking of the implant with the adjacent bone. The incorporation of organic molecules (biochemicals) such as proteins and peptides into the surface of the implant is also an accelerated bone-healing method. Therefore, surface engineering is designed to improve biological performance, such as biocompatibility, bioactivity, corrosion, and wear resistance of the implants, which can modulate and control the living tissue response. Surface modifications, including mechanical, chemical, and physical methods, are the basic methods used to improve the stability of the implants. 182 , 183
6.1 Mechanical methods
Mechanical methods like machining, grinding, polishing and blasting are common mechanical surface modifications. Mechanical methods improve surface topologies and roughness, remove contaminations on the surface and increase the surface area, increase the cell adhesion onto the surface and augment bone tissue interlocking with implants. The grit-blasting technique involves the bombardment of the implant surface with the use of silica, Al2O3 or TiO2, which is also called sandblasting. 184
6.2 Ion implantation
It involves the introduction of atoms onto the surface of the material in a small amount with a beam of high-velocity ions without modifying the properties or surface of the material. These techniques produce meta-stable solid solutions without limitations and enhance new bone formation under in-vivo conditions. 185
6.3 Laser nitriding
The laser nitriding technique can modify the surface topology of the materials. It is the process of increasing the hardness of the surface by laser excitation under a stream of nitrogen, nitrogen-argon mixture. Geetha et al., carried out laser nitriding for Ti–13Nb–13Zr alloy in a nitrogen atmosphere using Nd: YAG laser. As a result, surface-treated alloys exhibit high corrosion resistance without any cracks. 186
6.4 Chemical method
Chemical methods include chemical treatments and coatings (sol-gel, chemical vapor deposition). Acid etching treatment was performed using hydrofluoric, nitric and sulphuric acids. Dohan Ehrenfest et al., reviewed the surface modification of implants and its applications. 187
6.4.1 Coatings
In bone tissue, the inorganic phase is made up of carbonate-rich hydroxyapatite. Hydroxyapatites have been widely used for coating deposition on the implant surface. This hydroxyapatite was shown to be bioactive and allow the formation of new bone tissue. Calcium phosphate coating has been explored to modify the surface. Different types of calcium phosphate used are Sr-HAP, Mg–Mg-substituted HAP, bisphosphate carbonated HAP, fluorinated HAP and Ag-HAP. 188
There are various types of coating deposition on implants: electrophoretic coating, thermal spray (plasma spray and high-velocity oxy-fuel combustion spray), electrophoretic deposition, laser deposition, biomimetic deposition and sol-gel deposition. 59 , 189 , 190
6.4.1.1 Deposition techniques
Among various coatings, physical deposition techniques are the most commonly preferred method. It includes thermal spraying, radio frequency magnetron sputtering, and pulsed laser and ion beam-assisted deposition.
6.4.1.2 Thermal spraying
It is a process in which heated material or melted soft material is sprayed onto the surface of the implants. The coating precursor is heated by means of a high-temperature flame or plasma jet. The thermal spray provides a coating deposition of a 20 µm thick layer than other techniques. The coating precursor is fed in powder form, heated to a molten or semi-molten state, and activated towards the surface of the implants, forming uniform micrometer-size particles. By increasing the particle velocity, the quality of coatings, layers, and films can be improved. 191
The substrates are heated as a result of the extremely high temperatures used in thermal spraying. In certain instances, this could lead to the near surface zones recrystallizing and undergoing phase change. For instance, following the application of a plasma-sprayed HA coating, it was discovered that a martensitic transformation and recrystallization occurred at the near surface of a low-modulus Ti–24Nb–4Zr–7.9Sn alloy substrate. According to Zhao et al., the combination of temperature and cooling process was responsible for both the events. Certainly, such occurrences result into further ambiguities concerning the mechanical and adhesive characteristics of the deposited coatings, films, and layers. 192
6.4.1.3 Plasma spraying
The plasma spraying process is the most commonly used surface modification technique on the implant surface. In plasma spraying, precursor materials are deposited as powder, liquid, fibre or suspension, which is introduced into a plasma jet, emanating from a plasma torch or plasma arc at atmospheric pressure under vacuum pressure, and a stream of gas passes through the torch. This torch turns gases into ionized plasma at a very high temperature (20,000 K), with a high speed of 400 m/s. In the jet, the precursor material is either in the form of a melted solid or a partially melted solid. The cooling techniques are to keep the temperature at 100–150 °C. 193 Plasma-sprayed HAP was first used by Herman in 1988. 194 HAP is sprayed onto the substrates by heating HAP to the temperature of 1,200–1,600 °C in a plasma flame formed by an electric arc containing argon gas. In 1980 Groot et al., developed a plasma sprayed HAP coating on the implant surface; 195 later, Herman et al., modified it in 1988. HAP is sprayed onto the substrates by heating HAP at the temperature of 1,200–1,600 °C in a plasma flame formed by an electric arc using an argon gas. Plasma guns produce layer thicknesses of 5–15 lamellae. Once the layer is applied to the substrate, the plasma gun returns to its original position and produces another layer, as reported by Quek et al. 196 The current value for plasma spraying of HAP on the substrate ranged from 350 to 1,000 A and was optimized. The schematic representation of plasma spray is shown in Figure 11.
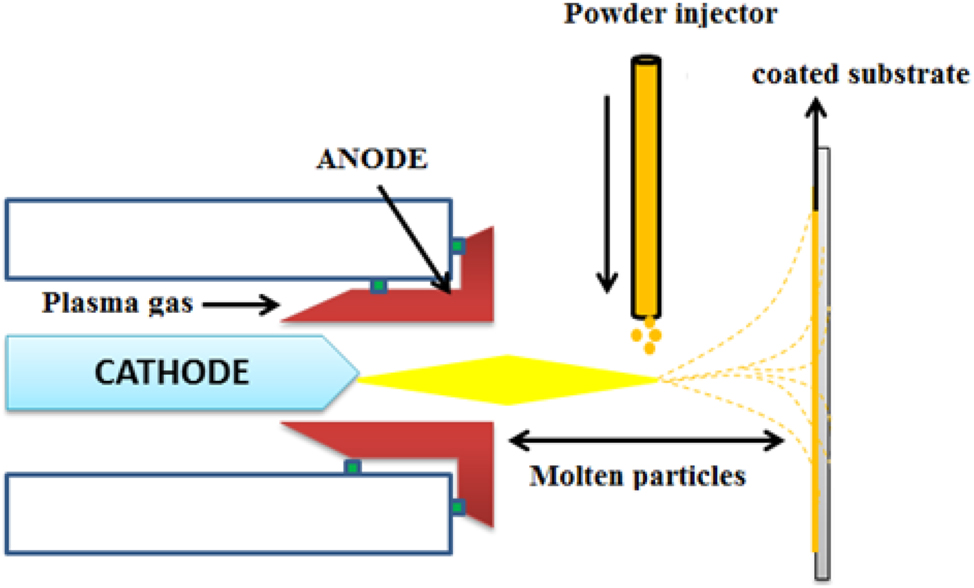
Schematic representation of plasma spray coatings.
6.4.1.4 Radiofrequency (RF) magnetron sputtering
Radiofrequency (RF) magnetron sputtering is a frequently used physical technique to deposit HAP powder into the implant. Sputter is a process where an atom or molecules of some materials are ejected in a vacuum chamber and turned into precursors onto the substrate for coating due to the bombardment of ions with high energy. Nelea et al., investigated the deposition of HAP film on the Ti–5Al–2.5Fe using RF magnetron sputtering. The deposition was performed at a low temperature of 550 °C, and as a result, smooth and uniform coating was obtained with less amount of crystalline phase. 197 Various types of deposition, such as HAP, β-TCP, and β-calcium pyrophosphate powders, can be performed using RF Magnetron sputtering. The chemical composition depends on the types of target materials. A combined RF Magnetron sputtering deposition technique with other deposition techniques, such as plasma-assisted RF Magnetron co-sputtering deposition that is used to deposit calcium orthophosphate on Ti–6Al–4V alloys, and further reviews on RF Magnetron sputtering was carried out by Surmenev. 198 The diagram of RF magnetron sputtering is shown in Figure 12.
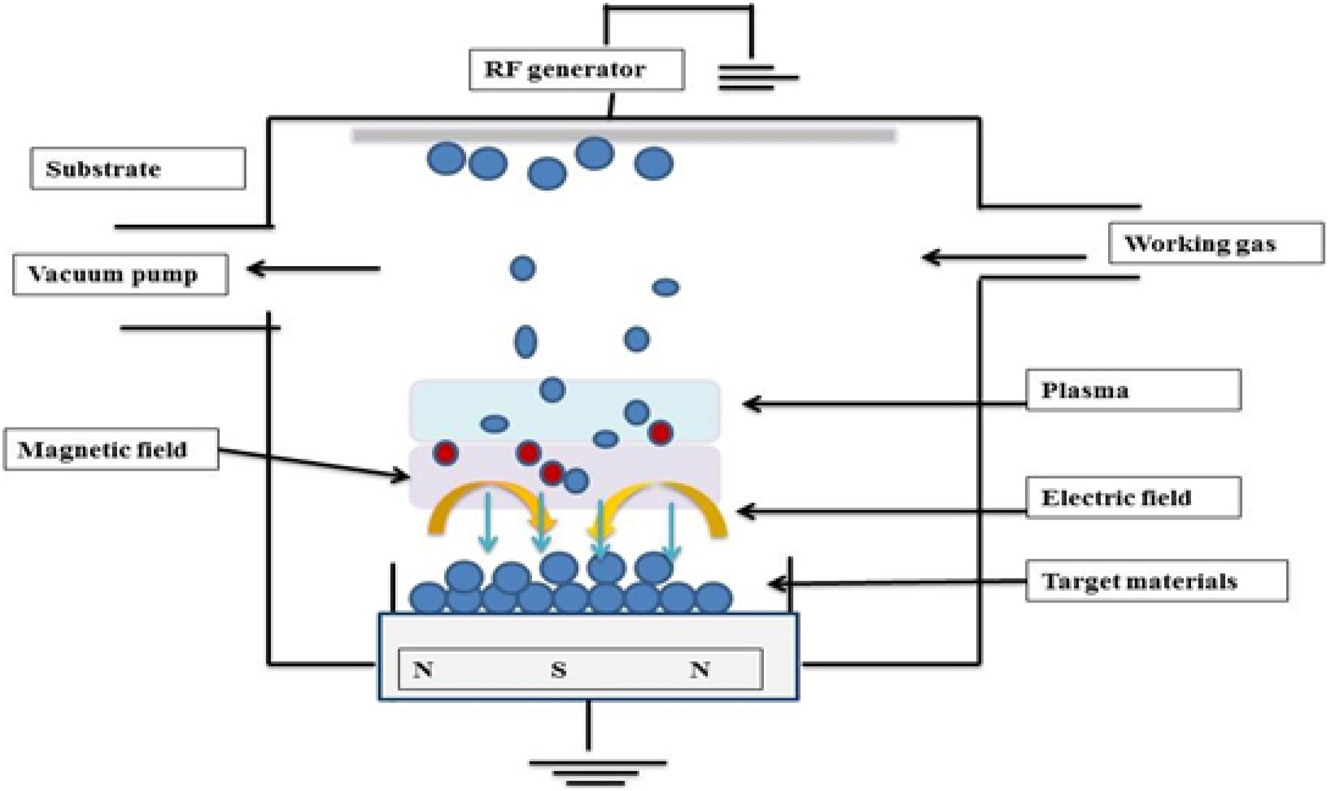
Schematic representation of RF magnetron sputtering. 197
6.4.1.5 Pulsed laser deposition
The pulsed laser deposition technique was first discovered by Cotell, for depositing HAP coating onto the substrate. It comprises glasses like KrF laser source; high vacuum deposition chambers attached with rotating target and to the holder substrate are fixed. This process involves irradiation of feedstock (solid) by a focused pulsed laser, and this interaction results in the formation of compounds like Ca4P2O9, Ca3(PO4), CaO, P2O5, and H2O. The plasma cloud is composed of ions, atoms, electrons, molecules, droplets, etc. The deposition of solid on the substrate with temperature ranges from 350 to 600 °C produces a thin adherent film onto the substrate. 199 Schematic representation of Pulsed Laser Deposition is shown in Figure 13.
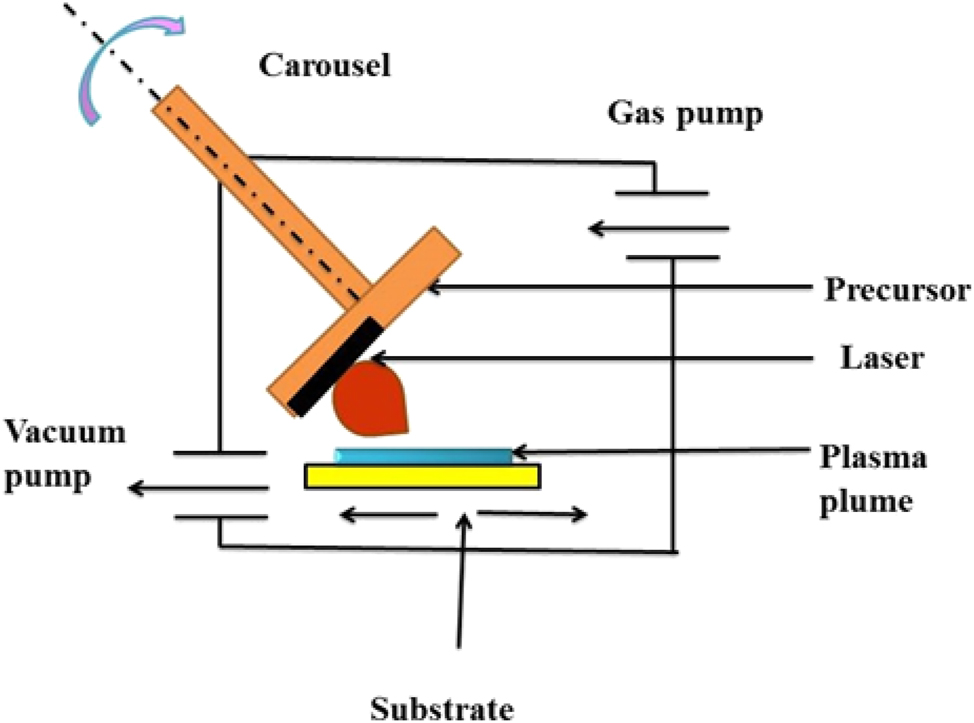
Schematic representation of pulsed laser deposition. 200
Ion beam-assisted deposition (IBAD) techniques are used to deposit thin ceramics and polymers. It consists of electrons or ion bombardment that vaporizes the precursor materials, forming an element cloud on the substrate. Ion gun irradiates the substrate with gases like argon or reactive oxygen at high-energy gas ions. Deposition of pure HAP films on Ti–6Al–4V alloys by electron beam vaporization and simultaneous bombardment using Argon ion beam were performed by Choi et al., the bond strength of coatings can be increased with an increase in the current. 201
6.5 Wet-chemical deposition
These techniques involve mild chemical preparation conditions and also form a three-dimensional geometry coating onto the substrate, which cannot be done by using physical techniques.
Types of wet-chemicals
(1) Biomimetic
(2) Sol-gel
Recently, Nijhuis et al., reviewed the wet chemical deposition of biomaterial coatings for biomedical applications. 202 Kokubo et al., first discovered this technique and worked under physiological conditions of (37 °C, pH-7.4, P(CO2)-0.05) atmosphere. It is a simple technique that involves the immersion of a metal substrate into an SBF solution to obtain a coating on the surface and form a biologically active bone-like calcium phosphate layer. 203 Habibovic et al., conducted biomimetic studies on HAP coating, and the result showed a thick and homogenous crystalline HAP coating on all pores. They also studied the influence of biomimetically octo-calcium phosphates on porous titanium alloys. They utilized a more concentrated SBF solution to produce crystalline coatings to increase the biomimetic coating. An in vivo study revealed that the coated OCP showed an inductive behavior. 204
6.5.1 Sol-gel techniques
It is a colloidal suspension of solid particles (1–500 nm) in a liquid medium that is referred to as sol. Sol can be deposited onto the substrate by spraying, dip coating or spin coating technique (Figure 14). It is in the form of gel and forms a thin layer onto the substrate when the substrate is heated or dried. In these techniques, calcium orthophosphate layers are prepared by inserting the sample in calcium and phosphorus gel at low temperatures. As the formed layer is porous and less dense, the coated layer can be annealed at 400–1,000 °C. In order to improve the bond strength, another coating can be applied as multi-layered coatings. Vijayalakshmi et al., synthesized HAP by sol-gel methods and coated it on 316L SS. The results have indicated that the sol-gel–derived HAP coatings exhibited excellent resistance to the localized attack on pristine 316L SS. 205 The corrosion resistance behavior of the coating was assessed through electrochemical studies. The sol-gel process is shown in Figure 15. Precipitation of HAP of thickness 10 µm on the surface of the sol-gel titania coating was observed by Li et al. 206 They deposited TiO2 film on the surface of NiTi alloys using the sol-gel method to enhance biocompatibility. The film was compact, smooth and 205 nm thick. TiO2 film on NiTi showed effective corrosion resistance from the electrochemical corrosion studies. It also showed improved blood compatibility, which was demonstrated by dynamic clotting time and blood platelet adhesions. Using the sol-gel method, Kim et al., 2004, deposited HAP on the surface of Ti in a titania buffer solution. Proliferation of cells was observed on the surface of HAP/TiO2 coating in human osteoblasts. Potentiodynamic polarization tests confirmed improved corrosion resistance of TiO2. 207
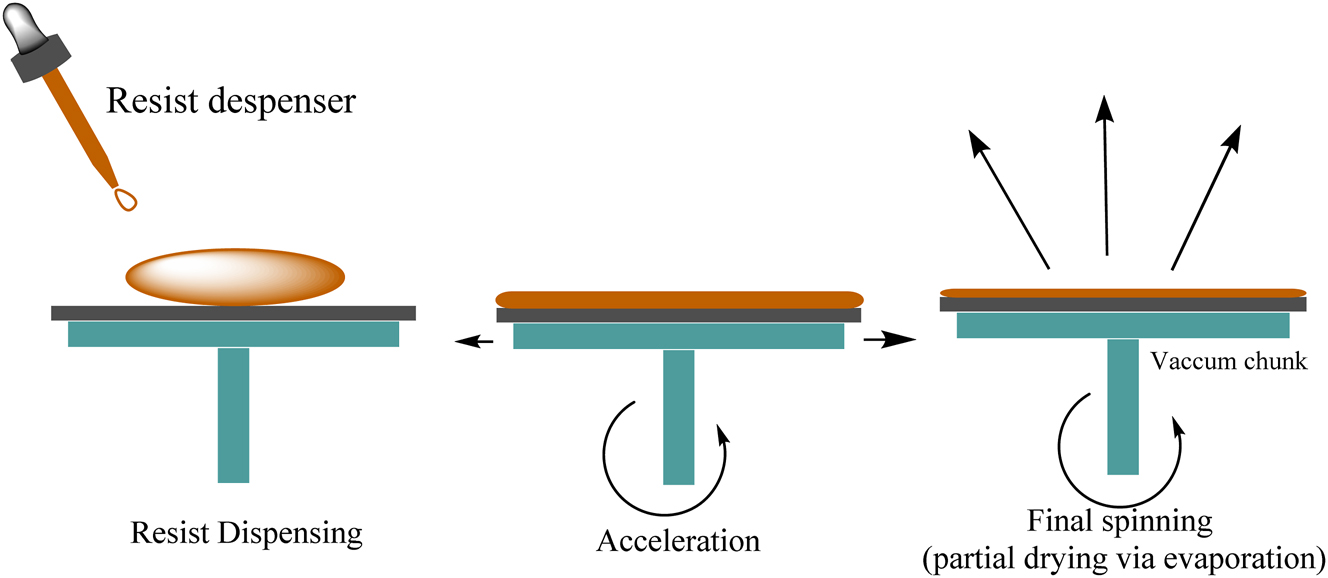
Spin coating process.

Sol-gel method of coatings.
Gan et al., studied the development of coating using different precursors. He applied both inorganic and organic precursors to produce a film on the implant surface using calcium nitrate tetrahydrate and ammonium dihydrogen phosphate as inorganic precursor and Calcium nitrate tetrahydrate and triethyl phosphate as an organic precursor. The thickness of organic and inorganic precursors is 1 and 1.5 µm, respectively. Compared with inorganic precursors, organic precursors showed a dense, thick film with high crystallinity. 208
6.5.2 Electrochemical deposition
It is performed at ambient temperature and pressure, and the feedstock (precursor) carries the electrical charges dispersed in the electrolytic solution. Generally, coatings obtained by electrochemical deposition are uniform in structure, formed through nucleation, and operated at low temperatures. To produce apatite coatings, non-apatite calcium orthophosphate is deposited, followed by calcination at 800 °C temperature, which improves its bonding to the surface of the implant. 209
6.5.3 Electrophoretic deposition
An essential method for depositing different materials and composites from colloidal suspensions or macromolecule solutions is electrophoretic deposition (EPD). On large-surface-area substrates, it provides the benefits of a high deposition rate and the potential for homogenous film development. Film thickness and deposition rate can be superbly controlled with EPD. It can be applied to thick coatings, patterned films, and thin films. For the deposition of polymers, ceramics, metals, hydroxides, and other organic and inorganic materials, EPD offers a flexible and affordable method. The potential for uniform deposition on substrates with complicated forms and the excellent purity of the deposits make EPD particularly appealing for biomedical applications. 210 , 211
This technique involves the migration of charged particles in the electrolytic solution by the precursor particles towards the surface of the implants under the influence of an electric field. Isopropanol or ethanol is the medium used for electrophoretic depositions (Figure 16). It consists of two electrodes: (1) a working electrode (Ti or SS, substrate), (2) a counter electrode (Platinum panel placed at a distance of 1.5 cm).
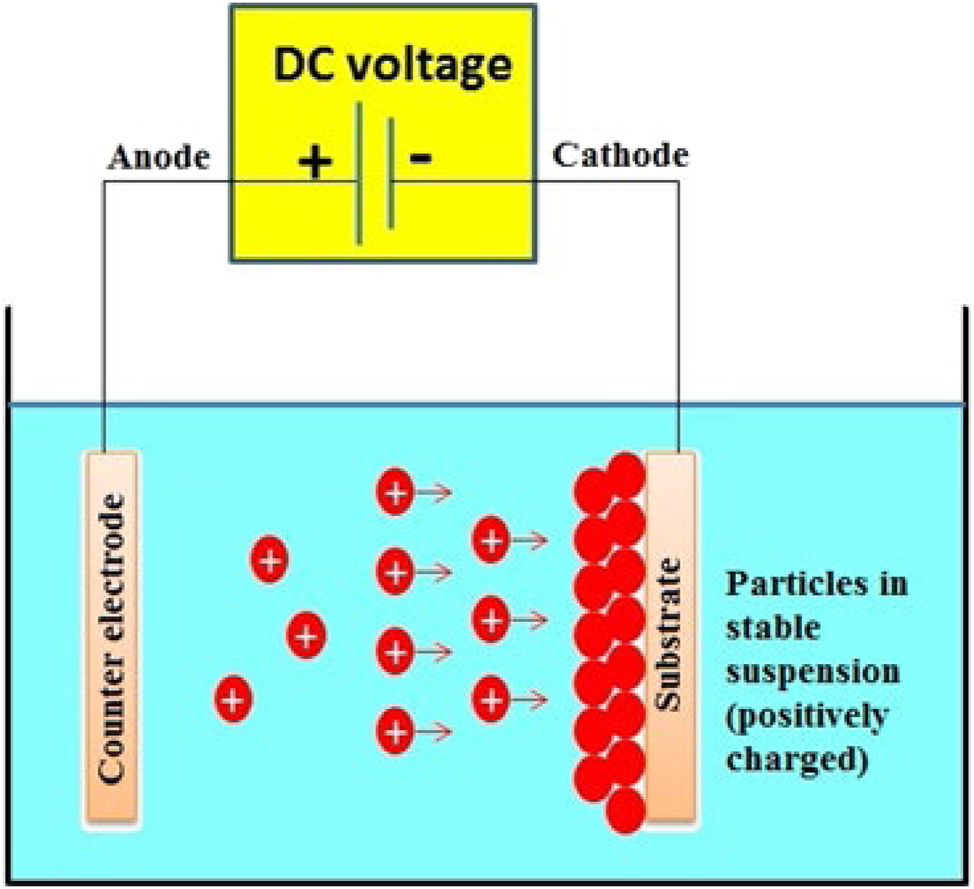
Schematic representation of electrochemical deposition on metal substrates.
Electrophoretic deposition of calcium phosphate onto the porous titanium alloys was demonstrated by Lopez-Heredia et al., 212 Calcium phosphate was homogenously coated on a titanium surface, and the thickness of the coating was found to be 25 µm with a bond strength of 25 MPa. Using the electrolyte technique, Garbuz et al., coated calcium phosphate and alendronate of 5 µm thick, where the calcium phosphate and alendronate were reported to enhance the ingrowth of bone. 213 Using electrochemical etching and cathodic deposition, Adamek et al., coated HAP on the surface of the Ti–6Al–4V titanium alloy. Large pores and nano lamellas were observed in the HAP layer. The chemical deposition method was a promising route for enhancing the bioactivity of the surface. 214
6.5.4 Electrospray deposition
Solutions containing the precursor materials are sprayed on the surface of the implants under the influence of an electric field that creates an aerosol of similar charge (i.e., micron-sized droplets). It controls the thickness and chemical composition of the coatings. 209 A schematic representation of electrospray deposition of coatings is shown in Figure 17. The main advantage of electrospray techniques is the deposition of small droplets of 10 nm on the surface of materials. The charges and size of the droplets can be controlled by an electric field. Jaworek, 215 reviewed the electrospray method and devices for thin film deposition using a metal ion source. This technique was applied to modern materials technology, microelectronics and nanotechnology. Leeuwenburgh et al., performed ESD using a soluble calcium salt (nitrate or chloride) and phosphoric acid, which are dissolved in the alcohol. The prepared solution was sprayed onto the substrate and heated to 300 °C. The chemical morphology of the film was found to be strong depending on the precursor solution. 216
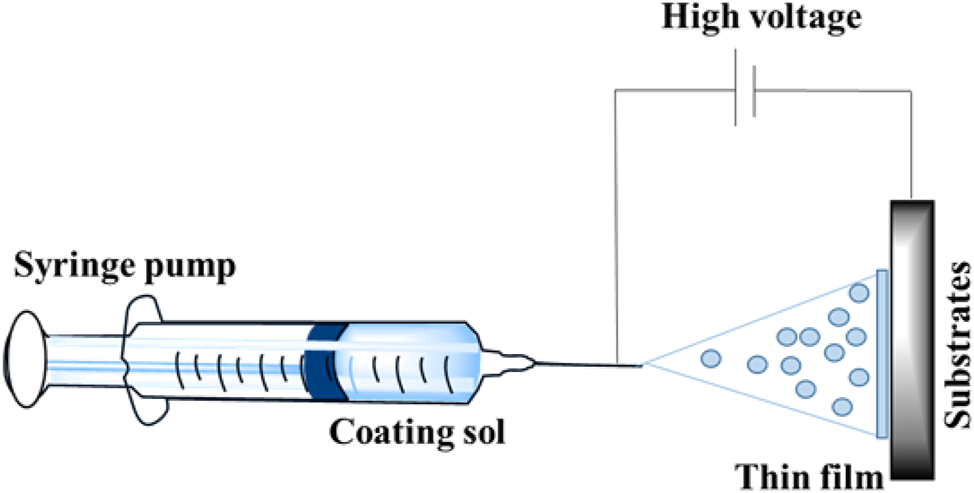
Electrospray deposition of coatings.
7 The interface of bioimplant-tissues
7.1 Contact of implants with blood and protein
When implants are incorporated into the human body, they ensure the formation of injury with bleeding. These bio-implants primarily interact with blood, which has serum, plasma (protein), platelets and other cells. Proteins comprise 20 different amino acids with linkage of peptide bonds, and they have extreme functions in the human body. The proteins can act as enzymes to catalyze the number of chemical reactions that are most crucial in normal life. Proteins are the main groups of molecules that behave as cell signaling for immigration, proliferation and differentiation of cells. Also, these proteins are involved in generating the extracellular matrix for several tissues. The changes in structure and levels of proteins can affect the normal function of the body and be liable for different diseases. However, this can be identified by the blood screening and results as a state of disease. 217
Proteins play a vital role in recognizing the nature of implants’ interface with tissues. When implantation takes place in the human body, it can slightly alter the human environment by a change in ionic concentration and pH, which leads to modifying the function of proteins. In addition, the proteins change their biological activity with structural properties by contact with solid materials after implantation. The most abundant protein in the blood is albumin, and in the immune system are immunoglobulins, which are absorbed on the implants, followed by the formation of an absorbed protein layer that may be fibrose. These proteins exhibit superior affinity towards the surface of biomaterials.
The coagulation of blood was directed by the attachment of factor XII-protein clotting agents, which exist in blood, followed by platelets, which can attach to the surface of implants. This leads to the clot formation of fibrin. Therefore, the contact of blood can help the cytokines and cells to be involved in the interaction of with bio-implants in a biological system. 218
7.2 The wound curing
Biomaterial implantation develops the interruption of normal cellular activity of tissues, and the human body highly exhibits the healing response of the wound; this can be instantly activated by the implantation of biomaterials. The wound creation initiates the transpiration of cellular actions that can provide information about the interaction of biomaterials and tissues. During the interaction period of tissue and implant, four sub-reactions occur: hemostasis, inflammation, initial repair/proliferation, and remodeling, 219 as shown in Figure 18.
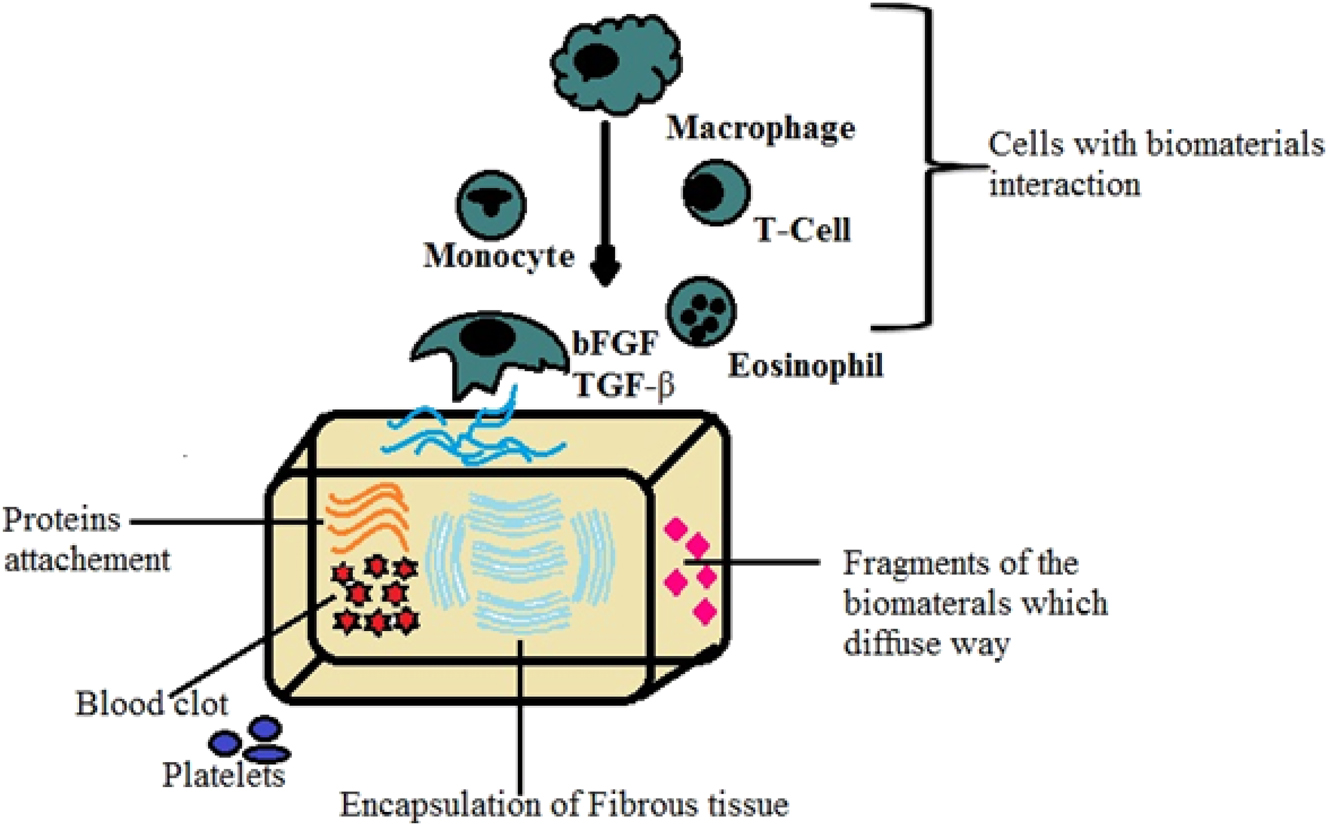
The interface of bioimplant-tissues.
7.2.1 Hemostasis
Blood bleeding can usually be controlled by the platelets through the coagulation process by the attachment of clot-forming proteins, which are adhered to the surface of bio-implants. The clot of blood is the extreme step for the initiation of repairing tissues and fills the cracks around the implantation. 220
7.2.2 Inflammation after implantation
The cytokines (cell signaling molecules) can be generated after the clot formation of blood that can recruit the inflammation cells from the bloodstream. Phagocytosis can take place by these inflammatory cells by digesting tissues and biomaterials. The inflammatory cells can release growth factors at the wound position and start the cell replication of connective tissues at the wound spot. 221
7.2.3 Cell proliferation and initial tissue repair
After biomaterial implantation, it promotes cell attachment, proliferation and population. This nature of bio-implants can regenerate the damaged and vanished tissues in the bone defect area. When non-degradable implants are placed in the injury sites, have encapsulated by the fibrous tissue. This encapsulated tissue can isolate the implant from the biological system to defend the host. The physical and chemical properties of biomaterials, rate of apatite formation, and exchange of ions have also influenced the thickness of fibrous capsules. If permanent implants are inserted into a body, then a little fibrous capsulation is left all over the life of the bio-implant. This cannot happen in the bone because of the direct bone apposition occurring on the surface of implants. 222 , 223
7.2.4 Remodel designing
The newly formed tissues will be rapidly remolded by the transformation of cells into functional tissues, and the wound marks remain in the diseased part of the body. 224
7.3 Degradation and resorption of biomaterials
Biomaterials are degradable and non-degradable materials, in which degradable materials can undergo degradation by chemical processes or cells. The bioresorbable materials are designed according to the rate of degradation in a human environment with respect to time and act to replace the damaged tissue with a complete resemblance of natural tissue. Bioresorbable materials are developed using polymers such as polylactic acid (PLLA), polyglycolic acid (PGA), etc. These polymers can degrade easily at a lower level of molecular weight and release the fragments that elicit an immune response from macrophages. The degradation rate of these polymers can depend on the crystallinity and molecular weight, which has a degradation period from 6 months to a few years. The faster degradation materials create the non-curing wounds. After the degradation of materials, they can release the fragments and cause inflammation. Therefore, this inflammation action creates problems for the implants and requires surgery to remove them from the host. 225 , 226
7.4 Immunological response
Immunogenicity is the affinity of an entity to stimulate the immune response. The immune system protects the human body through a combination of chemical and physical barriers, such as enzymes and skin, respectively, and cellular barriers, like T cells (cytotoxic T lymphocytes). The immune system proteins are activated while bio-implantation takes place in the host, and these proteins direct the cell’s behaviour on the surface of implants. Hence, the surface chemistry of bio-implants plays a crucial role in the attachment of various immune proteins that further leads to promoting the cell’s adhesion with differentiation. 227
8 Application of biomaterial implants
8.1 Soft tissue applications
Polymers like silicone, Dacron, Teflon, polyurethanes, polyacrylate, polyethylene, Nylon, etc., are widely used for soft tissue applications; these polymers can be made into various physical forms such as liquid, foam, film, rod, fabric, and suture materials. Five groups of implants used for soft tissue applications based on their function are
Shunts – fluid transport in the body.
Percutaneous devices – pass from the outside through the epidermis into the body.
Space fillers.
Scaffolds for tissue growth (sutures and wound dressing).
Electrodes – neuromuscular stimulation. 228
8.1.1 Maxillofacial implants
There is an increased number of procedures to improve facial plastic surgery, which corrects the genetics, traumatic and cosmetic deformities. Either natural or synthetic biomaterials can be used for a number of facial plastic surgery. These biomaterials are used to change the defects in the face, nose, lips and forehead. 229
Polyvinyl chloride and acetate (5–20 %) copolymers, PMMA, silicon, Dacron, Teflon, and polyurethanes are widely used materials for maxillofacial implants. Polymers such as PMMA or silicone rubber were used for augmentation in soft tissue applications like gum and chain.
A wide range of tissues, which include cartilage auto graft, cartilage homograft, and bone graft, are collected and used to augment, re-contour and replace defective structures found in the face. 230
8.1.2 Cartilage autograft
Glasgold and Glasgold, in 1991, revealed that cartilage autografts are the most commonly used implants for the face, although the use of synthetic materials is restricted to the nose due to its complex factors. The nasal (septal and lateral cartilage), lobe of the ear (concha cartilage) and rib (costal cartilage) are the major sources of cartilage. In nasal tip surgery, the lower lateral cartilages are removed to fill the defect in the other part of the nose. To reconstruct malar, mandibular defects and auricular defects, the coastal cartilage derived from the sixth and seventh rib is used. 231
Donald and Boride have reviewed the use of human cartilage homograft, which can be preserved under sterile and frozen at −70° for 30 min and then freeze-dried at 40 °C for 36 h. Before surgery, the freeze-dried material is rehydrated. 232
8.1.3 Other implants materials
Churukian et al., reported that collagen could be used as an injectable form to remove wrinkles and acne scars. These materials require continuous preservation due to the limited life of the implants. The usage of injectable siloxanes, which polymerize in situ, has been partly successful in correcting facial deformities. 233
8.1.4 Biomaterials in urological applications
The most commonly used biomaterial for urological applications is silicone. Biomaterials found increased application in urethral catheters to relieve urinary retention to the refined and complex percutaneous urinary damage system, indwelling stent devices for correction of urinary incontinence, urinary bladder, testicular and penile prostheses. 234
Silicone rubber catheters are well accepted with crystal deposition on their smooth lumens, permitting prolonged use of an indwelling drainage system. Nylon, polyurethane, and polyethylene are biomaterials used for the fabrication of stents. After renal surgery, indwelling urethral stents provide internal splintage to enable the healing of the pelvis and ureter, in addition to consenting to uninterrupted urine drainage from the kidney to the bladder. The treatment for urinary incontinence has remained one of the challenging problems in urology. However, various medical techniques have been successfully employed. 235
8.2 Hard tissue applications
8.2.1 Cardiovascular application
Cardiovascular systems include the heart and blood vessels, which concern blood circulation throughout the body. Problems associated with heart valves include structural changes, opening or closing of valves or diseased valve metals. Various metallic implants, ceramics, and polymers are most commonly used for the fabrication of heart valves. Fatty acid blockage of coronary arteries and blood vessels can be replaced by artificial arteries. 236
Lee and Harston, carried out several in vitro tests for the estimation of the thrombogenic potential, consisting of first exposing some blood to contact surfaces of numerous materials and consequently determining the clotting time within this group of materials, which is associated with silicate glasses for standard reference. Kaolin, metakaolin, ZrO2, feldspars, and SiO2 displayed a relative degree of thrombogenicity, whereas Al2O3 and calcium phosphate bones appeared to cause comparatively little thrombogenicity. 237
Ischemic heart disease caused 180,000 deaths in the U.K. and 500,000 deaths in the USA. It is a premature heart disease which causes death in middle age. An artificial heart can be replaced by using seamless polyurethane for a rigid outer shape with a cardiac ventricle and auricle. The implant should ensure blood biocompatibility in order to prevent the rejection of the device by thrombogenicity blood response. Fabrication of materials like pyrolytic carbon-coated graphite, pyrolytic carbon-coated titanium, Co–Ni-alloys, and Co–Cr alloys SS coated with polyprosynthetic implants can improve biocompatibility. Ti, 316L SS and CO–Cr are the most commonly used metallic materials used for the stents. Among these, 316L SS is widely used for stents, and Co–Cr with high elastic modulus has been used for the development of stents. Lately, Mg alloys have been used for cardiovascular implants for temporary or local delivery services.
Recent cardiovascular therapies are controlled by loss of endothelium restenosis and thrombosis. A hybrid nano matrix associated with electrospun PCL amalgamate nanofibers was reported by Andukuri. From this, it is concluded that hybrid nanofibers were found to have wider applications in cardiovascular implants. 238 Figure 19 illustrates artificial heart valves.

Artificial heart valves. 239
8.2.2 Orthopaedic implant
Orthopaedic implants are augmented into the skeletal system, such as bone plates, screws, total hip joints, knee joints, elbow joints, shoulder joints, and reattachments of ligaments of the body for healing purposes. And, in case of accidents, it is permanently placed into the body, which is most common in recent decades. Osteoarthritis and rheumatoid arthritis are a form of joint disorders, for instance, hip, knee, shoulder, ankle and elbow. It has been accessible to alter the joint with prosthesis, plates and screws, hip, knee, spinal, and shoulder. Figure 20 depicts a total hip replacement. There are two types of orthopaedic implants:
Temporary implant. e.g., plates and screws.
Permanent implant. e.g., hip, knee, spinal, shoulder.
Total hip replacement.
Corrosion is the main reason for the failure of the implants, which results from wear. Ceramics, Co–Cr of high wear resistance adopted to construct the implant for orthopaedic application. Femoral components comprise titanium and balls made up of Co–Cr with or without coating. Femoral components fabricated from Ti–6Al–4V and Ti–6Al–7Nb cause corrosion, which was reported by Waller et al. 240 Nakagawa et al., acknowledged the in vivo corrosion behavior of the titanium with plasma deposition to prevent corrosion on the implant surface. 241 Khan et al., carried out corrosion tests using titanium metals like Cp-Ti, Ti–Nb–Zr, and Ti–Mo alloys. Despite the corrosion and toxicity of the implant, which causes adverse effects remains undetermined. 242
8.2.2.1 Sutures materials
The suture has been classified into various types based on their origin, as shown in Figure 21.

Classification of sutures.
8.2.2.2 Monofilaments
According to Guttmann et al., monofilament sutures have a smooth surface that provides tissue and blood cell passages without any inflammation or contamination, whereas braided sutures are highly flexible, have lower memory, and are easily handled. Natural sutures are made from silk, cotton or linen. The use of silk sutures has been reduced due to its low tensile strength and higher tissue reaction, which in turn leads to tissue ingrowth and finally causes inflammations. Henceforth, synthetic non-absorbable sutures such as polyester, polyamide, polypropylene, etc., have been developed. 243
Stainless steel BS 3521, tantalum or silver are the metallic sutures wires used as single-strand monofilament sutures. These surgical suture wires are utilized in orthopaedic, thoracic surgery and nerve-ending clipping. In neuro and thoracic surgery, metal ligatures are made from silver tantalum wires, which are most commonly preferred for medical applications.
8.2.2.3 Drug delivery sutures
The degree of infection or inflammation caused near the wound closure can be decreased by the antimicrobial drug delivery systems that improve the healing process. Anti-bacterial agents such as tetracycline and gentamicin can be coated or doped onto the polymers (PCL) or nanomaterials for drug delivery. 244
8.2.2.4 Sensor application
Biomaterials can be used in sensor applications, such as electrodes, to measure the bioelectric signal that monitors the function of body parts or organs, e.g. ECG (electrocardiogram). ECG records the heart’s electrical activity by extracellular potential generated across the cell membranes. Platinum and its alloys are more acceptable electrodes for biomedical applications.
8.2.3 Metals in dentistry
Metals in dentistry are primarily used to construct crowns, inlays and orthodontic wires. The metals are generally in the form of an alloy to emphasize their strength and aesthetic value. Gold alloys (containing silver, copper, palladium, platinum, and zinc), cobalt-chromium, titanium–aluminum–vanadium, stainless steel and nitinol are used for dentistry. 245
8.2.4 Oral implants
The adults over the age of 50 years were found to have upper and lower dentures, whereas others have partial dentures to replace the teeth. Without any serious illnesses concerning aesthetics, function and retention, these false teeth serve the individuals. Subperiosteal and end osseous devices are the most commonly used dental implants. These implant devices are used to repair damaged and diseased mandibles, support the reconstruction of the alveolar ridge, and stimulate the growth of bone to change lesions accompanying periodontal disease. Therefore, successful long-term dental implants for these applications would solve many problems and provide aesthetic appeal for a large number of people. 246
8.2.5 Dental implant
Bacterial disease is the main cause of damage to tooth and gum tissues. Demineralization and dissolution of teeth caused by metabolism of bacteria may result in dental cavities, tooth decay or damage. Biomaterial implants can be used to replace the teeth. Different types of alloys are used for dental implantations, such as restoration, amalgam implants, solders and orthodontic materials. The basic requirement for the implant is high flexibility and the ability to adapt to the shape. End osseous and subperiosteal implants are used for dental applications such as root blades to secure the crowns and retain dentures. The end osseous is located within the bone, and the subperiosteal is located on the top bone. A schematic diagram of the dental implant is shown in Figure 22.

Human dental implant.
Corrosion is the major problem for metallic dental implants where the pH of saliva ranges from 5.2 to 7.8, which leads to the failure of implants. Temperature, quantity and quality of saliva, plague, pH, protein, and food are the main causes of corrosion. Chaturvedi reassessed the corrosion of metallic implants for dental applications. 247 Galvanic corrosion occurs in between metallic alloys, such as Co–Cr alloys, Ni–Cr, sliver palladium, gold ternary Ti. Galvanic corrosion is the major problem associated with density. It occurs when two metals or dissimilar alloys come into contact, and there is a flow of electric current. Pitting corrosion caused by Co alloys is highly carcinogenic, and titanium alloys are resistant to corrosion. Hence, Ti alloys are commonly used for dental implants. Changes in temperature, pH of saliva, brushing, and food lead to the failure of implants even though implants are highly corrosion resistant. 248
8.2.6 Dental fillings and restorations
According to Boyne, dental amalgam is conventionally used for dental filling. The amalgam consists of silver-tin copper alloy with mercury. The presence of mercury in amalgam hardens and causes the tooth to crack. Alternatively, composite, ceramics and glass ionomer polymer filling are employed. A composite filling is made of resin and plastic. Inorganic fillings containing a minimal quantity of silica-alumina glasses and calcium fluoride have been used recently for cavity restoration with high hardness, low polymerization shrinkage and wear resistance. 249
8.2.6.1 Drug delivery system
The drug delivery system comprises carriers like polymers or ceramics, which incorporate bioactive agents with controlled therapeutic limits. The carrier used for loading the drug should be non-toxic, non-carcinogenic, non-inflammatory or non-immunogenic. The bioactive agents used to release on the target site to breakdown the cells or tissues and elimination of the products. Based on the properties (pH, ionic strength, concentration and wettability) of bioactive agents, the rate of drug release takes place. Drug release is classified as chemical-controlled and diffusion-controlled. In diffusion-controlled, the drug diffuses out of the carrier into the surroundings in the form of a matrix or reservoir. Chemically controlled release occurs either by polymer biodegradation or by chemical bond cleavages. Polymers such as PVA, PGA, PLA, Poly [2-hydroxy-ethyl methyl acrylate PHEMA], PAA and cellulose derivatives are the most commonly used carriers for controlled drug release. 250
Bioceramics carriers such as HAP, tricalcium phosphate and magnetic materials are usually used in drug delivery applications. For cancer treatment, a collagen-based microsphere is used to release IL-2, polyline and cisplatin on the targeted site. In the case of prostate cancer treatment, Zoladex (Zeneca Pharmaceuticals USA) was used, which consists of LH-RH group agonist in the polylactic polymer. 251 Figure 23 illustrates the application of biomaterials in the human body.
Applications of biomaterials.
9 Future directions
During the last 60 years, the biomaterial field has changed significantly in the development of biomedical devices with improved applications in the direction of future tissue engineering. However, in this present period, stem cell transplantation is one of the most trending clinical applications in normal human life. So, biomaterials have been used in the enhancement of stem cell therapy with an appropriate biological response.
An alternate method for the restoration of damaged tissues and treatment for degenerative disease is stem-cell transplantation. Stem cells and regeneration medicine is a fast-growing field that focuses on human health. Three important features for the successful use of stem cell therapy are stem cells grow indefinitely, differentiate into specified cell types, and can be delivered to the site of biomaterials with excellent biocompatibility. Tiago Pereira, developed biomaterials associated with cellular systems using mesenchymal cells that are isolated from Wharton’s jelly of the umbilical cord. They studied different biomaterials associated with MCSs in rat muscles. 252
Transplantation of stem cells to the heart can improve cardiovascular functions. Vincent and Richard, 253 reviewed how biomaterials can enhance the stem cell for heart functions. Biomaterials can assist in angiogenesis engraftment and differentiation of stem cells. Biomaterials can accelerate the electrochemical integration of stem cells and assist in the angiogenesis, engraftment and differentiation of stem cells. Along with stem cells, biomaterials can deliver proteins, genes and RNA. Many approaches require biomaterials and stem cell biology in regenerative medicine. 254 , 255
However, concepts like healing, dynamics and adaptation, hierarchy, and complexity are beginning to characterize the design of soft biomaterials from a future perspective. To attain this potential and acquire knowledge about the creation and design of complex biomaterials systems, enhanced bone modeling and experimental methods are needed. Contemporary chemistry has made it feasible to synthesize polymers with controlled molecular weights, distinct sequences, and integrated biological functionality; nonetheless, the functionality and performance of biomaterial systems ultimately rely on how these structural components combine and interact at complex biological interfaces.
Numerous novel technologies, including 3D and 4D bioprinting, electronic beam melting, and robocasting, have been developed by combining various production techniques from fields of study not typically related to biomaterials or medicine. Customized biomaterials can now be produced at a degree that was previously impossible with these new technologies. The field of biomaterials will progress and assist in addressing the difficulties that lie ahead if researchers from several disciplines not only engineering and medicine continue their interdisciplinary cooperation. In order for the general public to benefit from the materials’ unparalleled abilities to heal, repair, and regenerate, a concentrated effort must be made to move smart biomaterials from laboratory research and development into clinical practice.
10 Conclusions
In this present research review, we have provided fundamental information about synthetic biomaterials and their applications towards biomedical and tissue engineering technology. There is an increase in interest in research and development in the field of nano-biomedical applications.
Synthetic biomaterials are highly recommended for use in orthopaedic and dentistry surgery due to characteristic features such as biocompatibility, bone-bonding ability, non-inflammatory and non-toxic, etc. In these synthetic biomaterials, more sub-class of biomaterials are present; they are bioceramics, composites, polymers and metallic alloys. These biomaterials have their own advantages and disadvantages when applied in clinical applications such as drug delivery, total hip replacement, and cardiovascular valves. The design and clinical trials of developed synthetic biomaterials are an interdisciplinary area that requires the contributions of scientists, pathologists, and engineers. These biomaterials are mainly implemented in the surgery with proper interaction of tissues in the form of biomechanics, biochemical and physiology.
However, bioceramics and their composites are limited to use in large bone defects and fractures due to their poor mechanical properties. Hence, metallic alloys such as 316L SS, Co–Cr alloys, Ti-alloys, etc. act as substituting materials for ceramics and their composites in high load-bearing areas. In these, 316L SS was reported as 70 % failure due to pitting, crevice attack, and crack. Titanium and its alloys play a promising role in the biomedical field, and titanium implants are more advantageous in terms of blood clot formations due to their reduced surface roughness and moderate wettability. Ti-etched surfaces are essential for osteoblast proliferation and suitable for bone healing tissues. Titanium implants are generally surface modified to increase the osteoconductivity and osteointegration with an increase in the corrosion and wear resistance. Surface modification implants protect the metallic implants from corrosion and wear and improve biocompatibility. The bioactive ceramic coatings on metallic alloys play a crucial role in the formation of chemical bonds with natural bone and act as a barrier to prevent the leaching of ions from bio-implants with improved corrosion resistance.
In the medical field, 3D printing technology has become a potent instrument that makes it possible to produce intricate implants and medical devices that are both affordable and tailored to each patient’s unique requirements. The technique has a number of benefits, including shorter lead times, better patient outcomes, and lower costs. Tissue engineering, aided by 3D printing and decellularization techniques, will undoubtedly provide a revolutionary answer for tissue/organ regeneration and, eventually, for patient treatments that are both safe and effective.
Furthermore, the current researchers need to investigate the bio-implants by applying advanced manufacturing methods with highly sophisticated coatings techniques to improve the osteo-conduction, biocompatibility, and corrosion resistance properties of biomaterials also require focus on the in vivo studies for future tissue engineering applications.
Award Identifier / Grant number: DBT-RA/2023 / January /N/3756
Acknowledgments
The authors would like to thank the VELLORE INSTITUTE OF TECHNOLOGY University, Vellore, Tamil Nadu, for rendering the necessary facilities.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Credit author statement: I am herewith submitting a Review titled “A systematic review on biomaterials and their recent progress in biomedical applications: bone tissue engineering” by Priyadarshini Baskaran, Balasubramanian Muthiah and Vijayalakshmi Uthirapathy for publication. Priyadarshini Baskaran -writing, Balasubramanian Muthiah- drafting original review, Vijayalakshmi Uthirapathy - drafting original review. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. As the corresponding author, U. Vijayalakshmi is responsible for ensuring that the descriptions are accurate and agreed by all authors.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
-
Research funding: This work was supported by Department of Biotechnology, Ministry of Science and Technology, India (https://doi.org/10.13039/501100001407, DBT-RA/2023 / January /N/375).
-
Data availability: Not applicable.
References
1. Sukhwinder, K. B.; Neeta, L. L.; Seeram, R. Smart Biomaterials – A Review. Rev. Adv. Mater. Sci. 2015, 40, 303–314.Search in Google Scholar
2. Williams, D. Definitions in Biomaterials. In Proceedings of a Consensus Conference of the European Society for Biomaterials; European Society for Biomaterials: Chester, UK, 1986.Search in Google Scholar
3. Azagarsamy, M. A.; Anseth, K. S. Bioorthogonal Click Chemistry: An Indispensable Tool to Create Multifaceted Cell Culture Scaffolds. ACS Macro Lett. 2013, 2, 5–9; https://doi.org/10.1021/mz300585q.Search in Google Scholar PubMed PubMed Central
4. Hoffman, A. S. Stimuli-responsive Polymers: Biomedical Applications and Challenges for Clinical Translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16; https://doi.org/10.1016/j.addr.2012.11.004.Search in Google Scholar PubMed
5. Buchanan, F.; Gallagher, L.; Jack, V.; Dunne, N. Short Fiber Reinforcement of Calcium Phosphate Bone Cement. Proc. IME H J. Eng. Med. 2007, 221, 203–211; https://doi.org/10.1243/09544119jeim235.Search in Google Scholar
6. Sharma, B.; Williams, C. G.; Kim, T. K.; Sun, D.; Malik, A.; Khan, M.; Elisseeff, J. H. Designing Zonal Organization into Tissue-Engineered Cartilage. Tissue Eng. 2007, 13 (2), 405–414; https://doi.org/10.1089/ten.2006.0068.Search in Google Scholar PubMed
7. Hench, L. L.; Ethridge, E. C. Biomaterials: An Interfacial Approach. J. Biosci. Bioeng. 1982, 4, 62–86.Search in Google Scholar
8. Gaspare, T.; Zimbler, M. S. Renaissance Surgeon. Arch. Facial Plast. Surg. 2001, 3 (4), 283–284; https://doi.org/10.1001/archfaci.3.4.283.Search in Google Scholar PubMed
9. Sun, L.; Berndt, C. C.; Gross, K. A.; Kucuk, A. Material Fundamentals and Clinical Performance of Plasma-Sprayed Hydroxyapatite Coatings: A Review. J. Biomed. Mater. Res. B. 2001, 58, 570–592; https://doi.org/10.1002/jbm.1056.Search in Google Scholar PubMed
10. Long, M.; Rack, H. J. Titanium Alloys in Total Joint Replacement—A Materials Science Perspective. Biomaterials 1998, 19, 1621–1639; https://doi.org/10.1016/s0142-9612(97)00146-4.Search in Google Scholar PubMed
11. Suzuki, A.; Kanetaka, H.; Shimizu, Y.; Tomizuka, R.; Hosoda, H.; Miyazaki, S.; Okuno, O.; Igarashi, K.; Mitani, H. Orthodontic Buccal Tooth Movement by Nickel-Free Titanium-Based Shape Memory and Superelastic Alloy Wire. Angle Orthod. 2006, 76, 1041–1046; https://doi.org/10.2319/083105-306.Search in Google Scholar PubMed
12. Driessens, F. C. M.; Boltong, M. G.; De Maeyer, E. A. P.; Wenz, R.; Nies, B.; Planell, J. A. The Ca/P Range of Nanoapatitic Calcium Phosphate Cement. Biomaterials 2002, 23, 4011–4017; https://doi.org/10.1016/s0142-9612(02)00151-5.Search in Google Scholar PubMed
13. Hench, L. L.; Polak, J. M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017; https://doi.org/10.1126/science.1067404.Search in Google Scholar PubMed
14. Ambrosio, L.; Netti, P.; Iannace, S.; Huang, S. J.; Nicolais, L. Composite Hydrogels for Intervertebral Disc Prostheses. J. Mater. Sci. Mater. Med. 1996, 7, 525–530; https://doi.org/10.1007/bf00058561.Search in Google Scholar
15. Rajendran, V. Engineering Physics; New Delhi: Tata McGraw-Hill Publishing Company Limited, 2011, ISBN 978-0-07-132897-5.Search in Google Scholar
16. Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158; https://doi.org/10.1098/rsif.2008.0151.Search in Google Scholar PubMed PubMed Central
17. Rezwan, K.; Chen, Q. Z.; Ju Blaker, J.; Roberto Boccaccini, A. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431; https://doi.org/10.1016/j.biomaterials.2006.01.039.Search in Google Scholar PubMed
18. Rodrigues, A. S.; Andelman, S. J.; Bakarr, M. I.; Boitani, L.; Brooks, T. M.; Cowling, R. M.; Fishpool, L. D.; Fonseca, A.; Gaston, K. J.; Hoffmann, M.; Long, J. S.; Marquet, P. A.; Pilgrim, J. D.; Pressey, R. L.; Schipper, J.; Sechrest, W.; Stuart, S. N.; Underhill, L. G.; Waller, R. W.; Watts, M. E. J.; Yan, X. Effectiveness of the Global Protected Area Network in Representing Species Diversity. Nature 2004, 428, 640–643; https://doi.org/10.1038/nature02422.Search in Google Scholar PubMed
19. Bothe, R. T. Reaction of Bone to Multiple Metallic Implants. Surg. Gynecol. Obstet. 1940, 71, 59–602.Search in Google Scholar
20. Lee, L. B.; Solanki, A.; Kim, J. D.; Jung, J. Nanomedicine: Dynamic Integration of Nanotechnology with Biomedical Science. In Nanomedicine; Pan Stanford Publisher: Singapore, 2019; pp 1–38.10.1201/9780429065767-1Search in Google Scholar
21. Narayan, R. J.; Kumta, P. N.; Sfeir, C.; Lee, D. H.; Choi, D.; Olton, D. Nanostructured Ceramics in Medical Devices: Applications and Prospects. JOM 2004, 56, 38–43; https://doi.org/10.1007/s11837-004-0289-x.Search in Google Scholar
22. Ovsianikov, A.; Chichkov, B.; Adunka, O.; Pillsbury, H.; Doraiswamy, A.; Narayan, R. J. Rapid Prototyping of Ossicular Replacement Prostheses. Appl. Surf. Sci. 2007, 253, 6603–6607; https://doi.org/10.1016/j.apsusc.2007.01.062.Search in Google Scholar
23. Ducheyne, P. Bioactive Ceramics. J. Bone Joint Surg. 1994, 76, 861–862; https://doi.org/10.1302/0301-620x.76b6.7983107.Search in Google Scholar
24. Doraiswamy, A.; Jin, A. C.; Narayan, R. J.; Mageswaran, P.; Mente, P.; Modi, R.; Auyeung, R.; Chrisey, D. B.; Ovsianikov, A.; Chichkov, B. Two Photon Induced Polymerization of Organic–Inorganic Hybrid Biomaterials for Microstructured Medical Devices. Acta Biomater. 2006, 2, 267–275; https://doi.org/10.1016/j.actbio.2006.01.004.Search in Google Scholar PubMed
25. Williams, D. F. The Williams Dictionary of Biomaterials; Liverpool: Liverpool, UK, 1999.10.5949/UPO9781846314438Search in Google Scholar
26. Hummer, C. D.; Rothman, R. H.; Hozack, W. J. Catastrophic Failure of Modular Zirconia Ceramic Femoral Head Components after Total Hip Arthroplasty. J. Arthritis 1995, 10, 848–850; https://doi.org/10.1016/s0883-5403(05)80085-3.Search in Google Scholar PubMed
27. Verma, D.; Katti, K. S.; Katti, D. R. Osteoblast Adhesion, Proliferation and Growth on Polyelectrolyte Complex–Hydroxyapatite Nanocomposites. Phil. Trans. R. Soc. A 2010, 368, 2083–2097; https://doi.org/10.1098/rsta.2010.0013.Search in Google Scholar PubMed
28. Venugopal, J.; Prabhakaran, M. P.; Zhang, Y.; Low, S.; Choon, A. T.; Ramakrishna, S. Biomimetic Hydroxyapatite-Containing Composite Nanofibrous Substrates for Bone Tissue Engineering. Phil. Trans. R. Soc. A 2010, 368, 2065–2081; https://doi.org/10.1098/rsta.2010.0012.Search in Google Scholar PubMed
29. Ha, T. L. B.; Quan, T. M.; Vu, D. N.; Si, D. M. Naturally Derived Biomaterials: Preparation and Application. Regen. Med. Tissue Eng. 2013, 22, 247.Search in Google Scholar
30. Eswarappa, V.; Bhatia, S. K. Introduction to Global Health, Biomaterials, and Therapeutics Springer Briefs in Public Health. In Naturally Based Biomaterials and Therapeutics: The Case of India; Springer New York: New York, NY, Vol. 16, 2013; pp 3–13.10.1007/978-1-4614-5386-4_1Search in Google Scholar
31. Suh, J. K. F.; Matthew, H. W. T. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598; https://doi.org/10.1016/s0142-9612(00)00126-5.Search in Google Scholar PubMed
32. Mohamed, A. H.; Abdul, S. M.; Naser, A. A. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768; https://doi.org/10.3390/ma8052749.Search in Google Scholar
33. Hench, L. L. Sol-Gel Silica for Precision and Multifunctional Optics. Ceram. Int. 1991, 17, 209–216; https://doi.org/10.1016/0272-8842(91)90015-r.Search in Google Scholar
34. Dorozhkin, S. V. Calcium Orthophosphate-Based Bioceramics. Materials 2013, 6 (9), 3840–3942; https://doi.org/10.3390/ma6093840.Search in Google Scholar PubMed PubMed Central
35. Peltier, L. F. The Use of Plaster of Paris to Fill Defects in Bone. Clin. Orthop. Relat. Res. 1961, 21, 1–31.Search in Google Scholar
36. Shegarfi, H.; Reikeras, O. Bone Transplantation and Immune Response. Orthop. Surg. 2009, 17 (2), 206–211; https://doi.org/10.1177/230949900901700218.Search in Google Scholar PubMed
37. Chevalier, J.; Gremillard, L.; Virkar, A. V.; Clarke, D. R. The Tetragonal‐monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92 (9), 1901–1920; https://doi.org/10.1111/j.1551-2916.2009.03278.x.Search in Google Scholar
38. Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. 2013, 29 (6), 503–513; https://doi.org/10.1016/j.jmst.2013.03.002.Search in Google Scholar
39. Baino, F. Functionally Graded Bioactive Glass-Derived Scaffolds Mimicking Bone Tissue. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing: United Kingdom, Vol. 1, 2019; pp 443–466.10.1016/B978-0-08-102196-5.00016-1Search in Google Scholar
40. Carrodeguas, R. G.; De Aza, S. α-Tricalcium Phosphate: Synthesis, Properties and Biomedical Applications. Acta Biomater. 2011, 7 (10), 3536–3546; https://doi.org/10.1016/j.actbio.2011.06.019.Search in Google Scholar PubMed
41. Park, J. B.; Lakes, R. S. Biomaterials: An Introduction; Plenum Publishing Corporation: New York, 2007.Search in Google Scholar
42. Park, J. B.; Bronzino, J. B. Biomaterials Principles and Applications; CRC Press: Boca Raton, 2002.10.1201/9781420040036Search in Google Scholar
43. Thamaraiselvi, T. V.; Rajeswari, S. Trends Biomaterial – A Review. Artif. Organ. 2004, 18, 9.Search in Google Scholar
44. Nitesh, R. P.; Piyush, P. G. A Review on Biomaterials: Scope, Applications & Human Anatomy Significance. Int. J. Emerg. Tech. Adv. Eng. 2012, 2, 91–101.Search in Google Scholar
45. Sergey, V. D. Bioceramics of Calcium Orthophosphates. Biomaterials 2010, 31, 1465–1485; https://doi.org/10.1016/j.biomaterials.2009.11.050.Search in Google Scholar PubMed
46. Noiri, F.; Hoshi, H.; Murakami, H.; Sato, K.; Kawai, S. I.; Kawai, K. Biocompatibility of a Mobile Alumina-Ceramic Orbital Implant. Folia Ophthalmol. Jpn. 2002, 53, 476–480.Search in Google Scholar
47. Yuhta, T.; Kikuta, Y.; Mitamura, Y.; Nakagane, K.; Murabayashi, S.; Nishimura, I. Direct Bone Formation on Alumina Bead Composite. J. Biomed. Mater. Res. 1994, 28, 217–224; https://doi.org/10.1002/jbm.820280212.Search in Google Scholar PubMed
48. Lawson, S. Environmental Degradation of Zirconia Ceramics. J. Eur. Ceram. Soc. 1995, 15, 485–502; https://doi.org/10.1016/0955-2219(95)00035-s.Search in Google Scholar
49. Patel, B. N. Acrylic Removable Prosthesis – An Integral Part of Modern-Day Dentistry. Famdent 2005, 6, 62–64.Search in Google Scholar
50. Lilley, E. Review of Low Temperature Degradation in Zirconia. In Corrosion and Corrosive Degradation of Ceramics. Ceramic Transaction; American Ceramic Society: Westerville, Ohio, Vol. 10, 1990.Search in Google Scholar
51. Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25; https://doi.org/10.1016/s0142-9612(98)00010-6.Search in Google Scholar PubMed
52. Chevalier, J.; Drouin, J. M.; Cale, S. B. Low‐temperature Aging of Y‐TZP Ceramics. J. Am. Ceram. Soc. 1999, 82, 2150–2154.10.1111/j.1151-2916.1999.tb02055.xSearch in Google Scholar
53. Mathina, M.; Shinyjoy, E.; Ramya, S.; Kavitha, L.; Gopi, D. Multifunctional Crab Shell Derived Hydroxyapatite/Metal Oxide/Polyhydroxybutyrate Composite Coating on 316L SS for Biomedical Applications. Mater. Lett. 2022, 313, 131701; https://doi.org/10.1016/j.matlet.2022.131701.Search in Google Scholar
54. Verket, A.; Tiainen, H.; Lyngstadaas, H. J.; Haugen, S. P.; Nilsen, O.; Reseland, J. E. Enhanced Osteoblast Differentiation on Scaffolds Coated with TiO2 Compared to SiO2 and CaP Coatings. Biointerphases 2021, 7, 1; https://doi.org/10.1007/s13758-012-0036-8.Search in Google Scholar PubMed
55. Vijayalakshmi, U.; Chellappa, M.; Anjaneyulu, U.; Manivasagam, G.; Sethu, S. Influence of Coating Parameter and Sintering Atmosphere on the Corrosion Resistance Behaviour of Electrophoretically Deposited Composite Coatings. Mater. Manuf. Process. 2016, 31, 95–106; https://doi.org/10.1080/10426914.2015.1070424.Search in Google Scholar
56. Saenz, A. Ceramic Biomaterials: An Introductory Overview. J. Mater. Educ. 1999, 21, 267–276.Search in Google Scholar
57. Anjaneyulu, U.; Pattanayak, D. K.; Vijayalakshmi, U. The Facile and Phase Pure Evaluations of Nano Hydroxyaptite Powder by Sol-Gel Method. Int. J. ChemTech Res. 2014, 7, 1516–1520.Search in Google Scholar
58. Anjaneyulu, U.; Vijayalakshmi, U. Influence of Particle Size and Morphology of Chemically Modified Hydroxyapatite by Sol-Gel Method. Int. J. ChemTech Res. 2015, 7 (3), 1426–1432.Search in Google Scholar
59. Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive Coating as a Surface Modification Technique for Biocompatible Metallic Implants: A Review. J. Asian Ceram. Soc. 2019, 7, 397–406.10.1080/21870764.2019.1669861Search in Google Scholar
60. Ruan, J.; Grant, M. H. Biocompatibility Evaluation In Vitro. Part I: Morphology Expression and Proliferation of Human and Rat Osteoblasts on the Biomaterials. J. Central South University of Tech. 2001; 8, 1–8.10.1007/s11771-001-0015-6Search in Google Scholar
61. Yuan, H.; Zou, P.; Yang, Z.; Zhang, X.; De Bruijn, J. D.; De Groot, K. Bone Morphogenetic Protein and Ceramic-Induced Osteogenesis. J. Mater. Sci. Mater. Med. 1998, 9, 717–721; https://doi.org/10.1023/a:1008998817977.10.1023/A:1008998817977Search in Google Scholar PubMed
62. Best, S. M.; Porter, A. E.; Thian, E. S.; Huangc, J. Bioceramics: Past, Present and for the Future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327; https://doi.org/10.1016/j.jeurceramsoc.2007.12.001.Search in Google Scholar
63. Elangomannan, S.; Louis, K.; Dharmaraj, B. M.; Kandasamy, V. S.; Soundarapandian, K.; Gopi, D. Carbon Nanofiber/Polycaprolactone/Mineralized Hydroxyapatite Nanofibrous Scaffolds for Potential Orthopedic Applications. ACS Appl. Mater. Interfaces 2017, 9 (7), 6342–6355; https://doi.org/10.1021/acsami.6b13058.Search in Google Scholar PubMed
64. Anjaneyulu, U.; Priyadarshini, B.; Arul Xavier Stango, S.; Chellappa, M.; Geetha, M.; Vijayalakshmi, U. Preparation and Characterisation of Sol–Gel-Derived Hydroxyapatite Nanoparticles and its Coatings on Medical Grade Ti-6Al-4V Alloy for Biomedical Applications. Mater. Technol. 2017, 32 (13), 800–814; https://doi.org/10.1080/10667857.2017.1364476.Search in Google Scholar
65. Mathina, M.; Shinyjoy, E.; Kavitha, L.; Manoravi, P.; Gopi, D. A Comparative Study of Naturally and Synthetically Derived Bioceramics for Biomedical Applications. Mater. Today 2020, 26, 3600–3603; https://doi.org/10.1016/j.matpr.2019.08.222.Search in Google Scholar
66. Sadat-Shojai, M.; Khorasani, M. T.; Dinpanah Khoshdargi, E.; Jamshid, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621; https://doi.org/10.1016/j.actbio.2013.04.012.Search in Google Scholar PubMed
67. Xilin, Y.; Stott, M. J. α and β Tricalcium Phosphate: A Density Functional Study. Phys. Rev. B 2003, 68, 205.Search in Google Scholar
68. Priyadarshini, B.; Vijayalakshmi, U. In Vitro Bioactivity, Biocompatibility and Corrosion Resistance of Multi-Ionic (Ce/Si) Co-doped Hydroxyapatite Porous Coating on Ti-6Al-4 V for Bone Regeneration Applications. Mater. Sci. Eng. C 2021, 119, 111620; https://doi.org/10.1016/j.msec.2020.111620.Search in Google Scholar PubMed
69. Anjaneyulu, U.; Deepak, K. P.; Vijayalakshmi, U. Snail Shell Derived Natural Hydroxyapatite: Effects on NIH-3T3 Cells for Orthopaedic Applications. Mater. Manufac. Process. 2016, 31, 206–216.10.1080/10426914.2015.1070415Search in Google Scholar
70. Mathina, M.; Shinyjoy, E.; Ramya, S.; Kavitha, L.; Gopi, D. Multifunctional Crab Shell Derived Hydroxyapatite/Metal Oxide/Polyhydroxybutyrate Composite Coating on 316L SS for Biomedical Applications. Mater. Lett. 2022, 313, 131701; https://doi.org/10.1016/j.matlet.2022.131701.Search in Google Scholar
71. Prabhakaran, K.; Balamurugan, A.; Rajeswari, S. Development of Calcium Phosphate-Based Apatite from Hen’s Egg Shell. Bull. Mater. Sci. 2005, 28, 115; https://doi.org/10.1007/bf02704229.Search in Google Scholar
72. Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K. A. Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105; https://doi.org/10.3390/polym13071105.Search in Google Scholar PubMed PubMed Central
73. Verestiuc, L.; Spataru, M. C.; Baltatu, M. S.; Butnaru, M.; Solcan, C.; Sandu, A. V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti-Mo-Si Materials for Bone Prosthesis Applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198; https://doi.org/10.1016/j.jmbbm.2020.104198.Search in Google Scholar PubMed
74. Thanigaivel, S.; Priya, A. K.; Balakrishnan, D.; Dutta, K.; Rajendran, S.; Soto-Moscoso, M. Insight on Recent Development in Metallic Biomaterials: Strategies Involving Synthesis, Types and Surface Modification for Advanced Therapeutic and Biomedical Applications. Biochem. Eng. J. 2022, 187, 108522; https://doi.org/10.1016/j.bej.2022.108522.Search in Google Scholar
75. Arifin, A.; Sulong, A. B.; Muhamad, N.; Syarif, J.; Ramli, M. I. Material Processing of Hydroxyapatite and Titanium Alloy (HA/Ti) Composite as Implant Materials Using Powder Metallergy: A Review. Mater. Des. 2014, 55, 165–175; https://doi.org/10.1016/j.matdes.2013.09.045.Search in Google Scholar
76. Fernandes, J. S.; Gentile, P.; Pires, R. A.; Reis, R. L.; Hatton, P. V. Multifunctional Bioactive Glass and Glass-Ceramic Biomaterials with Antibacterial Properties for Repair and Regeneration of Bone Tissue. Acta Biomater. 2017, 59, 2–11; https://doi.org/10.1016/j.actbio.2017.06.046.Search in Google Scholar PubMed
77. Zhou, Y.; Wu, C.; Chang, J. Bioceramics to Regulate Stem Cells and Their Microenvironment for Tissue Regeneration. Mater. Today 2018, 24, 41–56; https://doi.org/10.1016/j.mattod.2018.07.016.Search in Google Scholar
78. Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(lactic Acid) Composites in Tissue Engineering and Drug Delivery. Composites, Part B 2020, 199, 108238; https://doi.org/10.1016/j.compositesb.2020.108238.Search in Google Scholar
79. Jimenez-Marcos, C.; Mirza-Rosca, J. C.; Baltatu, M. S.; Vizureanu, P. Experimental Research on New Developed Titanium Alloys for Biomedical Applications. Bioengineered 2022, 9, 686; https://doi.org/10.3390/bioengineering9110686.Search in Google Scholar
80. Levin, D. A.; James, J. H.; John, A. B. Adjacent Segment Degeneration Following Spinal Fusion for Degenerative Disc Disease. Bull.-Hosp. Jt. Dis. New York 2007, 65, 1–29.Search in Google Scholar
81. Clarke, S. A.; Walsh, P.; Maggs, C.; Buchanan, F. Designs from the Deep: Marine Organisms for Bone Tissue Engineering. Biotech. Adv. 2011, 29, 610–617; https://doi.org/10.1016/j.biotechadv.2011.04.003.Search in Google Scholar
82. Nery, E. B.; Lynch, K. L.; Rooney, G. E. Alveolar Ridge Augmentation with Tricalcium Phosphate Ceramic. J. Prosthet. Dent. 1978, 40, 668–675; https://doi.org/10.1016/0022-3913(78)90067-7.Search in Google Scholar
83. Hench, L. L.; Kokubo, T. Properties of Bioactive Glasses and Glass-Ceramics. In Handbook of Biomaterial Properties; Black, J.; Hastings, G., Eds.; Chapman & Hall: London, 1998; pp 355–363.10.1007/978-1-4615-5801-9_22Search in Google Scholar
84. Kohn, D. H.; Ducheyne, P. Microstructural Refinement of β-sintered and Ti-6Al-4V Porous-Coated by Temporary Alloying with Hydrogen. J. Mater. Sci. 1991, 26, 534; https://doi.org/10.1007/bf00576555.Search in Google Scholar
85. Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce In Vivo Surface‐structure Changes in Bioactive Glass‐ceramic A‐W3. J. Biomed. Mater. Res. 1990, 24, 721–734; https://doi.org/10.1002/jbm.820240607.Search in Google Scholar
86. Dharmaraj, B. M.; Subramani, R.; Dhanaraj, G.; Louis, K. Multifunctional Halloysite Nanotube Based Composite Coatings on Titanium as Metal Implant for Orthopedic Applications. Compos. C: Open Access 2020, 3, 100077; https://doi.org/10.1016/j.jcomc.2020.100077.Search in Google Scholar
87. Vijayalakshmi, U.; Balamurugan, A.; Rajeswari, S. Synthesis and Characterization of Porous Silica Gels for Biomedical Applications. Trends Biomater. Artif. Organs 2005, 18, 101–105.Search in Google Scholar
88. Anjaneyulu, U.; Vijayalakshmi, U. Preparation and Characterization of Novel Sol-Gel Derived Hydroxyapatite/Fe3O4 Composites Coatings on Ti-6Al-4V for Biomedical Applications. Mater. Lett. 2017, 189, 118–121; https://doi.org/10.1016/j.matlet.2016.11.078.Search in Google Scholar
89. Peltola, T.; Jokinen, M.; Rahiala, H.; Levanen, E.; Rosenholm, J. B.; Kangasniemi, I.; Yli‐Urpo, A. Calcium Phosphate Formation on Porous Sol‐gel‐derived SiO2 and CaO‐P2O5‐SiO2 Substrates In Vitro. J. Biomed. Mater. Res. 1999, 44, 12–21; https://doi.org/10.1002/(sici)1097-4636(199901)44:1<12::aid-jbm2>3.0.co;2-e.10.1002/(SICI)1097-4636(199901)44:1<12::AID-JBM2>3.0.CO;2-ESearch in Google Scholar
90. Rounan, L. C.; Hench, L. L. An Investigation of Bioactive Glass Powders by Sol-Gel Processing. J. Appl. Biomater. 1991, 2, 231–239; https://doi.org/10.1002/jab.770020403.Search in Google Scholar PubMed
91. Vijayalakshmi, U.; Rajeswari, S. Synthesis and Characterization of Sol-Gel Derived Glass-Ceramic and Its Corrosion Protection on 316L SS. J. Sol. Gel Sci. Technol. 2007, 43, 251–258; https://doi.org/10.1007/s10971-007-1576-0.Search in Google Scholar
92. Litkowski, L. J.; Hack, G. D.; Sheaffer, H. B.; Greenspan, D. C. Occlusion of Dentin Tubules by 45S5 Bioglass, In Bioceramics 10, Proceedings of 10th International Symposium on Ceramics In Medicine, Paris, France; Sedel, L., Rey, C., Eds.; 1997.10.1016/B978-008042692-1/50098-4Search in Google Scholar
93. Sridevi, S.; Sutha, S.; Kavitha, L.; Gopi, D. Physicochemical and Biological Behaviour of Biogenic Derived Hydroxyapatite and Carboxymethyl Cellulose/Sodium Alginate Biocomposite Coating on Ti6Al4V Alloy for Biomedical Application. Mater. Chem. Phys. 2020, 254, 123455; https://doi.org/10.1016/j.matchemphys.2020.123455.Search in Google Scholar
94. Priyadarshini, B.; Vijayalakshmi, U. Development of Cerium and Silicon Co-doped Hydroxyapatite Nanopowder and its In Vitro Biological Studies for Bone Regeneration Applications. Adv. Powder Technol. 2018, 29 (11), 2792–2803; https://doi.org/10.1016/j.apt.2018.07.028.Search in Google Scholar
95. Zhang, Z. Y.; Xu, Y. D.; Ma, Y. Y.; Qiu, L. L.; Wang, Y.; Kong, J. L.; Xiong, X. M. Biodegradable ZnO@ Polymer Core–Shell Nanocarriers: pH-Triggered Release of Doxorubicin In Vitro. Angew. Chem. 2013, 125, 4221–4225; https://doi.org/10.1002/ange.201300431.Search in Google Scholar
96. Yadav, N.; Singh, S. Polyoxometalate-Mediated Vacancy-Engineered Cerium Oxide Nanoparticles Exhibiting Controlled Biological Enzyme-Mimicking Activities. Inorg. Chem. 2021, 60, 7475–7489; https://doi.org/10.1021/acs.inorgchem.1c00766.Search in Google Scholar PubMed
97. Priyadarshini, B.; Anjaneyulu, U.; Vijayalakshmi, U. Preparation and Characterization of Sol-Gel Derived Ce4+ Doped Hydroxyapatite and It’s In Vitro Biological Evaluations for Orthopedic Applications. Mater. Des. 2017, 119, 446–455; https://doi.org/10.1016/j.matdes.2017.01.095.Search in Google Scholar
98. Younis, A.; Chu, D.; Li, S. Cerium Oxide Nanostructures and Their Applications. Funct. Nanomater. 2016, 3, 53–68.10.5772/65937Search in Google Scholar
99. Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26.10.2174/1871526518666180628124323Search in Google Scholar PubMed
100. Priyadarshini, B.; Ramya, S.; Shinyjoy, E.; Kavitha, L.; Gopi, D.; Vijayalakshmi, U. Structural, Morphological and Biological Evaluations of Cerium Incorporated Hydroxyapatite Sol–Gel Coatings on Ti–6Al–4V for Orthopaedic Applications. J. Mater. Res. Technol. 2021, 12, 1319–1338; https://doi.org/10.1016/j.jmrt.2021.03.009.Search in Google Scholar
101. Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K. W.; Chu, P. K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678; https://doi.org/10.1002/advs.201700678.Search in Google Scholar PubMed PubMed Central
102. Gopi, D.; Ramya, S.; Rajeswari, D.; Karthikeyan, P.; Kavitha, L. Strontium, Cerium Co-substituted Hydroxyapatite Nanoparticles: Synthesis, Characterization, Antibacterial Activity towards Prokaryotic Strains and In Vitro Studies. Colloids Surf., A: Physicochem. Eng. 2014, 451, 172–180; https://doi.org/10.1016/j.colsurfa.2014.03.035.Search in Google Scholar
103. Lin, W.; Huang, Y. W.; Zhou, X. D.; Ma, Y. Toxicity of Cerium Oxide Nanoparticles in Human Lung Cancer Cells. Int. J. Toxicol. 2006, 25, 451–457; https://doi.org/10.1080/10915810600959543.Search in Google Scholar PubMed
104. Ashwini, D. Nano Medicine – An Emerging Field in Nanotechnology. Nano 2008, 18, 1–5.Search in Google Scholar
105. Wu, W.; He, Q. G.; Jiang, C. Z. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397; https://doi.org/10.1007/s11671-008-9174-9.Search in Google Scholar PubMed PubMed Central
106. Gupta, A. K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995; https://doi.org/10.1016/j.biomaterials.2004.10.012.Search in Google Scholar PubMed
107. Priyadarshini, B.; Stango, A. X.; Balasubramanian, M.; Vijayalakshmi, U. In Situ Fabrication of Cerium-Incorporated Hydroxyapatite/Magnetite Nanocomposite Coatings with Bone Regeneration and Osteosarcoma Potential. Nanoscale Adv. 2023, 5 (18), 5054–5076; https://doi.org/10.1039/d3na00235g.Search in Google Scholar PubMed PubMed Central
108. Wu, W.; Xiao, X. H.; Ren, F.; Zhang, S. F.; Jiang, C. Z. A Comparative Study of the Magnetic Behavior of Single and Tubular Clustered Magnetite Nanoparticles. J. Low Temp. Phys. 2012, 168, 306; https://doi.org/10.1007/s10909-012-0634-3.Search in Google Scholar
109. Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2010, 115, 390; https://doi.org/10.1021/jp1084982.Search in Google Scholar
110. Cheng, X. L.; Jiang, J. S.; Hu, M.; Mao, G. Y.; Liu, Z. W.; Zeng, Y.; Zhang, Q. H. Liquid-Liquid Interface-Assisted Solvothermal Synthesis of Durian-like α-Fe2O3 Hollow Spheres Constructed by Nano-Polyhedrons. CrystEngComm 2012, 14, 3056; https://doi.org/10.1039/c2ce06411a.Search in Google Scholar
111. Ratner, B. D.; Hoffman, A. S.; Schoen, F. J.; Lemons, J. E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Academic Press: San Diego, CA, USA, 2004.Search in Google Scholar
112. Xynos, I. D.; Edgar, A. J.; Buttery, L. D.; Hench, L. L.; Polak, J. M. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and Induce Insulin-like Growth Factor II mRNA Expression and Protein Synthesis. J. Biomed. Mater. Res. 2001, 55, 151; https://doi.org/10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d.10.1002/1097-4636(200105)55:2<151::AID-JBM1001>3.0.CO;2-DSearch in Google Scholar
113. Konduk, B. A.; Ahmet, H. U. On the Structural Changes and Mechanical Stability of Polymeric Hollow Fibers Used in Hemodialysis. World Congr. Med. Phys. Biomed. Eng. 2006, 5, 3268; https://doi.org/10.1007/978-3-540-36841-0_825.Search in Google Scholar
114. Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and Challenge for Magnesium Alloys as Biomaterials. Adv. Eng. Mater. 2008, 10, 3–14; https://doi.org/10.1002/adem.200800035.Search in Google Scholar
115. Chellappa, M.; Vijayalakshmi, U. Electrophoretic Deposition of Silica and Its Composite Coatings on Ti-6Al-4V, and It’s In Vitro Corrosion Behaviour for Biomedical Applications. Int. J. ChemTech Res. 2015, 7, 1259.Search in Google Scholar
116. Rogojan, R. Synthesis and Characterization of Alumina Nano-Powder Obtained by Sol-Gel Method. UPB Buletin Stiintific, Series B. Chem. Mater. Sci. 2011, 73, 67–76.Search in Google Scholar
117. Bellucci, D.; Valeria, C.; Antonella, S. A New Highly Bioactive Composite for Scaffold Applications: A Feasibility Study. Materials 2012, 4, 339–354.10.3390/ma4020339Search in Google Scholar
118. Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Polymeric Scaffolds in Tissue Engineering Application: A Review. Inter. J. Poly. Sci. 2011, 1–19; https://doi.org/10.1155/2011/290602.Search in Google Scholar
119. Patel, H.; Bonde, M.; Srinivasan, G. Biodegradable Polymer Scaffold for Tissue Engineering. Trends Biomater. Artif. Organs 2011, 25, 20–29.Search in Google Scholar
120. Zhang, Y.; Zhang, M. Synthesis and Characterization of Macroporous Chitosan/Calcium Phosphate Composite Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. 2001, 55, 304–312; https://doi.org/10.1002/1097-4636(20010605)55:3<304::aid-jbm1018>3.0.co;2-j.10.1002/1097-4636(20010605)55:3<304::AID-JBM1018>3.0.CO;2-JSearch in Google Scholar
121. Lee, L. Y.; Nam, S. H.; Im, S. Y.; Park, Y. J.; Lee, Y. M.; Seol, Y. J.; Chung, C. P.; Lee, S. J. Enhanced Bone Formation by Controlled Growth Factor Delivery from Chitosan-Based Biomaterials. J. Contr. Release 2002, 78, 187–197; https://doi.org/10.1016/s0168-3659(01)00498-9.Search in Google Scholar
122. Du, J.; Hu, X.; Su, Y.; Wei, T.; Jiao, Z.; Liu, T.; Wang, H.; Nie, Y.; Li, X.; Song, K. Gelatin/Sodium Alginate Hydrogel-Coated Decellularized Porcine Coronary Artery to Construct Bilayer Tissue Engineered Blood Vessels. Int. J. Biol. Macromol. 2022, 209, 2070–2083; https://doi.org/10.1016/j.ijbiomac.2022.04.188.Search in Google Scholar
123. Zulkiflee, I.; Fauzi, M. B. Gelatin-Polyvinyl Alcohol Film for Tissue Engineering: A Concise Review. Biomedicines 2021, 9, 979; https://doi.org/10.3390/biomedicines9080979.Search in Google Scholar
124. Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601; https://doi.org/10.1098/rsif.2006.0124.Search in Google Scholar
125. Singh, M. R.; Patel, S.; Singh, D. Natural Polymer-Based Hydrogels as Scaffolds for Tissue Engineering. Nanobiomater. Soft Tissue Eng. 2016, 1, 231–260. https://doi.org/10.1016/b978-0-323-42865-1.00009-xSearch in Google Scholar
126. Mondal, M. I. H. Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2019, ISBN 978-3-319-77829-7.Search in Google Scholar
127. Irastorza-Lorenzo, A.; Sanchez-Porras, D.; Ortiz-Arrabal, O.; De Frutos, M. J.; Esteban, E.; Fernández, J.; Janer, A.; Campos, A.; Campos, F.; Alaminos, M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 41923; https://doi.org/10.3390/ijms22041923.Search in Google Scholar PubMed PubMed Central
128. Salati, M. A.; Khazai, J.; Tahmuri, A. M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J. D.; Habibzadeh, S.; Seidi, F.; Saeb, M. R.; Mozafari, M. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150; https://doi.org/10.3390/polym12051150.Search in Google Scholar PubMed PubMed Central
129. Varoni, E.; Tschon, M.; Palazzo, B.; Nitti, P.; Martini, L.; Rimondini, L. Agarose Gel as Biomaterial or Scaffold for Implantation Surgery: Characterization; Histological and Histomorphometric Study on Soft Tissue Response. Connect. Tissue Res. 2012, 53, 548–554; https://doi.org/10.3109/03008207.2012.712583.Search in Google Scholar PubMed
130. Elvassore, N.; Bertucco, A.; Caliceti, P. Production of Insulin‐loaded Poly(Ethylene Glycol)/Poly(l‐lactide)(PEG/PLA) Nanoparticles by Gas Antisolvent Techniques. J. Pharmaceut. Sci. 2001, 90 (10), 1628–1636; https://doi.org/10.1002/jps.1113.Search in Google Scholar PubMed
131. Xu, X.; Xiao, L.; Xu, Y.; Zhuo, J.; Yang, X.; Li, L.; Xiao, N.; Tao, J.; Zhong, Q.; Li, Y.; Chen, Y.; Du, Z.; Luo, K. Vascularized Bone Regeneration Accelerated by 3D-Printed Nanosilicate-Functionalized Polycaprolactone Scaffold. Regen. Biomater. 2021, 8 (6), rbab061; https://doi.org/10.1093/rb/rbab061.Search in Google Scholar PubMed PubMed Central
132. Liu, J.; Lin, S.; Dang, J.; Wang, S.; Cheng, W.; Ran, Z.; Zhu, H.; Den, H.; Xiong, C.; Xu, W.; Huang, Z.; Xu, P.; Xu, H. Anticancer and Bone-Enhanced Nano-Hydroxyapatite/Gelatin/Polylactic Acid Fibrous Membrane with Dual Drug Delivery and Sequential Release for Osteosarcoma. Int. J. Biol. Macromol. 2023, 240, 124406; https://doi.org/10.1016/j.ijbiomac.2023.124406.Search in Google Scholar PubMed
133. Brennan, M.; Layrolle, P.; Mooney, D. J. Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration. Adv. Funct. Mater. 2020, 30 (37), 1909125; https://doi.org/10.1002/adfm.201909125.Search in Google Scholar PubMed PubMed Central
134. Yuan, Q.; Zhang, Y.; Chen, Q. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles: Potential Therapeutics as MSC Trophic Mediators in Regenerative Medicine. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 303, 1735–1742; https://doi.org/10.1002/ar.24186.Search in Google Scholar PubMed PubMed Central
135. Huang, H.; Liu, J.; Hao, H.; Chen, D.; Zhizhong, L.; Li, M.; Song, H.; Xiang, R.; Jiang, C.; Fu, X.; Han, W. Preferred M2 Polarization by ASC‐Based Hydrogel Accelerated Angiogenesis and Myogenesis in Volumetric Muscle Loss Rats. Stem Cell. Int. 2017, 1, 2896874; https://doi.org/10.1155/2017/2896874.Search in Google Scholar PubMed PubMed Central
136. Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhou, Y.; Wu, G. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. Appl. Mater. Interfaces. 2018, 10 (6), 5240–5254; https://doi.org/10.1021/acsami.7b17620.Search in Google Scholar PubMed
137. Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; Ning, L.; Shi, Y.; Fang, C.; Fan, S.; Lin, X. Desktop-Stereolithography 3D Printing of a Radially Oriented Extracellular Matrix/Mesenchymal Stem Cell Exosome Bioink for Osteochondral Defect Regeneration. Theranostics 2019, 9 (9), 2439; https://doi.org/10.7150/thno.31017.Search in Google Scholar PubMed PubMed Central
138. Cheng, W.; Xu, C.; Su, Y.; Shen, Y.; Yang, Q.; Zhao, Y.; Zhao, Y.; Liu, Y. Engineered Extracellular Vesicles: A Potential Treatment for Regeneration. iScience 2023; https://doi.org/10.1016/j.isci.2023.108282.Search in Google Scholar PubMed PubMed Central
139. Weinstein, A.; Gibbons, D.; Brown, S.; Ruff, W. Implant Retrieval: Material and Biological Analysis. In Proceedings of a Conference Held at the National Bureau of Standards, Gaithersburg, MD 20234, May 1–3; US Department of Commerce, National Bureau of Standards: Gaithersburg, MD, Vol. 13, 1980.Search in Google Scholar
140. Lemons, J. E.; Lucas, L. C. Properties of Biomaterials. Arthroplasty 1986, 1, 143–147; https://doi.org/10.1016/s0883-5403(86)80053-5.Search in Google Scholar PubMed
141. Teoh, S. H. Fatigue of Biomaterials: A Review. Int. J. Fatig. 2000, 22, 825–837; https://doi.org/10.1016/s0142-1123(00)00052-9.Search in Google Scholar
142. Alvarado, J. Biomechanics of Hip and Knee Prostheses. In Applications of Engineering Mechanics in Medicine; Woodhead Publisher: United Kingdom, 2003.Search in Google Scholar
143. Bahraminasab, M.; Jahan, A. Material Selection for Femoral Component of Total Knee Replacement Using Comprehensive VIKOR. Mater. Des. 2011, 32, 4471–4477; https://doi.org/10.1016/j.matdes.2011.03.046.Search in Google Scholar
144. Williams, D. F. On the Nature of Biomaterials. Biomaterials 2009, 30 (30), 5897–5909; https://doi.org/10.1016/j.biomaterials.2009.07.027.Search in Google Scholar PubMed
145. Nair, L. S.; Laurencin, C. T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32 (8–9), 762–798; https://doi.org/10.1016/j.progpolymsci.2007.05.017.Search in Google Scholar
146. Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells, Nanomed. Biotechnol. 2017, 45 (2), 185–192; https://doi.org/10.3109/21691401.2016.1146731.Search in Google Scholar PubMed
147. Burg, K. J.; Porter, S.; Kellam, J. F. Biomaterial Developments for Bone Tissue Engineering. Biomaterials 2000, 21 (23), 2347–2359; https://doi.org/10.1016/s0142-9612(00)00102-2.Search in Google Scholar PubMed
148. Agrawal, S.; Srivastava, R. Osteoinductive and Osteoconductive Biomaterials. In Racing for the Surface, Vol. 1, 2020; pp. 355–395.10.1007/978-3-030-34471-9_15Search in Google Scholar
149. Liu, Y.; Lim, J.; Teoh, S. H. Development of Clinically Relevant Scaffolds for Vascularised Bone Tissue Engineering. Biotechnol. Adv. 2013, 31 (5), 688–705; https://doi.org/10.1016/j.biotechadv.2012.10.003.Search in Google Scholar PubMed
150. Kohn, J. New Approaches to Biomaterials Design. Nat. Mater. 2004, 3 (11), 745–747; https://doi.org/10.1038/nmat1249.Search in Google Scholar PubMed
151. Yang, S.; Leong, K. F.; Du, Z.; Chua, C. K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8 (1), 1; https://doi.org/10.1089/107632702753503009.Search in Google Scholar PubMed
152. Peters, F.; Groisman, D.; Davids, R.; Hänel, T.; Dürr, H.; Klein, M. Comparative Study of Patient Individual Implants from β‐tricalcium Phosphate Made by Different Techniques Based on CT Data. Mater. Sci. Eng. Technol. 2006, 37 (6), 457–461; https://doi.org/10.1002/mawe.200600019.Search in Google Scholar
153. An, Y. H.; Draughn, R. A. Mechanical Testing of Bone and the Bone-Implant Interface; CRC Press: Boca Raton, 1999.10.1201/9781420073560Search in Google Scholar
154. Roohani-Esfahani, S. I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D Printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468; https://doi.org/10.1038/srep19468.Search in Google Scholar PubMed PubMed Central
155. Laptev, A.; Vyal, O.; Bram, M.; Buchkremer, H. P.; Stöver, D. Green Strength of Powder Compacts provided for Production of Highly Porous Titanium Parts. Powder Metall. 2005, 48, 358–364; https://doi.org/10.1179/174329005X73838.Search in Google Scholar
156. Wally, Z. J.; Van Grunsven, W.; Claeyssens, F.; Goodall, R.; Reilly, G. C. Porous Titanium for Dental Implant Applications. Metals 2015, 5, 1902–1920; https://doi.org/10.3390/met5041902.Search in Google Scholar
157. Zhang, Z.; Fan, Y.; Dunne, N.; Li, X. Effect of Microporosity on Scaffolds for Bone Tissue Engineering. Regen. Biomater. 2018, 5, 115–124; https://doi.org/10.1093/rb/rby001.Search in Google Scholar PubMed PubMed Central
158. Dhiman, S.; Sidhu, S. S.; Bains, P. S.; Bahraminasab, M. Mechanobiological Assessment of Ti-6Al-4V Fabricated via Selective Laser Melting Technique: a Review. Rapid Prototyp. J. 2019, 25 (7), 1266–1284; https://doi.org/10.1108/rpj-03-2019-0057.Search in Google Scholar
159. Williams, D. F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 20, 2941–2953; https://doi.org/10.1016/j.biomaterials.2008.04.023.Search in Google Scholar PubMed
160. Viceconti, M.; Muccini, R.; Bernakiewicz, M.; Baleani, M.; Cristofolini, L. Large-Sliding Contact Elements Accurately Predict Levels of Bone-Implant Micromotion Relevant to Osseointegration. J. Biomech. 2000, 33, 1611–1618; https://doi.org/10.1016/s0021-9290(00)00140-8.Search in Google Scholar PubMed
161. Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial Surface Design of Biomedical Titanium Materials for Orthopedic Applications. J. Mater. Sci. Technol. 2021, 78, 51–67; https://doi.org/10.1016/j.jmst.2020.10.066.Search in Google Scholar
162. Nielsen, K. Corrosion of Metallic Implants. Br. Corrosion J. 1987, 22, 272–278; https://doi.org/10.1179/000705987798271352.Search in Google Scholar
163. Tengvall, P.; Lunstrom, I. Physico-chemical Considerations of Titanium as a Biomaterial. Clin. Mater. 1992, 9, 115–134; https://doi.org/10.1016/0267-6605(92)90056-y.Search in Google Scholar PubMed
164. Singh, R.; Kurella, A.; Dahotre, N. B. Laser Surface Modification of Ti-6Al-4V: Wear and Corrosion Characterization in Simulated Biofluid. J. Biomater. Appl. 2006, 1, 49; https://doi.org/10.1177/0885328206055998.Search in Google Scholar PubMed
165. Kruger, J. Fundamental Aspects of the Corrosion of Metallic Implants. Am. Soc. Test. Mater. 1979, 1, 107; https://doi.org/10.1520/stp35940s.Search in Google Scholar
166. Kamachi, M. U.; Dayal, R. K.; Venkadesan, S.; Gnanamoorthy, J. B. Influence of Titanium Addition on Pitting, Crevice Corrosion and Intergranular Corrosion Resistances of Type 316 Stainless Steels. Met. Mater. Int. 1996, 8, 139–146.Search in Google Scholar
167. Kirkland, N. T.; Lespagnol, J.; Birbilis, N.; Staiger, M. P. A Survey of Bio-corrosion Rates of Magnesium Alloys. Corrosion Sci. 2010, 52, 287–291; https://doi.org/10.1016/j.corsci.2009.09.033.Search in Google Scholar
168. Fontana, M. G. Corrosion Engineering; Tata McGraw-Hill Publishing Company Ltd: New Delhi, Vol. 1, 2005; p 16.Search in Google Scholar
169. Mudali, U. K.; Sridhar, T. M.; Baldev, R. Corrosion of Bio Implants. Sadhana 2003, 28, 601–637; https://doi.org/10.1007/bf02706450.Search in Google Scholar
170. Bates, J. B. Cathodic Protection to Prevent Crevice Corrosion of Stainless Steels in Halide Media. Corrosion 1973, 29, 28–32; https://doi.org/10.5006/0010-9312-29.1.28.Search in Google Scholar
171. Ciszewski, A.; Baraniak, M.; Urbanek, B. M. Corrosion by Galvanic Coupling between Amalgam and Different Chromium-Based Alloys. Dent. Mater. 2007, 23, 1256–1261; https://doi.org/10.1016/j.dental.2006.11.006.Search in Google Scholar PubMed
172. Hamoon, Z.; Mohammad, E.; Hamid, R. S. Galvanic Corrosion Behavior of Dental Alloys. Environm. Ind. Corrosion 2012, 7, 157–168.Search in Google Scholar
173. Hu, J.; Zhano, Z. J.; Li, L. X. Corrosion Fatigue Resistances of Surgical Implant Stainless Steels and Titanium Alloy. Corrosion Sci. 1993, 35, 587–597; https://doi.org/10.1016/0010-938x(93)90193-k.Search in Google Scholar
174. Mudali, U. K.; Shankar, P.; Ningshen, S.; Dayal, R. K.; Khatak, H. S.; Raj, B. On the Pitting Corrosion Resistance of Nitrogen Alloyed Cold Worked Austenitic Stainless Steels. Corrosion Sci. 2002, 44, 2183–2198.10.1016/S0010-938X(02)00035-5Search in Google Scholar
175. Dearnley, P. A. Brief Review of Test Methodologies for Surface-Engineered Biomedical Implant Alloys. Surf. Coating. Technol. 2005, 98, 483–490.10.1016/j.surfcoat.2004.10.067Search in Google Scholar
176. Gayakwad, N. An Overview of Corrosion and Smart Healing Coatings. In Encapsulated Corrosion Inhibitors for Eco-benign Smart Coatings, Vol. 1, 2024, pp 1–6.10.1201/9781032677255-1Search in Google Scholar
177. Tanwer, S.; Shukla, S. K. Recent Advances in the Applicability of Drugs as Corrosion Inhibitor on Metal Surface: A Review. Curr. Res. Green Sustain. 2022, 5, 100227; https://doi.org/10.1016/j.crgsc.2021.100227.Search in Google Scholar
178. Ige, O. O.; Umoru, L. E.; Adeoye, M. O.; Adetunji, A. R.; Olorunniwo, O. E.; Akomolafe, I. I. Monitoring, Control and Prevention Practices of Biomaterials Corrosion – An Overview. Trends Biomater. Artif. Organs 2009, 23 (2), 93–104.Search in Google Scholar
179. Yu, J. Corrosion Fatigue Resistance of Surgical Implants Steels and Titanium Alloy. Corrosion Sci. 1993, 35, 587–589.10.1016/0010-938X(93)90193-KSearch in Google Scholar
180. Singh, R.; Dahotre, N. B. Corrosion Degradation and Prevention by Surface Modification of Bio Metallic Materials. J. Mater. Sci. Mater. Med. 2001, 18, 725–751; https://doi.org/10.1007/s10856-006-0016-y.Search in Google Scholar PubMed
181. Virtanen, S.; Milošev, I.; Gomez-Barrena, E.; Trebše, R.; Salo, J.; Konttinen, Y. Special Modes of Corrosion under Physiological and Simulated Physiological Conditions. Acta Biomater. 2008, 4 (3), 468–476; https://doi.org/10.1016/j.actbio.2007.12.003.Search in Google Scholar PubMed
182. Rahul, B.; Shaily, B.; Brajendra, M.; David, L. O. Corrosion in Titanium Dental Implants/Prostheses–A Review. Trends Biomater. Artif. Organs 2011, 25, 34–46.Search in Google Scholar
183. Lu, L.; Zhou, X.; Mohanty, P. In Vitro Immersion Behavior of Cold Sprayed Hydroxyapatite/Titanium Composite Coatings. J. Biosci. Med. 2014, 2, 10–16; https://doi.org/10.4236/jbm.2014.22002.Search in Google Scholar
184. Ige, O. O.; Umoru, L. E.; Adeoye, M. O.; Adetunji, A. R.; Olorunniwo, O. E.; Akomolafe, I. I. Monitoring, Control and Prevention Practices of Biomaterials Corrosion – An Overview. Trends Biomater. Artif. Organs 2009, 23, 93–104.Search in Google Scholar
185. Ukai, H.; Murakami, K. Surface Characterization of Calcium Implanted Titanium for Biomaterials. In The 2nd Biomaterials Interface Topical Conference, October 24−25, Denver, Colorado, USA; 1994.Search in Google Scholar
186. Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants – A Review. Prog. Mater. Sci. 2009, 54, 397–425; https://doi.org/10.1016/j.pmatsci.2008.06.004.Search in Google Scholar
187. Dohan Ehrenfest, D. M.; Coelho, P. G.; Kang, B.; Albrektsson, T. Classification of Osseointegrated Implant Surfaces: Materials, Chemistry and Topography. Trends Biotechnol. 2010, 28, 198–206; https://doi.org/10.1016/j.tibtech.2009.12.003.Search in Google Scholar PubMed
188. Ruggero, B.; Jeroen, V. D.; Sander, L.; John, J. Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces. Coatings 2012, 2, 95–119; https://doi.org/10.3390/coatings2030095.Search in Google Scholar
189. Gross, K. A.; Babovic, M. Influence of Abrasion on the Surface Characteristics of Thermally Sprayed Hydroxyapatite Coatings. Biomaterials 2002, 23, 4731–4737; https://doi.org/10.1016/s0142-9612(02)00222-3.Search in Google Scholar PubMed
190. Fauchais, P.; Armelle, V. Thermal Sprayed Coatings Used Against Corrosion and Corrosive Wear; Intech publisher: Croatia, 2012.10.5772/34448Search in Google Scholar
191. Young, E. J.; Mateeva, E.; Moore, J. J.; Mishra, B.; Loch, M. Low Pressure Plasma Spray Coatings. Thin Solid Films 2000, 377, 788–792; https://doi.org/10.1016/s0040-6090(00)01452-8.Search in Google Scholar
192. Zhao, G. L.; Wen, G.; Song, Y.; Wu, K. Near Surface Martensitic Transformation and Recrystallization in a Ti-24Nb-4Zr-7.9Sn Alloy Substrate after Application of a HA Coating by Plasma Spraying. Mater. Sci. Eng. C 2011, 31, 106–113; https://doi.org/10.1016/j.msec.2010.08.003.Search in Google Scholar
193. Freidberg, J. P. Plasma Physics and Fusion Energy; Cambridge University Press: Cambridge, UK, 2007.10.1017/CBO9780511755705Search in Google Scholar
194. Herman, H. Plasma-Sprayed Coatings. Sci. Am. 1988, 259 (3), 112–117; https://doi.org/10.1038/scientificamerican0988-112.Search in Google Scholar
195. De Groot, K.; Geesink, R.; Klein, C. P.; Serekian, P. Plasma Sprayed Coatings of Hydroxylapatite. J. Biomed. Mater. Res. 1987, 21 (12), 1375–1381; https://doi.org/10.1002/jbm.820211203.Search in Google Scholar PubMed
196. Quek, C. H.; Khor, K. A.; Cheang, P. Influence of Processing Parameters in the Plasma Spraying of hydroxyapatite/Ti–6Al–4V Composite Coatings. J. Mater. Process. Technol. 1999, 89, 550; https://doi.org/10.1016/s0924-0136(99)00062-x.Search in Google Scholar
197. Nelea, V.; Morosanu, C.; Bercu, M.; Mihailescu, I. N. Interfacial Titanium Oxide between Hydroxyapatite and TiAlFe Substrate. J. Mater. Sci. Mater. Med. 2007, 18, 2347–2354; https://doi.org/10.1007/s10856-007-3135-1.Search in Google Scholar PubMed
198. Surmenev, R. A. Review of Plasma-Assisted Methods for Calcium Phosphate-Based Coatings Fabrication. Surf. Coating. Technol. 2012, 206, 2035–2056; https://doi.org/10.1016/j.surfcoat.2011.11.002.Search in Google Scholar
199. Cotell, C. M. Pulsed Laser Deposition and Processing of Biocompatible Hydroxyapatite Thin Films. Appl. Surf. Sci. 1993, 69, 140–148; https://doi.org/10.1016/0169-4332(93)90495-w.Search in Google Scholar
200. Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11 (7), 811; https://doi.org/10.3390/coatings11070811.Search in Google Scholar
201. Choi, J. M.; Kim, H. E.; S Lee, I. Ion-Beam-Assisted Deposition (IBAD) of Hydroxyapatite Coating Layer on Ti-Based Metal Substrate. Biomaterials 2000, 21, 469–473; https://doi.org/10.1016/s0142-9612(99)00186-6.Search in Google Scholar PubMed
202. Nijhuis, A. W. G.; Leeuwenburgh, S. C. G.; Jansen, J. A. Wet-Chemical Deposition of Functional Coatings for Bone Implantology. Macromol. Biosci. 2010, 10, 1316–1329; https://doi.org/10.1002/mabi.201000142.Search in Google Scholar PubMed
203. Kokubo, T.; Miyaji, F.; Kim, H. M.; Nakamura, T. Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129; https://doi.org/10.1111/j.1151-2916.1996.tb08561.x.Search in Google Scholar
204. Habibovic, P.; Barrere, F.; Vanblitterswijk, C. A.; Degroot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2002, 85, 517–522; https://doi.org/10.1111/j.1151-2916.2002.tb00126.x.Search in Google Scholar
205. Vijayalakshmi, U.; Prabakaran, K.; Rajeswari, S. Preparation and Characterization of Sol–Gel Hydroxyapatite and its Electrochemical Evaluation for Biomedical Applications. J. Biomed. Mater. Res. A. 2008, 87A, 739–749; https://doi.org/10.1002/jbm.a.31773.Search in Google Scholar PubMed
206. Li, F.; Feng, Q. L.; Cui, F. Z.; Li, H. D.; Schubert, H. A Simple Biomimetic Method for Calcium Phosphate Coating. Surf. Coating. Technol. 2002, 154, 88–93; https://doi.org/10.1016/s0257-8972(01)01710-8.Search in Google Scholar
207. Kim, H. W.; Koh, Y. H.; Li, L. H.; Lee, S.; Kim, H. E. Hydroxyapatite Coating on Titanium Substrate with Titania Buffer Layer Processed by Sol–Gel Method. Biomaterials 2004, 25, 2533–2538; https://doi.org/10.1016/j.biomaterials.2003.09.041.Search in Google Scholar PubMed
208. Gan, L.; Wang, J. Calcium Phosphate Sol–Gel-Derived Thin Films on Porous-Surfaced Implants for Enhanced Osteoconductivity. Part I: Synthesis and Characterization. Biomaterials, 2004, 25, 5303–5312.10.1016/j.biomaterials.2003.12.038Search in Google Scholar PubMed
209. Duan, K.; Fan, Y.; Wang, R. Electrochemical Deposition and Patterning of Calcium Phosphate Bioceramic Coating. Ceram. Trans. 2003, 147, 53–61; https://doi.org/10.1002/9781118406069.ch6.Search in Google Scholar
210. Besra, L.; Liu, M. A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52 (1), 1–61; https://doi.org/10.1016/j.pmatsci.2006.07.001.Search in Google Scholar
211. Heavens, S. N. Electrophoretic Deposition as a Processing Route for Ceramics. Adv. Ceram. Prog. 1990, 1, 255–283.Search in Google Scholar
212. Lopez-Heredia, M. A.; Weiss, P.; Layrolle, P. An Electrodeposition Method of Calcium Phosphate Coatings on Titanium Alloy. J. Mater. Sci. Mater. Med. 2007, 18, 381–390; https://doi.org/10.1007/s10856-006-0703-8.Search in Google Scholar PubMed
213. Garbuz, D. S.; Hu, Y.; Kim, W. Y.; Duan, K.; Masri, B. A.; Oxland, T. R.; Burt, H.; Wang, R.; Duncan, C. P. Enhanced Gap Filling and Osteoconduction Associated with Alendronate-Calcium Phosphate Coated Porous Tantalum. J. Bone. Joint. Surg. Am. 2008, 90, 1090–1100; https://doi.org/10.2106/jbjs.g.00415.Search in Google Scholar PubMed
214. Adamek, G.; Jakubowicz, J. Mechano Electrochemical Synthesis and Properties of Porous Nano-Ti-6Al-4V Alloy with Hydroxyapatite Layer for Biomedical Applications. Electrochem. Commun. 2010, 12, 653–656; https://doi.org/10.1016/j.elecom.2010.02.023.Search in Google Scholar
215. Jaworek, A. Electrospray Droplet Sources for Thin Film Deposition. J. Mater. Sci. 2007, 42, 266–297; https://doi.org/10.1007/s10853-006-0842-9.Search in Google Scholar
216. Leeuwenburgh, S. C. G.; Wolke, J. G. C.; Siebers, M. C.; Schoonman, J.; Jansen, J. A. In Vitro and In Vivo Reactivity of Porous, Electrosprayed Calcium Phosphate Coatings. Biomaterials 2006, 27, 3368–3378; https://doi.org/10.1016/j.biomaterials.2006.01.052.Search in Google Scholar
217. Rho, J. Y.; Tsui, T. Y.; Pharr, G. M. Elastic Properties of Human Cortical and Trabecular Lamellar Bone Measured by Nanoindentation. Biomaterials 1997, 18, 1325–1330; https://doi.org/10.1016/s0142-9612(97)00073-2.Search in Google Scholar
218. Rho, J. Y.; Roy, M. E.; Tsui, T. Y.; Pharr, G. M. Elastic Properties of Microstructural Components of Human Bone Tissue as Measured by Nanoindentation. J. Biomed. Mater. Res. 1999, 45, 48–54; https://doi.org/10.1002/(sici)1097-4636(199904)45:1<48::aid-jbm7>3.0.co;2-5.10.1002/(SICI)1097-4636(199904)45:1<48::AID-JBM7>3.0.CO;2-5Search in Google Scholar
219. Hengsberger, S.; Kulik, A.; Zysset, P. Nanoindentation Discriminates the Elastic Properties of Individual Human Bone Lamellae under Dry and Physiological Conditions. Bone 2002, 30, 178–184; https://doi.org/10.1016/s8756-3282(01)00624-x.Search in Google Scholar
220. Huiskes, R. Failed Innovation in Total Hip Replacement: Diagnosis and Proposals for a Cure. Acta Orthop. Scand. 1993, 64, 699–716; https://doi.org/10.3109/17453679308994602.Search in Google Scholar
221. Dalton, J. E.; Cook, S. D.; Thomas, K. A.; Kay, J. F. The Effect of Operative Fit and Hydroxyapatite Coating on the Mechanical and Biological Response to Porous Implants. J. Bone. Joint. Surg. Am. 1995, 77, 97–110; https://doi.org/10.2106/00004623-199501000-00012.Search in Google Scholar
222. Brånemark, P. I.; Zarb, G. A.; Albrektsson, T.; Rosen, H. M. Tissue-integrated Prostheses: Osseointegration in Clinical Dentistry. Plast. Reconstr. Surg. 1985, 77, 496–497; https://doi.org/10.1097/00006534-198603000-00037.Search in Google Scholar
223. Areva, S.; Paldan, H.; Peltola, T.; Närhi, T.; Jokinen, M.; Linden, M. Use of Sol–Gel‐derived Titania Coating for Direct Soft Tissue Attachment. J. Biomed. Mater. Res. A. 2004, 70, 169–178; https://doi.org/10.1002/jbm.a.20120.Search in Google Scholar
224. Kasemo, B.; Lausmaa, J. Material-Tissue Interfaces: The Role of Surface Properties and Processes. Environ. Health Perspect. 1994, 102, 41; https://doi.org/10.2307/3432057.Search in Google Scholar
225. Michaels, G. C.; Carr, A. B.; Larsen, P. E. Effect of Prosthetic Superstructure Accuracy on the Osteointegrated Implant Bone Interface. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 198–205; https://doi.org/10.1016/s1079-2104(97)90006-8.Search in Google Scholar
226. Ryhanen, J. Biocompatibility Evaluation of Nickel-Titanium Shape Memory Metal Alloy. Acta Univ. Ouluensis D 1999, 518, 117.Search in Google Scholar
227. Urban, R. M.; Jacobs, J. J.; Sumner, D. R.; Peters, C. L.; Voss, F. R.; Galante, J. O. The Bone-Implant Interface of Femoral Stems with Non-circumferential Porous Coating. A Study of Specimens Retrieved at Autopsy. J. Bone Joint Surg. Am. 1996, 78, 1068–1081; https://doi.org/10.2106/00004623-199607000-00012.Search in Google Scholar PubMed
228. Frank, C.; Woo, S. L. Y.; Andriacchi, T.; Brond, R.; Oakes, B.; Dahers, L.; Dehave, K.; Lewis, J.; Sabiston, T. In Injury and Repair of the Musculoskeletal Soft Tissues; S. L.-Y. Woo; Buckwalter, J., Eds.; Vol. 45, 1988. J. Am. Acad. Orthop. Surg.Search in Google Scholar
229. Glasgold, I.; Silver, F. H. Cartilage Autografts. In Applications of Biomaterials in Facial Plastic Surgery; CRC Press: Boca Raton, FI, 1991.Search in Google Scholar
230. Sujata, V. B. Biomaterials; Narosa Publishers: New Delhi, 2002; pp 112–129.Search in Google Scholar
231. Glasgold, A.; Glasgold, M. J. Cartilage Autografts. In Applications of Biomaterials in Facial Plastic Surgery; Glasgold, A.; Silver, F. H., Eds.; CRC Press: Boca Raton, 1991, Chapter 8.10.1097/00006534-199209000-00033Search in Google Scholar
232. Donald, P. J.; Brodie, H. A. Cartilage Autografts. In Applications of Biomaterials in Facial Plastic Surgery; Glasgold, A. I.; Silver, E. H., Eds.; CRC Press: Boca, Raton, Fl, 1991, Chapter 9.Search in Google Scholar
233. Churukian, M. M.; Cohen, A.; Kamer, F. M.; Lefkoff, L.; Palmer, F. R.; Ross, C. A. Injectable Collagen: A Ten-Year Experience. In Applications of Biomaterials in Facial Plastic Surgery; Glasgold, A. I.; Silver, F. H., Eds.; CRC Press: Boca Raton, Fl, 1991, Ch. 15.Search in Google Scholar
234. Wadhwa, S. N.; Shenoy, S. P. Biomaterials in Urological Practice in Biomechanics; Sahay, K. B., Saxena, R. K., Eds.; Wiley-Eastern, India, 1989.Search in Google Scholar
235. Warren, J. W. Infections Associated with Urological Devices. In Infections Associated with Prosthetic Devices; Sugarman, B.; Young, E. J., Eds.; CRC Press: Boca Raton, 1984; pp 23–56.Search in Google Scholar
236. Weng, Y.; Chen, J.; Tu, Q.; Li, Q.; Maitz, M. F.; Huang, N. Biomimetic Modification of Metallic Cardiovascular Biomaterials: From Function Mimicking to Endothelialization In Vivo. Interface Focus 2012, 2, 356–365; https://doi.org/10.1098/rsfs.2011.0126.Search in Google Scholar PubMed PubMed Central
237. Lee, W. M.; Harston, P. Cardiovascular Implant State of the Art and Potential for Bioceramics. In Use of Ceramics in Surgical Implants; Hulbert, S. F.; Young, F. A., Eds.; Grodon and Breach: N.Y., Vol. 8, 1978; pp 127.Search in Google Scholar
238. Andukuri, A. A Bioinspired Multifunctional Nanomatrix for Cardiovascular Applications; Diss, The University of Alabama at Birmingham, 2013.Search in Google Scholar
239. Peter, Z.; Johan, B.; Paul, H.; Deon, B. Prosthetic Heart Valves: Catering for the Few. Biomaterials 2008, 29, 385–406; https://doi.org/10.1016/j.biomaterials.2007.09.033.Search in Google Scholar PubMed
240. Waller, B. F.; Gering, L. E.; Branyas, N. A.; Slack, J. D. Anatomy, Histology, and Pathology of the Cardiac Conduction System Part V. Clin. Cardiol. 1993, 16, 565–569; https://doi.org/10.1002/clc.4960160710.Search in Google Scholar PubMed
241. Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of Fluoride Concentration and pH on Corrosion Behavior of Titanium for Dental Use. J. Dent. Res. 1999, 78, 1568–1572; https://doi.org/10.1177/00220345990780091201.Search in Google Scholar PubMed
242. Khan, S. A.; Kumar, A.; Varshney, M.; Trikha, V.; Yadav, C. Dextrose Prolotherapy for Recalcitrant Coccygodynia. J. Orthop. Surg. 2008, 16, 27–29; https://doi.org/10.1177/230949900801600107.Search in Google Scholar PubMed
243. Gutmann, H.; Török, M.; Fricker, G.; Huwyler, J.; Beglinger, C.; Drewe, J. Modulation of Multidrug Resistance Protein Expression in Porcine Brain Capillary Endothelial Cells In Vitro. Drug Metabol. Dispos. 1999, 27 (8), 937–941.10.1016/S0090-9556(24)15245-2Search in Google Scholar
244. Dunn, E. J.; Polk, A.; Scarrett, D. J.; Olivier, G.; Lall, S.; Goosen, M. F. Vaccines in Aquaculture: the Search for an Efficient Delivery System. Aquacult. Eng. 1990, 9 (1), 23–32; https://doi.org/10.1016/0144-8609(90)90009-o.Search in Google Scholar
245. Hallan, G.; Lie, S. A.; Furnes, O.; Engesaeter, L. B.; Vollset, S. E.; Havelin, L. I. Medium and Long-Term Performance of 11,516 Uncemented Primary Femoral Stems from the Norwegian Arthroplasty Register. J. Bone Joint Surg. 2007, 89B, 1574–1580; https://doi.org/10.1302/0301-620x.89b12.18969.Search in Google Scholar
246. Cranin, A. N. Oral Implantology; Thomas: Springfield, Illinois, 1970; pp 74–79.Search in Google Scholar
247. Chaturvedi, T. P. Allergy Related to Dental Implant and its Clinical Significance. Clin. Cosmet. Invest. Dent. 2013, 57–61; https://doi.org/10.2147/ccide.s35170.Search in Google Scholar
248. Stanley, H. R.; Hench, L. L.; Bennet, C. G.; Chellemi, S. J.; J King, C.; Going, R. E.; Ingersoll, N. J.; Ethridge, E. C.; Kreutiziger, K. L.; Loeb, L.; Clark, A. E. The Implantation of Natural Tooth Form Bioglass in Baboons Long-Term Results. Int. I. Oral Implants 1982, 2, 26–36.Search in Google Scholar
249. Boyne, R.; Rattansi, A. The Theory and Politics of Postmodernism: By Way of an Introduction. In Postmodernism and Society (Communications and Culture); Palgrave: London, 1990; pp 1–45.10.1007/978-1-349-20843-2_1Search in Google Scholar
250. Basavaraj, K. H.; Johnsy, G.; Navya, M. A.; Rashmi, R. Biopolymers as Transdermal Drug Delivery Systems in Dermatology Therapy. Crit. Rev. Ther. Drug Carrier Syst. 2010, 27 (2); https://doi.org/10.1615/critrevtherdrugcarriersyst.v27.i2.20.Search in Google Scholar PubMed
251. Joseph, J.; Parameswaran, R.; Gopalakrishna Panicker, U. Recent Advancements in Blended and Reinforced Polymeric Systems as Bioscaffolds. Int. J. Polym. Mater. Polym. Biomater. 2023, 72 (11), 834–855; https://doi.org/10.1080/00914037.2022.2066666.Search in Google Scholar
252. Pereira, T. Biomaterials and Stem Cell Therapies for Injuries Associated to Skeletal Muscular Tissues; Intech publisher: Croatia, 2013.10.5772/53335Search in Google Scholar
253. Vincent, F. M.; Richard, T. L. Biomaterials to Enhance Stem Cell Function in the Heart. Circ. Res. 2011, 109, 907–922.10.1161/CIRCRESAHA.111.249052Search in Google Scholar PubMed
254. Furth, M. E.; Atala, A.; Van Dyke, M. E. Smart Biomaterials Design for Tissue Engineering and Regenerative Medicine. Biomaterials 2007, 28 (34), 5068–5073; https://doi.org/10.1016/j.biomaterials.2007.07.042.Search in Google Scholar PubMed
255. Montoya, C.; Du, Y.; Gianforcaro, A. L.; Orrego, S.; Yang, M.; Lelkes, P. I. On the Road to Smart Biomaterials for Bone Research: Definitions, Concepts, Advances, and Outlook. Bone Res. 2021, 9 (1), 12; https://doi.org/10.1038/s41413-020-00131-z.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

